The interlayer proton capture and transport mechanism in oxygen electrodes boosts proton ceramic electrolysis
Meijuan
Fei
ab,
Zhaohui
Cai
b,
Peng
Chen
ab,
Dongliang
Liu
b,
Cheng
Huang
ab,
Jianqiu
Zhu
c,
Linjuan
Zhang
 c,
Wei
Wang
c,
Wei
Wang
 ab,
Chuan
Zhou
*ab,
Wei
Zhou
ab,
Chuan
Zhou
*ab,
Wei
Zhou
 *ab and
Zongping
Shao
*ab and
Zongping
Shao
 *d
*d
aState Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering, Nanjing Tech University, Nanjing 211800, China. E-mail: zhouwei1982@njtech.edu.cn; zhouc@szlab.ac.cn
bSuzhou Laboratory, Suzhou 215000, China
cKey Laboratory of Interfacial Physics and Technology, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China
dCentre for Advanced Energy Material and Technologies, WA School of Mines, Curtin University, Perth, WA 6102, Australia. E-mail: zongping.shao@curtin.edu.au
First published on 10th December 2025
Abstract
The synergistic regulation of steam utilization and proton transport at the oxygen electrode is crucial for proton ceramic electrolysis cells (PCECs). Ruddlesden–Popper (RP) perovskites leverage interlayer water intercalation features to achieve rapid proton uptake even under low-steam conditions. Herein, an RP-type oxygen electrode capable of reversible phase transitions and hydrated oxyhydroxide formation under high-temperature steam was constructed, successfully transcending the hydration limits of single perovskites. By integrating the structural analysis employing microcrystal electron diffraction (MicroED) and density functional theory (DFT) calculations, it is revealed that the interlayer proton-trapping sites significantly boost the steam adsorption/hydration and lower the energy barrier for proton migration across layers. The Sr3(Co0.8Fe0.1Nb0.1)2O7−δ (SCFN-RP) electrode demonstrates excellent catalytic activity, reaching 1.01 A cm−2@1.3 V at 550 °C. This work emphasizes the crucial role of reversible hydrated oxyhydroxides in RP perovskites and offers a novel conception for the design of high-performance oxygen electrodes for PCECs.
Broader contextAs hydrogen emerges and plays a central role in clean energy strategies, protonic ceramic electrolysis cells (PCECs) have gained increasing attention for their high energy efficiency. Achieving durable performance at intermediate temperatures, however, requires oxygen electrodes with rapid proton conduction, efficient steam management, and excellent catalytic activity. Conventional perovskite cathodes fail to meet these requirements due to poor water tolerance and limited proton conductivity. Here, we introduce a Ruddlesden–Popper (RP) oxide, Sr3(Co0.8Fe0.1Nb0.1)2O7−δ (SCFN-RP), as a promising oxygen electrode. Its excellent water uptake enables a reversible steam-induced phase transition, which generates additional ion migration pathways to accelerate proton transport and boost electrocatalytic activity. The study demonstrates not only the design of a highly active and stable oxygen electrode but also reveals the structural mechanism of water intercalation and proton transport. This work underscores the structural tunability of RP-layered oxides as a powerful strategy to optimize proton adsorption and migration, confirming their great promise as next-generation oxygen electrodes. |
Introduction
Governments around the world are currently pushing aggressively for the development of clean energy. Hydrogen, as a renewable energy source with zero carbon emissions, will play a significant role in the future sustainable energy framework. Protonic ceramic electrolysis cells (PCECs) are an efficient and all-solid-state technology for generating green hydrogen. The low activation energy for proton conduction leads to excellent electrochemical performance at intermediate to low temperatures, meeting the demands for sustainable hydrogen utilization and production.1,2Serving as the main reaction interface for the protonation reaction of water and the oxygen evolution reaction (OER), the oxygen electrode requires efficient water transport pathways and excellent proton conductivity to enable rapid adsorption and dissociation of water, thereby accelerating steam surface exchange and proton migration.3 In addition to producing high-purity and dry hydrogen in the PCEC mode, this system can also operate efficiently in the protonic ceramic fuel cell (PCFC) mode using hydrogen, ammonia, or hydrocarbon fuels, demonstrating excellent fuel flexibility. These two modes involve the consumption and generation of steam, respectively. Accordingly, abundant water transport channels in the oxygen electrode can also facilitate steam diffusion, prevent local accumulation, and assist in the release of active sites for the OER or the oxygen reduction reaction (ORR).4 Such extensive features are scarcely achievable with conventional oxygen electrode materials possessing single-phase cubic perovskite structures.
Currently, a research focus is the construction of multiphase composite or layered oxide oxygen electrodes, which improve electrode activity by leveraging the synergistic effects between multiple complexes or the ion transport properties of the layered structure. High-performance oxygen electrodes, such as the triphasic BaCo0.7(Ce0.8Y0.2)0.3O3−δ,5 the cubic–hexagonal composite Ba1.5Sr1.5Co1.6Fe0.4O7−δ,6 Na0.3Sr0.7Ti0.1Fe0.9O3−δ containing a super hydrating β-NaFeO2 phase,7 and Pr2Ni0.6Co0.4O4−δ enriched with catalytically active Pr6O11,8 typically enhance ORR and OER catalytic activity and proton transport by incorporating nanoscale oxygen-active or proton-affinitive phases. Although multiphase composite oxide electrodes demonstrate outstanding performance, finding universality in their electrode design principles is difficult. Ruddlesden–Popper (RP) perovskites (An+1BnO3n+1), which are characterized by an ordered layered structure with alternating stacks of ABO3 perovskite layers and AO rock–salt layers, have been extensively investigated for their ionic transport behaviour. The unique AO layers are believed to contain multiple potential ion migration pathways, contributing to enhanced overall ionic conductivity.9,10 Notably, the natural interlayer spacing between the AO layer and perovskite layer provides sufficient structural flexibility to accommodate additional structural units. Most studies on RP-type oxygen electrodes have focused on K2NiF4-type layered oxides (e.g., La2NiO4+δ and Pr2NiO4+δ), which exhibit excellent mixed ionic–electronic conductivity.11 However, their proton conductivity under intermediate temperatures or low humidity has rarely been reported. Certain RP-type layered oxides, such as Sr3Co2O7−δ, exhibit excellent water adsorption capabilities in ambient humid air. They can undergo water intercalation between the rock-salt and perovskite layers, resulting in a phase transition to hydrated hydroxide Sr3Co2O5(OH)2·xH2O, accompanied by an expansion of the c-axis lattice parameter.12–15 Compared to RP-type perovskites with Pr or La at the A-site, Sr-based RP oxides feature weaker Sr–O bond covalency within the rock-salt layers, resulting in oxide ions with higher local electron density and basicity.16 These characteristics make the AO layers more favourable for water adsorption and subsequent proton incorporation. This structural feature endows the material with excellent water adsorption capability, potentially increasing the local proton concentration and facilitating proton transport, thereby offering a new strategy for designing efficient oxygen electrodes for PCECs.
