Sodium-compensating electrolyte additives stabilize interfaces for highly reversible anode-free sodium batteries
Chunlin
Xie
ab,
Shengfang
Liu
a,
Jin
Wang
a,
Xianghui
Meng
c,
Shuyi
Yu
d,
Jiaming
Zhang
a,
Haijun
Peng
a,
Dan
Sun
 a,
Yougen
Tang
a,
Yougen
Tang
 a and
Haiyan
Wang†
a and
Haiyan
Wang†
 *a
*a
aHunan Provincial Key Laboratory of Chemical Power Sources, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: wanghy419@csu.edu.cn
bGuangdong DFP New Material Group Co., Ltd, Shantou, 515064, P.R. China
cShandong All Grand Battery Co., Ltd, Dezhou, 253000, P.R. China
dInstitute for Advanced Study, Central South University, Changsha 410083, China
First published on 12th November 2025
Abstract
Anode-free sodium batteries (AFSBs) offer superior cost, energy density, and manufacturing advantages over sodium-ion batteries (SIBs) by eliminating the conventional anode. However, their development is fundamentally hindered by dead sodium formation and interfacial side reactions that severely limit the cycle life. To address these issues, for the first time, we report sodium bis(trimethylsilyl)amide (NaHMDS) as a non-invasive, gas-free electrolyte additive for sodium compensation, which operates at a low compensation potential of 2.82 V (vs. Na+/Na). This multifunctional additive enables Ah-level AFSB pouch cells to retain 89.6% capacity after 500 cycles at a rate of 1C by compensating active sodium loss, stabilizing electrode interphases, and improving sodium stripping/plating reversibility. In contrast, the sodium-compensating additives sodium thiocyanate (NaSCN) and NaNO2 induce irreversible side reactions, electrode degradation, and dendrite growth while failing to provide effective sodium compensation for AFSBs. This work presents a promising sodium-compensating additive with commercial viability, redefining AFSB additive design principles by prioritizing interfacial stability over conventional metrics such as compensation capacity and potential.
Broader contextAnode-free sodium batteries (AFSBs) offer a compelling alternative to conventional sodium-ion batteries (SIBs) by eliminating anode materials, thereby achieving higher energy density and lower cost. However, their practical viability is fundamentally limited by dead sodium formation and interfacial side reactions, which irreversibly deplete the finite sodium inventory, a critical challenge in AFSBs. While sodium-compensating additives have been explored in SIBs, their direct transfer to AFSBs often fails due to uncontrolled side reactions and dendritic growth, highlighting the need for additive design criteria tailored to AFSBs’ unique interfacial chemistry. In this work, we elucidate the stabilization mechanisms of sodium-compensating additives in AFSBs through comprehensive tracking of interfacial chemistry and morphological evolution at both the anode and cathode. We introduce, for the first time, sodium bis(trimethylsilyl)amide (NaHMDS) as a non-invasive, gas-free electrolyte additive capable of sodium compensation at a low potential of 2.82 V (vs. Na+/Na). Notably, this additive enables the Ah-level AFSB pouch cell to retain 89.6% capacity after 500 cycles at a rate of 1C by compensating for sodium loss, stabilizing electrode interphases, and improving sodium stripping/plating reversibility. This work establishes interfacial stability as the governing criterion for sodium-compensating additive design in AFSBs, superseding conventional metrics such as compensation capacity and potential. |
Introduction
Sodium-ion batteries (SIBs) have emerged as a viable complement to lithium-ion batteries (LIBs) for large-scale energy storage, owing to sodium's significantly higher natural abundance, widespread availability, and lower resource costs.1–3 However, the high production cost and limited theoretical capacity (<330 mAh g−1) of conventional hard carbon anodes impose significant constraints on both the achievable energy density and cost competitiveness of SIBs relative to commercial LIB technologies.4 Sodium metal anodes exhibit compelling intrinsic properties for high-energy-density batteries, including a low redox potential (−2.71 V vs. SHE) and a high theoretical capacity (1166 mAh g−1).5 Nevertheless, their commercial viability faces two fundamental constraints: (i) substantial manufacturing costs associated with ultrathin sodium fabrication and inert-atmosphere processing, and (ii) the inherent compromise of maintaining the excess anode metal that reduces practical energy density and increases safety risks.6 The anode-free sodium battery (AFSB) configuration completely eliminates conventional anode materials (both metallic sodium and hard carbon), employing only a bare current collector as the initial anode substrate.7 During the initial charging cycle, sodium ions extracted from the cathode are electrochemically deposited onto the collector surface, forming a functional sodium metal anode. This architecture reduces manufacturing costs while improving the energy density and safety performance.8 Importantly, it maintains full compatibility with existing LIB production infrastructure, thereby establishing a new paradigm to circumvent the inherent limitations of conventional SIBs.In AFSBs, the cathode serves as the sole sodium source, presenting an inherently limited sodium inventory.9 Without protective carbon matrices, sodium metal is repeatedly exposed to the electrolyte during plating/stripping cycles, leading to persistent side reactions and continuous solid electrolyte interphase (SEI) reformation. Furthermore, the substrate's poor sodium affinity leads to irreversible dead sodium formation.10,11 These cumulative degradation mechanisms progressively deplete the limited sodium supply, constituting the primary obstacle to achieving long-term cyclability in AFSBs.12 To address these challenges, a combined strategy of stabilizing cathode/anode interfaces and enabling reversible sodium plating/stripping through precisely engineered electrolytes and optimized current collectors is essential for enhancing cycling stability and Coulombic efficiency.13,14 For instance, Hu et al.15 demonstrated that synergistic modulation of the sodium deposition morphology via NaBF4 additives and carbon nanofiber modification effectively suppressed both parasitic reactions and dead sodium formation. Their anode-free sodium pouch cells retained 84% capacity after 260 cycles. In a complementary approach, Wang et al.16 developed a single-solvent high-entropy electrolyte system that concurrently stabilizes cathode–electrolyte and anode–electrolyte interfaces, enabling long-cycle-life anode-free sodium pouch cells with wide temperature operability.
Sodium-compensating additives have been widely investigated in SIBs to compensate for active sodium loss caused by interfacial side reactions during initial cycling. However, conventional sodium-compensating additives (e.g., sodium oxalate, sodium nitrite, and sodium citrate) present critical challenges in AFSBs.17–19 Their decomposition voltage ranges exhibit poor compatibility with ether-based electrolytes, and when incorporated into cathodes, the gas evolution during decomposition may compromise electrode structural integrity. Furthermore, the evolved gases may diffuse to the anode and react with metallic sodium, which not only depletes active sodium but also forms thick passivation layers that hinder ion transport.20 These fundamental limitations significantly restrict the practical application of such additives in AFSBs. The newly developed electrolyte-soluble sodium-compensating additive, recently reported in Nature, has generated significant research interest.21 This innovative approach enables direct sodium compensation in assembled battery systems through simple electrolyte introduction, allowing non-destructive operation without cell disassembly. However, such additives still face challenges related to gas evolution during operation. Notably, the researchers developed a non-gassing electrolyte additive (NaSCN) for sodium compensation, which enhances the initial coulombic efficiency (ICE) in sodium-ion batteries while maintaining superior electrochemical performance.22 Nevertheless, its potential application in AFSBs requires further evaluation. To address these challenges, systematic investigation of stabilization mechanisms for diverse sodium-compensating additives in AFSBs, along with the development of gas-free, electrolyte-soluble sodium-compensating additives that can concurrently stabilize both cathode–electrolyte and anode–electrolyte interfaces, constitutes a crucial step toward the practical implementation of AFSBs.
In this work, we report a gas-free sodium-compensating additive with a low compensation potential. Spectroscopic analysis of the compensation byproducts reveals an HMDS−-mediated mechanism where hydrogen abstraction from ether solvents during oxidation initiates their short-chain polymerization. The stabilization mechanisms of sodium-compensating additives are investigated by tracking anode/cathode interfacial chemistry and morphological evolution using in situ/ex situ characterization during cycling. NaHMDS provides dual sodium compensation and interfacial stabilization functions, enhancing both energy density and cycling stability in AFSBs. For reference, the NaSCN additive induces interfacial side reactions that deactivate sodium despite its gas-free operation, whereas the conventional cathode additive (NaNO2) generates NO2 that migrates to form thick SEI layers and compromises cathode integrity, rendering both approaches unsuitable for AFSBs. Notably, anode-free sodium coin-cells employing NaHMDS achieve 92.1% capacity retention over 320 cycles at 1C, far surpassing cells with NaSCN or NaNO2, which fail within 5 cycles. This work establishes a sodium-compensating electrolyte additive strategy that synergistically improves energy density and cycling stability, providing a new design paradigm for practical AFSBs.
Results and discussion
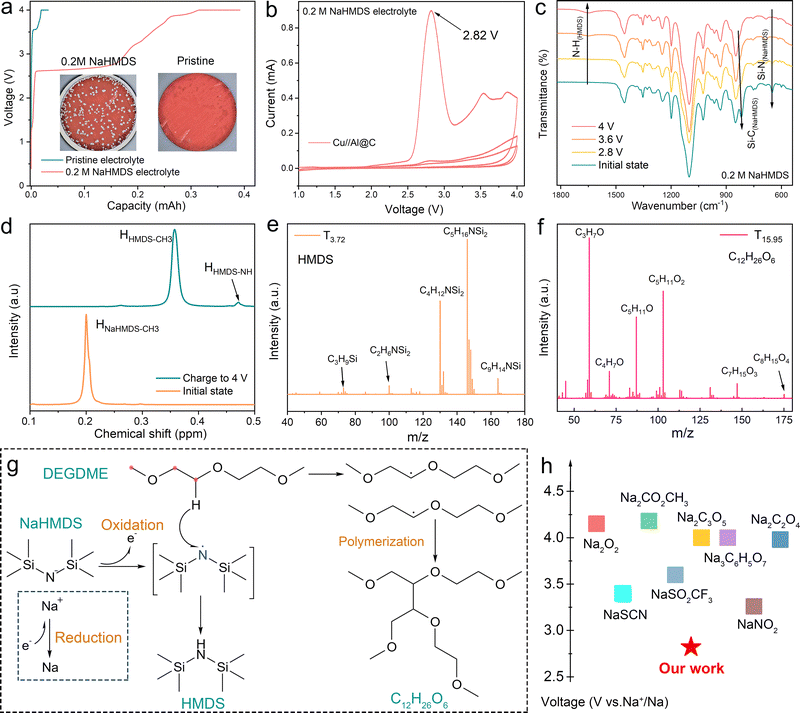
Coin cells (Cu‖Al@C configuration) were assembled using a copper foil as the anode and a carbon-coated aluminum foil (Al@C) as the cathode. Upon charging to 4.0 V, the cell employing a 0.2 M NaHMDS electrolyte exhibits a charge capacity of 0.39 mAh, whereas the cell using the pristine electrolyte delivers a significantly lower charge capacity of only 0.03 mAh (Fig. 1a). Subsequent disassembly and optical microscopy characterization reveal distinct morphological differences: the copper anode cycled in the NaHMDS electrolyte shows metallic sodium deposition (Fig. 1a, inset), while no deposition is observed on the pristine electrolyte counterpart. This confirms that the deposited sodium originates from the electrochemical reduction of NaHMDS, not the cathode (Al@C does not contain a sodium source). The surface of metallic sodium deposited using a 0.2 M NaHMDS electrolyte is dense (Fig. S1). Cyclic voltammetry (CV) measurements show that NaHMDS decomposes at 2.82 V (vs. Na+/Na), with near-complete compensation achieved by the first cycle (Fig. 1b). In anode-free pouch cells, no swelling is observed after charging to 3.9 V, confirming gas-free reduction (Fig. S2). Differential electrochemical mass spectrometry also indicated no significant gas generation from the NaHMDS electrolyte during the first cycle, similar to the pristine electrolyte (Fig. S3). Notably, NaHMDS exhibits a solubility exceeding 2 M in the NaPF6 electrolyte (Fig. S4). With a theoretical sodium compensation capacity of 143.1 mAh g−1, the addition of 1 mL of 2 M NaHMDS electrolyte can provide 52.5 mAh of sodium source. Several classes of sodium compensation additives are systematically evaluated in ether-based electrolyte systems.21,22 While NaSCN demonstrates sodium compensation capability without gas evolution, this additive shows clear incompatibility with metallic sodium, as evidenced by the tarnished appearance of deposited sodium (Fig. S5). Additionally, its relatively high compensation potential (3.44 V) leads to incomplete electrochemical conversion, leaving considerable unreacted species after the initial cycle (Fig. S6). Other reported additives like NaNO2 exhibit lower decomposition potentials (3.41 V) but generate NO2 gas,18,23,24 and their suitability for AFSB applications remains poorly understood (Fig. S5 and S6). We employed 0.2 M NaHMDS and 0.1 M NaSCN as electrolyte additives, while using 10 wt% NaNO2 in the Na4Fe3(PO4)2P2O7 (NFPP) cathode as a conventional solid-phase additive, with all three systems providing similar total sodium compensation capacities for comparative studies. In this work, we will focus on investigating the effects of these sodium-compensating additives on the interfacial chemistry in AFSBs.To elucidate the sodium-compensating mechanism of NaHMDS, we conducted systematic electrochemical characterization using Cu‖Al@C cells containing the 0.2 M NaHMDS/DEGDME electrolyte (without NaPF6). The cells were charged to progressively higher potentials, followed by electrolyte extraction at each state of charge for spectroscopic analysis. Fourier transform infrared spectroscopy (FTIR) evolution (Fig. 1c) demonstrates clear potential-dependent behavior: the N–H stretching vibration intensifies progressively with an increase in charging potential, while the characteristic Si–N and Si–C vibrations of the HMDS− anion diminish concomitantly.25 Remarkably, the spectrum of the electrolyte charged to 4.0 V matches exactly with that of a 0.2 M bis(trimethylsilyl)amine (HMDS) reference solution (Fig. S7), confirming complete conversion to neutral HMDS species through electrochemical oxidation of HMDS− coupled with hydrogen abstraction from DEGDME. Complementary electrochemical impedance spectroscopy (EIS) measurements show a substantial impedance increase corresponding to the transformation from ionic-conductive NaHMDS to non-conductive HMDS solution (Fig. S8). This transition is further verified by 1H NMR spectroscopy, where new proton signals at δ 0.47 ppm emerge, unambiguously assigned to the N–H group of neutral HMDS (Fig. 1d).26 To identify volatile compensation products, we performed headspace solid-phase microextraction gas chromatography-mass spectrometry (HS-SPME-GC-MS) analysis of electrolytes charged to 4.0 V (Fig. S9). The analysis identifies characteristic HMDS fragment peaks at a retention time of 3.72 min (TR = 3.72 min), providing definitive evidence that HMDS is a primary compensation product (Fig. 1e). Additionally, mass spectrometric characterization reveals fragment peaks corresponding to C12H26O6, a short-chain polymerization product formed through hydrogen abstraction from DEGDME (Fig. 1f and Fig. S10). Based on the spectroscopic and chromatographic evidence of NaHMDS compensation products, we propose a sodium compensation mechanism (Fig. 1g) as follows: (i) Na+ is reduced to metallic sodium at the anode, concurrent with (ii) cathodic oxidation of HMDS− to form radical intermediates that abstract α-hydrogens from DEGDME, yielding neutral HMDS. The conversion product HMDS remains highly stable when in contact with metallic sodium (Fig. S11). This additive overcomes the high decomposition potential of conventional sodium compensation agents, offering a promising solution to the persistent low ICE in sodium batteries (Fig. 1h and Table S1).
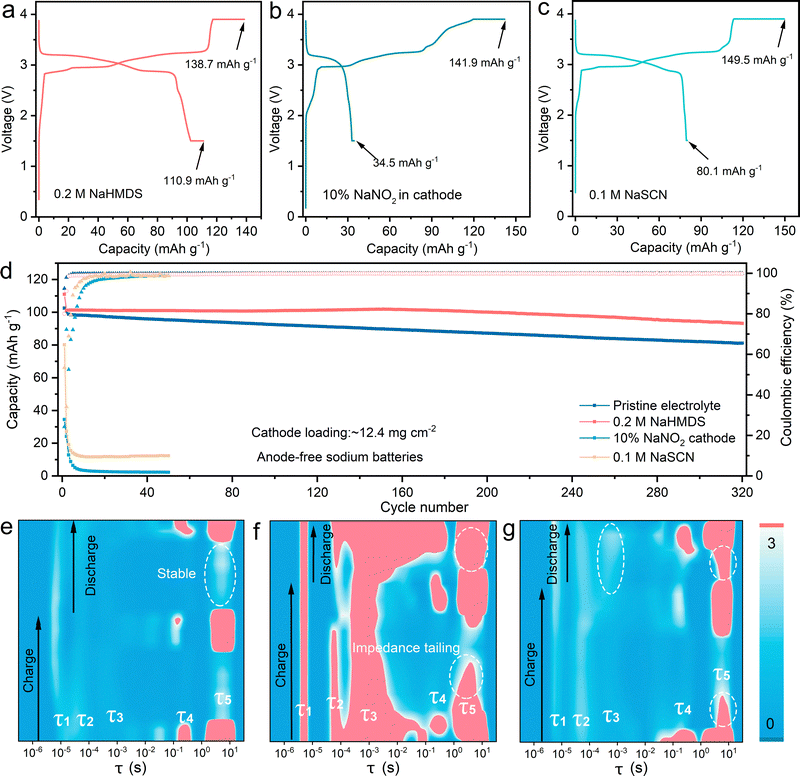
We assembled AFSBs using graphite-coated aluminum foil and a high-loading NFPP cathode (12.4 mg cm−2), evaluating the electrochemical performance of different sodium-compensating additives. As shown in Fig. 2a, the electrolyte containing 0.2 M NaHMDS delivers a charge capacity of 138.7 mAh g−1 and an enhanced discharge capacity of 110.9 mAh g−1, significantly outperforming the pristine electrolyte (110.8 mAh g−1 and 102.7 mAh g−1, respectively) (Fig. S12). This performance demonstrates the effectiveness of NaHMDS in compensating for active sodium loss from the cathode, enabling the AFSB to achieve discharge capacities approaching the cathode's theoretical charge capacity (Fig. S13). Notably, while NaNO2 and NaSCN exhibit higher charge capacities (141.9 and 149.1 mAh g−1, respectively), they are less practical due to substantially lower discharge capacities (34.5 and 80.1 mAh g−1) (Fig. 2b–d). The anode-free sodium battery employing a 0.2 M NaSCN additive also exhibited rapid capacity decay (Fig. S14). These findings underscore that for sodium compensation in AFSBs, additive selection must consider not only theoretical capacity but, more critically, system compatibility. In situ EIS measurements were performed during the initial charge/discharge cycles to evaluate interfacial charge transfer resistance with different sodium-compensating additives (Fig. S15 and S16). The distribution of relaxation time (DRT) was derived from impedance spectra via Tikhonov-based deconvolution, yielding relaxation time distributions (τ; relaxation time).27 The relaxation time peaks τ1 and τ2 correspond to contact impedance at the current collector interfaces, τ3 reflects ionic transport through the SEI layer on sodium metal, τ4 represents sodium ion transfer to the electrode surface, and τ5 signifies kinetic limitations governed by sodium plating/stripping energy barriers and state-dependent sodium (de)intercalation kinetics at the cathode.28 As shown in Fig. 2e, the gas-free NaHMDS additive maintains low interfacial contact resistance at both the anode and cathode without degradation and sustains minimal SEI impedance. Moreover, it critically suppresses the τ5 kinetic limitation by enabling low-barrier sodium plating/stripping with negligible tailing. In contrast, the NaNO2 additive, due to gas evolution and incorporation into the cathode, significantly increases both interfacial contact resistance and SEI impedance. These effects collectively lead to severely deteriorated kinetics in the sodium plating/stripping processes (Fig. 2f and Fig. S17). Similarly, the gas-free electrolyte additive NaSCN maintains unchanged interfacial contact resistance but exhibits increased SEI impedance and deteriorated sodium plating/stripping kinetics (Fig. 2g). Furthermore, the conversion products of NaHMDS do not significantly degrade the electrochemical performance of the anode-free sodium battery (Fig. S18 and 19). These findings reveal that sodium-compensating additives must overcome compromised interfacial reversibility in AFSBs, manifested by deteriorated contact resistance, elevated SEI impedance, and aggravated kinetic barriers to sodium plating/stripping. Notably, NaHMDS serves as an effective sodium-compensating additive that simultaneously ensures stable sodium compensation and highly reversible plating/stripping. Its analogue, LiHMDS, also functions effectively as a lithium compensator to enhance discharge capacity in anode-free lithium batteries. As shown in Fig. S20, the Cu‖LFP cell with 0.2 M LiHMDS delivers a high discharge capacity of 169.5 mAh g−1, significantly outperforming the pristine electrolyte (134.7 mAh g−1). These results demonstrate the broad applicability of HMDS−-containing salts in compensating for alkali metal loss at electrode interfaces.
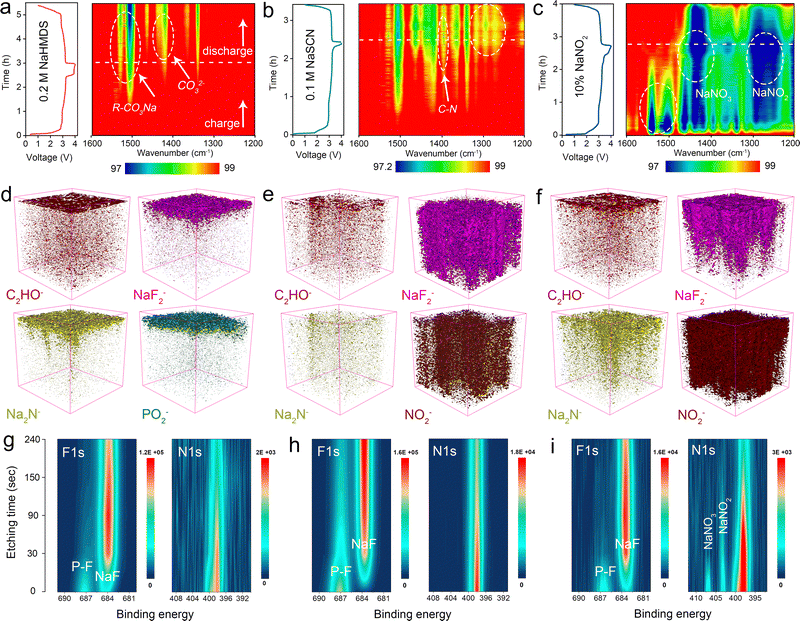
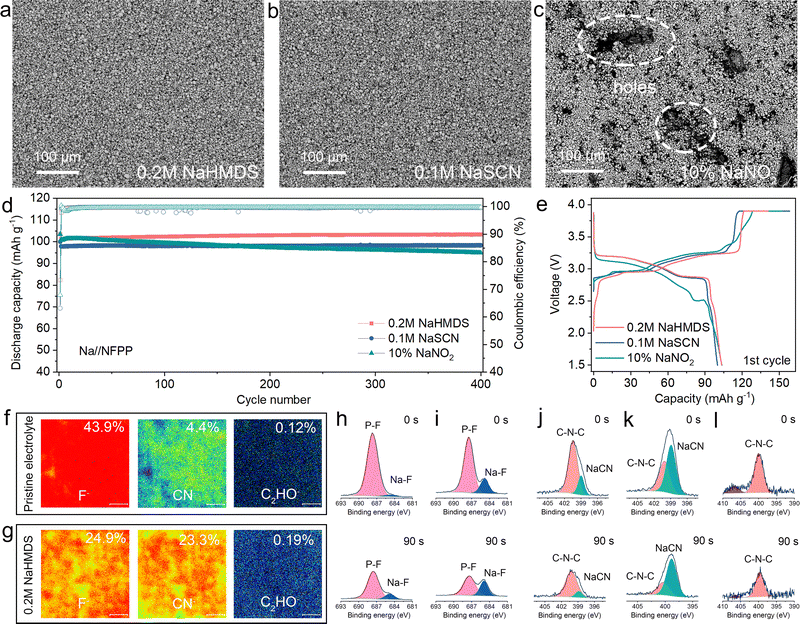
We used in situ FTIR to analyze the chemical composition of the anode interface during the initial charge/discharge processes, investigating the influence of sodium-compensating additives on interfacial evolution.29,30 For in situ FTIR testing, the battery was first placed in the in situ cell for 1 hour to equilibrate under ambient conditions. Subsequently, we collected a stable infrared spectrum of the anode interface to serve as the background reference. All subsequent infrared spectra were automatically background-subtracted. Enrichment or depletion of infrared-active SEI components at the interface will manifest as changes in spectral transmittance. As shown in Fig. S21–S23, the consistent presence of C–O–C and Na2CO3 species with low intensity variations at the anode interface (pristine electrolyte) suggests the formation of a thin SEI layer during cycling.31 The SEI derived from the NaHMDS electrolyte contains higher concentrations of R-CO3Na components but shows significantly fewer characteristic peaks than that from the NaSCN system, indicating less severe side reactions at the sodium metal interface (Fig. 3a, b and Fig. S24). Critically, NaNO2-containing cells exhibit substantial formation of NaNO3 and NaNO2 at the anode interface during cycling.32 R-CO3Na species form rapidly but subsequently degrade, destabilizing organic SEI components. This rapid formation of N–O species directly correlates with NO2 diffusion from the cathode, where NaNO2 decomposes during sodium compensation (Fig. 3c and Fig. S25). These severe side reactions significantly accelerate capacity fading. Time-of-flight secondary ion mass spectrometry (TOF-SIMS) was employed to characterize the spatial distribution of the SEI on cycled anodes. As shown in Fig. S26, the anode interface with the pristine electrolyte forms a thin but porous hybrid organic–inorganic SEI layer. In contrast, the NaHMDS-derived SEI exhibits a thin yet dense structure, consisting of an outer thin organic layer and an inner inorganic-dominated region. This inner region contains organic species with Si–C and C–O bonds alongside inorganic components such as NaF, Na3N, and sodium nitrogen oxide salt (Fig. 3d and Fig. S27–S29).33 The SEI fragments containing nitrogen and silicon may originate from the conversion product HMDS, which participates in the sodium-ion solvation structure and preferentially decomposes at the anode interface (Fig. S30). The compact SEI architecture, featuring an ion-conductive NaF/Na3N inner phase and a stabilizing organic outer layer, effectively suppresses electrolyte compensation while facilitating efficient sodium deposition/stripping.9 As shown in Fig. 3e, the NaSCN-derived SEI exhibits a thick, heterogeneous structure with discontinuous organic outer layers and inner inorganic-dominated regions comprising NaF/NaNO2 phases alongside abundant sulfur-containing species (Fig. S31). This thick SEI growth irreversibly consumes active sodium and establishes severe ionic transport barriers, significantly impairing sodium stripping.34 Although NaSCN electrochemically compensates for sodium in anode-free systems, these combined effects directly cause severe capacity degradation.16 This discharge-limiting effect from thick SEI formation is also observed in NaNO2-containing battery systems (Fig. 3f and Fig. S32). These results emphasize the critical need to balance sodium compensation with SEI stability when designing compensation additives. While such additives can effectively supply active sodium, their interfacial side reactions may render the supplemented sodium electrochemically inaccessible. X-ray photoelectron spectroscopy (XPS) with varying etching times was used to probe the chemical evolution of electrolyte-derived SEI components. As shown in Fig. 3g, the NaF signal intensity initially increases and subsequently decreases with prolonged sputtering time, while the nitrogen signal steadily declines, confirming the formation of a thin SEI layer containing coexisting fluorine and nitrogen species facilitated by NaHMDS. A small amount of silicon–carbon was also detected at the interface (Fig. S33).35 The resulting NaF layer is even thinner than that in the pristine electrolyte (Fig. S34). Notably, NaHMDS outperforms conventional additives by not only compensating for sodium loss but also forming a stable SEI with rapid ion transport, thereby collectively improving the cycle life and energy density. This dual functionality contrasts sharply with NaSCN, which generates a thick NaF interphase with persistent polysulfide species (evidenced by S 2p signals detected after 240 s sputtering) (Fig. 3h and Fig. S35). Similarly, a thick SEI layer enriched in nitrogen and oxygen species is formed in the Al@C‖NaNO2-NFPP cell (Fig. 3i and Fig. S36). These XPS data demonstrate that sodium-compensating additives fundamentally alter the SEI, influencing battery performance through interfacial chemistry beyond solely acting as a sodium source.
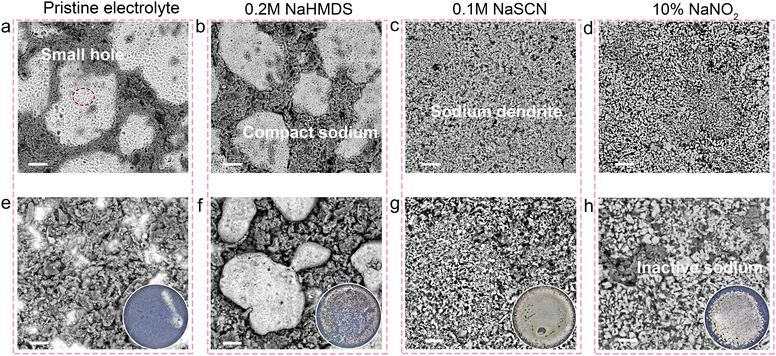
We used scanning electron microscopy (SEM) equipped with a backscattered electron (BSE) detector and a non-destructive sample transfer device to examine the deposition/stripping morphology at the interface of cycled AFSBs. As shown in Fig. 4a, in the charged state, sodium deposits uniformly as individual nuclei in the pristine electrolyte, though small pores are visible on the magnified surface (Fig. S37). Upon discharging, no residual sodium remains at the interface due to the lack of excess sodium for compensation (Fig. 4e and Fig. S38). With the 0.2 M NaHMDS electrolyte, sodium deposition also occurs via uniform growth of individual nuclei, and surface porosity is significantly reduced. This indicates that NaHMDS not only facilitates the formation of a thin, compact SEI but also promotes denser sodium deposition, mitigating side reactions between sodium and the electrolyte (Fig. 4b and Fig. S39). Critically, NaHMDS compensates for active sodium, leaving residual sodium particles on the current collector surface after full discharge (Fig. 4f and Fig. S40). In contrast, microscopic examination of the electrode from the cell with the 0.1 M NaSCN electrolyte reveals dendritic features on the surface (Fig. 4c and Fig. S41). Significantly, substantial continuous bulk sodium deposits during charging, with dendritic sodium residues persisting on the substrate after discharge, demonstrating irreversible plating behavior (Fig. 4g and Fig. S42). This irreversible stripping and dendritic morphology are exacerbated in the NaNO2 system (Fig. 4d, h and Fig. S43, S44). These high-surface-area dendritic structures accelerate parasitic reactions, concurrently depleting sodium inventory and degrading deposition/stripping kinetics, thereby generating dead sodium. The observed chemical composition and morphological evolution demonstrate that NaHMDS not only compensates for irreversible sodium loss but also enables a thin, compact, highly conductive SEI while promoting dense sodium deposition, thus extending the cycle life of AFSBs. Conversely, both NaSCN and NaNO2 additives induce thick SEI layers and dendritic morphologies, which fail to provide effective sodium compensation and further compromise AFSB performance.
The influence of sodium-compensating additives extends beyond anode stabilization to critically govern cathode structural evolution and CEI formation in AFSBs. As shown in Fig. 5a and b, cells with electrolyte-additive-type sodium compensators (NaHMDS and NaSCN) maintain the structural integrity of NFPP cathodes after cycling (Fig. S45–S47). In contrast, cells containing cathode-additive-type NaNO2 compensators exhibit extensive pore formation caused by gas evolution during solid-phase decomposition at the cathode (Fig. 5c and Fig. S48). These gas-induced defects, coupled with electrode structural imperfections, result in cathode material delamination and impaired ionic transport, consequently deteriorating battery performance. As shown in Fig. 5d and e, the Na‖NFPP half-cell containing the NaHMDS additive demonstrates lower charge/discharge polarization and improved cycling stability compared to those with NaSCN or NaNO2 additives. Notably, the half-cell with the NaNO2 cathode additive retains only 91.6% capacity after 400 cycles, while the NaHMDS-based cell exhibits no capacity degradation. The NaSCN-containing cells demonstrate progressively unstable charging behavior during extended cycling (Fig. S49 and S50), which we attribute to cumulative side reactions involving the additive. As shown in Fig. 5f and g, the NaHMDS electrolyte exhibits significantly suppressed fluoride fragments and enriched organic components (CN− and C2HO−) at the cathode compared to the pristine electrolyte. This indicates additive participation in forming a more homogeneous CEI. Despite the reduced total fluorine content, the derived NaF concentration exceeds that in the pristine electrolyte (Fig. 5h and i). Comparative analysis shows that the NaSCN-derived CEI contains significant sulfur and nitrogen species (Fig. 5j, k and Fig. S51), consistent with electrolyte reactions at the cathode interface. In contrast, NaHMDS produces only trace silicon signals at the CEI (Fig. S52), while NaNO2 shows minimal CEI modification (Fig. 5l). These results demonstrate the profound influence of sodium-compensating additives on both cathode integrity and CEI composition. NaHMDS emerges as the superior additive among those examined, owing to its unique ability to simultaneously form a homogeneous CEI and maintain cathode structural stability during prolonged cycling. This dual functionality accounts for the exceptional cycling performance of NaHMDS compared to NaNO2 and NaSCN alternatives.
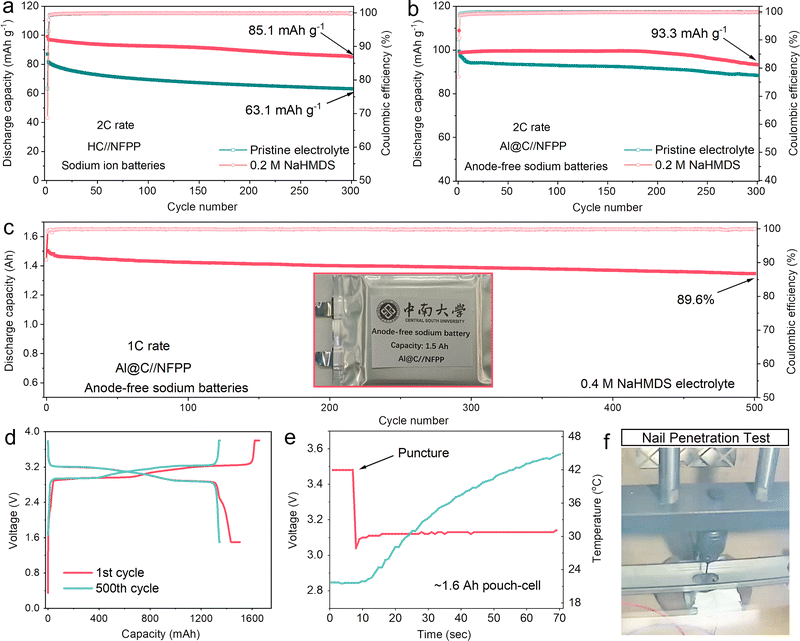
To assess the universal applicability of NaHMDS as a sodium-compensating additive, we evaluated its performance in both conventional SIB and AFSB configurations. In standard SIBs employing hard carbon anodes and NFPP cathodes, the NaHMDS-containing electrolyte demonstrated superior cycling stability, delivering a discharge capacity of 85.1 mAh g−1 after 300 cycles compared to 63.1 mAh g−1 for the pristine electrolyte (Fig. 6a and Fig. S53). In AFSBs, the NaHMDS additive enables a stable discharge capacity of 93.3 mAh g−1 at 2C for 300 cycles, maintaining zero capacity fading during the initial 200 cycles and thereby confirming its dual enhancement of both capacity and cycling stability (Fig. 6b and Fig. S54). To optimize sodium compensation efficacy, pouch cells were fabricated with the 0.4 M NaHMDS electrolyte (3 mL Ah−1). Following a 48-hour rest period after electrolyte injection (without formation protocols), the cells were cycled at a rate of 1C (1.5–3.8 V, 30 °C) under 0.5 MPa stack pressure. With the NaHMDS additive, the anode-free pouch cell achieves a marked improvement in capacity retention (89.6%) over 500 cycles compared to the baseline (84.3%), demonstrating its effectiveness in compensating for sodium loss and enhancing cyclability (Fig. 6c, d and Fig. S55). Notably, this compensation strategy delivers cycling performance that surpasses previous reports for AFSBs (Table S2). Most impressively, the pouch cells can withstand a nail penetration test at full charge voltage without any thermal runaway or smoke emission (Fig. 6e, f and Movie S1). These findings demonstrate the ability of sodium compensators to enable AFSBs with extended cycle life, highlighting significant potential for cost-effective, high-energy-density energy storage.
Conclusions
In summary, we report a novel sodium-compensating electrolyte additive, NaHMDS, that significantly enhances the cycling stability and energy density of AFSBs. This additive enables gas-free operation, preserves cathode integrity, and offers injectable compensation. Interfacial analysis reveals that the anode interface with NaHMDS can form a robust SEI with a thin NaF/Na3N inner layer and an organic-rich outer phase, enabling dense sodium deposition. Furthermore, the compensation products remain electrochemically stable at the cathode while facilitating a homogeneous CEI. The AFSB with NaHMDS retains 92.1% of its capacity after 320 cycles (normalized to the second cycle) with no capacity fading during the first 200 cycles. The LiHMDS analog replicates this compensation mechanism, enhancing specific capacity in anode-free lithium batteries. Our strategy provides a non-invasive solution to the long-standing challenge of low ICE in alkali-metal anode-free batteries.Author contributions
H.-Y. W. designed the experiment and participated in the analysis of results. C. X. participated in the experimental design, conducted the characterization, and wrote the manuscript. S. L. and J. W. conducted the characterization. X. M., S. Y., J. Z., H. P., Y. T., and D. S. provided helpful discussions and experimental conditions. All authors have read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ee04804d.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 22579191), the Hunan Provincial Science and Technology Plan Projects of China (No. 2017TP1001) and the Hunan Provincial Innovation Foundation for Postgraduate (CX20240020). We gratefully acknowledge Shandong All Grand Battery Co., Ltd and Guangdong DFP New Material Group Co., Ltd for their collaboration in fabricating the anode-free sodium pouch cells. The authors would like to thank Hunan Navi New Materials Technology for the XPS test.Notes and references
- W. Zhang, J. Lu and Z. Guo, Mater. Today, 2021, 50, 400–417 CrossRef CAS
.
- S. Zhang, S. Wu, J. Hwang, K. Matsumoto and R. Hagiwara, J. Am. Chem. Soc., 2024, 146, 8352–8361 CrossRef CAS
.
- X. Huang, H. Sun, X. Li, W. Zhu, L. Chen, T. Ma, S. Ding, T. Ma, Y. Dong, K. Zhang, F. Cheng, Q. Wei, L. Gao, J. Zhao, W. Zhang and J. Chen, J. Am. Chem. Soc., 2024, 146, 29391–29401 CrossRef CAS PubMed
.
- A. Yao, S. M. Benson and W. C. Chueh, Nat. Energy, 2025, 10, 404–416 CrossRef
.
- F. Huang, P. Xu, G. Fang and S. Liang, Adv. Mater., 2024, 36, 2405310 CrossRef CAS
.
- J. Ge, C. Ma, Y. Zhang, P. Ma, J. Zhang, Z. Xie, L. Wen, G. Tang, Q. Wang, W. Li, X. Guo, Y. Guo, E. Zhang, Y. Zhang, L. Zhao and W. Chen, Adv. Mater., 2024, 2413253 Search PubMed
.
- J. Ruan, J. Hu, Q. Li, S. Luo, J. Yang, Y. Liu, Y. Song, S. Zheng, D. Sun, F. Fang and F. Wang, Nat. Sustainability, 2025, 8, 530–541 CrossRef
.
- Z. Hu, L. Liu, X. Wang, Q. Zheng, C. Han and W. Li, Adv. Funct. Mater., 2024, 34, 2313823 CrossRef CAS
.
- C. Xie, H. Wu, K. Liang, Z. Ding, J. Dai, R. Zhang, Q. Zhang, D. Sun, Y. Ren, Y. Li, Y. Tang and H. Wang, Energy Environ. Sci., 2024, 17, 4228–4237 RSC
.
- C. Xie, K. Liang, H. Wu, Z. Xie, Y. Ren, J. Dai, J. Lu, Y. Tang and H. Wang, Adv. Energy Mater., 2025, 15, 2500351 CrossRef CAS
.
- J.-C. Liu, T. You, Y.-F. Zhao, F.-Q. Liu, J.-D. Li, L.-L. Wang, C. Wang and L. Li, Rare Met., 2025, 44, 3817–3826 CrossRef CAS
.
- Q. Zhu, D. Yu, J. Chen, L. Cheng, M. Tang, Y. Wang, Y. Li, J. Yang and H. Wang, Joule, 2024, 8, 482–495 CrossRef CAS
.
- Z. Wang, R. Tian, H. Jiang, G. Chen, Z. Shen, C.-Z. Zhao, F. Du and Q. Zhang, Adv. Mater., 2025, 2504760 CrossRef CAS
.
- S. Wu, T. Wada, H. Shionoya, J. Hwang, K. Matsumoto and R. Hagiwara, Energy Storage Mater., 2023, 61, 102897 CrossRef
.
- Y. Li, Q. Zhou, S. Weng, F. Ding, X. Qi, J. Lu, Y. Li, X. Zhang, X. Rong, Y. Lu, X. Wang, R. Xiao, H. Li, X. Huang, L. Chen and Y.-S. Hu, Nat. Energy, 2022, 7, 511–519 CrossRef CAS
.
- Y. Li, J. Wang, Y. Wang, S. Wang, L. Wu, B. Zhou, D. Yang, L. Jiang, L. Kan, Q. Zhu, M. Kurbanov and H. Wang, Adv. Mater., 2025, 37, 2419764 CrossRef CAS
.
- Y.-Y. Zhang, C.-H. Zhang, Y.-J. Guo, M. Fan, Y. Zhao, H. Guo, W.-P. Wang, S.-J. Tan, Y.-X. Yin, F. Wang, S. Xin, Y.-G. Guo and L.-J. Wan, J. Am. Chem. Soc., 2023, 145, 25643–25652 CrossRef CAS
.
- J. Martínez De Ilarduya, L. Otaegui, M. Galcerán, L. Acebo, D. Shanmukaraj, T. Rojo and M. Armand, Electrochim. Acta, 2019, 321, 134693 CrossRef
.
- R. Zhang, Z. Tang, D. Sun, R. Li, W. Yang, S. Zhou, Z. Xie, Y. Tang and H. Wang, Chem. Commun., 2021, 57, 4243–4246 RSC
.
- Z. Tang, S. Zhou, Y. Huang, H. Wang, R. Zhang, Q. Wang, D. Sun, Y. Tang and H. Wang, Electrochem. Energy Rev., 2023, 6, 8 CrossRef CAS
.
- S. Chen, G. Wu, H. Jiang, J. Wang, T. Chen, C. Han, W. Wang, R. Yang, J. Zhao, Z. Tang, X. Gong, C. Li, M. Zhu, K. Zhang, Y. Xu, Y. Wang, Z. Hu, P. Chen, B. Wang, K. Zhang, Y. Xia, H. Peng and Y. Gao, Nature, 2025, 638, 676–683 CrossRef CAS
.
- S. Chen, G. Wu, P. Wang, Z. Zheng, W. Wang and Y. Gao, Adv. Mater., 2025, 37, 2502251 CrossRef CAS
.
- Y.-B. Niu, Y.-J. Guo, Y.-X. Yin, S.-Y. Zhang, T. Wang, P. Wang, S. Xin and Y.-G. Guo, Adv. Mater., 2020, 32, 2001419 CrossRef CAS
.
- C.-H. Jo, J. U. Choi, H. Yashiro and S.-T. Myung, J. Mater. Chem. A, 2019, 7, 3903–3909 RSC
.
- C. Carteret and A. Labrosse, J. Raman Spectrosc., 2010, 41, 996–1004 CrossRef CAS
.
- D. R. Armstrong, E. Herd, D. V. Graham, E. Hevia, A. R. Kennedy, W. Clegg and L. Russo, Dalton Trans., 2008, 1323–1330, 10.1039/B716494G
.
- Y. Lu, C.-Z. Zhao, J.-Q. Huang and Q. Zhang, Joule, 2022, 6, 1172–1198 CrossRef CAS
.
- Z. Li, P. Huang, J. Zhang, Z. Guo, Z. Liu, L. Chen, J. Zhang, J. Luo, X. Tao, Z. Miao, H. Jiang, C. Wang, X. Ye, X. Wu, W.-D. Liu, R. Liu, Y. Chen and W. Hu, Energy Environ. Sci., 2025, 18, 2962–2972 RSC
.
- D. Wang, Revealing SEI Formation and Evolution at the Li Anode/Liquid Electrolyte Interface in Li-ion Batteries by in situ Fourier Transform Infrared Spectroscopy, Massachusetts Institute of Technology, 2024.
- S. Xiang, L. Zhu, L. Fu, M. Wang, X. Zhang, Y. Tang, D. Sun and H. Wang, eScience, 2025, 5, 100291 CrossRef
.
- H. Liu, Y. Chen, W. Wang, X. He, Z. He, L. Li, S. Zeng, R. Cao and G. Zhang, EES Catal., 2023, 1, 495–503 RSC
.
- M. Trivedi, A. Branton, D. Trivedi, G. Nayak, K. Bairwa and S. Jana, J. Chromatogr. Sep. Tech., 2015, 6, 1000282 CrossRef
.
- L. Li, T. Yang, K. Ren, X. Song, S. Wu, H.-W. Li, Z. Jiang, M. Sun and Y. Li, Mater. Today, 2025, 90, 249–257 CrossRef CAS
.
- Z. Cheng, Z. Zhang, M. Wu, M. Jia, X. Du, Z. Gao, S. Tong, T. Wang, X. Yan, X. Zhang and H. Zhou, Angew. Chem., Int. Ed., 2025, e202503864 CAS
.
- S. Chen, L. Huang, J. Liang, H. Huang, Y. Fu, J. Li, X. Chen, H. Chen, Y. Liao, J. He and W. Li, Energy Storage Mater., 2025, 81, 104501 CrossRef
.
| This journal is © The Royal Society of Chemistry 2026 |