Open Access Article
Open Access ArticleNutritional value, phytochemical richness, pharmacological potential, and culinary uses of monkey jackfruit
Raj
Singh
 a,
C.
Nickhil
a,
C.
Nickhil
 *a,
Shweta
a,
Sankar Chandra
Deka
*a,
Shweta
a,
Sankar Chandra
Deka
 a and
R.
Nisha
*b
a and
R.
Nisha
*b
aDepartment of Food Engineering and Technology, School of Engineering, Tezpur University, Assam, India. E-mail: nickhil@tezu.ernet.in
bDepartment of Food Technology, School of Agricultural and Food Technology, Assam Skill University, Assam, India
First published on 11th December 2025
Abstract
Monkey jackfruits are rich in vital nutrients, including carbohydrates, fiber, vitamins, and essential minerals like potassium and magnesium, establishing them as a valuable dietary resource. A comprehensive phytochemical analysis reveals the presence of various bioactive compounds, including flavonoids, phenolics, and saponins, imparting potent antioxidant, anti-inflammatory, and anticancer properties to the fruit. Furthermore, this review highlights the fruit's pharmacological promise, demonstrating its efficacy in managing diabetes, promoting cardiovascular health, and addressing skin disorders due to its anti-inflammatory and antimicrobial attributes. Beyond their medicinal significance, monkey jackfruits have earned a place in culinary arts, finding utility in a diverse range of dishes, from savory curries to delectable desserts. Their unique flavor profile and meat-like texture position them as a compelling meat substitute for vegetarians and vegans. This study underscores the multifaceted nature of monkey jackfruits, emphasizing their nutritional richness, phytochemical potential, pharmacological applications, and culinary versatility.
Sustainability spotlightBeing a sustainable food source, monkey jackfruit is highly nutritious, contains different phytochemicals, and exhibits various pharmacological activities. The culinary uses, both traditional and modern, show its importance in diet diversification. It also demonstrates potential for biodiversity and the enhancement of local livelihoods. The utilization of monkey jackfruit can strengthen food systems for sustainable nutrition and contribute to improving health and food diversity. |
1. Introduction
Artocarpus lakoocha Roxb., commonly known as the monkey jackfruit, belongs to the Moraceae family and is a tropical fruit originating from India.1 The generic name Artocarpus is derived from the Greek words “artos” (“bread”) and “karpos” (“fruit”), and the specific name “lakoocha” is derived from the Hindi name of the tree, perhaps as a tribute to its Indian origin.2 The tree has not been observed growing outside its native range, indicating that it has extremely specific climatic requirements for survival. In India, this tree is widespread in the peninsular tropical region. Artocarpus lakoocha is known by various names. Lakoocha, Lakooch, Lakoochi, Badhal, Dhau, Dahua, and Dephal are the terms used in Hindi and local dialects. Lakoocha also has multiple names in the Sanskrit language, like Airawata, Amlaka, Dahu, Dridhavalkala, and Granthimatphala, which point towards the native status of the tree species in India.The genus Artocarpus, which belongs to the Moraceae family, consists of jackfruit (Artocarpus heterophyllus), lakoocha or monkey jack (A. lakoocha), chempedak (A. integer), breadfruit or breadnut (A. altilis), and marang (A. odoratissima), comprising over 64 distinct species of the monoecious evergreen trees.3 A comparison of the major Artocarpus species is demonstrated in Table 1. Artocarpus lakoocha is a medium or large deciduous or evergreen tree. The deciduous period is very short in the eastern region of India, generally occurring in February and ending in early March. Artocarpus lakoocha seedling trees take five years to bear fruit. It can grow up to 15 m in height and is a handsome ornamental plant. The crown is usually well spread, making it an excellent shade and avenue tree. The leaves of the plant are oblong, acute, alternate, 10–25 cm long, glossy green on the upper side when young, rough when old, and leathery. Artocarpus lakoocha is a species bearing orange-yellow color for the male flowers and greenish color for the female flowers on the same tree. A single fruit weighs approximately between 250 and 300 g and contains 10–30 seeds of irregular shapes and various sizes. The seeds contain a white sticky latex that is highly recalcitrant, and the seed coat is white and thin. Seeds are dispersed by birds and monkeys.2,10 The fruits are nearly round or irregular and 5–12 cm in diameter, and they have velvety surfaces. Artocarpus lakoocha is a native of the sub-Himalayan humid regions of India and grows up to an altitude of 1200 m above MSL.11Artocarpus lakoocha can be sustained in both outdoor and underwater environments. It can also be found across the Indian subcontinent, especially in Uttar Pradesh, Jharkhand, Bihar, Assam, West Bengal, Tamil Nadu, and Kerala.12,13 Monkey jackfruit is one of the most important evergreen trees. The major region they inhabit is Asian nations, such as Bangladesh, Bhutan, Nepal, Malaysia, Singapore, and other neighbouring countries.14
| Species | Common name | Key nutritional highlights | Major phytochemicals | Bioactivity evidence | Culinary uses |
|---|---|---|---|---|---|
| Artocarpus lakoocha 4,5 | Monkey jackfruit | Moisture: ∼75.5%, protein: ≈0.24%, fat: ≈0.63%, crude fiber: ≈2.47%, and vitamin C: ≈13.4 mg per 100 g | Phenolics, flavonoids, and tannins; oxyresveratrol and related stilbenoids reported in heartwood/other extracts | In vitro/animal studies report antioxidant, antimicrobial, anti-inflammatory and hepatoprotective activities; traditional medicinal uses are widely documented | Eaten fresh, used in traditional preparations, cosmetics and ethnomedicine |
| Artocarpus heterophyllus 6,7 | Jackfruit | High carbohydrate (major energy source); seed composition shows substantial starch and notable protein depending on cultivar (values vary) | Phenolics, flavonoids, carotenoids, and prenylated flavonoids; bioactives reported across pulp, seed, leaf and latex | Numerous reports of antioxidant, antimicrobial, anti-inflammatory, and metabolic-benefit potentials in vitro and in animal studies; seeds and leaves frequently show activities in bioassays | Very versatile culinary uses: ripe fruit fresh; unripe used as a vegetable (meat substitute); seeds boiled, roasted or processed into flours and snacks |
| Artocarpus altilis 8 | Breadfruit | High in complex carbohydrates (starch); good dietary fiber; variable protein; important minerals (K, Ca, and Mg) | Phenolics and other antioxidant compounds present, content influenced by maturity and processing; carotenoids are reported in some cultivars | Extracts (esp. leaves) show antioxidant/anti-inflammatory properties in lab studies; functional/processing research focuses on food applications | Widely used as boiled, baked, and fried staples; processed into flour and novel food products |
| Artocarpus camansi 9 | Breadnut | Seeds are relatively nutrient-dense: representative proximate values reported—protein: ≈4.9%, fat: ≈3.5%, and carbohydrate: ≈26.1% | Seeds and other tissues contain phenolics and flavonoids; phytochemical profiling exists but is less extensive than that for jackfruit | In vitro antioxidant activity reported; some studies indicate potential antihyperglycemic activities and other bioactivities in model systems, but data remain limited | Seeds are commonly consumed, boiled or roasted; recent processing studies explore seed flour for bakery and value-added products |
It has commercial and medicinal value and is a source of livelihood and nutritional support for the local population. In Bangladesh, this plant is occasionally cultivated in backyards and homestead gardens. The dried aqueous extract made from the heartwood of this plant, also known as “Puaghaad,” has been traditionally used as an antihelminthic. Unripe fruit and male flower spikes are used as vegetables and to make pickles, sauces, and chutneys. When mature, the fruits have an irregular shape, sweet-sour flavor, and a yellow-to-orange hue.15 The Artocarpus lakoocha tree is also used for feed and timber. The hardwood sold as lakoocha is comparable to the famous teak wood. As Artocarpus lakoocha is durable outdoors and underwater, it can be used for construction, furniture, boat making, and cabinet work. The tree bark contains 8.5% tannin, is chewed like betel nut, and treats skin ailments. It yields a durable fiber that is good for cordage. The wood and roots have a lavish color dye.
Recently, studies have focused on monkey jackfruit, an underappreciated and largely unknown crop with potential use as food for both humans and animals. The structure of the fruit is illustrated in Fig. 1. The compounds in this fruit play an important role in treating gastric and liver disorders.10,16 The benefits of fruit and vegetable consumption include a greater life span,17,18 improved mental health,19 better cardiovascular health,20 reduced risk of some cancers,21 and weight management.22 In a study conducted in the USA, a relatively low risk of obesity was observed among healthy middle-aged women who consumed fruits and vegetables.23
Artocarpus lakoocha has a unique flavor and is an excellent source of carbohydrates, lipids, proteins, dietary fiber, minerals (phosphorus and iron), and vitamins (A, C, and B1).24 The three main sugars in monkey jackfruit are fructose, glucose, and sucrose. Additionally, it is an excellent source of β-carotene, a precursor of vitamin A, and ascorbic acid (vitamin C). Ascorbic acid and β-carotene share antioxidant properties that support healthy eyesight. This plant-based antioxidant plays an important role in maintaining normal health, protecting against coronary heart disease, and combating cancer. It is a top-notch iron source, that is, 28 to 80 mg per 100 g higher than the daily required value in the human diet.25 The pulp is edible and has hepatoprotective properties due to its antioxidant properties. Various secondary metabolites, such as alkaloids, phenols, flavonoids, tannins, and steroids, have been reported for monkey jackfruit.13
For generations, fruits from this species have played a significant role in supplementing human diets, garnering particular attention for their nutritional qualities, phytochemical contents, and bioactive substances, which are recognized as the vital components of overall well-being.26–28 Monkey jackfruit has gained recognition among researchers and scientists for its potential nutritional, nutraceutical, and pharmacological attributes, making it valuable in various fields, including food, pharmacology, and new product development. According to a study by the World Health Organization (WHO), over two-thirds of the global population in impoverished regions rely on primary or secondary plant metabolites for their health benefits.29
Further research on monkey jackfruit is crucial to increase the public awareness of its health benefits and potential as a common fruit crop. Despite the use of micropropagation technology to develop high-quality planting materials, the dissemination of this knowledge is lacking, highlighting the need for improved propagation methods.30 This review provides a comprehensive summary of the fruit's nutritional value, phytochemical compounds, culinary uses, traditional usage, and validated pharmacological properties. This review offers a comprehensive exploration of monkey jackfruits (Artocarpus lakoocha), focusing on their underreported nutritional and phytochemical profiles, pharmacological applications, and unique culinary uses. This study bridges existing knowledge gaps by integrating traditional insights with modern scientific perspectives for a holistic understanding.
2. Methodology
A comprehensive literature review was conducted to gather and analyze relevant information on Artocarpus lakoocha (commonly known as monkey jackfruit) to understand its nutritional value, phytochemical richness, pharmacological potential, and culinary applications. Renowned scientific databases, such as PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar, were used to retrieve the data. The keywords employed during the search included “Artocarpus lakoocha,” “monkey jackfruit,” “nutritional composition,” “phytochemicals,” “bioactive compounds,” “health benefits,” and “applications.” The review primarily focused on studies published between 1984 and 2024, although some early publications from the early 20th century were also examined to trace the historical evolution of research on this species. The collected literature included peer-reviewed journal articles, book chapters, and review papers, with a preference for works published in reputable journals with high impact factors and significant citation counts. Both quantitative and qualitative data were extracted, compared, and discussed to provide a holistic understanding of the nutritional profile, phytochemical constituents, pharmacological properties, and culinary applications of the monkey jackfruit. The information obtained was categorized thematically to encompass nutritional composition, phytochemical diversity, pharmacological and health benefits, and traditional and modern culinary uses. Furthermore, a critical analysis of the available literature was conducted to identify emerging research trends, highlight existing knowledge gaps, and outline potential future research directions aimed at unlocking the full nutritional and therapeutic potential of Artocarpus lakoocha.3. Nutritional composition and medicinal importance of monkey jackfruits
Monkey jackfruit is a very important species from a nutritional point of view, as shown in Table 2. Beyond basic nutrients, monkey jackfruit has various health-promoting ingredients, such as fiber, polyphenols, vitamins A and C, and minerals. According to Jahan,24 it has high vitamin C content (171.07 mg per 100 g), beta-carotene (3718.16 µg per 100 g), and an adequate amount of minerals, including zinc (19.82–24.92 ppm), manganese (3.76 mg per 100 g), copper (1.31 mg per 100 g), and iron (15.09 mg per 100 g). The observations for the proximate composition showed the values of moisture, ash, protein, crude fiber, and carbohydrates to be 81.32–86.95%, 1.5–5.33%, 0.12–0.51%, 1.84–10.21%, 3.17–15%, and 1.32–8%, respectively. Different researchers have observed diverse results regarding the nutritional profile. Biochemical analysis showed an ascorbic acid content of approximately 20.86 mg per 100 g the edible portion. The macro mineral content of the fruit includes sodium (1.0 mg per 100 g), potassium (181.0 mg per 100 g), calcium (13.0 mg per 100 g), manganese (42.0 mg per 100 g), and phosphorus (41.0 mg per 100 g).31 According to a few studies by Gupta,16Lakoocha fruit contains a significant amount of minerals, such as calcium (66.6 mg), magnesium (23.6 mg), potassium (350 mg), phosphorus (22.1 mg), iron (778 µg), zinc (3981 µg), copper (7974 µg), and manganese (2025 µg). Because of the adequate amount of vitamin C and beta carotenoid in monkey jackfruit, it plays an important role in the human diet.32 Therefore, it can be stated that Lakoocha is an important species from a nutritional perspective.| Elements | Values |
|---|---|
| Moisture (%)25 | 81.32–86.95 |
| Carbohydrate (%)25 | 1.32–8.62 |
| Fat (%)25 | 3.17–15 |
| Protein (%)25 | 0.12–0.51 |
| Fibre (%)25 | 1.84–10.21 |
| Vitamin A (IU)16 | 416–423 |
| Thiamin (µg%)16 | 0.01–0.02 |
| Riboflavin (µg%)16 | 0.09–0.15 |
| Niacin (µg%)16 | 0.15–0.3 |
| Sodium (mg per 100 g DW)31 | 22.02–22.75 |
| Potassium (mg per 100 g DW)31 | 165.14–195.38 |
| Calcium (mg per 100 g DW)31 | 47.71–54.53 |
| Magnesium (mg per 100 g DW)31 | 47.71–55.05 |
| Phosphorus (mg per 100 g DW)31 | 196.41–175.25 |
| Iron (mg per 100 g DW)31 | 2.26–2.88 |
| Zinc (mg per 100 g DW)31 | 0.09–0.16 |
| Copper (ppm)31 | 7.81–12.84 |
| Manganese (%)31 | 0.098–0.15 |
The popularity of herbal medications is on the rise in India, with people across all demographics embracing their use, a trend that is expected to continue in the foreseeable future. Ethnomedicinal formulations from South and Southeast Asia utilize nearly every part of the Maackia amurensis tree. The immature fruit is valued for its medicinal properties, addressing issues such as tridosa impotence, appetite loss, and blood complaints. Conversely, ripe fruits are considered liver tonics because of their sour-sweet taste. Additionally, the powdered bark of the tree serves as a remedy for skin ailments and acts as a vermifuge. The latex of this plant is utilized for its purgative properties. Beyond its medicinal benefits, jackfruit holds cultural and economic significance, serving as a vital food source and offering various utilitarian uses, including in traditional medicine and industry, underscoring its multifaceted importance to communities where it is cultivated.15,33,34
4. Essential phytochemical and biological activities in monkey jackfruits
Monkey jackfruit contains flavonoids, tannins, saponins, steroids, glycosides, triterpenoids, proteins, phenolic compounds, resins, and squalene. According to Krishnamurthy,13 screening the secondary metabolites of monkey jackfruit in various organic solvents, including petroleum ether, chloroform, ethanol, and water, showed that the levels of alkaloids (high) and tannins (low) were considerably more dependent on altitude than those of the other metabolites. The therapeutic characteristics of monkey jackfruit include antidiarrheal, antiglycation, antiaging, anti-inflammatory, analgesic, antibacterial, cytotoxic, insecticidal, and pancreatic lipase inhibitory activities.15Table 3 shows the various compounds present in the monkey jackfruit plant. In addition, the combination of monkey jackfruit with goat milk and herbs (neem, holy basil, etc.) can treat arthritic edema, prevent skin diseases, cleanse wounds, and treat dysentery. A separate study investigated the antioxidant and antiglycation properties of the phytooxyresveratrol extracted from monkey jackfruit. The antiglycation activity of the ethanolic extract of monkey jackfruit exhibited high antiglycation activity. The similar features between the two substances (isolated and standard) suggest their potential use in anti-aging products.16 Monkey jackfruit (Artocarpus lakoocha) contains various phytochemicals, each with potential health benefits.| Section of the tree | Compound | Molecular formula | Function |
|---|---|---|---|
| Heartwood35–39 | Oxyresveratrol | C14H12O4 | Antiviral, anti-poliovirus, anti-measles virus, anti-HSV and cytotoxic activities; significantly slowed the development of skin lesions and saved mice from death |
| Lakoochin A and B | C26H30O4 and C29H34O4 | The structures of Lakoochin A and B were elucidated by the analysis of their spectral data. Lakoochin A and B exhibited antimycobacterial activity. While Lakoochin A was cytotoxic against the breast cancer cell line but inactive toward nasopharyngeal carcinoma cells, compound Lakoochin B possessed cytotoxicity against both the breast cancer and nasopharyngeal carcinoma cells | |
| Norartocarpin | C15H10O6 | Norartocarpetin may be used as a skin whitening agent in the treatment of hyperpigmentation illnesses and in skin whitening cosmetics due to its inhibitory effects on antimelanogenesis | |
| Artocarpin | C26H28O6 | Artocarpin has been shown to have cytotoxic, antiplasmodial, anti-inflammatory, and antibacterial activities | |
| Cycloartocarpin and Cudraflavone C | C26H26O6 and C25H26O6 | It has exhibited antitubercular and antiplasmodial activities, while exhibiting moderate cytotoxic activities towards human oral epidermoid carcinoma and human breast cancer | |
| Fruit pericarp40,41 | Cycloartenone | C30H48O | It determines antibacterial, antioxidant, anthelmintic and insecticidal efficacy |
| α-Amyrin acetate and β-amyrin acetate | C32H52O2 | It treats various inflammatory disorders. Mechanical hypersensitivity, mechanical sensitization, and oedema, as well as antioxidant, cardiotonic, and antihyperlipidemic properties | |
| Leaves42–45 | Lupeol acetate | C32H52O2 | It can assist in managing the overall myocardium processes by lowering total cholesterol, triglyceride, and phospholipid levels |
| Phenols (vanillic acid) | C8H8O4 | Antioxidant and potential anti-cancer properties | |
| Gallic acid | C7H6O5 | ||
| Cinnamic acid | C9H8O2 | ||
| Ferulic acid | C10H10O4 | ||
| Tannin (tannic acid) | C76H52O46 | Antioxidant and antimicrobial properties | |
| Eicosane | C20H42 | Antifungal | |
| Diethyl phthalate | C12H14O4 | Antimicrobial and antioxidant | |
| Flower46,47 | Beta-carotenoids | C40H56 | Antioxidant properties, immune support, skin health, anti-inflammatory effects and cancer prevention |
| Flavonoids (alpha-amyrin) | C30H50O | Antioxidant properties, anti-inflammatory effects and cardiovascular health | |
| Beta-amyrin | |||
| Bark34,45,48–50 | Lakoochanone | C29H26O8 | Anti-inflammatory and antioxidant properties |
| Lakoochanosides | — | Antioxidant properties | |
| Catechin | C15H14O6 | Antioxidant and potential cardiovascular benefits | |
| Moracin | C19H18O4 | Antioxidant, anti-inflammatory, antiviral and antimalarial properties | |
| Cyclocommunin | C25H24O6 | Antioxidant | |
| Artocarpesin | C20H18O6 | Antioxidant and anti-inflammatory properties | |
| Artocarpanone | C16H14O6 | Anti-inflammatory, cytotoxic and antidiarrheal properties | |
| Engeletin | C21H22O10 | Anti-inflammatory and antioxidant properties | |
| Heterophyllene B | C13H16N2O5 | Antioxidant, antiplasmodia and anti-inflammatory properties | |
| Isogemichalcone B | C26H30O6 | Potential antioxidant and anti-inflammatory properties | |
| Albanin A | C20H18O6 | Skin whitening agents |
Only a few studies have been conducted on the phytochemicals of the monkey jackfruit. A recent study by Saleem51 showed that the HPLC chromatograms of the methanolic extract showed the presence of chromatotropic acid (21.6 µg g−1), quercetin (2.5 µg g−1), gallic acid (33.4 µg g−1), vanillic acid (40.9 µg g−1), cinnamic acid (11.1 µg g−1), ferulic acid (23.7 µg g−1), and kaempferol (2124.7 µg g−1). The polyphenol content was also observed as 325.63 mg per 100 g GAEs, flavonoid as 521.98 mg per 100 g CEs, and tannin content as 124.03 mg per 100 g TEs.
The phytoconstituents present in the fruit barks are lakoochanosides, lakoochanone, Moracin C, integrin, cyclommunin, engeletin, isogemichalcone B, morachalcone A, het erophyllene B, albanin A, Moracin M, artocarpesin, norartocarpin, resveratrol, artocarpanone, and oxyresveratrol, whereas flavones, phenols, tannins, eicosane, and diethyl phthalate are the compounds found in the leaves.48,52,53 The leaves contain sufficient amounts of kaempferol and quercetin. Albanin A,54 isoartocarpesin,55 cudraflavone C,56 norartocarpin,57 cudraflavone B,58 and catechin A57 were reportedly observed in various parts of the same plant.59
Extraction and analytical methods provide a comprehensive approach for separating and identifying bioactive compounds from Artocarpus lakoocha, contributing to its applications in pharmacology and nutraceuticals. Most of the extractions were conducted using ethanol and methanol as the solvents.60–62 Ultrasound-assisted extraction has also been used to extract compounds such as oxyresveratrol, which is also considered a green method.63 Soxhlet extraction is the traditional method for isolating phytochemicals. The identification of active compounds in crude extracts is generally initiated using thin-layer chromatography. The free radical scavenging activity of the extract was identified using antioxidant methods, such as the DPPH and ABTS assays. Chaurasia and Pandey45 conducted a brief analysis. UV-Vis spectrophotometry is generally used to analyze the antioxidant activities, total phenols, flavonoids, and tannins.5
The main bioactive compounds in monkey jackfruit that exhibit biological activities are oxyresveratrol, flavonoids, stilbenoids, tannins, and phenolic compounds. Oxyresveratrol is a major bioactive compound in the fruit, which exhibits antioxidant activities by neutralizing free radicals and reducing oxidative stress through electron donation. It also inhibits reactive oxygen species (ROS) production, contributing to its anti-inflammatory and anti-aging effects.
The standardization of bioactive compound extraction from monkey jackfruit (Artocarpus lakoocha) is essential for ensuring the reproducibility, quality control, and industrial scalability of oxyresveratrol (ORV)-rich formulations. The extraction efficiency of ORV and related phenolics depends on solvent polarity, temperature, particle size, and extraction time. Conventional solvent extraction using ethanol or methanol (60–80%) has been widely reported, but greener alternatives, such as deep eutectic solvents (DES) and aqueous ethanol under ultrasound or microwave assistance, significantly improve the yield and purity while minimizing solvent residues.4,63 Optimization studies using response surface methodology (RSM) demonstrated that extraction at 60–70 °C for 30–45 min with a solvent-to-solid ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/w) provides the highest ORV recovery from heartwood, achieving concentrations of 50–80 mg per g extract.64 Post-extraction purification using column chromatography or macroporous resin further enhances the ORV content and removes pigments and polysaccharides. Analytical standardization relies on the HPLC and LC-MS quantification of ORV using authentic standards, along with total phenolic and flavonoid content assays for the quality benchmarking of the extracts. The adoption of validated analytical protocols and solvent recycling enhances both the environmental and economic sustainability of large-scale extraction. Such standardization enables reproducible pharmacological testing and regulatory compliance for nutraceutical and cosmeceutical applications.4,63,64
1 (v/w) provides the highest ORV recovery from heartwood, achieving concentrations of 50–80 mg per g extract.64 Post-extraction purification using column chromatography or macroporous resin further enhances the ORV content and removes pigments and polysaccharides. Analytical standardization relies on the HPLC and LC-MS quantification of ORV using authentic standards, along with total phenolic and flavonoid content assays for the quality benchmarking of the extracts. The adoption of validated analytical protocols and solvent recycling enhances both the environmental and economic sustainability of large-scale extraction. Such standardization enables reproducible pharmacological testing and regulatory compliance for nutraceutical and cosmeceutical applications.4,63,64
Artocarpus lakoocha, particularly the heartwood-derived stilbenoid oxyresveratrol (ORV), triterpenoids, such as lupeol/lupeol acetate, and assorted triterpenoid/steroidal saponins, produces a coherent mechanistic story that links anti-melanogenic action, eicosanoid (arachidonic acid) signalling, pharmacokinetics, mitochondrial protection, and redox modulation. ORV is the most directly documented anti-melanogenic compound. In cellular melanocyte models, it lowers melanin synthesis by inhibiting tyrosinase activity and downregulating the MC1R → cAMP → MITF axis (thereby reducing tyrosinase and TRP expression), effects that are closely associated with the suppression of cellular oxidants and the restoration of redox balance in melanocytes.65,66 Lupeol and its acetate derivative predominantly act as the anti-inflammatory modulators of the arachidonic acid cascade. They reduce COX-2 expression and prostaglandin production and interfere with upstream TLR/NF-κB signalling in inflammatory models, thereby diminishing prostaglandin-driven proliferation and paracrine signals that can promote pigmentary and tumor-promoting microenvironments.67 Pharmacokinetically, ORV is rapidly absorbed but undergoes extensive first-pass metabolism (notably glucuronidation/sulfation) and has relatively low oral bioavailability. Animal studies have shown markedly altered plasma AUC and clearance when ORV is co-administered with bioenhancers (e.g., piperine) or formulated as nano-carriers (NLC/SMEDDS). These are strategies that substantially increase systemic exposure and tissue delivery—a critical point when linking in vitro potencies to any in vivo therapeutic hypothesis.68 At the organelle level, ORV and specific Artocarpus extracts exhibit dual redox behaviors. At physiological/sub-cytoprotective concentrations, they scavenge ROS, preserve mitochondrial membrane potential, limit mitochondrial cytochrome-c release, and inhibit apoptosis in UVB-stressed keratinocytes or toxin models. However, at high concentrations (or in sensitive cancer cells), they may behave as pro-oxidants (including inducing ferroptosis or mitochondrial dysfunction), thereby triggering cell death. This mechanistic bifurcation explains both the cytoprotection and selective anticancer cytotoxicity observed in separate studies.69 Triterpenoid and steroidal saponins present in Artocarpus spp. further support mitochondrial integrity and redox homeostasis in non-neoplastic models by restoring GSH, elevating antioxidant enzymes (SOD, CAT, and GSH-Px), stabilizing mitochondrial networks, and inhibiting MAPK-mediated apoptotic signalling. These actions have been reported for saponins across multiple plant sources and are likely to be operative in jackfruit relatives, where saponins have been detected.70 Taken together, a recent real-time mechanistic study of oxyresveratrol from Artocarpus showed the clear inhibition of melanogenesis through MC1R/cAMP/MITF suppression, along with a concurrent reduction in cellular oxidants, thereby experimentally linking ORV's biochemical (tyrosinase and signalling) targets to its antioxidant and mitochondrial-stabilizing effects in skin cells. Meanwhile, separate pharmacokinetic studies have demonstrated that the meaningful systemic or topical delivery of ORV depends on formulation strategies to overcome rapid metabolism.
5. Pharmacological importance of monkey jackfruit
Artocarpus lakoocha has emerged as a pharmacologically important species, largely because its heartwood, bark, leaves, and fruit contain a concentrated amount of oxyresveratrol (ORV) and a suite of phenolics and flavonoids that show reproducible bioactivities in vitro and in vivo.4 It has shown promising pharmacological activities in various studies (Table 4). The cytotoxicity test is an in vitro test based on a cell culture that is used to evaluate the safety of pharmaceuticals, cosmetics, food additives, and pesticides, as well as to determine the presence of antineoplastic activities in a chemical. In vitro, cytotoxic chemicals are toxic to cancer cells and can also be hazardous to rapidly reproducing normal cells. These compounds will exhibit antitumor activities if this toxicity is transferred across tumors in vivo. Monkey jackfruit plants exhibit numerous biological activities, including cytotoxicity. Based on the collected data, a comparison of the effects of various doses of Artocarpus lakoocha leaf extract with ethanol (10, 20, 40, 60, 80, and 160 g mL−1)72 and pericarp methanol extract (10–1000 g mL−1)32 was performed to determine the IC50 of the antioxidant activities via regression analysis. Artocarpus lakoocha extract was found to destroy brine shrimp in a dose-dependent manner. The saponin, alkaloid, and cardiac glycoside content of the extract may explain its lethality to brine shrimp.32 The anti-inflammatory effects of Artocarpus lakoocha leaves extracted in methanol were shown to be dose-dependent and statistically significant (p ≤ 0.05). At a dosage of 200 mg kg−1, Artocarpus lakoocha was more effective than indomethacin in reducing inflammation (64.90%). The dosage of the Artocarpus lakoocha leaf methanol extract reduced writhing by 29.63% and 57.41% (p ≤ 0.05). The consequence of arachidonic acid breakdown is one item that might induce inflammation. Arachidonic acid is an unsaturated fatty acid with 20 carbon atoms. Arachidonic acid is produced from phospholipids when cell phospholipases are activated by physical, chemical, or biological stimuli.75 The leaves of the Artocarpus lakoocha plant (100 mg kg−1) have a modest antidiarrheal effect. This is based on the information gathered. However, at a dosage of 200 mg kg−1, the effect is halted (68.11%), which is comparable to what the usual medicine, loperamide, can accomplish (71.1%). Thus, the extract given prevented diarrhea via an antisecretory mechanism. This was also demonstrated by a decrease in the number of wet stools in the experimental test group. Diarrhea treatments aim to correct the diet, halt excessive water and electrolyte loss, correct acid–base imbalances, treat the symptoms, address the causes of diarrhea, and treat the illnesses that produce diarrhea as a side effect. As a result, the most crucial part of managing diarrhea is food control.81 Additionally, the pharmacological properties of the Artocarpus lakoocha plants include liver and nerve cell protection. The puaghaad and oxyresveratrol present in fruits will increase cell survival, notably ROS levels and lipid peroxidation, the neuroprotective impact of Artocarpus lakoocha wood extract with aqueous suggests that mitochondrial protection is difficult to detect. Because of the action of oxyresveratrol on the synthesis and pharmacokinetics of redox-sensitive antioxidant enzymes, ingesting puaghaad orally might be a great method for preventing transient neurological problems.71| Sample | Extraction | Technique | Function | Finding |
|---|---|---|---|---|
| Heart wood46,71 | Aqueous | Bovine serum albumin (BSA) | Antiglycation | AGE-BSA was suppressed by the extract |
| Aqueous | H2O2-induced oxidative stress in SH-SY5Y cells | Neuroprotective | The extract possesses neuroprotective properties | |
| Fruit51 | Methanol | In vivo-induced paracetamol in mice | Hepatoprotective | The extract inhibited elevations in liver function tests as well as histological changes caused by paracetamol |
| Leaf5,72–76 | Methanol | Brine shrimp lethality bioassay | Cytotoxic | The extract has a high toxicity level. The extract's LC50 value was 2.83–2.94 g mL−1 |
| Carrageenan-induced paw edema test in mice | Anti-inflammatory | At a dosage of 200 mg kg−1, the extract had anti-inflammatory properties | ||
| Acetic acid-induced writhing test | Analgesic | The extract showed inhibitions of 29.63% and 57.41% | ||
| Castor oil-induced diarrhea | Antidiarrhoeal | The extract decreased castor oil-induced diarrhoea in test animals at doses of 100 and 200 mg kg−1 | ||
| In vivo, hyperlipidemia-induced rats were given an extract | Anticholesterol | The extract significantly increased serum high-density lipoprotein (HDL) while lowering serum total cholesterol, triglycerides, and LDL | ||
| Ethanol | MTT test with NIH-3T3 cells in mice in vivo | Proliferative and wound healing | The extract was shown to have proliferative and wound-healing effects | |
| Methanol | 1,1-Diphenyl-2 picryl-hydrazil (DPPH) | Antioxidant and antimicrobial properties | IC50 value is 111.98–146.18 µg mL−1 | |
| 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) | IC50 value is 138.26–138.92 µg mL−1 | |||
| 1,1-Diphenyl-2 picryl-hydrazil (DPPH) | IC50 value is 26.95–26.959 µg mL−1 | |||
| n-Hexane, ethyl acetate, and ethanol | 1,1-Diphenyl-2 picryl-hydrazil (DPPH) | Antioxidant | The IC50 values are as follows: n-hexane (1062.03–1063.45 g mL−1), ethyl acetate (323.18–323.20 g mL−1), and ethanol (99.23–99.30 g mL−1) | |
| Heart pericarp77 | Methanol | Brine shrimp lethality bioassay | Cytotoxic | The extract proved toxic, having an IC50 of 427.74 g mL−1 |
| Bark78 | Hydro-methanolic | Tests for induced nociception using formalin; paw edema caused by carrageenan and acetic acid; tail immersion and hot plate tests | Antinociceptive | There is an antinociceptive action in the extract |
| Crude79 | Aqueous | Schistosoma mansoni-infected in vivo mice | Schistosomicidal | At a concentration of 250 g mL−1, the extract inhibited motility |
| Dry fruit80 | Methanol | 1,1-Diphenyl-2 picryl-hydrazil (DPPH) | Antioxidative properties | IC50 value is 6.72–10.97 mg ascorbic acid per g |
| 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) | IC50 value is 2.32–3.64 mg ascorbic acid per g | |||
| Hydroxyl | IC50 value is 41.35–53.1 mg BHT per g | |||
| Superoxide anion | IC50 value is 0.47–0.60 mg ascorbic acid per g | |||
| Seeds49 | Methanol | 1,1-Diphenyl-2 picryl-hydrazil (DPPH) | Antioxidant and anticancer activities | 18.28–22.50 µg mL−1 |
| Ferric reducing antioxidant potential | 31.22–32.11 µg mL−1 |
6. Applications of monkey jackfruit
Artocarpus lakoocha has a sweet and slightly tangy flavor, and its flesh is often compared to that of the regular jackfruit. Fruit pulp is often sweet and sour and is eaten fresh, but is also frequently cooked into curries. Curry, pickles, and sauce are made from the unripe fruit and male flower spike of Artocarpus lakoocha. The fruits and male flowers of this tree are among the portions that may be consumed fresh, cooked, steamed, or roasted. The wide, flat seeds may be eaten. They also have applications in the cosmetic industry as they can be used as a skin-whitening ingredient. The presence of flavones and flavonoids is responsible for anti-cancer activities and can help in the formulation of chemotherapeutic agents. Lectin in the plant can be used for the treatment of HIV, suggesting the wide application in biochemistry. The value-added products developed from the fruits are dehydrated raw jackfruit slices, dehydrated raw jackfruit flour, jackfruit mixture, candy, squash, jam, jelly, jackfruit ganache, jackfruit leather, chocolate, cake, muffins, honey, pudding, and jackfruit wine. Monkey jackfruit pulp can also be used to make pickles, curry, sauce, and juice with sugar, cardamom, and black pepper, depending on the taste profile of the individual. The raw fruits are widely used for the purpose of pickle preparation.82 Apart from these, Artocarpus lakoocha holds immense potential for the development of value-added products, such as fermented beverages, nutraceutical extracts, anti-aging cosmeceuticals, and functional snacks, owing to its rich nutrient profile and high oxyresveratrol (ORV) content. The pulp and seeds, abundant in fermentable sugars and phenolic compounds, can be transformed into probiotic or alcoholic fermented beverages through controlled enzymatic saccharification, followed by lactic acid or yeast fermentation, enhancing antioxidant activity, polyphenol bioavailability, and shelf stability. This is similar to the optimized jackfruit seed drink fermentations reported by Hoang.83 The heartwood and bark, rich in ORV, can be used to produce standardized nutraceutical extracts via green solvent or deep eutectic extraction, yielding ORV concentrations up to 80% w/w with potent anti-inflammatory, antioxidant, and neuroprotective effects.4,63 Given ORV's low oral bioavailability, encapsulation in nanoparticles or cyclodextrin complexes or co-administration with piperine can enhance systemic absorption.68 The topical formulations of Artocarpus lakoocha extracts show significant tyrosinase inhibition, melanin suppression, and reactive-oxygen-species scavenging activities, validating their use in anti-aging and skin-brightening cosmeceuticals.64 These effects stem from ORV's dual mechanism of direct enzyme inhibition and the down-regulation of melanogenic genes (MITF, TRP-1, and TRP-2), complemented by NF-κB modulation that suppresses inflammatory cytokine expression. Beyond extracts, the seeds of Artocarpus lakoocha represent a valuable raw material for functional snacks, as seed flours provide resistant starch, dietary fiber, and proteins suitable for cookies, extruded chips, and nutrient bars. Prior trials with jackfruit and breadnut seed flours confirm improved textural and nutritional properties without sensory compromise.84 The integration of ORV microcapsules into seed-based snacks could yield antioxidant-enriched products for preventive nutrition markets. To commercialize these products, process standardization, safety profiling, and consumer sensory evaluations are essential, along with the sustainable sourcing and quality control of raw materials. Collectively, these value-added innovations could transform monkey jackfruit from an underutilized tropical species into a high-value resource for functional foods, nutraceuticals, and cosmeceutical applications, contributing simultaneously to nutrition security, circular bioeconomy, and rural livelihoods. A paper is also available on the preparation of powder, juice, sambhar, papad, hulli, and various local meals, such as fish curry and chicken curry, from monkey jackfruit.13 The processing challenges faced during the use of latex due to its sticky texture have led to mechanical handling complications. As the fruit is highly perishable, post-harvest losses occur. Therefore, the development of preservation techniques, such as drying and freezing, is required. The bioactive compounds may vary with the change in environment, such as soil and climate.7. Potential health benefits of monkey jackfruit
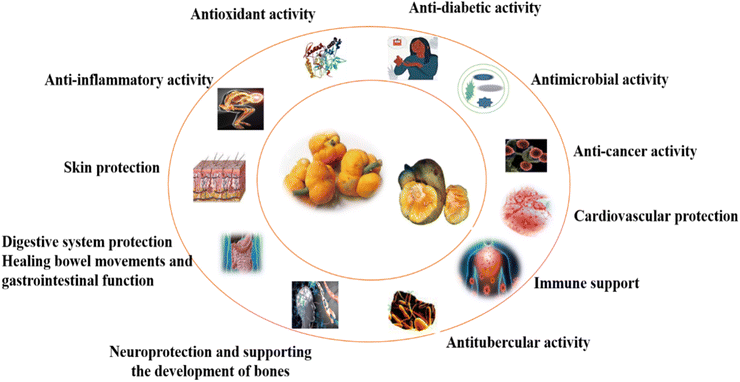
The health benefits of monkey jackfruit are shown in Fig. 2. This unique fruit is rich in essential nutrients, making it a valuable addition to a balanced diet. Monkey jackfruit is a good source of dietary fiber, which can aid in digestion and help prevent constipation. It also contains vitamins and minerals, including vitamin C, which supports the immune system, and potassium, which is important for heart health and maintaining proper blood pressure.1 Some pharmacological activities are found in the monkey jackfruit plant: anti-inflammatory, antiviral, anticancer, and anti-HIV properties. The presence of bioactive components in different plant parts leads to specific health benefits. The bioactive compounds such as cycloartenone, cycloartenol, α-amyrin acetate, and β-amyrin acetate, are present in fruit pericap. Hypolipidemic, and antiatherosclerotic, act as first precursors for the synthesis of sterols and stanols, anti-inflammatory activity, hypersensitivity, oedema, and radical scavenging.40,41 The leaves contain two bioactive compounds, i.e., lupeol acetate, which is responsible for antioxidant activity; decreasing cholesterol, phospholipid, and triglyceride levels; and interrupting cardiovascular disease,42 and oxyresveratrol, which helps with the significantly delayed development of skin lesions and is anti-viral, cytotoxic, anti-HSV, and anti-HIV.35,85 However, the seed contains lectin, which helps in carbohydrate (mannose) binding and antigen detection. The immature fruit is hot, sour, and sweet and causes the loss of appetite and blood complaints. The mature fruit tastes sour and sweet and is a tonic for the liver.15 The potential health benefit is discussed in detail below.7.1 Cytotoxic and anticancer activities
The species of Artocarpus, particularly Artocarpus lakoocha, have emerged as promising sources of cytotoxic phytochemicals, with most existing data derived from in vitro studies using crude extracts and purified compounds. The heartwood, bark, and fruit of A. lakoocha are rich in stilbenoids, especially oxyresveratrol, along with other phenolics, triterpenoids (such as cycloartenone and α-amyrin derivatives), and lectins, all of which exhibit antiproliferative effects against various cancer cell lines.50 Mechanistic studies have indicated that oxyresveratrol induces cytotoxicity via several interconnected mechanisms.86 It triggers apoptosis by modulating apoptotic gene expression and cell cycle checkpoints, increases intracellular ROS levels and DNA damage signals, and affects the regulation of genes related to metastasis and DNA repair, and these are effects observed in MCF-7 breast cancer and other models.87 Recent research suggests that oxyresveratrol can also induce ferroptosis, an iron-dependent, lipid peroxidation-driven cell death pathway, in breast cancer cells by inhibiting the EGFR → PI3K → AKT → GPX4 cascade, emphasizing its role in redox-based anticancer mechanisms.88 Crude methanolic and ethanolic extracts of Artocarpus tissues consistently exhibit micromolar to low microgram-per-milliliter IC50 values across cancer types (breast, colon, cervical, and lung), and bioassay-guided fractionation confirms that cytotoxicity is concentrated in polyphenol-rich fractions, highlighting the role of specific small molecules rather than general extract toxicity.89 Macromolecular components, including water-soluble polysaccharides from A. heterophyllus, have also demonstrated biological effects in colon cancer models by modulating inflammatory mediators, indicating diverse mechanisms across the genus.90 However, most available studies are limited to in vitro assays or simple organismal screens, use inconsistent extraction protocols, and rarely assess the selectivity toward non-malignant cells. In addition, pharmacokinetic and in vivo toxicity data are scarce.4Therefore, although oxyresveratrol and related Artocarpus constituents are strong anticancer leads, systematic in vivo efficacy, safety, and mechanistic studies, including dose–response relationships, molecular target validation, and combinatorial treatment assessments, are crucial for clinical translation. Future research should focus on standardized extraction, comparative potency profiling, and well-designed animal models to elucidate the underlying mechanisms and therapeutic potential of these compounds.
7.2 Anti-inflammatory and analgesic properties
Artocarpus lakoocha is a nutritionally rich, yet underutilized tropical fruit. Its edible parts and traditional formulations provide carbohydrates, dietary fiber, essential vitamins and minerals, and a complex array of phytochemicals, such as phenolics, flavonoids, stilbenoids (notably oxyresveratrol), and prenylated flavones like artonins. These compounds collectively underpin the anti-inflammatory and analgesic actions of the plant. Mechanistically, both crude extracts and isolated constituents exhibit potent antioxidant effects by scavenging ROS and suppressing lipid peroxidation, alongside the direct modulation of inflammatory signaling pathways, including the inhibition of pro-inflammatory enzymes and mediators (e.g., prostaglandins and nitric oxide), membrane stabilization, and the suppression of neutrophil and macrophage activity, all of which help alleviate inflammation and pain. In vivo validation in rodent models revealed that methanolic and ethanolic extracts significantly reduced carrageenan-induced paw edema and produced notable analgesic effects in hot-plate, tail-flick, and acetic-acid-induced writhing tests, with some doses approaching those of the standard drugs. These findings showed a strong association with high levels of phenolic and flavonoid compounds.78 Recent research isolating oxyresveratrol has confirmed its significant anti-inflammatory properties in skin inflammation models by reducing oxidative stress and decreasing inflammatory cytokine levels, while initial toxicological assessments indicate favorable safety margins.91,92 Overall, the nutritional and phytochemical varieties of Artocarpus lakoocha highlight its dual potential as both a functional food and a pharmacological agent, offering basic antioxidant and anti-inflammatory benefits through diet and more pronounced effects through concentrated extracts. However, important research priorities include standardizing phytochemical characterization across different cultivars; understanding the structure–activity relationships of artonins and stilbenoids; and conducting pharmacokinetic, chronic toxicity, and clinical studies to support their therapeutic use.937.3 Gastroprotective and antidiarrheal effects
Artocarpus lakoocha combines moderate nutritional content, including fiber, carotenoids, vitamins, and minerals, with a high concentration of bioactive phytochemicals, such as oxyresveratrol, flavonoids, tannins, and phenolic acids. These compounds collectively support the traditional use of the species and experimentally validate gastroprotective and antidiarrheal properties.94 The strong antioxidant and anti-inflammatory effects of these polyphenols contribute to mucosal protection against oxidative and inflammatory damage, which are key factors in ulcer and secretory diarrhea.63 Tannins and other astringent compounds reduce intestinal secretion and toxin binding, whereas flavonoids and oxyresveratrol regulate gut motility, suppress inflammatory mediators, and neutralize ROS, collectively resulting in measurable antidiarrheal and gastroprotective outcomes in vivo.4,95 Studies on the Artocarpus lakoocha extracts have demonstrated a wide range of biological activities, including antidiarrheal, antioxidant, hepatoprotective, and antimicrobial activities, suggesting their value as both functional food ingredients and phytotherapeutic candidates.51 In representative animal experiments, extract doses of 100–200 mg kg−1 significantly reduced the incidence and severity of diarrhea, decreased intestinal fluid accumulation, and preserved mucosal integrity; these are effects that correlated with phenolic content and antioxidant indices.72 These findings link nutritional composition and phytochemical richness to antioxidant and antisecretory mechanisms that provide gastroprotection. However, to advance clinical use, standardized extraction, dose–response, safety profiling, and constituent identification are essential.5 Therefore, Artocarpus lakoocha is a nutritionally beneficial and phytochemically potent species with consistent preclinical gastroprotective and antidiarrheal efficacy. Targeted research on chemical standardization, mechanistic pathways, and clinical validation will define its future use in dietary and medicinal contexts.7.4 Neuroprotective and hepatoprotective activities
Artocarpus lakoocha, valued nutritionally and medicinally, offers edible pulp, seeds, and heartwood extracts rich in carbohydrates, fiber, vitamins (especially vitamin C), minerals, and a diverse array of bioactives, including polyphenols, flavonoids, stilbenoids (notably oxyresveratrol), and polysaccharides. These compounds collectively explain the observed neuroprotective and hepatoprotective effects of Artocarpus lakoocha. Multiple studies have indicated strong antioxidant and anti-inflammatory potential in vitro and in vivo, which are mechanisms that underpin both neuronal and hepatic protection.4 In neuroprotection, the ethanolic extracts of Artocarpus lakoocha have been shown to reduce oxidative stress, suppress inflammatory mediators, and enhance neuronal survival in cellular and rodent models, effects likely mediated by free radical scavenging, the inhibition of pro-inflammatory signaling, and the stabilization of mitochondrial functions.96 For hepatoprotection, extracts from Artocarpus species normalize serum enzyme levels, restore hepatic antioxidant defenses, and reduce histopathological liver damage in toxin-induced injury models (e.g., CCl4, paracetamol, and diet-induced stress). In some cases, these protective effects are comparable to those of silymarin, and contributions from both phenolic compounds and immunomodulatory polysaccharides have been identified.51,97 These findings collectively validate traditional uses and justify advancing standardized extracts and isolated compounds, such as oxyresveratrol, into pharmacokinetic, toxicity, and dose-ranging studies, followed by disease-specific preclinical trials (e.g., Parkinson's, Alzheimer's, or NAFLD models) and, ultimately, clinical testing.8. Challenges
There are key challenges that impede the advancement of research and the practical use of Artocarpus lakoocha. Initially, compositional and phytochemical data are fragmented and often non-standardized; studies use different tissues, extraction methods, and reporting units, making cross-study comparisons and nutrition labeling unreliable.5 Compounds such as oxyresveratrol and other phenolics have been identified; systematic untargeted metabolomic and chemotype mapping across cultivars and environments are lacking, limiting discovery, quality control and the reproducibility of bioactivity claims.98 Third, safety and translational evidence remain insufficient; promising in vitro and animal results have not been matched by rigorous toxicology, ADME, or human clinical studies required for nutraceutical or therapeutic development. Recent preliminary toxicology work highlights the need for broad safety profiling.92 Furthermore, poor postharvest handling, short shelf life, and the paucity of scalable processing protocols hinder value-addition, market integration and smallholder uptake.99 Finally, limited socioeconomic and agronomic studies mean there is little evidence on the best agroforestry models, cultivar selection for yield/nutrition, and market pathways needed to incentivize farmers. Addressing these challenges requires coordinated interdisciplinary research, standardized analytical methods, and targeted translational funding.49. Future perspective and research gap
Artocarpus lakoocha holds clear and specific promise as a multifunctional crop; its fruit pulp delivers a noteworthy proximate and micronutrient profile (e.g., high moisture, low fat and protein, and measurable vitamin C and fiber), indicating a real potential to diversify diets and help address micronutrient shortfalls in undernourished communities when integrated into local food systems. Its heartwood, bark, leaves and fruit are rich in phenolics, flavonoids and distinctive bioactives, such as oxyresveratrol, which are compounds that have shown strong antioxidant, antibacterial and anti-inflammatory properties in laboratory studies and make Artocarpus lakoocha a promising source for nutraceuticals, topical cosmeceuticals and standardized botanical extracts. Because Artocarpus lakoocha is already used in agroecosystems (including as a fodder tree) and related Artocarpus species have demonstrated climate-resilience traits, the deliberate integration of monkey jackfruit into agroforestry and diversified production systems could enhance on-farm biodiversity, improve soil health, increase carbon storage, and provide farmers with an additional, climate-resilient income stream, all of which support food-security goals in low-latitude regions.100 Practically, the edible pulp, flowers and seeds of the species lend themselves to a wide range of value-added food products (fresh, preserved, flours and seed-based ingredients), while the heartwood and leaf extracts already show enough bioactivity to justify translational R&D into safe topical and oral formulations.However, realizing this potential for monkey jackfruit requires targeted scientific and development actions because important evidence gaps remain. Standardized, cultivar and tissue-specific compositional datasets are sparse (limiting reliable nutrition claims and product labeling), and phytochemical research remains fragmented (few untargeted metabolomics or chemotyping studies exist to map chemical diversity across cultivars and environments).4 Toxicological and safety data are limited despite encouraging in vitro and animal results; recent toxicology and in vivo assessments underscore the urgent need for systematic acute/chronic toxicology, ADME profiling, and dose-finding work before human applications proceed. Likewise, mechanistic pharmacology and well-designed human clinical studies are essentially absent, and applied research on postharvest handling, shelf-life extension, sensory optimization and low-cost value-addition is insufficient.99 To unlock monkey jackfruit's role in nutrition, sustainability and rural livelihoods, an interdisciplinary program is needed that combines standardized compositional and metabolomic mapping across cultivars and tissues, rigorous toxicology and translational pharmacology, phased human trials for priority indications, applied postharvest and product-development studies tailored to low-resource contexts, and agroecological and socioeconomic research to design farmer-friendly agroforestry and value-chain models.92,100
10. Consumer studies and toxicity
Comprehensive research on the consumer acceptance, toxicity, and dosage control of Artocarpus lakoocha remains limited, yet emerging evidence provides valuable insights into its nutritional and pharmacological potential. Consumer acceptance studies focusing exclusively on Artocarpus lakoocha are scarce; however, regional evaluations from India, Thailand, and Bangladesh indicate encouraging results when the fruit pulp is incorporated into blended food products, such as jams, beverages, or fermented foods, improving sensory scores for color, flavor, and mouthfeel. These studies, though preliminary, highlight the potential of Artocarpus lakoocha to be developed into functional and value-added foods, provided that standardized sensory evaluation protocols and broad demographic testing are employed.101,102 Comparable sensory work on related Artocarpus species (e.g., A. heterophyllus and A. altilis) demonstrates that texture, sweetness, and aroma strongly influence consumer preference, suggesting similar drivers for Artocarpus lakoocha-based products.Toxicological studies on Artocarpus lakoocha extracts reveal relatively low acute toxicity but highlight the need for systematic dose–response and chronic toxicity evaluations. Recent investigations on ethanolic and ethyl acetate extracts from Artocarpus lakoocha bark and leaves show no mortality or significant biochemical alterations in rodents at doses up to 2000 mg kg−1, suggesting a wide safety margin in acute exposure. However, subchronic and genotoxicity data remain scarce, and the pharmacokinetic behaviour of its major bioactive compound, oxyresveratrol, shows moderate oral absorption (∼10–15%) and rapid systemic clearance, indicating a need for controlled dosing and formulation optimization to enhance bioavailability.103In vitro studies demonstrate that oxyresveratrol exerts strong antioxidant and anti-inflammatory activities but may exhibit pro-oxidant or genotoxic effects at high concentrations or under metal-rich conditions, necessitating careful safety evaluations before clinical applications.68,104
No standardized human dosage guidelines currently exist for Artocarpus lakoocha or its bioactive constituents. Translational studies should therefore determine the no-observed-adverse-effect levels (NOAELs) and lowest-observed-adverse-effect levels (LOAELs) in well-designed animal trials, followed by human pharmacokinetic and tolerability assessments. Establishing controlled dose ranges is vital for potential nutraceutical or therapeutic formulations to ensure safety and reproducibility. Furthermore, standardized extraction methods and compositional characterization are essential to maintain consistency between batches and across studies. Until robust clinical data become available, recommending safe intake levels remains premature.105 Therefore, it is a potentially nutritious and bioactive food resource and is safe for human consumption.
11. Conclusion
Monkey jackfruits, with their impressive nutritional content and adaptability, have emerge as a crucial resource in addressing global challenges. Rich in essential vitamins, minerals, and fiber, they combat malnutrition and diet-related health issues while offering a sustainable solution for food security and climate resilience. As a meat substitute, they support the shift towards plant-based diets, and their historical use in traditional medicine hints at numerous health benefits, from antioxidant to potential cancer-fighting properties. This intersection of nutrition, sustainability, and health underscores their potential as innovative pharmaceuticals and nutraceuticals, requiring continued research, investment, and awareness to fully leverage their benefits in building a more resilient world.Declarations
This review paper, titled “Nutritional Value, Phytochemical Richness, Pharmacological Potential, and Culinary Uses of Monkey Jackfruit ”, has been prepared solely by the author(s), and all contributors have been appropriately credited.Author contributions
Raj Singh: conceptualization, data curation and writing-review and editing; C. Nickhil: investigation, writing and editing; Shweta: writing and editing; Sankar Chandra Deka: investigation and final checking of the manuscript; R. Nisha: investigation and final checking of the manuscript.Conflicts of interest
The authors declare that there is no conflict of interest.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.References
- M. Yadav, K. Srilekha and K. U. Maheswari, Potential health benefit of underutilized fruits: a review, J. Pharmacogn. Phytochem., 2018, 7(5), 1417–1420 CAS.
- C. Orwa, A. Mutua, R. Kindt, R. Jamnadass, S. Anthony and L. Arbutus unedo, Agroforestree Database: A Tree Reference and Selection Guide, Version 4, 2009 Search PubMed.
- R. A. Drew, The application of biotechnology to the conservation and improvement of tropical and subtropical fruit species, Seed and Plant Genetic Resources Service, Food and Agriculture Organization of the Unite Nations, 1997 Search PubMed.
- P. Sitorus, J. M. Keliat, V. Asfianti, M. Muhammad and D. Satria, A literature review of Artocarpus lacucha focusing on the phytochemical constituents and pharmacological properties of the plant, Molecules, 2022, 27(20), 6940 CrossRef CAS PubMed.
- E. Bhattacharya, R. Dutta, S. Chakraborty and S. M. Biswas, Phytochemical profiling of Artocarpus lakoocha leaf methanol extract and its antioxidant and antimicrobial activities, Asian Pac. J. Trop. Biomed., 2019, 9(11), 484–492 CrossRef CAS.
- D. Pertiwi, R. Hartati, E. Julianti and I. Fidrianny, Antibacterial and antioxidant activities in various parts of Artocarpus lacucha Buch.-Ham ethanolic extract, Biomed. Rep., 2024, 20(4), 66 CrossRef CAS PubMed.
- R. A. S. N. Ranasinghe, S. D. T. Maduwanthi and R. A. U. J. Marapana, Nutritional and health benefits of jackfruit (Artocarpus heterophyllus)—a review, Int. J. Food Sci., 2019, 2019, 4327183 CrossRef CAS PubMed.
- K. A. Mehta, Y. C. R. Quek and C. J. Henry, Breadfruit (Artocarpus altilis): Processing, nutritional quality and food applications, Front. Nutr., 2023, 10, 1156155 CrossRef PubMed.
- R. O. Adeleke and O. A. Abiodun, Nutritional composition of breadnut seeds (Artocarpus camansi), Afr. J. Agric. Res., 2010, 5(11), 1273–1276 Search PubMed.
- S. M. Islam, F. M. Hasan, M. Ali, M. Robbani and T. M. Hossain, Socioeconomic potential of Monkey Jack: an underutilized fruit in Bangladesh, Int. J. Innovative Res., 2016, 1(1), 40–44 Search PubMed.
- D. H. Dwivedi, V. Mishra, N. Singh and S. K. Dwivedi, Genetic Variability Studies in Barhal (Artocarpus lakoocha Roxb.), in Central Uttar Pradesh. InXXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): III International Symposium on 918, 2010, vol. 22, pp. 419–425 Search PubMed.
- S. K. Bishnoi, J. B. Tomar, K. K. Saini and P. Kumar, Artocarpus lakoocha: a potential tree species for forest and agricultural diversification in Jharkhand, India, in 3rd National Symposium on Agricultural Production and Protection in Context of Climate Change, 2012, pp. 3–5 Search PubMed.
- S. R. Krishnamurthy and P. Sarala, Phytochemical studies of Artocarpus gomezianus Wall. ex Trecul. var. lakoocha Roxb. fruits collected from various altitudes of Central Western Ghats, Indian J. Nat. Prod. Resour., 2013, 4(4), 398–411 Search PubMed.
- M. F. Hossain, M. A. Islam, S. Akhtar and S. M. Numan, Nutritional value and medicinal uses of Monkey Jack fruit (Artocarpus lakoocha), Int. Res. J. Biol. Sci., 2016, 5(1), 60–63 Search PubMed.
- P. Gautam and R. Patel, Artocarpus lakoocha Roxb.: An overview, Eur. J. Complementary Altern. Med., 2014, 1(1), 10–14 Search PubMed.
- A. K. Gupta, U. Pathak, M. Medhi, A. Mastinu, M. S. Sikarwar and P. Mishra, Botanical, chemical and pharmacological properties of Artocarpus lakoocha (monkey fruit): a review, Agric. Rev., 2020, 41(4), 305–316 Search PubMed.
- R. Singh, C. Nickhil, R. Nisha, K. Upendar, B. Jithender and S. C. Deka, A comprehensive review of advanced deep learning approaches for food freshness detection, Food Eng. Rev., 2024, 1–34 Search PubMed.
- A. Bellavia, S. C. Larsson, M. Bottai, A. Wolk and N. Orsini, Fruit and vegetable consumption and all-cause mortality: a dose–response analysis, Am. J. Clin. Nutr., 2013, 98(2), 454–459 CrossRef CAS PubMed.
- T. S. Conner, K. L. Brookie, A. C. Carr, L. A. Mainvil and M. C. Vissers, Effect of fruit and vegetable consumption on psychological well-being in young adults, PLoS One, 2017, 12(2), e0171206 CrossRef PubMed.
- O. Oyebode, V. Gordon-Dseagu, A. Walker and J. S. Mindell, Fruit and vegetable consumption and all-cause, cancer and CVD mortality: analysis of Health Survey for England data, J. Epidemiol. Community Health, 2014, 68(9), 856–862 CrossRef PubMed.
- P. Boffetta, E. Couto, J. Wichmann, P. Ferrari, D. Trichopoulos and H. B. Bueno-de-Mesquita, et al., Fruit and vegetable intake and cancer risk in EPIC, J. Natl. Cancer Inst., 2010, 102(8), 529–537 CrossRef CAS PubMed.
- B. J. Rolls, J. A. Ello-Martin and B. C. Tohill, What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management?, Nutr. Rev., 2004, 62(1), 1–17 CrossRef PubMed.
- K. He, F. B. Hu, G. A. Colditz, J. E. Manson, W. C. Willett and S. Liu, Changes in intake of fruits and vegetables and risk of obesity and weight gain among middle-aged women, Int. J. Obes., 2004, 28(12), 1569–1574 CrossRef CAS PubMed.
- S. Jahan, T. Gosh, M. Begum and B. K. Saha, Nutritional profile of some tropical fruits in Bangladesh, Bangladesh J. Med. Sci., 2011, 10(2), 95–100 CrossRef.
- P. Sarala and S. R. Krishnamurthy, Monkey jack: Underutilized edible medicinal plant, nutritional attributes and traditional foods of Western Ghats, Karnataka, India, Indian Journal of Traditional Knowledge, 2014, 13(3), 508–518 Search PubMed.
- A. Kumar, M. Premoli, F. Aria, S. A. Bonini, G. Maccarinelli and A. Gianoncelli, et al., Cannabimimetic plants: New cannabinoidergic modulators?, Planta, 2019, 249, 1681–1694 CrossRef CAS PubMed.
- A. Mastinu, S. A. Bonini, W. Rungratanawanich, F. Aria, M. Marziano and G. Maccarinelli, et al., Gamma-oryzanol prevents LPS-induced brain inflammation and cognitive impairment in mice, Nutrients, 2019, 11(4), 728 CrossRef CAS PubMed.
- W. S. D. Yamika, N. Aini and B. Waluyo, Physalis peruviana L. growth, yield and phytochemical content: a review, Agric. Rev., 2019, 40(4), 324–328 Search PubMed.
- S. Khalid, N. Khalid, R. S. Khan, H. Ahmed and A. Ahmad, A review on chemistry and pharmacology of Ajwa date fruit and pit, Trends Food Sci. Technol., 2017, 63, 60–69 CrossRef CAS.
- R. Singh, R. Nisha, R. Naik, K. Upendar, C. Nickhil and S. C. Deka, Sensor fusion techniques in deep learning for multimodal fruit and vegetable quality assessment: a comprehensive review, J. Food Meas. Charact., 2024, 18(9), 8088–8109 CrossRef.
- M. M. Hossain, M. A. Rahim and M. R. Haque, Biochemical properties of some important underutilized minor fruits, J. Agric. Food Res., 2021, 5, 100148 CAS.
- T. R. Prashith Kekuda, H. L. Raghavendra, N. Mallikarjun, T. M. Venugopal and H. S. Anil Kumar, Elemental composition, anticariogenic, pancreatic lipase inhibitory and cytotoxic activity of Artocarpus lakoocha Roxb pericarp, Int. J. Drug Dev. Res., 2012, 4(1), 330–336 CAS.
- C. Nickhil, Development of Mechanical Tool to Separate Bulbs from Whole Jackfruit, Master's thesis, University of Agricultural Sciences, Bengaluru, GKVK, 2015.
- A. Hari, K. G. Revikumar and D. Divya, Artocarpus: A review of its phytochemistry and pharmacology, Journal of Pharma Search, 2014, 9(1), 7–12 Search PubMed.
- T. Chuanasa, J. Phromjai, V. Lipipun, K. Likhitwitayawuid, M. Suzuki and P. Pramyothin, et al., Anti-HSV-1 activity of oxyresveratrol and therapeutic efficacy in mice, Antiviral Res., 2008, 80(1), 62–70 CrossRef CAS PubMed.
- A. Puntumchai, P. Kittakoop, S. Rajviroongit, S. Vimuttipong, K. Likhitwitayawuid and Y. Thebtaranonth, Lakoochins A and B: New antimycobacterial stilbene derivatives from Artocarpus lakoocha, J. Nat. Prod., 2004, 67(3), 485–486 CrossRef CAS PubMed.
- H. H. Ko, Y. T. Tsai, M. H. Yen, C. C. Lin, C. J. Liang and T. H. Yang, et al., Norartocarpetin from Artocarpus communis acts as a melanogenesis inhibitor without cytotoxicity, BMC Complementary Altern. Med., 2013, 13, 348 CrossRef.
- M. H. Tsai, J. F. Liu, Y. C. Chiang, S. C. S. Hu, L. F. Hsu and Y. C. Lin, et al., Artocarpin, an isoprenyl flavonoid, induces p53-dependent or independent apoptosis via ROS-mediated MAPKs and Akt activation in non-small cell lung cancer cells, Oncotarget, 2017, 8(17), 28342 CrossRef PubMed.
- M. S. Sikarwar, B. J. Hui, K. Subramaniam, B. D. Valeisamy, L. K. Yean and K. Balaji, A review on Artocarpus altilis (Parkinson) Fosberg (breadfruit), J. Appl. Pharm. Sci., 2014, 4(8), 91–97 Search PubMed.
- M. S. Kumar, M. R. Kumar, A. C. Bharath, H. V. Kumar, T. P. Kekuda and K. C. Nandini, et al., Screening of selected biological activities of Artocarpus lakoocha Roxb fruit pericarp, J. Basic Clin. Pharm., 2010, 1(4), 239 Search PubMed.
- K. Krishnan, L. E. Mathew, N. R. Vijayalakshmi and A. Helen, Anti-inflammatory potential of β-amyrin, a triterpenoid isolated from Costus igneus, Inflammopharmacology, 2014, 22, 373–385 CrossRef CAS PubMed.
- N. Han and M. Bakovic, Biologically active triterpenoids and their cardioprotective and anti-inflammatory effects, J. Bioanal. Biomed., 2015, S12, 005 Search PubMed.
- T. Z. Shoshe, Quantification of total phenol, in vitro investigation of antioxidant activity, cytotoxic effect and phytochemical screening of the leaf extract of Artocarpus lacucha, 2017 Search PubMed.
- M. T. Islam Shajib, M. E. M. Ferdous, A. Naz Shah, U. Habiba and S. N. Islam, Monkey Jack (Artocarpus lakoocha), in Handbook of Phytonutrients in Indigenous Fruits and Vegetables, 2022, pp. 311–322 Search PubMed.
- S. Chaurasia and A. Pandey, Phytochemistry and pharmacology of genus Artocarpus, Russ. J. Bioorg. Chem., 2023, 49(3), 481–514 CrossRef CAS.
- S. Pandey and A. Poonia, Monkey jackfruit (Artocarpus lakoocha Roxb.): The lesser-known fruit, Indian Food Ind., 2021, 6, 38–45 Search PubMed.
- M. Vanajakshi, J. H. Virupaksha and S. Maria, A review on pharmacological actions of Artocarpus lacoocha Roxb, Res. J. Pharmacol. Pharmacodyn., 2016, 8(4), 181–184 CrossRef.
- S. Boonyaketgoson, V. Rukachaisirikul, S. Phongpaichit and K. Trisuwan, Deoxybenzoin and flavan derivatives from twigs of Artocarpus lakoocha, Phytochem. Lett., 2019, 31, 96–100 CrossRef CAS.
- T. Ahamad, M. A. Khan, W. A. Ansari and M. F. Khan, Antioxidant and anticancer activities of selected Indian medicinal plants viz., Artocarpus lakoocha, Kigelia pinnata, and Amaranthus viridis, Era's J. of Med. Res., 2020, 7(1), 46–51 CrossRef.
- S. Krupa, C. S. Karigar and K. S. Murthy, Pharmacology and phytochemistry of Artocarpus family: A review, Indo Global J. Pharm. Sci., 2020, 10(3), 48–55 CrossRef.
- M. Saleem, A. Asif, M. F. Akhtar and A. Saleem, Hepatoprotective potential and chemical characterization of Artocarpus lakoocha fruit extract, Bangladesh J. Pharmacol., 2018, 13(1), 90–97 CrossRef.
- P. Hankittichai, P. Buacheen, P. Pitchakarn, M. Na Takuathung, N. Wikan and D. R. Smith, et al., Artocarpus lakoocha extract inhibits LPS-induced inflammatory response in RAW 264.7 macrophage cells, Int. J. Mol. Sci., 2020, 21(4), 1355 CrossRef CAS PubMed.
- A. K. Buddhisuharto, H. Pramastya, M. Insanu and I. Fidrianny, Updated review of phytochemicals and pharmacological activities of Artocarpus genus, Biointerface Res. Appl. Chem., 2021, 11(6), 14898–14905 CAS.
- F. Ferrari, I. Messana and M. D. C. M. de Araujo, Structures of three new flavone derivatives from Brosimopsis oblongifolia, Planta Med., 1989, 55(1), 70–72 CrossRef CAS PubMed.
- Z. P. Zheng, K. W. Cheng, J. T. K. To, H. Li and M. Wang, Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent, Mol. Nutr. Food Res., 2008, 52(12), 1530–1538 CrossRef CAS PubMed.
- Y. Hano, Y. Yamagami, M. Kobayashi, R. Isohata and T. Nomura, Artonins E and F, two new prenylflavones from the bark of Artocarpus communis Forst, Heterocycles, 1990, 31(5), 877–882 CrossRef CAS.
- E. T. Arung, K. Shimizu and R. Kondo, Inhibitory effect of artocarpanone from Artocarpus heterophyllus on melanin biosynthesis, Biol. Pharm. Bull., 2006, 29(9), 1966–1969 CrossRef CAS.
- T. Fujimoto, Y. Hano, T. Nomura and J. Uzawa, Components of root bark of Cudrania tricuspidata: two new isoprenylated flavones, Planta Med., 1984, 50(2), 161–163 CrossRef CAS PubMed.
- S. Maneechai, W. De-Eknamkul, K. Umehara, H. Noguchi and K. Likhitwitayawuid, Flavonoid and stilbenoid production in callus cultures of Artocarpus lakoocha, Phytochemistry, 2012, 81, 42–49 CrossRef CAS PubMed.
- U. Neog, P. Dhar, T. Kumari, C. Nickhil, S. C. Deka and R. Pandiselvam, Optimization of microwave-assisted extraction of phytochemicals from norabogori fruit (Prunus persica L. Batsch) and application in fruit leather, Biomass Convers. Biorefin., 2023, 1–15 Search PubMed.
- B. D. Maibam, S. Chakraborty, C. Nickhil and S. C. Deka, Effect of Euryale ferox seed shell extract on starch digestibility and predicted glycemic index of wheat bread, Int. J. Biol. Macromol., 2023, 226, 1066–1078 CrossRef CAS PubMed.
- B. Jithender, D. M. Vyas, C. Nickhil and P. J. Rathod, Development of juice extraction process for pomegranate (Punica granatum): evaluation and physicochemical aspects, Indian J. Agric. Sci., 2021, 91(6), 936–938 CrossRef.
- K. Saesue, P. Thanomrak, W. Prompan, W. Punan, N. Khorana and W. Juprasert, et al., Development of a ready-to-use oxyresveratrol-enriched extract from Artocarpus lakoocha Roxb. using greener and deep eutectic solvents for whitening applications, Cosmetics, 2024, 11(2), 58 CrossRef CAS.
- K. Likhitwitayawuid, Oxyresveratrol: Sources, production, biological activities, pharmacokinetics and delivery systems, Molecules, 2021, 26(14), 4212 CrossRef CAS PubMed.
- J. Zhang, S. Liu, W. Guo, Y. Huang and N. Li, Oxyresveratrol suppressed melanogenesis, dendrite formation and melanosome transport in melanocytes via regulation of the MC1R/cAMP/MITF pathway, Sci. Rep., 2025, 15(1), 20400 CrossRef.
- T. Rodboon, P. Puchadapirom, S. Okada and P. Suwannalert, Oxyresveratrol from Artocarpus lakoocha Roxb. inhibits melanogenesis in B16 melanoma cells by regulating cellular oxidants, Walailak J. Sci. Technol., 2016, 13(4), 261–270 Search PubMed.
- D. L. Lucetti, E. C. Lucetti, M. A. M. Bandeira, H. N. Veras, A. H. Silva and L. K. A. Leal, et al., Anti-inflammatory effects and mechanism of action of lupeol acetate isolated from Himatanthus drasticus, J. Inflammation, 2010, 7(1), 60 CrossRef CAS PubMed.
- D. Junsaeng, T. Anukunwithaya, P. Songvut, B. Sritularak, K. Likhitwitayawuid and P. Khemawoot, Comparative pharmacokinetics of oxyresveratrol alone and in combination with piperine as a bioenhancer in rats, BMC Complementary Altern. Med., 2019, 19(1), 235 CrossRef PubMed.
- K. Malaniyom, P. Ratanachamnong, P. Namchaiw, U. Namdaung, S. Suksamrarn and Y. Jaisin, Suppression of inflammatory response by oxyresveratrol from Artocarpus lakoocha root bark against UV-B-induced keratinocytes, Heliyon, 2024, 10(20), e38962 CrossRef CAS PubMed.
- Y. Cui, F. Li, X. Zhu, J. Xu, A. Muhammad and Y. Chen, et al., Alfalfa saponins inhibit oxidative stress-induced apoptosis via MAPK pathway, Redox Rep., 2022, 27(1), 1–8 CrossRef CAS PubMed.
- W. O. M. Hasriadi, P. Lapphanichayakool and N. Limpeanchob, Neuroprotective effect of Artocarpus lakoocha extract and oxyresveratrol against hydrogen peroxide-induced toxicity in SH-SY5Y cells, Int. J. Pharm. Sci., 2017, 9, 229–233 Search PubMed.
- M. L. Nesa, S. Munira, A. S. Bristy, M. M. Islam and H. Chayan, Cytotoxic, anti-inflammatory, analgesic, CNS depressant and antidiarrhoeal activities of methanolic extract of Artocarpus lakoocha leaves, World. J. Pharm. Sci., 2015, 167–174 Search PubMed.
- S. Seiki and W. H. Frishman, Pharmacologic inhibition of squalene synthase and other downstream enzymes of the cholesterol synthesis pathway: a new therapeutic approach to treatment of hypercholesterolemia, Cardiol. Rev., 2009, 17(2), 70–76 CrossRef PubMed.
- N. Nazliniwaty, O. A. Hanafiah, D. Pertiwi, M. Muhammad and D. Satria, Ethanolic extract of Artocarpus lacucha and Anredera cordifolia leaves improves wound healing in NIH-3T3 cells, Open Access Maced. J. Med. Sci., 2022, 10(A), 807–811 CrossRef.
- M. G. Uddin, M. M. Islam, A. Ahmed, S. A. Siddiqui and A. Debnath, Evaluation of anthelmintic, antioxidant and anti-inflammatory potential of methanolic extract of Artocarpus lacucha leaves, Discovery Phytomed., 2020, 7(1), 27–32 CrossRef.
- P. A. Z. Hasibuan, Antioxidant activity of n-hexane, ethyl acetate, and ethanol extracts from Artocarpus lacucha leaves using DPPH method, Indones. J. Pharm. Clin. Res., 2018, 1(2), 41–47 CrossRef.
- H. Raghavendra, N. Mallikarjun, T. Venugopal and H. S. Anil Kumar, Elemental composition, anticariogenic, pancreatic lipase inhibitory and cytotoxic activity of Artocarpus lakoocha Roxb pericarp, Int. J. Drug Dev. Res., 2012, 4(1), 330–336 Search PubMed.
- S. Islam, M. S. Shajib, R. B. Rashid, M. F. Khan, M. A. Al-Mansur and B. K. Datta, et al., Antinociceptive activities of Artocarpus lacucha and its isolated phenolic compound catechin in mice, BMC Complementary Altern. Med., 2019, 19, 1–13 CrossRef CAS PubMed.
- N. Preyavichyapugdee, M. Sangfuang, S. Chaiyapum, S. Sriburin, Y. Pootaeng-on and P. Chusongsang, et al., Schistosomicidal activity of crude extract of Artocarpus lakoocha, Southeast Asian J. Trop. Med. Public Health, 2016, 47, 1–15 Search PubMed.
- S. Tarbiat, In vitro antioxidant, anti-inflammatory and lipid-lowering activities of Artocarpus lakoocha fruit extract and its implication on treatment of dyslipidemia, Int. J. Pharm. Sci. Rev. Res., 2018, 52(2), 75–82 CAS.
- W. Iskandar, O. W. K. Handayani and W. H. Cahyati, Analysis of family income factors on diarrhea incidence through behavior in Tapalang, Public Health Perspect. J., 2019, 4(3), 206–213 Search PubMed.
- P. E. Thomas and B. Dharmapalan, Value added products from jackfruit (Artocarpus heterophyllus) fruit, Acta Sci. Nutr. Health, 2020, 4(2), 105–110 Search PubMed.
- B. Q. Hoang, H. T. Nguyen and D. N. T. Duong, Development of lactic acid fermentation of jackfruit (Artocarpus heterophyllus) seed drink and its physicochemical and sensory properties, J. Food Sci. Technol., 2024, 61(6), 1180–1187 CrossRef CAS PubMed.
- S. Mohammed, P. K. Dubey, A. A. Mishra and S. Rahman, Valorisation of jackfruit seed flour in extrusion and bakery products: A review, Food Sci. Biotechnol., 2024, 33(14), 3167–3180 CrossRef CAS PubMed.
- S. Maneechai, K. Likhitwitayawuid, B. Sritularak, C. Palanuvej, N. Ruangrungsi and P. Sirisa-Ard, Quantitative analysis of oxyresveratrol in Artocarpus lakoocha and ‘Puag-Haad’, Med. Princ. Pract., 2009, 18(3), 223–227 CrossRef PubMed.
- M. T. Islam, S. K. Barman, M. Saha, M. S. Islam, and M. I. Sahid, Phytochemical Screening and Evaluation of Antioxidant Potential of Ethanolic Extract of Artocarpus lacucha Leaves In-vitro, 2024, pp. 156–170 Search PubMed.
- S. Radapong, K. Chan, S. D. Sarker and K. J. Ritchie, Oxyresveratrol modulates genes associated with apoptosis, cell cycle control and DNA repair in MCF-7 cells, Front. Pharmacol, 2021, 12, 694562 CrossRef CAS PubMed.
- L. Xiang, Q. Li, Z. Guan, G. Wang, X. Yu and X. Zhang, et al., Oxyresveratrol as a novel ferroptosis inducer exhibits anticancer activity against breast cancer via the EGFR/PI3K/AKT/GPX4 signalling axis, Front. Pharmacol, 2025, 15, 1527286 CrossRef PubMed.
- M. J. Haruni, M. S. Parvin, S. Munira and M. E. Islam, Phytochemical profile and evaluation of antioxidant, anticancer, and antimicrobial potential of Artocarpus lacucha bark extracts: in vitro and in silico studies, J. Food Biochem., 2025, 2025(1), 8016598 CrossRef CAS.
- A. Wiater, R. Paduch, S. Trojnar, A. Choma, M. Pleszczyńska and P. Adamczyk, et al., Effect of water-soluble polysaccharide from jackfruit (Artocarpus heterophyllus Lam.) on human colon carcinoma cells cultured in vitro, Plants, 2020, 9(1), 103 CrossRef CAS PubMed.
- N. Wongkattiya, P. Sanguansermsri, I. Fraser, A. Shuayprom, S. Pongmuangmul and R. Koranee, et al., Oxyresveratrol derived from Artocarpus lakoocha extract represents a potential natural agent for treating acne and skin inflammation, J. Appl. Pharm. Sci., 2025, 15(11), 50–57 Search PubMed.
- T. Banerjee, A. Sarkar, A. De, S. Sar, A. Mandal and P. Panda, et al., Toxicological evaluation of Artocarpus lacucha ethyl acetate extract: in vitro and in vivo assessment, J. Ethnopharmacol., 2025, 347, 119804 CrossRef CAS PubMed.
- A. T. Shafaq, H. M. Ishaq and M. Shahzad, Pharmacological effects of Artocarpus lakoocha methanol extract on inhibition of squalene synthase and other downstream enzymes of the cholesterol synthesis pathway, Pharm. Biol., 2022, 60(1), 840–845 CrossRef PubMed.
- S. Singhatong, D. Leelarungrayub and C. Chaiyasut, Antioxidant and toxicity activities of Artocarpus lakoocha Roxb. heartwood extract, J. Med. Plants Res., 2010, 4(10), 947–953 CAS.
- L. Xu, C. Liu, W. Xiang, H. Chen, X. Qin and X. Huang, Advances in the study of oxyresveratrol, Int. J. Pharmacol., 2014, 10(1), 44–54 CAS.
- P. Jantaharn, T. Sornda, N. Limpeanchob, T. Pitaksuteepong, W. Tojaroen and K. Chootip, et al., Neuroprotective activity of Artocarpus lacucha extract in SH-SY5Y cells and lifespan extension in C. elegans, Ind. Crops Prod., 2025, 237, 122203 CrossRef CAS.
- S. Zeng, Y. Chen, C. Wei, L. Tan, C. Li and Y. Zhang, et al., Protective effects of polysaccharide from Artocarpus heterophyllus Lam. (jackfruit) pulp on non-alcoholic fatty liver disease in high-fat diet rats via PPAR and AMPK signalling pathways, J. Funct.Foods, 2022, 95, 105195 CrossRef CAS.
- D. K. Joung, S. H. Mun, S. H. Choi, O. H. Kang, S. B. Kim and Y. S. Lee, et al., Antibacterial activity of oxyresveratrol against methicillin-resistant Staphylococcus aureus and its mechanism, Exp. Ther. Med., 2016, 12(3), 1579–1584 CrossRef PubMed.
- M. N. Hossain, M. S. Hasan, M. I. Keya, M. G. Rabby, R. Tarannum and R. Parvin, et al., Effects of Artocarpus lacucha fruit pulp extracts on physicochemical characteristics and microbial stability of raw chicken meatballs during refrigeration, Appl. Food Res., 2025, 101253 CrossRef CAS.
- H. J. Borah, R. Singhal and S. Hazarika, Artocarpus lakoocha Roxb.: An untapped bioresource of resveratrol from NE India and its antioxidant activity, Ind. Crops Prod., 2017, 95, 75–82 CrossRef CAS.
- S. K. Bishnoi, R. Shinde and P. K. Sarkar, Monkey Jack (Artocarpus Lakoocha Roxb.): Hope for Sustaining Livelihood, 2017 Search PubMed.
- R. Verma, R. Sharma and S. Bhama, Standardization, preparation and evaluation of dheu (Artocarpus lakoocha)-based toffee, Himachal J. Agric. Res., 2021, 228–234 Search PubMed.
- D. Satria, P. Sitorus, A. Dalimunthe, S. B. Waruwu and V. Asfianti, Oral acute toxicity study of ethanol extract of Mobe leaves (Artocarpus lacucha Buch.-Ham) in Wistar rats, Pharmacia, 2024, 71, 1–8 Search PubMed.
- S. Radapong, S. D. Sarker and K. J. Ritchie, Oxyresveratrol possesses DNA-damaging activity, Molecules, 2020, 25(11), 2577 CrossRef CAS PubMed.
- N. Alam, H. Najnin, M. Islam, S. Shakya, I. M. Khan and R. Zaidi, Biochemical and histopathological analysis after sub-chronic administration of oxyresveratrol in Wistar rats, Drug Chem. Toxicol., 2023, 46(1), 166–175 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |