Sustainable moisture-induced electricity from wood through asymmetric hygroscopic design and radiative cooling
Chenyue
Guo
 a,
Huajie
Tang
a,
Decheng
Kong
a,
Qixiang
Chen
a,
Xin
Wu
a,
Fan
Fan
a,
Xinyu
Zhao
a,
Renhao
Ding
a,
Wenqi
Zhong
*a and
Dongliang
Zhao
a,
Huajie
Tang
a,
Decheng
Kong
a,
Qixiang
Chen
a,
Xin
Wu
a,
Fan
Fan
a,
Xinyu
Zhao
a,
Renhao
Ding
a,
Wenqi
Zhong
*a and
Dongliang
Zhao
 *abc
*abc
aSchool of Energy and Environment, Southeast University, Nanjing, 211189, China
bInstitute of Science and Technology for Carbon Neutrality, Southeast University, Nanjing, 210096, China
cInstitute for Carbon Neutral Development, Southeast University, Nanjing, 210096, China. E-mail: dongliang_zhao@seu.edu.cn; wqzhong@seu.edu.cn
First published on 1st December 2025
Abstract
As an emerging sustainable energy technology, moisture-electric generators (MEGs) can spontaneously harvest electricity from ubiquitous water vapor. Natural wood, with its abundant oxygen-containing functional groups and anisotropic microchannels, is an ideal material for MEG fabrication. However, most wood-based generators rely on streaming potential driven by evaporation, requiring an external water supply to ensure continuous operation, which significantly limits their practical applications. Here, we present an asymmetric hygroscopic structure based on delignified natural wood, with LiCl and carbon black incorporated into the hygroscopic and hydrophobic sides, respectively. This design maintains a stable internal water content gradient through the dynamic equilibrium of moisture sorption–desorption, enabling continuous directional ion migration and stable output for over 220 h. Delignification enhances hydrophilicity and surface charge density by exposing cellulose nanofibrils. Additionally, the radiative cooling effect of the hygroscopic layer induced by delignification promotes moisture sorption and prevents the collapse of the water content gradient under solar heating. A single device can continuously generate an open-circuit voltage of ∼0.94 V and a short-circuit current of ∼43 µA at 25 °C and 70% RH, with a maximum output power density of ∼29 µW cm−3. This work provides a sustainable strategy for developing efficient bio-based MEGs.
Broader contextThe accelerating energy crisis and climate change demand innovative strategies for sustainable power generation. While conventional hydropower harnesses concentrated water flow, a vast amount of energy stored in dispersed forms such as atmospheric moisture remains largely untapped. Recent advances in hydrovoltaic technology demonstrate the possibility of electricity generation directly from water–solid interactions without external energy input, offering a new paradigm for green energy harvesting. Among these approaches, moisture-electric generators (MEGs) are particularly promising, as they leverage ubiquitous moisture to produce electricity continuously without fuel consumption or additional stimuli. Their potential aligns closely with the growing demand for self-powered sensors and internet of things (IoT) devices, which require scalable, low-cost, and environmentally friendly energy solutions. However, real-world deployment of MEGs is hindered by environmental fluctuations, including variations in humidity, temperature, and solar radiation, which affect their stability and output. Addressing these challenges is crucial for advancing MEGs from laboratory prototypes to practical technologies. This study contributes to this effort by developing a sustainable, bio-based MEG capable of stable operation under natural conditions, providing new opportunities for clean energy generation in diverse environments. |
Introduction
The escalating challenges of the energy crisis and climate change demand low-carbon and sustainable energy harvesting technologies to replace fossil fuels.1 Water, as a vital energy carrier, annually stores, transfers, and converts trillions of kilowatts of energy through the hydrological cycle.2,3 Traditional hydropower collects concentrated energy from rivers or tides by converting mechanical energy into electricity based on electrodynamic principles.4 Substantial amounts of energy stored and converted in dispersed forms such as moisture, raindrops, and evaporation remain largely untapped.5,6 Recent advances in nanoscience have enabled the development of hydrovoltaic technology that directly harvests electricity from diverse forms of water through water–solid interface interactions without additional energy input.7,8 Various nanostructured functional materials, including carbon nanoparticles,9,10 graphene oxide,11–13 metal oxides,14,15 polymers,16–18 and MXenes,19 have been employed in hydrovoltaic devices. However, the fabrication of these materials is often complex, costly, and reliant on non-biodegradable components.20 From a sustainability perspective, developing low-cost, renewable, and eco-friendly alternatives is imperative.Wood is an abundant and widely distributed renewable natural resource. Owing to its intrinsic hierarchically porous structure, highly aligned cellulose nanofibers, and abundant dissociable oxygen-containing functional groups, natural wood can efficiently transport water, ions, and nutrients.21 Based on these properties, when water molecules pass through the wood channels, polar functional groups are ionized and ultimately form an electric double layer (EDL) at the solid–liquid interface.22 Selective ion transport occurs in partial channels with dimensions comparable to the Debye length of the solid–liquid interface, thereby generating streaming current within the channels and streaming potential across their ends.23 These attributes make wood a promising candidate for hydrovoltaic generation. However, due to the dense cell walls that restrict water penetration and the relatively low surface charge density, devices based on natural wood exhibit limited output.24,25 Strategies including surface modification,26,27 polyelectrolyte incorporation,28,29 and cell wall nanoengineering30 have been developed to improve output performance, but most wood-based generators operate as evaporation-induced electricity generators (EEGs), relying on directional water flow driven by evaporation.25,26,30 Such devices require external water supply and suitable environmental conditions to sustain operation, constraining their practical applicability.
Moisture-electric generators (MEGs) harvest energy from ambient water vapor by converting the chemical potential released during the state transition of water molecules into electricity.31,32 Due to the ubiquity of moisture and the operation without additional stimuli, MEGs hold great promise for self-powered electronics and the internet of things (IoT) applications.33,34 To satisfy practical utilization requirements, it is essential to enhance their energy conversion efficiency while also improving environmental adaptability and long-term output stability.35 Recent studies have integrated moisture sorption and desorption processes to maintain water and ion flow by establishing an artificial hydrological cycle,36,37 enabling continuous electricity generation under controlled laboratory conditions. However, not only humidity but also fluctuations in temperature and solar radiation significantly affect the sorption–desorption equilibrium. While moderate solar heating can accelerate desorption and ion transport, continuous sunlight absorption (especially by carbon-based materials with high solar absorptance) inevitably inhibits moisture sorption, leading to the collapse of the water content gradient and subsequent output decay.28,38 Therefore, considering radiative heat transfer between the device and environment is necessary to ensure stable moisture-electric generation in real-world applications.
To address these challenges, we propose a self-sustained wood-based moisture-electric generator (WMEG) enabled by an asymmetric hygroscopic structure and radiative cooling. The WMEG is fabricated from delignified natural wood and consists of a LiCl-loaded hygroscopic top layer and a carbon black-loaded hydrophobic bottom layer. Radiative cooling is an energy-free thermal management strategy that reflects sunlight while emitting mid-infrared radiation through the atmospheric window to outer space.39–41 By leveraging the subambient radiative cooling effect of the hygroscopic layer to regulate heat transfer with the environment,42,43 the WMEG enables stable power generation through a dynamic equilibrium of moisture sorption–desorption in fluctuating natural environments. Additionally, delignification exposes more oriented cellulose nanofibrils, increasing surface charge density and facilitating ion transport. Benefiting from the spontaneously formed water content gradient and the well-aligned nanochannels that enhance ion migration, the WMEG achieves continuous and efficient output for over 220 h. A single WMEG (2 cm × 2 cm × 1 mm) can generate a stable open-circuit voltage (Voc) of ∼0.94 V and a short-circuit current (Isc) of ∼43 µA at 25 °C and 70% relative humidity (RH), with a maximum output power density of ∼29 µW cm−3. This work presents a sustainable strategy for developing bio-based MEGs and demonstrates their potential for efficient operation in hot, arid environments with intense solar radiation.
Results and discussion
Structural design and characterization of the WMEG
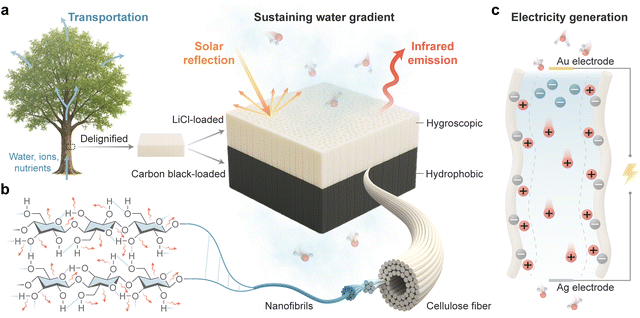
Wood exemplifies how nature employs highly oriented cellulose nanofibrils to control anisotropic mass transport properties. Structurally, the wood cell wall comprises a primary layer and three secondary layers (S1, S2 and S3), of which the S2 layer is predominant.44 The S2 layer consists of elongated wood tracheids responsible for water transport, surrounded by densely packed cellulose nanofibrils aligned along the tree's growth direction.45 The hierarchically porous structure and anisotropic three-dimensional interconnected microchannels enable directional transport of water, ions, and nutrients from roots to the leaves during photosynthesis (Fig. 1a). At the microscale, the layered wood cell wall is composed of paracrystalline cellulose nanofibril aggregates embedded in a matrix of amorphous heteropolysaccharide hemicellulose and polyphenolpropane-based branched lignin.46 Lignin functions as a binding agent, imparting stiffness and mechanical strength to maintain the structural integrity of the cell wall.47 However, its inherent hydrophobicity and chemical inertness limit water–solid interface interactions, which adversely affects the utilization of natural wood in moisture-electric generation. To address this issue, we employed a “top-down” strategy to remove lignin and partially dissolve hemicellulose via simple chemical treatment,48 thereby preserving the naturally aligned channels within the wood while enhancing its hydrophilicity and surface charge density.The bilayer structure WMEG is fabricated by loading LiCl and carbon black onto the delignified wood to form a hygroscopic top layer and a hydrophobic bottom layer (Fig. S1, SI), with copper and silver electrodes attached to each side, respectively. The abundant, interconnected hierarchical pores of the delignified wood allow both LiCl and carbon black to effectively infiltrate and adhere to cellulose fiber surfaces (Fig. S2 and S3, SI). When exposed to air, hydrophilic functional groups in the cellulose molecular chains (Fig. 1b), together with the strong hygroscopicity of LiCl, enable the hygroscopic layer to spontaneously sorb ambient moisture. The phase transition from vapor to adsorbed water releases chemical potential energy, which drives proton dissociation and leaves behind negatively charged cellulose nanofibrils (Fig. 1c). The asymmetric hygroscopic structure of the WMEG facilitates the formation of internal gradients in water content and ion concentration through a dynamic equilibrium between moisture sorption and desorption. Driven by these gradients, water molecules and dissociated ions migrate directionally through the vertically aligned nanochannels from the hygroscopic side to the hydrophobic side. The negatively charged nanochannel walls attract cations, forming electric double layers at the solid–liquid interface. Due to the ion selectivity of the nanochannels, anions are repelled and tend to accumulate on one side, while the majority of cations and water molecules are allowed to transport through the channels, thereby generating streaming potential and current. In addition, the multiscale fibers and hierarchical pores of the hygroscopic layer enable effective backscattering of visible light,49 while the molecular stretching vibrations in cellulose contribute to strong infrared radiation emission in the atmospheric window (Fig. 1b). Since the emitted heat flux exceeds absorbed solar radiation, the hygroscopic layer achieves passive subambient radiative cooling. This effect promotes water sorption and prevents excessive evaporation caused by solar heating, sustaining continuous water and ion flow even under fluctuating environmental conditions.
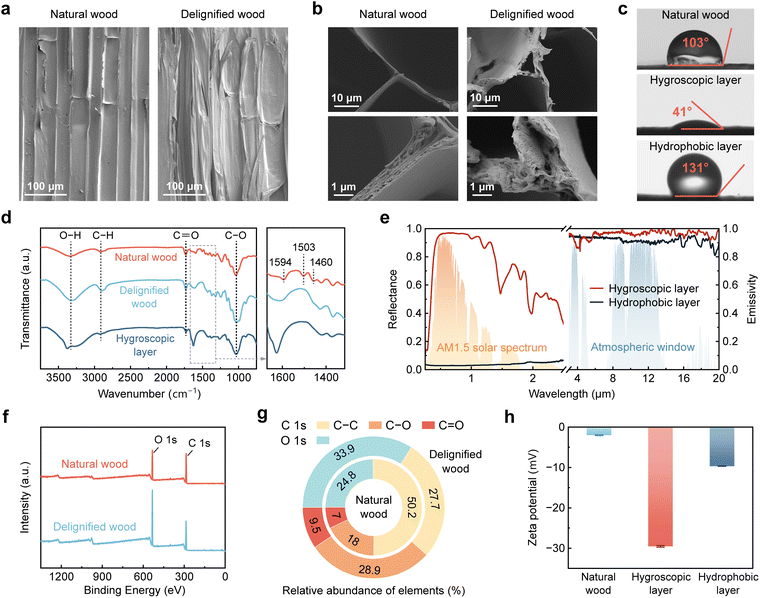
Fig. 2a and b present the side and top view scanning electron microscopy (SEM) images of natural wood and delignified wood, respectively, illustrating the structural changes in the cell walls after delignification. Natural wood exhibits a compact, well-organized layered structure, with vessels and fiber tracheids vertically aligned along the growth direction of the tree. Cellulose nanofibril aggregates are embedded within this structure. Following delignification, although partial rupture of the cell walls is observed, the freeze-drying process preserves the structural framework and prevents collapse of the softened cell walls. Consequently, the delignified wood retains its naturally aligned porous structure. Removal of lignin leads to thinner cell walls and more open cellular structures, exposing a greater number of cellulose nanofibrils and thereby providing additional nanochannels available for ion transport. Combined with the elimination of the hydrophobic structural polymer lignin, these morphological changes significantly enhance the hydrophilicity of the wood. The water contact angle on the cross-section of natural wood tracheids is 103° (Fig. 2c), whereas the hygroscopic layer shows a much smaller water contact angle of 41°, indicating strong hydrophilicity. In contrast, the carbon black-loaded hydrophobic layer displays a large water contact angle of 131°. This pronounced contrast in hydrophilicity between the two layers facilitates the formation of a stable internal water content gradient, enabling directional water transport.
 | ||
| Fig. 2 Characterization of the WMEG. Side (a) and top view (b) SEM images of natural wood and delignified wood, respectively. (c) Water contact angles of the natural wood, hygroscopic layer, and hydrophobic layer. (d) FTIR spectra of the natural wood, delignified wood, and hygroscopic layer. (e) Solar reflectance and mid-infrared emissivity spectra of the hygroscopic layer and hydrophobic layer. The normalized solar irradiance spectrum (orange area) and the atmospheric transmittance (blue area) are also plotted. (f) XPS spectra of the natural wood and delignified wood. (g) The relative abundance of C and O elements calculated from XPS spectra in Fig. 2f. (h) Zeta potential of the natural wood, hygroscopic layer, and hydrophobic layer. | ||
Fourier transform infrared (FTIR) spectra show distinct absorption peaks corresponding to O–H (∼3340 cm−1), C–H (2920–2890 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1735–1725 cm−1), and C–OH (∼1030 cm−1) stretching vibrations in natural wood, delignified wood, and the hygroscopic layer (Fig. 2d). Cellulose exhibits strong infrared absorbance by O–H and C–O,50 while lignin displays characteristic peaks at 1594, 1503, and 1460 cm−1 due to aromatic skeletal vibrations.51 These lignin-related peaks are absent in the spectra of the delignified wood and hygroscopic layer, confirming effective lignin removal. Fig. 2e presents the solar reflectance and mid-infrared emissivity spectra of the hygroscopic and hydrophobic layers, alongside the normalized solar irradiance spectrum and atmospheric transmittance for reference. The hygroscopic layer exhibits a high solar-weighted average reflectance of ∼0.90, attributed to multiscale fibers and hierarchical pores formed after delignification, which serve as randomized and disordered scattering elements at visible wavelengths.52 Due to the strong absorption of ultraviolet and part of the visible light by lignin, natural wood exhibits a solar-weighted average reflectance of ∼0.57 (Fig. S4, SI). The bright white appearance of the hygroscopic layer (Fig. S1, SI) further supports complete lignin removal and high diffusive reflectance in the sunlight range. In contrast, the hydrophobic layer shows a high solar absorptance of ∼0.97 due to the incorporation of carbon black. Furthermore, the hygroscopic layer demonstrates an average mid-infrared emissivity of ∼0.97 in the atmospheric transparency window (8–13 µm), primarily owing to the strong infrared absorption from cellulose-associated C–O–C, C–O, and C–H stretching vibrations between 1250 and 770 cm−1. It is worth noting that these spectral measurements were conducted in the hydrated (operational) state. Due to the inherent infrared absorption of water, the near-infrared reflectance of the hygroscopic layer is slightly lower than that of dry delignified wood without LiCl loading (Fig. S4, SI), though both retain comparable reflectance in the visible range. As a result, their difference in solar-weighted average reflectance is minimal (∼0.03), while the hygroscopic layer possesses superior mid-infrared emissivity than delignified wood.
O (1735–1725 cm−1), and C–OH (∼1030 cm−1) stretching vibrations in natural wood, delignified wood, and the hygroscopic layer (Fig. 2d). Cellulose exhibits strong infrared absorbance by O–H and C–O,50 while lignin displays characteristic peaks at 1594, 1503, and 1460 cm−1 due to aromatic skeletal vibrations.51 These lignin-related peaks are absent in the spectra of the delignified wood and hygroscopic layer, confirming effective lignin removal. Fig. 2e presents the solar reflectance and mid-infrared emissivity spectra of the hygroscopic and hydrophobic layers, alongside the normalized solar irradiance spectrum and atmospheric transmittance for reference. The hygroscopic layer exhibits a high solar-weighted average reflectance of ∼0.90, attributed to multiscale fibers and hierarchical pores formed after delignification, which serve as randomized and disordered scattering elements at visible wavelengths.52 Due to the strong absorption of ultraviolet and part of the visible light by lignin, natural wood exhibits a solar-weighted average reflectance of ∼0.57 (Fig. S4, SI). The bright white appearance of the hygroscopic layer (Fig. S1, SI) further supports complete lignin removal and high diffusive reflectance in the sunlight range. In contrast, the hydrophobic layer shows a high solar absorptance of ∼0.97 due to the incorporation of carbon black. Furthermore, the hygroscopic layer demonstrates an average mid-infrared emissivity of ∼0.97 in the atmospheric transparency window (8–13 µm), primarily owing to the strong infrared absorption from cellulose-associated C–O–C, C–O, and C–H stretching vibrations between 1250 and 770 cm−1. It is worth noting that these spectral measurements were conducted in the hydrated (operational) state. Due to the inherent infrared absorption of water, the near-infrared reflectance of the hygroscopic layer is slightly lower than that of dry delignified wood without LiCl loading (Fig. S4, SI), though both retain comparable reflectance in the visible range. As a result, their difference in solar-weighted average reflectance is minimal (∼0.03), while the hygroscopic layer possesses superior mid-infrared emissivity than delignified wood.
To further validate the chemical composition changes induced by delignification, X-ray photoelectron spectroscopy (XPS) measurements were performed (Fig. 2f). XPS analysis and high-resolution deconvolution of the C 1s and O 1s peaks (Fig. S5, SI) reveal the relative abundance of C and O elements in both natural (inner ring) and delignified wood (outer ring), as shown in Fig. 2g. The oxygen-to-carbon (O/C) atomic ratio increases from 0.33 in natural wood to 0.51 after delignification, further confirming the effective removal of lignin.53 Moreover, structural alterations in the cell walls and oxidation by sodium chlorite (NaClO2) during delignification increase the abundance of hydrophilic groups,25 enhancing the ionization capacity of the delignified wood during moisture sorption and providing more charge carriers. Zeta potentials reflect the interaction between ionizable groups and water, as well as the surface charge properties of the material. The absolute value of the zeta potential increases from 1.97 mV of natural wood to 29.5 mV of the hygroscopic layer (Fig. 2h), indicating a significant enhancement in surface charge density after delignification. Furthermore, a distinct surface charge density gradient between the hygroscopic and hydrophobic layers reduces ionic diffusion resistance and contributes to the formation of an internal electric field,54 which is essential for efficient power generation in the WMEG.
Electrical generation performance of the WMEG
Benefiting from its asymmetric hygroscopic structure, the WMEG sustains a stable internal water content gradient and directional ion migration through a dynamic equilibrium of moisture sorption and desorption. A single WMEG unit (2 cm × 2 cm × 1 mm) delivers a steady Voc of approximately 0.91 V and an Isc of ∼39 µA over 220 h of continuous operation under controlled laboratory conditions (∼25 °C and 65% RH) (Fig. 3a). As a moisture-driven energy harvesting device, the electrical output performance of the WMEG is intrinsically coupled to ambient temperature and relative humidity. By adjusting the internal water content gradient through appropriate moisture sorption and release, the WMEG achieves stable electrical output over a broad range of relative humidity (30%–90% RH at 25 °C) and temperature (10–50 °C at 75% RH), indicating its strong environmental adaptability (Fig. S6 and S7, SI). As shown in Fig. 3b, both Voc and Isc initially increase and subsequently decline as the RH rises from 30% to 90%, reaching peak values of 0.94 V and 43 µA at 70% RH. Extremely high or low humidity levels weaken the internal water content gradient, hindering rapid ion transport and reducing electrical output. Similarly, elevating the temperature from 10 °C to 30 °C rises the Voc from 0.42 V to 0.89 V and Isc from 18 µA to 35 µA, followed by a decline with further temperature increase (Fig. 3c). These trends indicate the necessity of moderate temperature and humidity conditions to sustain long-term moisture sorption–desorption equilibrium, which underpins efficient water and ion migration within the device.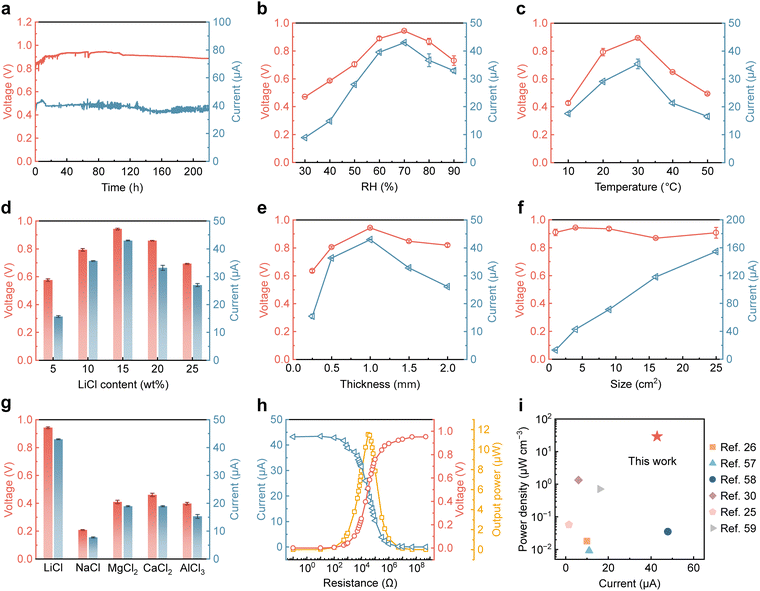 | ||
| Fig. 3 Power generation performance of the WMEG. (a) A continuous recording of Voc and Isc from the WMEG for 220 h in an ambient environment (25 °C, 65% RH). (b) Electrical output in response to RH at a temperature of 25 °C. (c) The variation of stable Voc and Iscversus temperature change at 75% RH. (d) Voc and Isc of WMEGs with hygroscopic layers treated with LiCl solutions of different concentrations. The testing conditions are 25 °C and 70% RH. (e) Voc and Isc output of the WMEG with different thicknesses (25 °C, 70% RH). (f) Electrical output plotted against the size of the 1-mm-thick device (25 °C, 70% RH). (g) Electrical output performance of WMEGs containing various hygroscopic salts in the hygroscopic layer (25 °C, 70% RH). (h) Power output of the WMEG with varying external resistances ranging from 0.1 Ω to 500 MΩ (25 °C, 70% RH). (i) Comparison of the electrical output performance of the WMEG in this work and the reported wood-based generators.25,26,30,57–59 | ||
The impact of LiCl solution concentration used to treat the hygroscopic layer on the output performance and mass variation of the WMEG is shown in Fig. 3d and Fig. S8 (SI). Higher LiCl content enhances moisture sorption capacity, allowing the device to rapidly establish internal water content and ion concentration gradients upon exposure to moisture, thus improving electrical output. However, excessively high LiCl content results in increased water transfer from the hygroscopic to the hydrophobic layer (Fig. S8, SI), diminishing the interlayer water gradient and consequently weakening the driving force for ion transport. In addition, the thickness of the material also influences the ion concentration gradient and diffusion distance. Both Voc and Isc increase with thickness up to an optimal value of 1 mm, after which the output performance declines (Fig. 3e). As the device size increases from 1 cm2 to 25 cm2, there is minimal change in Voc, while Isc gradually increases (Fig. 3f). Enlarging the device size can be regarded as connecting multiple units in parallel. Therefore, adjusting the device size allows tuning of the power output to meet diverse application requirements. Furthermore, we compared the electrical output performance of devices incorporating different hygroscopic salts with the same concentration on the hygroscopic layer (Fig. 3g). The WMEG loaded with LiCl exhibits the highest performance, as the small ionic radius and low valence of Li+ reduce diffusion resistance and electrostatic repulsion,55 thereby promoting efficient ion migration. In contrast, devices with salts of larger ionic radius and higher valence display lower performance, with the NaCl-loaded device showing the lowest output due to its relatively weak hygroscopicity.
Fig. 3h displays the output voltage and current of the WMEG (2 cm × 2 cm × 1 mm) under external load resistances ranging from 0.1 Ω to 500 MΩ, along with the corresponding power output calculated from these values. A maximum of approximately 29 µW cm−3 is achieved at a load resistance of ∼30 kΩ. The electrochemical impedance spectroscopy (EIS) plot of the WMEG exhibits an almost linear Nyquist response without a semicircular arc, confirming good ohmic contact between the electrode and the wood,56 which minimizes interface-related power loss (Fig. S9, SI). To evaluate the electrical output performance of the WMEG developed in this study, its output metrics are compared with those of recently reported wood-based hydrovoltaic generators (Fig. 3i and Table S1, SI).25,26,30,57–59 The WMEG achieves a maximum Isc of ∼43 µA and power density of ∼29 µW cm−3, showcasing remarkable energy output. Furthermore, the comparison of output performance between the WMEG and a typical wood-based evaporation-induced electricity generator25 under low RH and strong solar irradiation shows that the latter requires additional water supply to compensate for accelerated evaporation due to solar heating (Fig. S10, SI), whereas the WMEG operates solely on ambient moisture, offering greater versatility and superior environmental adaptability.
Verification of the power generation mechanism
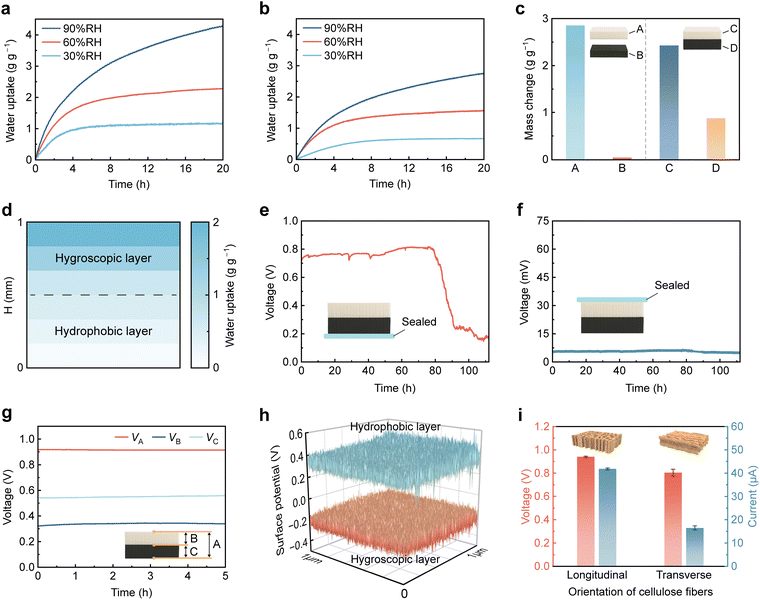
To verify the directional water transport from the hygroscopic to the hydrophobic side within the WMEG, we investigated the moisture sorption behavior of the entire device as well as its individual layers. Due to the abundant hydrophilic functional groups in delignified wood and the strong hygroscopicity of LiCl, the dried hygroscopic layer achieves sorption capacities of 1.18, 2.28, and 4.27 g g−1 after 20 h at 30%, 60%, and 90% RH, respectively (Fig. 4a). In comparison, the bilayer structure composed of both hygroscopic and hydrophobic layers exhibits lower water uptakes of 0.68, 1.56, and 2.75 g g−1 under the same conditions (Fig. 4b). For instance, at 25 °C and 30% RH, the water uptake of the hygroscopic layer increases rapidly within the first 4 h and reaches saturation at around 6 h, whereas the bilayer structure shows a slower mass increase and stabilizes after approximately 10 h. As shown in Fig. 4c, the mass change after 20 h at 25 °C and 70% RH is 2.85 g g−1 for the standalone hygroscopic layer (A) and negligible for the standalone hydrophobic layer (B). In the bilayer structure, the hygroscopic and hydrophobic layers (C and D) exhibit mass changes of 2.43 and 0.88 g g−1, respectively. These results confirm that, during the WMEG operation, moisture is spontaneously sorbed from ambient air by the hygroscopic layer and directionally transported toward the hydrophobic layer. As depicted in Fig. 4d, this asymmetric hydrophilicity establishes a distinct internal water content gradient, which facilitates continuous water and ion migration through the nanochannels under a stable gradient.Consistent with the previously proposed electricity generation mechanism, the WMEG enables directional transport of water and ions through a dynamic equilibrium of moisture sorption and desorption. To further elucidate the role of the asymmetric hygroscopic structure in sustaining continuous power generation, we sealed either the hygroscopic or hydrophobic side of the device to alter its water exchange behavior and monitored the resulting electrical output (Fig. 4e and f). When the hygroscopic side is sealed, the device can no longer sorb moisture effectively through the hydrophobic side. Since moisture serves as the energy source for the WMEG, the output voltage drops to nearly zero. In contrast, the device with the hydrophobic side sealed maintains a voltage output of ∼0.8 V for 80 h, which subsequently declines to ∼0.2 V over the next 10 h as the sorbed water reaches saturation. These findings demonstrate the importance of the asymmetric hygroscopic design in maintaining a long-term internal water content gradient essential for continuous power generation.
The electrical outputs of different layers within the device are shown in Fig. 4g and Fig. S11 (SI). The hygroscopic layer generates a voltage of 0.33 V (VB), while the hydrophobic layer produces a higher voltage of 0.54 V (VA), which can be attributed to the combined effects of desorption and ion-selective transport.55 These differences in output further support the presence of stable internal gradients in water content and ion concentration, with both sorption and desorption processes contributing to the continuous migration of water and ions. Kelvin probe force microscopy (KPFM) was employed to measure the surface potential changes of the hygroscopic and hydrophobic layers during device operation. As shown in Fig. 4h, the directional migration of water and dissociated ions from the hygroscopic to the hydrophobic side results in an obvious surface potential difference. The hydrophobic layer exhibits a higher positive potential, while the hygroscopic layer shows a reduced potential due to ion dissociation induced by water sorption, consistent with the proposed electricity-generation mechanism. Moreover, the orientation of cellulose fibers in wood significantly influences the electrical output. The wood-based generator with longitudinally aligned channels demonstrates superior performance compared to that with horizontal alignment, particularly in current output (Fig. 4i). This enhancement is attributed to the continuous and straight pathways provided by vertically aligned channels, which reduces ion diffusion distances and transport resistance,21,26 thereby accelerating water and ion migration.
Electrical output performance in outdoor environments
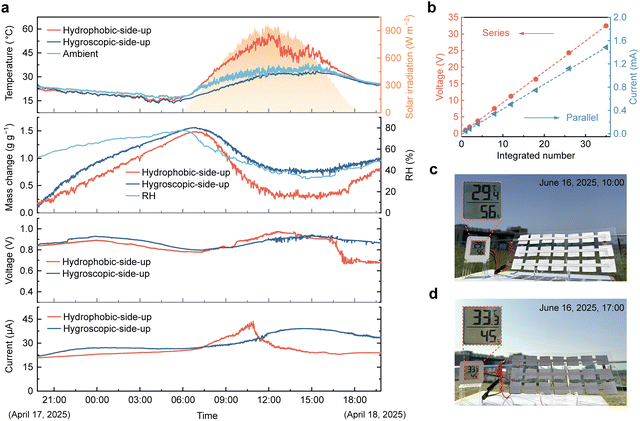
For MEGs integrating both moisture sorption and desorption processes, the inherent asymmetric structure prevents water saturation and current decay typically caused by a single sorption process.55,60 However, when exposed to sunlight, the photothermal effect of the materials significantly accelerates water desorption, ultimately disrupting the sorption–desorption equilibrium and leading to performance degradation.28,38 Therefore, for practical outdoor operation, improving and effectively utilizing radiative heat exchange between the generator and the environment is of great importance.To investigate the influence of spectral properties on outdoor performance, we compared the voltage and current outputs, as well as the temperature and mass variations, of WMEGs positioned with either the hygroscopic or the hydrophobic side facing the sky (Fig. 5a). The experimental setup is shown in Fig. S12 (SI), with the corresponding environmental conditions during the tests provided in Fig. 5a and Fig. S13 (SI). Owing to the high infrared emissivity of hygroscopic and hydrophobic surfaces, the radiative heat loss to outer space offsets the exothermic heat of sorption and promotes water uptake at night, resulting in similar electrical outputs for both configurations. As shown in the temperature curves in Fig. 5a and the infrared thermal images in Fig. S14 (SI), the hydrophobic-side-up device exhibits a pronounced photothermal effect as solar irradiance increases during the day, with an average daytime temperature 10.2 °C higher than the ambient. In contrast, the hygroscopic layer reflects most incoming solar radiation. Even under intense solar irradiance of ∼900 W m−2, the hygroscopic-side-up device remains slightly cooler than ambient, with an average daytime temperature decrease of 3.1 °C. Under strong daytime irradiation and low ambient humidity, the hydrophobic-side-up device experiences a sharp loss of water content. Although rapid water desorption briefly accelerates ion transport and increases current output, persistent solar absorption inevitably causes a mismatch between sorption and desorption, leading to the collapse of the water content gradient and a decline in streaming potential. In comparison, the radiative cooling effect of the hygroscopic layer suppresses excessive daytime water desorption, maintaining the dynamic sorption–desorption equilibrium and enabling the device to deliver stable, high-efficiency electrical output throughout the test period.
In addition, the ability to flexibly tailor power output for different scenarios is crucial for the practical applications of MEGs. As shown in Fig. 5b, integrating 30 WMEG units in series or parallel produces a voltage of ∼32.4 V and a current of ∼1.48 mA, respectively. The nearly linear scaling of output with series or parallel connection confirms the excellent scalability of the WMEG. A 3 × 7 series–parallel array of WMEG units can continuously power a small electronic device for 7 h in a hot outdoor environment under high solar irradiance (Fig. 5c and d), with environmental parameters during the demonstration shown in Fig. S15 (SI). The strong environmental adaptability and stable DC output of the WMEG allow it to function directly as a portable outdoor power source without requiring additional rectification or energy storage circuits.
Conclusions
In summary, we developed a moisture-electric generator derived from natural wood, offering facile fabrication, environmental sustainability, and high output performance. Delignification preserves the naturally aligned hierarchical porous channels of wood while exposing more cellulose nanofibrils, thereby enhancing its surface charge density and hydrophilicity. Additionally, the altered surface structure and chemical composition endow the delignified wood with radiative cooling capability, attributed to its high solar reflectance and strong mid-infrared emissivity. The asymmetric hygroscopic structure and highly oriented nanochannels sustain a long-lasting water content gradient and promote efficient directional ion transport, enabling stable operation of the WMEG for over 220 h. A single WMEG unit produces a sustained Voc of ∼0.94 V and an Isc of ∼43 µA, achieving a maximum power density of 29 µW cm−3 at 25 °C and 70% RH. The subambient radiative cooling effect of the hygroscopic layer improves and utilizes heat exchange with the environment, accelerating moisture sorption and mitigating the disruption of the water content gradient caused by solar heating. Consequently, the WMEG maintains efficient and stable energy conversion even under fluctuating outdoor conditions.Methods
Materials
Balsa wood (Ochroma pyramidale) was obtained from an online marketplace (Alibaba, China). Lithium chloride (LiCl, 99.9%), sodium chlorite (NaClO2, >80%), acetylene carbon black, ethyl cellulose, and α-terpineol (>98%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Glacial acetic acid (≥99.8%) and ethanol (≥99.8%) were bought from Shanghai Macklin Biochemical Technology Co., Ltd. All reagents were used without further purification. Deionized water was used in all experiments.Preparation of delignified wood
First, 3 g NaClO2 was dissolved in 297 ml deionized water to prepare a 1 wt% NaClO2 solution. The pH of NaClO2 solution was adjusted to 4.6 using glacial acetic acid as a buffer. Balsa wood slices were then immersed in the solution and heated in an oil bath at 80 °C for 12 h until fully bleached. The wood slices were subsequently washed repeatedly with deionized water to remove residual ions. Finally, the delignified wood was freeze-dried for 12 h for later use.Fabrication of the WMEG
Firstly, 4.5 g LiCl was added into 25.5 ml deionized water and stirred until completely dissolved. A delignified wood slice was immersed in the LiCl solution for 8 h, then freeze-dried for 12 h to prepare the hygroscopic layer. Subsequently, a mixture of 0.8 g acetylene black, 1.5 g ethyl cellulose, 4.5 g α-terpineol, and 50 ml ethanol was stirred thoroughly and sonicated for 1 h. A delignified wood slice was then immersed in the carbon black suspension and subjected to vacuum treatment for 20 min to ensure complete infiltration into the wood pores. After drying at 80 °C for 2 h, the hydrophobic layer was obtained. Then, copper and silver electrodes were placed on the hygroscopic and hydrophobic sides, respectively. Finally, the electrodes, hygroscopic layer, and hydrophobic layer were pressed together under 1 MPa pressure to assemble the WMEG.Characterization
The side and top view morphologies of natural wood, delignified wood, hygroscopic layer, and hydrophobic layer were observed by SEM (Zeiss Sigma 300, Germany). Energy-dispersive X-ray spectroscopy (EDS) in SEM was employed to characterize the elemental distribution of the hygroscopic and hydrophobic layers. The static water contact angles of natural wood, hygroscopic layer, and hydrophobic layer were measured using a contact angle goniometer (DataPhysics OCA20, Germany). The chemical structures of natural wood, delignified wood, and hygroscopic layer were characterized by FTIR spectroscopy (Thermo Fisher Nicolet iS50, USA) in an attenuated total-reflection (ATR) model. A UV-vis-NIR spectrophotometer (Shimadzu UV-3600i Plus, Japan) with an integrating sphere was used to measure the solar reflectance of delignified wood, hygroscopic layer, and hydrophobic layer. Infrared emissivity was measured using an FTIR spectrometer (Thermo Fisher Nicolet iS50, USA) equipped with a gold integrating sphere. XPS spectra of the natural wood and delignified wood were recorded using a Thermo Scientific K-Alpha photoelectron spectrometer (USA). The zeta potential of natural wood, hygroscopic layer, and hydrophobic layer was measured using a particle size and zeta potential analyzer (Malvern Zetasizer Nano ZS90, UK) based on electrophoretic light scattering (ELS). Each sample was tested in triplicate to ensure accuracy. The electrochemical impendence spectroscopy (EIS) plot was measured using an electrochemical workstation (CH Instruments CHI660D, USA). The surface potential variation was measured using KPFM (Bruker Dimension Icon, Germany).Electrical output performance measurements
The indoor electrical output performance tests were conducted in a constant temperature and humidity chamber (Shanghai Yiheng LHS-250HC, China). Unless otherwise specified, indoor electrical output tests were conducted at 25 °C and 70% RH. Error bars represent the standard deviation. Sample and ambient temperatures were monitored using T-type thermocouples. Temperature and electrical output signals were recorded using a data acquisition system (Keysight DAQ970A, USA). The real-time mass changes of the samples were monitored using an electronic balance (Ohaus PR224ZH, USA). The ambient relative humidity, solar radiation intensity, and wind speed during outdoor experiments were recorded using a high-precision digital weather station.Author contributions
Conceptualization: D. Z., C. G.; funding acquisition: D. Z., W. Z.; investigation: C. G., H. T., D. K., Q. C., R. D., X. W., F. F., X. Z.; methodology: D. Z., C. G., H. T.; project administration: D. Z.; supervision: D. Z., W. Z.; visualization: C. G.; writing – original draft: C. G., D. Z.; writing – review & editing: C. G., D. Z.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting the findings of this study are included in the main text and supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ee05073a.Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 52421003 and 52276178), the Natural Science Foundation of Distinguished Young Scholars of Jiangsu Province (No. BK20240075), the Southeast University Interdisciplinary Research Program for Young Scholars, the Fundamental Research Funds for the Central Universities (2242025F10008), the SEU Innovation Capability Enhancement Plan for Doctoral Students (No. CXJH_SEU 25063), and the New Cornerstone Science Foundation through the XPLORER PRIZE.References
- D. Welsby, J. Price, S. Pye and P. Ekins, Nature, 2021, 597, 230–234 CrossRef CAS.
- K. E. Trenberth and J. T. Fasullo, Science, 2010, 328, 316–317 CrossRef CAS PubMed.
- G. L. Stephens, J. Li, M. Wild, C. A. Clayson, N. Loeb, S. Kato, T. L’Ecuyer, P. W. Stackhouse, M. Lebsock and T. Andrews, Nat. Geosci., 2012, 5, 691–696 CrossRef CAS.
- E. F. Moran, M. C. Lopez, N. Moore, N. Muller and D. W. Hyndman, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 11891–11898 CrossRef CAS.
- B. Zhang, W. Xu, L. Peng, Y. Li, W. Zhang and Z. Wang, Nat. Rev. Electr. Eng., 2024, 1, 218–233 CrossRef.
- K. von Schuckmann, M. D. Palmer, K. E. Trenberth, A. Cazenave, D. Chambers, N. Champollion, J. Hansen, S. A. Josey, N. Loeb, P. P. Mathieu, B. Meyssignac and M. Wild, Nat. Clim. Change, 2016, 6, 138–144 CrossRef.
- B. Shao, Y. Song, Z. Song, Y. Wang, Y. Wang, R. Liu and B. Sun, Adv. Eng. Mater., 2023, 13, 2204091 CAS.
- G. Zan, S. Li, K. Zhao, H. Kim, E. Shin, K. Lee, J. Jang, G. Kim, Y. Kim, W. Jiang, T. Kim, W. Kim and C. Park, Energy Environ. Sci., 2025, 18, 53–96 RSC.
- G. Xue, Y. Xu, T. Ding, J. Li, J. Yin, W. Fei, Y. Cao, J. Yu, L. Yuan, L. Gong, J. Chen, S. Deng, J. Zhou and W. Guo, Nat. Nanotechnol., 2017, 12, 317–321 CrossRef CAS PubMed.
- K. Liu, T. Ding, J. Li, Q. Chen, G. Xue, P. Yang, M. Xu, Z. L. Wang and J. Zhou, Adv. Eng. Mater., 2018, 8, 1702481 Search PubMed.
- Y. Huang, H. Cheng, C. Yang, P. Zhang, Q. Liao, H. Yao, G. Shi and L. Qu, Nat. Commun., 2018, 9, 4166 CrossRef.
- H. Cheng, Y. Huang, L. Qu, Q. Cheng, G. Shi and L. Jiang, Nano Energy, 2018, 45, 37–43 CrossRef CAS.
- Y. Huang, H. Cheng, C. Yang, H. Yao, C. Li and L. Qu, Energy Environ. Sci., 2019, 12, 1848–1856 RSC.
- D. Shen, M. Xiao, G. Zou, L. Liu, W. W. Duley and Y. N. Zhou, Adv. Mater., 2018, 30, 1705925 CrossRef.
- L. Li, S. Feng, Y. Bai, X. Yang, M. Liu, M. Hao, S. Wang, Y. Wu, F. Sun, Z. Liu and T. Zhang, Nat. Commun., 2022, 13, 1043 CrossRef CAS PubMed.
- S. Yang, L. Zhang, J. Mao, J. Guo, Y. Chai, J. Hao, W. Chen and X. Tao, Nat. Commun., 2024, 15, 3329 CrossRef CAS PubMed.
- S. Guo, S. Patel, J. Wang, Z. Yu, H. Qu, S. Zhang, K. Yu, S. Liu, J. J. Koh, X. Q. Koh, Z.-E. Ooi, D. H. L. Seng, W. Sun, L. Yang, Y. Zhang, J. Wang, S. K. Ravi, C. Yu and S. C. Tan, Sci. Adv., 2025, 11, eadw5991 CrossRef CAS.
- P. Li, Y. Hu, H. Wang, T. He, H. Cheng and L. Qu, Nat. Commun., 2025, 16, 6600 CrossRef CAS PubMed.
- H. Xia, W. Zhou, X. Qu, W. Wang, X. Wang, R. Qiao, Y. Zhang, X. Wu, C. Yang, B. Ding, L.-Y. Hu, Y. Ran, K. Yu, S. Hu, J.-F. Li, H.-M. Cheng, H. Qiu, J. Yin, W. Guo and L. Qiu, Nat. Nanotechnol., 2024, 19, 1316–1322 CrossRef CAS.
- M. Li, L. Zong, W. Yang, X. Li, J. You, X. Wu, Z. Li and C. Li, Adv. Funct. Mater., 2019, 29, 1901798 CrossRef.
- T. Li, X. Zhang, S. D. Lacey, R. Mi, X. Zhao, F. Jiang, J. Song, Z. Liu, G. Chen, J. Dai, Y. Yao, S. Das, R. Yang, R. M. Briber and L. Hu, Nat. Mater., 2019, 18, 608–613 CrossRef CAS PubMed.
- X. Li, Z. L. Wang and D. Wei, Adv. Funct. Mater., 2024, 34, 2405520 CrossRef CAS.
- F. H. J. van der Heyden, D. Stein and C. Dekker, Phys. Rev. Lett., 2005, 95, 116104 CrossRef PubMed.
- J. Zhang, X. Zhang, Y. Zhu, H. Chen, Z. Chen and Z. Hu, Int. J. Biol. Macromol., 2024, 279, 135258 CrossRef CAS PubMed.
- J. Lin, Z. Zhang, X. Lin, X. Cai, S. Fu, X. Fang, Y. Ding, X. Wang, G. Sèbe and G. Zhou, Adv. Funct. Mater., 2024, 34, 2314231 CrossRef CAS.
- X. Zhou, W. Zhang, C. Zhang, Y. Tan, J. Guo, Z. Sun and X. Deng, ACS Appl. Mater. Interfaces, 2020, 12, 11232–11239 CrossRef CAS PubMed.
- K. Zhang, L. Cai, A. Nilghaz, G. Chen, X. Wan and J. Tian, Nano Energy, 2022, 98, 107288 CrossRef CAS.
- Y. Chen, C. Ye, J. He, R. Guo, L. Qu and S. Tang, Energy Environ. Sci., 2025, 18, 6063–6075 RSC.
- J. Zhang, Z. Hu, Y. Hou, C. Wu and W. Ding, ACS Appl. Polym. Mater., 2024, 6, 8856–8865 CrossRef CAS.
- J. Garemark, F. Ram, L. Liu, I. Sapouna, M. F. Cortes Ruiz, P. T. Larsson and Y. Li, Adv. Funct. Mater., 2022, 33, 2208933 CrossRef.
- J. Xu, P. Wang, Z. Bai, H. Cheng, R. Wang, L. Qu and T. Li, Nat. Rev. Mater., 2024, 9, 722–737 CrossRef CAS.
- T. Xu, X. Ding, H. Cheng, G. Han and L. Qu, Adv. Mater., 2024, 36, e2209661 CrossRef PubMed.
- Y. Xiao, C. Guo, H. Yan, D. Zhao, P. Tan and R. Qi, Innovation Energy, 2025, 2, 100099 CrossRef CAS.
- Z. Feng, T. Yin, C. Liu, X. Yin, T. Wan, Y. Huang, J. Wang, B. Wen, M. Li, L. Hu, C. H. Lin, D. Chu, J. Chen and Z. Li, Adv. Mater., 2025, 37, e2506046 CrossRef PubMed.
- P. Wang, J. Xu, R. Wang and T. Li, Matter, 2023, 6, 19–22 CrossRef.
- L. Li, X. Sun, J. Miao, H. Wang, Y. Song and D. Tang, Energy Environ. Sci., 2025, 18, 1176–1190 RSC.
- Y. Zhang, Z. Yu, H. Qu, S. Guo, J. Yang, S. Zhang, L. Yang, S. Cheng, J. Wang and S. C. Tan, Adv. Mater., 2024, 36, e2208081 CrossRef PubMed.
- Y. Zhang, S. Guo, Z. G. Yu, H. Qu, W. Sun, J. Yang, L. Suresh, X. Zhang, J. J. Koh and S. C. Tan, Adv. Mater., 2022, 34, 2201228 CrossRef CAS.
- H. Tang, C. Guo, F. Fan, H. Pan, Q. Xu and D. Zhao, Energy Environ. Sci., 2024, 17, 4498–4507 RSC.
- F. Xie, W. Jin, J. R. Nolen, H. Pan, N. Yi, Y. An, Z. Zhang, X. Kong, F. Zhu, K. Jiang, S. Tian, T. Liu, X. Sun, L. Li, D. Li, Y.-F. Xiao, A. Alu, S. Fan and W. Li, Science, 2024, 386, 788–794 CrossRef CAS.
- J. Fei, X. Zhang, D. Han, Y. Lei, F. Xie, K. Zhou, S.-W. Koh, J. Ge, H. Zhou, X. Wang, X. Wu, J.-Y. Tan, Y. Gu, Y. Long, Z. H. Koh, S. Wang, P. Du, T. Mi, B.-F. Ng, L. Cai, C. Feng, Q. Gan and H. Li, Science, 2025, 388, 1044–1049 CrossRef CAS.
- C. Guo, H. Tang, P. Wang, Q. Xu, H. Pan, X. Zhao, F. Fan, T. Li and D. Zhao, Nat. Commun., 2024, 15, 6100 CrossRef CAS.
- H. Tang, C. Guo, X. Zhao, F. Fan, R. Lu and D. Zhao, Energy Convers. Manage., 2025, 325, 119385 CrossRef CAS.
- S. Ling, D. L. Kaplan and M. J. Buehler, Nat. Rev. Mater., 2018, 3, 18016 CrossRef CAS PubMed.
- P. Fratzl and R. Weinkamer, Prog. Mater. Sci., 2007, 52, 1263–1334 CrossRef CAS.
- T. Li, J. Song, X. Zhao, Z. Yang, G. Pastel, S. Xu, C. Jia, J. Dai, C. Chen, A. Gong, F. Jiang, Y. Yao, T. Fan, B. Yang, L. Wågberg, R. Yang and L. Hu, Sci. Adv., 2018, 4, eaar3724 CrossRef PubMed.
- A. Kumar, T. Jyske and M. Petrič, Adv. Sustainable Syst., 2021, 5, 2000251 CrossRef CAS.
- X. Han, Y. Ye, F. Lam, J. Pu and F. Jiang, J. Mater. Chem. A, 2019, 7, 27023–27031 RSC.
- T. Li, Y. Zhai, S. He, W. Gan, Z. Wei, M. Heidarinejad, D. Dalgo, R. Mi, X. Zhao, J. Song, J. Dai, C. Chen, A. Aili, A. Vellore, A. Martini, R. Yang, J. Srebric, X. Yin and L. Hu, Science, 2019, 364, 760–763 CrossRef CAS.
- H. Chen, C. Ferrari, M. Angiuli, J. Yao, C. Raspi and E. Bramanti, Carbohydr. Polym., 2010, 82, 772–778 CrossRef CAS.
- B. Richard, F. Quilès, C. Carteret and O. Brendel, Chem. Geol., 2014, 381, 168–179 CrossRef CAS.
- D. S. Wiersma, Nat. Photonics, 2013, 7, 188–196 CrossRef CAS.
- X. Bao, M. Yang, J. Zhu, H. Xu, H. Dang, K. Guo, D. Long, X. Guan, X. Chen and J. Lin, J. Wood Sci., 2025, 71, 44 CrossRef CAS.
- N. He, H. Wang, F. Li, B. Jiang, D. Tang and L. Li, Energy Environ. Sci., 2023, 16, 2494–2504 RSC.
- J. Tan, S. Fang, Z. Zhang, J. Yin, L. Li, X. Wang and W. Guo, Nat. Commun., 2022, 13, 3643 CrossRef CAS.
- H. Jia, S. Shahi, L. K. Shrestha, K. Ariga and T. Michinobu, RSC Adv., 2023, 13, 21502–21509 RSC.
- J. Zhang, Y. Hou, Y. Li and S. Hu, J. Mater. Res. Technol., 2022, 17, 1822–1830 CrossRef.
- Y. Ding, S. Li, J. Tian, F. Wang, Y. Shi, X. Tao, X. Wang, R. Lei and X. Chen, ACS Appl. Electron. Mater., 2021, 3, 5287–5295 CrossRef CAS.
- T. Cai, L. Lan, B. Peng, C. Zhang, S. Dai, C. Zhang, J. Ping and Y. Ying, Nano Lett., 2022, 22, 6476–6483 CrossRef CAS PubMed.
- S. Guo, Y. Zhang, Z. Yu, M. Dai, X. Liu, H. Wang, S. Liu, J. J. Koh, W. Sun, Y. Feng, Y. Chen, L. Yang, P. Sun, G. Lu, C. Yu, W. Chen, S. De Wolf, Z. Wang and S. C. Tan, Nat. Commun., 2025, 16, 5267 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |