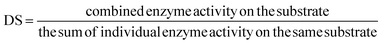Open Access Article
Open Access ArticleEffective valorisation of cereal lignocellulosic waste: a review of pretreatment techniques to enhance microstructural modification
Smriti
Ale
 ab,
Pramesh
Dhungana
ab,
Pramesh
Dhungana
 a,
Janet
Howieson
ab and
Rewati Raman
Bhattarai
a,
Janet
Howieson
ab and
Rewati Raman
Bhattarai
 *a
*a
aSchool of Molecular and Life Sciences, Faculty of Science and Engineering, Curtin University, Bentley 6102, Australia. E-mail: 19292935@student.curtin.edu.au; pramesh.dhungana@curtin.edu.au; j.howieson@curtin.edu.au; r.bhattarai@curtin.edu.au
bEnd Food Waste Australia Cooperative Research Centre, Wine Innovation Central Building Level 1, Waite Campus, Urrbrae, SA 5064, Australia
First published on 26th November 2025
Abstract
Food waste biomass from agro-food processing, such as grain husks, represents an abundant and renewable resource with significant potential for valorisation through enzyme hydrolysis. The enzyme hydrolysis process can convert lignocellulosic waste into valuable end products like biofuel, bioethanol, biochemicals, bioplastics, and bio-fertilisers. However, the complex structure of lignocellulose, including tightly bonded cellulose, hemicellulose, and lignin, poses significant barriers to enzymatic hydrolysis and conversion into valuable products. Hence, structural modification, as a pretreatment step before hydrolysis, is crucial in facilitating the efficient conversion of lignocellulosic waste biomass into valuable end products. This review examines the role of various pretreatment methods, including milling, extrusion, chemicals (acid & alkali), steam explosion, ammonia expansion, and biological processes (bacteria, fungi, and enzymes), in breaking down the recalcitrant nature of lignocellulose through structural modification in cereal husk. This review also discusses the resulting changes in microstructure (crystallinity, porosity, and surface morphology) due to various pre-treatments and their aligned effects on the hydrolysis rate and production of high-value fermentable sugars. The novelty of this review lies in the focus on incorporating microstructure-based pretreatment strategies for the underexplored cereal husk, offering new insights into the structure–function relationship that influences the enzyme hydrolysis and valorisation potential. By identifying the gaps in current research and highlighting the fermentation potential of pretreated cereal husks, this review provides a guide to biomass-specific, synergistic and nanotechnology approaches and environmentally sustainable valorisation strategies that support circular economy goals.
Sustainability spotlightCereal husks are generated in significant quantities as by-products of grain processing and are often underutilised or disposed of as low-value waste. Addressing this challenge is vital for reducing environmental impacts and promoting resource efficiency. This review highlights different pretreatment strategies to valorise cereal husks that enhance structural modification and conversion into high-value products such as fermentable sugars, functional food ingredients, and bio-packaging. Reducing agricultural waste through value-added industrial applications contributes to UN Sustainable Development Goals (SDGs) 9: Industry, Innovation and Infrastructure, 12: Responsible Consumption and Production, and 13: Climate Action, supporting the development of sustainable food systems and bio-based industries. |
1. Introduction
Agro-food processing wastes, such as straw, pulp, peels and husks, represent a significant proportion of the total food waste, accounting for approximately 12.9%.1,2 These wastes are generated from agricultural activities during harvest (leaves, roots, stalks, straws, stems) and industrial processing (husks, peels, pulps).3 All these wastes share a common composition, primarily made up of cellulose, hemicellulose, and lignin, collectively referred to as lignocellulose biomass.2 Lignocellulose waste biomass (LWB) is readily available, cost-effective, and renewable, and therefore has long been exploited commercially, as seen in the production of bioethanol from sugarcane bagasse by companies like Raizen and GranBio.4,5 Other notable examples include the conversion of corn stover into ethanol by Poet-DSM and the processing of wheat straw by Beta Renewals, as well as Clariant and Valmet.6 Additionally, woodchips are used commercially in the paper industry by International Paper and Stora Enso,7 and DuPont uses corn stover to transform it into biochemicals and ethanol.8 Recent advancements in lignocellulose biorefinery are paving the way for the sustainable production of energy, biofuels, biochemicals, bio-packaging, bioethanol, biofertiliser, biochar, and bio-enzymes.9–11 Whilst recognising the broad spectrum of LWB and its extended applications, this review will focus primarily on the cereal husk structure, chemical composition, and valorisation potential.Around 63.5–73.5 million metric tonnes of cereal husks from wheat, rice, oats, and barley are produced yearly as processing waste.12 The amount of husk residues produced from cereal processing depends on environmental, economic, processing and cultural factors.13,14 Lignocellulosic waste accumulated during the manufacturing and processing of cereals, if improperly disposed of, incinerated, or landfilled, can have environmental effects due to greenhouse gas (GHG) emissions.15 This leads to global warming, soil acidification and water contamination and hence, the sustainable development of waste valorisation is compromised.15,16 Agricultural waste has been reported to be the second-largest (19.9%) contributor to greenhouse gases in the environment.17 The adverse effects of improper waste management, particularly lignocellulose waste, can be addressed through the implementation of effective and eco-friendly valorisation processes to produce simple fermentable sugars.3
Cereal grains are inherently designed to remain physically, chemically, and biologically inert until proper conditions prompt germination. This is primarily due to the protective hull that shields cereal seeds from adverse environmental conditions. If consumed unprocessed, the seed can go through the digestive tract undigested.18 Food Processing operations like dehulling and milling are essential and play a pivotal role in transforming agricultural commodities into consumer-friendly foods, enhancing shelf life, nutrient bio-accessibility, colour, flavour, economic value, and ease of production.19 Consequently, a large quantity of by-products is generated, especially in the form of husks, which are lignocellulosic in nature.20 Lignocellulose in cereal husk is primarily composed of cellulose, hemicellulose and lignin with varying proportions depending on the type of biomass source, growth conditions, climatic conditions, harvesting and storage processes.5,21 Variances in the lignocellulose composition of different cereal crop husks are depicted in Table 1.
| Cereals | Scientific names | Lignocellulose | Ash | Extractives | References | |||
|---|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | ||||||
| By-product (husk) | Oats | Avena sativa | 23–48% | 16–35% | 16–25% | 2–10% | 2.1–13.94% | 22–25 |
| Wheat | Triticum aestivum | 36% | 18% | 16% | 1–19% | 20.89% | 23 and 26–28 | |
| Rice | Oryza sativa | 28–35% | 12–33% | 15–23% | 20% | 6.8% | 2, 9 and 29–34 | |
| Corn | Zea mays | 28–38% | 28–35% | 9–20% | 5–7% | 13.9–19.55% | 35–38 | |
| Barley | Hordeum vulgare | 34–39% | 23–30% | 12–20% | 5.35% | 3.82–16.53% | 24, 39 and 40 | |
The microfibrils of crystalline and amorphous cellulose are interconnected by hydrogen bonds, along with amorphous chains of hemicellulose embedded within the lignin matrix, providing a compact and stable structure.41,42 Lignocellulose biomass generally requires breakdown to convert into simpler forms of sugars for its commercial use in a biorefinery.43
In recent years, the potential applications of lignocellulose-derived sugars to produce biofuels, alcohols, biogas, and bio-packaging have been discussed.10 Likewise, previous investigations have revealed various potential uses of cereal husk waste, including the preparation of biodegradable packaging,44,45 production of biofuels46,47 and treatment of wastewater.48–52 However, the lignocellulose biomass forms a complex, polymerised structure through intermolecular hydrogen bonds, covalent linkages, and interactions between its monomers and polymers, contributing to the recalcitrance property and making the isolation of key components challenging.
Recent studies described the correlation between microstructural properties and possible valorisation techniques for different LWBs, highlighting the feasibility of various applications in the food industry. The feasibility of using fungal-assisted valorisation of lignocellulose due to structural degradation or depolymerisation, the role of chemical pretreatment, and the potential of using acid or alkali to break down the complex lignocellulose structure have been discussed in various research articles.53–58
This review will describe the fundamental aspects of different lignocellulosic cereal husk waste, its structural constituents, the underlying challenges in depolymerisation and the potential for valorisation through modification of microstructural properties. This review will also discuss the treatments carried out to modify the structural and chemical properties of lignocellulose, thereby converting it into valuable products. Finally, it will provide an overview of waste valorisation efficiency and profitability through microstructural modification.
The scope of this paper focuses on exploring how microstructural modifications can improve the efficiency of waste valorisation while addressing the challenges associated with treatments.
2. Composition and general structure of lignocellulose
Lignocellulose biomass from cereal waste is mainly composed of structural polymers – cellulose, hemicellulose and lignin with varying proportions depending on the type of biomass source, growth conditions, climatic conditions, and harvesting and storage processes.5,59 Minor proportions of pectin, proteins, extractives and inorganic compounds may also be present.60Cellulose (C6H10O5)n is the dominant polymer in cereal husks (see Table 1). Cellulose is an amphiphilic polysaccharide composed of linear chains of D-glucopyranose units linked together by β-(1-4) glycosidic bonds. This structure forms a compact microfibril in crystalline and amorphous states.61 The other common components, hemicellulose and lignin (see below), envelop cellulose microfibrils through non-covalent hydrogen bonding. These microfibrils are insoluble and are bound together by hydrogen bonds and van der Waals forces, comprising 36 glucan chains, each containing 4000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 glucose molecules.5 The hydroxyl group at the axis forms intra- and intermolecular hydrogen bonds, resulting in a stable microfibril with a hydrophobic core and a hydrophilic exterior.62 Cellulose is relatively heat-stable, insoluble in water, alcohol, and ether, and cannot be digested by the human body.63 This suggests that highly crystalline forms of cellulose can resist physical, chemical, or enzymatic treatment, requiring a pretreatment to reduce cellulose's crystallinity and increase its digestibility.15
000 glucose molecules.5 The hydroxyl group at the axis forms intra- and intermolecular hydrogen bonds, resulting in a stable microfibril with a hydrophobic core and a hydrophilic exterior.62 Cellulose is relatively heat-stable, insoluble in water, alcohol, and ether, and cannot be digested by the human body.63 This suggests that highly crystalline forms of cellulose can resist physical, chemical, or enzymatic treatment, requiring a pretreatment to reduce cellulose's crystallinity and increase its digestibility.15
Hemicellulose (C5H8O4)n is a heterogeneous polymer with randomly branched structures with a degree of polymerisation ranging from 50–300 monosaccharide units. Unlike cellulose, hemicellulose lacks crystallinity due to its branching and the presence of acetyl groups, making it more easily degradable.60,62,64 The structure and chemical composition of hemicellulose, however, vary among different plant species with varying proportions of arabinose, xylose, rhamnose, mannose, glucose, and galactose, along with glucuronic acid side chains.65 These chains are linked together by β-(1-4) and β-(1-3) glycosidic bonds. The hemicellulose acts as a binding agent between cellulose and lignin, providing strong structural support.66 It is hydrophilic due to hydroxyl groups, allowing for high water absorption and influencing mechanical properties.67
Lignin consists of phenylpropane units: p-coumaric alcohol (C9H10O2), coniferyl alcohol (C10H12O3) and sinapyl alcohol (C11H14O4), each differing in the number of (OCH3) methoxyl group substitutions on the aromatic ring.68,69 They are linked together by ether (C–O) and carbon–carbon (C–C) bonds, creating lignin's three-dimensional structure.62 Upon polymerisation, these alcohol units form p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) units, respectively, linked through β-O-4 and α-O-4 linkages.62,70 The structure of lignin, including its type and proportion of H, G & S units (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G
G![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio), plays a crucial role in its degradability.71 Lignin rich in syringyl (S) units is more soluble in organic solvents, whereas guaiacyl (G) and p-hydroxyphenyl (H) rich lignin is recalcitrant due to higher crosslinking.72 The relative ratios of H, G, and S units in different cereal husks are summarised in Table 2; however, no literature data were available for wheat and barley husks.
S ratio), plays a crucial role in its degradability.71 Lignin rich in syringyl (S) units is more soluble in organic solvents, whereas guaiacyl (G) and p-hydroxyphenyl (H) rich lignin is recalcitrant due to higher crosslinking.72 The relative ratios of H, G, and S units in different cereal husks are summarised in Table 2; however, no literature data were available for wheat and barley husks.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G
G![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S lignin composition in cereal husks
S lignin composition in cereal husks
Lignin provides strength and rigidity to the lignocellulose structure, acting as a defence against microbial and enzymatic breakdown. The hydrophobic nature and amorphous matrix of lignin interact with cellulose and hemicellulose, providing high mechanical strength and recalcitrance by limiting the accessibility of chemical or enzyme attack.41,55 This structural complexity of lignocellulose makes the separation of individual components difficult, and the recovery of glucose is often challenging, requiring a pretreatment of lignocellulose biomass.75
3. Microstructural properties of husk lignocellulose
Besides the broader compositional characteristics, lignocellulosic cereal husk biomass possesses distinct microstructural characteristics that can significantly influence its recalcitrance property76 and, ultimately, the enzymatic hydrolysis process and valorisation potential.77,78 Therefore, it is essential to understand the microstructures of lignocellulosic biomass and how these structures contribute to its recalcitrance behaviour.79 Traditionally, commercial enzymes such as cellulase, hemicellulase and ligninase have been used to break down the complex lignocellulose biomass into fermentable sugars for downstream valorisation processes.80 The following sections provide a brief overview of each physical property, its implications for recalcitrance behaviour and subsequent enzyme hydrolysis.3.1. Crystallinity
Cellulose in cereal husks can exist in both crystalline and amorphous forms.81 Cellulose with high crystallinity is less susceptible to enzyme degradation as opposed to those in amorphous form, and this crystalline region in cellulose microfibrils is formed through hydrogen-bonded β-(1-4) glycosidic bonds, leaving little space for amorphous nature.82 The amorphous cellulose is 3–30 times faster to hydrolyse than the crystalline forms of cellulose.76In lignocellulosic cereal husk biomass, cellulose is the only component responsible for the crystalline contribution, whereas hemicellulose and lignin are considered amorphous components.83,84 The multilayered structures of the cell wall, inter- and intramolecular hydrogen bonds, and the strong hydrophobic nature of cellulose give it a recalcitrant property, increasing its resistance to breakdown methods (e.g., enzymes) and thereby hindering the effective breakdown of cellulose into fermentable sugars.68,78 The crystalline structure of cellulose can be distorted when subjected to chemical or physicochemical pretreatments.85–88 These treatments cause cellulose to swell and transition into a disordered state, reducing the crystallinity index (CrI), a measure of cellulose crystallinity. The CrI can be calculated using eqn (1):
| CrI (%) = (I002 − Iam)/I002 × 100% | (1) |
3.2. Degree of polymerisation
Cellulose is the homopolymer of glucose units linked together by β-1,4 glycosidic bonds, and the number of these glucose units is the degree of polymerisation (DP). The DP of cellulose plays a pivotal role in providing mechanical strength to the biomass.95 Long cellulose chains contain more hydrogen bonds, making them resistant to hydrolysis.96 Conversely, short cellulose chains possess fewer hydrogen bonds and form a weaker network, making them more susceptible to enzyme hydrolysis.97 Therefore, reducing the degree of polymerisation provides more binding sites for enzymes to act, exposes the cellulose ends, and speeds up the enzyme hydrolysis rate.77 Altering the DP changes structural properties like crystallinity and porosity.98 The DP can be modified through various pretreatment methods, physical, chemical, physicochemical, and biological, resulting in varying lengths of cellulose chains and supporting the production of soluble and insoluble polymer chains. Soluble chains have a DP ranging from 2–12, whereas insoluble chains can have a DP between 100 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.96,99 Additionally, cellulose with a DP > 1000 possesses a rigid crystalline structure, requiring a high enzyme load to break through its crystalline matrix. In contrast, cellulose with medium DP (300–1000) combines crystalline and amorphous regions, balancing resistance and accessibility characteristics.100 The lower DP cellulose (<300) is more easily accessible, requiring minimal pretreatment and lower enzyme concentration due to increased substrate availability.98
000.96,99 Additionally, cellulose with a DP > 1000 possesses a rigid crystalline structure, requiring a high enzyme load to break through its crystalline matrix. In contrast, cellulose with medium DP (300–1000) combines crystalline and amorphous regions, balancing resistance and accessibility characteristics.100 The lower DP cellulose (<300) is more easily accessible, requiring minimal pretreatment and lower enzyme concentration due to increased substrate availability.98
3.3. Available surface area
The available surface area of lignocellulose biomass is a critical factor affecting digestibility as it largely depends on the biomass particle size and porosity. A reduction in particle size or an increase in porosity increases the available surface area.76,101,102 The available surface area can be classified into two types: exterior surface area, influenced by length and width and interior surface area, influenced by pores and cracks within biomass.103 Studies have revealed that the interior surface area of cellulose is 2-fold larger compared to the exterior.104,105 The higher the available surface area, the higher the adsorption rate of enzymes onto the substrate, resulting in higher hydrolytic activity.763.4. Porosity
The pore size of lignocellulose biomass can vary widely, influencing the enzyme–cellulose interaction.106 The narrow pores can restrict enzyme–substrate interaction, affecting the hydrolysis rate.77 Enzymes like cellulases are trapped if the pore size is too small, affecting hydrolysis.99 Research indicates that the pore sizes in the 5–10 nm range are too narrow for the enzymes to diffuse. In contrast, biomass with a pore size greater than 10 nm showed a positive correlation with the hydrolysis rate, allowing better penetration and diffusion of enzymes.1063.5. Hemicellulose composition and amorphous structure
Hemicellulose has a lower DP (100–200 units) than cellulose but contains many complex substitutes like xylans, xyloglucans, mannans, and glucomannans.107,108 Although hemicellulose is more readily hydrolysable than cellulose, it protects cellulose fibres from enzyme attack through hydrogen bonds and covalently links with lignin.82,109 Also, the acetyl groups in the hemicellulose structure limit enzyme activity by interfering with the formation of hydrogen bonds between cellulose and cellulose-binding domains of an enzyme.76 Hemicellulose is also covalently linked to lignin, forming a lignin-carbohydrate complex that gives a rigid wood-like structure and acts as a barrier limiting enzyme access to cellulose.110,111 Removal of hemicellulose during pretreatment processes has been reported to improve fibre porosity and surface area.1123.6. Lignin content and distribution
Lignin is the outermost structural component of lignocellulose biomass, containing heteropolymeric phenylpropanoid units of p-coumaryl, coniferyl, and sinapyl alcohol.68 The presence of lignin hinders enzyme activity by limiting physical contact between the enzyme and cellulose77,98 and provides mechanical support by covalently linking to hemicellulose.113 The lignin structure and its proportion distributed throughout lignocellulose biomass determine the extent of recalcitrance and the rate of enzyme hydrolysis. The rate of enzyme hydrolysis in the presence of lignin also depends on the type of enzyme used for treatment, with cellulase being the least effective compared to xylanase and glucosidases.114 Lignin adsorbs enzymes irreversibly, thereby reducing enzyme activity and hydrolysis efficiency.82 Removal of lignin can disrupt the lignin-hemicellulose matrix, increase porosity, reduce the number of lignin-enzyme binding sites, and expose the hemicellulose surface area, thereby facilitating the hydrolysis rate.112,1154. Strategies for microstructural modification to enhance valorisation
The recalcitrant nature of lignocellulosic biomass towards enzymatic and microbial breakdown, the crystalline structure of cellulose, and the hemicellulose matrix embedded within highly polymerised phenolic lignin present a significant barrier to potential industrial valorisation.116 As discussed in the previous section, the extent of lignocellulose recalcitrance is influenced by its chemical composition, complex cross-linking between polymers, physical interactions, structural heterogeneity, including crystallinity, degree of polymerisation (DP), accessible surface area, and porosity.117Pretreatment of lignocellulose biomass is considered an effective strategy to overcome the microstructural barriers and recalcitrance for valorisation by disrupting the physical structure, breaking the chemical bonds within the lignocellulose biomass or eliminating the hemicellulose and lignin content.118–121 The damage to the crystal structure increases the accessibility of enzymes (both microbial and commercial), enhancing the breakdown of components and facilitating the separation of individual components into useful forms for upcycling.122,123 The pretreatment process is also reported to improve saccharification by up to 90%, a key step in sugar-based biorefinery operations.124–126 Different pretreatment strategies like physical, chemical, physicochemical and biological have been developed to act on cellulose crystallinity, DP, porosity and surface area, and lignin-hemicellulose matrices, thereby enhancing the enzyme accessibility and breakdown of lignocellulose biomass.127,128 To illustrate, a comparative summary of strategic methods to induce microstructural modification and their impact on hydrolysis yields is presented in Table 4, including their advantages and limitations.
| Treatment methods | Type | Mode of action | Biomass type | Modifications | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| Physical pretreatment | Milling | Reduces the size and cellulose lowers DP, and increases surface area | Corn stover | - Particle size 53–75 µm vs. 425–710 µm: 1.5-fold increase in enzyme hydrolysis rate | No toxic or inhibitor formation, eco-friendly | High power consumption, high capital cost of equipment | 129–131 |
| Commercial cellulose | - Exposed surface area: 1.3 m2 g−1 (untreated) vs. 2.2 m2 g−1 (treated) | 132 | |||||
| - DP: 191.6 units (untreated) vs. 136.6 units (treated) & CrI 77.5% (untreated) vs. 0.59% (treated) | |||||||
| - Enzyme hydrolysis rate: 93.8% | |||||||
| Extrusion | Defibrillation | Rice straw, wheat bran | - Total reducing sugar yield: 83.7% & 60–73% respectively | Short processing time, no inhibitor formation, easy scale-up | High energy consumption | 133–135 | |
| Microwave irradiation | Molecular collision disrupts the hydrogen bond | Corn stover | - Enzyme hydrolysis rate: 59.96% (untreated) vs. 85.4% (treated) | Short processing time, high uniformity and selectivity, and less energy input | High temperature and energy requirement, high operation cost | 136–139 | |
| Ultrasound | Molecular collision disrupts the hydrogen bond | Wheat straw | - CrI 62.42% (untreated) vs. 61.36% at 200 W & 54.56% at 700 W (treated) | Clean process, short extraction time, simple operation | Prolonged treatment can induce unwanted changes, and high equipment cost | 140–142 | |
| Rice husk | - CrI 62.5% (untreated) vs. 68.3% (treated) | 143 and 144 | |||||
| - Husk surface corrosion with exposed surface area: 2.42 m2 g−1 (untreated) vs. 2.82 m2 g−1 (treated) vs. 3.22 m2 g−1 (treated) at 20 kHz frequency for 0.5 h and 1 h, respectively | |||||||
| Chemical pretreatment | Acid | Hemicellulose depolymerisation, swelling of fibres, and redistribution of lignin | Barley hulls and oat hulls | - Glucose yield: 76.28% and 59.63% respectively | High efficiency, low energy consumption | Toxic, corrosive, and hazardous, inhibitor formation | 145–147 |
| Rice hulls | - Enzyme hydrolysis rate: 60% | 148 | |||||
| Rice hulls cellulose | - CrI: 39.3% (untreated) vs. 42% at 1 M HNO3 (treated), 37% for 1 M HCl (treated), 63.9% for 2 M HNO3 (treated), and 52.2% for 2 M HCl (treated) | 90 | |||||
| Rice straw | - Glucose yield: 83%, pore volume: 0.02 cm3 g−1 (untreated) vs. 0.04 cm3 g−1 (treated) | 149 | |||||
| Alkali | Fractionation of hemicellulose and lignin | Rice hulls | - Total reducing sugar yield: 37.1% | Effective in lignin hydrolysis or removal, partially hydrolysis of hemicellulose requires simple reactor equipment | Time-consuming and requires a large amount of alkali, treated biomass requires a neutralisation step, and proper waste disposal management is also required | 150–153 | |
| - Lignin reduction: 55% (untreated) vs. 14.9% (treated) | |||||||
| - Hemicellulose hydrolysis: 10.7% (untreated)vs. 33.1% (treated) | |||||||
| - Increased cellulose concentration: 32.65% (untreated) vs. 51.65% (treated) | |||||||
| - CrI: 45% (untreated) vs. 69% (treated) | |||||||
| Ozonolysis | Dissolves lignin and hemicellulose | Wheat straw | - Lignin reduction rate: 29% & 60% respectively | No inhibitor formation, water soluble, can be performed at ambient conditions | Expensive, as a large amount of ozone is required | 137, 154 and 155 | |
| - Enzyme hydrolysis rate: 57% & 5-fold higher | |||||||
| Organosolv | Acts on α-aryl and β-aryl ether linkages in lignin | Rice husk | - Lignin removal: 77.5% | Short processing time, recyclable, easy recovery of solvents, separation of pure lignin | Expensive solvents, the recovery process is energy-intensive, highly flammable and volatile | 137 and 155–157 | |
| - Lignin recovery: 49.8% | |||||||
| - Xylan removal: 86% | |||||||
| - Xylose recovery: 67.6% | |||||||
| Ionic liquid | Dissolves H-bond in cellulose, ether/ester bond between lignin-carbohydrate, and β-O-4 bonds within lignin | Wheat straw rice husk | - Enzyme hydrolysis rate: 54.8% | Non-toxic, non-volatile, thermal stability, green solvent, recyclable | Inactivates the cellulase enzyme, expensive solvent, and is energy-intensive to recycle | 137, 155, 158 and 159 | |
| - Glucose yield: 70% | |||||||
| Steam/CO2/ammonia | Swelling of fibres, hydrolysis of lignin & hemicellulose | Rice husk | - Glucose yield: 53.87% | Steam – minimal energy and chemical usage, cost-effective | Steam – formation of toxic component | 131, 155, 160 and 161 | |
| Increases surface area and enzyme accessibility | - Hemicellulose hydrolysis: 38.81% (untreated) vs. 2.6% (treated) | CO2 – no inhibitor formation, cost-effective, environment-friendly, non-flammable, easy recovery | CO2 – requires a facility to withstand high-pressure conditions | ||||
| - Lignin hydrolysis: 24.48% (untreated) to 15.65% (treated) | Ammonia – no inhibitor formation for downstream processes | Ammonia – not efficient for biomass with high lignin, corrosive, and high operating costs | |||||
| Corn stover | - 2.5-fold increase in glucose concentration | 162 | |||||
| Bacteria/fungi | Depolymerisation of lignin | Rice husk & sorghum husk | - Reducing sugars yield: 447.95 mg mL−1 g−1 & 103 mg g−1 respectively | No chemical requirement, environment-friendly, low energy input | Time-consuming, large space requirement, and need for continuous monitoring of microorganism growth | 54, 163 and 164 |
4.1. Physical pretreatment
Research has shown that corn stover with a particle size of 53–75 µm is approximately 1.5 times more susceptible to enzymatic hydrolysis than material with a larger particle size of 425–710 µm.129 Further evidence demonstrates that superfine grinding of steam-exploded rice straw to 60 µm improved the hydrolysis rate, with a significant yield of reducing sugar concentration of 61.4%.167 Another study, which combined grinding, heating, and chemical treatment (using a 10 mm particle size, 110 °C, and 2% ammonia) on rice straw, resulted in a 17.5% increase in biogas yield compared to untreated straw.168
Different milling techniques, like ball milling, two-roll milling, hammer milling, and disk milling, are commonly used to reduce the particle size of lignocellulosic biomass.169 Ball milling is reported to yield higher fermentable sugars than wet milling.137 This is consistent with the findings that compared the enzymatic hydrolysis of rice straw pretreated with both wet and ball milling methods, reporting glucose and xylose yields of 78.5% and 41.5% for wet milling, and 89.4% and 54.3%, respectively, for ball milling.170 Ball milling of oil palm empty fruit bunch for 120 minutes significantly reduced the crystallinity index from 56.1% to 9.3%, resulting in high glucose (67.5%) and xylose (80.1%) yields after enzymatic hydrolysis at 50 °C for 72 h. In comparison, the untreated sample yielded only 15.9% glucose and 5.4% xylose.171 In another study, 90 minutes of ball milling significantly increased the surface area of cellulose from 1.3 m2 g−1 to 2.2 m2 g−1, decreased the degree of polymerisation (DP) from 191.6 to 136.6 units, and reduced the CrI from 77.5% to 0.59%.132 The same study also demonstrated a strong correlation between structural properties, CrI, DP, surface area, and the enzymatic hydrolysis rate, which improved by 93.8%.132 Despite the effectiveness of grinding pretreatment, one of the major drawbacks of this process is its high energy consumption and the high capital cost of the equipment.130,155,165 Energy consumption of 0.5–2.5 kWh kg−1 for 7–30 minutes of ball milling at 270 rpm has been reported, to increase glucose yield by 59.02% and xylose yield by 49.95% during the enzymatic hydrolysis of wood fibre.172 Similarly, the size reduction of lignocellulosic biomass, such as corn stover, required considerable energy with reported values of up to 0.011 kWh kg−1, highlighting the energy-intensive nature of the milling pretreatment to reduce crystallinity by 73%.173
Ultrasound pretreatments are often used in conjunction with chemical pretreatment methods to achieve more effective results.189 Reported evidence includes a 45% removal of lignin from coffee husk when ultrasonicated at 47 kHz for 20 minutes in combination with 4% potassium permanganate, and 80–100% lignin removal from groundnut shells, coconut coir, and pistachio shells, which were dipped in a 1% NaOH solution, followed by ultrasonication at 20 kHz.190
4.2. Chemical pretreatment
Chemicals like acids, alkalis, organic solvents, and ionic liquids have been widely used to enhance the biodegradation of complex lignocellulosic biomass, resulting in significant structural modifications.116,194Barley husk, oat husk and rice straw pretreated with 1.86% (v/v) [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8% w/v, 130 °C, 30 min], 1% (v/v) [1.8% w/v], (130 °C, 19 min), and 1% (w/w) [160–180 °C for 1–5 min] H2SO4 resulted in a maximum glucose yield of 76.28%, 59.63% and 83% respectively, after enzyme hydrolysis.145,149 Additionally, the pore volume of rice straw was observed to increase from 0.02 cm3 g−1 to 0.04 cm3 g−1 with the associated sugar yield and the rearrangement of lignin structure, as well as C–H deformation at an elevated temperature of 190 °C, consistent with the findings of previous studies.203,204
8% w/v, 130 °C, 30 min], 1% (v/v) [1.8% w/v], (130 °C, 19 min), and 1% (w/w) [160–180 °C for 1–5 min] H2SO4 resulted in a maximum glucose yield of 76.28%, 59.63% and 83% respectively, after enzyme hydrolysis.145,149 Additionally, the pore volume of rice straw was observed to increase from 0.02 cm3 g−1 to 0.04 cm3 g−1 with the associated sugar yield and the rearrangement of lignin structure, as well as C–H deformation at an elevated temperature of 190 °C, consistent with the findings of previous studies.203,204
FTIR investigation of the pretreated rice husk with HCl (0.5% w/v, 125 °C, 1.5 h) also exhibited a high cellulose content, with intensified bands at 1046–2924 cm−1, increased crystallinity, and a smooth surface, as observed through SEM micrographs.58 The crystallinity index of rice husk has been reported to vary with different acid treatments, showing values of 39.3% for untreated, 42% for 1 M HNO3-treated, 37% for 1 M HCl-treated, 63.9% for 2 M HNO3-treated, and 52.2% for 2 M HCl-treated samples.90 This increase in crystalline values was attributed to the solubilisation of amorphous cellulose regions by acid.91 Structural characterisation using C-NMR demonstrated that treatment of rice husk cellulose with H2SO4 (64% 45 °C, 30 min) yielded a rod-like cellulose nanocrystal and increased crystallinity from 22.63% to 67.16%, which was reflected as a sharp peak in the XRD pattern.205
Both dilute and concentrated sulphuric acids are hazardous, toxic and corrosive, requiring corrosive-resistant non-metallic equipment.43,131,206
Unlike acid hydrolysis, alkaline hydrolysis is reported to be more effective in breaking the ester bond between lignin, hemicellulose and cellulose.211–214 However, the dissolution of lignin by alkali treatment is not fully selective; it can decompose carbohydrates, mainly hemicellulose and cellulose, which affects cellulose crystallinity.79 A significant removal of lignin and hemicellulose has been reported in rice husk, yielding up to 51.65% cellulose using 2% w/w NaOH (121 °C, 40 min, 0.25–0.623 mm particle size).150 The treated sample also exhibited a CrI of 69% compared to 45% in the native rice husk. FTIR spectra of the rice husk, wheat straw, rice straw and corn stover treated with 2% w/w NaOH (50 °C, 3 h) revealed the exposed cellulose, partial removal of hemicellulose, broken ester bond and reduced crystallinity.215 However, this method is time-consuming, and the use of chemicals alters the pH of the biomass, requiring an additional step to neutralise the pH level, depending on the target and nature of the end product.206
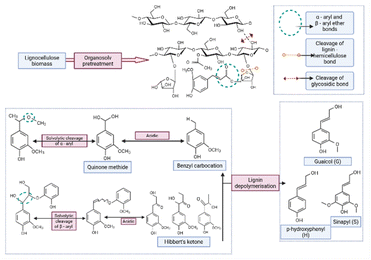
The mechanism of organosolv pretreatment involves the cleavage of α-aryl and β-aryl ether linkages within lignin, leading to its depolymerisation and solubilization.220 The reaction mechanism is illustrated in Fig. 2. The α-aryl ether bonds are reported to break 100 times faster than the β-aryl ether bonds.217 Under acidic conditions, the cleavage of α-aryl linkage follows three pathways: (i) solvolytic cleavage via SN2 nucleophilic substitution; (ii) quinone methide formation, and (iii) benzyl carbocation formation.221 Likewise, β-aryl ether cleavage occurs via (i) solvolytic cleavage with formaldehyde elimination, (ii) homolytic and solvolytic cleavage forming Hibbert's ketones, and (iii) benzyl carbocation formation.221,222
Structural changes resulting from organosolv pretreatment are well captured by NMR, XRD and FTIR analyses, demonstrating their effects on both the physical and chemical properties. For example, lignin extracted from rice husk using liquid 1,4-butanediol (70–90%, 200–220 °C, 1–3 h) revealed low molecular weight fragments (1939 g mol−1), with β-O-4 & β-5 carbon linkage identified as dominant structures, by 13C-NMR, highlighting the selective extraction of lignin using such methods.223 Similarly, ethanol-based organosolv pretreatment of coffee husk slightly increased the surface area (from 567 m2 g−1 to 568 m2 g−1) and pore volume (from 0.13 cm3 g−1 to 0.14 cm3 g−1).224
The major drawback of the organosolv pretreatment is its high flammability, volatility, and low boiling point, which limit its use in high-pressure applications. This necessitates the recovery and recycling of the process to reduce operational costs and prevent inhibitory effects on microbial fermentation.225 Additionally, residual lignin condensation is an undesirable side reaction that can lower delignification efficiency by repolymerising the dissolved lignin fragments onto the biomass surface.221
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively. Considerable changes in morphology were noticed in the form of wave-shaped, amorphous cellulose, porous and disorganised microfibrils, as opposed to the compact structures of the untreated rice husk when observed through a scanning electron microscope.228–230
5, respectively. Considerable changes in morphology were noticed in the form of wave-shaped, amorphous cellulose, porous and disorganised microfibrils, as opposed to the compact structures of the untreated rice husk when observed through a scanning electron microscope.228–230
4.3. Physicochemical pretreatment
This strategy combines factors like temperature or pressure with chemical processes to break down the lignocellulosic waste through approaches like steam explosion, CO2 explosion, and ammonia pretreatment.169 The use of steam or CO2 is a hybrid approach that affects both physical parameters (discussed in Section 3), chemical bonding (bond cleavage) and intermolecular interactions to fractionate the lignocellulose components effectively.121,231![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the aromatic rings of lignin are weakened.233 When the pressure is released and the hydrolysate is cooled down, the dissolved lignin and hemicellulose transition to the liquid phase while cellulose remains in the solid phase, thereby exposing surface area to enzymes and ultimately increasing the digestibility of cellulose.1 Additionally, acid is generated during the explosion through partial hydrolysis of cellulose, which then acts on hemicellulose to hydrolyse into smaller components,43 a process known as autohydrolysis.146 The effectiveness of steam explosion is influenced by moisture content, steam temperature, and substrate size, with high moisture content requiring longer treatment times.234–236 A pretreatment study on rice husks using a steam pressure of 2.5 MPa reported maximum lignin reduction from 24.48% to 15.65%, along with significant structural damage to lignin, affecting its barrier functionality.160 Another study reported an increase in cellulose and lignin content, but a reduction in hemicellulose content, from 38.81% to 2.6%, in rice husk treated at a steam temperature of 220 °C for 10 minutes.237 This study also observed significant changes in structure, where the smooth, lengthy, and ordered microfibril arrangement became rough, irregular, and distorted, with porous holes, due to the removal of hemicellulose, pectin, wax, and other impurities. This structural modification enhanced enzyme saccharification and improved sugar yield by 53.87%.237
C bonds in the aromatic rings of lignin are weakened.233 When the pressure is released and the hydrolysate is cooled down, the dissolved lignin and hemicellulose transition to the liquid phase while cellulose remains in the solid phase, thereby exposing surface area to enzymes and ultimately increasing the digestibility of cellulose.1 Additionally, acid is generated during the explosion through partial hydrolysis of cellulose, which then acts on hemicellulose to hydrolyse into smaller components,43 a process known as autohydrolysis.146 The effectiveness of steam explosion is influenced by moisture content, steam temperature, and substrate size, with high moisture content requiring longer treatment times.234–236 A pretreatment study on rice husks using a steam pressure of 2.5 MPa reported maximum lignin reduction from 24.48% to 15.65%, along with significant structural damage to lignin, affecting its barrier functionality.160 Another study reported an increase in cellulose and lignin content, but a reduction in hemicellulose content, from 38.81% to 2.6%, in rice husk treated at a steam temperature of 220 °C for 10 minutes.237 This study also observed significant changes in structure, where the smooth, lengthy, and ordered microfibril arrangement became rough, irregular, and distorted, with porous holes, due to the removal of hemicellulose, pectin, wax, and other impurities. This structural modification enhanced enzyme saccharification and improved sugar yield by 53.87%.237
Unlike the AFEX, the ARP method uses an aqueous ammonia solution (5–15%) to run through a packed biomass bed in a column reactor at elevated temperatures (150–180 °C).239 The aqueous ammonia solution flows through the biomass at a rate of 1–5 mL min−1 with a retention time of 10–90 min.241 This process hydrolyses lignin and hemicellulose, reducing the crystallinity of cellulose. The excess ammonia solution can be recovered and reused, and the resulting hydrolysates are free from fermentation inhibitors due to the mild process conditions (approximately 100 °C, pH below 12, and a short retention time of 10–90 min).243
Pretreatment with ammonia selectively removes lignin and hemicellulose, increasing the cellulose hydrolysis rate even at low enzyme loadings, compared to other pretreatment methods.155 The effectiveness of ammonia treatment is superior to dilute acid treatment due to its ability to achieve maximum sugar recovery at low enzyme loading, minimal sugar loss, and minimal formation of inhibitors.244 For example, the cellulose content was higher in rice husk samples treated with ammonia than in those treated with sodium hydroxide. This variation is attributed to the sodium hydroxide dissolving cellulose into β-cellulose and γ-cellulose, whereas the ammonia treatment preserves the cellulose integrity through selective lignin removal.63
4.4. Biological pretreatment
Structural modification of lignocellulose biomass through biological strategies relies on the enzymes produced by microorganisms (bacteria/fungi) to degrade lignin and hemicellulose, while offering the advantages of low operational cost and environmental friendliness.245 This method does not require high temperatures or pressure, does not generate any inhibitory products, and neutralisation after treatment is not required.246 The process, however, is time-consuming and requires a sterile environment.247 Various bacteria and fungi have been reported to be involved in the biological treatment of lignocellulose biomass. Bacteria such as Paenibacillus campinasensis, Thermomonospora fusca, Bacillus subtilis, and Azospirillum lipoferum can degrade lignocellulose biomass. Thermomonospora fusca and Cellulomonas fimi are cellulolytic bacteria capable of secreting cellulase enzymes predominantly.248 Likewise, Paenibacillus campinasensis can tolerate harsh environmental conditions and break down the lignocellulosic biomass.249 Cellulase enzymes produced by some anaerobic cellulolytic bacteria, such as Bacteroides cellulosolvens and Clostridium thermocellum, possess high activity, although they are secreted in smaller amounts.250 Few bacterial strains, like Bacillus subtilis and Azospirillum lipoferum, have been widely reported to produce bacterial laccases, which cause lignin depolymerisation.251 At least 30 predominant rumen cellulolytic bacterial species are known to be used in pretreatment through their own specific mechanisms of adhesion to cellulose and hydrolysis.206 However, the rate of hydrolysis by bacteria depends on the substrate type, incubation time, temperature, nutrients, aeration, pH, and type of bacterial strains used in delignification.252Fungal species like white rots (Pleurotus ostreatus, Trametes versicolor, Phanerochaete chrysosporium), brown rots (Gloeophyllum trabeum, Coniophora puteana) and soft rots (Trichoderma reesei) are the most commonly used microorganisms in the treatment of lignocellulose biomass owing to their ability to release lignin-degrading enzymes like lignin peroxidase, manganese peroxidase and laccase.246,252 Brown rots mainly attack cellulose, whereas white and soft rots attack cellulose and lignin.178 During the fungal treatment of lignocellulose biomass, mycelium penetration increases pore size and breaks down the bonds between lignin, hemicellulose, and cellulose, thus facilitating structural degradation.253 However, the rate of hydrolysis by fungi depends on the substrate type, incubation time, temperature, nutrients, aeration, pH, and the type of fungal strains used in delignification.252
Lignin degradation by fungi can occur through selective and non-selective mechanisms. Selective degradation involves the localised breakdown of lignin and hemicellulose with minimal degradation on the cellulose fraction, whereas non-selective degradation involves the simultaneous breakdown of all the lignocellulosic components.252 This mechanism is particularly characteristic of white-rot fungi, which first degrade lignin during their vegetative phase, followed by a productive phase in which polysaccharides are broken down.242,254,255 Non-selective degradation simultaneously breaks down all the lignocellulosic components.252
In a fungal pretreatment of rice husk, significant enzyme activity was observed in lignin-degrading enzymes, yielding 447.95 mg mL−1 g−1 of reducing sugars. The pretreatment of rice husk and sorghum husk with Phanerochaete chrysosporium not only yielded a reducing sugar content of 447.95 mg g−1 and 103 mg g−1, respectively, but also induced morphological changes like reduced crystallinity and increased pore size due to lignin removal.54,163 These changes in the surface morphology are due to the activity of ligninolytic enzymes produced by P. chrysosporium. Additionally, pretreatment with Pleurotus ostreatus has been reported to increase the enzymatic hydrolysis of rice straw through partial lignin degradation.256 Thus, the biological pretreatment process can bring extensive structural modification whilst improving the efficiency of enzymatic hydrolysis and increasing the yield of fermentable sugars.
Biological pretreatment also includes the use of commercial microbial enzymes to break down crystalline cellulose and hemicellulose into fermentable sugars, while minimising inhibitor formation and energy use.43,131 Commercial cellulase, hemicellulase and ligninase enzymes are commonly used to hydrolyse cellulose, hemicellulose, and lignin. Cellulase, like endoglucanase, exoglucanase and β-glucanase/β-glucosidase, adsorbs onto the cellulose surface and degrades it into simple sugars, whereas hemicellulose, like xylanase, acts on glycosidic linkages in xylan during saccharification, producing xylose.131,257,258 Ligninases, such as laccase peroxidases, serve as accessory enzymes in the effective hydrolysis of lignocellulosic biomass by removing the xylan layer, facilitating the dissociation of cellulose–hemicellulose, and disrupting the crystalline structure of cellulose.75,259,260
Although the primary focus has been on utilising cellulose through enzyme breakdown, current research is shifting towards the aligned use of hemicellulase enzymes to enhance theoretical yield and significantly improve process feasibility.261 A combination of enzymes is used for efficient breakdown, as these enzymes work synergistically to degrade the substrate, achieving higher performance than when acting individually. This phenomenon is called enzyme synergy.262 One study reported a significant synergistic effect of a cellulase–xylanase enzyme cocktail on dilute acid-pre-treated sorghum stover, yielding glucose and ethanol concentrations of 40.7% and 41.1%, respectively.263
The degree of synergy (DS) can be measured mathematically as:
A DS > 1 indicates synergistic action, while DS = 1 means enzymes degrade substrate independently, and a DS < 1 indicates enzymes are not synergistic but inhibit each other by competing for the same binding sites.264 However, a high DS does not always result in the maximum conversion yield. For example, the combination of enzymes laccase and ferulic acid esterase gave the highest DS of 88, but the lowest conversion yield of 17% in oat hulls. Conversely, the highest conversion yield of 57.2% was achieved in oat hulls when subjected to ferulic acid esterase + xylanase + arabinoxylanase with a DS of 1.52.265
5. Cereal husk applications, future opportunities and perspectives
The valorisation of cereal husk biomass extends beyond its pretreatment stage, with growing research focusing on sustainable strategies to repurpose agro-processing waste into renewable biofuels, value-added products through bioconversion, and eco-friendly solutions to minimise environmental pollution.266 Once cereal husks undergo pretreatment, the resulting modified biomass has different applications in the food industry. Post-treatment applications focus on utilising the structurally modified cellulose, hemicellulose, and lignin fractions to create safe, functional, and sustainable food ingredients and materials.123 These applications support the principles of a circular food economy by reducing waste, improving sustainability, and generating high-value products from underutilised by-products.One of the most direct applications of treated cereal husk is as a dietary fibre ingredient.267 The micronised cereal husks are suitable for incorporation into bakery products, extruded snacks, plant-based meat and functional beverages.268–271 The fibres are associated with health benefits, including cholesterol reduction, improved gut health, and blood sugar regulation.267 Cereal husk can also serve as a substrate for bioethanol production. Enzymatic saccharification of cellulose and hemicellulose releases fermentable sugars that can be converted into ethanol.272 Hydrolysed hemicellulose can also be used to produce prebiotic oligosaccharides, which selectively stimulate beneficial gut bacteria, such as Bifidobacterium and Lactobacillus, making them suitable for incorporation into functional foods.273 Additionally, hemicellulose can also be used to produce flavour enhancers, such as furfural, and sweeteners, including xylitol, as well as nutraceuticals and food packaging.274–276 Lignin also offers opportunities for upcycling due to its antioxidant, antibacterial, and UV-blocking properties.277,278
In addition to food ingredients, cereal husk has significant potential in the development of bioplastics and edible coatings, an environmentally friendly alternative to conventional plastics.21,279 These bioplastics, prepared by extracting cellulose fibres from cereal husks, are biodegradable, durable, and have improved tensile strength.12,280,281 Considering the wide availability, low cost, and underutilised nature of agro-processing residues, there is potential to add value to cereal husk waste through sustainable processing pathways.282 These innovations highlight the versatile applications of cereal husks, including the production of renewable biofuels, the development of functional foods, and environmentally friendly packaging. Such advancements reinforce the role of cereal husk valorisation in achieving a circular economy while reducing the environmental footprint associated with husk waste.
The diversity of pretreatment strategies discussed (Section 5) clearly demonstrates that no single method is effective across all biomass types. Moreover, the lignocellulose biomass derived from different plant sources greatly varies in composition and structure. Therefore, the success of down-streaming waste byproducts depends on aligning pretreatment with the dominant microstructural barriers of each biomass. For instance, lignin-rich biomass benefits from a delignification-focused pretreatment method such as alkali, while hemicellulose-rich biomass is best liberated through acid or steam explosion.
A biomass-specific structure-guided framework can significantly improve the efficiency of enzymatic hydrolysis and downstream conversion. This framework will provide a basis for pretreatment design, where combinations of methods will be selected to overcome the multiple barriers simultaneously. Such an approach not only enhances hydrolysis yields but also reduces the energy and chemical inputs, as well as the formation of inhibitors often associated with non-targeted pretreatments. Additionally, future studies could employ a meta-bibliographic analysis approach to systematically map emerging trends in pretreatment strategies, identify research clusters, and reveal knowledge gaps across the unexplored cereal husks.
6. Challenges and limitations
Commercially feasible utilisation of lignocellulose biomass requires addressing technical, operational and economic challenges. These include the high cost of pretreatment technologies, energy input, biomass composition and quality, the formation of inhibitory compounds, the environmental impact of the processing technique and the energy requirement.67 Additionally, the high cost of hydrolytic enzymes and the complexity of developing efficient enzyme cocktails for cellulose and hemicellulose hydrolysis further complicate the process.10,283As the lignocellulose biomass derived from different plant sources greatly varies in composition, standardising the pretreatment technique for each biomass type is difficult.284 Lignin recalcitrance also poses a major barrier to biomass conversion, as its rigid structure resists enzymatic degradation and necessitates higher energy and chemical inputs during pretreatment. Operational costs associated with energy-intensive equipment, expensive chemicals, and enzymes are a significant bottleneck in many industrial applications.67
During chemical pretreatment, undesirable by-products or derivatives, such as furans, hydroxymethylfurfural, organic acids, soluble sugars, and phenols, are released, negatively affecting microbial growth, substrate utilisation, fermentation, and impacting the environment.82,124,285,286 Due to their small size, these inhibitors can easily penetrate cell membranes, causing damage to internal structures, altering cell morphology and inhibiting RNA and protein synthesis. They are also reported to reduce sugar consumption by microbes, subsequently reducing the production of end products.124,287
During enzyme hydrolysis, accumulated sugars such as cellobiose and glucose can inhibit enzyme activity, resulting in feedback inhibition.288 During fermentation, substrate inhibition occurs due to high feedstock concentrations, resulting in low water activity, increased osmotic pressure and cell lysis. End-product inhibition occurs when high substrate concentration penetrates microbial cell membranes, disrupting their intracellular processes and leading to cell death.10
7. Conclusion
A large amount of organic waste is generated in the agro-industrial cereal processing cycle. The efficient valorisation of lignocellulosic components present in cereal biomass is pivotal in advancing sustainable biorefinery practices. However, structural recalcitrance of lignocellulose hinders the valorisation into high-value products. Pretreatment methods can effectively break down the recalcitrant structure of biomass, thereby enhancing further enzymatic hydrolysis and converting it into fermentable sugars and other high-value products.This review highlights the importance of pretreatment strategies in enhancing the hydrolysis efficiency of lignocellulose biomass by targeting microstructural barriers, including crystallinity, degree of polymerisation, porosity, surface area, and the lignin–hemicellulose ratio. A biomass-specific structure-guided framework can significantly improve the efficiency of enzymatic hydrolysis and downstream conversion.
However, challenges such as scalability, process integration, reducing energy consumption, processing cost and the environmental impact of certain pretreatments exist. Despite extensive research on developing numerous pretreatment techniques, only a few methods, like dilute acid, have been commercialised for bioconversion.
Future research should focus on developing synergistic pretreatment approaches to address the limitations of individual treatment methods. However, significant research specific to target raw materials is still required to establish and optimise these combined techniques for optimal efficiency. While numerous novel pretreatment methods have been proposed, scaling these processes from laboratory research to industrial applications remains a major challenge. Addressing this issue should be another key focus for future studies. Structural modification of lignocellulosic biomass holds significant potential for reducing waste and transforming it into valuable resources, supporting economic growth and the achievement of the United Nations Sustainable Development Goals.
Author contributions
Smriti Ale: writing – original draft, writing – reviewing & editing, conceptualization, visualization, Pramesh Dhungana: writing – reviewing & editing, conceptualization, supervision, validation. Janet Howieson: writing – reviewing & editing, supervision, validation, funding acquisition, Rewati Raman Bhattarai: writing – reviewing & editing, conceptualization, supervision, validation.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Acknowledgements
This work was funded by the End Food Waste Australia (EFWA), whose activities are funded by the Australian Government's Cooperative Research Centre Program.References
- K. Kucharska, P. Rybarczyk, I. Hołowacz, R. Łukajtis, M. Glinka and M. Kamiński, Molecules, 2018, 23, 2937 CrossRef PubMed.
- J. Kumla, N. Suwannarach, K. Sujarit, W. Penkhrue, P. Kakumyan, K. Jatuwong, S. Vadthanarat and S. Lumyong, Molecules, 2020, 25, 2811 CrossRef CAS PubMed.
- P. Singh, Agriculture Waste Management and Bioresource: the Circular Economy Perspective, John Wiley & Sons, Hoboken, New Jerseyedn, 2023 Search PubMed.
- A. C. Neto, M. J. O. C. Guimarães and E. Freire, J. Clean. Prod., 2018, 184, 168–178 CrossRef.
- S. K. Bhatia, S. S. Jagtap, A. A. Bedekar, R. K. Bhatia, A. K. Patel, D. Pant, J. Rajesh Banu, C. V. Rao, Y.-G. Kim and Y.-H. Yang, Bioresour. Technol., 2020, 300, 122724 CrossRef CAS.
- M. B. W. Saad and A. R. Gonçalves, Biomass Bioenergy, 2024, 190, 107426 CrossRef CAS.
- H. Pihkola, E. Hylkilä, E. Paronen, A. Markkula and H. Liirus, Int. J. Life Cycle Assess., 2024, 30, 1435–1450 CrossRef.
- D. P. William, DuPont's journey to build a global cellulosic biofuel business enterprise, https://www.energy.gov/sites/prod/files/2014/11/f19/provine_biomass_2014.pdf, accessed 13/02/2025.
- L. Jia, L. Zhao, B. Qin, F. Lu, D. Liu and F. Liu, Enzyme Microb. Technol., 2023, 171, 110319 CrossRef CAS PubMed.
- A. Ojo, Fermentation, 2023, 9, 990 CrossRef CAS.
- N. A. Sagar, M. Pathak, H. Sati, S. Agarwal and S. Pareek, Trends Food Sci. Technol., 2024, 147, 104413 CrossRef CAS.
- Z. Chen, P. Li, Q. Ji, Y. Xing, X. Ma and Y. Xia, Mater. Today Commun., 2023, 34, 105090 CrossRef CAS.
- G. Šelo, M. Planinić, M. Tišma, S. Tomas, D. Koceva Komlenić and A. Bucić-Kojić, Foods, 2021, 10, 927 CrossRef PubMed.
- A. C. Fărcaş, S. A. Socaci, S. A. Nemeş, L. C. Salanţă, M. S. Chiş, C. R. Pop, A. Borşa, Z. Diaconeasa and D. C. Vodnar, Foods, 2022, 11, 2454 CrossRef.
- M. J. Taherzadeh and K. Karimi, Int. J. Mol. Sci., 2008, 9, 1621–1651 CrossRef CAS.
- S. Babu, S. Singh Rathore, R. Singh, S. Kumar, V. K. Singh, S. K. Yadav, V. Yadav, R. Raj, D. Yadav, K. Shekhawat and O. Ali Wani, Bioresour. Technol., 2022, 360, 127566 CrossRef CAS PubMed.
- W. F. Lamb, T. Wiedmann, J. Pongratz, R. Andrew, M. Crippa, J. G. J. Olivier, D. Wiedenhofer, G. Mattioli, A. A. Khourdajie, J. House, S. Pachauri, M. Figueroa, Y. Saheb, R. Slade, K. Hubacek, L. Sun, S. K. Ribeiro, S. Khennas, S. de la Rue du Can, L. Chapungu, S. J. Davis, I. Bashmakov, H. Dai, S. Dhakal, X. Tan, Y. Geng, B. Gu and J. Minx, Environ. Res. Lett., 2021, 16, 73005 CrossRef CAS.
- S. O. Serna Saldivar, Cereal Grains: Properties, Processing, and Nutritional Attributes, CRC Press, Boca Raton, FL, 5th edn, 2016 Search PubMed.
- E. A. Decker, D. J. Rose and D. Stewart, Br. J. Nutr., 2014, 112, S58–S64 CrossRef CAS.
- A. Guimarães, A. C. Mota, A. S. Pereira, A. M. Fernandes, M. Lopes and I. Belo, Materials, 2024, 17, 935 CrossRef.
- S. A. Hassan, M. Abbas, W. Mujahid, W. Ahmed, S. Ahmad, A. A. Maan, A. Shehzad, Z. F. Bhat and R. M. Aadil, Trends Food Sci. Technol., 2023, 140, 104166 CrossRef CAS.
- A. L. Perruzza, ProQuest Dissertations & Theses, 2010 Search PubMed.
- A. N. Arzami, T. M. Ho and K. S. Mikkonen, Food Res. Int., 2022, 151, 110818 CrossRef CAS PubMed.
- N. Neitzel, M. Eder, R. Hosseinpourpia, T. Walther and S. Adamopoulos, Mater. Today Commun., 2023, 36, 106602 CrossRef CAS.
- R. W. Welch, M. V. Hayward and D. I. H. Jones, J. Sci. Food Agric., 1983, 34, 417–426 CrossRef.
- M. Mirjalili, M. B. Tabatabai and L. Karimi, Afr. J. Biotechnol., 2011, 10, 14478–14484 CrossRef CAS.
- M. F. Al Rawi, G. Y. Al Kindi, J. K. Al Refaae, T. A. Hussain and H. A. Al-Haidri, IOP Conf. Ser. Earth Environ. Sci., 2023, 1222, 12012 CrossRef.
- V. K. Gupta, R. Jain and S. Varshney, J. Hazard Mater., 2007, 142, 443–448 CrossRef CAS PubMed.
- E. P. Dagnino, E. R. Chamorro, S. D. Romano, F. E. Felissia and M. C. Area, Ind. Crops Prod., 2013, 42, 363–368 CrossRef CAS.
- N. Bisht, P. C. Gope and N. Rani, J. Mech. Behav. Mater., 2020, 29, 147–162 CrossRef.
- N. Johar, I. Ahmad and A. Dufresne, Ind. Crops Prod., 2012, 37, 93–99 CrossRef CAS.
- K. Wannaporn, P. Alisa, P. Suphat, U. a. Niramon, L. Thunnop, T. Pipat, W. Tri Indrarini and J. Pannapapol, Sci. Rep., 2022, 12 Search PubMed.
- P. Brites, M. I. S. Aguiar, J. Gonçalves, P. Ferreira and C. Nunes, Int. J. Biol. Macromol., 2024, 271, 132489 CrossRef CAS PubMed.
- S. M. L. Rosa, N. Rehman, M. I. G. de Miranda, S. M. B. Nachtigall and C. I. D. Bica, Carbohydr. Polym., 2012, 87, 1131–1138 CrossRef CAS.
- T. Korotkova, S. Ksandopulo, A. Donenko, S. Bushumov and A. Danilchenko, Orient. J. Chem., 2016, 32, 3213–3219 CrossRef CAS.
- M. D. Hazrol, S. M. Sapuan, R. A. Ilyas, E. S. Zainudin, M. Y. M. Zuhri and N. I. Abdul, Heliyon, 2023, 9, e15153 CrossRef CAS PubMed.
- S. S. Pattanayak, S. H. Laskar and S. Sahoo, J. Mater. Sci. Mater. Electron., 2022, 33, 5149–5160 CrossRef CAS.
- P. T. H. Anh and D. M. Tai, Chem. Eng. Technol., 2024, 47, e202300379 CrossRef CAS.
- T. H. Kim, F. Taylor and K. B. Hicks, Bioresour. Technol., 2008, 99, 5694–5702 CrossRef CAS PubMed.
- G. Garrote, J. M. Cruz, H. Domínguez and J. C. Parajó, J. Food Eng., 2008, 84, 544–552 CrossRef CAS.
- A. Bichot, J.-P. Delgenès, V. Méchin, H. Carrère, N. Bernet and D. García-Bernet, Rev. Environ. Sci. Biotechnol., 2018, 17, 707–748 CrossRef CAS.
- B. Volynets, F. Ein-Mozaffari and Y. Dahman, Green Process. Synth., 2017, 6, 1–22 CrossRef CAS.
- A. T. W. M. Hendriks and G. Zeeman, Bioresour. Technol., 2009, 100, 10–18 CrossRef CAS PubMed.
- A. Sirichalarmkul and S. Kaewpirom, J. Appl. Polym. Sci., 2021, 138, 50652 CrossRef CAS.
- X. Tan, Q. Peng, K. Yang, T. Yang, J. Saskova, J. Wiener, M. Venkataraman, J. Militky, W. Xiong and J. Xu, Alex. Eng. J., 2022, 61, 4529–4540 CrossRef.
- N. H. Embong, N. Hindryawati, P. Bhuyar, N. Govindan, M. H. A. Rahim and G. P. Maniam, Appl. Nanosci., 2023, 13, 2241–2249 CrossRef CAS.
- K. Shankar, N. S. Kulkarni, S. K. Jayalakshmi and K. Sreeramulu, Biomass Bioenergy, 2019, 127, 105298 CrossRef CAS.
- U. Habiba, S. Mutahir, M. A. Khan, M. Humayun, M. S. Refat and K. S. Munawar, Catalysts, 2022, 12, 1063 CrossRef CAS.
- Y. Dan, L. Xu, Z. Qiang, H. Dong and H. Shi, Chemosphere, 2021, 262, 127940 CrossRef CAS PubMed.
- P. M. Sanka, M. J. Rwiza and K. M. Mtei, Water, Air, Soil Pollut., 2020, 231 CrossRef CAS.
- E. Akhayere, A. Vaseashta and D. Kavaz, Sustainability, 2020, 12, 1–16 CrossRef.
- A. K. Prajapati, P. Verma, S. Singh, M. K. Mondal and M. R. R. Kooh, Adsorpt. Sci. Technol., 2022, 2022, 3956977 CrossRef.
- K. Dashora, M. Gattupalli, G. D. Tripathi, Z. Javed, S. Singh, M. Tuohy, P. K. Sarangi, D. Diwan, H. B. Singh and V. K. Gupta, Catalysts, 2023, 13, 149 CrossRef CAS.
- R. Potumarthi, R. R. Baadhe, P. Nayak and A. Jetty, Bioresour. Technol., 2013, 128, 113–117 CrossRef CAS PubMed.
- S. J. A. Van Kuijk, A. S. M. Sonnenberg, J. J. P. Baars, W. H. Hendriks and J. W. Cone, Biotechnol. Adv., 2015, 33, 191–202 CrossRef CAS PubMed.
- H. Wang, Y. Pu, A. Ragauskas and B. Yang, Bioresour. Technol., 2019, 271, 449–461 CrossRef CAS PubMed.
- Y. Wang, L. Leng, M. K. Islam, F. Liu, C. S. K. Lin and S. Y. Leu, Int. J. Mol. Sci., 2019, 20, 3354 CrossRef CAS PubMed.
- T. N. Ang, G. C. Ngoh and A. S. M. Chua, Bioresour. Technol., 2013, 135, 116–119 CrossRef CAS PubMed.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 55–583 RSC.
- G. De Bhowmick, A. K. Sarmah and R. Sen, Bioresour. Technol., 2018, 247, 1144–1154 CrossRef CAS PubMed.
- A. Woiciechowski, L. C. J. Dalmas Neto, L. Porto de Souza Vandenberghe, D. P. de Carvalho Neto, A. C. Novak Sydney, L. A. J. Letti, S. G. Karp, L. A. Zevallos Torres and C. R. Soccol, Bioresour. Technol., 2020, 304, 122848 CrossRef PubMed.
- I. Sumantri, Y. Yordianto, P. I. Ratnasari, H. Hadiyanto and S. Suherman, AIP Conf. Proc., 2023, 2667, 040007 CrossRef CAS.
- C. Bonechi, M. Consumi, A. Donati, G. Leone, A. Magnani, G. Tamasi and C. Rossi, Bioenergy Systems for the Future: Prospects for Biofuels and Biohydrogen, 2017, pp. 3–42, DOI:10.1016/B978-0-08-101031-0.00001-6.
- W. Schutyser, T. Renders, G. Van den Bossche, S. Van den Bosch, S. F. Koelewijn, T. Ennaert and B. F. Sels, in Catalysis in Lignocellulosic Biorefineries: The Case of Lignin Conversion, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germanyedn, 2017, pp. 537–584, DOI:10.1002/9783527699827.ch23.
- Y. Lu, Q. He, G. Fan, Q. Cheng and G. Song, Green Process. Synth., 2021, 10, 779–804 CrossRef CAS.
- M. Joshi and S. Manjare, Environ. Sci. Pollut. Res. Int., 2024, 31, 48928–48954 CrossRef CAS PubMed.
- R. Ravindran and A. K. Jaiswal, Bioresour. Technol., 2016, 199, 92–102 CrossRef CAS PubMed.
- M. J. Rosado, J. Rencoret, G. Marques, A. Gutiérrez and J. C. del Río, Front. Plant Sci., 2021, 12, 640475 CrossRef PubMed.
- N. H. A. Latif, N. Brosse, I. Ziegler-Devin, L. Chrusiel, R. Hashim and M. H. Hussin, Int. J. Biol. Macromol., 2023, 253, 127210 CrossRef CAS PubMed.
- J. H. Grabber, Crop Sci., 2005, 45, 820–831 CrossRef CAS.
- R. S. Abolore, S. Jaiswal and A. K. Jaiswal, Ind. Crop. Prod., 2025, 235, 121696 CrossRef CAS.
- R. Bhadane, L. Vahasalo, C. Xu and P. Eklund, Biomass Bioenergy, 2026, 205, 108490 CrossRef.
- X. Zhang, J. Zhu, L. Sun, Q. Yuan, G. Cheng and D. S. Argyropoulos, Ind. Crop. Prod., 2019, 133, 241–249 CrossRef CAS.
- Y.-J. Liu, B. Li, Y. Feng and Q. Cui, Biotechnol. Adv., 2020, 40, 107535–107514 CrossRef CAS PubMed.
- X. Zhao, L. Zhang and D. Liu, Biofuel Bioprod. Biorefining, 2012, 6, 465–482 CrossRef CAS.
- P. Alvira, E. Tomás-Pejó, M. Ballesteros and M. J. Negro, Bioresour. Technol., 2010, 101, 4851–4861 CrossRef CAS PubMed.
- R. B. Melati, F. L. Shimizu, G. Oliveira, F. C. Pagnocca, W. de Souza, C. Sant'Anna and M. Brienzo, Bioenergy Res., 2019, 12, 1–20 CrossRef CAS.
- M. Broda, C. M. Popescu, S. F. Curling, D. I. Timpu and G. A. Ormondroyd, Materials, 2022, 15, 2348 CrossRef CAS PubMed.
- B. Zhao, H. Al Rasheed, I. Ali and S. Hu, Bioresour. Technol., 2021, 319, 124115 CrossRef CAS PubMed.
- D. P. Maurya, A. Singla and S. Negi, 3 Biotech, 2015, 5, 597–609 CrossRef PubMed.
- D. Kim, Molecules, 2018, 23, 309 CrossRef PubMed.
- C. A. Mendes, F. A. Adnet, A. M. Leite, C. R. Furtado and A. M. Sousa, Cellul. Chem. Technol., 2014, 49, 727–735 Search PubMed.
- E. Ahmed, A. Zeitoun, G. Hamad, M. A. M. Zeitoun, A. Taha, S. A. Korma and T. Esatbeyoglu, Foods, 2022, 11, 3149 CrossRef CAS PubMed.
- H. S. Hafid, F. N. Omar, J. Zhu and M. Wakisaka, Carbohydr. Polym., 2021, 260, 117789 CrossRef CAS PubMed.
- S. K. Jang, H. Jeong and I. G. Choi, Sustainability, 2023, 15, 5869 CrossRef CAS.
- A. S. Nur Hanani, A. Zuliahani, W. I. Nawawi, N. Razif and A. R. Rozyanty, IOP Conf. Ser. Mater. Sci. Eng., 2017, 204, 12025 CrossRef.
- J. Zhang, Y. Wang, L. Zhang, R. Zhang, G. Liu and G. Cheng, Bioresour. Technol., 2014, 151, 402–405 CrossRef CAS PubMed.
- R. G. Huamani-Palomino, S. Mayta, B. M. Córdova, M. Yáñez-S, T. Venâncio, E. Rivera and M. Quintana, Carbohydr. Polym., 2024, 346, 122593 CrossRef CAS PubMed.
- Z. Ahmad, N. N. Roziaizan, R. Rahman, A. F. Mohamad, W. I. N. Wan Ismail, S. N. Rahmat, N. Abd Rahman, Z. Mohd Jaini, R. Yunus, Isolation and characterization of microcrystalline cellulose (MCC) from rice husk (RH), in MATEC Web of Conferences, EDP Sciences, 2016, vol. 47, p. 05013 Search PubMed.
- N. Samsalee, J. Meerasri and R. Sothornvit, Carbohydr. Polym. Technol. Appl., 2023, 6, 100353 CAS.
- I. Pasha, F. Ahmad and M. Usman, J. Food Biochem., 2021, 45, e13768 CrossRef CAS PubMed.
- S. Dehkhoda, M. Bagheri, M. Heydari and S. Rabieh, Int. J. Biol. Macromol., 2022, 212, 165–171 CrossRef CAS PubMed.
- G. B. Paschoal, C. M. O. Muller, G. M. Carvalho, C. A. Tischer and S. Mali, Quim. Nova, 2015, 38, 478–482 CAS.
- Z. Fang, B. Li, Y. Liu, J. Zhu, G. Li, G. Hou, J. Zhou and X. Qiu, Matter, 2020, 2, 1000–1014 CrossRef.
- K. Karimi and M. J. Taherzadeh, Bioresour. Technol., 2016, 203, 348–356 CrossRef CAS PubMed.
- B. Yang, Z. Dai, S.-Y. Ding and C. E. Wyman, Biofuels, 2011, 2, 421–449 CrossRef CAS.
- A. Zoghlami and G. Paës, Front. Chem., 2019, 7, 874 CrossRef CAS PubMed.
- Y. H. P. Zhang and L. R. Lynd, Biotechnol. Bioeng., 2004, 88, 797–824 CrossRef CAS PubMed.
- P. Basera, S. Chakraborty and N. Sharma, Discov. Sustain., 2024, 5, 311–316 CrossRef.
- A. O. Converse, H. Ooshima and D. S. Burns, Appl. Biochem. Biotechnol., 1990, 24–5, 67–73 CrossRef.
- A.-I. Yeh, Y.-C. Huang and S. H. Chen, Carbohydr. Polym., 2010, 79, 192–199 CrossRef CAS.
- R. P. Chandra, R. Bura, W. E. Mabee, A. Berlin, X. Pan and J. N. Saddler, Substrate Pretreatment: The Key to Effective Enzymatic Hydrolysis of Lignocellulosics?, in Biofuels edi. L. Olsson, Advances in Biochemical Engineering/Biotechnology, SpringerBerlin, Heidelberg, Germanyedn, 2007, vol. 108, pp. 67–93, DOI:10.1007/10_2007_064.
- R. Huang, R. Su, W. Qi and Z. He, Biotechnol. Prog., 2010, 26, 384–392 CrossRef CAS PubMed.
- M. Chang, T. Chou and G. Tsao, in Advances in Biochemical Engineering, 1981, vol. 20, pp. 15–42 Search PubMed.
- M. Herbaut, A. Zoghlami, A. Habrant, X. Falourd, L. Foucat, B. Chabbert and G. Paës, Biotechnol. Biofuels, 2018, 11, 52 CrossRef PubMed.
- P. McKendry, Bioresour. Technol., 2002, 83, 37–46 CrossRef CAS PubMed.
- T. Rodrigues Mota, D. Matias de Oliveira, R. Marchiosi, O. Ferrarese-Filho and W. Dantas dos Santos, AIMS Bioeng., 2018, 5, 63–77 Search PubMed.
- W. Jiang, H. Peng, H. Li and J. Xu, Biomass Bioenergy, 2014, 71, 294–298 CrossRef CAS.
- D. Tarasov, M. Leitch and P. Fatehi, Biotechnol. Biofuels, 2018, 11, 269 CrossRef PubMed.
- N. Giummarella, Y. Pu, A. J. Ragauskas and M. Lawoko, Green Chem., 2019, 21, 1573–1595 RSC.
- J. Kruyeniski, P. J. T. Ferreira, M. d. G. Videira Sousa Carvalho, M. E. Vallejos, F. E. Felissia and M. C. Area, Ind. Crops Prod., 2019, 130, 528–536 CrossRef CAS.
- Biofuels, ed. L. Olsson, Springer Berlin, Heidelberg, Germanyedn, 2007, vol. 108, pp. 67–93 Search PubMed.
- A. R. Esteghlalian, V. Srivastava, N. Gilkes, D. J. Gregg and J. N. Saddler, in American Chemical Society, Washington, DCedn, 2000, DOI:10.1021/bk-2001-0769.ch006.
- V. Pihlajaniemi, M. H. Sipponen, H. Liimatainen, J. A. Sirviö, A. Nyyssölä and S. Laakso, Green Chem., 2016, 18, 1295–1135 RSC.
- A. Nidhi, R. Bhatnagar and S. S. Yazdani, Biomass for Bioenergy and Biomaterials, CRC Press, Boca Raton, Florida, 2022 Search PubMed.
- L. Petridis and J. C. Smith, Nat. Rev. Chem., 2018, 2, 382–389 CrossRef CAS.
- N. S. Ab Rasid, A. Shamjuddin, A. Z. Abdul Rahman and N. A. S. Amin, J. Clean. Prod., 2021, 321, 129038 CrossRef CAS.
- S. Liu and G. Cheng, Ind. Crops Prod., 2024, 208, 117926 CrossRef CAS.
- R. Kumar, T. H. Kim, B. Basak, S. M. Patil, H. H. Kim, Y. Ahn, K. K. Yadav, M. M. S. Cabral-Pinto and B.-H. Jeon, J. Clean. Prod., 2022, 333, 130180 CrossRef CAS.
- B. Basak, R. Kumar, A. V. S. L. Sai Bharadwaj, T. H. Kim, J. R. Kim, M. Jang, S.-E. Oh, H.-S. Roh and B.-H. Jeon, Bioresour. Technol., 2023, 369, 128413 CrossRef CAS PubMed.
- Z. Wu, K. Peng, Y. Zhang, M. Wang, C. Yong, L. Chen, P. Qu, H. Huang, E. Sun and M. Pan, Mater. Today Bio, 2022, 16, 100445 CrossRef CAS PubMed.
- M. Mujtaba, L. Fernandes Fraceto, M. Fazeli, S. Mukherjee, S. M. Savassa, G. Araujo de Medeiros, A. do Espírito Santo Pereira, S. D. Mancini, J. Lipponen and F. Vilaplana, J. Clean. Prod., 2023, 402, 136815 CrossRef CAS.
- L. J. Jonsson and C. Martin, Bioresour. Technol., 2016, 199, 103–112 CrossRef PubMed.
- M. S. Singhvi, S. Chaudhari and D. V. Gokhale, RSC Adv., 2014, 4, 8271–8277 RSC.
- G. J. M. Rocha, A. R. Gonçalves, S. C. Nakanishi, V. M. Nascimento and V. F. N. Silva, Ind. Crops Prod., 2015, 74, 810–816 CrossRef CAS.
- T. A. L. Silva, H. D. Z. Zamora, L. H. R. Varão, N. S. Prado, M. A. Baffi and D. Pasquini, Waste Biomass Valoriz., 2018, 9, 2191–2201 CrossRef CAS.
- S. Sun, S. Sun, X. Cao and R. Sun, Bioresour. Technol., 2016, 199, 49–58 CrossRef CAS PubMed.
- M. Zeng, N. S. Mosier, C.-P. Huang, D. M. Sherman and M. R. Ladisch, Biotechnol. Bioeng., 2007, 97, 265–278 CrossRef CAS PubMed.
- J. A. Müller, Chem. Eng. Technol., 2003, 26, 207–217 CrossRef.
- P. Kumar, D. M. Barrett, M. J. Delwiche and P. Stroeve, Ind. Eng. Chem. Res., 2009, 48, 3713–3729 CrossRef CAS.
- M. Lu, J. Li, L. Han and W. Xiao, Bioresour. Technol., 2019, 273, 1–7 CrossRef CAS PubMed.
- W.-H. Chen, Y.-Y. Xu, W.-S. Hwang and J.-B. Wang, Bioresour. Technol., 2011, 102, 10451–10458 CrossRef CAS PubMed.
- B. Lamsal, J. Yoo, K. Brijwani and S. Alavi, Biomass Bioenergy, 2010, 34, 1703–1710 CrossRef CAS.
- C. Karunanithy and K. Muthukumarappan, Biochem. Eng. J., 2011, 54, 71–82 CrossRef CAS.
- L. Wang, J. Fan, L. Gan, X. Zeng, L. Lin and J. Liu, Biomass Bioenergy, 2024, 188, 107343 CrossRef CAS.
- S. Haghighi Mood, A. Hossein Golfeshan, M. Tabatabaei, G. Salehi Jouzani, G. H. Najafi, M. Gholami and M. Ardjmand, Renewable Sustainable Energy Rev., 2013, 27, 77–93 CrossRef CAS.
- D. Mikulski and G. Kłosowski, Biotechnol. Biofuels Bioprod., 2023, 16, 19 CrossRef CAS PubMed.
- H. Li, Y. Qu, Y. Yang, S. Chang and J. Xu, Bioresour. Technol., 2016, 199, 34–41 CrossRef CAS PubMed.
- X. Wang, G. Fang, C. Hu and T. Du, J. Appl. Polym. Sci., 2008, 109, 2762–2767 CrossRef CAS.
- A. Carreira-Casais, P. Otero, P. Garcia-Perez, P. Garcia-Oliveira, A. G. Pereira, M. Carpena, A. Soria-Lopez, J. Simal-Gandara and M. A. Prieto, Int. Res. J. Publ. Environ. Health, 2021, 18, 9153 CrossRef CAS PubMed.
- N. Y. A. Prempeh, X. Nunekpeku, A. Murugesan and H. Li, Foods, 2025, 14, 2057 CrossRef CAS PubMed.
- S. Gea, R. Bulan, E. Zaidar, A. F. Piliang, N. Oktari, S. Rahayu, Y. A. Hutapea and R. M. Sari, J. Wood Chem. Technol., 2023, 43, 117–128 CrossRef CAS.
- W. Shi, J. Jia, Y. Gao and Y. Zhao, Bioresour. Technol., 2013, 146, 355–362 CrossRef CAS PubMed.
- F. Demirel, M. Germec, H. B. Coban and I. Turhan, Cellulose, 2018, 25, 6377–6393 CrossRef CAS.
- J. Singh, M. Suhag and A. Dhaka, Carbohydr. Polym., 2015, 117, 624–631 CrossRef CAS PubMed.
- A. T. Hoang, S. Nizetic, H. C. Ong, C. T. Chong, A. E. Atabani and V. V. Pham, J. Environ. Manag., 2021, 296, 113194 CrossRef CAS PubMed.
- B. C. a. Saha, L. B. Iten, M. A. Cotta and Y. V. Wu, Biotechnol. Prog., 2005, 21, 816–822 CrossRef CAS PubMed.
- T.-C. Hsu, G.-L. Guo, W.-H. Chen and W.-S. Hwang, Bioresour. Technol., 2010, 101, 4907–4913 CrossRef CAS PubMed.
- M. Shahabazuddin, T. Sarat Chandra, S. Meena, R. K. Sukumaran, N. P. Shetty and S. N. Mudliar, Bioresour. Technol., 2018, 263, 199–206 CrossRef CAS PubMed.
- C. Wan, Y. Zhou and Y. Li, Bioresour. Technol., 2011, 102, 6254–6259 CrossRef CAS PubMed.
- H. Wu, X. Dai, S.-L. Zhou, Y.-Y. Gan, Z.-Y. Xiong, Y.-H. Qin, J. Ma, L. Yang, Z.-K. Wu, T.-L. Wang, W.-G. Wang and C.-W. Wang, Bioresour. Technol., 2017, 241, 70–74 CrossRef CAS PubMed.
- P. Kaur, H. B. Bohidar, F. M. Pfeffer, R. Williams and R. Agrawal, Cellulose, 2023, 30, 4247–4261 CrossRef CAS.
- P. F. Vidal and J. Molinier, Biomass, 1988, 16, 1–17 CrossRef CAS.
- J. Baruah, B. K. Nath, R. Sharma, S. Kumar, R. C. Deka, D. C. Baruah and E. Kalita, Front. Energy Res., 2018, 6, 141 CrossRef.
- K. Zhang, Z. Pei and D. Wang, Bioresour. Technol., 2016, 199, 21–33 CrossRef CAS PubMed.
- T. H. Kim, H. J. Ryu and K. K. Oh, Energies, 2019, 12, 1800 CrossRef CAS.
- X. Yu, J. Tian, H. Xie, H. Shen and Q. Wang, Bioresour. Technol., 2014, 153, 403–407 CrossRef CAS PubMed.
- Q. Li, Y.-C. He, M. Xian, G. Jun, X. Xu, J.-M. Yang and L.-Z. Li, Bioresour. Technol., 2009, 100, 3570–3575 CrossRef CAS PubMed.
- F. Yan, S. Tian, K. Du and X. Wang, Bioresources, 2021, 16, 6910–6920 CAS.
- A. Shukla, D. Kumar, M. Girdhar, A. Sharma, A. Mohan and B. Qi, Int. J. Energy Res., 2023, 2023, 1–13 CrossRef.
- N. Narayanaswamy, A. Faik, D. J. Goetz and T. Gu, Bioresour. Technol., 2011, 102, 6995–7000 CrossRef CAS PubMed.
- P. R. Waghmare, R. V. Khandare, B. H. Jeon and S. P. Govindwar, Biofuel Res. J., 2018, 5, 846–853 CrossRef CAS.
- C. E. Wyman, B. E. Dale, R. T. Elander, M. Holtzapple, M. R. Ladisch and Y. Y. Lee, Bioresour. Technol., 2005, 96, 1959–1966 CrossRef CAS PubMed.
- S. Chuetor, A. Barakat, X. Rouau and T. Ruiz, Powder Technol., 2017, 310, 74–79 CrossRef CAS.
- Y. W. Sitotaw, N. G. Habtu, A. Y. Gebreyohannes, S. P. Nunes and T. Van Gerven, Biomass Convers. Biorefinery, 2023, 13, 15593–15616 CrossRef CAS.
- S. Jin and H. Chen, Biochem. Eng. J., 2006, 30, 225–230 CrossRef CAS.
- R. Zhang and Z. Zhang, Bioresour. Technol., 1999, 68, 235–245 CrossRef CAS.
- M. Taylor, H. Alabdrabalameer and V. Skoulou, Sustainability, 2019, 11, 3604 CrossRef CAS.
- A. Hideno, H. Inoue, K. Tsukahara, S. Fujimoto, T. Minowa, S. Inoue, T. Endo and S. Sawayama, Bioresour. Technol., 2009, 100, 2706–2711 CrossRef CAS PubMed.
- M. R. Zakaria, S. Fujimoto, S. Hirata and M. A. Hassan, Appl. Biochem. Biotechnol., 2014, 173, 1778–1789 CrossRef CAS PubMed.
- B.-J. Gu, J. Wang, M. P. Wolcott and G. M. Ganjyal, Bioresour. Technol., 2018, 251, 93–98 CrossRef CAS PubMed.
- K. Rajendran, E. Drielak, V. Sudarshan Varma, S. Muthusamy and G. Kumar, Biomass Convers. Biorefinery, 2018, 8, 471–483 CrossRef CAS.
- Z. Wang, X. He, L. Yan, J. Wang, X. Hu, Q. Sun and H. Zhang, Ind. Crops Prod., 2020, 143, 111960 CrossRef CAS.
- J. Yoo, S. Alavi, P. Vadlani and V. Amanor-Boadu, Bioresour. Technol., 2011, 102, 7583–7590 CrossRef CAS PubMed.
- B. Kumar, N. Bhardwaj, K. Agrawal, V. Chaturvedi and P. Verma, Fuel Process. Technol., 2020, 199, 106244 CrossRef CAS.
- S. I. Mussatto, Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery, Elsevier, Amsterdam, Netherlandsedn, 2016 Search PubMed.
- S. Roy, Pretreatment Methods of Lignocellulosic Biomass for Biofuel Production, ROUTLEDGE, S.l, 1st edn, 2021 Search PubMed.
- J. Azuma, F. Tanaka and T. Koshijima, J. Ferment. Technol., 1984, 62, 377–384 CAS.
- H. Ma, W.-W. Liu, X. Chen, Y.-J. Wu and Z.-L. Yu, Bioresour. Technol., 2009, 100, 1279–1284 CrossRef CAS PubMed.
- S. Zhu, Y. Wu, Z. Yu, C. Wang, F. Yu, S. Jin, Y. Ding, R. a. Chi, J. Liao and Y. Zhang, Biosyst. Eng., 2006, 93, 279–283 CrossRef.
- A. Richel and N. Jacquet, Biomass Convers. Biorefinery, 2015, 5, 115–124 CAS.
- J. Cheng, H. Su, J. Zhou, W. Song and K. Cen, Int. J. Hydrogen Energy, 2011, 36, 2093–2101 CrossRef CAS.
- M. Ishfaq Bhat, N. C. Shahi, U. C. Lohani, S. Singh, Q. Sidique and R. Sirohi, Bioresour. Technol., 2022, 351, 127029 CrossRef CAS PubMed.
- H. Chandel, P. Kumar, A. K. Chandel and M. L. Verma, Biomass Convers. Biorefinery, 2024, 14, 2959–2981 CrossRef PubMed.
- A. K. Kumar and S. Sharma, Bioresour. Bioprocess., 2017, 4, 7 CrossRef PubMed.
- J. Yu, J. Zhang, J. He, Z. Liu and Z. Yu, Bioresour. Technol., 2009, 100, 903–908 CrossRef CAS PubMed.
- P. F. Zhang, Z. J. Pei, D. H. Wang, X. R. Wu, W. L. Cong, M. Zhang and T. Deines, J. Manuf. Sci. Eng., 2011, 133, 011012 CrossRef.
- R. Ravindran, S. Jaiswal, N. Abu-Ghannam and A. K. Jaiswal, Bioresour. Technol., 2017, 224, 680–687 CrossRef CAS PubMed.
- P. B. Subhedar, P. Ray and P. R. Gogate, Ultrason. Sonochem., 2018, 40, 140–150 CrossRef CAS PubMed.
- B. Kumari, B. K. Tiwari, M. B. Hossain, N. P. Brunton and D. K. Rai, Food Bioprocess Technol., 2018, 11, 223–241 CrossRef CAS.
- Y.-Z. Wang, L. Zhang, T. Xu and K. Ding, Ind. Crops Prod., 2017, 109, 404–409 CrossRef CAS.
- P. Kumar, D. M. Barrett, M. J. Delwiche and P. Stroeve, Ind. Eng. Chem. Res., 2011, 50, 10996–11001 CrossRef CAS.
- D. Fengel and G. Wegener, Wood: Chemistry, Ultrastructure, Reactions, Walter de Gruyter, Berlin, 1989, Reprint 2011 Search PubMed.
- R. A. Silverstein, Y. Chen, R. R. Sharma-Shivappa, M. D. Boyette and J. Osborne, Bioresour. Technol., 2007, 98, 3000–3011 CrossRef CAS PubMed.
- L. Zhao, Z.-F. Sun, C.-C. Zhang, J. Nan, N.-Q. Ren, D.-J. Lee and C. Chen, Bioresour. Technol., 2022, 343, 126123 CrossRef CAS PubMed.
- J. du Pasquier, G. Paës and P. Perré, Bioresour. Technol., 2023, 369, 128439 CrossRef CAS PubMed.
- J. P. Lancha, P. Perré, J. Colin, P. Lv, N. Ruscassier and G. Almeida, Sci. Rep., 2021, 11, 8444 CrossRef CAS PubMed.
- Z. Zhou, D. Liu and X. Zhao, Renewable Sustainable Energy Rev., 2021, 146, 111169 CrossRef CAS.
- A. R. Mankar, A. Pandey, A. Modak and K. K. Pant, Bioresour. Technol., 2021, 334, 125235 CrossRef CAS PubMed.
- J.-H. Park, H.-C. Cheon, J.-J. Yoon, H.-D. Park and S.-H. Kim, Int. J. Hydrogen Energy, 2013, 38, 6130–6136 CrossRef CAS.
- R. R. Gonzales, P. Sivagurunathan and S.-H. Kim, Int. J. Hydrogen Energy, 2016, 41, 21678–21684 CrossRef CAS.
- E. Ruiz, C. Cara, P. Manzanares, M. Ballesteros and E. Castro, Enzyme Microb. Technol., 2008, 42, 160–166 CrossRef CAS PubMed.
- P. Sassner, C. G. Mårtensson, M. Galbe and G. Zacchi, Bioresour. Technol., 2008, 99, 137–145 CrossRef CAS PubMed.
- A. N. Vu, L. H. Nguyen, H.-C. V. Tran, K. Yoshimura, T. D. Tran, H. Van Le and N.-U. T. Nguyen, RSC Adv., 2024, 14, 248–226 Search PubMed.
- B. Barati, F. F. Zafar, P. F. Rupani and S. Wang, Environ. Technol. Innovat., 2021, 21, 101362 CrossRef CAS.
- G. Brodeur, E. Yau, K. Badal, J. Collier, K. B. Ramachandran and S. Ramakrishnan, Enzym. Res., 2011, 2011, 787532–787517 Search PubMed.
- S. McIntosh and T. Vancov, Bioresour. Technol., 2010, 101, 6718–6727 CrossRef CAS PubMed.
- V. K. Ponnusamy, D. D. Nguyen, J. Dharmaraja, S. Shobana, J. R. Banu, R. G. Saratale, S. W. Chang and G. Kumar, Bioresour. Technol., 2019, 271, 462–472 CrossRef CAS PubMed.
- N. Bleyen, V. Van Gompel, S. Eyley, D. Durce, G. Verpoucke, W. Thielemans and E. Valcke, Cellulose, 2025, 32, 4363–4385 CrossRef CAS.
- M. Gaspar, G. Kalman and K. Reczey, Process Biochem., 2007, 42, 1135–1139 CrossRef CAS.
- Z. Benkő, A. Andersson, Z. Szengyel, M. Gáspár, K. Réczey and H. Stålbrand, Appl. Biochem. Biotechnol., 2007, 137–140, 253–265 CrossRef PubMed.
- J. X. Sun, X. F. Sun, R. C. Sun and Y. Q. Su, Carbohydr. Polym., 2004, 56, 195–204 CrossRef CAS.
- R. C. Sun, J. Tomkinson, Y. X. Wang and B. Xiao, Polymer, 2000, 41, 2647–2656 CrossRef CAS.
- E. Menya, P. W. Olupot, H. Storz, M. Lubwama, Y. Kiros and M. J. John, J. Therm. Anal. Calorim., 2020, 139, 1681–1691 CrossRef CAS.
- Y. Sun and J. Cheng, Bioresour. Technol., 2002, 83, 1–11 CrossRef CAS PubMed.
- S. C. Rabelo, P. Y. S. Nakasu, E. Scopel, M. F. Araújo, L. H. Cardoso and A. C. D. Costa, Bioresour. Technol., 2023, 369, 128331 CrossRef CAS PubMed.
- L. Mesa, E. González, C. Cara, M. González, E. Castro and S. I. Mussatto, Chem. Eng. J., 2011, 168, 1157–1162 CrossRef CAS.
- B.-W. Koo, H.-Y. Kim, N. Park, S.-M. Lee, H. Yeo and I.-G. Choi, Biomass Bioenergy, 2011, 35, 1833–1840 CrossRef CAS.
- R. El Hage, N. Brosse, P. Sannigrahi and A. Ragauskas, Polym. Degrad. Stabil., 2010, 95, 997–1003 CrossRef CAS.
- L. G. Nair, K. Agrawal and P. Verma, Bioresour. Bioprocess., 2023, 10, 50–29 CrossRef PubMed.
- D. Wei Kit Chin, S. Lim, Y. L. Pang and M. K. Lam, Biofuel Bioprod. Biorefining, 2020, 14, 808–829 CrossRef CAS.
- Y.-P. Chen and X.-S. Cheng, J. For. Res., 2008, 19, 159–163 CrossRef CAS.
- G. M. Tessera, N. G. Habtu and M. K. Abera, J. Nat. Fibers, 2023, 20 CAS.
- F. Sun and H. Chen, Bioresour. Technol., 2008, 99, 5474–5479 CrossRef CAS PubMed.
- D. J. Hayes, Catal. Today, 2009, 145, 138–151 CrossRef CAS.
- S. H. Lee, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2009, 102, 1368–1376 CrossRef CAS PubMed.
- P. D. Bohn, C. G. Anchieta, K. R. Kuhn, E. I. Muller, F. D. Mayer and R. C. Kuhn, Clean Technol. Environ. Policy, 2022, 24, 2117–2128 CrossRef CAS.
- Y. Wang, J.-Y. Liu, J. Sun, S. Shangdiar, K. T. T. Amesho, Y.-C. Lin, Y.-P. Peng and K.-L. Chang, Environ. Sci. Pollut. Res. Int., 2021, 28, 40715–40723 CrossRef CAS PubMed.
- T. N. Ang, G. C. Ngoh, A. S. M. Chua and M. G. Lee, Biotechnol. Biofuels, 2012, 5, 67 CrossRef CAS PubMed.
- W. Zhao, R. Yang, Y. Zhang and L. Wu, Green Chem., 2012, 14, 3352–3336 RSC.
- I. Alawad and H. Ibrahim, Biomass Convers. Biorefinery, 2024, 14, 6155–6183 CrossRef CAS.
- D. Trache, A. Donnot, K. Khimeche, R. Benelmir and N. Brosse, Carbohydr. Polym., 2014, 104, 223–230 CrossRef CAS PubMed.
- H. H. Brownell, E. K. C. Yu and J. N. Saddler, Biotechnol. Bioeng., 1986, 28, 792–801 CrossRef CAS PubMed.
- N. Jacquet, G. Maniet, C. Vanderghem, F. Delvigne and A. Richel, Ind. Eng. Chem. Res., 2015, 54, 2593–2598 CrossRef CAS.
- H. Rabemanolontsoa and S. Saka, Bioresour. Technol., 2016, 199, 83–91 CrossRef CAS PubMed.
- M. O. Kazeem, U. K. Md Shah, A. S. Baharuddin and N. A. A. Rahman, Bioresources, 2017, 12, 6207–6236 CrossRef CAS.
- V. B. Agbor, N. Cicek, R. Sparling, A. Berlin and D. B. Levin, Biotechnol. Adv., 2011, 29, 675–685 CrossRef CAS PubMed.
- L. Capolupo and V. Faraco, Appl. Microbiol. Biotechnol., 2016, 100, 9451–9467 CrossRef CAS PubMed.
- S. M. M. Islam, Q. Li, A. A. Loman and L.-K. Ju, Enzyme Microb. Technol., 2017, 106, 18–27 CrossRef CAS PubMed.
- N. Mosier, C. Wyman, B. Dale, R. Elander, Y. Y. Lee, M. Holtzapple and M. Ladisch, Bioresour. Technol., 2005, 96, 673–686 CrossRef CAS PubMed.
- E. Shirkavand, S. Baroutian, D. J. Gapes and B. R. Young, Renewable Sustainable Energy Rev., 2016, 54, 217–234 CrossRef CAS.
- F. Teymouri, L. Laureano-Perez, H. Alizadeh and B. E. Dale, Bioresour. Technol., 2005, 96, 2014–2018 CrossRef CAS PubMed.
- A. K. Mathew, B. Parameshwaran, R. K. Sukumaran and A. Pandey, Bioresour. Technol., 2016, 199, 13–20 CrossRef CAS PubMed.
- R. Sankaran, R. A. Parra Cruz, H. Pakalapati, P. L. Show, T. C. Ling, W.-H. Chen and Y. Tao, Bioresour. Technol., 2020, 298, 122476 CrossRef CAS PubMed.
- M. Andlar, T. Rezić, N. Marđetko, D. Kracher, R. Ludwig and B. Šantek, Eng. Life Sci., 2018, 18, 768–778 CrossRef CAS PubMed.
- V. Chaturvedi and P. Verma, 3 Biotech, 2013, 3, 415–431 CrossRef PubMed.
- N. C. Joshi, S. Sinha, P. Bhatnagar, Y. Nath, B. Negi, V. Kumar and P. Gururani, Curr. Res. Microb. Sci., 2024, 6, 100237 CAS.
- J. Miron, D. Ben-Ghedalia and M. Morrison, J. Dairy Sci., 2001, 84, 1294–1309 CrossRef CAS PubMed.
- B. S. Dien, M. A. Cotta and T. W. Jeffries, Appl. Microbiol. Biotechnol., 2003, 63, 258–266 CrossRef CAS PubMed.
- L. Bandounas, N. J. Wierckx, J. H. de Winde and H. J. Ruijssenaars, BMC Biotechnol., 2011, 11, 94 CrossRef CAS PubMed.
- J. Kainthola, A. Podder, M. Fechner and R. Goel, Bioresour. Technol., 2021, 321, 124397 CrossRef CAS PubMed.
- M. Tisma, M. Planinic, A. Bucic-Kojic, M. Panjicko, G. D. Zupancic and B. Zelic, Bioresour. Technol., 2018, 253, 220–226 CrossRef CAS PubMed.
- F. Kempken, in The Mycota, Springer Berlin, Heidelberg, Germanyedn, 2002, vol. 11 Search PubMed.
- J. M. Palmer and C. S. Evans, Philos. Trans. R. Soc., B, 1983, 300, 293–303 CAS.
- M. Taniguchi, H. Suzuki, D. Watanabe, K. Sakai, K. Hoshino and T. Tanaka, J. Biosci. Bioeng., 2005, 100, 637–643 CrossRef CAS PubMed.
- Q. K. Beg, M. Kapoor, L. Mahajan and G. S. Hoondal, Appl. Microbiol. Biotechnol., 2001, 56, 326–338 CrossRef CAS PubMed.
- F. Zadrazil, A. K. Puniya and K. Singh, in Biological upgrading of feed and feed components, Wiley-VCH Verlag GmbH, Weinheim, Germanyedn, 1995, pp. 55–70, DOI:10.1002/9783527615353.ch4.
- J. Hu, R. Chandra, V. Arantes, K. Gourlay, J. Susan van Dyk and J. N. Saddler, Bioresour. Technol., 2015, 186, 149–153 CrossRef CAS PubMed.
- M. Adsul, S. K. Sandhu, R. R. Singhania, R. Gupta, S. K. Puri and A. Mathur, Enzyme Microb. Technol., 2020, 133, 109442 CrossRef CAS PubMed.
- S. T. Merino and J. Cherry, Progress and Challenges in Enzyme Development for Biomass Utilization, in Biofuels, ed. L. Olsson, Advances in Biochemical Engineering/Biotechnology, Springer Berlin Heidelberg, Berlin, Heidelberg, 2007, vol. 108, pp. 95–120, DOI:10.1007/10_2007_066.
- S. Malgas, M. Thoresen, J. S. van Dyk and B. I. Pletschke, Enzyme Microb. Technol., 2017, 103, 1–11 CrossRef CAS PubMed.
- A. K. Pandey, G. Edgard and S. Negi, Renewable Energy, 2016, 98, 51–56 CrossRef CAS.
- J. S. Van Dyk and B. I. Pletschke, Biotechnol. Adv., 2012, 30, 1458–1480 CrossRef CAS PubMed.
- E. Schmitz, S. Leontakianakou, S. Norlander, E. Nordberg Karlsson and P. Adlercreutz, Bioresour. Technol., 2022, 343, 126114 CrossRef CAS PubMed.
- M. Bilal, M. Z. Nawaz, H. M. N. Iqbal, J. Hou, S. Mahboob, A. K. Ghanim and C. Hairong, Protein Pept. Lett., 2018, 25, 108–119 CrossRef CAS PubMed.
- D. Dziki, W. Tarasiuk and U. Gawlik-Dziki, Materials, 2021, 14, 5443 CrossRef CAS PubMed.
- E. C. Pichler, R. Schönlechner, R. Różyło, D. Dziki and M. Świeca, J. Cereal. Sci., 2024, 118, 103981 CrossRef CAS.
- R. Różyło, R. Schönlechner, E. C. Pichler, D. Dziki, A. Matwijczuk, B. Biernacka and M. Świeca, Food Chem., 2023, 428, 136782 CrossRef PubMed.
- B. Biernacka, D. Dziki, R. Różyło, U. Gawlik-Dziki, R. Nowak and W. Pietrzak, Molecules, 2023, 28, 7197 CrossRef CAS PubMed.
- D. Dziki, K. Lisiecka, U. Gawlik-Dziki, R. Różyło, A. Krajewska and G. Cacak-Pietrzak, Appl. Sci., 2022, 12, 12512 CrossRef CAS.
- J. Wu, A. Elliston, G. Le Gall, I. J. Colquhoun, S. R. A. Collins, I. P. Wood, J. Dicks, I. N. Roberts and K. W. Waldron, Biotechnol. Biofuels, 2018, 11, 62 CrossRef PubMed.
- U. K. Jana and N. Kango, Int. J. Biol. Macromol., 2020, 149, 931–940 CrossRef CAS PubMed.
- L. Jiang, A. Zheng, Z. Zhao, F. He, H. Li and W. Liu, Bioresour. Technol., 2015, 182, 364–367 CrossRef CAS PubMed.
- Y. Luo, Z. Li, X. Li, X. Liu, J. Fan, J. H. Clark and C. Hu, Catal. Today, 2019, 319, 14–24 CrossRef CAS.
- G. Liao, E. Sun, E. B. G. Kana, H. Huang, I. A. Sanusi, P. Qu, H. Jin, J. Liu and L. Shuai, Carbohydr. Polym., 2024, 341, 122351 CrossRef CAS PubMed.
- O. A. T. Dias, D. R. Negrão, D. F. C. Gonçalves, I. Cesarino and A. L. Leão, Mol. Cryst. Liq. Cryst., 2017, 655, 204–223 CrossRef CAS.
- P. Karagoz, S. Khiawjan, M. P. C. Marques, S. Santzouk, T. D. H. Bugg and G. J. Lye, Biomass Convers. Biorefinery, 2024, 14, 26553–26574 CrossRef CAS PubMed.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, Ind. Crop. Prod., 2019, 139, 111526 CrossRef CAS.
- A. E. Karaca, C. Özel, A. C. Özarslan and S. Yücel, Polym. Compos., 2022, 43, 6838–6853 CrossRef CAS.
- D. Datta, S. Samanta and G. Halder, Polym. Test., 2019, 77, 105878 CrossRef.
- P. M. B. Fernandes, A. A. R. Fernandes, R. S. de Biasi, T. Carneiro, L. Favarato, C. Turbay, M. Bolivar-Telleria, A. M. C. Santos and Z. Lei, BioMed Res. Int., 2018, 2018, 1–20 Search PubMed.
- B. Singh, J. Korstad, A. Guldhe and R. Kothari, Front. Energy Res., 2022, 10 Search PubMed.
- J. Wang, D. Ma, Y. Lou, J. Ma and D. Xing, Sci. Total Environ., 2023, 905, 166992 CrossRef CAS PubMed.
- P. T. Adeboye, M. Bettiga, F. Aldaeus, P. T. Larsson and L. Olsson, Microb. Cell Factories, 2015, 14, 149 CrossRef PubMed.
- H. B. Klinke, A. B. Thomsen and B. K. Ahring, Appl. Microbiol. Biotechnol., 2004, 66, 10–26 CrossRef CAS PubMed.
- L. J. Jönsson, B. Alriksson and N.-O. Nilvebrant, Biotechnol. Biofuels, 2013, 6, 16 CrossRef PubMed.
- A. O. Ojo and D. S. Olga, Processes, 2023, 11, 688 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |