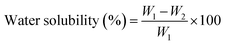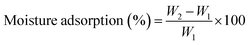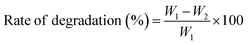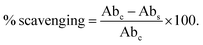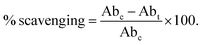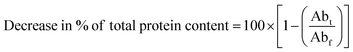Open Access Article
Open Access ArticleTradescantia pallida extract incorporated chitosan/pullulan intelligent biodegradable films: an eco-friendly packaging to preserve the freshness of chicken
Manjunath P.
Eelager
 ab,
Saraswati P.
Masti
ab,
Saraswati P.
Masti
 *a,
Suhasini
Madihalli
a,
Ravindra B.
Chougale
*a,
Suhasini
Madihalli
a,
Ravindra B.
Chougale
 c,
Nagarjuna Prakash
Dalbanjan
d and
S. K.
Praveen Kumar
c,
Nagarjuna Prakash
Dalbanjan
d and
S. K.
Praveen Kumar
 d
d
aDepartment of Chemistry, Karnatak Science College, Dharwad-580 001, Karnataka, India. E-mail: dr.saraswatimasti@yahoo.com
bResearch and Development Department, Indian Institute of Packaging, Mumbai-400 093, Maharashtra, India
cPG Department of Studies in Chemistry, Karnatak University, Dharwad-580 003, Karnataka, India
dPG Department of Studies in Biochemistry, Karnatak University, Dharwad-580 003, Karnataka, India
First published on 10th November 2025
Abstract
Herein, biodegradable active films were developed using a polysaccharide based blend of chitosan (CS) and pullulan (PU), incorporated with the ethanolic extract of Tradescantia pallida (TP), to function as intelligent food packaging materials. FTIR confirmed intermolecular hydrogen bonding between the polyphenolic constituents of TP extract and the CS/PU matrix. The resulting CPT intelligent biodegradable films exhibited an impressive tensile strength of 45.84 MPa, surpassing that of conventional plastic films. SEM micrographs revealed a uniform and compact surface morphology, demonstrating successful blending and structural integrity. The incorporation of polyphenolic compounds not only enhanced the antimicrobial efficacy of the films against foodborne pathogens but also imparted remarkable antioxidant potential, as evidenced by DPPH (89.81%) and ABTS (85.14%) radical scavenging activities. A distinct color shift from purple to yellow upon spoilage enabled real-time monitoring of freshness. Chicken samples packaged in polyethylene (PE) and CPT-2 intelligent biodegradable films were stored under ambient conditions. Analyses of microbial growth, ammonia release, free fatty acid degradation, and the effects of UV exposure revealed that CPT-2 intelligent biodegradable films offered superior protection against microbial contamination and oxidative deterioration, thereby extending the chicken's shelf life compared to PE packaging. The CPT intelligent biodegradable films also showed biodegradation potential, addressing the persistence, landfill accumulation, and greenhouse gas emissions associated with single-use plastics. Thereby mitigating the adverse impacts of SUPs on human health and the ecosystem. Overall, the physicochemically enhanced intelligent films offer a sustainable and effective alternative to plastic packaging, improving food safety and environmental health.
Sustainability spotlightSustainability has become an imperative across all sectors due to the growing environmental burden caused by SUPs and their persistent pollution. In the food sector, the challenges of resource scarcity and rapid spoilage demand innovative and eco-friendly packaging solutions. Herein, a chitosan/pullulan blend was developed, both polymers being biodegradable and currently at a Technology Readiness Level (TRL) of 5–6, indicating strong potential for commercial advancement. The incorporation of anthocyanin-rich Tradescantia pallida extract introduces both active and intelligent functionalities to the film, making it a sustainable alternative to conventional synthetic packaging materials. The prepared intelligent film demonstrated the ability to extend the shelf life of chicken by 48 hours at room temperature, effectively doubling that of polyethylene (PE) packaging under similar conditions. Beyond its superior preservation capacity, the film's complete biodegradability ensures that, after disposal, it does not contribute to landfill accumulation or microplastic pollution. Thus, the developed film not only addresses food preservation challenges but also aligns with circular economy principles by minimizing environmental impact as a promising step toward a sustainable, pollution-free packaging ecosystem and a greener future. |
1. Introduction
Excessive plastic consumption has become a significant environmental threat, with waste disposal and management presenting substantial global challenges. Plastics in landfills contribute to global warming and microplastic pollution. Across its life cycle, from extraction to manufacturing, plastic production emits large amounts of greenhouse gases, generating approximately 1.96 gigatons in 2015, according to the Minderoo-Monaco Commission on Plastics and Human Health (2023). The food sector significantly contributes to this problem through the use of single-use, non-biodegradable packaging, driven by modern lifestyles. Valued at $338.34 billion in 2023, the global food packaging industry is projected to reach $478.18 billion by 2028. Although recycled plastic is considered a better packaging alternative, it often contains harmful substances such as BPA, phthalates, flame retardants, and heavy metals, which limit its suitability for food-related uses.1–3 The rise in demand for food packaging, combined with the detrimental effects of single-use and recycled plastics, has prompted the development of environmentally acceptable and sustainable alternatives. Bioplastics degrade in the environment with minimal hazardous environmental impact and are receiving greater attention from researchers. There are three primary categories in bioplastics: partially bio-based, such as Bio-PET, which is non-biodegradable, and another group generated from conventional fossil resources that are biodegradable, such as CPL and PBHT. Conversely, biopolymers are naturally occurring polysaccharides, lipids, and proteins that are biodegradable and eco-friendly.4–6Polysaccharide based films are gaining popularity due to their natural origin. The deacetylation of β-(1–4)-poly-N-acetyl-D-glucosamine (chitin) leads to the formation of the second most abundant polysaccharide, i.e., chitosan (CS). Chitosan consists of β-1,4-linked 2-amino-2-deoxy-β-D-glucose (deacetylated D-glucosamine) and N-acetyl-D-glucosamine units, a copolymer that has received FDA certification for food-related studies. The biodegradable, biocompatible, non-toxic, and antimicrobial properties, along with the film-forming capacity of chitosan, have received considerable attention from a commercial perspective in food packaging and biomedical applications.7–9 Emulsion formation capability, good tensile strength, and antimicrobial activity suggest that CS can be considered for food packaging applications. However, the brittleness and high water vapour permeability of pure CS films restrict their applicability as a food packaging material.10
The extracellular polysaccharide obtained from Aureobasidium spp. Fungi are known to produce pullulan (PU). Pullulan is a biopolymer that received GRAS status from the FDA in 2004 and was registered by the European Union (EU) as a food additive (E1204) in the same year.11 The characteristic structural flexibility in pullulan is due to α-1,6-glucosidic linkages present between the linearly polymerized maltotriose units.12,13 Pullulan films are transparent, possess thermal capacity, and exhibit resistance to oil and grease, while also showing good oxygen barrier properties.14 Despite all these excellent tensile strength and physicochemical qualities, pullulan film's lack of water resistance properties limits its usage as a food packaging material.
In the present study, a polymer blend of CS/PU is considered to overcome the limitations of chitosan and pullulan neat films. Glycerol was added to the CS/PU blend as the plasticizing agent to enhance the elastic nature of the polymer blend. The glycerol's acceptor and donor hydrogen bonding ability suggests it as a better plasticizing agent for chitosan.15 Adding glycerol as a plasticizer increases the free volume in the polymer matrix, thereby enhancing the flexibility of the brittle CS/PU blend. Food freshness is crucial, as every product has a defined shelf life. To monitor this, intelligent packaging materials have been developed that can sense spoilage through various detection methods. Among these, pH-based sensing is effective in identifying food deterioration. Anthocyanins, naturally occurring polyphenols in fruits and vegetables, are pH-sensitive pigments responsible for their vivid colors. They appear red in acidic conditions due to the flavylium cation and yellow in basic conditions due to the chalcone structure.16
Hence, the anthocyanin content in plant and fruit extracts was utilized intelligently in packaging to identify pH variations by changing the color of the films. Yan Qin et al. reported that betalains from red pitaya (Hylocereus polyrhizus) peel extract are extracted into starch/PVA to fabricate biodegradable films for intelligent packaging applications.17 Tilak Gasti et al. fabricated chitosan/methylcellulose films to monitor fish fillet freshness, incorporating Phyllanthus reticulatus as an anthocyanin source.18 Thus, Tradescantia pallida (also known as purple heart or wandering jew) is an extremely popular, inexpensive, and readily available ornamental groundcover worldwide. This accessibility, stemming from its ease of growth, high nursery availability, drought tolerance, and simple propagation via cuttings, makes it a cost-effective alternative for medicinal cultivation. Historically, its leaves have been used as an anti-inflammatory, antitoxic, and circulatory tonic. Modern pharmacological interest, particularly in the leaf extract, is driven by its rich phytochemical profile of phenolics, flavonoids, and anthocyanins, making it suitable for active and intelligent packaging applications.19 Traditionally, TP treated venereal diseases, mucosal infections, and gastrointestinal disorders.20 The polyphenolic constituents of the Tradescantia pallida (TP) extract include phenolic acids such as p-coumaric acid, syringic acid, and chlorogenic acid; flavonoids such as naringenin, morin, catechin, epicatechin, and quercetin; and anthocyanins such as cyanidin-3,7,3′-triglucoside and petunidin, which are primarily responsible for the colouration of TP leaves. These phenolic acids, flavonoids, and anthocyanins collectively contribute to the film's antimicrobial and antioxidant properties, while also enabling real-time color variation in response to pH changes, making them suitable for intelligent food packaging applications.21 Due to all these considerations, TP extract is considered the active agent in the present study because polyphenolic extract consists of anthocyanin content capable of identifying pH variation on spoilage.
According to the UN's Food and Agriculture Organization (FAO), worldwide chicken meat production reached 137 million tons in 2020.22 Chicken meat is susceptible to microbial assault, perishable at room temperature, and challenging to preserve. Plastic is the primary packaging material used in the meat industry. In India, meat packaging is dependent on 10µ PET/45µ HDPE – LDPE, LDPE – BA – Nylon – BA – LLDPE, HDPE – LDPE – HDPE, HDPE – LLDPE, and HDPE + LDPE (blended) plastics, according to the Indian Centre for Plastic in the Environment.23 These plastics, derived from fossil fuels, are the primary source of environmental contamination because they persist in the environment for extended periods.24,25 Therefore, the current study hypothesized that polysaccharides based CS/PU films incorporated with TP extract should be employed instead of using single-use plastic for chicken meat packaging because CPT intelligent biodegradable films enhanced physicochemical, superior antibacterial and antioxidant properties, colour changing capabilities due to pH variation and biodegradable nature made CPT intelligent biodegradable films an appealing alternative to microplastic generating tradition plastic packaging and serve as active and intelligent packaging materials for eco-friendly and sustainable future.
2. Materials and methods
2.1. Materials
Chitosan (viscosity: 200–600 mPa s, 0.5% in 0.5% acetic acid, deacetylation value: min. 80% after drying, product no.: C0831, CAS: 9012-76-4, MW = 19–31 kDa) and pullulan (product no.: P0978, CAS: 9057-02-7, MW ≈ 200 kDa) procured from TCI, Tokyo. Mueller–Hinton agar (MHA), yeast extract, peptone, sodium chloride L-ascorbic acid Hi-Media, methanol SD Fines, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2-[(2,6-dichlorophenyl)amino]benzene acetic acid sodium salt (diclofenac sodium salt) from SRL.2.2. Extraction of anthocyanin content from Tradescantia pallida (TP)
The leaves of the Tradescantia pallida plant, also called purple heart, were collected from the Karnatak Science College botanical garden, Dharwad. The anthocyanin content was extracted according to the procedure suggested by Valdir Aniceto Pereira et al., with some modifications.26 Distilled water was used to wash the collected leaves. A 100 g sample of finely chopped TP leaves was placed in a 150 ml ethanolic solution (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 2
water = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and 1 mol l−1 HCl was added dropwise to adjust the pH of the solution to 2. The sample was protected from the sunlight and stored at 4 °C for 48 h. After filtering the solution, the extract was centrifuged for 10 min at 7000 rpm. After filtering, the resultant supernatant pH was adjusted to 7 using 2.5 ml l−1 of NaOH and dried at 40 °C. The dried powder extract was then stored at 4 °C. The total phenolic content of ethanolic leaf extract was 134.44 ± 0.29 mg g−1 of leaf, and the total flavonoid content in ethanolic leaf extract was 117.78 ± 0.57 mg g−1 of leaf.
1), and 1 mol l−1 HCl was added dropwise to adjust the pH of the solution to 2. The sample was protected from the sunlight and stored at 4 °C for 48 h. After filtering the solution, the extract was centrifuged for 10 min at 7000 rpm. After filtering, the resultant supernatant pH was adjusted to 7 using 2.5 ml l−1 of NaOH and dried at 40 °C. The dried powder extract was then stored at 4 °C. The total phenolic content of ethanolic leaf extract was 134.44 ± 0.29 mg g−1 of leaf, and the total flavonoid content in ethanolic leaf extract was 117.78 ± 0.57 mg g−1 of leaf.
2.3. Fabrication of CPT intelligent films
Chitosan (CS) and pullulan (PU) polysaccharides are the polymers considered in the study. 1.5 g of CS and 0.5 g of PU were dissolved in 1% acetic acid and distilled water, respectively. After achieving a homogenous blend of CS and PU, 10 ml of 1% glycerol was added as a plasticizer. The blend solution was poured into a Petri dish and dried at room temperature for 6 days to obtain the control CP film. To the control blend solution, 100 and 200 mg of TP extract dissolved in ethanol were added and stirred on a magnetic stirrer for 4–5 h at 350 rpm. The CPT-1 and CPT-2 intelligent biodegradable films fabricated from the solvent casting method poured in Petri plates were peeled after 6 d and stored in a desiccator.3. Characterizations
3.1. Fourier transform infrared (FTIR) spectroscopy
Fundamental alterations induced by the CS/PU blend and the incorporation of TP extract were characterized using attenuated total reflection (ATR) mode with a Shimadzu IR spectrometer (Japan). Samples measuring 4 × 1 cm2 were placed directly in the spectrometer, and spectral analysis was performed across the 400–4000 cm−1 range at a resolution of 4 cm−1.273.2. Mechanical properties
The mechanical properties were assessed using the Universal Testing Machine (series 7200-1 kN) manufactured by Dak System Inc., as per the ASTM-D882 standard test (ASTM, 1992). The films consider a grip separation of 50 mm, a crosshead speed of 0.1 mm min−1, and film sample parameters of 10 cm in length and 2.5 cm in width, carried out at RH of 50–55% at RT.28,293.3. Morphological analysis
To examine the surface morphology, 2 cm × 2 cm control CP and CPT intelligent films were considered. The 10 kV acceleration voltage JEOL JSM-6360 (Japan) was used for SEM imaging. A thin layer of gold is sputtered onto films to prevent charging.303.4. Water contact angle (WCA)
A contact angle analyzer (model DMs-401, Kyowa Interface Science Co., Ltd, Tokyo) was used to study hydrophilic or hydrophobic behavior. Each time, a liquid drop volume of 1 µl was injected onto 1 × 5 cm2 films to capture the nature of contact in 5 different positions.313.5. Water solubility (WS)
The water solubility of CP and CPT intelligent films is determined according to the ASTM standard D570-98. After pre-drying 2 × 2 cm2 film samples for 24 h at 60 °C in a hot air oven, the initial weight (W1) was recorded. After immersing the films in 30 milliliters of double-distilled water for a day at room temperature, the excess water from the immersed films was removed using filter paper. Following another day of drying at 100 °C, the films were weighed, and the final weight was recorded (W2). The percentage of water solubility was determined using the equation below.323.6. Moisture adsorption (MA)
The intelligent film samples, measuring 2 × 2 cm2, were subjected to a drying process in an oven at a temperature of 100 °C for 24 h, and the initial weight (W1) was recorded. The films were reweighed after exposure to atmospheres with relative humidity levels of 19%, 50%, and 75% for 24 hours, yielding the final weight (W2). Each sample underwent three separate measurements, and MA was calculated as follows,333.7. Water vapour transmission rate (WVTR)
The cylindrical vials, with an inner diameter of 1.7 cm, were filled with 10 ml of distilled water. The objective was to assess the films' water vapor transmission rate (WVTR). The measurement was done gravimetrically using the ASTM E00996-00 method. A 3 × 3 cm2 film was used to cover the opening of the vail, and Teflon tape was wrapped around it to ensure airtightness. The vials were subjected to a temperature of 40 °C in a hot air oven for 24 hours. The WVTR capacity of each film was determined by calculating the weight fluctuations of vials at 24 h using the equations given below,343.8. Soil burial test
The investigation measured the degradation ability of a 2 × 2 cm2 control CP and CPT intelligent films, initially weighing as W1. The films were buried 5 cm deep in the soil on mesh and watered daily. After 10, 20, and 30 days, the films were removed, cleaned with double-distilled water, dried at 45 °C, and weighed to obtain a decreased final weight of W2. The rate of degradation of active films was calculated using the following equation.353.9. Anti-microbial sensitivity
The antimicrobial property of CPT-intelligent films was determined against Escherichia coli (ATCC 10799), Staphylococcus aureus (ATCC 6538), Bacillus subtilis, and Candida albicans (ATCC 24433). Pure bacterial cultures were subcultured on Luria–Bertani (LB) medium until the optical density (OD) at 600 nm reached 0.5. A swab was used to smear the microorganisms onto the MHA (4 mm thickness) plates. 100 µl of the solution was gently dropped into the wells created using a gel puncher (8 mm diameter). The plates were then incubated overnight at 37 °C in the incubator. The inhibition zone around the drop was measured using a vernier scale.3.10. Anti-oxidant activity
The free radical scavenging activity was expressed in terms of percent inhibition, which was calculated using the formula shown below,
3.11. Microbiological analysis of chicken
3.12. Evaluation of chicken meat spoilage activities after incubation time
Soon after the treatment, chicken meat was blended with 18 ml of 0.9% saline using a tissue homogenizer (Labico, LT-451, Labtech Instruments, India) for 0.5 min and then vortexed for 0.5 min. The blend was filtered through an individual four-layered muslin cloth. The chicken meat broth solution (CMBS) was further subjected to biochemical characterizations to evaluate nutritional levels.Abc denotes the absorbance of the control, and Abt represents the absorbance of CMBS.
The total protein content of the buffered CMBS was quantified using the FCR method with slight modifications.44 Briefly, 40 µl of buffered CMBS was diluted to 400 µl with water, followed by the addition of 1 ml of alkaline–copper sulfate solution. The mixture was incubated for 10 min, 100 µl of 50% FCR reagent was added, and incubated for 30 min in the dark at RT. After incubation, absorbance was measured in a UV-visible spectrophotometer (Labman, LMSP UV-1200) with 1.5 ml glass cuvettes of 1 cm path length at 750 nm. A solution with 0.4 ml buffer was used as a set blank. The total protein content was estimated using the standard calibration curve of bovine serum albumin. The relative decrease in % of protein content compared to the fresh chicken was expressed using the following formula.
3.13. Applications of UV treatment for packaged meat
A meat packaging experiment was conducted to assess the feasibility of implementing UV-blocking properties in prepared active film in comparison to traditional PE plastic and unpacked chicken, with a focus on its performance in the context of preserving meat. 2 g of sliced fresh chicken meat was kept in a sterile Petri plate (unpacked meat and meat packed with CPT-2, PEP). The experiment was conducted in a laminar airflow chamber with UV light. A broad-spectrum UV lamp was used as the UV source, with a distance of 30 cm between the source and the meat treatment. The samples were exposed to UV light for 20, 40, and 60 min at RT. CMBS, after UV treatment, was prepared as described above and was subjected to microbiological and biochemical analysis. The biochemical analysis involved the assay of antioxidants by DPPH free radical scavenging activity (as described above) and the estimation of free thiol groups using Ellman's test.where Abf denotes the absorbance of fresh CMBS and Abt denotes the absorbance of UV-treated samples.
3.14. Statistical analysis
Using the Origin 9.0 program, statistical analysis was performed using ANOVA (One-Way Analysis of Variance). The results of each experiment were obtained in triplicate and are presented as the mean ± SD. The Tukey test (p ≤ 0.05) was used to assess the difference between the mean values.4. Results and discussions
4.1. FTIR analysis
FTIR spectroscopy, using the Attenuated Total Reflectance (ATR) method, is a primary tool for analyzing the interactions between the functional groups of chitosan, pullulan, and TP extract in control CP and CPT intelligent biodegradable films. The control PC film exhibits the characteristic –OH stretching band at 3284 cm−1, as well as C–H symmetric and asymmetric stretching at 2932 and 2889 cm−1, respectively.48 Due to chitosan and pullulan polysaccharides, peaks observed at 1642 and 1533 cm−1 are due to C–O (amide-I) stretching and N–H (amide-II) bending. 1376 and 1152 cm−1 because of –OH bending and C–O–C bridge by asymmetric stretching, respectively.49,50 The characteristic peak observed at 850 cm−1 is due to the α-glucopyranose units of pullulan,51 as represented in Fig. 1(a). | ||
| Fig. 1 (a) FTIR analysis, mechanical properties (b) stress vs. strain curve and (c) TS and EB of control CP and CPT intelligent biodegradable films. | ||
Incorporating TD extract into the CS/PL matrix results in the CPT intelligent biodegradable films. The change in –OH stretching frequency to a higher wavelength of 3289–3294 cm−1 and the variation in the wavelength of polysaccharide peaks suggested intermolecular hydrogen bond formation, attributed to the interaction between the polymer and the active agent.52 The disappearance of characteristic α-glucopyranose units at 850 cm−1 in the case of CPT-2 intelligent biodegradable films indicated homogeneity between the polymer blend and the active agent.53 Further, the homogenous nature was confirmed by the SEM analysis of CPT intelligent biodegradable films. By forming intermolecular hydrogen bonds, this interaction between the CS/PL and TD confirms the compatibility between the active agent and the polymer matrix.
4.2. Mechanical properties
The CPT intelligent films are fabricated by incorporating Tradescantia pallida (TP) anthocyanin extract as an active and intelligent agent in the CS/PU polymer blend. The tensile strength (TS) and elongation at break (EB) of CP and CPT intelligent films were evaluated. The mechanical properties of films used for food packaging become particularly significant as they must withstand the stress of various environmental factors during transportation while preserving the food from mechanical damage. The TS and EB of the control CP film are 28.19 ± 0.23 MPa and 28.51 ± 0.19%, respectively. Adding TP anthocyanin extract as an active agent increases the tensile strength of CPT-1 and CPT-2 intelligent biodegradable films by 36.45 ± 0.13 and 45.84 ± 0.56 MPa, respectively. However, the elongation at break decreased to 19.22 ± 0.13% and 13.35 ± 0.17%, respectively, as demonstrated in Fig. 1(c).The incorporation of anthocyanin-rich polyphenols resulted in a 79% increase in tensile strength (TS) of the CPT-2 intelligent biodegradable film compared to the control CP film. This improvement can be attributed to the interactions between the phenolic groups of the TP extract and the –NH2 and –OH functional groups of chitosan (CS), as well as the –OH groups of pullulan (PU).18 However, a 47% reduction in elongation at break was observed, likely due to the reduced flexibility of the CPT biodegradable films resulting from these intermolecular interactions.54 The CPT intelligent biodegradable films exhibit the TS in the range of 36–45 MPa, superior to the microplastic generating conventional polyethylene (22–31 MPa) and polypropylene (31–38 MPa) plastics, which are widely used commercially in everyday life.39
The mechanical robustness and flexibility of CPT-2 biodegradable films, with a tensile strength (TS) of 45.84 ± 0.56 MPa and an elongation at break (EB) of 13.35 ± 0.17%, indicate a balanced performance suitable for food packaging applications. The TS value suggests the film can withstand considerable stress without breaking, ensuring durability during storage and handling. Meanwhile, the EB reflects moderate flexibility. Together, these properties enable the film to maintain structural integrity and adapt to the mechanical stresses encountered during packaging, transportation, and consumer use, making it a promising alternative to conventional plastics in sustainable packaging solutions.
4.3. Scanning electron microscopy (SEM) and water contact angle (WCA)
Scanning electron microscopy is the most adaptable and prominent method for determining a biopolymer's homogeneous nature, surface morphology, and phase separation, which is essential for fabricating polymer films.55 The micrographs from the SEM analysis were correlated with the mechanical characteristics of the produced films and used to evaluate how active additives cause agglomeration, which further affects the physicochemical characteristics of the polymer films.56,57 In the present study (Fig. 2(a)), the control CP films had uniform surfaces with a few white spots resulting from the plasticizing effect of glycerol. As the TP extract's anthocyanin content increased in CPT intelligent biodegradable films, the white spots on the film surface became less noticeable. This may be due to the formation of intermolecular hydrogen bonds between the polyphenols in the TP extract and the CS, PU, and glycerol components of the polymer blend. Similar results were obtained in the vanillic acid-incorporated CS/PVA blend.39 These findings indicate that homogenous dense surface morphology was relevant in enhancing the tensile strength of CPT intelligent biodegradable films. | ||
| Fig. 2 (a) SEM micrographs and (b) water contact images of control CP and CPT intelligent biodegradable films. | ||
The hydrophobic properties of the CPT intelligent biodegradable film were assessed using water contact angle analysis. The films must exhibit a hydrophobic nature to be suitable for use in the food packaging industry. Food deterioration is frequently caused by increased water activity in packaging because it promotes the growth of microorganisms. The control CP films exhibited a contact angle of 89.1° ± 0.12°. It may be due to the availability of excess hydroxyl groups through unbonded glycerol molecules. Generally, hydrophilic films exhibit a water contact angle of less than 90°, while hydrophobic films display a water contact angle of more than 90°.48 The WCA values of CPT-1 and CPT-2 intelligent biodegradable films rise by 95.8° ± 0.16a and 99.0° ± 0.13c, respectively, as the concentration of TP extract increases, as represented in Fig. 2(b). The lesser availability of –OH groups to form the hydrogen bond with water molecules caused a decrease in the adhesive nature of intelligent biodegradable films. A similar enhancement in the contact angle was reported by Li-Ting Wu et al. by incorporating Clitoria ternatea (CT) anthocyanin extracts into gellan gum (G) film.58
4.4. Water solubility (WS) and moisture adsorption (MA)
The prepared films were water-resistant, which influenced the physico-mechanical characteristics in food packaging applications. The fabricated intelligent biodegradable film's water solubility values are presented in Table 1; the control CP films demonstrated a solubility of 22.29 ± 0.15% during 24 h. With the addition of the TP anthocyanin extract, the solubility of CPT-1 and CPT-2 intelligent biodegradable films decreased by 17.61 ± 0.13% and 15.31 ± 0.12%, respectively. The reduced solubility of films involving CS and PU may be due to the enhanced surface area of the CS upon contact with PU, which results in intermolecular hydrogen bonding.59 However, free unbonded glycerol may lead to greater solubility in the CP film. In contrast, a 7% decrement in the CPT-2 intelligent biodegradable films' solubility compared to the control CP film interaction of polyphenolic groups of TP extract with –NH2 and –OH groups of chitosan, pullulan, and unbonded glycerol.| CP | CPT-1 | CPT-2 | |
|---|---|---|---|
| Thickness (µm) | 80 ± 4a | 80 ± 3.5b | 90 ± 2c |
| Water solubility (%) | 22.29 ± 0.16a | 17.602 ± 0.13b | 15.313 ± 0.12c |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Moisture adsorption (%) | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RH 19% RH 19% |
3.16 ± 0.12b | 2.76 ± 0.13a | 2.24 ± 0.11c |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RH 50% RH 50% |
4.53 ± 0.11c | 3.86 ± 0.14a | 3.13 ± 0.13b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RH 75% RH 75% |
5.51 ± 0.14c | 5.09 ± 0.17a | 4.61 ± 0.15b |
| WVTR (g per m2 per 24 h) | 34.61 ± 0.25c | 30.02 ± 0.31a | 26.55 ± 0.28b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Degradation in soil (%) | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Day-10 Day-10 |
5.32 ± 0.13a | 4.79 ± 0.11b | 3.23 ± 0.12c |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Day-20 Day-20 |
16.34 ± 0.21a | 10.02 ± 0.18b | 8.91 ± 0.15c |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Day-30 Day-30 |
25.34 ± 0.24a | 19.64 ± 0.20c | 16.82 ± 0.21b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Antioxidant activity (%) | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPH DPPH |
58.26 ± 1.59c | 75.16 ± 1.55b | 89.81 ± 1.47a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ABTS ABTS |
54.97 ± 1.52a | 69.73 ± 1.48c | 85.14 ± 1.46b |
Packaged food must resist deterioration from moisture adsorption. Therefore, the packing material is crucial for protecting food from microbial harm caused by excessive moisture absorption, which is conducive to microbial development.60 Relative humidity is essential for moisture absorption. The fabricated films were evaluated at three relative humidity levels: 19%, 50%, and 75%. According to the data in Table 1, compared to CPT intelligent biodegradable films, the control CP has a significant moisture uptake under all relative humidity levels. The reduction in moisture absorption may be attributed to the addition of TP extract, which is due to the dense surface morphology and reduced availability of –OH groups in CPT intelligent biodegradable films. The findings on water solubility and moisture adsorption suggest that CPT intelligent biodegradable films could serve as a suitable food packaging material.
4.5. Water vapour transmission rate (WVTR) and soil burial test
Water vapour transmission significantly depends on the interaction between polymers and active agents. The homogeneous and dense surface of the fabricated film ensures the entrapment of moisture in packed food and prevents water vapor from the environment from entering the package. In the present study, the control CP film showed the highest transmission rate of water, 34.61 ± 0.25 g per m2 per 24 h, due to the presence of polar groups in CS (–NH2 and –OH), PU (–OH), and glycerol (–OH). As the anthocyanin content of TP extract increased in CPT-1 and CPT-2 intelligent biodegradable films, intermolecular hydrogen bonding reduced the availability of free polar groups, thereby restricting water molecules from passing through the film layer. Hence, CPT-1 and CPT-2 intelligent biodegradable films exhibit lower WVTR values of 30.05 ± 0.31a and 26.55 ± 0.28b g per m2 per 24 h is due to filling the structural void between polymers with the TP anthocyanin extract as the active agent. A similar decline in the WVTR value resulted in chitosan films with the addition of starchy powder from white turmeric rhizomes.61The packaging materials in the market are primarily made of plastic, and after their usefulness, they end up in landfills, generating microplastics that have profound health implications. Hence, the fabricated intelligent biodegradable film should be degradable and eco-friendly. Soil burial tests were conducted for 10, 20, and 30 d to evaluate the biodegradability of the CP and CPT intelligent biodegradable films. The soil microbes initially colonize the film after burial, generating film fragments. The enzymatic function of extracellular hydrolases depolymerized the polymer content, resulting in the creation of CO2 and water. These benefits of microbial activity in soil promote degradation and improve the nutrient cycle.62 After 30 d, control CP films exhibited a 25.34 ± 0.24% degradation. It is higher than the degradation rates of CPT-1 (19.64 ± 0.2%) and CPT-2 (16.82 ± 0.21%) in intelligent biodegradable films, as mentioned in Table 1. CPT intelligent biodegradable films express enhanced antibacterial activity due to the incorporated anthocyanin content of TP extract, restrict the colonization of microorganisms, and slow down the degradation rate compared to control CP films. The rate of degradation of fabricated CP and CPT intelligent films is indicative of the environmental sustainability, considering CPT intelligent biodegradable films as an alternative to conventional PE packaging.
4.6. Antimicrobial activity against foodborne pathogens
The antimicrobial ability of packaging materials is essential from a consumer's health perspective. According to the World Health Organisation (WHO) report, over 200 diseases are caused by unsafe food, i.e., contaminated by harmful foodborne bacteria and fungi.63 In the present study, S. aureus (ATCC 6538), B. subtilis (MTCC 13343) (Gram +ve), E. coli (ATCC 10799) (Gram −ve), and C. albicans (ATCC 24433) (fungi) are considered for analysis of the antimicrobial activity of the CP and CPT intelligent biodegradable films. Literature studies show evidence that S. aureus causes foodborne illness mainly due to consuming ready-to-eat foods contaminated from the nasopharynx, which produces enterotoxins leading to vomiting and diarrhea. Bacillus pathogens often cause food poisoning by contaminating food.64,65Similarly, toxins produced by spoilage due to E. coli can affect the human central nervous system and kidneys upon consumption.65 For all these reasons, food contaminated by foodborne microorganisms is dangerous for consumption. Intelligent biodegradable packaging films that exhibit activity against foodborne pathogens are necessary to prevent spoilage and ensure consumer health. The control CP films demonstrated antimicrobial activity against all tested pathogens, attributed to the inherent antimicrobial nature of chitosan. The inhibition zones measured 18 ± 0.17 mm and 14 ± 0.11 mm for Gram-positive Bacillus subtilis and Staphylococcus aureus, 14 ± 0.17 mm for Gram-negative Escherichia coli, and 13 ± 0.11 mm for the fungal strain Candida albicans.39,48 As shown in Fig. 3, the CPT-2 intelligent biodegradable films exhibited enhanced antimicrobial efficacy, with inhibition zones of 26 ± 0.24 mm and 17.5 ± 0.18 mm for B. subtilis and S. aureus, 18.5 ± 0.15 mm for E. coli, and 15 ± 0.12 mm for C. albicans, respectively.
 | ||
| Fig. 3 Zone of inhibition and CFU against S. aureus, B. subtilis, E. coli, and C. albicans for control CP and CPT intelligent biodegradable films. | ||
The extensive zone of inhibition resulting from the CPT-2 intelligent biodegradable films compared to the control CP film is due to the polyphenolic proportion present in the CPT-2, specifically the anthocyanin content of the TP extract. Gram-positive and Gram-negative bacteria have well-protected cell walls composed of peptidoglycan, which protects against osmotic pressure. The anthocyanin (polyphenol) content of TP extract in CPT-2 intelligent biodegradable active films inhibits bacterial growth by disrupting phospholipid or lipid bilayers in the cell wall, thereby enhancing membrane permeability and altering ion transport.66,67 Hence, the growth suppression of pathogenic cells was ensured by an excellent zone of inhibition exhibited by the CPT-2.
These findings demonstrate that the fabricated CPT-2 intelligent biodegradable films may be employed in food packaging to extend shelf life without the risk of food deterioration and foodborne pathogen-related illnesses.
4.7. Antioxidant activity
The imbalance between the antioxidant defense and free radical generation is the cause of oxidative stress. Oxidative stress initiates damage to molecular species, such as lipids and proteins.68 Free radicals are highly reactive species capable of causing cell damage in biologically relevant carbohydrates, lipids, and proteins, and are also a contributing factor to homeostatic disruption.69,70 During fresh meat processing, antioxidants are added to counteract oxidation, which can cause spoilage.71 Hence, antioxidant activity is necessary for food packaging materials to avoid directly incorporating antioxidants into food. DPPH and ABTS scavenging assays were used to evaluate the antioxidant activity of CP and CPT intelligent biodegradable active films.In Table 1, control CP films exhibit 58.26 ± 1.59% and 54.9 ± 1.55% of antioxidant activity in DPPH and ABTS assays, respectively. Due to the anthocyanin content present in the TP extract, CPT-2 intelligent biodegradable films exhibit activity of 89.81 ± 1.47% and 85.14 ± 1.52%. The polyphenolic content in CPT-2 intelligent film may initiate the chain-breaking mechanism, fulfilling the additional requirement of electrons for free radicals as they act as a primary antioxidant donor.72 Through this phenomenon, polyphenols help maintain a balanced oxidative stress, and DPPH and ABTS assay analysis confirmed that the fabricated CPT intelligent biodegradable films effectively preserve packed food from oxidative deterioration. Similar enhancements in antioxidant activity have been reported by Amina Sadi et al.73 for incorporating anthocyanin content from red cabbage into a CMC/gelatin polymer blend51 and by Gabriel da Silva Filipini et al. for methylcellulose films with Syzygium cumini skin extract.74
4.8. Preservation of chicken meat and biochemical assessment of its nutrients
Around the world, most people regularly include eggs and chicken in their diet due to their rich source of protein. According to a United Nations report by the Food and Agriculture Organization, 73 million tons of eggs and 100 million tons of chicken were produced worldwide in 2016.75 Due to human lifestyle changes, urbanization, and growing populations, demand increases rapidly each year.76,77 Poultry is the industry's second-largest source of meat, meaning that poultry products are a significant contributor to foodborne illnesses. Meeting the market's demand for well-preserved chicken meat in the face of microbial attacks that cause spoilage is a primary concern nowadays. Therefore, the current study focuses on using sustainable CPT-2 intelligent biodegradable film packaging as an alternative to plastic packaging to protect chicken meat from foodborne microbe spoilage.The chicken was packed in plastic (PE), control-CP film, and CPT intelligent biodegradable film to evaluate the effectiveness of packaging. The chicken had been packaged and stored at room temperature, including with S. aureus and E. coli foodborne pathogens for 24, 48, and 72 h. Following the incubation period, 2 g of chicken flesh was blended for 0.5 min using a tissue homogenizer (Labico, LT-451, Labtech Instruments, India) with 18 ml of 0.9% saline, and then for an additional 0.5 min using a vortex. The mixture was passed through a single, four-layered muslin fabric filter. The CMBS was further subjected to biochemical characterizations to evaluate nutritional levels.
4.8.1.1. Total bacterial count (TBC). Since chicken meat is very perishable and readily contaminated by microorganisms, it undergoes decontamination by modifying its nutritional value. Plastics are mainly used for packaging meat products, and packaging waste accounts for a significant portion of municipal waste. Single-use plastic packaging degrades meat products since it lacks antibacterial and antioxidant properties. This investigation employed plastic, control CP, and CPT-2 intelligent biodegradable packaging film to package chicken meat. Instead of refrigeration to prolong its shelf life, the packaged chicken flesh was kept at room temperature for 24, 48, and 72 h to evaluate the CPT intelligent biodegradable film's preservation capacity. The overall bacterial growth was expressed as log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g of chicken, with a fresh chicken bacterial count of 4.956
CFU per g of chicken, with a fresh chicken bacterial count of 4.956![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g. According to the International Commission on Microbiological Specifications for Foods (ICMSF), the permissible microbiological limit for edible meat is 7
CFU per g. According to the International Commission on Microbiological Specifications for Foods (ICMSF), the permissible microbiological limit for edible meat is 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g.78
CFU per g.78
Microbial growth data for three packaging types are presented in Fig. 4 and Table S3. After 24 hours of incubation at room temperature, chicken packed in conventional plastic showed a bacterial count of 6.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g. This elevated count, attributed to plastic's inability to inhibit microbial activity, led to spoilage within a day. In contrast, CP films, leveraging the natural antibacterial properties of chitosan, preserved chicken freshness for a longer duration. Notably, chicken packed with CPT-2 intelligent biodegradable films, which incorporate polyphenolic TP extract and anthocyanins, achieved an extended shelf life of up to two days, with microbial growth reaching 6.99
CFU per g. This elevated count, attributed to plastic's inability to inhibit microbial activity, led to spoilage within a day. In contrast, CP films, leveraging the natural antibacterial properties of chitosan, preserved chicken freshness for a longer duration. Notably, chicken packed with CPT-2 intelligent biodegradable films, which incorporate polyphenolic TP extract and anthocyanins, achieved an extended shelf life of up to two days, with microbial growth reaching 6.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g. The enhanced preservation effect is likely due to altered ion transport and lipid bilayer disruption in microbial cells. Comparable results have been reported by S. R. Kanatt et al., demonstrating that the inclusion of amaranthus leaf extract (ALE) anthocyanins in PVA packaging films extends the shelf life of chicken meat.79
CFU per g. The enhanced preservation effect is likely due to altered ion transport and lipid bilayer disruption in microbial cells. Comparable results have been reported by S. R. Kanatt et al., demonstrating that the inclusion of amaranthus leaf extract (ALE) anthocyanins in PVA packaging films extends the shelf life of chicken meat.79
 | ||
| Fig. 4 Total bacterial count (TBC) of chicken meat preservation carried out in PE, control CP, and CPT-2 intelligent biodegradable films. | ||
4.8.1.2. Total bacterial count by inclusion of foodborne pathogens. To analyze packaging effectiveness against contamination involving aerobic mesophilic bacteria, such as S. aureus and E. coli, in the present study. Chicken packaging was preserved at room temperature, and the aerobic mesophilic bacteria grew and thrived in the moderate temperature range of 20–45 °C.80 When contaminated food is consumed, these microorganisms can cause severe health issues.81 When chicken, seafood, and red meat are contaminated with S. aureus, it frequently leads to food poisoning.82 The plastic packaging has a log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g of chicken count ranging from 7.36 to 10.89. However, the CPT-2 intelligent biodegradable films packaging has a log
CFU per g of chicken count ranging from 7.36 to 10.89. However, the CPT-2 intelligent biodegradable films packaging has a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g of chicken ranging from 5.67 to 8.84 for 24 to 72 h. Similarly, E. coli also negatively affects human health and is used as a hygiene indicator in poultry-related food manufacturers.83 After 72 hours of incubation, CPT-2 intelligent biodegradable packaging showed 8.97
CFU per g of chicken ranging from 5.67 to 8.84 for 24 to 72 h. Similarly, E. coli also negatively affects human health and is used as a hygiene indicator in poultry-related food manufacturers.83 After 72 hours of incubation, CPT-2 intelligent biodegradable packaging showed 8.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g compared to plastic packaging, which had 10.87
CFU per g compared to plastic packaging, which had 10.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU per g.
CFU per g.
Both pathogenic results suggested that the CPT-2 intelligent biodegradable packaging is superior to single-use plastic packaging. The anthocyanin content in the active agent successfully inhibits microbial growth. The active agent in the polymer network effectively acted against foodborne pathogens by penetrating the cell wall, resulting in suppression of pathogenic growth. Intelligent biodegradable packaging successfully prolongs the shelf life by ensuring the quality of the meat.84
 | ||
| Fig. 5 Visual identification of spoilage of chicken meat using CPT-2 intelligent biodegradable film as an intelligent indicator of pH variation. | ||
The primary sources impacted by the microbial attack that causes spoilage during storage include the availability of lactic acid, glucose, amino acids, and other nitrogenous substances used as energy sources in meat.86,87 Ammonia (NH3) and methylamines are the amine sources obtained by biomolecules due to microbial and enzyme decomposition of meat products.88 As a result, as meat spoilage is initiated, its total volatile basic nitrogen (TVB-N) value increases.89 In the current investigation, the TVB-N variation in chicken meat packed in plastic, control CP, and CPT-2 intelligent biodegradable films is presented in Fig. 6. During a 24 h packing period, chicken packaged in plastic has a TVB-N value of 13.52 mg per 100 g. However, chicken packaged in CPT-2 intelligent biodegradable film has a value of 8.49 mg per 100 g. It may be due to the anthocyanin content in CPT-2 intelligent biodegradable film's significant antibacterial and barrier qualities, which prevent microbial spoiling and protein degradation, attributed to a reduced TVB-N value.87 Senapati and Sahu (2020)90 state that the acceptable TVB-N levels for dietary chicken meat fall between 25 to 28 mg per 100 g. After 72 h of preservation, the CPT-2 intelligent packaging chicken meat has a TVB-N value of 23.47 mg per 100 g. At the same time, plastic packaging contains 38.86 mg per 100 g, while control CP film contains 30.42 mg per 100 g. Considering these findings, it can be concluded that CPT-2 intelligent biodegradable packaging effectively extends the shelf life of chicken meat by preventing spoilage due to the production of ammonia and ammonium salts over 72 h at room temperature.
 | ||
| Fig. 6 Spoilage analysis (TVB-N, acidity index, and impact of UV light) of chicken by packaging in PE, control CP, and CPT-2 intelligent biodegradable films. | ||
The impact of foodborne microorganisms, specifically Staphylococcus aureus and Escherichia coli, on total volatile basic nitrogen (TVB-N) levels, an indicator of spoilage, was investigated in chicken meat packaged using plastic, control CP, and CPT-2 intelligent biodegradable films. E. coli is a Gram-negative bacteria well known for food spoilage on contamination; these pathogens utilize mixed acids and trimethylamine oxide (TMAO) to produce slime production with an off odour by producing formic acid, ethanol, and trimethylamine (TMA) as spoilage products.86,91 Hence, the plastic packaging lacked resistance against microbial growth, and chicken meat spoiled within a day at room temperature (19.47 mg per 100 g). Due to their inherent antimicrobial properties, CPT-2 intelligent biodegradable films restrict microbial growth and extend the shelf life to 2 days at room temperature (19.57 mg per 100 g). In the case of S. aureus contamination, these pathogens utilize glucose, amino acids, and TMAO to produce souring and off-odour in meat by producing acetate, pyruvate, succinate, and TMA as spoilage products.87,92 Similar TBN-V values are observed with plastic packaging, acceptable freshness up to a day (20.94 mg per 100 g), and extension up to 48 h with CPT-2 intelligent biodegradable packaging (21.28 mg per 100 g). The slight enhancement in the TVB-N values with S. aureus compared to E. coli may be due to the thick outer peptidoglycan layers of Gram +ve bacteria, which make it difficult to penetrate the cell of bacteria by an active agent to neutralize the spoilage action.39
The rapid increase in chicken meat's TVB-N value reduces the meat's glucose content.93 The microbial deterioration of meat products is associated with the conversion of TMAO, which results in the formation of spoilage-initiating byproducts such as TMA, DMA, and formaldehyde. Hence, packaging material should have antimicrobial properties to avoid spoilage. Foodborne pathogens (S. aureus and E. coli) adapt to facultative anaerobic growth conditions. In the absence of oxygen, it switches to fermentation and proliferates in the presence of oxygen. Hence, the packaging material has good barrier properties. Incorporated anthocyanin content creates a compact structure with a polymer blend and enhances the barrier properties.94 Therefore, due to the improved antimicrobial ability and barrier nature of CPT-2 intelligent biodegradable films with the incorporation of anthocyanin content, TP extract is an active agent, extending the shelf life of packed chicken meat compared to traditional plastic packaging at room temperature.
Oleic acid is a monounsaturated omega-9 fatty acid (C18:1) commonly found in animal fats and vegetable oils.97 The plastic packaging, which is similar to normal chicken packaging and includes foodborne pathogens, exhibits a comparatively higher acidity index of total free soluble fat than chicken packed in CP and CPT-2 intelligent biodegradable films, respectively. Lipid oxidation primarily occurs due to photo- and enzyme-catalyzed oxidation.97,98 Photooxidation mainly occurs in the presence of light and oxygen. Enzymatically catalyzed oxidation is caused by the action of lipases produced by microorganisms, such as bacteria and molds, which arises due to spoilage. Lipolysis forms glycerol and fatty acids by the hydrolysis of triglycerides.87 Hence, the lack of antimicrobial properties of plastic packaging attributed to an increase in the acidity index of total free soluble fat by 1.61 g of oleic acid equivalents per 100 g of meat in normal chicken packaging, and by including foodborne pathogens, it increased by 1.71 and 1.69 g of oleic acid equivalents per 100 g of meat, respectively. In the case of CP and CPT-2 intelligent biodegradable packaging, the inherent antimicrobial properties, due to the anthocyanin content in the packaging environment, adversely impact pathogen growth. Hence, the acidity index is restricted by 1.56 and 1.38 g of oleic acid equivalents per 100 g of meat in normal packaging. On inclusion of S. aureus 1.64 and 1.44 g of oleic acid equivalents per 100 g of meat, while with E. coli 1.63 and 1.49 g of oleic acid equivalents per 100 g of meat, respectively. Therefore, reducing lipid oxidation in CPT-2 intelligent packaging prolongs the shelf life of packed chicken compared to control CP as well as plastic packaging.
4.9. Impact of UV treatment on packaged chicken meat
The UV radiation present in food is detrimental to consumption because it activates free radicals through the initiation of photochemical reactions. These undesirable photochemical reactions damage food products by digesting protein, oxidizing lipids, causing color changes, reducing vitamin content, and producing foul odors.99,100 The packaging films should have better UV shielding properties to protect the food from these unwanted photochemical reactions caused by UV impact. The impact of UV radiation on chicken meat was analyzed by determining the free thiol (–SH) groups present after 20, 40, and 60 min of irradiation.Sulfur containing amino acids, cysteine, and glutathione are the compounds that act as antioxidants in meat products, consisting of the thiol groups (–SH). These protect meat products from oxidative stress and prevent lipid and protein oxidation.101 Hence, by assessing the impact of UV radiation on thiol groups present in the meat as uncovered, covered with plastic, and CPT-2 intelligent packaging film. According to Fig. 6, which illustrates the percentage of UV radiation transmitted through plastic and CPT-2 intelligent biodegradable films, UV-C penetration at 280 nm was 65% in plastic and 5% in CPT-2 intelligent biodegradable films. Similarly, the CPT-2 intelligent biodegradable film's UV-B (at 320 nm) and UV-A (at 400 nm) area penetration of around 6% and 33%, respectively, was noticeably lower than that of the plastic film, which was 85% and 88%, respectively. UV-B and UV-A radiation frequently cause lipid oxidation, vitamin breakdown, and decolorization of meat products.102 According to this finding, the CPT-2 intelligent biodegradable films prevented the meat items from spoiling by effectively acting as a UV barrier.
UV irradiation affected the free thiol groups, and Ellman's test was used to determine the thiol groups in the chicken meat after 20, 40, and 60 minutes of irradiation. As a result of successive thiol groups oxidizing when exposed to UV radiation, thiol groups oxidized in proteins led to the formation of –S–S– (disulfide) bonds and also caused the protein crosslinking.103 The protein network is disrupted by the polymerization of proteins, which significantly affects softness, and by the uncontrolled oxidation of thiol caused by UV light, which causes food to spoil.104 The percentage of decrease in the thiol groups after UV irradiation was presented in Fig. 6. After 60 minutes of UV irradiation, the uncovered chicken meat declined by 50% in free thiol groups; in contrast, the chicken enclosed in PE packaging was almost as close to the uncovered chicken, with 43.75% of the drop indicating impotent behaviour of PE packaging against UV radiation. After 60 minutes of radiation, the CPT-2 intelligent biodegradable film packaging effectively blocks UV penetration, reducing the oxidation of thiol groups by only 15.63%. These findings confirm the optical properties of CPT-2 intelligent biodegradable films, demonstrating their suitability as a packaging material with UV barrier properties.
In the biological system, UV light produces reactive oxygen species (ROS), which interact with free thiol groups to initiate oxidative damage. The depletion of thiol groups correlates with the antioxidant capacity of meat products. In the current investigation, the DPPH scavenging assay revealed that fresh chicken meat has an antioxidant activity of 20.42%. After 60 minutes of UV irradiation, the antioxidant capacity of uncovered meat remained at only 3%, while PE-covered meat reached 5.25%. In the case of CPT-2 intelligent biodegradable films covering chicken meat, it retained its antioxidant capacity at 16.49%. Antioxidants, including phenol components, shield proteins and lipids from oxidation, which might disrupt the disulfide network.105,106 The polyphenolic content present in CPT-2 intelligent biodegradable film, as well as the anthocyanin content of TP extract, is also capable of protecting proteins and lipids from oxidation. Therefore, chicken meat covered with CPT-2 intelligent biodegradable films showed a slight decrease in antioxidant capacity and a percentage decline in thiol content compared to PE packaging. According to Sisse Jongberg et al., a phenolic extract from green tea can prevent thiol oxidation in chicken meat.104
The enhancement in physicochemical properties and the superior capability of CPT-2 intelligent biodegradable films against microbial attacks to preserve the nutritional value of packed meat at room temperature confirm that the fabricated biodegradable film of a chitosan and pullulan blend incorporating TP extract as an active agent is a suitable alternative to PE packaging and holds promise for a sustainable future.
5. Conclusions
Poultry represents one of the significant sources of meat production, where effective preservation is essential to satisfy consumer demand. However, the meat packaging industry continues to rely predominantly on single-use plastic materials, contributing substantially to environmental pollution. In this context, the present study developed chitosan/pullulan-based CPT-2 intelligent biodegradable films incorporating 200 mg of Tradescantia pallida (TP) extract as an active and intelligent packaging alternative to conventional polyethylene (PE) films. The CPT-2 intelligent biodegradable films demonstrated enhanced preservation performance by suppressing bacterial growth, the release of ammonia and ammonium salts, and lipid oxidation regarding free fatty acid content at room temperature, thereby extending the shelf life of chicken up to 2 days at room temperature, which is an environment favourable for foodborne pathogen activity compared to refrigeration temperature. In contrast, PE packaging maintained quality for only 1 day under similar conditions.Despite their promising functionality and complete biodegradability, cost optimization remains a significant challenge for the commercialization of CPT-2 intelligent biodegradable films. The current development corresponds to a Technology Readiness Level (TRL) of 4–5, indicating successful laboratory validation but requiring further investigation to advance toward TRL-9 for industrial-scale application as a cost-effective substitute for single-use plastics (SUP). Considering that all polymers used are food-grade and that Tradescantia pallida (TP) is a readily available, drought-tolerant, widely cultivated, and simple-to-propagate plant through cuttings, it offers a sustainable and economical source of active agents. Therefore, future research should focus on optimizing fabrication techniques and production costs to enhance scalability and economic feasibility. Overall, this study confirms that T. pallida extract serves as a viable and cost-effective intelligent component in biodegradable packaging films, highlighting the need for continued innovation to replace SUPs and ensure a sustainable future for the upcoming generation.
Consent for publication
All authors agreed to publish the paper.Author contributions
Manjunath P. Eelager: conceptualization; data curation; formal analysis; methodology; visualization; resources; funding; software; roles: writing – original draft; review & editing. Saraswati P. Masti: the corresponding author; investigation; supervision; validation; writing – review & editing, visualization. Suhasini Madihalli: investigation; formal analysis. Ravindra B. Chougale: formal analysis. Nagarjuna Prakash Dalbanjan: data curation, resources, formal analysis. S. K. Praveen Kumar: formal analysis.Conflicts of interest
The authors declare that they have no competing interests.Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.Supplementary information: tables presenting the optimization parameters for the base matrix (CS/PU), composition details of the control (CP) and CPT intelligent biodegradable films, zone of inhibition and colony-forming unit (CFU) analysis of CP and CPT films against various foodborne pathogens, total bacterial count (TBC) of chicken meat (normal and inoculated with S. aureus and E. coli) packaged in PE, CP, and CPT-2 films, and a comparative assessment of tensile strength, antimicrobial, and antioxidant properties among different intelligent compounds incorporated into the polymer blend films. Additionally, it also consists of the results and discussion related to the total protein content, antioxidant capability of chicken meat after the incubation period, and the tentative cost analysis and life cycle assessment (LCA) of the CPT-2 intelligent packaging film. See DOI: https://doi.org/10.1039/d5fb00349k.
Acknowledgements
Manjunath P. Eelager, one of the authors, is thankful to “KSTEPS, DST, GOVT. OF KARNATAKA” for the DST PhD Fellowship-2021 (DST/KSTePS/Ph. D. Fellowship/CHE-04:2021-22/1005, Manjunath P. Eelager) for their financial assistance and BioRender application to optimise the graphical abstract. All authors are grateful to DST-SERB for aid in the purchase of UTM (Dr Saraswati P. Masti, Principal investigator, under project sanction letter no. SB/EMEQ-213/2014, dated 29-01-2016) for mechanical characteristics measurements, other laboratory equipment, and chemicals. The authors also thank the principal and the head of the chemistry department at Karnatak Science College, Dharwad-580 001, for providing the necessary infrastructure facilities. The Director of the University Scientific Instrumentation Centre (USIC) and the DST PURSE Phase II program Coordinator, K. U. Dharwad, provide an instrumental facility.References
- P. J. Landrigan, H. Raps, M. Cropper, C. Bald, M. Brunner, E. M. Canonizado, D. Charles, T. C. Chiles, M. J. Donohue and J. Enck, Ann. Glob. Health, 2023, 89, 23 CrossRef PubMed.
- S. Food Packaging Market Size, Trends & Growth [2030], Market Research Report, https://www.fortunebusinessinsights.com/industry-reports/food-packaging-market-101941 Search PubMed.
- B. Dewangan, M. Mittal and M. P. Eelager, Sustainable Chem. Pharm., 2025, 46, 102092 CrossRef CAS.
- S. Ayu Rafiqah, A. Khalina, K. Zaman, I. Tawakkal, A. Harmaen and N. M. Nurrazi, Bio-Based Packaging: Material, Environmental and Economic Aspects, 2021, pp. 335–351, DOI:10.1002/9781119381228.ch19.
- S. Sid, R. S. Mor, A. Kishore and V. S. Sharanagat, Trends Food Sci. Technol., 2021, 115, 87–104 CrossRef CAS.
- M. P. Eelager, N. P. Dalbanjan, S. Madihalli, M. Madar, N. P. Agadi, K. Korganokar and B. K. Kiran, Sustainable Futures, 2025, 10, 101208 CrossRef.
- S. Madihalli, S. P. Masti, M. P. Eelager, R. B. Chougale, L. K. Kurabetta, A. A. Hunashyal, N. P. Dalbanjan and S. K. Praveen Kumar, Food Biosci., 2024, 62, 105492 CrossRef CAS.
- S. Madihalli, S. P. Masti, M. P. Eelager, R. B. Chougale, B. M. Anilkumar and A. N. Priyadarshini, Int. J. Biol. Macromol., 2025, 303, 140611 CrossRef CAS.
- N. P. Dalbanjan, K. Korgaonkar, M. P. Eelager, B. N. Gonal, A. J. Kadapure, S. B. Arakera and S. K. Praveen Kumar, Nano TransMed, 2025, 4, 100091 CrossRef.
- V. D. Hiremani, N. Goudar, S. Khanapure, T. Gasti, M. P. Eelager, S. S. Narasagoudr, S. P. Masti and R. B. Chougale, J. Food Meas. Charact., 2023, 17, 1548–1561 CrossRef.
- K. Kraśniewska, K. Pobiega and M. Gniewosz, Int. J. Food Eng., 2019, 15, 20190030 Search PubMed.
- G. Mugnaini, C. Resta, G. Poggi and M. Bonini, J. Colloid Interface Sci., 2021, 592, 430–439 CAS.
- N. P. Dalbanjan, M. P. Eelager and S. S. Narasagoudr, Food Humanity, 2024, 3, 100366 Search PubMed.
- P. R. Vuddanda, M. Montenegro-Nicolini, J. O. Morales and S. Velaga, Eur. J. Pharm. Sci., 2017, 96, 290–298 Search PubMed.
- M. Chen, T. Runge, L. Wang, R. Li, J. Feng, X.-L. Shu and Q.-S. Shi, Carbohydr. Polym., 2018, 200, 115–121 Search PubMed.
- V. Shukla, G. Kandeepan, M. R. Vishnuraj and A. Soni, Agric. Res., 2016, 5, 205–209 CAS.
- Y. Qin, Y. Liu, X. Zhang and J. Liu, Food Hydrocolloids, 2020, 100, 105410 CAS.
- T. Gasti, S. Dixit, O. J. D'souza, V. D. Hiremani, S. K. Vootla, S. P. Masti, R. B. Chougale and R. B. Malabadi, Int. J. Biol. Macromol., 2021, 187, 451–461 CAS.
- N. Hidayah, M. S. Hawa, B. Purwono and S. N. Rahmadhia, Trends Sci., 2025, 22, 10077 Search PubMed.
- M. Butnariu, Á. Fernández Ochoa, A. Segura Carretero and M. d. l. L. Cádiz Gurrea, Front. Biosci., 2022, 27, 197, DOI:10.31083/j.fbl2706197.
- F. Imtiaz, M. Islam, H. Saeed, A. Ahmed, F. K. Hashmi, K. M. Khan, U. I. Dar, K. Ullah, S. M. Rana and B. Saleem, Pharmaceutics, 2022, 14, 2578 CAS.
- D. Yıldız, Global Poultry Industry and Trends, https://www.feedandadditive.com/global-poultry-industry-and-trends/ Search PubMed.
- ICPE, Packaging of Meat and Poultry Products, https://www.icpe.in/icpefoodnpackaging/pdfs/19_meat.pdf Search PubMed.
- L. Cabernard, S. Pfister, C. Oberschelp and S. Hellweg, Nat. Sustainability, 2022, 5, 139–148 Search PubMed.
- M. Madar, V. Srinivasan, M. P. Eelager, N. P. Dalbanjan, K. N. Kummur, V. Nayak, M. M. Basanagouda and A. Sidarai, Tetrahedron, 2025, 185, 134796 CrossRef CAS.
- V. A. Pereira Jr, I. N. Q. de Arruda and R. Stefani, Food Hydrocolloids, 2015, 43, 180–188 CrossRef.
- M. P. Eelager, S. P. Masti, S. Madihalli, N. Gouda, L. K. Kurbetta, M. N. Gunaki, A. A. Hunashyal and R. B. Chougale, J. Environ. Chem. Eng., 2025, 13, 116029 CrossRef CAS.
- S. Madihalli, S. P. Masti, M. P. Eelager, R. B. Chougale, N. P. Dalbanjan and S. K. Praveen Kumar, Int. J. Biol. Macromol., 2025, 302, 140926 CrossRef CAS PubMed.
- R. Mirji, B. Lobo, D. Dutta, S. P. Masti and M. P. Eelager, Appl. Radiat. Isot., 2023, 196, 110773 CrossRef CAS PubMed.
- S. Madihalli, S. P. Masti, M. P. Eelager, M. N. Gunaki, R. B. Chougale, N. P. Dalbanjan and S. K. Praveen Kumar, Sustainable Food Technol., 2025, 1035–1052 RSC.
- M. P. Eelager, S. P. Masti, N. P. Dalbanjan, S. Madihalli, M. N. Gunaki, L. K. Kurbetta, S. K. Praveen Kumar and R. B. Chougale, Prog. Org. Coat., 2024, 192, 108510 CrossRef CAS.
- M. P. Eelager, S. P. Masti, R. B. Chougale, N. P. Dalbanjan and S. K. Praveen Kumar, Int. J. Biol. Macromol., 2024, 269, 132270 CrossRef CAS PubMed.
- L. K. Kurabetta, S. P. Masti, M. P. Eelager, M. N. Gunaki, S. Madihalli, A. A. Hunashyal, R. B. Chougale, S. K. Praveen Kumar and A. J. Kadapure, Int. J. Biol. Macromol., 2023, 253, 127552 CrossRef CAS.
- L. K. Kurabetta, S. P. Masti, M. N. Gunaki, A. A. Hunashyal, M. P. Eelager, R. B. Chougale, N. P. Dalbanjan and S. K. Praveen Kumar, Int. J. Biol. Macromol., 2024, 277, 134191 CrossRef CAS PubMed.
- M. N. Gunaki, S. P. Masti, O. J. D'Souza, M. P. Eelager, L. K. Kurabetta, R. B. Chougale, A. J. Kadapure and S. K. Praveen Kumar, Food Hydrocolloids, 2024, 152, 109937 CrossRef CAS.
- M. S. Blois, Nature, 1958, 181, 1199–1200 CrossRef CAS.
- N. P. Dalbanjan, L. Bheemayya, A. J. Kadapure, M. P. Eelager, T. M. C. Swamy, R. R. Kamble and S. K. Praveen Kumar, ASPET Discovery, 2025, 1, 100004 CrossRef.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radicals Biol. Med., 1999, 26, 1231–1237 CrossRef CAS.
- M. P. Eelager, S. P. Masti, R. B. Chougale, V. D. Hiremani, S. S. Narasgoudar, N. P. Dalbanjan and P. K. SK, Int. J. Biol. Macromol., 2023, 232, 123499 CrossRef CAS.
- I. Borovuk and N. Zazharska, J. Adv. Vet. Anim. Res., 2022, 9, 155 CrossRef PubMed.
- L. Zhou and C. E. Boyd, Aquaculture, 2016, 450, 187–193 CrossRef CAS.
- S. Guštin and R. Marinšek-Logar, Process Saf. Environ. Prot., 2011, 89, 61–66 CrossRef.
- B. Jiang, R. Tsao, Y. Li and M. Miao, Food Safety: Food Analysis Technologies/Techniques, in Encyclopedia of Agriculture and Food Systems, Elsevier, 2014, pp. 273–288 Search PubMed.
- O. Lowry, N. Rosebrough, A. L. Farr and R. Randall, J. Biol. Chem., 1951, 193, 265–275 CrossRef CAS PubMed.
- P. Cunniff, Official Methods of Analysis of AOAC International, AOAC International, Arlington, 1995, vol. 1 Search PubMed.
- V. Feddern, L. Kupski, E. P. Cipolatti, G. Giacobbo, G. L. Mendes, E. Badiale-Furlong and L. A. de Souza-Soares, Eur. J. Lipid Sci. Technol., 2010, 112, 1277–1284 CrossRef CAS.
- N. P. Dalbanjan, A. J. Kadapure, P. Huded, V. B. Chachadi, S. Nayaka and S. K. Kumar, Ukr. Biochem. J., 2022, 94, 61–66 CrossRef CAS.
- S. S. Narasagoudr, V. G. Hegde, V. N. Vanjeri, R. B. Chougale and S. P. Masti, Carbohydr. Polym., 2020, 236, 116049 CrossRef CAS PubMed.
- T. Gasti, S. Dixit, V. D. Hiremani, R. B. Chougale, S. P. Masti, S. K. Vootla and B. S. Mudigoudra, Carbohydr. Polym., 2022, 277, 118866 CrossRef CAS PubMed.
- M. F. Queiroz, K. R. Teodosio Melo, D. A. Sabry, G. L. Sassaki and H. A. O. Rocha, Mar. Drugs, 2014, 13, 141–158 CrossRef PubMed.
- S. Roy and J.-W. Rhim, Polymers, 2020, 12, 2665 CrossRef CAS.
- J. Ghaderi, S. F. Hosseini, N. Keyvani and M. C. Gómez-Guillén, Food Hydrocolloids, 2019, 95, 122–132 CrossRef CAS.
- L. H. Gaabour, AIP Adv., 2021, 11(10), 105120 CrossRef CAS.
- C. M. Yoshida, V. B. V. Maciel, M. E. D. Mendonça and T. T. Franco, LWT – Food Sci. Technol., 2014, 55, 83–89 CrossRef CAS.
- H. Saari, C. Fuentes, M. Sjöö, M. Rayner and M. Wahlgren, Carbohydr. Polym., 2017, 157, 558–566 CrossRef CAS PubMed.
- S. Mafirad, M. R. Mehrnia, P. Zahedi and S. N. Hosseini, Polym. Compos., 2018, 39, 4452–4466 CrossRef CAS.
- Z.-H. Zhang, Z. Han, X.-A. Zeng, X.-Y. Xiong and Y.-J. Liu, Int. J. Biol. Macromol., 2015, 81, 638–643 CrossRef CAS PubMed.
- L.-T. Wu, I.-L. Tsai, Y.-C. Ho, Y.-H. Hang, C. Lin, M.-L. Tsai and F.-L. Mi, Carbohydr. Polym., 2021, 254, 117410 CrossRef CAS PubMed.
- J. Wu, F. Zhong, Y. Li, C. Shoemaker and W. Xia, Food Hydrocolloids, 2013, 30, 82–91 CrossRef CAS.
- L. Vermeiren, F. Devlieghere, M. van Beest, N. de Kruijf and J. Debevere, Trends Food Sci. Technol., 1999, 10, 77–86 CrossRef CAS.
- V. D. Hiremani, S. Khanapure, T. Gasti, N. Goudar, S. K. Vootla, S. P. Masti, R. B. Malabadi, B. S. Mudigoudra and R. B. Chougale, Int. J. Biol. Macromol., 2021, 193, 2192–2201 CrossRef CAS.
- Y. Sun, W. Yang, H. Shi, S. K. Tanveer and J. Hai, Front. Bioeng. Biotechnol., 2022, 10, 1006388 CrossRef.
- WHO, Food Safety, https://www.who.int/news-room/fact-sheets/detail/food-safety Search PubMed.
- P. C. B. Turnbull, Medical Microbiology, 4th edn, 1996 Search PubMed.
- L. H. ALManseeqanaa and R. H. Ogaili, Acad. Int. J. Med. Sci., 2023, 1(2), 7–10 CrossRef.
- H. Kumar, K. Bhardwaj, N. Cruz-Martins, E. Nepovimova, P. Oleksak, D. S. Dhanjal, S. Bhardwaj, R. Singh, C. Chopra and R. Verma, Molecules, 2021, 26, 3447 CrossRef CAS PubMed.
- F. Nazzaro, F. Fratianni, L. De Martino, R. Coppola and V. De Feo, Pharmaceuticals, 2013, 6, 1451–1474 CrossRef PubMed.
- A. B. Falowo, P. O. Fayemi and V. Muchenje, Food Res. Int., 2014, 64, 171–181 CrossRef CAS PubMed.
- V. Lobo, A. Patil, A. Phatak and N. Chandra, Pharmacogn. Rev., 2010, 4, 118 CrossRef CAS.
- C. Kh, Br. Med. Bull., 1993, 49, 481–493 CrossRef PubMed.
- M. A. Shah, S. J. D. Bosco and S. A. Mir, Meat Sci., 2014, 98, 21–33 CrossRef CAS PubMed.
- A. M. Pisoschi, A. Pop, F. Iordache, L. Stanca, G. Predoi and A. I. Serban, Eur. J. Med. Chem., 2021, 209, 112891 CrossRef CAS PubMed.
- A. Sadi and H. Ferfera-Harrar, Int. J. Biol. Macromol., 2023, 242, 124964 CrossRef CAS PubMed.
- G. da Silva Filipini, V. P. Romani and V. G. Martins, Food Hydrocolloids, 2020, 109, 106139 CrossRef CAS.
- K. Wessels, D. Rip and P. Gouws, Foods, 2021, 10, 1742 CrossRef CAS PubMed.
- P. Magdelaine, M. Spiess and E. Valceschini, World's Poult. Sci. J., 2008, 64, 53–64 CrossRef.
- N. Alexandratos and J. Bruinsma, World Agriculture towards 2030/2050: The 2012 Revision, 2012, DOI:10.22004/ag.econ.288998.
- R. Priyadarshi, S.-M. Kim and J.-W. Rhim, Sustainable Mater. Technol., 2021, 29, e00325 CrossRef CAS.
- S. R. Kanatt, Food Packag. Shelf Life, 2020, 24, 100506 CrossRef.
- A. Wisal, A. Ullah, W. Anwar, C. M. Morel and S. S. Hassan, Genomics Inf., 2023, 21(3), e34 CrossRef PubMed.
- C. E. Manyi-Loh and R. Lues, Microorganisms, 2023, 11, 2484 CrossRef CAS PubMed.
- W. Yuan and H.-G. Yuk, Food Microbiol., 2018, 72, 176–184 CrossRef PubMed.
- Ç. Soysal, H. Bozkurt, E. Dirican, M. Güçlü, E. D. Bozhüyük, A. E. Uslu and S. Kaya, Food Control, 2015, 54, 294–299 CrossRef.
- J. H. Han, Antimicrobial Food Packaging, 2003 Search PubMed.
- E. Balbinot-Alfaro, D. V. Craveiro, K. O. Lima, H. L. G. Costa, D. R. Lopes and C. Prentice, Food Eng. Rev., 2019, 11, 235–244 CrossRef.
- R. K. Robinson, Encyclopedia of Food Microbiology, Academic Press, 2014 Search PubMed.
- A. E.-D. A. Bekhit, B. W. Holman, S. G. Giteru and D. L. Hopkins, Trends Food Sci. Technol., 2021, 109, 280–302 CrossRef CAS.
- K. Dong, X. Luo, L. Liu, F. An, D. Tang, L. Fu, H. Teng and Q. Huang, Int. J. Food Sci. Technol., 2021, 56, 1597–1607 CrossRef CAS.
- X. Lu, Y. Zhang, L. Zhu, X. Luo and D. L. Hopkins, Meat Sci., 2019, 149, 79–84 CrossRef CAS.
- M. Senapati and P. P. Sahu, Food Chem., 2020, 324, 126893 CrossRef CAS PubMed.
- F. Li and L. L. Guan, Appl. Environ. Microbiol., 2017, 83, e00061 CAS.
- A. E.-D. A. Bekhit, B. W. B. Holman, S. G. Giteru and D. L. Hopkins, Trends Food Sci. Technol., 2021, 109, 280–302 CrossRef CAS.
- L. C. Umuhumuza and X. Sun, Eur. Food Res. Technol., 2011, 232, 425–431 CrossRef CAS.
- Q. Ma, L. Du and L. Wang, Sens. Actuators, B, 2017, 244, 759–766 CrossRef CAS.
- A. J. Pellissery, P. G. Vinayamohan, M. A. R. Amalaradjou and K. Venkitanarayanan, in Meat Quality Analysis, Elsevier, 2020, pp. 307–334 Search PubMed.
- C. Papuc, G. V. Goran, C. N. Predescu and V. Nicorescu, Compr. Rev. Food Sci. Food Saf., 2017, 16, 96–123 CrossRef CAS PubMed.
- Y. Yu, G. Wang, X. Yin, C. Ge and G. Liao, Food Res. Int., 2021, 149, 110696 CrossRef CAS PubMed.
- F. S. Fedorov, A. Yaqin, D. V. Krasnikov, V. A. Kondrashov, G. Ovchinnikov, Y. Kostyukevich, S. Osipenko and A. G. Nasibulin, Food Chem., 2021, 345, 128747 CrossRef CAS PubMed.
- J. Csapó, J. Prokisch, C. Albert and P. Sipos, Acta Univ. Sapientiae, Aliment., 2019, 12, 21–41 Search PubMed.
- S. E. Duncan and H.-H. Chang, Adv. Food Nutr. Res., 2012, 67, 25–73 CAS.
- M. Parcheta, R. Świsłocka, S. Orzechowska, M. Akimowicz, R. Choińska and W. Lewandowski, Materials, 2021, 14, 1984 CrossRef CAS PubMed.
- A. Khan, R. Priyadarshi, T. Bhattacharya and J.-W. Rhim, Food Bioprocess Technol., 2023, 1–15, DOI:10.1007/s11947-023-03048-7.
- M. N. Lund, R. Lametsch, M. S. Hviid, O. N. Jensen and L. H. Skibsted, Meat Sci., 2007, 77, 295–303 CrossRef CAS PubMed.
- S. Jongberg, A. M. Racanicci and L. H. Skibsted, Food Chem., 2019, 300, 125134 CrossRef CAS PubMed.
- Y. Li, S. Jongberg, M. L. Andersen, M. J. Davies and M. N. Lund, Free Radicals Biol. Med., 2016, 97, 148–157 CrossRef CAS PubMed.
- S. Jongberg, L. d. S. Terkelsen, R. Miklos and M. N. Lund, Meat Sci., 2015, 100, 2–9 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |