Open Access Article
Open Access ArticleSolvent-less environmentally benign amino ester, amide, and peptide synthesis enabled by resonant acoustic mixing
Nassim
Maarouf-Mesli
 a,
Justin
Desjardins-Michaud
a,
Justin
Desjardins-Michaud
 a,
Zeynab
Imani
a,
Zeynab
Imani
 a,
Kinshuk
Ghosh
a,
Kinshuk
Ghosh
 a,
Felix
Polyak
a,
Felix
Polyak
 *ab and
William D.
Lubell
*ab and
William D.
Lubell
 *a
*a
aDépartement de Chimie, Université de Montréal, Complexe des Sciences, B-3015, 1375 Avenue Thérèse-Lavoie-Roux Montréal, Québec H2 V 0B3, Canada. E-mail: nassim.maarouf@umontreal.ca; justin.desjardins-michaud@umontreal.ca; zeynab.imani@outlook.fr; kinshuk.ghosh.kinshuk.ghosh@umontreal.ca; felix.polyak@studiobioscience.com; william.lubell@umontreal.ca
bStudio Bioscience Inc., 111 Rue Terry Fox, Verdun, QC H3E 1L4, Canada
First published on 6th November 2025
Abstract
The demand for sustainable methods for peptide synthesis is growing imperatively with their escalating use in therapeutic and biomedical applications. The cornerstone technology of solid-phase peptide synthesis has an unsustainable environmental impact due to its high process mass intensity and reliance on hazardous solvents. The reported study presents pioneering use of a resonant acoustic mixer (RAM) for sustainable peptide bond formation at elevated concentration with minimal liquid waste. Effective amide and ester bond formation was achieved rapidly under mild conditions using the RAM in “green solvents” such as ethyl acetate and 1,2-dimethoxyethane (DME), with minimal levels of epimerization. Peptide coupling was systematically studied under various conditions, indicating the influences of liquid viscosity and base solubility on conversion efficiency. A preliminary comparison with conventional and ultrasonic stirring methods suggested that better conversions were obtained using the RAM at and above 60 g0. Assessment in different peptide model systems prone to racemization and fragment couplings confirmed comparable conversion and minimal epimerization to that observed using solution-phase and mechanochemical methods. The RAM approach proved to be robust and scalable providing an environmentally friendly platform for the synthesis of amino esters, amides, and peptides, thereby presenting novel opportunities for the sustainable production of peptide-based therapeutics.
Green foundation1. This study contributes to the field of green chemistry by introducing the use of a resonant acoustic mixer (RAM) in scalable, solvent-minimized approaches for amide, ester, and peptide bond formation. Avoiding toxic solvents, such as DMF, the RAM approach decreased process mass intensity compared to conventional solid-phase peptide synthesis.2. Among notable accomplishments reported herein, high-yielding peptide syntheses were achieved at ≤1.0 μL mg−1 concentrations in green solvents (EtOAc and DME) and rapid reactions times (≤15 min) with minimal epimerization. Aqueous workup procedures obviated the need for chromatographic purification. 3. Greener synthesis could be achieved by replacing non-atom-economical protecting groups (e.g., Fmoc) and corrosive TFA cleavages with sustainable alternatives. Future research on integrating RAM into solid- and liquid-phase platforms may further minimize waste and enable synthesis of longer peptide sequences under sustainable conditions. |
Introduction
Peptide science has made a significant impact in various fields with special success in therapeutic and biomedical applications.1–4 The advent of solid-phase peptide synthesis (SPPS) revolutionized peptide assembly.5,6 Application of SPPS for industrial peptide manufacturing has however a large environmental footprint due to its relatively high process mass intensity (PMI) and poor atom economy.7 Increased peptide demand has spurred the need for environmentally benign “greener” synthetic strategies.8 Notably, the use of commonly employed reprotoxic and toxic solvents such as N,N-dimethylformamide (DMF) and dichloromethane, as well as corrosive acids such as trifluoroacetic acid (TFA), all need to be avoided to improve sustainability.9–12 Alternative approaches for peptide production offer potential for reducing PMI and improving atom economy, including liquid-phase supported peptide synthesis12 and solvent-less mechanochemical processes,13,14 as well as recombinant15 and chemoenzymatic approaches.16Resonant acoustic mixing couples the effects of high-intensity vibrational motion and acoustic frequency agitation which generate, respectively, systemic circulation and microcirculation flow fields to realize a uniform dispersion throughout the mixed system.17,18 To maximize mixing efficiency, the resonant acoustic mixer (RAM) leverages a moderate frequency (58–62 Hz contingent upon mixing vessel and contents) over a relatively long effective range (1.4 cm) to generate exceptional energy as measured in gravitational force [g0 force (1 g0 = 9.806 m s−2)]. Avoiding milling and crushing, mixing with the RAM has emerged as a powerful tool recently applied in cocrystal, nanoparticle and metal/organic framework preparation,19–23 metal-catalyzed reactions,24,25 short DNA and RNA fragment synthesis,26 and the sustainable preparation of active pharmaceutical ingredients,27 but, to our knowledge, it has not yet been used for making peptides.28,29 Offering efficient mixing at high concentration, the RAM enables potential for rapid reaction kinetics using minimal excess of reagents.
Aiming to determine the merits of using the RAM for environmentally sound and sustainable peptide synthesis, a systematic examination was performed to assess reactivity in green liquids and to ascertain limits for forming ester and amide bonds without racemization. Robust means for assembling amino esters, amino amides and polyamides have been developed using minimal amounts of environmentally benign liquid, e.g., ≤1.0 µL mg−1 of EtOAc and 1,2-dimethoxyethane (DME). Rapid reaction completion was determined to occur typically within 10 minutes without significant racemization (epimerization) as ascertained in the synthesis of various challenging peptides.30 Focusing on carboxylate activation and reactions with nucleophiles, arguably the most important challenge in peptide synthesis, this study has narrowly employed the combination of water-soluble carbodiimide and ethyl cyanohydroxyiminoacetate (Oxyma) as an additive31 to generally enable effective isolation of pure product without the use of significant amounts of organic solvent or chromatography.
Results
Peptide synthesis was performed on a Resodyn LabRAM II acoustic mixer in 20 mL borosilicate scintillation vials. The synthesis of dipeptide Cbz-Phe-Val-Ot-Bu [(S,S)-1] from Cbz-Phe-OH (2) and Val-Ot-Bu (3) as coupling partners using RAM was selected to investigate different parameters: liquid, base, and concentration (η, µL mg−1, Chart 1). Subsequently, the corresponding acid, Cbz-Phe-Val-OH [(S,S)-4], was coupled to H-Pro-NH2 (5) to prepare Cbz-Phe-Val-Pro-NH2 [(S,S)-6] as a critical means for assessing epimerization during peptide coupling (Fig. 1).32 The coupling agent 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI, 120 mol%) and additive Oxyma (120 mol%) were employed to enable aqueous removal of the urea side product and to avoid potentially explosive triazole additives. The hydrochloride salt of H-Val-Ot-Bu (5) was used to assess in situ free basing, for which triethylamine was initially selected in part as a liquid medium with low viscosity (μ = 0.36 mPa s). After dissolving the reaction mixture in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN
1 CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, conversion was monitored using LC-MS (method A, λ = 214 nm) by examining peak ratios for Cbz-Phe-OH (2, Rt = 7.8 min), Oxyma (Rt = 5.2 min), and dipeptide (S,S)-1 (Rt = 10.2 min).
H2O, conversion was monitored using LC-MS (method A, λ = 214 nm) by examining peak ratios for Cbz-Phe-OH (2, Rt = 7.8 min), Oxyma (Rt = 5.2 min), and dipeptide (S,S)-1 (Rt = 10.2 min).
 | ||
| Chart 1 Model dipeptide synthesis with the RAM to evaluate the effects of liquid and base on conversion. | ||
 | ||
| Fig. 1 Couplings employed to study epimerization in the RAM: isolated yields; dr assessed by HPLC at λ = 214 nm and 205 nm (details presented in Tables S2–S4). | ||
Without any liquid, the RAM-mediated coupling at 60 g0 gave 80% conversion to dipeptide (S,S)-1 after 4 min, due likely in part to the use of Et3N as a non-viscous base (Table 1). Conversion was increased to 96% after 4 min by employing the reprotoxic and toxic liquid DMF (η = 0.45 µL mg−1, μ = 0.79 mPa s, εr = 0.39) as the control.33 Examining environmentally friendly alternatives,34 the importance of liquid viscosity and to a lesser extent, polarity was demonstrated along with the significance of concentration (η = 0.90 µL mg−1) and reaction time (10 min) for conversion: DME (μ = 0.46 mPa s, εr = 0.23), 98%; THF (μ = 0.46 mPa s, εr = 0.21), 95%; EtOAc (μ = 0.42 mPa s, εr = 0.23), 92%; 2-MeTHF (μ = 0.46 mPa s, εr = 0.18), 90%; NBP (μ = 1.20 mPa s, εr = 0.32), 84%.12,35–38 Conversion was improved by adding into EtOAc 20 vol% of a co-liquid: NBP, 98%; propylene carbonate (μ = 2.5 mPa s, εr = 0.47), 96%; sulfolane (μ = 6.28 (50 °C) 10.34 mPa s, εr = 0.41), 95%.39 Although distinct correlations did not exist, reaction conversion was observed to increase using liquids having lower viscosity and higher polarity. Rheological properties other than viscosity, such as liquid elasticity and plasticity, have also been suggested to influence mixing in the RAM and may have consequences on conversion.40
| Liquid | Base | Time [min] | η, µL mg−1 | % conversion |
|---|---|---|---|---|
| a Conversion was assessed by LC-MS analysis at λ = 214 nm [method A in the SI] measuring peak intensity ratios: Cbz-Phe-OH (2, Rt = 7.8 min), Oxyma (Rt = 5.2 min), and dipeptide (S,S)-1 (Rt = 10.2 min). | ||||
| DME | (i-Pr)2NEt | 10 | 0.90 | 100 |
| DME | Et3N | 10 | 0.90 | 98 |
| DME | Et3N | 4 | 0.90 | 97 |
| DME | Et3N | 4 | 0.45 | 92 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 NBP/EtOAc 4 NBP/EtOAc |
(i-Pr)2NEt | 10 | 0.90 | 100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 NBP/EtOAc 4 NBP/EtOAc |
Et3N | 10 | 0.90 | 98 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 NBP/EtOAc 4 NBP/EtOAc |
Et3N | 4 | 0.45 | 93 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PPC/EtOAc 4 PPC/EtOAc |
Et3N | 10 | 0.90 | 96 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 sulfolane/EtOAc 4 sulfolane/EtOAc |
Et3N | 10 | 0.90 | 95 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 NBP/THF 4 NBP/THF |
Et3N | 4 | 0.45 | 93 |
| DMF | Et3N | 4 | 0.45 | 96 |
| THF | Et3N | 10 | 0.90 | 95 |
| EtOAc | Et3N | 10 | 0.90 | 92 |
| 2-MeTHF | Et3N | 10 | 0.90 | 90 |
| NBP | Et3N | 4 | 0.45 | 87 |
| NBP | Et3N | 10 | 0.90 | 84 |
| No liquid | Et3N | 4 | — | 80 |
In a study comparing organic and inorganic bases (Table S1), conversion varied with solubility (η = 0.45 µL mg−1) after 4 min at 60 g0; Et3N, K2CO3 and NaHCO3 gave conversions of 92%, 89% and 83%, respectively, in DME and 87%, 70% and 78% in NBP. Enhanced conversion was obtained employing Hünig's base [(i-Pr)2EtN, 120 mol%] instead of Et3N (98%) in DME as well as in 20% NBP in EtOAc (η = 0.90 µL mg−1) for 10 min, likely due in part to the greater lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ≈ 2.2 and 1.4) and weaker basicity (pKa of the conjugate acids 18.1 and 18.46 in acetonitrile) of the former.41,42 Ionic pair solubility has also been shown to improve peptide coupling.43
P ≈ 2.2 and 1.4) and weaker basicity (pKa of the conjugate acids 18.1 and 18.46 in acetonitrile) of the former.41,42 Ionic pair solubility has also been shown to improve peptide coupling.43
Optimized conditions were consequently used to prepare dipeptide (S,S)-1 on a gram scale. In DME (η = 0.90 µL mg−1), Cbz-Phe-OH (2, 100 mol%, 1.00 g) and HCl·H-Val-Ot-Bu (3, 150 mol%, 1.05 g) were coupled using Oxyma (120 mol%), (i-Pr)2EtN, (120 mol%) and EDCI (120 mol%) at 60 g0 in the RAM for 10 min. After aqueous work-up, Cbz-Phe-Val-Ot-Bu (1, 1.50 g) was isolated in 99% yield.
Comparisons of mixing methods
Under RAM conditions yielding high conversion, a set of control experiments were performed to distinguish the advantages of agitation at 60 g0 and high concentration in DME (η = 0.90 µL mg−1). The synthesis of dipeptide (S,S)-1 from acid 2 (100 mol%) and the hydrochloride of amine 3 (150 mol%) was examined using the same EDCI (120 mol%), Oxyma (120 mol%) and (i-Pr)2EtN (120 mol%) conditions employing conventional stirring at 1500 rpm as well as agitation by sonication (40 kHz) which after 10 min gave, respectively, 53% and 61% conversion. Moreover, agitation of the same reaction with the RAM at 1, 15 and 30 g0 gave, respectively, 64%, 71% and 68% conversion. In sum, the control experiments demonstrated that high concentration alone with conventional, ultrasonic, and lower g0 mixing was insufficient to attain the high levels of conversion obtained using the RAM at 60 g0. In subsequent studies of Fmoc protected amino acid couplings (vide infra), agitation with the RAM at 80 g0 proved effective. Moreover, in couplings of sterically hindered hydrochloride salt with RAM agitation above 80 g0 (90 and 100 g0), significant amounts of tert-butyl ester solvolysis were observed.Green chemistry metrics
A comparison of the PMI was made for the synthesis of Cbz-Phe-Val-Ot-Bu [(S,S)-1] using previous methods employing solution-phase44 and ball-milling methods.14 All three methods were performed on a similar (≈1 g) scale and gave yields ranging from 60 to 99% (SI Table S7) from which PMIs were calculated. In solution, dichloromethane was used as a solvent, and in ball-milling and RAM, EtOAc was employed; moreover, DME was also used in RAM. Before purification, comparable PMI values were calculated for RAM (4.1, DME; 4.6 EtOAc) and ball-milling (4.2, EtOAc), which were 3–5 fold better than the solution-phase method (13.5–19.2 CH2Cl2). Accounting for purification, the RAM method had about a 2-fold better PMI (59.9, DME; 66.2, EtOAc) than the solution-phase (127.9–181.2) and ball-milling (115.8) methods.Epimerization studies
Epimerization is a key issue commonly encountered in amide bond formation during peptide synthesis and minimized through optimization of coupling conditions. Employing the EDCI and Oxyma coupling with environmentally benign solvents under conditions which were described above as a point of departure, three different systems were explored to study epimerization (Fig. 1). The coupling of Cbz-Phe-L-Val-OH [(S,S)-4] and H-Pro-NH2 (5) to prepare Cbz-Phe-Val-Pro-NH2 [(S,S,S)-6] was examined because this system has previously been used to assess epimerization during EDCI couplings in DMF in which different additives were shown to diminish the amount of Cbz-Phe-D-Val-Pro-NH2 [(S,R,S)-6] diastereomer to levels between 9.2 and 17.2% (Table S2).34In a second model, Cbz-Ala-Phg-Ile-OMe [(S,S,S)-7] was prepared from Cbz-Ala-Phg-OH [(S,S)-8] and HCl·Ile-OMe (9) because the former had previously been made using ball milling in 88–93% yield after 10 min employing EDCI and different additives (Oxyma, HOAt and HOBt·H2O) in DMF with epimerization and formation of Cbz-Ala-D-Phg-Ile-OMe [(S,R,S)-7] varying from 0 to 25%; moreover, similar conditions over 30 min in EtOAc were claimed to give the diastereomerically pure tripeptide (S,S,S)-7 in 96% yield.14 Finally, the dipeptides Cbz-D- and L-Phg-Pro-NH2 [(R,S)- and (S,S)-10] were synthesized because earlier studies had shown that Oxyma resulted in less epimerization (about 1%) than HOBt and HOAt as additives for DIC couplings in the “green solvents” 2-MeTHF and cyclopentyl methyl ether, respectively.33,45
For the synthesis of the tripeptide amide (S,S,S)-6, dipeptide diastereomers Cbz-Phe-D- and L-Val-Ot-Bu [(S,R and S,S)-4] were first shown to have >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr by 1H NMR examination of the iso-propyl methyl doublets at 0.87 and 0.84 ppm. The purity of the corresponding tripeptide diastereomers (S,R,S)- and (S,S,S)-6 was ascertained by LC-MS (method A, λ = 214 nm) and integration of the UV intensity of the corresponding peaks at Rt = 7.5 and 7.12 min. Employing excess proline amide (150 mol%) and Et3N (120 mol%) in DME (η = 0.90 µL mg−1) at 60 g0 for 10 min afforded 13.6% of the (S,R,S)-isomer (S,R,S)-6, due likely in part to base-mediated deprotonation of an oxazolone intermediate.46 In the absence of Et3N, lower amounts of the (S,R,S)-isomer were observed contingent on the amount of proline amide [(5), 150, 120 and 100 mol% gave 7.2%, 9.1% and 10.0%, respectively]. Excess proline amide (5) is expected to facilitate interception of the oxazolone intermediate.46 Epimerization was minimized further to 6.8% and 6.6% with a slight drop in conversion by reducing the amount of EDCI or Oxyma from 120 to 100 mol%, respectively. Epimerization was less significant in the synthesis of Cbz-Phe-D-Val-Pro-NH2 [(S,R,S)-6]; only 4.8% of the (S,S,S)-isomer (S,S,S)-6 was observed after coupling using Cbz-Phe-D-Val-OH [(S,R)-4] and H-Pro-NH2 (5) in the presence of Et3N (120 mol%).
1 dr by 1H NMR examination of the iso-propyl methyl doublets at 0.87 and 0.84 ppm. The purity of the corresponding tripeptide diastereomers (S,R,S)- and (S,S,S)-6 was ascertained by LC-MS (method A, λ = 214 nm) and integration of the UV intensity of the corresponding peaks at Rt = 7.5 and 7.12 min. Employing excess proline amide (150 mol%) and Et3N (120 mol%) in DME (η = 0.90 µL mg−1) at 60 g0 for 10 min afforded 13.6% of the (S,R,S)-isomer (S,R,S)-6, due likely in part to base-mediated deprotonation of an oxazolone intermediate.46 In the absence of Et3N, lower amounts of the (S,R,S)-isomer were observed contingent on the amount of proline amide [(5), 150, 120 and 100 mol% gave 7.2%, 9.1% and 10.0%, respectively]. Excess proline amide (5) is expected to facilitate interception of the oxazolone intermediate.46 Epimerization was minimized further to 6.8% and 6.6% with a slight drop in conversion by reducing the amount of EDCI or Oxyma from 120 to 100 mol%, respectively. Epimerization was less significant in the synthesis of Cbz-Phe-D-Val-Pro-NH2 [(S,R,S)-6]; only 4.8% of the (S,S,S)-isomer (S,S,S)-6 was observed after coupling using Cbz-Phe-D-Val-OH [(S,R)-4] and H-Pro-NH2 (5) in the presence of Et3N (120 mol%).
Dipeptide diastereomers Cbz-Ala-D- and L-Phg-Ot-Bu [(S,R)- and (S,S)-12] were, respectively, prepared from Cbz-Ala-OH (13) and HCl·H-D- and L-Phg-Ot-Bu (D- and L-14) under the representative RAM conditions (60 g0, 10 min) with (i-Pr)2EtN in DME and were shown to have >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr by measuring the intensities of the corresponding tert-butyl signals at 17.6 and 17.8 ppm in the 13C{1H} NMR spectra. After ester solvolysis using 4 M HCl in 1,4-dioxane, Cbz-Ala-D- and L-Phg-OH [(S,R)- and (S,S)-8] were coupled to HCl·H-Ile-OMe (9) and epimerization and conversion were ascertained by measuring the UV intensity of the corresponding peaks for Cbz-Ala-D- and L-Phg-Ile-OMe [(S,R,S)- and (S,S,S)-7] at Rt = 11.2/12.9 and 11.5/13.3 min using LC-MS analysis (methods B and C, λ = 205 nm, Table S3). Complete and 77% conversion with 1.55% and 0.97% of Cbz-Ala-D-Phg-Ile-OMe [(S,R,S)-7] in (S,S,S)-diastereomer (S,S,S)-7 was obtained using (i-Pr)2EtN and Et3N as bases (120 mol%) in DME (η = 0.90 µL mg−1) at 60 g0 for 10 min. On the other hand, the weaker bases 2,4,6-collidine and N-methyl morpholine gave lower conversion (54% and 52%) and more epimerization (3% and 5% (S,R,S)-7). The phosphate additive, NaH2PO4, reduced epimerization but compromised conversion. Employing 120 and 400 mol% NaH2PO4 gave conversions of 26% and 81% with less than 1% of the (S,R,S)-diastereomer (S,R,S)-7. Similarly, Cbz-Ala-D-Phg-Ile-OMe [(S,R,S)-7] was prepared in 88% and 84% conversion using (i-Pr)2EtN and Et3N, respectively, with about 1% of the (S,S,S)-diastereomer (S,S,S)-7.
1 dr by measuring the intensities of the corresponding tert-butyl signals at 17.6 and 17.8 ppm in the 13C{1H} NMR spectra. After ester solvolysis using 4 M HCl in 1,4-dioxane, Cbz-Ala-D- and L-Phg-OH [(S,R)- and (S,S)-8] were coupled to HCl·H-Ile-OMe (9) and epimerization and conversion were ascertained by measuring the UV intensity of the corresponding peaks for Cbz-Ala-D- and L-Phg-Ile-OMe [(S,R,S)- and (S,S,S)-7] at Rt = 11.2/12.9 and 11.5/13.3 min using LC-MS analysis (methods B and C, λ = 205 nm, Table S3). Complete and 77% conversion with 1.55% and 0.97% of Cbz-Ala-D-Phg-Ile-OMe [(S,R,S)-7] in (S,S,S)-diastereomer (S,S,S)-7 was obtained using (i-Pr)2EtN and Et3N as bases (120 mol%) in DME (η = 0.90 µL mg−1) at 60 g0 for 10 min. On the other hand, the weaker bases 2,4,6-collidine and N-methyl morpholine gave lower conversion (54% and 52%) and more epimerization (3% and 5% (S,R,S)-7). The phosphate additive, NaH2PO4, reduced epimerization but compromised conversion. Employing 120 and 400 mol% NaH2PO4 gave conversions of 26% and 81% with less than 1% of the (S,R,S)-diastereomer (S,R,S)-7. Similarly, Cbz-Ala-D-Phg-Ile-OMe [(S,R,S)-7] was prepared in 88% and 84% conversion using (i-Pr)2EtN and Et3N, respectively, with about 1% of the (S,S,S)-diastereomer (S,S,S)-7.
Performing the stringent test for epimerization, complete conversion of Cbz-Phg-OH (11, 100 mol%) and H-Pro-NH2 (5, 150 mol%) to Cbz-Phg-Pro-NH2 [(S,S)-10] was achieved with production of up to 3% of the (R,S)-diastereomer (R,S)-10 using Oxyma (120 mol%) and EDCI (120 mol%) without base in DME and in 20 vol% NBP in EtOAc, respectively. The diastereomeric ratio was assessed by measuring the UV intensity of the corresponding peaks at Rt = 4.96 [(S,S)-10] and 5.35 min [(R,S)-10] by LC-MS (method C, λ = 205 nm). In sum, epimerization was generally minimized using the RAM protocols and the diastereomer was kept at levels similar to those previously achieved in the three systems employing earlier methods.14,34
Ester, amide, and dipeptide synthesis
In pursuit of using the RAM for the synthesis of challenging targets under investigation in our laboratory, environmentally sound methods were innovated for making orthogonally protected amino ester, amide, and dipeptide building blocks (Schemes 1 and 2). Tolerance was demonstrated for various amino acid protecting groups that are commonly employed in peptide synthesis.42 Initially, 2,4-dimethoxybenzyl carboxylates were synthesized as representative esters under the EDCI and Oxyma conditions in different liquids. Subsequently, allyl esters were synthesised under similar conditions. | ||
| Scheme 1 Ester and amide synthesis with the RAM. Enantiomeric ratios determined using chiral supercritical fluid chromatography (SFC). | ||
Dimethoxybenzyl esters, Fmoc-D- and Fmoc-L-Trp(Boc)-ODmb [(R)- and (S)-21], were synthesized in 98% and 99% yield, respectively, with er > 99.5:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 using the corresponding acid, 2,4-dimethoxybenzyl alcohol (120 mol%) and (i-Pr)2NEt (120 mol%) in DME (η = 0.90 µL mg−1) at 60 g0 for 10 min. Allyl ester 22 was synthesized in 98% yield from Fmoc-Lys(Boc)-OH (16) using allyl alcohol (150 mol%) under similar conditions in EtOAc (η = 1.0 µL mg−1) at 80 g0 for 12 min and aqueous workup. Moreover, H-D- and L-Val-ODmb [(R)- and (S)-24] were synthesized in 72% and 75% yields, respectively, from Fmoc-D- and L-Val-OH (D- and L-17) by a two-step approach employing EtOAc (η = 1.0 µL mg−1) as the liquid medium at 80 g0 featuring (i-Pr)2NEt (200 mol%) mediated esterification (8 min), followed by Fmoc group removal from esters 23 with 5 vol% 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 12 min) and aqueous workup.
0.5 using the corresponding acid, 2,4-dimethoxybenzyl alcohol (120 mol%) and (i-Pr)2NEt (120 mol%) in DME (η = 0.90 µL mg−1) at 60 g0 for 10 min. Allyl ester 22 was synthesized in 98% yield from Fmoc-Lys(Boc)-OH (16) using allyl alcohol (150 mol%) under similar conditions in EtOAc (η = 1.0 µL mg−1) at 80 g0 for 12 min and aqueous workup. Moreover, H-D- and L-Val-ODmb [(R)- and (S)-24] were synthesized in 72% and 75% yields, respectively, from Fmoc-D- and L-Val-OH (D- and L-17) by a two-step approach employing EtOAc (η = 1.0 µL mg−1) as the liquid medium at 80 g0 featuring (i-Pr)2NEt (200 mol%) mediated esterification (8 min), followed by Fmoc group removal from esters 23 with 5 vol% 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 12 min) and aqueous workup.
Amides and dipeptides (e.g., 30–34) were synthesized employing RAM with DME and EtOAc as liquid media, respectively. Couplings were conducted with the RAM in DME (η = 0.9–1.0 μL mg−1) typically at 60 g0 for 10 min (Fig. 2) and in EtOAc (η = 1.0 μL mg−1) at 80 g0 (Fig. 3).
Employing ammonium chloride in DME, Boc-Leu-OH (18) and Boc-Lys(Tfa)-OH (19)47 were converted into Boc-Leu-NH2 [(S)-25] and Boc-Lys(Tfa)-NH2 [(S)-26] in 84% and 95% yields. Dipeptide o-Nps-Ala-Trp(Boc)-ODmb [(S,S)-30] was synthesized from o-Nps-Ala-OH (35, 105 mol%)48 and H-Trp(Boc)-ODmb [(S)-29, 100 mol%] in 98% yield after aqueous workup. A protected dipeptide, Fmoc-Arg(Pbf)-Gly-OEt (31), from a fragment used in the solution-phase supported synthesis of semaglutide49 was prepared in 89% yield by coupling Fmoc-Arg(Pbf)-OH (36, 100 mol%) and HCl·Gly-OEt (37, 150 mol%).
A two-step process was developed to prepare H-D-Leu-NHCH2CCH (28) and H-D-Glu(t-Bu)-D-Leu-NHCH2CCH (33) in 87% and 69% yields, respectively, without detectable racemization from the respective Fmoc-amino acids (D-20 and 40) with propargylamine and H-D-Leu-NHCH2CCH (28) in EtOAc (η = 1.0 µL mg−1) at 80 g0 featuring coupling (8 min), followed by Fmoc group removal from amide 27 with 5 vol% DBU (12 min) and aqueous workup. The Fmoc group was also removed from Fmoc-Trp(Boc)-ODmb [(S)-21] using a combination of diethylamine (1000 mol%) and DBU (40 mol%) in DME (η = 0.90 µL mg−1) at 60 g0 for 10 min. After evaporation of the volatiles, H-Trp(Boc)-ODmb [(S)-29] was isolated in 98% yield by aqueous workup, followed by filtration through a silica gel pad to remove non-polar dibenzofulvene and its corresponding diethylamine adduct.
The challenging coupling of two α-amino iso-butyric acid (H-Aib-OH) residues was also examined by the synthesis of Fmoc-Aib-Aib-Ot-Bu (32) in 82% yield from the reaction of Fmoc-Aib-OH (38, 100 mol%) and H-Aib-OtBu (39, 150 mol%) employing EDCI (200 mol%), Oxyma (300 mol%) and Et3N (200 mol%) in DME at 80 g0 for 40 min. The challenging coupling of two β-substituted amino acids was also explored by the synthesis of Fmoc-D-Thr(t-Bu)-D- and L-Val-ODmb [(R,R- and R,S)-34] in 95% and 94% yields respectively from Fmoc-D-Thr(t-Bu)-OH (42) and H-D- and L-Val-ODmb [(R)- and (S)-24] using EDCI (200 mol%) and Oxyma (300 mol%) in EtOAc (η = 1.0 µL mg−1) at 80 g0 for 40 min, followed by aqueous workup and chromatographic purification.
Tetrapeptide synthesis by fragment coupling
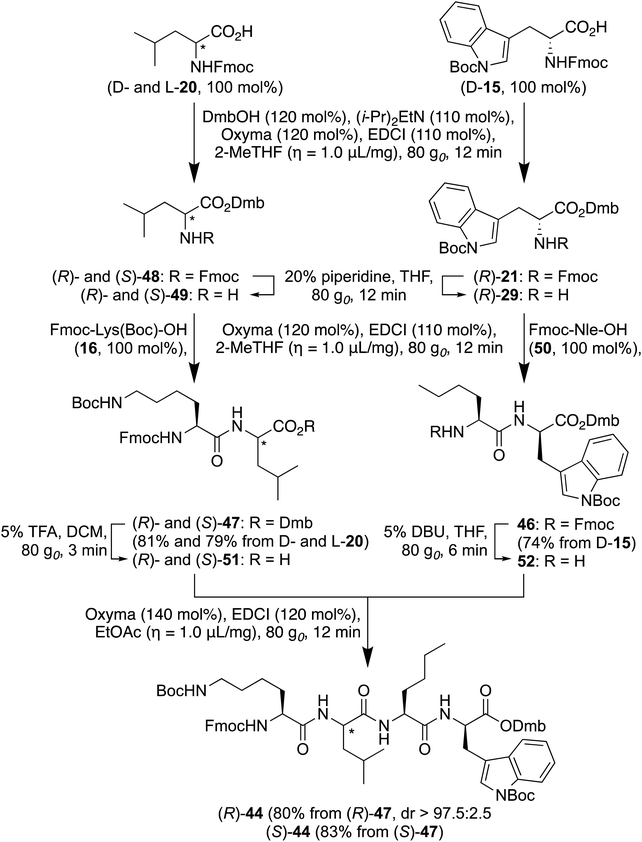
The synthesis of two tetrapeptides, Fmoc-D-Thr(t-Bu)-D-Val-D-Glu(t-Bu)-D-Leu-NHCH2CCH [(R)-43] and Fmoc-Lys(Boc)-D-Leu-Nle-D-Trp(Boc)-ODmb [(R)-44], from their respective dipeptide precursors was explored to examine the RAM in fragment coupling. Tetrapeptide (R)-43 possesses a component of an interleukin-1 receptor antagonist under study, because of its potential to suppress inflammatory activation, prolong gestation and improve neonatal outcomes induced in mice by group B streptococcus.50 Tetrapeptide (R)-44 is a protected part of an anti-amyloidogenic cyclic D/L-α-peptide analog under investigation for the early detection and treatment of Alzheimer's disease.51 In addition to their interesting utility for making medicinally relevant peptide targets, tetrapeptides 43 and 44 offer synthetic challenges because of issues in coupling side chain protected and β-branched residues as well as epimerization in coupling fragments to make sequences with alternating D- and L-residue configurations.52,53 In the synthesis of tetrapeptides 43 (Chart 2) and 44, an Fmoc/t-Bu strategy was employed using the environmentally friendly solvents 2-MeTHF and EtOAc without (i-Pr)2NEt as bases in couplings of free amines.Commencing with Fmoc-D-Thr(t-Bu)-D- and L-Val-ODmb [(R)- and (S)-34], the Dmb esters were solvolyzed with 5 vol% TFA in CH2Cl2 at 80 g0 for 4 min. After aqueous workup, the corresponding acids [(R)- and (S)-45, 100 mol%] were coupled to H-D-Glu(t-Bu)-D-Leu-NHCH2CCH (33) using EDCI (200 mol%) and Oxyma (300 mol%) in EtOAc (η = 1.0 µL mg−1) at 80 g0 for 40 min. After purification on silica gel, Fmoc-D-Thr(t-Bu)-D- and L-Val-D-Glu(t-Bu)-D-Leu-NHCH2CCH [(R)- and (S)-43] were isolated in 79% and 77% yields, respectively. Before chromatography, tetrapeptides (R)- and (S)-43 were shown to, respectively, possess 4% and 3% of the corresponding (S)- and (R)-diastereomer by LC-MS analysis.
For the synthesis of tetrapeptides Fmoc-Lys(Boc)-D- and L-Leu-Nle-D-Trp(Boc)-ODmb [(R)- and (S)-44], orthogonally protected dipeptide fragments Fmoc-Nle-D-Trp(Boc)-ODmb (46) and Fmoc-Lys(Boc)-D- and L-Leu-ODmb [(R)- and (S)-47] were, respectively, synthesized in three steps without isolation of intermediates. Esters of Fmoc-D-Trp(Boc)-OH (D-15) and Fmoc-D- and L-Leu-OH (D- and L-20) were prepared using 2,4-dimethoxybenzyl alcohol with EDCI, (i-Pr)2NEt, and Oxyma in 2-MeTHF (η = 1.0 μL mg−1) at 80 g0 for 12 min in 87–97% conversion with minor amounts of unreacted starting acid as the only detectable impurity by LC-MS analysis. Without further purification, the Fmoc group was removed from Dmb ester (R)-21 and Fmoc-D- and L-Leu-ODmb [(R)- and (S)-48] using 20% piperidine in THF at 80 g0 for 12 min, and residual amino acid impurity was removed by aqueous wash. Although most of the dibenzofulvene and its piperidine adduct were removed by precipitation from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeCN
1 MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution, followed by extraction of the amine into EtOAc, residual amounts were detected by LC-MS and carried forward into the subsequent coupling reactions.
H2O solution, followed by extraction of the amine into EtOAc, residual amounts were detected by LC-MS and carried forward into the subsequent coupling reactions.
Amine (R)-29 and H-D- and L-Leu-ODmb [(R)- and (S)-49] were amino acylated using an excess (105 mol%) of Fmoc-Nle-OH (50) and Fmoc-Lys(Boc)-OH (16) employing EDCI and Oxyma in 2-MeTHF (η = 1.0 μL mg−1) at 80 g0 for 12 min to provide protected dipeptides 46, (R)-47 and (S)-47 in 74%, 81% and 79% overall yields after precipitation from Et2O using a minimum amount of hexanes. Similar yields of dipeptides 46, (R)-47 and (S)-47 were obtained by performing the coupling reaction in EtOAc.
Prior to fragment coupling, Dmb esters (R)- and (S)-47 were solvolyzed to Fmoc-Lys(Boc)-D- and L-Leu-OH [(R)- and (S)-51] using a 5 vol% solution of TFA in CH2Cl2 (η = 1.0 μL mg−1) at 80 g0 for 3 min. Higher and lower TFA concentrations resulted in partial loss of the Boc protection and extended reaction times. After neutralization of the excess TFA with carbonate, filtration, evaporation, and digestion of the residue with methanol, acids (R)- and (S)-51 were obtained upon evaporation of the volatiles and estimated to have >98% purity by LCMS analysis. Removal of the Fmoc group from Fmoc-Nle-D-Trp(Boc)-ODmb (46) using the mentioned RAM conditions with piperidine gave a side product showing a molecular ion consistent with diketopiperazine. The latter was avoided by employing 5 vol% DBU in EtOAc (η = 1.0 μL mg−1) at 80 g0 for 6 min, and H-Nle-D-Trp(Boc)-ODmb (52) contaminated with dibenzofulvene was obtained after aqueous wash and employed in the subsequent fragment couplings.
In the fragment couplings, dipeptide acids (R)- and (S)-51 (105 mol%) were reacted with amine 52 (100 mol%) using EDCI (120 mol%) and Oxyma (140 mol%) in EtOAc (η = 1.0 μL mg−1) at 80 g0 for 12 min with ≥97% conversion. The diastereomeric purity of Fmoc-Lys(Boc)-D-Leu-Nle-D-Trp(Boc)-ODmb [(R)-44] was assessed using chiral SFC, the limits of detection of which demonstrated that <2.5% of the (S)-diastereomer [(S)-44] was present in the crude peptide. Purification by chromatography on silica gel gave tetrapeptides (R)- and (S)-44 in 80% and 83% overall yields, respectively, from protected dipeptides (R)- and (S)-47.
Synthesis of Aib-enkephalin
The synthesis and analysis of the enkephalin amide analog, H-Tyr-Aib-Aib-Phe-Met-NH2, demonstrated that steric constraints could enhance conformational rigidity favouring a consecutive β-turn structure as well as improved activity in a behavioural assay in mice.54 Consequently, Aib-enkephalin (H-Tyr-Aib-Aib-Phe-Leu-NH2, 54) has become a testing ground for demonstrating technology, including coupling agents, benign liquids, and the use of ultrasound for enhanced sustainable solid-phase peptide synthesis.55–57 Targeting Aib-enkephalin 54 to validate the synthesis of a challenging peptide with the RAM, a sequence was pursued from Boc-Leu-NH2 [(S)-25]. Acid labile Boc and t-butyl groups were removed by conventional solvolysis using 4 M hydrochloride in 1,4-dioxane. Couplings were typically performed using Boc-protected amino acids (100 mol%) and resulting hydrochloride salts (100 mol%) employing the RAM with EDCI, Oxyma and base (100 mol%) in DME (η = 0.90 µL mg−1) at 80 g0 for 15–20 min (Scheme 3). Isolation and characterization of each hydrochloride salt (e.g., 54) and the protected penultimate peptide 62 were performed without chromatography using aqueous workup, trituration, and precipitation.Discussion
Sustainable peptide manufacturing will require minimizing PMI with attention to the overhaul of contemporary use of toxic solvents (e.g., DMF and CH2Cl2), corrosive acids (e.g., TFA), and atom-intensive protecting groups (e.g., the Fmoc group).7,58 Solvent use should also be minimized in peptide product isolation and purification. Among efforts to reduce waste in solid-phase synthesis, microwave heating and ultrasound agitation have been used to accelerate reaction times and minimize resin washings, which account for 90% of solvent use.59,60 In solution-phase peptide synthesis, mechanochemistry by solvent-less ball milling and twin-screw extrusion methods has provided short peptides employing conventional protection and coupling methods.13,61 For example, the dipeptide sweetener aspartame has been made by milling and extrusion methods.61 Moreover, by activation of C-terminal glycine fragments, ball milling has been used to synthesize octapeptides, e.g., Boc-Tyr(OBn)-D-Ala-Phe-Gly-Gly-Tyr-Pro-OMe.62 Collisional and frictional interactions from mechanochemical methods impart however significant stress to the reaction material and may lead to irregular rises in local temperature and risk of contamination from wear of the milling media.63,64Resonant acoustic mixing does not rely on high-impact mechanical force; instead, low-frequency resonance and acoustic energy are transferred effectively to achieve mixing.17,18 With the objective of exploring the merits of the RAM in solution-phase peptide synthesis, several notable results have been revealed. Rapid coupling of various protected amino acids and dipeptide fragments was achieved with minimal epimerization and relatively high yields. Stoichiometric amounts of coupling partners were reacted effectively in environmentally friendly solvents, such as EtOAc, DME and 2-MeTHF. Difficult couplings between sterically hindered amino acids were accomplished efficiently. High concentration (η ≤ 1 μL mg−1) minimized solvent waste. Focussing on EDCI and Oxyma as coupling agents enabled the removal of most impurities by aqueous workup and trituration after the reaction. In addition to amide bond formation, effective esterification was achieved under related conditions. Mixing with the RAM at forces of 60 g0 and 80 g0 at high concentration proved critical for good reaction conversion. Gram-scale reactions were also demonstrated for the syntheses of esters [e.g., (S)-21 and 22] and dipeptides [e.g., (S,S)-30, 31, 46, and (R)- and (S)-47, see the SI].
In the present study, conventional protecting groups lacking atom economy (e.g., Fmoc) and cleavage conditions with corrosive acids (e.g., TFA) in undesirable solvents (e.g., CH2Cl2) were employed in certain cases primarily because the focus was on coupling chemistry. Cursory investigations have also indicated that other amide-bond forming conditions were effective using the RAM. Beyond solution-phase synthesis, the RAM may likely hold promise for supported liquid-phase and solid-phase synthesis. Further experimentation with the RAM is ongoing to address the above issues for the preparation of longer and more arduous sequences and will be reported in due time.
In sum, application of the RAM has enabled efficient formation of amino esters, amino amides, and peptides under mild conditions with minimal solvent usage. Operating the RAM at 60–80 g0 and employing high concentration in environmentally benign liquid (η ≤ 1 µL mg−1) gave rapidly high conversions. Low liquid viscosity and judicious use of organic bases emerged as critical factors for effective mixing, minimizing epimerization and superior reaction outcomes. Across multiple coupling models, including sterically hindered, epimerization-prone and fragment assembly systems, the RAM gave successful high conversion, rapid reaction rates and quality peptides comparable to modern mechanochemical and solution-phase methods. Collectively, the RAM method has proven to be a pragmatic means for assembling ester and amide bonds offering striking potential for overcoming synthetic obstacles and meeting environmental requirements for sustainable and resource-efficient peptide production.
Author contributions
N. M. M. conceived and performed initial experiments. N. M. M., J. D. M., Z. I., and K. G. conceived and executed experiments; W. D. L. and F. P. supervised the studies; W. D. L. and F. P. provided resources; N. M. M., J. D. M., Z. I., and K. G. analyzed the data. N. M. M., J. D. M., Z. I., K. G., and W. D. L. wrote the manuscript; W. D. L. edited the manuscript; and N. M. M., J. D. M., Z. I., K. G., F. P., and W. D. L. made manuscript revisions. All authors approved the final version of this manuscript.Conflicts of interest
The authors declare no competing interests.Data availability
Supplementary information (SI): all experimental procedures, characterization data (NMR, MS, HPLC, and LC-MS analyses) and supporting analyses. See DOI: https://doi.org/10.1039/d5gc04619j.Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada (NSERC) for funding a Discovery Research Projects (RGPIN-2019-04079 and RGPIN-2025-04423), the Canadian Institutes of Health Research (CIHR) for the project PJT-186296 “Treating Age-Related Macular Degeneration by Mitigating Inflammation, Oxidative Stress, and Lipid Accumulation through CD36 Modulation”, and the Fonds de Recherche Nature et Technologie Québec for support of project #328969 “Étude des interactions supramoléculaires de feuillets bêta de l'amyloïde modulée par les azapeptides pour mieux comprendre la base structurelle des maladies amyloïdes et faciliter leur détection précoce”, and the Centre in Green Chemistry and Catalysis (CGCC, FRQNT-2020-RS4-265155-CCVC). Operation of the NMR spectrometers at the Regional Centre for Magnetic Resonance in the Department of Chemistry at the Université de Montréal was made possible through funding from the Canada Foundation for Innovation and the Institute Courtois. The authors acknowledge the assistance of members of the Université de Montréal facilities: Dr P. Aguiar, Dr C. Malveau and N. Baho (NMR spectroscopy), and Dr A. Fürtös, Dr M.-A. Vaudreuil and K. Gilbert (mass spectrometry). NMM and JDM thank respectively NSERC and FRQNT for graduate fellowships.References
- B. G. de la Torre and F. Albericio, Peptide Therapeutics 2.0, Molecules, 2020, 25(10), 2293 CrossRef CAS PubMed
.
- D. J. Drucker, Advances in oral peptide therapeutics, Nat. Rev. Drug Discovery, 2020, 19(4), 277–289 CrossRef CAS PubMed
.
- K. Fosgerau and T. Hoffmann, Peptide therapeutics: current status and future directions, Drug Discovery Today, 2015, 20(1), 122–128 CrossRef CAS PubMed
.
- L. B. Knudsen and J. Lau, The discovery and development of liraglutide and semaglutide, Front. Endocrinol., 2019, 10, 155 CrossRef PubMed
.
- R. B. Merrifield, Solid phase peptide synthesis. I. The synthesis of a tetrapeptide, J. Am. Chem. Soc., 1963, 85(14), 2149–2154 CrossRef CAS
.
- A. Isidro-Llobet, M. N. Kenworthy, S. Mukherjee, M. E. Kopach, K. Wegner and F. Gallou,
et al., Sustainability challenges in peptide synthesis and purification: from R&D to production, J. Org. Chem., 2019, 84(8), 4615–4628 CrossRef CAS PubMed
.
- I. Kekessie, K. Wegner, I. Martinez, M. E. Kopach, T. D. White and J. K. Tom,
et al., Process Mass Intensity (PMI): A Holistic Analysis of Current Peptide Manufacturing Processes Informs Sustainability in Peptide Synthesis, J. Org. Chem., 2024, 89(7), 4261–4282 CrossRef CAS PubMed
.
- S. B. Lawrenson, R. Arav and M. North, The greening of peptide synthesis, Green Chem., 2017, 19(7), 1685–1691 RSC
.
- J. Sherwood, F. Albericio and B. G. de la Torre, N, N–Dimethyl Formamide European Restriction Demands Solvent Substitution in Research and Development, ChemSusChem, 2024, 17(8), e202301639 CrossRef CAS PubMed
.
- K. G. Varnava and V. Sarojini, Making solid–phase peptide synthesis greener: a review of the literature, Chem. – Asian J., 2019, 14(8), 1088–1097 CrossRef CAS PubMed
.
- L. Ferrazzano, D. Corbisiero, G. Martelli, A. Tolomelli, A. Viola and A. Ricci,
et al., Green solvent mixtures for solid-phase peptide synthesis: A dimethylformamide-free highly efficient synthesis of pharmaceutical-grade peptides, ACS Sustainable Chem. Eng., 2019, 7(15), 12867–12877 CrossRef CAS
.
- Y. E. Jad, G. A. Acosta, T. Govender, H. G. Kruger, A. El-Faham and B. G. de la Torre,
et al., Green solid-phase peptide synthesis 2. 2-Methyltetrahydrofuran and ethyl acetate for solid-phase peptide synthesis under green conditions, ACS Sustainable Chem. Eng., 2016, 4(12), 6809–6814 CrossRef CAS
.
- V. Declerck, P. Nun, J. Martinez and F. Lamaty, Solvent-free synthesis of peptides, Angew. Chem., Int. Ed., 2009, 48, 9318–9321 CrossRef CAS PubMed
.
- Y. Yeboue, M. Jean, G. Subra, J. Martinez, F. Lamaty and T.-X. Métro, Epimerization-free C-term activation of peptide fragments by ball milling, Org. Lett., 2021, 23(3), 631–635 CrossRef CAS PubMed
.
- S. L. Wenski, S. Thiengmag and E. J. Helfrich, Complex peptide natural products: biosynthetic principles, challenges and opportunities for pathway engineering, Synth. Syst. Biotechnol., 2022, 7(1), 631–647 CrossRef CAS PubMed
.
- J. Pawlas, T. Nuijens, J. Persson, T. Svensson, M. Schmidt and A. Toplak,
et al., Sustainable, cost-efficient manufacturing of therapeutic peptides using chemo-enzymatic peptide synthesis (CEPS), Green Chem., 2019, 21(23), 6451–6467 RSC
.
- I. U. Khan, R. Guo, U. Farooq, S. Adhikari and H. Zhou, Parametric effects on the mixing efficiency of resonant acoustic mixing technology for high-viscosity mixture: A numerical study, Processes, 2023, 11(1), 266 CrossRef
.
- D. Liao, H. Cao, S. Li, W. Cheng, X. Yan and C. An, Study on the control of flow field by resonance acoustic mixing technology for purification of high performance spherical HATO crystals, Sep. Purif. Technol., 2024, 339, 126688 CrossRef CAS
.
- M. R. Rasouli and M. Tabrizian, An ultra-rapid acoustic micromixer for synthesis of organic nanoparticles, Lab Chip, 2019, 19(19), 3316–3325 RSC
.
- D. J. am Ende, S. R. Anderson and J. S. Salan, Development and scale-up of cocrystals using resonant acoustic mixing, Org. Process Res. Dev., 2014, 18(2), 331–341 CrossRef CAS
.
- D. Yalcin, S. Rajesh, J. White, S. C. Howard, P. J. Pigram and N. Tran,
et al., Resonant acoustic mixing method to produce lipid-based liquid-crystal nanoparticles, J. Phys. Chem. C, 2021, 125(19), 10653–10664 CrossRef CAS
.
- A. S. Makarov and M. Rueping, Scalable depolymerizing transesterification and amidation of (poly) lactic acid (PLA) enabled by resonant acoustic mixing (RAM), Green Chem., 2025, 27, 716–721 RSC
.
- E. Hamzehpoor, F. Effaty, T. H. Borchers, R. S. Stein, A. Wahrhaftig-Lewis and X. Ottenwaelder,
et al., Mechanochemical Synthesis of Boroxine–linked Covalent Organic Frameworks, Angew. Chem., 2024, 136(51), e202404539 CrossRef
.
- L. Gonnet, C. B. Lennox, J. L. Do, I. Malvestiti, S. G. Koenig and K. Nagapudi,
et al., Metal-Catalyzed Organic Reactions by Resonant Acoustic Mixing, Angew. Chem., Int. Ed., 2022, 61(13), e202115030 CrossRef CAS PubMed
.
- A. Nanni, D. Kong, C. Zhu and M. Rueping, Nickel-catalyzed cross-coupling aminations via high-throughput mechanochemistry enabled by resonant acoustic mixing, Green Chem., 2024, 26(14), 8341–8347 RSC
.
- J. D. Thorpe, J. Marlyn, S. G. Koenig and M. J. Damha, Synthesis of short DNA and RNA fragments by resonant acoustic mixing (RAM), RSC Mechanochem., 2024, 1, 244–249 RSC
.
- C. Chatzigiannis, S. Scalzullo, E. Colacino and M. E. Rivas-Velazco, Multigram–scale organic synthesis by Resonant Acoustic Mixing. Application to the Sustainable Preparation of Active Pharmaceutical Ingredients, ChemSusChem, 2025, e202500110 CrossRef CAS PubMed
.
- N. Maarouf-Mesli, J. Desjardins-Michaud, Z. Imani, K. Ghosh, F. Polyak and W. D. Lubell, Solvent–Less Environmentally Benign Amino Ester, Amide, and Peptide Synthesis Enabled by Resonant Acoustic Mixing, ChemRxiv, 2025 DOI:10.26434/chemrxiv-2025-67qlj
.
- During review of the manuscript, the following publication was brought to our attention: A. Nanni, P. Bilalis and M. Rueping, Resonant acoustic mixing enables solvent-less amide coupling in solid-phase peptide synthesis, Green Chem, 2025 10.1039/D5GC04067A
.
- A. El-Faham, R. S. Funosas, R. Prohens and F. Albericio, COMU: a safer and more effective replacement for benzotriazole–based uronium coupling reagents, Chem. – Eur. J., 2009, 15(37), 9404–9416 CrossRef CAS PubMed
.
- R. Subirós-Funosas, R. Prohens, R. Barbas, A. El-Faham and F. Albericio, Oxyma: An Efficient Additive for Peptide Synthesis to Replace the Benzotriazole–Based HOBt and HOAt with a Lower Risk of Explosion [1], Chem. – Eur. J., 2009, 15(37), 9394–9403 CrossRef PubMed
.
- L. A. Carpino, A. El-Faham and F. Albericio, Efficiency in peptide coupling: 1-hydroxy-7-azabenzotriazole vs 3, 4-dihydro-3-hydroxy-4-oxo-1, 2, 3-benzotriazine, J. Org. Chem., 1995, 60(11), 3561–3564 CrossRef CAS
.
- D. Prat, J. Hayler and A. Wells, A survey of solvent selection guides, Green Chem., 2014, 16(10), 4546–4551 RSC
.
- Y. E. Jad, S. N. Khattab, B. G. de la Torre, T. Govender, H. G. Kruger and A. El-Faham,
et al., EDC· HCl and Potassium Salts of Oxyma and Oxyma–B as Superior Coupling Cocktails for Peptide Synthesis, Eur. J. Org. Chem., 2015, 2015(14), 3116–3120 CrossRef CAS
.
- J. Lopez, S. Pletscher, A. Aemissegger, C. Bucher and F. Gallou, N-butylpyrrolidinone as alternative solvent for solid-phase peptide synthesis, Org. Process Res. Dev., 2018, 22(4), 494–503 CrossRef CAS
.
- A. Kumar, M. Alhassan, J. Lopez, F. Albericio and B. G. de la Torre, N-Butylpyrrolidinone for solid–phase peptide synthesis is environmentally friendlier and synthetically better than DMF, ChemSusChem, 2020, 13(19), 5288–5294 CrossRef CAS PubMed
.
- J. Sherwood, H. L. Parker, K. Moonen, T. J. Farmer and A. J. Hunt, N-Butylpyrrolidinone as a dipolar aprotic solvent for organic synthesis, Green Chem., 2016, 18(14), 3990–3996 RSC
.
- C. Reichardt, Solvatochromic dyes as solvent polarity indicators, Chem. Rev., 1994, 94(8), 2319–2358 CrossRef CAS
.
- J. Pawlas and J. H. Rasmussen, Environmentally Sensible Organocatalytic Fmoc/t-Bu Solid-Phase Peptide Synthesis, Org. Lett., 2022, 24(9), 1827–1832 CrossRef CAS PubMed
.
- Q. Huo and X. Wang, Mixing mechanism of power-law non-Newtonian fluids in resonant acoustic mixing, Phys. Fluids, 2024, 36(2), 024121 CrossRef CAS
.
- H. Reich and F. Bordwell, Equilibrium pKa Table (DMSO Solvent and Reference), ACS Div. Org. Chem., 2021 Search PubMed
.
- A. M. Kelly-Rowley, V. M. Lynch and E. V. Anslyn, Molecular recognition of enolates of active methylene compounds in acetonitrile. The interplay between complementarity and basicity and the use of hydrogen bonding to lower guest pKas, J. Am. Chem. Soc., 1995, 117(12), 3438–3447 CrossRef CAS
.
- A. El-Faham and F. Albericio, Peptide coupling reagents, more than a letter soup, Chem. Rev., 2011, 111(11), 6557–6602 CrossRef CAS PubMed
.
- U. Filp, A. Pekošak, A. J. Poot and A. D. Windhorst, Stereocontrolled [11C] Alkylation of N–Terminal Glycine Schiff Bases To Obtain Dipeptides, Eur. J. Org. Chem., 2017, 2017(37), 5592–5596 CrossRef CAS
.
- Y. E. Jad, G. A. Acosta, S. N. Khattab, B. G. de la Torre, T. Govender and H. G. Kruger,
et al., 2-Methyltetrahydrofuran and cyclopentyl methyl ether for green solid-phase peptide synthesis, Amino Acids, 2016, 48, 419–426 CrossRef CAS PubMed
.
- M. Goodman and W. McGahren, Mechanistic studies of peptide oxazolone racemization, Tetrahedron, 1967, 23(5), 2031–2050 CrossRef CAS PubMed
.
- J. Rizo, F. Albericio, E. Giralt and E. Pedroso, Reversible protection of lysine to facilitate the purification of protected peptide segments, Tetrahedron Lett., 1992, 33(3), 397–400 CrossRef CAS
.
- K. S. Kanyiva, M. Tane and T. Shibata, Iodine-catalyzed synthesis of chiral 4-imidazolidinones using α-amino acid derivatives via dehydrogenative N–H/C (sp3)–H coupling, J. Org. Chem., 2019, 84(20), 12773–12783 CrossRef CAS PubMed
.
- X. Liu, N. Zhang, X. Gu, Y. Qin, D. Song and L. Zhang,
et al., Total synthesis of semaglutide based on a soluble hydrophobic-support-assisted liquid-phase synthetic method, ACS Comb. Sci., 2020, 22(12), 821–825 CrossRef CAS PubMed
.
- P. Y. Chin, L. M. Moldenhauer, W. D. Lubell, D. M. Olson, S. Chemtob and J. A. Keelan,
et al., Inhibition of interleukin-1 signaling protects against Group B streptococcus-induced preterm birth and fetal loss in mice, J. Reprod. Immunol., 2025, 169, 104520 CrossRef CAS PubMed
.
- M. Habashi, S. Vutla, K. Tripathi, S. Senapati, P. S. Chauhan and A. Haviv-Chesner,
et al., Early diagnosis and treatment of Alzheimer's disease by targeting toxic soluble Aβ oligomers, Proc. Natl. Acad. Sci. U. S. A., 2022, 119(49), e2210766119 CrossRef CAS PubMed
.
- N. L. Benoiton, K. Kuroda and F. M. Chen, The relative susceptibility to racemization of L-and D-residues in peptide synthesis, Tetrahedron Lett., 1981, 22(35), 3359–3360 CrossRef CAS
.
- M. Paradís-Bas, J. Tulla-Puche and F. Albericio, The road to the synthesis of “difficult peptides”, Chem. Soc. Rev., 2016, 45(3), 631–654 RSC
.
- T. Sudha and P. Balaram, Stabilization of β-turn conformations in enkephalins: α-Aminoisobutyric acid analogs, Int. J. Pept. Protein Res., 1983, 21(4), 381–388 CrossRef CAS PubMed
.
- R. Subiros-Funosas, G. A. Acosta, A. El-Faham and F. Albericio, Microwave irradiation and COMU: a potent combination for solid-phase peptide synthesis, Tetrahedron Lett., 2009, 50(45), 6200–6202 CrossRef CAS
.
- A. Kumar, Y. E. Jad, A. El-Faham, B. G. de la Torre and F. Albericio, Green solid-phase peptide synthesis 4. γ-Valerolactone and N-formylmorpholine as green solvents for solid phase peptide synthesis, Tetrahedron Lett., 2017, 58(30), 2986–2988 CrossRef CAS
.
- S. Mottola, A. Del Bene, V. Mazzarella, R. Cutolo, I. Boccino and F. Merlino,
et al., Sustainable Ultrasound-Assisted Solid-Phase peptide synthesis (SUS-SPPS): Less Waste, more efficiency, Ultrason. Sonochem., 2025, 107257 CrossRef CAS PubMed
.
- K. Ghosh and W. D. Lubell, N–to C–Peptide Synthesis, Arguably the Future for Sustainable Production, J. Pept. Sci., 2025, 31(6), e70019 CrossRef CAS PubMed
.
- F. Merlino, S. Tomassi, A. M. Yousif, A. Messere, L. Marinelli and P. Grieco,
et al., Boosting Fmoc solid-phase peptide synthesis by ultrasonication, Org. Lett., 2019, 21(16), 6378–6382 CrossRef CAS PubMed
.
- J. M. Collins, S. K. Singh, T. A. White, D. J. Cesta, C. L. Simpson and L. J. Tubb,
et al., Total wash elimination for solid phase peptide synthesis, Nat. Commun., 2023, 14(1), 8168 CrossRef CAS PubMed
.
- T. Mohy El-Dine, M. Lavayssiere, H. Adihou, G. Subra, T. X. Métro and O. Ludemann-Hombourger,
et al., Synthesis of peptides by reactive extrusion. Application to the continuous and solventless preparation of aspartame, ChemistryEurope, 2024, 2(3–4), e202400007 CrossRef CAS
.
- A. Wróblewska, I. I. Bak-Sypien, P. Paluch, E. Wielgus, J. Zając and A. Jeziorna,
et al., Solvent-Free Mechanosynthesis of Oligopeptides by Coupling Peptide Segments of Different Lengths-Elucidating the Role of Cesium Carbonate in Ball Mill Processes, Chem. – Eur. J., 2024, 30(44), e202400177 CrossRef PubMed
.
- J. Jablan, E. Marguí, L. Posavec, D. Klarić, D. Cinčić and N. Galić,
et al., Product contamination during mechanochemical synthesis of praziquantel co-crystal, polymeric dispersion and cyclodextrin complex, J. Pharm. Biomed. Anal., 2024, 238, 115855 CrossRef CAS PubMed
.
- L. Vugrin, C. Chatzigiannis, E. Colacino and I. Halasz, In situ Raman spectroscopy for comparing ball milling and resonant acoustic mixing in organic mechanosynthesis, RSC Mechanochem., 2025, 2(3), 482–487 RSC
.
| This journal is © The Royal Society of Chemistry 2026 |