Open Access Article
Open Access ArticleA comprehensive review on the nutritional value, anti-nutritional factors, acridity, medicinal properties, and culinary applications of Alocasia macrorrhizos
Olivia
Das
,
C.
Nickhil
 and
Sankar
Chandra Deka
and
Sankar
Chandra Deka
 *
*
Department of Food Engineering and Technology, Tezpur University, Napaam, Sonitpur, Tezpur, Assam, India. E-mail: sankar@tezu.ernet.in
First published on 28th November 2025
Abstract
Alocasia macrorrhizos is an ornamental plant, often called giant taro or elephant ear. The plant is native to tropical regions of the Asia and Pacific and is highly valued for its nutritional and therapeutic qualities. This review aims to present its nutritional and health benefits, pharmacological benefits, traditional uses, and identification of phytochemicals. Alocasia macrorrhizos is a staple food in many societies due to its high content of nutritional fibres, carbohydrates, vitamins, and minerals- particularly calcium, iron, and potassium. A. macrorrhizos has long been used in traditional medicine to treat several diseases such as chronic constipation, mouth ulcer, rheumatic arthritis, haemorrhoids, and cough and combat malaria and jaundice. On the other hand, it is an essential staple food that must be prepared properly to reduce the presence of toxic substances, like calcium oxalate and other antinutrient substances such as phytates, tannins, polyphenols, amylase inhibitors, and hydrogen cyanide, via several conventional treatments, such as fermentation, frying, soaking, and ethanol extraction, and non-conventional treatments such as microwave and ultrasound. This plant is popular all over the world for its large and eye-catching leaves. Despite its medicinal value, there is still a lack of knowledge regarding its nutritional, health, and commercial benefits. The review emphasizes the requirement for more research into its commercial and culinary applications, along with its nutritional and health benefits.
Sustainability spotlightAlocasia macrorrhizos holds substantial potential as a sustainable food and medicinal resource due to its adaptability to diverse agro-climatic conditions and high biomass yield. Despite challenges related to acridity and anti-nutritional factors, suitable processing can unlock its nutritional and therapeutic benefits. Promoting its utilization not only diversifies food sources but also supports food security, livelihood opportunities, and circular use of underutilized plant resources. |
1. Introduction
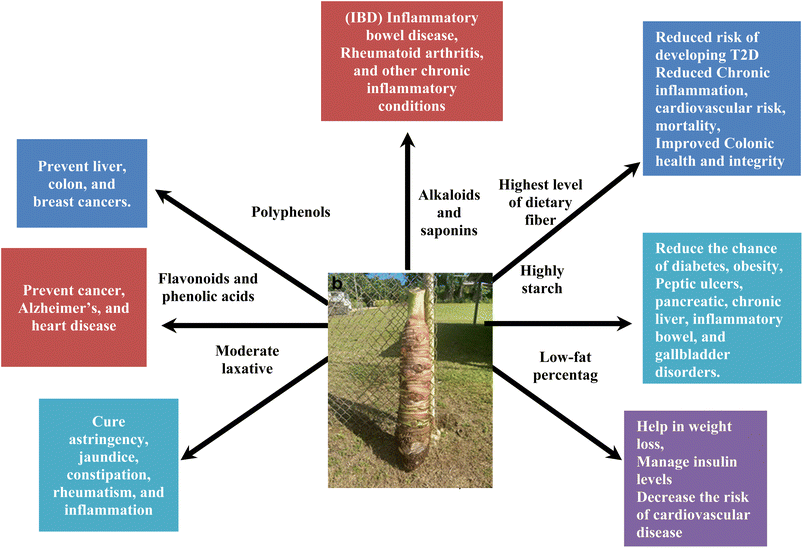
Alocasia macrorrhizos, also called the giant taro or elephant ear plant, is a flowering plant indigenous to tropical regions of the Asia and Pacific, and it serves as a staple food in various communities. A. macrorrhizos (L.) G.Don. Var. Macrorrhiza (Malaysia to Pacific areas) and A. macrorrhizos (L.) G.Don. Var. Violacea (India to Malaysia) are the two varieties that are categorized according to their origin. A. macrorrhizos (L.) G.Don belongs to the Aroid family that consists of 107 genera along with 3700 species that are distributed worldwide, characterized by its extreme heterogeneity.1 There are numerous popular names for A. macrorrhizos (Alocasia macrorrhizos (L.) G.Don) used locally throughout its cultivated area, including man kachu, giant Alocasia, metallic taro, and giant elephant ear taro.1 This genotype is divided into two categories: cultivated and wild, which are non-edible plants. The two different species named Alocasia indica (Lour.) Spach and Alocasia macrorrhizos (L.) G.Don are frequently confused or used interchangeably. In Bangladesh, A. macrorrhizos is referred to as ‘man kachu’, while Alocasia indica is known as ‘mugur kachu’. A. macrorrhizos, also called giant taro, is a dark green plant with an ovato-sagittate shape and a leathery surface that is glossy or coriaceous, with distinct primary veins.2 The above-ground stem of the giant taro, in cultivation, can reach lengths of up to 1 m and diameters up to 20 cm, and in the Philippines and the Asian continent, it can reach up to a height of 15 feet and a width of 10 feet.3In folk medicine, A. macrorrhizos, a plant with secondary metabolites, is frequently used to treat various ailments. Traditionally, it has been applied to cure constipation, snake bites, jaundice, diabetes, and ear infections.1,4 It is an analgesic that reduces pain in joints, stomach, and head in India, Bangladesh, and parts of Indonesia, and for cough and toothache in Malaysia.4 Moreover, A. macrorrhizos is used to treat abscesses, eczema, and inflammation in Vietnam. It has long been used in the Philippines to heal animal wounds, snake bites, and toothaches. The rootstock is a mild laxative used to relieve astringency, jaundice, constipation, rheumatism, and inflammation.
Additionally, it is a cooling, diuretic, and astringent agent (Fig. 1). It has also been demonstrated to have a starch-rich stem, which is an essential source of carbohydrates and makes the crop significant, particularly in areas where food scarcity is a concern.1 The plant stems are mainly consumed as staple food, while the leaves are consumed as a substitute food during famine in Asia due to their valuable sources of protein, fiber, and minerals.5 The A. macrorrhizos plant has been retained as an ornamental plant worldwide due to its large, eye-catching leaves, which enhance the garden's appearance. Because it exhibits a unique blend of unity and beauty, Alocasia macrorrhizos is a valuable plant in horticulture and agriculture. In Bangladesh and India, the tuber's stem is peeled, cut, chopped, cooked, baked, or roasted, and then consumed like a vegetable. Besides this, A. macrorrhizos has been cultivated for over 3000 years and used as an animal feed. The leaves can be sautéed with garlic and onion. However, it has been noted that before consumption, the tubers are fully cooked to dissolve the irritating calcium oxalate crystals. The ability of oxalate to bind to dietary calcium and form calcium oxalate crystals makes it an antinutritional substance.6 Due to the presence of exceptionally huge amounts of calcium oxalate crystals in wild plant stems, they are not consumed as food.1 Therefore, it has been identified that A. macrorrhizos is a versatile plant that may be used for a variety of purposes including producing bioethanol, as animal feed, and as a subsistence crop. Thus, it has been noted that the primary reason for the underutilization of the food crop is the lack of awareness of the nutritional and commercial value of taro.
The existing literature on A. macrorrhizos primarily focuses on its traditional uses, botanical characteristics, and some preliminary phytochemical studies.1 However, one major concern is the lack of comprehensive studies on the nutritional composition and bioactive compounds of giant taro, particularly compared to other taro species. The toxicological profile of A. macrorrhizos remains largely unexplored. A comprehensive safety assessment and identification of effective treatments to reduce the antinutritional factor are crucial for their potential use in the food industry and culinary applications.
2. Nutritional component of A. macrorrhizos
The nutritional value of Alocasia microrrhizos, compared to daily nutrient requirements, and the biological significance of each component are presented in Table 1. The Indian Council of Medical Research is responsible for regularly updating the recommended dietary allowances (RDAs) and nutrient requirements. This provides information on nutrients and helps develop nutrient requirement policies that lead to a healthy population. Taro is an essential source of carbohydrates and starch that can be obtained from the underground parts of plants such as stems and corms.7 Furthermore, the leaves and flowers of the plant can serve as a nutritious vegetable. Thus, it can be determined that nutritional components may vary based on the age and development of the plant.| Name of nutrient | Quantity % | Reference daily intake | Biological significance | Health benefits | Reference |
|---|---|---|---|---|---|
| Water (%) | 16.25–17.32 | 32–56 mL per kg body mass | Essential for metabolism, temperature regulation, nutrient, oxygen transport, and cellular homeostasis | Water helps to regulate body temperature, flush out waste, and lubricate joints | 19–21 |
| 82.79–85.45 | 10 | ||||
| Calories (kJ 100 g DM) | 1636.96 | 2200–2400 kcal | Provide energy for different tasks | Calorie intake helps to active lifestyle | 10 |
| Crude protein (%) | 2.61 | 46–56 gm | Provide structural elements for cells and tissues, regulate the concentrations of acids and bases in blood, many hormones are proteins | Vital roles in growth, metabolism, immune function, and development, highlighting their indispensable contribution to overall health and well-being | 14 and 19 |
| 4.35–4.57 | 10 | ||||
| Crude fat (%) | 2.98 | 20–35% of total calories | Concentrated source of energy, helps with brain function, and supports cell growth | Low fat help in weight loss, manage insulin levels, decrease the risk of cardiovascular disease | 19 |
| 3.28–3.34 | 10 | ||||
| Carbohydrate content (%) | 74.85 | 130 gm | It is the primary source of energy, helps to regulate nerve tissue, provides mechanical support to cell walls | High starch content reduces the chance of peptic ulcers, pancreatic, chronic liver, inflammatory bowel, and gallbladder disorders | 19 and 22 |
| Fibre content (%) | 1.49 | 25–30 gm | Improved insulin sensitivity, improved glycaemic status and lipid profiles, slow digestion and absorption | Reduced risk of developing T2D, reduced chronic inflammation, cardiovascular risk, mortality, improved colonic health and integrity | 13 and 19 |
| 3.75–3.81 | 10 | ||||
| Ascorbic acid content mg/100 gm | 0.64 | 65–90 mg | Antioxidant helps in tissue growth repair, wound healing | Help in the growth and repair of the tissue, promoting wound healing, and serving as a potential antioxidant | 16 and 19 |
| 10.97–11.99 | 10 | ||||
| Total phenol content (g/100 g) | 0.61–0.83 | It acts as an antioxidant, anti-inflammatory, cancer prevention, and antimicrobial agent | Prevent liver, colon, and breast cancers, rheumatoid arthritis, inflammatory bowel disease, and other chronic inflammatory conditions | 10 and 23 | |
| Antioxidant activity (IC50) | 141.74–143.68 µg mL−1 | 500 gm | Protects cells from oxidative damage | 23 | |
| Flavonoid content | 4.11–4.57 µg QE/mg | It acts as an antioxidant, anti-inflammatory, cancer prevention, and antimicrobial agent | Prevent cancer, Alzheimer's, and heart disease | 23 | |
| Calcium (mg/100 g) | 642.9–708.9 | 1200 mg | Supports bone health, blood clotting, metabolic regulation, muscle contraction, and nerve signalling | It helps form and maintain healthy bones and teeth, maintains muscle function and a normal heartbeat, and is involved in blood clotting | 16 and 19 |
| 576.14–580.66 | 10 | ||||
| Iron (mg/100 g) | 56.3–56.5 | 8–18 mg | It is a key component of haemoglobin, prevents anaemia, is involved in energy metabolism, is involved in the synthesis of collagen, also found in myoglobin | Crucial for oxygen transport, brain health, immune support, healthy pregnancy outcome, energy production, and muscle function | 10 and 16 |
| Magnesium (mg/100 g) | 182.66–185.34 | 310–420 mg | Essential for processing ATP and for bone health, supports energy production, DNA synthesis, nerve and muscle function, enzyme activity, electrolyte balance | Support heart health, inflammatory reduction, nerve signalling, muscle performance, sleep quality, metabolic function, cardiovascular health and stress management | 16 |
| Potassium (mg/100 g) | 1719.9–1851.7 | 4700 mg | It helps nerves function and muscles contract, essential in co-regulating ATP with sodium, contributes to the overall electrolyte balance | Potassium helps manage hypertension | 16 and 19 |
| 1831.42–1833.1 | 10 | ||||
| Sodium (mg/100 g) | 183.41–185.83 | 2000 mg | It helps the body maintain fluid balance, and regulate blood pressure, essential![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) in co-regulating ATP with potassium and regulating pH and hormones in co-regulating ATP with potassium and regulating pH and hormones |
Vital for fluid regulation, brain health, heart function, prevention of cramps, and nerve transmission | 10, 16 and 24 |
| Zinc (mg/100 g) | 2.19–2.33 | 8–11 mg | It is required for cell division and growth, necessary for the proper functioning of the body's immune system, pervasive, and required for many enzymes | Support in immune function, enhancing cognitive function, maintaining taste and smell, and providing antioxidant protection | 10 and 16 |
2.1 Carbohydrates
Starch is the major component in taro (73–80%).8 Moreover, it has been recognized that variety, cultivation conditions, soil type, use of fertilizers and moisture treatment, harvest maturity, and post-harvest handling and storage all influence its composition.9 Specifically, 74.85% carbohydrate is found in giant taro and 52.35–52.37 mg/100 g starch is present in that plant.10 Taro starch is easily digestible, making it an ideal ingredient for infant foods. Because of its gluten-free nature and small granule size, this research is widely used in infant formula, for kids with milk sensitivity and cereal allergies. Its granules have a characteristic amylose/amylopectin ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and range in size from 1.5 to 6.6 micrometres.7 Taro is a unique food because its small granules render it hypoallergenic and easily digestible.6A. macrorrhizos has high carbohydrate content, recognized for its starchy quality.10 According to the RDA, the daily requirement for carbohydrates is 130 grams per person. High carbohydrate content contributes to its superior caloric value, providing energy and supporting various bodily functions.12 Moreover, carbohydrates are the primary source of the body, fuelling brain function along with physical activity. However, higher consumption of added fructose and glucose is associated with decreased global cognition.13 Thus, it highlights the vital role of carbohydrates in maintaining the overall function and health of the body. The tubers, corms, and rhizomes of Alocasia macrorrhizos contains 52.36 grams of starch per 100 grams, making them a significant source of dietary carbohydrate.11
7 and range in size from 1.5 to 6.6 micrometres.7 Taro is a unique food because its small granules render it hypoallergenic and easily digestible.6A. macrorrhizos has high carbohydrate content, recognized for its starchy quality.10 According to the RDA, the daily requirement for carbohydrates is 130 grams per person. High carbohydrate content contributes to its superior caloric value, providing energy and supporting various bodily functions.12 Moreover, carbohydrates are the primary source of the body, fuelling brain function along with physical activity. However, higher consumption of added fructose and glucose is associated with decreased global cognition.13 Thus, it highlights the vital role of carbohydrates in maintaining the overall function and health of the body. The tubers, corms, and rhizomes of Alocasia macrorrhizos contains 52.36 grams of starch per 100 grams, making them a significant source of dietary carbohydrate.11
2.2 Fibre
In addition to starch, A. macrorrhizos is a notable source of dietary fibre, especially 1.3% glucomannan, a soluble fibre found in giant taro, which shows numerous health benefits and potential applications.11 Glucomannan lowers blood pressure, triglyceride, cholesterol, and glucose levels, improves health, and aids in weight loss.11 Moreover, it exhibits anticancer, anti-inflammatory, and prebiotic properties. Approximately 13.5% and 3.21% fibres are present in raw and cooked taro corms, respectively.7 In addition, the chemical makeup of taro's non-starch polysaccharides represents immunomodulatory properties. A. macrorrhizos provides approximately 3.75 to 3.81 mg of dietary fibre per 100 grams of edible parts, contributing to its nutritional value as a source of fibre.10 Studies have shown that frequent higher fibre consumption or high-fibre diets are associated with improved cognitive function.12 The high-fibre diets are linked to ideal cerebral glucose metabolism, and ultimately, improved long-term results. Table 1 indicates that crude fibre has high nutritional benefits such as supporting healthy blood glucose management, promoting the absorption and transportation of essential micronutrients, help in reducing cholesterols (LDL), reducing insuline and blood glucose level, improving water-holding capacity in food and shortening the intestinal transit time.13 This highlights the potential of A. macrorrhizos to mitigate health conditions such as diabetes and cardiovascular diseases.2.3 Proteins
On a dry-weight basis, taro contains roughly 11% protein. The protein fraction is high in essential amino acids such as valine, leucine, phenylalanine, arginine, and threonine. Among the essential amino acids, the leaf possesses higher concentrations of lycine, leucine, methionine, cysteine, and phenylalanine than the corm. The protein concentration in corms is higher across the periphery than in the middle.6A. macrorrhizos constitutes a very low amount of protein, as shown in Table 1. Protein is a complex nitrogen-containing organic compound. The daily protein requirement according to the ICMR (Indian Council of Medical Research) is presented in Table 1. The essential amino acids such as trionine, leucine, arginine, valine, and phenylalanine are abundant in this type of taro. In addition, it has been mentioned that the protein content of the corm is higher than that of the leaf, which comprises about 23% protein. Protein acts as a crucial structural component for tissues and cells. It plays a vital role in building and repairing body tissues such as muscle, skin, bones, and organs.14 Besides that, it functions as an enzyme, antibody, hormone, and protein contributing to numerous physiological processes essential for life. However, protein plays vital roles in growth, metabolism, immune function, and development, highlighting their indispensable contribution to overall health and well-being. Two noteworthy protein types found in taro are trypsin inhibitors and mannose-binding lectin (MBL). Nevertheless, trypsin inhibitors help to produce hypoallergenic proteins, and MBL deficiencies have been associated with autoimmune disorders. As a result, taro acts as a substitute for people with food allergies.2.4 Fat
Similar to the other tuber crops, A. macrorrhizos has a very low fat content; the range is presented in Table 1. This fat content is low compared to the daily fat requirement. Therefore, the low-fat percentage, combined with carbohydrates and dietary fibre, makes the plant or vegetable a suitable choice for people looking to maintain a healthy diet with low-calorie intake from fat. Biologically, fats are the concentrated energy source; it has been identified in several studies that fat provides more than double the energy per gram compared to carbohydrates and protein. They play a crucial role in brain function, the improvement of cognitive processes, and enhance overall mental health. In addition, fat supports cell growth, maintains cell membrane integrity, and helps absorb fat-soluble vitamins, including vitamins A, D, E, and K, which are crucial for the body's function.152.5 Moisture content
The moisture content is an essential factor as it impacts the texture, taste, and spoilage susceptibility. The moisture content can soften the texture but simultaneously enhance spoilage risk due to enzymatic activity and microbial growth in that product. A. macrorrhizos have a medium moisture content, which is presented in Table 1, leading to bulkiness and vulnerability to physical damage, making storage and transport challenging.10 Consequently, it has been identified that shelf life and preservation can be improved by using refrigerated storage. For example, research indicates that taro corms can be successfully preserved for approximately 5 to 6 weeks at 15 °C and 85% relative humidity or for at least 8 weeks at 7–12 °C in a well-ventilated chamber without experiencing any quality deterioration. Similarly, taro root can be kept for approximately 8 weeks at 0 °C to 2 °C and 2 to 4 weeks at 20 °C.7 Other research shows that taro's moisture content varies with species, growing environment, and harvest season, with a range of 60–83%.6 The dry matter content varies from 27.50% to 17.17% at harvest.2.6 Vitamins
Transition to vitamins, taro corms are comparatively poor sources of ascorbic acid and carotene; however, they have twice as much carotene quality as potatoes and are on par with cabbage.7 Vitamins are biologically active substances that are essential for the regular physiological function of our body. Vitamin C, for example, is crucial for wound healing, tissue development, and repair.16 Furthermore, this vitamin acts as an antioxidant in our body that protects our cells from free radicals. A human has to consume 65–90 mg of vitamin C daily. Biologically, ascorbic acid is essential for the growth and repair of tissue, promoting wound healing and serving as a potential antioxidant. It helps protect cells from damage that free radicals can cause, supports immune function, and helps synthesise collagen, which is essential for maintaining skin health, along with cartilage and bone health.16 However, taro leaves have folic acid, iron, and beta-carotene, all of which aid in preventing anaemia. Higher carotenoid-containing foods are effective in treating chronic diseases, including diabetes, heart disease, and cancer.2.7 Minerals
The mineral profile of A. macrorrhizos further enhances its dietary value. The amount of minerals such as iron, calcium, phosphorus, zinc, sodium, and magnesium is demonstrated in Table 1. Taro is mineral-rich, with concentrations ranging from 3.54 to 7.78%.6,17 A high potassium-to-sodium ratio makes it particularly beneficial for individuals with high blood pressure, as diets high in potassium help to manage hypertension. Furthermore, it has been mentioned that one of the most common nutritional issues in the world, zinc deficiency, is associated with stunning and cognitive impairment. In that context, it has been identified that Alocasia is an excellent non-animal source of zinc, and the consumption of taro influences the mitigation of zinc deficiency.It has been identified that minerals are crucial for various physiological activities in the body. For example, calcium is crucial for forming and maintaining healthy bones and teeth, ensuring proper muscle function, supporting a normal heartbeat, and aiding in blood clotting. Iron, a key component of haemoglobin, carries oxygen throughout the body, supports energy metabolism and collagen synthesis, and is present in myoglobin for muscle oxygen storage (Godswill et al., 2020). Magnesium is essential for processing ATP as well as for bones. Potassium is essential for nerve function and muscle contraction, as well as for co-regulating ATP with sodium and ensuring overall electrolyte balance.16 In addition, sodium helps the body maintain fluid balance, regulates blood pressure, and is vital for the proper functioning of muscles and nerves. Zinc plays a vital role in many physiological processes, including cell division and growth, as well as in the immune system. The interaction of mineral richness in giant taro, moisture control, and vitamin retention demonstrates its several nutritional advantages. Significant storage practices not only help to extend the shelf life but also retain bioactive components, making it a resilient crop to meet nutritional requirements across several environmental and health contexts.
2.8 Phenolic compounds
Phenolic compounds are major class of plant secondary metabolites and they play a crucial role in defense mechanisms by protecting against oxidative stress, UV-radiation, and microorganisms.4 This compound mainly contributes astringency, bitterness, and pigmentation in most plants. A. macrorrhizos has phenolic compounds such as phenolic acids and flavonoids that have a high contribution to therapeutic properties. Phenolic compounds serve as neuroprotective, antiepileptic, and anxiety, antidepressant, and prevent cancer by acting as antioxidants that help to mitigate oxidative stress as well as inflammation, which shields neurons from damage and degeneration. Their protective effects are mediated through different mechanisms, such as scavenging free radicals, improving the activity of antioxidant enzymes, influencing key signalling pathways including AKT and ERK, and suppressing enzymes like cholinesterase that are essential in the context of neurodegenerative disorders like Alzheimer's.23 However, the biological impact of this substance is influenced by the consumption level, bioavailability, digestion, and absorption. Once in the colon, phenolic compounds are transformed with the help of gut microbiota; these substances can combat lifestyle as well as neurodegenerative diseases.18Flavonoid is the major compound of the phenolic compounds. Among all subgroups of flavonoids, Flavan-3-ols are prevalent in different foods such as fruits, cocoa, and tea. This compound is constituted by monomers including catechins and epicatechins. The monomeric forms possess antioxidant properties by the help of their phenolic hydroxyl groups that neutralize reactive oxygen species (ROS) as well as chelate iron ions to reduce lipid peroxidation.18 This study has shown that phenolic compounds exhibit antibacterial properties against harmful bacteria such as Clostridium perfingens, Escherichia coli, Salmonella, C. difficile, and Pseudomonas. Simultaneously, they influence the growth and function of the beneficial probiotics, including Bifidobacterium along with Lactobacillus species, which positively impact the gut microbiota to maintain balance.
Corms generally have low protein and fat contents, but are rich in carbohydrates, fibres, and minerals.25 Moreover, taro is a rich source of essential nutrients such as calcium, iron, phosphorus, thiamine, riboflavin, and niacin.6Fig. 1 illustrates a comprehensive overview of the health benefits, highlighting both the advantages of macronutrients for our health and the additional benefits provided by bioactive compounds. Furthermore, A. macrorrhizos is rich in essential nutrients, including iron, magnesium, starch, zinc, carbohydrates, and vitamin C, necessary for good health. By including A. macrorrhizos in the diet, it can leverage its potential to enhance the overall well-being through its diverse and potent health-promoting properties. A. macrorrhizos is a prospective food for further study and development because of its nutritional value and versatility, which makes it a viable substitute for people with allergies. In addition, it has been mentioned that due to the gluten-free nature of the plant, the consumption of A. macrorrhizos is beneficial for people with celiac disease or other allergic reactions.7 Therefore, the nutritional properties of this plant can help to prevent several diseases.
3. Comparison with other tuber corms and rhizomes
Alocasia macrorrhizos, also known as giant taro, is a highly nutritious food source compared to other corms, tubers, and rhizomes, including Colocasia esculenta, Asparagus racemosus, and Maranta arundinacea. A. macrorrhizos is a good source of energy since it has a higher carbohydrate content than that of other corms.19 This particular corm also exhibits moderate levels of fat, moisture, and fibre, contributing to overall nutrition and digestive health. Regarding mineral content, A. macrorrhizos has the highest iron level among corms. The moisture content of the A. macrorrhizos is higher than that of other corms and tubers (Table 2). Its crude protein content is comparable to Colocasia but lower than Asparagus. Table 2 shows that the crude fat of this corm is slightly lower than that of Asparagus racemosus but higher than that of Colocasia and Maranta arundinacea. It has been noted that Alocasia macrorrhizos is rich in vitamin C, calcium, and potassium, making it beneficial for skin, health, and antioxidants.| Scientific name | Common name | Type | Moisture (mg/100 g) | Crude protein (mg/100 g) | Crude fat (mg/100 g) | Dietary fibre (mg/100 g) | Starch (mg/100 g) g/100 gm−1 | Vitamin C (mg/100 g) | Calcium (mg/100 g) | Potassium (mg/100 g) | Iron (mg/100 g) | Total oxalate g/100 gm−1 | Tannin g/100 gm−1 | Total phenolics (g/100 gm) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colocasia esculenta | Taro or A. macrorrhizos | Corm | 44.19–44.53 | 4.82–5.14 | 4.3–4.36 | 3.93–3.99 | 23.29–23.63 | 9.27–9.45 | 557.64–559.08 | 147.97–148.31 | 72.28–72.52 | 0.22–0.26 | 0.12–0.16 | 0.29–0.35 | 10 |
| Asparagus racemosus | Shatavari | Tubers | 80.8–82.44 | 7.16–7.32 | 7.25–7.47 | 6.36–6.48 | 29.1–29.82 | 31.2–31.6 | 135.61–137.23 | 89.55–89.97 | 30.9–31.5 | 0.09–0.11 | 0.19–0.23 | 0.33–0.43 | 10 |
| Maranta arundinacea | Arrowroot | Rhizome | 77.64–79.72 | 12.54–12.74 | 2.71–2.73 | 3.77–3.79 | 62.22–62.5 | 7.31–7.43 | 384.11–384.53 | 96.04–96.56 | 22.01–22.19 | 0.29–0.37 | 0.51–0.53 | 0.14–0.18 | 10 |
| Ipomeabatatas | Sweet potato | Tuber | 61.80–62.30 | 8.90 | 2.00 | 7.00 | 35.710 | 17.29 | 300.02 | 0.25 | 30 | ||||
| Solanum tuberosum | Potato | Tuber | 70.18–72.02 | 2.88–2.9 | 0.15–0.19 | 8.11–8.29 | 15.54–16.86 | 0.04–0.64 | 6.04–7.02 | 74.64–78.56 | 0.57–0.61 | 31 | |||
| Alocasia macrorrhizos | Giant taro | Corm | 82.79–85.45 | 4.35–4.57 | 3.28–3.34 | 3.75–3.81 | 52.35–52.37 | 10.97–11.99 | 576.14–580.66 | 128.7–130.22 | 56.3–56.52 | 0.64–0.72 | 0.34–0.5 | 0.61–0.83 | 10 |
Furthermore, its oxalate and tannin contents are higher than those of other vegetables, which can impact nutrient absorption. Therefore, compared to other corms and rhizomes, A. macrorrhizos stands out for having high moisture and starch contents and a balanced profile of protein, dietary fibre, and fats. This makes it a valuable food source in various diets, especially in Asia, where it is grown for both culinary and medicinal purposes.
4. Antinutritional factors
Antinutrients, which are naturally occurring substances found in plant-based foods, can hinder nutrient absorption in the human body.26 Moreover, a plant-based diet provides health benefits such as antioxidant properties, but may also have bioavailability and nutrient availability challenges. Thus, these substances cause nutrient deficiency and other health issues if used in excess amounts over an extended period. Taro, for example, contains oxalates, protease inhibitors, tannins, phytates, and polyphenols that can affect the nutritional value of the taro. A previous report of different studies shows that the corms of A. macrorrhizos have more trypsin inhibitors and amylose inhibitor activity than other edible tubers, corms, and rhizomes. Moreover, various amounts of antinutritional factors in A. macrorrhizos, their toxicity, chemical nature, eduction methods and analytical techniques to quantify antinutritional factors are illustrated in Table 3. The chemical nature, toxicity, cause, and reduction methods are also presented in this Figure.| Antinutritional factor | Amount present in giant taro | Chemical nature | Toxicity | Cause | Reduction method | Analysis method | Reference |
|---|---|---|---|---|---|---|---|
| Total oxalate (g/100 g) | 0.64 to 0.72 | Insoluble oxalate salts (CaOx), soluble oxalate strong oxidation in nature mineral chelating properties present | Under 50 mg per day | Hypocalcaemia gastric haemorrhaging renal or kidney stone | Soaking sun drying cooking, boiling, fermentation | Permanganates titration/using UV–vis spectrophotometer/using HPLC | 10, 19 and 27 |
| Tannin g/100 g | 0.34–0.5 | Astringent and bitter plant polyphenol compound, heat stable bind or precipitate proteins, amino acids, and alkaloids, hinders dietary iron absorption | Intestinal damage hinders iron absorption, inhibits digestive enzymes, enhances excretion of endogenous protein, causes toxicity of absorbed tannin, depression of food intake, and carcinogenic effects | Reduce the bioavailability of different minerals, particularly iron and proteins | Boiling, fermentation sun drying | Folin-ciocalteu method/Vanillin HCL assay | 27, 28, 32 and 33 |
| Trypsin inhibitors (TIU) | 10.36 | Heat-labile, protein-based substances dimeric proteins | Impaired protein digestion | Inhibiting the effect of trypsin and chymotrypsin enzymes hinders protein absorption | Boiled for 20 minutes, autoclave treatment | Enzymatic inhibition assay | 26 |
| Hydrogen cyanide (mg/100 g) | 0.2–0.26 | Present in the form of cyanogenic glucosides cyanogenesis stabilized by glycosylation (combined with sugar) | Between 0.5 and 3.5 mg for an adult lethal dose | Cytochrome oxidase inhibition is the main reason reduce the utilization of oxygen in tissues immediate effect on the heart and respiratory system death cerebral damage and lethargy in humans as well as animals | Drying, soaking, and fermentation | Colorimetric method | 19 and 27 |
| Amylase inhibitor (AIU) | 9.84 | Starch blocker hypoglycaemic effect heat labile active over a pH range of 4.5 to 9.5 | Low toxicity | Reduce glucose absorption and reduce starch digestion | Microwave, flour processing, boiling, baking | Dinitro salicylic acid assay | 27 |
4.1 Oxalate
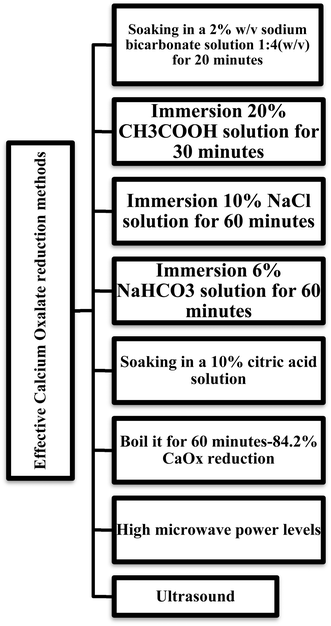
Taro leaves and corms contain oxalates, which can crystallize into insoluble matter that may cause kidney stones and impair calcium absorption.27 Calcium is bound by oxalic acid and forms the insoluble calcium oxalate. For instance, calcium oxalate crystals, which can make up to 2.05 to 4.21% of the dry matter of taro, typically as raphides, make it toxic if consumed uncooked. As oxalate can bind to dietary calcium and form calcium oxalate crystals, they are classified as an antinutritional component. Due to this mechanism, the body cannot absorb and utilize calcium, leading to diseases such as rickets and osteomalacia.28 The quantity of soluble oxalates in the cooked tissue was decreased after boiling the taro corms for around forty minutes. A. macrorrhizos contains 0.64 to 0.72 g/100 g total oxalate.10 In contrast, the corm of A. macrorrhizos contains a higher level of total oxalate, ranging from 8.5% to 9%.19 However, these antinutritional factors can be decreased by using suitable cooking techniques, including fermentation, boiling, and soaking.29 According to reports, among all techniques, boiling has the highest impact on oxalate reduction (Fig. 2). Calcium oxalate dissolves readily in acids but is insoluble at neutral or alkaline pH values.4.2 Tannin
Tannin is a bitter, astringent plant polyphenol compound that may bind or precipitate proteins and several other organic compounds such as amino acids and alkaloids. The molecular weight of this compound ranges from 500 to 3000 daltons.27 It is heat-stable and reduces the digestibility of proteins in both animals and humans, most probably by blocking digestive enzymes, making protein unavailable, or enhancing faecal nitrogen. Tannins are known to hinder the activities of amylase, trypsin, chymotrypsin, and lipase enzymes, reduce the protein quality of food, and interfere with iron absorption from the diet. It affects nutritional health through damaging the intestinal barrier, mitigating iron absorption, and possesses carcinogenic effects.29 This study has recognized that tannin can reduce the bioavailability of different minerals, particularly iron and proteins. Boiling lowered the tannin concentration by 6.69%, while fermentation reduced it by 43.52%. Enzyme activation could be the reason for the most significant decrease in tannin caused by the fermentation of taro flour.28,29 Thus, it has been identified that foods with high tannin contents have low nutritional values as they precipitate proteins, impair iron absorption, and reduce the utilization of vitamins and minerals from meals.4.3 Amylase inhibitors
Amylose inhibitors are also called starch blockers due to the chemicals that prevent the absorption of dietary starches. The digestive enzyme amylase and other secondary enzymes break down starch, a complex carbohydrate, before it can be absorbed. Thus, the study shows that the α-amylase inhibitors, in combination with α-amylase, make it unavailable in the process of starch digestion. It has been discovered that this inhibitor is heat labile, and it is found to be active in the pH range of 4.5 to 9.5.27,29 It has hypoglycaemic effects, but when used as a starch blocker tablet, it decreases insulin responses and enhances the caloric output of foods.4.4 Trypsin inhibitors
Trypsin inhibitors are found in raw soybeans that inhibit protease enzyme activity in the digestive tract, reduce protein digestion, and result in diarrhoea and excessive gas formation. Additionally, it has been mentioned that 10 to 20% of active trypsin is present in human pancreatic juice, which can bind to protease, a digestion-resistant substance in the small intestine, removed through excretion. The formation of irreversible enzyme-trypsin inhibitor complex diminishes intestinal trypsin activity, resulting in reduced protein digestibility and consequent growth reterdation. This substance can easily be destroyed by heat; thus, autoclave treatment or a boiling process can decrease the quantity of this substance.27 It has been identified in that paper that when the tubers are sufficiently boiled, all of the protease inhibitors are rendered inactive, making it soft.Additionally, trypsin inhibitors can obstruct the digestion and absorption of protein. In corms, the inhibitors constitute 1–4% of the total crude protein, whereas in leaves, they are either missing or inactive.6 Initially, trypsin inhibitor activity rises when corms are cooked, but then it falls. Therefore, it has been mentioned that several types of taro may be boiled for 20 minutes, which is enough to minimize trypsin activity and acridity.
4.5 Hydrogen cyanide
Plant species produced hydrogen cyanide (HCN) from cyanogenic glycosides, which are glycosides of sugar, often glucose, attached to cyanide-containing aglycone, classified as phytoanticipins. Cyanogenesis, the formation of free hydrogen cyanide, is the key characteristic of the particular toxins that can combine with cyanohydrins that are stabilized by glycosylation to produce cyanogenic glycosides. HCN inhibits cytochrome oxidation in the mitochondria of the cell by binding to Fe3+/Fe2+ present in the enzyme. Thus, it results in less utilization of oxygen in the tissues. Cyanide causes a drop in the ATP/ADP ratio, signifying a change from aerobic to anaerobic metabolism and enhancing blood glucose and lactic acid levels, and consuming high levels of this ANF can lead to death.27,295. Acridity of taro
Due to the presence of acrid substances in taro-based foods, some consumers experience tissue inflammation and itching. The fresh A. macrorrhizos corm has 471.15 mg/100 g of calcium oxalate, which has been noted as more than what has been documented in the literature for raw taro Colocasia corm.34 The calcium oxalate concentration from the skin reduces towards the centre of the A. macrorrhizos corm. Since food can only contain 71 mg of calcium oxalate per 100 g, the corm of A. macrorrhizos must be processed to lower the concentration. For six weeks, the safe limit for an adult to consume calcium oxalate is between 0.60 and 1.25 g per day.35
A. macrorrhizos produces oxalic acid by different metabolic pathways, such as ascorbate, glyoxylate, or hydrolysis of oxaloacetate. The oxalic acid subsequently combines with bioavailable calcium to develop insoluble calcium oxalate crystals. This particular biomineralization can be controlled by genetic factors, crystal forms such as raphide and druses, which vary by type of species and tissues within specialized iodoblast cells. When plant tissue is injured, raphide bundles that are stored in vacuoles might be damaged and amplify the acridity.36 Potassium oxalate crystals are also found in A. macrorrhizos, which might irritate the tongue and throat, partly due to protease and its inhibitor.6 Recent studies show that genes involved in oxalate biosynthesis, along with actin and profilin proteins, play a crucial role in the formation and assembly of raphide crystal bundles, while oxalate oxidase is responsible for the breakdown of oxalate.37 Acrid sensation occurs mainly due to mechanical damage caused by raphide crystals that puncture tissue and release cell contents, in that way exposing the skin or mucous membranes to sharp and irritating particles.38 In taro, abundant acridity components can make it challenging to swallow, and they can cause severe irritation and a burning sensation in the mouth and throat when people consume it.6 In a recent case report, it has been identified that a 63-year-old woman was poisoned by the intentional ingestion of 200–300 mL of the liquid extract of Alocasia amzonica roots due to the oxalate content of the plant.39 The poisoning led to several oral and gastrointestinal injuries, along with systemic complications such as encephalopathy, pulmonary oedema, liver dysfunction, and metabolic acidosis. However, it has been noted that several techniques used to lessen the acrid substance include peeling, processing, grinding, soaking, and fermentation. It needs to be cooked for a long time, and a significant portion of the taro skin should be removed to reduce the bitter taste. The processes of fermentation, baking, and ethanol extraction can also be used to eliminate acridity. From a technical as well as economic standpoint, the percentage of calcium oxalate in A. macrorrhizos can decrease to below the permissible threshold by soaking the corm in a 2% w/v sodium bicarbonate solution at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (w/v) for 20 minutes at room temperature.1 It was identified that there are different concentrations of acrid factor present in all parts of the plant, and it seems to be transported on the surface of the needle-like calcium oxalate raphide crystals. It has been believed that either a glycoside or cysteine proteinase is responsible for significant irritation or swelling of the infected tissues. Moreover, the arid component of taro-based foods can be caused by different materials, such as cooked petioles, leaves, or environmental stress, which can cause itching and extreme skin irritation. For this substance, the mouth and throat experience intense itching, stinging, and burning, followed by swelling or mild skin irritation or itching. In that case, using sodium bicarbonate and washing with an acidic solution are two procedures that help to reduce acridity. Different studies have mentioned that the aridity of different taros can be reduced by various common processes such as fermentation, frying, peeling, grading, heating, soaking, and ethanol extraction. The remaining calcium oxalate is found deep within the pore structure or in intact cells, where it is less accessible. While the leaching of less accessible calcium oxalate from intact cells is significantly slower and has a high mass transfer barrier, leaching of readily accessible calcium oxalate occurs extremely quickly, with a rate regulated by its diffusion in the sodium bicarbonate solution.
4 (w/v) for 20 minutes at room temperature.1 It was identified that there are different concentrations of acrid factor present in all parts of the plant, and it seems to be transported on the surface of the needle-like calcium oxalate raphide crystals. It has been believed that either a glycoside or cysteine proteinase is responsible for significant irritation or swelling of the infected tissues. Moreover, the arid component of taro-based foods can be caused by different materials, such as cooked petioles, leaves, or environmental stress, which can cause itching and extreme skin irritation. For this substance, the mouth and throat experience intense itching, stinging, and burning, followed by swelling or mild skin irritation or itching. In that case, using sodium bicarbonate and washing with an acidic solution are two procedures that help to reduce acridity. Different studies have mentioned that the aridity of different taros can be reduced by various common processes such as fermentation, frying, peeling, grading, heating, soaking, and ethanol extraction. The remaining calcium oxalate is found deep within the pore structure or in intact cells, where it is less accessible. While the leaching of less accessible calcium oxalate from intact cells is significantly slower and has a high mass transfer barrier, leaching of readily accessible calcium oxalate occurs extremely quickly, with a rate regulated by its diffusion in the sodium bicarbonate solution.
Research in Central Vietnam found various methods for reducing soluble oxalate levels in taro. Wilting resulted in a reduction of 5.9%; soaking in water heated to 36 to 38 °C decreased levels by 26.2%, while boiling it for 60 minutes was identified as the best approach, achieving 84.20% reduction. It has been noted that when taro was baked and boiled with cow's milk, there was also another significant reduction in soluble oxalate. This experiment shows that soluble oxalate in taro can be reduced by soaking it in baking soda for 2 hours and then boiling it at 90° for six minutes. Another research about juice processing of carambola fruits, which have a high calcium oxalate concentration, alcohol fermentation with Saccharomyces cerevisiae for one to two weeks lowered the soluble oxalate content by 39–59% and 37–58% total oxalate content. Thus, prolonged fermentation led to a greater reduction in oxalate.40 The present study uses four household procedures, including soaking (8–12 hours), cooking (30–60 minutes), blanching (1–3 minutes), and microwave heating (2–6 minutes) to reduce antinutritional factors. Among them, soaking for 8 to 10 hours is recommended as the most effective household treatment.34 Using an acidic heating treatment considerably reduced the amount of phytate as well as oxalate in taro flour. The elimination of the itching sensation can be possible after 10 and 25 minutes of cooking in lemon solution and water, respectively.34 Moreover, it has been noted that as the microwave power level increased, the oxalate content of taro flour samples fairly reduced. This could be the result of the oxalate crystal partially breaking down at such high microwave power levels. Different concentrations of citric acid (0%, 2.5%, 5%, 7.5%, and 10%) have been used in a study to investigate the reduction of calcium oxalate in flour. According to this study, 10% citric acid decreased the CaOx level from 250 mg/100 g to 35.70 mg/100 g, which is around 85.72%. Thus, soaking in a 10% citric acid solution produced the optimal physical and chemical result.41 Boiling and NaCl treatment are two methods that researchers have used to mitigate calcium oxalate. By retaining its natural phytoconstituents, a novel technique such as ultrasound is a promising technology for the effective reduction of antinutritional factors.26Fig. 2 summarize several techniques to reduce calcium oxalate . After 30 minutes of immersion in a 20% acetic acid (CH3COOH) solution, the concentration of CaOx in malanga can be decreased from 1313 to 443 mg/100 g, or 66%. The immersion of purple yams for 60 minutes in a 10% NaCl solution can reduce CaOx content from 1024.4 to 807.7 mg/100 g, which is 20.96%.35 Furthermore, white taro can have its CaOx level decreased by 44% from 1096 to 483.2 mg/100 g by immersing it in a 6% sodium bicarbonate solution for 60 minutes.
6. Therapeutic benefits of taro
Several bioactive substances like alkaloids, flavonoids, and phenolic compounds are found in Alocasia extracts. These substances have substantial therapeutic benefits including antioxidant, anti-cancer, antibacterial, anti-inflammatory, antihyperglycemic, anti-diarrhea, and anti-diabetic properties, and these substances support people to fight against several chronic illnesses.23A. macrorrhizos possesses various phytochemicals, including alkaloids in its tubers, saponins in its leaves and roots, glycosides in its leaves and roots, and triterpenoids in its tubers and roots.3 The substances control different cellular functions at different phases, such as protein phosphorylation, gene expression modulation, and enzyme inhibition.4 Furthermore, various types and concentrations of bioactive metabolites are found in various parts of plants, as well as in extracts. For example, the stem of A. indica has a higher level of phenolic contents than the leaf sample; however, the rhizome extract of A. macrorrhizos is high in flavonoid contents.4 The phenol content and antioxidant activity reflect the protective property of A. macrorrhizos. The quantities of the bioactive substances such as total phenol, antioxidant, and flavonoid are illustrated in Table 1. Biologically, these substances act as antioxidants that reduce oxidative stress in cells. They also exhibit anti-inflammatory properties, aiding in preventing chronic inflammatory diseases.4 Moreover, they contribute to cancer prevention by neutralizing free radicals and may serve as antimicrobial agents, protecting against infections. The human biological system contains reactive oxygen species (ROS), also called free radicals, that have the potential to damage different molecules, including DNA, and hamper entire cell function, resulting in the development of diseases.4,23 Many plants, fruits, and vegetables contain some bioactive compounds or secondary metabolites that can treat several chronic diseases including cardiovascular disease, gout, diabetes, obesity, cancer, hyperuricemia, and inflammatory disease by scavenging the ROS in the human body.4 According to previous phytochemical studies, A. macrorrhizos has a wide range of biochemical substances, including various flavonoids, aloceramides, cyanogenic glycosides, indole alkaloids, piperidine alkaloids, and other alkaloids. Rhizomes of this plant contain alkaloids, fatty acids, phenolics, lignans, and phytosterols. Table 4 represent several numbers of significant bioactive substances of Alocasia macrorrhizos, their extraction methods, and medical properties.| Bioactive compound | Extraction method | Properties | Reference |
|---|---|---|---|
| Alkaloid, alocasin | Ethanolic or methanolic extraction of rhizome | Antifungal, antioxidant, anticancer, hepatoprotective | 4, 23 and 42 |
| Indole alkaloids | Ethanolic extraction of rhizome | Antifungal, anticancer | 4 and 45 |
| Piperidine alkaloid | Ethanolic extraction of rhizome | Anti-inflammatory, antioxidant, anticancer | 4 |
| Lignan amides | Ethanolic extraction of rhizome | Anti-inflammatory, antiproliferative | 4 and 45 |
| Anthocyanins | Ethanolic extraction | Anti-inflammatory, antioxidant, neuroprotective | 4 |
| Phenolic compounds and flavonoids | Diethyl ether/water extracts (DPPH) | Antioxidant, antidiabetic, hepatoprotective | 4 |
| Sterols, phytosterols | Methanolic extraction of rhizome | Hepatoprotective | 4 |
| Cyanogenic glycosides | Leaves | Anticancer | 4 |
| Sphingolipids | Methanolic extraction of rhizome | Anti-inflammatory | 4 |
| Ceramide | Ethanolic extraction of rhizome | Anti-aging | 4 |
| Tannin and polyphenolics | Ethanol, methanol, and acetone extraction | Antimicrobial, antidiabetic | 4, 44 and 45 |
The plant's antioxidant qualities make it edible, and diethyl ether extracts are responsible for this. These ethanolic extracts of this plant also have hepatoprotective, anti-inflammatory, and antinociceptive qualities. Alocasin, which is extracted from this plant, gives it its antifungal qualities.42 Polyphenols are categorised as secondary metabolites and show excellent antioxidant activities.43 In general, ethanol, methanol, and acetone solutions can be used to extract tannin from plants.44,45 Recent research shows that the bioactive compounds are associated with not only antibacterial but also anthelmintic properties.46 The ethanolic extract from the leaf of A. macrorrhizos demonstrated considerable antibacterial activity, especially against Gram-negative bacteria compared to Gram-positive bacteria. Furthermore, the particular extract possesses strong anthelmintic potential, such as helping to treat parasitic infections like Ascariasis and hookworm. Thus, these findings of the study highlight the necessity of exploring plant-based sources to develop novel, potent, and eco-friendly antimicrobial therapies.
6.1 Antioxidant activity
Antioxidants prevent and protect against oxidation of various biomolecules such as DNA, RNA, protein, and lipids. Consuming foods high in antioxidants, such as vegetables, fruits, tubers, and grains, can be helpful to prevent oxidative stress and maintain health.6 The total antioxidant capacity (TAC) of these foods can be supportive in improving a functional diet.7 Specifically, flavonoids and phenolic acids are natural antioxidants from plants, which help reduce oxidative stress and free radicals. Free radicals, which can arise from metabolism or environmental factors like radiation and pollution, are unstable molecules that can damage cells. Therefore, antioxidants have been found to stabilize these molecules by providing electrons for reducing cell damage and contributing to chronic diseases like heart disease, Alzheimer's, and cancer. Numerous components, such as tannins, flavonoids, alkaloids, saponins, vitamins, carotenoids, and fatty acids, provide taro with its antioxidant properties.47 The leaf ethanol extract had a total phenolic content of 9.18 mg/100 mg and flavonoids at 2.30 mg/100 mg.48 Furthermore, the ethanolic and aqueous extracts of the Alocasia macrorrhizos leaf sample contained flavonoids, phenols, tannins, carbohydrates, glycosides, and proteins, while the chloroform and ethyl acetate extracts lacked all phytoconstituents. Taro's antioxidant activity and phenolic content are natural antioxidant sources because it possesses a bioactive compound.49This plant's antioxidative qualities and its value in herbal medicine were facilitated by flavonoids and phenolic substances.4 Polar solvents, such as water, or hydrophobic solvents such as hexane, are inappropriate for extracting bioactive antioxidants from aroid rhizomes, roots, and stolons. However, water extract of plant leaves might have the highest level of antioxidant activity. Moreover, it has been identified that the antioxidant efficacy of plant extracts is assessed using stable free radicals found in the molecule 2,2-diphenyl-1-picrylhydrazyl (DPPH).23 The IC50 value (µg ml−1) represents the scavenging activity of the plant extract.
6.2 Anticancer potential
Cancer is a disease characterized by uncontrollable cell proliferation and division in the body, leading to the development of a mass of abnormal cells known as tumors.7 Moreover, studies determine that free radicals are a crucial element that plays an important role in the development of certain cancers. Free radicals are extremely reactive chemicals that damage cells, including DNA, cell membranes, and proteins. It has been noted that at that time, free radicals damage DNA, leading to genetic change and mutation in the cell, which can develop cancer. Henceforth, A. macrorrhizos is a plant with several bioactive compounds such as polyphenols, carotenoids, and flavonoids with potential anticancer properties. It has been demonstrated that these bioactive compounds contain anticancer properties by causing cell death and preventing the development and spread of cancer cells in the body.7 According to various studies, taro polyphenols may help prevent liver, colon, and breast cancers. A. macrorrhizos is an herbaceous plant that grows quickly and spreads easily in tropical regions. It has been identified in the in vitro study of A. macrorrhizos, at a dose of 400 µg ml−1, and it inhibited cell proliferation and induced apoptosis in human hepatocellular cells (SMMC-7721). In addition, A. macrorrhizos tuber's cytotoxic action against human hepatocarcinomatous, human throat cancer, and human nasal carcinoma epithelial cells identified a mild antiproliferative effect.6.3 Antimicrobial activity
The potential anti-microbial properties of taro have also been studied. Several investigations have demonstrated that the antibacterial properties of taro enable it to effectively combat a range of pathogens such as bacteria, fungi, and viruses. In addition, it has been noted that the bioactive compounds include alkaloids, flavonoids, and phenolic compounds, which are principally responsible for antibacterial properties. Another researcher investigated the antibacterial properties of taro leaves and recommended using them to make natural antimicrobial products and effective medicinal compounds for healing burn and wound conditions instead of pharmaceutical medications.50 The leaf extract of taro has antibacterial agents that can treat certain infections and reduce bacterial growth. For antifungal studies, it has been identified that Alocasin may be the antifungal present in the rhizomes of A. macrorrhizos.4 Alternatively, the antimicrobial activity of A. indica leaf extracts was largely attributed to bioactive components such as tannin and polyphenols. Therefore, taro has an abundance of potential for use as a natural antibacterial agent to treat various bacterial-induced food-borne diseases.6.4 Anti-inflammatory activity
Numerous compounds are found in taro as anti-inflammatory agents, such as flavonoids, anthocyanins, and polyphenols, which are recognized for their antioxidant properties.51 Moreover, several studies show that taro includes substances such as alkaloids and saponins with anti-inflammatory qualities that make it a promising natural material for treating several inflammatory disorders including rheumatoid arthritis, inflammatory bowel disease, and other chronic inflammatory conditions. The ethanolic extract of dried rhizome from Alocasia indica has been investigated for anti-inflammatory and analgesic properties. This finding supports Bangladesh for traditional use of the plant for curing inflammation. The anti-inflammatory properties of A. macrorrhizos may be associated with ligananamides and monoindoles, which may also be effective anti-inflammatory options.7. Medicinal uses in traditional systems
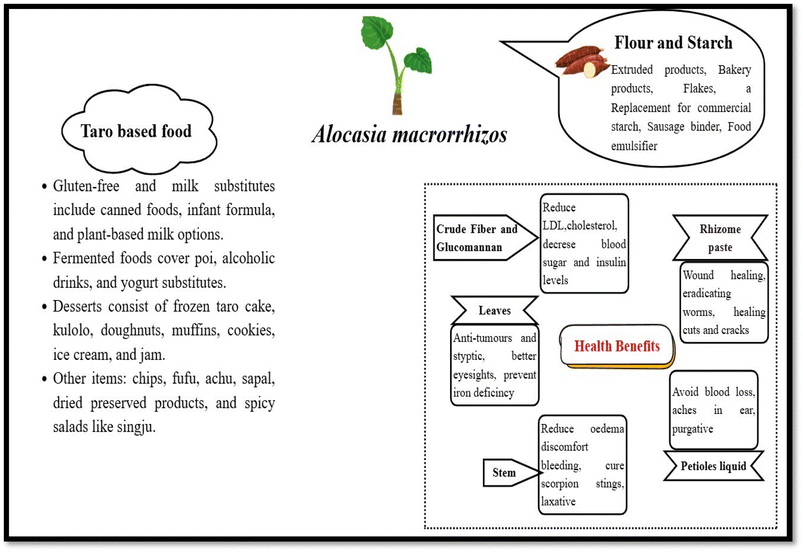
A. macrorrhizos is traditionally used to cure diabetes, jaundice, ear pus, and constipation. Furthermore, it has been demonstrated that A. macrorrhizos is traditionally used to accelerate wound healing and as an anti-inflammatory remedy. Several parts of the plant, such as the tuber, leaf, stem, and rhizome, can be treated with plant extract to treat various types of disease. A. macrorrhizos is an essential medical plant in the tropical and subtropical regions of South America and Asia. Additionally, this plant is recommended in Ayurvedic medicine to prevent disease of the abdomen and spleen, and inflammation, and used as an antimicrobial, antifungal, antioxidant, anticancer, analgesic, hepatorenal, and hepatoprotective agent. Particularly, it has been noted that the plant has alocasin that acts as an antifungal agent.4 Therefore, it has been concluded that the plant has been used in treating numerous diseases since ancient times.It has been mentioned that women who experience complications during delivery can benefit from boiling and consuming taro corms and leaves. Fig. 3 demonstrates that taro leaves are used for better eyesight and to prevent iron deficiency, while stem juice is applied to cuts and wounds to reduce oedema, discomfort, and bleeding.4 Taro is used in Chinese medicine to cure gastroenteritis after tumour removal. Due to its excellent nutritional content and digestive qualities, taro has been included in hospitals to incorporate nutrition in meals for senior citizens.51 The traditional residents of Cikondang village in West Java consume the leaf stalk of the particular plant to cure coughs by boiling it in water or eating it raw. People in Thailand's Chawan district may additionally utilize the bulb of A. macrorrhizos as a treatment to alleviate constipation. Usually, the tubers, petioles, and stems are properly cooked to eliminate the raphides of calcium oxalate along with hydrocyanic acid and preserve the parts of the plant that can be kept for months and consumed in curries. Additionally, the leaves of the plants are anti-tumour and styptic, and tubers are used to cure abdominal tumours and spleen enlargement.
A. macrorrhizos is used to treat rheumatic arthritis and is widely used as an astringent, diuretic, and digestive laxative.4 Moreover, leaves and stems are boiled and served with ghee for three days in successive weeks to treat constipation and colic diseases. As a rubefacient, the chopped leaves and roots are applied externally. Conjee, produced by boiling the rootstock or dried stem with rice flour, is used to treat haemorrhoids, generalized oedema, and chronic constipation.4 While the dried and powdered tuber is consumed orally with milk and sugar regularly for rheumatism and gout, it is also heated and used directly in painful areas. Moreover, the rootstock can be applied to the mouth with honey to treat mouth ulcers. Furthermore, the powdered tuber is consumed orally for piles and constipation. Petiole juice is used drop by drop in the ears of children to treat cough and otorrhea. The stem is used to cure scorpion stings and is used as a laxative. Future studies should focus on the chemical and pharmacological data of Alocasia macrorrhizos, a species used to combat malaria.52 This is a powerful remedy for malaria; thus, this should not be used in large amounts, especially as a decoction.
8. Culinary applications
Nearly half a billion people, or 10% of the world's population, depend on root crops like taro as their main source of nutrition.6,53 Due to the presence of high protein, carbohydrate, minerals, and vitamins in the corms of the taro, they can be suitable for bread, noodles, and gluten-free products.54 Taro leaves can be used in stew, purees, soup, and sauces. Taro flour retains 70% to 80% carbohydrate, a reliable by-product in the healthy food market. Due to the high moisture content, tubers have a limited shelf life; nevertheless, the tubers can be preserved by making them into starch or flour.25 Because of their small grain size, the taro starch microcapsules provide exceptional protection to Lactobacillus paracasei subsp. Paracasei.50 In addition, they offer Pickering emulsion stability, which depends on amphiphilicity and the starch's particle size. Thus, the spherical taro starch could be applied as an encapsulation.Giant taro, also called man kachu in the local dialect, is mainly cultivated as a homestead crop in Bangladesh, but it also has some commercial value and is offered for sale in the local farmer market. The leaf, petiole, and corm of Alocasia indica are edible and can be consumed in various delicacies. The petiole and leaf are typically cooked and consumed, while the corm is typically consumed fresh in a spicy salad called Singju, which can also be consumed as a cooked vegetable. Because this species is thought to be high in iron, the cooked petiole is given after childbirth to aid in a faster recovery, and the leaf lamina is applied externally to minimize boils and swellings.
In gluten-free products, extruded snacks, noodles, and bread pieces, taro serves as the main ingredient with particular properties such as gelatinization, solubility, retrogradation, freeze-thaw, thermal, and swelling.55 Due to its high fibre content and capacity to add functional qualities to products derived from this tuber flour, taro flour and starch are effective ways of producing by-products for healthy food markets and specific uses. With a 70–80% starch-based carbohydrate content and characteristics that indicate very fine grains, this flour has a 98.8% digestibility.6 However, dried taro pieces are thick and hard, and hence, the manufacturing of taro flour may be slow; nevertheless, the pre-gelatinization procedure helps to change the properties of the starch. They are widely used in food formulations like taro bread, taro pancakes, and kulolo. Moreover, it is used as a nutritional component in canned foods, extruded products, and fermented alcoholic beverages. It has been identified that technological development could improve the appeal of consumers to taro-based products. Different types of taro-based products are made in different nations. For instance, taro flour is frequently used in Ghana, whereas taro flakes and frozen cakes are popular in Taiwan. America uses taro in infant formula, while China creates taro pieces. Fig. 3 illustrates various uses of taro. Henceforth, it has been identified that these food products derived from taro can serve as an inspiration and a guide for developing food products from Alocasia macrorrhizos, utilizing its unique properties for similar or innovative applications. Therefore, this is an excellent substitute for milk and cereal allergic people. Considering all its advantages, A. macrorrhizos is a versatile and nutritious food that offers both medical and nutritional benefits, making it a promising component for further study and advancement.56A. macrorrhizos starch contains 1.3% glucomannan, forming a gel with a relatively high viscosity when exposed to air. The glucomannan is used as a supplement and food additive.
9. Conclusion
Numerous micro- and macro-nutrients are abundantly present in taro leaves and corms. Several vitamins and minerals are also found in taro, which are necessary for the body to function normally. Different clinical investigations support biological and pharmacological characteristics. It is necessary to recognize the growing popularity of A. macrorrhizos consumption as a nutritional intervention strategy that may be used to improve an individual's overall health. The interventional strategy should be implemented to mitigate the quantity of allergenic or toxic components and antinutritional factors such as oxalates, protease inhibitors, tannins, phytates, and polyphenols. In a nutshell, the taro leaves, corms, and plants offer possible uses in the food sector, including various drinks and value-added food items.Conflicts of interest
The authors declare that they have no conflict of interest.Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.References
- J. V. Müller and F. Guzzon, The forgotten giant of the Pacific: a review on giant taro (Alocasia macrorrhizos (L.) G.Don), Genet. Resour. Crop Evol., 2024, 71, 519–527 CrossRef.
- M. S. Smita Tarun Raut, Zenodo, 2023 Search PubMed.
- J. O. Osuji and S. C. Ihenko, Comparative Morphology, Anatomy and Phytochemistry of Cyrtosperma senegalense (Schott) Engl. and Alocasia macrorrhizos L. (Araceae), Int. J. Environ. Clim. Change, 2023, 13, 3862–3872 CrossRef.
- D. Arbain, L. M. R. Sinaga, M. Taher, D. Susanti, Z. A. Zakaria and J. Khotib, Traditional Uses, Phytochemistry and Biological Activities of Alocasia Species: A Systematic Review, Front. Pharmacol, 2022, 13, 849704 CrossRef CAS.
- T. D. Kularatna, N. Q. Arancon and J. A. Eiben, Comparative Growth of Elephant Ear Taro (Alocasia macrorrhiza) and Giant Swamp Taro (Cyrtosperma merkusii) in Hawai‘i, Crops, 2024, 4, 55–71 CrossRef.
- K. D. Nath, C. Nickhil, D. Borah, R. Pandiselvam and S. C. Deka, Preparation, characterization, and nutritional analysis of Napham: An Indian traditional smoke-dried-fermented fish paste, J. Food Biochem., 2024, 2024(1), 7410065 Search PubMed.
- Md. J. Ferdaus, E. Chukwu-Munsen, A. Foguel and R. C. Da Silva, Taro Roots: An Underexploited Root Crop, Nutrients, 2023, 15, 3337 CrossRef CAS PubMed.
- M. C. Momin, S. Mitra, A. R. Jamir and M. Kongbrailatpam, Evaluation of physicochemical properties in different cultivars of Taro [Colocasia esculenta (L.) Schott]: A comparative study, The Pharma Innovation Journal, 2021, 40(14), 1–9 Search PubMed.
- C. K. Nagar, S. K. Dash, K. Rayaguru, U. S. Pal and M. Nedunchezhiyan, Isolation, characterization, modification and uses of taro starch: A review, Int. J. Biol. Macromol., 2021, 192, 574–589 CrossRef CAS PubMed.
- T. S. Tresina Soris, A. Doss and V. R. Mohan, Nutritional and Antinutritional Assessment of Some Underutilized Corms, Rhizomes and Tubers, Trop. Subtrop. Agroecosyst., 2020, 23(1) DOI:10.56369/tsaes.2907.
- D. U. Kapoor, H. Sharma, R. Maheshwari, A. Pareek, M. Gaur, B. G. Prajapati, G. R. Castro, K. Thanawuth, S. Suttiruengwong and P. Sriamornsak, Konjac glucomannan: A comprehensive review of its extraction, health benefits, and pharmaceutical applications, Carbohydr. Polym., 2024, 339, 122266 CrossRef CAS PubMed.
- A.-K. Muth and S. Q. Park, The impact of dietary macronutrient intake on cognitive function and the brain, Clin. Nutr., 2021, 40, 3999–4010 CrossRef CAS PubMed.
- T. M. Barber, S. Kabisch, A. F. H. Pfeiffer and M. O. Weickert, The Health Benefits of Dietary Fibre, Nutrients, 2020, 12, 3209 CrossRef CAS PubMed.
- D. Tomé, S. Benoit and D. Azzout-Marniche, Protein metabolism and related body function: mechanistic approaches and health consequences, Proc. Nutr. Soc., 2021, 80, 243–251 CrossRef.
- H. Schmieder, C. Leischner, A. Piotrowsky, L. Marongiu, S. Venturelli and M. Burkard, Exploring the link between fat-soluble vitamins and aging-associated immune system status: a literature review, Immun. Ageing, 2025, 22, 8 CrossRef.
- A. G. Godswill, I. V. Somtochukwu, A. O. Ikechukwu and E. C. Kate, Health Benefits of Micronutrients (Vitamins and Minerals) and their Associated Deficiency Diseases: A Systematic Review, Int. J. Food Sci., 2020, 3, 1–32 Search PubMed.
- B. Mieso Buta, Evaluation of Oxalate Content in Boyna and Taro Roots Grown in Areka (Ethiopia), World Sci. Res., 2020, 7, 12–16 Search PubMed.
- Y. Matsumura, M. Kitabatake, S. Kayano and T. Ito, Dietary Phenolic Compounds: Their Health Benefits and Association with the Gut Microbiota, Antioxidants, 2023, 12, 880 CrossRef CAS.
- O. O. Arogundade and O. Adedeji, The importance of nutritive parameters in the taxonomy of some corm-producing members of the family Araceae, Not. Sci. Biol., 2020, 12, 318–333 CrossRef CAS.
- C. Munteanu, D. Teoibas-Serban, L. Iordache, M. Balaurea and C.-D. Blendea, Water intake meets the Water from inside the human body – physiological, cultural, and health perspectives - Synthetic and Systematic literature review, Balneo PRM Res. J., 2021, 12, 196–209 Search PubMed.
- Understanding Fever and Body Temperature: A Cross-Disciplinary Approach to Clinical Practice, ed. E. Grodzinsky and M. Sund Levander, Springer International Publishing, Cham, 2020 Search PubMed.
- J. Tondt, W. S. Yancy and E. C. Westman, Application of nutrient essentiality criteria to dietary carbohydrates, Nutr. Res. Rev., 2020, 33, 260–270 CrossRef CAS PubMed.
- F. Abdulhafiz, S. Ibrahim, M. F. H. Reduan, Z. Hamzah, L. V. Reddy and A. Mohammed, Phytochemical analysis and antimicrobial activity of the fruit and petiole extracts of Alocasia longiloba against Escherichia coli and Staphylococcus aureus, AIP Publishing, 2022, 2454(1), 020026 CAS.
- N. R. Cook, F. J. He, G. A. MacGregor and N. Graudal, Sodium and health—concordance and controversy, BMJ, 2020, m2440 CrossRef.
- M. Sharma, A. Shankar, A. K. Delta and A. Kumar, Visiting Taro from a Botanical and Phytochemical Perspective, The Pharma Innovation Journal, 2021, 10(8), 1446–1451 Search PubMed.
- S. Srivastava, V. K. Pandey, P. Singh, G. V. S. Bhagya Raj, K. K. Dash and R. Singh, Effects of microwave, ultrasound, and various treatments on the reduction of antinutritional factors in elephant foot yam: A review, eFood, 2022, 3, e40 CrossRef.
- A. Thakur, V. Sharma and A. Thakur, An overview of anti-nutritional factors in food, Int. J. Chem. Stud., 2019, 7(1), 2472–2479 CAS.
- S. Mitharwal, A. Kumar, K. Chauhan and N. K. Taneja, Nutritional, phytochemical composition and potential health benefits of taro (Colocasia esculenta L.) leaves: A review, Food Chem., 2022, 383, 132406 CrossRef CAS PubMed.
- A. Saqib, A. Asghar, F. Saeed, M. Afzaal, Y. Abbas Shah, A. W. Wani, H. Ahmad, A. Ijaz, N. Akram and C. T. Ndagire, Anti-nutritional and allergic components of taro: recent updates & perspectives, Food Agric. Immunol., 2025, 36, 2485896 CrossRef.
- Department of Food Science and Nutrition, CSC &RI, TNAU, Madurai and R. Senthilkumar, Nutrient Analysis of Sweet Potato and Its Health Benefits, Ind. J. Pure App. Biosci., 2020, 8, 614–618 Search PubMed.
- D. A. L. Chyne, R. Ananthan and T. Longvah, Food compositional analysis of Indigenous foods consumed by the Khasi of Meghalaya, North-East India, J. Food Compos. Anal., 2019, 77, 91–100 CrossRef CAS.
- E. Wada, T. Feyissa and K. Tesfaye, Proximate, Mineral and Antinutrient Contents of Cocoyam ( Xanthosoma sagittifolium (L.) Schott) from Ethiopia, Int. J. Food Sci., 2019, 2019, 1–7 CrossRef.
- S. Abera, W. Yohannes and B. S. Chandravanshi, Effect of Processing Methods on Antinutritional Factors (Oxalate, Phytate, and Tannin) and Their Interaction with Minerals (Calcium, Iron, and Zinc) in Red, White, and Black Kidney Beans, Int. J. Anal. Chem., 2023, 2023, 1–11 CrossRef.
- A. Kumar, K. Gupta, Md. A. Islam Apu, G. S. Abrol and V. Tomer, Effect of household processing on nutritional and antinutritional composition, mineral-mineral ratios, and functional properties of Colocasia leaves, Heliyon, 2023, 9, e17137 CrossRef CAS.
- Z. F. Rozali, Z. Zulmalisa, I. Sulaiman, Y. M. Lubis, S. Noviasari, K. Eriani and C. W. Asrizal, Decreased of calcium oxalate levels in the purple taro flour (Colocasia esculenta) from Aceh Province, Indonesia using three immersion methods, IOP Conf. Ser. Earth Environ. Sci., 2021, 711, 012022 CrossRef.
- N. S. Lawrie, N. M. Cuetos, F. Sini, G. A. Salam, H. Ding, A. Vancolen, J. M. Nelson, R. H. J. Erkens and G. Perversi, Systematic review on raphide morphotype calcium oxalate crystals in angiosperms, AoB Plants, 2023, 15, plad031 CrossRef PubMed.
- O. Gómez-Espinoza, D. Rojas-Villalta, A. M. Zúñiga-Pereira, C. Chacón-Díaz, L. A. Bravo and M. Reyes-Díaz, Plant oxalate oxidases: key enzymes in redox and stress regulation, J. Exp. Bot., 2025, eraf317 Search PubMed.
- R. E. Paull, D. Zerpa-Catanho, N. J. Chen, G. Uruu, C. M. J. Wai and M. Kantar, Taro raphide-associated proteins: Allergens and crystal growth, Plant Direct, 2022, 6, e443 CrossRef CAS.
- S. Stoeva-Grigorova, S. Dragomanova, M. Radeva-Ilieva, G. Kehayova, S. Dimitrova, S. Marinov, P. Marinov, M. Yovcheva, D. Ivanova and S. Zlateva, Poisoning from Alocasia × amazonica Roots: A Case Report, Toxins, 2025, 17, 189 CrossRef PubMed.
- Z. H. Malik, R. A. Mir, M. S. Dar and R. Somasundaram, Crystalline Chronicles : Navigating the Wonders and Worries of Plant Calcium Oxalate - A Review, Indian J. Nat. Sci., 2024, 15(85) Search PubMed.
- U. Ulyarti, G. Sahendra and M. Mursyid, The Effect Citric Acid Solution on Physical and Chemical Properties of Porang Flour (Amorphophallus Oncophyllus): Porang flour, Jur. Bio-Geo. Mat. E, 2024, 4, 1–8 CrossRef.
- W. Gao, Y. Wang, R. Wang, Y.-H. Wang, J.-W. Xu and X.-J. He, Antiproliferative piperidine alkaloids from giant taro (Alocasia macrorrhiza), Chin. J. Nat. Med., 2022, 20, 541–550 CAS.
- A. K. Das, Md. N. Islam, Md. O. Faruk, Md. Ashaduzzaman and R. Dungani, Review on tannins: Extraction processes, applications and possibilities, S. Afr. J. Bot., 2020, 135, 58–70 CrossRef CAS.
- R. Sirisangsawang and N. Phetyim, Optimization of tannin extraction from coconut coir through response surface methodology, Heliyon, 2023, 9, e13377 CrossRef CAS PubMed.
- U. Sriwijaya, S. Sudirman, L. J. A. Situngkir, U. Sriwijaya, H. Herpandi, U. Sriwijaya, I. Widiastuti, U. Sriwijaya, M. Janna and U. Sriwijaya, Antioxidant Activity Of Tannin-Rich Extract From Water Lettuce (Pistia Stratiotes) Leaf, J. Sustainability Sci. Manage., 2024, 19, 1–7 Search PubMed.
- C. Dubey, Analysis of Phytochemical Profile and Evaluation of Pharmacological Effects of Alocasia Macrorrhizos, International Journal of Research Publication and Reviews, 2025, 6, 5934–5941 CrossRef.
- M. Akyüz, Determination of Antioxidant Activity of Ethanol Extract of Gölevez [(Colocasia esculenta (L.)] Tubers, ksutarimdoga, 2019, 22, 388–394 Search PubMed.
- S. K. Singh, J. R. Patel and A. Dangi, Physicochemical, Qualitative and Quantitative Determination of Secondary Metabolites and Antioxidant Potential of Kalanchoe Pinnata (Lam.) Pers. Leaf Extracts, J. Drug Delivery Ther., 2019, 9, 220–224 CrossRef CAS.
- K. Nur-Hadirah, M. Arifullah, A. A. Nazahatul, S. Klaiklay, P. Chumkaew, M. Z. Norhazlini and H. Zulhazman, Total phenolic content and antioxidant activity of an edible Aroid, Colocasia esculenta (L.) Schott, IOP Conf. Ser. Earth Environ. Sci., 2021, 756, 012044 CrossRef.
- O. Alfaro-Galarza, E. O. López-Villegas, N. Rivero-Perez, D. Tapia- Maruri, A. R. Jiménez-Aparicio, H. M. Palma-Rodríguez and A. Vargas-Torres, Protective effects of the use of taro and rice starch as wall material on the viability of encapsulated Lactobacillus paracasei subsp. Paracasei, LWT–Food Sci. Technol., 2020, 117, 108686 CrossRef CAS.
- S. M. Saxby, The Potential of Taro (Colocasia Esculenta) as a Dietary Prebiotic Source for the Prevention of Colorectal Cancer, PhD thesis, University of Hawaii at Manoa, 2020.
- E. Elliott, F. Chassagne, A. Aubouy, E. Deharo, O. Souvanasy, P. Sythamala, K. Sydara, V. Lamxay, C. Manithip, J. A. Torres and G. Bourdy, Forest Fevers: traditional treatment of malaria in the southern lowlands of Laos, J. Ethnopharmacol., 2020, 249, 112187 CrossRef CAS PubMed.
- K. H. M. Siddique, X. Li and K. Gruber, Rediscovering Asia's forgotten crops to fight chronic and hidden hunger, Nat. Plants, 2021, 7, 116–122 CrossRef.
- A. Paixão, J. Cardoso, A. Loução, D. Embaló, C. Costa, A. M. A. F. Pereira, L. Luzidia Filemone, C. Simões and J. Morais, Obtention of Taro Flour (Colocasia esculenta L.) and Its Potential in Bread Making, Int. J. Food Sci. Agric., 2021, 5, 393–398 Search PubMed.
- D. Singla, A. Singh, S. B. Dhull, P. Kumar, T. Malik and P. Kumar, Taro starch: Isolation, morphology, modification and novel applications concern - A review, Int. J. Biol. Macromol., 2020, 163, 1283–1290 CrossRef CAS.
- R. Rubiyanti, N. Aji, M. T. Anwari and N. R. Azzahrah, in Proceedings of the 1st UMSurabaya Multidisciplinary International Conference 2021 (MICon 2021), ed. S. Februanti, M. Mundakir, Y. Levani, P. L. Ghazali, J. Saputra and M. Mujiarto, Atlantis Press SARL, Paris, 2023, vol. 708, pp. 965–972 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |