Direct allylic C(sp3)–H acylation of alkenes via metallaphotoredox catalysis using carboxylic acids
Xin
Ge
a,
Can-Quan
Ye
a,
Mei
Huang
a,
Meng-Ning
Wang
a,
Jie-Yu
Chen
a,
Ri-Yuan
Tang
 *abc and
Bo
Yang
*abc and
Bo
Yang
 *ab
*ab
aKey Laboratory for Biobased Materials and Energy of Ministry of Education, College of Materials and Energy, South China Agricultural University, Guangzhou 510642, P. R. China. E-mail: boyang@scau.edu.cn; rytang@scau.edu.cn
bKey Laboratory of Advanced Materials for Facility Agriculture, Ministry of Agriculture and Rural Affairs, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641, P. R. China
cNational Key Laboratory of Green Pesticide, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641, P. R. China
First published on 24th November 2025
Abstract
The direct catalytic coupling of feedstock chemicals to construct complex scaffolds is a central challenge in synthetic chemistry. Here, we report a metallaphotoredox strategy enabling direct allylic C(sp3)–H acylation of alkenes using carboxylic acids as versatile acylating agents. This nickel/photoredox dual catalytic system operates under mild conditions and merges two abundant substrates, carboxylic acids and unactivated alkenes, to construct β,γ-unsaturated ketones, privileged motifs in bioactive molecules, via a mechanistically distinct pathway that bypasses traditional prefunctionalization requirements. Key to this transformation is the synergistic generation of bromine radicals from a nickel-bound bromide, which selectively abstract allylic hydrogen atoms, and the subsequent interception of transient allyl radicals by acyl–Ni(II) intermediates. The reaction exhibits broad scope and enables the programmable synthesis of bis(β,γ-unsaturated ketones) from dicarboxylic acids. This work establishes a platform for C(sp3)–H acylation with high atom- and step-economy, offering a general disconnection for carbonyl–alkene linkages.
Green foundation1. Our metallaphotoredox catalysis enables direct allylic C(sp3)–H acylation using abundant carboxylic acids and unactivated alkenes under mild conditions, eliminating prefunctionalization and reducing waste. Polar aprotic solvents (e.g., DMA, DME, and DMF), frequently used in metallaphotoredox cross-coupling reactions, are under increased regulatory pressure, and are generally considered undesirable as green solvents. Moreover, these solvents participate in undesired solvent coupling reactions, which complicates the overall reaction profile and reduces the overall yield. In contrast, the less polar iPrOAc utilized in our system is considered a process-friendly greener solvent.2. Our nickel/photoredox dual catalytic system enables direct allylic C(sp3)–H acylation of unactivated alkenes with free carboxylic acids. This method operates without exogenous hydrogen atom transfer reagents and requires no pre-activation of substrates, providing a more straightforward and versatile approach to valuable β,γ-unsaturated ketone scaffolds. 3. In future work, considering the high cost and scarcity of iridium photocatalysts, further exploration of additional cheap Ir-free photocatalysts as alternatives is highly needed. |
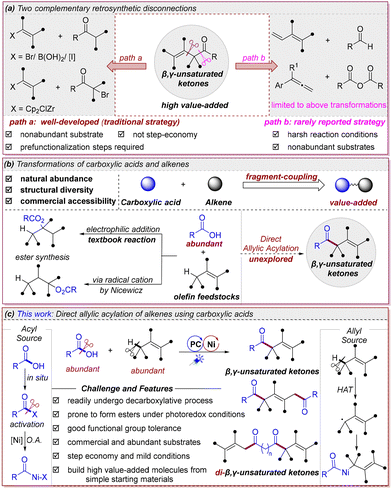
The β,γ-unsaturated ketone scaffold represents a privileged structural motif in organic chemistry, featuring prominently in natural products, bioactive molecules, and as versatile building blocks in synthetic chemistry.1 Despite its ubiquity, the development of efficient and selective methods for its construction remains a significant challenge in modern synthetic chemistry. Conventional approaches for its synthesis predominantly rely upon enolate or enolate equivalent α-alkenylation strategies (Scheme 1a, left),2 including the Ni-catalyzed cross-coupling of ketone enolates with alkenyl halides reported by Helquist3 and Cu/chiral amine-catalyzed α-alkenylation of aldehydes reported by MacMillan.4 While powerful and providing reliable access, these methods universally require less accessible and prefunctionalized coupling partners (alkenyl halides and sensitive organometallics) and focus on forming the α,β-bond of the target scaffold. Crucially, they suffer from inherent limitations in step economy, functional group tolerance, and substrate scope, thereby limiting their broader applications in synthetic chemistry. More fundamentally, the retro-synthetically distinct disconnection via Cα-CO bond formation (Scheme 1a, right) remains underexplored,5 which has hampered their wider application and represents both a synthetic gap and an opportunity for innovation. To address these problems, we sought to develop a step-economic and robust platform to afford β,γ-unsaturated ketones from readily accessible and commercially available feedstock chemicals under mild conditions.
Carboxylic acids and alkenes, owing to their structural diversity, natural abundance, and commercial accessibility, represent privileged synthetic building blocks.6 Their pervasive presence in natural products and functional molecular architectures further establishes them as ideal coupling partners for organic synthesis. In recent years, various transformations have been developed by enabling the activation and subsequent cross-coupling of carboxylic acids7 and alkenes8via transition-metal catalysis in a separate context. Nevertheless, the coupling of these two prevalent structural motifs to build value-added molecules is significantly rare but of high interest. Traditionally, their direct coupling typically yields esters through conventional electrophilic additions9 or nucleophilic attack on radical cations pioneered by Nicewicz10 (Scheme 1b, left). Motivated by the synthetic importance of β,γ-unsaturated ketone architectures and our ongoing exploration of biomass-derived small molecule (carboxylic acid) valorization,7a we sought to investigate whether diverse β,γ-unsaturated ketones could be constructed from the coupling of carboxylic acids and alkenes, in which carboxylic acids were used as acylating agents and alkenes were utilized as allyl fragment donors through allylic C(sp3)–H bond functionalization (Scheme 1b, right). In this regard, the only example of β,γ-unsaturated ketone formation via carboxylic acid/alkene coupling was reported by Wang through a multi-catalytic system involving photoredox and NHC catalysis.11 While pioneering, this system is restricted to aromatic acids, requires exogenous HAT reagents and prefunctionalization of aromatic acids, which therefore limits the synthetic applicability of such a method. This highlights the unmet need for developing a new and efficient catalytic system that enables the direct synthesis of β,γ-unsaturated ketones from free carboxylic acids and unactivated alkenes under mild conditions.
The development of such reactions faces significant challenges, including the scission of the inert C–O bond of the carboxylic acid moiety, control of regioselectivity during alkene functionalization, and the suppression of undesired side reactions such as decarboxylative transformations.7f,12
In recent years, stemming from the pioneering work of MacMillan,13 Molander,14 and Doyle,15 metallaphotoredox catalysis has evolved into a robust platform that may enable the facile assembly of acyl groups at the allylic position of alkenes, providing the desired β,γ-unsaturated ketone architectures. As demonstrated by Molander,16 Doyle17 and their co-workers, photoexcited Ni(II) or Ni(III) complexes can generate halogen radicals, which then act as HAT catalysts in several C–H bond functionalizations.18 Capitalizing on these advances, we proposed that nickel catalysis could uniquely address these challenges by: (i) in situ carboxylic acid activation through transient anhydride formation; (ii) generating halogen radicals in situ for allylic C–H abstraction; (iii) merging both processes via Ni-mediated radical capture. We proposed that a nickel halide (or its ligated species) could serve as both a HAT reagent to convert alkenes into allyl radicals and a transition-metal catalyst to engage sequentially with acyl electrophiles, formed in situ from carboxylic acids,19 and allyl radicals through oxidative addition20 and radical capture.21 The resulting diorganonickel intermediate would then undergo reductive elimination, delivering valuable ketone products with high efficiency (Scheme 1c). Such a method would not only streamline the synthesis of these valuable compounds but also expand the synthetic utility of carboxylic acids as versatile acylating reagents. Furthermore, this transformation provides an orthogonal strategy to traditional esterification, offering a novel pathway for the synthesis of complex molecular architectures. In this work, we developed a nickel/photoredox catalytic system that enables direct allylic C(sp3)–H acylation of unactivated alkenes with free aliphatic carboxylic acids without exogenous HAT reagent, which mutually complements Wang's work.11
Reaction development
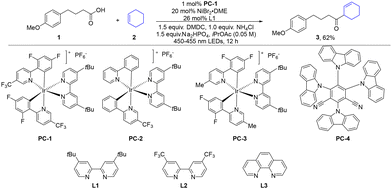
To evaluate the feasibility of our strategy, we initiated our investigations by exploring the reaction of the commercially available 4-(4-methoxyphenyl)butyric acid 1 and cyclohexene 2 under photoredox conditions. After extensive optimization of all reaction parameters, we were pleased to find that the combination of 20 mol% NiBr2·DME, 26 mol% 4,4′-Di-tert-butyl-2,2′-bipyridine (L1), 1 mol% Ir[dF(CF3)ppy]2(dtbbpy)PF6 (PC-1), 1.5 equiv. DMDC, 1.0 equiv. NH4Cl, and 1.5 equiv. Na2HPO4 in iPrOAc under blue LED irradiation provided the desired product 3 in 62% isolated yield (entry 1, Table 1). Among the common photocatalysts tested, only PC-1 and PC-3 proved effective, while others gave negligible yields (entries 1–4). Alternative bases (K2CO3, Cs2CO3, and 2,6-lutidine, entries 5–7) led to significantly reduced or undetectable product formation, confirming Na2HPO4 as optimal. The evaluation of various solvents showed that the process-friendly green solvent (iPrOAc)22 outperformed other solvents, likely due to its compatibility with the reaction system (entries 8–10). Replacing L1 or NiBr2·DME with other ligands/nickel sources drastically diminished the yield of targeted molecule 3, highlighting their critical roles (entries 11–14). While omission of NH4Cl (entry 15), which has been previously employed to facilitate the formation of a mixed anhydride,19a,21c led to a modest yield drop (53% NMR yield), its inclusion improved efficiency, suggesting a beneficial but non-essential role. The reaction failed in the absence of a photocatalyst, nickel catalyst, or light (entries 16–18), confirming their indispensability for this transformation.| Entry | Variation from optimized conditions | Yielda (%) |
|---|---|---|
| a Reaction conditions: 1 (0.3 mmol), 2 (0.6 mmol), 1 mol% Ir[dF(CF3)ppy]2(dtbbpy)PF6 (0.003 mmol), 20 mol% NiBr2·DME (0.06 mmol), 26 mol% L1 (dtbbpy) (0.078 mmol), Na2HPO4 (0.45 mmol), DMDC (0.45 mmol, 1.5 equiv.), NH4Cl (0.3 mmol, 1.0 equiv.), iPrOAc (6 mL), and 450–455 nm LEDs. b Yields of 3 were determined by 1H NMR spectroscopy with mesitylene as an internal standard and isolated yield is shown in parentheses. PC-4, 4CzIPN. | ||
| 1 | None | 56 (62%b) |
| 2 | PC-2 instead of PC-1 | Trace |
| 3 | PC-3 instead of PC-1 | 22 |
| 4 | PC-4 instead of PC-1 | Trace |
| 5 | K2CO3 instead of Na2HPO4 | Trace |
| 6 | Cs2CO3 instead of Na2HPO4 | 0 |
| 7 | 2,6-Lutidine instead of Na2HPO4 | 30 |
| 8 | MeCN instead of iPrOAc | 17 |
| 9 | DCM instead of iPrOAc | 14 |
| 10 | Dioxane instead of iPrOAc | 32 |
| 11 | L2 instead of L1 | 25 |
| 12 | L3 instead of L1 | Trace |
| 13 | Ni(acac)2 instead of NiBr2·DME | 0 |
| 14 | NiCl2 instead of NiBr2·DME | 0 |
| 15 | No NH4Cl | 53 |
| 16 | No PC | 0 |
| 17 | No nickel catalyst | 0 |
| 18 | No light | 0 |
Substrate scope investigation
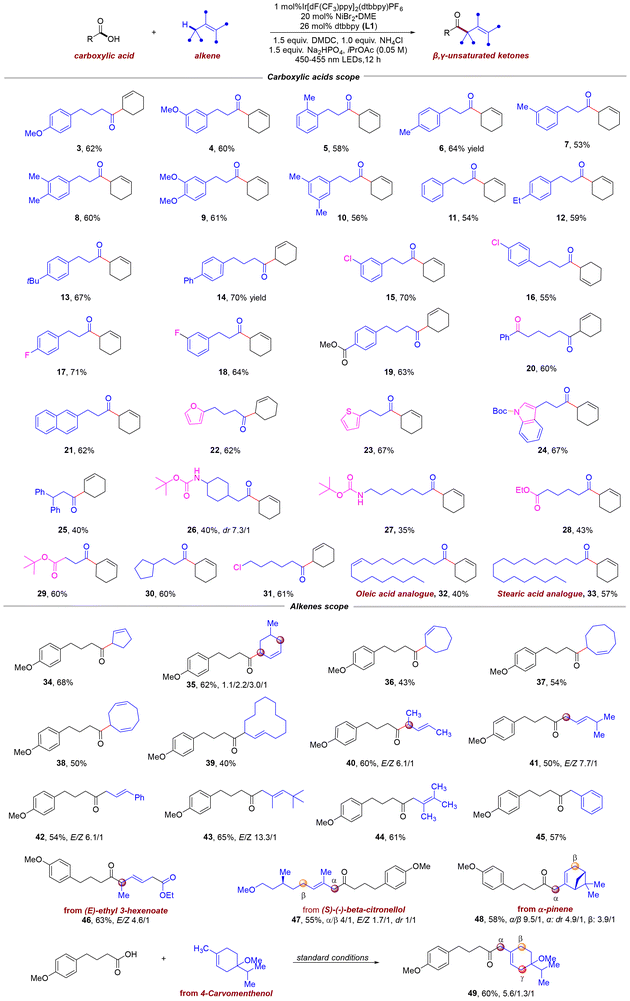
With the optimized reaction conditions in hand, we then investigated the scope of carboxylic acids and alkenes (Scheme 2). First, we examined the ability of various carboxylic acids to undergo this fragment cross-coupling in our system. The developed catalytic system exhibits good applicability to aliphatic carboxylic acids containing aromatic rings, enabling efficient conversion to the corresponding β,γ-unsaturated ketones (3–21). The substituents on the aromatic ring can be either electron-withdrawing or electron-donating groups (3–19). In particular, carboxylic acids with synthetic handles, such as chloride and fluoride (15–18), were readily incorporated into the accessible ketone scaffolds, which highlights the potential applications for the incorporation of these scaffolds into more complex targets. Remarkably, carboxylic acids with additional functionalities were also compatible with this protocol (19–20, 26–29, and 31). For example, various functional groups, such as ester, amide, ketone, aldehyde and alkyl chloride, remain intact to furnish the corresponding cross-coupling products, potentially allowing for the subsequent orthogonal functionalization. Aliphatic carboxylic acids bearing a naphthalene moiety were amenable substrates, providing the cross-coupling product 21 in 62% yield. In addition, the developed protocol also allows for the construction of heterocycle-containing β,γ-unsaturated ketones in a single step from readily available substrates. For example, a range of five-membered heteroaryl-containing aliphatic acids, such as furan, thiophene and N-protected indole-derived substrates, were functionalized with high efficiency (22–24). When 3,3-diphenylpropanoic acid was subjected to the standard conditions, a reaction occurred, affording the desired product 25 in moderate yield. Amino acids are fundamental building blocks of life, serving as precursors for proteins and bioactive molecules; their derivatization enables diverse applications in drug discovery, biomaterials, and chemical biology. As a result, we used N-Boc amino acids to test the feasibility of our developed strategy, and the results showed that N-Boc amino acids were suitable for this transformation, yielding the corresponding amino ketone molecules in moderate yield (26 and 27). In addition to aliphatic carboxylic acids with acyclic substituents, this catalytic system is also applicable to those containing cyclic substituents (30). Alkenyl acids derived from natural sources, such as oleic acid analogue (32) and stearic acid (33), were also well tolerated. These results highlight the potential for structural diversification and sustainable utilization of naturally occurring carboxylic acids.Subsequently, the substrate scope of this reaction with respect to alkenes for β,γ-unsaturated ketone synthesis was then examined. As shown in Scheme 2, this cross-coupling protocol permits the direct coupling of aliphatic acids with a wide range of unfunctionalized alkenes. Importantly, both cyclic and acyclic alkenes were readily accommodated in this transformation. For example, a series of simple cyclic olefins with various ring sizes afforded the corresponding β,γ-unsaturated ketones in moderate to good yields (34–39). Notably, cyclic substrates bearing alkyl substituents also provide high levels of coupling efficiency, though minor regio-isomers may form due to competing hydrogen abstraction at secondary sites. The observed selectivity arises from preferential bromine radical abstraction at the most hydridic hydrogen, followed by coupling at the less sterically hindered position. Notably, for the reaction leading to 38, the allyl radical has two sites of reactivity, which would lead to two different structural isomers. However, only the major isomer 38 was obtained and the minor isomer might be ignored during isolation. Evaluation of various acyclic alkenes revealed that alkenes with long or short alkyl chains could be used as substrates and afforded the corresponding products (40–44) in moderate to good yields. Not surprisingly, a high level of regiocontrol was observed in the formation of the branched acylated product 40 arising from hydrogen atom abstraction of the more hydridic site. In contrast, implementation of (E)-4-methyl-2-pentene leads predominantly to the formation of the linear product 41, resulting from coupling at the least hindered terminus of the allylic radical, with only trace quantities of branched ketone adducts being detected. Exposure of substituted aryl alkene to this developed catalytic system could also afford the corresponding ketone 42 in moderate yield.
Notably, more hindered trisubstituted and tetrasubstituted alkenes were amenable substrates. For example, the trisubstituted alkene reacted smoothly and provided the only E-type acylated product 43 in 65% yield. Good efficiency was also observed when a tetrasubstituted alkene was used (44). Additionally, a benzylic C(sp3)–H substrate was successfully employed in this coupling reaction, delivering corresponding ketone 45 in 57% yield. The exceptionally mild conditions and broad functional group tolerance of our method encouraged its application to the late-stage functionalization of natural products. Indeed, this approach readily furnished a diverse array of β,γ-unsaturated ketones incorporating natural product fragments (46–49).
Remarkably, naturally derived substrates, such as (E)-ethyl 3-hexenoate, (s)-(−)-β-citronellol, a molecule with pesticidal activity, and a-pinene, which have multiple allylic C(sp3)–H sites, dominantly reacted at the specialized carbon to deliver corresponding products 46–48 in 55–63% yields. The choice of the acylated site is different from Wang's work.11 It is worth noting that in the reaction leading to compound 46, the steric hindrance of the corresponding allyl radical drove the selective formation of the major product 46, while the minor isomer was disregarded. No competitive ring-opening product of the four-membered ring was observed in the reaction with α-pinene, which could be attributed to the following reasons: (1) the major isomer formed via hydrogen atom transfer (HAT) at the more accessible α-position, suppressing β-HAT pathways that would produce a β-position allyl radical; (2) ring-opening of the β-position allyl radical is kinetically disfavored compared to the rapid Ni-mediated radical capture, due to steric hindrance in coupling process. Consequently, the ring-opening product was formed in negligible amounts and could not be isolated by column chromatography. A 4-carvomenthenol-derived alkene was tested under photoredox conditions, affording the allylacylated product 49 in 60% yield, accompanied by a mixture of regioisomers.
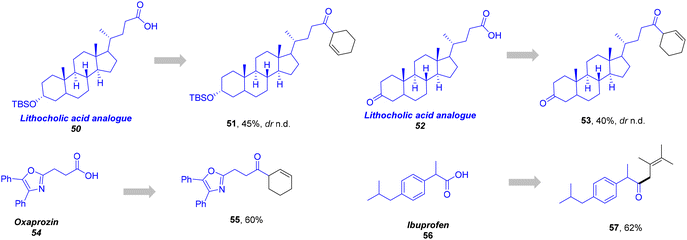
To further demonstrate the synthetic utility of this strategy, we investigated whether this protocol could be applied to the late-stage functionalization of biologically active molecules (Scheme 3). A range of pharmaceuticals or bioactive molecule-derived substrates were successfully deployed in the reaction, including lithocholic acid analogues (51, 45% yield; 53, 40% yield) and oxaprozin, a nonsteroidal anti-inflammatory drug (55, 60% yield), forming the desired products in good yields. Notably, a commercially available drug Ibuprofen, a secondary carboxylic acid, could also be transformed into corresponding ketone 57 in 62% yield. These findings demonstrate significant potential for structural modification across a broad spectrum of complex biomolecules, with particular relevance to medicinal chemistry applications.
Considering that bis(β,γ-unsaturated ketone) scaffolds are highly desirable building blocks in drug discovery, we also evaluated a terminal dicarboxylic acid that would provide access to this kind of compounds. As illustrated in Scheme 4a, our developed protocol could be extended to the valuable transformations of dicarboxylic acids through simple modification of reaction conditions. Terminal dicarboxylic acids can well participate in transformations with a range of alkenes in one step, furnishing diverse bis(β,γ-unsaturated ketone) scaffolds in 50–70% yields (58–61), which traditionally required multistep syntheses.
The successful implementation of these examples highlights the significant potential of this methodology for constructing valuable bis(β,γ-unsaturated ketone) derivatives. Importantly, the presence of allylic C–H bonds in β,γ-unsaturated ketone scaffolds is particularly important, as it permits these molecules to behave as alkene reactants toward carboxylic acids, ultimately forming other kinds of di-β,γ-unsaturated ketone architectures in 40–64% yields (62–66) (Scheme 4b). From the standpoint of the overall outcome, the alkene plays a role of a bridge to connect two molecules of carboxylic acids, providing an efficient and flexible pathway to rapidly increase molecular complexity. To further showcase the synthetic utility of this developed strategy, a large-scale experiment was conducted, delivering the desired ketone 44 in 53% yield from electron-rich substrates, comparable to the yield we observed on a small scale. Additionally, a similar molecule 67 could also be constructed in 57% yield using electron-withdrawing substrates (Scheme 4c). There is no significant yield erosion in yield, suggesting that large-scale chemical production might be possible.
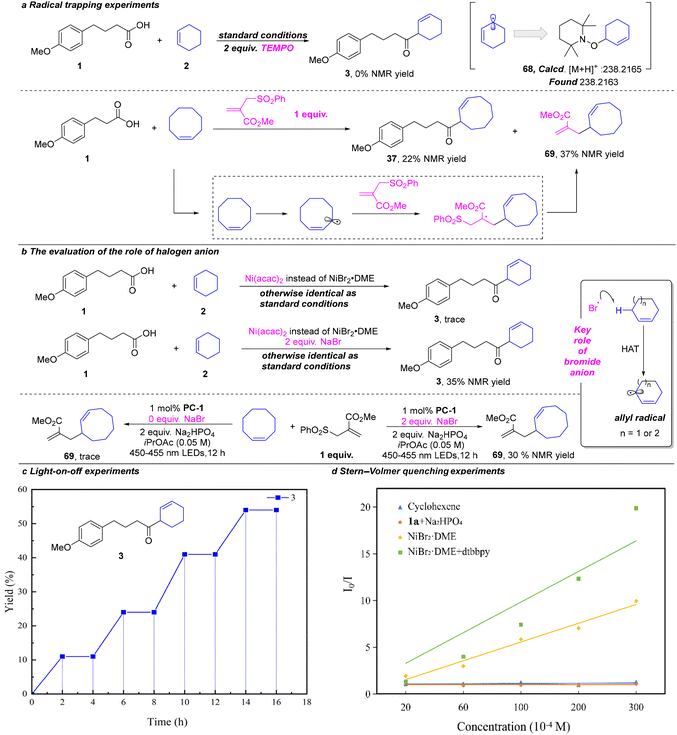
To better understand the detailed mechanism of the reaction, a series of mechanistic studies were performed (Scheme 5). In the presence of radical trap TEMPO, the model reaction was completely shut down (Scheme 5a, top), indicating that a radical intermediate might be involved in this transformation. More importantly, allyl-trapped product 68 was observed by HRMS, further supporting that the reaction proceeds through a radical hydrogen atom abstraction pathway and the intermediacy of an allyl radical. Moreover, addition of 1.0 equiv. of allylic sulfone to the reaction of carboxylic acid 1 and cyclooctene led to a adduct 69 in 37% NMR yield, accompanied by the formation of common β,γ-unsaturated ketone 37 in 22% NMR yield (Scheme 5a, bottom). This observation further suggested that the reaction may involve the formation of allyl radicals. According to the reported literature,23 photocatalytic oxidation of the bromide anion, a dissociable ligand on nickel, could generate bromine radicals for hydrogen atom abstraction from C(sp3)–H, so we explored the role of the bromide anion in our system. As shown in Scheme 5b, nearly no corresponding ketone 3 was detected when Ni(acac)2 was used in place of NiBr2·DME. However, when the reaction was performed in the presence of NaBr, 35% NMR yield of 3 could be detected. These results indicated the key role of the bromide anion during the reaction. Similarly, when cyclooctene was reacted with an allylic sulfone in the presence of NaBr, 30% NMR yield of allyl-adduct 69 could be detected. In contrast, no obvious corresponding allyl-adduct 69 could be detected in the absence of NaBr through 1H NMR analysis. Taking these results together, a reactive bromine radical might be involved in the reaction system. The light-on–off experiment indicates that light irradiation is necessary but the radical chain mechanism cannot be ruled out (Scheme 5c).24 Finally, we performed Stern–Volmer quenching experiments using carboxylic acid 1, alkene 2, NiBr2·DME, and a combination of NiBr2·DME with dtbbpy L1 as quenchers (Scheme 5d). The quenching plot with NiBr2·DME and dtbbpy L1 has an upward deviation from linearity (Scheme 5d, green line), indicating that both dynamic quenching (the excited photocatalyst undergoes efficient reduction by NiBr2·DME in the presence of dtbbpy L1) and static quenching (the ligand exchange between the Ir photocatalyst and nickel complex) take place. Unsurprisingly, carboxylic acid 1 and alkene 2 exhibited no quenching effects on the excited photocatalyst. Notably, the single NiBr2·DME also exhibited efficient quenching effects, though not as strong as that of the combination of NiBr2·DME with dtbbpy. This phenomenon may be attributable to the oxidative capability of the photoexcited state.
Based on the previously reported literature23 and the aforementioned mechanistic studies, a plausible mechanism for this transformation is proposed in Scheme 6. The reaction begins with the oxidative addition of the Ni(0) intermediate I to an in situ-generated acyl electrophile 70, forming the Ni(II) intermediate II. Simultaneously, a bromine radical—oxidatively generated from the bromide anion or nickel-bound bromide 71 (E1/2[Ir(III*/II)] = +1.21 V vs. SCE in CH3CN; bromide anion oxidation: E1/2 = +0.80 V vs. SCE in DME)23b—abstracts a hydrogen atom from the allylic C–H bond of alkene 72, producing a reactive radical 73. This radical is rapidly captured by II, yielding the Ni(III) intermediate III, which undergoes reductive elimination to furnish the final coupling product 74 along with the Ni(I) intermediate IV. A subsequent single-electron transfer (SET) reduction of IV by the reduced photocatalyst Ir(II) (Ered1/2[Ir(II/III)] = −1.37 V vs. SCE in CH3CN)25 regenerates the Ni(0) species I, completing the catalytic cycle. This mechanism, featuring a critical hydrogen atom transfer (HAT) step, aligns well with our experimental observation.
Conclusions
Our study demonstrates a synergistic nickel/photoredox catalytic system that directly couples carboxylic acids with allylic C(sp3)–H bonds, circumventing classical limitations in allylic acylation. By leveraging the dual role of nickel as a radical mediator and cross-coupling catalyst, this method achieves selective C–C bond formation without exogenous oxidants or directing groups. The mechanistic elucidation – combining spectroscopic and trapping experiments – provides a blueprint for designing related metallaphotoredox reactions involving halogen-radical-mediated HAT. Beyond its synthetic versatility (spanning drug derivatives to natural products), this transformation offers a paradigm for leveraging feedstock chemicals in complex fragment couplings, as exemplified by the modular assembly of bis(β,γ-unsaturated ketones). The operational simplicity, scalability, and functional group tolerance of this approach position it as a practical tool for diversifying molecular architectures in medicinal chemistry and materials science. Future efforts will explore enantioselective variants and applications in polymer chemistry.Author contributions
B. Y. designed the experiments. X. G., C. Y. and M. H. performed experiments. X. G. wrote the SI. M. W. and J. C. helped isolate some compounds. B. Y. and R. T. directed the whole project. B. Y. wrote the paper. B. Y. and R. T. revised, reviewed and edited the paper. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details and characterization of all compounds, and copies of 1H and 13C NMR spectra. See DOI: https://doi.org/10.1039/d5gc04541j.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (22571098), funding by the Science and Technology Projects in Guangzhou (2025A04J0056) and the National Key Research and Development Program of China (No. 2024YFD2201500).References
- (a) H. Irschik, et al., Isolation, structure elucidation, and biological activity of maltepolides: remarkable macrolides from myxobacteria, Angew. Chem., Int. Ed., 2013, 52, 5402–5405 CrossRef CAS PubMed; (b) C. Fu, et al., The natural product carolacton inhibits folatedependent C1 metabolism by targeting fold/MTHFD, Nat. Commun., 2017, 8, 1529–1537 CrossRef PubMed; (c) S. Zhang, W. Duan and W. Wang, Efficient, enantioselective organocatalytic synthesis of Trichostatin A, Adv. Synth. Catal., 2006, 348, 1228–1234 CrossRef CAS; (d) T. Asaba, Y. Katoh, D. Urabe and M. Inoue, Total synthesis of crotophorbolone, Angew. Chem., Int. Ed., 2015, 54, 14457–14461 CrossRef CAS PubMed; (e) N. S. Radin, Drug design: hiding in full view, Drug Dev. Res., 2008, 69, 15–25 CrossRef CAS.
- (a) T. Ooi, R. Goto and K. Maruoka, Fluorotetraphenylbismuth: a new reagent for efficient regioselective α-phenylation of carbonyl compounds, J. Am. Chem. Soc., 2003, 125, 10494–10495 CrossRef CAS PubMed; (b) Y. Huang, R. Huang and Y. Zhao, Cobalt-catalyzed enantioselective vinylation of activated ketones and imines, J. Am. Chem. Soc., 2016, 138, 6571–6576 CrossRef CAS PubMed; (c) M. Grigalunas, T. Ankner, P. Norrby, O. Wiest and P. Helquist, Palladium-catalyzed alkenylation of ketone enolates under mild conditions, Org. Lett., 2014, 16, 3970–3973 CrossRef CAS PubMed; (d) T. Ankner, C. C. Cosner and P. Helquist, Palladium- and nickelcatalyzed alkenylation of enolates, Chem. – Eur. J., 2013, 19, 1858–1871 CrossRef CAS PubMed; (e) J. Guo, et al., Nickel(II)-catalyzed enantioselective α-vinylation of β-keto amides/esters with hypervalent iodine salts, Org. Lett., 2016, 18, 5540–5543 CrossRef CAS PubMed.
- M. Grigalunas, T. Ankner, P. Norrby, O. Wiest and P. Helquist, Ni-catalyzed alkenylation of ketone enolates under mild conditions: catalyst identification and optimization, J. Am. Chem. Soc., 2015, 137, 7019–7022 CrossRef CAS PubMed.
- (a) J. M. Stevens and D. W. C. MacMillan, Enantioselective α-alkenylation of aldehydes with boronic acids via the synergistic combination of copper(II) and amine catalysis, J. Am. Chem. Soc., 2013, 135, 11756–11759 CrossRef CAS PubMed; (b) E. Skucas and D. W. C. MacMillan, Enantioselective α-vinylation of aldehydes via the synergistic combination of copper and amine catalysis, J. Am. Chem. Soc., 2012, 134, 9090–9093 CrossRef CAS PubMed; (c) H. Kim and D. W. C. MacMillan, Enantioselective organo-SOMO catalysis: the α-vinylation of aldehydes, J. Am. Chem. Soc., 2008, 130, 398–399 CrossRef CAS PubMed.
- (a) Y. Yuan, X. Zhang, H. Qian and S. Ma, Catalytic enantioselective allene–anhydride approach to β,γ-unsaturated enones bearing anα-all-carbon-quaternary center, Chem. Sci., 2020, 11, 9115–9121 RSC; (b) H. Liu, et al., Cooperative N-heterocyclic carbene/nickel-catalyzed hydroacylation of 1,3-dienes with aldehydes in water, ACS Catal., 2022, 12, 1657–1663 CrossRef CAS.
- (a) P. Ertl and T. Schuhmann, A Systematic Cheminformatics Analysis of Functional Groups Occurring in Natural Products, J. Nat. Prod., 2019, 82, 1258–1263 CrossRef CAS PubMed; (b) T. Henkel, R. M. Brunne, H. Müller and F. Reichel, Statistical Investigation into the Structural Complementarity of Natural Products and Synthetic Compounds, Angew. Chem., Int. Ed., 1999, 38, 643–647 CrossRef CAS; (c) G. Ni, Q.-J. Zhang, Z.-F. Zheng, R.-Y. Chen and D.-Q. Yu, 2-arylbenzofuran derivatives from Morus cathayana, J. Nat. Prod., 2009, 72, 966–968 CrossRef CAS PubMed; (d) H. J. Knolker and K. R. Reddy, Isolation and synthesis of biologically active carbazole alkaloids, Chem. Rev., 2002, 102, 4303–4427 CrossRef PubMed.
- (a) B. Yang and R.-Y. Tang, Direct synthesis of dialkyl ketones from deoxygenative cross-coupling of carboxylic acids and alcohols, Chem. Sci., 2024, 15, 18405–18410 RSC; (b) X.-B. Yan, Y.-Q. Liu, N. Wang, T. Zhang, D. Li, Z. Wang, Y. Lin and K. Zhang, Decarboxylative Cross-Acyl Coupling of Carboxylic Acids with Aldehydes Enabled by Nickel/Photoredox Catalysis, J. Am. Chem. Soc., 2025, 147, 15929–15935 CrossRef CAS PubMed; (c) E. Lin, et al., Aryl Acid-Alcohol Cross-Coupling: C(sp3)–C(sp2) Bond Formation from Nontraditional Precursors, J. Am. Chem. Soc., 2025, 147, 14905–14914 CrossRef CAS PubMed; (d) C. P. Johnston, R. T. Smith, S. Allmendinger and D. W. C. MacMillan, Metallaphotoredox-catalysed sp3–sp3 cross-coupling of carboxylic acids with alkyl halides, Nature, 2016, 536, 322–325 CrossRef CAS PubMed; (e) Y. Liang, X. Zhang and D. W. C. MacMillan, Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis, Nature, 2018, 559, 83–88 CrossRef CAS PubMed; (f) S. B. Beil, T. Q. Chen, N. E. Intermaggio and D. W. C. MacMillan, Carboxylic Acids as Adaptive Functional Groups in Metallaphotoredox Catalysi, Acc. Chem. Res., 2022, 55, 3481–3494 CrossRef CAS PubMed; (g) J. A. Kautzky, T. Wang, R. W. Evans and D. W. C. MacMillan, Decarboxylative Trifluoromethylation of Aliphatic Carboxylic Acids, J. Am. Chem. Soc., 2018, 140, 6522–6526 CrossRef CAS PubMed; (h) A. Y. Chan, I. B. Perry, N. B. Bissonnette, B. F. Buksh, G. A. Edwards, L. I. Frye, O. L. Garry, M. N. Lavagnino, B. X. Li, Y. Liang, E. Mao, A. Millet, J. V. Oakley, N. L. Reed, H. A. Sakai, C. P. Seath and D. W. C. MacMillan, Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis, Chem. Rev., 2022, 122, 1485–1542 CrossRef CAS PubMed; (i) X. Shu, L. Huan, Q. Huang and H. Huo, Direct Enantioselective C(sp3)–H Acylation for the Synthesis of α-Amino Ketones, J. Am. Chem. Soc., 2020, 142, 19058–19064 CrossRef CAS PubMed; (j) L. Huan, X. Shu, W. Zu, D. Zhong and H. Huo, Asymmetric benzylic C(sp3)–H acylation via dual nickel and photoredox catalysis, Nat. Commun., 2021, 12, 3536 CrossRef CAS PubMed; (k) A. V. Tsymbal, L. D. Bizzini and D. W. C. MacMillan, Nickel Catalysis via SH2 Homolytic Substitution: The Double Decarboxylative Cross-Coupling of Aliphatic Acids, J. Am. Chem. Soc., 2022, 144, 21278–21286 CrossRef CAS PubMed; (l) A. Whyte and T. P. Yoon, Selective Cross-Ketonization of Carboxylic Acids Enabled by Metallaphotoredox Catalysis, Angew. Chem., Int. Ed., 2022, e202213739 CAS; (m) X. Wu, J. Han, S. Xia, W. Li, C. Zhu and J. Xie, Decarboxylative Acylation of Carboxylic Acids: Reaction Investigation and Mechanistic Study, CCS Chem., 2022, 4, 2469–2480 CrossRef CAS.
- (a) H. Huang, P. Bellotti, P.-P. Chen, K. N. Houk and F. Glorius, Allylic C(sp3)–H arylation of olefins via ternary catalysis, Nat. Synth., 2022, 1, 59–68 CrossRef CAS; (b) Z. Dong, Z. Ren, S. J. Thompson, Y. Xu and G. Dong, Transition-Metal-Catalyzed C–H Alkylation Using Alkenes, Chem. Rev., 2017, 117, 9333–9403 CrossRef CAS PubMed; (c) T. E. Müller and M. Beller, Metal-Initiated Amination of Alkenes and Alkynes, Chem. Rev., 1998, 98, 675–704 CrossRef PubMed.
- N. M. Bortnick, Process of making esters of acrylic and methacrylic acids, US, 3087962, 1963 Search PubMed.
- A. Perkowski and D. A. Nicewicz, Direct Catalytic Anti-Markovnikov Addition of Carboxylic Acids to Alkenes, J. Am. Chem. Soc., 2013, 135, 10334–10337 CrossRef CAS PubMed.
- X. Wang, et al., Direct allylic acylation via cross-coupling involving cooperative N–heterocyclic carbene, hydrogen atom transfer, and photoredox catalysis, Nat. Commun., 2023, 14, 2951 CrossRef CAS PubMed.
- M. D. Palkowitz, M. A. Emmanuel and M. S. Oderinde, A Paradigm Shift in Catalysis: Electro- and Photomediated Nickel-Catalyzed Cross-Coupling Reactions, Acc. Chem. Res., 2023, 56, 2851–2865 CrossRef CAS PubMed.
- (a) Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle and D. W. C. MacMillan, Merging Photoredox with Nickel Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides, Science, 2014, 345, 437–440 CrossRef CAS PubMed; (b) J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, The Merger of Transition Metal and Photocatalysis, Nat. Rev. Chem., 2017, 1, 0052 CrossRef CAS.
- J. C. Tellis, D. N. Primer and G. A. Molander, Single-Electron Transmetalation in Organoboron Cross-Coupling by Photoredox/Nickel Dual Catalysis, Science, 2014, 345, 433–436 CrossRef CAS PubMed.
- C. L. Joe and A. G. Doyle, Direct Acylation of C(sp3)–H Bonds Enabled by Nickel and Photoredox Catalysis, Angew. Chem., Int. Ed., 2016, 55, 4040–4043 CrossRef CAS PubMed.
- D. R. Heitz, J. C. Tellis and G. A. Molander, Photochemical nickel-catalyzed C–H arylation: synthetic scope and mechanistic investigations, J. Am. Chem. Soc., 2016, 138, 12715–12718 CrossRef CAS PubMed.
- B. J. Shields and A. G. Doyle, Direct C(sp3)–H cross coupling enabled by catalytic generation of chlorine radicals, J. Am. Chem. Soc., 2016, 138, 12719–12722 CrossRef CAS PubMed.
- (a) L. Huang and M. Rueping, Direct Cross-Coupling of Allylic C(sp3)–H Bonds with Aryl-and Vinylbromides by Combined Nickel and Visible-Light Catalysis, Angew. Chem., Int. Ed., 2018, 57, 10333–10337 CrossRef CAS PubMed; (b) S. K. Kariofillis and A. G. Doyle, Synthetic and mechanistic implications of chlorine photoelimination in nickel/photoredox C(sp3)–H cross-coupling, Acc. Chem. Res., 2021, 54, 988–1000 CrossRef CAS PubMed; (c) X. Cheng, H. Lu and Z. Lu, Enantioselective benzylic C–H arylation via photoredox and nickel dual catalysis, Nat. Commun., 2019, 10, 3549 CrossRef PubMed.
- (a) L. J. Gooßen and K. Ghosh, Palladium-catalyzed synthesis of aryl ketones from boronic acids and carboxylic acids or anhydride, Angew. Chem., Int. Ed., 2001, 40, 3458–3460 CrossRef; (b) Y. Wei, J. Lam and T. Diao, Synthesis of C-acyl furanosides via the cross-coupling of glycosyl esters with carboxylic acids, Chem. Sci., 2021, 12, 11414–11419 RSC.
- (a) R. Kakino, H. Narahashi, I. Shimizu and A. Yamamoto, General and Greener Route to Ketones by Palladium-Catalyzed Direct Conversion of Carboxylic Acids with Organoboronic Acids, Chem. Lett., 2001, 30, 1242–1243 CrossRef; (b) L. J. Gooben, N. Rodríguez and K. Gooben, Carboxylic Acids as Substrates in Homogeneous Catalysis, Angew. Chem., Int. Ed., 2008, 47, 3100–3120 CrossRef PubMed.
- (a) G. Bergonzini, C. Cassani and C.-J. Wallentin, Acyl Radicals from Aromatic Carboxylic Acids by Means of Visible-Light Photoredox Catalysis, Angew. Chem., Int. Ed., 2015, 54, 14066–14069 CrossRef CAS PubMed; (b) S. O. Badir, A. Dumoulin, J. K. Matsui and G. A. Molander, Synthesis of Reversed C-Acyl Glycosides through Ni/Photoredox Dual Catalysis, Angew. Chem., Int. Ed., 2018, 57, 6610–6613 CrossRef CAS PubMed; (c) C. Zhao, X. Jia, X. Wang and H. Gong, Ni-Catalyzed Reductive Coupling of Alkyl Acids with Unactivated Tertiary Alkyl and Glycosyl Halides, J. Am. Chem. Soc., 2014, 136, 17645–17651 CrossRef CAS PubMed; (d) L. Huan, X. Shu, W. Zu, D. Zhong and H. Huo, Asymmetric benzylic C(sp3)–H acylation via nickel and photoredox catalysis, Nat. Commun., 2021, 12, 3536 CrossRef CAS PubMed.
- (a) J. Bohlke, C. Armstrong, T. Knauber, M. González-Esguevillas and D. F. Fernández, Sustainable solvents in metallaphotoredox catalysis, ACS Sustainable Chem. Eng., 2024, 12, 8998–9002 CrossRef CAS; (b) P. Delgado, R. J. Glass, G. Geraci, R. Duvadie, D. Majumdar, R. I. Robinson, I. Elmaarouf, M. Mikus and K. L. Tan, Use of green solvents in metallaphotoredox cross-electrophile coupling reactions utilizing a lipophilic modified dual Ir/Ni catalyst system, J. Org. Chem., 2021, 86, 17428–17436 CrossRef CAS PubMed.
- (a) T. Kawasaki, N. Ishida and M. Murakami, Dehydrogenative Coupling of Benzylic and Aldehydic C–H Bonds, J. Am. Chem. Soc., 2020, 142, 3366–3370 CrossRef CAS PubMed; (b) P. Zhang, C. C. Le and D. W. C. MacMillan, Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling, J. Am. Chem. Soc., 2016, 138, 8084–8087 CrossRef CAS PubMed; (c) T. Kawasaki, N. Ishida and M. Murakami, Photoinduced Specific Acylation of Phenolic Hydroxy Groups with Aldehydes, Angew. Chem., Int. Ed., 2020, 59, 18267–18271 CrossRef CAS PubMed; (d) X. Shu, L. Huan, Q. Huang and H. Huo, Direct Enantioselective C(sp3)–H Acylation for the Synthesis of α-Amino Ketones, J. Am. Chem. Soc., 2020, 142, 19058–19064 CrossRef CAS PubMed; (e) L. Huang, M. Szewczyk, R. Kancherla, B. Maity, C. Zhu, L. Cavallo and M. Rueping, Modulating stereoselectivity in allylic C(sp3)–H bond arylation via nickel and photoredox catalysis, Nat. Commun., 2023, 14, 548 CrossRef CAS PubMed.
- M. Cismesia and T. P. Yoon, Characterizing chain processes in visible light photoredox catalysis, Chem. Sci., 2015, 6, 5426–5434 RSC.
- C. Prier, D. Rankic and D. W. C. MacMillan, Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |