Open Access Article
Open Access ArticleOzone in gaseous and aqueous phases as a sanitizing agent for grapes used in winemaking and its impact on the implantation of Lachancea thermotolerans
Yaiza
Rodríguez
 a,
Juan Manuel
del Fresno
a,
Juan Manuel
del Fresno
 a,
Carmen
González
a,
Carmen
González
 a,
María Antonia
Bañuelos
a,
María Antonia
Bañuelos
 b and
Antonio
Morata
b and
Antonio
Morata
 *a
*a
aUniversidad Politécnica de Madrid, ETSIAAB, Chemistry and Food Technology Dept., enotecUPM, Avenida Puerta de Hierro, 2, 28040, Madrid, Spain. E-mail: antonio.morata@upm.es
bUniversidad Politécnica de Madrid, ETSIAAB, Biotechnology-Plant Biology Dept., Avenida Puerta de Hierro, 228040, Madrid, Spain
First published on 7th October 2025
Abstract
Ozonation is an effective and sustainable method for grape sanitation, facilitating the implementation of non-Saccharomyces yeasts such as Lachancea thermotolerans. This study evaluated gaseous (28 g h−1 for 30 min) and aqueous ozone (0.5 g h−1 for 30 min) treatments on L. thermotolerans implantation in Red Globe grapes. Fermentations with L. thermotolerans and Saccharomyces cerevisiae were conducted at different inoculation rates, assessing must acidification, sugar consumption and volatile compounds. Ozone treatments increased lactic acid production, lowered pH and enhanced L. thermotolerans metabolic activity. Volatile analysis revealed a higher production of 2-phenyl ethanol, a characteristic compound of L. thermotolerans. These findings suggest ozonation as a potential alternative to sulfitation, improving yeast implantation and modulating wine acidity and aroma.
Sustainability spotlightThe use of ozone (O3) to sanitize grapes is a sustainable and environmentally friendly technology. Its powerful oxidizing capacity allows the effective elimination of microorganisms without generating toxic waste, as it quickly decomposes into oxygen. This feature significantly reduces the need for chemicals and minimizes the environmental impact of the wine industry. Furthermore, the implementation of ozonation systems requires minimal infrastructure and does not require additional products for cleaning or maintenance, which simplifies its application and reduces the consumption of resources. Its use contributes directly to the United Nations Sustainable Development Goals (SDGs), in particular Goal 12 (responsible production and consumption) and Goal 13 (climate action) by reducing the use of chemicals and the environmental footprint of wine production. Moreover, the application of Lachancea thermotolerans in winemaking presents an innovative biotechnological strategy for the sustainable acidification of wine. This non-Saccharomyces yeast naturally produces significant amounts of L-lactic acid during alcoholic fermentation, which leads to a controlled reduction of pH and an increase in total acidity. Unlike traditional acidification methods, which often rely on the addition of exogenous acids or chemical agents, L. thermotolerans allows for a more sustainable alternative. Furthermore, bioacidification through L. thermotolerans aligns with sustainable production goals by decreasing chemical inputs and improving wine stability under conditions of overripe grapes and low acidity induced by climate change. |
Introduction
In recent years, there has been an increasing interest in the use of non-Saccharomyces yeasts to improve the quality and complexity of wines.1 In addition to this, there is a trend occurring in hot climates, where wines often have excess ethanol but lack acidity. The yeast Lachancea thermotolerans offers a solution to this problem, as it is capable of partially converting sugars into lactic acid during alcoholic fermentation.2 The reduction in pH occurs naturally during fermentation and avoids the addition of tartaric acid or the use of resins, which are very effective in reducing pH, but have undesirable effects on wine quality. By consuming sugars for the production of lactic acid, this yeast also contributes to a slight reduction in the alcohol content of the wine.3Due to this natural acidification capacity, several trials have been done to determine the potential of L. thermotolerans in winemaking. It has been shown that both pure and mixed fermentations with Saccharomyces cerevisiae not only improved acidity, reducing pH by 0.3 or even more than 0.5 units from an initial pH of 3.8–4 (ref. 3 and 4) but also reducing volatile acidity and increasing the concentration of beneficial aromatic compounds, such as 2-phenyl ethanol.5
However, many non-Saccharomyces yeasts have limited fermentative capacity; in the case of L. thermotolerans, pure cultures have reported a moderate fermentative power with 38.8–54.73 g of residual unfermented sugar and ethanol production of 7.58–10.46% v/v.1,6 Furthermore, they also exhibit low resistance to sulfur dioxide (SO2). As a result, the presence of this compound limits the proliferation of this yeast and lactic acid is not produced.5,7
Sulfitation with sulfur dioxide is the most used treatment in the wine industry for its antimicrobial and antioxidant properties. However, its use has certain disadvantages, such as the previously mentioned one regarding the implantation of certain yeasts, as well as alterations in the sensory properties of the wine, including the neutralization of aromas and the appearance of organoleptic defects.8
In this context, emerging technologies for food preservation have become very important because they allow the control of pathogenic or spoilage microorganisms without compromising sensory quality. These technologies include high-pressure treatments (HHP and UHPH), pulsed electric fields (PEF), pulsed light (PL), ultraviolet (UV) and electron beam irradiation, electrolyzed water and ozone.9
Ozone (O3) is a penetrating odor gas formed by the rearrangement of oxygen atoms when subjected to high energy input. Its high oxidation–reduction potential (2.07 V) makes it a potent antimicrobial agent so that molecular ozone or its decomposition products (e.g. hydroxyl radical) inactivate microorganisms by reacting with their intracellular enzymes, nucleic material and cell envelope components.10 Ozone has a natural instability that allows it to decompose rapidly without generating toxic subproducts, making it a safe alternative for the food industry.11 In 2001, the FDA approved its use as a food additive for the treatment, storage and processing of food in gaseous and aqueous states.12
Several studies have proved the efficacy of ozone as an antimicrobial agent in foods, both in a gaseous state and in aqueous solution.13–15 Its activity depends on environmental factors such as the pH of the medium, temperature, humidity or the amount of organic matter present.16 However, it has been reported that the environmental conditions of a winery do not significantly reduce its efficacy.17
Other studies have confirmed that ozone is able to control the microorganisms present in grapes without negatively altering their aromatic profile.18 In addition, the application of ozonated water to grapevines had a positive effect on parameters related to ripening, phenolic compound content and free terpenoids in grapes.19,20
While previous studies have demonstrated the antimicrobial efficacy of ozone and its limited impact on grape aromatic profiles,14,21,22 much remains to be investigated regarding the potential of ozone treatments to specifically enhance the implantation and fermentative performance of non-Saccharomyces yeasts like L. thermotolerans during winemaking. In particular, the influence of different yeast inoculum concentrations on implantation success under ozone sanitization has not been systematically investigated. This study addresses these gaps by evaluating both gaseous and aqueous ozone applications as grape sanitization methods and assessing their effects on L. thermotolerans implantation across varying inoculum levels. These findings provide novel insights into integrating emerging sanitization technologies with non-Saccharomyces yeast management strategies to improve wine quality and fermentation reliability.
The goal of this study is to evaluate the effectiveness of ozone, both in its gaseous form and in aqueous solution, as a method of grape sanitization and its effect on the implantation of the yeast L. thermotolerans. For this purpose, tests were conducted to analyze growth indicators of this yeast, such as alcohol content, acidity and the concentration of different volatile compounds.
Materials and methods
Ozone application
A total of 6 kg of Red Globe variety grapes were used. The grapes were destemmed and divided into three groups of 2 kg each before being exposed to the different ozonation treatments. The ozone used for the trials was generated by using a JOBYNA JB-OZ-S28 air purifier (JOBYNA, Dongguan City, China), operating under conditions already optimized and tested in previous trials.For the first treatment (O3G), ozone in gaseous form was used, introducing the grapes in a 5 L container in which an ozone-saturated atmosphere had been previously reached. Once the grapes were introduced, a constant ozone flow of 28 g h−1 was maintained for a period of 30 minutes.
For the second treatment (O3L), the grapes were immersed in previously ozonated water using a diffuser connected to the ozone generator. The ozone flow rate was 0.5 g h−1 for 30 min, allowing it to circulate as homogeneously as possible.
Once the treatments were completed, the treated grapes were transferred to sterilized jars for pressing and obtaining the must, and later divided into 100 mL flasks in which the fermentations were performed.
In the case of the control samples (C), the grapes were pressed directly without being treated previously.
From the musts obtained, YPD medium plates were seeded in triplicate to estimate the yeast population present after the different treatments prior to inoculation. The plates were incubated for 48 hours and then the colonies were counted.
Fermentations
Two different yeasts were used to inoculate the must obtained with the different treatments: Saccharomyces cerevisiae (Sc) and Lachancea thermotolerans (Lt). The inoculants were made from active dry yeast, and three different concentrations were added: 5, 10 and 20 g hL−1 (grams per hectoliter) (Fig. 1). Also, for each of the conditions, triplicates of the fermentations were performed.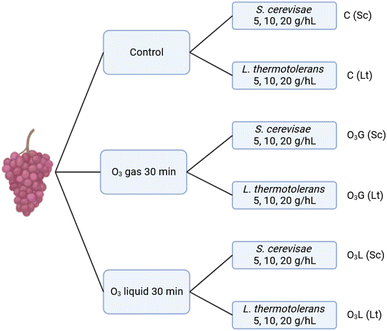 | ||
| Fig. 1 Experimental procedure and sample codes for the application of different ozonation treatments and their subsequent inoculation. | ||
Fermentation control was performed by monitoring the concentration of reducing sugars in the must using an OenoFoss spectrophotometer (FOSS Iberia, Barcelona, Spain). This equipment uses Fourier transform infrared spectroscopy (FTIR) to identify and quantify different compounds previously calibrated for must in fermentation and finished wine. Along with the concentration of sugars, the final alcohol degree was also obtained by this method.
Evolution of lactic acid and pH during alcoholic fermentation
To measure lactic acid concentration, daily samples were taken from each of the fermentations in order to monitor the production of this compound. The analysis was made with a Y25 multienzymatic analyzer (BioSystems, Barcelona, Spain), and the method of analysis is based on the use of the enzyme lactate dehydrogenase.For pH, a portable pH meter METRIA model M22 (Labbox Labware S.L., Premià de Dalt, Spain) was used to make an initial measurement before inoculation and a final measurement when the fermentations were finished. This made it possible to calculate the pH variation throughout the process.
Fermentative volatile analysis
After finishing the fermentations, samples were taken from all of them to analyze the concentration of different volatile compounds present using gas chromatography coupled to a flame ionization detector (GC-FID) with Agilent Technologies 6850 equipment (J&W Scientific, Folsom, CA, USA) according to the method described by ref. 23. Before being analyzed, the samples were filtered using a 0.45 μm membrane. After this, 100 μL of internal standard (4-methyl-2-pentanol) was added to 1 mL of each filtered sample. A DB-624 column (60 m × 250 μm × 1.40 μm) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 split was used to separate the different compounds for further analysis. The temperature program started with an initial value of 40 °C followed by an increase of 10 °C per minute until reaching 250 °C which was maintained for 5 minutes. The gas used as the mobile phase was hydrogen with a flow rate of 2.2 mL min−1. The detector was programmed at a temperature of 300 °C and allowed the quantification of the following compounds: acetaldehyde, methanol, 1-propanol, diacetyl, ethyl acetate, isobutanol, acetoin, 3-methyl-1-butanol, 2-methyl-1-butanol, isobutyl acetate, ethyl butyrate, 2,3-butanediol, 2-phenylethyl alcohol and 2-phenylethyl acetate. All these compounds were previously calibrated in the equipment.
10 split was used to separate the different compounds for further analysis. The temperature program started with an initial value of 40 °C followed by an increase of 10 °C per minute until reaching 250 °C which was maintained for 5 minutes. The gas used as the mobile phase was hydrogen with a flow rate of 2.2 mL min−1. The detector was programmed at a temperature of 300 °C and allowed the quantification of the following compounds: acetaldehyde, methanol, 1-propanol, diacetyl, ethyl acetate, isobutanol, acetoin, 3-methyl-1-butanol, 2-methyl-1-butanol, isobutyl acetate, ethyl butyrate, 2,3-butanediol, 2-phenylethyl alcohol and 2-phenylethyl acetate. All these compounds were previously calibrated in the equipment.
Statistical analysis
All calculations, including means, standard deviations and other statistics, were obtained using Rstudio software (Posit, PBC, Boston, USA). Analysis of variance (ANOVA) was used to evaluate the effects of the different factors and to determine if they were significant. In cases where significant differences were observed, pairwise comparisons were performed to determine the level of significance among the different factors. Principal Component Analysis (PCA), which was applied to the analysis of volatiles, was obtained using XLSTAT software (Addinsoft, Paris, France), which provides the corresponding graph with all the information necessary for its interpretation.Results and discussion
Plate count
A remarkable reduction of the initial yeast population was observed after applying both treatments, since the plate count of the control samples showed a population higher than 5 × 104 CFU mL−1 compared to 9 × 103 CFU mL−1 and 5 × 103 CFU mL−1 for O3L and O3G, respectively. This indicates that with the ozonation methodology applied, it was possible to reduce the yeast population of the grapes by an order of magnitude.The reduction of the yeast population using ozonation treatments has been previously studied, obtaining decreases of around 0.5–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 of the initial population in the grape.14,24 A similar result was observed in this study, with the decrease in the O3G treatment being 1
CFU mL−1 of the initial population in the grape.14,24 A similar result was observed in this study, with the decrease in the O3G treatment being 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 while in the case of O3L it was smaller, being only 0.6
CFU mL−1 while in the case of O3L it was smaller, being only 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1. The variability present in the different methods may be due to the fact that, as previously described by other authors, the efficiency of ozone can change taking into account different factors such as the species and strain of organisms in the grape, the density of the microbiota or the methodology used for ozone application.13,25 A recent study on the application of gaseous ozone on this same grape variety, Red Globe, also highlights the potential of this treatment to be used as a safe and effective fungicide.26
CFU mL−1. The variability present in the different methods may be due to the fact that, as previously described by other authors, the efficiency of ozone can change taking into account different factors such as the species and strain of organisms in the grape, the density of the microbiota or the methodology used for ozone application.13,25 A recent study on the application of gaseous ozone on this same grape variety, Red Globe, also highlights the potential of this treatment to be used as a safe and effective fungicide.26
Fermentative kinetics
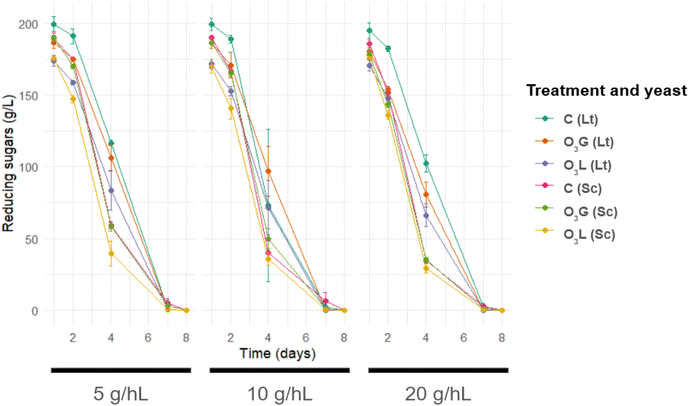
All fermentations were completed without difficulty in 8 days, consuming all the sugars present in the starting must. It can be observed that, especially in the case of the L. thermotolerans yeast, the sugar consumption of the control sample during the initial days was less pronounced than in the case of the O3G and O3L treated samples (Fig. 2). This could be the first indicator that the ozone treatment has facilitated the implantation of this yeast. This effect is likely related to the higher initial microbial load in the control grapes, which did not undergo any sanitizing treatment. Such microbial competition can hinder the proliferation and implantation of L. thermotolerans, resulting in slower sugar consumption. In contrast, S. cerevisiae is less affected due to its greater fermentative capacity, which allows it to grow efficiently even under these conditions.By analyzing the alcoholic content reached in each of the fermentations (Table 1), it can be observed that in all the treated samples (O3G and O3L) there are significant differences between the two yeasts used, with the amount of ethanol always being higher in the case of S. cerevisiae fluctuating between 10.36 and 11.87% vol, while for L. thermotolerans the maximum value was 10.66% vol. On the other hand, in the control samples (C) the alcoholic content is similar in all fermentations and no differences can be established between the inoculated yeasts.
| Inoculum concentration | Treatment | Yeast | Ethanol (%v/v) |
|---|---|---|---|
| 5 g hL−1 | C | Lt | 11.62 ± 0.29c |
| Sc | 11.84 ± 0.09c | ||
| O3G | Lt | 10.66 ± 0.08a | |
| Sc | 11.56 ± 0.13c | ||
| O3L | Lt | 9.61 ± 0.29b | |
| Sc | 10.59 ± 0.17a | ||
| 10 g hL−1 | C | Lt | 11.73 ± 0.12c |
| Sc | 11.77 ± 0.39c | ||
| O3G | Lt | 10.52 ± 0.14a | |
| Sc | 11.58 ± 0.08c | ||
| O3L | Lt | 9.73 ± 0.21b | |
| Sc | 10.60 ± 0.25a | ||
| 20 g hL−1 | C | Lt | 11.56 ± 0.29c |
| Sc | 11.87 ± 0.06c | ||
| O3G | Lt | 10.09 ± 0.35ab | |
| Sc | 11.27 ± 0.22c | ||
| O3L | Lt | 9.72 ± 0.14a | |
| Sc | 10.36 ± 0.22b |
The difference in the alcoholic degree of the fermentations is the first indicator of the success of the implantation of the L. thermotolerans yeast. Other studies that compared enological parameters of pure fermentations carried out with S. cerevisiae and L. thermotolerans resulted in significant differences in the ethanol production of these two yeasts.1 The absence of this difference in alcoholic degree in the C samples could indicate that other yeasts, already present in the grape, had a major role in the fermentation and, as a consequence, L. thermotolerans could not be correctly implanted.
Lactic acid evolution during alcoholic fermentation
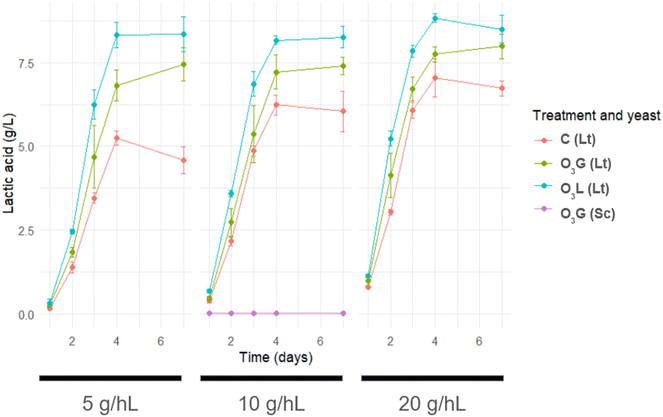
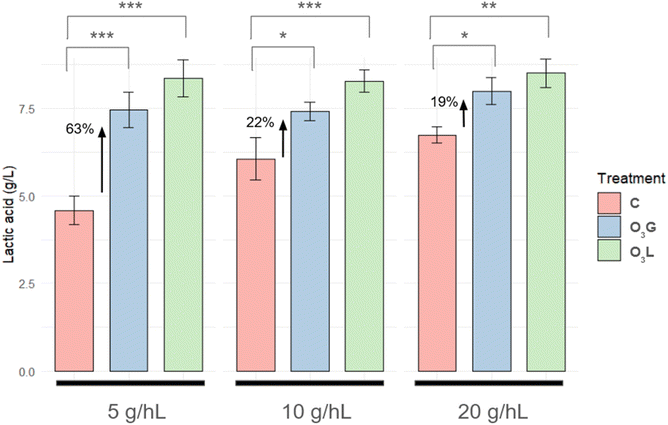
Differences can be seen in the evolution of lactic acid production during fermentation, considering the treatment applied and the concentration of the L. thermotolerans inoculum used (Fig. 3). In general, it seems that the O3L samples produced a higher amount of lactic acid during all the days of fermentation, followed by the O3G samples, and finally the C samples. Furthermore, the difference in the production of this compound between treated samples and control samples is higher in those where the L. thermotolerans inoculum was lower (5 g hL−1). In the samples where S. cerevisiae was inoculated, in any case it didn't exceed 0.02 g L−1, while for the samples inoculated with L. thermotolerans, final concentration values between 4.15 and 8.85 g L−1 were recorded.A more detailed analysis of the final lactic acid concentration data confirms the trend previously described in relation to lactic acid evolution (Fig. 3). A significant increase in lactic acid production is detected in all the samples treated with ozone, both O3G and O3L, in comparison with the control samples (Fig. 4). After calculating the percentage increase in lactic acid production with respect to the control samples, it can be seen that, for the O3G treatment, production is 62.7%, 22.3% and 18.6% higher in the samples with an inoculum of 5, 10 and 20 g hL−1, respectively. In the case of the O3L treatment, the difference is even more noticeable, reaching an increase of 82.3%, 36.7% and 26.3% for the inoculum concentrations of 5, 10 and 20 g hL−1, respectively. It should be noted that the difference between samples C with respect to O3G and O3L progressively decreases as the concentration of the L. thermotolerans inoculum increases.
The production of lactic acid indicates the presence of L. thermotolerans since it is a compound characteristic of its metabolism. This formation of lactic acid is produced from pyruvate in the glycolytic metabolism of sugars in which the enzyme lactate dehydrogenase is involved. The acidification occurs at the beginning of the alcoholic fermentation, at 3–5 days of fermentation.27 Its production range can oscillate to around 1–16 g L−1 of concentration in wine depending on the strain used.28 All these data are consistent with those obtained in this study and allow correlating the concentration of lactic acid present in the samples with the degree of L. thermotolerans implantation: the highest production of lactic acid occurred during the first 4 days of fermentation (Fig. 3) and the final concentration reached 4.15–8.85 g L−1 (Fig. 4).
As mentioned previously, significant differences were recorded between the control and treatment groups, indicating that ozonation facilitated the implantation of L. thermotolerans, allowing for greater production of lactic acid during fermentation. However, it should also be considered that this difference was not similar for all the inoculum concentrations used. Bigger differences were observed in the samples with the lowest inoculum concentrations, which confirms that the treatments had a clear influence on yeast implantation. This means that even under the most unfavourable conditions (when there was a lower ratio of inoculum to the yeasts that could be present in the original grapes), it was possible to achieve lactic acid concentrations similar to those of the samples with the highest inoculum concentrations.
pH
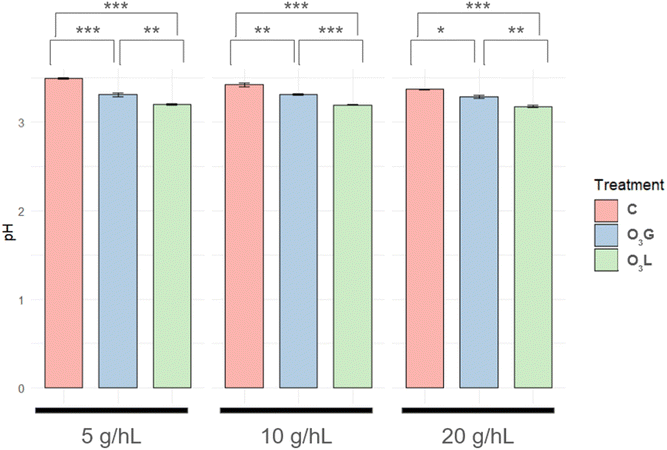
For all samples in which L. thermotolerans was inoculated, there was a decrease in the pH of the wine compared to the measurements conducted initially before fermentation. These values correlate with the lactic acid concentrations observed in these fermentations (Fig. 4). Furthermore, this decrease showed significant differences when comparing the pH values of the O3G and O3L samples with the C samples, being higher in the treatment samples (Fig. 5). An influence of the concentration of the added inoculum is also observed: for both treatments, the difference in acidification, when compared with the C samples, is more pronounced when the concentration of the added inoculum is lower. This difference progressively reduces as the concentration of inoculum increases. For the O3G treatment, acidification is 28.7% higher for a concentration of 5 g hL−1 of inoculum, 17.9% higher for 10 g hL−1 and 15% higher for 20 g hL−1. Similarly, in the O3L samples, acidification increases by 32.2% with 5 g hL−1 of inoculum, 22.11% with 10 g hL−1 and 17% with 20 g hL−1.In previous studies that used L. thermotolerans in both pure and mixed fermentations, the decrease in pH is associated with the presence of lactic acid, a significant increase in total acidity and a reduction in volatile acidity.1,5 It has also been documented how high lactic acid production can decrease the pH of wine by 0.5 units or more during fermentation.3 Significant differences in the decrease in pH (Fig. 5) were recorded between the control samples and the two treatments (O3G and O3L), which is consistent with what has already been discussed for lactic acid concentration. Moreover, the same tendency is observed when analyzing the concentration of inoculum added, with greater differences in the samples with less yeast added (5 g L−1).
Volatile fermentation compounds
Regarding the data obtained for the aromas of fermentative origin present in each sample at the end of fermentation. The concentration values recorded for each compound analyzed are included, as well as the summations of these compounds grouped into esters and higher alcohols. The sum of all the compounds is also included to obtain the value of total volatiles present in each sample. To compare the different samples, the results have been divided according to the concentration of inoculated yeast (Table 2).| 5 g hL−1 | ||||||
|---|---|---|---|---|---|---|
| Lt | Sc | |||||
| C | O3G | O3L | C | O3G | O3L | |
| Methanol | 38.60 ± 4.85ab | 38.71 ± 4.01ab | 12.60 ± 3.26b | 37.77 ± 4.48ab | 34.88 ± 1.69ab | 40.87 ± 22.00a |
| 1-Propanol | 99.04 ± 4.39a | 82.33 ± 10.46a | 80.40 ± 20.66a | 79.11 ± 5.20a | 95.98 ± 4.00a | 89.15 ± 12.53a |
| Diacetyl | 0.00 ± 0.00b | 1.87 ± 0.44a | 1.45 ± 0.08ab | 0.00 ± 0.00b | 0.97 ± 0.84ab | 1.53 ± 1.35ab |
| Ethyl acetate | 53.15 ± 3.18a | 29.79 ± 3.93a | 37.67 ± 19.91a | 59.50 ± 17.88a | 44.52 ± 13.70a | 62.68 ± 15.91a |
| 2-Butanol | nd | nd | nd | nd | nd | nd |
| Isobutanol | 30.35 ± 0.64b | 38.11 ± 1.75a | 36.68 ± 0.54a | 18.93 ± 0.46c | 19.90 ± 1.17c | 20.79 ± 1.63c |
| 1-Butanol | 3.20 ± 5.54a | 1.52 ± 2.63a | 4.79 ± 0.49a | 4.20 ± 0.27a | 5.24 ± 0.25a | 3.96 ± 0.17a |
| Acetoin | 42.11 ± 23.36a | 27.26 ± 24.82a | 17.10 ± 17.90a | 15.54 ± 8.24a | 10.05 ± 0.84a | 12.33 ± 13.58a |
| 3-Methyl-1-butanol | 137.38 ± 22.38a | 132.48 ± 29.05a | 94.40 ± 8.15a | 97.47 ± 3.09a | 99.02 ± 3.87a | 95.83 ± 9.08a |
| 2-Methyl-1-butanol | 34.35 ± 1.32bc | 37.39 ± 2.78abc | 29.13 ± 2.81c | 39.99 ± 3.53ab | 45.39 ± 3.85a | 34.59 ± 3.45ac |
| Isobutyl acetate | nd | 1.26 ± 2.19a | 1.87 ± 3.23a | nd | 3.75 ± 3.43a | 1.91 ± 3.31a |
| Ethyl butyrate | nd | nd | nd | 1.68 ± 0.15a | nd | nd |
| 2,3-Butanediol | 371.84 ± 16.83a | 328.32 ± 32.66a | 279.04 ± 45.77a | 648.74 ± 114.77a | 674.67 ± 60.81a | 514.25 ± 44.64a |
| Hexanol | nd | nd | nd | nd | nd | nd |
| 2-Phenyl ethanol | 10.89 ± 0.36b | 15.20 ± 6.90ab | 22.69 ± 0.41a | 9.46 ± 0.07b | 9.82 ± 0.21b | 13.42 ± 4.52b |
| 2-Phenylethyl acetate | 4.73 ± 0.01b | 4.87 ± 0.19b | 5.86 ± 0.03a | nd | 5.03 ± 0.04b | 5.01 ± 0.31b |
| Esters | 57.87 ± 3.18a | 35.92 ± 5.40a | 45.40 ± 16.67a | 61.18 ± 17.73a | 53.30 ± 12.69a | 69.60 ± 13.08a |
| Higher alcohols | 315.21 ± 31.50a | 307.01 ± 30.50ab | 268.09 ± 30.36ab | 249.17 ± 5.86b | 275.35 ± 8.75ab | 257.74 ± 5.42ab |
| Total volatiles | 882.03 ± 78.15bc | 804.95 ± 29.16c | 713.29.12 ± 46.78c | 1065.98 ± 55.40a | 1067.89 ± 49.74a | 1017.94 ± 106.05ab |
| 10 g hL−1 | ||||||
|---|---|---|---|---|---|---|
| Lt | Sc | |||||
| C | O3G | O3L | C | O3G | O3L | |
| Methanol | 35.67 ± 1.36ab | 26.35 ± 2.29bc | 16.06 ± 2.30c | 42.66 ± 5.35a | 39.66 ± 10.07ab | 49.85 ± 6.47a |
| 1-Propanol | 97.83 ± 2.26a | 80.17 ± 13.29a | 80.02 ± 11.52a | 82.54 ± 8.36a | 107.07 ± 9.11a | 90.93 ± 10.87a |
| Diacetyl | 1.42 ± 0.01a | 0.51 ± 0.88ab | nd | nd | 0.94 ± 0.81ab | nd |
| Ethyl acetate | 41.33 ± 6.43a | 33.24 ± 11.86a | 32.55 ± 9.48a | 57.91 ± 30.23a | 57.31 ± 21.15a | 54.54 ± 18.80a |
| 2-Butanol | nd | nd | nd | nd | nd | nd |
| Isobutanol | 27.11 ± 0.31b | 40.27 ± 5.62a | 38.47 ± 5.15a | 21.07 ± 0.76b | 22.91 ± 1.96b | 22.37 ± 2.34b |
| 1-Butanol | 5.08 ± 0.33a | 4.28 ± 0.44a | 4.45 ± 0.18a | 4.25 ± 0.23a | 1.58 ± 0.08a | 4.46 ± 0.56a |
| Acetoin | 19.43 ± 16.98a | 55.00 ± 38.43a | 27.79 ± 28.82a | 11.27 ± 1.85a | 33.05 ± 27.41a | 19.09 ± 12.37a |
| 3-Methyl-1-butanol | 123.52 ± 4.30a | 109.78 ± 11.81ab | 100.24 ± 2.20ab | 101.93 ± 2.12ab | 108.03 ± 14.57ab | 92.38 ± 8.30b |
| 2-Methyl-1-butanol | 35.38 ± 4.77ab | 36.96 ± 2.81ab | 29.25 ± 5.22b | 37.58 ± 0.76ab | 34.78 ± 1.71ab | 40.46 ± 1.44a |
| Isobutyl acetate | 3.16 ± 2.76a | 0.70 ± 1.21a | nd | nd | nd | nd |
| Ethyl butyrate | nd | 0.44 ± 0.76a | nd | nd | nd | nd |
| 2,3-Butanediol | 308.96 ± 39.13a | 263.93 ± 47.92a | 292.25 ± 34.84a | 573.78 ± 99.53a | 622.62 ± 68.15a | 627.40 ± 92.08a |
| Hexanol | 1.71 ± 2.96a | nd | nd | nd | nd | nd |
| 2-Phenyl ethanol | 10.91 ± 0.11bcd | 17.90 ± 6.18ab | 21.21 ± 1.02a | 9.94 ± 0.11d | 14.43 ± 0.60cd | 17.30 ± 1.23abc |
| 2-Phenylethyl acetate | 4.77 ± 0.03a | 4.95 ± 0.13a | 6.08 ± 0.36a | 4.73 ± 0.01a | 4.82 ± 0.07a | 3.45 ± 3.02a |
| Esters | 49.26 ± 5.79a | 39.32 ± 12.89a | 38.62 ± 9.52a | 62.65 ± 30.24a | 62.13 ± 21.11a | 57.99 ± 15.79a |
| Higher alcohols | 301.55 ± 12.41a | 289.36 ± 30.89a | 273.64 ± 15.09a | 257.32 ± 10.77a | 288.80 ± 20.85a | 267.91 ± 5.54a |
| Total volatiles | 778.40 ± 82.70b | 723.89 ± 38.94b | 752.48 ± 78.43b | 1067.06 ± 36.02a | 1083.02 ± 121.92a | 1059.59 ± 113.66a |
| 20 g hL−1 | ||||||
|---|---|---|---|---|---|---|
| Lt | Sc | |||||
| C | O3G | O3L | C | O3G | O3L | |
| Methanol | 38.84 ± 4.12bc | 35.22 ± 3.36bc | 48.26 ± 5.22b | 64.44 ± 9.50a | 33.02 ± 7.47bc | 29.06 ± 1.19c |
| 1-Propanol | 115.00 ± 8.05a | 81.13 ± 5.94c | 75.14 ± 6.29c | 86.39 ± 9.20bc | 103.19 ± 7.18ab | 104.11 ± 3.92ab |
| Diacetyl | nd | 2.02 ± 0.26a | 1.64 ± 0.30ab | 1.39 ± 0.00ab | 0.95 ± 0.83bc | 1.47 ± 0.05ab |
| Ethyl acetate | 40.69 ± 6.52ab | 34.92 ± 11.07ab | 27.39 ± 4.68b | 58.04 ± 19.92a | 47.72 ± 8.79ab | 57.60 ± 7.26a |
| 2-Butanol | nd | 0.83 ± 1.44b | 2.80 ± 0.55a | nd | nd | nd |
| Isobutanol | 33.12 ± 1.80b | 38.67 ± 4.76ab | 39.92 ± 2.67a | 22.11 ± 1.45c | 23.61 ± 0.58c | 25.31 ± 0.92c |
| 1-Butanol | 5.03 ± 0.25a | nd | 2.96 ± 2.56a | 2.94 ± 2.55a | 1.49 ± 2.59a | 4.28 ± 0.13a |
| Acetoin | 4.17 ± 7.22a | 46.10 ± 31.58a | 13.56 ± 7.46a | 6.75 ± 5.91a | 29.53 ± 37.30a | 25.65 ± 24.70a |
| 3-Methyl-1-butanol | 136.98 ± 18.88a | 102.27 ± 6.29a | 130.66 ± 37.23a | 111.67 ± 11.86a | 97.48 ± 4.12a | 104.55 ± 15.71a |
| 2-Methyl-1-butanol | 36.95 ± 3.85a | 40.55 ± 16.69a | 40.00 ± 16.94a | 39.45 ± 4.34a | 38.29 ± 4.62a | 37.05 ± 6.05a |
| Isobutyl acetate | 1.62 ± 2.81a | nd | 1.86 ± 3.23a | 0.81 ± 1.41a | nd | 0.60 ± 1.04a |
| Ethyl butyrate | nd | nd | nd | 1.35 ± 0.15ab | nd | 2.13 ± 1.53a |
| 2,3-Butanediol | 352.82 ± 103.06a | 300.35 ± 22.07a | 381.89 ± 48.73a | 614.09 ± 95.11a | 613.92 ± 100.92a | 534.37 ± 62.44a |
| Hexanol | 1.87 ± 3.24a | 3.93 ± 0.22a | 1.14 ± 1.98a | 3.54 ± 3.11a | nd | nd |
| 2-Phenyl ethanol | 10.98 ± 0.31c | 11.04 ± 0.44c | 22.35 ± 1.46a | 10.37 ± 0.10c | 16.87 ± 1.35b | 17.71 ± 0.44b |
| 2-Phenylethyl acetate | 4.90 ± 0.05a | 4.85 ± 0.10a | 6.57 ± 0.65a | 4.73 ± 0.01a | 4.79 ± 0.10a | 3.30 ± 2.86a |
| Esters | 47.22 ± 9.14a | 39.77 ± 11.16a | 35.82 ± 4.25a | 64.93 ± 21.27a | 52.51 ± 8.76a | 63.63 ± 5.33a |
| Higher alcohols | 339.93 ± 24.03a | 278.41 ± 26.38b | 314.98 ± 21.17ab | 276.47 ± 19.26b | 280.93 ± 10.64ab | 293.02 ± 23.93ab |
| Total volatiles | 880.62 ± 81.79ab | 763.68 ± 38.31b | 932.31 ± 42.97ab | 1063.22 ± 104.61a | 1071.92 ± 81.90a | 1065.48 ± 100.90a |
In general, the compound with the highest concentration in all samples is 2,3-butanediol. Among the esters, the most abundant in all fermentations is ethyl acetate. The total of volatiles, higher alcohols and esters shows hardly any differences between samples, which indicates similarity between the aromatic profiles of the different fermentations.
The aromatic profile of the wine obtained was quite similar for all samples, as described in other studies in which ozonation was used.24,29 Alcohols were the main compounds in all the samples analyzed, and these results were similar to other studies also made with table grapes.30
Ethyl acetate is generally the major ester in wine, which is consistent with results observed in this study. At low concentrations (<100 mg L−1) it contributes a desirable fruity character, but at higher concentrations it can give an aroma of solvent/nail varnish.31 In this case, none of the samples exceeded 65 mg L−1, so the presence of this compound does not imply aromatic defects.
From the table corresponding to 5 g hL−1 there are significant differences in the concentration of isobutanol, which is higher in the ozonated samples (O3G and O3L) of the L. thermotolerans fermentations when compared with their respective control. Something similar also occurs in the case of 2-phenylethanol, but only with the O3L treatment. For 10 g hL−1 this pattern is repeated. On the other hand, for 20 g hL−1 the differences between the samples are reduced and, for isobutanol, there is only a significant increase with the O3L treatment.
It is worth noting the increase in the concentration of 2-phenylethanol in the samples inoculated with L. thermotolerans that were treated with ozonation (O3G and O3L) since, generally, an increase in this compound is attributed to mixed fermentations with strains of L. thermotolerans, due to its role as a signaling molecule.1,2,32 Furthermore, this compound together with its derived acetate ester has a positive floral impact (rose petals) on the aroma and freshness of the wine27 but no significant differences could be established between samples for the ester. A pattern is also observed in the difference between controls and treatments similar to what happened with the results obtained for lactic acid and pH; the lower the concentration of the inoculum used, the greater the difference between these samples.
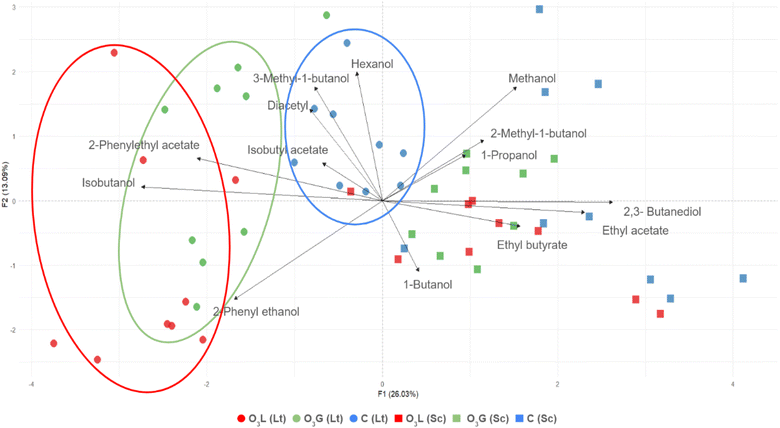
Based on the aroma data mentioned above, a Principal Component Analysis (PCA) was performed. Due to the lack of significant differences, the axes only explain 39.12% of the variability but still allow the samples to be grouped according to the yeast inoculated and the treatment used (Fig. 6). Of this variability, 26.03% is attributed to the first component (F1) and the remaining 13.09% to the second component (F2).
First, all the inoculations carried out with S. cerevisiae are in the positive values of the first component, so compounds such as 2,3-butanediol and ethyl acetate are more representative of these samples, while, in the case of L. thermotolerans, they would be more related to isobutanol and 2-phenylethyl acetate. Furthermore, within the L. thermotolerans samples, they can be grouped according to the treatment applied. The C samples are clearly separated from the O3L samples, although in the case of O3G, it overlaps with the other treatment and it is closer to the C samples, remaining in an intermediate position. Thus, for the C samples, being more grouped in the center of the axes, the values obtained were more similar to each other and close to the general average of all the samples. Instead, in the case of both treatments (particularly for O3L), they are further away from the center and more dispersed from each other, which indicates greater variability with respect to the rest of the sample groups and also greater internal variability.
Although the aromatic profiles do not present significant differences, the samples can be clearly divided according to the yeast inoculated, which could be another indication of the successful implantation of these yeasts. In addition, the possibility of grouping the L. thermotolerans samples according to the treatment applied shows that ozonation has had an influence on the production of certain compounds.
Conclusions
Ozone has proven to be an effective method for sanitizing grapes, both in its gaseous form and in aqueous solution, significantly reducing the population of microorganisms present on the surface of the grapes. In addition, its application has favored the implantation of the yeast Lachancea thermotolerans.All the parameters analyzed indicate that ozonation has facilitated the implantation of the inoculated yeasts, allowing, in the case of L. thermotolerans, the wines obtained from their fermentations to show characteristics specific of this yeast, such as acidification or the presence of certain aromatic compounds such as 2-phenyl ethanol.
Another relevant aspect was the impact of inoculum concentration, as the lower the concentration of yeast inoculated, the greater the differences between the controls and the ozone treatments. This proves that both treatments allow for successful yeast implantation, even under unfavorable conditions.
These results indicate that ozonation has the potential to be considered a viable alternative to sulphitation, especially when used with non-Saccharomyces yeasts. However, future research should focus on comparing its effectiveness with other sanitization methods and on evaluating its scaling under winery conditions.
This work provides new evidence that ozone treatments, both in gaseous and aqueous forms, effectively sanitize grapes while significantly facilitating the implantation and metabolic activity of L. thermotolerans during fermentation. The study demonstrates that the impact of ozone is particularly pronounced at lower yeast inoculum concentrations, where significant differences were observed between ozone-treated and control samples. Compared to traditional sulphitation, ozone emerges as a promising alternative sanitization method, especially in combination with non-Saccharomyces yeasts to enhance wine acidity and aroma profiles. By linking ozone sanitization directly with yeast ecology and inoculum-dependent implantation dynamics, these findings expand current understanding and offer valuable directions for more sustainable and targeted microbial management practices in the wine industry. Future research should further compare ozone with other sanitization approaches and assess scalability under real winery conditions.
Author contributions
Yaiza Rodríguez: writing – original draft, investigation. Juan Manuel del Fresno: writing – review & supervision. Carmen González: writing – review & editing. María Antonia Bañuelos: writing – review & editing. Antonio Morata: writing – review & editing, validation, project administration, methodology, funding acquisition, conceptualization.Conflicts of interest
There are no conflicts to declare.Data availability
The authors declare that most of the main data are included in the tables and figures of the article. Some of the raw data as Excel files or statistic files will be available upon request.Acknowledgements
This article is part of the project “Optimización del proceso de fermentación mediante pretratamiento de la materia prima y la mejora de los protocolos y condiciones de generación de biomasa” (PAG-020100-2023-274 – PP09), Plan de Recuperación Transformación y Resiliencia. NextGenerationEU funding. Ministerio de Industria, Comercio y Turismo.Notes and references
- M. Gobbi, F. Comitini, P. Domizio, C. Romani, L. Lencioni, I. Mannazzu and M. Ciani, Food Microbiol., 2013, 33, 271–281 CrossRef CAS.
- A. Hranilovic, W. Albertin, D. L. Capone, A. Gallo, P. R. Grbin, L. Danner, S. E. P. Bastian, I. Masneuf-Pomarede, J. Coulon, M. Bely and V. Jiranek, Food Chem., 2021, 349, 129015 CrossRef CAS PubMed.
- A. Morata, I. Loira, W. Tesfaye, M. A. Bañuelos, C. González and J. A. Suárez Lepe, Fermentation, 2018, 4, 53 CrossRef.
- A. Morata, M. A. Bañuelos, C. Vaquero, I. Loira, R. Cuerda, F. Palomero, C. González, J. A. Suárez-Lepe, J. Wang, S. Han and Y. Bi, Eur. Food Res. Technol., 2019, 245, 885–894 CrossRef CAS.
- F. Comitini, M. Gobbi, P. Domizio, C. Romani, L. Lencioni, I. Mannazzu and M. Ciani, Food Microbiol., 2011, 28, 873–882 CrossRef CAS PubMed.
- K. Kapsopoulou, A. Kapaklis and H. Spyropoulos, World J. Microbiol. Biotechnol., 2005, 21, 1599–1602 CrossRef CAS.
- F. Sizzano, V. Bianconi, M. Blackford, S. Bieri, F. Vuichard, C. Monnard, L. Amiet, J.-L. Spring, E. Dorsaz, N. Pfenninger-Bridy, S. Simonin, B. Bach and G. Bourdin, Fermentation, 2024, 10, 458 CrossRef CAS.
- R. Raposo, M. J. Ruiz-Moreno, T. Garde-Cerdán, B. Puertas, J. M. Moreno-Rojas, P. Zafrilla, A. Gonzalo-Diago, R. F. Guerrero and E. Cantos-Villar, LWT–Food Sci. Technol., 2016, 65, 214–221 CrossRef CAS.
- A. Morata, I. Loira, R. Vejarano, C. González, M. J. Callejo and J. A. Suárez-Lepe, Trends Food Sci. Technol., 2017, 67, 36–43 Search PubMed.
- M. a. Khadre, A. E. Yousef and J.-G. Kim, J. Food Sci., 2001, 66, 1242–1252 Search PubMed.
- A. Baggio, M. Marino, N. Innocente, M. Celotto and M. Maifreni, Eur. Food Res. Technol., 2020, 246, 669–692 Search PubMed.
- Secondary Direct Food Additives Permitted in Food for Human Consumption, https://www.federalregister.gov/documents/2001/06/26/01-15963/secondary-direct-food-additives-permitted-in-food-for-human-consumption, accessed March 20, 2025.
- A. J. Brodowska, A. Nowak and K. Śmigielski, Crit. Rev. Food Sci. Nutr., 2018, 58, 2176–2201 CrossRef CAS PubMed.
- F. Cravero, V. Englezos, K. Rantsiou, F. Torchio, S. Giacosa, S. R. Segade, V. Gerbi, L. Rolle and L. Cocolin, Food Res. Int., 2016, 87, 134–141 CrossRef CAS PubMed.
- E. Sarron, P. Gadonna-Widehem and T. Aussenac, Foods, 2021, 10, 605 CrossRef CAS.
- J.-G. Kim, A. E. Yousef and S. Dave, J. Food Prot., 1999, 62, 1071–1087 CrossRef CAS PubMed.
- R. Guzzon, T. Nardin, O. Micheletti, G. Nicolini and R. Larcher, Aust. J. Grape Wine Res., 2013, 19, 180–188 CrossRef CAS.
- R. Guzzon, E. Franciosi, S. Moser, I. Carafa and R. Larcher, J. Appl. Microbiol., 2018, 125, 513–527 CrossRef CAS PubMed.
- A. Campayo, K. Serrano de la Hoz, M. M. García-Martínez, M. R. Salinas and G. L. Alonso, Biomolecules, 2020, 10, 213 CrossRef CAS PubMed.
- P. Mostashari, M. Gavahian, S. Jafarzadeh, J.-H. Guo, M. Hadidi, R. Pandiselvam, E. Huseyn and A. Mousavi Khaneghah, Compr. Rev. Food Sci. Food Saf., 2022, 21, 3129–3152 CrossRef CAS.
- A. Campayo, K. Serrano de la Hoz, M. M. García-Martínez, M. R. Salinas and G. L. Alonso, Agronomy, 2020, 10, 1218 CrossRef CAS.
- R. Botondi, F. De Sanctis, N. Moscatelli, A. M. Vettraino, C. Catelli and F. Mencarelli, Food Chem., 2015, 188, 641–647 CrossRef CAS.
- D. Abalos, R. Vejarano, A. Morata, C. González and J. A. Suárez-Lepe, Eur. Food Res. Technol., 2011, 232, 663–669 CrossRef CAS.
- A. Morata, C. Escott, C. Vaquero, J. M. del Fresno, I. Loira, R. Cuerda and C. González, Food Biosci., 2025, 63, 105700 CrossRef CAS.
- R. Guzzon, T. Nardin, O. Micheletti, G. Nicolini and R. Larcher, Aust. J. Grape Wine Res., 2013, 19, 180–188 CrossRef CAS.
- J. Li, J. Xi, R. Wang, K. Jiang, X. Li, Q. Zhang, H. Xue and Y. Bi, Postharvest Biol. Technol., 2025, 224, 113490 CrossRef CAS.
- A. Morata, C. Escott, M. A. Bañuelos, I. Loira, J. M. del Fresno, C. González and J. A. Suárez-Lepe, Biomolecules, 2020, 10, 34 CrossRef CAS PubMed.
- G. Banilas, G. Sgouros and A. Nisiotou, Microbiol. Res., 2016, 193, 1–10 CrossRef CAS PubMed.
- M. Modesti, S. Brizzolara, R. Forniti, B. Ceccantoni, A. Bellincontro, C. Catelli, F. Mencarelli and P. Tonutti, Aust. J. Grape Wine Res., 2023, 2023, 8244309 Search PubMed.
- S. Canturk, S. Tangolar, S. Tangolar and M. Ada, Appl. Fruit Sci., 2024, 67, 12 CrossRef.
- K. M. Sumby, P. R. Grbin and V. Jiranek, Food Chem., 2010, 121, 1–16 CrossRef CAS.
- M. E. Beckner Whitener, S. Carlin, D. Jacobson, D. Weighill, B. Divol, L. Conterno, M. Du Toit and U. Vrhovsek, LWT–Food Sci. Technol., 2015, 64, 412–422 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |