Visible light-driven heterogeneous semiconductor CdS photocatalyzed defluorinative acylation reaction for the synthesis of γ,γ-difluoroallylic ketones
Kashif
Hussain
ab,
Fukai
Xie
a and
Wen
Dai
 *a
*a
aGreen Oxidation & New Materials Synthesis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
bUniversity of Chinese Academy of Sciences, Beijing, 101408, China
First published on 16th December 2025
Abstract
We describe an efficient and feasible visible-light-driven photocatalytic protocol for the synthesis of γ,γ-difluoroallylic ketones from α-trifluoromethyl alkenes. A recyclable and photostable heterogeneous CdS photocatalyst is employed for the first time to promote this transformation via a radical–polar crossover pathway using readily available aliphatic and aromatic aldehydes as acyl radical precursors. The method proceeds under mild conditions, exhibits a broad substrate scope, and demonstrates excellent tolerance to various functional groups. Notably, the protocol enables the late-stage functionalization of biologically active molecules, offering a sustainable and metal-free approach to access valuable difluoroalkylated carbonyl compounds.
Green foundation1. This work employs visible light as a renewable energy source to drive a CdS nanomaterial-mediated heterogeneous semiconductor photocatalytic transformation under mild conditions. The reaction proceeds without the need for external oxidant, toxic reagents, or pre-functionalized substrates, thereby achieving high atom economy, reduced waste generation, and improved efficiency.2. This study presents an efficient and sustainable organic synthesis by integrating the use of recyclable and environmentally friendly photocatalysts as an alternative to traditional photocatalytic systems. The use of earth-abundant, recyclable, and photostable CdS nanomaterial-based photocatalysts eliminates the need for costly, toxic, and complex catalytic systems, enabling a greener and more practical route for organic synthesis. 3. This work contributes to developing a bridge between organic synthesis and heterogeneous semiconductors, thereby providing a sustainable approach to organic synthesis. The mild reaction conditions enable the functionalization of diverse and biologically active molecules, highlighting the potential for scalable and industrially applicable green transformations. |
Introduction
Organofluorine compounds have garnered substantial attention across agrochemistry, medicinal chemistry, and materials science, owing to their unique physicochemical properties.1–3 Approximately 30% of agrochemicals and 20% of modern pharmaceutical drugs contain at least one or more fluorine atoms. Generally, fluorine substitution enhances the therapeutic efficiency of biologically active drugs by improving their metabolic stability, absorbability, and lipophilicity, thereby driving a rapid expansion of organofluorine compounds in diverse biological applications.4–6 Among organofluorine compounds, gem-difluoroalkenes represent intriguing structural motifs that have been widely used in medicinal chemistry.7–9 This unit acts as a bioisostere of carbonyl groups, enhancing their bioactivity and biological performance,10–12i.e., artemisinin (Fig. 1A). Over the years, various strategies have been reported for the synthesis of gem-difluoroalkenes, including difluoroolefination of carbonyl and diazo compounds, as well as reductive defluorination of the trifluoromethyl group (Fig. 1B).13,14 The utilization of α-trifluoromethyl alkenes as a cost-effective precursor for organofluorine compounds has been extensively studied. In this context, the methods are generally classified into two main approaches: (1) the addition of strong nucleophiles (such as Grignard reagents) to α-trifluoromethyl alkenes, followed by β-fluoride elimination via SN2′ reaction.15–18 (2) Reductive allylic defluorination of α-trifluoromethyl alkenes can be achieved using transition metal-based catalysts such as Ni, Pd, or Fe (Fig. 1C).19–24 Among the structurally diverse gem-difluoroalkenes, the γ,γ-difluoroallylic ketone moiety represents a significant and versatile building block in organic synthesis. Subsequently, these compounds exhibit distinctive reactivity in intramolecular SNV reactions and serve as valuable synthons for the construction of a variety of fluorinated molecules.25,26 However, only a few methods have been reported for their preparation. In 2016, the Zhou group reported a visible-light-driven synthesis of γ,γ-difluoroallylic ketones using an iridium-based photocatalyst. However, despite offering mild conditions, the substrate scope is limited to pre-synthesized aryl α-keto acids, which limits late-stage modification of bioactive molecules.27 In 2020, Wang reported a method to synthesize γ,γ-difluoroallylic ketones with aromatic benzoic acid.28 Notably, in 2024, Wang employed acyl oxime esters to access γ,γ-difluoroallylic ketones.29 In 2024, Sun and co-workers demonstrated the synthesis of γ,γ-difluoroallylic ketones from aryl thionic acids via a desulfurative and defluorinative alkylation of α-trifluoromethyl alkenes; however, the reaction is limited to aryl thionic acids.30 More recently, in 2024, the Yang group reported the synthesis of γ,γ-difluoroallylic ketones via selective β-scission of β-ketone alcohols in the presence of α-trifluoromethyl alkenes. The method is limited to β-ketone alcohol substrates and employs an iridium-based photocatalyst31 (Fig. 1).To date, most reported methods for synthesizing γ,γ-difluoroallylic ketones require a homogenous photocatalyst and pre-functionalized substrates, resulting in reduced atom economy, increased synthetic complexity, and limited suitability for late-stage modification of complex or bioactive molecules. Moreover, all these methods rely on precious metal photocatalysts, particularly iridium, which is very expensive and not economically viable for large-scale or commercial applications.
In our previous work, we explored the application of CdS nanomaterials as a heterogeneous photocatalyst for organo-borane reactions.32 Heterogeneous photocatalysts offer several significant advantages, including being highly economical, recyclable, less toxic, and sustainable, compared to traditional homogeneous photocatalysts that contain organic dyes and transition metals.32 Additionally, commercially available aldehydes can serve as a valuable source of acyl radicals. However, the conversion of an aldehyde into acyl radicals to synthesize γ,γ-difluoroallylic ketones is still unknown. Based on the CdS heterogeneous photocatalysis, which utilizes band energy theory, photoexcited electron–hole pairs facilitate reduction and oxidation reactions.33 We postulated that the potential of valence band maxima of CdS (≈1.7 V versus SHE) oxidizes the aldehyde into an acyl radical by dehydrogenation. Herein, we describe γ,γ-difluoroallylic ketones that could be synthesized from readily available aldehydes using a recyclable CdS nanostrips photocatalyst through a visible-light-driven radical–polar crossover addition–elimination approach.
Results and discussion
The XRD diffraction pattern confirmed the crystalline phase composition of the CdS nanostrips. As shown in Fig. 2A, the diffraction peaks are in good agreement with those of greenockite CdS (JCPDS card no. 41-1049), indicating the formation of the hexagonal phase.34 The characteristic peaks observed at 2θ values of 24.807° and 26.507° can be indexed to the (100) and (002) crystal planes of hexagonal CdS, respectively. Furthermore, no additional peaks were detected, confirming the high phase purity and crystallinity of the obtained product.33 The morphology and structure of the CdS nanostrips were examined using SEM and TEM, as shown in Fig. 2B and C. The images confirm that the nanostrips are uniformly distributed throughout the sample. In addition, the lattice fringes with a spacing of 0.336 nm observed in Fig. 2D correspond to the [002] plane of hexagonal CdS. The FFT pattern shows well-ordered bright spots with regular spacing, indicating the single-crystalline nature of the CdS nanostrips. XPS analysis was employed further to probe the surface chemical composition of the CdS nanostrips. The survey spectrum confirmed the presence of Cd, S, and O, with no additional peaks corresponding to any other elements (Fig. 2E), indicating the purity of the CdS nanostrips. The oxygen signal originates from molecular oxygen due to exposure to air during storage. The high-resolution XPS spectra of S 2p and Cd 3d are shown in Fig. 2F and G. The peaks appeared at 161.4 and 162.5 eV, corresponding to S 2p3/2 and S 2p1/2, respectively, confirming the presence of sulphur in the normal S2− state. Meanwhile, the peaks at 404.7 and 411.5 eV are attributed to Cd 3d5/2 and Cd 3d3/2, respectively, consistent with the presence of Cd2+. Fig. 2H depicts the XPS VB of CdS nanostrips.35 The BET surface area of the CdS nanostrips was measured to be 31.9 m2 g−1. EDS was used to visualise the elemental mapping and distribution of cadmium (Cd) and sulphur (S) in the CdS nanostrips (Fig. 2J–L).The DRS UV–visible spectrum (Fig. 2M) showed an absorption edge near 550 nm. The band gap energy of the CdS nanostrips was determined to be 2.37 eV from Tauc plots based on the UV–vis spectrum (Fig. 2N). To further evaluate the photoelectrochemical properties, including charge separation, transient photocurrent response (IT), and electrochemical impedance spectroscopy (EIS), a Nyquist plot was recorded (Fig. 2O and P). The CdS nanostrips exhibited strong photocurrent responses and small arc radii in EIS, indicating efficient charge separation and low resistance, which are beneficial for photocatalytic performance. The Mott–Schottky plot of CdS nanostrips (Fig. 2Q) revealed a flat-band potential of −1.35 eV (vs. Ag/AgCl, pH 7). The valence band (VB) potential was calculated by combining the flat-band potential with the band gap energy (1.7 eV), and this value was further confirmed by XPS-VB analysis, which yielded a value of 1.67 eV.
The best conditions for this reaction were found in the presence of α-trifluoromethyl alkene 1a (0.15 mmol, 1.0 equiv.), butyraldehyde 2a (0.2 mmol, 1.3 equiv.), NaOAc (0.075 mmol, 0.05 equiv.), and 15 mg CdS nanostrips in DMSO (1.0 mL) at ambient temperature (25 °C), the desired 3a was obtained in 96% yield (determined by 19F NMR) under irradiation of blue LEDs (Table 1, entry 1). The other types of photocatalysts were also screened for this reaction; however, TiO2 gave a comparatively low yield. In contrast, g-C3N4 did not afford the anticipated product (entries 2 and 3). The reaction proceeded in MeCN with a 39% yield (entry 4). Another base, such as Na2HPO4, yielded a moderate result (55%). The control experiment also indicated that blue light, CdS nanostrips, and an Ar atmosphere were essential for the reaction (entries 6–8; see the SI for detailed optimization experiments).
| Entry | Variation from the standard conditions | Yieldb (%) |
|---|---|---|
| a Standard conditions: 1a (0.15 mmol, 1 equiv.), 2a (0.2 mmol, 1.3 equiv.), CdS nanostrips (15 mg), NaOAc (0.075 mmol, 0.05 equiv.), DMSO (1.0 mL), 10 W blue LED (455–460 nm), Ar, r.t., 18 h. b Yield of crude product was determined by 19F NMR using trifluoromethylbenzene as an internal standard. c Isolated yield. | ||
| 1 | None | 96 (94)c |
| 2 | TiO2 instead of CdS nanostrips | 18 |
| 3 | g-C3N4 instead of CdS nanostrips | Trace |
| 4 | MeCN instead of DMSO | 39 |
| 5 | Na2HPO4 instead of NaOAc | 55 |
| 6 | Under air | Trace |
| 7 | Without CdS | n.r. |
| 8 | without blue LEDs | n.r. |
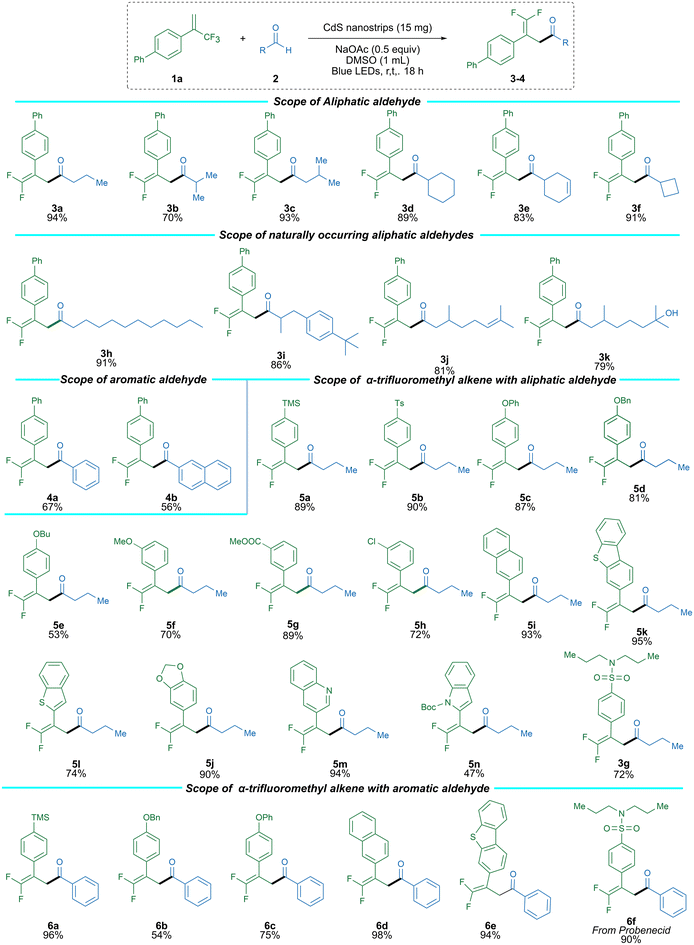
With the optimized reaction conditions in hand, we explored the substrate scope of various alkyl and aryl aldehydes for this reaction (Scheme 1). The linear alkyl aldehyde afforded an excellent yield (3a, 94%), and the β-substituted alkyl aldehyde also didn't show any considerable effect on the yield (3c, 93%). Still, the substituent at the α-position afforded a high yield (3b, 70%). Moreover, the cyclic alkyl aldehyde afforded high to excellent yields (3d–3f, 89–91%); nevertheless, the unsaturated cyclohexene afforded a high yield (3e, 83%). In addition, the drug molecule probenecid also transformed into product 3g with 72% yield. Moreover, to demonstrate the efficiency of this reaction system on the transformation of drug-like natural products, we employed this method on some naturally occurring aldehydes, i.e., Laurinaldehyde, Citronellal, Hydroxycitronellal, and Lilial. These natural products afforded desired products in good to excellent yields (3h–3k, 79–91%) (Scheme 1).
The aromatic aldehydes were also used to cover and expand the substrate scope for aryl aldehydes. The flat benzaldehyde (4a) and naphthaldehyde (4b) afforded moderate to high yields. Subsequently, we explored the substrate scope of different α-trifluoromethyl alkene. The variety of functional groups at the para-position of α-trifluoromethyl alkene, including TMS, Ts, OPh, OBn, and n-Bu, afforded good to excellent yields (5a–5e, 53–90%). The reaction was also compatible with different functional groups at the meta-position, including electron-donating and electron-withdrawing groups such as OMe, COOMe, and Cl, which afforded high to excellent yields (5f–5h, 72–89%). In addition, fused ring aromatic substrates such as naphthalene (5i), dioxoline (5j), different benzothiophene (5k, 5l), quinoline (5m), and N-Boc-indole (5n) based α-trifluoromethyl alkene reacted efficiently. They afforded γ,γ-difluoroallylic ketones in correspondingly moderate to high yields (Scheme 1). Furthermore, the various substituted α-trifluoromethyl alkenes, including TMS OPh and OBn, afforded moderate to excellent yields (6a–6c, 54–96%) with aromatic aldehyde; meanwhile, the naphthalene (6d) and fused thiophene (6e) afforded excellent yields 98% and 94%, respectively. We employed this method on the medicinal molecule Probenecid (6f), which afforded the desired product with a 90% yield (Scheme 1).
