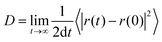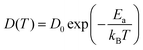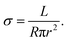Nitrogen-triggered amorphization enables high-performance solid-state electrolytes
Bolong
Hong
abc,
Lei
Gao
 *d,
Bingkai
Zhang
*d,
Bingkai
Zhang
 e,
Pengfei
Nan
f,
Ruishan
Zhang
e,
Yuhang
Li
g,
Zhihao
Lei
g,
Ming
Liu
e,
Pengfei
Nan
f,
Ruishan
Zhang
e,
Yuhang
Li
g,
Zhihao
Lei
g,
Ming
Liu
 g,
Jing
Wu
h,
Longbang
Di
d,
Haijin
Ni
c,
Songbai
Han
g,
Jing
Wu
h,
Longbang
Di
d,
Haijin
Ni
c,
Songbai
Han
 *c and
Jinlong
Zhu
*c and
Jinlong
Zhu
 *ac
*ac
aDepartment of Physics, Southern University of Science and Technology, Shenzhen 518055, China. E-mail: zhujl@sustech.edu.cn
bCollege of Semiconductors (National Graduate College for Engineers), Southern University of Science and Technology, Shenzhen 518055, China
cShenzhen Key Laboratory of Solid State Batteries, Guangdong Provincial Key Laboratory of Energy Materials for Electric Power, Guangdong-Hong Kong-Macao Joint Laboratory for Photonic Thermal-Electrical Energy Materials and Devices, Southern University of Science and Technology, Shenzhen 518055, China
dSchool of Advanced Materials, Peking University, Shenzhen Graduate School, Shenzhen 518055, China
eSchool of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, 510006, China
fInformation Materials and Intelligent Sensing Laboratory of Anhui Province, Leibniz International Joint Research Center of Materials Sciences of Anhui Province, Institutes of Physical Science and Information Technology, Anhui University, Hefei 230601, China
gShenzhen Geim Graphene Center, Tsinghua University, Shenzhen International Graduate School, Shenzhen 518055, China
hCryo-EM Center, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China
First published on 19th November 2025
Abstract
Amorphous solid-state electrolytes (SSEs) hold great promise for advancing the application of all-solid-state batteries (ASSBs), owing to their favorable ionic conductivity, structural tunability, and promising electrochemical performance. However, the absence of universal design principles for amorphous SSEs limits their development. By fundamentally re-evaluating the amorphization ability of amorphous SSE systems, this study establishes a nitrogen-driven universal strategy to convert diverse metal chlorides into amorphous xLi3N–MCly (0.3 ≤ 3x ≤ 1.9; M denotes a metal element; 2 ≤ y ≤ 4) SSEs. Nitrogen synergistically disrupts crystalline order via distorted coordination polyhedra and N-bridged networks, while dynamic bond reorganization enables rapid Li+ migration, achieving an ionic conductivity of 2.02 mS cm−1 for 0.533Li3N–HfCl4 at 25 °C. Structure–property relationships reveal that high charge density and bridging capability of N3− enhance network disorder, shorten metal-coordinating atom distances, and optimize Li+ diffusion pathway connectivity. ASSBs employing 0.533Li3N–HfCl4 retain 81.87% capacity after 2000 cycles at 1000 mA g−1 with high cathode loading (6.24 mg cm−2), demonstrating engineering viability. This work provides a paradigm for rational design of high-performance amorphous SSEs.
Broader contextThe development of all-solid-state batteries relies critically on the development of high-performance solid-state electrolytes (SSEs). Recently reported amorphous Cl-based and dual-anion-based SSEs exhibit combined properties of high ionic conductivity, broad electrochemical windows, softness, and easy fabrication, demonstrating significant application potential. However, the lack of universal synthesis strategies and a limited understanding of Li+ transport mechanisms have hindered their advancement. This work systematically reveals the intrinsic relationship between composition and amorphous formation ability (AFA), confirming that AFA is jointly determined by the bonding characteristics of network formers and the charge density of bridging anions. Establishing a universal N-driven strategy has been demonstrated to successfully transform a series of metal chlorides into amorphous xLi3N–MCly (0.3 ≤ 3x ≤ 1.9; M denotes a metal element; 2 ≤ y ≤ 4) SSEs, achieving ionic conductivities of up to 2.02 mS cm−1 (0.533Li3N–HfCl4). Furthermore, a Li+ transport mechanism involving synergistic “polymer-like” dissociation and “dynamic monkey-bar” hopping was proposed and elucidates the structure–property relationship in xLi3N–MCly SSEs. This research provides important insights for the design of novel amorphous SSEs. |
Introduction
The pursuit of high-performance solid-state electrolytes (SSEs) is a cornerstone in the development of next-generation all-solid-state batteries (ASSBs) featuring high safety and energy density.1,2 In the past decade, inorganic crystalline SSEs have attracted significant research attention owing to their promising properties. However, intrinsic limitations, including grain boundary resistance, mechanical deformability, interface compatibility, and scalable synthesis, continue to hinder their applications.3–5 For instance, crystalline oxide-based SSEs (e.g., garnet and NASICON-type) are limited by high synthesis temperatures, high grain boundary impedance, and poor wettability by Li metal.6 Although sulfide-based SSEs (e.g., Li10GeP2S12 and Li6PS5Cl) present ionic conductivities comparable to those of liquid electrolytes, the narrow electrochemical stability windows and poor stability against moisture in the air limit their applications in ASSBs.7Recent advances in amorphous SSEs offer a promising alternative to overcome the limitations hindering the development of ASSBs.7,8 Specifically, amorphous SSEs feature isotropic ion transport without grain boundary constraints,9,10 leading to higher conductivity and lower temperature sensitivity.11 In terms of interfacial compatibility, their low interfacial heterogeneity favors compatibility with electrodes.12 Moreover, the absence of grain boundaries contributes to the higher density, improved mechanical strength, and better processability of amorphous SSEs.13 Still, fundamental limitations persist across a wide range of amorphous SSE systems, despite recent notable progress in their development. Sulfide-based amorphous SSEs (e.g., Li2S–P2S5 glasses) achieve exceptional ionic conductivities (>1 mS cm−1 at 25 °C) via disordered thiophosphate networks, but their narrow electrochemical stability (<3.5 V vs. Li+/Li) hinders integration with high-voltage cathodes.14 Oxide amorphous systems (e.g., Li3BO3–Li2SO4 glasses) exhibit enhanced oxidative stability (>4.0 V) and mechanical robustness,15 yet their ionic conductivities remain below 0.1 mS cm−1 at room temperature (RT).
Recently, a new class of amorphous SSEs, including Cl-based and dual-anion-based systems (labeled as LixA–MCly, where x = 1, 2, 3; A = Cl, O, N; M denotes a metal element; y = 2, 3, 4, 5), has attracted considerable interest due to their structural diversity and promising electrochemical properties (Fig. 1).7,16 During synthesis, ion exchange occurs between lithium-containing precursors and metal chlorides at specific molar ratios, resulting in the distortion of coordination polyhedra and the formation of a disordered network. This configuration generates an amorphous structure with fast Li+ diffusion channels, leading to enhanced performance compared with crystalline chloride-based SSEs.17 For instance, in O–Cl based SSEs (e.g., xLi2O–ZrCl413,18 and xLi2O–TaCl519), diverse polyhedral units like [ZrOaClb](2a+b−4)− (0 ≤ b/a < 6) are connected randomly via bridging O/Cl into a corner-sharing disorder network. This inherent disorder creates a distorted Li-site environment and abundant terminal Cl−, enabling rapid Li+ migration through providing collective diffusion pathways and lowering the energy barriers, which collectively lead to breakthrough ionic conductivities (>1 mS cm−1 at 25 °C). However, such strategies remain limited to a narrow subset of metal chlorides (e.g., TaCl5, NbCl5, ZrCl4, and HfCl4).12,19,20 In addition, in our recent work a new amorphous N–Cl based SSE was identified, in which 0.417Li3N–TaCl5 exhibited a high ionic conductivity of 7.34 mS cm−1 at 30 °C and retained performance at low temperature.11,21 Critically, although new amorphous SSEs continue to be reported, the synthesis and formation principles of amorphous halides and dual-anion based SSEs, as well as the specific mechanism of Li+ migration, remain to be fully established.22 A key challenge is the lack of a universal methodology for systematically synthesizing amorphous SSEs and establishing a systematic framework for the design of amorphous SSEs. Large-scale systematic modeling is also essential for unveiling the mechanisms underlying the formation of amorphous SSEs and providing profound insights into Li+ transport dynamics. This understanding will be critical for a rational design and synthesis of next-generation high-performance amorphous SSEs.
 | ||
| Fig. 1 Roadmap for the recent advancement of amorphous LixA–MCly (x = 1, 2, 3; A = Cl, O, N; M denotes a metal element; y = 2, 3, 4, 5) SSEs synthesized by high energy ball milling. SSE compositions and conductivity data are from this work and prior literature.18,20,21,23 Ball milling time data for 0.5Li2O–TaCl5 are from Fig. S1, while other data are from the literature.11 The formation energy data were obtained from the Materials Project database.24 | ||
Here, we unveil an amorphization mechanism in LixA–MCly SSEs driven by covalent interactions and bridging structures (Fig. 1), and establish a universal N-driven strategy for the formation of amorphous xLi3N–MCly SSEs (0.3 ≤ 3x ≤ 1.9; M denotes a metal element; 2 ≤ y ≤ 4). Benefiting from the high amorphization ability (AFA) of the xLi3N–MCly, N3− bridged networks induce structural disorder and enable fast Li+ migration through dynamic bond reorganization, achieving ionic conductivities of up to 2.02 mS cm−1 (0.533Li3N–HfCl4). Based on the ionic conductivity trends of a series of amorphous xLi3N–MCly SSEs, the structure–property relationship of amorphous SSEs is comprehensively investigated. In addition, ASSBs with the configuration of Li–In|Li5.5PS4.5Cl1.5|0.533Li3N–HfCl4|LiNi0.83Mn0.05Co0.12O2 employing high-loading cathodes (6.24 mg cm−2) exhibit a capacity retention of 81.87% after 2000 cycles at a high current density of 1000 mA g−1. This approach of nitrogen-driven amorphization defines a universal design principle for high-performance amorphous SSEs.
Results and discussion
Role of network modifiers and formers in facilitating amorphization
High-energy ball milling is currently the primary method for preparing amorphous SSEs, leveraging localized mechanical stress and thermal effects to drive particle comminution and solid-phase reactions25,26 (Fig. S2). However, the complex interplay of stress, heat, and atomic interactions within the milling process makes it challenging to observe and investigate the mechanisms of amorphous phase formation in real time. Despite these challenges, well-defined starting materials and synthesis conditions enable systematic studies of amorphization mechanisms through comparative analysis. Theoretical frameworks, notably the continuous random network (CRN) model proposed by Zachariasen,27 elucidate the structural principles governing amorphous phase formation, emphasizing the need for a continuous disordered atomic network for glass structures. This model highlights the critical role of AFA or glass-forming ability, which is defined as the ease with which a material system transforms to an amorphous state.28 Fundamentally, AFA depends on the contributions of amorphous network formers and modifiers in stabilizing the disordered structure.29AFA plays a vital role in the design and synthesis of high-performance amorphous SSEs. However, the understanding of AFA in the formation of amorphous LixA–MCly systems remains insufficiently explored. During ball milling synthesis of LixA–MCly SSEs, metal chlorides dissociate lithium-containing precursors and release free Li+ ions.30 Concurrently, anions (from LixA) are incorporated into the metal chloride, forming coordination polyhedra, such as [TaCl6]− in LiCl–TaCl5.20,31 Within the resultant amorphous network, Li+ ions function as network modifiers that disrupt lattice symmetry and confer ionic conductivity to the structure, while these coordination polyhedra act as network formers that establish the continuous and disordered framework.32 As fundamental building units of the disordered network, the bonding nature of the network formers is a core factor influencing AFA. When comparing covalent and ionic bonds, which are commonly present in SSEs, structures dominated by covalent bonds exhibit significantly stronger AFA.33 This is primarily due to the tendency of covalent compounds to form three-dimensional network structures, such as SiO2 glass. In such a system, the Si–O bonds prefer corner-sharing tetrahedral configurations, and the random distortions in Si–O–Si bond angles promote structural disorder.34 Furthermore, covalent bonds readily form long chains or extended networks (e.g., polymers), whose inherent folding and bending characteristics easily induce long-range disorder, which explains the strong AFA typically observed in polymers.35Fig. 1 indicates that Cl-based amorphous LiCl–MCly SSEs are rare, and tend to form only in the presence of covalent pentavalent metal chlorides,36 such as TaCl5, which act as starting materials. The covalent Ta–Cl bonds provide a critical bonding framework for the formation of the network former [TaCl6]−. In contrast, the tetravalent metal chloride HfCl4 exhibits more ionic character, attributed to the lower electronegativity of Hf. Consequently, the xLiCl–HfCl4 system possesses weaker AFA, leading to the formation of crystalline Li2HfCl6.37 Similarly, ionic crystals such as YCl3 and MgCl2 resist amorphization upon milling with LiCl,38,39 as their highly ordered ionic coordination creates stable crystalline structures that hinder the long-range disorder required for amorphous phases.33
Moreover, introducing high charge density bridging anions (e.g., O2−) into metal chlorides offers a strategy to enhance the AFA of the SSE system. These anions distort metal-centered coordination polyhedra and bridge multiple polyhedral units, thereby generating diverse network formers (e.g., [TaCl5−aOa]a−; 1 ≤ a < 5) that stabilize amorphous structures.19 In particular, the number of O–Cl dual-anion amorphous SSEs is twice that of chloride-based SSEs (Fig. 1). The formation of amorphous xLi2O–HfCl4/ZrCl4 SSEs relies on the synergistic effect between the residual covalency in Hf/Zr–Cl bonds and the oxygen bridging, which extends to tetravalent chlorides but not ionic trivalent chlorides, such as YCl3 and LaCl3. For instance, ball milling Li2O with YCl3 forms crystalline Li3YCl3O1.5 with low Li+ conductivity.18 The AFA imparted by a network former is governed by the charge density of its bridging anion. Bridging anions with higher charge density for bonding can concurrently coordinate more metal centers and generate more complex network formers, thereby imparting stronger AFA to the system while requiring fewer such bridging anions. Consequently, O2− does not have sufficient bridging capability to induce amorphization in ionic divalent/trivalent metal chlorides. Interestingly, our prior work demonstrated that N3− (in Li3N), possessing higher charge density, readily forms amorphous 0.33Li3N–LaCl3 SSEs when milled with ionic LaCl3,21 indicating that the xLi3N–MCly system has strong AFA. Furthermore, when TaCl5 and lithium-containing precursors are used as the starting materials, the Li3N can significantly improve the efficiency of amorphous SSEs compared with LiCl or Li2O (Fig. 1).11 This is due to the higher formation energy of Li3N, which allows for easier decomposition and efficient ion exchange with TaCl5, with the assistance of high energy ball milling to cross its kinetic energy barrier, thus accelerating the formation of the amorphous phase.20
Taken together, both theoretical analysis and experimental results demonstrate the dual advantages of xLi3N–MCly systems: its superior AFA, driven by the high charge density N3− bridging, and its facile synthesis, enabled by high formation energy of Li3N (Fig. 1). These attributes suggest the potential for developing a series of novel N–Cl amorphous SSEs through the incorporation of Li3N into various metal chlorides, including ionic chlorides that are typically challenging to be amorphized. This N-driven synthesis approach offers a valuable material platform for conducting a systematic and in-depth study of the relationship between the performance and composition of amorphous SSEs.
Nitrogen-driven synthesis of amorphous solid-state electrolytes
By employing the electronegativity of the metal element (e.g., Mg, Y, and Zr) in MCly as a key indicator, the AFA of the xLi3N–MCly system was evaluated (Fig. S3). Furthermore, to investigate the AFA of the xLi3N–MCly system, Li3N and a range of metal chlorides with varying ionic characteristics (e.g., HfCl4, CeCl3, and MgCl2) were selected as starting materials through the high energy ball milling synthesis of amorphous xLi3N–MCly SSEs. Unlike other amorphous SSEs, formed via extended ball milling, such as those in LiCl–TaCl5 systems20,23 or hazardous gas-generating reactions in LiOH–ACl5 (A = Ta, Nb)40 and MAlCl4−2xOx (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li, Na)41 routes, the N-driven protocol developed in this work efficiently converts diverse metal chlorides into amorphous SSEs. Various metal chlorides were successfully employed, including TaCl5, HfCl4, ZrCl4, CeCl3, LaCl3, YCl3, MgCl2, and MnCl2 (Fig. 1), with over 30 theoretically compatible candidates identified (Fig. S3). Remarkably, pentavalent metal chlorides (e.g., TaCl5) exhibit intrinsic amorphization tendencies with diverse lithium-containing precursors due to their high covalent character (Fig. 1).23,42 However, the inherent characteristic obscures the individual roles of the bridging function and the covalency in AFA. Based on this fact, to more clearly investigate the attribution of the amorphous phase formation to N-mediated coordination engineering, we focus on MCly (M = metal elements, 2 ≤ y ≤ 4) systems with inherently ionic character.
Li, Na)41 routes, the N-driven protocol developed in this work efficiently converts diverse metal chlorides into amorphous SSEs. Various metal chlorides were successfully employed, including TaCl5, HfCl4, ZrCl4, CeCl3, LaCl3, YCl3, MgCl2, and MnCl2 (Fig. 1), with over 30 theoretically compatible candidates identified (Fig. S3). Remarkably, pentavalent metal chlorides (e.g., TaCl5) exhibit intrinsic amorphization tendencies with diverse lithium-containing precursors due to their high covalent character (Fig. 1).23,42 However, the inherent characteristic obscures the individual roles of the bridging function and the covalency in AFA. Based on this fact, to more clearly investigate the attribution of the amorphous phase formation to N-mediated coordination engineering, we focus on MCly (M = metal elements, 2 ≤ y ≤ 4) systems with inherently ionic character.
XRD patterns of xLi3N–MCly (0.3 ≤ 3x ≤ 1.9; M = Hf, Ce, Mg; 2 ≤ y ≤ 4) shown in Fig. S4, along with representative 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4 in Fig. 2a–c, all exhibit amorphous features, contrasting with the crystalline SSEs (Li2MgCl4, LiCeCl4, and Li2HfCl6) that display sharp diffraction features. Subsequently, as representative cases, we investigated the synthesis process of xLi3N–MCly, focusing on ball-milling conditions (Fig. S5). The results confirm that 0.1Li3N–MgCl2 and 0.533Li3N–HfCl4 reach the highest degree of amorphization at 30 h, while 0.3Li3N–CeCl3 achieves and maintains complete amorphization from as early as 10 h up to 30 h. Also, this amorphous feature is observed in other N–Cl based SSEs—including 0.1Li3N–MnCl2, 0.3Li3N–YCl3, and 0.533Li3N–ZrCl4—as evidenced by the lack of sharp diffraction peaks in XRD profiles (Fig. S6). Notably, the amorphous SSEs derived from MgCl2 and CeCl3 represent the first reported instances in their respective material classes, expanding the compositional landscape of amorphous halide SSEs (Fig. 1). Cryo-transmission electron microscopy (Cryo-TEM) further supports these findings, as 0.3Li3N–CeCl3 and 0.533Li3N–HfCl4 reveal disordered lattice structures and lacking crystalline fringes. The corresponding fast Fourier transform (FFT) patterns indicate the absence of diffraction spots (Fig. 2d). 0.1Li3N–MgCl2 exhibits minimal residual crystallinity manifested as sporadic diffraction spots, likely attributable to the low formation energy of MgCl2 (Fig. S7). This thermodynamic stability limits its interaction with Li3N, imposing kinetic barriers to complete amorphization.20 The chemical homogeneity of these phases is further confirmed by 7Li solid-state NMR spectra (Fig. 2e), which show a single Lorentzian peak, excluding residual lithium-containing impurities or structural inhomogeneities. Obviously, the AFA of the N-mediated strategy surpasses that of O- or Cl-based approaches, enabling the amorphization of a broader range of metal chlorides (Fig. 1 and Fig. S3). In addition, similar to other reported amorphous SSEs, 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4 exhibit good compactness. Scanning electron microscopy (SEM) images of cold-pressed pellets at ∼500 MPa (Fig. 2f and Fig. S8) reveal smooth surfaces with minimal void formation. Complementary X-ray computed tomography (CT) analysis (Fig. 2g and h) quantifies the three-dimensional porosity as 9.4% for 0.1Li3N–MgCl2, 10.0% for 0.3Li3N–CeCl3, and 7.9% for 0.533Li3N–HfCl4, all notably lower than that of Li3InCl6 (∼18%) and Li6PS5Cl (∼15%).11
 | ||
| Fig. 2 Synthesis and structure analysis of amorphous xLi3N–MCly SSEs. (a–c) XRD patterns of representative 0.1Li3N–MgCl2 and Li2MgCl4 (a), 0.3Li3N–CeCl3 and LiCeCl4 (b), 0.533Li3N–HfCl4 and Li2HfCl6 (c). The structural data for the crystalline SSEs were obtained from the ICSD and OQMD databases.43,44 (d) Cryo-TEM images and corresponding FFT patterns of amorphous 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4. (e) 7Li solid-state NMR spectra of the amorphous 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4. (f) SEM images of cold-pressed xLi3N–MCly SSEs pellets. (g) 3D volume-rendered images from an XCT scan. (h) Porosity analyzed by XCT for 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4. | ||
Decoding the role of nitrogen in coordination modification and bridging function
To further elucidate the promoting role of nitrogen in amorphous phase formation, we conducted a comprehensive analysis of the structural characteristics and amorphization mechanisms of xLi3N–MCly SSEs. Fig. 3a presents the Raman spectra of xLi3N–MCly SSEs alongside those of the corresponding crystalline metal chlorides. In crystalline MgCl2, a sharp peak at 239.3 cm−1 reflects well-defined metal–Cl lattice vibrations, arising from its long-range translational symmetry. Similarly, crystalline CeCl3 exhibits distinct peaks at 101.1, 183.2, and 208.6 cm−1, and HfCl4 shows peaks at 79.0, 104.7, 121.2, 144.9, 284.3, and 389.7 cm−1, all indicative of ordered metal–Cl vibrational modes characteristic of their crystalline lattices.12,45,46 Upon Li3N incorporation, these sharp peaks broaden into diffuse bands, signaling a loss of long-range periodicity. This peak broadening reflects increased structural disorder, with a diversification of short-range coordination environments, bond angles, and lengths, resulting in a broader distribution of vibrational frequencies.47,48 In amorphous 0.3Li3N–CeCl3 and 0.533Li3N–HfCl4, new broad features emerge at 300–500 cm−1 and 400–650 cm−1 (Fig. S9), respectively, reflecting metal–N coordination formation,49,50 while 0.1Li3N–MgCl2 lacks these signals, due to fluorescence interference obscuring the Mg–N vibrational signal.Moreover, ab initio molecular dynamics (AIMD) simulations were employed to model the amorphous structure of 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4 (Fig. S10). Experimentally, pair distribution function (PDF) analysis, including partial PDF for M–Cl and M–N distances (M = Mg, Ce, Hf), was conducted to reveal the local coordination structure (Fig. 3b and c). In crystalline LiAMClB (A = 1 or 2, M = Mg, Ce, Hf, B = 4 or 6), the M–Cl pairs correspond to the nearest distances. In contrast, the M–Cl pairs in amorphous SSEs exhibit expanded ranges, reflecting N-induced distortion of coordination geometries and disruption of crystalline periodicity. Specifically, the M–Cl distances range from 2.10 to 3.30 Å in 0.1Li3N–MgCl2, 2.42 to 3.67 Å in 0.3Li3N–CeCl3, and 2.17–3.46 Å in 0.533Li3N–HfCl4, respectively. PDF analysis further underscores the direct bonding interactions between nitrogen and metal cations (Fig. 3c), with nearest distances ranging from 1.9 to 2.32 Å for Mg–N, 2.08 to 2.49 Å for Ce–N, and 1.80 to 2.29 Å for Hf–N. Notably, the characteristic vibrational frequencies observed in Raman spectroscopy correlate well with the interatomic distances obtained from PDF, both reflecting variations in bond lengths.51,52 The metal–N stretching vibrations exhibit a blue shift relative to the metal–Cl peaks in Raman spectroscopy, consistent with the shorter metal–N first-neighbor distances observed in the PDF analysis. Also, the local coordination details of polyhedra in xLi3N–MCly, revealed by AIMD simulations, highlight the role of nitrogen in promoting the formation of amorphous phases (Fig. 3d). In crystalline Li2MgCl4, LiCeCl4, and Li2HfCl6, rigid polyhedral units ([MgCl6]4−, [CeCl8]5−, [HfCl6]2−) maintain fixed coordination numbers. In contrast, their amorphous counterparts, with nitrogen incorporation, form dynamic bridged networks with fluctuating coordination numbers, ranging from 4 to 6 in 0.1Li3N–MgCl2, 5 to 8 in 0.3Li3N–CeCl3, and 4 to 7 in 0.533Li3N–HfCl4 (Fig. S11). In short, Li3N drives amorphization through two synergistic mechanisms. First, by partially substituting Cl, nitrogen introduces coordination asymmetry and forms distorted polyhedra in xLi3N–MCly, disrupting short-range structural periodicity. Second, N3− acts as a bridge, linking adjacent polyhedra via corner- or edge-sharing configurations to generate random amorphous networks. Collectively, Raman, AIMD, and PDF analysis converge on a single conclusion: N3− acts as the key driver, proliferating a wide range of network formers. Its elevated charge density and pronounced polarizability diversify both coordination geometries and inter-polyhedral linkages, generating a highly cross-linked yet aperiodic framework that substantially elevates AFA.
Dynamic Li+ transport in amorphous xLi3N–MCly SSEs.
Ionic conductivities of xLi3N–MCly were evaluated through electrochemical impedance spectroscopy (EIS; Fig. S12, S13, and S14). 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4 exhibit the conductivities of 3.97 × 10−4 mS cm−1, 0.0217 mS cm−1, and 2.02 mS cm−1, respectively, at 25 °C (Fig. 4a). Their electronic conductivities, measured by direct current (DC) methods, are 2.30 × 10−9, 2.30 × 10−9, and 1.19 × 10−8 S cm−1 (Fig. S15), respectively, confirm the dominance of ionic conduction in xLi3N–MCly SSEs. Nyquist and Arrhenius plots for xLi3N–MCly, shown in Fig. S16 and S17, illustrate the temperature dependence of ionic conductivity. The activation energies, derived from the Arrhenius equation σ = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/kBT), where σ denotes ionic conductivity, A the preexponential parameter, Ea the activation energy, T the absolute temperature, and kB the Boltzmann constant, decrease from 0.572 eV in 0.1Li3N–MgCl2 to 0.346 eV in 0.3Li3N–CeCl3 and 0.307 eV in 0.533Li3N–HfCl4 (Fig. 4b). At 1200 K, the mean-squared displacement (MSD) analysis based on AIMD simulations reveals that the Li+ diffusion coefficients are 3.48 × 10−5, 9.33 × 10−5, and 1.37 × 10−4 cm2 s−1 for 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4, respectively, showing a gradual increase consistent with experimental ionic conductivity trends (Fig. 4c). For 0.533Li3N–HfCl4, temperature-dependent diffusion coefficients (Fig. S18) yield an MSD-derived activation energy of 0.335 ± 0.032 eV, closely matching the experimental value of 0.307 eV.
exp(−Ea/kBT), where σ denotes ionic conductivity, A the preexponential parameter, Ea the activation energy, T the absolute temperature, and kB the Boltzmann constant, decrease from 0.572 eV in 0.1Li3N–MgCl2 to 0.346 eV in 0.3Li3N–CeCl3 and 0.307 eV in 0.533Li3N–HfCl4 (Fig. 4b). At 1200 K, the mean-squared displacement (MSD) analysis based on AIMD simulations reveals that the Li+ diffusion coefficients are 3.48 × 10−5, 9.33 × 10−5, and 1.37 × 10−4 cm2 s−1 for 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4, respectively, showing a gradual increase consistent with experimental ionic conductivity trends (Fig. 4c). For 0.533Li3N–HfCl4, temperature-dependent diffusion coefficients (Fig. S18) yield an MSD-derived activation energy of 0.335 ± 0.032 eV, closely matching the experimental value of 0.307 eV.
 | ||
| Fig. 4 Ionic transport properties, structural dynamics, and morphological features of amorphous xLi3N–MCly SSE. (a) Ionic conductivities of amorphous xLi3N–MCly at 25 °C. (b) The activation energies of amorphous xLi3N–MCly. (c) MSD and diffusivities of Li+ in xLi3N–MCly at 1200 K. (d) PDF of Li–Li based on amorphous xLi3N–MCly. (e) Schematic of the N-centered short-range structure in xLi3N–MCly. (f) PDF of Hf–N/O, Hf–Hf, and Cl–Cl based on amorphous N–Cl or O–Cl based SSEs. The PDF data of O–Cl based SSE from the Sun group's previous work.12 (g) Schematic of Li+ diffusion mechanism involving N-bridged networks and variable Cl coordination. | ||
PDF analysis, coupled with structure modeling, elucidates the relationship between local structure and Li+ transport in amorphous xLi3N–MCly SSEs (Fig. 4d–g). First, the disordered network in amorphous xLi3N–MCly supports a “dynamic monkey bar” mechanism,21,31 where Li+ migrates through transient bonding with Cl−. The incorporation of higher-valence cations (e.g., Hf4+ in 0.533Li3N–HfCl4) favors the formation of Cl−-rich amorphous networks, thereby enhancing Li+ mobility by providing more dynamic pathways. Second, Li+ concentration is a key factor in determining ionic conductivity. In the xLi3N–MCly system, a polymer-like dissociation mechanism allows the amorphous matrix to release free Li+ from Li3N.30 Higher-valence cations form more M–N bonds, which facilitate more Li+ release, leading to higher ionic conductivity in 0.533Li3N–HfCl4. Third, shorter distances between neighboring Li sites typically facilitate ion transport by enhancing pathway connectivity. Partial PDF analysis (Fig. 4d) reveals a gradual contraction of Li–Li spacing from 2.99 Å (0.1Li3N–MgCl2) to 2.47 Å (0.3Li3N–CeCl3) and 2.32 Å (0.533Li3N–HfCl4), which aligns with the observed increase of ionic conductivity. Last but not least, the amorphous 0.533Li3N–HfCl4 exhibits a more compact disordered network. PDF analysis (Fig. 4f) shows a shorter Hf–N distance (1.90 Å) compared to Hf–O (2.02 Å),53,54 due to stronger covalent Hf–N bonding.55 The higher bridging capability of N3− also leads to shorter Hf–Hf and Cl–Cl distances, indicating enhanced network densification and a continuous Li+ channel that favors Li+ transportation (Table S1).18,19,37,56–58
High-voltage all-solid-state batteries with unprecedented stability
The amorphous 0.533Li3N–HfCl4 SSE, which shows the highest ionic conductivity of 2.0 mS cm−1 at RT, was selected to evaluate the electrochemical performance in high-voltage ASSBs. These ASSBs demonstrate good interfacial stability, rate capability, and cycling durability under diverse practical conditions. ASSB configurations employing Li–In anodes and high-loading cathodes (LiCoO2 (LCO) and LiNi0.83Mn0.05Co0.12O2 (NCM83), ∼6.24 mg cm−2) demonstrated ultralow area resistance (< 20 Ω cm2 at 25 °C), as confirmed by Nyquist analysis (Fig. 5a), indicative of superior ionic transport kinetics and negligible charge-transfer barriers, indicating favorable application prospects.59 This remarkable performance is attributed to the tailored SSE bilayer design: the amorphous 0.533Li3N–HfCl4 layer establishes intimate contact with oxide cathodes, promoting efficient charge transfer, as evidenced by energy-dispersive spectroscopy (EDS) results showing uniform coating of 0.533Li3N–HfCl4 on the cathode particles surface and effective filling of interparticle voids (Fig. S19), while the Li5.5PS4.5Cl1.5 (LPSC) layer mitigate the incompatibility between 0.533Li3N–HfCl4 and the Li–In anode (Fig. S20 and S21). Rate capability tests underscored outstanding high-current resilience, with the Li–In|LPSC|0.533Li3N–HfCl4|LCO cell delivering a discharge capacity of 135 mAh g−1 after switching from 910 mA g−1 to 28 mA g−1 within a potential range of 2.5–4.2 V vs. Li+/Li (Fig. 5b and c) and the Li–In|LPSC|0.533Li3N–HfCl4|NCM83 cell achieving 180.3 mAh g−1 upon transitioning from 1000 mA g−1 to 40 mA g−1 within a potential range of 2.5–4.3 V vs. Li+/Li (Fig. 5d and e). Long-term cycling tests further highlighted the robustness of the electrolyte, with the LCO-based ASSB retaining 83.29% capacity (105 mAh g−1) after 200 cycles at 140 mA g−1 within a potential range of 2.5–4.2 V vs. Li+/Li, and preserving 84.06% capacity (91.3 mAh g−1) after 300 cycles at 280 mA g−1 (Fig. 5f and g and Fig. S22 and S23). Strikingly, the NCM83 configuration exhibited unparalleled stability at ultrahigh rates of 1000 mA g−1, maintaining discharge capacities of 98.9, 89.0, and 82.7 mAh g−1 after 1000, 2000, and 3000 cycles, respectively, corresponding to 90.98%, 81.87%, and 76.05% capacity retention (Fig. 5h and Fig. S24). Compared to previously reported SSEs19,37,56,57 (Fig. S25 and Table S2), the ASSBs based on amorphous 0.533Li3N–HfCl4 SSE demonstrate distinct advantages in rate capability and long-term cycling stability, underscoring the critical role of the amorphous 0.533Li3N–HfCl4 SSE in enabling robust interfacial dynamics and high ionic conductivity.Conclusions
In conclusion, through an in-depth examination of the AFA of amorphous SSE systems, this study establishes a nitrogen-driven universal strategy. Leveraging the strong bridging ability of N3−, this strategy induces amorphization in multi-component systems and enables the synthesis of diverse amorphous xLi3N–MCly (0.3 ≤ 3x ≤ 1.9; M denotes a metal element; 2 ≤ y ≤ 4) SSEs, achieving ionic conductivities of up to 2.02 mS cm−1 for 0.533Li3N–HfCl4. By combining AIMD simulations with PDF analysis, we reveal a synergistic effect between the “polymer-like” and “dynamic monkey-bar” migration models in governing the ionic transport mechanism. Within the disordered networks of amorphous xLi3N–MCly SSEs constructed by N-bridging, Li+ migrates rapidly through the dynamic breaking and reformation of Li–Cl bonds. The ionic conductivity is cooperatively regulated by the Li+ concentration, Cl− concentration, and the topological compactness of the network. Moreover, both Li–In||LCO and Li–In||NCM83 ASSBs employing the amorphous 0.533Li3N–HfCl4 SSE demonstrate excellent rate capability and long-term cycling stability. This study deepens the fundamental understanding of amorphous SSE formation and ion transport, offering a universal design principle and a theoretical foundation for developing high-performance SSEs. Looking forward, scaling up the synthesis and enhancing the compatibility with Li metal anodes are crucial for the practical application of amorphous xLi3N–MCly SSEs.Experimental
Material synthesis
Amorphous xLi3N–MCly (0.3 ≤ 3x ≤ 1.9; M denotes a metal element; 2 ≤ y ≤ 4) SSEs were synthesized via mechanochemical processing under an argon atmosphere. Li3N (>99.4%, Alfa Aesar) and metal chlorides (HfCl4, ZrCl4, CeCl3, YCl3, MgCl2, MnCl2; >99.9%, Macklin or Aladdin) were mixed in stoichiometric molar ratios corresponding to the target composition. The resulting mixture was loaded into zirconia jars (45 mL) with zirconia milling balls (5–10 mm) at a 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ball-to-powder mass ratio and processed using a planetary ball mill (Fritsch Pulverisette 7). The material was first low-speed ball milling at 100 rpm for 3 hours, followed by high-energy ball milling at 500 rpm for 5–70 hours. The milling protocol employed an intermittent bidirectional rotation pattern, with 15 minutes of counterclockwise rotation, a 5-minute pause, and 15 minutes of clockwise rotation, repeated throughout the process. All handling and milling operations were conducted under continuous argon protection to prevent atmospheric contamination.
1 ball-to-powder mass ratio and processed using a planetary ball mill (Fritsch Pulverisette 7). The material was first low-speed ball milling at 100 rpm for 3 hours, followed by high-energy ball milling at 500 rpm for 5–70 hours. The milling protocol employed an intermittent bidirectional rotation pattern, with 15 minutes of counterclockwise rotation, a 5-minute pause, and 15 minutes of clockwise rotation, repeated throughout the process. All handling and milling operations were conducted under continuous argon protection to prevent atmospheric contamination.
Material characterization studies and analysis
Structural characterization of the synthesized xLi3N–MCly SSE was performed using multiple analytical techniques. Amorphous phase identification was executed via X-ray diffraction analysis (Empyrean, Malvern Panalytical) employing monochromatic Cu Kα radiation (λ = 1.5406 Å), with specimens encapsulated within Kapton polyimide membranes under inert conditions to prevent air exposure. The tests were conducted at a scan rate of 4.5° min−1 from 10 to 90°. Morphological evaluation and elemental mapping were conducted through field-emission scanning electron microscopy (SEM, JEOL-JSM7610) coupled with a cryogenically cooled silicon drift detector for energy-dispersive X-ray spectroscopy (EDS). The XCT measurements were conducted at Nanovoxel 3432E (Sanying, China). The visualization and segmentation of XCT images were performed using Avizo software (Thermo Fisher Scientific, USA). Chemical bonding was analyzed using a confocal Raman microscope (Horiba LabRAM Odyssey) equipped with a 532 nm laser excitation source. To ensure the integrity of the air-sensitive samples, they were loaded into Ar-filled quartz capillaries (1.0 mm internal diameter) and sealed with UV-curable glue to create an airtight environment. All sample preparation steps were performed under an Ar atmosphere. The cryogenic transmission electron microscopy (cryo-TEM) characterization studies were performed using an aberration-corrected Titan Krios TEM operated at 300 kV. The 7Li solid-state NMR (SSNMR) experiments were performed on a Bruker Avance NEO 400 spectrometer equipped with a 9.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T wide-bore magnet using a 3.2
T wide-bore magnet using a 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mm HXY double-resonance MAS probe. The 7Li MAS NMR spectra were acquired using a one-pulse sequence with a π/2 pulse length of 3.0 µs and a recycle delay of 2.0 s at a spinning rate of 10 kHz. 7Li chemical shifts with respect to a 1 M LiCl solution at 0.0 ppm.
mm HXY double-resonance MAS probe. The 7Li MAS NMR spectra were acquired using a one-pulse sequence with a π/2 pulse length of 3.0 µs and a recycle delay of 2.0 s at a spinning rate of 10 kHz. 7Li chemical shifts with respect to a 1 M LiCl solution at 0.0 ppm.
Construction of amorphous structures and pair distribution function simulation
The crystalline structure of Li2MgCl4 was obtained from the inorganic crystal structure database (ICSD),43 while those of LiCeCl4 and Li2HfCl6 were retrieved from the open quantum materials database (OQMD).44Ab initio molecular dynamics (AIMD)60 simulations were then performed in the NPT61 ensemble to thermally treat these crystalline structures. The systems were gradually heated to 1500 K, inducing a transition from the ordered crystalline phase to a fully molten state. Following melting, rapid quenching was applied to generate the corresponding amorphous structures. The melt–quench protocol was carried out using a Langevin thermostat, with a total simulation time of 10 ps and a time step of 1 fs. The resulting amorphous configurations are presented in Fig. S9, and the corresponding cell parameters are provided in Table S3.In this study, AIMD simulations were performed on the constructed amorphous structures within the NVT62 ensemble, with the system temperature regulated using a Nosé–Hoover thermostat. The simulations were conducted with a time step of 1 fs for a total duration of 50 ps. All AIMD calculations were carried out using the Vienna Ab initio Simulation Package (VASP),60 employing the projector augmented-wave (PAW)63 method together with the Perdew–Burke–Ernzerhof (PBE)64 generalized gradient approximation (GGA)65 for the exchange–correlation functional. A plane-wave energy cutoff of 340 eV was used, and the Brillouin zone was sampled at the Γ point. The total number of atoms in the 0.1Li3N–MgCl2, 0.3Li3N–CeCl3, and 0.533Li3N–HfCl4 systems was 148, 208, and 228, respectively. In addition, the pair correlation functions G(r) were calculated using PDFgui software, based on CIF files generated from AIMD simulations.66
AIMD data analysis: ionic diffusion and activation energy
For 0.1Li3N·MgCl2, 0.3Li3N·CeCl3, and 0.533Li3N·HfCl4, AIMD simulations were conducted at 800, 1000, 1200, 1300, and 1500 K to examine ionic transport properties. The mean square displacement (MSD) of ions was calculated as follows:| MSD(t) = 〈|r(t) − r(0)|2〉 |
where d = 3 for three-dimensional systems. The temperature dependence of D was then analysed using the Arrhenius relation:
where D(T) is the diffusion coefficient at temperature T, D0 is the pre-exponential factor reflecting the intrinsic structural and dynamic characteristics of the system, Ea is the activation energy for diffusion, kB is Boltzmann's constant (8.617 × 10−5 eV K−1), and T is the absolute temperature. This relationship highlights the exponential enhancement of D with increasing temperature and the crucial role of Ea in governing ionic mobility. By plotting ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D versus
D versus (Arrhenius plots) and performing linear fits, the slope
(Arrhenius plots) and performing linear fits, the slope 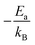 was obtained, allowing quantitative evaluation of the activation energies and thus the ionic transport behavior across the studied systems.
was obtained, allowing quantitative evaluation of the activation energies and thus the ionic transport behavior across the studied systems.
Electrochemical measurements and fabrication of batteries
The ionic conductivity of synthesized xLi3N–MCly SSEs was quantitatively assessed through EIS measurements conducted under potentiostatic mode. The xLi3N–MCly SSE powder (100 mg) was prepared by cold-pressing within a polyether–ether–ketone (PEEK) model cell (10 mm inner diameter) under ∼500 MPa with two stainless steel rods as blocking electrodes, producing compacted pellets with controlled thicknesses (0.4–0.7 mm). EIS test using a Solartron 1470E impedance analyser, with measurements spanning a frequency range of 0.1 Hz–1 MHz (10 mV voltage amplitude) at different temperatures. Each impedance spectrum comprised 71 discrete frequency points, from which ionic conductivity (σ) was calculated via the following equation:where L, R, and r denote pellet thickness, ohmic resistance from Nyquist plot extrapolation, and electrode radius, respectively. The cell assembly process for DC measurements was similar to EIS tests. To determine the electronic conductivity, the current responses of the cell were measured at 0.2 V for 1800 s.
A multi-layered architecture was engineered for solid-state battery construction through sequential powder compaction processes under an inert atmosphere (< 0.1 ppm H2O/O2). The composite cathode was prepared by mixing LiCoO2 (LCO, Guangdong Canrd New Energy Technology Co., Ltd) or LiNi0.83Co0.12Mn0.05O2 (NCM83, GEM Co., Ltd), amorphous 0.533Li3N–HfCl4 electrolyte, and vapor-grown carbon nanofibers (VGCF, Guangdong Canrd New Energy Technology Co., Ltd) in a mass ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, homogenized through an agate mortar. The cells were then assembled in a PEEK mold with an inner diameter of 10 mm. First, 65 mg of 0.533Li3N–HfCl4 SSE layer was evenly spread in the mold and pressed under ∼125 MPa of pressure, followed by layering 35 mg of Li5.5PS4.5Cl1.5 (LPSC, Guangdong Canrd New Energy Technology Co., Ltd) on top of the 0.533Li3N–HfCl4 surface. The bilayer electrolyte was then consolidated under ∼250 MPa of pressure. Subsequently, 7 mg of the composite cathode was uniformly spread on the 0.533Li3N–HfCl4 side of the bilayer pellet and pressed at ∼375 MPa. The anode was formed by attaching high-purity indium foil (ϕ10 mm) and lithium foil (ϕ8 mm, weight ratio Li/In = 1
3, homogenized through an agate mortar. The cells were then assembled in a PEEK mold with an inner diameter of 10 mm. First, 65 mg of 0.533Li3N–HfCl4 SSE layer was evenly spread in the mold and pressed under ∼125 MPa of pressure, followed by layering 35 mg of Li5.5PS4.5Cl1.5 (LPSC, Guangdong Canrd New Energy Technology Co., Ltd) on top of the 0.533Li3N–HfCl4 surface. The bilayer electrolyte was then consolidated under ∼250 MPa of pressure. Subsequently, 7 mg of the composite cathode was uniformly spread on the 0.533Li3N–HfCl4 side of the bilayer pellet and pressed at ∼375 MPa. The anode was formed by attaching high-purity indium foil (ϕ10 mm) and lithium foil (ϕ8 mm, weight ratio Li/In = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to the LPSC layer. Finally, the cell assembly was completed by inserting stainless steel current collectors on both sides and applying a consistent stack pressure of ∼62.5 MPa, which was maintained throughout electrochemical testing to optimize the interfacial contact. All electrochemical performance tests were performed using the Land and Neware battery testing system within temperature-controlled chambers to maintain isothermal operating conditions.
40) to the LPSC layer. Finally, the cell assembly was completed by inserting stainless steel current collectors on both sides and applying a consistent stack pressure of ∼62.5 MPa, which was maintained throughout electrochemical testing to optimize the interfacial contact. All electrochemical performance tests were performed using the Land and Neware battery testing system within temperature-controlled chambers to maintain isothermal operating conditions.
The electrochemical stability was evaluated via cyclic voltammetry (Solartron 1470E) using a Li–In|LPSC + 0.533Li3N–HfCl4|0.533Li3N–HfCl4 + VGCF cell, where the working electrode consisted of 0.533Li3N–HfCl4 mixed with VGCF (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 by weight). The scan was performed from 0.6 to 5.6 V (vs. Li+/Li) at a rate of 0.1 mV s−1.
5 by weight). The scan was performed from 0.6 to 5.6 V (vs. Li+/Li) at a rate of 0.1 mV s−1.
Author contributions
Conceptualization: conceptualization: B. Hong, L. Gao, S. Han, J. Zhu. methodology: B. Hong, L. Gao, M. Liu, S. Han, J. Zhu. Visualization: B. Hong, L. Di, H. Ni, P. Nan, Y. Li, Z. Lei. Funding acquisition: S. Han, J. Zhu. Project administration: S. Han, J. Zhu. Supervision: S. Han, J. Zhu. Writing – original draft: B. Hong, S. Han. Writing – review & editing: L. Gao, S. Han, J. Zhu. All authors contributed to the discussion about the manuscript.Conflicts of interest
The authors declare no competing interests.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ee05943g.Acknowledgements
This study was supported by the Guangdong Grants (2021ZT09C064), the Guangdong Basic and Applied Basic Research Foundation (2024B1515120042), the National Natural Science Foundation of China (12426301, 12275119, 52227802, and 12405343), the Shenzhen Science and Technology Program (KQTD20200820113047086), the Shenzhen Key Laboratory of Solid State Batteries (SYSPG20241211173726011), the Guangdong-Hong Kong-Macao Joint Laboratory for Photonic-Thermal-Electrical Energy Materials and Devices (2019B121205001), the Natural Science Foundation of Guangdong Province (2024A1515030261), and the Guangdong Provincial Key Laboratory of Energy Materials for Electric Power (2018B030322001). We also acknowledge the Major Science and Technology Infrastructure Project of Material Genome Big-science Facilities Platform supported by the Municipal Development and Reform Commission of Shenzhen, and the Center for Computational Science and Engineering at the Southern University of Science and Technology.Notes and references
- Q. Zhao, S. Stalin, C.-Z. Zhao and L. A. Archer, Nat. Rev. Mater., 2020, 5, 229–252 CrossRef CAS
.
- P. Albertus, V. Anandan, C. Ban, N. Balsara, I. Belharouak, J. Buettner-Garrett, Z. Chen, C. Daniel, M. Doeff, N. J. Dudney, B. Dunn, S. J. Harris, S. Herle, E. Herbert, S. Kalnaus, J. A. Libera, D. Lu, S. Martin, B. D. McCloskey, M. T. McDowell, Y. S. Meng, J. Nanda, J. Sakamoto, E. C. Self, S. Tepavcevic, E. Wachsman, C. Wang, A. S. Westover, J. Xiao and T. Yersak, ACS Energy Lett., 2021, 6, 1399–1404 CrossRef CAS
.
- T. Lee, J. Qi, C. A. Gadre, H. Huyan, S.-T. Ko, Y. Zuo, C. Du, J. Li, T. Aoki, R. Wu, J. Luo, S. P. Ong and X. Pan, Nat. Commun., 2023, 14, 1940 CrossRef CAS PubMed
.
- J. A. Dawson, P. Canepa, T. Famprikis, C. Masquelier and M. S. Islam, J. Am. Chem. Soc., 2018, 140, 362–368 CrossRef CAS PubMed
.
- W. Li, J. A. Quirk, M. Li, W. Xia, L. M. Morgan, W. Yin, M. Zheng, L. C. Gallington, Y. Ren, N. Zhu, G. King, R. Feng, R. Li, J. A. Dawson, T.-K. Sham and X. Sun, Adv. Mater., 2024, 36, e2302647 CrossRef PubMed
.
- S.-Y. Lee, J. Rawal, J. Lee, J. Gautam, S. Kim, G.-L. Xu, K. Amine and S.-J. Park, Electrochem. Energy Rev., 2025, 8, 9 CrossRef CAS
.
- T. Yu, Y. Liu, H. Li, Y. Sun, S. Guo and H. Zhou, Chem. Rev., 2025, 125, 3595–3662 CrossRef CAS PubMed
.
- Z. Wang, S.-H. Luo, X. Zhang, S. Guo, P. Li and S. Yan, J. Non Cryst. Solids, 2023, 619, 122581 CrossRef CAS
.
- J. Ou, I. Senevirathna, V. Tatagari, A. Saleem, C. Segre and L. Shaw, Energy Mater., 2025, 5, 500088 CAS
.
- C. Wang, M. Aykol and T. Mueller, Chem. Mater., 2023, 35, 6346–6356 CrossRef CAS
.
- B. Hong, L. Gao, C. Li, G. Lai, J. Zhu, D. Huang, Y. Zuo, W. Yin, M. Sun, S. Zhao, J. Zheng, S. Han and R. Zou, Nat. Commun., 2025, 16, 143 CrossRef PubMed
.
- X. Lin, S. Zhang, M. Yang, B. Xiao, Y. Zhao, J. Luo, J. Fu, C. Wang, X. Li, W. Li, F. Yang, H. Duan, J. Liang, B. Fu, H. Abdolvand, J. Guo, G. King and X. Sun, Nat. Mater., 2025, 24, 83–91 CrossRef CAS PubMed
.
- L. Hu, J. Wang, K. Wang, Z. Gu, Z. Xi, H. Li, F. Chen, Y. Wang, Z. Li and C. Ma, Nat. Commun., 2023, 14, 3807 CrossRef CAS PubMed
.
- Q. Zhang, D. Cao, Y. Ma, A. Natan, P. Aurora and H. Zhu, Adv. Mater., 2019, 31, e1901131 CrossRef PubMed
.
- M. Tatsumisago, R. Takano, K. Tadanaga and A. Hayashi, J. Power Sources, 2014, 270, 603–607 CrossRef CAS
.
- C. Wu, Z. Wang, Z. Jia, J. Cui, C. Shu, X. Wang, Y. Wu and W. Tang, EES Batter., 2025, 1, 364–384 RSC
.
- J. Yang, J. Lin, T. Brezesinski and F. Strauss, ACS Energy Lett., 2024, 9, 5977–5990 CrossRef CAS
.
- S. Zhang, F. Zhao, L.-Y. Chang, Y.-C. Chuang, Z. Zhang, Y. Zhu, X. Hao, J. Fu, J. Chen, J. Luo, M. Li, Y. Gao, Y. Huang, T.-K. Sham, M. D. Gu, Y. Zhang, G. King and X. Sun, J. Am. Chem. Soc., 2024, 146, 2977–2985 CrossRef CAS PubMed
.
- S. Zhang, F. Zhao, J. Chen, J. Fu, J. Luo, S. H. Alahakoon, L.-Y. Chang, R. Feng, M. Shakouri, J. Liang, Y. Zhao, X. Li, L. He, Y. Huang, T.-K. Sham and X. Sun, Nat. Commun., 2023, 14, 3780 CrossRef PubMed
.
- F. Li, X. Cheng, G. Lu, Y.-C. Yin, Y.-C. Wu, R. Pan, J.-D. Luo, F. Huang, L.-Z. Feng, L.-L. Lu, T. Ma, L. Zheng, S. Jiao, R. Cao, Z.-P. Liu, H. Zhou, X. Tao, C. Shang and H.-B. Yao, J. Am. Chem. Soc., 2023, 145, 27774–27787 CrossRef CAS PubMed
.
- B. Hong, L. Gao, P. Nan, Y. Li, M. Liu, R. Zou, J. Gu, Q. Xu, J. Zhu and S. Han, Angew. Chem., Int. Ed., 2025, 64, e202415847 CrossRef CAS PubMed
.
- X. Zhu, J. Wu and J. Lu, Adv. Funct. Mater., 2024, 34, 2409547 CrossRef CAS
.
- Y. Ishiguro, K. Ueno, S. Nishimura, G. Iida and Y. Igarashib, Chem. Lett., 2023, 52, 237–241 CrossRef CAS
.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, APL Mater., 2013, 1, 011002 CrossRef
.
- C. Welch, K. T. Cho and V. Srinivasan, Chem. Mater., 2024, 36, 6748–6764 CrossRef CAS
.
- M. Hofer, M. Grube, C. F. Burmeister, P. Michalowski, S. Zellmer and A. Kwade, Adv. Powder Technol., 2023, 34, 104004 CrossRef CAS
.
- W. H. Zachariasen, J. Am. Chem. Soc., 1932, 54, 3841–3851 CrossRef CAS
.
- C. Chattopadhyay, K. S. N. Satish Idury, J. Bhatt, K. Mondal and B. S. Murty, Mater. Sci. Technol., 2016, 32, 380–400 CrossRef CAS
.
- K.-H. Sun, J. Am. Ceram. Soc., 1947, 30, 277–281 CrossRef CAS
.
- Y. Song, S. Xue, Z. Xu, J. Fang, Z. Zhan, Y.-H. Wang, C. Chen, S. Li, T. Liu, Y. Yang, L. Yang and F. Pan, Adv. Energy Mater., 2025, 15, 2500913 CrossRef CAS
.
- M. Lei, B. Li, H. Liu and D.-E. Jiang, Angew. Chem., Int. Ed., 2024, 63, e202315628 CrossRef CAS PubMed
.
- Y. Zhu, Z. D. Hood, H. Paik, P. B. Groszewicz, S. P. Emge, F. N. Sayed, C. Sun, M. Balaish, D. Ehre, L. J. Miara, A. I. Frenkel, I. Lubomirsky, C. P. Grey and J. L. M. Rupp, Matter, 2024, 7, 500–522 CrossRef CAS
.
- J. Robertson, Phys. Status Solidi B, 2008, 245, 1026–1032 CrossRef CAS
.
- X. Yuan and A. N. Cormack, J. Non Cryst. Solids, 2003, 319, 31–43 CrossRef CAS
.
-
W. Wang, Amorphous Matter The Fourth Conventional Matter, Science Press, Beijing, China, 2023 Search PubMed
.
- A. H. Reddoch, J. Chem. Phys., 1961, 35, 1085–1089 CrossRef CAS
.
- K. Tuo, F. Yin, F. Mi and C. Sun, J. Energy Chem., 2023, 87, 12–23 CrossRef CAS
.
- T. Asano, A. Sakai, S. Ouchi, M. Sakaida, A. Miyazaki and S. Hasegawa, Adv. Mater., 2018, 30, e1803075 CrossRef PubMed
.
- H.-Y. Tan, M.-Y. Zhou, Z. Huang, J.-D. Luo, J.-T. Yang, J.-P. Wang, Y.-C. Wu, X.-B. Cheng, Z.-W. Wang, X.-D. Hao, L. Wang, K. Gong, Y.-C. Yin, Y. Xiao and H.-B. Yao, Nano Res., 2024, 17, 8826–8833 CrossRef CAS
.
- Y. Tanaka, K. Ueno, K. Mizuno, K. Takeuchi, T. Asano and A. Sakai, Angew. Chem., Int. Ed., 2023, 62, e202217581 CrossRef CAS PubMed
.
- T. Dai, S. Wu, Y. Lu, Y. Yang, Y. Liu, C. Chang, X. Rong, R. Xiao, J. Zhao, Y. Liu, W. Wang, L. Chen and Y.-S. Hu, Nat. Energy, 2023, 8, 1221–1228 CrossRef CAS
.
- A. Türler, B. Eichler, D. T. Jost, D. Piguet, H. W. Gäggeler, K. E. Gregorich, B. Kadkhodayan, S. A. Kreek, D. M. Lee, M. Mohar, E. Sylwester, D. C. Hoffman and S. Hübener, Radiochim. Acta, 1996, 73, 55–66 CrossRef
.
- A. Belsky, M. Hellenbrandt, V. L. Karen and P. Luksch, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 364–369 CrossRef PubMed
.
- J. E. Saal, S. Kirklin, M. Aykol, B. Meredig and C. Wolverton, Jom, 2013, 65, 1501–1509 CrossRef CAS
.
- N. Pistawala, L. Harnagea, S. Karmakar, R. Rawat and S. Singh, Phys. Rev. Mater., 2024, 8, 076201 CrossRef CAS
.
- H. Sano, H. Miyaoka, T. Kuze, H. Mori, G. Mizutani, N. Otsuka and M. Terano, Surf. Sci., 2002, 502–503, 70–74 CrossRef CAS
.
- F. Marchini, B. Porcheron, G. Rousse, L. Albero Blanquer, L. Droguet, D. Foix, T. Koç, M. Deschamps and J. M. Tarascon, Adv. Energy Mater., 2021, 11, 2101111 CrossRef CAS
.
- D. Tuschel, Spectroscopy, 2017, 32, 26–33 CAS
.
- H. Zhang, Y. Zhang, Y. Wang, M. Sui, L. Yue, S. Liu, Q. Li, Z. Liu, Z. Yao, P. Wang and B. Liu, Adv. Funct. Mater., 2024, 34, 2411470 CrossRef CAS
.
- A. Salamat, A. L. Hector, B. M. Gray, S. A. J. Kimber, P. Bouvier and P. F. McMillan, J. Am. Chem. Soc., 2013, 135, 9503–9511 CrossRef CAS PubMed
.
- C. A. Young and A. L. Goodwin, J. Mater. Chem., 2011, 21, 6464 RSC
.
- F. D. Hardcastle and I. E. Wachs, J. Phys. Chem., 1991, 95, 5031–5041 CrossRef CAS
.
- N. Umezawa, K. Shiraishi, Y. Akasaka, A. Oshiyama, S. Inumiya, S. Miyazaki, K. Ohmori, T. Chikyow, T. Ohno, K. Yamabe, Y. Nara and K. Yamada, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 165130 CrossRef
.
- S. K. Kim, Y.-S. Kim, Y.-A. Jeon, J. Choi and K.-S. No, J. Electroceram., 2006, 17, 197–203 CrossRef CAS
.
- W. Shan, A. Shi, Z. Xin, X. Zhang, B. Wang, Y. Li and X. Niu, Adv. Funct. Mater., 2024, 35, 2412773 CrossRef
.
- K. Tuo, F. Yin and C. Sun, ACS Sustainable Chem. Eng., 2024, 12, 7012–7025 CrossRef CAS
.
- F. Li, X. Cheng, L.-L. Lu, Y.-C. Yin, J.-D. Luo, G. Lu, Y.-F. Meng, H. Mo, T. Tian, J.-T. Yang, W. Wen, Z.-P. Liu, G. Zhang, C. Shang and H.-B. Yao, Nano Lett., 2022, 22, 2461–2469 CrossRef CAS PubMed
.
- R. Li, P. Lu, X. Liang, L. Liu, M. Avdeev, Z. Deng, S. Li, K. Xu, J. Feng, R. Si, F. Wu, Z. Zhang and Y.-S. Hu, ACS Energy Lett., 2024, 9, 1043–1052 CrossRef CAS
.
- S. Randau, D. A. Weber, O. Kötz, R. Koerver, P. Braun, A. Weber, E. Ivers-Tiffée, T. Adermann, J. Kulisch, W. G. Zeier, F. H. Richter and J. Janek, Nat. Energy, 2020, 5, 259–270 CrossRef CAS
.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS PubMed
.
- Z. Huang, Q. Wang, X. Liu and X. Liu, Phys. Chem. Chem. Phys., 2023, 25, 2349–2358 RSC
.
- L. Gao, X. Liu, J. Bai, L. Kong, Z. Bai and W. Li, J. Phys. Chem. C Nanomater. Interfaces, 2024, 128, 17756–17766 CrossRef CAS
.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS
.
- A. E. Mattsson, R. Armiento, P. A. Schultz and T. R. Mattsson, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 195123 CrossRef
.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed
.
- C. L. Farrow, P. Juhas, J. W. Liu, D. Bryndin, E. S. Božin, J. Bloch, T. Proffen and S. J. L. Billinge, J. Phys.: Condens. Matter, 2007, 19, 335219 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2026 |