Inspired by the above research findings, we synthesized an RP-structured perovskite oxide, Sr3(Co0.8Fe0.1Nb0.1)2O7−δ (SCFN-RP), by adjusting the stoichiometry of the single perovskite SrCo0.8Fe0.1Nb0.1O3−δ (SCFN-P) to serve as the oxygen electrode for PCECs. In this study, microcrystal electron diffraction (MicroED) was employed for the first time to analyse the structure of RP-type perovskites, successfully revealing the oxygen site distribution between layers. The water-intercalation-induced phase transition of SCFN-RP under high-temperature and humid conditions led to the formation of a hydrated oxyhydroxide phase, Sr3(Co0.8Fe0.1Nb0.1)2O17−x(OH)x·αH2O, accompanied by an expansion of the c-axis lattice parameter. The appearance and disappearance of low-angle diffraction peaks under various conditions in high-temperature X-ray diffraction (HT-XRD) confirm the reversibility of this phase transition. Furthermore, the density functional theory (DFT) computational model constructed on the basis of MicroED structural analysis indicates that the formation of hydrated oxyhydroxides introduces additional sites for interlayer proton transport, effectively reducing the energy barrier for proton transport and thus accelerating proton migration. Accordingly, a current density of 1.01 A cm−2@1.3 V at 550 °C was achieved in the PCEC mode, along with excellent operational stability. Owing to their unique water intercalation capability and outstanding electrocatalytic activity, this class of RP-structured materials holds great promise as ideal oxygen electrode candidates for PCECs, demonstrating significant potential for future development in this field.
Results and discussion
Water intercalation phenomenon and phase transition behaviour
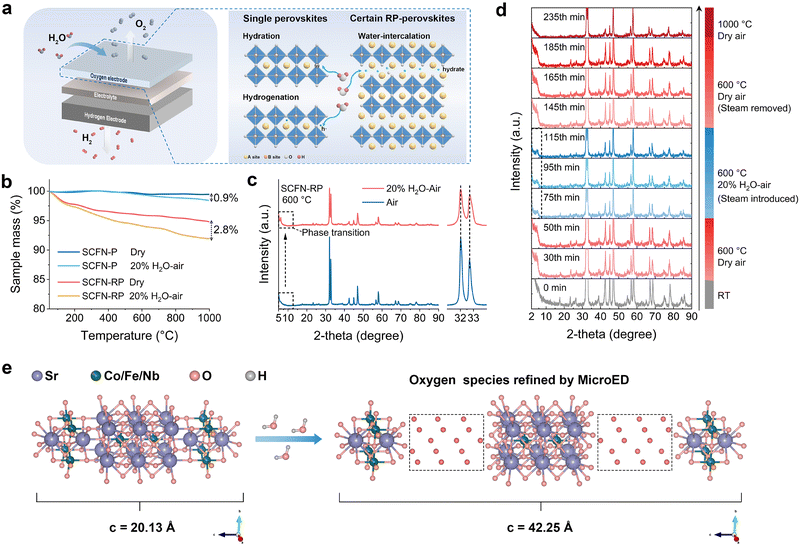
The water adsorption and desorption capabilities of the oxygen electrode in PCECs (Fig. 1a) are of crucial importance in maintaining high electrochemical performance. Water adsorbed and dissociated on the electrode serves as the essential source of protons. Protons in single-phase perovskites are mainly introduced through hydration or hydrogenation reactions. During the hydration process, water molecules dissociate into protons that bind to lattice oxygen, forming protonic defects and migrating through the oxygen vacancies or among the oxygen ions. During the hydrogenation process, water molecules participate in a reaction with lattice oxygen by consuming electronic holes, during which protons are absorbed and oxygen is produced.1 The active sites for hydration and hydrogenation reactions in single perovskite oxygen electrodes are typically confined to the surface exposed to steam, resulting in the confinement of water adsorption and proton formation to the surface, which restricts bulk activity and overall proton transport. Moreover, to guarantee the long-term functionality of the oxygen electrode, a more rapid and thorough protonation reaction ability under low-load operating conditions (low steam pressure below 30 vol% H2O–air) is indispensable. In addition to the two mechanisms mentioned above, certain RP-type structures, owing to their superior interlayer water storage capability, can further induce proton formation within the bulk of the material. The enhanced proton uptake capacity promotes higher carrier concentrations and optimized transport channels, facilitating improved proton conductivity.The SCFN-RP and SCFN-P samples were synthesized using a sol–gel method (Fig. S1 and S2). Thermogravimetric analysis (TGA) was employed to preliminarily investigate the weight loss of both fresh and steam-treated SCFN-RP and SCFN-P samples. As shown in Fig. 1b, the weight loss of the fresh SCFN-RP and SCFN-P samples was 5.2% and 0.6%, respectively. The mass loss results from the reduction of Co and Fe, accompanied by the release of lattice oxygen. Compared with the fresh sample, the steam-treated SCFN-RP sample clearly exhibited greater weight loss, with a 2.8% difference, whereas the SCFN-P sample exhibited minimal change, with a 0.9% difference. This phenomenon preliminarily demonstrates the excellent oxygen activation and water adsorption capabilities of SCFN-RP. To investigate whether the excellent water absorption ability of SCFN-RP has an impact on its phase structure, X-ray diffraction (XRD) analysis was performed after treating the powders at 600 °C for 2 h under dry air and 20 vol% H2O–air atmospheres. The diffraction peaks of the SCFN-P sample shifted to lower angles following steam exposure, indicating the chemical expansion of the crystal structure upon the incorporation of H2O into the single perovskite lattice (Fig. S3). However, the main peak of the SCFN-RP sample did not significantly shift, whereas a small diffraction peak appeared at 6.2° (Fig. 1c).17 Notably, the diffraction peak was still observed for the SCFN-RP sample after being treated at 600 °C for 1 h in a low-humidity atmosphere (5 vol% H2O–air) (Fig. S4). This observation confirms that a pronounced structural phase transition occurs, leading to the formation of hydrated oxyhydroxides in high-temperature steam environments.15 To explore the effect of H2O on SCFN-RP at high temperatures more precisely, Fig. 1d shows the HT-XRD spectrum of the SCFN-RP sample. The fresh SCFP-RP sample did not exhibit any distinct diffraction peaks in the 2°–10° low-angle range of the XRD patterns in ambient air. No noticeable peaks were apparent when the temperature was raised to 600 °C. Only an increase in the background signal was observed, which is likely attributed to the fluorescence effects stemming from the sample composition, variations in the incident depth, or scattering effects at the elevated temperatures.18 Then, a tiny diffraction peak formed immediately after the SCFN-RP sample was subjected to steam (20 vol% H2O–air) for 5 min, confirming the incorporation of hydrated oxyhydroxides into the bulk phase. Moreover, the low-angle diffraction peaks remained stable under steam conditions. XRD analysis was also performed on the SCFN-RP sample after 10 min of steam removal. As expected, the diffraction peaks disappeared. Furthermore, when the temperature was increased to 1000 °C, the original phase structure of SCFN-RP was retained. This result indicates that the main phase of SCFN-RP retains its crystalline integrity without noticeable distortion, even after repeated intercalation and deintercalation of hydrated oxyhydroxides at high temperatures, thereby ensuring the structural robustness of the oxygen electrode under practical operating conditions (Table S1).
A structural diagram of SCFN-RP prior to steam treatment is shown in Fig. 1e. The Rietveld refinement results confirm that SCFN-RP adopts a typical RP-type perovskite structure (space group I4![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), with lattice parameters of a = b = 3.86 Å and c = 20.13 Å (Fig. S1). HT-XRD analysis initially indicated that the introduction of steam at elevated temperatures induces a structural transformation in SCFN-RP, resulting in the formation of a reversible hydrated oxyhydroxide. To gain deeper insight into the structural evolution of SCFN-RP following the phase transition, structural analysis was conducted via MicroED. The key change observed in the refined structure (Tables S2–S6) was a clear expansion of the c-axis to 40.25 Å, with the a and b axes remaining nearly constant at 3.84 Å. This lattice expansion, corresponding to a Δc/c0 value of 1.1, is a consequence of the formation of hydrated oxyhydroxides during the phase transition, which ultimately increased the interlayer spacing.13 Structural parameters derived from MicroED analysis indicate that dissociated oxygen species from water molecules under steam conditions occupy the interlayer space between the perovskite and rock-salt layers of SCFN-RP, thereby promoting the formation of hydrated oxyhydroxide Sr3(Co0.8Fe0.1Nb0.1)2O17−x(OH)x·αH2O. As a result, the phase transition induced in SCFN-RP could create high-density proton storage sites within the bulk phase, thereby ensuring a sustained proton supply and efficient transport in PCECs.
m), with lattice parameters of a = b = 3.86 Å and c = 20.13 Å (Fig. S1). HT-XRD analysis initially indicated that the introduction of steam at elevated temperatures induces a structural transformation in SCFN-RP, resulting in the formation of a reversible hydrated oxyhydroxide. To gain deeper insight into the structural evolution of SCFN-RP following the phase transition, structural analysis was conducted via MicroED. The key change observed in the refined structure (Tables S2–S6) was a clear expansion of the c-axis to 40.25 Å, with the a and b axes remaining nearly constant at 3.84 Å. This lattice expansion, corresponding to a Δc/c0 value of 1.1, is a consequence of the formation of hydrated oxyhydroxides during the phase transition, which ultimately increased the interlayer spacing.13 Structural parameters derived from MicroED analysis indicate that dissociated oxygen species from water molecules under steam conditions occupy the interlayer space between the perovskite and rock-salt layers of SCFN-RP, thereby promoting the formation of hydrated oxyhydroxide Sr3(Co0.8Fe0.1Nb0.1)2O17−x(OH)x·αH2O. As a result, the phase transition induced in SCFN-RP could create high-density proton storage sites within the bulk phase, thereby ensuring a sustained proton supply and efficient transport in PCECs.
Hydrated oxyhydroxide-induced interlayer proton transport and electrochemical performance
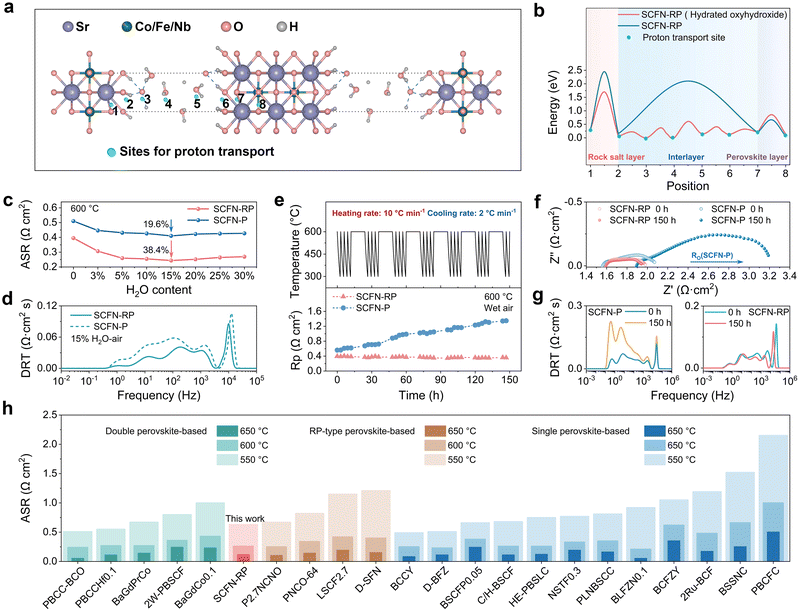
To verify whether the formation of hydrated oxyhydroxides is beneficial for proton transport, we performed a comparative analysis of the proton migration barriers before and after the phase transition in SCFN-RP on the basis of MicroED structural insights and DFT calculations (Fig. 2a and b). It is clearly seen that proton migration from site 1 to site 2 in the rock-salt layer corresponds to the highest energy barrier. The formation of oxyhydroxides facilitates proton transport via hydrogen bond-assisted hopping between –OH groups, thereby reducing the migration barrier to 1.7009 eV. Without the interlayer water uptake, proton migration between the rock-salt and perovskite layers is hindered by the lack of a continuous pathway, with a direct jump from site 2 to site 7 requiring an energy barrier as high as 2.1024 eV. The hydrated structure formed upon phase transition establishes a hydrogen-bonded network across the interface, with a new proton transfer site formed at site 3. Protons can migrate rapidly along this hydrogen bond chain via a multistep hopping route from site 4 through sites 5 and 6 to reach site 7, with a reduced barrier of 0.6077 eV. As protons migrate from site 7 to site 8, the local structural changes in the perovskite layer due to the phase transition extend the c-axis transport distance, leading to an increase in the maximum migration barrier from 0.6688 eV to 0.8570 eV. In summary, the steam environment induces a phase transition in SCFN-RP that introduces interlayer proton trapping sites. This not only facilitates the formation of interlayer H–O bonds but also effectively lowers the proton migration barrier, thereby overcoming the limitation of proton transport being confined to two-dimensional intralayer pathways in the RP phase, accelerating proton transport and hydration processes.The area-specific resistance (ASR) of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb)-supported symmetrical cells was measured under dry air and humidified conditions to evaluate the electrocatalytic activity of oxygen electrodes, thereby verifying the influence of interlayer-constructed proton transport channels on electrochemical performance (Fig. S8 and S9). The SCFN-RP electrode under 5 vol% H2O–air showed the ASRs of 0.06, 0.12, 0.26, 0.63 and 2.07 Ω cm2 at 700, 650, 600, 550, and 500 °C, respectively, while the SCFN-P electrode exhibited higher ASRs of 0.09, 0.18, 0.43, 0.97 and 2.50 Ω cm2 at the same temperatures. Additionally, the ASRs containing SCFN-RP and SCFN-P electrodes were also measured at 600 °C under different steam pressures. Fig. 2c shows that the ASRs of both SCFN-RP and SCFN-P electrodes tend to decrease, followed by an increase, as the steam content increases, reaching minimum values at a steam content of 15 vol% H2O–air. When the steam content increased from 0 to 15 vol%, the ASR of the SCFN-RP electrode clearly decreased by 38.4%, from 0.39 Ω cm2 to 0.24 Ω cm2, whereas the SCFN-P electrode experienced a reduction of only 19.6%, decreasing from 0.51 Ω cm2 to 0.41 Ω cm2. To gain a deeper understanding of the electrochemical reaction processes occurring at the electrode surface, the distribution of relaxation times (DRT) technique was applied to analyse the electrochemical impedance spectroscopy (EIS) data. Fig. 2d presents the DRT analysis of SCFN-RP and SCFN-P electrodes under 15 vol% H2O–air at 600 °C. The SCFN-RP electrode shows lower peak areas across all frequency ranges, suggesting more efficient mass transport and reaction of oxygen and steam, which in turn facilitates enhanced surface reaction kinetics. In general, the SCFN-RP electrode exhibits the exceptional bulk-phase ionic transport properties of both O2− and H+, coupled with the highly uniform dispersion of active sites for oxygen and steam activation, which is consistent with the DFT calculation results.
To further evaluate its thermal expansion compatibility and structural stability with the electrolyte under practical operating conditions, thermal cycling tests were conducted on symmetric cells in wet air (3 vol% H2O–air) from 300 °C to 600 °C. After 26 thermal cycles lasting for 150 h, a pronounced and sustained increase in the ASR for the SCFN-P electrode was observed starting from the 9th cycle, as shown in Fig. 2e, whereas the ASR for the SCFN-RP electrode gradually decreased throughout all cycles. The results indicate that the cell structure of the SCFN-RP oxygen electrode under steam conditions is stable, further confirming the critical role of the continuous hydration channels in enhancing proton transport. Fig. 2f presents the initial and final EIS curves during thermal cycling. Both the ohmic and polarization resistances of the SCFN-P electrode clearly significantly increase. The areas of the two gas diffusion peaks in the low-frequency ranges of the DRT analysis in Fig. 2g significantly increased, which could be attributed to the competitive adsorption and diffusion of oxygen and steam due to the limited active sites in SCFN-P.4 The SCFN-RP electrode maintained stable ohmic resistance with slightly reduced polarization resistance, and DRT analysis revealed a reduction in both intermediate and high frequency peak areas after thermal cycling, indicating excellent ion diffusion and enhanced proton migration at the perovskite/rock–salt interfaces in moisture. The ASR, serving as a critical indicator, is compared among various oxygen electrode materials reported in recent studies on symmetrical cells. (Table S7). As shown in Fig. 2h, current research is predominantly focused on single perovskite structures, whereas studies on double perovskites and RP-type structures remain relatively limited.3,5–8,19–34 Oxygen electrodes such as BaCo0.7(Ce0.8Y0.2)0.3O3−δ (BCCY) and PrBa0.8Ca0.2Co2O5+δ (PBCC–BCO) demonstrate outstanding electrochemical performance, likely owing to the superior catalytic activity of multiphase nanocomposites, but their design strategies are not yet universally applicable. The SCFN-RP oxygen electrode has a lower ASR than most reported materials and demonstrates the best performance among RP-structured electrodes, confirming the crucial role of hydrated oxyhydroxides in enhancing proton conduction.
Water adsorption capability and the proton capture mechanism
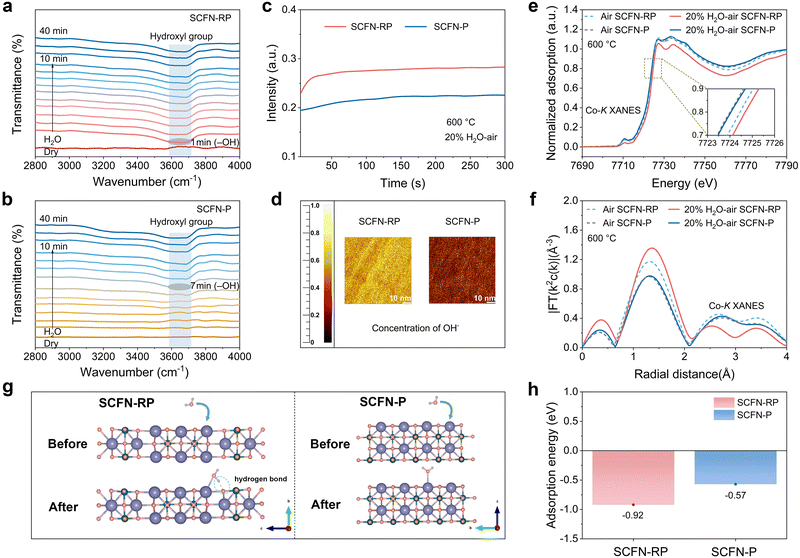
Given that water adsorption is a prerequisite for effective hydration, the proton uptake behaviours of the SCFN-RP and SCFN-P samples were further examined via in situ Fourier transform infrared (FTIR) spectroscopy.8 Both samples were subjected to 40 min of isothermal steam treatment at 600 °C in an atmosphere consisting of 20 vol% H2O–air (Fig. 3a and b). The SCFN-RP sample exhibited a rapid emergence and sustained presence of hydroxyl absorption bands in the 3500–3800 cm−1 region within 1 min of steam exposure. In contrast, the SCFN-P sample showed a significantly slower hydroxyl response, stabilizing only after 7 min, suggesting limited water adsorption capacity and slower proton incorporation kinetics (Fig. S10). Time-of-flight secondary ion mass spectrometry (TOF-SIMS) was employed to perform depth profiling on steam-treated SCFN-RP and SCFN-P samples, further monitoring the variation in characteristic ion signals, such as OH−, with depth to evaluate their enrichment on the surface and within the bulk of the materials (the normalized value is defined as the ratio of OH− intensity to the total ion count). As shown in Fig. 3c, the normalized OH− signal intensities in the SCFN-RP and SCFN-P samples tend to increase and then stabilize with the depth augmentation. The OH− signal intensity and its increasing rate from the surface to the bulk are significantly higher in SCFN-RP than in SCFN-P. The relatively weak OH− signal at the immediate surface is attributable to desorption of physically adsorbed water and weakly bound hydroxyl species under the ultra-high vacuum conditions required for TOF-SIMS measurement. The detected OH− signal originates predominantly from hydration reactions that generate additional protonic defects. The markedly stronger and more rapidly increasing OH− signal in SCFN-RP provides direct evidence of its higher bulk hydroxyl concentration. This enhancement mechanism stems from hydrated oxyhydroxide formed during the phase transition of SCFN-RP, which exhibits a much greater propensity for bulk hydration. This structure facilitates the efficient diffusion of water molecules from the surface into the bulk. In contrast, the perovskite structure of SCFN-P possesses a limited inherent tendency for such bulk hydration.7,35,36 The TOF-SIMS ion distribution map clearly visualizes the concentration distribution of OH− on the electrode surfaces (Fig. 3d). Both samples display relatively uniform OH− distributions, and the SCFN-RP sample has the higher OH− concentration, further indicating a fast water adsorption capability that facilitates hydration reactions and enhances proton conduction.To further investigate the hydration mechanism and its correlation with oxygen vacancy compensation, X-ray absorption spectroscopy (XAS) was employed to analyse the changes in the absorption edge energy of the metal cations before and after steam treatment. Fig. 3e presents the Co K-edge X-ray absorption near-edge structure (XANES) spectra under different conditions. The results revealed a distinct shift of the Co K-edge towards higher energy upon the introduction of steam in SCFN-RP, indicating an increased electron binding energy of Co ions induced by hydration, whereas the Co valence state in SCFN-P changed negligibly after steam treatment, indicating a weaker hydration response.7 The Fe K-edge XANES spectra exhibit a similar trend in binding energy variations (Fig. S13). The extended X-ray absorption fine structure (EXAFS) of the Co K-edge was also obtained by Fourier transformation for a deeper exploration of the spatial structure of Co. The first and most prominent coordination peak in the figure corresponds to the Co–O bond (Fig. 3f). The figure shows that the coordination number of Co–O in the SCFN-RP sample increases after the introduction of steam, indicating that more oxygen ions surround the Co in the SCFN-RP sample lattice, which implies fewer bulk oxygen vacancies and pronounced hydration capability. The intensity of the Co–O peak in the SCFN-P sample remains almost unchanged before and after steam treatment, indicating a weaker hydration reaction.
DFT calculations were also performed to evaluate the H2O binding energies of SCFN-RP and SCFN-P, confirming the superior water adsorption capability of SCFN-RP. Water adsorption on SCFN-RP and SCFN-P occurs primarily through the bonding of the oxygen atom to the A-site Sr. This is primarily due to its deeper 3d orbitals and pronounced charge transfer capability, which promote stable electrostatic interactions with lone pair electrons on oxygen in water molecules (Fig. S14). Additionally, the large energy gap between Sr and oxygen enhances electron transfer and bond stability. In contrast, the 3d orbitals of Co tend to form antibonding states that weaken bonding with water, while the higher metallicity of Sr facilitates stronger interactions with polar water molecules.37 Benefiting from the undercoordinated oxygen sites in RP-type rock-salt layers, the hydrogen atoms in H2O can also form hydrogen bonds (Fig. 3g). This dual bonding mechanism not only stabilizes the adsorption configuration but also facilitates the formation of hydrated oxyhydroxides. As a result, SCFN-RP exhibits a markedly lower water binding energy (−0.92 eV) than SCFN-P (−0.57 eV) (Fig. 3h), thereby accelerating water activation and dissociation and enhancing the reaction kinetics.
Electrolysis performance and durability
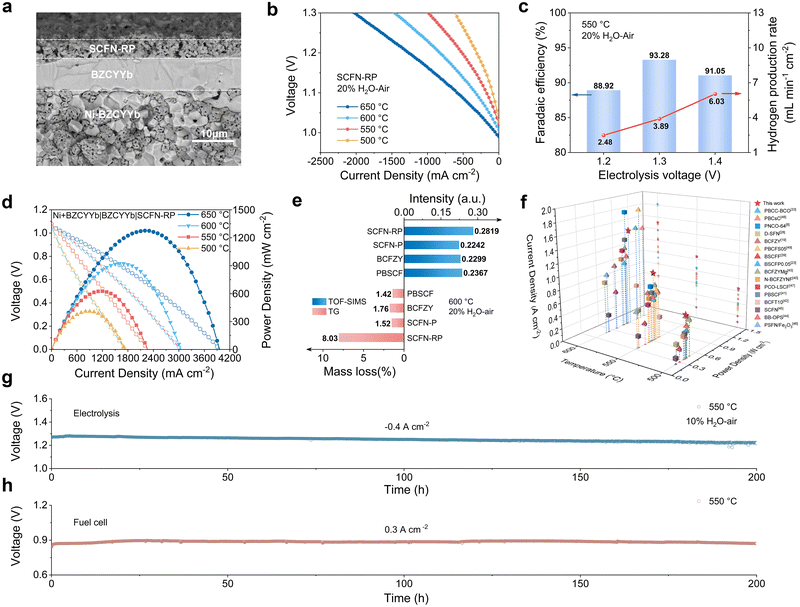
A laboratory-scale single cell with the configuration of Ni-BZCYYb/BZCYYb/SCFN-RP was fabricated to evaluate the performance of the SCFN-RP oxygen electrode. As shown in the cross-sectional SEM image (Fig. 4a), the dense BZCYYb electrolyte layer has a thickness of approximately 10 µm. The excellent OER activity of the SCFN-RP oxygen electrode under the PCEC mode was further demonstrated. As shown in Fig. 4b, the I–V curves measured at different temperatures (650–500 °C) indicate that at a polarization voltage of 1.3 V, current densities of 2.13, 1.54, 1.01, and 0.64 A cm−2 were achieved at 650, 600, 550 and 500 °C, respectively. A comparison of the electrolysis performance under identical conditions (Fig. S15) revealed that the SCFN-RP electrode exhibited significantly enhanced performance over the SCFN-P electrode. EIS at 1.3 V (Fig. S16 and S17) further demonstrated that the SCFN-RP electrode possesses lower ohmic and polarization resistances than the SCFN-P electrode, indicating that its superior electronic and protonic conductivities effectively reduce the interfacial charge transfer resistance and synergistically enhance the overall electrochemical reaction kinetics. Fig. 4c presents the faradaic efficiency (FE) of the SCFN-RP electrode at 550 °C in 20 vol% H2O–air under various applied voltages. The efficiency increased to 93.28% at 1.3 V but decreased to 91.05% when the voltage was further increased to 1.4 V. This decrease is attributed to the elevated oxygen partial pressure at higher voltages, which correlates positively with the electron–hole concentration, resulting in lower faradaic efficiency.38Fig. 4g further shows the operational stability of the cell under a constant current density of −0.4 A cm−2 at 550 °C in a 10 vol% H2O–air atmosphere. The cell with the SCFN-RP electrode maintained stable operation for more than 200 h, with a gradual improvement in performance, confirming its excellent long-term durability. Furthermore, the power output performance of the SCFN-RP electrode was evaluated in the PCFC mode. The peak power densities (PPDs) of 1.28, 0.92 and 0.63 W cm−2 were achieved at 650, 600 and 550 °C, respectively (Fig. 4d). In addition, the electrochemical stability of the SCFN-RP oxygen electrode was assessed at 550 °C under a constant current density of 0.3 A cm−2. The electrode also demonstrated excellent catalytic durability, as evidenced by its stable operation over 200 hours (Fig. 4h).In this study, the conventional cobalt-based single perovskite BaCo0.4Fe0.4Zr0.1Y0.1O3−δ (BCFZY) and double perovskite PrBa0.5Sr0.5Co1.5Fe0.5O5+δ (PBSCF) were selected as reference materials. TGA and TOF-SIMS were performed to compare the weight loss and the normalized OH− content stabilized with depth (Fig. 4e). The SCFN-RP sample exhibited a significantly increased OH− concentration and pronounced mass loss, confirming its superior water adsorption and hydration capability attributed to the formation of hydrated oxyhydroxides induced by steam. The electrochemical performance of several representative oxygen electrode materials under both PCFC and PCEC modes was systematically evaluated at 600, 550 and 500 °C (Fig. 4f).8,19,23,28,33,39–49 SCFN-RP exhibited relatively superior performance in the PCFC mode and even more remarkable activity in the PCEC mode. While the RP-type Pr2Ni0.6Co0.4O4−δ (PNCO-64) and single perovskite Pr0.5Ba0.5Co0.7Fe0.25Sn0.05O3+δ (PBCFS05) electrodes demonstrated high electrocatalytic activity at 600 °C, their performance decreased at lower temperatures, likely due to hindered hydration and proton transport (Tables S8 and S9). In contrast, the SCFN-RP electrode maintained excellent PCEC performance even at 550 °C and 500 °C, which further confirms that the formation of hydrated oxyhydroxides enhances the adsorption and dissociation of water, provides active sites for proton migration and facilitates proton transport.
Conclusions
In this study, an RP-type perovskite SCFN-RP was constructed by adjusting the stoichiometry of the single perovskite SCFN-P, successfully transcending the hydration limits of single perovskites. Benefiting from its unique interlayer water intercalation capability, SCFN-RP exhibits excellent steam adsorption and protonation capabilities, as confirmed by FTIR, TOF-SIMS and XAS. This behavior originates from the synergistic effect of hydrogen bonding with lattice oxygen in the rock–salt layers and the formation of stable Sr–O bonds in the perovskite layers. This dual bonding mechanism enables reversible structural phase transitions under high-temperature steam conditions and leads to the formation of hydrated oxyhydroxides, as further verified by HT-XRD. By integrating structural analysis employing MicroED and DFT calculations, it is demonstrated that the formation of the hydrated oxyhydroxide compound Sr3(Co0.8Fe0.1Nb0.1)2O17−x(OH)x·αH2O effectively facilitates interlayer proton capture, overcomes the limitation of proton transport being confined to two-dimensional intralayer pathways in the RP phase, and establishes a hydrogen-bonding network enabling proton conduction along the c-axis. This significantly lowers the energy barrier for proton migration across layers. As a result, the SCFN-RP electrode achieves a current density of 1.01 A cm−2 @1.3 V at 550 °C, exhibiting excellent electrocatalytic activity and stability and outperforming most of previously reported RP-type oxygen electrodes. This work emphasizes the crucial role of reversible hydrated oxyhydroxides in RP perovskites and offers a novel conception for the design of high-performance oxygen electrodes for PCECs.Author contributions
Conceptualization: Meijuan Fei, Chuan Zhou, and Wei Zhou; methodology: Zhaohui Cai and Dongliang Liu; investigation: Peng Chen and Cheng Huang; visualization: Jianqiu Zhu; supervision: Linjuan Zhang, Wei Wang, and Zongping Shao; writing – original draft: Meijuan Fei and Chuan Zhou; writing – review & editing: Meijuan Fei, Chuan Zhou, and Wei Zhou.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting the findings of this study are available within the article and supplementary information (SI) or from the authors upon reasonable request. Supplementary information: powder synthesis, cell fabrication, materials and microstructure characterization, and electrochemical measurements. See DOI: https://doi.org/10.1039/d5ee05802c.Acknowledgements
This work was supported from the National Natural Science Foundation of China (No. 22278203 and No. 22279057) and funded by Basic Research Program of Jiangsu (BK20250535).References
- X. Chen, Y. Tan, Z. Li, T. Liu, Y. Song, S. Zhai, N. Yu, Z. Shao and M. Ni, Adv. Mater., 2025, 2418620 CrossRef CAS PubMed.
- F. Liu, D. Ding and C. Duan, Adv. Sci., 2023, 10, 2206478 CrossRef CAS PubMed.
- F. He, Y. Zhou, T. Hu, Y. Xu, M. Hou, F. Zhu, D. Liu, H. Zhang, K. Xu, M. Liu and Y. Chen, Adv. Mater., 2023, 35, 2209469 CrossRef CAS PubMed.
- C. Zhou, D. Liu, M. Fei, X. Wang, R. Ran, M. Xu, W. Wang, W. Zhou, R. O'Hayre and Z. Shao, J. Power Sources, 2023, 556, 232403 CrossRef CAS.
- Y. Song, Y. Chen, W. Wang, C. Zhou, Y. Zhong, G. Yang, W. Zhou, M. Liu and Z. Shao, Joule, 2019, 3, 2842–2853 CrossRef CAS.
- Z. Liu, Y. Bai, H. Sun, D. Guan, W. Li, W.-H. Huang, C.-W. Pao, Z. Hu, G. Yang, Y. Zhu, R. Ran, W. Zhou and Z. Shao, Nat. Commun., 2024, 15, 472 CrossRef CAS PubMed.
- C. Zhou, X. Wang, D. Liu, M. Fei, J. Dai, D. Guan, Z. Hu, L. Zhang, Y. Wang, W. Wang, R. O'Hayre, S. P. Jiang, W. Zhou, M. Liu and Z. Shao, Energy Environ. Mater., 2023, 7, e12660 CrossRef.
- J. Xia, F. Zhu, F. He, K. Xu, Y. Choi and Y. Chen, Adv. Energy Mater., 2023, 13, 2302964 CrossRef CAS.
- I. Kagomiya, K. Jimbo, K.-I. Kakimoto, M. Nakayama and O. Masson, Phys. Chem. Chem. Phys., 2014, 16, 10875–10882 RSC.
- Y. Zhu, H. A. Tahini, Z. Hu, Y. Yin, Q. Lin, H. Sun, Y. Zhong, Y. Chen, F. Zhang, H. J. Lin, C. T. Chen, W. Zhou, X. Zhang, S. C. Smith, Z. Shao and H. Wang, EcoMat, 2020, 2, e12021 CrossRef CAS.
- R. Dutta, A. Maity, A. Marsicano, M. Ceretti, D. Chernyshov, A. Bosak, A. Villesuzanne, G. Roth, G. Perversi and W. Paulus, J. Mater. Chem. A, 2020, 8, 13987–13995 RSC.
- S. Tashiro, Y. Miyahara, C. Lee, H. Kiuchi, T. Abe and K. Miyazaki, Chem. Mater., 2023, 35, 7039–7048 CrossRef CAS.
- M. Lehtimäki, H. Yamauchi and M. Karppinen, J. Solid State Chem., 2013, 204, 95–101 CrossRef.
- N. B. D. Pelloquin, D. Flahaut, V. Caignaert and A. Maignan, Chem. Mater., 2005, 17, 773–780 CrossRef.
- D. Pelloquin, N. Barrier, A. Maignan and V. Caignaert, Solid State Sci., 2005, 7, 853–860 CrossRef CAS.
- H. Sonoki, D. Mori, S. Taminato, Y. Takeda, O. Yamamoto and N. Imanishi, Chem. Commun., 2019, 55, 7454–7457 RSC.
- M. Lehtimäki, A. Hirasa, M. Matvejeff, H. Yamauchi and M. Karppinen, J. Solid State Chem., 2007, 180, 3247–3252 CrossRef.
- L. Musílek, R. Prokeš and T. Trojek, Radiat. Phys. Chem., 2022, 200, 110388 CrossRef.
- C. Duan, J. Tong, M. Shang, S. Nikodemski, M. Sanders, S. Ricote, A. Almansoori and R. O’Hayre, Science, 2015, 349, 1321–1326 CrossRef CAS PubMed.
- J. Gong, L. Xu, W. Zhu, L. Xie, X. Chen and X. Liu, ACS Sustainable Chem. Eng., 2024, 12, 3757–3765 CrossRef CAS.
- X. Li, Z. Jin, C. Wang, R. Peng, Y. Zha, J. Cao, Y. Ji and Z. Shao, Adv. Energy Mater., 2024, 14, 2400319 CrossRef CAS.
- Y. Li, C. Yang, X. Liu, C. Yang, K. Xia, Y. Tian, S. He and B. Chi, ACS Sustainable Chem. Eng., 2025, 13, 4740–4749 CrossRef CAS.
- Z. Liu, D. Cheng, Y. Zhu, M. Liang, M. Yang, G. Yang, R. Ran, W. Wang, W. Zhou and Z. Shao, Chem. Eng. J., 2022, 450, 137787 CrossRef CAS.
- Z. Liu, Z. Tang, Y. Song, G. Yang, W. Qian, M. Yang, Y. Zhu, R. Ran, W. Wang, W. Zhou and Z. Shao, Nano-Micro Lett., 2022, 14, 217 CrossRef CAS PubMed.
- H. Shi, C. Su, X. Xu, Y. Pan, G. Yang, R. Ran and Z. Shao, Small, 2021, 17, 2101872 CrossRef CAS PubMed.
- Z. Wang, Y. Wang, J. Wang, Y. Song, M. J. Robson, A. Seong, M. Yang, Z. Zhang, A. Belotti, J. Liu, G. Kim, J. Lim, Z. Shao and F. Ciucci, Nat. Catal., 2022, 5, 777–787 CrossRef CAS.
- K. Xu, H. Zhang, Y. Xu, D. Liu, F. Zhu, F. He, Y. Liu, H. Wang and Y. Chen, Adv. Powder Mater., 2024, 3, 100187 CrossRef.
- N. Yu, I. T. Bello, X. Chen, T. Liu, Z. Li, Y. Song and M. Ni, Nano-Micro Lett., 2024, 16, 177 CrossRef CAS PubMed.
- X. Yu, L. Ge, Y. Mi, B. Wu, Z. Yu, Z. Jin, Z. Zhao, B. He, H. Chen, Y. Zheng and S. Cui, Small, 2025, 21, 177 Search PubMed.
- Y. Zhang, A. Zhu, Y. Guo, C. Wang, M. Ni, H. Yu, C. Zhang and Z. Shao, Appl. Energy, 2019, 238, 344–350 CrossRef CAS.
- S. Zhao, W. Ma, B. He, Y. Ling, Y. Huang, F. Hu, Z. Shu and L. Zhao, Adv. Mater., 2025, 37, 2501387 CrossRef CAS PubMed.
- S. Zhao, W. Ma, W. Wang, Y. Huang, J. Wang, S. Wang, Z. Shu, B. He and L. Zhao, Adv. Mater., 2024, 36, 2405052 CrossRef CAS PubMed.
- Y. Zhou, E. Liu, Y. Chen, Y. Liu, L. Zhang, W. Zhang, Z. Luo, N. Kane, B. Zhao, L. Soule, Y. Niu, Y. Ding, H. Ding, D. Ding and M. Liu, ACS Energy Lett., 2021, 1511–1520 CrossRef CAS.
- F. Zhu, F. He, D. Liu, H. Zhang, Y. Xu, K. Xu and Y. Chen, Energy Storage Mater., 2022, 53, 754–762 CrossRef.
- Y. Shin, M. D. Sanders, E. Truong, S. Majumder, B. Cladek, M. Walker, B. Ogbolu, R. Zhang, G. A. Evmenenko, Y.-Y. Hu, M. J. Bedzyk, K. Page, S. M. Haile and R. O'Hayre, Solid State Ionics, 2025, 429, 116962 CrossRef CAS.
- A. Seong, J. Kim, D. Jeong, S. Sengodan, M. Liu, S. Choi and G. Kim, Adv. Sci., 2021, 8, 2004099 CrossRef CAS PubMed.
- X. Li, H. Zhao, J. Liang, Y. Luo, G. Chen, X. Shi, S. Lu, S. Gao, J. Hu, Q. Liu and X. Sun, J. Mater. Chem. A, 2021, 9, 6650–6670 RSC.
- F. Liu, H. Deng, D. Diercks, P. Kumar, M. H. A. Jabbar, C. Gumeci, Y. Furuya, N. Dale, T. Oku, M. Usuda, P. Kazempoor, L. Fang, D. Chen, B. Liu and C. Duan, Nat. Energy, 2023, 8, 1145–1157 CrossRef CAS.
- X. Chen, N. Yu, I. T. Bello, D. Guan, Z. Li, T. Liu, T. Liu, Z. Shao and M. Ni, Energy Storage Mater., 2023, 63, 103056 CrossRef.
- X. Chen, N. Yu, Y. Song, T. Liu, H. Xu, D. Guan, Z. Li, W. H. Huang, Z. Shao, F. Ciucci and M. Ni, Adv. Mater., 2024, 36, 2403998 CrossRef CAS PubMed.
- S. Choi, T. C. Davenport and S. M. Haile, Energy Environ. Sci., 2019, 12, 206–215 RSC.
- Y.-S. Feng, Y. Shen, F. Wang, C. Tian, Q.-Y. Hu, Q. Duan, J.-W. Li and D. Bao, Rare Met., 2024, 43, 3055–3065 CrossRef CAS.
- M. Liang, Y. Song, D. Liu, L. Xu, M. Xu, G. Yang, W. Wang, W. Zhou, R. Ran and Z. Shao, Appl. Catal., B, 2022, 318, 121868 CrossRef CAS.
- Z. Liu, Y. Chen, G. Yang, M. Yang, R. Ji, Y. Song, R. Ran, W. Zhou and Z. Shao, Appl. Catal., B, 2022, 319, 121929 CrossRef CAS.
- Y. Song, Y. Chen, M. Xu, W. Wang, Y. Zhang, G. Yang, R. Ran, W. Zhou and Z. Shao, Adv. Mater., 2020, 32, 1906979 CrossRef CAS PubMed.
- Z. Wang, Y. Xiao, Y. Zhang, Y. Wang, X. Wang, F. Wang and T. He, Chem. Eng. J., 2024, 490, 151911 CrossRef CAS.
- L. Wu, J. Sun, H. Qi, B. Tu, C. Xiong, F. Chen and P. Qiu, J. Mater. Chem. A, 2024, 12, 25979–25987 RSC.
- Y. Xu, K. Xu, F. Zhu, F. He, H. Zhang, C. Fang, Y. Liu, Y. Zhou, Y. Choi and Y. Chen, ACS Energy Lett., 2023, 8, 4145–4155 CrossRef CAS.
- M. Fu, W. Hu, H. Tong, X. Ling, L. Tan, F. Chen and Z. Tao, J. Adv. Ceram., 2024, 13, 63–72 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |