Disruption of short-range π–π stacking via a disordered spatial architecture for energy storage at 250 °C
Deng
Hu
a,
Hang
Luo
 *a,
Guanghu
He
a,
Xi
Chen
a,
Yuting
Wan
a,
Fan
Wang
a,
Xiaona
Li
a,
Huan
Wang
a,
Haoran
Xie
*b and
Dou
Zhang
*a,
Guanghu
He
a,
Xi
Chen
a,
Yuting
Wan
a,
Fan
Wang
a,
Xiaona
Li
a,
Huan
Wang
a,
Haoran
Xie
*b and
Dou
Zhang
 *a
*a
aState Key Laboratory of Powder Metallurgy, Central South University, Changsha, Hunan 410083, China. E-mail: hangluo@csu.edu.cn; dzhang@csu.edu.cn
bKey Laboratory of Renewable Energy Electric-Technology of Hunan Province, School of Energy and Power Engineering, Changsha University of Science and Technology, Changsha, Hunan 410114, China. E-mail: hrxie88@csust.edu.cn
First published on 9th December 2025
Abstract
Film capacitors are indispensable in electrical engineering; however, balancing the insulation and thermal stability of polymer dielectrics remains a key challenge for high-temperature energy storage. Aromatic polyimide (PI) exhibits a high glass transition temperature (Tg, >300 °C), facilitating the formation of charge transfer complexes (CTCs). Semi-aromatic PIs mitigate this order, but insufficient thermal stability leads to poor performance above 200 °C. To resolve this contradiction, we incorporated a sp3-centered monomer, tris(4-aminophenyl)methane (TAPM), into a semi-aromatic PI (MPD-PI), constructing a spatially disordered architecture that suppresses short-range π–π stacking and CTCs, enhancing dielectric insulation and thermal stability. The resulting copolymer specifically achieves a discharged energy density of 7.13 J cm−3 at 200 °C with 90% efficiency and 5.18 J cm−3 at 250 °C, representing 341% and 280% improvements compared to those of MPD-PI, respectively. The improved thermal stability also imparts excellent cycling stability (3 × 105 cycles at 200 °C and 300 MV m−1) and a state-of-the-art breakdown strength of 596.2 MV m−1 at 250 °C. The conformational-engineering strategy of this work provides a versatile route for high-temperature polymer dielectrics.
Broader contextWith the rapid growth of energy conservation and renewable energy, advanced power systems demand dielectric films that combine high efficiency, reliability, and flexibility. Polyimides (PIs), valued for their thermal stability and processability, are widely used for high-field energy storage. However, fully aromatic PIs suffer from pronounced dielectric loss and leakage at high temperatures due to strong π-conjugation, meanwhile introducing alicyclic segments to improve insulation often compromises chain rigidity and thermal stability, creating a trade-off between insulating performance and high-temperature robustness. Here, we present a molecular design strategy that employs monomers with a sp3-centered geometry to build spatially disordered charge-confinement networks, thereby disrupting π-conjugation along the polymer backbone and simultaneously enhancing insulation and thermal stability in semi-aromatic PIs. As a result, the materials deliver excellent high-temperature energy storage capability and dielectric breakdown strength, outperforming most commercial and reported dielectric films. This approach offers new molecular-level insight into designing high-temperature polymer dielectrics and may accelerate the development of next-generation energy storage technologies. |
1. Introduction
Film capacitors are indispensable components for rapid energy conversion and pulsed-power delivery in the modern electrical era.1–5 Owing to their low density, mechanical flexibility, microsecond-scale charge–discharge capability, and exceptional voltage endurance (>3 kV), dielectric polymer films are widely deployed in aerospace satellites, rail transit, high-voltage inverters, and deep-well petroleum exploration—applications that demand reliable operation under extreme high-temperature and high-electric-field conditions.6–11 In such environments, capacitors must simultaneously deliver high discharged energy density (Ud) and high energy efficiency (η), while maintaining performance stability over prolonged cycling. For linear or quasi-linear dielectrics, the discharged energy density can be approximated using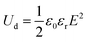 , where ε0 is the permittivity of free space, εr is the dielectric constant, and E is the operating electric field. Therefore, achieving high Ud requires the concurrent enhancement of both εr and E.
, where ε0 is the permittivity of free space, εr is the dielectric constant, and E is the operating electric field. Therefore, achieving high Ud requires the concurrent enhancement of both εr and E.
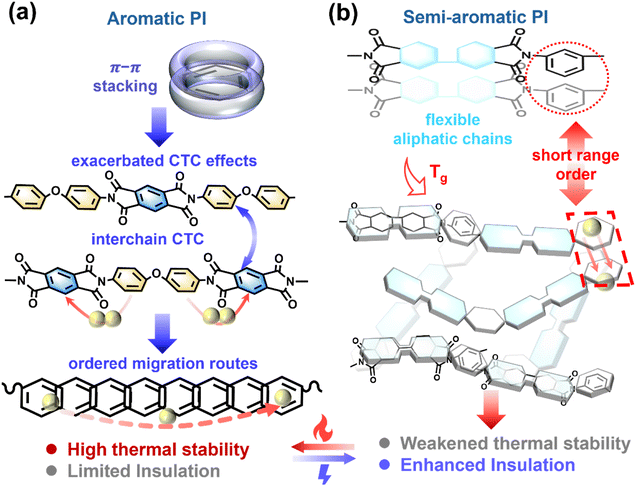
However, existing polymer dielectrics generally suffer from severe performance bottlenecks at elevated temperatures.9,12–14 A typical commercial polymer dielectric, biaxially oriented polypropylene (BOPP), undergoes thermal relaxation of its oriented structure, restricting its maximum operating temperature to 120 °C.15–17 Aromatic polyimides (PIs) are regarded as promising candidates for high-temperature energy storage owing to their outstanding thermal stability.18–20 Yet, under high-temperature, high-field operation conditions, their reliability is ultimately constrained by two intrinsic degradation pathways: (i) in fully aromatic PIs such as Kapton, pronounced π–π stacking between adjacent chains facilitates the formation of interchain charge–transfer complexes (CTCs), which evolve into long-range ordered pathways for carrier migration (Fig. 1a), thereby markedly increasing leakage current;21,22 and (ii) thermally activated conduction,23 thermal energy lowers the potential barriers for carrier hopping, while local chain fluctuations further enhance the motion of excited carriers, progressively reducing bulk resistivity. In contrast, semi-aromatic PIs—produced by incorporating alicyclic dianhydride or diamine units into the polymer backbone—attenuate π–π stacking and CTC interactions, thereby widening the electronic bandgap (Eg) and lowering dielectric loss, which endow them with superior insulation compared to fully aromatic PIs under high-temperature, high-field conditions.24–27 Nevertheless, the introduction of flexible alicyclic segments reduces chain rigidity and lowers the glass-transition temperature (Tg), thereby diminishing thermomechanical stability.28,29 In addition, residual short-range π–π stacking persists, permitting localized carrier hopping and ultimately limiting stability and breakdown strength above 200 °C (Fig. 1b). Conventional modification strategies, including inorganic/organic doping,30–37 fluorination,38–40 or increasing crosslink density,39,41 can partially mitigate these issues, but often at the expense of interfacial compatibility, premature breakdown, or elevated dielectric loss.42,43
Here, we focus on the geometric architecture of polymer chain segments. Copolymerization with the trifunctional monomers tris(4-aminophenyl)amine (TAPA) and tris(4-aminophenyl)methane (TAPM) induces conformational disorder, thereby disrupting local π–π stacking and electron transport. This process generates a disordered spatial architecture with a rigid center, which increases the optical bandgap of the film from 4.27 to 4.58 eV, weakening carrier excitation. It also enhances thermal stability at elevated temperatures, increasing Tg from 264.66 to 270.54 °C. As a result, TAPM-based semi-aromatic PI films retain excellent high-temperature energy storage performance, achieving an Eb of 699.4 MV m−1 and an Ud of 7.13 J cm−3 at 200 °C with an efficiency of 90%, while at 250 °C sustaining an Eb of 596.2 MV m−1 with an Ud of 5.18 J cm−3, superior to those of most reported and commercial dielectric polymers. The films also endure over 3 × 105 charge–discharge cycles at 200 °C under 300 MV m−1 and demonstrate microsecond-scale rapid discharge. These results demonstrate promising potential for scalable fabrication and practical applications.
2. Results and discussion
2.1. Design of PI chains and a spatially disordered architecture
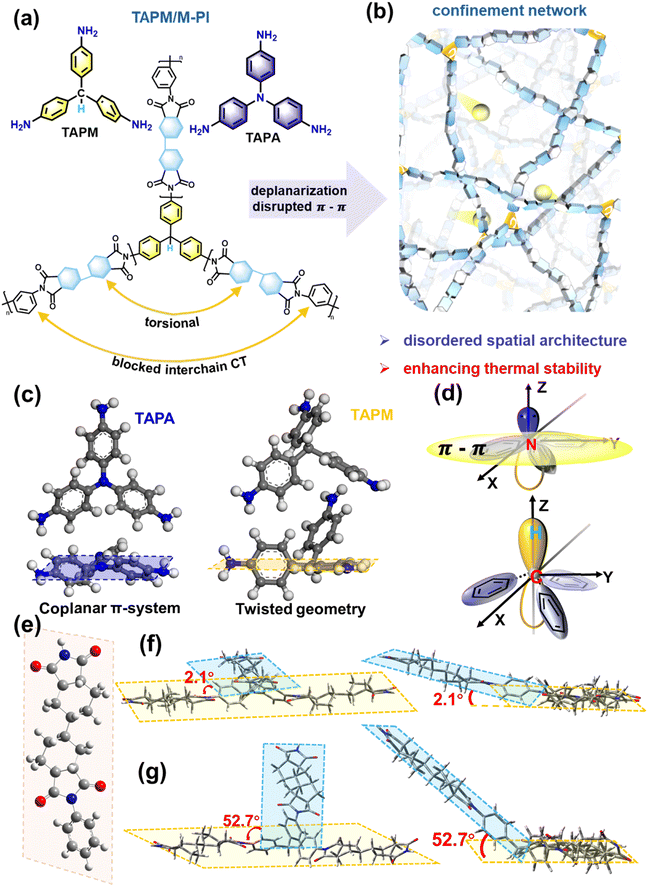
Both tris(4-aminophenyl)amine (TAPA) and tris(4-aminophenyl)methane (TAPM) feature three para-positioned reactive amine groups (Fig. S1). In the synthetic route, 1,3-phenylenediamine (MPD) and dicyclohexyl-3,4,3′,4′-tetracarboxylic dianhydride (HBPDA) first undergo prepolymerization to form poly(amic acid) (PAA) oligomers. Subsequently, the amino groups of TAPA or TAPM react with the residual anhydride units to form a terpolymerized polyimide (PI) chain structure (Fig. 2a and Fig. S2). By embedding these trifunctional units into the semi-aromatic backbone, the resulting PI chains experience local steric hindrance and increased segmental torsion. This torsion disrupts the continuity of π-electron pathways and blocks short-range ordered π–π stacking interactions, thereby suppressing charge transfer complex (CTC) formation and improving the intrinsic insulation of the PI. More critically, the TAPM-induced tri-directional divergence of chain segments constructs a spatially disordered network that forms a molecular-scale charge-confinement architecture and enhances the thermal robustness of the PI (Fig. 2b).We further validated the optimization effects of this structural design through multiscale computations. The molecular geometries of TAPA and TAPM were investigated through geometry optimization in materials studio (MS) (Fig. 2c). TAPA possesses a central sp2-hybridized nitrogen atom, which arranges the three aminophenyl rings in a nearly coplanar fashion, giving rise to an extended π-conjugated system with pronounced electron delocalization. In contrast, TAPM features a central sp3-hybridized carbon atom and assumes a twisted non-coplanar geometry, which separates the phenyl rings and disrupts internal π-conjugation (Fig. 2d).
The density functional theory (DFT) optimized structure of the pure semi-aromatic polyimide unit (MPD-PI) reveals a partially coplanar backbone with a bending angle of approximately 120° (Fig. 2e). Further calculations were performed to examine how introducing TAPA or TAPM modifies the chain conformation and insulating properties. Density of states (DOS) analyses indicate that the alicyclic dianhydride segment contributes significantly to the wide theoretical bandgap (Eg) (Fig. S3). For MPD-PI, the calculated Eg is 5.45 eV, with the LUMO at −1.38 eV mainly from the anhydride and the HOMO at −6.83 eV dominated by the diamine (Fig. S3a). Incorporating TAPA strengthens intramolecular π-conjugation and raises the HOMO to −5.39 eV while leaving the LUMO nearly unchanged, producing a deep hole trap (1.44 eV) expected to hinder carrier hopping and migration (Fig. S4). In contrast, incorporating TAPM slightly increases the bandgap by interrupting the π-conjugation. Meanwhile, the elevated electrostatic potential of the donor units weakens the intermolecular interactions between polymer chains, as evidenced in Fig. S5.
More importantly, TAPM strongly distorts the backbone and enhances the conformational disorder. As shown in Fig. 2f and g, TAPA-PI remains nearly planar, with a dihedral angle of only 2.1° between adjacent conjugated units, resulting in extended π-orbital overlap and coherent charge delocalization. This strong conjugation promotes long-range carrier migration under high electric fields, leading to higher leakage current and polarization loss. By contrast, TAPM-PI adopts a markedly twisted geometry with a dihedral angle of 52.7°, caused by the tetrahedral sp3-hybridized carbon at the TAPM core. Such three-dimensional divergence generates conformational tension, disrupts interchain π–π stacking, and effectively breaks short-range orbital coherence.
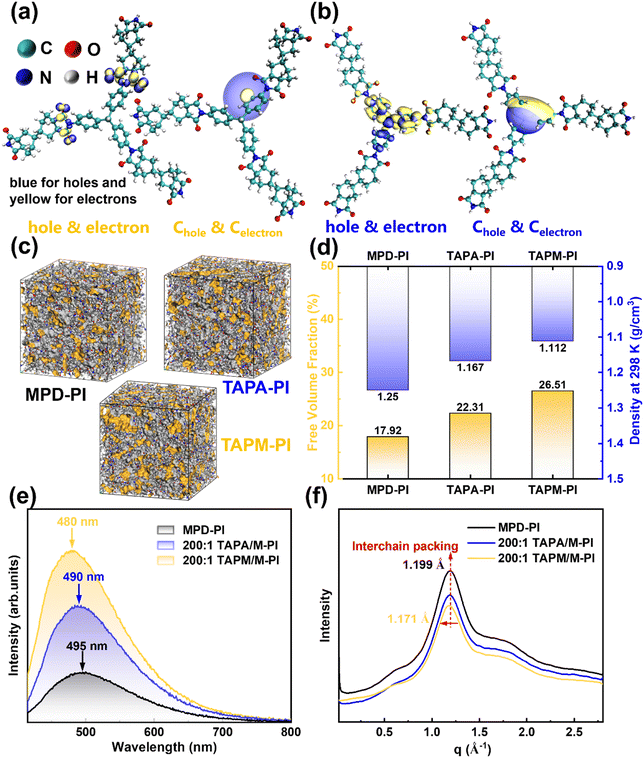
Using time-dependent density functional theory (TDDFT), the spatial characteristics of the excited states of PI segments incorporating TAPA and TAPM units were systematically investigated. Key descriptors, including the centroid distance between holes and electrons (D), the overlap integral (Sr), the standard deviation difference (Δσ), and the extension index (t), were employed to quantitatively evaluate the exciton localization of different systems. For example, the S1–S3 states of the MPD-PI system primarily exhibit localized excitations, with electronic transitions largely confined to the imide units (Table S1 and Fig. S6), indicating a certain degree of spatial localization. However, the suppression of charge migration is less effective than in the TAPM-PI system. The TAPM-PI system exhibits extremely small and consistent D values (0.118–0.137 Å) and Δσ values approaching zero for the S1–S3 states (Table S2), indicating highly overlapped electron–hole distributions and strong spatial localization in the excited states (Fig. 3a and Fig. S7). This pronounced spatial localization of the excited states effectively shortens the available carrier hopping pathways, thereby impeding long-range charge transport. As a result, leakage current is markedly reduced, enabling improved insulation stability and energy-storage reliability under high-temperature, high-field conditions. In contrast, the S1 and S2 states of the TAPA-PI segment display typical unidirectional charge-transfer (CT) characteristics, while S3 corresponds to a centrosymmetric CT excitation (Table S3, Fig. 3b and Fig. S8). A moderate introduction of TAPA can enhance the carrier trapping effect and shorten the effective transport distance, thereby mitigating the risk of leakage. However, excessive incorporation of TAPA induces multiple low-energy excitations, leading to local charge accumulation and premature dielectric breakdown. Atom-resolved contribution heatmaps of the excited states further visualize the spatial distribution of electrons and holes and clearly depict the direction and magnitude of charge transfer processes (Fig. S9).
To further elucidate the effect of spatially disordered structures, we combined experiments and molecular dynamics simulations to evaluate their influence on the aggregation state of PI. Amorphous-cell MD models were constructed (Fig. 3c), and the free volume fractions (FFVs) and densities of the three representative PIs were compared (Fig. 3d). The results show that the three-dimensionally divergent geometry introduced by TAPM effectively disrupts interchain packing, leading to a pronounced increase in internal voids (FFV of TAPM-PI increased to 26.51%) accompanied by a slight decrease in density (down to 1.112 g cm−3). This trend is consistent with the experimentally observed density reduction of the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPM/M-PI film (Fig. S10). Meanwhile, as shown in Fig. S11 and S12, both the simulated volumetric thermal expansion coefficients (αV) and the experimentally measured linear thermal expansion coefficients (αL) exhibit similar differences: MPD-PI shows the highest αV (1.12 × 10−4 K−1), whereas TAPM/M-PI exhibits a distinctly lower value (9.47 × 10−5 K−1). In-plane thermal expansion measurements further confirm this behavior, with αL decreasing from 47.2 ppm K−1 for MPD-PI to 36.6 ppm K−1 for TAPM/M-PI, and MPD-PI displaying a more pronounced high-temperature expansion tendency (increased dL/L0 slope). Overall, these results demonstrate that the TAPM-induced spatial disorder significantly enhances the dimensional stability of PI films at elevated temperatures.
1 TAPM/M-PI film (Fig. S10). Meanwhile, as shown in Fig. S11 and S12, both the simulated volumetric thermal expansion coefficients (αV) and the experimentally measured linear thermal expansion coefficients (αL) exhibit similar differences: MPD-PI shows the highest αV (1.12 × 10−4 K−1), whereas TAPM/M-PI exhibits a distinctly lower value (9.47 × 10−5 K−1). In-plane thermal expansion measurements further confirm this behavior, with αL decreasing from 47.2 ppm K−1 for MPD-PI to 36.6 ppm K−1 for TAPM/M-PI, and MPD-PI displaying a more pronounced high-temperature expansion tendency (increased dL/L0 slope). Overall, these results demonstrate that the TAPM-induced spatial disorder significantly enhances the dimensional stability of PI films at elevated temperatures.
Building upon TDDFT and MD simulations, the impact of TAPA- and TAPM-induced disordered spatial architectures on excited-state behavior and aggregation was further confirmed by steady-state photoluminescence (PL) and wide-angle X-ray scattering (WAXS) measurements. Compared to MPD-PI, both TAPA/M-PI and TAPM/M-PI systems exhibit significantly enhanced fluorescence intensity, pronounced blue shifts in emission peaks (Fig. 3e, from 495 nm to 490 nm and 480 nm, showing a clear Stokes shift arising from excited-state relaxation and polarization in the polyimide backbone44), and suppressed excited-state quenching.45 The photoluminescence quantum yield (PLQY) decreases slightly from 2.85% (MPD-PI) to 2.48% for TAPA/M-PI but increases to 3.44% for TAPM/M-PI (Fig. S13). The non-coplanar configuration and torsion induced by TAPA shorten the effective conjugation length, causing a small blue shift. However, its sp2-conjugated triarylamine core enhances intramolecular charge coupling, creating more trap states and non-radiative pathways, thus lowering the PLQY. In contrast, the sp3-centered tetrahedral geometry of TAPM twists the PI backbone, effectively breaking interchain π–π stacking and suppressing CTC formation. Consequently, TAPM/M-PI exhibits a more pronounced blue shift and higher PLQY, confirming that the introduction of TAPM disrupts π-conjugation and improves the dielectric insulation performance.
The WAXS patterns (Fig. 3f) reveal broad scattering peaks centered at q ≈ 1.2 Å−1 for all three films, corresponding to an interchain distance d of 5.2 Å. To precisely determine the diffraction features, the WAXS data were baseline-corrected and fitted using a Pseudo-Voigt function. As shown in Fig. S14 and Table S4, peak 1 is assigned to interchain packing and peak 2 to π–π stacking. Compared with MPD-PI, both TAPA/M-PI and TAPM/M-PI exhibit a shift of peak 1 toward lower q values (from 1.199 to 1.171 Å−1), indicating looser chain packing and enlarged interchain spacing.46 Furthermore, peak 2 gradually shifts from 1.777 Å−1 for MPD-PI to 1.690 Å−1 for TAPM/M-PI, corresponding to d ≈ 3.7 Å, confirming the weakening of short-range π–π stacking.47 Combined with the PL results, these findings suggest that the tri-directional architecture introduced by TAPM disrupts the ordered π–π stacking and charge-transfer complexes (CTCs), leading to a more disordered insulating microstructure that stabilizes the excited states and suppresses high-field conduction losses.
2.2. Structural characterization and thermal stability evaluation
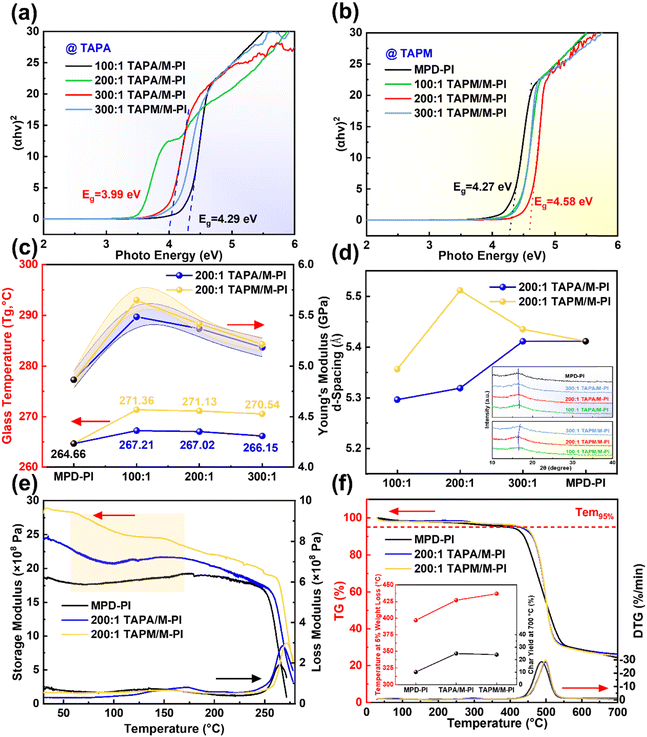
The chemical structure and thermal stability of the copolymerized films were subsequently examined. All films exhibited a uniform cross-sectional morphology, and the TAPM/M-PI films showed a slightly greater average thickness (Fig. S15). The FTIR spectra showed no new peaks, confirming no side reactions during copolymerization (Fig. S16). The UV-Vis absorption spectra revealed a redshift in the TAPA system (Fig. S17a) and a blueshift in the TAPM system (Fig. S17b), reflecting opposite trends in the optical bandgap (Eg). Specifically, the Eg value of the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPA/M-PI film decreased to 3.99 eV and further reduced with increasing TAPA content (Fig. 4a), while the Eg value of the 200
1 TAPA/M-PI film decreased to 3.99 eV and further reduced with increasing TAPA content (Fig. 4a), while the Eg value of the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPM/M-PI film increased to 4.58 eV (Fig. 4b). The narrowed Eg in the TAPA system is consistent with enhanced d–π conjugation and HOMO-level elevation, which form deep hole traps that reduce leakage current density. The widened Eg in the TAPM system arises from disrupted π-conjugation and enhanced spatial confinement of excited-state carriers, induced by disordered chain segments.
1 TAPM/M-PI film increased to 4.58 eV (Fig. 4b). The narrowed Eg in the TAPA system is consistent with enhanced d–π conjugation and HOMO-level elevation, which form deep hole traps that reduce leakage current density. The widened Eg in the TAPM system arises from disrupted π-conjugation and enhanced spatial confinement of excited-state carriers, induced by disordered chain segments.
Both types of PI films showed a progressive increase in glass transition temperature (Tg) and Young's modulus (Fig. 4c and Fig. S18), confirming that the spatial network organized by tri-functional groups efficiently constrained segmental dynamics. Notably, the Tg value of the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPM/M-PI film reached 271.13 °C, the highest among all samples, which is advantageous for maintaining dimensional and electrical stability under high-field, high-temperature conditions.48 X-ray diffraction (XRD) analysis was employed to investigate changes in the interchain packing distance (d-spacing) of the films (Fig. 4d). In the TAPA system, the coplanar divergent geometry enables tighter chain packing, leading to a slight decrease in the d-spacing with increasing TAPA content. By contrast, the TAPM system exhibited a substantially enlarged d-spacing due to the introduction of the rigid tetrahedral sp3 center, which enforces spatial disorder of chain segments and disrupts short-range π–π stacking.
1 TAPM/M-PI film reached 271.13 °C, the highest among all samples, which is advantageous for maintaining dimensional and electrical stability under high-field, high-temperature conditions.48 X-ray diffraction (XRD) analysis was employed to investigate changes in the interchain packing distance (d-spacing) of the films (Fig. 4d). In the TAPA system, the coplanar divergent geometry enables tighter chain packing, leading to a slight decrease in the d-spacing with increasing TAPA content. By contrast, the TAPM system exhibited a substantially enlarged d-spacing due to the introduction of the rigid tetrahedral sp3 center, which enforces spatial disorder of chain segments and disrupts short-range π–π stacking.
Within the temperature range from 35 °C to Tg, both PI films exhibited significantly enhanced storage modulus compared to the MPD-PI film (Fig. 4e and Fig. S19). Such reinforcement arises from the restriction of chain mobility by the spatial confinement architecture, which delays the onset of thermomechanical softening. The TAPM/M-PI film showed the highest modulus values (2.4 GPa at 150 °C, 2.2 GPa at 200 °C, and 1.9 GPa at 250 °C) together with the highest Tg. This combination of high stiffness and thermal resistance is particularly advantageous for sustaining high-field energy storage performance at elevated temperatures. Moreover, thermogravimetric analysis (TGA) revealed that TAPM/M-PI possesses the highest weight-loss temperature (Tem5%) and char yield (Fig. 4f), indicating superior resistance to thermal decomposition and oxidative degradation. The TAPM-induced spatial confinement network not only enhances the intrinsic structural and thermal stability of the PI matrix but also establishes a robust physical framework that supports high-temperature dielectric performance.
2.3. Insulation mechanisms and breakdown reinforcement
Stable dielectric polarization at elevated temperatures is critical to evaluating the thermal operating limit of dielectric films. As evidenced by the temperature-dependent dielectric spectra (Fig. S20 and S21), the tri-directional polymer network suppresses εr and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ as a typical feature of trifunctional structures.49 Below 100 °C, the drop in εr is attributed to moisture desorption—a typical feature of semi-aromatic PIs. The εr value remains nearly constant up to 255 °C, whereas tan
δ as a typical feature of trifunctional structures.49 Below 100 °C, the drop in εr is attributed to moisture desorption—a typical feature of semi-aromatic PIs. The εr value remains nearly constant up to 255 °C, whereas tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ increases around 150 °C due to β-relaxation and rises sharply over 255 °C owing to α-relaxation. With its high Tg the TAPM-based system delays α-relaxation to 270 °C and maintains stable dielectric performance at 250 °C. At high temperatures (Fig. S22–S24), εr and tan
δ increases around 150 °C due to β-relaxation and rises sharply over 255 °C owing to α-relaxation. With its high Tg the TAPM-based system delays α-relaxation to 270 °C and maintains stable dielectric performance at 250 °C. At high temperatures (Fig. S22–S24), εr and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ decline steadily with increasing frequency due to limited dipole response. At room temperature, moisture contributes to noticeable dielectric loss and relaxation polarization. A higher confinement center density further reduces εr and slightly increases tan
δ decline steadily with increasing frequency due to limited dipole response. At room temperature, moisture contributes to noticeable dielectric loss and relaxation polarization. A higher confinement center density further reduces εr and slightly increases tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, while both systems exhibit a minimum tan
δ, while both systems exhibit a minimum tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at the 200
δ at the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Importantly, the spatially non-conjugated TAPM enhances dipole-orientation freedom and local polarization to increase εr, whereas carrier confinement suppresses conduction loss to reduce tan
1 ratio. Importantly, the spatially non-conjugated TAPM enhances dipole-orientation freedom and local polarization to increase εr, whereas carrier confinement suppresses conduction loss to reduce tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ.
δ.
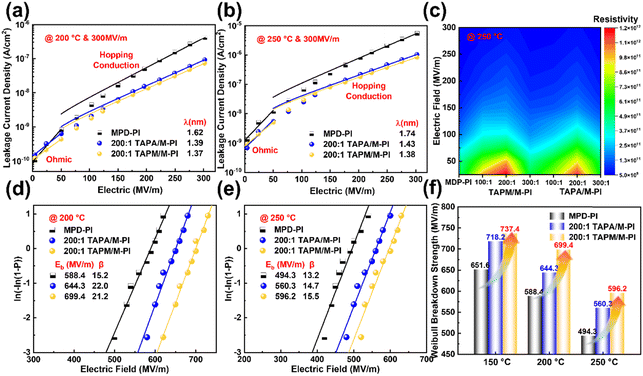
Benefiting from a relatively wide Eg, the PI films exhibit a typical Ohmic conduction behavior below 50 MV m−1, while transitioning to a hopping conduction mechanism at higher electric fields (Fig. 5a and 6b and Fig. S25, S26). Unlike the linear conduction at low fields, high-temperature hopping transport becomes strongly restricted by the aggregate-state microstructure.50,51 Compared with MPD-PI, the TAPA-based systems show a moderately reduced hopping distance (λ), attributed to the introduction of intermediate trap states and the limited spatial confinement network. More notably, the incorporation of TAPM induces segmental spatial divergence and spatial confinement through its rigid sp3-centered molecular geometry, significantly shortening the effective carrier-hopping path. As a result, the TAPM/M-PI films exhibit a remarkably low leakage current of 8.2 × 10−7 A cm−2 at 250 °C and 300 MV m−1, nearly one order of magnitude lower than that of MPD-PI (5.5 × 10−6 A cm−2). λ decreases to 1.37 nm and 1.38 nm at 200 °C and 250 °C, respectively. This structural feature contributes to reduced leakage-related conduction losses and enhances the dielectric stability of the film. Furthermore, the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPM/M-PI film exhibits the highest bulk resistance (Fig. 5c), further confirming its strong suppression of thermally activated electrical conduction.
1 TAPM/M-PI film exhibits the highest bulk resistance (Fig. 5c), further confirming its strong suppression of thermally activated electrical conduction.
Weibull analysis reveals that the introduction of the spatial architecture significantly improves the breakdown strength (Eb) of semi-aromatic PI films. The optimal Eb is achieved at a comonomer ratio of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in both the TAPA and TAPM systems (Fig. 5d, e and Fig. S27, S28). In contrast, the TAPA-based films exhibit limited improvement due to restricted segmental disorders arising from their planar π-conjugated structure. As shown in Fig. 5f, the TAPM/M-PI films achieve Eb values of 737.4, 699.4, and 596.2 MV m−1 at 150, 200, and 250 °C, respectively, along with the highest Weibull shape parameter (β) that indicates the most stable breakdown behavior. This enhanced reliability is closely linked to the film's superior high-temperature mechanical and insulating properties. The performance enhancement arises from the rigid deplanarized structure of TAPM, which twists chain segments and induces a spatially disordered architecture in the PI. This spatial confinement network effectively suppresses carrier migration and stabilizes the aggregated structure of PI under high-temperature and high-field conditions.
1 in both the TAPA and TAPM systems (Fig. 5d, e and Fig. S27, S28). In contrast, the TAPA-based films exhibit limited improvement due to restricted segmental disorders arising from their planar π-conjugated structure. As shown in Fig. 5f, the TAPM/M-PI films achieve Eb values of 737.4, 699.4, and 596.2 MV m−1 at 150, 200, and 250 °C, respectively, along with the highest Weibull shape parameter (β) that indicates the most stable breakdown behavior. This enhanced reliability is closely linked to the film's superior high-temperature mechanical and insulating properties. The performance enhancement arises from the rigid deplanarized structure of TAPM, which twists chain segments and induces a spatially disordered architecture in the PI. This spatial confinement network effectively suppresses carrier migration and stabilizes the aggregated structure of PI under high-temperature and high-field conditions.
2.4. High-temperature capacitive energy storage performance
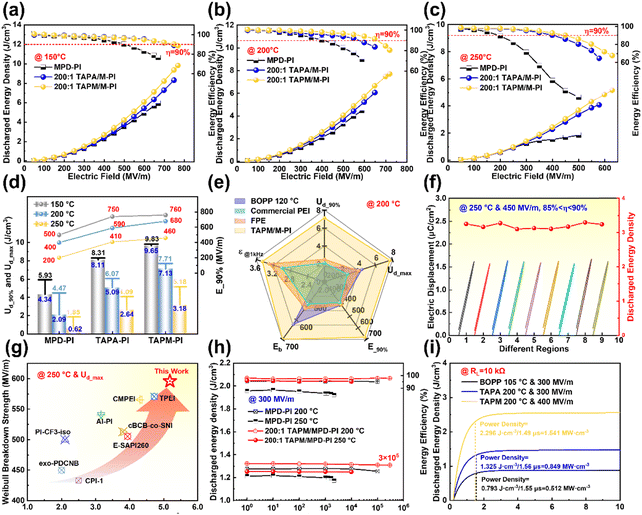
Fig. S29–S34 illustrate the electric displacement–electric field (D–E) loops of all samples. Although the MPD-PI system exhibits the highest maximum displacement (Dm), the incorporation of hole-trapping energy levels and a certain degree of a confinement network in the TAPA copolymer effectively captures charge carriers, thereby significantly suppressing conduction losses induced by polarization processes. In contrast, the spatial chain-segment disorder introduced by TAPM further suppresses CTC formation and significantly blocks carrier hopping pathways, thereby leading to more pronounced loss suppression. As a result, both systems display notably narrower D–E loops. Fig. 6a–c and Fig. S35 further evaluate the energy storage performance of all PI films at elevated temperatures. Notably, performance enhancement as a function of comonomer ratio is non-linear, with 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 emerging as the optimal formulation. At η = 90% efficiency, two critical metrics were examined: discharged energy density (Ud_90%) and the corresponding electric field (E_90%). The 200
1 emerging as the optimal formulation. At η = 90% efficiency, two critical metrics were examined: discharged energy density (Ud_90%) and the corresponding electric field (E_90%). The 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPA/M-PI film shows intermediate performance, whereas the 200
1 TAPA/M-PI film shows intermediate performance, whereas the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPM/M-PI film consistently delivers the best performance across all tested temperatures (Fig. 6d). Specifically, the TAPM/M-PI film achieves E_90% values of 760, 680, and 460 MV m−1 and the corresponding Ud_90% values of 9.65, 7.13, and 3.18 J cm−3 at 150, 200, and 250 °C, respectively, with the 250 °C value representing a 513% enhancement compared to that of MPD-PI. More importantly, the TAPM/M-PI film shows a remarkable maximum discharge energy density (Ud_max) of 5.18 J cm−3 at 250 °C. Compared to the representative commercial polymer dielectric films such as biaxially oriented polypropylene (BOPP), polyetherimide (PEI), and fluorene polyester (FPE), the 200
1 TAPM/M-PI film consistently delivers the best performance across all tested temperatures (Fig. 6d). Specifically, the TAPM/M-PI film achieves E_90% values of 760, 680, and 460 MV m−1 and the corresponding Ud_90% values of 9.65, 7.13, and 3.18 J cm−3 at 150, 200, and 250 °C, respectively, with the 250 °C value representing a 513% enhancement compared to that of MPD-PI. More importantly, the TAPM/M-PI film shows a remarkable maximum discharge energy density (Ud_max) of 5.18 J cm−3 at 250 °C. Compared to the representative commercial polymer dielectric films such as biaxially oriented polypropylene (BOPP), polyetherimide (PEI), and fluorene polyester (FPE), the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPM/M-PI exhibits significant advantages in Ud_90%, Ud_max, E_90%, Eb and εr (Fig. 6e and Fig. S36). In addition, this film demonstrates excellent flexibility and film-forming ability (Fig S37). Random sampling from nine different regions of a large-area membrane (Fig. 6f) further confirms that even under harsh conditions of 250 °C and 450 MV m−1, the discharged energy density remains stable around 2.4 J cm−3, highlighting its outstanding performance stability and spatial uniformity.
1 TAPM/M-PI exhibits significant advantages in Ud_90%, Ud_max, E_90%, Eb and εr (Fig. 6e and Fig. S36). In addition, this film demonstrates excellent flexibility and film-forming ability (Fig S37). Random sampling from nine different regions of a large-area membrane (Fig. 6f) further confirms that even under harsh conditions of 250 °C and 450 MV m−1, the discharged energy density remains stable around 2.4 J cm−3, highlighting its outstanding performance stability and spatial uniformity.
Encouragingly, as shown in Fig. 6g and Fig. S37, the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPM/M-PI copolymer film exhibits remarkable advantages in both energy storage and dielectric breakdown strength at 250 °C and 200 °C, outperforming most state-of-the-art polymer dielectrics reported.21,22,27,52–56 As shown in Fig. 6h, the film retains stable charge–discharge performance over 104 cycles at 250 °C (MPD-PI only 2145 cycles) and endures more than 3 × 105 cycles at 200 °C under 300 MV m−1, far exceeding the 105-cycle endurance of MPD-PI. To further evaluate its power density potential, we conducted rapid discharge tests (Fig. 6i and Fig. S39). Under 200 °C and 400 MV m−1, the 200
1 TAPM/M-PI copolymer film exhibits remarkable advantages in both energy storage and dielectric breakdown strength at 250 °C and 200 °C, outperforming most state-of-the-art polymer dielectrics reported.21,22,27,52–56 As shown in Fig. 6h, the film retains stable charge–discharge performance over 104 cycles at 250 °C (MPD-PI only 2145 cycles) and endures more than 3 × 105 cycles at 200 °C under 300 MV m−1, far exceeding the 105-cycle endurance of MPD-PI. To further evaluate its power density potential, we conducted rapid discharge tests (Fig. 6i and Fig. S39). Under 200 °C and 400 MV m−1, the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPM/M-PI film achieved a fast discharge time (τ0.9, the time required to release 90% of the maximum stored energy) of 1.49 µs and a power density of 1.541 MW cm−3. At 300 MV m−1, τ0.9 and power density were 1.53 µs and 0.876 MW cm−3, respectively. In comparison, the 200
1 TAPM/M-PI film achieved a fast discharge time (τ0.9, the time required to release 90% of the maximum stored energy) of 1.49 µs and a power density of 1.541 MW cm−3. At 300 MV m−1, τ0.9 and power density were 1.53 µs and 0.876 MW cm−3, respectively. In comparison, the 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TAPA/M-PI film exhibited a τ0.9 of 1.56 µs and a power density of 0.849 MW cm−3 under 200 °C and 300 MV m−1. Meanwhile, the commercial BOPP film showed inferior performance with a power density of only 0.512 MW cm−3 and τ0.9 of 1.55 µs at 120 °C. These results underscore the excellent energy responsiveness of TAPM-based polymer films under extreme conditions, highlighting their great potential for high-temperature pulsed capacitor and next-generation energy storage applications.
1 TAPA/M-PI film exhibited a τ0.9 of 1.56 µs and a power density of 0.849 MW cm−3 under 200 °C and 300 MV m−1. Meanwhile, the commercial BOPP film showed inferior performance with a power density of only 0.512 MW cm−3 and τ0.9 of 1.55 µs at 120 °C. These results underscore the excellent energy responsiveness of TAPM-based polymer films under extreme conditions, highlighting their great potential for high-temperature pulsed capacitor and next-generation energy storage applications.
3. Conclusions
In this work, trifunctional monomers were incorporated into semi-aromatic MPD-PI via copolymerization, enabling molecular-level control over chain-segment disorder and thereby enhancing high-temperature dielectric performance. TAPM, with its sp3-centered tri-directional geometry, induces chain divergence and enhances conformational disorder in PI, thereby disrupting short-range π–π ordering and suppressing carrier migration. At the same time, the rigid TAPM centre constructs a spatial confinement network that not only markedly widens the bandgap but also significantly improves high-temperature thermal stability. In summary, TAPM/M-PI films demonstrate remarkable high-temperature energy storage capability. At 200 °C, they deliver 7.13 J cm−3 with 90% efficiency, representing a 341% enhancement compared to that of MPD-PI, while enduring 3 × 105 charge–discharge cycles at 300 MV m−1. Even under the more demanding condition of 250 °C, the films retain an Eb of 596 MV m−1 and achieve 5.18 J cm−3 (280% improvement). These results far surpass the performance benchmarks of conventional fully aromatic PIs and PEI.Author contributions
D. H., H. L., H. R. X, and D. Z. conceived and developed the ideas. D. H. designed the experiments, and synthesized and purified the materials. D. H. performed device fabrication and conducted the measurement. G. H. H., X. C., Y. T. W., F. W., X. N. L., and H. W. supported the experimental process and data analysis. D. H. wrote the manuscript and D. H. supervised by H. L., H. R. X., and D. Z. conceived and directed the project. All authors commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ee05932a.
Acknowledgements
The authors acknowledge the support provided by the National Natural Science Foundation of China (52172265 and 52002404), Excellent Youth Science Foundation of Hunan Province (2022JJ20067), the Science and Technology Innovation Program of Hunan Province (2022RC1074), and the State Key Laboratory of Powder Metallurgy, Central South University, Changsha, China.Notes and references
- J. Chen, Y. Zhou, X. Huang, C. Yu, D. Han, A. Wang, Y. Zhu, K. Shi, Q. Kang, P. Li, P. Jiang, X. Qian, H. Bao, S. Li, G. Wu, X. Zhu and Q. Wang, Nature, 2023, 615, 62–66 CrossRef CAS PubMed.
- L. Zhou, S. Zhao, P. Xie, X. Miao, S. Liu, N. Sun, M. Guo, Z. Xu, T. Zhong and Y. Shen, Appl. Phys. Rev., 2023, 10, 031310 CAS.
- J.-W. Zha, M. Xiao, B. Wan, X. Wang, Z.-M. Dang and G. Chen, Prog. Mater. Sci., 2023, 140, 101208 CrossRef CAS.
- M. Feng, Y. Liu, X. Wu, Y. Xing and Q. Chi, Prog. Mater. Sci., 2025, 152, 101458 CrossRef CAS.
- Y. Liu, H. Luo, H. Xie, Z. Xiao, F. Wang, X. Jiang, X. Zhou and D. Zhang, Microstructures, 2023, 3, 2023008 CAS.
- H. Pan, S. Lan, S. Xu, Q. Zhang, H. Yao, Y. Liu, F. Meng, E.-J. Guo, L. Gu, D. Yi, X. Renshaw Wang, H. Huang, J. L. MacManus-Driscoll, L.-Q. Chen, K.-J. Jin, C.-W. Nan and Y.-H. Lin, Science, 2021, 374, 100–104 CrossRef CAS PubMed.
- Y. Wang, Z. Bao, S. Ding, J. Jia, Z. Dai, Y. Li, S. Shen, S. Chu, Y. Yin and X. Li, Adv. Mater., 2024, 36, 2308597 CrossRef CAS PubMed.
- T. Zhu, H. Zhao, N. Zhang, C. Zhang, L. Yin, Z.-M. Dang and J. Bai, Adv. Energy Mater., 2023, 13, 2203587 CrossRef CAS.
- Z. Xie, L. Fan, H. Li, Z. Ran, S. Lai, X. Liu, A. Deatherage, Y. Wang, Q. Li, Y. Yin and Y. Liu, Prog. Polym. Sci., 2025, 164, 101957 CrossRef CAS.
- W. Xu, C. Zhou, W. Ji, Y. Zhang, Z. Jiang, F. Bertram, Y. Shang, H. Zhang and C. Shen, Angew. Chem., Int. Ed., 2024, 63, e202319766 CrossRef CAS PubMed.
- M. Yang, C. Wan, L. Zhou, X. Li, J. Pan, H. Li, J. Wang, W. Ren, B. Sun, E. Xu, Y. Xiao, M. Guo, M. Zhang, X. Li, J. Jiang, P. Hu, L. Duan, C.-W. Nan, Z. Shen, X. Wang and Y. Shen, Nat. Energy, 2025, 10(11), 1323–1333 CrossRef CAS.
- X. Dong, B. Wan and J.-W. Zha, Chem. Rev., 2024, 124, 7674–7711 CrossRef CAS PubMed.
- G. He, X. Li, H. Luo, D. Zhang and S. Zhang, Adv. Mater., 2025, 37, 2419563 CrossRef CAS PubMed.
- J. Chen, Z. Pei, Y. Liu, K. Shi, Y. Zhu, Z. Zhang, P. Jiang and X. Huang, Adv. Mater., 2023, 35, 2306562 CrossRef CAS PubMed.
- Z. Li, C. Wu, L. Chen, Y. Wang, Z. Mutulu, H. Uehara, J. Zhou, M. Cakmak, R. Ramprasad and Y. Cao, Adv. Mater., 2024, 36, 2310497 CrossRef CAS PubMed.
- J. Li, S. Wang, Y. Zhu, Z. Luo, Y.-R. Zhang, Q. Shao, H. Quan, M. Wang, S. Hu, M. Yang, J. Fu, R. Wang, J. Hu, H. Yuan, J. He and Q. Li, J. Mater. Chem. A, 2023, 11, 10659–10668 RSC.
- Y. Zhou, Y. Chen, Y. Cui, Y. Li, Z. Li, C. Zhou, L. Cheng and W. Liu, Energy Storage Mater., 2024, 72, 103715 CrossRef.
- L. Sun, F. Zhang, L. Li, J. Liang, J. Dong, Z. Pan, Y. Niu, J. Chen, Y. Liu, Y. Lu, K. Wu, Q. Li, J. Li, Q. Wang and H. Wang, Adv. Mater., 2025, 37, 2412561 CrossRef CAS PubMed.
- X. Li, P. Hu, J. Jiang, J. Pan, C.-W. Nan and Y. Shen, Adv. Mater., 2025, 37, 2411507 CrossRef CAS PubMed.
- Q.-K. Feng, S.-L. Zhong, J.-Y. Pei, Y. Zhao, D.-L. Zhang, D.-F. Liu, Y.-X. Zhang and Z.-M. Dang, Chem. Rev., 2022, 122, 3820–3878 CrossRef CAS PubMed.
- Y. Wan, H. Luo, Z. Yan, S. Shen, J. Peng, X. Li, G. He, D. Zhang and J.-W. Zha, Nat. Commun., 2025, 16, 6242 CrossRef CAS PubMed.
- G. He, H. Luo, Y. Liu, Y. Wan, B. Peng, D. Hu, F. Wang, X. Li, J. Peng, H. Wang and D. Zhang, Energy Environ. Sci., 2025, 18, 2405–2414 RSC.
- K. Fan, X. Li, X. Liu, X. He and Z.-M. Dang, Adv. Mater., 2025, 37, 2417181 CrossRef CAS PubMed.
- R. Gurnani, S. Shukla, D. Kamal, C. Wu, J. Hao, C. Kuenneth, P. Aklujkar, A. Khomane, R. Daniels, A. A. Deshmukh, Y. Cao, G. Sotzing and R. Ramprasad, Nat. Commun., 2024, 15, 6107 CrossRef CAS PubMed.
- M. Yang, W. Ren, Z. Jin, E. Xu and Y. Shen, Nat. Commun., 2024, 15, 8647 CrossRef CAS PubMed.
- W. Ren, H. Tong, S. Cao, S. Zhao, M. Yang, X. Li, J. Pan, N. Sun, Y. Xiao, E. Xu, C.-W. Nan and Y. Shen, Adv. Mater., 2025, e05296, DOI:10.1002/adma.202505296.
- W. Ren, M. Yang, M. Guo, L. Zhou, J. Pan, Y. Xiao, E. Xu, C.-W. Nan and Y. Shen, Energy Storage Mater., 2024, 65, 103095 CrossRef.
- B. Wan, J.-W. Zha and Z.-M. Dang, Prog. Polym. Sci., 2025, 169, 102014 CrossRef CAS.
- W. Sima, Y. Mai, P. Sun, M. Yang, T. Yuan, B. Chen and Y. Yang, Energy Storage Mater., 2025, 74, 103974 CrossRef.
- D. Ai, H. Li, Y. Zhou, L. Ren, Z. Han, B. Yao, W. Zhou, L. Zhao, J. Xu and Q. Wang, Adv. Energy Mater., 2020, 10, 1903881 CrossRef CAS.
- M. Yang, Y. Zhao, Z. Wang, H. Yan, Z. Liu, Q. Li and Z.-M. Dang, Energy Environ. Sci., 2024, 17, 1592–1602 RSC.
- B. Zhang, X.-m Chen, Z. Pan, P. Liu, M. Mao, K. Song, Z. Mao, R. Sun, D. Wang and S. Zhang, Adv. Funct. Mater., 2023, 33, 2210050 CrossRef CAS.
- M. Yang, F. Yuan, W. Shi, W. Ren, M. Guo, C. Ouyang, L. Zhou, N. Sun, Y. Xiao, E. Xu, X. Zhang, Y. Wei, X. Deng, C. Nan, X. Wang and Y. Shen, Adv. Funct. Mater., 2023, 33, 2214100 CrossRef CAS.
- M. Liu, J. Song, H. Qin, S. Qin, Y. Zhang, W. Xia, C. Xiong and F. Liu, Adv. Funct. Mater., 2024, 34, 2313258 CrossRef CAS.
- H. Jiang, D. Zheng, H. Ye and L. Xu, Adv. Funct. Mater., 2025, 35, 2418466 CrossRef CAS.
- L. Li, Z. Han, H. P. Yennawar, Y. Cheng, T. Han, R. Feng, Y. Zhang, G. Zhao, Q. Wang and L. Dong, Adv. Sci., 2025, 12, 2414380 CrossRef CAS PubMed.
- L. Li, J. Cheng, Y. Cheng, T. Han, Y. Liu, Y. Zhou, G. Zhao, Y. Zhao, C. Xiong, L. Dong and Q. Wang, Adv. Mater., 2021, 33, 2102392 CrossRef CAS PubMed.
- H. Ye, H.-j Kwon, Y. Kim, S. B. Park, R. Wang, H. Benliang, J.-e Gwon, K. Wu, Y. Wu, H. Zhang, D. W. Chang, B. Lim, S. W. Lee and S. H. Kim, Adv. Funct. Mater., 2025, 35, 2412418 CrossRef CAS.
- H. Li, M. R. Gadinski, Y. Huang, L. Ren, Y. Zhou, D. Ai, Z. Han, B. Yao and Q. Wang, Energy Environ. Sci., 2020, 13, 1279–1286 RSC.
- B. Zou, S. Zhao, F. Bao, L. Zhou, B. He, Y. Zhao, J. Zhang, W. Peng, Y. Shen, M. Huang and C.-W. Nan, Adv. Funct. Mater., 2025, 35, 2505254 CrossRef CAS.
- Y. Wan, P. Hu, B. Sun, S. Shen, H. Luo, S. Zhang, D. Zhang, C.-W. Nan and Y. Shen, Adv. Funct. Mater., 2025, 2506635, DOI:10.1002/adfm.202506635.
- S. Fratini, M. Nikolka, A. Salleo, G. Schweicher and H. Sirringhaus, Nat. Mater., 2020, 19, 491–502 CrossRef CAS PubMed.
- J. Dong, L. Li, P. Qiu, Y. Pan, Y. Niu, L. Sun, Z. Pan, Y. Liu, L. Tan, X. Xu, C. Xu, G. Luo, Q. Wang and H. Wang, Adv. Mater., 2023, 35, 2211487 CrossRef CAS PubMed.
- K. Kanosue, R. n Augulis, D. Peckus, R. Karpicz, T. Tamulevičius, S. Tamulevičius, V. Gulbinas and S. Ando, Macromolecules, 2016, 49, 1848–1857 CrossRef CAS.
- R. Wang, Y. Zhu, S. Huang, J. Fu, Y. Zhou, M. Li, L. Meng, X. Zhang, J. Liang, Z. Ran, M. Yang, J. Li, X. Dong, J. Hu, J. He and Q. Li, Nat. Mater., 2025, 24, 1074–1081 CrossRef CAS PubMed.
- Y. Wang, T. Hasegawa, H. Matsumoto, T. Mori and T. Michinobu, Adv. Funct. Mater., 2017, 27, 1604608 CrossRef.
- Y. Wang, T. Hasegawa, H. Matsumoto and T. Michinobu, J. Am. Chem. Soc., 2019, 141, 3566–3575 CrossRef CAS PubMed.
- F. Wang, H. Wang, X. Shi, C. Diao, C. Li, W. Li, X. Liu, H. Zheng, H. Huang and X. Li, Chem. Eng. J., 2024, 485, 149972 CrossRef CAS.
- B. Wan, M. Xiao, X. Dong, X. Yang, M.-S. Zheng, Z.-M. Dang, G. Chen and J.-W. Zha, Adv. Mater., 2024, 36, 2304175 CrossRef CAS PubMed.
- J.-Y. Pei, L.-J. Yin, S.-L. Zhong and Z.-M. Dang, Adv. Mater., 2023, 35, 2203623 CrossRef CAS PubMed.
- M. Yang, Y. Zhao, H. Yan, Z. Wang, C. Xu, C. Zhang, E. Bilotti, J. Li and Z.-M. Dang, Energy Environ. Sci., 2024, 17, 7627–7648 RSC.
- Y. Tian, M.-S. Zheng, Y. Li, C. Xu, Y. Zhang, W. Liu, Z.-M. Dang and J.-W. Zha, Mater. Horiz., 2023, 10, 5835–5846 RSC.
- J. Hao, S. Shukla, R. Gurnani, M. Mukherjee, H. Sahu, A. Khomane, P. Aklujkar, M. Desai, C. Wu, R. Ramprasad, G. Sotzing and Y. Cao, Adv. Mater., 2025, 37, 2417625 CrossRef CAS PubMed.
- R. Wang, Y. Zhu, J. Fu, M. Yang, Z. Ran, J. Li, M. Li, J. Hu, J. He and Q. Li, Nat. Commun., 2023, 14, 2406 CrossRef CAS PubMed.
- Y. Wan, H. Luo, Z. Yan, J. Peng, G. He, X. Li, F. Wang, Z. Ran, D. Zhang and Q. Li, Adv. Mater., 2025, e10122, DOI:10.1002/adma.202510122.
- W. Xu, F. Yang, G. Zhao, S. Zhang, G. Rui, M. Zhao, L. Liu, L.-Q. Chen and Q. Wang, Energy Environ. Sci., 2024, 17, 8866–8873 RSC.
| This journal is © The Royal Society of Chemistry 2026 |