We conducted a series of control experiments to understand the mechanism of the insight reaction. The light-on/off experiments aligned with the control reaction, confirming that no product 3a was formed in the absence of light, thereby demonstrating that the photocatalyst initiates the reaction under illumination. In particular, when TEMPO (2,2,6,6-tetramethyl-piperidine nitroxide) was subjected to the model reaction system, the desired product was almost inhibited. The corresponding radical trapping product was confirmed by high-resolution mass spectrometry, indicating that the reaction proceeded via a radical-based mechanism (Fig. 3B-I). To validate the role of active species in defluorinative acylation reaction, we performed an electron, hole, and carbon-centered radical inhibition experiment. The addition of K2S2O8, an electron scavenger, significantly suppressed the yield of the desired product, while triethanolamine (TEOA), serving as a hole scavenger, completely inhibited the reaction. Furthermore, the addition of BHT reduced the yield of the desired product, indicating that electron, hole, and carbon-centered radicals play crucial roles in the reaction (Fig. 3B-II).36 Additionally, in situ EPR measurements were performed to confirm the generation of active species using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as the radical-trapping agent. A strong signal corresponding to a carbon-centered radical was observed exhibiting the g value of 2.002 (Fig. 3C). UV–vis spectral analysis of the reaction components showed that CdS nanostrips were the only species exhibiting significant absorption near 550 nm (Fig. 3A).
Additionally, we assumed that CdS immersion of the SET-driven radical-mediated events in the defluorinative acylation process, Stern–Volmer quenching experiments were conducted. The photoluminescence of CdS was efficiently quenched by increasing concentrations of the α-trifluoromethyl alkene 1a, whereas the 2a showed no measurable quenching effect (Fig. 3E and SI Fig. S2).37–39 The combined results of the dark condition EPR spectrum and hole scavenger (TEOA) demonstrated that photogenerated holes play a decisive role and are responsible for the formation of acyl radicals.32,33 Moreover, the kinetic profile of the standard reaction between 1a and 2a displayed rapid conversion without any observable induction period. The viability of the synthetic utility in the production of γ,γ-difluoroallylic ketones was demonstrated by a gram-scale reaction (Fig. 3G–I). Following standard conditions, a 4.5 mmol reaction scale yielded an excellent isolated product of 92%, highlighting the scalability of the reaction method. Additionally, a further transformation of 3a was performed to enhance its practical application. We used a Pd/C catalyst for the hydrogenation of 3a, leading to product 7 in 77% yield (Fig. 3G-II). The recyclability of CdS nanostrips was recovered simply by centrifuging the reaction mixture; the recycled catalyst could be utilized four times with excellent yields (Fig. 3G-III).40 No noticeable structural or electronic changes were detected compared with fresh CdS nanostrips, confirming that the catalyst retained its crystal and electronic structure after reuse (SI Fig. S3). Different light sources were also screened to optimize the reaction conditions (Fig. 3H, see SI for details).
Based on our mechanistic studies and existing literature reports,27,40 our proposed mechanism is outlined in Fig. 3I. The reaction is initiated from the oxidative dehydrogenation of aldehyde 2 by a photogenerated hole on the VB of CdS nanostrips upon photoexcitation, with the formation of acyl radical I. The photo-generated electron reduces the α-trifluoromethyl alkene 1a to radical anion IV.41 Then, radical addition of I to the α-trifluoromethyl alkene 1 to produce α-trifluoromethyl carbon radical II. Subsequently, the photogenerated electron on the CB of CdS reduces II and forms a trifluoromethyl carbanion intermediate III. Finally, a β-fluoride elimination of III completes the synthesis of the γ,γ-difluoroallylic ketone product 3.42 In path B, the photo-generated electron reduces the α-trifluoromethyl alkene 1a to radical anion IV,41 which couples with radical I and forms carbanion intermediate III by radical anion and radical coupling, and after a β-fluoride elimination, affords γ,γ-difluoroallylic ketone product 3.
Conclusion
In conclusion, we have successfully developed a mild and efficient protocol for the facile synthesis of γ,γ-difluoroallylic ketones by visible-light-driven photocatalytic defluorination reactions. We utilize an inexpensive and readily available organofluorine source to produce value-added fluorinated organic compounds using a highly sustainable, recyclable, and eco-friendly heterogeneous semiconductor CdS photocatalyst. The reaction proceeds efficiently with a wide range of aliphatic, aromatic, and cyclic aldehydes, as well as trifluoromethyl alkene, underscoring its broad applicability. The mild condition, with outstanding tolerance to functional groups, broadens the substrate scope of this method, making it suitable for the late-stage derivatization of bioactive molecules.Experimental
The reaction was generally added inside the glove box. The CdS nanostrips (15 mg), α-trifluoromethyl alkenes (0.15 mmol, 1.0 equiv.), butyraldehyde (0.2 mmol, 1.3 equiv.), sodium acetate (0.075 mmol, 0.5 equiv.), and dimethyl sulfoxide (1.0 mL) were added to an oven-dried reaction tube under an Ar atmosphere. The reaction tubes were closed with a rubber septum and sealed with parafilm tape. The reaction mixture was placed on a photoreactor, fixed the stirring at 700 rpm, and irradiated with a 10 W blue LED at room temperature (25 °C) for 18 h. When the reaction was completed, 5 (mL) water was added to the reaction and extracted with ethyl acetate, washed with brine, dried over anhydrous sodium sulphate, concentrated in a vacuum rotatory evaporator, and purified by column chromatography (hexane/ethyl acetate) to afford the γ,γ-difluoroallylic ketones (see SI for further details).Author contributions
Kashif Hussain conducted the experiments, curated the data, analyzed the results, and drafted the manuscript. Fukai Xie analyzed the data and reviewed the draft. Dai Wen guided and directed the project. All authors contributed to the analysis and interpretation of the data.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data that support the findings of this study are available in the supplementary information (SI) of this article. Supplementary information: experimental, optimization details, compound characterization, and copies of spectra. See DOI: https://doi.org/10.1039/d5gc05892a.Acknowledgements
Financial support from Dalian Institute of Chemical Physics (DICP) is acknowledged.References
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed.
- K. Muller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed.
- J. Fried and E. F. Sabo, J. Am. Chem. Soc., 1954, 76, 1455–1456 CrossRef CAS.
- C. Alonso, E. Martinez de Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847–1935 CrossRef CAS PubMed.
- J. Nie, H.-C. Guo, D. Cahard and J.-A. Ma, Chem. Rev., 2011, 111, 455–529 CrossRef CAS PubMed.
- Y. Pan, J. Qiu and R. B. Silverman, J. Med. Chem., 2003, 46, 5292–5293 CrossRef CAS PubMed.
- J.-M. Altenburger, G. Y. Lassalle, M. Matrougui, D. Galtier, J.-C. Jetha, Z. Bocskei, C. N. Berry, C. Lunven, J. Lorrain and J.-P. Herault, Bioorg. Med. Chem., 2004, 12, 1713–1730 CrossRef CAS.
- M. Bobek, I. Kavai and E. De Clercq, J. Med. Chem., 1987, 30, 1494–1497 CrossRef CAS.
- N. A. Meanwell, J. Med. Chem., 2011, 54, 2529–2591 CrossRef CAS.
- G. Magueur, B. Crousse, M. Ourévitch, D. Bonnet-Delpon and J.-P. Bégué, J. Fluorine Chem., 2006, 127, 637–642 CrossRef CAS.
- C. Leriche, X. He, C.-w. T. Chang and H.-w. Liu, J. Am. Chem. Soc., 2003, 125, 6348–6349 CrossRef CAS PubMed.
- G. Chelucci, Chem. Rev., 2012, 112, 1344–1462 CrossRef CAS PubMed.
- H. Amii and K. Uneyama, Chem. Rev., 2009, 109, 2119–2183 CrossRef CAS PubMed.
- H. M. Park, T. Uegaki, T. Konno, T. Ishihara and H. Yamanaka, Tetrahedron Lett., 1999, 40, 2985–2988 CrossRef CAS.
- S. Watanabe, K. Sugahara, T. Fujita, M. Sakamoto and T. Kitazume, J. Fluorine Chem., 1993, 62, 201–206 CrossRef CAS.
- H. Yamanaka, T. Uegaki, T. Ishihara, T. Kubota and J. T. Gupton, J. Fluorine Chem., 1999, 97, 101–108 CrossRef CAS.
- T. Miura, Y. Ito and M. Murakami, Chem. Lett., 2008, 37, 1006–1007 CrossRef CAS.
- M. Yokota, D. Fujita and J. Ichikawa, Org. Lett., 2007, 9, 4639–4642 CrossRef CAS.
- T. Ichitsuka, T. Fujita and J. Ichikawa, ACS Catal., 2015, 5, 5947–5950 CrossRef CAS.
- Y. Liu, Y. Zhou, Y. Zhao and J. Qu, Org. Lett., 2017, 19, 946–949 CrossRef CAS.
- T. Ichitsuka, T. Fujita, T. Arita and J. Ichikawa, Angew. Chem., Int. Ed., 2014, 53, 7564–7568 CrossRef CAS PubMed.
- D. Ding, Y. Lan, Z. Lin and C. Wang, Org. Lett., 2019, 21, 2723–2730 CrossRef CAS.
- Y. J. Jang, D. Rose, B. Mirabi and M. Lautens, Angew. Chem., 2018, 130, 16379–16383 CrossRef.
- T. Fujita, K. Sakoda, M. Ikeda, M. Hattori and J. Ichikawa, Synlett, 2012, 57–60 Search PubMed.
- T. Fujita, M. Ikeda, M. Hattori, K. Sakoda and J. Ichikawa, Synthesis, 2014, 1493–1505 Search PubMed.
- T. Xiao, L. Li and L. Zhou, J. Org. Chem., 2016, 81, 7908–7916 CrossRef CAS.
- Y.-Q. Guo, R. Wang, H. Song, Y. Liu and Q. Wang, Org. Lett., 2020, 22, 709–713 CrossRef CAS PubMed.
- C. Ai, T. Wang, Y. Bao, S. Yan, Y. Zhang and J.-Y. Wang, Org. Biomol. Chem., 2024, 22, 9197–9202 RSC.
- Y. Liu, X. Zhou, R. Li and Z. Sun, Org. Lett., 2024, 26, 6424–6427 CrossRef CAS.
- Q. Zhu, Z. Zhang, H. Yu, C. Yuan, L. Wang, Z. Wang, T. Liu, H. Xu and Y. Yang, Eur. J. Org. Chem., 2025, e202401149 CrossRef CAS.
- F. Xie, Z. Mao, D. P. Curran, H. Liang and W. Dai, Angew. Chem., 2023, 135, e202306846 CrossRef.
- X. Ning, S. Meng, X. Fu, X. Ye and S. Chen, Green Chem., 2016, 18, 3628–3639 RSC.
- F. Yang, X. Tian, K. Zhang, X. Zhang and L. Liu, ECS J. Solid State Sci. Technol., 2018, 7, P311 CrossRef CAS.
- B. Liu, Z. Luo, W. Wu, Y. Qi, Y. Qin and X. Qiu, Carbon Res., 2025, 4, 1–13 RSC.
- M. Li, L. Ma, L. Luo, Y. Liu, M. Xu, H. Zhou, Y. Wang, Z. Li, X. Kong and H. Duan, Appl. Catal., B, 2022, 309, 121268 CrossRef CAS.
- Y. Li, S. Y. Guo, H. Gu, B. Wang, P. Su, X. Zhang, H. Zhang, S. Zhang, F. Yang and J. Liu, Angew. Chem., Int. Ed., 2025, e202425601 CAS.
- Y. Liu, X.-X. Zhang, X.-T. Li, S.-Y. Xu, D.-W. Ji and Q.-A. Chen, Org. Biomol. Chem., 2025, 23, 3619–3628 RSC.
- X.-X. Zhang, S.-T. Xu, X.-T. Li, T.-T. Song, D.-W. Ji and Q.-A. Chen, J. Am. Chem. Soc., 2025, 147, 11533–11542 CrossRef CAS.
- Y.-Q. Guo, Y. Wu, R. Wang, H. Song, Y. Liu and Q. Wang, Org. Lett., 2021, 23, 2353–2358 CrossRef CAS.
- Q. Chen, R. Fan, X. Deng, J. Chen, W. Wang, Q. Yan and F.-E. Chen, J. Org. Chem., 2024, 89, 18571–18584 CrossRef CAS.
- Y. Chen, X. Mao, M.-M. Li and W. Ding, J. Org. Chem., 2025, 90, 3391–3403 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |