 Open Access Article
Open Access ArticleAdvancing biosecurity: progress and prospects of volatile organic compound (VOC) detection for invasive pest and pathogen surveillance
Farjana
Haque
a,
Fatema Zerin
Farhana
a,
Milkiyas Toru
Tantu
a,
Md Akeruzzaman
Shaon
 a,
Geoff M.
Gurr
*b and
Muhammad J. A.
Shiddiky
a,
Geoff M.
Gurr
*b and
Muhammad J. A.
Shiddiky
 *a
*a
aRural Health Research Institute, Charles Sturt University, Orange, NSW 2800, Australia. E-mail: mshiddiky@csu.edu.au
bGulbali Institute, School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Orange, NSW 2800, Australia. E-mail: ggurr@csu.edu.au
First published on 4th November 2025
Abstract
Biosecurity protects the health of people, animals, plants, and ecosystems by preventing the introduction and spread of invasive species that threaten agriculture, biodiversity, and the economy. With increasing global trade and travel, the movement of pests and pathogens has intensified, causing major agricultural losses estimated at more than one trillion dollars between 1970 and 2017. Australia has established one of the world's most effective biosecurity systems, built on pre-border, border, and post-border measures and supported by national frameworks such as the Emergency Plant Pest Response Deed and PLANTPLAN. However, the continuing rise in invasive species highlights the need for portable and rapid diagnostic tools that can be applied consistently across regions. This review examines the historical, ecological, and economic impacts of invasive species, focusing on Australia's biosecurity practices and the detection of the brown marmorated stink bug (Halyomorpha halys). Conventional laboratory methods such as DNA-based assays, PCR, and ELISA remain reliable but are slow and costly. In contrast, new strategies that detect volatile organic compounds (VOCs) emitted by pests and infested plants show promise for on-site surveillance. These VOCs act as chemical fingerprints that can be captured and analyzed using techniques such as headspace sampling, solid-phase microextraction, and electrochemical sensing. The review evaluates the benefits and limitations of VOC-based detection and highlights opportunities for improving early pest diagnostics in agriculture and biosecurity through further development of these technologies.
Sustainability spotlightThis review advances sustainable food systems by exploring novel, chemistry-driven solutions for early detection of invasive pests like the brown marmorated stink bug. By leveraging volatile organic compound (VOC) emissions for non-invasive, real-time detection, the technologies discussed, such as electronic noses and portable electrochemical sensors, offer scalable, low-energy, and low-cost alternatives to conventional methods. This work directly supports the UN Sustainable Development Goals (SDGs), particularly SDG 2 (Zero Hunger), SDG 12 (Responsible Consumption and Production), and SDG 15 (Life on Land), by promoting resilient agriculture, minimizing food loss, and safeguarding biodiversity. It aligns with the journal's mission by integrating chemical ecology with sustainable biosecurity strategies that reduce environmental impact while enhancing global food security and agricultural productivity. |
1. Introduction
Pests and pathogens pose significant threats to global food security, causing substantial economic and agricultural losses. The economic impact of invasive species exceeds $423 billion annually, with this burden having quadrupled every decade since 1970.1 In Australia, biosecurity breaches caused by invasive pests and pathogens disrupt agriculture, forestry, and biodiversity, endangering food production and export markets. For instance, the eradication of the Asian papaya fruit fly (Bactrocera dorsalis) in Queensland cost AUD 33.5 million, while trade bans linked to the outbreak resulted in an additional AUD 100 million in losses.2,3 Globally, invasive species contribute significantly to biodiversity loss, accounting for 60% of plant and animal extinctions. According to the Food and Agriculture Organization (FAO), yield losses in key crops such as wheat, maize, and rice, exacerbated by invasive pathogens, contributed to rising global food insecurity in 2022, and are currently affecting over 2.4 billion people.4,5Volatile organic compounds (VOCs) emitted by living organisms serve as chemical fingerprints and present a promising avenue for detecting pests and pathogens. These compounds function as semiochemical signals or defense responses under biotic and abiotic stress.6,7 Specific VOCs released by infected plants or herbivorous insects, such as tridecane and E-2-decenal,can indicate infestation. The brown marmorated stink bug (BMSB, Halyomorpha halys), an invasive pest affecting over 300 plant species, exemplifies this potential. This hitchhiking pest emits distinctive VOCs that may facilitate its early detection during freight inspections, helping to prevent incursions into pest-free regions like Australia and New Zealand.7,8 Current VOC detection methods for H. halys include gas chromatography-mass spectrometry (GC-MS)8 and trained odor-detecting biosecurity dogs.9 While GC-MS offers high sensitivity and specificity, its size and cost make it impractical for field deployment.10 Canine detection, first employed in the 1970s to identify gypsy moth (Lymantria dispar), remains effective but is labor-intensive and dependent on trained personnel and dogs.9 Furthermore, Morrison III et al. (2017) suggested that pheromone-based traps can be used to monitor H. halys incursions,11 though these tools often lack the capacity for real-time, high-throughput data collection necessary for large-scale monitoring. These limitations underscore the need for portable, rapid, and cost-effective technologies to enhance pest surveillance and management.
Early detection and prompt response to invasive pests like H. halys are vital for minimizing agricultural losses and maintaining economic stability. Delays in detection can lead to uncontrolled pest spread, reduced eradication potential, increased management costs, biodiversity damage, and disruptions to food production and trade.7 Strengthening biosecurity systems through the integration of advanced technologies for on-site pest identification can lower quarantine costs, support sustainable agriculture, and promote global trade stability.12 Australia's proactive biosecurity framework, including robust pre-border and post-border surveillance, highlights the importance of integrating innovative technologies into existing protocols.13
Previous studies on major agricultural pests, such as Scirpophaga incertulas (yellow stem borer), have demonstrated the potential of VOC-based strategies for mitigating crop losses.14 Similarly, the economic impact of VOC emissions from H. halys, including documented declines in wine quality, illustrates the broader implications of pest-emitted volatiles on agricultural production.15 Research has also revealed that plants emit specific VOCs in response to pest invasion, offering novel avenues for early pest detection.16 The development of airborne semiochemical sensing technologies for real-time and sustainable monitoring has further advanced this field.17 Concurrently, recent reviews have explored the utility of electronic nose (e-nose) technologies in profiling insect semiochemicals, including their structural classification and detection performance.18 In this context, we provide a critical overview of key VOCs emitted by H. halys, focusing on their potential as unique chemical fingerprints for species-specific detection. We also synthesize current progress in VOC detection platforms relevant to biosecurity and sustainable agricultural systems. Despite encouraging developments, the application of VOC-based technologies for in-field pest and pathogen management remains at an early stage, requiring further technological refinement and wider field validation.
This narrative review presents a detailed analysis of the historical background and economic impact of invasive species, with a focus on the role of VOCs in biosecurity. It critically evaluates VOC-based detection technologies using H. halys as a case study of current international biosecurity concern, highlighting their capabilities, strengths, and limitations. Finally, it outlines a future perspective on how rapid, portable VOC detection tools could revolutionize pest monitoring and management. By enabling real-time, on-site identification, these technologies hold significant promise for transforming pest control strategies, strengthening biosecurity systems, and enhancing food security resilience in the face of increasing global challenges.
1.1 Literature search strategy and selection criteria
This review is based on a structured literature search conducted across Web of Science, PubMed, Scopus, and Google Scholar to identify studies related to biosecurity, BMSB, and VOCs. The search covered all years up to September 2025. The following keywords and their combinations were used: “biosecurity,” “brown marmorated stink bug,” “pest,” “volatile organic compounds” “VOC,” “BMSB VOC,” “VOC isolation techniques,” and “VOC detection techniques.” Only full-length, peer-reviewed journal articles published in English were included. Studies were selected if they met one or more of the following criteria: (1) described or evaluated biosecurity practices; (2) examined BMSB detection or VOCs released by BMSB; (3) reported current pest detection methods or VOC-based detection strategies; or (4) described approaches for VOC collection and identification.2. Biosecurity: an interdisciplinary approach to protecting health and ecosystems
Biosecurity is a critical interdisciplinary framework that addresses the interconnected health of humans, animals, plants, and ecosystems. Its primary aim is to mitigate the adverse impacts of invasive alien species on agriculture, human health, and the environment.19 This framework requires a multifaceted approach, ranging from routine domestic monitoring to the deployment of advanced surveillance technologies. With globalization accelerating, biosecurity has gained increasing importance due to the growing movement of goods, people, and biological materials across borders.12 The rising frequency of invasive species introductions underscores the urgent need for robust systems that can effectively prevent and manage these incursions. Since 1980, biosecurity started to encompass a broad range of applications, including pest management, zoonotic disease control, and environmental conservation.20 The FAO defines biosecurity as a holistic concept that integrates food security, zoonoses, plant and animal health, freshwater and marine ecosystems, and biodiversity protection.21 This expanded scope reflects the increasing risks posed by invasive species and the pressing need for innovative strategies to address them.2.1 The burden of biosecurity threats: pests and pathogens in agriculture
The global agricultural sector faces significant challenges from pests and pathogens, which threaten food security by affecting crops of all types, including major staples such as wheat, rice, maize, potatoes, and soybeans. These five crops alone account for over 50% of the world's food supply, yet their productivity is continually undermined by invasive species. A global survey revealed alarming yield losses: rice suffered a 30% reduction, while other major crops experienced losses ranging from 17% to 23%.4 These figures highlight the persistent shortcomings of global crop protection systems in managing pests and disease-causing pathogens. The economic impact of invasive species is equally severe. According to InvaCost, a public database that tracks the economic costs of biological invasions, the total reported costs from 1970 to 2017 reached approximately US $1.288 trillion, with annual average costs estimated at $26.8 billion.22 These expenditures continue to rise due to the growing global movement of people and goods. Invertebrates have proven more financially burdensome than vertebrates, with insect invasions alone costing nearly $76 billion annually.22 Beyond monetary losses, invasive species degrade infrastructure, disrupt ecosystems, and hinder international trade, underscoring their broad and complex threats to society.2.2 Historical perspectives on pest and pathogen invasions
The historical spread of pests and pathogens offers valuable insights into the global dynamics of biological threats. For instance, the mid-19th century Irish Potato Famine, caused by Phytophthora infestans, originated from the Americas and led to widespread starvation and migration in Europe.23 Likewise, the late 19th-century invasion of European vineyards by the grape phylloxera insect (Daktulsphaira vitifoliae), brought from North America, devastated the wine industry.24 More recently, the 2016 outbreak of wheat blast in Bangladesh, caused by the fungus Magnaporthe oryzae, demonstrated the ongoing emergence of invasive pathogens with catastrophic outcomes. Genetic analyses traced this outbreak to strains originating in South America, illustrating the transcontinental nature of modern biosecurity threats.25 Other significant examples include the spread of Venturia inaequalis, the fungus responsible for apple scab, from Central Asia to Europe,26 the introduction of cassava mosaic virus disease from Africa to Southeast Asia,25 and the global distribution of the diamondback moth (Plutella xylostella), which originated in South America.27 These historical events highlight the enduring challenge of managing invasive species across borders and reinforce the need for comprehensive biosecurity frameworks to prevent future crises.2.3 Australia's role in global biosecurity
As an island nation with unique biodiversity and a substantial agricultural sector, Australia has implemented some of the most stringent biosecurity measures globally. These efforts are vital for protecting the country's economy, environment, and public health from invasive pests and diseases. Australia's biosecurity system operates across a continuum of activities, including pre-border, border, and post-border measures, designed to reduce the risk of exotic species introduction, establishment, and spread.28 The Department of Agriculture, Water, and the Environment (DAWE) leads national biosecurity initiatives, ensuring compliance with international phytosanitary standards and managing surveillance systems that help maintain pest-free status.29 Australia's biosecurity success is further supported by collaborative frameworks such as the Emergency Plant Pest Response Deed (EPPRD) and the national response plan, PLANTPLAN.30 Similarly, in the European Union, Regulation 2016/2031 (the “Plant Health Regulation”) sets out early measures to prevent the spread of plant pests across member states.31 The European Food Safety Authority develops pest risk assessment methods to evaluate possible threats and provide scientific advice on plant and food safety. The EU also supports projects such as PURPEST (Plant Pest Prevention through Technology-guided Monitoring and Site-specific Control), which is developing sensor platforms that detect pest invasions using pest-specific VOCs.32 In the United States, the National Plant Diagnostic Network (NPDN) and the Animal and Plant Health Inspection Service (USDA-APHIS) play similar roles by applying a range of surveillance techniques to identify and manage invasive species.33 Together, these examples show how regions adapt their biosecurity systems to local needs while increasingly adopting portable, technology-based tools to detect and control emerging pest and pathogen threats.2.4 The future of biosecurity
The ongoing battle against invasive pests and pathogens demands constant innovation and international collaboration.34 Future biosecurity efforts must harness emerging technologies, such as remote sensing, portable diagnostic tools, and artificial intelligence, to enhance detection, monitoring, and response capabilities. In parallel, biosecurity policies must evolve to address the growing complexity of global trade and the environmental changes that create new pathways for biological invasions.35 The effectiveness of future biosecurity systems will depend on integrating scientific research, cross-border cooperation, and heightened public engagement. By confronting the economic, ecological, and social impacts of invasive species, biosecurity can play a central role in building a more resilient and sustainable global food system.36 Australia's leadership in this arena offers a compelling model for other nations, demonstrating the value of proactive strategies and coordinated action in protecting ecosystems and livelihoods from biological threats.3. Brown marmorated stink bugs (BMSB): a global invasive threat
H. halys, is an invasive piercing-sucking pest native to East Asia, including Japan, the Republic of Korea, and China.37 Over the past few decades, it has spread extensively to the United States, Canada, Europe, Russia, and parts of South America.38–40 It is now emerging as a serious biosecurity threat in Africa, southern Asia, and Oceania, including Australia and New Zealand.41,42 Its success as an invader is largely attributed to its polyphagous feeding habits, allowing it to consume more than 300 species of agricultural and ornamental plants.11,43,44 This broad host range leads to significant damage to a variety of crops such as hazelnuts, peaches, apples, vegetables, small fruits, soybeans, and corn. In addition to agricultural harm, BMSBs pose a nuisance in residential areas.45,46 This stink bug is also known vector of pathogenic bacteria and yeasts, including Eremothecium coryli, which causes fruit rot, and phytoplasmas associated with Paulownia witches' broom disease.47 Moreover, H. halys exhibits high adaptability, facilitated by its capacity to travel long distances via imported goods such as personal belongings, machinery, and aircraft.48,49 This adaptability and mobility amplify its potential to invade new regions and complicate containment efforts. The development and reproductive cycles of H. halys are strongly influenced by temperature and photoperiod.50 In temperate regions, the bug completes one to two generations per year. As winter approaches, adults aggregate in human-made structures to overwinter, entering diapause, a dormant state that can be disrupted by favorable environmental conditions. This climatic adaptability highlights H. halys' potential to establish in a wide range of environments, further complicating its management.513.1 Climate change and geographic expansion
Like many biosecurity-relevant species, H. halys is significantly affected by climate change, which alters its distribution and population dynamics. Climate projections suggest that rising temperatures and extended growing seasons will facilitate its establishment in new regions.50 Areas at heightened risk include parts of eastern South America, central and southern Africa, Australia's eastern coast, and New Zealand's North Island—regions that offer climates conducive to the pest's survival and reproduction.42 The combination of climate suitability and a broad host range emphasizes the urgent need for proactive strategies to limit the spread and impact of H. halys. Anticipating its potential distribution under changing environmental conditions is crucial for effective biosecurity planning.3.2 Defensive volatiles: chemical ecology of H. halys
H. halys emits a complex blend of VOCs from specialized abdominal glands. These VOCs, —including tridecane, (E)-2-decenal, 4-oxo-(E)-2-hexenal, among others (Table 1), function as defensive secretions to deter predators and protect the insect from environmental stress. They are also released during diapause aggregation.52 Tridecane, the most abundant compound during diapause and a key component of the pheromone blend, is considered a promising marker for detecting infestations in international cargo.53 GC-MS has been employed to identify these volatiles, providing valuable insights into the species' chemical ecology.52,53 Notably, trained detection dogs have demonstrated the ability to identify these VOCs, suggesting their utility in biosecurity surveillance applications.9 Laboratory studies show that adult male stink bugs increase their production of tridecane and (E)-2-decenal during evening and nighttime hours, while nymphs emit consistently higher levels of (E)-2-decenal compared to adults.54 These dynamic emission patterns warrant further investigation due to their potential implications for developing reliable detection systems. A deeper understanding of the behavioral and ecological roles of these volatiles is essential for designing innovative and sustainable strategies to manage H. halys infestations.| VOC name | VOC detection source | Behavioral role |
|---|---|---|
| Tridecane8,15,53–55 | Egg masses, first instar nymphs, exuviae of first instar nymphs, second instar, exuviae of second instar, adult phase | (a) Emitted primarily during adult diapause aggregation and in response to threats (defensive mechanism) |
| (E)-2-Decenal8,15,53,54 | (b) Behavioral role unknown for earlier developmental stages | |
| 4-Oxo-(E)-2-hexenal53,56 | Exuviae of first instar nymphs, adult phase | (a) In adults: Defensive secretions |
| (b) Behavioral role unknown in earlier stages | ||
| Dodecane8,53 | Adult phase | Defensive secretions |
| 2(5H)-furanone, 5-ethyl52 | Adult phase | Defensive secretions |
| (E)-(E)-2-Decenyl acetate, Undecane; Tetradecane; Pentadecane; Decane52,56 | Adult phase | Defensive secretions |
| 3-Hexen-1-ol, octanal, 2-nonanone, 3,5-dimethyldodecane, 1-nonanol52 | Adult phase | Defensive secretions |
| Hexadecanal, octadecanal, eicosanal57 | Egg phase | Behavioral role unknown |
| Z-2-Decenal, E-4-decenal56 | Adult phase | Behavioral role unknown |
| 2-Undecenal56 | Exuvia of first instar nymphs | Behavioral role unknown |
| Hexanal, heptanal, 6-methyl-5-hepten-2-one, acetophenone, decanal, E,Z-2,4-decadienal, hexadecane, 2,6,10-trimethyl-pentadecane56 | Egg masses, first instar nymphs, exuviae of first instar nymphs, second instar, exuviae of second instar, adult phase | Behavioral role is unknown across all stages except adults |
3.3 Implications for biosecurity
The ongoing spread of H. halys highlights the urgent need for advanced biosecurity detection methods. Given its capacity to exploit multiple transport pathways, the identification of VOCs associated with diapause and stress responses presents a promising approach for early detection and surveillance.8 As H. halys continues to invade new regions, collaborative research becomes increasingly important to understand its complex interactions with host plants and environmental variables. By harnessing recent advances in chemical ecology, molecular biology, and predictive modeling, researchers and policymakers can work collaboratively to develop sustainable strategies to manage this invasive pest and mitigate the spread of the pathogens it may carry or exacerbate.4. Current pest detection methods in post-border biosecurity
Biosecurity surveillance can be broadly categorised into three primary activities: border surveillance, post-border surveillance, and containment.58,59 Border surveillance aims to prevent the introduction of non-native species into the importing country. In contrast, post-border surveillance involves the ongoing monitoring of alien or invasive species that have already entered a region. Containment focuses on controlling these species once they have been identified.58 These surveillance activities can be further divided into generic and specific approaches. Generic surveillance targets a broad range of potential invasive species, whereas specific surveillance is tailored to detect particular species of concern. Common methods used in generic biosecurity surveillance include visual inspections,60 remote sensing,61 aerial surveillance,12 genetic tools,62,63 and acoustic detection.64 Specific surveillance methods often incorporate technologies such as trained sniffer dogs9 and electronic noses (e-noses).65,66Numerous research efforts are currently underway to develop sensors and robotic systems for detecting pests in bulk carrier holds. However, none have yet matched the effectiveness of trained detection dogs. Detector dogs have been a vital component of Australia's biosecurity system since 1992 and are routinely deployed at international terminals, mail centers, and cargo routes. While highly effective, the use of trained dogs is relatively costly, involving significant investment in staff and dog recruitment, training, support, and operations. In addition, traditional laboratory-based techniques such as PCR,67 flow cytometry,68 immunofluorescence,69 ELISA, western blotting, immunostrip assays,70 dot-blot immune-binding assays, and GC-MS71 have been widely applied for pest and pathogen detection. Although these methods are known for their accuracy, they are often time-consuming, laboratory-dependent, require skilled technicians, and are expensive, highlighting the growing demand for faster, portable, and cost-effective alternatives.
To detect and mitigate the spread of H. halys, the DAWE, Australia, along with the Department of Agriculture and Fisheries, has implemented pheromone traps, light traps, and sticky traps in designated surveillance zones. However, pheromone traps are less effective at capturing diapausing individuals and newly emerged overwintering adults.72,73 A study conducted in Japan reported that wood-layered slit traps aided in the recovery of H. halys,74 although similar outcomes were not observed elsewhere in Asia.37 Furthermore, pheromone traps typically attract only adult males, and light traps, while useful, are seasonally effective and must remain stationary for extended periods, thereby limiting their portability. In addition, these trapping systems are associated with high shipping costs and complex manufacturing processes, posing challenges for large-scale deployment.75 In light of these limitations, recent advancements in volatile organic compound (VOC)-based methodologies offer promising opportunities to enhance pest detection systems, particularly for applications targeting H. halys.
4.1 VOC-based detection systems
VOCs are a diverse group of organic molecules characterised by their high vapour pressure at room temperature. These compounds, typically comprising carbon chains ranging from C2 to C20, are produced by both biogenic sources (e.g., plants, insects, microorganisms) and anthropogenic activities.76 In biological systems, VOCs serve essential functions in defence, communication, and ecological interactions. They span a wide range of chemical classes, including hydrocarbons, alcohols, aldehydes, organic acids, and terpenes.77 Due to their taxon-specific nature, VOCs have garnered significant interest as potential biomarkers for the detection of pests and diseases. A well-known example of VOC-based detection in agriculture is the monitoring of ethylene—a plant-emitted VOC involved in initiating fruit ripening.70 In forestry, VOC profiling has proven effective in distinguishing between healthy and diseased plants. For instance, the VOC emissions from birch trees have been shown to vary depending on pathogen exposure, while VOC analysis has successfully differentiated pathogenic fungi in horse chestnut trees Likewise, specific VOCs have been identified from asymptomatic trees78,79 infected with the pathogenic fungus Ceratocystis platani, demonstrating the potential of VOC-based methods for early and accurate disease detection.80,81GC-MS is widely regarded as the gold standard for profiling VOCs,82 as it enables gas-phase separation and detection, providing detailed structural and chemical information about individual compounds within complex VOC mixtures. Alternative mass spectrometry-based techniques, such as PTR (PTR-MS)83 and selected ion flow tube mass spectrometry (SIFT-MS),84 offer near real-time analysis and quantification of VOCs. However, these systems are typically large, complex, and lack the durability required for direct, in-field applications. Their potential for field deployment hinges on substantial miniaturisation and the development of rugged, portable housings equipped with adequate support systems. Currently, VOCs are collected using various sampling methods, with the samples subsequently transported to laboratories for chemical analysis and characterisation. Despite their promise, VOC-based detection systems for pest identification, particularly for the BMSB, remain underdeveloped. Further research is essential to optimise VOC sampling and analysis techniques and to unlock the full potential of these systems in operational biosecurity environments.
4.2 Techniques for VOC collection
The collection of volatile organic compounds (VOCs) is a critical initial step in characterizing their properties and harnessing their potential for pest detection. A variety of VOC sampling techniques have been developed, including solid-phase microextraction (SPME), headspace sampling (Fig. 1A), purge-and-trap (P&T), thermal desorption, and supercritical fluid extraction (SFE).88 These methods are specifically designed to capture the complex mixture of VOCs emitted by biological systems, while minimising contamination and preserving the integrity of the volatile compounds.89 Each technique offers distinct advantages and limitations (Table 2), making the choice of method highly dependent on the specific context and objectives of the analysis.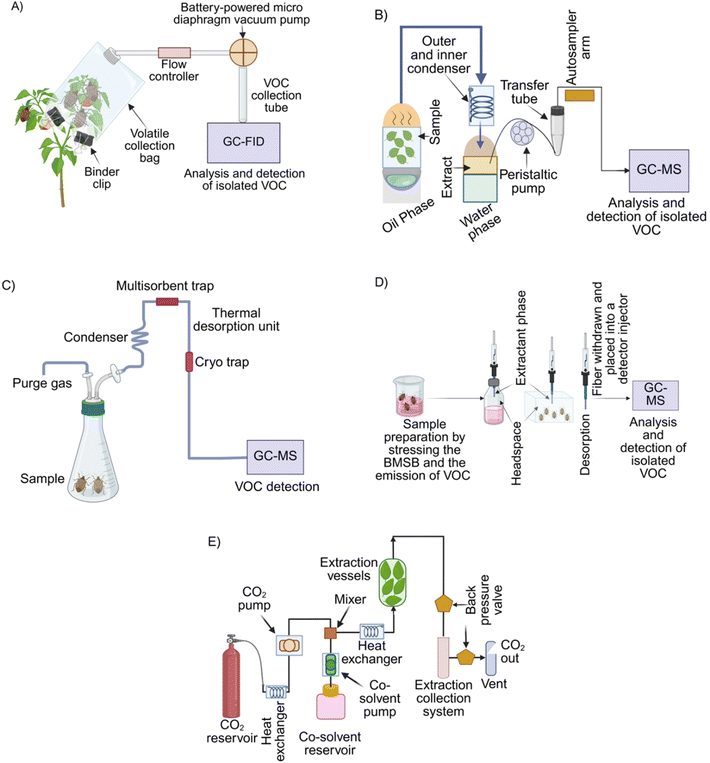 | ||
| Fig. 1 Schematic overview of VOC collection and analysis methods related to H. halys and agricultural matrices. (A) VOCs collected from materials infested with H. halys using a non-absorbent Vac-Pak volatile collection bag. The bag is sealed around the plant stem with binder clips, and VOCs are extracted via a glass tube connected to a vacuum pump and flow controller. Subsequently, the collected VOC was analyzed by GC-MS integrated with a flame ionization detector (FID).85 (B) Conventional SDE system for isolating VOCs from complex biological matrices.86 (C) VOC extraction from H. halys through purge-and-trap sampling, involving gradual asphyxiation and targeted gas purging over a controlled time period, and assessed with GC-MS.52 (D) Detection of VOCs emitted by H. halys using headspace solid-phase microextraction (HS-SPME) coupled with a multidimensional GC-MS detection system.15 (E) On-line supercritical fluid extraction of VOCs directly from agricultural samples.87 Illustrations were Created in BioRender. Haque, F. (2025) https://www.BioRender.com/patnvcs. | ||
| Technique name | Working principle | Advantages | Disadvantages |
|---|---|---|---|
| Steam distillation106 | Steam is passed through the sample to volatilize compounds, which are then condensed and collected | Effective for large-scale extraction of essential oils and thermally stable compounds | May promote hydrolysis and thermal degradation; low recovery of all VOCs; artifacts from antifoam agents; solvent-intensive; not ideal for small sample volumes |
| Simultaneous distillation extraction (SDE)95,106–109 | Combines steam distillation and solvent extraction in a single step using specialized apparatus (e.g., Likens–Nickerson) | High extraction efficiency; suitable for complex matrices; minimizes analyte loss when coupled directly with detection | Time-consuming; thermal degradation of unstable volatiles (e.g., alkenes, esters); complex setup; less suitable for low molecular weight VOCs and small samples |
| Purge-and-trap (P&T) or dynamic headspace extraction110,111 | VOCs are swept from the sample by an inert gas and adsorbed onto a trap material for subsequent analysis | High efficiency for aqueous samples; good for trace-level detection; minimal sample loss; easily coupled with GC-MS | Requires costly instrumentation; high operational costs; not ideal for small sample volumes |
| Headspace solid-phase microextraction (HS-SPME)101,107,112,113 | Analytes partition between sample headspace and a coated fiber; no solvents are used | Solvent-free; environmentally friendly; sensitive and rapid; applicable to solids, liquids, and gases; amenable to automation; minimal sample volume required | Requires method optimization for each matrix; fiber degradation over time; not suitable for semi-volatile or non-volatile analytes; accurate calibration needs internal standards |
| Supercritical fluid extraction (SFE)114,115 | VOCs are extracted using supercritical CO2 or other fluids at specific temperatures and pressures | Highly selective and pure extracts; reduced solvent use; efficient for non-polar compounds | High equipment and operational costs; limited efficiency for polar analytes; specialized solvents required |
| Headspace co-distillation (HCD)106 | Continuous inert gas flow through the sample headspace minimizes degradation; VOCs are trapped post-distillation | Suitable for low molecular weight, highly volatile VOCs; minimizes thermal decomposition | VOC recovery can be affected by environmental variables (e.g., temperature, humidity, light); reproducibility challenges |
| Thermal desorption116,117 | VOCs are trapped on adsorbent tubes and later released into a carrier gas stream using heat | Excellent repeatability and recovery; very low limits of detection; compatible with pre-concentration | Sorbent degradation possible; thermal instability of some VOCs; variability in tube flow rates complicates quantitative comparison |
5. VOC detection approaches
VOCs offer a diverse landscape for detection, with sensors relying on analyte–sensor interactions determined by the chemical characteristics of the VOCs. These interactions, which form the foundation of VOC detection, are primarily categorized into two distinct approaches: selective and cross-reactive sensing. In selective sensing, detection is narrowly focused on specific VOCs, enabling a high degree of specificity even amidst interfering gaseous species. This method is invaluable for applications demanding precision, such as environmental monitoring and clinical diagnostics.10 Conversely, cross-reactive sensing is inspired by biological olfactory systems. This approach employs sensor arrays that can respond to a broad spectrum of VOCs, capturing complex mixtures by mimicking human smell.118 When coupled with machine learning, cross-reactive sensors leverage patterns from broadly reactive sensor arrays to decode and identify VOC signatures. This bioinspired strategy is highly effective for analyzing complex samples, making it versatile for applications ranging from air quality monitoring to agricultural pest detection.9,105.1 Challenges in VOC detection and current analytical techniques
The identification and quantification of VOCs present unique challenges due to their nonreactive nature and typically low concentrations in biological or environmental samples. Despite these difficulties, advanced analytical techniques have been developed to detect VOCs with precision. GC-MS remains the gold standard for VOC analysis, offering unmatched sensitivity and selectivity.119,120 Other sophisticated methods such as gas chromatography-flame ionization detection (GC-FID), laser absorption spectrometry, and quartz crystal microbalance (QCM) sensors are widely used for VOC detection in various contexts. Additionally, commercial sensors, including metal oxide sensors,10 photoionization detectors, and electrochemical sensors, provide practical options for field detection.10,121 While these methods have demonstrated exceptional analytical capabilities, they are often constrained by high costs, slow detection speeds, and operational complexities. Such limitations (Table 3) hinder their deployment in scenarios requiring high-throughput analysis, such as border security or rapid disease diagnosis.| Technique name | Working principle | Advantages | Disadvantages |
|---|---|---|---|
| GC-MS119,122,123 | Separation of VOCs based on physicochemical interactions within a chromatographic column, followed by mass-to-charge (m/z) based identification via ionization. Offers semi-quantitative/quantitative analysis | Considered the gold standard; high sensitivity and selectivity; wide analyte range; enables both identification and quantification; excellent reproducibility | Requires preconcentration; expensive and non-portable; complex operation and data analysis; needs calibration with external/internal standards |
| PTR-MS124,125 | VOCs are ionized via proton transfer from H3O+ ions and analyzed in real time by MS without prior chromatographic separation | Real-time detection; does not require preconcentration; sensitive to VOCs at low ppb levels; minimal sample preparation | Less sensitive to semi-volatile or heavier compounds; compound-specific response factors needed; relatively high instrumentation cost |
| SIFT-MS126,127 | Uses soft chemical ionization (H3O+, NO+, O2+) in a flow tube reactor to ionize VOCs; product ions are detected via quadrupole MS | No need for authentic calibration standards; broad VOC range; real-time analysis; avoids preconcentration | Limited field applicability; reduced sensitivity for certain analytes; emerging method with limited standardization |
| E-nose Technology127,128 | Array of chemical sensors provides a pattern-based response to VOC mixtures, mimicking mammalian olfaction | Portable, low-cost, real-time analysis; user-friendly; applicable for rapid screening and point-of-care; minimal training required | Inability to quantify individual VOCs; low sensitivity and selectivity; interference from humidity; short sensor lifespan; lacks regulatory standardization |
| Electrochemical sensor71,129–131 | Detect VOCs through redox reactions, capacitance shifts, or electron transfer processes occurring at electrode surfaces | Fast response; potential for miniaturization and on-site applications; low power requirements | Prone to interference; limited sensitivity/selectivity without optimization; affected by environmental conditions; analyte specificity often lacking |
| Optical sensors132 | Based on optical transduction mechanisms such as colorimetry, fluorescence, interferometry, SPR, and photonic crystal interactions | Immune to electromagnetic noise; wide dynamic range; suitable for smart integration with AI and photonic circuits; non-contact detection | Complex fabrication; performance affected by temperature/humidity; relatively high cost for high-performance versions; possible matrix interferences |
| Nanomaterial based sensors133,134 | Exploit high surface-area nanomaterials that interact with VOCs via π–π stacking, charge transfer, hydrogen bonding, or dipolar interactions | High surface-to-volume ratio; low-cost; fast response; tunable properties using various nanomaterials; promising for portable platforms | Poor reproducibility and stability; reduced selectivity for weakly interacting VOCs; sensitive to humidity and temperature fluctuations; degradation over time |
5.2 Gas chromatography and mass spectrometry for VOC analysis
Gas chromatography has long been a cornerstone for VOC analysis, providing robust separation and detection of VOC components (Fig. 2A).119 GC-MS, in particular, has emerged as the preferred technique due to its unparalleled analytical power, enabling the detection of VOCs associated with diseases, microbial infections, plant VOC emissions, and pest-related compounds.135,136H. halys-related research has extensively utilized GC-MS to identify VOCs such as tridecane, (E)-2-decenal, and 5-ethyl-2(5H)-furanone, as well as host plant-emitted VOCs like phenol and caryophyllene.7,8,53,54 Despite its precision, GC-MS has significant drawbacks, including the requirement135,136 for skilled personnel, high equipment costs, and limited scalability for real-time applications at high-throughput locations like customs checkpoints. The main engineering limitation of GC-MS lies in its need for volatile and thermally stable compounds, restricting its use to small molecules that can withstand vaporization without decomposition.122 This requirement increases sample preparation complexity. PTR-MS lacks fragmentation patterns for complex mixtures, which hinders compound identification and differentiation of isomers.137 Improving mass accuracy and developing internal calibration strategies are essential for enhancing analytical performance. SIFT-MS faces product ion overlap, complicating the analysis of isomeric and closely related compounds.138 Challenges also include the large, non-portable flow systems and instability of precursor ions. While these methods continue to evolve, their portability remains limited.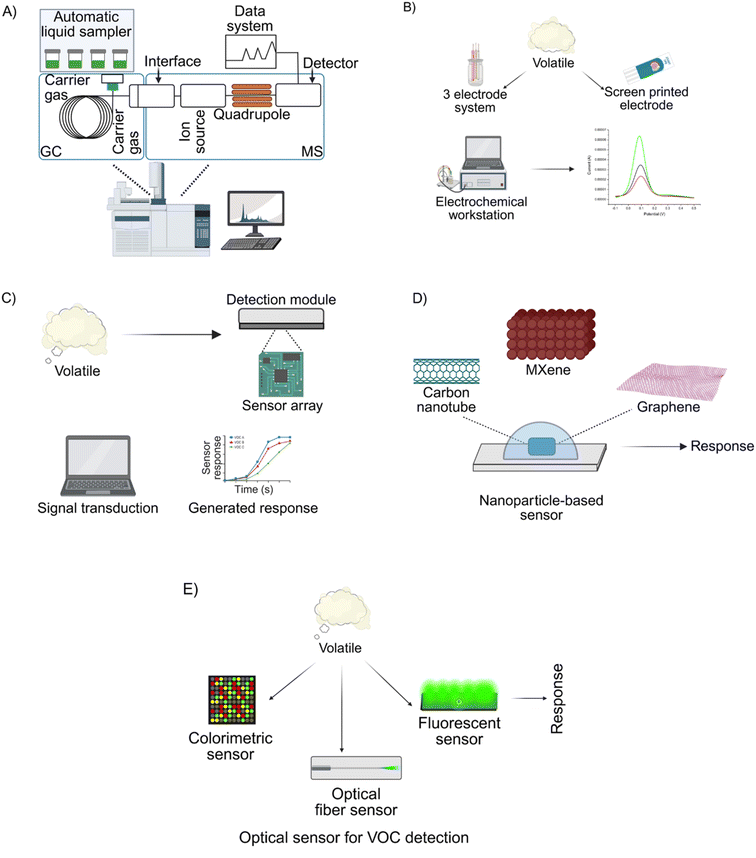 | ||
| Fig. 2 Graphical overview of VOC identification and quantification technologies. (A) GC-MS remains the gold standard for the robust separation, identification, and quantification of VOC components.7 (B) Electrochemical detection platforms for VOC sensing, offering high sensitivity and portability.130 (C) Electronic nose (e-nose) systems that generate complex VOC signatures for pattern recognition and analysis. (D) Nanoparticle-based sensors, leveraging functionalized nanomaterials for enhanced VOC capture and detection. (E) Optical sensing platforms, enabling rapid and non-invasive detection of VOCs through changes in light absorption, emission, or scattering. Illustrations were created in BioRender. Haque, F. (2025) https://www.BioRender.com/57b04hw. | ||
5.3 Electrochemical sensors: a promising alternative
Electrochemical sensors offer a versatile and cost-effective solution for VOC detection (Fig. 2B). These sensors operate by measuring the electrical current or potential generated from redox reactions involving VOC molecules on catalytic electrode surfaces. Techniques such as cyclic voltammetry further enhance specificity by identifying distinct oxidation or reduction peaks for individual VOCs.130,131 The reliability, portability, and capability to function in gaseous and liquid media make electrochemical sensors indispensable in fields like agriculture,131 disease detection,139 and environmental monitoring.140 For instance, hydrogen peroxide detection in breath samples at the parts-per-billion level has been achieved using advanced electrochemical systems, illustrating their potential for non-invasive medical diagnostics.1415.4 The potential of e-nose technology
Electronic nose (e-nose) systems are gaining traction as a highly efficient method for VOC detection (Fig. 2C). These devices utilize arrays of gas sensors combined with signal processing units and pattern recognition algorithms.142 When VOCs interact with the sensor array, they induce reversible physical or chemical changes, altering the electrical properties of the sensing material. These changes are analyzed through pattern recognition systems to generate unique VOC signatures.143 E-nose platforms employ various sensor technologies, including conductive polymer,144 metal oxide semiconductor,145 QCM,146 and surface acoustic wave sensors.147 Despite their utility, e-nose systems face challenges such as limited selectivity, environmental sensitivity, and the complexity of data interpretation, which may restrict their effectiveness for analyzing intricate VOC mixtures.128 Integrating an e-nose with a preconcentrator can improve its performance by enriching airborne VOCs to detectable levels before analysis. Preconcentrators typically use solid sorbents that capture VOCs and release them rapidly through thermal or photonic desorption.148,149 Future e-noses will likely integrate advanced sensing materials, optimized architectures, and data analysis based on machine learning. Combining e-noses with other non-destructive tools such as acoustic sensors, imaging systems, and artificial intelligence could enable accurate, autonomous, and affordable pest detection in agricultural settings.5.5 Nanomaterial-based VOC sensing
Carbon nanotubes (CNTs), graphene, MXenes, carbon dots and other carbon-based nanomaterials have emerged as versatile platforms for the development of VOC sensors (Fig. 2D).134 These sensors operate through a sequence of processes, including VOC capture, interaction with sensing-active centers, and subsequent diffusion dynamics. Depending on the nature of the VOC-material interaction, carbon-based nanomaterials have been engineered into optical, micro-gravimetric, and resistive sensor formats. Among carbon nanomaterials, single-walled carbon nanotubes and multi-walled carbon nanotubes are gaining increasing attention for VOC detection. Functional modifications, such as decorating CNTs with gold (Au) nanoparticles and further functionalizing with monolayers of 16-mercaptohexadecanoic acid or 1-hexadecanethiol,150 have enabled sensitive detection of alcohols, acetone, and aromatic VOCs. Furthermore, the incorporation of metal nanocomposites, such as palladium nanoparticles, into CNT frameworks has been shown to substantially enhance the sensing response toward formaldehyde.151Graphene, a two-dimensional (2D) single-atom-thick carbon material, offers an exceptionally high specific surface area, providing abundant active sites for gas adsorption.152 This material exhibit high carrier mobility and density, large surface area, exceptional mechanical strength, and low electrical noise, making them promising candidates for VOC sensing. Surface modifications with noble metal nanoparticles, such as Au, Ag, and Pt, further improve graphene's sensing performance by facilitating rapid charge transfer between the target gas molecules and the sensor. For instance, Pd-functionalized graphene has been used to detect trace levels of benzene and carbon monoxide, while a novel three-dimensional (3D) gas microsensor based on Au-modified, nanoporous nitrogen-doped graphene quantum dots integrated with TiO2 nanospheres demonstrated excellent sensitivity toward HCHO.153 MXenes, another emerging class of 2D materials (e.g., Ti3C2Tx), share structural similarities with graphene but exhibit a rich array of surface functional groups, including oxygen, hydroxyl, and fluorine. These functionalities enhance VOC adsorption and facilitate sensor fabrication. Owing to their high surface-to-volume ratio and remarkable electronic, optical, and mechanical properties, MXenes are considered highly promising materials for gas sensing. Chen et al. demonstrated that Au-decorated MXene-based sensors could effectively detect formaldehyde, acetone, and hexane.154 The sensing mechanism of these conductometric devices relies on changes in the electrical conductivity of the sensing material upon exposure to gas molecules with electron-donating or electron-withdrawing characteristics. Several of these materials remain underexplored for VOC detection and require further comparative studies to assess their performance relative to more established sensing materials.
5.6 Advanced optical sensors
Optical gas sensors utilize changes in optical properties, such as colorimetry, fluorescence, and scattering, to detect VOCs (Fig. 2E). These sensors are particularly suited for large-scale monitoring and can integrate with portable platforms like smartphones for on-site analysis. Recent developments include cost-effective, colorimetric sensor arrays for plant pathogen detection.155 However, optical sensors are often limited by high costs, operational complexity, and susceptibility to external factors like sunlight and physical damage, which hinder their miniaturization and portability.156,1575.7 Comparative analysis of VOC detection techniques
A comparative analysis of major VOC detection techniques is presented in Table 4, highlighting their key performance parameters such as sensitivity, selectivity, detection limits, cost, portability, response time, operational speed, and technology readiness level (TRL). This evaluation draws on representative literature data and manufacturer-reported performance metrics to clarify the practical trade-offs among available methods and to guide their use across biosecurity applications.| Technology | LOD | Sensitivity | Selectivity | Est. Cost (USD)* | Portability | Response time | Speed | TRL#158,159 |
|---|---|---|---|---|---|---|---|---|
| a GC-MS: gas chromatography mass spectroscopy; (PTR-MS): Proton transfer reaction-mass spectrometry; SIFT-MS: Selected ion flow tube-mass spectrometry, TRL- Technology Readiness Level, Pbbv-part per billion by volume.* The estimated cost was determined based on the online website of different companies. # The estimated TRL was determined based on the real-world application status of different detection methods. To date, no comprehensive review has evaluated both the analytical performance and legal readiness of VOC detection technologies. | ||||||||
| GC-MS137,138,160 | 0.31–0.49 ppbv | High | High | 100–200k | Low (lab-based) | Slow–moderate | Off-line | 9 |
| PTR-MS161,162 | 0.05–0.5 pbbv | High | High | >200k | Low (portable units emerging) | Slow–moderate | Real-time | 6–9 |
| SIFT-MS163,164 | 1 pbbv | High | High | >200k | Semi (benchtop, portable units emerging) | Slow–moderate | Real-time | 8–9 |
| E-nose Technology165,166 | 1 pbbv | Moderate to low | Moderate to low | ∼200 | High (handheld available) | Fast | Real-time | 4–9 |
| Electrochemical sensor130,167 | 0.5–2.4 ppbv | High | High | >10 | High | Fast | Real-time | 3–8 |
| Optical sensors168,169 | 0.627–14.7 ppmv | High to moderate | High | ∼1000 | Moderate to high | Fast | Real-time | 3–7 |
| Nanomaterial-based sensors134,170 | ∼35 ppbv | High to moderate | High to moderate | ∼200 | Moderate to high | Moderate to fast | Real-time | 1–7 |
GC-MS remains the benchmark for chemical specificity and sensitivity, achieving detection limits as low as 0.31–0.49 ppbv. Its high selectivity and broad compound coverage make it ideal for laboratory-based confirmation of VOC profiles from complex matrices. However, its high capital cost (USD 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–200
000–200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)137,160 due to complex vacuum systems and high-resolution detectors, limited portability, and long analysis times constrain its use for rapid or field-based diagnostics. GC-MS is most suitable for confirmatory testing and detailed compositional analysis in well-equipped laboratories. Portable GC-MS is developing, but it is still costly and needs consumables and periodic calibration, as well as the reproducibility and reliability need to be optimized.171 PTR-MS and SIFT-MS provide real-time VOC detection with comparable sensitivity (0.05–1 ppbv) and high selectivity. Both techniques require substantial investment (typically exceeding USD 200
000)137,160 due to complex vacuum systems and high-resolution detectors, limited portability, and long analysis times constrain its use for rapid or field-based diagnostics. GC-MS is most suitable for confirmatory testing and detailed compositional analysis in well-equipped laboratories. Portable GC-MS is developing, but it is still costly and needs consumables and periodic calibration, as well as the reproducibility and reliability need to be optimized.171 PTR-MS and SIFT-MS provide real-time VOC detection with comparable sensitivity (0.05–1 ppbv) and high selectivity. Both techniques require substantial investment (typically exceeding USD 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and are largely confined to benchtop or semi-portable systems, though compact units are emerging.161,162 Their fast response time and quantitative capability make them valuable for continuous monitoring or real-time atmospheric VOC analysis. However, their requirement for precise ion control, carrier gas purity, and high-voltage instrumentation limits their current use in field-based biosecurity surveillance. In contrast, e-nose technologies offer a low-cost (approximately USD 200),166 portable, and rapid alternative, providing real-time detection through sensor arrays that respond to characteristic VOC patterns. Although their sensitivity (around 1 ppbv) and selectivity are generally lower than mass spectrometric methods, their minimal sample preparation, compact design, and fast response make them highly attractive for on-site applications, including border inspections and early pest detection.165,166 Though they suffer from cross-sensitivity as they respond to classes of VOCs rather than unique molecules, and baseline drift due to humidity and temperature fluctuations. Addressing these issues requires an improved material design that can enhance selective adsorption and signal transduction.
000) and are largely confined to benchtop or semi-portable systems, though compact units are emerging.161,162 Their fast response time and quantitative capability make them valuable for continuous monitoring or real-time atmospheric VOC analysis. However, their requirement for precise ion control, carrier gas purity, and high-voltage instrumentation limits their current use in field-based biosecurity surveillance. In contrast, e-nose technologies offer a low-cost (approximately USD 200),166 portable, and rapid alternative, providing real-time detection through sensor arrays that respond to characteristic VOC patterns. Although their sensitivity (around 1 ppbv) and selectivity are generally lower than mass spectrometric methods, their minimal sample preparation, compact design, and fast response make them highly attractive for on-site applications, including border inspections and early pest detection.165,166 Though they suffer from cross-sensitivity as they respond to classes of VOCs rather than unique molecules, and baseline drift due to humidity and temperature fluctuations. Addressing these issues requires an improved material design that can enhance selective adsorption and signal transduction.
Electrochemical sensors demonstrate strong potential for practical deployment, with high sensitivity (0.5–2.4 ppbv), fast response, and excellent portability.130,167 These approaches can be adapted where redox chemistry is available or via indirect detection (e.g., functionalized electrode or converting inert VOC into a reacting derivative). Their low cost (typically under USD 10 per unit) and modest energy requirements enable large-scale or distributed monitoring networks. While selectivity remains a challenge in mixed-VOC environments, advances in electrode modification and nanomaterial integration are improving their analytical performance and stability. Optical sensors, which operate through spectroscopic interactions with target analytes, offer rapid, real-time detection and good selectivity. Their detection limits vary more widely (0.627–14.7 ppmv), and costs are moderate (around USD 1000).168,169 They provide a useful balance between analytical precision and field operability, though their performance can be affected by optical path length and environmental fluctuations such as humidity and temperature. Nanomaterial-based sensors represent the most promising and versatile platform for VOC detection. They combine relatively high sensitivity (∼35 ppbv) with moderate cost (∼USD 200) and portable designs, which are required for real-world applications. Their response and recovery times are improving through the use of advanced nanostructures with large surface areas and high carrier mobility with tunable electronic properties. Through surface functionalization and hybridization with metal nanoparticles, polymers, or MXenes, these sensors can achieve selective responses toward chemically diverse VOCs, addressing one of the major limitations of conventional chemiresistive devices.134 Furthermore, their compatibility with microfabrication and low-cost synthesis allows the development of lightweight, room-temperature, and low-power systems suitable for distributed biosecurity monitoring. However, the long-term stability, reproducibility, and environmental robustness of these sensors remain under investigation. Despite ongoing challenges, advances in material engineering and AI-based data interpretation are rapidly improving their robustness. Consequently, nanomaterial-based VOC sensors are emerging as the most feasible candidates for bridging laboratory precision and field operability.
Overall, high-precision mass spectrometric methods (e.g., GC-MS, PTR-MS, and SIFT-MS) set the analytical standard for sensitivity and selectivity but are constrained by cost, instrument size, and slow throughput. In contrast, low-cost and portable approaches such as e-noses, electrochemical sensors, and nanomaterial-based systems, offer significant promise for rapid, field-deployable detection. Optical sensors occupy an intermediate position, providing moderate precision with practical portability.
This comparative assessment demonstrates that no single technology is universally superior. The optimal choice depends on the intended biosecurity application: GC-MS and SIFT-MS for laboratory confirmation, PTR-MS for near-real-time monitoring, and e-noses, electrochemical, or nanomaterial-based sensors for border and field-based surveillance. As instrumentation continues to evolve toward miniaturization, integration, and data-driven interpretation, these platforms collectively provide a continuum of solutions bridging laboratory precision and real-world biosecurity readiness. Future developments are likely to focus on combining multiple sensing approaches into hybrid platforms that pair the high sensitivity of spectrometric methods with the portability and affordability of nanomaterial-based devices. Advances in fabrication and data processing will improve both selectivity and reliability. Sustainability, including low-energy operation, environmentally friendly materials, and recyclability, will also play an increasingly important role in guiding the next generation of VOC detection technologies.
5.8 Toward efficient and scalable VOC detection
VOCs are increasingly recognized as vital biomarkers for early-stage pest detection, infectious plant disease monitoring, identification of plant self-defense chemicals, interplant communication,172 human disease diagnostics,117 and environmental pollution surveillance.173 Despite their promise, the reliable identification and quantification of VOCs, particularly those emitted by pests such as H. halys, remains a complex and evolving field. Although GC–MS is the gold standard for VOC analysis, its practical limitations including high operational costs, complex instrumentation, slow detection speeds, and reliance on skilled operators, render it unsuitable for early-stage or frequent in-field applications.121 The massive and power-intensive nature of GC–MS equipment further restricts its scalability outside laboratory settings. To overcome these limitations, emerging technologies such as electrochemical sensors, nanomaterial-based sensors, optical platforms, and electronic nose (e-nose) systems are being explored as promising alternatives for cost-effective, scalable, and rapid VOC detection (Table 3). However, each approach faces unique limitations that must be addressed to achieve widespread adoption. These include the lack of standardized sampling protocols and the integration of sensor technologies into regulatory workflows. Future VOC detection systems must be engineered to meet multiple criteria: they should be cost-effective, mass-producible, highly sensitive and selective, rapidly responsive, reproducible, thermostable, humidity-tolerant, non-invasive, and portable for use in field conditions. Additionally, these systems must ensure long-term operational stability, low variability, robust analytical performance, secure data storage, and minimal reliance on complex sampling techniques.An ideal VOC sensor would match or surpass the sensitivity of biological olfactory systems, detecting VOCs at concentrations as low as 0.01 nM or in the parts-per-billion (ppb) range.174 To meet the challenge of detecting ultra-trace levels, advanced target marker-based strategies can be employed.175 However, isolating specific VOCs from complex environmental backgrounds remains a substantial challenge, as conventional isolation methods are often incompatible with field deployment. Consequently, direct detection strategies that eliminate sampling complexity are essential for enabling practical, on-site application. The current lack of comprehensive studies focused on VOC profiling for pest detection has hindered standardisation and cross-comparison between methods, further slowing technological advancement. Addressing this gap will require coordinated efforts to develop accessible, high-quality VOC databases and reference libraries.
To enable early diagnosis of plant pest and pathogen invasion, next-generation VOC sensors must overcome critical challenges related to sensitivity and selectivity.176 Discriminating target biomarkers from ambient environmental VOCs requires materials with enhanced chemical reactivity and high surface-to-volume ratios. Innovations such as nano-architectured sensing surfaces and advanced surface functionalisation strategies will be vital for increasing adsorption specificity. However, external variables, such as moisture, temperature fluctuations, and biological contamination, can impact sensor stability and longevity.177 To mitigate these effects, sensor surfaces should be inertly functionalised, and devices engineered for environmental resilience. Equally important is ensuring reproducibility and standardisation across different fabrication processes and research environments. Uniform manufacturing protocols, rigorous quality control, and validated performance benchmarks are essential for producing reliable sensor outputs across diverse settings. The integration of sophisticated data analytics, artificial intelligence, and bioinformatics can further improve the interpretation and utility of VOC datasets, enabling more informed and robust decision-making.10 Another major frontier is the development of self-powered or passive VOC sensors, leveraging emerging technologies such as near-field communication, disposable solar panels, or biofuel cells. Sustainable sensor designs must also prioritise environmental safety throughout their lifecycle, including the fabrication and disposal phases. This involves minimising the use of hazardous materials, conducting toxicity assessments of sensor components, and ensuring that single-use devices do not pose ecological risks. Adopting green engineering principles will be critical to support environmentally responsible agricultural and biosecurity practices.178
VOC-based detection approaches can also be extended to identify volatiles emitted by other pests and pathogens, including the boll weevil (Anthonomus grandis),179 the spotted lanternfly (Lycorma delicatula), the brown stink bug (Euschistus servus), as well as headspace volatiles from pathogen-infected fruits and vegetables impacted by fruit fly (Drosophila suzukii) or rice plants infested by the fall armyworm (Spodoptera frugiperda). Utilising VOCs as biomarkers in biosecurity will enable early detection of plant pests and pathogens, offering critical insights into the invasion dynamics of exotic species. This integrated VOC sensing platform could empower farmers to adopt more sustainable food production practices. Moreover, innovations such as multiplexed chemical sensor systems hold promise for overcoming the ongoing threat posed by exotic pests.
A field trial examined soil samples from a sheep paddock and a nearby vineyard with different levels of microbial biomass. The study found that VOC emissions reflected microbial activity, with higher emissions corresponding to higher microbial activity, measured using ten electronic noses, each fitted with six MQ-series gas sensors.180 The Tasmanian Institute of Agriculture, supported by the Tasmanian Government's Agricultural Development Fund, has developed a low-cost electronic nose called QUOLL® to monitor soil biological activity through soil gas emissions linked to changes in soil carbon. QUOLL® is currently being tested on farms across Tasmania, starting at a vegetable research facility and expanding to commercial farms.181 These examples provide concrete links between VOC sensing technologies and current commercial or field trials. The absence of standardized calibration protocols makes it difficult to compare and share VOC detection data, which limits reliable long-term use. Many current systems also detect only a narrow range of VOCs, highlighting the need for platforms that can measure and distinguish complex VOC mixtures in real-world settings. To move laboratory advances into practical applications, more large-scale trials at farms and ports are needed.
The path toward commercial viability and widespread adoption of VOC-based detection technologies requires a comprehensive and multi-faceted strategy. This includes sustained investment in research and development, integration of on-site detection modules, application of AI and machine learning for data analysis, use of Internet of Things (IoT) platforms for connectivity, and development of wearable VOC monitoring systems.10 Scalable manufacturing techniques, such as additive manufacturing and roll-to-roll printing, will also be essential to enable high-volume, cost-effective production. As advancements in affordable, miniaturised, and printable sensing technologies continue to accelerate, VOC detection systems are poised to evolve from specialised research tools into widely accessible diagnostic and monitoring platforms. Their potential impact extends well beyond agriculture and biosecurity, with transformative applications in healthcare, environmental monitoring, and public health surveillance through the early detection of critical VOC signatures.
6. Conclusion
We have reviewed the transformative potential of VOC sensing in biosecurity, emphasising its critical role in revolutionising agricultural diagnostics and pest management strategies. As global trade continues to expand, the threat posed by invasive pests and pathogens intensifies, underscoring the urgent need for advanced, field-deployable detection technologies. VOCs, as key biomarkers emitted by pests and pathogens, offer a unique opportunity for early detection, thereby reducing biosecurity costs and enhancing management efficiency. Despite notable progress in VOC detection, most existing methods, including GC–MS, remain expensive and lack the portability required for real-time, in-field applications. To address these limitations, we highlight the importance of future research focusing on the development of miniaturised, multifunctional, ultra-sensitive, eco-friendly, and portable VOC sensing technologies capable of selectively detecting target VOCs from complex environmental mixtures in real-time. The development of field-deployable VOC detectors remains a promising but nascent area, still challenged by interference from ambient air conditions and fluctuations in temperature and humidity, all of which can compromise sensor sensitivity. Incorporating protective sensor coatings and implementing motorised airflow through the detection unit may enhance both sensitivity and accuracy. In parallel, improvements in data acquisition, processing, and display systems will contribute to greater detection efficiency and user accessibility. Overcoming current limitations, particularly the challenges of multiplex detection and seamless integration into user-friendly, hand-held devices, will require a collaborative, multidisciplinary approach, bringing together expertise in materials science, engineering, chemistry, data science, and regulatory science. Looking ahead, the next generation of VOC sensing platforms will rely heavily on innovations in sensor engineering, automation, and data analytics. Achieving these advances will enable on-site, real-time monitoring of pests and pathogens, fundamentally transforming biosecurity strategies and pest management systems. Ultimately, such progress will play a vital role in safeguarding global agricultural productivity and food security.Author contributions
F. H., G. M. G., and M. J. A. S. conceptualized the manuscript. F. H. drafted the original manuscript. F. Z. F, M. T. T., M. A. S., G. M. G., M. J. A. S. critically revised the manuscript. F. H. prepared the figures. All authors read and approved the final manuscript.Conflicts of interest
The authors declare no competing financial interest.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was funded through a grant from the Commonwealth of Australia, represented by the Department of Health (Grant Activity 4-DGEJZ1O/4-CW7UT14).References
- H. Roy, A. Pauchard, P. Stoett, T. Renard Truong, S. Bacher and B. Galil, et al., Summary for policymakers of the thematic assessment report on invasive alien species and their control, IPBES Invasive alien species assessment., 2023, 1–56 Search PubMed.
- V. Australia Fruit Fly: a priority pest reviewed Vinehealth Australia & Government of South Australia, 2017, available from: https://vinehealth.com.au/2017/01/fruit-fly-priority-pest-reviewed/.
- Fay H., The Eradication Program for Papaya Fruit Fly (Bactrocera Papayae Drew and Hancock) in North Queensland. Aciar Proceedings, Australian Centre for International Agricultural Research, 1997 Search PubMed.
- S. Savary, L. Willocquet, S. J. Pethybridge, P. Esker, N. McRoberts and A. Nelson, The global burden of pathogens and pests on major food crops, Nat. Ecol. Evol., 2019, 3(3), 430–439 CrossRef.
- Nations FaAOotU, The State of Food Security and Nutrition in the World 2023, available from: https://openknowledge.fao.org/server/api/core/bitstreams/f1ee0c49-04e7-43df-9b83-6820f4f37ca9/content/state-food-security-and-nutrition-2023/food-security-nutrition-indicators.html Search PubMed.
- G. Vivaldo, E. Masi, C. Taiti, G. Caldarelli and S. Mancuso, The network of plants volatile organic compounds, Sci. Rep., 2017, 7(1), 11050 CrossRef PubMed.
- L. Nixon, I. I. Morrison, K. B. Rice, S. Goldson, E. G. Brockerhoff and A. Khrimian, et al., Behavioural responses of diapausing Halyomorpha halys (Hemiptera: Pentatomidae) to conspecific volatile organic compounds, J. Appl. Entomol., 2022, 146(3), 319–327 CrossRef CAS.
- L. J. Nixon, W. R. Morrison, K. B. Rice, E. G. Brockerhoff, T. C. Leskey and F. Guzman, et al., Identification of volatiles released by diapausing brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), PLoS One, 2018, 13(1), e0191223 CrossRef.
- A. Y. Moser, W. Y. Brown, L. A. Bizo, N. R. Andrew and M. K. Taylor, Biosecurity dogs detect live insects after training with odor-proxy training aids: scent extract and dead specimens, Chem. Senses, 2020, 45(3), 179–186 CrossRef CAS PubMed.
- M. Khatib and H. Haick, Sensors for volatile organic compounds, ACS Nano, 2022, 16(5), 7080–7115 CrossRef CAS PubMed.
- I. I. Morrison, P. Milonas, D. E. Kapantaidaki, M. Cesari, E. Di Bella and R. Guidetti, et al., Attraction of Halyomorpha halys (Hemiptera: Pentatomidae) haplotypes in North America and Europe to baited traps, Sci. Rep., 2017, 7(1), 16941 CrossRef.
- R. Jurdak, A. Elfes, B. Kusy, A. Tews, W. Hu and E. Hernandez, et al., Autonomous surveillance for biosecurity, Trends Biotechnol., 2015, 33(4), 201–207 CrossRef CAS PubMed.
- C. Anderson, S. Low-Choy, P. Whittle, S. Taylor, C. Gambley and L. Smith, et al., Australian plant biosecurity surveillance systems, Crop Prot., 2017, 100, 8–20 Search PubMed.
- K. P. Pattanaik, A. Mahanty, N. K. Panda, P. Bhavana, G. B. Gowda and N. K. Patil, et al., Prealighting and Postalighting Volatile Organic Compounds Emitted by Rice Influence the Behavior of Scirpophaga incertulas, J. Agric. Food Chem., 2024, 72(42), 23151–23159 Search PubMed.
- P. Mohekar, J. Osborne, N. G. Wiman, V. Walton and E. Tomasino, Influence of winemaking processing steps on the amounts of (E)-2-decenal and tridecane as off-odorants caused by brown marmorated stink bug (Halyomorpha halys), J. Agric. Food Chem., 2017, 65(4), 872–878 CAS.
- S. MacDougall, F. Bayansal and A. Ahmadi, Emerging methods of monitoring volatile organic compounds for detection of plant pests and disease, Biosensors, 2022, 12(4), 239 Search PubMed.
- P. Ivaskovic, B. E. Ainseba, Y. Nicolas, T. Toupance, P. Tardy and D. Thiéry, Sensing of airborne infochemicals for green pest management: what is the challenge?, ACS Sens., 2021, 6(11), 3824–3840 CrossRef CAS PubMed.
- M. Chen, M. Yazdani and K. Murugappan, Non-Destructive Pest Detection: Innovations and Challenges in Sensing Airborne Semiochemicals, ACS Sens., 2024, 9(11), 5728–5747 CAS.
- P. E. Hulme, J. R. Beggs, R. N. Binny, J. P. Bray, N. Cogger and M. K. Dhami, et al., Emerging advances in biosecurity to underpin human, animal, plant, and ecosystem health, IScience, 2023, 26(9), 107462 Search PubMed.
- V. Renault, M.-F. Humblet and C. Saegerman, Biosecurity concept: Origins, evolution and perspectives, Animals, 2021, 12(1), 63 Search PubMed.
- J. Waage and J. D. Mumford, Agricultural biosecurity, Philos. Trans. R. Soc., B, 2008, 363(1492), 863–876 CrossRef CAS PubMed.
- C. Diagne, B. Leroy, R. Gozlan, A.-C. Vaissière, C. Assailly and L. Nuninger, et al., InvaCost, a public database of the economic costs of biological invasions worldwide, Sci. Data, 2020, 7(1), 277 CrossRef CAS PubMed.
- T. Guillemaud, M. Ciosi, E. Lombaert and A. Estoup, Biological invasions in agricultural settings: Insights from evolutionary biology and population genetics, C. R. Biol., 2011, 334(3), 237–246 CrossRef.
- J. Granett, M. A. Walker, L. Kocsis and A. D. Omer, Biology and management of grape phylloxera, Annu. Rev. Entomol., 2001, 46(1), 387–412 CrossRef CAS.
- M. Carvajal-Yepes, K. Cardwell, A. Nelson, K. A. Garrett, B. Giovani and D. G. Saunders, et al., A global surveillance system for crop diseases, Science, 2019, 364(6447), 1237–1239 CrossRef CAS.
- P. Gladieux, X.-G. Zhang, D. Afoufa-Bastien, R.-M. Valdebenito Sanhueza, M. Sbaghi and B. Le Cam, On the origin and spread of the scab disease of apple: out of central Asia, PLoS One, 2008, 3(1), e1455 CrossRef.
- M. You, F. Ke, S. You, Z. Wu, Q. Liu and W. He, et al., Variation among 532 genomes unveils the origin and evolutionary history of a global insect herbivore, Nat. Commun., 2020, 11(1), 2321 CrossRef CAS PubMed.
- A. J. Carnegie and H. F. Nahrung, Post-border forest biosecurity in Australia: response to recent exotic detections, current surveillance and ongoing needs, Forests, 2019, 10(4), 336 CrossRef.
- A. J. Carnegie, F. Tovar, S. Collins, S. A. Lawson and H. F. Nahrung, A coordinated, risk-based, national forest biosecurity surveillance program for Australian forests, Front. For. Glob. Change., 2022, 4, 756885 CrossRef.
- P. H. Australia. The National Plant Biosecurity Status Report 2020. Plant Health Australia website; 2021 Search PubMed.
- E. Karamfilova. Plant Health: Revision of Regulation (EU) 2016/2031 on protective measures against plant pests January 2024, available from: https://eur-lex.europa.eu/eli/reg/2016/2031/oj/eng.
- Plant Pest Prevention through Technology-guided Monitoring and Site-specific Control (PURPEST) 5 December 2022, available from: https://www.cordis.europa.eu/project/id/101060634.
- J. Stack, K. Cardwell, R. Hammerschmidt, J. Byrne, R. Loria and K. Snover-Clift, et al., The national plant diagnostic network, Plant Dis., 2006, 90(2), 128–136 CrossRef CAS.
- P. E. Hulm, Trade, transport and trouble: managing invasive species pathways in an era of globalization, Appl. Ecol, 2009, 46, 10–18 CrossRef.
- R. Early, B. A. Bradley, J. S. Dukes, J. J. Lawler, J. D. Olden and D. M. Blumenthal, et al., Global threats from invasive alien species in the twenty-first century and national response capacities, Nat. Commun., 2016, 7(1), 12485 CrossRef CAS PubMed.
- A. Santini, L. Ghelardini, C. De Pace, M.-L. Desprez-Loustau, P. Capretti and A. Chandelier, et al., Biogeographical patterns and determinants of invasion by forest pathogens in Europe, New Phytol., 2013, 197(1), 238–250 CrossRef CAS PubMed.
- D.-H. Lee, B. D. Short, S. V. Joseph, J. C. Bergh and T. C. Leskey, Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea, Environ. Entomol., 2013, 42(4), 627–641 CrossRef.
- D. Ludwick, I. I. Morrison, A. L. Acebes-Doria, A. M. Agnello, J. C. Bergh and M. L. Buffington, et al., Invasion of the brown marmorated stink bug (Hemiptera: Pentatomidae) into the United States: Developing a national response to an invasive species crisis through collaborative research and outreach efforts, J. Integr. Pest Manag., 2020, 11(1), 4 CrossRef.
- R. E. Valentin, A. L. Nielsen, N. G. Wiman, D.-H. Lee and D. M. Fonseca, Global invasion network of the brown marmorated stink bug, Halyomorpha halys, Sci. Rep., 2017, 7(1), 9866 CrossRef PubMed.
- A. R. Boscolo, M. T. Vizzari, A. Benazzo and S. Ghirotto, Disentangling the worldwide invasion process of Halyomorpha halys through approximate Bayesian computation, Heredity, 2025, 134(1), 64–74 CrossRef.
- D. J. Kriticos, J. M. Kean, C. B. Phillips, S. D. Senay, H. Acosta and T. Haye, The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity, J. Pest Sci., 2017, 90, 1033–1043 CrossRef.
- E. J. Kistner, Climate change impacts on the potential distribution and abundance of the brown marmorated stink bug (Hemiptera: Pentatomidae) with special reference to North America and Europe, Environ. Entomol., 2017, 46(6), 1212–1224 CrossRef.
- D. Simberloff, J.-L. Martin, P. Genovesi, V. Maris, D. A. Wardle and J. Aronson, et al., Impacts of biological invasions: what's what and the way forward, Trends Ecol. Evol., 2013, 28(1), 58–66 CrossRef PubMed.
- T. C. Leskey and A. L. Nielsen, Impact of the invasive brown marmorated stink bug in North America and Europe: history, biology, ecology, and management, Annu. Rev. Entomol., 2018, 63(1), 599–618 CrossRef CAS PubMed.
- P. Milonas and G. Partsinevelos, First report of brown marmorated stink bug Halyomorpha halys Stål (Hemiptera: Pentatomidae) in Greece, Bull. OEPP, 2014, 44(2), 183–186 Search PubMed.
- I. Martinez-Sañudo, M. A. Perotti, D. Scaccini, A. Pozzebon, L. Marri and L. Mazzon, Co-haplotyping symbiont and host to unravel invasion pathways of the exotic pest Halyomorpha halys in Italy, Sci. Rep., 2020, 10(1), 18441 Search PubMed.
- K. B. Rice, C. J. Bergh, E. J. Bergmann, D. J. Biddinger, C. Dieckhoff and G. Dively, et al., Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae), J. Integr. Pest Manag., 2014, 5(3), A1–A13 CrossRef.
- D.-H. Lee and T. Leskey, Flight behavior of foraging and overwintering brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), Bull. Entomol. Res., 2015, 105(5), 566–573 CrossRef.
- J. Aigner and T. Kuhar, Lethal High Temperature Extremes of the Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) and Efficacy of Commercial Heat Treatments for Control in Export Shipping Cargo1, J. Agric. Urban Entomol., 2016, 32(1), 1–6 CrossRef.
- J. M. Fouani, M. Scala, V. Zaffaroni-Caorsi, V. Verrastro, G. Anfora and V. Mazzoni, The post-diapause vibrational behavior, motility, and survival of the brown marmorated stink bug Halyomorpha halys (Stål) adults at different temperatures, Sci. Rep., 2024, 14(1), 1198 CrossRef CAS PubMed.
- T. C. Leskey, G. C. Hamilton, A. L. Nielsen, D. F. Polk, C. Rodriguez-Saona and J. C. Bergh, et al., Pest status of the brown marmorated stink bug, Halyomorpha halys in the USA, Outlooks Pest Manage., 2012, 23(5), 218–226 CrossRef.
- D. Solomon, D. Dutcher and T. Raymond, Characterization of Halyomorpha halys (brown marmorated stink bug) biogenic volatile organic compound emissions and their role in secondary organic aerosol formation, J. Air Waste Manage. Assoc., 2013, 63(11), 1264–1269 CrossRef CAS.
- L. J. Nixon, A. Tabb, W. R. Morrison, K. B. Rice, E. G. Brockerhoff and T. C. Leskey, et al., Volatile release, mobility, and mortality of diapausing Halyomorpha halys during simulated shipping movements and temperature changes, J. Pest Sci., 2019, 92, 633–641 CrossRef.
- C. Harris, S. Abubeker, M. Yu, T. Leskey and A. Zhang, Semiochemical production and laboratory behavior response of the brown marmorated stink bug, Halyomorpha halys, PLoS One, 2015, 10(11), e0140876 CrossRef PubMed.
- R. Favaro, M. Berka, M. Pettersson, G. Thöming, C. C. Arce and M. L. Inacio, et al., The use of volatile organic compounds in preventing and managing invasive plant pests and pathogens, Front. Hortic., 2024, 3, 1379997 CrossRef.
- A. Karimi and J. Gross, Development and validation of an innovative headspace collection technique: volatile organic compound patterns emitted by different developmental stages of Halyomorpha halys, Front. Hortic., 2024, 3, 1380008 CrossRef.
- R. Tognon, J. Aldrich, J. Sant'Ana and F. Zalom, Conditioning Native Telenomus and Trissolcus (Hymenoptera: Scelionidae) egg parasitoids to recognize the exotic brown marmorated stink bug (Heteroptera: Pentatomidae: Halyomorpha halys), Environ. Entomol., 2019, 48(1), 211–218 CrossRef CAS.
- D. R. Paini, S. P. Worner, D. C. Cook, P. J. De Barro and M. B. Thomas, Threat of invasive pests from within national borders, Nat. Commun., 2010, 1(1), 115 CrossRef.
- S. Sharma, S. McKirdy and F. Macbeth, The biosecurity continuum and trade: Tools for post-border biosecurity, The Handbook of Plant Biosecurity: Principles and Practices for the Identification, Containment and Control of Organisms that Threaten Agriculture and the Environment Globally, Springer, 2013, pp. 189–206 Search PubMed.
- S. J. McKirdy, S. O'Connor, M. L. Thomas, K. L. Horton, A. Williams and D. Hardie, et al., Biosecurity risks posed by a large sea-going passenger vessel: challenges of terrestrial arthropod species detection and eradication, Sci. Rep., 2019, 9(1), 19339 CrossRef CAS.
- G. P. Bhattarai, R. B. Schmid and B. P. McCornack, Remote sensing data to detect hessian fly infestation in commercial wheat fields, Sci. Rep., 2019, 9(1), 6109 CrossRef.
- W. Mo, C. A. Vaiana and C. J. Myers, The need for adaptability in detection, characterization, and attribution of biosecurity threats, Nat. Commun., 2024, 15(1), 10699 CrossRef CAS.
- G. Lewis, J. L. Jordan, D. A. Relman, G. D. Koblentz, J. Leung and A. Dafoe, et al., The biosecurity benefits of genetic engineering attribution, Nat. Commun., 2020, 11(1), 6294 CrossRef CAS PubMed.
- B. Martinez, J. K. Reaser, A. Dehgan, B. Zamft, D. Baisch and C. McCormick, et al., Technology innovation: advancing capacities for the early detection of and rapid response to invasive species, Biol. Invasions, 2020, 22(1), 75–100 CrossRef.
- J. Laothawornkitkul, J. P. Moore, J. E. Taylor, M. Possell, T. D. Gibson, C. N. Hewitt and N. D. Paul, Discrimination of plant volatile signatures by an electronic nose: a potential technology for plant pest and disease monitoring, Environ. Sci. Technol., 2008, 42(22), 8433–8439 CrossRef CAS.
- I. Nordström, P. Sherwood, B. Bohman, S. Woodward, D. L. Peterson and J. Niño-Sánchez, et al., Utilizing volatile organic compounds for early detection of Fusarium circinatum, Sci. Rep., 2022, 12(1), 21661 CrossRef.
- L. Sanchez, S. Pant, K. Mandadi and D. Kurouski, Raman spectroscopy vs. quantitative polymerase chain reaction in early stage Huanglongbing diagnostics, Sci. Rep., 2020, 10(1), 10101 CrossRef CAS.
- P. Simonet, G. Duport, K. Gaget, M. Weiss-Gayet, S. Colella and G. Febvay, et al., Direct flow cytometry measurements reveal a fine-tuning of symbiotic cell dynamics according to the host developmental needs in aphid symbiosis, Sci. Rep., 2016, 6(1), 19967 CrossRef CAS PubMed.
- J. D. Janse and B. Kokoskova, Indirect immunofluorescence microscopy for the detection and identification of plant pathogenic bacteria (in particular for Ralstonia solanacearum), Plant Pathology: Techniques and Protocols, Springer, 2008, pp. 89–99 Search PubMed.
- F. Martinelli, R. Scalenghe, S. Davino, S. Panno, G. Scuderi and P. Ruisi, et al., Advanced methods of plant disease detection. A review, Agron. Sustainable Dev., 2015, 35(1), 1–25 CrossRef.
- Y. Fang and R. P. Ramasamy, Current and prospective methods for plant disease detection, Biosensors, 2015, 5(3), 537–561 CrossRef CAS PubMed.
- T. C. Leskey, A. Agnello, J. C. Bergh, G. P. Dively, G. C. Hamilton and P. Jentsch, et al., Attraction of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) to traps baited with semiochemical stimuli across the United States, Environ. Entomol., 2015, 44(3), 746–756 CrossRef CAS PubMed.
- I. I. Morrison, A. Acebes-Doria, E. Ogburn, T. P. Kuhar, J. F. Walgenbach and J. C. Bergh, et al., Behavioral response of the brown marmorated stink bug (Hemiptera: Pentatomidae) to semiochemicals deployed inside and outside anthropogenic structures during the overwintering period, J. Econ. Entomol., 2017, 110(3), 1002–1009 CrossRef PubMed.
- K. Watanabe, Characteristic damage of Lygocoris (Apolygus) lucorum (Meyer-Dür)(Heteroptera: Miridae) and Halyomorpha halys (Stål)(Heteroptera: Pentatomidae) on cherry, Annu. Rep. Soc. Plant Prot. North Jpn., 1996, 47, 143–144 Search PubMed.
- I. I. Morrison, C. R. Mathews and T. C. Leskey, Frequency, efficiency, and physical characteristics of predation by generalist predators of brown marmorated stink bug (Hemiptera: Pentatomidae) eggs, Biol. Control, 2016, 97, 120–130 CrossRef.
- K. Wilkins, K. Larsen and M. Simkus, Volatile metabolites from mold growth on building materials and synthetic media, Chemosphere, 2000, 41(3), 437–446 CrossRef CAS PubMed.
- V. Bitas, H.-S. Kim, J. W. Bennett and S. Kang, Sniffing on microbes: diverse roles of microbial volatile organic compounds in plant health, Mol. Plant-Microbe Interact., 2013, 26(8), 835–843 CrossRef CAS PubMed.
- A. B. Johne, B. Weissbecker and S. Schuetz, Approaching risk assessment of complex disease development in horse chestnut trees: a chemical ecologist's perspective, J. Appl. Entomol., 2008, 132(5), 349–359 CrossRef.
- T. Vuorinen, A.-M. Nerg, L. Syrjälä, P. Peltonen and J. K. Holopainen, Epirrita autumnata induced VOC emission of silver birch differ from emission induced by leaf fungal pathogen, Arthropod-Plant Interact., 2007, 1(3), 159–165 CrossRef.
- F. Brilli, N. Luchi, M. Michelozzi, L. Calamai, G. Cencetti and F. Pecori, et al., Volatile organic compounds (VOC) as biomarkers for detection of Ceratocystis platani, Eur. J. For. Pathol., 2020, 50(4), e12618 CrossRef.
- L. C. Vezzola, M. Michelozzi, L. Calamai, P. Gonthier, L. Giordano, P. Cherubini and M. Pelfini, Tree-ring volatile terpenes show potential to indicate fungal infection in asymptomatic mature Norway spruce trees in the Alps, Int. J. For. Res., 2019, 92(2), 149–156 Search PubMed.
- A. Lytovchenko, R. Beleggia, N. Schauer, T. Isaacson, J. E. Leuendorf and H. Hellmann, et al., Application of GC-MS for the detection of lipophilic compounds in diverse plant tissues, Plant Methods, 2009, 5(1), 4 CrossRef PubMed.
- R. S. Blake, P. S. Monks and A. M. Ellis, Proton-transfer reaction mass spectrometry, Chem. Rev., 2009, 109(3), 861–896 CrossRef CAS PubMed.
- D. Smith and P. Španěl, Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis, Mass Spectrom. Rev., 2005, 24(5), 661–700 CrossRef CAS PubMed.
- B. R. Blaauw, G. Hamilton, C. Rodriguez-Saona and A. L. Nielsen, Plant stimuli and their impact on brown marmorated stink bug dispersal and host selection, Front. Ecol. Evol., 2019, 7, 414 CrossRef.
- P.-H. Liao, H.-H. Yang, P.-C. Wu, N. H. Abu Bakar and P. L. Urban, On-Line Coupling of Simultaneous Distillation–Extraction Using the Likens–Nickerson Apparatus with Gas Chromatography, Anal. Chem., 2019, 92(1), 1228–1235 CrossRef PubMed.
- Q. Gros, J. Duval, C. West and E. Lesellier, On-line supercritical fluid extraction-supercritical fluid chromatography (SFE-SFC) at a glance: A coupling story, TrAC, Trends Anal. Chem., 2021, 144, 116433 CrossRef CAS.
- C. C. Rering, A. M. Gaffke, A. B. Rudolph, J. J. Beck and H. T. Alborn, A comparison of collection methods for microbial volatiles, Front. Sustain. Food Syst., 2020, 4, 598967 CrossRef.
- H. T. Alborn, R. G. Bruton and J. J. Beck, Sampling of volatiles in closed systems: a controlled comparison of three solventless volatile collection methods, J. Chem. Ecol., 2021, 47(12), 930–940 CrossRef CAS PubMed.
- M. A. Ferhat, N. Tigrine-Kordjani, S. Chemat, B. Meklati and F. Chemat, Rapid extraction of volatile compounds using a new simultaneous microwave distillation: solvent extraction device, Chromatographia, 2007, 65, 217–222 CrossRef CAS.
- P. Hashemi, M. Abolghasemi, A. Fakhari, S. N. Ebrahimi and S. Ahmadi, Hydrodistillation–solvent microextraction and GC–MS identification of volatile components of Artemisia aucheri, Chromatographia, 2007, 66, 283–286 CrossRef CAS.
- Q. Lin, H. Ni, L. Wu, S. Y. Weng, L. Li and F. Chen, Analysis of aroma-active volatiles in an SDE extract of white tea, Food Sci. Nutr., 2021, 9(2), 605–615 CrossRef CAS PubMed.
- J. H. Son, M. A. Islam, J. H. Hong, J. Y. Jeong, O. Y. Song and H. E. Kim, et al., Extraction of volatile organic compounds from leaves of Ambrosia artemisiifolia L. and Artemisia annua L. by headspace-solid phase micro extraction and simultaneous distillation extraction and analysis by gas chromatography/mass spectrometry, Food Sci. Biotechnol., 2021, 30(3), 355–366 CrossRef CAS.
- S. Teixeira, A. Mendes, A. Alves and L. Santos, Simultaneous distillation–extraction of high-value volatile compounds from Cistus ladanifer L, Anal. Chim. Acta, 2007, 584(2), 439–446 CrossRef CAS.
- P. Pollien and A. Chaintreau, Simultaneous Distillation− Extraction: Theoretical Model and Development of a Preparative Unit, Anal. Chem., 1997, 69(16), 3285–3292 CrossRef CAS.
- M. Zhu, E. Li and H. He, Determination of volatile chemical constitutes in tea by simultaneous distillation extraction, vacuum hydrodistillation and thermal desorption, Chromatographia, 2008, 68(7), 603–610 CrossRef CAS.
- N. Narain, M. de Sousa Galvao and M. S. Madruga, Volatile compounds captured through purge and trap technique in caja-umbu (Spondias sp.) fruits during maturation, Food Chem., 2007, 102(3), 726–731 CrossRef CAS.
- B. Webster, S. Gezan, T. Bruce, J. Hardie and J. Pickett, Between plant and diurnal variation in quantities and ratios of volatile compounds emitted by Vicia faba plants, Phytochemistry, 2010, 71(1), 81–89 CrossRef CAS PubMed.
- H. Lord, Y. Yu, A. Segal and J. Pawliszyn, Breath analysis and monitoring by membrane extraction with sorbent interface, Anal. Chem., 2002, 74(21), 5650–5657 CrossRef CAS.
- L. Wang, H. Lord, R. Morehead, F. Dorman and J. Pawliszyn, Sampling and monitoring of biogenic emissions by eucalyptus leaves using membrane extraction with sorbent interface (MESI), J. Agric. Food Chem., 2002, 50(22), 6281–6286 CrossRef CAS PubMed.
- A. Steffen and J. Pawliszyn, Analysis of flavor volatiles using headspace solid-phase microextraction, J. Agric. Food Chem., 1996, 44(8), 2187–2193 CrossRef CAS.
- F. Augusto, A. L. e Lopes and C. A. Zini, Sampling and sample preparation for analysis of aromas and fragrances, TrAC, Trends Anal. Chem., 2003, 22(3), 160–169 CrossRef CAS.
- P. Weintraub and J. Gross, Capturing Insect Vectors of Phytoplasmas, Phytoplasma: Methods and Protocols, Springer, 2012, pp. 61–72 Search PubMed.
- E. E. Stashenko, M. A. Puertas and M. Y. Combariza, Volatile secondary metabolites from Spilanthes americana obtained by simultaneous steam distillation-solvent extraction and supercritical fluid extraction, J. Chromatogr. A, 1996, 752(1–2), 223–232 CrossRef CAS.
- B. Huang, L. Qin, Q. Chu, Q. Zhang, L. Gao and H. Zheng, Comparison of headspace SPME with hydrodistillation and SFE for analysis of the volatile components of the roots of Valeriana officinalis var. latifolia, Chromatographia, 2009, 69, 489–496 CrossRef CAS.
- F. Peng, L. Sheng, B. Liu, H. Tong and S. Liu, Comparison of different extraction methods: steam distillation, simultaneous distillation and extraction and headspace co-distillation, used for the analysis of the volatile components in aged flue-cured tobacco leaves, J. Chromatogr. A, 2004, 1040(1), 1–17 CrossRef CAS.
- P. Farkaš, J. Sadecka, M. Kováč, B. Siegmund, E. Leitner and W. Pfannhauser, Key odourants of pressure-cooked hen meat, Food Chem., 1997, 60(4), 617–621 CrossRef.
- J. J. Young, I. M. Atikul, N. Khan, N. Jamila, J. H. Hong and K. S. Kim, Simultaneous Distillation Extraction (SDE) and Headspace Solid-Phase Microextraction (HS-SPME) for the Determination of Volatile Organic Compounds (VOCs) by Gas Chromatography–Mass Spectrometry (GC-MS) in Perilla Frutescens Foliage from South Korea, Anal. Lett., 2022, 55(10), 1605–1622 CrossRef.
- Z. Zhang and G. Li, A review of advances and new developments in the analysis of biological volatile organic compounds, Microchem. J., 2010, 95(2), 127–139 CrossRef CAS.
- M. D. Askari, M. P. Maskarinec, S. M. Smith, P. M. Beam and C. C. Travis, Effectiveness of purge-and-trap for measurement of volatile organic compounds in aged soils, Anal. Chem., 1996, 68(19), 3431–3433 CrossRef CAS.
- I. Ueta, T. Mitsumori, Y. Suzuki, S. Kawakubo and Y. Saito, Determination of very volatile organic compounds in water samples by purge and trap analysis with a needle-type extraction device, J. Chromatogr. A, 2015, 1397, 27–31 CrossRef CAS PubMed.
- N.-S. Kim and D.-S. Lee, Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography–mass spectrometry, J. Chromatogr. A, 2002, 982(1), 31–47 CrossRef CAS PubMed.
- S. Risticevic, H. Lord, T. Gorecki, C. L. Arthur and J. Pawliszyn, Protocol for solid-phase microextraction method development, Nat. Protoc., 2010, 5(1), 122–139 CrossRef CAS PubMed.
- V. Pilařová, K. Plachká, D. Herbsová, Š. Kosturko, F. Svec and L. Nováková, Comprehensive two-step supercritical fluid extraction for green isolation of volatiles and phenolic compounds from plant material, Green Chem., 2024, 26(11), 6480–6489 RSC.
- M. D. Macías-Sánchez, C. Mantell Serrano, M. Rodríguez Rodríguez, E. Martínez de la Ossa, L. M. Lubián and O. Montero, Extraction of carotenoids and chlorophyll from microalgae with supercritical carbon dioxide and ethanol as cosolvent, J. Sep. Sci., 2008, 31(8), 1352–1362 CrossRef PubMed.
- N. E. Rabaud, S. E. Ebeler, L. L. Ashbaugh and R. G. Flocchini, The application of thermal desorption GC/MS with simultaneous olfactory evaluation for the characterization and quantification of odor compounds from a dairy, J. Agric. Food Chem., 2002, 50(18), 5139–5145 CrossRef CAS PubMed.
- I. Belluomo, S. E. Whitlock, A. Myridakis, A. G. Parker, V. Converso and M. J. Perkins, et al., Combining Thermal Desorption with Selected Ion Flow Tube Mass Spectrometry for Analyses of Breath Volatile Organic Compounds, Anal. Chem., 2024, 96(4), 1397–1401 CrossRef CAS PubMed.
- H. W. Song, D. Moon, Y. Won, Y. K. Cha, J. Yoo, T. H. Park and J. H. Oh, A pattern recognition artificial olfactory system based on human olfactory receptors and organic synaptic devices, Sci. Adv., 2024, 10(21), eadl2882 CrossRef CAS PubMed.
- S. Van Bramer and K. R. Goodrich, Determination of plant volatiles using Solid Phase Microextraction GC–MS, J. Chem. Educ., 2015, 92(5), 916–919 CrossRef CAS.
- J. Dewulf, H. Van Langenhove and G. Wittmann, Analysis of volatile organic compounds using gas chromatography, TrAC, Trends Anal. Chem., 2002, 21(9–10), 637–646 CrossRef CAS.
- X. Huang, Y. Li, E. Witherspoon, R. He, G. Petruncio and M. Paige, et al., Species-selective detection of volatile organic compounds by ionic liquid-based electrolyte using electrochemical methods, ACS Sens., 2023, 8(9), 3389–3399 CrossRef CAS PubMed.
- J. Lisec, N. Schauer, J. Kopka, L. Willmitzer and A. R. Fernie, Gas chromatography mass spectrometry–based metabolite profiling in plants, Nat. Protoc., 2006, 1(1), 387–396 CrossRef CAS PubMed.
- R. Michishita, M. Shimoda, S. Furukawa and T. Uehara, Inoculation with black soldier fly larvae alters the microbiome and volatile organic compound profile of decomposing food waste, Sci. Rep., 2023, 13(1), 4297 CrossRef CAS PubMed.
- D. Materić, M. Peacock, M. Kent, S. Cook, V. Gauci, T. Röckmann and R. Holzinger, Characterisation of the semi-volatile component of dissolved organic matter by thermal desorption–proton transfer reaction–mass spectrometry, Sci. Rep., 2017, 7(1), 15936 CrossRef.
- E. Aprea, F. Biasioli, S. Carlin, I. Endrizzi and F. Gasperi, Investigation of volatile compounds in two raspberry cultivars by two headspace techniques: Solid-phase microextraction/gas Chromatography− Mass spectrometry (SPME/GC− MS) and proton-transfer Reaction− Mass spectrometry (PTR− MS), J. Agric. Food Chem., 2009, 57(10), 4011–4018 CrossRef CAS PubMed.
- J. La Nasa, F. Modugno, M. P. Colombini and I. Degano, Validation study of selected ion flow tube-mass spectrometry (SIFT-MS) in heritage science: characterization of natural and synthetic paint varnishes by portable mass spectrometry, J. Am. Soc. Mass Spectrom., 2019, 30(11), 2250–2258 CrossRef CAS PubMed.
- I. Belluomo, P. R. Boshier, A. Myridakis, B. Vadhwana, S. R. Markar, P. Spanel and G. B. Hanna, Selected ion flow tube mass spectrometry for targeted analysis of volatile organic compounds in human breath, Nat. Protoc., 2021, 16(7), 3419–3438 CrossRef CAS.
- A. Z. Berna, S. Trowell, W. Cynkar and D. Cozzolino, Comparison of metal oxide-based electronic nose and mass spectrometry-based electronic nose for the prediction of red wine spoilage, J. Agric. Food Chem., 2008, 56(9), 3238–3244 CrossRef CAS PubMed.
- Y. Fang, Y. Umasankar and R. P. Ramasamy, Electrochemical detection of p-ethylguaiacol, a fungi infected fruit volatile using metal oxide nanoparticles, Analyst, 2014, 139(15), 3804–3810 RSC.
- Y. Umasankar, G. C. Rains and R. P. Ramasamy, Electroanalytical studies on green leaf volatiles for potential sensor development, Analyst, 2012, 137(13), 3138–3145 RSC.
- Y. Umasankar and R. P. Ramasamy, Highly sensitive electrochemical detection of methyl salicylate using electroactive gold nanoparticles, Analyst, 2013, 138(21), 6623–6631 RSC.
- C. McDonagh, C. S. Burke and B. D. MacCraith, Optical chemical sensors, Chem. Rev., 2008, 108(2), 400–422 CrossRef CAS PubMed.
- G. Konvalina and H. Haick, Sensors for breath testing: from nanomaterials to comprehensive disease detection, Acc. Chem. Res., 2014, 47(1), 66–76 CrossRef CAS PubMed.
- N. Nath, A. Kumar, S. Chakroborty, S. Soren, A. Barik, K. Pal and Jr F. G. de Souza, Carbon nanostructure embedded novel sensor implementation for detection of aromatic volatile organic compounds: an organized review, ACS Omega, 2023, 8(5), 4436–4452 CrossRef CAS PubMed.
- J. M. Sanchez and R. D. Sacks, GC analysis of human breath with a series-coupled column ensemble and a multibed sorption trap, Anal. Chem., 2003, 75(10), 2231–2236 CrossRef CAS PubMed.
- C. A. Zini, T. F. De Assis, E. B. Ledford, C. Dariva, J. Fachel, E. Christensen and J. Pawliszyn, Correlations between pulp properties of eucalyptus clones and leaf volatiles using automated solid-phase microextraction, J. Agric. Food Chem., 2003, 51(27), 7848–7853 CrossRef CAS PubMed.
- B. Buszewski, T. Ligor, T. Jezierski, A. Wenda-Piesik, M. Walczak and J. Rudnicka, Identification of volatile lung cancer markers by gas chromatography–mass spectrometry: comparison with discrimination by canines, Anal. Bioanal. Chem., 2012, 404(1), 141–146 CrossRef CAS PubMed.
- Y.-H. Kim and K.-H. Kim, Ultimate detectability of volatile organic compounds: how much further can we reduce their ambient air sample volumes for analysis?, Anal. Chem., 2012, 84(19), 8284–8293 CrossRef CAS PubMed.
- J. R. Sempionatto, M. Lin, L. Yin, E. De la Paz, K. Pei and T. Sonsa-Ard, et al., An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers, Nat. Biomed. Eng., 2021, 5(7), 737–748 CrossRef CAS PubMed.
- G. Hanrahan, D. G. Patil and J. Wang, Electrochemical sensors for environmental monitoring: design, development and applications, J. Environ. Monit., 2004, 6(8), 657–664 RSC.
- D. Maier, E. Laubender, A. Basavanna, S. Schumann, F. Güder, G. A. Urban and C. Dincer, Toward continuous monitoring of breath biochemistry: a paper-based wearable sensor for real-time hydrogen peroxide measurement in simulated breath, ACS Sens., 2019, 4(11), 2945–2951 CrossRef CAS PubMed.
- K. Lee, I. Cho, M. Kang, J. Jeong, M. Choi and K. Y. Woo, et al., Ultra-low-power e-nose system based on multi-micro-led-integrated, nanostructured gas sensors and deep learning, ACS Nano, 2022, 17(1), 539–551 CrossRef PubMed.
- T. Arroyo, J. Lozano, J. M. Cabellos, M. Gil-Diaz, J. P. Santos and C. Horrillo, Evaluation of wine aromatic compounds by a sensory human panel and an electronic nose, J. Agric. Food Chem., 2009, 57(24), 11543–11549 CrossRef CAS PubMed.
- C. H. A. Esteves, B. A. Iglesias, T. Ogawa, K. Araki, L. Hoehne and J. Gruber, Identification of tobacco types and cigarette brands using an electronic nose based on conductive polymer/porphyrin composite sensors, ACS Omega, 2018, 3(6), 6476–6482 CrossRef CAS PubMed.
- T. Kim, Y. Kim, W. Cho, J.-H. Kwak, J. Cho and Y. Pyeon, et al., Ultralow-Power Single-Sensor-Based E-Nose System Powered by Duty Cycling and Deep Learning for Real-Time Gas Identification, ACS Sens., 2024, 9(7), 3557–3572 CrossRef CAS PubMed.
- T. Julian, S. N. Hidayat, A. Rianjanu, A. B. Dharmawan, H. S. Wasisto and K. Triyana, Intelligent mobile electronic nose system comprising a hybrid polymer-functionalized quartz crystal microbalance sensor array, ACS Omega, 2020, 5(45), 29492–29503 CrossRef CAS PubMed.
- M. Benetti, D. Cannatà, E. Verona, A. P. Papavlu, V. C. Dinca and T. Lippert, et al., Highly selective surface acoustic wave e-nose implemented by laser direct writing, Sens. Actuators, B, 2019, 283, 154–162 CrossRef CAS.
- M. Hu, J. Wang, H. Liu, Q. Chen, C. Xie and Q. Li, et al., Development of functional nucleic acid sensor for detection of locust pheromone 4-vinylanisole, Chem. Eng. J., 2023, 466, 143065 CrossRef CAS.
- K. Song and S.-K. Lee, Development of a compact sample pre-concentration system for the detection of a trace amount of volatile organic compounds (VOCs), Sens. Actuators, B, 2007, 125(1), 173–179 CrossRef CAS.
- N. Bohli, M. Belkilani, J. Casanova-Chafer, E. Llobet and A. Abdelghani, Multiwalled carbon nanotube based aromatic volatile organic compound sensor: sensitivity enhancement through 1-hexadecanethiol functionalisation, Beilstein J. Nanotechnol., 2019, 10(1), 2364–2373 CrossRef CAS PubMed.
- M. Yoosefian, H. Raissi and A. Mola, The hybrid of Pd and SWCNT (Pd loaded on SWCNT) as an efficient sensor for the formaldehyde molecule detection: A DFT study, Sens. Actuators, B, 2015, 212, 55–62 CrossRef CAS.
- Y. R. Choi, Y.-G. Yoon, K. S. Choi, J. H. Kang, Y.-S. Shim and Y. H. Kim, et al., Role of oxygen functional groups in graphene oxide for reversible room-temperature NO2 sensing, Carbon, 2015, 91, 178–187 CrossRef CAS.
- S. Shao, H. W. Kim, S. S. Kim, Y. Chen and M. Lai, NGQDs modified nanoporous TiO2/graphene foam nanocomposite for excellent sensing response to formaldehyde at high relative humidity, Appl. Surf. Sci., 2020, 516, 145932 CrossRef CAS.
- W. Y. Chen, X. Jiang, S.-N. Lai, D. Peroulis and L. Stanciu, Nanohybrids of a MXene and transition metal dichalcogenide for selective detection of volatile organic compounds, Nat. Commun., 2020, 11(1), 1302 CrossRef CAS PubMed.
- Z. Li, R. Paul, T. Ba Tis, A. C. Saville, J. C. Hansel and T. Yu, et al., Non-invasive plant disease diagnostics enabled by smartphone-based fingerprinting of leaf volatiles, Nat. Plants, 2019, 5(8), 856–866 CrossRef CAS PubMed.
- T. Beduk, C. Durmus, S. B. Hanoglu, D. Beduk, K. N. Salama and T. Goksel, et al., Breath as the mirror of our body is the answer really blowing in the wind? Recent technologies in exhaled breath analysis systems as non-invasive sensing platforms, TrAC, Trends Anal. Chem., 2021, 143, 116329 CrossRef CAS.
- S. Y. Park, Y. Kim, T. Kim, T. H. Eom, S. Y. Kim and H. W. Jang, Chemoresistive materials for electronic nose: Progress, perspectives, and challenges, InfoMat, 2019, 1(3), 289–316 CrossRef CAS.
- E. L. Macturk, K. Hayes, G. O'Sullivan and K. A. Perrault Uptmor, Are We Ready for It? A Review of Forensic Applications and Readiness for Comprehensive Two-Dimensional Gas Chromatography in Routine Forensic Analysis, J. Sep. Sci., 2025, 48(4), e70138 CrossRef CAS PubMed.
- Government A. Defence - Technology Readiness Level (TRL) Definition, available from: https://www.researchgrants.gov.au/resource-hub/defence-technology-readiness-level-trl-definition.
- J. Martínez-del-Río, J. M. Vidal and A. G. Frenich, Economic evaluation of pesticide-residue analysis of vegetables, TrAC, Trends Anal. Chem., 2013, 44, 90–97 CrossRef.
- K. Sekimoto and A. R. Koss, Modern mass spectrometry in atmospheric sciences: Measurement of volatile organic compounds in the troposphere using proton-transfer-reaction mass spectrometry, J. Mass Spectrom., 2021, 56(3), e4619 CrossRef CAS PubMed.
- C. Warneke, J. De Gouw, E. Lovejoy, P. Murphy, W. Kuster and R. Fall, Development of proton-transfer ion trap-mass spectrometry: On-line detection and identification of volatile organic compounds in air, J. Am. Soc. Mass Spectrom., 2005, 16(8), 1316–1324 CrossRef CAS.
- S. R. Markar, T. Wiggins, S. Antonowicz, S.-T. Chin, A. Romano and K. Nikolic, et al., Assessment of a noninvasive exhaled breath test for the diagnosis of oesophagogastric cancer, JAMA Oncol., 2018, 4(7), 970–976 CrossRef PubMed.
- M. J. Perkins and V. S. Langford, Standard validation protocol for selected ion flow tube mass spectrometry methods applied to direct headspace analysis of aqueous volatile organic compounds, Anal. Chem., 2021, 93(24), 8386–8392 CrossRef CAS PubMed.
- M. M. Macías, J. E. Agudo, A. G. Manso, C. J. G. Orellana, H. M. G. Velasco and R. G. Caballero, A compact and low cost electronic nose for aroma detection, Sensors, 2013, 13(5), 5528–5541 CrossRef PubMed.
- W. Yan, W. Liu, Z. Zhao, J. Wang, G. B. Nam and S. Cui, et al., Humidity-independent electronic nose of α-Fe2O3/ZnFe2O4 heterojunctions for trace detection of N-butanol exhalation in lung cancer screening, Sens. Actuators, B, 2023, 384, 133577 CrossRef CAS.
- W. Rosario, D. K. Avasthi and N. Chauhan, Graphene oxide and molecularly imprinted polymer-based sensor for the electrochemical detection of benzene: A novel tactic in early diagnosis of lung cancer, Talanta Open, 2025, 100506 CrossRef.
- D. Kou, Y. Zhang, S. Zhang, S. Wu and W. Ma, High-sensitive and stable photonic crystal sensors for visual detection and discrimination of volatile aromatic hydrocarbon vapors, Chem. Eng. J., 2019, 375, 121987 CrossRef CAS.
- J. Lim, J. Shim, I. Kim, S. K. Kim, H. Lim and S.-Y. Ahn, et al., Ultrasensitive mid-infrared optical gas sensor based on germanium-on-insulator photonic circuits with limit-of-detection at sub-ppm level, ACS Photonics, 2024, 11(10), 4268–4278 CrossRef CAS.
- L. Song, L. Luo, Y. Xi, J. Song, Y. Wang and L. Yang, et al., Reduced graphene oxide-coated Si nanowires for highly sensitive and selective detection of indoor formaldehyde, Nanoscale Res. Lett., 2019, 14(1), 97 CrossRef PubMed.
- P. E. Leary, K. L. Kizzire, R. Chan Chao, M. Niedziejko, N. Martineau and B. W. Kammrath, Evaluation of portable gas chromatography–mass spectrometry (GC–MS) for the analysis of fentanyl, fentanyl analogs, and other synthetic opioids, J. Forensic Sci., 2023, 68(5), 1601–1614 CrossRef CAS PubMed.
- J. Jin, M. Zhao, T. Gao, T. Jing, N. Zhang and J. Wang, et al., Amplification of early drought responses caused by volatile cues emitted from neighboring tea plants, Hortic. Res., 2021, 8, 263 CrossRef PubMed.
- E. David and V.-C. Niculescu, Volatile organic compounds (VOCs) as environmental pollutants: occurrence and mitigation using nanomaterials, Int. J. Environ. Res. Public Health, 2021, 18(24), 13147 CrossRef CAS PubMed.
- Y. Hirata, H. Oda, T. Osaki and S. Takeuchi, Biohybrid sensor for odor detection, Lab Chip, 2021, 21(14), 2643–2657 RSC.
- E. P. Ollé, J. Farré-Lladós and J. Casals-Terré, Advancements in microfabricated gas sensors and microanalytical tools for the sensitive and selective detection of odors, Sensors, 2020, 20(19), 5478 CrossRef PubMed.
- J. Martinazzo, A. N. Brezolin, R. T. Paschoalin, A. C. Soares, J. Steffens and C. Steffens, Sexual pheromone detection using PANI· Ag nanohybrid and PANI/PSS nanocomposite nanosensors, Anal. Methods, 2021, 13(35), 3900–3908 RSC.
- L. Yang, G. Zheng, Y. Cao, C. Meng, Y. Li and H. Ji, et al., Moisture-resistant, stretchable NOx gas sensors based on laser-induced graphene for environmental monitoring and breath analysis, Microsyst. Nanoeng., 2022, 8(1), 78 Search PubMed.
- R. K. Murali-Baskaran, P. Mooventhan, D. Das, A. Dixit, K. C. Sharma and S. Senthil-Nathan, et al., The future of plant volatile organic compounds (pVOCs) research: Advances and applications for sustainable agriculture, Environ. Exp. Bot., 2022, 200, 104912 CrossRef CAS.
- W. G. Henderson, A. Khalilian, Y. J. Han, J. K. Greene and D. C. Degenhardt, Detecting stink bugs/damage in cotton utilizing a portable electronic nose, Comput. Electron. Agric., 2010, 70(1), 157–162 CrossRef.
- I. Hunt, C. Boucher, B. Das, A. Martin and R. Hardy, Viticultural Soil Testing Using Electronic Noses, Aust. J. Grape Wine Res., 2025, 2025(1), 4224202 CrossRef CAS.
- Tasmania Uo. The QUOLL e-nose: Microbes against climate change: University of Tasmania, available from: https://www.utas.edu.au/tia/research/research-projects/project/agricultural-systems/the-quoll-e-nose-microbes-against-climate-change Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |






