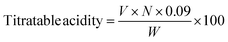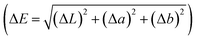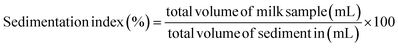Influence of thermosonication on the physicochemical, techno-functional, bioactive, microbiological and sensory properties of tiger nut (Cyperus esculentus L.) milk
Received
1st August 2025
, Accepted 12th November 2025
First published on 24th November 2025
Abstract
Emerging food processing techniques such as thermosonication (TS) for plant-based milk present a novel and promising research approach. In this study, tiger nut milk subjected to pasteurisation (90 °C for 60 s) and thermosonication (frequency: 45 kHz; power: 200 W; amplitude: 100%; temperature: 40, 50 and 60 °C; time: 5, 10 and 15 min) was analysed for its physicochemical, techno-functional, bioactive, antioxidant, microstructural, enzymatic, microbiological and sensory properties. Both treatments had no impact on the pH, total soluble solids and titratable acidity, while TS significantly (p < 0.05) reduced the sedimentation index (4.31–3.52%), residual enzyme peroxidase activity (100.0–6.30%) and polyphenol oxidase activity (100.0–5.14%). Comparatively, TS increased the contents of ascorbic acid (6.95–8.35 mg/100 mL), total phenols (22.50–28.03 mg GAE/100 mL), flavonoids (16.01–30.19 mg/100 mL), and carotenoids (8.05–13.04 mg/100 mL) and improved the antioxidant capacity (41.98–68.98%). Moreover, the optical micrograph showed reduced particle size to a non-visible level with increasing TS treatment, while the total bacterial and yeast/mould population reduced from 5.45 and 4.46 log CFU per mL to a non-detectable level, respectively. The sensory parameters slightly improved during TS treatment, with the mean scores above average. Notably, the TS treatment at 45 kHz and 50 °C presented the best thermosonication regime for the tiger nut milk sample, while treatment at 45 kHz and 60 °C elicited impaired changes to most of the milk properties. Hence, the use of thermosonication in enhancing the quality and economic value of tiger nut milk is technically feasible as an alternative to thermal processing.
Sustainability spotlight
This study shows that thermosonication is a sustainable, innovative food processing technique that improves the nutritional, functional, microbiological, and sensory quality of tiger nut milk while maintaining its compositional integrity. By reducing the residual enzymatic activity and microbial population below the FDA recommendation and enhancing the bioactive and antioxidant capacity, thermosonication provided a better alternative to thermal treatment. This outcome synchronises well with SDG 12 by encouraging cleaner production technologies and supports SDG 3 via the development of safer, healthier plant-based beverages. Furthermore, the valorisation of exotic rhizomes like tiger nut through the novel technology of thermosonication contributes to resilient food value chain and promotes sustainable dietary diversification.
|
1 Introduction
The rising consumers' demand for foods containing natural ingredients has driven the food industry into producing nutritious plant-sourced beverages in recent times.1 In this context, plant-based milk (PBM) containing liquid extracts from cereals, legumes, nuts and oilseeds, which are rich in nutrients and bioactive compounds, contributes immensely to a healthy lifestyle.2,3 Advantageously, PBM serves as an alternative to dairy milk in mitigating the prevalence of lactose intolerance, allergies and arteriosclerosis in vulnerable individuals.4,5 Given these benefits, the global retail sales of PBM have recorded for about $2.4 billion in 2020.6,7
However, the sensory and safety quality of plant-sourced beverages can easily deteriorate due to their high water activity, near-neutral pH and endogenous enzymes.8 To this end, the use of pasteurisation has been upheld to promote these qualities.9 While this thermal process enables the achievement of enzyme inactivation and a 5-log reduction, it also elicits significant loss in nutritional and sensory values.10 Hence, the emergence of alternative and novel techniques such as ultrasound has received research attention in recent times.11 The ultrasound mechanism involves the generation and propagation of sound waves (20–100 kHz) through a liquid (food) biomass to form bubbles that generate acoustic cavitation (sonolysis). These bubbles generate a vapour pressure difference, leading to their collapse and coalescence.12 The energy generated from this sonolytic coalescence (approximately 5000 k and 100 MPa) causes micro-streams, radical formation and shearing effect on cellular constituents.13 This phenomenon inactivates microbial and enzymatic activities within the food biomass, thereby promoting the physicochemical, bioactive, sensory, and safety quality of the food.14 The integration of this technique with controlled temperature, known as thermosonication (TS), has been found to be a promising approach in enhancing the quality and safety attributes of plant-sourced beverages.15 While a good number of PBM such as almond milk16 coconut milk17 and soy milk18 have been treated by this technique, other underutilized PBM sources like tiger nut milk is attracting an increasing interest due to their regional relevance in Africa and emerging niche markets globally.
Tiger nut (Cyperus esculentus L), also called “earth almond” is a sweet and nutrient-dense underground rhizome, cultivated initially around Egypt and the Mediterranean area.19 However, with the evolution of time, tiger nut cultivation and consumption attracted attention in different parts of America, Europe, Australia and African countries including Nigeria, as food and feedstocks.20 This global attention stemmed from its nutritional composition of protein (3.23–8.45%), lipids (22.14–44.92%), crude fibre (8.26–15.47%), mineral elements (1.60–2.60%) and carbohydrates (23.21–48.12%).21 Moreover, tiger nut contains a contributive content of phytochemicals, organic acids and other bioactive compounds.22 Interestingly, this excellent blend of nutritional and bioactive contents has enhanced its value-addition into milk, which is highly relished by the consumers.23 With a rising global market value of $481.2 million in 2024, the need to enhance the quality and safety attributes of tiger nut milk with emerging techniques becomes more relevant.24 Despite its nutritional potential, no studies to date have systematically evaluated the effect of thermosonication on tiger nut milk. It is hypothetically assumed that thermosonication will improve the quality of tiger nut milk based on the previous study on its application in enhancing other plant-based milk. Therefore, this study aims to investigate the effect of thermosonication on some quality and safety attributes of tiger nut milk.
2 Materials and method
2.1 Tiger nut preparation and milk extraction
The preparation and extraction were performed by the method of Ariyo.25 Tiger nut (Cyperus esculentus L) was obtained from Bodija commodity market (7.4350° N, 3.9143° E) in Ibadan, Nigeria. The nuts were cleaned and sorted to remove impurities and defective nuts, while the initial moisture content was determined to be 33% (wet basis) before extraction. The cleaned nut (one kilogram) was soaked with 3 L of distilled water for 12 h, with regular changes of water after 6 h. Soaked tiger nut was milled into a slurry with 2 L of distilled water in a high-speed motor warring blender (Model HGBTWT, Warring mill, Torrington, USA) having a speed of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The blended slurry was sieved using a muslin cloth to obtain a homogenised filtrate as tiger nut milk. The milk batch obtained was stored at 4 °C in plastic bottles and labelled for onward pasteurisation and thermosonication, while the untreated sample was labelled as a control. All chemicals applied in the current study were of analytical grade and obtained from Sigma-Aldrich, and all experiments were conducted in triplicates (n = 3).
000 rpm. The blended slurry was sieved using a muslin cloth to obtain a homogenised filtrate as tiger nut milk. The milk batch obtained was stored at 4 °C in plastic bottles and labelled for onward pasteurisation and thermosonication, while the untreated sample was labelled as a control. All chemicals applied in the current study were of analytical grade and obtained from Sigma-Aldrich, and all experiments were conducted in triplicates (n = 3).
2.2 Treatment protocol
2.2.1 Pasteurisation.
The tiger nut milk (TNM) samples were pasteurised to achieve approximately a 5-log microbial reduction in a laboratory-scale pasteuriser (JBN 26. Grant Inst., Ltd., Cambridge SG86GB, United Kingdom) at 90 °C for 60 s in accordance with the method reported by Santhirasegaram and Razali.26 The pasteurised TNM was subsequently cooled under a refrigerated condition (4 ± 2 °C for 30 min) for onward analysis.
2.2.2 Thermosonication.
The TS treatment of TNM was done according to a modified procedure reported by Qiu and Su27 in an ultrasonic bath (Elma Transsonic T1-H-10, Singen, Germany; power 200 W; frequency 45 kHz and amplitude 100%) with an acoustic energy density of 0.348 W cm−3. A 500 mL beaker containing the TNM sample was placed at the same water level in the sonicator bath. The TS treatment was performed in triplicates at different temperatures (40, 50 and 60 °C) and monitored using a calibrated digital thermometer with regular adjustment of the bath setting throughout the entire period (5, 10 and 15 min) for each sample, as shown in Table 1. Light interaction with the samples was prevented by maintaining a dark environment to prevent photodegradation of the sample. All samples were cooled to 4 °C and stored in a sterile plastic bottle for onward assay.
Table 1 Experimental design for raw, pasteurised and thermosonicated tiger nut milk samplesa
| Treatment |
Temperature (°C) |
Time (min) |
Ultrasonic power (W) |
Frequency (kHz) |
|
TNM: tiger nut milk, TS: thermosonicated milk samples, PTNM: pasteurised tiger nut milk sample.
|
| Raw TNM |
— |
— |
— |
— |
| PTNM |
90 |
1 |
— |
— |
| TS-1 |
40 |
5 |
200 |
45 |
| TS-2 |
40 |
10 |
200 |
45 |
| TS-3 |
40 |
15 |
200 |
45 |
| TS-4 |
50 |
5 |
200 |
45 |
| TS-5 |
50 |
10 |
200 |
45 |
| TS-6 |
50 |
15 |
200 |
45 |
| TS-7 |
60 |
5 |
200 |
45 |
| TS-8 |
60 |
10 |
200 |
45 |
| TS-9 |
60 |
15 |
200 |
45 |
2.3 Physicochemical properties (pH, total soluble solids and titratable acidity)
The pH of TNM samples was measured using a pH meter (Model 4120400, Auxilab, Spain) at ambient temperature. A hand-held refractometer was used for the total soluble solid (Atago Co. Ltd., Tokyo, Japan) with results expressed in °Brix, while titratable acidity was determined using 0.1 N sodium hydroxide and a phenolphthalein indicator, and the result is expressed as the percentage of lactic acid using the formula:| | 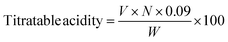 | (1) |
where V is the volume of NaOH, N is the normality of NaOH, W represents the equivalent weight of sample, 0.09 represents the multiplying factor for lactic acid.
2.4 Techno-functional properties
2.4.1 Cloudiness and browning index.
The cloudiness and browning index (BI) of TNM samples were determined by following the method of Cervantes-Elizarrarás and Piloni-Martini.28 First, 10 mL of TNM sample was centrifuged at 3000 rpm for 30 min, and the resulting supernatant was passed through a 0.45 µm filter. The absorbance at 660 nm was measured using a UV-Vis spectrophotometer (T70 PG instruments, Alma Park, UK). For BI, 5 mL of TNM mixed with 5 mL of ethanol was centrifuged, and the absorbance was observed at 420 nm. All measurements were performed in triplicate.
2.4.2 Viscosity.
The viscosity of TNM was evaluated by the method reported by Park and Olawuyi29 using a viscometer (DV I, Brookfield Amatek, Middleboro, MA, USA). About 10 mL of TNM sample was dispensed into a sterile 250 mL beaker at ambient temperature (25 ± 2 °C) using a digital thermometer for temperature control and positioned underneath the viscometer spindle (no. 63). The spindle was rotated at 100 rpm under ambient conditions, and the viscosity values are expressed in centipoise (cP).
2.4.3 Colour.
The colour (CIE L*a*b*) tristimulus values of TNM were analysed using a digital colourimeter (Chroma meter CR-400, Konika Minolta, USA) at ambient temperature (25 ± 2 °C).
The instrument was calibrated with standard white and black plates30 before measurement, while colour variation ΔE was derived using the following equation:
| | 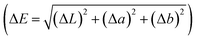 | (2) |
2.5 Bioactive compounds
2.5.1 Ascorbic acid.
The analysis for the value of ascorbic acid (AA) in TNM was performed following a method reported by Anaya-Esparza and Velázquez-Estrada31 with slight modifications. Briefly, 1 mL of TNM was added to 1 mL of 5% trichloroacetic acid (TCA) and centrifuged at 3500 rpm for 15 min to obtain a clear extract. Subsequently, 1 mL of 2% 2,4-dinitrophenyl hydrazine at 37 °C for 3 h was added to the clear extract for red complex formation with 1.0 M H2SO4. The degree of absorbance from the colour formation at 521 nm was read using a UV-Vis spectrophotometer, while AA was derived as milligram per 100 mL of TNM using an ascorbic acid standard calibration curve.
2.5.2 Total phenolic compound (TPC).
The analysis for the total phenolic compounds of TNM was performed by following the protocol presented by Rebaya and Belghith32 with slight modifications, using Folin–Ciocalteu's reagent. The absorbance observed at 760 nm using a spectrophotometer was extrapolated with a standard curve, and expressed in mg GAE/100 mL for each milk sample.
2.5.3 Carotenoid content.
The carotenoid content of TNM was analysed by the method of Lee and Castle.33 About 2 mL of TNM was added to 50 mL of n-butanol solvent and shaken thoroughly. The resulting mixture was kept away from light interference to adequate carotenoid extraction and centrifuged at 8000 rpm for 15 min. The supernatant obtained was read using a UV-Vis spectrophotometer at 440 nm, while beta-carotene was applied as the standard representative sample in the extrapolation curve.
2.5.4 Flavonoid content.
The flavonoid content of TNM was assayed by a procedure presented by Nayak and Chandrasekar.34 Briefly, 1.0 mL of TNM added to 0.3 mL of 5% NaNO3 was incubated at ambient temperature for 5 min. Subsequently, 0.3 mL of 10% AlCl3 was added to the mixture and allowed for another 6 min holding time. This was followed by the addition of 2.0 mL of 1 M NaOH and the volume was made up to 10 mL with distilled water. The resulting solution was shaken properly and allowed to stand for 15 min at ambient temperature. The absorbance measured at 510 nm using a spectrophotometer was used to obtain the flavonoid concentration in mg catechin equivalent/100 mL of TNM using a catechin reference calibration curve.
2.6 Antioxidant capacity
The value of antioxidant capacity (AC) in the TNM was determined following the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical procedure of Radha Krishnan and Azhagu Saravana Babu35 with slight modifications. The AC was derived using the following formula:| | | Antioxidant capacity (%) = [(Ac − As)/Ac] × 100 | (3) |
where Ac represents the absorbance of the blank mixture (containing DPPH void of beverage sample). As represents the absorbance of the TNM or standard.
2.7 Enzyme inactivation: peroxidase (POD) and polyphenoloxidase (PPO)
The POD and PPO activity of the treated TNM sample was analysed by following a modified procedure reported by Dars and Hu.36 The supernatant extract of TNM samples after centrifugation at 3500 rpm for 15 min under refrigeration (4 ± 2 °C) was used for the analysis. For POD analysis, 0.32 mL of the supernatant upheld at pH 6.8 was added to 0.32 mL of alkaline pyrogallol at 5% and 0.147 mol per L H2O2. A rise in the absorbance level in the spectrophotometer at 420 nm was observed within 3 min. For the PPO analysis, 1.5 ml of the TNM supernatant was added to 0.5 mL catechol and 3.0 mL K3PO4 phosphate buffer at 0.2 mol L−1 upheld at pH 6.8. A similar rise in the absorbance at 410 nm within 3 min was observed. The resulting outcome from the treated samples was expressed as the percentage of residual enzymatic activity, while the control sample was assumed to be 100%. The degree of residual enzymatic activity was derived using the following equation:| | | Residual enzymatic activity (%) = At/Au × 100 | (4) |
where At and Au stand for the enzymatic activity of the sample from TS-treated and untreated TNM, respectively.
2.8 Sedimentation index
The determination of sedimentation index (IS) was done according to the procedure reported by Rojas and Leite37 with minor modification. The TNM sample (50 mL) was dispensed into centrifuge tubes and stored for two days with the addition of 50% glycerol to inactivate the growth of microorganisms. The phase separation was observed and the results of sedimentation index were obtained as follows:| | 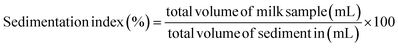 | (5) |
2.9 Optical microscopy
The optical microscopy of TNM was performed according to the method reported by Salve61 with minor modifications. About 10 mL of TNBM sample was taken in a covered measuring cylinder and shaken vigorously under ambient conditions. About 10 µL aliquot was pipetted onto a clean sterile glass slide and made into a thin smear using another glass slide. The thin smear was air-dried for 10 min, fixed using with 5% methanol for 1–2 min and stained with 0.3% Sudan black in 70% ethanol. The sample was observed using a digital microscope (ANDONSTAR AD409 Max-Es Guangdong, 518103. China.) using a 100× objective lens. The fitted Microscope measure software V3. 7.7 was used to capture the micrograph images of the milk samples.
2.10 Microbiological safety.
The microbiological analysis for bacterial, yeast and mould count population in pasteurised and thermosonicated TNM was performed using the total plate count (TPC) and yeast/mould count (YMC) spread plate method, respectively. Following the protocol reported by Nayak and Rayaguru,38 TPC was evaluated using plate count agar for bacterial population (37 °C for 48 h), while yeast and mould were evaluated after storage at 25 °C for 5 days with potato dextrose agar using the spread plate method. Furthermore, the detection limit used for the microbial population under the spread plate method was 1 CFU mL−1. Hence counts below this were expressed as non-detectable (ND). The mean count of microbial population over triplicates obtained was expressed as log CFU per mL.
2.11 Sensory evaluation.
The sensory properties of pasteurised and thermosonicated TNM were performed at the sensory laboratory by the protocol reported by Basu and Shivhare.39 This consists of 70 semi-trained panelists familiar with dairy products. An informed consent of the panellist was obtained based on the aim, protocol, risk and unconditional withdrawal from the process. The TNM samples produced hygienically were randomly coded to prevent bias, and panellists were instructed to rate the TNM based on a 9-point hedonic scale on specified sensory parameters (taste, flavour, colour, mouth feel and overall acceptability). Clean water was provided as a mouth rinser between the samples, and a similar lightning approach was maintained, while serenity and a cool atmosphere were maintained in getting optimal evaluation from the panelists.
2.12 Statistical analysis.
All analyses of samples were performed three times (n = 3), and the mean of results with the corresponding standard errors were subjected to analysis of variance (ANOVA) using Statistical Package for Social Statistics (SPSS) Version 22.0. The mean values were compared using Duncan's multiple range test at p < 0.05.
3 Results and discussion
3.1 pH, TSS and TA
The results of treatment on the pH, TSS and TA of TNM values in Table 2 show that pasteurisation and TS had no significant (p > 0.05) impact on these properties relative to the raw sample. This development could be due to the buffering capacity of milk with minimal impacts on sugar hydrolysis under the treatment conditions. Thus, the pH (6.52–6.62), TSS (3.00–3.30 °Brix) and TA (0.16–0.19%) of the treated sample were not different from the values of pH (6.68), TSS (3.40 °Brix) and TA (0.17%) observed in the raw sample. An earlier report of pasteurisation and TS observed on almond milk by Manzoor and Siddique16 supported this outcome. The pH indicates the potential for microbial stability, protein functionality, viscosity and colour stability. The TSS contributes to sensory sweetness and mouthfeel, and the TA shows relevance in flavour characteristics. Hence, the physicochemical properties represent an important parameter in the nutritional, safety and sensory quality of the milk sample.
Table 2 Effect of pasteurisation and thermosonication on the physicochemical and colour parameters of tiger nut milka,b
| Treatment |
pH |
Total soluble solids (oBrix) |
Titratable acidity (%) |
L* |
a* |
b* |
|
TNM: tiger nut milk; PTNM: pasteurised tiger nut milk; TS: thermosonicated milk samples.
Values are expressed as mean ± standard deviation of three replicate experiments. The mean values in the same column with the same superscripts are not significantly different (p < 0.05).
|
| Raw TNM |
6.68 ± 0.04a |
3.40 ± 0.11a |
0.17 ± 0.19a |
71.79 ± 0.14b |
3.94 ± 0.03b |
13.09 ± 0.02c |
| PTNM |
6.62 ± 0.03a |
3.20 ± 0.12a |
0.18 ± 0.18a |
73.79 ± 0.13b |
3.93 ± 0.02b |
9.84 ± 0.03b |
| TS-1 |
6.53 ± 0.03a |
3.30 ± 0.17a |
0.19 ± 0.21a |
72.74 ± 0.14b |
4.53 ± 0.04b |
10.63 ± 0.02b |
| TS-2 |
6.52 ± 0.03a |
3.20 ± 0.13a |
0.18 ± 0.11a |
74.71 ± 0.12b |
4.11 ± 0.04b |
12.44 ± 0.01b |
| TS-3 |
6.55 ± 0.02a |
3.00 ± 0.11a |
0.19 ± 0.18a |
74.76 ± 0.11b |
4.35 ± 0.05b |
13.80 ± 0.01b |
| TS-4 |
6.52 ± 0.03a |
3.20 ± 0.16a |
0.17 ± 0.17a |
75.89 ± 0.11b |
4.23 ± 0.04b |
13.17 ± 0.03a |
| TS-5 |
6.53 ± 0.02a |
3.30 ± 0.17a |
0.16 ± 0.19a |
75.73 ± 0.14b |
4.21 ± 0.02b |
12.71 ± 0.03b |
| TS-6 |
6.52 ± 0.04a |
3.30 ± 0.15a |
0.19 ± 0.15a |
76.04 ± 0.12a |
4.16 ± 0.03a |
13.75 ± 0.03a |
| TS-7 |
6.55 ± 0.03a |
3.00 ± 0.13a |
0.17 ± 0.13a |
69.77 ± 0.12b |
4.27 ± 0.02a |
13.60 ± 0.02b |
| TS-8 |
6.56 ± 0.03a |
3.10 ± 0.11a |
0.18 ± 0.15a |
67.24 ± 0.12b |
4.28 ± 0.01a |
13.02 ± 0.03b |
| TS-9 |
6.57 ± 0.03a |
3.20 ± 0.15a |
0.17 ± 0.17a |
64.82 ± 0.13b |
4.75 ± 0.02a |
13.53 ± 0.02a |
3.2 Cloudiness
The values of cloudiness from treated TNM in Fig. 2 show that the raw sample with a cloudiness value of 4.83 significantly reduced to 2.42 after pasteurisation. Thermosonicated samples significantly (p < 0.05) increased from 5.43 (TS-1) to 5.89 (TS-3) at 40 °C, 5.97 (TS-4) to 6.79 (TS-6) at 50 °C, and 7.05 (TS-7) to 8.19 (TS-9) at 60 °C. Cloudiness signifies the level of suspended particles like pectin, proteins and lipids in the tiger nut milk.40 Hence, the reduction with pasteurisation could be ascribed to the thermal denaturation of proteins, resulting in formation of aggregated colloids, which lowers the turbidity, while an increase in cloudiness after TS treatment could be ascribed to the dissociation of these suspended particles into finer colloidal particles by a shear force during sonolytic cavitation.41 An earlier report on the cloudiness (0.64–0.76) of freshly extracted tomato juice subjected to thermosonication (50–70 °C for 5 to 15 min) aligned with the present study.42 According to Atalar and Gul,43 improved colloidal stability in PBM resonates with reduced sedimentation and moderate viscosity, which indicates the positive impact of TS on the cloudiness value.
3.3 Browning index
The result of browning index (BI) of TNM in the raw sample was 0.160, but it significantly (p < 0.05) increased with pasteurisation to 0.178 (Table 3). However, TS treatment slightly increased the BI from 0.162 (TS-1) to 0.168 (TS-7). Elevated conditions of TS-8 and TS-9 showed a marked increase in BI from 0.170 to 0.171, respectively. The observed alterations in the BI have been ascribed to the development of the Maillard reaction during TS.44 According to the authors, the mild increase observed under moderate TS conditions could be attributed to the impact of acoustic cavitation with capacity to enhance the release of pigments associated with the Maillard formation. Meanwhile, this slight increase does not affect consumers' perception as the values of sensory colour in TS samples compare well with the raw sample but higher than the pasteurised sample, as shown in Table 5. Other authors have reported that this phenomenon is often activated above 50 °C, which explains the observed reduction in the lightness (L*) values of the TNM under elevated treatment conditions.45 Furthermore, elevated TS condition could lead to darker colour, resulting in the loss of quality and consumer acceptability.43 A similar pattern of result was reported in thermosonicated almond milk treated at 30–60 °C by Manzoor and Siddique.16 The Browning index in PBM represents quality and safety markers.
Table 3 Effect of pasteurisation and thermosonication on the browning, sedimentation index and enzyme activity of tiger nut milka,b
| Treatment |
Browning index |
Sedimentation index (%) |
POD (%) |
PPO (%) |
|
TNM: tiger nut milk; PTNM: pasteurised tiger nut milk; TS: thermosonicated milk samples; POD: peroxidase; PPO: polyphenol oxidase.
Values are expressed as mean ± standard deviation of three replicate experiments. The mean values in the same column with the same superscripts are not significantly different (p < 0.05).
|
| Raw TNM |
0.160 ± 0.11c |
4.31 ± 0.07b |
100.00 ± 0.00a |
100.00 ± 0.01a |
| PTNM |
0.178 ± 0.14a |
5.42 ± 0.12a |
7.13 ± 0.17h |
6.12 ± 0.16g |
| TS-1 |
0.162 ± 0.17bc |
4.27 ± 0.15b |
81.17 ± 0.19b |
78.16 ± 0.17b |
| TS-2 |
0.164 ± 0.12b |
4.23 ± 0.23b |
72.16 ± 0.13c |
66.15 ± 0.13c |
| TS-3 |
0.165 ± 0.14b |
4.19 ± 0.19b |
68.13 ± 0.19d |
60.17 ± 0.16d |
| TS-4 |
0.164 ± 0.13b |
4.25 ± 0.21b |
59.07 ± 0.17e |
51.12 ± 0.19e |
| TS-5 |
0.166 ± 0.17b |
4.13 ± 0.13b |
55.28 ± 0.12e |
44.18 ± 0.17e |
| TS-6 |
0.167 ± 0.07b |
4.09 ± 0.21bc |
18.11 ± 0.13f |
13.17 ± 0.13f |
| TS-7 |
0.168 ± 0.13b |
3.96 ± 0.13c |
11.25 ± 0.11g |
9.13 ± 0.11g |
| TS-8 |
0.170 ± 0.13a |
3.81 ± 0.09c |
8.12 ± 0.15h |
7.06 ± 0.18h |
| TS-9 |
0.171 ± 0.12a |
3.52 ± 0.17c |
6.30 ± 0.18i |
5.14 ± 0.11i |
3.4 Viscosity
The result of treatment on the viscosity of TNM is presented in Fig. 1, which shows an increase in the viscosity value (3.05–3.98 cP) after pasteurisation. However, during TS, the viscosity slightly (p < 0.05) increased at 40 °C (3.15–3.41 cP), 50 °C (3.35 to 3.59 cP) and 60 °C (3.78–4.01 cP). This increase in the viscosity of treated TNM could be ascribed to an increasing number of dispersed milk particles during sonolytic cavitation, which reduced the spatial distance. Furthermore, an increase at elevated temperatures could be ascribed to protein denaturation, which agglomerates to improve the TNM viscosity.46,47 According to Rojas and Leite,37 an increase in viscosity correlates with the reduction in sedimentation index based on the acoustic impact of TS on the dispersed particle size. According to the last cited author, a moderate increase in viscosity improves the colloidal stability and sensory acceptability, particularly with limited emulsifier and stabilisers, while high viscosity affects drinkability and consumer appeal. A similar increase in the viscosity (3.57–4.22 cP) of almond milk treated at 40 kHz, for 10–40 min at 30 to 60 °C aligned with the present study.16
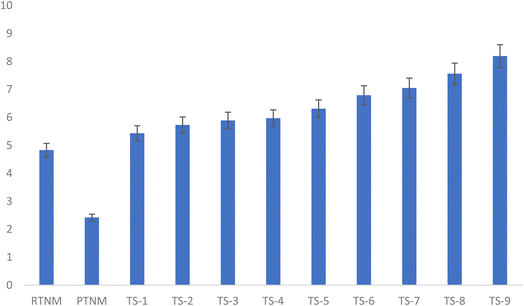 |
| | Fig. 1 Effect of pasteurisation and thermosonication on the cloudiness value of tiger nut milk. | |
3.5 Colour
The colour parameter is a veritable indicator for consumer appeal and acceptability of any processed food. Importantly, the lightness (L*) value in the raw sample slightly increased after pasteurisation (Table 2). In the TS-treated samples at 40–50 °C, the L* values significantly (p < 0.05) increased, while elevated treatment at 60 °C reduced the L* values. The observed increase in the colour parameters at moderate temperatures could be ascribed to the sonolytic cavitation of unstable suspended elements during TS, eliciting colour compounds via partial precipitation.48 Moreover, Chantapakul and Tao49 associated the increase in lightness (L*) values with dispersion and reduction in suspended fat globule size during cavitation, which increases the light-scattering capacity of the milk sample. Accordingly, an increase in the L* values under moderate TS conditions is desirable to consumer, as lighter appearance in milk is often linked to freshness and better quality. However, treatment under elevated TS conditions has been associated with the development of Maillard reaction and could inform the reduction in the L* values of the samples at 60 °C. This development imparts a yellow-brown colouration, which often impedes consumer acceptability.49 An earlier report on TS-treated Hazel nut milk aligned with the present study.43
3.6 Ascorbic acid
The result of ascorbic acid (AA) analysis presented in Table 4 was 6.95 mg/100 mL, but decreased to 4.53 mg/100 mL after pasteurisation. In contrast, TS treatment under moderate conditions significantly (p < 0.05) increased the AA level from (7.64 to 7.98 mg/100 mL) at 40 °C and from 8.06 to 8.35 mg/100 ml at 50 °C. However, treatment at 60 °C reduced AA from 7.35 to 4.40 mg/100 mL. The increase in AA under moderate conditions could be ascribed to expulsion of solubilised oxygen restricting oxidative loss during TS, thus enhancing the AA retention.50 A similar evidence of increase (2.97–3.56 mg mL−1) in TS-treated blackcurrant fruit juice supports the current study.51 Furthermore, the observed decline under extreme conditions could be ascribed to hydroxyl radical formation during extreme cavitation, as noted in thermosonicated blackcurrants mentioned earlier. Improved ascorbic acid in PBM via TS under moderate conditions of 50 °C depicts better shelf stability and promotes functional capacity of TNM, while elevated treatment can impair these qualities.52 Hence, the need to optimise the treatment condition for enhanced retention in TNM is very important from research and consumer's standpoint Table 5.
Table 4 Effect of pasteurisation and thermosonication on bioactive, antioxidant and microbiological properties of tiger nut milka,b
| Treatment |
Ascorbic acid (mg/100 ml) |
Total phenolic content (mg GAE/100 ml) |
Total flavonoid content (mg/100 ml) |
Total carotenoid content (mg/100 ml) |
Antioxidant capacity (%) |
Total bacterial count (Log CFU per ml) |
Yeast and mould count (Log CFU per ml) |
|
TNM: tiger nut milk; PTNM: pasteurised tiger nut milk; TS: thermosonicated milk samples.
Values are expressed as mean ± standard deviation of three replicate experiments. The mean values in the same column with the same superscripts are not significantly different (p < 0.05).
|
| Raw TNM |
6.95 ± 0.13c |
22.50 ± 0.02d |
16.01 ± 0.03d |
8.05 ± 0.02cd |
41.98 ± 0.06cd |
5.45 ± 0.21a |
4.46 ± 0.19a |
| PTNM |
4.53 ± 0.12d |
21.29 ± 0.04de |
13.33 ± 0.02e |
6.11 ± 0.02ef |
38.77 ± 0.12d |
ND |
ND |
| TS-1 |
7.64 ± 0.19b |
24.25 ± 0.05c |
19.23 ± 0.01 cd |
9.09 ± 0.01cd |
52.66 ± 0.31bc |
4.57 ± 0.13b |
3.28 ± 0.21b |
| TS-2 |
7.87 ± 0.13b |
25.57 ± 0.03bc |
22.15 ± 0.05c |
10.07 ± 0.01c |
54.02 ± 0.21b |
3.24 ± 0.19c |
3.07 ± 0.09b |
| TS-3 |
7.98 ± 0.01b |
26.01 ± 0.02b |
26.17 ± 0.03b |
11.14 ± 0.01bc |
55.80 ± 0.18b |
2.64 ± 0.13d |
2.18 ± 0.18c |
| TS-4 |
8.06 ± 0.01a |
25.43 ± 0.05bc |
24.16 ± 0.02bc |
11.53 ± 0.02bc |
63.91 ± 0.21ab |
1.35 ± 0.11e |
1.53 ± 0.17d |
| TS-5 |
8.17 ± 0.03a |
27.42 ± 0.03ab |
27.28 ± 0.04ab |
13.04 ± 0.02a |
67.05 ± 0.06a |
ND |
0.94 ± 0.23e |
| TS-6 |
8.35 ± 0.01a |
28.03 ± 0.01a |
30.19 ± 0.03a |
12.14 ± 0.01b |
68.98 ± 0.06a |
ND |
ND |
| TS-7 |
7.53 ± 0.01b |
23.65 ± 0.08cd |
26.18 ± 0.02b |
10.53 ± 0.02c |
60.15 ± 0.06ab |
ND |
ND |
| TS-8 |
6.37 ± 0.01c |
21.48 ± 0.02de |
18.16 ± 0.04cd |
7.92 ± 0.02e |
45.26 ± 0.06c |
ND |
ND |
| TS-9 |
4.40 ± 0.01d |
20.16 ± 0.02e |
11.35 ± 0.05f |
5.97 ± 0.01f |
36.30 ± 0.31d |
ND |
ND |
Table 5 Effect of pasteurisation and thermosonication on the sensory qualities of tiger nut milka,b
| Treatment |
Taste |
Colour |
Flavour |
Mouthfeel |
Overall acceptability |
|
TNM: tiger nut milk; PTNM: pasteurised tiger nut milk; TS: thermosonicated milk samples.
Values are expressed as mean ± standard deviation of three replicate experiments. The mean values in the same column with the same superscripts are not significantly different (p < 0.05).
|
| Raw TNM |
7.17 ± 0.18a |
7.23 ± 0.21a |
6.25 ± 0.13a |
7.19 ± 0.21a |
7.35 ± 0.19a |
| PTNM |
6.05 ± 0.15b |
6.18 ± 0.12b |
6.10 ± 0.24a |
6.19 ± 0.13b |
6.18 ± 0.21b |
| TS-1 |
7.47 ± 0.17a |
7.36 ± 0.13a |
6.39 ± 0.15a |
7.43 ± 0.21a |
7.55 ± 0.15a |
| TS-2 |
7.15 ± 0.19a |
7.31 ± 0.26a |
6.38 ± 0.23a |
7.41 ± 0.19a |
7.58 ± 0.12a |
| TS-3 |
7.31 ± 0.09a |
7.35 ± 0.14a |
6.31 ± 0.17a |
7.45 ± 0.25a |
7.47 ± 0.17a |
| TS-4 |
7.42 ± 0.16a |
7.32 ± 0.14a |
6.35 ± 0.15a |
7.47 ± 0.21a |
7.35 ± 0.25a |
| TS-5 |
7.40 ± 0.14a |
7.30 ± 0.16a |
6.33 ± 0.21a |
7.46 ± 0.13a |
7.41 ± 0.21a |
| TS-6 |
7.45 ± 0.19a |
7.12 ± 0.13a |
6.29 ± 0.14a |
7.43 ± 0.21a |
7.57 ± 0.11a |
| TS-7 |
7.18 ± 0.23a |
7.19 ± 0.26a |
6.31 ± 0.19a |
7.31 ± 0.21a |
7.54 ± 0.19a |
| TS-8 |
7.16 ± 0.17a |
7.13 ± 0.12a |
6.26 ± 0.13a |
7.28 ± 0.14a |
7.49 ± 0.21a |
| TS-9 |
7.08 ± 0.27a |
7.09 ± 0.22a |
6.14 ± 0.17a |
7.19 ± 0.28a |
7.46 ± 0.23a |
3.7 Bioactive compounds (TPC, TFC, and TCC)
The bioactive compounds of phenolic, flavonoid and carotenoid contents of TNM Fig. 2 were significantly affected by the treatment conditions (Table 4). The TPC, TFC and TCC in the raw sample were 22.50 mg GAE/100 ml, 16.01 and 8.05 mg/100 ml, respectively. Pasteurisation slightly reduced these compounds, while TS treatment at 40–50 °C significantly (p < 0.05) increased TPC (24.25–28.03 mg GAE/100 mL), TFC (19.23–30.19 mg/100 mL) and TCC (9.09–12.14 mg/100 mL), respectively. Conversely, TS treatment at 60 °C elicited a decline in all these bioactive compounds. According to Ghasemzadeh and Jaafar,53 the observed increase under moderate TS conditions may be due to sonolytic disruption, resulting in the leaching of bound phenolic and flavonoid compounds within the plant matrix. Other authors ascribed this increase to the formation of hydroxyl radicals in the aromatic phenolic structure during cavitation.54 However, the decline at 60 °C could be ascribed to induced degradation and isomerization due to extreme cavitation of these compounds.36 An earlier study on TS-treated almond milk reported a similar pattern in the bioactive compounds treated at 30–60 °C.16 According to Yu and Lu,21 tiger nut contains an appreciable repository of bioactive compounds, which can promote the health of consumers. Hence, the improvement of these compounds at 40–50 °C is beneficial in promoting the functional and health-contributing potential of tiger nut milk. However, the decline at 60 °C will affect the milk quality deleteriously, suggesting the need for optimisation during the industrial process.
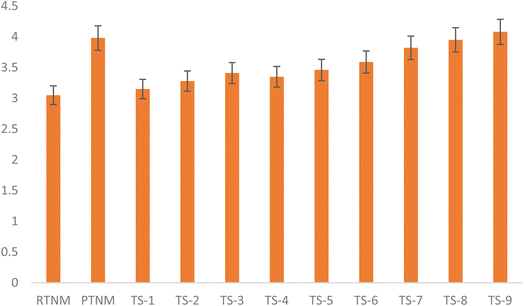 |
| | Fig. 2 Effect of pasteurisation and thermosonication on the viscosity (cP) value of tiger nut milk. | |
3.8 Antioxidant capacity
The antioxidant capacity (AC) of raw TNM presented in Table 4 was 41.98%, which decreased to 38.77% after pasteurisation. However, TS treatment significantly (p < 0.05) increased the AC of TNM at 40 °C (52.66–55.80%) and 50 °C (63.91–68.98%). However, a reduction from 60.15 to 36.30% was obtained after treatment at 60 °C. The increase in antioxidant capacity at 40 and 50 °C could be ascribed to the increase in the earlier-mentioned bioactive compounds in the milk matrix during the sonolytic cavitation.11 Moreover, lesser formation of hydroxyl radicals due to the stress response of the milk sample during cavitation has been linked to AC increase during sonolysis.55 For consumers, enhanced antioxidant capacity under moderate TS conditions presents a promising potential for TNM in scavenging for radicals and oxidative stress in certain diseases such as diabetes, cancer and heart-related diseases. However, increased formation of hydroxyl radicals at higher treatment has been reported to exhibit inhibitory effects on AC potentials.56 Hence, an optimised antioxidant capacity of TS-treated TNM depicts a promising and functional attribute with a potential role in reducing oxidative stress-related disorders.
3.9 Enzyme inactivation
The effect of treatment on the enzyme inactivation of POD and PPO in TNM is presented in Table 3. The raw samples both showed POD and PPO activity values of 100%, while pasteurisation reduced these residual enzymes to 7.13 and 6.12%, respectively. During TS, these enzyme activities reduced with extended treatment conditions. The POD activity was reduced from 81.17 to 68.13% at 40 °C, and from 59.07 to 18.11% at 50 °C. Elevated treatment conditions at 60 °C further reduced the POD from 11.25 to 6.30%. PPO followed a similar pattern of reduction from 78.16 to 60.17% at 40 °C, 51.12 to 13.17% at 50 °C and a notable reduction from 9.13 to 5.14% at 60 °C.
The increasing inactivation could be ascribed to acoustic cavitation, which disrupts the enzyme and protein structural network.57 A similar level of inactivation of the enzyme at 96.47% in thermosonicated soymilk at 70 °C for 12 min resonated with the present study. According to Umair and Jabeen,58 inactivation of POD and PPO is immensely beneficial as these enzymes elicit browning and off-flavour. Hence, TS presents a promising approach to maintain the freshness and quality of PBM without depending on the thermal method of pasteurisation.
3.10 Sedimentation index
Sedimentation remains one of the challenges associated with the colloidal stability of PBM. In the present study, the sedimentation index (SI) of the raw sample was significantly (p < 0.05) increased after pasteurisation. However, both moderate and elevated TS treatment slightly reduces the SI of the TNM. The reduction in the sedimentation index with TS depicts better stability in the particles of the thermosonicated samples. This phenomenon, which follows the Stokes law, has been ascribed to the dissociation of proteins and fat-based colloidal particles during sonolytic cavitation into microparticles, thus reducing the sedimentation by improving the colloidal stability.47 An earlier study on the sedimentation index of chick pea milk treated at 25 °C for 2–8 min reported a similar pattern of reduction.59 Accordingly, reduced sedimentation in PBM enhances visual appeal and mouthfeel, thus increasing its consumers' acceptability especially for those desiring smooth uniformity in milk products increases its market potentials.
3.11 Optical microscopy
The optical micrograph of treated TNM is shown in Fig. 3. Tiger nut milk is an emulsion embedded within a dispersed (fat globules and protein micelles) and continuous phase (water and other soluble compounds).60 In this study, the raw sample (Fig. 3: C) showed numerous fat globules and protein micelles, while both treatments influenced the number of these compounds. Consequently, treatment with pasteurisation (Fig. 3: PST) increased the number of fat globules and protein micelles, while progressive thermosonication load significantly reduced the number of these compounds (TS1–6), with practically no visible globules and droplets observed under elevated TS conditions (TS7–9). An increase in number with pasteurisation could be ascribed to protein denaturation and fat coalescence during thermal treatment, resulting in the formation of several protein complexes and fat droplets.60 Conversely, reduction with thermosonication could be ascribed to the sonolytic cavitation, which breakdown protein aggregates and fragment fat droplets within the milk sample.61 Further, this development increased the emulsion stability as shown in a reduced sedimentation index, resulting in a better homogenised tiger nut milk.47 An earlier report by the last author on sonicated peanut milk treated at different acoustic powers (200–400 W) supported this study.
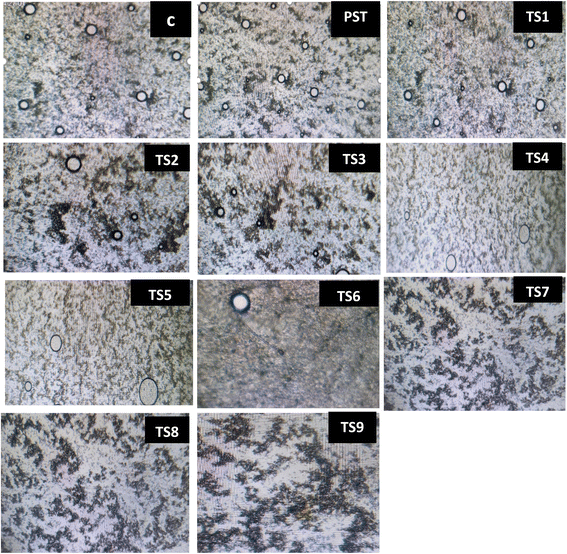 |
| | Fig. 3 Optical micrographs of raw (C), pasteurised (PST) and thermosonicated (TS1–TS9) tiger nut milk. | |
3.12 Microbiological safety
The microbial population of total bacteria and mould in the raw TNM was 5.45 and 4.46 log CFU per mL, respectively (Table 4). Meanwhile, complete inactivation was achieved with pasteurisation, as counts were reduced to a non-detectable level (<1.0 log CFU per mL). However, TS treatment at 40 °C resulted in a 1–2 log reduction for bacterial and mould count, while TS at ≥50 °C resulted in complete inactivation below detection limits, hence meeting the 5-log reduction requirement for microbiological safety by FDA. The complete inactivation of microorganism observed after pasteurisation could be ascribed to thermal disruption of nuclear constituents and cellular materials resulting in cell death.62 Noteworthy, under moderate TS conditions, the development of cavitation-based shear force and fragmentation could influence microbial inactivation. Furthermore, incomplete microbial inactivation observed under this condition could be ascribed to the survival of thermo-resistant spores as reported in some TS-treated fruit juice and nut-based milk under similar conditions.16,41 However, complete inactivation at ≥50 °C could be ascribed to acoustic cavitation, which generates thermo-physical implosion and free hydroxyl radicals, which causes cell membrane perforation (sonoporation) and intracellular matrix fragmentation.63 A similar reduction of bacteria (1.19 log CFU per mL) and yeast (0.9 log CFU per mL) in thermosonicated peanut milk has been reported.61
3.13 Sensory evaluation
The sensory values of raw and treated TNM under some selected parameters are presented in Fig. 4. The sensory scores in the raw samples include taste (7.17), colour (7.23), flavour (6.25). Mouth feel (7.19) and overall acceptability (7.35). Pasteurisation significantly (p < 0.05) reduced these sensory scores comparatively by 15 to 20% to 6.05, 6.18, 6.10, 6.19 and 6.18, respectively. Relative to the raw samples, treatment with TS improved consumer perception of taste (∼15%), colour (∼19%), flavour (4%), mouthfeel (∼17%) and overall acceptability (∼18%). The observed reduction in the flavour parameter could be ascribed to the loss of volatile compounds during higher cavitation conditions or thermally induced stress on the TNM matrix.64 Accordingly, this observation could also explain the earlier reduction in the TPC, TFC, and TCC of pasteurised samples and those treated under elevated TS conditions. Furthermore, similar loss of flavour volatiles has been reported in thermosonicated strawberry juice.14 Notably, the sensory scores of TS-treated samples were above 7.0 on the 9-point hedonic scale signifying a good preference by the consumers. This further indicates that TS enhances not only the quality of TNM, but also its sensory value in comparison to pasteurisation, thus promoting its functional and market potentials.
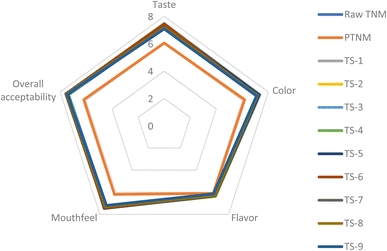 |
| | Fig. 4 Effect of pasteurisation and thermosonication on the sensory characteristics of tiger nut milk. | |
4 Conclusion
Given the global attention to nutritious foods and increased market value of plant-based milk from natural sources like tiger nut, the need to explore the use of emerging techniques (thermosonication) as an alternative to the traditional method (pasteurisation) becomes very imminent. In the present study, both thermosonication and pasteurisation had no significant impacts on the physicochemical properties of tiger nut milk; thermosonication (TS) improved the lightness (L*) value (71.79–76.04). Meanwhile, TS reduced the sedimentation index (4.31–3.52%) and enzyme activities of POD (100.0–6.30%) and PPO (100.0–5.14%) relative to the control sample. Importantly, TS increased the contents of ascorbic acid (6.95–8.35 mg/100 mL), total phenolic compounds (22.50–28.03 mg/100 mL), total flavonoids (16.01–30.19 mg/100 mL), and total carotenoids (8.05–13.04 mg/100 mL) and improved the antioxidant capacity (41.98–68.98%) relative to the control sample. Further to this, the optical micrograph showed reduced particle size with extended TS treatment, while both pasteurisation and thermosonication significantly inactivated microbial population assayed in the milk sample to a non-detectable level, respectively. The sensory parameters slightly improved with TS treatment, with the mean scores above average. Notably, TS treatment at 45 kHz, 50 °C presented the best thermosonication regime for tiger nut milk, while treatment at 45 kHz, 60 °C elicited impaired changes to most of the milk properties. However, studies on certain parameters such as zeta potentials, micro particle size, shelf stability and sensory parameter for different consumers based on location, during prolonged storage, are recommended for further investigation in thermosonicated tiger nut milk.
Author contributions
Oladunjoye A. O.: conceptualization, methodology, software and validation, editing of final writing, visualization and supervision, project administration. Idiat M. I.: formal analyses, investigation, resources and data curation, writing of original draft.
Conflicts of interest
The authors declare no conflicts of interest in this study.
Data availability
All data supporting this study have been shown in tables and figures.
Acknowledgements
The authors would like to acknowledge the technical support staff of the Department of Food Technology, University of Ibadan, for their technical assistance for this study.
References
- E. Medawar, S. Huhn, A. Villringer and A. Veronica Witte, The effects of plant-based diets on the body and the brain: a systematic review, Transl. Psychiatry, 2019, 9(1), 226, DOI:10.1038/s41398-019-0552-0.
- S. A. Siddiqui, T. Mehany, H. Schulte, R. Pandiselvam, A. A. Nagdalian and A. B. Golik,
et al., Plant-based Milk–thoughts of researchers and industries on what should be called as milk, Food Rev. Int., 2024, 40(6), 1703–1730 CrossRef.
- E. F. Aydar, S. Tutuncu and B. Ozcelik, Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects, J. Funct. Foods, 2020, 70, 103975, DOI:10.1016/j.jff.2020.103975.
- S. Moore, A. Costa, M. Pozza, T. Vamerali, G. Niero and S. Censi,
et al., How animal milk and plant-based alternatives diverge in terms of fatty acid, amino acid, and mineral composition, npj Sci. Food, 2023, 7(1), 50, DOI:10.1038/s41538-023-00227-w.
- W. Su, Y. Y. Zhang, S. Li and J. Sheng, Consumers' preferences and attitudes towards plant-based milk, Foods, 2023, 13(1), 2, DOI:10.3390/foods13010002.
-
Worldmetrics, Global plant-based milk industry statistics drive market expansion and innovation, https://worldmetrics.org/plant-based-milk-industry-statistics/, accessed. 2024.
- A. N. Mekanna, A. Issa, D. Bogueva and C. Bou-Mitri, Consumer perception of plant-based milk alternatives: systematic review, Int. J. Food Sci. Technol., 2024, 59(11), 8796–8805 Search PubMed.
- N. Sharma, B. D. Maibam and M. Sharma, Review on effect of innovative technologies on shelf-life extension of non-dairy sources from plant matrices, Food Chem. Adv., 2024, 5, 100781, DOI:10.1016/j.focha.2024.100781.
- A. Xie, Y. Dong, Z. Liu, Z. Li, J. Shao and M. Li,
et al., A review of plant-based drinks addressing nutrients, flavor, and processing technologies, Foods, 2023, 12(21), 3952, DOI:10.3390/foods12213952.
- S. C. B. Bavisetty and K. Venkatachalam, Physicochemical qualities and antioxidant properties of juice extracted from ripe and overripe wax apple as affected by pasteurization and sonication, J. Food Process. Preserv., 2021, 45(6), e15524, DOI:10.3390/foods12213952.
- L. M. Anaya-Esparza, R. M. Velázquez-Estrada, A. X. Roig, H. S. García-Galindo, S. G. Sayago-Ayerdi and E. Montalvo-González, Thermosonication: An alternative processing for fruit and vegetable juices, Trends Food Sci. Technol., 2017, 61, 26–37 Search PubMed.
- S. Wang, J. Kang, X. Zhang and Z. Guo, Dendrites fragmentation induced by oscillating cavitation bubbles in ultrasound field, Ultrasonics, 2018, 83, 26–32 CAS.
- D. M. Sotelo-Lara, G. G. Amador-Espejo, D. F. Álvarez-Araiza, A. K. Cordero-Rivera, K. G. Millán-Quintero and R. Campos-Vega,
et al., Ultrasound and Thermosonication as Promising Technologies for Processing Plant-Based Beverages: A Review, Food Technol. Biotechnol., 2024, 62(4), 538–552 Search PubMed.
- B. Xu, M. Feng, B. Chitrakar, J. Cheng, B. Wei and B. Wang,
et al., Multi-frequency power thermosonication treatments of clear strawberry juice: Impact on color, bioactive compounds, flavor volatiles, microbial and polyphenol oxidase inactivation, Innovative Food Sci. Emerging Technol., 2023, 84, 103295, DOI:10.1016/j.ifset.2023.103295.
- A. R. Abdulstar, A. B. Altemimi and A. R. Al-Hilphy, Exploring the power of thermosonication: a comprehensive review of its applications and impact in the food industry, Foods, 2023, 12(7), 1459, DOI:10.3390/foods12071459.
- M. F. Manzoor, R. Siddique, A. Hussain, N. Ahmad, A. Rehman and A. Siddeeg,
et al., Thermosonication effect on bioactive compounds, enzymes activity, particle size, microbial load, and sensory properties of almond (Prunus dulcis) milk, Ultrason. Sonochem., 2021, 78, 105705, DOI:10.1016/j.ultsonch.2021.105705.
- Y. Sun, H. Chen, W. Chen, Q. Zhong, M. Zhang and Y. Shen, Effects of ultrasound combined with preheating treatment to improve the thermal stability of coconut milk by modifying the physicochemical properties of coconut protein, Foods, 2022, 11(7), 1042, DOI:10.3390/foods11071042.
- G. Xie, J. Luo, F. Li, D. Li, Y. Han and Y. Tao, Comparison between hydrodynamic and ultrasound cavitation on the inactivation of lipoxygenase and physicochemical properties of soy milk, Ultrason. Sonochem., 2023, 101, 106692, DOI:10.1016/j.ultsonch.2023.106692.
- E. Sánchez-Zapata, J. Fernández-López and J. Angel Pérez-Alvarez, Tiger nut (Cyperus esculentus) commercialization: health aspects, composition, properties, and food applications, Compr. Rev. Food Sci. Food Saf., 2012, 11(4), 366–377 Search PubMed.
- O. A. Oluseye, O. I. Christianah, O. S. Barnabas and O. O. Bola, Cultivation, Nutrition, and Market Trends of the Multi-Faceted Tubers: Tiger Nuts, Int J Curr Microbiol App Sci, 2024, 13(06), 216–227 Search PubMed.
- Y. Yu, X. Lu, T. Zhang, C. Zhao, S. Guan and Y. Pu,
et al., Tiger nut (Cyperus esculentus L.): nutrition, processing, function and applications, Foods, 2022, 11(4), 601, DOI:10.3390/foods11040601.
- G. Nina, A. Goncharov, N. Batishcheva, S. Vlasov, E. Okuskhanova and M. CÃsarovÃ,
et al., Proximate, mineral and functional properties of tiger nut flour extracted from different tiger nuts cultivars, J. Microbiol., Biotechnol. Food Sci., 2019, 9(3), 653–656 CAS.
- Y. Tasiu, M. Atiku and O. Alalade, Processing and Preservation of Tiger nut (Cyprus esculentus) milk: an overview, J. Pure Appl. Sci., 2023, 9(3b), 136–143 Search PubMed.
-
CMR, CMR. Tigernut Milk Market Report 2025 (Global Edition), 2025, https://www.cognitivemarketresearch.com/ Search PubMed.
- O. Ariyo, O. Adetutu and O. Keshinro, Nutritional composition, microbial load and consumer acceptability of tiger nut (Cyperus esculentus), date (Phoenix dactylifera L.) and ginger (Zingiber officinale Roscoe) blended beverage, Agro-Science, 2021, 20(1), 72–79 Search PubMed.
- V. Santhirasegaram, Z. Razali and C. Somasundram, Effects of thermal treatment and sonication on quality attributes of Chokanan mango (Mangifera indica L.) juice, Ultrason. Sonochem., 2013, 20(5), 1276–1282 CAS.
- X. Qiu, J. Su, J. Nie, Z. Zhang, J. Ren and S. Wang,
et al., Effects of thermosonication on the antioxidant capacity and physicochemical, bioactive, microbiological, and sensory qualities of blackcurrant juice, Foods, 2024, 13(5), 809, DOI:10.3390/foods13050809.
- A. Cervantes-Elizarrarás, J. Piloni-Martini, E. Ramírez-Moreno, E. Alanís-García, N. Güemes-Vera and C. A. Gómez-Aldapa,
et al., Enzymatic inactivation and antioxidant properties of blackberry juice after thermoultrasound: Optimization using response surface methodology, Ultrason. Sonochem., 2017, 34, 371–379 Search PubMed.
- J. J. Park, I. F. Olawuyi and W. Y. Lee, Influence of Thermo-sonication and Ascorbic Acid Treatment on Microbial Inactivation and Shelf-Life Extension of Soft Persimmon (Diospyros kaki T.) Juice, Food Bioprocess Technol., 2021, 14(3), 429–440 CAS.
- L. E. Ordóñez-Santos, J. Martínez-Girón and M. E. Arias-Jaramillo, Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice, Food Chem., 2017, 233, 96–100 Search PubMed.
- L. M. Anaya-Esparza, R. M. Velázquez-Estrada, S. G. Sayago-Ayerdi, J. A. Sánchez-Burgos, M. V. Ramírez-Mares and M. de Lourdes García-Magana,
et al., Effect of thermosonication on polyphenol oxidase inactivation and quality parameters of soursop nectar, LWT, 2017, 75, 545–551 CrossRef CAS.
- A. Rebaya, S. I. Belghith, B. Baghdikian, V. M. Leddet, F. Mabrouki and E. Olivier,
et al., Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae), J. Appl. Pharm. Sci., 2014, 5(1), 52–57 Search PubMed.
- H. S. Lee and W. S. Castle, Seasonal changes of carotenoid pigments and color in Hamlin, Earlygold, and Budd Blood orange juices, J. Agric. Food Chem., 2001, 49(2), 877–882 CrossRef CAS.
- P. K. Nayak, C. M. Chandrasekar and R. K. Kesavan, Effect of thermosonication on the quality attributes of star fruit juice, J. Food Process Eng., 2018, 41(7), e12857, DOI:10.1111/jfpe.12857.
- K. Radha Krishnan, P. Azhagu Saravana Babu, S. Babuskin, M. Sivarajan and M. Sukumar, Modeling the kinetics of antioxidant extraction from Origanum vulgare and Brassica nigra, Chem. Eng. Commun., 2015, 202(12), 1577–1585 CrossRef CAS.
- A. G. Dars, K. Hu, Q. Liu, A. Abbas, B. Xie and Z. Sun, Effect of thermo-sonication and ultra-high pressure on the quality and phenolic profile of mango juice, Foods, 2019, 8(8), 298, DOI:10.3390/foods8080298.
- M. L. Rojas, T. S. Leite, M. Cristianini, I. D. Alvim and P. E. Augusto, Peach juice processed by the ultrasound technology: Changes in its microstructure improve its physical properties and stability, Food Res. Int., 2016, 82, 22–33 CrossRef CAS.
- P. K. Nayak, K. Rayaguru and K. Radha Krishnan, Quality comparison of elephant apple juices after high-pressure processing and thermal treatment, J. Sci. Food Agric., 2017, 97(5), 1404–1411 CrossRef CAS.
- S. Basu, U. Shivhare, T. Singh and V. Beniwal, Rheological, textural and spectral characteristics of sorbitol substituted mango jam, J. Food Eng., 2011, 105(3), 503–512 CrossRef CAS.
- T. Yu, Q. Wu, J. Wang, B. Liang, X. Wang and X. Shang, Physicochemical properties of tiger nut (Cyperus esculentus L) polysaccharides and their interaction with proteins in beverages, Food Chem.: X, 2023, 19, 100776, DOI:10.1016/j.fochx.2023.100776.
- B. Basumatary, P. K. Nayak, C. M. Chandrasekar, A. Nath, M. Nayak and R. K. Kesavan, Impact of thermo sonication and pasteurization on the physicochemical, microbiological and anti-oxidant properties of pomelo (Citrus maxima) juice, Int. J. Fruit Sci., 2020, 20(sup3), 2056–2073 CrossRef.
- L. Li, H. Su, L. Pang, Y. Pan, X. Li and Q. Xu,
et al., Thermosonication enhanced the bioactive, antioxidant, and flavor attributes of freshly squeezed tomato juice, Ultrason. Sonochem., 2025, 115, 107299, DOI:10.1016/j.ultsonch.2025.107299.
- I. Atalar, O. Gul, F. T. Saricaoglu, A. Besir, L. B. Gul and F. Yazici, Influence of thermosonication (TS) process on the quality parameters of high pressure homogenized hazelnut milk from hazelnut oil by-products, J. Food Sci. Technol., 2019, 56, 1405–1415 CrossRef CAS PubMed.
- A. O. Oladunjoye, F. O. Adeboyejo, T. A. Okekunbi and O. R. Aderibigbe, Effect of thermosonication on quality attributes of hog plum (Spondias mombin L.) juice, Ultrason. Sonochem., 2021, 70, 105316, DOI:10.1016/j.ultsonch.2020.105316.
- D. Bermudez-Aguirre and B. A. Niemira, Pasteurization of foods with ultrasound: The present and the future, Appl. Sci., 2022, 12(20), 10416, DOI:10.3390/app122010416.
- A. J. Vela, M. Villanueva, Á. G. Solaesa and F. Ronda, Impact of high-intensity ultrasound waves on structural, functional, thermal and rheological properties of rice flour and its biopolymers structural features, Food Hydrocoll., 2021, 113, 106480, DOI:10.1016/j.foodhyd.2020.106480.
- A. K. Sarangapany, A. Murugesan, A. S. Annamalai, A. Balasubramanian and A. Shanmugam, An overview on ultrasonically treated plant-based milk and its properties–A Review, Appl. Food Res., 2022, 2(2), 100130, DOI:10.1016/j.afres.2022.100130.
- N. Kutlu, R. Pandiselvam, A. Kamiloglu, I. Saka, N. Sruthi and A. Kothakota,
et al., Impact of ultrasonication applications on color profile of foods, Ultrason. Sonochem., 2022, 89, 106109, DOI:10.1016/j.ultsonch.2022.106109.
- T. Chantapakul, W. Tao, W. Chen, X. Liao, T. Ding and D. Liu, Manothermosonication: Inactivation and effects on soymilk enzymes, Ultrason. Sonochem., 2020, 64, 104961, DOI:10.1016/j.ultsonch.2020.104961.
- U. Roobab, A. Abida, G. M. Madni, M. M. A. N. Ranjha, X.-A. Zeng and A. M. Khaneghah,
et al., An updated overview of ultrasound-based interventions on bioactive compounds and quality of fruit juices, J. Agric. Food Res., 2023, 14, 100864, DOI:10.1016/j.jafr.2023.100864.
- C. N. D. Silva, J. R. D. Carmo, B. V. Nunes, F. Demoliner, V. R. D. Souza and S. C. Bastos, Synergistic Effect of Thermosonication on the Stability of Bioactive Compounds and Antioxidant Activity of Blackberry Juice, Foods, 2025, 14(5), 901, DOI:10.3390/foods14050901.
- T. Mehany, S. A. Siddiqui, B. Olawoye, O. Olabisi Popoola, A. Hassoun and M. F. Manzoor,
et al., Recent innovations and emerging technological advances used to improve quality and process of plant-based milk analogs, Crit. Rev. Food Sci. Nutr., 2024, 64(20), 7237–7267 CrossRef PubMed.
- A. Ghasemzadeh, H. Z. Jaafar, A. S. Juraimi and A. Tayebi-Meigooni, Comparative evaluation of different extraction techniques and solvents for the assay of phytochemicals and antioxidant activity of hashemi rice bran, Molecules, 2015, 20(6), 10822–10838 CrossRef CAS.
- P. K. Nayak, C. M. Chandrasekar, S. Gogoi and R. krishnan Kesavan, Impact of thermal and thermosonication treatments of amora (Spondius pinnata) juice and prediction of quality changes using artificial neural networks, Biosyst. Eng., 2022, 223, 169–181 Search PubMed.
- Y. Wu, L. Xu, X. Liu, K. F. Hasan, H. Li and S. Zhou,
et al., Effect of thermosonication treatment on blueberry juice quality: Total phenolics, flavonoids, anthocyanin, and antioxidant activity, LWT, 2021, 150, 112021, DOI:10.1016/j.lwt.2021.112021.
- T. M. Qureshi, M. Nadeem, F. Maken, A. Tayyaba, H. Majeed and M. Munir, Influence of ultrasound on the functional characteristics of indigenous varieties of mango (Mangifera indica L.), Ultrason. Sonochem., 2020, 64, 104987, DOI:10.1016/j.ultsonch.2020.104987.
- K. Rathnakumar, R. G. T. Kalaivendan, G. Eazhumalai, A. P. R. Charles, P. Verma and S. Rustagi,
et al., Applications of ultrasonication on food enzyme inactivation-recent review report (2017–2022), Ultrason. Sonochem., 2023, 96, 106407, DOI:10.1016/j.ultsonch.2023.106407.
- M. Umair, S. Jabeen, Z. Ke, S. Jabbar, F. Javed and M. Abid,
et al., Thermal treatment alternatives for enzymes inactivation in fruit juices: Recent breakthroughs and advancements, Ultrason. Sonochem., 2022, 86, 105999, DOI:10.1016/j.ultsonch.2022.105999.
- A. Vallath and A. Shanmugam, Study on model plant based functional beverage emulsion (non-dairy) using ultrasound–A physicochemical and functional characterization, Ultrason. Sonochem., 2022, 88, 106070, DOI:10.1016/j.ultsonch.2022.106070.
- O. Adedeji, K. Yohanna, O. Adedeji, B. Yunusa and A. Ango, Stability, nutritional composition, and antioxidant properties of surfactant-assisted enzymatically extracted tiger nut milk, Acta Aliment., 2022, 51(3), 413–423, DOI:10.1556/066.2022.00068.
- A. R. Salve, K. Pegu and S. S. Arya, Comparative assessment of high-intensity ultrasound and hydrodynamic cavitation processing on physico-chemical properties and microbial inactivation of peanut milk, Ultrason. Sonochem., 2019, 59, 104728, DOI:10.1016/j.ultsonch.2019.104728.
- J. Mandha, H. Shumoy, A. O. Matemu and K. Raes, Characterization of fruit juices and effect of pasteurization and storage conditions on their microbial, physicochemical, and nutritional quality, Food Biosci., 2023, 51, 102335, DOI:10.1016/j.fbio.2022.102335.
- J. Li, X. Zhang, M. Ashokkumar, D. Liu and T. Ding, Molecular regulatory
mechanisms of Escherichia coli O157: H7 in response to ultrasonic stress revealed by proteomic analysis, Ultrason. Sonochem., 2020, 61, 104835, DOI:10.1016/j.ultsonch.2019.104835.
- C. Cheng, Y. Wu and J. Yue, Effects of thermal and nonthermal processing techniques on aroma compounds in fruit juices: a meta-analysis, Food Bioeng., 2022, 1(3–4), 289–297 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2026 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article * and
Mopelola I.
Idiat
* and
Mopelola I.
Idiat
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The blended slurry was sieved using a muslin cloth to obtain a homogenised filtrate as tiger nut milk. The milk batch obtained was stored at 4 °C in plastic bottles and labelled for onward pasteurisation and thermosonication, while the untreated sample was labelled as a control. All chemicals applied in the current study were of analytical grade and obtained from Sigma-Aldrich, and all experiments were conducted in triplicates (n = 3).
000 rpm. The blended slurry was sieved using a muslin cloth to obtain a homogenised filtrate as tiger nut milk. The milk batch obtained was stored at 4 °C in plastic bottles and labelled for onward pasteurisation and thermosonication, while the untreated sample was labelled as a control. All chemicals applied in the current study were of analytical grade and obtained from Sigma-Aldrich, and all experiments were conducted in triplicates (n = 3).