Kinetic modulation enabling densely oriented electrodeposition of Zn anodes in aqueous batteries
Kuo
Wang†
a,
Hongtu
Zhan†
a,
Xiao-Xia
Liu
 abc and
Xiaoqi
Sun
abc and
Xiaoqi
Sun
 *ab
*ab
aDepartment of Chemistry, Northeastern University, Shenyang 110819, China. E-mail: sunxiaoqi@mail.neu.edu.cn
bNational Frontiers Science Center for Industrial Intelligence and Systems Optimization, 3-11 Wenhua Road, Shenyang, 110819, China
cKey Laboratory of Data Analytics and Optimization for Smart Industry (Northeastern University), Ministry of Education, China
First published on 28th November 2025
Abstract
Zn metal is a suitable anode in aqueous batteries, but it suffers from mossy deposition and side reactions. Herein, we systematically elucidate the kinetically controlled morphology evolution of Zn deposition in the conventional ZnSO4 electrolyte and accordingly present the 2-methoxyethyl acetate (MA) additive to enable a thermodynamically governed deposition behavior. The unique charge distribution of the MA molecule alters the Zn2+ solvation shells in the electrolyte as well as during the desolvation process. It helps with solvation water release to inhibit side reactions, and the controlled final removal of chelated MA leads to the formation of a thermodynamically favored plate morphology. The local enrichment of desolvated MA further shields the unique Zn crystal plane and allows dense packing. As a result, the lifespan of symmetric Zn cells reaches 5740 h after 1.6 vol% MA addition, which is around 8 months and more than 72 times that of the baseline system. With a 50% depth of discharge, the MA additive also extends the cycle life from 40 h to over 1580 h. A Zn//V6O13·H2O full cell with an N/P ratio of 1.8 maintains a high capacity of 302 mAh g−1 after 600 cycles at 5 A g−1, superior to only 90 mAh g−1 retained after 250 cycles with the baseline electrolyte.
Broader contextRechargeable aqueous Zn batteries are promising candidates for grid-scale applications, thanks to their high safety and low cost. Unfortunately, Zn metal anodes suffer from porous and non-uniform deposition morphology as well as hydrogen evolution reactions, which greatly limit the stability of Zn batteries. Currently, there is lack of systematic investigations into the underlying causes and fundamental understanding of the intrinsic relationships among modification mechanisms. Herein, we elucidate an internally modified cation depletion directed mossy deposition preference and accordingly engineer a 2-methoxyethyl acetate (MA) electrolyte additive with unique charge distributions. With preferential interactions using specific functional sites, the MA molecule alters the Zn2+ desolvation paths and the corresponding bonding properties and deposition kinetics. It not only enables thermodynamically favorable plate morphology but also accelerates solvation water removal. The local shielding of desolvated MA on the unique Zn crystal plane further allows dense and uniform packing of the plates. As a result, 1.6 vol% MA addition supports long-term stable cycling of symmetric cells with high discharge depth and full batteries with low N/P ratios. Our work provides fundamental insights into the successive functional mechanisms of electrolyte additive molecules, which would pave effective paths toward the applications of aqueous zinc batteries. |
Introduction
Rechargeable aqueous zinc batteries have attracted widespread attention as a promising energy storage technology due to their cost-effectiveness and environmental friendliness, as well as the high specific capacity of Zn metal anodes (820 mAh g−1 and 5855 mAh cm−3).1–9 However, their practical applications are severely hindered by the inherent limitations associated with the Zn anode, including porous and non-uniform deposition morphology as well as continuous hydrogen evolution reaction (HER).10–17 They can easily cause cell short-circuiting, irreversible Zn loss and surface passivation, thus deteriorating the reversibility and cycle life of Zn electrodes and also full batteries. Therefore, the development of effective and feasible strategies to avoid the aforementioned processes is urgently required.Recently, researchers have made some advancements in the modifications of Zn anode stabilities, with solutions mainly including protective layer construction, electrode alloying and electrolyte regulation. Protective layers isolate the underlying metal from the electrolyte and provide uniform Zn2+ flux, while alloy designs control Zn nucleation and growth behaviors, thereby improving the plating/stripping performance of Zn electrodes. Nevertheless, the electrode processing using these strategies could be complicated. In comparison, electrolyte optimizations provide more convenient and cost-effective paths to regulate the electrochemical behaviors of Zn electrodes. For instance, it has been shown that co-solvents/additives, such as propylene carbonate, diethyl carbonate, formamide, sulfolane, and hexamethylphosphoric triamide, modify the Zn surface environment, change Zn2+ solvation structures, and/or reduce water activity, thereby enhancing the cycling stability of Zn electrodes.18–22 Besides, some additives have been found to achieve specific adsorption on anisotropic zinc metal surfaces, e.g., H2SO4 + KMnO4, NaI and Bmim+.23–25 Nevertheless, it is essential to understand the intrinsic relationships among different mechanisms to realize efficient rational designs of additive molecules with synergistic effects.
Herein, we systematically study the Zn deposition evolution in the conventional 3 mol kg−1 ZnSO4 aqueous electrolyte, which reveals the initial formation of interwoven whiskers followed by irregular plates due to the internally modified cation depletion conditions at the interface. They result in porous structures, severe side reactions and poor cycle life of the Zn electrode. Accordingly, we introduce a 2-methoxyethyl acetate (MA) additive with a low volume fraction of 1.6% for efficient modifications. The unique charge distribution on the MA molecule allows its entrance into the inner solvation shell of Zn2+ with the double bond oxygen on the ester group. Meanwhile, the ether oxygen on the other side of the molecule forms hydrogen bonds with solvated water. Upon water removal during desolvation, on the other hand, the ether oxygen on MA also starts to coordinate with Zn2+, resulting in modified bonding properties and easier solvation water release. These features help to inhibit the HER. Meanwhile, the chelation of MA controls its final desolvation, which modulates the deposition kinetics and avoids cation depletion. The additive thus transforms the kinetically controlled deposition process into a thermodynamically favored behavior with plate morphology. The desolvated MA further locally accumulates at the interface, resulting in its effective shielding on the unique Zn crystal plane to enable dense and uniform packing of the plates with a preferred (100) orientation. Benefitting from these effects of the MA additive, the Zn electrode with a depth of discharge (DOD) of 50% reaches a long lifespan over 1580 h. A Zn//V6O13·H2O full cell with a low N/P ratio of 1.8 (calculated using theoretical capacities) also realizes stable cycling and maintains a high capacity of 302 mAh g−1 after 600 cycles at 5 A g−1. The performance of the Zn electrode and full cells far exceeds the one with the MA-free electrolyte.
Results and discussion
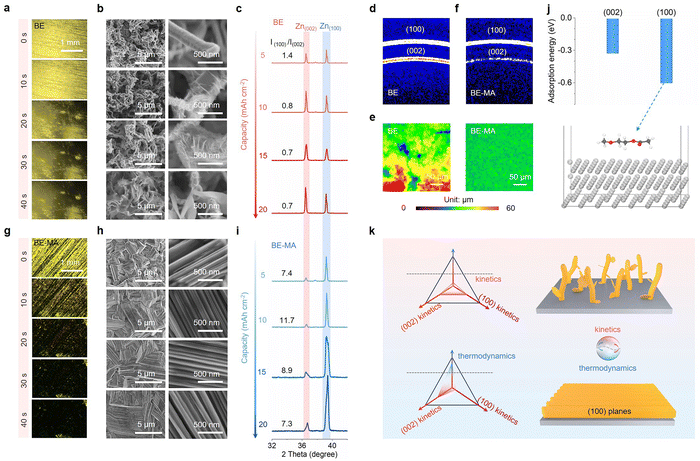
The electrochemical deposition behavior of Zn in the baseline 3 mol kg−1 ZnSO4 electrolyte (labelled as BE) is first examined. Fig. 1a shows the in situ optical microscopy images during plating at 10 mA cm−2. The surface of the Cu substrate was cleaned by sand paper prior to the test. Deposits are formed locally at the scratched areas, which is due to the higher electric field. They further aggregate as the deposition proceeds, whereas the other parts of the surface remain exposed. Further detailed morphological changes are monitored by scanning electron microscopy (SEM). As shown in Fig. 1b, the deposits first grow in the form of interwoven whiskers at a capacity of 5 mAh cm−2. This is due to the rapid depletion of local interface cations under the relatively facile reaction kinetics, and the deposits tend to extend towards the electrolyte to reach more cations. This morphology then generates a higher surface area for subsequent Zn deposition, which on the other hand alleviates cation depletion at each local area. Thus, plates start to form on the surface of whiskers upon further deposition over 10 mAh cm−2. The whiskers and loosely packed plates do not show preferred orientations, as demonstrated by the peak intensities in the X-ray diffraction (XRD) and grazing-incidence wide-angle X-ray scattering (GIWAXS) patterns (Fig. 1c and d). This morphology leads to significant height variations as shown in the confocal laser scanning microscopy (CLSM) image (Fig. 1e). Due to the protuberant points and porous structure in the deposited Zn, it easily leads to short-circuiting and uncontrolled side reactions in batteries.To modulate the Zn deposition morphology, it is important to control the reaction kinetics. Accordingly, we introduce 1.6 vol% of the MA additive with multiple polar groups in the ZnSO4 electrolyte (labelled as BE-MA). In contrast to the BE electrolyte, the initial nucleation of Zn does not show any site preference after MA addition, enabling a uniform coverage after only 30 s as shown in the in situ optical microscopy images (Fig. 1g). The SEM and CLSM images also demonstrate a dense surface and uniform height obtained upon deposition at different capacities (Fig. 1h). A closer examination suggests the formation of compactly stacked plates vertically covering the substrate, which is the thermodynamically favored morphology of Zn considering its crystal structure. Meanwhile, XRD suggests the domination of the (100) peak over (002), with the peak intensity ratios remaining above 7 at different capacities (Fig. 1i). The GIWAXS pattern also shows a strong intensity of the (100) diffraction ring and a minimal intensity of the (002) ring (Fig. 1f). According to density functional theory (DFT) calculations, the MA molecule presents almost doubled adsorption energy on the (100) plane of Zn than that on the (002) plane (Fig. 1j). It shields the growth of the former and results in a strong preferred orientation.26,27 Therefore, the MA electrolyte additive effectively modifies the Zn deposition preference, which successfully transforms the kinetically controlled porous morphology into a thermodynamically favored plate morphology. Its unique shielding on the specific Zn plane further leads to the preferred deposition orientation to realize dense and uniform packing (Fig. 1k).
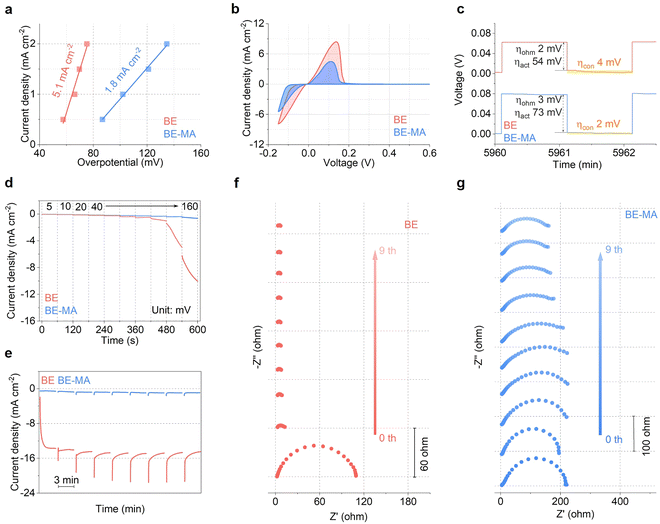
The MA additive modulation on the reaction kinetics of the Zn electrode is evaluated in detail. Fig. 2a compares the exchange current density of the Zn electrode. It decreases largely from 5.1 mA cm−2 in the BE electrolyte to 1.8 mA cm−2 upon 1.6% MA addition, corresponding to the reduced deposition kinetics of the latter. This is also confirmed by the negative shift of deposition overpotential from cyclic voltammetry (CV) curves (Fig. 2b). The source of overpotential is identified by electrochemical impedance spectroscopy (EIS, Fig. S1) and the galvanostatic intermittent titration technique (GITT, Fig. 2c and Fig. S2).28 Basically, the overall kinetic polarization is divided into ohmic polarization, activation polarization, and concentration polarization. During relaxation, the instantaneous voltage rise upon current interruption corresponds to ohmic polarization and activation polarization, owing to their current dependence. The subsequent gradual increase until stabilization at the thermodynamic potential is attributed to concentration polarization. EIS analysis suggests close ohmic polarization following MA addition. After subtracting its contribution in GITT curves, the remaining activation polarization is 54 mV in the BE electrolyte and increases to 73 mV after MA addition. Meanwhile, the concentration polarization reduces from 4 mV to 2 mV. It demonstrates that the MA additive suppresses deposition kinetics while ensuring sufficient Zn2+ supply. Chronoamperometric (CA) deposition is further carried out at various voltages from −5 mV to −160 mV (Fig. 2d). In the BE electrolyte, the current densities of the Zn electrode show apparent increases within each voltage range, which become severer with the voltage reaching over −120 mV. This represents a significant accumulation of electrochemical active area caused by unstable deposition, especially at high rates. In contrast, after the addition of 1.6% MA, the current density remains stable in all voltage ranges, demonstrating a stable and uniform deposition surface. Alternatively, we carried out CA analysis under a constant voltage of −150 mV in the two systems, and EIS is performed 3-min intervals (Fig. 2e–g). Similar to the increasing voltage test discussed above, the current density in the BE electrolyte increases rapidly during initial deposition and remains high as the deposition proceeds. In the corresponding Nyquist plots, the charge transfer resistance (Rct) significantly decreases and becomes negligible after the first CA process. The results suggest the rapidly expanded active area, which reaches its limit within the first 3 minutes and remains high during subsequent deposition. In contrast, following 1.6% MA addition, the current density remains low and steady, and the semi-circles in the Nyquist plots also show stable evolution. It demonstrates the controlled reaction activity, which ensures the thermodynamically favored deposition morphology in the BE-MA electrolyte.
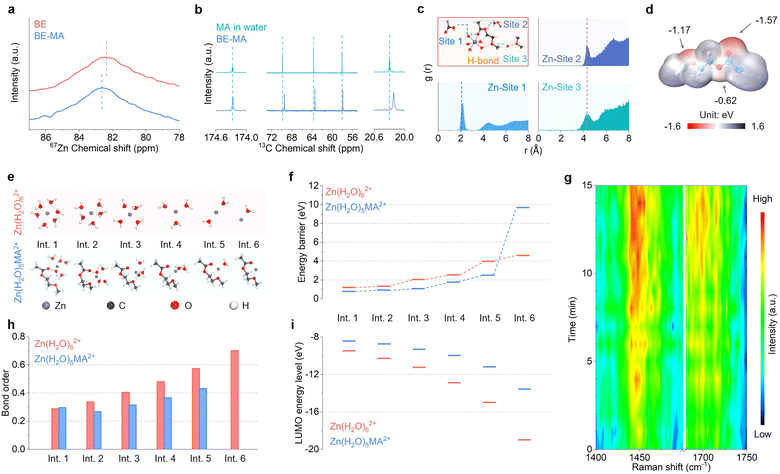
The functional mechanisms of the MA additive on the Zn deposition kinetics are investigated. Fig. 3a shows the 67Zn nuclear magnetic resonance (NMR) spectra of BE and BE-MA solutions. The Zn peak presents a down-field shift upon MA addition, indicating a change in its solvation structure. This is confirmed by 13C NMR. When the ZnSO4 salt is introduced into the MA-water mixed solvent, as shown in Fig. 3b, the carbon peaks exhibit an upfield shift, indicating the participation of the MA molecule in the reconstruction of the Zn2+ solvation shell. The detailed solvation structure of Zn2+ in the BE-MA electrolyte is studied by molecular dynamics (MD) simulations (Fig. S3). Fig. 3c shows the radial distribution function (RDF) and representative structure taken from the simulation box. The inner solvation shell of Zn2+ is composed of five water and one MA molecules on its double bond oxygen from the ester group, with the Zn2+–O(MA) interaction distance at around 2 Å. Meanwhile, the first interaction distances between Zn2+ and the other two oxygen sites of MA are both around ∼4.3 Å. This geometry allows the formation of hydrogen bonds between the ether site oxygen of MA and water in the inner solvation shell. The above interaction preference of the MA molecule can be explained by its electrostatic potential (ESP) map. As shown in Fig. 3d, the negative charge on the oxygen sites increases in the order of single bond O on ester (−0.62 eV), O on ether (−1.17 eV) and double bond O on ester (−1.57 eV), demonstrating the increasing tendency to form hydrogen bonds and then coordinate with Zn2+.
The unique solvation behavior of Zn2+ modulates the desolvation process during deposition. According to our further calculations, the five solvated water molecules are first removed from the Zn(H2O)5MA2+ structure and MA is finally released (Fig. 3e). The last process presents the highest energy barrier of 9.7 eV and is identified as the rate determining step (Fig. 3f). This more than doubles the highest barrier of 4.6 eV for the conventional Zn(H2O)62+ structure in the BE electrolyte. The MA additive thus ensures a more controlled Zn deposition kinetics, which inhibits rapid cation depletion and promotes a thermodynamically favored process with homogeneous nucleation and growth. Fig. 3g shows the in situ Raman spectra of the electrode/electrolyte interface. As the deposition proceeds, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1700 cm−1 and the CH2/CH3 deformation vibration at 1450 cm−1 intensify gradually, which is attributed to the locally accumulated MA after desolvation. Detailed analysis also demonstrates the preferential adsorption of the MA molecule on the surface of Zn over water (Fig. S4). This enrichment of MA at the interface allows effective shielding on the unique Zn plane (Fig. 1j), thereby enabling preferentially oriented dense packing of deposit plates.
O stretching vibration at 1700 cm−1 and the CH2/CH3 deformation vibration at 1450 cm−1 intensify gradually, which is attributed to the locally accumulated MA after desolvation. Detailed analysis also demonstrates the preferential adsorption of the MA molecule on the surface of Zn over water (Fig. S4). This enrichment of MA at the interface allows effective shielding on the unique Zn plane (Fig. 1j), thereby enabling preferentially oriented dense packing of deposit plates.
A closer examination of the energy barriers during the desolvation processes, on the other hand, demonstrates the much easier release of solvation water in the Zn(H2O)5MA2+ structure than in Zn(H2O)62+, i.e. 0.7–2.5 eV vs. 1.2–4.6 eV. It contributes to the suppressed HER in the former. In order to uncover these differences, we examine the desolvation structures in further detail. It reveals that the ether site on MA also starts to coordinate with Zn2+ after the release of the first water, which forms a chelation structure. It explains the difficult final removal of solvated MA discussed above. Meanwhile, the charge distributions on the solvation structure are modified, which changes the bonding properties among Zn2+ and solvated water. According to the Zn–H2O bond order calculations, they present similar values initially, whereas those in Zn(H2O)x−1MA2+ are apparently lower than those in conventional Zn(H2O)x2+ during the subsequent dehydration process (Fig. 3h). This verifies that the solvated water in the former is more easily released. Besides, the calculation of the lowest unoccupied molecular orbital (LUMO) results in a higher energy level in the Zn(H2O)x−1MA2+ structures during desolvation (Fig. 3i and Fig. S5), corresponding to the enhanced reduction resistance. Therefore, the introduction of MA into the solvation shell of Zn not only ensures a thermodynamically favored deposition behavior to realize uniform and dense plating, but also contributes to inhibiting side reactions.
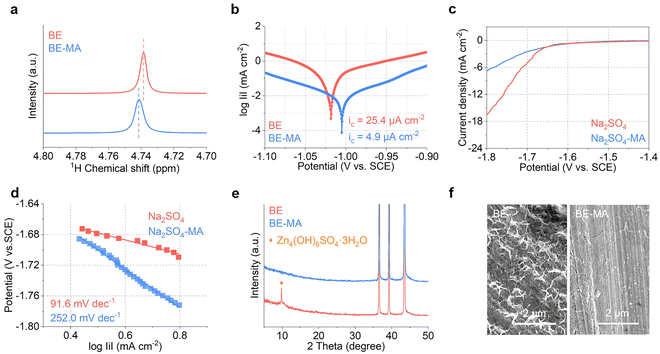
The side reactions in the two electrolytes are further evaluated experimentally. Fig. 4a shows the 1H NMR spectra. A shift of the water peak is observed after MA addition, which demonstrates its reduced activity. Fig. 4b compares the Tafel plots of the Zn electrode in the BE and BE-MA electrolytes. The corrosion current density decreases from 25.4 µA cm−2 to 4.9 µA cm−2 upon the introduction of 1.6% MA, suggesting the inhibition of corrosion processes.29,30 The HER is studied in the 3 mol kg−1 Na2SO4 electrolyte without Zn2+ to eliminate the current from Zn2+ reduction. The presence of MA in the electrolyte effectively decreases the cathodic current density and increases the Tafel slope in comparison to the BE electrolyte (Fig. 4c and d). Zn foil is further soaked in BE and BE-MA solutions for 48 h and characterized by XRD and SEM (Fig. 4e and f). In the BE electrolyte, the surface of Zn foil is covered by corrosion by-products, which are recognized as Zn4(OH)6SO4·3H2O. This results from the local pH increase after the parasitic reaction on the Zn electrode. In contrast, following MA addition, these by-products are eliminated as demonstrated by XRD and the clean surface in the SEM image. The results confirm that 1.6% MA addition effectively inhibits the side reactions on the Zn electrode in aqueous solutions. This is also essential to maintain a clean and flat Zn surface, which in turn ensures stable deposition behavior.
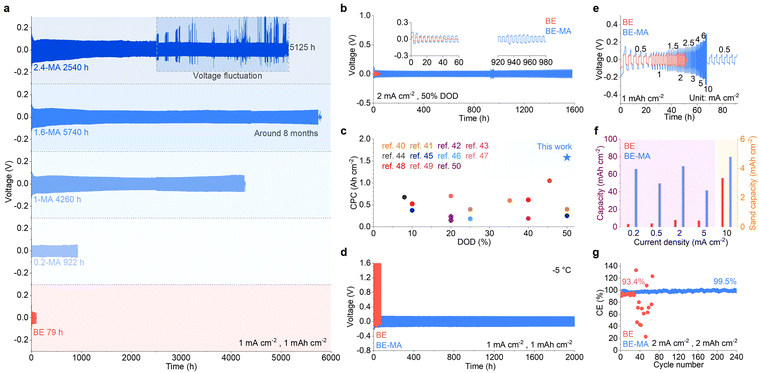
The electrochemical performance of the Zn electrode in different electrolytes is studied. Fig. 5a shows the repeated plating/stripping behaviors in symmetric Zn cells at a current density of 1 mA cm−2 and a capacity of 1 mAh cm−2. The cell with the BE electrolyte exhibits a cycle life of 79 h, after which short-circuiting takes place. In comparison, the cycling stability extends to 922 h and 4260 h upon the addition of 0.2% and 1% MA, respectively. The longest cycle life of 5740 h is achieved with 1.6% MA additive, which is around 8 months and more than 72 times that of the BE system. A further increase of the MA amount to 2.4% results in severe voltage fluctuations after 2540 h, yet still far longer than that in BE. This suggests that low amounts of MA additive could effectively improve the cycling stability of the Zn electrode, with 1.6% being the optimal amount. It also shows an apparent improvement in comparison to the recently reported electrolyte additives in zinc cells (Fig. S6 and Table S1).31–39 Repeated long-term cycling is further carried out at a high current density of 20 mA cm−2 (Fig. S7). The cell with the BE-MA electrolyte stably operates for over 700 h, which is superior to the MA-free cell showing rapid short-circuiting during the first few cycles. The cycling capability at high DOD is important for practical applications and is evaluated at 50% DOD (Fig. 5b). In comparison to the short-circuiting at 40 h in the BE electrolyte, the cell containing MA additive delivers excellent long-term cycling for over 1580 h. This is also among the best of representative publications (Fig. 5c and Table S2).40–50 We further carried out cycling tests at −5 °C to evaluate low-temperature behaviors (Fig. 5d). The BE electrolyte shows large overpotential from the beginning of the test. In contrast, upon the introduction of MA, stable cycling is achieved for over 2000 hours.
Fig. 5e compares the rate performance of symmetric cells at current densities ranging from 0.5 mA cm−2 to 10 mA cm−2. The cell short-circuits at a current density of 2.5 mA cm−2 in the BE electrolyte. In contrast, the cell with MA additive functions properly at all current densities. We further evaluated the continuous single plating/stripping behaviors at different current densities (Fig. 5f and Fig. S8). In the BE electrolyte, the cells experience short-circuiting at capacities of 3.6, 4.2, 8.1, and 7.4 mAh cm−2 at current densities of 0.3, 0.5, 2, and 5 mA cm−2, respectively. On the other hand, at a high current density of 10 mA cm−2, a sudden voltage increase corresponding to the Sand behavior is observed at a capacity of 3.4 mAh cm−2. With the addition of 1.6% MA, the capacities at all current densities are significantly increased, i.e., 66.3, 49.8, 69.4, 41.8 and 4.8 mAh cm−2 at 0.3, 0.5, 2, 5 and 10 mA cm−2, respectively. The reversibility of Zn plating/stripping is further evaluated in Zn//Cu cells. As depicted in Fig. 5g, the coulombic efficiency of the cell with the BE-MA electrolyte reaches 99.5% over 240 cycles, whereas the cell using the BE electrolyte experiences short-circuiting after only 28 cycles. Besides, the MA additive is also capable of extending the cycle life of the Zn electrode with a Zn(TFSI)2-based electrolyte by more than 90 times (Fig. S9). The above results confirm the wide applicability of the MA additive with only 1.6% volume fraction to significantly enhance the electrochemical stability of the Zn electrode.
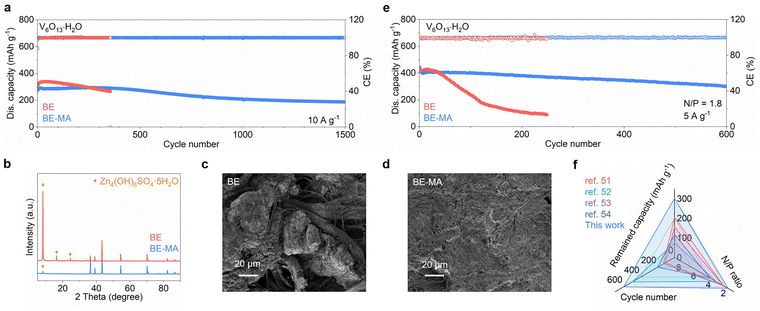
The MA additive is finally evaluated in full cells. Fig. 6a compares the long-term cycling performance of a V6O13·H2O cathode in the two electrolytes at 10 A g−1. The cell employing the BE electrolyte suffers soft short-circuiting at the 350th cycle, whereas the MA additive extends the lifespan to 1500 cycles. Notably, the XRD pattern of the cycled Zn anode from BE shows strong diffractions from basic zinc salt by-products (Fig. 6b), demonstrating severe side reactions. Meanwhile, the SEM image presents locally aggregated large particles, which even grow into separators (Fig. 6c). In contrast, with the BE-MA electrolyte, the by-product formation is significantly suppressed, and the anode surface remains dense and flat (Fig. 6d). This promoted stability of the Zn anode enables an extended cycle life of full cells. The cycling stability is further evaluated using a limited anode with N/P = 1.8 (based on theoretical capacities, Fig. 6e). The capacity with the BE electrolyte decays rapidly during the initial 100 cycles, and only 90 mAh g−1 is retained after 250 cycles. In contrast, the MA additive enables stable capacity retention during cycling, with a high capacity of 302 mAh g−1 retained after 600 cycles. This cycling performance is also better than representative Zn full cells with limited anodes (Fig. 6f and Table S3).51–54 Alternatively, we test the cycling performance of a commercial MnO2 cathode (Fig. S10). The MA additive helps to modulate the activation process during early cycles, which would be attributed to its regulation of the solvation structure of Mn2+ in the electrolyte. Importantly, MA helps to extend the lifespan from 79 to 350 cycles at 0.5 A g−1 and an N/P ratio of 1.8. Besides, a long cycle life of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 is achieved at a high current density of 5 A g−1 with an excess anode. The results confirm that the MA additive ensures the high stability and reversibility of Zn plating/stripping at the anode, which further promotes the cycling stability of full cells.
000 is achieved at a high current density of 5 A g−1 with an excess anode. The results confirm that the MA additive ensures the high stability and reversibility of Zn plating/stripping at the anode, which further promotes the cycling stability of full cells.
Conclusions
In conclusion, we elucidate the kinetically induced unstable and porous Zn deposition preference in conventional aqueous batteries and present an effective regulation strategy to realize a thermodynamically favored and preferentially oriented densely packed plate morphology. The MA molecule with multiple oxygen sites is selected as a low percentage (1.6 vol%) additive for the 3 mol kg−1 ZnSO4 baseline electrolyte. The double bond oxygen on the ester site enters the inner solvation shell of Zn2+, and the oxygen on the ether site also starts to coordinate upon the release of solvated water during the desolvation processes. The charge distributions on the desolvated structures are therefore modified, which lowers Zn–H2O bond orders, enables easier solvated water removal and inhibits side reactions. Meanwhile, the resulting MA chelation elevates the energy barrier for its final release. It effectively transforms the kinetically controlled deposition to a thermodynamically favored process, which leads to the formation of Zn plates. Furthermore, MA locally accumulates at the interface after desolvation and experiences almost doubled adsorption energy on the Zn(100) face in comparison to (002). This unique shielding results in preferred orientation deposition, thereby enabling dense and uniform packing of the plates. Benefitting from the above effects of the MA additive, the Zn electrode realizes stable plating/stripping for 5740 h at 1 mA cm−2 and 1 mAh cm−2, which is more than 72 times that in the BE electrolyte. Even with 50% DOD, the cycle life in the BE-MA electrolyte also reaches 1580 h, far exceeding 40 h in BE. Moreover, a Zn//V6O13·H2O full cell with the BE-MA electrolyte and N/P = 1.8 achieves stable cycling with a high capacity of 302 mAh g−1 retained after 600 cycles at 5 A g−1. This is again superior to the facile capacity decay within the initial 100 cycles and only 90 mAh g−1 capacity is retained after 250 cycles in the MA-free electrolyte. Our results reveal the fundamental relationships among kinetically/thermodynamically dominant processes, desolvation steps, preferential orientations, as well as their successive effects to realize densely packed deposits and stable cycling of the Zn electrode. It would pave efficient paths for the design of additive molecules for high-performance aqueous energy storage devices.Experimental section
Synthesis
V6O13·H2O was synthesized according to a previous method.55 In a typical synthesis, 2.73 g V2O5 and 4.52 g H2C2O4 were added into 40 mL of deionized water. The mixture was stirred at 90 °C for 1 h and transferred into a Teflon-lined autoclave. Subsequently, 10 mL of H2O2 and 30 mL of ethanol were added. The autoclave was heated at 180 °C for 3 h. After cooling down to room temperature, the product was filtered, washed with deionized water and ethanol, and dried at 60 °C under vacuum overnight.Characterization
The in situ Zn deposition optical microscopy images were recorded using a metallurgical microscope (CMM-90AE, China). The morphologies were characterized using an SU8010 scanning electron microscope (HITACHI, Japan) and an OLS4100 3D laser scanning microscope (Olympus, Japan). XRD was carried out on a PANalytical Empyrean diffractometer with Cu Kα radiation. The GIWAXS patterns were measured using a Bruker D8 (Germany). In situ Raman spectroscopy was performed on a BWS465-532S (B&WTEK, USA). 67Zn, 13C and 1H NMR analyses were carried out on a Bruker 600 MHz NMR spectrometer (Germany).Electrochemical measurements
The electrochemical performances of Zn//Zn, Zn//Cu, Zn//V6O13·H2O and Zn//MnO2 cells were tested in 2032 coin-type cells. The tests were conducted at a constant temperature of 27 °C. A 3 mol kg−1 ZnSO4 aqueous solution was used as the BE electrolyte, and the BE-MA electrolyte was obtained by adding 1.6 vol% MA to the BE electrolyte. The MA additive did not show strong effects on the basic properties of the electrolyte (Fig. S11). An additional 0.1 mol kg−1 MnSO4 was added for Zn//MnO2 cells. During battery assembly, 100 µL of the electrolyte was added, and no additional electrolyte was added afterward. The separators consisted of two layers of filter paper with an average thickness of 0.10 mm. V6O13·H2O and MnO2 cathodes were prepared by mixing the active material, Super P and PVDF at a mass ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in N-methyl pyrrolidone (NMP) and drop-casting on a graphite foil substrate. CV, CA and EIS measurements were performed in Zn//Zn Swagelok® cells. The electrochemical double layer capacitance (EDLC) was measured by linear fitting of i–v plots according to the equation: Cdl = i/v. All electrochemical measurements were performed on Bio-logic VMP3 or LANHE CT2001A battery test systems.
1 in N-methyl pyrrolidone (NMP) and drop-casting on a graphite foil substrate. CV, CA and EIS measurements were performed in Zn//Zn Swagelok® cells. The electrochemical double layer capacitance (EDLC) was measured by linear fitting of i–v plots according to the equation: Cdl = i/v. All electrochemical measurements were performed on Bio-logic VMP3 or LANHE CT2001A battery test systems.
Computational methods
Theoretical calculations were carried out using the DMol3 program package in Materials Studio. The exchange and correlation terms were determined using generalized gradient approximation (GGA) in the form proposed by Perdew, Burke, and Ernzerhof (PBE). The energy convergence criterion was set to 10−6 Hartree. In the Z direction, a vacuum of about 15 Å was applied to eliminate the effect of periodic boundary conditions in the slab model. An 18.65 × 18.65 × 24.95 Å3 supercell was used to study the adsorption on the Zn(002) surface, and an 18.65 × 24.73 × 25.39 Å3 supercell was used to study the adsorption on the Zn(100) surface. The bottom layer was kept fixed to maintain bulk properties. For MD simulations, the models were constructed using 5556 H2O, 300 ZnSO4 and 14 MA molecules. The COMPASS II force field was selected to assign charges for Zn2+, SO42−, and MA. The geometry optimization was carried out in the Forcite module, in which the lattice geometry was optimized based on the convergence of total energy (0.001 kcal mol−1) with a force of 0.5 kcal mol−1 Å−1. MD simulations were then conducted using NVT and NPT ensembles at 298 K. The cutoff distance for van der Waals and electrostatic interactions was 12.5 Å and 12 Å, respectively. All simulations were carried out under the standard periodic boundary conditions and the simulation time was long enough to ensure the equilibrium states of electrolyte systems.Conflicts of interest
The authors declare no competing financial interests.Data availability
The data supporting this article have been included in the main text and the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ee04200c.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52174276), the Central Guidance for Local Science and Technology Development Foundation (Youth Science Program Type A of Liaoning Province; 2025JH6/101100007), the Fundamental Research Funds for the Central Universities (N25QNR011) and the 111 Project (B16009). Special thanks are due to the Analytical and Testing Center, Northeastern University for the instrumental analysis.References
- A. Duan, S. Luo, Y. Tang, Y. Feng, M. Li, B. Zhang and W. Sun, Adv. Energy Mater., 2025, 15, 2404693 CrossRef CAS.
- X. Zhang, J. Li, Y. Liu, B. Lu, S. Liang and J. Zhou, Nat. Commun., 2024, 15, 2735 CrossRef CAS.
- Q. Yang, Q. Li, Z. Liu, D. Wang, Y. Guo, X. Li, Y. Tang, H. Li, B. Dong and C. Zhi, Adv. Mater., 2020, 32, 2001854 CrossRef CAS.
- W. Zhong, Z. Shen, J. Mao, S. Zhang, H. Cheng, Y. Kim and Y. Lu, Energy Environ. Sci., 2024, 17, 2059–2068 RSC.
- S. Chen, D. Ji, Q. Chen, J. Ma, S. Hou and J. Zhang, Nat. Commun., 2023, 14, 3526 CrossRef CAS PubMed.
- X. Ma, Q. Wang, X. Zhang, Y. Lin, F. Zhang, J. Huang and Y. Wang, Energy Environ. Sci., 2025, 18, 982–990 RSC.
- A. Chen, C. Zhao, J. Gao, Z. Guo, X. Lu, J. Zhang, Z. Liu, M. Wang, N. Liu, L. Fan, Y. Zhang and N. Zhang, Energy Environ. Sci., 2023, 16, 275–284 RSC.
- Q. Nian, X. Luo, D. Ruan, Y. Li, B.-Q. Xiong, Z. Cui, Z. Wang, Q. Dong, J. Fan, J. Jiang, J. Ma, Z. Ma, D. Wang and X. Ren, Nat. Commun., 2024, 15, 4303 CrossRef CAS.
- Y. Zhang, Z. Liu, X. Li, L. Fan, Y. Shuai and N. Zhang, Adv. Energy Mater., 2023, 13, 2302126 CrossRef CAS.
- Q. Ren, X. Tang, Y. Guo, X. Liao, C. Zhang, Z. Zhu, P. Wang, W. Wang, Y. Li, W. Song, S. Wang, K. He, Z.-B. Wang and Y. Yuan, Adv. Energy Mater., 2025, 15, 2403961 CrossRef CAS.
- Q. Li, D. Luo, Q. Ma, Z. Zheng, S. Li, Y. Xie, L. Xue, M. Lin, Y. Nie, G. Feng, H. Dou, J. Chen, X. Wang and Z. Chen, Energy Environ. Sci., 2025, 18, 1489–1501 RSC.
- H. Du, Y. Wang, X. Liu, X. Wu, Z. Yan and J. Chen, Adv. Energy Mater., 2024, 14, 2304144 CrossRef CAS.
- Y. Shang, P. Kumar, T. Musso, U. Mittal, Q. Du, X. Liang and D. Kundu, Adv. Funct. Mater., 2022, 32, 2200606 CrossRef CAS.
- K. Wang, T. Qiu, L. Lin, H. Zhan, X.-X. Liu and X. Sun, Energy Storage Mater., 2024, 70, 103516 CrossRef.
- H. Jiang, L. Tang, Y. Fu, S. Wang, S. K. Sandstrom, A. M. Scida, G. Li, D. Hoang, J. J. Hong, N.-C. Chiu, K. C. Stylianou, W. F. Stickle, D. Wang, J. Li, P. A. Greaney, C. Fang and X. Ji, Nat. Sustainability, 2023, 6, 806–815 CrossRef.
- D. Hoang, Y. Li, M. S. Jung, S. K. Sandstrom, A. M. Scida, H. Jiang, T. C. Gallagher, B. A. Pollard, R. Jensen, N.-C. Chiu, K. Stylianou, W. F. Stickle, P. A. Greaney and X. Ji, Adv. Energy Mater., 2023, 13, 2301712 CrossRef CAS.
- R. Zhao, X. Dong, P. Liang, H. Li, T. Zhang, W. Zhou, B. Wang, Z. Yang, X. Wang, L. Wang, Z. Sun, F. Bu, Z. Zhao, W. Li, D. Zhao and D. Chao, Adv. Mater., 2023, 35, 2209288 CrossRef CAS.
- F. Ming, Y. Zhu, G. Huang, A.-H. Emwas, H. Liang, Y. Cui and H. N. Alshareef, J. Am. Chem. Soc., 2022, 144, 7160–7170 CrossRef CAS PubMed.
- L. Miao, R. Wang, S. Di, Z. Qian, L. Zhang, W. Xin, M. Liu, Z. Zhu, S. Chu, Y. Du and N. Zhang, ACS Nano, 2022, 16, 9667–9678 CrossRef CAS.
- C. You, R. Wu, X. Yuan, L. Liu, J. Ye, L. Fu, P. Han and Y. Wu, Energy Environ. Sci., 2023, 16, 5096–5107 RSC.
- M. Li, X. Wang, J. Hu, J. Zhu, C. Niu, H. Zhang, C. Li, B. Wu, C. Han and L. Mai, Angew. Chem., Int. Ed., 2023, 62, e202215552 CrossRef CAS PubMed.
- D. Wang, D. Lv, H. Peng, C. Wang, H. Liu, J. Yang and Y. Qian, Angew. Chem., Int. Ed., 2023, 62, e202310290 CrossRef CAS PubMed.
- S. Chen, K. Ouyang, Y. Liu, M. Cui, G. Pu, Y. Wang, K. Zhang and Y. Huang, Angew. Chem., Int. Ed., 2024, 63, e202409303 CAS.
- W. Yuan, X. Nie, Y. Wang, X. Li, G. Ma, Y. Wang, S. Shen and N. Zhang, ACS Nano, 2023, 17, 23861–23871 CrossRef CAS.
- G. Ma, W. Yuan, X. Li, T. Bi, L. Niu, Y. Wang, M. Liu, Y. Wang, Z. Shen and N. Zhang, Adv. Mater., 2024, 36, 2408287 CrossRef CAS PubMed.
- T. Wei, Y. Ren, Y. Wang, L. E. Mo, Z. Li, H. Zhang, L. Hu and G. Cao, ACS Nano, 2023, 17, 3765–3775 CrossRef CAS.
- S. Tang, Q. Wei, B. Liu, J. Yang, H. Jiang, Y. Ge, D. Wu, J. Li, T. Qiu, H. Zhang and X. Tian, Angew. Chem., Int. Ed., 2025, 64, e202510252 CrossRef CAS.
- Z.-X. Chen, Q. Cheng, X.-Y. Li, Z. Li, Y.-W. Song, F. Sun, M. Zhao, X.-Q. Zhang, B.-Q. Li and J.-Q. Huang, J. Am. Chem. Soc., 2023, 145, 16449–16457 CrossRef CAS.
- Z. Zhao, J. Zhao, Z. Hu, J. Li, J. Li, Y. Zhang, C. Wang and G. Cui, Energy Environ. Sci., 2019, 12, 1938–1949 RSC.
- H. Yu, D. Chen, Q. Li, C. Yan, Z. Jiang, L. Zhou, W. Wei, J. Ma, X. Ji, Y. Chen and L. Chen, Adv. Energy Mater., 2023, 13, 2300550 CrossRef CAS.
- Z. Xing, P. Ye, X. Shi, L. Shan, S. Guo, M. Chen, Y. Xia, Y. Gao, H. K. Thabet, T. F. Altamimi, Z. M. El-Bahy, X. Tian and J. Zhou, Angew. Chem., Int. Ed., 2025, e202516974 CAS.
- X. Yu, M. Chen, J. Wang, S. Li, H. Zhang, Q. Zhao, H. Luo, Y. Deng, H. Liang, J. Zhou, F. Wang, D. Chao, Y. Zou, G. Feng, Y. Qiao and S.-G. Sun, Nat. Commun., 2025, 16, 3820 CrossRef CAS.
- Y. Wang, Y. Cui, M. Zhao, J. Wang, X. Liu, Y. Zhu and Y. Yang, Nat. Commun., 2025, 16, 5565 CrossRef.
- Y. Wang, S. Li, P. Cui, K. Ye, X. Wang, D. Cao and K. Zhu, Adv. Energy Mater., 2025, 15, 2500593 CrossRef CAS.
- W. Guo, L. Xu, Y. Su, L. Zhao, Y. Ding, Y. Zou, G. Zheng, T. Cheng and J. Sun, Angew. Chem., Int. Ed., 2025, 64, e202417125 CrossRef CAS.
- Z. Peng, L. Tang, S. Li, L. Tan and Y. Chen, Angew. Chem., Int. Ed., 2025, 64, e202418242 CrossRef CAS PubMed.
- Z. Li, Z. Wang, W. Sun, Y. Ma, W. Guo and Y. Fu, Adv. Mater., 2025, 37, 2420489 CrossRef CAS.
- Q. Zong, R. Li, J. Wang, Q. Zhang and A. Pan, Angew. Chem., Int. Ed., 2024, 63, e202409957 CrossRef CAS.
- J. Luo, L. Xu, Y. Zhou, T. Yan, Y. Shao, D. Yang, L. Zhang, Z. Xia, T. Wang, L. Zhang, T. Cheng and Y. Shao, Angew. Chem., Int. Ed., 2023, 62, e202302302 CrossRef CAS.
- L. Wang, W. Huang, W. Guo, Z. H. Guo, C. Chang, L. Gao and X. Pu, Adv. Funct. Mater., 2022, 32, 2108533 CrossRef CAS.
- P. X. Sun, Z. Cao, Y. X. Zeng, W. W. Xie, N. W. Li, D. Luan, S. Yang, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2022, 61, e202115649 CrossRef CAS.
- R. Sun, D. Han, C. Cui, Z. Han, X. Guo, B. Zhang, Y. Guo, Y. Liu, Z. Weng and Q.-H. Yang, Angew. Chem., Int. Ed., 2023, 62, e202303557 CrossRef CAS.
- H. Yu, H. Yao, Y. Zheng, D. Liu, J. S. Chen, Y. Guo, N. W. Li and L. Yu, Adv. Funct. Mater., 2024, 34, 2311038 CrossRef CAS.
- P. Sun, L. Ma, W. Zhou, M. Qiu, Z. Wang, D. Chao and W. Mai, Angew. Chem., Int. Ed., 2021, 60, 18247–18255 CrossRef CAS PubMed.
- Z. Yang, F. Lai, Q. Mao, C. Liu, R. Wang, Z. Lu, T. Zhang and X. Liu, Adv. Mater., 2024, 36, 2311637 CrossRef CAS.
- X. Guo, K. Zhang, D. Han, C. Cui, A. Liu, Y. Guo, J. Gao, R. Sun, C. Wei, L. Yin, G. He, Z. Weng and Q.-H. Yang, Adv. Energy Mater., 2025, 2501180, DOI:10.1002/aenm.202501180.
- T. Li, H. Yang, X. Dong, H. Ma, J. Cai, C. Wei, T. Zhang, S. Zhang, F. Huang and T. Lin, Angew. Chem., Int. Ed., 2025, 64, e202501183 CrossRef CAS PubMed.
- M. Kwon, J. Lee, S. Ko, G. Lim, S.-H. Yu, J. Hong and M. Lee, Energy Environ. Sci., 2022, 15, 2889–2899 RSC.
- J. Zhang, W. Huang, L. Li, C. Chang, K. Yang, L. Gao and X. Pu, Adv. Mater., 2023, 35, 2300073 CrossRef CAS.
- D. Tang, X. Zhang, D. Han, C. Cui, Z. Han, L. Wang, Z. Li, B. Zhang, Y. Liu, Z. Weng and Q.-H. Yang, Adv. Mater., 2024, 36, 2406071 CrossRef CAS.
- Y. Chen, G. Zhou, X. Huang, Y. Liu, X. Tian, L. Wang, X. Liu, X. Ning, D. Zhu, Z. Bai, N. Wang, X. Ren and S. Dou, Energy Storage Mater., 2025, 78, 104247 CrossRef.
- X. Zhang, C. Qu, X. Zhang, X. Peng, Y. Qiu, Y. Su, J. Zeng, Z. Liu, X. Liu, W. Qi, H. Wang and F. Xu, Adv. Energy Mater., 2024, 14, 2401139 CrossRef CAS.
- X. Song, L. Bai, C. Wang, D. Wang, K. Xu, J. Dong, Y. Li, Q. Shen and J. Yang, ACS Nano, 2023, 17, 15113–15124 CrossRef CAS PubMed.
- Y. Zeng, Z. Pei, D. Luan and X. W. D. Lou, J. Am. Chem. Soc., 2023, 145, 12333–12341 CrossRef CAS PubMed.
- Z. Zhao, R. Wang, C. Peng, W. Chen, T. Wu, B. Hu, W. Weng, Y. Yao, J. Zeng, Z. Chen, P. Liu, Y. Liu, G. Li, J. Guo, H. Lu and Z. Guo, Nat. Commun., 2021, 12, 6606 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |