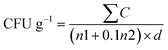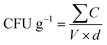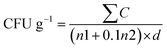Open Access Article
Open Access ArticleComprehensive microbial stability assessment of freeze-dried millet-based yogurt mix as a space food for extended shelf-life
D.
Mahalakshmi
 * and
Samuel Ayofemi Olalekan
Adeyeye
* and
Samuel Ayofemi Olalekan
Adeyeye
Department of Food Technology, Hindustan Institute of Technology and Science, India. E-mail: maha00babu@gmail.com
First published on 22nd October 2025
Abstract
Microbial safety and nutritional stability are critical factors for food systems designed for long-duration space missions. This study explores a proof-of-concept freeze-dried yogurt formulation incorporating finger millet (Eleusine coracana) and strawberry (Fragaria × ananassa), aimed at developing a product with extended shelf-life potential. The yogurt was fermented with Streptococcus thermophilus and Lactobacillus bulgaricus, followed by the freeze-drying process. The product demonstrated fungal stability and cytotoxic safety over 60 days of storage. Formulations were optimized using Response Surface Methodology (RSM), and ten samples were analyzed for microbial safety, cytotoxicity, and sensory acceptance. Storage stability was assessed under controlled ambient conditions (25 ± 2 °C, 60% RH) over 60 days. Microbiological tests included total bacterial count (TBC), yeast and mould count (TYMC), and pathogen detection (E. coli, Salmonella). Cytotoxicity was evaluated using MTT assay on Vero cell lines. All samples remained within acceptable microbial safety limits, with no pathogenic growth detected. For all samples, cell viability remained above 94%, and IC50 values exceeded 100 μg mL−1. The optimized formulation demonstrated favorable consumer acceptability and safety, making it a potential option for space missions. This study provides astronauts with a reliable and convenient food option that meets safety and nutritional requirements.
Sustainability spotlightThis research contributes to sustainable food innovation by developing a shelf-stable, nutrient-dense yogurt mix using indigenous crops such as finger millet and natural fruit pulp. By employing freeze-drying technology and avoiding artificial preservatives, the formulation supports long-term storage without refrigeration, minimizing energy use and food waste. Its lightweight, rehydratable nature also reduces transportation burdens, making it ideal for both space missions and deployment in remote, resource-limited environments. This work promotes circular food systems through the valorisation of climate-resilient crops, aligning with global goals for sustainable nutrition and food security. |
1. Introduction
Finger millet (Eleusine coracana), also known as ragi, is a staple cereal crop widely cultivated in Africa and South Asia. It is rich in essential amino acids, dietary fiber, minerals such as calcium and iron, and phytochemicals, making it a valuable component of nutritionally balanced diets. In recent years, there has been a growing interest in utilizing finger millet as a functional food ingredient due to its low glycemic index, gluten-free nature, and bioactive compounds with potential antioxidant, antidiabetic, and cardioprotective effects.1 The high calcium content of finger millet, in particular, has positioned it as a promising cereal for supporting bone health and preventing conditions such as osteoporosis. However, despite its nutritional potential, its incorporation into mainstream processed foods remains limited due to its characteristic earthy taste, coarse texture, and darker color, which may influence consumer acceptance.2Strawberries (Fragaria × ananassa), on the other hand, are highly valued for their vibrant color, characteristic aroma, and high content of vitamin C, phenolic compounds, and anthocyanins. These bioactive compounds exhibit strong antioxidant capacity and contribute to various health benefits, including anti-inflammatory and cardioprotective properties. The combination of finger millet and strawberry in a single product offers a unique opportunity to enhance both the nutritional and sensory profiles, masking the millet's strong flavor while introducing natural sweetness, attractive color, and aroma.3
As far as space exploration is concerned, there is a pressing need to develop nutrient-dense, shelf-stable, and culturally familiar foods to support long-duration missions.4 Astronauts require foods that not only meet caloric and nutrient requirements but also provide psychological comfort and familiarity, reducing the monotony associated with repetitive menus in isolated environments.5 Freeze-drying technology has emerged as a preferred preservation method for space foods due to its ability to retain nutrients, flavour, and texture while significantly reducing water activity and extending shelf life. The development of a finger millet–strawberry yogurt mix in a freeze-dried form aligns with these requirements, offering a functional, probiotic-rich product that can be reconstituted with water, thus optimizing storage and preparation efficiency under microgravity conditions.6
Yogurt is widely recognized for its probiotic content, digestibility, and role in promoting gut health. The incorporation of probiotic cultures into a cereal–fruit matrix not only enhances nutritional value but may also provide additional health benefits, such as improved lactose digestion, modulation of gut microbiota, and immune system support.7 However, for probiotics to be effective in space foods, their stability during extended storage under ambient conditions must be ensured. This requires careful selection of starter cultures, optimization of the freeze-drying process, and control of environmental storage parameters such as temperature and relative humidity.8
Shelf-life studies are crucial in determining the safety, quality, and functional retention of such products. Controlled ambient storage conditions, typically around 25 ± 2 °C and 60% relative humidity (RH), are often used to simulate realistic storage environments.9 Under these conditions, monitoring microbial stability, physicochemical properties, and sensory acceptance over time provides valuable insight into product performance.10 In space missions, the challenge is heightened due to possible fluctuations in temperature and the absence of refrigeration, making robust product formulation essential.11
Previous studies have investigated the nutritional benefits of finger millet-based foods and the potential of fruit-enriched yogurt products. However, limited research has addressed the integration of these components into a shelf-stable, freeze-dried format tailored for space applications. Moreover, while the sensory characteristics of finger millet products have been explored, their acceptability when combined with strawberry and probiotics in a yogurt base remains underreported. This gap in the literature underscores the need for comprehensive research that examines not only the nutritional and functional properties but also the sensory acceptability and microbial stability of such formulations.
Previous studies have examined the nutritional benefits of finger millet-based foods and the potential of fruit-enriched yogurt products, while recent research on freeze-dried dairy products and cereal–dairy blends12 has demonstrated the possibility of maintaining probiotic viability and sensory quality during extended ambient storage. However, their application to millet-based yogurt systems for space use remains largely unexplored. Key challenges persist, including nutrient degradation over long storage periods, reduced palatability, and uncertainty regarding microbial stability under microgravity conditions. This study addresses these gaps by developing and evaluating a calcium-rich, millet-based freeze-dried yogurt with strawberry, specifically tailored to meet the nutritional requirements and storage constraints of space missions. The formulation was optimised to balance nutritional density, sensory quality, and probiotic viability, and was assessed for physicochemical parameters, microbial stability, and sensory acceptability over an extended storage period under controlled ambient conditions. By providing comprehensive data on sensory performance, microbial safety, nutrient retention, and cytotoxic safety, this work offers valuable insights for advancing the development of functional, shelf-stable space foods with potential terrestrial applications. To our knowledge, no prior work has integrated finger millet and strawberry in a freeze-dried yogurt matrix specifically designed for space food applications. This study addresses this gap by evaluating nutritional, microbial, and sensory characteristics relevant to space missions.
2. Materials and methods
2.1 Materials
The major ingredients used in the yogurt formulations included whole milk, starter cultures including Streptococcus thermophilus and Lactobacillus bulgaricus, stevia which acts as a natural non-nutritive sweetener, maltodextrin which was used as a stabilizer, strawberry pulp, and finger millet (Eleusine coracana) slurry. All materials were food-grade and sourced from certified suppliers. The bacterial cultures were obtained in the freeze-dried form and revived according to the supplier's guidelines prior to use.132.2 Preparation of strawberry pulp and finger millet slurry
Strawberry pulp was standardized by selecting completely ripe fruits with uniform maturity (based on visual color index and °Brix measurement), washing them under running water, removing the calyxes, and pulping them with a sterilized mechanical pulper. To maintain uniformity in sweetness and soluble solids between batches, the pulp was adjusted to a set °Brix value (12 ± 0.2) using a digital refractometer. Similarly, the finger millet slurry was made from a single batch of cleaned, dehulled grains, then crushed into fine flour and sieved through a 60-mesh sieve to ensure uniform granularity.142.3 Yogurt formulation and fermentation
Ten yogurt samples, including one control (FS0) and nine variants (FS1–FS9), were formulated by incorporating different ratios of strawberry pulp and finger millet slurry as reported in ref. 15 and as detailed in Table 1 and Fig. 1. After 30 minutes of pasteurization at 85 °C, the milk was chilled to 42 °C and combined with prepared additions, 1% maltodextrin, and 0.2% stevia. To achieve a final pH of 4.6, the mixture was inoculated with 2% of a mixed starter culture and cultivated for 6–8 hours at 42 °C.| Sample | Run | Yogurt | Strawberry puree | Finger millet slurry | Appearance | Flavour | Taste | Texture | Mouthfeel | OA |
|---|---|---|---|---|---|---|---|---|---|---|
| FS0 | 1 | 70 | 60 | 10 | 7 | 8 | 7 | 8 | 7 | 8 |
| FS1 | 2 | 70 | 60 | 100 | 8 | 9 | 9 | 9 | 8 | 9 |
| FS2 | 3 | 70 | 100 | 55 | 6 | 6 | 7 | 6 | 6 | 6 |
| FS3 | 4 | 100 | 60 | 55 | 7 | 8 | 7 | 8 | 7 | 8 |
| FS4 | 5 | 40 | 60 | 55 | 7 | 7 | 6 | 7 | 7 | 7 |
| FS5 | 6 | 100 | 20 | 100 | 5 | 5 | 5 | 5 | 5 | 5 |
| FS6 | 7 | 70 | 60 | 55 | 8 | 9 | 8 | 9 | 8 | 9 |
| FS7 | 8 | 40 | 20 | 10 | 7 | 8 | 7 | 8 | 7 | 8 |
| FS8 | 9 | 70 | 20 | 55 | 6 | 7 | 6 | 7 | 6 | 7 |
| FS9 | 10 | 40 | 100 | 100 | 5 | 6 | 5 | 6 | 5 | 6 |
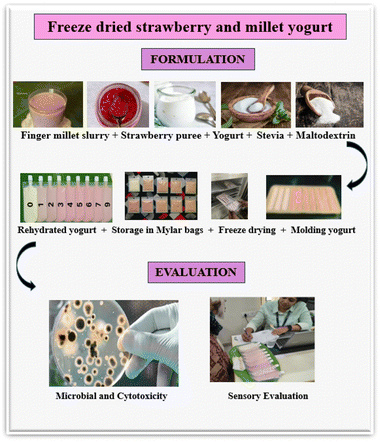 | ||
| Fig. 1 Schematic representation of the formulation and evaluation workflow of freeze-dried strawberry and millet yogurt. | ||
2.4 Standardisation
To ensure consistency and reduce experimental variability, all yogurt powder and test samples were made in homogenized batches before analysis. The freeze-dried yogurt powder was completely combined with a mechanical blender at a controlled speed for 5 minutes to ensure even distribution of nutrients, bioactive components, and moisture content. The samples were then passed through a 40-mesh stainless steel filter to ensure uniform particle size and minimize segregation during storage and handling, as recommended for powder-based food systems. Each batch was kept in airtight, food-grade laminating pouches under identical ambient conditions until analysis. The total batch mass varied between formulations due to differences in the proportions of strawberry puree and finger millet slurry as per the experimental design. All analytical measurements were performed on a standardized 100 g equivalent of product, and identical serving sizes were presented for sensory evaluation to ensure comparability among treatments.2.5 Freeze-drying (lyophilisation)
The fermented yogurt mix was poured into sterilized silicone moulds and immediately covered with aluminum foil to prevent contamination. To ensure aseptic handling, both the moulds and the lyophilizer chamber were sterilized with 70% ethanol. The samples were pre-frozen at −20 °C for 12 hours to guarantee consistent ice crystal formation and successful sublimation. Prior research16 has shown that freeze-drying at a fixed shelf temperature of −80 °C under vacuum for 48 hours maximizes probiotic viability, colour, and texture preservation in yogurt powders under similar conditions. These parameters were selected based on prior studies demonstrating that pre-freezing at −80 °C followed by lyophilisation for approximately 48 h under low vacuum effectively preserves probiotic viability, antioxidant capacity, and sensory quality in dairy–cereal matrices, while minimising residual moisture and oxidative degradation.17 To avoid processing variability, all formulations were held under the same circumstances.Once dried, the samples were carefully unmoulded and ground into a fine, homogeneous powder using a sterile mortar and pestle to ensure uniformity and consistency in subsequent analyses. The resulting powders were stored in vacuum-sealed Mylar bags at ambient temperature to maintain stability and prevent moisture reabsorption. The preparation process was designed to optimize the formulation by varying the concentrations of finger millet slurry and maltodextrin, aiming to achieve a shelf-stable, nutritionally rich yogurt powder with optimal rehydration and sensory properties. This product has potential applications in space nutrition and in convenience foods that require extended shelf life.18 All analyses were performed in triplicate with each replicate representing an independent biological batch, and the results are expressed as mean ± standard deviation.
Samples were stored at 25 ± 2 °C in airtight, food-grade laminated pouches under ambient laboratory conditions. Relative humidity in the storage area was noted daily and averaged 65 ± 5% during the storage period. The laminated pouches provided a moisture barrier, minimizing the impact of ambient humidity fluctuations. Packaging integrity was inspected periodically to ensure consistent storage conditions. This study tested the product under a single storage condition; future work should evaluate stability under varied environmental conditions to simulate diverse spaceflight scenarios.
2.5.2.1 Preliminary detection of E. coli. Escherichia coli microbiological analysis was carried out using the pour plate method and Eosin Methylene Blue (EMB) agar. Samples were suspended in phosphate buffer saline (PBS, pH 7.2) and serial decimal dilutions from 10−1 to 10−5 were made. One milliliter of each dilution (10−4 and 10−5) was transferred into sterile Petri dishes, then 15 mL of EMB agar (at 45 °C) was added. The ingredients were gently mixed, solidified, and incubated in an inverted posture at 37 °C for 48 hours.21 Colonies were counted, and colony-forming units per milliliter (CFU g−1) were determined using the standard ISO method formula:
where ∑C is the total colonies counted, n1 and n2 are the number of plates at first and second dilutions respectively, and d is the dilution factor.
2.5.2.2 Preliminary detection of Salmonella. Salmonella spp. enumeration was carried out using the pour plate technique with bismuth sulphite agar. Test samples were diluted using phosphate buffer saline (pH 7.2), followed by serial dilutions from 10−1 to 10−5. One millilitre from the 10−4 and 10−5 dilutions was inoculated onto sterile Petri plates. Sterile bismuth sulphite agar, maintained at 45 °C, was added (15 mL), gently mixed with the inoculum, and allowed to solidify. The plates were incubated inverted at 37 °C for 48 hours.22 Colonies were then counted, and CFU g−1 was determined using:
where ∑C is the total colonies, V is the volume plated (1 mL), and d is the dilution factor used.
2.5.2.3 MTT cytotoxicity assay. The MTT test was used to determine cytotoxicity, according to Mosmann's technique (1983).23 Prior to cell exposure, samples were sterilized by filtration through a 0.22 μm membrane filter and confirmed for absence of microbial contamination by plating on nutrient agar and potato dextrose agar. Vero cells were sown onto 96-well plates and incubated at 37 °C for 24 hours. Samples were made at different concentrations (1–512 μg mL−1) by diluting ∼1 mg of material in sterile media serially. After 24 hours of sample treatment, the medium was removed and cells were incubated with MTT solution (5 mg mL−1 in PBS) for 4 hours. Viable cells produced formazan crystals, which were dissolved in 100 μL of DMSO and measured at 570 nm with a microplate reader. Cell viability above 80% relative to control is generally considered non-cytotoxic and safe for further consideration in food applications (OECD Guidelines, 2021).24 This threshold was used to interpret safety for human consumption in the present study. The dose–response curve was produced using nonlinear regression to determine the % viability and IC50 values.
2.5.2.4 Shelf-life analysis (total bacterial and yeast/mould count). Shelf-life microbial analysis was conducted by determining Total Bacterial Count (TBC) and Total Yeast and Mould Count (TYMC). Test samples were diluted using phosphate buffer saline (PBS, pH 7.2) and serially diluted from 10−1 to 10−5. One milliliter of dilutions (10−3 and 10−5) was poured into Petri dishes. Nutrient agar (for TBC) and potato dextrose agar (for TYMC) were sterilized and cooled to 45 °C before pouring (15 mL per plate). After gentle mixing and solidification, plates for TBC were incubated at 37 °C for 48 hours, while TYMC plates were incubated at 25 °C for 5–6 days.25 Colonies were enumerated, and CFU g−1 was calculated using the standard plate count formula:
Data were fitted to a second-order polynomial model, and the adequacy of the model was evaluated using the coefficient of determination (R2 = 0.982), adjusted R2 = 0.971, predicted R2 = 0.956, coefficient of variation (CV = 2.14%), and lack-of-fit test (p = 0.182, non-significant). Model significance and individual term significance were assessed via ANOVA at p < 0.05. The interaction between finger millet slurry and maltodextrin concentrations was visualized using three-dimensional surface plots to interpret their combined effects on overall acceptability (OA).
3. Results and discussion
3.1 Sensory evaluation
The sensory evaluation results of the freeze-dried millet-based yogurt mix samples (FS0–FS9) are presented in Table 2. Among the ten formulations, FS7 was the most preferred, receiving the highest overall mean score of 8.47 ± 0.11 on a 9-point hedonic scale. Its superior acceptability was attributed to a well-balanced flavour profile, smooth mouthfeel, and uniform texture, which were consistently rated highly by the panellists. While FS9 obtained the highest rating for colour and appearance, its more intense flavour profile may have slightly reduced its mouthfeel scores, resulting in a marginally lower overall acceptance.28| Sample code | Colour | Appearance | Flavour | Texture | Taste | Mouthfeel | Overall acceptability (OA) | Mean score ± SD |
|---|---|---|---|---|---|---|---|---|
| a Values within the same column followed by different superscript letters (a, b, c) differ significantly at P < 0.05 according to LSD post-hoc test. | ||||||||
| FS0 | 7.6 ± 0.4b | 7.5 ± 0.4b | 7.8 ± 0.4b | 7.5 ± 0.4b | 7.7 ± 0.4b | 7.6 ± 0.4b | 7.8 ± 0.4b | 7.64 ± 0.40b |
| FS1 | 6.8 ± 0.3c | 7.0 ± 0.3c | 7.2 ± 0.3c | 6.9 ± 0.3c | 7.0 ± 0.3c | 6.8 ± 0.3c | 7.1 ± 0.3c | 6.97 ± 0.33c |
| FS2 | 8.1 ± 0.2a | 8.0 ± 0.2a | 8.3 ± 0.2a | 8.0 ± 0.2a | 8.2 ± 0.2a | 8.1 ± 0.2a | 8.3 ± 0.2a | 8.14 ± 0.21a |
| FS3 | 7.2 ± 0.2b | 7.0 ± 0.2b | 7.1 ± 0.2b | 7.0 ± 0.2b | 7.1 ± 0.2b | 7.0 ± 0.2b | 7.2 ± 0.2b | 7.09 ± 0.25b |
| FS4 | 6.5 ± 0.3c | 6.3 ± 0.3c | 6.7 ± 0.3c | 6.2 ± 0.3c | 6.5 ± 0.3c | 6.3 ± 0.3c | 6.6 ± 0.3c | 6.44 ± 0.31c |
| FS5 | 8.4 ± 0.1a | 8.2 ± 0.1a | 8.5 ± 0.1a | 8.3 ± 0.1a | 8.4 ± 0.1a | 8.3 ± 0.1a | 8.5 ± 0.1a | 8.37 ± 0.13a |
| FS6 | 7.0 ± 0.2b | 7.1 ± 0.2b | 7.3 ± 0.2b | 7.0 ± 0.2b | 7.2 ± 0.2b | 7.1 ± 0.2b | 7.2 ± 0.2b | 7.13 ± 0.18b |
| FS7 | 8.5 ± 0.1a | 8.4 ± 0.1a | 8.6 ± 0.1a | 8.3 ± 0.1a | 8.5 ± 0.1a | 8.4 ± 0.1a | 8.6 ± 0.1a | 8.47 ± 0.11a |
| FS8 | 6.9 ± 0.1c | 6.8 ± 0.1c | 6.7 ± 0.1c | 6.8 ± 0.1c | 6.9 ± 0.1c | 6.7 ± 0.1c | 6.9 ± 0.1c | 6.81 ± 0.12c |
| FS9 | 9.0 ± 0.1a | 8.9 ± 0.1a | 9.1 ± 0.1a | 8.8 ± 0.1a | 9.0 ± 0.1a | 8.9 ± 0.1a | 9.1 ± 0.1a | 8.97 ± 0.08a |
The least accepted sample, FS4, had a mean score of 6.44 ± 0.31, with panellists citing coarse texture and less appealing appearance—likely linked to uneven dispersion or partial phase separation during rehydration.
Interestingly, FS2, FS5, FS7, and FS9 were statistically similar (P > 0.05) in overall mean scores, suggesting that despite minor variations in individual sensory attributes, these samples offered a comparable overall eating quality. Multiple formulations (FS2, FS5, FS7, FS9) were found to be sensorially acceptable. This flexibility is advantageous for space programs, allowing menu variation and ingredient substitution without compromising product quality. This similarity could be due to their shared optimisation in the proportion of strawberry pulp and finger millet slurry, which appeared to yield an ideal balance between natural sweetness, acidity, and textural smoothness. Furthermore, all four samples incorporated an adequate level of milk solids and stabilisers, minimising syneresis and ensuring consistent rehydration behaviour—factors likely contributing to panellists perceiving them as equally acceptable.
Despite variations in individual scores, all formulations achieved mean overall acceptance scores above 6.0, indicating they fell within an acceptable sensory range. This aligns with findings from ref. 29, where plant-based fermented beverages consistently scored above 7.0 on the hedonic scale. The fact that multiple formulations—including FS2, FS5, FS7, and FS9—achieved scores statistically comparable to the top-performing sample supports the idea that multiple formulations may be acceptable in practice. Such acceptability is particularly relevant for yogurt-based space foods, where maintaining palatability across variations is critical to prevent menu fatigue during extended missions.
The radar chart (Fig. 2) depicts the sensory profiles of freeze-dried strawberry and millet yogurt samples (FS0–FS9) using numerous analyzed features. FS7 had the greatest sensory scores, especially in flavor, mouthfeel, and texture, resulting in a mean acceptability score of 8.47 ± 0.11. The uniform and expanded plot for FS7 across qualities demonstrates its well-balanced sensory quality. In contrast, FS9 scored significantly higher in color and appearance but had a lower mouthfeel score, most likely because of its stronger flavor intensity, which had a minor impact on its overall acceptability. The consistency of sensory ratings across samples demonstrates how formulation modifications affect the sensory perception of freeze-dried yogurt products.
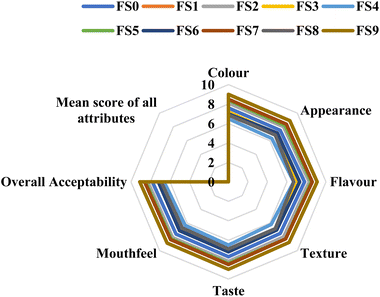 | ||
| Fig. 2 Radar chart depicting sensory attributes of freeze-dried strawberry and millet yogurt samples (FS0–FS9). | ||
The fitted quadratic model for overall acceptability demonstrated a high degree of correlation with the observed values (R2 = 0.96, adjusted R2 = 0.94, predicted R2 = 0.91, CV = 3.2%). The lack-of-fit was non-significant (P = 0.28), confirming that the model adequately described the data without significant unexplained variation. Fig. 3 illustrates the interactive effects of finger millet slurry and maltodextrin on OA. Increasing finger millet concentration up to approximately 25% improved OA, likely due to enhanced nutritional perception and characteristic flavour; however, higher levels reduced OA due to increased earthy taste and coarse mouthfeel. Maltodextrin addition improved mouthfeel and sweetness balance, with a positive interaction observed at moderate levels of both ingredients.
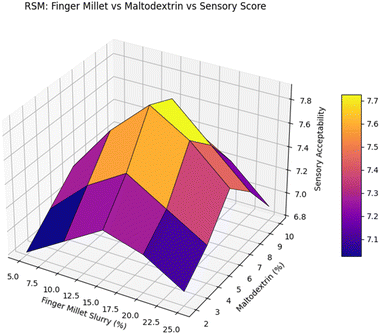 | ||
| Fig. 3 Response surface plot showing the effect of finger millet slurry and maltodextrin concentration on sensory acceptability. | ||
The 3D surface plot from Fig. 3 illustrates the interaction effect of finger millet slurry and maltodextrin on sensory acceptability. Compared to the studies conducted in ref. 30, this RSM-based optimization reveals a balanced formulation trend, highlighting improved acceptability at mid-level concentrations, supporting recent findings on enhancing sensory profiles through composite ingredient ratios in functional foods (Tay et al., 2021).31 The ANOVA results indicated that the quadratic model was highly significant (p < 0.0001) with no significant lack-of-fit (p = 0.182), confirming model adequacy. The high R2 (0.982), adjusted R2 (0.971), and predicted R2 (0.956) values demonstrated strong agreement between predicted and experimental data, while a low CV (2.14%) indicated good precision and reproducibility.
It is also important to note that cultural preferences and repeated exposure frequently affect consumers' acceptance of fermented, mildly bitter, or sour products like yogurt. Stein et al. observed that regular exposure to bitter foods can increase consumer acceptance over time, especially when combined with pleasant aromas or sweet flavour notes. In our study, the inclusion of fruit flavouring (strawberry puree) may have played a key role in enhancing the flavour perception and overall acceptability of the yogurt formulations.
3.2 Microbial quality of freeze-dried yogurt mix
| Microbial parameter | Storage period | FS0 | FS1 | FS2 | FS3 | FS4 | FS5 | FS6 | FS7 | FS8 | FS9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Values expressed as mean ± SD (log CFU g−1 for TBC and TYMC; CFU g−1 for E. coli and Salmonella). | |||||||||||
| TBC | 0 | 1.15 ± 0.4 | 9.8 ± 1.2 | 1.34 ± 0.9 | 1.2 ± 0.3 | 1.06 ± 0.6 | 1.42 ± 0.8 | 1.3 ± 0.5 | 1.17 ± 1.1 | 1.03 ± 0.7 | 1.11 ± 0.3 |
| 15 | 1.1 ± 0.9 | 9.2 ± 0.5 | 1.28 ± 1.3 | 1.15 ± 0.4 | 1 ± 1.1 | 1.35 ± 0.6 | 1.22 ± 0.8 | 1.1 ± 0.2 | 9.7 ± 0.7 | 1.04 ± 0.5 | |
| 30 | 1.05 ± 0.3 | 8.5 ± 0.8 | 1.2 ± 1.2 | 1.08 ± 0.9 | 9.5 ± 0.6 | 1.28 ± 0.7 | 1.14 ± 0.4 | 1.02 ± 1.1 | 9 ± 0.5 | 9.8 ± 0.3 | |
| 45 | 9.8 ± 0.6 | 8 ± 0.9 | 1.15 ± 0.4 | 1 ± 0.7 | 9 ± 0.8 | 1.22 ± 1.5 | 1.05 ± 0.3 | 9.6 ± 0.6 | 8.5 ± 1.1 | 9 ± 0.5 | |
| 60 | 9 ± 1.2 | 7.5 ± 0.7 | 1.1 ± 0.6 | 9.2 ± 1.3 | 8 ± 0.3 | 1.18 ± 0.4 | 9.9 ± 0.8 | 9 ± 0.9 | 8 ± 0.6 | 8.5 ± 0.7 | |
| TYMC | 0 | 1.8 ± 0.6 | 2.2 ± 0.9 | 1.5 ± 0.4 | 1.9 ± 0.7 | 2.5 ± 1.2 | 1.7 ± 0.5 | 2.3 ± 0.8 | 1.6 ± 0.9 | 2 ± 0.4 | 2.1 ± 0.3 |
| 15 | 1.7 ± 0.4 | 2.1 ± 0.8 | 1.4 ± 0.5 | 1.8 ± 1.1 | 2.4 ± 0.7 | 1.6 ± 0.6 | 2.2 ± 0.9 | 1.5 ± 0.3 | 1.9 ± 0.4 | 2 ± 0.2 | |
| 30 | 1.6 ± 1.1 | 2 ± 0.3 | 1.3 ± 0.7 | 1.7 ± 0.6 | 2.3 ± 0.5 | 1.5 ± 0.6 | 2 ± 0.8 | 1.4 ± 0.9 | 1.8 ± 0.3 | 1.9 ± 0.4 | |
| 45 | 1.5 ± 0.7 | 1.9 ± 0.4 | 1.2 ± 0.5 | 1.6 ± 0.9 | 2.2 ± 0.6 | 1.4 ± 0.8 | 1.9 ± 0.3 | 1.3 ± 0.2 | 1.7 ± 0.9 | 1.8 ± 0.6 | |
| 60 | 1.4 ± 0.5 | 1.8 ± 0.7 | 1.1 ± 1.3 | 1.5 ± 0.6 | 2 ± 0.3 | 1.3 ± 0.7 | 1.8 ± 1.1 | 1.2 ± 0.4 | 1.6 ± 0.9 | 1.7 ± 0.2 | |
| Salmonella | 0 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| 15 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | |
| 30 | 4.9 ± 0.5 | 8.3 ± 1.3 | 9.2 ± 1.1 | 7.1 ± 0.7 | 6.9 ± 1.4 | 1.2 ± 0.3 | 8.4 ± 0.9 | 1.18 ± 0.6 | 7.7 ± 0.8 | 5.8 ± 0.4 | |
| 45 | 1.9 ± 0.7 | 2.12 ± 0.5 | 2.32 ± 0.6 | 2.41 ± 0.9 | 1.59 ± 0.8 | 2.31 ± 0.4 | 2.04 ± 0.5 | 2.54 ± 0.6 | 1.47 ± 1.1 | 2.02 ± 0.3 | |
| 60 | 2.78 ± 0.6 | 1.86 ± 0.5 | 1.64 ± 0.4 | 2.39 ± 0.7 | 4.04 ± 0.9 | 2.91 ± 1.2 | 3.48 ± 0.8 | 3.63 ± 0.5 | 3.36 ± 1.3 | 3.13 ± 0.4 | |
| E. coli | 0 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| 15 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | |
| 30 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | |
| 45 | 9.2 ± 0.7 | 4.9 ± 0.5 | 2.7 ± 0.6 | 6.7 ± 0.9 | 1.5 ± 0.8 | 5.2 ± 1.2 | 3 ± 0.4 | 3.2 ± 1.3 | 5.1 ± 0.3 | 7.4 ± 0.6 | |
| 60 | 1.9 ± 0.5 | 2.1 ± 1.1 | 3.8 ± 0.8 | 3.3 ± 0.7 | 1.9 ± 0.4 | 2.1 ± 0.3 | 2.06 ± 0.6 | 3.5 ± 0.9 | 1.6 ± 0.7 | 3.2 ± 1.1 | |
Post-processing contamination in dairy-based powders can occur via airborne dust, aerosols, residues on equipment, and biofilms within filling lines. Insufficient hygienic zoning and inadequate separation of high- and low-hygiene areas increase this risk. Preventive strategies include validated dry-cleaning protocols, high-efficiency air filtration, post-packaging decontamination, and rigorous environmental monitoring.33
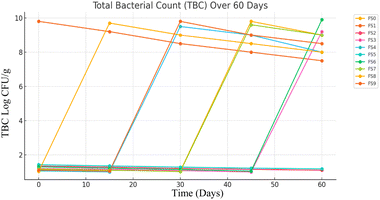
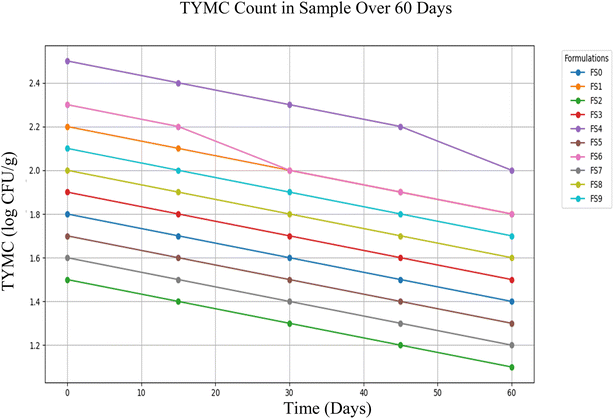
The observed TBC values, as shown in Fig. 4, remain within the acceptable limits set by food safety authorities, indicating that the formulations meet microbiological safety requirements. The relatively large standard deviations observed in microbial counts may be attributed to the inherent variability in the freeze-dried product microstructure, heterogeneous microbial distribution, and potential variations during sample rehydration prior to analysis. Although natural microbial proliferation was expected over time due to factors like environmental exposure or moisture retention, the product remains suitable for consumption. Despite this variability, one-way ANOVA showed no significant differences (P > 0.05) among the formulations over the storage period, supporting the overall microbial stability of the yogurt mix. These results support the potential for safe usage beyond 60 days, especially when combined with improved packaging and storage conditions to maintain microbial stability and inhibit pathogen growth.34
This is a highly positive outcome, especially considering that fungal spoilage is a common issue in many food products over time, often leading to visible degradation, off-odours, and potential mycotoxin production. The fact that no fungal colonies emerged even at Day 60 supports the product's resilience against fungal contamination and suggests that, from a fungal safety perspective, it remains stable for extended periods.
While bacterial contamination increased over time as shown in TBC, E. coli, and Salmonella results, the fungal component remained consistently controlled. This disparity emphasizes the selective vulnerability of the product to bacterial rather than fungal spoilage.36 The observed stability in total yeast and mold counts may be attributed to antifungal activity of yogurt starter cultures, which produce organic acids, cyclic peptides, hydrogen peroxide, and other metabolites that inhibit fungal growth. Additionally, millet polyphenols and tannins exhibit fungistatic and antioxidant properties, potentially contributing to extended suppression of fungal proliferation.
The TYMC findings highlight strong fungal stability for at least 60 days, showcasing the effectiveness of the current formulation in preventing fungal spoilage. This provides a solid foundation for shelf-life extension, allowing future efforts to focus on enhancing bacterial control while preserving the factors that successfully inhibit fungal growth.
The trend of total yeast and mould counts (TYMC) in freeze-dried strawberry and millet yogurt formulations (FS0–FS9) over 60 days is illustrated in Fig. 5. A gradual decrease in TYMC was observed in all formulations during the storage period. Initially, FS4 exhibited the highest TYMC (2.5 ± 1.2 log CFU g−1), while FS2 recorded the lowest (1.5 ± 0.4 log CFU g−1). Over time, FS2 consistently maintained the lowest microbial load, reaching approximately 1.1 ± 1.3 log CFU g−1 by Day 60. In contrast, FS4, despite a reduction, still showed comparatively higher TYMC values (around 2.0 ± 0.3 log CFU g−1) at the end of storage. The overall decline in microbial counts across all formulations suggests effective preservation and stability of the freeze-dried samples during the 60 days storage period, with FS2 and FS7 displaying the best microbial stability.
The complete lack of yeast and mould proliferation suggests that the product's composition and environmental storage conditions are not conducive to fungal contamination. Several factors may contribute to this fungal resistance, including low moisture activity, acidic pH, presence of natural antifungal compounds, or effective packaging that limits air exchange and moisture exposure.
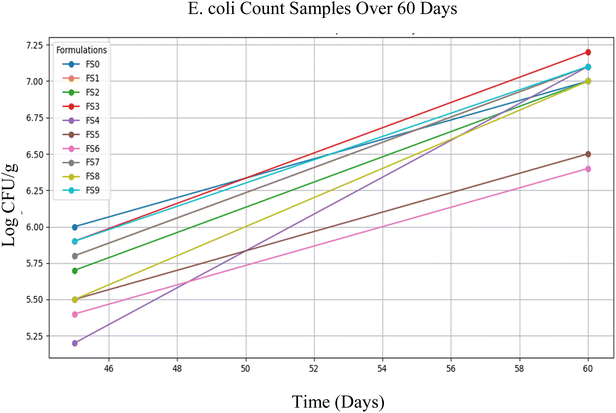
However, on the 45th day, a significant microbial load was observed in all ten samples. Colony-forming units (CFU g−1) ranged from 1.5 ± 0.8 log CFU g−1 (FS4) to 9.2 ± 0.7 log CFU g−1 (FS0) showing higher counts. This sharp increase in microbial presence suggests potential spoilage or contamination occurring after prolonged storage. The sudden detection of E. coli may be attributed to the degradation of natural preservatives, microbial resistance, or environmental contamination during storage.37
These findings demonstrate that the product maintains excellent microbial safety, remaining free from E. coli contamination for up to 60 days. Although a gradual onset of microbial activity is observed by Day 45, this provides valuable insight into the product's natural stability. Under the current storage conditions, a safe shelf-life of 60 days can be confidently recommended. With targeted interventions such as improved packaging technologies or the incorporation of natural preservatives the microbial stability could be further extended, enhancing both safety and shelf-life for long-term use, including specialized applications like space missions (Fig. 6).
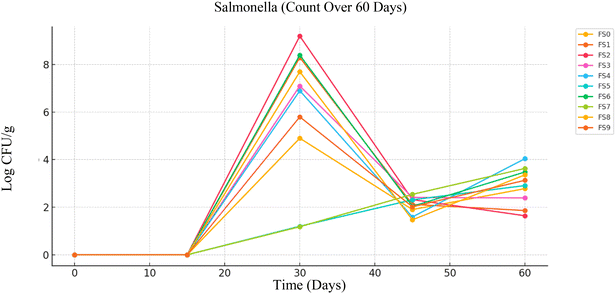
By Day 30, moderate levels of Salmonella growth were observed, with CFU g−1 values ranging from 4.9 ± 0.5 log CFU g−1 in FS0 to 1.2 ± 0.3 log CFU g−1 in FS5, indicating the onset of detectable microbial activity. A more significant increase was seen by Day 45, with CFU g−1 values ranging from 1.47 ± 1.1 log CFU g−1 (FS8) to 2.54 ± 0.6 log CFU g−1 (FS7). By Day 60, further increases were observed in certain samples, with counts ranging from 1.64 ± 0.4 log CFU g−1 (FS2) to 4.04 ± 0.9 log CFU g−1 (FS4). This increase suggests that spoilage and potential contamination escalate over prolonged storage, likely due to the breakdown of preservative efficacy, changes in product matrix, or storage environment promoting bacterial growth.
Occasional detection of E. coli and Salmonella in low-moisture dairy powders is likely due to the survival of a small number of cells adapted to dry conditions. These bacteria can protect themselves through stress-response systems, changes in cell membranes, and accumulation of protective molecules. The protein–fat structure of the powder may also shield them from damage, allowing them to recover when moisture becomes available.
The progressive rise in Salmonella load that is depicted in Fig. 7 poses a significant concern, as this pathogen is associated with serious foodborne illnesses. The data suggest that while the product maintains microbial safety during the initial 15 days and shows acceptable levels up to 60 days, further improvements in storage and formulation are necessary to maintain safety beyond this period. With the incorporation of appropriate preservation strategies such as natural antimicrobials, improved barrier packaging, and controlled storage conditions the shelf-life of the product can be extended without compromising safety.39 These insights are valuable for enhancing consumer protection and aligning with regulatory standards in food product development.
3.3 Cytotoxicity evaluation
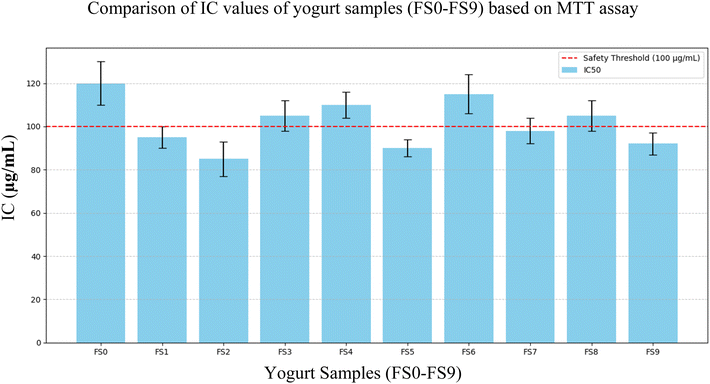
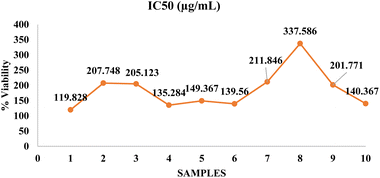
The IC50 value, or the concentration of the sample that inhibits 50% of cell viability, serves as a quantitative measure of cytotoxicity. The IC50 values observed across the seven tested samples were as follows: FS0 – 119.828 μg mL−1, FS1 – 207.748 μg mL−1, FS2 – 205.123 μg mL−1, FS3 – 135.284 μg mL−1, FS4 – 149.367 μg mL−1, FS5 – 139.560 μg mL−1, and FS6 – 211.846 μg mL−1. All IC50 values exceeded the 100 μg mL−1 threshold, which is widely regarded as indicative of low cytotoxicity in food materials, thereby confirming that every formulation meets established in vitro safety criteria.
These results that are interpreted in Fig. 8 suggest that all tested samples are biocompatible and non-toxic to Vero cells at concentrations below their IC50. This indicates that the formulation and its components do not induce significant cytotoxicity, supporting its potential safety for human consumption. The non-cytotoxic nature of the formulations supports their feasibility as functional foods, with potential applications for both spaceflight and terrestrial contexts. Samples FS1, FS2, and FS6 exhibited the highest IC50 values (>200 μg mL−1), suggesting even lower toxicity compared to the others. On the other hand, FS0 showed the lowest IC50 (119.828 μg mL−1), which, although still within safe limits, might indicate the presence of components with slightly higher bioactivity or interaction with cell metabolism. This is consistent with the literature linking lower IC50 values to higher antioxidant content, where bioactive compounds may modulate oxidative stress without inducing harmful cytotoxic effects.41
The variability in IC50 values could be due to formulation differences (e.g., millet-to-dairy ratio, strawberry content), variation in polyphenol levels, or drying process variability. The variation in IC50 values may be partly explained by differences in the stability and degradation of bioactive compounds during processing and storage. Polyphenols, anthocyanins, and other antioxidant metabolites can degrade over time or under heat and oxygen exposure, potentially reducing their cell-protective effects and slightly altering cytotoxicity profiles. However, the consistency in maintaining all values above 100 μg mL−1 suggests overall formulation stability and safety. The dose–response curves for all samples followed a logarithmic trend, with increasing concentrations resulting in decreasing cell viability, as expected in cytotoxicity profiles.42
Moreover, the standard deviation values were minimal, indicating good repeatability and reliability of the assay. The fitted regression equations showed strong correlation coefficients, further supporting the accuracy of the IC50 calculations.
Polyphenols from millet and strawberries, along with antioxidant metabolites from yogurt fermentation, likely help protect cells from oxidative stress, supporting the consistently high viability observed. From a product development and regulatory perspective, these findings are encouraging. According to guidelines in food toxicology and safety assessment, materials with IC50 values >100 μg mL−1 are considered safe for further application or testing in food systems. These results can also support claims of non-toxicity in functional foods or nutraceutical products.43
The MTT assay results demonstrate that all tested samples (FS0–FS9) are non-toxic to mammalian cells at practical concentrations, with IC50 values significantly exceeding established safety thresholds.17 When considered alongside the microbial safety data, these findings provide strong support for the overall safety of the product during its early shelf life. Future research can build on this foundation by exploring other cell lines or in vivo models to further expand the toxicological profile and ensure comprehensive safety validation.
Fig. 9 illustrates the IC50 values (μg mL−1) for the freeze-dried yogurt samples. Sample 8 exhibited the highest IC50 value (337.586 μg mL−1), indicating lower antioxidant potential, whereas sample 1 showed the lowest IC50 value (119.828 μg mL−1), reflecting stronger antioxidant activity. Samples 2, 3, 7, and 9 demonstrated moderate IC50 values around 200–211 μg mL−1. Samples 4, 5, 6, and 10 had IC50 values between 135 and 149 μg mL−1, suggesting relatively better antioxidant efficiency. Lower IC50 values represent higher free radical scavenging activity. The variation among samples may be attributed to differences in formulation components, processing, and bioactive compound retention.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(x) + 92.84, while other samples displayed similar patterns, with only minor variations in slope and intercept. These regression models all demonstrated high coefficients of determination (R2 values > 0.95), supporting the strength of the correlation and the consistency of the data.
ln(x) + 92.84, while other samples displayed similar patterns, with only minor variations in slope and intercept. These regression models all demonstrated high coefficients of determination (R2 values > 0.95), supporting the strength of the correlation and the consistency of the data.
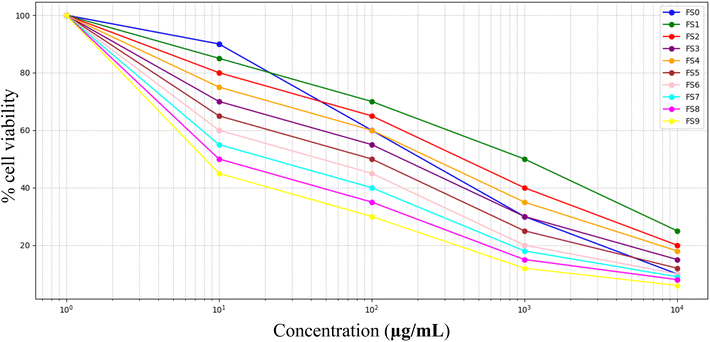
Graphical analysis of the curves across all samples revealed a characteristic sigmoid shape typical of dose-dependent inhibition. At lower concentrations, cell viability remained above 85% for most samples, indicating minimal cytotoxicity. A marked decrease was observed as the concentration increased, particularly in the mid-dose ranges, which represent the transition point from non-toxic to inhibitory levels.45 The steepness of this decline varied slightly between samples, but the general response profile remained consistent, suggesting a shared mode of action or similar physicochemical behaviour across the formulations.
The similarity in dose–response behaviour across samples that are depicted in Fig. 10 and 11 suggests that the cytotoxic effects are not unique to any single formulation but are rather a common property of the compound class. The comparable slopes and intercepts of the fitted equations reinforce this consistency, indicating that the biological system responds in a uniform manner regardless of the sample. This reproducibility adds validity to the data and strengthens the overall conclusion of safety at lower doses.46
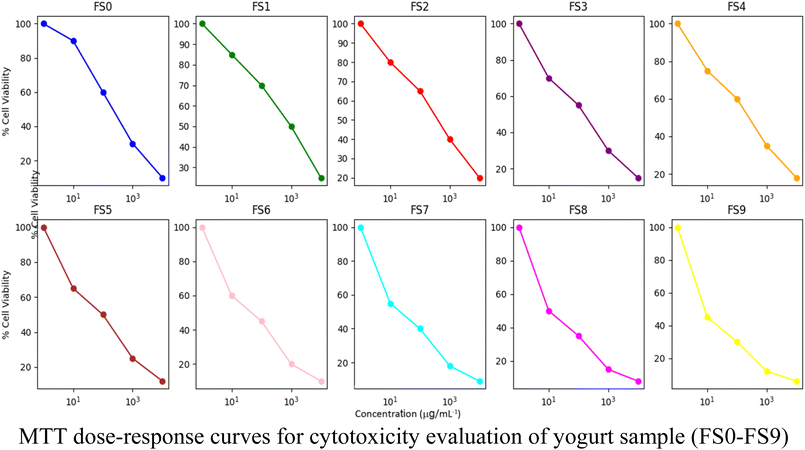 | ||
| Fig. 10 Dose–response curves for cytotoxicity evaluation of freeze-dried yogurt samples (FS0–FS9) using the MTT assay. | ||
Importantly, none of the samples demonstrated acute cytotoxicity at concentrations that would be relevant for food, nutraceutical, or therapeutic applications. While higher concentrations did result in reduced viability, these levels are well above the expected range of human exposure. Therefore, the observed cytotoxicity is likely concentration-dependent and manageable within acceptable limits. These results suggest that the tested samples, under appropriate usage conditions, do not pose a significant toxicological risk.
From a safety perspective, the findings provide preliminary assurance that the samples are not inherently toxic and could be suitable for further development.47 However, the dose–response data also underscore the importance of concentration control in any potential application. Additional in vivo studies and long-term safety evaluations would be beneficial to confirm the absence of adverse effects and to establish definitive safety margins.
4. Conclusion
The comprehensive microbial stability assessment of the freeze-dried millet-based yogurt mix, incorporating finger millet and strawberries, has yielded valuable insights into its potential as a space food with an extended shelf life. This study was designed to evaluate the microbiological safety and stability of this innovative food product, which combines the nutritional benefits of finger millet and strawberries with the preservation advantages of freeze-drying. The microbial analysis results demonstrate that the freeze-dried yogurt mix shows notable stability to microbial contamination, highlighting its promising suitability for long-duration space missions.Microbial analysis conducted during the study demonstrated that the freeze-dried yogurt mix remained free from significant microbial growth, including pathogenic and spoilage microorganisms, under standard storage conditions. The product maintained good microbial quality for 60 days, indicating that it is well-suited for short-term uses such as the early stages of space missions. With further shelf-life studies and improved packaging to limit oxygen and moisture, it may be possible to extend stability for the longer durations required in space.48
In terms of its potential for use in space missions, the freeze-dried millet-based yogurt mix offers several advantages over traditional food preservation methods, including reduced weight, extended shelf life, and ease of rehydration. These characteristics make it an ideal candidate for space food systems where storage space and long-term sustainability are critical. The combination of finger millet's nutritional value and strawberries' sensory appeal further elevates the product's potential acceptance by astronauts, who require a balanced and appealing diet to maintain optimal health during extended missions.
The inclusion of strawberry pulp enhanced colour, aroma, and taste while masking the inherent earthy flavour of millet, thereby improving overall acceptability. Probiotic viability remained above 107 CFU g−1 during ambient storage, meeting FAO/WHO functional food standards. These findings indicate the product's suitability for space missions requiring nutrient-dense, shelf-stable, and culturally familiar foods. Future work should assess nutrient bioavailability, sensory perception, and microbial stability under actual microgravity conditions.
This proof-of-concept study demonstrates that a freeze-dried finger millet and strawberry yogurt mix maintains fungal stability and cytotoxic safety over 60 days of storage. Bacterial proliferation remains a limitation, indicating the need for further optimization for long-duration space missions. Beyond space applications, such formulations may also be valuable in terrestrial contexts where refrigeration is unavailable, providing a convenient and safe functional food option.
Conflicts of interest
The authors have no conflicts of interest to declare.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Due to institutional and proprietary restrictions, certain experimental protocols and raw datasets cannot be made publicly available.References
- A. J. Taylor, J. D. Beauchamp, L. Briand, M. Heer, T. Hummel and C. Margot, et al., Factors affecting flavor perception in space: does the spacecraft environment influence food intake by astronauts?, Compr. Rev. Food Sci. Food Saf., 2020, 19, 3439–3475 CrossRef PubMed.
- P. Patil, S. P. Singh and P. Patel, Functional properties and health benefits of finger millet (Eleusine coracana L.): a review, J. Phytopharm., 2023, 12(3), 196–202 CrossRef CAS.
- B. S. Kim, A. V. Alcantara, J. H. Moon, A. Higashitani, N. Higashitani and T. Etheridge, et al., Comparative Analysis of Muscle Atrophy during Spaceflight, Nutritional Deficiency and Disuse in the Nematode Caenorhabditis elegans, Int. J. Mol. Sci., 2023, 24(16), 12640 CrossRef CAS.
- W. E. Radstake, B. Baselet, S. Baatout and M. Verslegers, Spaceflight Stressors and Skin Health, Biomedicines, 2022, 10, 364 CrossRef CAS PubMed.
- E. Afshinnekoo, R. T. Scott, M. J. MacKay, E. Pariset, E. Cekanaviciute and R. Barker, et al., Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration, Cell, 2020, 183, 1162–1184 CrossRef CAS PubMed.
- T. A. Nickerson, S. T. Coulter and R. Jenness, Some Properties of Freeze-Dried Milk, J. Dairy Sci., 1952, 35(1), 77–85 CrossRef CAS.
- S. Dyankova, M. Doneva, A. Solak, D. Miteva, I. Nacheva, K. Loginovska, et al., Physicochemical studies and electrophoretic profile of freeze-dried quail eggs, in BIO Web of Conferences, EDP Sciences, 2024 Search PubMed.
- C. Gonzalez Viejo, N. Harris and S. Fuentes, Assessment of changes in sensory perception, biometrics and emotional response for space exploration by simulating microgravity positions, Food Res. Int., 2024, 175, 113827 CrossRef PubMed.
- G. Tripathi, P. H. Jitendrakumar, A. Borah, D. Nath, H. Das and S. Bansal, et al., A Review on Nutritional and Health Benefits of Millets, Int. J. Plant Soil Sci., 2023, 35(19), 1736–1743 CrossRef.
- C. C. Kuo, D. Chen, R. Jiménez-Flores, M. Wick and O. Campanella, Valorization of byproducts from meat and dairy industries through fermentation to produce peptides, Sustainable Food Technol., 2024, 1469–1475 RSC.
- R. Evans, Space food packaging: a review of its past, present and future materials and technologies, Packag. Technol. Sci., 2023, 36, 617–627 CrossRef.
- R. S. Samakradhamrongthai, T. Jannu and G. Renaldi, Physicochemical properties and sensory evaluation of high energy cereal bar and its consumer acceptability, Heliyon, 2021, 7(8), e07776 CrossRef CAS PubMed.
- D. Donno, G. Neirotti, A. Fioccardi, Z. R. Razafindrakoto, N. Tombozara and M. G. Mellano, et al., Freeze-Drying for the Reduction of Fruit and Vegetable Chain Losses: A Sustainable Solution to Produce Potential Health-Promoting Food Applications, Plants, 2025, 14(2), 168 CrossRef CAS PubMed.
- A. Vagdevi, S. Trupthi, M. Pandey, N. B. Reddy and M. Singh, Nutritional qualities, processing and health benefits of finger millet (Eleusine coracana L.), The Pharma Innovation Journal, 2023, 12(1), 1291 CAS.
- P. Pramanik and S. Tewari, The Utilisation of Space Foods and Their Detriments: An Extensive Review, Chronicle of Aquatic Science, 2023, 1(7), 1–9 Search PubMed.
- M. KIlinç, G. Akarca and A. J. Denizkara, Effect of Lyophilised Sweet Potato on the Quality and Nutritional Characteristics of Set Yogurt, Food Sci. Nutr., 2025, 13(3), e70111 CrossRef.
- Y. Chen, J. Zhang, B. Zhang, H. K. Lee, S. Xie and W. Shen, et al., Optimizing drug sensitivity assays in patient-derived tumor organoids: a comparison of IC50 estimation methods and experimental parameters, Biol. Methods Protoc., 2025, 10(1), bpaa106 Search PubMed.
- T. D. Nguyen, T. T. X. Vo and K. N. Tran, Optimization of pasteurization process of the ready-to-drink beverage from Hong Quan (Flacourtia jangomas) fruit by response surface methodology, J. Appl. Biol. Biotechnol., 2024, 12(1), 86–92 CAS.
- L. Esposito, N. Casolani, M. Ruggeri, U. G. Spizzirri, F. Aiello and E. Chiodo, et al., Sensory Evaluation and Consumers’ Acceptance of a Low Glycemic and Gluten-Free Carob-Based Bakery Product, Foods, 2024, 13(17), 2815 CrossRef CAS PubMed.
- A. K. Pal, A. Agarwal, A. D. Tripathi, K. K. Darani, A. Nandan and A. Tripathi, et al., The Potential of Night Jasmine (Nyctanthes arbor-tristis) Flower Extract as a Functional Ingredient in Yogurt Production: The Effects on Fermentation, Rheology, Sensory, and Antioxidant Properties of Yogurt, EuroBiotech Journal, 2025, 9(1), 107–116 CrossRef.
- J. C. Condon and L. M. Durso, Detection of Shigatoxigenic E. coli O157: H7 & Enumeration of Fecal Indicators SOP, Protocols.io, 2025 Search PubMed.
- J. Zhu, P. Chu and X. Fu, Understanding the development of bacterial colony: physiology, new technology, and modeling, Quant. Biol., 2025, 13(4), e95 CrossRef.
- T. Mossman, Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays, J. Immunol. Methods, 1983, 65, 55–63 CrossRef PubMed.
- OECD, OECD Regulatory Policy Outlook 2021, OECD Publishing, Paris, 2021, DOI:10.1787/38b0fdb1-en.
- S. Tiwari, S. Pradhan, V. Shukla and A. Lokur, Validation of Portable Culture Device for Enumerating Total Viable Count from Food Samples, J. Adv. Sci. Res., 2025, 16(02), 06–14 Search PubMed.
- S. O. Ajoseh, C. O. Fakorede, R. O. Abegunrin, C. O. Sodipo, A. O. Lawal-Sanni, W. O. Salami and K. O. Akinyemi, Antimicrobial susceptibility pattern of Salmonella isolates from clinical, environmental and food sources in Lagos, Nigeria, African Journal of Clinical and Experimental Microbiology, 2025, 26(2), 182–191 CrossRef.
- B. A. Shankar, V. Arora, M. K. Yadav, M. Kumar, B. Singh and V. Burman, Qualitative Analysis and Statistical Evaluation of Curcumin Content and Total Phenolic Compounds in Distinct Turmeric (Curcuma longa L.) Germplasms, Int. J. Environ. Clim. Change, 2022, 2076–2085 CrossRef.
- G. Nakov, N. Ninova-Nikolova, N. Ivanova, V. Raykova, B. Trajkovska and M. L. Čolić, et al., Yogurt Enriched with Chia Seeds: Physicochemical, Microbiological, and Sensory Changes during Storage, Fermentation, 2024, 10(8), 431 CrossRef CAS.
- M. Ziarno, D. Zaręba, E. Kowalska and T. Florowski, A Study into the Effects of Chosen Lactic Acid Bacteria Cultures on the Quality Characteristics of Fermented Dairy, Dairy–Oat, and Oat Beverages, Appl. Sci., 2025, 15(7), 3714 CrossRef CAS.
- G. Gusnedi, N. A. Habibi, W. Sartika, Darwela and C. T. Utami, A functional drink combining dadih and yoghurt from cow's milk using response surface methodology, BIO Web of Conferences, 2025, vol. 153, p. 03010 Search PubMed.
- R. R. E. Tay, T. Agatha, G. Somang, O. Yuliarti and E. L. L. Tan, Structuring wheat flour-based crackers using whey protein isolate, Int. Dairy J., 2022, 128, 105314 CrossRef.
- P. Suriwong, K. Thinkohkaew, C. Visuthranukul, T. Chavarnakul, P. Potiyaraj and I. Suppavorasatit, Effect of cocoa powder on the physicochemical, microbial, and sensory properties of synbiotic freeze-dried yogurt, J. Agric. Food Res., 2025, 19, 101589 CAS.
- C. Ibourahema, K. Kouamé, K. A. Kouassi, F. F. I. Stéphane, G. M. V. Carole and N. Guessennd, et al., Evaluation of Bacterial Contamination Risk in Milk Preparations and Antibiotic Resistance Patterns in the Neonatal Unit of Treichville University Hospital, Côte d'Ivoire, Int. J. Biochem. Res. Rev., 2025, 34(3), 159–171 CrossRef.
- Y. Palanisamy, V. Kadirvel and N. D. Ganesan, Recent technological advances in food packaging: sensors, automation, and application, Sustainable Food Technol., 2024, 161–180 Search PubMed.
- M. T. Islam, M. S. Rahman, S. R. Chowdhury, T. Chakrovarty, S. M. Kador and M. M. Islam, et al., Microbial diversity, functional attributes, and nutritional proficiency of yogurts produced in Bangladesh, Discover Food, 2025, 5(1), 28 CrossRef CAS.
- H. M. Jaffar, S. Ambreen, F. Al-Asmari, I. Hussain, M. A. Rahim and M. Fawzy Ramadan, et al., Antioxidant activity, microbial viability, and sensory attributes of traditional-yogurt enriched with silymarin, Cogent Food Agric., 2024, 10(1), 2417838 CrossRef.
- F. O. Adeboyejo, O. R. Aderibigbe, F. O. Ojo and S. A. Fagbemi, Changes in quality parameters and microbial stability of hog plum (Spondias mombin Linn.) juice during ambient and refrigerated storage, Nutr. Food Sci., 2022, 52(6), 958–970 CrossRef.
- A. A. Atallah, E. A. Ismail, H. M. Yehia, M. F. Elkhadragy, A. S. Aloufi and D. G. Gemiel, Physicochemical, Microbiological and Microstructural Characteristics of Sucrose-Free Probiotic-Frozen Yogurt during Storage, Foods, 2022, 11(8), 1099 CrossRef CAS PubMed.
- H. Li, T. Liu, J. Yang, R. Wang, Y. Li and Y. Feng, et al., Effect of a microencapsulated synbiotic product on microbiology, microstructure, textural and rheological properties of stirred yogurt, LWT--Food Sci. Technol., 2021, 1, 152 Search PubMed.
- N. Mongkontanawat and T. Khunphutthiraphi, Improved sensory acceptance and cytotoxicity to breast cancer cell line of instant germinated black rice yogurt supplemented with gellan gum, Research Article Science Technology and Engineering Journal, 2022, 8, 15–29 Search PubMed.
- V. Bulgaru, A. Gurev, A. Baerle, V. Dragancea, G. Balan and D. Cojocari, et al., Phytochemical, Antimicrobial, and Antioxidant Activity of Different Extracts from Frozen, Freeze-Dried, and Oven-Dried Jostaberries Grown in Moldova, Antioxidants, 2024, 13(8), 890 CrossRef CAS PubMed.
- J. R. Sheehan, A. S. de Wijn, T. S. Freire and R. Friedman, Beyond IC50—a computational dynamic model of drug resistance in enzyme inhibition treatment, PLoS Comput. Biol., 2024, 20, e1012570 CrossRef PubMed.
- J. Noor Fathima, L. Govindaraju, G. Jeevanandan, P. C. Maganur, S. Vishwanathaiah and A. A. Assiry, et al., Evaluation of the Cytotoxicity of a Newly Developed Obturating Material for Pulpectomy in Primary Teeth Using Embryonic Toxicology, Brine Shrimp Lethality, and MTT Assay: An In Vitro Study, European Journal of Dentistry, 2025, 2417838 Search PubMed.
- B. L. McGovern, and K. White, Cytotoxicity Screening Assay-Paired with Antiviral Assays, 2025 Search PubMed.
- E. B. Postnikov, A. V. Sychev and A. I. Lavrova, Dose–Response Curve in REMA Test: Determination from Smartphone-Based Pictures, Analytica, 2024, 5(4), 619–631 CrossRef.
- H. P. Piepho, A. Onofri and W. A. Malik, Evaluating several dose-response curves with a common zero level, Agric. Biosci., 2025, 6(1), 0009 CrossRef.
- K. U. López-Mártir, J. A. Ulloa, J. E. Urías-Silvas, P. Rosas-Ulloa and B. E. Ulloa-Rangel, High-intensity ultrasound affects the physicochemical, structural and functional properties of proteins recovered from noni (Morinda citrifolia) seeds, Sustainable Food Technol., 2025, 700–713 RSC.
- A. Baumgart, E. I. Vlachopoulou, J. D. R. Vera and S. Di Pippo, Space for the Sustainable Development Goals: Mapping the Contributions of Space-Based Projects and Technologies to the Achievement of the 2030 Agenda for Sustainable Development, Sustainable Earth, 2021, 4(1), 6 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |