Dielectric molecular-bridges enable durable inverted perovskite solar cells with 26.60% efficiency and a high reverse breakdown voltage
Chuan
Luo
a,
Yu
Chen
*a,
Xing
Wu
a,
Yang
Peng
a,
Jing
Zhou
a,
Yuwei
Duan
a,
Yang
Shen
b,
Hongxiang
Li
c,
Yihui
Wu
 d and
Qiang
Peng
d and
Qiang
Peng
 *ad
*ad
aCollege of Materials, Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu, 610059, P.R. China. E-mail: yuchen7@cdut.edu.cn; qiangpeng@scu.edu.cn
bSchool of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, P. R. China
cCollege of Polymer Science and Engineering, Sichuan University, Chengdu, 610065, P. R. China
dSchool of Chemical Engineering and National Key Laboratory of Advanced Polymer Materials, Sichuan University, Chengdu 610065, P. R. China
First published on 26th November 2025
Abstract
Halogen-induced defects originating from the soft lattices of perovskites are an important factor affecting the quality and stability of perovskite films, especially at buried interfaces. Herein, we propose a dielectric molecular-bridge strategy, which employs bis(4-fluorophenyl)chlorophosphine (F-CPP) to tailor the crystallization of perovskites, inhibit ion migration, regulate the interfacial band arrangement and passivate nonradiative recombination. Interestingly, this strategy can also improve the dielectric constants of perovskites and the reverse-bias stability. The champion device achieves a power conversion efficiency (PCE) of 26.60% with a maximum transient reverse breakdown voltage of −6.6 V, whereas large-area and wide-bandgap devices also exhibit PCEs of 24.08% (1 cm2), 22.56% (1.68 eV), 20.40% (1.73 eV) and 20.19% (1.78 eV). Moreover, under −1 V reverse-bias testing conditions, unencapsulated devices maintain 90.5%, 82.7% and 93.5% of their initial efficiencies after long-term storage, continuous thermal aging, and light soaking, respectively. This work demonstrates a feasible dielectric molecular-bridge strategy for improving the efficiency and stability of perovskite solar cells.
Broader contextIn the actual operation of solar cells, solar cells encounter shady conditions due to the presence of obstructions, causing them to be connected in series as loads in a circuit and thus subjected to reverse bias voltage. When the reverse bias voltage reaches a certain value, it will seriously affect the efficiency of solar cells and even cause irreversible damage. In order to enable perovskite solar cells to be widely applied in commercial photovoltaics, there are still some key challenges that need to be addressed, especially to improve their reverse breakdown voltages and operational stability. Herein, we judiciously screen and introduce a multifunctional dielectric molecule, namely bis(4-fluorophenyl)chlorophosphine (F-CPP), into the buried interface to address this challenge. The results showed that by using F-CPP, the dielectric constant of the perovskite film and the dielectric capacitance of a device were both increased two-fold, and the efficiency and stability of the corresponding device were greatly improved, especially in terms of the reverse-bias stability. Specifically, the interaction between the functional molecule with high electronegativity and the buried perovskite leads to a change in the dielectric properties. |
Introduction
With the development of photovoltaic (PV) devices, organic–inorganic hybrid perovskite solar cells (PSCs) have received widespread attention due to their low cost, high efficiency and solution-processable fabrication methods, with the certified power conversion efficiencies (PCEs) of champion devices being pushed above 26%.1–5 High-quality perovskite films are critical for the preparation of efficient and stable PSCs. However, the defects and interstices are mainly abundant at interfaces, especially at the neglected buried interface. The buried perovskite interface possesses a higher defect density and deviated stoichiometry compared with the bulk, which has a profound effect on the crystallinity and physicochemical properties of perovskite films.6–8As previously reported, trap-assisted defects at the buried interface are concentrated in the form of halide vacancies, Pb clusters, undercoordinated I−, undercoordinated Pb2+, Pb–I antisite defects (PbI3−) and so on.9 Among these, undercoordinated I− is easily oxidized to volatile bimolecular iodine (I2), which can be consumed to form irreversible iodine vacancies and trigger more undercoordinated Pb2+.10 It can also be seen that these defects are caused by halide migration or volatilization, resulting in halogen-induced defects that pose a major threat to all perovskite films. Additionally, the presence of halogen-induced defects in perovskites often leads to the formation of a large number of migration channels at the charge transport layer/perovskite interface. It is easy for electrons to tunnel when a reverse bias is applied to the device, resulting in a large current and damage to the target device.11 These factors can lead to severe chemical chain reactions and accelerate the degradation of perovskite films, and they are not conducive to further improvements in the efficiency and stability of resulting devices. To overcome the above issues, a passivator with diverse functional units and dielectric properties is desirable. In general, passivators with chloride (Cl) units can alter the electronic properties of target films and repair defects at the interface and/or grain boundaries.12,13 Hu et al. optimized the molecular configuration of tris-(4-chlorophenyl)benzene to enable multiple Cl atoms in optimal spatial positions to maximize their binding with surface defects.14 Sargent et al. employed 4-chlorobenzenesulfonate (4Cl-BZS) to enable better dual-site Pb2+ passivation compared with other benzenesulfonate ligands, arising from the stronger adsorption energy induced by the contained Cl functional units.1 However, the binding force between Cl units in molecules and perovskites was still insufficient, which is not conducive to lattice stabilization and halogen-induced defect passivation.12
Compared with I and Cl units, fluorine units (F) feature higher electronegativity, which can enhance the binding strength with the perovskite surface.15 Sargent et al. demonstrated that the increased electronegativity of F units was accompanied by stronger binding energies and higher anion migration barriers.16 Chen et al. adopted perfluorinated perfluorotriethylamine (PFTEA) to immobilize lead and passivate the surface defects of perovskite films, thereby promoting defect passivation and improving device performance.17 Meanwhile, F units could increase the dielectric constant of the target film due to the fact that the electronegativity and dielectric properties were positively correlated, which is expected to raise the transient reverse breakdown voltage (Vbv) and improve the long-term reverse-bias stability of devices.18–20 Unfortunately, there were no relevant studies in PSCs. When combining the characteristics of Cl and F units in search of a passivator containing dual-halogen units as a dielectric molecular-bridge at the buried interface, it is of great significance that halogen-induced defects are synergically passivated to improve the photovoltaic performance and device stability.
Herein, we developed bis(4-fluorophenyl)chlorophosphine (F-CPP) to serve as a dielectric molecular-bridge at the buried interface. Due to the Lewis-base properties, F-CPP can effectively link to the substrate, passivate halogen-induced defects, and tailor perovskite crystallization. Interestingly, the insertion of F units significantly improves the dielectric properties of perovskite films, thereby regulating the interfacial band arrangement and increasing transient Vbv to −6.6 V. As a result, the corresponding inverted PSCs deliver an outstanding PCE of 26.60% (certified as 26.17%), along with excellent long-term operational and reverse-bias stability. This strategy can also be extended to large-area devices and other perovskite systems, delivering PCEs of 24.08% (1 cm2), 22.56% (1.68 eV), 20.40% (1.73 eV) and 20.19% (1.78 eV).
Results and discussion
Functional molecule selection and interaction mechanism
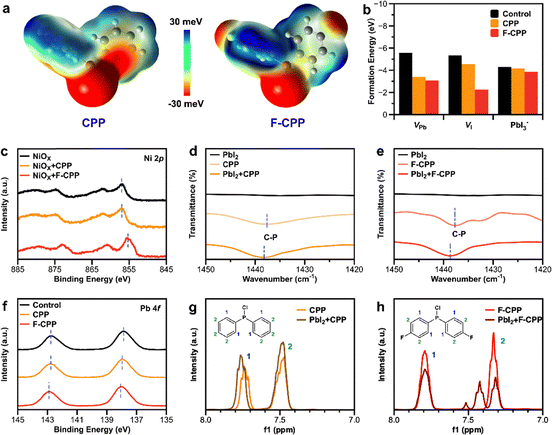
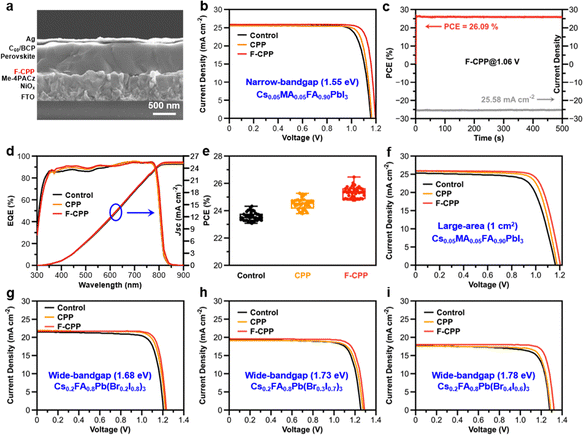
As the binding energy between P units and the perovskite was found to be the strongest among the functional units tested, we selected chlorodiphenylphosphine (CPP) and F-CPP to pre-treat the buried interface, respectively. Density functional theory (DFT) calculations were first used to study the electrostatic potentials (ESPs) of both molecules.21Fig. 1a shows that F-CPP exhibited more negative ESP (red) and more positive ESP (blue) due to its higher electronegativity, and the area containing Cl atoms corresponded to the negative dipole (Fig. S1, SI). This meant that F-CPP with Lewis-base properties had a stronger ability to donate electron pairs to the Lewis-acid sites in perovskites. In order to study the passivation ability of defects, DFT calculations were used again to calculate the various defect formation energies (Eform) of both molecules adsorbed on the surface of perovskites (Fig. S2 and S3, SI). The target molecules were adsorbed on the (100) plane of the perovskite. Fig. 1b shows the corresponding calculated point-defect formation energies: the F-CPP sample presented smaller Eform values than the control and CPP samples for VPb, VI and PbI3−. This meant that F-CPP had the most effective passivation ability.X-ray photoelectron spectroscopy (XPS) analysis was further conducted to uncover molecular bridging; the characteristic peak of C 1s located at 284.8 eV was used as the calibration peak to calibrate the binding energy scale (Fig. S4, SI). We first demonstrated the interactions between the substrate and as-selected functional molecules. The characteristic Ni 2p peak for the CPP- and F-CPP-treated substrate films exhibited an obvious shift in both cases (Fig. 1c) compared with the control substrate film. More XPS data also confirmed this interaction (Fig. S5 and Table S1, SI), where the positions of the Ni 2p peak were calculated to be 857.03 ± 0.0374, 856.91 ± 0.0286 and 855.27 ± 0.0460 eV for the NiOx, NiOx + CPP and NiOx + F-CPP substrates, respectively. Additionally, the characteristic O 1s peak could be further divided into lattice oxygen and adsorbed oxygen. The lattice oxygen peak exhibited a slight shift from 530.68 eV for NiOx to 530.63 and 530.58 eV for CPP- and F-CPP-treated substrate films (Fig. S6, SI), respectively, whereas the adsorbed oxygen peak also showed a similar shift. These results indicated that CPP and F-CPP were involved in a chemical interaction with exposed nickel oxide (NiOx) on the substrate, mainly relying on Ni2+.22 Due to the direct contact between (4-(3,6-dimethyl-9H-carbazole-9-yl)butyl)phosphonic acid (Me-4PACz) and the target molecules, we further conducted Fourier-transform infrared (FT-IR) and liquid-state hydrogen proton nuclear magnetic resonance (1H NMR) analyses to study this interaction. Fig. S7 and S8 (SI) show that there were no obvious shifts at 1158 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 950 cm−1 (P–OH) for Me-4PACz + F-CPP compared with Me-4PACz, which was indicative of almost no interaction between Me-4PACz and the target molecules. Meanwhile, we used atomic force microscopy-infrared spectroscopy (AFM-IR) to observe the distribution of Me-4PACz on the surface of substrate films with the architecture of FTO/NiOx/Me-4PACz. The IR stretching peak recorded at 1158 cm−1 corresponded to P
O) and 950 cm−1 (P–OH) for Me-4PACz + F-CPP compared with Me-4PACz, which was indicative of almost no interaction between Me-4PACz and the target molecules. Meanwhile, we used atomic force microscopy-infrared spectroscopy (AFM-IR) to observe the distribution of Me-4PACz on the surface of substrate films with the architecture of FTO/NiOx/Me-4PACz. The IR stretching peak recorded at 1158 cm−1 corresponded to P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups uniformly anchored on the substrate film (Fig. S9, SI), while some areas were still exposed due to weak adsorption and thermal annealing effects. These could provide bridging active sites for F-CPP and exposed NiOx.
O groups uniformly anchored on the substrate film (Fig. S9, SI), while some areas were still exposed due to weak adsorption and thermal annealing effects. These could provide bridging active sites for F-CPP and exposed NiOx.
Then, the bridging effect between both molecules and the buried perovskite film was further investigated. Upon peeling off the buried interface, the preserved characteristic peak of F 1s indicated that F-CPP was not washed away with subsequent solutions (Fig. S10, SI). FT-IR spectroscopy also confirmed that the characteristic P–C stretching vibration in F-CPP located at 1437.61 cm−1 was shifted to 1438.64 cm−1 for PbI2 + F-CPP (Fig. 1d and e). Additionally, the characteristic Pb 4f XPS peak for CPP- and F-CPP-treated buried-perovskite films showed a significant shift (Fig. 1f), and similar results could be found for the characteristic I 3d peak (Fig. S11, SI). The characteristic I 3d peak showed a shift from 630.16 eV for the control perovskite film to 630.27 and 630.43 eV for CPP- and F-CPP-treated buried-perovskite films, respectively. These results suggested that the as-selected molecules could effectively link to the buried perovskite and passivate halogen-induced defects (such as undercoordinated Pb2+ and PbI3−).23 H NMR spectra for different samples were subsequently measured. The characteristic proton signal from the ortho-hydrogen in CPP showed a significant shift from 7.74 ppm to 7.76 ppm, whereas the meta-hydrogen at 7.78 ppm had no shift (Fig. 1g). Meanwhile, the characteristic proton signal from the meta-hydrogen in F-CPP was split into two characteristic peaks, but ortho-hydrogen did not shift, which can be attributed to the strong electronegativity of F atoms stabilizing the vibration of ortho-hydrogen. This result demonstrated the efficient interaction between PbI2 and both molecules. Therefore, the as-selected functional molecules could effectively serve as a functional molecular bridge and passivate defects at the buried perovskite surface.
Morphologies and crystallinities of perovskite films
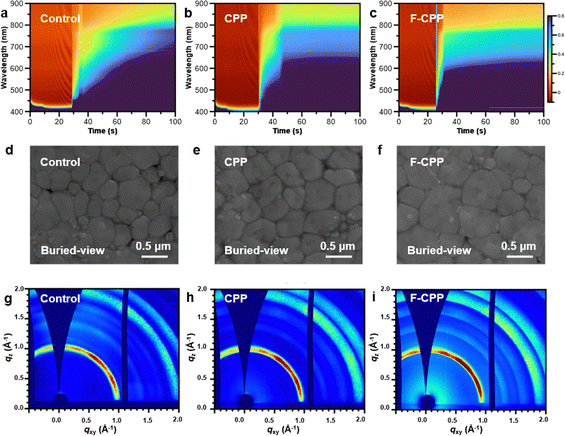
To investigate the effect on perovskite crystallization in detail, we conducted in situ ultraviolet-visible (UV-vis) absorption spectroscopy analysis. Fig. 2a–c and Fig. S12 (SI) show that the sharpest absorption-increase rate is seen for the F-CPP-treated perovskite film after dropping the antisolvent. Calculations of nucleation rates of control and F-CPP-treated samples using first derivatives further demonstrated that the nucleation rate of the F-CPP-treated perovskite film improved during the spin-coating process (Fig. S13, SI), which was favourable for achieving high-quality perovskite films with a large grain size.24 We subsequently observed the morphologies of buried perovskite films via scanning electron microscope (SEM) and an atomic force microscope (AFM) images. Fig. 2d–f and Fig. S14, S15 (SI) show that the CPP- and F-CPP-treated buried-perovskite films all exhibited a larger average grain size than the control buried-perovskite film. As shown in Fig. S16 and S17 (SI), similar results could be observed for the top-surface perovskite films. The corresponding cross-section SEM images (Fig. S18, SI) further displayed that the monolithic crystal of the F-CPP-treated perovskite film grew on the substrate without interstitial traps. Then, depth-resolved grazing-incidence wide-angle X-ray scattering (GIWAXS) was employed to evaluate the crystallinity of different perovskite films.25 From the perspective of the buried interface (Fig. 2g–i and Fig. S19, SI), the diffraction rings of the F-CPP-treated perovskite film at q values of 1 and 2 Å−1 showed gradually increased diffraction brightness compared with the control and CPP-treated perovskite films, corresponding to the (100) and (200) crystal planes of α-phase perovskites, respectively.26 This result demonstrated that perovskite films with F-CPP treatment had enhanced crystallinity.X-ray diffraction (XRD) further confirmed the enhancement of crystallinity and grain size for the F-CPP-treated perovskite film (Fig. S20, SI), matching well the above results. To obtain depth-dependent crystal structure information for different perovskite films, we measured the grazing-incidence X-ray diffraction (GIXRD) patterns.27 The control perovskite film showed a significant shift to lower angles in the range of 10° to 50°, where the diffraction peak at 2θ = 28.2° corresponded to the (200) plane of perovskites. Meanwhile, the CPP-treated perovskite film showed a slight shift and the F-CPP-treated film had no sign of shifts, indicating that residual stress in the F-CPP-treated perovskite film was efficiently released.28 Fig. S21 (SI) shows plots of 2θ vs. sin2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ψ extracted from different perovskite films. The corresponding residual strain (σM) values were calculated to be −47.11, 17.89 and 6.24 MPa for the control, CPP- and F-CPP-treated perovskite films (Note S1, SI), respectively. The GIXRD results revealed that residual stress could be effectively regulated through the dual-halogen passivation effect of F-CPP. Overall, the F-CPP-treated perovskite films featured large grain size, no interstitial traps, enhanced crystallinity and released residual stress, which might be the main reasons for obtaining high-quality perovskite films.
ψ extracted from different perovskite films. The corresponding residual strain (σM) values were calculated to be −47.11, 17.89 and 6.24 MPa for the control, CPP- and F-CPP-treated perovskite films (Note S1, SI), respectively. The GIXRD results revealed that residual stress could be effectively regulated through the dual-halogen passivation effect of F-CPP. Overall, the F-CPP-treated perovskite films featured large grain size, no interstitial traps, enhanced crystallinity and released residual stress, which might be the main reasons for obtaining high-quality perovskite films.
The effects of dielectric molecular bridges
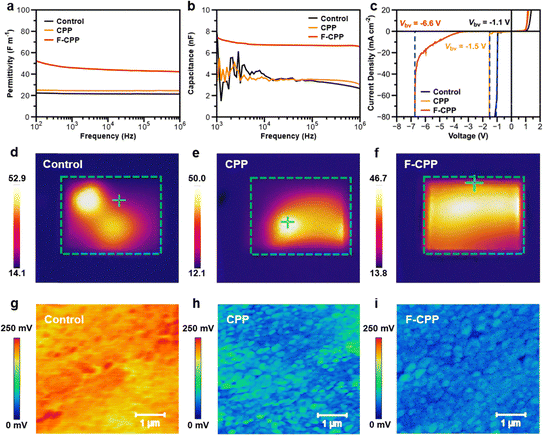
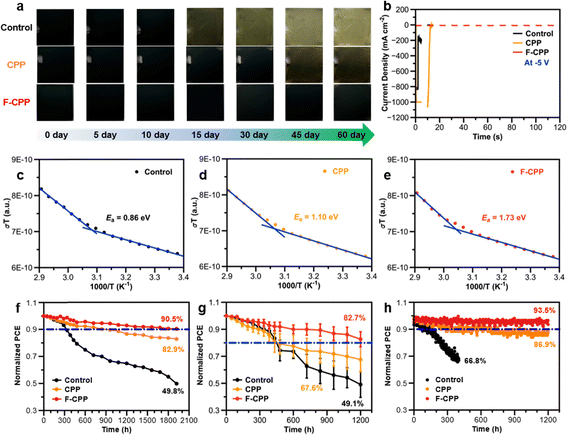
Electronegativity is a scale that measures the ability of an atom to attract electrons in a compound, and the dielectric constant represents the degree of polarization of the dielectric, which is the ability to bind electric charges. There is a certain positive correlation between these two parameters.18–20 Thus, after introducing F-CPP with high electronegativity induced by dual F units, the dielectric constant of perovskite with F-CPP treatment would be altered due to the interaction between F-CPP and buried perovskite. We performed broadband dielectric constant measurements to obtain the relative dielectric constants (εr) of perovskite films.19 The corresponding εr value for the F-CPP-treated perovskite film was determined to be 45.5 (Fig. 3a), which was approximately twice those of the control (21.6) and CPP-treated (25.5) films. Capacitance vs frequency plots (Fig. 3b) confirmed that the F-CPP-treated device achieved the largest dielectric capacitance.29 It was speculated that the F-CPP-treated device had a higher load voltage tolerance, which was beneficial to raise the transient Vbv and improve the reverse-bias stability.18–20Fig. 3c showed the dark current density–voltage (J–V) curves for different devices. As expected, the F-CPP-treated device delivered a maximum transient Vbv of −6.6 V, which is one of the largest Vbv values for silver-based PSCs to date (Table S2, SI). In contrast, the control and CPP-treated devices only had transient Vbv values of −1.1 V and −1.5 V, respectively. Meanwhile, perovskites with different bandgaps also showed a similar increase in the device transient Vbv values (Fig. S22, SI). Additionally, we directly observed the breakdown conditions of the whole device via IR thermography after applying a −5 V reverse bias (Fig. 3d–f).30 The F-CPP-treated device showed a uniform IR signal, whereas the control and CPP-treated devices both showed different breakdown signals at different points. We also measured the dark-state leakage current (J0) situation of the relevant devices. The values of J0 from the forward scan were calculated to be 4.8 × 10−3, 1.9 × 10−3 and 6.8 × 10−4 mA cm−2 for the control, CPP- and F-CPP-treated devices (Fig. S23, SI), respectively. This indicated that the F-CPP-treated device had better stability during electrical excitation, which was beneficial for improving Vbi and promoting charge transfer. These results proved that F-CPP was an excellent dielectric molecular-bridge that could protect devices from high reverse load voltages.We subsequently carried out Kelvin probe force microscope (KPFM) measurements to examine the surface potentials of different substrate films, where the values of contact potential difference (CPD) were extracted to be 228, 64 and 52 mV for control, CPP- and F-CPP-treated films (Fig. 3g–i and Fig. S24, SI), respectively.31 This indicates that the F-CPP-treated substrate film exhibited stronger p-type character, which helps accelerate hole transport and decrease the energy-band barrier at the buried interface.32 We then carried out ultraviolet photoelectron spectroscopy (UPS) together with UV-vis absorption spectroscopy to study the detailed energy band alignment at the buried interface (Fig. S25 and S26, SI).33 The corresponding valence band maximum (EVB), conduction band minimum (ECB) and Fermi level (EF) values are illustrated in Fig. S27 (SI), where the energy differences between EF and EVB were then calculated to be 0.91, 0.82 and 0.72 eV for control, CPP- and F-CPP-treated films, respectively. The lower energy difference indicated that the surface tended to be more p-type, which was in line with the results from KPFM. Overall, these results suggested that F-CPP treatment could not only raise the reverse-bias stability but could also regulate the interfacial band arrangement.
Carrier transport and recombination dynamics
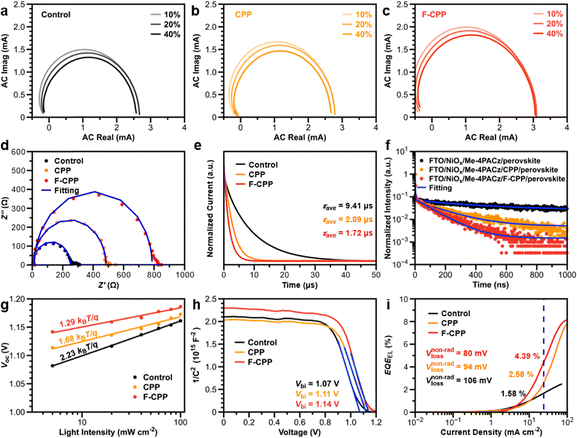
We also studied the carrier transport and recombination dynamics for different devices. Intensity-modulated photocurrent spectroscopy (IMPS) and intensity-modulated photovoltage spectroscopy (IMVS) were used to obtain the carrier collection time (τc) and recombination time (τr).34Fig. 4a–c and Fig. S28 (SI) show typical semicircles in the complex plane at 10%, 20% and 40% irradiation intensities for the different devices, where τc for the F-CPP-treated device increased from 7.7 µs at 10% irradiation intensity to 8.9 µs at 40% irradiation intensity. In contrast, the control and CPP-treated devices all displayed larger values, whether exposed to weak or strong light irradiation. This meant that the photogenerated carriers in F-CPP-treated devices could be more efficiently transported over time compared with the control and CPP-treated devices.35 In addition, at different irradiation intensities (Fig. S29, SI), the increase in τr values for different devices showed the same trend, where the F-CPP-treated device presented a lower level of increase in the τr value than the control and CPP-treated devices. The corresponding charge collection efficiency (ηcc) values at various light intensities were further calculated, and the F-CPP-treated device exhibited maximum values in all cases (Note S2 and Table S3, SI).Electrochemical impedance spectroscopy (EIS) analysis was subsequently conducted, where the F-CPP-treated device (Fig. 4d and Table S4, SI) had the lowest series resistance (Rs) and the largest recombination resistance (Rrec).36 Transient photocurrent (TPC) and transient photovoltage (TPV) measurements also demonstrated similar results (Fig. 4e and Fig. S30, SI): the F-CPP-treated device showed a decreased average charge transport time (τave-tr) and an increased charge recombination time (τave-re) compared with the control and CPP-treated devices. This was confirmed by steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) measurements. The F-CPP-treated perovskite film showed faster quenching of the PL peak (Fig. S31, SI) and a steeper TRPL decay curve (Fig. 4f and Table S5, SI) compared with the control and CPP-treated perovskite films. Additionally, the average PL decay lifetime decreased from 402.34 ns for the FTO/NiOx/Me-4PACz/perovskite film to 170.11 ns and 107.99 ns for the FTO/NiOx/Me-4PACz/CPP/perovskite and FTO/NiOx/Me-4PACz/F-CPP/perovskite films (Note S3, SI), respectively.37 PLQYs were further measured to quantify the effect of interface passivation on reducing the interface loss and improving carrier transport. The PLQY value of F-CPP-treated perovskite film was larger than those of the control and CPP-treated perovskite films (Fig. S32, SI). Quasi-Fermi-level splitting (QFLS) was further estimated (Note S4, SI), and the QFLS value of the F-CPP-treated device increased by 32 meV compared with the control device, whereas the measured VOC of the device increased by 36 meV. These results indicated that reduced nonradiative recombination in the F-CPP-treated device was the primary reason for the improvement in photovoltaic efficiency, rather than the optimization of interfacial band alignment.
To gain insight into the reasons for the improvements in VOC and FF, we conducted Mott–Schottky measurements to extract the value of built-in potential (Vbi). The F-CPP-treated device showed a maximum Vbi value of 1.14 V (Fig. 4h), indicative of enhanced carrier separation driving force.38 Space charge-limited current (SCLC) measurements were conducted to estimate the defect-state density (Ntrap) in the hole-only device (Fig. S33 and Note S5, SI). The F-CPP-treated device exhibited an Ntrap value of 5.83 × 1015 cm−3, which was significantly lower than those of the control (1.01 × 1016 cm−3) and CPP-treated (7.10 × 1015 cm−3) devices.39 It has been proved that enhanced Vbi and reduced Ntrap values are beneficial for obtaining high VOC and FF. Plots of VOC and JSCversus the logarithm of the light intensity were obtained to evaluate the ideality factors of different devices (Fig. 4g, Note S6 and Fig. S34, SI). The F-CPP-treated device displayed a slope of 1.29kT/q (here, k is Boltzmann's constant, T is the temperature, and q is the elementary charge), whereas the control and CPP-treated devices presented steep slopes of 2.23 and 1.68kT/q, respectively, indicative of suppressed nonradiative recombination in F-CPP-treated devices. We subsequently calculated the VOC loss derived from nonradiative recombination based on the external quantum efficiency of electroluminescence (EQE-EL).40Fig. 4i shows the EL spectra of the control, CPP- and F-CPP-treated devices, and the corresponding values of EQEEL were detected to be 1.58%, 2.58% and 4.39%, respectively. According to the detailed balance theory, the value of VOC loss for the F-CPP-treated device was calculated to be 80 mV (Note S7 and Table S6, SI), which was superior to the control (106 mV) and CPP-treated (94 mV) devices. The reduction of VOC loss inevitably leads to a great improvement in device efficiency.
Photovoltaic performance of devices
To assess the effect on the PV performance of devices, we constructed inverted PSCs with the configuration of FTO/NiOx/Me-4PACz/F-CPP/Cs0.05FA0.95PbI3/C60/BCP/Ag (Fig. 5a). It was remarkable that the F-CPP-treated device delivered a champion PCE of 26.60% (certified as 26.17%) with a VOC of 1.196 V, a short-circuit current (JSC) of 25.91 mA cm−2 and a FF of 85.88% (Fig. 5b and Fig. S35, SI). In contrast, the control and CPP-treated devices exhibited PCEs of 24.46% and 25.23%, respectively. The detailed parameters are shown in Table S7 (SI). The optimal concentration of F-CPP was determined to be 1 mg mL−1 (Fig. S36, SI). The corresponding hysteresis effect index (HEI) for the F-CPP-treated (3.8%) device was much lower than those of the control (9.8%) and CPP-treated (5.6%) devices (Note S8, Fig. S37 and Table S8, SI).41 Meanwhile, the steady-state output for the F-CPP-treated device achieved a PCE of 26.09% (certified as 25.96%) at 1.06 V (Fig. 5c and Fig. S38, SI), which was higher than the control (24.52%) and CPP-treated (25.17%) devices. Fig. 5d shows the external quantum efficiencies (EQEs) for different devices, where the integrated photocurrent densities were calculated to be 25.01, 25.19 and 25.50 mA cm−2, respectively. We also verified the reproducibility of different devices: the F-CPP-treated device showed a narrower distribution compared with the control and CPP-treated devices, especially in terms of VOC and FF (Fig. 5e and Fig. S39, SI). To verify the universality of this dielectric molecular-bridge strategy, we created large-area and wide-bandgap devices. Fig. 5f shows a champion PCE of 24.08% for the F-CPP-treated device with a 1 cm2 aperture area. At the same time, F-CPP-treated devices delivered high PCEs of 22.56%, 20.40% and 20.19% based on different perovskite bandgaps of 1.68 eV, 1.73 eV and 1.78 eV (Fig. 5g–i and Table S9, SI), respectively. We also constructed inverted PSCs without the Me-4PACz layer to assess the necessity of Me-4PACz. It was found that PSCs without the Me-4PACz layer exhibited lower PCEs than those of the F-CPP-treated devices (Fig. S40, SI), which might be attributed to the reduced photoelectric performance of hole-transport layers and the mismatched interfacial energy alignment.Device stability
Stability is another important factor for devices. We first presented surface water contact angle measurements to estimate the moisture infiltration, and Fig. S41 (SI) shows a gradually enlarged contact angle from 39.5° for the control film to 48.3° and 50.8° for the CPP- and F-CPP-treated perovskite films, respectively. Additionally, there was almost no signal from the P–OH group located at 950 cm−1 and the P–C group did not shift in F-CPP-treated substrate film spectra under moist and humid conditions (Fig. S42, SI), indicative that Cl ions did not form. It was obvious that the perovskite films with F-CPP treatment could effectively insulate the surface from moisture, which was consistent with the results from exposed perovskite films in air at a relative humidity (RH) of 40–80% and a temperature of 25 °C. As shown in Fig. 6a, the F-CPP-treated perovskite film remained in the black crystalline phase after 60 d, whereas the control and CPP-treated films both showed significant degradation. To examine the continuous reverse-bias stability, we performed pulse cycle measurements (Fig. 6b). The F-CPP-treated device showed a steady and consistent output current after applying a −5 V pulse reverse-bias for 120 s, whereas the control and CPP-treated devices were directly punctured. Fig. S43 (SI) shows that a PCE of 24.46% was retained by the F-CPP-treated device after undergoing pulse cycle measurements, while the PCEs of the control and CPP-treated devices approached 0%. In addition, we measured the ion migration activation energy (Ea) to reflect the stability of different devices (Fig. 6c–e).42 The corresponding Ea value for the control device was calculated to be 0.86 eV, whereas the CPP- and F-CPP-treated devices showed Ea values of 1.10 eV and 1.73 eV, respectively. This indicated that inhibited ion migration behaviour existed in the F-CPP-treated device, which was in line with the increase in the transient Vbv and the thus-improved continuous reverse-bias stability.Later, under −1 V reverse-bias testing conditions, we exposed different devices to a variety of external situations to evaluate the long-term operational and reverse-bias stability of the related devices. The storage stability (N2-filled glove box at a temperature of 25 °C) of all devices was examined, and the unencapsulated F-CPP-treated device retained 90.5% of its initial efficiency over 2000 h (Fig. 6f), outperforming the control (82.9%) and CPP-treated (49.8%) devices. Fig. 6g shows the light stability (temperature: 25 °C, AM 1.5G illumination) under continuous output maximum power point (MPP) tracking. The unencapsulated F-CPP-treated device maintained over 93.5% of its initial efficiency over 1200 h, in comparison with 66.8% and 86.9% for the control and CPP-treated devices, respectively. Testing the thermal-aging stability (N2-filled glove box at a continuous temperature of 85 °C) of devices also presented similar results (Fig. 6h), where the preserved average efficiency of the unencapsulated F-CPP-treated device (82.7%) was much higher than those of the control (49.1%) and CPP-treated (67.6%) devices. We reasoned that the admirable PCE and excellent stability of F-CPP-treated devices were mainly contributed to by the following aspects: (1) the existence of Cl functional units could tailor the crystallization of perovskites, thus obtaining high-quality perovskite films with large grain size and released residual stress; (2) the F functional units in F-CPP could offer higher electronegativity and increase the dielectric constant of perovskite films, thereby raising the transient Vbv and improving the reverse-bias stability of devices; and (3) the synergistic passivation of Lewis-base and halogen units could significantly reduce defect formation energy, passivate nonradiative recombination and inhibit ion migration.
Conclusions
This study proposed an effective dielectric molecular-bridge strategy to passivate halogen-induced defects, accelerate hole transport and tailor perovskite crystallization by inserting a multifunctional molecule at the buried interface. This strategy could also regulate interfacial energy band alignment, inhibit ion migration and increase the transient Vbv to −6.6 V. As a result, the champion F-CPP-treated device achieved an outstanding PCE of 26.60% (certified as 26.17%) along with excellent long-term storage, thermal-aging, MPP tracking and reverse-bias stability. Moreover, corresponding large-area and wide-bandgap devices also delivered high PCEs of 24.08% (1 cm2), 22.56% (1.68 eV), 20.40% (1.73 eV) and 20.19% (1.78 eV). This work demonstrated a feasible dielectric molecular-bridge strategy for improving the efficiency and stability of PSC devices.Experimental procedures
Materials
All chemicals were used as received. Lead chloride (PbCl2, 99.9%), formamidinium iodide (FAI, 99.5%), lead iodide (PbI2, 99.999%), cesium iodide (CsI, 99.99%) and magnesium fluoride (MgF2, 99.99%) were obtained from Xi’an Yuri Solar Corp., Ltd. Nickel oxide nanoparticles (NiOx, 99.999%), fullerene-C60 (99.9%) and bathocuproine (BCP) were obtained from Advanced Electronic Technology Co., Ltd (4-(3,6-dimethyl-9H-carbazole-9-yl)butyl)phosphonic acid (Me-4PACz, 99%) was purchased from TCI (Shanghai) Development Corp., Ltd. Chlorodiphenylphosphine (CPP, 97%), bis(4-fluorophenyl)chlorophosphine (F-CPP, 99.9%), N,N-dimethylformamide (DMF, 99.9%), 1,3-diaminopropane dihydroiodide (PDAI2), dimethyl sulfoxide (DMSO, 99.9%), anisole (99.7%) and isopropyl alcohol (IPA, 99.5%) were purchased from Adamas-beta.Perovskite precursor solutions
(1) Narrow-bandgap perovskite (Cs0.05MA0.05FA0.90PbI3): 19.49 mg of CsI, 11.92 mg of MAI, 232.16 mg of FAI, 691.52 mg of PbI2 and 5 mol% MACl and PbCl2 additive were added to a mixed solution of 1 mL of DMF and DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and the mixed solution was stirred for 6 h at room temperature and filtered with a 0.22-µm polytetrafluoroethylene membrane before use. (2) Wide-bandgap perovskite (Cs0.2FA0.8Pb(BrxI1−x)3): FAI, FABr, PbI2, PbBr2, and CsI were added to DMF and DMSO (4
1, v/v), and the mixed solution was stirred for 6 h at room temperature and filtered with a 0.22-µm polytetrafluoroethylene membrane before use. (2) Wide-bandgap perovskite (Cs0.2FA0.8Pb(BrxI1−x)3): FAI, FABr, PbI2, PbBr2, and CsI were added to DMF and DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixed solvent, and the mixed solution was stirred for 6 h at room temperature and filtered with a 0.22-µm polytetrafluoroethylene membrane before use. (Note: Eg = 1.68 eV, x = 0.6; Eg = 1.73 eV, x = 0.9; Eg = 1.78 eV, x = 1.2).
1, v/v) mixed solvent, and the mixed solution was stirred for 6 h at room temperature and filtered with a 0.22-µm polytetrafluoroethylene membrane before use. (Note: Eg = 1.68 eV, x = 0.6; Eg = 1.73 eV, x = 0.9; Eg = 1.78 eV, x = 1.2).
Device fabrication
The glass/FTO substrate was ultrasonically cleaned with glass cleaning solution, deionized water and ethanol for 20 min. After that, the substrate was rinsed with nitrogen and treated with UV ozone for 15 min before use. Next, a NiOx (10 mg mL−1 in H2O) nanoparticle dispersion was dynamically spin-coated on the substrate at 2000 rpm for 30 s and annealed in air at 150 °C for 10 min. After annealing, the substrate was transferred to a nitrogen glovebox, and the rest of the process was done in the nitrogen glovebox. Me-4PACz (0.5 mg mL−1 in ethanol) was dynamically spin-coated on NiOx film at 5000 rpm for 30 s and annealed at 100 °C for 10 min. Then, 70 µL of CPP or F-CPP solution with different concentrations in IPA was dynamically spin-coated on the Me-4PACz substrate at 5000 rpm for 30 s and annealed at 100 °C for 10 min. The perovskite precursor solution was subsequently spin-coated in a continuous two-step process at 1000 and 5000 rpm for 8 s and 30 s, respectively. With 6 s remaining in the second spin-coating step, 140 µL of anisole was added dropwise to the substrate, followed by annealing at 120 °C for 20 min. Then, PDAI2 (1 mg mL−1 in IPA) was spin-coated onto the perovskite film and annealed at 100 °C for 10 min. Lastly, a vacuum evaporation instrument was used to evaporate C60 (20 nm), BCP (6 nm) and Ag (100 nm) in turn to complete the overall device fabrication. Except for the spin-coating of NiOx, all the remaining device fabrication processes were carried out in a N2-filled glovebox.Characterization
A scanning electron microscope (SEM, Thermo Scientific Apreo 2C) and atomic force microscope (AFM, Bruker NanoIR3) were used to observe the morphologies of different perovskite films. Kelvin probe force microscopy (KPFM, Asylum Research MFP-3D) was employed to measure the surface potential of substrates. Ultraviolet-visible (UV-vis, UV-3600i Plus) absorption spectroscopy was used to study the spectral absorption of different perovskite films. X-ray diffraction (XRD, Bruker AXS D8 Advance) patterns of the samples were obtained by using Cu-Kα radiation (λ = 1.5416 Å). Grazing-incidence wide-angle X-ray scattering (GIWAXS) spectra were obtained by examining samples via incident X-ray diffraction. In situ UV spectroscopy (Spec-vision) was used to measure changes in absorption during perovskite spin coating. Contact angle (CA, SDC-350) measurements were used to calculate the water contact angles of different samples. Fourier-transform infrared (FT-IR) spectra were obtained using a Nicolet iS50 instrument. Nuclear magnetic resonance spectroscopy (NMR, Bruker Ascend 400) showed the changes in the hydrogen chemical environment. Photoluminescence (PL) and transient photoluminescence (TRPL) spectra were obtained using a PicoQuant FluoTime apparatus under 510 nm laser excitation. X-ray photoelectron spectroscopy (XPS) data were obtained using a Thermo Fisher Scientific ESCALAB 250Xi apparatus. Ultraviolet photoelectron spectroscopy (UPS, Kratos AXIS SUPRA) measurements involved a 21.22-eV emission line. The permittivity spectra were obtained using a broadband permittivity tester (Novocontrol Concept 42). The current density–voltage (J–V) characteristic curves were collected using a Keithley 2400 apparatus under 100 mW cm−2 illumination and a simulated AM 1.5G spectrum. External quantum efficiency (EQE) data were obtained using an Enliterch QE-R system. Infrared thermography images were acquired with a hand-held thermographic camera (HIKMICRO). Maximum power point (MPP) analysis tracked the operation of unencapsulated devices under a −1 V reverse bias and 1 sun (white light-emitting diode lamp). Electrochemical impedance spectroscopy (EIS), Mott–Schottky (M-S), intensity-modulated photocurrent spectroscopy (IMPS), intensity-modulated photovoltage spectroscopy (IMVS), transient photovoltage (TPC) and transient photovoltage (TPV) data were collected using a Paios system (Fluxim). SEM, XPS and UPS measurements were all conducted under vacuum.Simulation methods
The calculations were executed using density functional theory (DFT) with the projector augmented wave (PAW) method, as implemented in the Vienna ab initio simulation package (VASP). The exchange–correlation potential was parameterized by the generalized gradient approximation (GGA) in the form of Perdew–Burke–Ernzerhof (PBE) functionals. We adopted slab models to probe the influence of impurities on diverse perovskite surfaces. For geometry optimization and self-consistent field calculations, a plane-wave expansion cutoff of 300 eV and a 3 × 3 × 1 k-point mesh for sampling the Brillouin zone were employed. The atomic positions within the structures were optimized until the maximum residual forces were below 0.03 eV Å−1. Furthermore, van der Waals corrections to the electronic energies were integrated using the D2 method.Author contributions
Y. C. conceived the idea and proposed the experimental design. C. L. and Y. C. performed device fabrication and technology innovation. C. L. characterized the material samples (AFM, SEM, KPFM, in situ UV-vis and MPP tests, etc.). X. W., Y. P., H. C., S. L. L. and J. Z. assisted with the measurements. H. X. L., Y. S., and Y. W. D. helped perform the GIWAXS, DFT and GIXRD measurements. Y. C. drafted the original manuscript. Y. C., Y. H. W. and Q. P. helped with the manuscript preparation. Y. C. and Q. P. supervised the project. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details, XPS spectra, UPS spectra, SEM images, AFM-IR images, UV-vis absorption spectra, FT-IR spectra, XRD, GIXRD, PL and TRPL spectra, hysteresis behaviour of devices, certification report, steady I–T, SCLC, and contact angle data, J–V curves, and tables that summarize the TRPL, EIS, IMPS, IMVS, EL, and J–V parameters. See DOI: https://doi.org/10.1039/d5ee02610e.Acknowledgements
The authors thank the National Key Research and Development Program of China (Grant No. 2022YFB4200500), the National Natural Science Foundation of China (Grant No. 22409020, 22379101 and 22075190), the Natural Science Foundation of Sichuan Province (Grant No. 2024NSFSC1163 and 2024NSFSC0001) and the Discipline Leading Innovation Team Project of Chengdu University of Technology (11400-000525-08). A portion of this work is based on data obtained at Beijing Synchrotron Radiation Facility (BSRF) and Shanghai Synchrotron Radiation Facility (SSRF). The authors gratefully acknowledge the cooperation of the beamline scientists at BSRF-1W1A, SRRF-BL16B1, SRRF-BL02U2 and SRRF-BL03HB.Notes and references
- H. Chen, C. Liu, J. Xu, A. Maxwell, W. Zhou, Y. Yang, Q. Zhou, A. S. R. Bati, H. Wan, Z. Wang, L. Zeng, J. Wang, P. Serles, Y. Liu, S. Teale, Y. Liu, M. I. Saidaminov, M. Li, N. Rolston, S. Hoogland, T. Filleter, M. G. Kanatzidis, B. Chen, Z. Ning and E. H. Sargent, Science, 2024, 384, 189 CrossRef CAS PubMed.
- X. Luo, X. Lin, F. Gao, Y. Zhao, X. Li, L. Zhan, Z. Qiu, J. Wang, C. Chen, L. Meng, X. Gao, Y. Zhang, Z. Huang, R. Fan, H. Liu, Y. Chen, X. Ren, J. Tang, C.-H. Chen, D. Yang, Y. Tu, X. Liu, D. Liu, Q. Zhao, J. You, J. Fang, Y. Wu, H. Han, X. Zhang, D. Zhao, F. Huang, H. Zhou, Y. Yuan, Q. Chen, Z. Wang, S. Liu, R. Zhu, J. Nakazaki, Y. Li and L. Han, Sci. China: Chem., 2022, 65, 2369 CrossRef CAS.
- Y. Peng, Y. Chen, J. Zhou, C. Luo, W. Tang, Y. Duan, Y. Wu and Q. Peng, Nat. Commun., 2025, 16, 1252 CrossRef CAS PubMed.
- Y. Peng, Y. Chen, Y. Shen, J. Zhou, Y. Duan, H. Li, S. Pu, Y. Jiang, Y. Wu and Q. Peng, Adv. Funct. Mater., 2025, 35, e21121 CrossRef.
- J. Zhou, Y. Chen, Y. Shen, Y. Peng, X. Wu, Z. Xie, C. Luo, M. Deng, Y. Duan, Y. Wu and Q. Peng, Angew. Chem., Int. Ed., 2025, 64, e202511042 CrossRef CAS PubMed.
- K. Park, J.-H. Lee and J.-W. Lee, ACS Energy Lett., 2022, 7, 1230 CrossRef CAS.
- Y. Wu, Q. Wang, Y. Chen, W. Qiu and Q. Peng, Energy Environ. Sci., 2022, 15, 4700 RSC.
- X. Chen, Q. Wang, H. Wei, J. Yang, Y. Yao, W. Tang, W. Qiu, X. Xu, L. Song, Y. Wu and Q. Peng, Energy Environ. Sci., 2024, 17, 7342 RSC.
- B. Chen, P. N. Rudd, S. Yang, Y. Yuan and J. Huang, Chem. Soc. Rev., 2019, 48, 3842 RSC.
- B. Li, Q. Dai, S. Yun and J. Tian, J. Mater. Chem. A, 2021, 9, 6732 RSC.
- F. Jiang, Y. Shi, T. R. Rana, D. Morales, I. E. Gould, D. P. McCarthy, J. A. Smith, M. G. Christoforo, M. Y. Yaman, F. Mandani, T. Terlier, H. Contreras, S. Barlow, A. D. Mohite, H. J. Snaith, S. R. Marder, J. D. MacKenzie, M. D. McGehee and D. S. Ginger, Nat. Energy, 2024, 9, 1275 CrossRef CAS.
- X. Liu, Y. Guo, Y. Cheng, S. Lu, R. Li and J. Chen, Chem. Commun., 2023, 59, 13394 RSC.
- B. Li, W. Pu, M. Zhang, Y. Liu and M. Li, Small, 2025, 21, 2407186 CrossRef PubMed.
- J. Wu, M.-H. Li, J.-T. Fan, Z. Li, X.-H. Fan, D.-J. Xue and J.-S. Hu, J. Am. Chem. Soc., 2023, 145, 5872 CrossRef CAS PubMed.
- T. Xiao, M. Hao, T. Duan, Y. Li, Y. Zhang, P. Guo and Y. Zhou, Nat. Energy, 2024, 9, 999 CrossRef CAS.
- J. Xu, H. Chen, L. Grater, C. Liu, Y. Yang, S. Teale, A. Maxwell, S. Mahesh, H. Wan, Y. Chang, B. Chen, B. Rehl, S. M. Park, M. G. Kanatzidis and E. H. Sargent, Nat. Mater., 2023, 22, 1507 CrossRef CAS PubMed.
- Z. Zhang, J. Ding, H. Liu, C. Li, M. Li, T. Pauporté, J.-X. Tang, J. Chen and C. Chen, Adv. Mater., 2025, 37, 2508126 CrossRef CAS PubMed.
- T. Kabemura, S. Ueda, Y. Kawada and K. Horio, IEEE Trans. Electron Devices, 2018, 65, 3848 CAS.
- H. Chen, Q. Cheng, H. Liu, S. Cheng, S. Wang, W. Chen, Y. Shen, X. Li, H. Yang, H. Yang, J. Xi, Z. Chen, X. Lu, H. Lin, Y. Li and Y. Li, Sci. Bull., 2022, 67, 1243 CrossRef CAS PubMed.
- P. Boufflet, G. Bovo, L. Occhi, H.-K. Yuan, Z. Fei, Y. Han, T. D. Anthopoulos, P. N. Stavrinou and M. Heeney, Adv. Electron. Mater., 2018, 4, 1700375 CrossRef.
- Q. Wang, Y. Chen, X. Chen, W. Tang, W. Qiu, X. Xu, Y. Wu and Q. Peng, Adv. Mater., 2024, 36, 2307709 CrossRef CAS PubMed.
- J. Liu, J. Chen, P. Xu, L. Xie, S. Yang, Y. Meng, M. Li, C. Xiao, M. Yang and Z. Ge, Adv. Energy Mater., 2024, 14, 2303092 CrossRef CAS.
- Y. Chen, Y. shen, W. Tang, Y. Wu, W. Luo, N. Yuan, J. Ding, S. Zhang and W.-H. Zhang, Adv. Funct. Mater., 2022, 32, 2206703 CrossRef CAS.
- Z. Xie, Y. Duan, M. Cheng, Y. Li, Z. Liu, H. Li, Y. Chen, Z. Zang, S. Liu and Q. Peng, Angew. Chem., Int. Ed., 2024, 63, e202419070 Search PubMed.
- Y. Li, Y. Duan, Z. Liu, L. Yang, H. Li, Q. Fan, H. Zhou, Y. Sun, M. Wu, X. Ren, N. Yuan, J. Ding, S. Yang and S. Liu, Adv. Mater., 2024, 36, 2310711 CrossRef CAS PubMed.
- H. Zhou, W. Wang, Y. Duan, R. Sun, Y. Li, Z. Xie, D. Xu, M. Wu, Y. Wang, H. Li, Q. Fan, Y. Peng, Y. Yao, C. Liao, Q. Peng, S. Liu and Z. Liu, Angew. Chem., Int. Ed., 2024, 63, e202403068 CrossRef CAS PubMed.
- J. Wu, S.-C. Liu, Z. Li, S. Wang, D.-J. Xue, Y. Lin and J.-S. Hu, Nat. Sci. Rev., 2021, 8, nwab047 CrossRef CAS PubMed.
- H. Zhang, Z. Chen, M. Qin, Z. Ren, K. Liu, J. Huang, D. Shen, Z. Wu, Y. Zhang, J. Hao, C. S. Lee, X. Lu, Z. Zheng, W. Yu and G. Li, Adv. Mater., 2021, 33, e2008487 CrossRef PubMed.
- Z. Hu, Y. Lv, C. Zhao, Q. Feng, Z. Feng, K. Dang, X. Tian, Y. Zhang, J. Ning, H. Zhou, X. Kang, J. Zhang and Y. Hao, IEEE Electron Device Lett., 2020, 41, 441 CAS.
- C. Wang, L. Huang, Y. Zhou, Y. Guo, K. Liang, T. Wang, X. Liu, J. Zhang, Z. Hu and Y. Zhu, Adv. Energy Mater., 2023, 13, 2203596 CrossRef CAS.
- D. Wang, Y. Li, W. Li, W. Pan, R. Li, S. Wang, F. Liu, Z. Lan, J. Wu, E. Huang, X. Guo, X. Liu and Q. Li, Adv. Funct. Mater., 2024, 34, 2412068 Search PubMed.
- H. Tang, Z. Shen, Y. Shen, G. Yan, Y. Wang, Q. Han and L. Han, Science, 2024, 383, 1236 CrossRef CAS PubMed.
- Y. Chen, Z. Yang, S. Wang, X. Zheng, Y. Wu, N. Yuan, W. H. Zhang and S. F. Liu, Adv. Mater., 2018, 30, 1805660 CrossRef PubMed.
- Y. Lv, R. Yuan, B. Cai, B. Bahrami, A. H. Chowdhury, C. Yang, Y. Wu, Q. Qiao, S. F. Liu and W. H. Zhang, Angew. Chem., Int. Ed., 2020, 59, 11969 CrossRef CAS PubMed.
- R. Yuan, B. Cai, Y. Lv, X. Gao, J. Gu, Z. Fan, X. Liu, C. Yang, M. Liu and W.-H. Zhang, Energy Environ. Sci., 2021, 14, 5074 RSC.
- Y. Chen, Z. Yang, X. Jia, Y. Wu, N. Yuan, J. Ding, W.-H. Zhang and S. Liu, Nano Energy, 2019, 61, 148 CrossRef CAS.
- Y. Chen, J. Yang, S. Wang, Y. Wu, N. Yuan and W. H. Zhang, iScience, 2020, 23, 100762 CrossRef CAS PubMed.
- Y. Chen, W. Tang, Y. Wu, X. Yu, J. Yang, Q. Ma, S. Wang, J. Jiang, S. Zhang and W.-H. Zhang, Chem. Eng. J., 2021, 425, 131499 CrossRef CAS.
- Y. Chen, W. Tang, Y. Wu, R. Yuan, J. Yang, W. Shan, S. Zhang and W.-H. Zhang, Sol. RRL, 2020, 4, 2000344 CrossRef CAS.
- Z. Li, B. Li, X. Wu, S. A. Sheppard, S. Zhang, D. Gao, N. J. Long and Z. Zhu, Science, 2022, 376, 416 CrossRef CAS PubMed.
- K. Chen, W. Tang, Y. Chen, R. Yuan, Y. Lv, W. Shan and W.-H. Zhang, J. Energy Chem., 2021, 61, 553 CrossRef CAS.
- S. Dong, Z. Fan, W. Wei, S. Tie, R. Yuan, B. Zhou, N. Yang, X. Zheng and L. Shen, Light: Sci. Appl., 2024, 13, 174 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |