Open Access Article
Open Access ArticleSustainable valorization of porcine placenta through microencapsulation: a circular economy approach to functional ingredient development
Pornpassorn
Chulalaksananukul
a,
Saeid
Jafari
a,
Khursheed Ahmad
Shiekh
a,
Isaya
Kijpatanasilp
a,
Nutthida
Pholmanee
b,
Phurt
Harnvoravongchai
c,
Tavan
Janvilisri
d and
Kitipong
Assatarakul
 *a
*a
aDepartment of Food Technology, Faculty of Science, Chulalongkorn University, Bangkok, 10330, Thailand. E-mail: Kitipong.A@chula.ac.th; Tel: +0-2218-5328
bDepartment of Biochemistry, Faculty of Science, Mahidol University, Bangkok, 10400, Thailand
cDepartment of Biology, Faculty of Science, Mahidol University, Bangkok, 10400, Thailand
dDepartment of Microbiology, Faculty of Science, Chulalongkorn University, Bangkok, 10330, Thailand
First published on 15th September 2025
Abstract
The increasing volume of porcine placenta, a nutrient-rich slaughterhouse byproduct, poses waste management challenges in Thailand's expanding pork industry. This study aimed to valorize porcine placenta by extracting bioactive compounds and developing shelf-stable microcapsules for functional food and pharmaceutical applications. Hexane-based fat removal reduced protein content by 24% and antioxidant activity (DPPH: 2.61 ± 0.08 mM TE/g db; FRAP: 4.80 ± 0.27 mM FeSO4/g db) but preserved protein profiles (25–100 kDa). Spray drying with gum arabic (40% w/v, 165 °C inlet temperature) achieved the highest protein retention (3.93 mg/g) and encapsulation efficiency (97.99%), producing microcapsules with low water activity (0.08–0.11), moisture content (2.28–4.08%), and glass transition temperatures (44.1–61.5 °C). Vacuum-sealed aluminum foil packaging maintained quality over 90 days at room temperature. This novel approach upcycles animal-derived waste into a stable, bioactive ingredient, advancing circular economy principles by reducing waste and offering sustainable applications in food and nutraceutical sectors.
Sustainability spotlightThis research advances sustainable food technology by transforming porcine placenta, a typically discarded slaughterhouse byproduct, into a stable, functional ingredient through green extraction and microencapsulation techniques. By repurposing animal-derived waste and extending its shelf life using environmentally responsible processing, the study supports circular economy principles and contributes to waste reduction, offering new value streams for the food and pharmaceutical industries. |
Introduction
In the last decade, Thailand has undergone significant progress and transformation in its animal farming sector, transitioning from conventional practices to advanced livestock management. This shift has led to a notable increase in pork production. However, the expansion of pig farms has concurrently brought about a rise in waste and residues post-mass processing (e.g., placenta and other organs), thereby impacting waste management costs and labor considerations.1 Swine placenta (porcine placenta) is an important organ in the maternal–fetal relationship, characterized by its nutrient richness and the presence of active peptides possessing antioxidant properties.2 These bioactive peptides exhibit anti-aging and anti-inflammatory properties, and play a role in wound healing, management of hypersensitivity and prevention of oxidation and mutation. Porcine placenta extract hydrolysates, a reservoir of bioactive peptides and amino acids, have been found to have antioxidant and antibacterial properties3,4 and also to exhibit antioxidant effects in porcine placenta after extraction. The use of placenta in various products requires careful handling, due to its limited shelf life and potential pathogenic contamination. To further process the placenta and prevent the growth of pathogens, extraction methods and drying techniques are applied.Encapsulation is a method that surrounds rapidly degradable materials with a protective shell or barrier layer to shield them from the external environment. Additionally, it facilitates the absorption of antioxidant components within the body, resulting in a more even dispersion of beneficial compounds throughout the microcapsule particles.5–7 Encapsulation technology is generally employed in the food industry's production processes due to its feasibility in incorporating bioactive substances like antioxidants, vitamins, and minerals. Upon entering the body, an encapsulated product can maintain crucial components without being disrupted by the digestive process, while also retaining its fragrance. The original color, flavor, and texture of the raw materials are also preserved.8 The microcapsules are intended for use as functional ingredients in food products (e.g., fortified beverages, supplements) and pharmaceutical formulations (e.g., antioxidant capsules). An advantage of spray drying for encapsulation lies in its continuous drying method, producing microcapsule powders with high retention of bioactive ingredients and drying yields. A recent study found that encapsulating porcine placenta extract in alginate enhances its antioxidant properties and significantly prolongs its release rate under gastrointestinal conditions compared to unencapsulated porcine placenta extract.9
To ensure the practical application of porcine placenta microcapsules, their bioactive compounds must be protected from environmental degradation. Packaging plays a critical role in maintaining the stability of encapsulated ingredients by preventing exposure to oxygen, moisture, and light, which can degrade proteins and antioxidants.10 This study evaluated vacuum-sealed aluminum foil and high-density polyethylene (HDPE) packaging to optimize the shelf life of microcapsules, aligning with sustainable food system goals.
Despite the potential, research on porcine placenta valorization remains limited, with challenges including protein loss during processing and a lack of optimized encapsulation methods. This study hypothesized that porcine placenta can be valorized into a shelf-stable functional ingredient through optimized extraction, microencapsulation, and packaging, preserving its bioactive properties for food and nutraceutical applications. Thus, this study aimed to address these gaps by extracting bioactive compounds from porcine placenta, optimizing microencapsulation via spray drying, and evaluating storage stability to develop a sustainable, value-added ingredient for food and nutraceutical applications, aligning with circular economy principles.
Materials and methods
Chemicals
2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma Aldrich, USA), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) (Sigma Aldrich, USA), 6-hydroxy-2,5,7,8-tretamethylchroman-2-carboxylic acid (Trolox) (Sigma Aldrich, USA), ethanol (CH3CH2OH) (DEPTAL-AX, Thailand), glacial acetic acid (CH3COOH) (A. R. grade, Qrec, New Zealand), 0.1 M hydrochloric acid (HCL) (Kemaus, Australia), hexane (A. R. grade, aNaPure, New Zealand), iron(II) sulphate 7-hydrate (FeSO4. 7H20) (KemAus, Australia), iron(III) chloride (FeCl3) (POCH S. A., Poland), methanol (CH3OH) (Fisher Scientific, UK), sodium acetate trihydrate (CH3COONa. 3H20) (Glentham, UK), sodium chloride (NaCl) (GHP, Thailand), sodium hypochlorite (NaOCl) (Loba, India).Porcine placenta processing
Porcine placentas were obtained from a pig farm in Ratchaburi province, Thailand. After this point, all sample processing were conducted in the laboratory. Blood and mucus were thoroughly cleaned with ice-cold sterile saline (0.9% w/v sodium chloride). The fetal vein, fetal artery, and umbilical cord were then separated. The remainder was divided into pieces, each measuring 3–5 mm, rinsed with ice-cold saline, and stored at 4 °C before use. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) hexane was added to the samples and agitated on ice for 3 h to remove fat content. The mixture was loaded into a separation cone, and kept at 4 °C for 16 h. The porcine placenta fat-removed extracts (PPFEs) from the lower phase were collected and kept at −20 °C for short-term and at −80 °C for long-term storage. The study was approved by the Faculty of Science, Mahidol University Animal Care and Use Committee (MUSC66-018-648). The proximate composition of raw porcine placenta was previously determined as follows: moisture (72.5 ± 1.2%), protein (15.8 ± 0.4%), fat (8.2 ± 0.3%), ash (2.1 ± 0.1%), and carbohydrates (1.4 ± 0.1%) (wet basis), with an initial fat content of 8.2% (unpublished data).
1 (v/v) hexane was added to the samples and agitated on ice for 3 h to remove fat content. The mixture was loaded into a separation cone, and kept at 4 °C for 16 h. The porcine placenta fat-removed extracts (PPFEs) from the lower phase were collected and kept at −20 °C for short-term and at −80 °C for long-term storage. The study was approved by the Faculty of Science, Mahidol University Animal Care and Use Committee (MUSC66-018-648). The proximate composition of raw porcine placenta was previously determined as follows: moisture (72.5 ± 1.2%), protein (15.8 ± 0.4%), fat (8.2 ± 0.3%), ash (2.1 ± 0.1%), and carbohydrates (1.4 ± 0.1%) (wet basis), with an initial fat content of 8.2% (unpublished data).
Analyses of porcine placenta extract's characteristics
The SDS-polyacrylamide gel electrophoresis technique (SDS-PAGE) was used to analyze the protein size and pattern of the sample. The protein concentration was quantified using the Bradford assay with bovine serum albumin as the standard. Antioxidant activity was analyzed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method according to.11 The absorbance difference at 515 nm was calculated by subtracting the DPPH solution's absorption value from the sample's absorption value and by creating a standard graph between the concentration of the standard solution Trolox (x-axis) and the absorbance difference (y-axis). The results were presented as mM TE/g db.The ferric ion reducing antioxidant power (FRAP) assay was conducted as described by.12 In brief, the FRAP solution was heated to 37 °C in a water bath until it turned reddish-brown. The sample (250 μL) and FRAP solution (4750 μL) were then pipetted together and allowed to stand at room temperature in the dark for 30 min. Using distilled water as the blank, the absorbance was measured at 593 nm. A standard curve of FeSO4 was used to determine the FRAP, which was then expressed as mM FeSO4 equivalents/g db.
Analyses of spray-dried microcapsule's properties
The PPFE was used in the encapsulation experiment. As adapted from,4 three factors were considered when conducting the microencapsulation: the type of encapsulating substance including resistant maltodextrin/RMD (Fibersol®-2, DE12; Matsutani, Japan) and gum arabic/GA (Agrigum, UK), the percentage of encapsulation concentration (40 and 45% w/v), and the inlet temperature (165 and 175 °C) of the spray dryer (Buchi B-290, Buchi Labortechnik AG, Switzerland). The spray dryer (Buchi B-290, Switzerland) was equipped with a two-fluid nozzle atomizer (0.7 mm diameter). The outlet temperatures corresponding to inlet temperatures of 165 °C and 175 °C were 85 ± 2 °C and 90 ± 2 °C, respectively. The PPFE was mixed with encapsulants (GA or RMD) at 40% or 45% w/v using a high-shear homogenizer (Ultra-Turrax T25, IKA, Germany) at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to form a stable emulsion. The feed stability was assessed by visual inspection and sedimentation tests, confirming no phase separation for 2 h at room temperature. Additionally, the sample flow rate (8 mL/min) was maintained throughout all experiments. The microcapsule powders were kept in vacuum-filled aluminum foil bags before further experiments.
000 rpm for 5 min to form a stable emulsion. The feed stability was assessed by visual inspection and sedimentation tests, confirming no phase separation for 2 h at room temperature. Additionally, the sample flow rate (8 mL/min) was maintained throughout all experiments. The microcapsule powders were kept in vacuum-filled aluminum foil bags before further experiments.
The analyses of protein properties and antioxidant properties (DPPH and FRAP assays) were explained in the previous section. The moisture content (%) was determined as described by.13 Water activity was measured by a water activity analyzer (MS1, Novasina, Switzerland) at a temperature of 25 °C. The color values were determined with a Minolta CR-400 colorimeter, which uses the illuminant D65 to display the color values in the CIE system (L*, a* and b*). L* is the lightness of 0–100 (0 is black and 100 is white), a* represents the axis from green (−a*) to red (+a*), b* is the blue axis value (−b*) to yellow (+b*). The encapsulation yield (%) was determined by calculating the weight of the microcapsule powder obtained after drying by a spray dryer, divided by the total solid weight of the extract obtained before spray drying, and multiplying the resulting value by 100.14 The encapsulation efficiency was adapted from15 and the surface bioactive compounds were determined. In brief, 0.1 g of microcapsule was dissolved in a mixed solution (ethanol: methanol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and shaken with a vortex mixer for 1 min. Then, the encapsulation efficiency was calculated using the following equation (eqn (1)):
1 v/v) and shaken with a vortex mixer for 1 min. Then, the encapsulation efficiency was calculated using the following equation (eqn (1)):
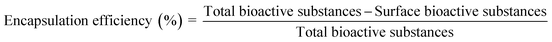 | (1) |
Water solubility (%) was determined as described by.12 In brief, 100 mL of distilled water was combined with 1 g of each powder sample, and the mixture was then homogenized using a magnetic stirrer (400 rpm for 4 min). The resultant mixture was centrifuged at 4000×g for 4 min. A 25 mL amount of the supernatant was placed in an aluminum cup that had been weighed before being dried for 5 h at 105 °C. The weight of the dried supernatant as a percentage of the starting powder was used to calculate the water solubility. A 3 mg sample of powder was placed in an aluminum pan and allowed to sit in a hygroscopic jar with saturated salt at a temperature of 25 °C to determine the glass transition temperature (Tg), which was adapted from.16 With an empty tray serving as a reference, the sample was then programmed to −70 °C and increased to 100 °C at a heating rate of 10° per min with a purge gas flow rate of 25 mL/min.
Surface morphology of microcapsule powder using a scanning electron microscope (SEM)
The analysis of the surface morphology of microcapsules was conducted at Chulalongkorn University's Scientific and Technological Research Equipment Centre (STREC) using a scanning electron microscope equipped with an energy-dispersive X-ray spectrometer (JEOL JSM-IT300 with Oxford X-MaxN 20, Japan) at 30 kV with magnification of 500× and 1000×.Effect of packaging type and packing condition on the properties of the microcapsule powder during storage
For the storage study, the microcapsule powders were packaged under different storage conditions. Two types of packaging materials (aluminum foil laminated bags and HDPE plastic bags) and two packaging conditions (vacuum-packed and packed under atmospheric pressure) were varied and tested at room temperature to monitor changes every 15 days for 90 days. The characteristics of the packed samples were analyzed as described in the previous section.Statistical analysis
Statistical analysis was performed using the paired-sample t-test for the investigation of fat extraction from porcine placenta. The encapsulation study by spray drying with two different inlet temperatures was carried out using a 3 × 2 × 2 factorial design in completely randomized design (CRD), and the study of various packaging types and conditions was designed using a 2 × 2 factorial design in a completely randomized design (CRD). Both experiments used analysis of variance (ANOVA) and Tukey's honestly significant difference (HSD) test to compare the means at a 95% confidence level.Results and discussion
Effects of fat extraction on protein concentration, yield, protein molecular mass and antioxidant activity of porcine placenta extract
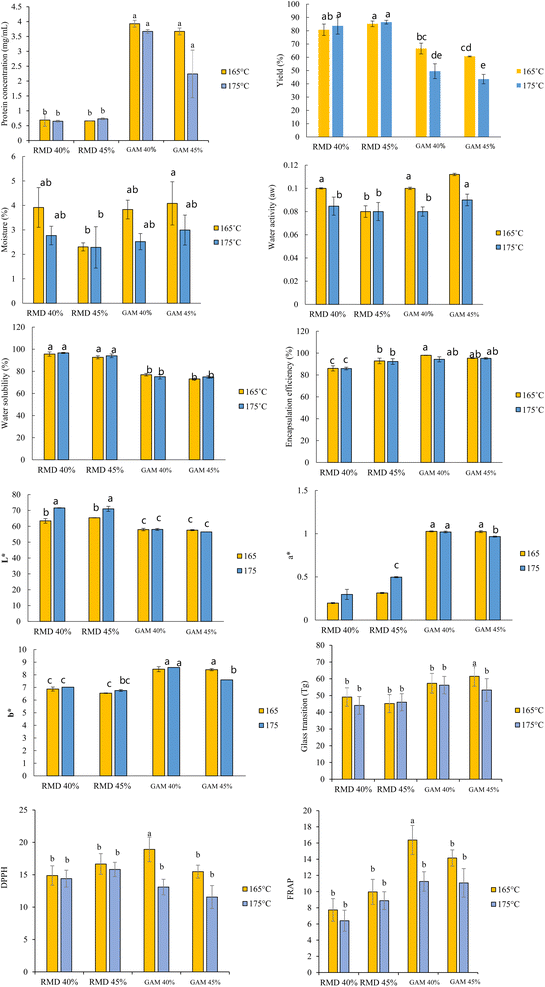
Hexane-based fat extraction reduced protein concentration by 24% (from 1.60 ± 0.01 to 1.22 ± 0.02 mg/mL) and antioxidant activity (DPPH: 26.1% reduction, from 3.53 ± 0.11 to 2.61 ± 0.08 mM TE/g db; FRAP: 8.7% reduction, from 5.26 ± 0.16 to 4.80 ± 0.27 mM FeSO4/g db) (Table 1). This loss is attributed to the association of hydrophobic peptides or lipoproteins with the hexane layer, as non-polar solvents can extract lipid-bound proteins.17 SDS-PAGE analysis showed that proteins in both types of extract were detected in the range of 25 to 100 kDa with a comparable pattern (Fig. 1). Therefore, the fat removal process affected the total amount of proteins but not the protein pattern, indicating that bioactive compounds likely persisted within the post-processed sample.| Parameters | Sample | |
|---|---|---|
| Porcine placenta extracts (PPE) | Porcine placenta fat-removed extracts (PPFE) | |
| a Mean ± standard deviation. Superscript letters (a and b) within each row of the DPPH and FRAP assays denote statistically significant differences (p ≤ 0.05). DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate: mM Trolox/g db); FRAP (ferric reducing antioxidant power assay: mM FeSO4/g db). | ||
| Protein concentration (mg/mL) | 1.60 ± 0.01 | 1.22 ± 0.02 |
| Yield (%) | 100.00 ± 0.00 | 58.3 ± 0.18 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Antioxidant activity | ||
| DPPH assay | 3.53 ± 0.11a | 2.61 ± 0.08b |
| FRAP assay | 5.26 ± 0.16a | 4.80 ± 0.27b |
SDS-PAGE analysis (Fig. 1) showed proteins in the 25–100 kDa range, likely including hydrophilic globular proteins and hydrophobic lipoproteins, as porcine placenta contains lipid-associated proteins.3 The 24% protein loss during hexane extraction suggests partial removal of lipoproteins or hydrophobic peptides, which are associated with the non-polar hexane layer.17
Antioxidant activity decreased by 26.1% (DPPH: from 3.53 ± 0.11 to 2.61 ± 0.08 mM TE/g db) and 8.7% (FRAP: from 5.26 ± 0.16 to 4.80 ± 0.27 mM FeSO4/g db) in PPFE compared to PPE (Table 1). Reduced antioxidant activity was observed in PPFE, which was consistent with findings by,17 regarding the impact of lipid removal from flaxseed antioxidant activity. It was reported that the antioxidant activity of lipid-extracted flaxseeds was lower than that of their lipid-containing counterparts, as assessed by DPPH and FRAP techniques. Given that fat is the primary catalyst for oxidation, a process affecting the texture, quality, color, and nutritional content of stored food products, removing fat from the sample served the fundamental purpose of enhancing sample stability. Therefore, porcine placenta fat-removed extracts (PPFE) was selected for the subsequent step of the experiment.
Effects of encapsulation by spray drying on characteristics of porcine placenta microcapsules
There was no significant difference (p > 0.05) in protein pattern and molecular mass (Fig. 2). This indicated that differences in drying temperatures (165 vs. 175 °C), encapsulation type (GA vs. RMD), and encapsulation concentration (40 or 45%) did not impact the molecular mass or protein transformation within the microcapsule powders. These findings were consistent with those of,18 who investigated the effect of spray drying techniques using various spray dryer inlet temperatures (77, 107, 155, and 178 °C) on the protein profiles of cow milk encapsulated within wood hemicellulose. That study found that encapsulation concentration and various drying inlet temperatures had no impact on the protein profiles of the microcapsule powders.The microcapsule powders employing resistant maltodextrin (RMD) as the encapsulating agent had lower protein concentrations (mg/g) than those encapsulated with gum arabic (p ≤ 0.05). In contrast, GA demonstrated maximum protein concentration (3.93 mg/g) at a concentration of 40% w/v and an inlet temperature of 165 °C (Fig. 3). The higher protein retention with GA (3.93 mg/g at 40% w/v, 165 °C) compared to RMD aligns with the findings of,19 who reported gum arabic's superior emulsifying properties enhance protein encapsulation efficiency in spray-dried systems.
The cost-efficiency and productivity of the production process directly correlate with the yield of spray drying technique. This indicator holds substantial importance when determining production conditions for industrial applications. However, other parameters also affect the percentage yield, including spray dryer variables (such as inlet temperature, outlet temperature, and sample flow rate) and drying condition variables (such as encapsulation type and concentration).20 The yield of the microcapsules ranged from 43.58 to 86.45% (Fig. 3). The percentage yield of the microcapsules was significantly influenced by the inlet temperature, the type of encapsulant, and the concentration of encapsulant (p ≤ 0.05). Increasing the inlet temperature from 165 to 175 °C and using RMD as an encapsulant agent both led to increased percentage yield. Additionally, increasing the gum arabic concentration from 40 to 45% resulted in a significant drop in yield percentage (p ≤ 0.05) when the co-influence of the encapsulation method and concentration was examined. Our findings showed that using gum arabic (GA) as an encapsulant resulted in lower microcapsule yields compared to resistant maltodextrin (RMD). This difference may be influenced by the surface activity of proteins in the porcine placenta extract, which could promote their migration to the microcapsule surface during spray drying, potentially contributing to reduced yields. Gum arabic increases the solution viscosity more than RMD (e.g., 115 cP vs. 40 cP), leading to coarser droplets and greater adhesion to drying equipment walls.21,22 Additionally, GA's higher water absorption capacity (3.57–4.56 g/g vs. ∼3.57 g/g for maltodextrin) can concentrate surface-active proteins at the droplet interface, creating surface tension gradients that enhance stickiness.23–25 Specific measurements of protein surface activity and viscosity changes before and after adding encapsulants (GA or RMD) were not conducted in this study. During spray drying, protein migration to the surface likely contributed to the formation of overlapping films and thicker microcapsule walls, reducing the overall yield due to powder adhesion to the drying equipment.7,24 In contrast, using RMD as an encapsulant increased the percentage yield when the spray dryer inlet temperature was raised from 165 to 175 °C. This aligns with the findings of,21 who reported lower microcapsule yields with gum arabic.
Additionally,26 noted that elevating the inlet temperature in spray drying with maltodextrin as an encapsulant improved microcapsule powder yield. In the current study, the temperature (80 °C outlet at 165 °C inlet; 85 °C outlet at 175 °C inlet) influenced yield and moisture content. Higher temperatures at 175 °C increased yield (86.45% for RMD) due to faster drying rates but reduced encapsulation efficiency (85.88% for GA), likely due to rapid water evaporation causing particle collapse.27
Moisture content is a crucial physicochemical attribute affecting microcapsule quality, stability, and shelf life. The moisture content of microcapsule powders ranged from 2.28 to 4.08% (wet basis), remaining below the 5% threshold. A statistically significant synergistic effect of encapsulant type and encapsulant concentration was observed (p ≤ 0.05). Increasing the concentration of RMD encapsulate from 40 to 45% w/v resulted in a decrease in the moisture content of the microcapsule powder while increasing the concentration of the encapsulating GA from 40 to 45% w/v resulted in an increase in the moisture content of the microcapsules. Moreover, using RMD as the encapsulant material and increasing the spray drying temperature from 165 to 175 °C reduced the moisture content of the microcapsule powder from 4.08 to 2.99%.
The moisture content of gum arabic-encapsulated powders varied from 2.28 to 4.08%. The only variable that had no significant impact (p > 0.05) on the moisture content of the porcine placenta microcapsule powder was the encapsulation concentration. High-temperature drying accelerates the heat transfer rate between the porcine placenta extract and the hot air inside the machine, leading to an increase in the rate of water evaporation. Consequently, as the drying temperature increased, the microcapsule moisture content decreased. This was consistent with,28 who investigated the spray drying temperature of melon seed protein microcapsule powder by adjusting the spray dryer's input temperature from 150 to 180 °C and discovered that the inlet temperature of 180 °C led to the microcapsule powder's lowest moisture content. Resistant maltodextrin lacks the same water absorption capacity as gum arabic, leading to higher moisture content in the GA-encapsulated microcapsules.
A key element influencing food product shelf life is water activity (aw). To avoid bacterial growth and metabolic reactions that could lead to the spoilage of dry food products, the water activity should be less than 0.6.29 The water activity of the microcapsule powders, under all conditions, ranged from 0.08 to 0.11, which contributed to the stability of the microcapsule powder. A decrease in water activity was observed with an increase in the inlet temperature from 165 to 175 °C and an increase in encapsulation concentration from 40 to 45%. The water activity of the microcapsules when utilizing RMD was lower compared to those using GA. RMD consistently inhibited lower water activity than GA encapsulated in amaranth (Amaranthus viridis L.) microcapsule powder, according to.30 Lower water activity in RMD-encapsulated microcapsules (0.08–0.11) compared to GA is attributed to RMD's lower hygroscopicity, as its branched D-glucose structure reduces water-binding capacity.25
The water solubility of microcapsule powder products is a defining quality characteristic when used as an ingredient in various food products. It demonstrates both the high level of solubility of the microcapsule powder in water and its ability to release antioxidant compounds. Among the microcapsules, the range of water solubility ranged from 73.17 to 96.67%. With RMD encapsulation, the water solubility improved by over 90%, ranging from 92.70 to 96.67%, while GA showed lower values, ranged from 73.17 to 76.97% (Fig. 3). The enhanced water solubility of RMD-encapsulated microcapsules is attributed to its resistance to digestion, achieved by structurally modifying the original maltodextrin with increased D-glucose branching in its primary structure. This modification facilitates binding to water molecules, thereby increasing the water solubility of the porcine placenta microcapsule powder containing indigestible maltodextrin. In contrast, gum arabic is a substance with a complex heteropolysaccharide structure, containing roughly 2% of protein with hydrophobic qualities, rendering it less water-soluble. Gum arabic can create viscous emulsions due to its polysaccharide-protein composition. These outcomes align with the findings of,25 indicating that RMD as an encapsulation produced higher water solubility compared to gum arabic. Additionally,31 reported that Annona muricata L. leaf extract microcapsule powders produced with RMD had better solubility than those encapsulated with gum arabic.
Color is a fundamental aspect that defines the product quality. While a* values ranged from green (−a*) to red (+a*) and b* values can represent blue (−b*) to yellow (+b*), the L* value, which denotes brightness, spans from 0 to 100, where 0 represents black and 100 represents white. The values of L*, a*, and b* of all samples ranged from 71.61 to 56.47, 0.20 to 1.03, and 6.55 to 8.58, respectively. The maximum L* value (71.61) was achieved in RMD at a 40% w/v concentration, while the lowest L* value (56.47) was observed with GA at a 45% w/v concentration at 175 °C. The microcapsules with the greatest red value (+a* = 1.03) were obtained in GA at a concentration of 40% w/v at 165 °C. The maximum b* value (8.58) was recorded in GA at 175 °C. Considering the a* and b* values of the microcapsules under all conditions, the samples exhibited a reddish-yellow appearance.
The stability of spray-dried microcapsule powders in long-term storage is significantly influenced by the glass transition temperature (Tg). The microcapsule powder becomes viscous, leading to an agglutination issue that degrades the powder's quality when the temperature of the powders surpasses the glass transition temperature (Tg).32 The glass transition temperature ranged from 44.1 and 61.5 °C, with the maximum observed at 61.5 °C. Due to the larger molecular mass of gum arabic (617![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 g/mol) compared to maltodextrin (4230 g/mol), which increases the glass transition temperature, GA had a higher glass transition temperature than RMD.
320 g/mol) compared to maltodextrin (4230 g/mol), which increases the glass transition temperature, GA had a higher glass transition temperature than RMD.
The ability of the microcapsules to bind or retain components inside is known as encapsulation efficiency. In general, various parameters that influence the antioxidant retention also affect encapsulation efficiency in microcapsule powder. When using GA at a concentration of 40% w/v at an inlet temperature of 165 °C, the encapsulation efficiency ranged from 85.88 to 97.99%. Various factors, including drying temperature, emulsifier properties of bioactive compounds, and encapsulation type affect the efficiency of encapsulation. It was observed that GA had a stronger retention efficiency than RMD.19 Encapsulation efficiency for RMD microcapsules ranged from 85.88 to 92.34%, lower than GA (97.99%) due to RMD's weaker barrier-forming properties.12 Resistant maltodextrin, characterized by its indigestible nature, is not as effective at creating a barrier to protect antioxidant compounds within the particles compared to gum arabic.12
The antioxidant activities of the microcapsules were assessed by DPPH and FRAP assays, yielding values ranging from 11.56 to 18.92 mM TE/g db and 6.40 to 16.37 mM FeSO4/g db, respectively. The maximum antioxidant activity was observed in GA at a concentration of 40% w/v and a an inlet temperature of 165 °C (18.92 ± 0.84 mM TE/g db and 16.37 ± 0.85 mM FeSO4/g db for DPPH and FRAP, respectively). In terms of antioxidant activity, our findings were consistent with those of,33 who reported that the spray-dried encapsulation led to increased antioxidant activity compared to the initial extract. The spray-dried mashitake mushroom encapsulates displayed higher antioxidant activity than the initial extract, according to34 (1.44 vs. 12.02 mM Fe(II)/g db). The reduction in antioxidant activity post-spray drying (DPPH: 11.56–18.92 mM TE/g db; FRAP: 6.40–16.37 mM FeSO4/g db) at higher inlet temperatures (175 °C) is attributed to the thermal degradation of heat-sensitive bioactive compounds, such as peptides and phenolic compounds, as reported by.28
Characterization of the microcapsule's surface morphology by scanning electron microscopy (SEM)
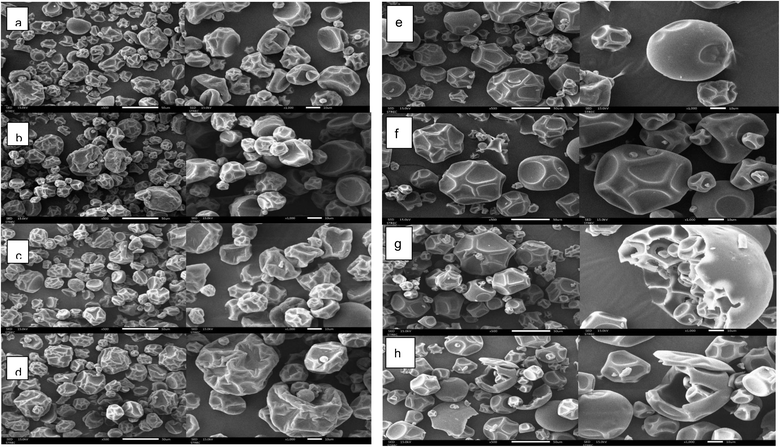
The particle shape of the microcapsules varies depending on factors such as the inlet temperature, encapsulation type, encapsulation concentration, flow properties, and solution viscosity.35 SEM revealed microcapsules with spherical shapes, dents, and cap-shaped structures (Fig. 4). GA microcapsules exhibited fewer dents and smoother surfaces due to better film-forming properties, while RMD microcapsules showed more pronounced dents and blow-holes, particularly at 175 °C, due to higher delta temperatures (85 °C) causing rapid water evaporation.27 This was consistent with findings from14 and,21 suggesting that the use of gum arabic produced smoother spherical particle walls and higher encapsulation integrity. The smoother morphology of GA microcapsules (Fig. 4) is also attributed to its film-forming properties, which create a uniform protective matrix during spray drying, reducing surface dents compared to RMD.21 Upon examining the inlet temperature component, an increase of inlet temperature from 165 to 175 °C led to more pronounced wrinkles and indentations. The rate of heat and mass transfer during drying affects the encapsulation pace. Rapid water evaporation during the drying process causes particles to develop irregular structures, including noticeable wrinkles and dents.27 In other words, during particle wall formation, the hot air vapor flow inside the particle encounters resistance due to the obstructing wall, leading to bubble formation inside the particle due to increased pressure. This results in hollow air holes inside the particles, and cavitation can be created during the spray drying process by the condensation and expansion of air bubbles inside the particles.8The investigation of antioxidant properties of porcine placenta microcapsule powder under various production conditions employing spray drying techniques led to the identification of the optimal conditions for producing microcapsule powder with the highest antioxidant activity (DPPH and FRAP assays). The optimized production conditions involved using gum arabic at a concentration of 40% w/v and an inlet temperature of 165 °C. This specific microcapsule powder was then utilized to assess the impact of different packaging types (aluminum foil laminated bag and HDPE plastic bag) and packing conditions (vacuum-packed and atmospheric-packed) on various properties of the microcapsules, including moisture content, water activity, solubility, color values, and antioxidant activity (DPPH and FRAP assays). The changes in these properties were monitored at intervals of 15 days over a period of 90 days.
Effect of packaging type and packing condition on microcapsule properties during the storage
Changes in food properties after storage are important indicators of product quality. The factors that affect the storage quality can be divided into three components, namely the specific product characteristics, storage conditions and containers. Therefore, the choice of packaging and the storage environment play a pivotal role in maintaining product quality over an extended period, reducing production costs and increasing the value of the product.36 Different types of food packaging have different protective properties against environmental factors, such as gas permeability, water vapor permeability rate and heat sealing. In addition, packing conditions significantly impact food quality. Temperature, humidity, oxygen, and light affect the stability and quality of food products.37Proteins are significant macromolecules that build up in the placenta and are used by the developing fetus as well as being a significant antioxidant. Initial protein concentrations for microcapsules packed in aluminum bag under vacuum (Al-vac), aluminum bag under atmospheric conditions (Al-atm), HDPE plastic bag under vacuum (HDPE-vac), and HDPE plastic bag under atmospheric conditions (HDPE-atm) were 4.10, 4.10, 3.90, and 3.80 mg/g, respectively (Fig. 5). When kept at ambient temperature for 90 days, the protein quantity in the microcapsules exhibited significant variation (p ≤ 0.05).
The degeneration of microorganisms might be influenced by the stability of active compounds within microcapsules. Moisture contents in all samples tended to rise throughout the course of storage, with HDPE-atm, HDPE-vac, Al-atm, and Al-vac showing the highest to lowest moisture contents, respectively, (p ≤ 0.05). It was discovered that an aluminum foil bag had better ability to prevent water vapor permeability compared to a HDPE plastic bag, attributed to their low water vapor permeability at high humidity. This was based on the ability of each type of packaging to prevent permeation of water vapor, gas, moisture, and light. According to Tyagi et al. (2021),38 PET (polyethylene terephthalate) plastic had less ability to prevent the penetration of oxygen and water vapor than aluminum foil bag, which led to lower moisture content of samples when using aluminum foil bag for the preservation of papaya powder by the spray drying technique. According to,39 aluminum foil bags with a thickness of 6 to 9 μm provided superior environmental protection than any alternative packaging materials like PET.
The product's stability and shelf life are influenced by the water activity value, a measure of the sample's free water content. Microorganism development, chemical reactions, and the loss of protein in dietary products are all linked to water activity. Therefore, the shelf life increases as water activity decreases. The water activity of samples tended to increase during storage, except for the samples packed in Al-vac (p > 0.05). HDPE-atm, HDPE-vac, Al-atm, and Al-vac, which exhibited the highest to lowest water activity values, respectively. Water activity tended to align with moisture content. This was consistent with40 a study on the shelf life and discoloration of aloe vera gel powder in different packaging in which an aluminum foil had longer shelf life than PP bags.
The desired dissolution property of microcapsule powder is its ability to completely dissolve in water without leaving residue. For powdered food products, water solubility is a crucial quality trait. The solubility of microcapsules during storage ranked from the highest to the lowest as HDPE-atm, HDPE-vac, Al-atm, and Al-vac. The moisture content, water activity, and water solubility followed a similar trend.41 studied the preservation of papaya microcapsules using various packaging (aluminum foil bag and PET bag), and found that the microcapsules stored in aluminum foil bag had higher solubility than those stored in PET bag over a 7 week period, consistent with our findings.
In terms of color values, the results showed no statistically significant impact on the brightness or L* value (p > 0.05). However, after 45 days of storage, packaging type and packing condition significantly affected the L* value (p ≤ 0.05). The brightness value of Al-vac was lower than that of HDPE-atm. The factors of packaging type and packing condition did not significantly (p > 0.05) affect the a* value. The color difference value (ΔE) represents the variation in color between the sample before and after storage. If the value is large, the color value has changed significantly from its starting value. Our experiments demonstrated that the samples packed in HDPE-atm exhibited a greater color difference compared to those in Al-vac. Storage temperature, sugar content, protein content, water activity value, and storage time are additional elements influencing color changes in microcapsule powders during storage.
Antioxidant activity in samples from 0 to 60 days of storage did not differ significantly (p > 0.05) as seen in Table 2. However, substantial impacts were noticed (p ≤ 0.05) after 60 days. DPPH and FRAP values of all samples tended to drop over the course of storage (p ≤ 0.05). While HDPE plastic bag allowed gas to pass through, aluminum foil bag had better ability to prevent gas, oxygen, steam, moisture, and odor from entering the container. These elements had an impact on the oxidation reaction, producing free radicals that destroyed the biomolecules inside, thereby lowering the quality of the food.
| Parameter | Storage (day) | Sample | |||
|---|---|---|---|---|---|
| Aluminum foil laminated bag | HDPE plastic bag | ||||
| Packed under vacuum | Packed under atmospheric conditions | Packed under vacuum | Packed under atmospheric conditions | ||
| a Mean ± standard deviation. Different superscript letters (A–C) within each column of the DPPH and FRAP assays indicate statistically significant differences (p ≤ 0.05) over the 0–90 day storage period. Different superscript letters (a–c) within each row indicate the effect of packaging at each time point (p ≤ 0.05). DPPH: 2,2-diphenyl-1-picryl-hydrazyl-hydrate (mM Trolox/g db); FRAP: ferric reducing antioxidant power assay (mM FeSO4/g db); HDPE: high-density polyethylene. | |||||
| DPPH | 0 | 13.65 ± 1.51A,a | 13.65 ± 1.51A,a | 13.65 ± 1.51A,a | 13.65 ± 1.51A,a |
| 15 | 12.67 ± 1.55AB,a | 11.94 ± 0.61ABC,a | 13.03 ± 2.03A,a | 11.45 ± 0.10AB,a | |
| 30 | 11.97 ± 0.47AB,a | 12.08 ± 0.49AB,a | 12.25 ± 0.45AB,a | 11.41 ± 0.41AB,a | |
| 45 | 11.76 ± 0.54AB,a | 11.44 ± 0.09ABCD,a | 11.73 ± 0.04AB,a | 11.59 ± 0.09AB,a | |
| 60 | 10.45 ± 0.69AB,a | 10.58 ± 0.83BCD,a | 9.74 ± 0.11ABC,a | 8.75 ± 0.17BC,a | |
| 75 | 10.35 ± 0.56B,a | 9.22 ± 0.30CD,b | 8.31 ± 0.30BC,c | 8.20 ± 0.01C,c | |
| 90 | 9.96 ± 0.49B,a | 9.14 ± 0.41D,a | 7.58 ± 0.81C,b | 7.37 ± 0.10C,b | |
| FRAP | 0 | 10.83 ± 0.17A,a | 10.83 ± 0.17A,a | 10.83 ± 0.17A,a | 10.83 ± 0.17A,a |
| 15 | 9.78 ± 0.67AB,a | 9.06 ± 0.47B,a | 10.45 ± 0.76A,a | 9.17 ± 0.73AB,a | |
| 30 | 9.76 ± 1.01AB,a | 8.70 ± 0.55B,a | 8.90 ± 0.49B,a | 8.69 ± 0.06AB,a | |
| 45 | 8.21 ± 1.47ABC,a | 8.11 ± 0.74B,a | 8.16 ± 0.46BC,a | 8.00 ± 0.28BC,a | |
| 60 | 7.83 ± 0.86BC,a | 7.78 ± 1.19BC,a | 6.91 ± 0.71CD,a | 6.11 ± 0.99CD,a | |
| 75 | 6.12 ± 0.62C,a | 6.07 ± 0.21CD,a | 6.04 ± 0.11D,a | 6.04 ± 1.10CD,a | |
| 90 | 5.84 ± 0.64C,a | 5.22 ± 0.11D,a | 5.57 ± 0.16D,a | 5.30 ± 0.71D,a | |
SDS-PAGE analysis of protein patterns within microcapsules for each type is depicted in Fig. 6. The quality of the microcapsules remained unchanged as storage time extended. Different packaging type and packing condition had no effect on protein deformation and molecular mass in the microcapsule powders, consistent with42 a study on the protein stability of partially fat-extracted walnut flour (Juglans regia L.). The study found that storage in aluminum and plastic bags had no effect on the protein molecular mass and form after an 8 month storage period.
The microcapsule powder, when stored in vacuum-sealed aluminum foil laminated bag, exhibited minimal changes in quality concerning water activity, moisture content, water solubility, and antioxidant activity (as determined by DPPH and FRAP assays). This storage condition proves to be the most effective in preserving the quality of the porcine placenta extract microcapsule powder.
Conclusions
This study successfully valorized porcine placenta, a slaughterhouse byproduct, into a shelf-stable functional ingredient through hexane-based fat extraction and spray drying with GA at 40% w/v and 165 °C inlet temperature. The process achieved high protein retention (3.93 mg/g), encapsulation efficiency (97.99%), and stability (low water activity: 0.08–0.11; moisture content: 2.28–4.08%) over 90 days in vacuum-sealed aluminum foil packaging. These findings align with circular economy principles by upcycling waste into a bioactive ingredient for food and nutraceutical applications. This work also offers an integrated approach combining optimized encapsulation and sustainable packaging. However, limitations include the 24% protein loss during fat extraction and the lack of in vivo studies to confirm bioactivity. Future research should explore alternative extraction methods to minimize protein loss, investigate the bioavailability of encapsulated compounds, and assess their efficacy in food and pharmaceutical formulations. Additionally, scaling up the process and evaluating its economic feasibility for industrial applications merit further investigation.Author contributions
Pornpassorn Chulalaksananukul: investigation, formal analysis, data curation and writing – original draft. Khursheed Ahmad Shiekh: investigation, formal analysis, data curation and writing – original draft. Saeid Jafari: data curation and writing – original draft. Isaya Kijpatanasilp: data curation and writing – original draft. Nutthida Pholmanee: investigation. Phurt Harnvoravongchai: investigation and writing – review & editing. Tavan Janvilisri: funding acquisition, project administration, writing – review & editing. Kitipong Assatarakul: conceptualization, data curation, funding acquisition, project administration, supervision, writing – original draft and writing – review & editing.Conflicts of interest
Authors have no conflict of interest.Data availability
Data are available from the authors upon reasonable request.Acknowledgements
This research project was supported by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), Chulalongkorn University and the Program Management Unit for Competitiveness (PMU C; C10F640021 and C02F670173). The postdoctoral fellowship was sponsored by the Second Century Fund (C2F) at Chulalongkorn university for Dr Saeid Jafari and Dr Khursheed Ahmad Shiekh.References
- W. Thanapongtharm, C. Linard, P. Chinson, S. Kasemsuwan, M. Visser, A. E. Gaughan, M. Epprech, T. P. Robinson and M. Gilbert, BMC Vet. Res., 2016, 12, 218 CrossRef PubMed.
- H. Teng, Y. Chen, X. Lin, Q. Lv, T.-T. Chai, F.-C. Wong, L. Chen and J. Xiao, Food Chem. Toxicol., 2019, 129, 138–143 CrossRef CAS PubMed.
- P. Laosam, W. Panpipat, G. Yusakul, L.-Z. Cheong and M. Chaijan, PLoS One, 2021, 16, e0258445 CrossRef CAS PubMed.
- W.-L. Tang, M. Zhang, B. Adhikari and A. S. Mujumdar, Dry. Technol., 2013, 31, 1600–1610 CrossRef CAS.
- S. Insang, I. Kijpatanasilp, S. Jafari and K. Assatarakul, Ultrason. Sonochem., 2022, 82, 105806 CrossRef CAS.
- Y. P. Timilsena, M. A. Haque and B. Adhikari, Food Nutr. Sci., 2020, 11, 481–508 CAS.
- S. Chheng, M. Fikry, S. Jafari, D. K. Mishra and K. Assatarakul, J. Stored Prod. Res., 2025, 112, 102660 CrossRef CAS.
- V. Nedovic, A. Kalusevic, V. Manojlovic, S. Levic and B. Bugarski, Procedia Food Sci., 2011, 1, 1806–1815 CrossRef CAS.
- A. Taweechaipaisankul, N. Thumrongsiri, W. Chonniyom, P. Dana, P. Tanyapanyachon, M. Rattanatayarom, W. Chinchoosak and N. Saengkrit, Food Hydrocolloids Health, 2025, 7, 100194 CrossRef CAS.
- K. Marsh and B. Bugusu, J. Food Sci., 2007, 72, R39–R55 CrossRef CAS PubMed.
- S. Chheng, M. Fikry, S. Jafari, S. K. Mehta, D. K. Mishra and K. Assatarakul, ES Food Agrofor., 2025, 19, 1434 Search PubMed.
- S. Jafari, Z. Karami, K. A. Shiekh, I. Kijpatanasilp, R. W. Worobo and K. Assatarakul, Foods, 2023, 12, 412 CrossRef CAS PubMed.
- AOAC International, Official methods of analysis of AOAC International, AOAC International, Gaithersburg, MD, USA, 17th edn, 2000 Search PubMed.
- Y. Ramakrishnan, N. M. Adzahan, Y. A. Yusof and K. Muhammad, Powder Technol., 2018, 328, 406–414 CrossRef CAS.
- C. Saénz, S. Tapia, J. Chávez and P. Robert, Food Chem., 2009, 114, 616–622 CrossRef.
- J. C. Silva, S. Rodrigues, X. Feás and L. M. Estevinho, Food Chem. Toxicol., 2012, 50, 1790–1795 CrossRef CAS PubMed.
- K. Brodowska, R. Catthoor, A. J. Brodowska, M. Symonowicz and E. Lodyga-Chruscinska, Albanian J. Agric. Sci., 2014, 13, 16 Search PubMed.
- Y. Fang, S. Rogers, C. Selomulya and X. D. Chen, Biochem. Eng. J., 2012, 62, 101–105 CrossRef CAS.
- R. V. D. B. Fernandes, S. V. Borges and D. A. Botrel, Food Sci. Technol., 2013, 33, 171–178 CrossRef.
- J. K. Rutz, C. D. Borges, R. C. Zambiazi, M. M. Crizel-Cardozo, L. S. Kuck and C. P. Noreña, Food Chem., 2017, 220, 59–66 CrossRef CAS PubMed.
- K. Sarabandi, S. M. Jafari, A. S. Mahoonak and A. Mohammadi, Int. J. Biol. Macromol., 2019, 140, 59–68 CrossRef CAS PubMed.
- S. Kausadikar, A. D. Gadhave and J. Waghmare, Adv. Appl. Sci. Res., 2015, 6, 69–78 CAS.
- E. J. Vernon-Carter and C. I. Beristain, Food Sci. Technol. Int., 2001, 7(6), 423–437 Search PubMed.
- T. Ahad, F. A. Masoodi, A. Gull, S. M. Wani and M. N. Shafi, J. Food Process. Preserv., 2021, 45, e15190 CAS.
- L. Pudziuvelyte, M. Marksa, V. Jakstas, L. Ivanauskas, D. M. Kopustinskiene and J. Bernatoniene, Molecules, 2019, 24, 1461 CrossRef CAS PubMed.
- J. Gawałek, E. Domian, A. Ryniecki and S. Bakier, Int. J. Food Sci. Technol., 2017, 52, 1933–1941 CrossRef.
- D. Nkurunziza, S. P. Sivagnanam, J.-S. Park, Y.-J. Cho and B. S. Chun, Algal Res., 2021, 58, 102381 CrossRef.
- C. Cao, X. Zhao, C. Zhang, Z. Ding, F. Sun and C. Zhao, J. Food Sci., 2020, 85, 3442–3449 CrossRef CAS.
- A. Sharifi, M. Niakousari, A. Maskooki and S. Mortazavi, Int. Food Res. J., 2015, 22, 2364 CAS.
- S. F. M. Amin, R. Karim, Y. A. Yusof and K. Muhammad, Int. J. Food Sci., 2021, 2021, 1819104 Search PubMed.
- O. Jordán-Suárez, P. Glorio-Paulet and L. Vidal, J. Encapsulation Adsorpt. Sci., 2018, 8, 178–193 CrossRef.
- S. F. Subtil, G. A. Rocha-Selmi, M. Thomazini, M. A. Trindade, F. M. Netto and C. S. Fávaro-Trindade, J. Food Sci. Technol., 2014, 51, 2014–2021 CrossRef CAS PubMed.
- L. Lima, Investigadora Titular del Centro Nacional de Medicina Natural y Tradicional, Universidad de la Habana. Cuba. Recuperado de, 2008 Search PubMed.
- P. Krittalak, B. Panida, L. Supaporn, N. Nowwapan, T. Takunrat and S. Ubolwanna, Chiang Mai J. Sci., 2018, 45, 762–773 CAS.
- A. I. Alves, M. Z. Rodrigues, M. R. M. Ribeiro Pinto, E. S. Lago Vanzela, P. C. Stringheta, Í. T. Perrone and A. M. Ramos, Int. J. Food Prop., 2017, 20, 1298–1305 CAS.
- S. Chen, S. Brahma, J. Mackay, C. Cao and B. Aliakbarian, J. Food Sci., 2020, 85, 517–525 CrossRef CAS.
- E. Ephrem, A. Najjar, C. Charcosset and H. Greige-Gerges, J. Funct.Foods, 2018, 48, 65–84 CrossRef CAS.
- P. Tyagi, K. S. Salem, M. A. Hubbe and L. Pal, Trends Food Sci. Technol., 2021, 115, 461–485 CrossRef CAS.
- L. Y. Yian and P. L. Phing, Malays. J. Anal. Sci., 2020, 24, 657–669 Search PubMed.
- C. Ramachandra and P. S. Rao, J. Food Sci. Technol., 2013, 50, 747–754 CrossRef CAS PubMed.
- C. Wong and W. Lim, Int. Food Res. J., 2016, 23, 1887 CAS.
- D. Labuckas, D. Maestri and A. Lamarque, Int. J. Food Sci. Technol., 2011, 46, 1388–1397 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |