Open Access Article
Open Access ArticleOptimizing Wolffia globosa protein extraction by ultrasonic pretreatment and enhancing protein attributes through LAB fermentation
Nontikarn
Taramark
a,
Daniel
Rice
 a,
Atikorn
Panya
a,
Atikorn
Panya
 b and
Anil Kumar
Anal
b and
Anil Kumar
Anal
 *a
*a
aFood Engineering and Bioprocess Technology, Food Innovation, Nutrition, and Health, Department of Food, Agriculture, and Bioresources, Asian Institute of Technology, Pathum Thani 12120, Thailand. E-mail: anilkumar@ait.ac.th
bBiotech, National Science and Technology Development Agency, 12120, Thailand. E-mail: atikorn.pan@biotec.or.th
First published on 20th November 2025
Abstract
The rising global population is generating food security issues, particularly in protein demands and nutritional quality. Many plant-based foods have been explored to meet this demand, although they often lack total protein or protein quality. Therefore, underutilized water crops, such as Wolffia globosa, offer great potential for alternative protein production. This study investigated the parameters of ultrasonic-assisted alkaline extraction and probiotic fermentation to extract and enhance the qualities of Wolffia protein. Response surface methodology was utilized to optimize ultrasound-assisted alkaline extraction, resulting in a maximum soluble protein yield of 118.44 ± 13.60 mg g−1 under conditions of 95% amplitude, 16 minutes extraction time, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 g mL−1 solid-to-liquid ratio. This method increased total protein content by 127%. Subsequent fermentation with Lactobacillus plantarum 2075 then improved nutritional quality by raising digestibility from 55.82% to 70.45%, increasing essential amino acid content, and raising antioxidant activity by 15.24% and phenolic content by 98.89% relative to the raw sample. Fermentation also modified technofunctional properties of the extracted sample, reducing foaming capacity and emulsion activity but improving the foam and emulsion stability. These findings highlight that ultrasonic-assisted alkaline extraction improves protein yield, and sequential fermentation further modifies protein characteristics and significantly enhances nutritional quality. The dual processing strategy for Wolffia globosa thus presents a promising sustainable alternative for quality plant protein in diverse food applications.
20 g mL−1 solid-to-liquid ratio. This method increased total protein content by 127%. Subsequent fermentation with Lactobacillus plantarum 2075 then improved nutritional quality by raising digestibility from 55.82% to 70.45%, increasing essential amino acid content, and raising antioxidant activity by 15.24% and phenolic content by 98.89% relative to the raw sample. Fermentation also modified technofunctional properties of the extracted sample, reducing foaming capacity and emulsion activity but improving the foam and emulsion stability. These findings highlight that ultrasonic-assisted alkaline extraction improves protein yield, and sequential fermentation further modifies protein characteristics and significantly enhances nutritional quality. The dual processing strategy for Wolffia globosa thus presents a promising sustainable alternative for quality plant protein in diverse food applications.
Sustainability spotlightGlobal food security is under strain due to the rapidly increasing population, and the need for sustainable protein production is paramount. While many alternative proteins are being explored to mitigate this situation, Wolffia globosa, a fast-growing aquatic plant, offers potential as a novel protein and nutrient resource. While W. globosa is protein and nutrient rich, processing is required to improve the quality for human consumption. Ultrasonication and fermentation are green processes that can improve W. globosa quality without additional environmental burdens. This study highlights green processing strategies to improve the nutritional and functional properties of W. globosa, addressing SDG 2 (Zero Hunger), SDG 3 (Good Health and Well-being), SDG 12 (Responsible Consumption and Production), and SDG 13 (Climate Action). |
1 Introduction
Food supply changes and global food security are stressed by the growing population, which is projected to reach 10 billion by 2050.1 The overall strain is largely impacting protein demands for sustainable production systems, which necessitate alternative protein production from non-traditional sources. Many alternative protein sources are rising in demand in production, including plant-based, fermentation, insect, and cellular proteins. The most widely developed and understood are plant-based systems, but these systems are also coming under direct strain due to climate impacts. In particular, the global mean temperatures are expected to reach beyond legume growing thresholds by 2050, projecting yield reductions from 10 to 49%.2 Thus, alternative climate-resilient plant-based foods are critical for meeting the evolving protein demands.Aquatic plants are gaining recognition for their nutritional quality and environmental sustainability, among which duckweed is grown throughout the globe, exhibiting environmental adaptation and high nutritional quality for potential food production.3 Furthermore, Wolffia globosa, commonly known as watermeal, has gained attention due to its high protein content, rapid growth, and minimal environmental impact.4 However, despite its nutritional potential, the utilization of Wolffia globosa as a food ingredient is limited by the presence of antinutritional factors such as phytic acid and tannins, which reduce protein digestibility and mineral bioavailability.5
Various processing techniques have been explored to enhance the nutritional quality of Wolffia globosa. Ultrasonic-assisted extraction (UAE), which utilizes high frequency sound waves to create cavitation bubbles and disrupt plant cell walls, emerged as a promising method to improve extraction efficiency of proteins and phenolic compounds.6 UAE shows great progress in natural compound extraction with minimal techno-functional impact or environmental consequences, while recent advances and strategies have shown great potential for scaling up the process to industrial scale.7 Additionally, fermentation has been demonstrated as an effective industrial scale processing method for reducing antinutritional factors and increasing nutrient bioavailability in plant products.8
While several studies have investigated either the extraction or fermentation of Wolffia globosa protein, few have explored the integration of these approaches to enhance both yield and quality. This study aimed to optimize UAE conditions and evaluate the effects of subsequent lactic acid bacteria (LAB) fermentation. Protein yield, composition, bioactivities, and techno-functional properties were assessed across treatment conditions. The findings highlight the potential of combining UAE and fermentation to improve the nutritional and functional qualities of Wolffia globosa protein, supporting its development as a sustainable alternative protein source.
2 Methodology
2.1 Raw material preparation
Fresh Wolffia globosa (WG) was purchased from three different farms: Ta Saeng farm (Khay Buk Wan District, Nong Khai, Thailand), Raja Wolffia farm (Bang Pa-In District, Phra Nakhon Si Ayutthaya, Thailand), and Ban Pum Krun Mind (Ban Klang Mun District, Kalasin, Thailand). Fresh WG underwent a thorough washing process with water, repeated three times until the water ran clear. Subsequently, the WG was subjected to drying at 50 °C for 20 hours using a hot air oven. The dried WG was then finely ground and passed through a 150-mesh sieve to obtain WG powder.2.2 Proximate analysis
Moisture content, ash, crude protein, crude fiber, crude fat, and carbohydrate were analyzed based on AOAC 2022.2.3 Alkaline extraction parameters
The WG powder was extracted under alkaline conditions (pH 10) for 30 minutes. Subsequently, the protein was precipitated by acidification to pH 3.5.2.4 Ultrasonic-assisted extraction (UAE)
Based on the method outlined by Inguanez et al., the study was conducted using an ultrasonic probe (UP200S, 200 W, Hielscher, Teltow, Germany) under 17 different conditions: varying extraction times (15, 25, and 35 minutes), amplitudes (80%, 90%, and 100%), and solid-to-liquid ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 1
20, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, and 1
30, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40).9 Amplitude is the percentage of the maximum tip displacement (∼120 microns) during vibration. After extraction, the supernatant and biomass were collected, and soluble protein content was measured from the supernatant.
40).9 Amplitude is the percentage of the maximum tip displacement (∼120 microns) during vibration. After extraction, the supernatant and biomass were collected, and soluble protein content was measured from the supernatant.
2.5 Box–Behnken design
Design Expert software (Trial Version 9.0.3, Stat-Ease Inc., Minneapolis, MN, USA) was used to optimize the ultrasonication conditions for the extraction of proteins from Wolffia globosa. The effects of amplitude (A), extraction time (B), and solid-to-liquid ratio (C) were analyzed using a Box–Behnken design (BBD) with each factor at three levels. A total of 17 experiments, including three center points, were carried out. The data were analyzed using a second-order polynomial model with Design Expert software. Y = β0 + ∑βiXi + ∑βiiXi2 + ∑βijXiXj.2.6 Quantification of protein
The soluble protein concentration of the protein hydrolysates was determined by the Bradford method using an UV/VIS spectrophotometer (UNICAM, Alva, U.K) at a wavelength of 595 nm. BSA was used as the standard.2.7 Fermentation process
The prepared protein extract was then prepared at 1% (w/v) in sterilized water, and 1 mL of Lactobacillus plantarum 2075 was added at 1 × 108 CFU mL−1. Then, the sample was incubated while shaking at 37 °C for 72 hours. Both aqueous and solid phases were collected and freeze-dried at −53 °C for 24 hours before further analysis.2.8 Total phenolic and total flavonoid content analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and agitated at 50 °C for 2 hours in a water bath. The resulting supernatants were centrifuged at 3800 × g for 10 minutes. The crude WG extracts were then stored at 20 °C until the total phenolic and flavonoid content could be determined.
30 and agitated at 50 °C for 2 hours in a water bath. The resulting supernatants were centrifuged at 3800 × g for 10 minutes. The crude WG extracts were then stored at 20 °C until the total phenolic and flavonoid content could be determined.
2.9 Anti-nutritional element analysis
2.10 Antioxidant properties analysis
To prepare the DPPH solution, 7.8 mg of DPPH powder was dissolved in 100 mL of 95% ethanol and protected from light with aluminum foil. The sample was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 minutes, and 1 mL of the supernatant was mixed with 1 mL of the DPPH solution. The mixture was incubated in the dark at room temperature for 30 minutes. For the control, ethanol was used instead of the sample. Absorbance was measured at 517 nm using a spectrophotometer, and DPPH radical scavenging activity was calculated using eqn (1):
000 rpm for 30 minutes, and 1 mL of the supernatant was mixed with 1 mL of the DPPH solution. The mixture was incubated in the dark at room temperature for 30 minutes. For the control, ethanol was used instead of the sample. Absorbance was measured at 517 nm using a spectrophotometer, and DPPH radical scavenging activity was calculated using eqn (1): | (1) |
2.11 In vitro digestibility
To assess in vitro protein digestibility, the method was adapted from Phongthai et al.13 A 1% protein solution was prepared and adjusted to pH 1.5. After incubating at 37 °C for 5 minutes, pepsin (1 g per 100 g protein) was added, and digestion continued for 120 minutes. The solution was then neutralized with 0.1 N NaOH to inactivate pepsin. Then trypsin (1 g per 100 g protein) was added, and digestion proceeded for another 120 minutes. After this period, the solution was heated at 95 °C for 10 minutes to inactivate trypsin. Trichloroacetic acid was added, and the solution was mixed and centrifuged at 5000 rpm for 10 minutes. The precipitate was freeze-dried at −53 °C for 24 hours, and protein content was analyzed using the Kjeldahl method. Protein digestibility was calculated according to eqn (2).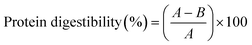 | (2) |
2.12 Amino acid profiles
The type and amino acid profile were analyzed following AOAC, (2000) with slight modifications. The protein concentrate was dissolved in 6 M hydrochloric acid (HCl), subjected to incubation at 110 °C for 24 hours, and diluted with sodium acetate. Subsequently, the pH was modified to 2.2 before separating and identifying the solution. Amino acids were quantified using GC-MS, expressing their amounts in units of mg per 100 g of protein, with norleucine employed as an internal standard.2.13 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was adapted from Khampratueng and Anal.14 The cell-free supernatant was concentrated using a protein concentrator fitted with a 10k molecular weight cutoff membrane (Thermo Fisher Scientific, US). SDS-PAGE was used to measure extracellular protein weight. The molecular weight of the extracellular enzyme was determined by using a 12% resolving gel and a 4% stacking gel. The proteins were stained with a 0.2% Coomassie brilliant blue R-250 solution in 30% ethanol and 10% glacial acetic acid and de-stained with a 30% ethanol solution in 10% glacial acetic acid.2.14 Fourier transform infrared (FTIR) spectroscopy
Infrared spectra transmission of protein samples was obtained through the utilization of an FTIR spectrometer (Nicolet iS50, Thermo Scientific, USA) by OMNIC software to separate the spectrum of amide I into multicomponent peaks at the wavelength range 4000–400 cm−1, 64 no. of scans and 4 cm−1 resolution. By analyzing the structure of α-helix at the wavelength 1655 cm−1, β-sheet at the wavelength 1638 cm−1, β-turn at the wavelength 1670 cm−1, and random coils at the wavelength 1655 cm−1.2.15 Functional properties
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 minute. The resulting samples were transferred to graduated cylinders, and the total volume was measured at 0 and 30 minutes. Foaming capacity and foam stability were determined using eqn (3) and (4), respectively.
000 rpm for 1 minute. The resulting samples were transferred to graduated cylinders, and the total volume was measured at 0 and 30 minutes. Foaming capacity and foam stability were determined using eqn (3) and (4), respectively.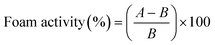 | (3) |
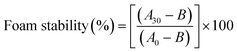 | (4) |
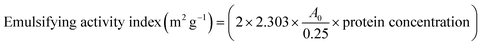 | (5) |
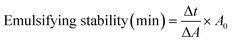 | (6) |
 | (7) |
2.16 Statistical analysis
Response Surface Methodology (RSM) was used to optimize ultrasonic treatment for concentrating soluble proteins with Design Expert software. The Box–Behnken design evaluated three factors: amplitude (A), extraction time (B), and solid-to-liquid ratio (C) over seventeen experiments, performed in triplicate, assessed varying soluble protein concentrations. The data were analyzed using SPSS, with significance tested by one-way ANOVA and Tukey's multiple comparison test (p ≤ 0.05).3 Results and discussion
3.1 Proximate analysis of Wolffia globosa
The proximate analysis of WG indicated protein at 26.75 ± 2.71% with carbohydrates making up the majority at 37.74 ± 1.34%, while crude fiber was found to be 8.92 ± 1.31%. The fat content was relatively low at 4.64 ± 0.18%. This is comparable to the work by Appenroth et al., which demonstrated protein between 20 and 30% and fat between 1 and 5% across 11 duckweed species, although the fiber content was higher than in this study at ∼25%.17 Additionally, WG comprised a moisture content and ash content of 8.96 ± 1.55% and 12.99 ± 0.02%, respectively.3.2 Determining optimal protein extraction conditions
Although WG shows potential as a food ingredient, processing is essential for increasing protein availability, digestibility, amino acid content, and technofunctional characteristics for downstream product formulation. Initially protein extraction was compared using untreated, ultrasound, and alkaline extraction (Table 1). Both ultrasound and alkaline extraction showed higher soluble and total protein than the untreated sample. Alkaline extraction showcased the highest soluble protein and thus combining both systems showed great potential. Thus, alkaline ultrasound-assisted extraction (AUAE) presents as an effective extraction method for protein from WG and was subsequently optimized using the Box–Behnken design (BBD).6,18| Sample | Soluble protein (mg g−1) | Total protein (%) |
|---|---|---|
| Untreated sample | 31.95 ± 0.33 | 26.75 ± 2.71 |
| Ultrasound extraction | 55.93 ± 6.42 | 53.35 ± 1.17 |
| Alkaline extraction | 94.59 ± 15.57 | 55.30 ± 0.73 |
Optimal protein extraction conditions were determined using a Box–Behnken design with 17 trials (Table 2), evaluating amplitude, extraction time, and solid-to-liquid ratio. ANOVA indicated that a quadratic model was suitable, with significant linear (A, B, C) and curved relationships between the variables (p < 0.05), which is depicted in eqn (8) below. The solid-to-liquid ratio significantly impacts the response value due to its larger coefficient compared to amplitude and extraction time. The lack of fit value was 0.7829 (p > 0.05), and the R2 value was 0.9806. The model effectively predicts optimal protein extraction conditions, as demonstrated by the provided prediction equations. The maximum soluble protein and total protein were 118.44 ± 13.60 mg g−1 and 60.74 ± 0.30%, respectively. The soluble protein from optimized AUAE was 1.25 and 2.12 times higher than UAE or alkaline extraction, respectively. Additionally, the total protein was higher in the AUAE treatment, and each treatment exhibited total protein higher than the ∼30% shown by Duangjarus et al. and Nitiwuttihorn et al. using UAE.6,18
| Y = 4.25 + 0.6611A − 0.2158B − 1.02C − 0.7027AB + 0.1250AC + 0.0237BC − 1.17A2 + 0.0637B2 + 0.4129C2 | (8) |
| Run | Extraction conditions | |||
|---|---|---|---|---|
| Amplitude (%) | Extraction time (min) | Solid-to- liquid ratio (g mL−1) | Protein (mg mL−1) | |
| Control | — | — | — | 1.06 |
| 1 | 90 | 25 | 30 | 3.98 |
| 2 | 80 | 25 | 40 | 1.60 |
| 3 | 100 | 35 | 30 | 2.92 |
| 4 | 90 | 25 | 30 | 4.68 |
| 5 | 100 | 15 | 30 | 4.72 |
| 6 | 80 | 15 | 30 | 1.97 |
| 7 | 90 | 25 | 30 | 4.21 |
| 8 | 90 | 25 | 30 | 4.02 |
| 9 | 90 | 35 | 20 | 5.39 |
| 10 | 80 | 25 | 20 | 4.10 |
| 11 | 90 | 15 | 40 | 4.02 |
| 12 | 90 | 25 | 30 | 4.36 |
| 13 | 90 | 35 | 40 | 3.60 |
| 14 | 80 | 35 | 30 | 2.98 |
| 15 | 100 | 25 | 20 | 5.14 |
| 16 | 90 | 15 | 20 | 5.90 |
| 17 | 100 | 25 | 40 | 3.14 |
The optimal extraction parameters were determined to be conditions of 95% amplitude, 16 minutes of extraction time, and a solid-to-liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 g mL−1. Furthermore, the RSM shown in Fig. S1 highlights the relationship between paired parameters toward soluble protein content. The findings reveal that the soluble protein content increases when the amplitude is higher and the extraction time is lower. The maximum soluble protein content is achieved at an amplitude of 95% and an extraction time of 16 minutes. The findings reported by Ochoa Rivas et al. showed that it was possible to achieve an increase of 136% in yield and an 86% improvement in purity when increasing the amplitude from 20 to 100% and reducing the time from 40 to 15 minutes.19 Furthermore, the findings indicate that the soluble protein content increases with higher amplitude and lower solid-to-liquid ratio. The maximum soluble protein content is attained at an amplitude of 95% and a solid-to-liquid ratio of 1 g solid sample with 20 mL of liquid solution. This finding is consistent with the research outlined by Siriwat et al.20
20 g mL−1. Furthermore, the RSM shown in Fig. S1 highlights the relationship between paired parameters toward soluble protein content. The findings reveal that the soluble protein content increases when the amplitude is higher and the extraction time is lower. The maximum soluble protein content is achieved at an amplitude of 95% and an extraction time of 16 minutes. The findings reported by Ochoa Rivas et al. showed that it was possible to achieve an increase of 136% in yield and an 86% improvement in purity when increasing the amplitude from 20 to 100% and reducing the time from 40 to 15 minutes.19 Furthermore, the findings indicate that the soluble protein content increases with higher amplitude and lower solid-to-liquid ratio. The maximum soluble protein content is attained at an amplitude of 95% and a solid-to-liquid ratio of 1 g solid sample with 20 mL of liquid solution. This finding is consistent with the research outlined by Siriwat et al.20
Additionally, soluble protein content increases with low extraction time and low solid-to-liquid ratio at moderate amplitudes. The maximum soluble protein content is observed at an extraction time of 16 minutes and a solid-to-liquid ratio of 1 g solid sample with 20 mL of liquid solution. This is attributed to the phenomenon where a reduced ratio of solid to liquid enhances extraction productivity. This occurs by creating a concentration disparity between the internal cellular environment of Wolffia globosa and the external alkaline solvent, consequently boosting the rate of mass transfer of soluble proteins and ultimately leading to a higher extraction yield.20
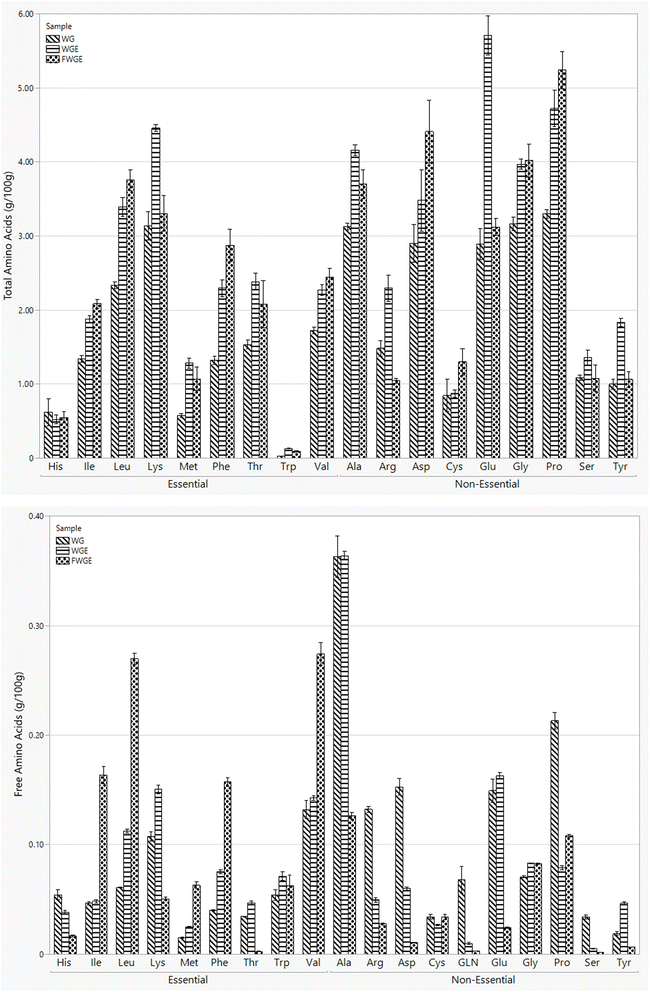
3.3 Nutritional comparison of Wolffia globosa treatments
The optimal extract from AUAE or WGE was then fermented for 72 hours utilizing L. plantarum 2075 to evaluate the effect on key nutritional components. A 72-hour period was selected because L. plantarum entered the death phase after this stage, and the pH, soluble protein, and total free amino acids remained stable. This fermented WGE (FWGE), along with the WGE and WG, was compared across key nutritional and bioactive qualities, including phenolic content, flavonoid content, antinutritional factors, antioxidant levels, digestibility, and amino acid profile, as shown in Table 3. The phenolics and flavonoids increased from the raw WG to the WGE and increased further after fermentation. The final phenolic value increased from 7.23 in WG to 14.37 mg GAE per g in the FWGE, and the flavonoids from 2.26 to 4.18 mg Q per g. This aligns with previous work, which demonstrated that ultrasonic amplitude improved total phenolic content (TPC) and total flavonoid content (TFC) in grapefruit peels and algae, respectively.21,22 Fermentation further enhances the TPC and TFC by enzymatically releasing bound molecules.23| WG | WGE | FWGE | |
|---|---|---|---|
| a WG is dried Wolffia globosa, WGE is AUAE Wolffia globosa, and FWGE is WGE fermented by L. plantarum 2075. The values are depicted as the mean ± SD, n = 3. Significant difference is indicated by letters using one-way ANOVA and Tukey's HSD post hoc test. | |||
| Phenolic (mg GAE per g DW) | 7.23 ± 0.22b | 13.83 ± 0.06a | 14.37 ± 0.88a |
| Flavonoid (mg Q per g DW) | 2.26 ± 0.03c | 3.38 ± 0.17b | 4.18 ± 0.02a |
| Tannin (mg TAE per g DW) | 5.73 ± 0.39c | 12.90 ± 0.18a | 10.85 ± 0.16b |
| Phytic acid (mg per g DW) | 0.18 ± 0.003a | 0.03 ± 0.008b | 0.01 ± 0.003c |
| DPPH (%) | 51.15 ± 2.23c | 62.88 ± 0.19a | 58.94 ± 0.99b |
| Digestibility (%) | 55.82 ± 0.57b | 70.45 ± 4.80a | |
| TAA (g/100 g) | 34.30 ± 0.91c | 49.22 ± 1.68a | 44.81 ± 1.68b |
| TFAA (g/100 g) | 2.23 ± 0.08a | 1.97 ± 0.03b | 1.59 ± 0.04c |
Tannins and phytic acid are antinutrients that disrupt the body's ability to uptake nutrients and reduce the bioavailability and digestibility of plant products. In this instance, the tannins increased from 5.73 to 12.90 after AUAE, which aligns with the previous work from ref. 24 demonstrating that UAE improved the extraction of the phenol tannin. Alternatively, after the WGE was fermented, the tannin content was reduced to 10.85, showcasing the breakdown of tannins by LAB, which was similarly observed in Lemna polyrhiza.25 The other major antinutrient tested showed a steep decline after UAE and a further drop after fermentation, starting from 0.18 mg g−1 in WG to 0.03 mg g−1 in WGE and 0.01 mg g−1 in FWGE.
The bioavailability and digestibility of plant proteins vary among different plant sources, and these properties can be improved by reducing or removing antinutritional factors—phytic acid, tannins, and protease inhibitors—that hinder protein digestion.26 This likely explains the significant increase in digestibility for the fermented sample FWGE at 70.45% compared to the 55.82% of the WGE, which had higher tannin and phytic acid concentrations. Furthermore, the 70.45% aligns with other studies showing an increase in digestibility in soybean flour from 75% to 88% and chickpea flour from 70.5% to 77.2% after fermentation with LAB.27,28 The WGE digestibility is lower than the untreated samples of the legumes; however, the fermentation of the Wolffia showed a more substantial change than the others. This shows even more potential for improving the base extraction or improving the fermentation parameters to further improve the protein.
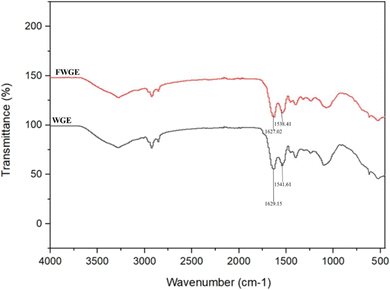 | ||
| Fig. 1 FTIR spectroscopy of extract and fermented extract. *WGE = Wolffia globosa extract by AUAE and FWGE = fermented Wolffia globosa extract by L. plantarum 2075. | ||
The results indicate that proteins undergoing fermentation with L. plantarum 2075 undergo slight secondary structural changes. Specifically, there is an increase in the quantity of α-helix and a decrease in β-turn and random coils, while the change in β-sheet is not obvious. The generation of small peptides during bacterial enzyme-mediated protein hydrolysis likely induced the structural changes from β-turn and random coils to α-helix in FWGE.30 In a study of Siriwat et al. (2023), the protein concentrate exhibited a rise in α-helix and β-turn content, while β-sheet and random coils decreased. These structural changes may lead to improved functional properties of the protein hydrolysate influencing solubility, digestibility, or stability, among others.19
 | ||
| Fig. 2 Determination of protein mass for the Wolffia samples. SDS-PAGE protein patterns of extracted proteins in (1) WG, (2) WGE, (3) FWGE, (4) WGE + lecithin, and (5) FWGE + lecithin. | ||
Meanwhile, FWGE consisted of five main bands, namely 20, 30, 48, 63, and 69 kDa. From the experimental results, it was observed that WGE had an average molecular size smaller than that of FWGE. This could possibly be attributed to proteins undergoing aggregation or polymerization during fermentation, resulting in the formation of larger protein complexes. This aggregation can occur due to changes in pH, temperature, or the presence of specific ions or molecules in the fermentation medium.31
For the samples mixed with lecithin to improve water solubility, changes in molecular size were observed. For WGE, initially consisting of three main bands (2), upon mixing with lecithin, an additional band with a molecular weight of 35 kDa appeared (4). Similarly, in FWGE, only two bands remained that reflect the changes that have occurred, with molecular weights of 48 and 69 kDa (5). These changes may be ascribed to aggregation or complex formation that potentially interferes with SDS protein interactions, explaining the new band for WGE and the loss of bands for FWGE.
After AUAE, the most abundant amino acid was glutamic acid (5.71 g/100 g), followed by lysine (4.46 g/100 g) and proline (4.72 g/100 g). Similarly, Dhamaratana et al. observed similar amino acid profiles in autoclave-extracted Wolffia protein, reporting glutamic acid (3.62 g/100 g), aspartic acid (2.94 g/100 g), and proline (2.81 g/100 g) as the highest fractions.5 However, this study exhibited higher TAA concentration for the same AA using AUAE. Additionally, lysine, which is nutritionally significant as it is an essential amino acid often limited in plant-based proteins, represented a higher percentage of the TAA in this study. Alternatively, both lysine (4.45 g/100 g) and glutamic acid (3.12 g/100 g) declined after fermentation, suggesting hydrolysis by the LAB, while proline (5.24 g/100 g) increased further as the most abundant AA for FWGE. This aligns with the presence of L-glutamate decarboxylase or lysine decarboxylase enzymes found in some strains of L. plantarum, which utilize glutamic acid and lysine, respectively.32,33
The TFAA shown in Fig. 3(b) gives some indication of the hydrolysis and polymerization of proteins under these extraction conditions. The WG and WGE samples exhibited abundant free alanine at 0.36 g/100 g for both, while in FWGE alanine was at 0.13 g/100 g, indicating the microbial uptake or degradation of alanine by L. plantarum 2075. Alternatively, fermentation increased the free EAA levels for isoleucine, leucine, phenylalanine, and valine, potentially exhibiting the value of the extract solution. Despite this, the overall TFAA decreased from WG for both WGE and FWGE, with FWGE showing the lowest TFAA at 1.59 g/100 g. The decline in TFAA for the FWGE likely results from direct uptake and utilization by L. plantarum 2075 to support the catabolism of other key metabolites.
Although the TAA of some of the EAAs still remains below the necessary limits for a complete protein, the increased levels of EAA are critical for the potential of Wolffia globosa as an alternative protein solution. UAE and subsequent fermentation demonstrate significant potential as an alternative protein processing to obtain not only high yield but quality protein. Beyond the EAA, further exploration and optimization of key flavor amino acids such as glutamate or aspartic acid are important toward future product development.
3.4 Techno-functional properties
The FWGE showed a similar trend to the unfermented samples across the pH values but showed markedly lower overall solubility. This is likely caused by the aggregation of proteins during fermentation as a result of heterologous expression in bacteria leading to insoluble deposits.34 Despite this, the trend indicates that the pI of the proteins was not markedly altered during the process. Lecithin can improve protein solubility by acting as spacers between proteins, which is critical for improving functional properties.35 This study observes that lecithin improved solubility for both WGE and FWGE when added, increasing solubility at pH 7 from 66.62 ± 0.76% to 75.21 ± 7.35% and from 45.22 ± 1.01% to 62.38 ± 4.57% respectively. The addition of lecithin at neutral pH showcases the potential for the fermented sample in high protein beverages, as the increased solubility improves beverage applications, and the higher digestibility compared to WGE is essential for optimal protein products. Although at higher pH the difference between lecithin and non-lecithin samples for WGE is reduced, this is likely due to the proteins reaching near maximum solubility.
| WGE | FWGE | |
|---|---|---|
| a WGE is AUAE Wolffia globosa, and FWGE is WGE fermented by L. plantarum 2075. The values are depicted as the mean ± SD, n = 3. Significant difference is indicated by letters using one-way ANOVA and Tukey's HSD post hoc test. | ||
| Water holding capacity (g g−1) | 8.60 ± 0.22a | 7.26 ± 0.07b |
| Foaming capacity (%) | 41.07 ± 1.03a | 35.16 ± 1.62b |
| Foam stability (%) | 53.37 ± 5.32b | 69.05 ± 2.38a |
| Emulsifying activity index (m2 g−1) | 80.99 ± 11.12a | 47.61 ± 9.31b |
| Emulsion stability (min) | 12.39 ± 0.56a | 13.31 ± 0.75a |
3.4.2.1 Water holding capacity. Based on the experimental results, it was found that the protein concentrate has a higher water holding capacity compared to the fermented protein concentrate, with values of 8.60 ± 0.22 g g−1 and 7.26 ± 0.07 g g−1, respectively. While there is limited work in duckweed species for comparison, these values are higher than the 4.28 g g−1 and 4.06 g g−1 observed in the microalgae A. platensis and H. pluvialis, respectively.36 The drop in WHC from WGE to FWGE is likely correlated with protein hydrolysis and reduced WHC of smaller peptides.
3.4.2.2 Foaming properties. WGE exhibits higher foam capacity and lower foam stability compared to FWGE. Specifically, WGE has a foam capacity of 41.07 ± 1.03%, which is similar to the foam capacity reported by Siriwat et al.20 which is 42.21%, and higher than the foam capacity of FWGE at 35.16 ± 1.62%. On the other hand, in terms of foam stability, WGE demonstrates a stability of 53.37 ± 5.32%, which is lower than the foam stability of FWGE at 69.05 ± 2.38%. This aligns with the expectation that fermentation of WGE with Lactobacillus spp. induces structural changes in proteins.37 These changes can impact the surface properties of proteins, affecting their ability to adsorb at the liquid–air interface and stabilize foam structures.38 Additionally, L. plantarum possesses proteolytic enzymes that are capable of breaking down proteins into smaller peptides and amino acids during fermentation.39 This proteolysis can lead to a decrease in the ability of proteins to form foams by disrupting their structural integrity.
The decrease in foaming capacity in fermented protein concentrate could be attributed to the increased stability of the protein structure, particularly with the increase in α-helix content. Proteins with stable networks form more stable foams compared to flexible proteins such as casein, which exhibit higher unfolding and disruption needed for foam formation.40 A more stable protein structure can indeed reduce unfolding, enhancing foam stability by limiting side chain movement and maintaining structural integrity.41 Thus, the lower foam capacity and higher foam stability in the FWGE are likely due to the increased α-helix content and decreased β-turn and random coil content.
3.4.2.3 Emulsion properties. The emulsion properties (Table 4) shows that WGE exhibits higher emulsion activity to FWGE with values of 80.99 ± 11.12 and 47.61 ± 9.31 m2 g−1, respectively. Conversely, WGE shows slightly lower emulsion stability compared to FWGE, with values of 12.39 ± 0.56 and 13.31 ± 0.75 minutes, respectively. Similarly, Çabuk et al., observed a reduction in emulsion activity for fermented samples of pea protein and a higher emulsion stability compared to the control.42 The decrease in emulsifying activity in fermented protein concentrate was likely influenced by the decrease in flexible regions, such as β-turns and random coils, which hinder the protein's ability to effectively interact with and stabilize emulsion droplets. Meanwhile, the increase in emulsifying stability results from the more stable protein structure, which can better withstand environmental stress and maintain the integrity of the emulsion interface.
The techno-functional properties for WGE and FWGE differ in key components, suggesting key differences in potential applications. The higher solubility and emulsifying activity index in WGE suggest clear potential as a plant protein beverage. At the same time, the FWGE shows minimal solubility but higher foam stability and less foam capacity, which suggests applicability in certain bakery products. The foaming capacity and stability of WGE and FWGE show similarity to commercial protein powders of fava bean and mung bean, respectively, according to a study by Jakobson et al.43 Additionally, this presents great potential for meat analogs, as similar work with fava and mung bean proteins was used to create functional meat analogs using 3D printing.44 Further studies are now essential for determining direct applications.
4 Conclusion
This study successfully demonstrated that the combination of ultrasonic-assisted extraction and probiotic-based fermentation significantly enhances the nutritional and bioactive properties of Wolffia globosa. The AUAE process improved protein yield by 125%, and subsequent fermentation with Lactobacillus plantarum 2075 reduced phytic acid and tannin contents by 15.9% and 66.7% respectively. Furthermore, while fermentation reduced the total antioxidant activity, WGE and FWGE still increased by 22.9 and 15.3% compared to WG. Perhaps the most critical advantage of the secondary fermentation stage was the increase in digestibility by 26.2%, which is essential for improving plant-based protein products. Moreover, the fermentation also significantly reduced the antinutrient content of the protein highlighting the improvement in bioavailability and nutritional quality. The AUAE effectively extracts Wolffia protein while also improving nutritional attributes, but fermentation further enhances this by improving digestibility and reducing antinutrients. These results indicate that Wolffia globosa can be developed into a functional food ingredient with superior nutritional quality and bioactive properties. The enhanced protein content, improved digestibility, and increased antioxidant activity position processed Wolffia globosa as a promising candidate for various food applications, particularly in the context of sustainable and health-promoting diets. This study contributes valuable insights into the potential of advanced processing techniques to enhance the nutritional and functional properties of plant-based foods, aligning with current trends in food innovation and engineering. Future studies on product formulation and sensory evaluation are critical toward advancing the treated Wolffia toward marketable and sustainable products.Author contributions
Nontikarn Taramark: writing-original draft, data curation, formal analysis, investigation, methodology. Daniel Rice: writing-original draft, writing-review & editing, data curation. Dr Atikorn Panya: conceptualization, supervision. Prof. Anil Kumar Anal: conceptualization, supervision, writing-review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available within the article.Supplementary information (SI): shows the response surface methodology of the alkaline ultrasound assisted extraction of the Wolffia globosa samples. See DOI: https://doi.org/10.1039/d5fb00550g.
Acknowledgements
The master's degree scholarship was funded by HM Queen's scholarship, Asian Institute of Technology, Thailand.References
- J. N. O'Sullivan, Demographic Delusions: World Population Growth is Exceeding Most Projections and Jeopardising Scenarios for Sustainable Futures, World, 2023, 4(3), 545–568, DOI:10.3390/world4030034.
- K. Dave, A. Kumar, N. Dave, M. Jain, P. S. Dhanda, A. Yadav and P. Kaushik, Climate Change Impacts on Legume Physiology and Ecosystem Dynamics: A Multifaceted Perspective, Sustainability, 2024, 16, 6026 CrossRef CAS.
- A. Ujong, J. Naibaho, S. Ghalamara, B. K. Tiwari, S. Hanon and U. Tiwari, Duckweed: exploring its farm-to-fork potential for food production and biorefineries, Sustainable Food Technol., 2025, 3, 54–80 RSC.
- P. Boonarsa, A. Bunyatratchata, T. Chumroenphat, P. Thammapat, T. Chaikwang, T. Siripan, H. Li and S. Siriamornpun, Nutritional Quality, Functional Properties, and Biological Characterization of Watermeal (Wolffia globosa), Horticulturae, 2024, 10, 1171 CrossRef.
- S. Dhamaratana, P. Methacanon and S. Charoensiddhi, Chemical composition and in vitro digestibility of duckweed (Wolffia globosa) and its polysaccharide and protein fractions, Food Chem. Adv., 2025, 6, 100867 CrossRef.
- C. Nitiwuttithorn, S. Wongsasulak, P. Vongsawasdi and J. Yongsawatdigul, Effects of alkaline and ultrasonication on duckweed (Wolffia arrhiza) protein extracts’ physicochemical and techno-functional properties, Front. Sustain. Food Syst., 2024, 8, 1343615, DOI:10.3389/fsufs.2024.1343615.
- V. H. Cauduro, G. Gohlke, N. W. da Silva, A. G. Cruz and E. M. Flores, A review on scale-up approaches for ultrasound-assisted extraction of natural products, Curr. Opin. Chem. Eng., 2025, 48, 101120 CrossRef.
- R. Mahoney, R. Weeks, Q. Huang, W. Dai, Y. Cao, G. Liu, Y. Guo, V. A. Chistyakov, A. M. Ermakov, D. Rudoy, A. Bren, I. Popov and M. L. Chikindas, Fermented Duckweed as a Potential Feed Additive with Poultry Beneficial Bacilli Probiotics, Probiotics Antimicrob. Proteins, 2021, 13, 1425–1432, DOI:10.1007/s12602-021-09794-4.
- L. Inguanez, X. Zhu, J. de Oliveira Mallia, B. K. Tiwari and V. P. Valdramidis, Extractions of Protein-Rich Alaria esculenta and Lemna minor by the Use of High-Power (Assisted) Ultrasound, Sustainability, 2023, 15(10), 8024, DOI:10.3390/su15108024.
- N. On-Nom, P. Promdang, W. Inthachat, P. Kanoongon, Y. Sahasakul, C. Chupeerach, U. Suttisansanee and P. Temviriyanukul, Wolffia globosa-Based Nutritious Snack Formulation with High Protein and Dietary Fiber Contents, Foods, 2023, 12(14), 2647, DOI:10.3390/foods12142647.
- N. Ngamkhae, O. Monthakantirat, Y. Chulikhit, C. Boonyarat, C. Khamphukdee, J. Maneenat, P. Kwankhao, S. Pitiporn and S. Daodee, Optimized Extraction Method for Kleeb Bua Daeng Formula with the Aid of the Experimental Design, J. Chem., 2021, 1, 1457729, DOI:10.1155/2021/1457729.
- O. Monthakantirat, Y. Chulikhit, J. Maneenet, C. Khamphukdee, Y. Chotritthirong, S. Limsakul, T. Punya, B. Turapra, C. Boonyarat and S. Daodee, Total Active Compounds and Mineral Contents in Wolffia globosa, J. Chem., 2022, 1, 9212872, DOI:10.1155/2022/9212872.
- S. Phongthai, S. T. Lim and S. Rawdkuen, Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties, J. Cereal Sci., 2016, 70, 146–154, DOI:10.1016/j.jcs.2016.06.001.
- P. Khampratueng and A. K. Anal, Enhancing the biodegradation of low-density polyethylene (LDPE) using novel bacterial consortia: Bacillus sp. AS3 and Sphingobacterium sp. AS8, J. Environ. Sci., 2026, 159, 263–270 CrossRef PubMed.
- S. Jain and A. K. Anal, Optimization of extraction of functional protein hydrolysates from chicken egg shell membrane (ESM) by ultrasonic assisted extraction (UAE) and enzymatic hydrolysis, LWT, 2016, 69, 295–302, DOI:10.1016/j.lwt.2016.01.057.
- S. Phongthai, S. T. Lim and S. Rawdkuen, Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties, J. Cereal Sci., 2016, 70, 146–154 CrossRef CAS.
- K. J. Appenroth, K. Sowjanya Sree, M. Bog, J. Ecker, C. Seeliger, V. Böhm, S. Lorkowski, K. Sommer, W. Vetter, K. Tolzin-Banasch, R. Kirmse, M. Leiterer, C. Dawczynski, G. Liebisch and G. Jahreis, Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human food, Front. Chem., 2018, 6, 483, DOI:10.3389/fchem.2018.00483.
- N. Duangjarus, W. Chaiworapuek, C. Rachtanapun, P. Ritthiruangdej and S. Charoensiddhi, Antimicrobial and Functional Properties of Duckweed (Wolffia globosa) Protein and Peptide Extracts Prepared by Ultrasound-Assisted Extraction, Foods, 2022, 11(15), 2348, DOI:10.3390/foods11152348.
- A. Ochoa-Rivas, Y. Nava-Valdez, S. O. Serna-Saldívar and C. Chuck-Hernández, Microwave and Ultrasound to Enhance Protein Extraction from Peanut Flour under Alkaline Conditions: Effects in Yield and Functional Properties of Protein Isolates, Food Bioprocess Technol., 2017, 10(3), 543–555, DOI:10.1007/s11947-016-1838-3.
- W. Siriwat, S. Ungwiwatkul, K. Unban, T. Laokuldilok, W. Klunklin, P. Tangjaidee, S. Potikanond, L. Kaur and S. Phongthai, Extraction, Enzymatic Modification, and Anti-Cancer Potential of an Alternative Plant-Based Protein from Wolffia globosa, Foods, 2023, 12(20), 3815, DOI:10.3390/foods12203815.
- M. Islam, S. Malakar, U. Dwivedi, N. Kumar, P. K. Prabakar, A. Kishore and A. Kumar, Impact of different drying techniques on grapefruit peels and subsequent optimization of ultrasonic extraction conditions for bioactive compounds, J. Food Process Eng., 2023, 46(6), e14331, DOI:10.1111/jfpe.14331.
- S. Braspaiboon, S. Osiriphun, S. Surawang, W. Jirarattanarangsri, N. Kanha and T. Laokuldilok, Ultrasound-assisted alkaline extraction of proteins in several algae and their nutritional characteristics, Int. J. Food Sci. Technol., 2022, 57(9), 6143–6154, DOI:10.1111/ijfs.15975.
- N. Liu, Y. Wang, X. An, J. Qi, B. Wang, J. Du and W. Wang, Composition, antioxidant and development-promoting activity of fermentation modified crude polysaccharides from stem and leaves of Chenopodium album L., Chem. Biol. Technol. Agric., 2024, 11, 88 CrossRef CAS.
- M. M. de Souza Ribeiro, J. Viganó, N. S. de Novais, L. M. de Souza Mesquita, R. C. Kamikawachi, W. Vilegas, P. S. Lopes, C. Soares da Silva, M. A. Rostagno and P. C. Veggi, The effect of ultrasound on improving the extraction of tannins from the Stryphnodendron adstringens bark, Sustainable Chem. Pharm., 2023, 33, 101044, DOI:10.1016/j.scp.2023.101044.
- A. Bairagi, K. Sarkar Ghosh, S. K. Sen and A. K. Ray, Duckweed (Lemna polyrhiza) leaf meal as a source of feedstuff in formulated diets for rohu (Labeo rohita Ham.) fingerlings after fermentation with a fish intestinal bacterium, Bioresour. Technol., 2002, 85(1), 17–24, DOI:10.1016/S0960-8524(02)00067-6.
- M. Li, X. Duan, J. Zhou, J. Li, B. K. Amrit and H. A. R. Suleria, Plant proteins and their digestibility, Processing Technologies and Food Protein Digestion, 2023, pp. 209–232, DOI:10.1016/B978-0-323-95052-7.00009-1.
- E. Bartkiene, G. Juodeikiene and D. Vidmantiene, Nutritional quality of fermented defatted soya and flaxseed flours and their effect on texture and sensory characteristics of wheat sourdough bread, Int. J. Food Sci. Nutr., 2012, 63(6), 722–729, DOI:10.3109/09637486.2011.649248.
- M. V. Chandra-Hioe, C. H. M. Wong and J. Arcot, The Potential Use of Fermented Chickpea and Faba Bean Flour as Food Ingredients, Plant Foods Hum. Nutr., 2016, 71, 90–95, DOI:10.1007/s11130-016-0532-y.
- Q. Zhang, X. Tong, B. Qi, Z. Wang, Y. Li, X. Sui and L. Jiang, Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport, J. Funct. Foods, 2018, 42, 298–305 CrossRef CAS.
- Y. Zhu, X. Zhao, X. Zhang, H. Liu and Q. Ao, Amino acid, structure and antioxidant properties of Haematococcus pluvialis protein hydrolysates produced by different proteases, Int. J. Food Sci. Technol., 2021, 56(1), 185–195, DOI:10.1111/ijfs.14618.
- M. Vázquez-Rey and D. A. Lang, Aggregates in monoclonal antibody manufacturing processes, Biotechnol. Bioeng., 2011, 108(7), 1494–1508, DOI:10.1002/bit.23155.
- Z. Sun, Y. Zhang, X. Lin, S. Zhang, Y. Chen and C. Ji, Inhibition Mechanism of Lactiplantibacillus plantarum on the Growth and Biogenic Amine Production in Morganella morganii, Foods, 2023, 12(19), 3625, DOI:10.3390/foods12193625.
- M. Iorizzo, G. Paventi and C. Di Martino, Biosynthesis of Gamma-Aminobutyric Acid (GABA) by Lactiplantibacillus plantarum in Fermented Food Production, Curr. Issues Mol. Biol., 2023, 46, 200–220, DOI:10.3390/cimb46010015.
- S. Li, W. Liu, K. D. Rausch, M. E. Tumbleson and V. Singh, Comparison of protein concentrate, protein isolate and wet sieving processes for enriching DDGS protein, J. Am. Oil Chem. Soc., 2014, 91(5), 867–874, DOI:10.1007/s11746-014-2411-8.
- H. L. Lin, W. T. Cheng, L. C. Chen, H. O. Ho, S. Y. Lin and C. M. Hsieh, Honokiol/magnolol-loaded self-assembling lecithin-based mixed polymeric micelles (lbMPMs) for improving solubility to enhance oral bioavailability, Int. J. Nanomed., 2021, 651–665, DOI:10.2147/IJN.S290444.
- O. K. Mosibo, G. Ferrentino and C. C. Udenigwe, Microalgae Proteins as Sustainable Ingredients in Novel Foods: Recent Developments and Challenges, Foods, 2024, 13(5), 733, DOI:10.3390/foods13050733.
- W. Wen-Qiong, Z. Jie-Long, Y. Qian, Z. Ji-Yang, L. Mao-Lin, G. Rui-Xia and H. Yujun, Structural and compositional changes of whey protein and blueberry juice fermented using Lactobacillus plantarum or Lactobacillus casei during fermentation, RSC Adv., 2021, 11(42), 26291–26301, 10.1039/d1ra04140a.
- F. Han, Q. Shen, W. Zheng, J. Zuo, X. Zhu, J. Li, C. Peng, B. Li and Y. Chen, The Conformational Changes of Bovine Serum Albumin at the Air/Water Interface: HDX-MS and Interfacial Rheology Analysis, Foods, 2023, 12(8), 1601, DOI:10.3390/foods12081601.
- Q. Mo, S. You, H. Fu, D. Wang, J. Zhang, C. Wang and M. Li, Purification and Identification of Antioxidant Peptides from Rice Fermentation of Lactobacillus plantarum and Their Protective Effects on UVA-Induced Oxidative Stress in Skin, Antioxidants, 2022, 11(12), 2333, DOI:10.3390/antiox11122333.
- K. Gräff, S. Stock, L. Mirau, S. Bürger, L. Braun, A. Völp, N. Willenbacher and R. von Klitzing, Untangling effects of proteins as stabilizers for foam films, Front. Soft Matter, 2022, 2, 1035377, DOI:10.3389/frsfm.2022.1035377.
- D. Aguilar-Farrera, J. I. Morales-Camacho, E. Espinosa-Hernández, C. G. Benítez-Cardoza, G. J. Jara-Romero and S. Luna-Suárez, Foaming and Structural Studies on the Acidic Subunit of Amaranth 11S Globulin Modified with Antihypertensive Peptides as a Function of pH and Ionic Strength, Molecules, 2022, 27(11), 3538, DOI:10.3390/molecules27113538.
- B. Çabuk, A. K. Stone, D. R. Korber, T. Tanaka and M. T. Nickerson, Effect of Lactobacillus plantarum fermentation on the surface and functional properties of pea protein-enriched flour, Food Technol. Biotechnol., 2018, 56(3), 411, DOI:10.17113/ftb.56.03.18.5449.
- K. Jakobson, A. Kaleda, K. Adra, M. L. Tammik, H. Vaikma, T. Kriščiunaite and R. Vilu, Techno-Functional and Sensory Characterization of Commercial Plant Protein Powders, Foods, 2023, 12(14), 2805, DOI:10.3390/foods12142805.
- S. Ghimire, C. Gamonpilas, M. Umar and A. K. Anal, Production of 3D-printed meat analogues using pea, fava, and mung bean proteins: A comparison study, Food Struct., 2025, 44, 100419 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |