A regenerable redox mediator for all-air processed wide-bandgap perovskite solar cells under high-humidity conditions
Yuting
Song
a,
Xinhang
Cai
a,
Haoyu
Ge
a,
Xuelian
Liu
a,
Ziyan
Liu
a,
Aijun
Li
a,
Naoyuki
Shibayama
 b and
Xiao-Feng
Wang
b and
Xiao-Feng
Wang
 *a
*a
aKey Laboratory of Physics and Technology for Advanced Batteries (Ministry of Education), College of Physics, Jilin University, Changchun, 130012, People's Republic of China. E-mail: xf_wang@jlu.edu.cn; Fax: +86-0431-81334301; Tel: +86-0431-81334301
bGraduate School of Engineering, Toin University of Yokohama, 1614 Kurogane-cho, Aoba, Yokohama, Kanagawa 225-8503, Japan
First published on 1st December 2025
Abstract
The commercialization of wide-bandgap (WBG) perovskite solar cells (PSCs) faces critical challenges in high-humidity fabrication environments and long-term operational stability. To address these issues, this study introduced 4-mercaptophenylacetic acid (MPAA) as a redox mediator into the perovskite. MPAA facilitates cyclic regeneration through the reversible conversion of thiol-disulfide, simultaneously reducing I2 and oxidizing Pb0, thereby effectively suppressing phase separation. Furthermore, its benzene ring's hydrophobic structure forms moisture barrier, significantly improving the fabrication adaptability of the perovskite in a high-humidity environment. Benefiting from these characteristics, the device fabricated by blade coating in high-humidity ambient air (≈65% relative humidity, RH) achieves a power conversion efficiency (PCE) of 23.16%, which is the state-of-the-art result for the WBG (≥1.68 eV) PSCs fabricated in ambient air. The fabricated mini-module (13 cm2) achieves a PCE of 18.46%, demonstrating the scaling potential of this strategy. Meanwhile, the MPAA-doped device retained 90.2% of its initial PCE after aging for 500 hours under the ISOS-L-3 protocol (85 °C, 50% RH), while the control device exhibited almost complete degradation. This strategy overcomes the limitations of high-humidity fabrication and long-term operational stability problems of WBG PSCs, thus providing significant support for the industrialization of perovskite photovoltaics.
Broader contextTandem solar cells (TSCs) have shown great promise in overcoming the Shockley–Queisser (SQ) limit of single-junction solar cells by minimizing thermalization losses. In particular, perovskite/silicon tandem solar cells have achieved efficiencies that exceed the SQ limit. However, as the front unit, high-performance wide-bandgap (WBG) perovskite solar cells (PSCs) are still typically fabricated in glove boxes, hindering the large-scale commercialization of TSCs. Therefore, there is a need to overcome the long-standing challenge of fabricating high-performance and highly stable WBG PSCs in ambient air. Herein, we successfully demonstrated a facile strategy to fabricate WBG PSCs in ambient air (∼65% relative humidity) via incorporating 4-mercaptophenylacetic acid (MPAA) as a redox mediator. Moreover, we achieved a remarkable PCE of 23.16% in WBG PSCs utilizing MPAA. This study facilitates the fabrication of WBG PSCs in ambient air and lays a key foundation for the future commercialization of perovskite solar technology. |
Introduction
Metal–organic halide perovskites have attracted extensive attention due to their excellent optoelectronic properties, such as a high absorption coefficient, long diffusion length, and high carrier mobility.1–3 To date, the power conversion efficiency (PCE) of single-junction perovskite solar cells (PSCs) has exceeded 27%, and when used in tandem configurations with silicon solar cells, the PCE even exceeds 34%, demonstrating great commercial potential.4–8 However, high-efficiency wide-bandgap (WBG) PSCs, which are crucial for tandem structures, not only suffer from limited stability due to the phase separation, but also face technical bottlenecks in their fabrication in high-humidity ambient air, posing significant challenges to commercialization.9During the ambient air fabrication process, iodine ion oxidation readily occurs in WBG perovskites. This process is further exacerbated by the presence of moisture, especially in high-humidity ambient air, as it promotes iodine oxidation. The resulting oxidation products (such as I2, I3− and I2Br−) form local concentration gradients, which in turn drive halide migration.10–13 Consequently, this leads not only to halide segregation in WBG perovskites, reducing device stability, but also to the formation of charge traps and halide vacancies in the perovskite film, hindering charge transport and degrading device performance.14 In addition, heating or illumination can reduce Pb2+ to metallic Pb0, forming deep-level defects that adversely affect device efficiency and stability.15,16 It is particularly noteworthy that ever since halide demixing behavior was first defined, various explanatory models have been proposed.17,18 Among them, the halide oxidation mechanism most reasonably explains the halide segregation phenomenon observed in perovskites.10,19,20 Therefore, effectively suppressing the regeneration of Pb0 and I2 and preventing moisture erosion of the perovskite film not only enable the fabrication of high-quality WBG perovskite films in high-humidity ambient air, but also significantly inhibit the phase separation phenomenon.
However, commonly used reducing agents lose their reduction capability after eliminating certain defects, which poses a challenge to permanent and sustainable defect removal.21,22 Therefore, this study proposes the addition of 4-mercaptophenylacetic acid (MPAA) as a redox mediator in the perovskite film. The reversible conversion mechanism between the thiol group and the disulfide bond enables the redox mediator to regenerate itself while eliminating I2 and Pb0. Our atomic analysis revealed a significant reduction in Pb0 and I2 content within the perovskite, thus confirming the beneficial impact of the cyclic redox process on suppressing halide segregation. Furthermore, MPAA imparted excellent hydrophobicity to the perovskite film, effectively preventing moisture-induced degradation during fabrication in high-humidity ambient air, making it suitable for fabricating high-quality WBG perovskite films under such conditions. Taking advantage of these synergistic effects, all active layers were fabricated using the blade coating method, and the resulting WBG PSC, fabricated in ambient air at ≈65% relative humidity (RH), achieved a PCE of 23.16%. To our knowledge, this is the first time that WBG PSCs have been fabricated using the full blade coating method. It represents not only the highest PCE achieved for WBG (≥1.68 eV) PSCs fabricated in ambient air, but also the highest PCE attained for WBG PSCs fabricated by blade coating. Simultaneously, the PCE of mini-modules (an active area of 13 cm2) fabricated using the same process reached 18.46%, demonstrating the potential of this strategy for large-scale applications. Through this multi-dimensional protection mechanism, the unencapsulated MPAA-doped device maintains 92.1% of its initial PCE after being stored for 2000 hours at 60% RH and room temperature. Furthermore, it retains 90.8% of its initial PCE after 1000 hours of maximum power point (MPP) tracking at room temperature (ISOS-L-1). More notably, it exhibits remarkable stability under the rigorous ISOS-L-3 testing protocol, retaining 90.2% of its initial PCE after 500 hours of MPP tracking at 85 °C and 50% RH. In contrast, the control device completely degraded. This method not only enhances the performance of fabricated devices in high-humidity environments but also provides a crucial guarantee for their long-term operational stability under such conditions.
Results and discussion
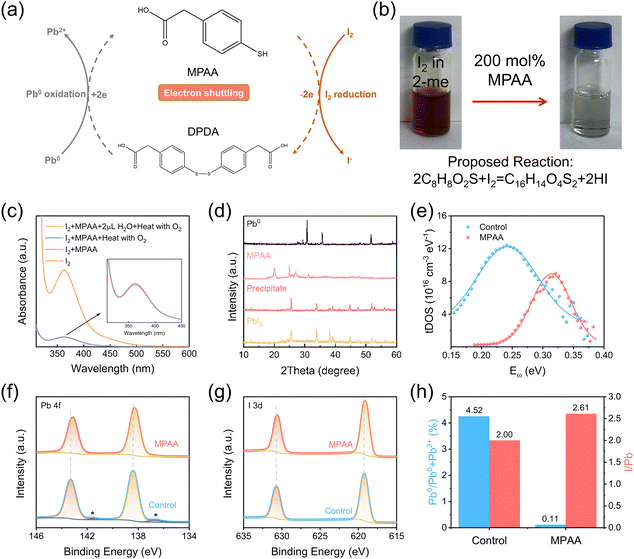
Fig. 1a illustrates the redox shuttle mechanism of MPAA. Initially, MPAA was mixed into an iodine solution (solid iodine dissolved in 2-methoxyethanol) at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and allowed to stand for one hour. Subsequently, a color change was observed in the MPAA and iodine solution mixture, transitioning from dark brown to a transparent state (Fig. 1b). Furthermore, as shown in the absorption spectra in Fig. 1c, the color of iodine still disappears when I2 and MPAA solutions are heated at a constant temperature of 100 °C in either an oxygen-only or a water-and-oxygen-containing environment. Moreover, the reduction capability of MPAA in the simultaneous presence of water and oxygen is extremely close to that observed in the absence of water and oxygen, proving its effectiveness in reducing I2 even in high-humidity ambient air, thus enabling the fabrication of high-quality perovskite films in high-humidity ambient air. We also analyzed the mass spectra of MPAA and its mixture with iodine dissolved in 2-methoxyethanol (2-me). As shown in Fig. S1, a peak with a mass-to-charge ratio (m/z) of 167.01 corresponding to MPAA was detected in the 2-me solution containing only dissolved MPAA. In Fig. S2, the mixed solution of MPAA and I2 showed signals at m/z 333.02 for dithiobis(4,1-phenylene)diacetic acid (DPDA) and at m/z 126.90 for iodine ions, confirming that DPDA is formed when MPAA reduces I2. Subsequently, the reversible transformation between the thiol group and the disulfide bond and its feasibility in simultaneously eliminating I2 and Pb0 while enabling regeneration were investigated by adding equimolar amounts of MPAA, Pb0, and I2 powders into 2-me. As shown in Fig. S3, the control solution (without MPAA) remained dark brown, whereas the solution containing MPAA became colorless at room temperature, accompanied by the formation of a yellow precipitate (Fig. S4). The yellow precipitate was analyzed by X-ray diffraction (XRD) (Fig. 1d), which confirmed that it was lead iodide. Meanwhile, the supernatant of the mixed solution of the three substances was sampled and subjected to mass spectrometry to identify the reaction products. As shown in Fig. S5, both MPAA and DPDA were detected, with mass-to-charge ratios of 167.01 and 333.02, respectively. These findings collectively support the proposed reversible transformation mechanism of the redox mediator, which enables its regeneration while concurrently eliminating I2 and Pb0. Therefore, the defect repair process facilitated by redox shuttling can be described by the following two chemical reactions:
1 molar ratio and allowed to stand for one hour. Subsequently, a color change was observed in the MPAA and iodine solution mixture, transitioning from dark brown to a transparent state (Fig. 1b). Furthermore, as shown in the absorption spectra in Fig. 1c, the color of iodine still disappears when I2 and MPAA solutions are heated at a constant temperature of 100 °C in either an oxygen-only or a water-and-oxygen-containing environment. Moreover, the reduction capability of MPAA in the simultaneous presence of water and oxygen is extremely close to that observed in the absence of water and oxygen, proving its effectiveness in reducing I2 even in high-humidity ambient air, thus enabling the fabrication of high-quality perovskite films in high-humidity ambient air. We also analyzed the mass spectra of MPAA and its mixture with iodine dissolved in 2-methoxyethanol (2-me). As shown in Fig. S1, a peak with a mass-to-charge ratio (m/z) of 167.01 corresponding to MPAA was detected in the 2-me solution containing only dissolved MPAA. In Fig. S2, the mixed solution of MPAA and I2 showed signals at m/z 333.02 for dithiobis(4,1-phenylene)diacetic acid (DPDA) and at m/z 126.90 for iodine ions, confirming that DPDA is formed when MPAA reduces I2. Subsequently, the reversible transformation between the thiol group and the disulfide bond and its feasibility in simultaneously eliminating I2 and Pb0 while enabling regeneration were investigated by adding equimolar amounts of MPAA, Pb0, and I2 powders into 2-me. As shown in Fig. S3, the control solution (without MPAA) remained dark brown, whereas the solution containing MPAA became colorless at room temperature, accompanied by the formation of a yellow precipitate (Fig. S4). The yellow precipitate was analyzed by X-ray diffraction (XRD) (Fig. 1d), which confirmed that it was lead iodide. Meanwhile, the supernatant of the mixed solution of the three substances was sampled and subjected to mass spectrometry to identify the reaction products. As shown in Fig. S5, both MPAA and DPDA were detected, with mass-to-charge ratios of 167.01 and 333.02, respectively. These findings collectively support the proposed reversible transformation mechanism of the redox mediator, which enables its regeneration while concurrently eliminating I2 and Pb0. Therefore, the defect repair process facilitated by redox shuttling can be described by the following two chemical reactions:| 2MPAA + I2 → DPDA + 2I− | (1) |
| DPDA + Pb0 → 2MPAA + Pb2+ | (2) |
To further verify whether the oxidation of iodide ions in perovskite films is effectively suppressed, the trap density of states (tDOS) was evaluated using thermal admittance spectroscopy (TAS) to detect the presence of I2 and I− in perovskites.23,24 TAS measurements revealed a significant reduction in tDOS in the shallow trap depth region (0.19–0.30 eV) for the MPAA-doped device (Fig. 1e). The trap peak located in the 0.20–0.35 eV depth range has been identified as a characteristic signal of I3− formation, which results from the interaction between I2 and I−.25 Therefore, the reduced tDOS suggests that MPAA effectively suppresses the oxidation of I− in perovskite films. Subsequently, Fourier transform infrared (FTIR) spectroscopy was used to determine whether MPAA in the perovskite films can be effectively regenerated. As shown in Fig. S6, the presence of –SH groups in the perovskite film fabricated from MPAA-containing perovskite precursor solution indicates that the MPAA-doped film is capable of inhibiting the oxidation of iodide ions. After the MPAA-doped film was aged in high-humidity air for 30 days, FTIR testing confirmed the continued presence of –SH groups in the aged MPAA-doped film (Fig. S7), indicating that MPAA in the perovskite film can be effectively regenerated and can continuously reduce oxidized iodide ions within the perovskite film.
To investigate the interaction between MPAA and the perovskite, X-ray photoelectron spectroscopy (XPS) analysis was performed on perovskite films with and without MPAA. Fig. S8 shows the high-resolution XPS spectra of S 2p in the perovskite films, indicating that trace amounts of MPAA are still present in the MPAA-doped film. Fig. S9 displays the high-resolution XPS spectra of S 2p for the MPAA-doped perovskite thin film, as well as the spectra of its internal region after 10 nm etching treatment, indicating that MPAA is mainly concentrated on the surface of the thin film and at the grain boundary regions. As shown in Fig. 1f, the high-resolution XPS spectra of Pb 4f reveal a shift in binding energy toward lower values, which is attributed to the formation of coordination bonds between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and Pb2+. This coordination is further supported by the FTIR results in Fig. S6, where a slight shift in the vibrational signal of the C
O groups and Pb2+. This coordination is further supported by the FTIR results in Fig. S6, where a slight shift in the vibrational signal of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of MPAA is observed in the MPAA-doped film. Additionally, the Pb0 characteristic peaks marked with asterisks at 136.7 eV and 141.6 eV in Fig. 1f nearly disappear after the addition of MPAA. The substantial presence of Pb0 further indicates the existence of iodide vacancies in the perovskite lattice of the control film, and these Pb0 species are likely to serve as recombination centers, thereby adversely affecting device performance.26 Quantitative analysis reveals that the intensity ratio of Pb0 to (Pb0 + Pb2+) in the control film is 4.52%, while this ratio is nearly undetectable in the MPAA-doped film (Fig. 1h), suggesting that the formation of Pb0 is effectively suppressed by MPAA. Due to the volatility of I2, accurately determining the I2
O group of MPAA is observed in the MPAA-doped film. Additionally, the Pb0 characteristic peaks marked with asterisks at 136.7 eV and 141.6 eV in Fig. 1f nearly disappear after the addition of MPAA. The substantial presence of Pb0 further indicates the existence of iodide vacancies in the perovskite lattice of the control film, and these Pb0 species are likely to serve as recombination centers, thereby adversely affecting device performance.26 Quantitative analysis reveals that the intensity ratio of Pb0 to (Pb0 + Pb2+) in the control film is 4.52%, while this ratio is nearly undetectable in the MPAA-doped film (Fig. 1h), suggesting that the formation of Pb0 is effectively suppressed by MPAA. Due to the volatility of I2, accurately determining the I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (I2 + I−) ratio through XPS peak fitting is challenging. Therefore, the oxidation state of iodide ions was indirectly monitored by analyzing changes in the theoretical stoichiometric ratio of I/Pb. The I/Pb atomic ratio of the control film was estimated to be 2.00, which falls short of the theoretical value of 2.7. This suggests that iodide ions were oxidized to form volatile I2 and iodine vacancy defects.27 In contrast, the I/Pb atomic ratio in the MPAA-doped film increased to 2.61, indicating that MPAA effectively suppresses the oxidation of iodide ions and the formation of iodine vacancies. As shown in Fig. 1g, the binding energy of the I 3d orbital in the MPAA-doped film exhibits a slight shift compared to that in the control film, suggesting that the interaction between MPAA and Pb2+ increases the electron cloud density around iodine, thereby reducing the binding energy. These results confirm that MPAA can simultaneously reduce I2 and oxidized Pb0 in perovskite films while being regenerated, thereby suppressing halide segregation and significantly improving the lattice integrity and structural stability of the film. Fig. 2a illustrates the interaction mechanism between MPAA and the perovskite.
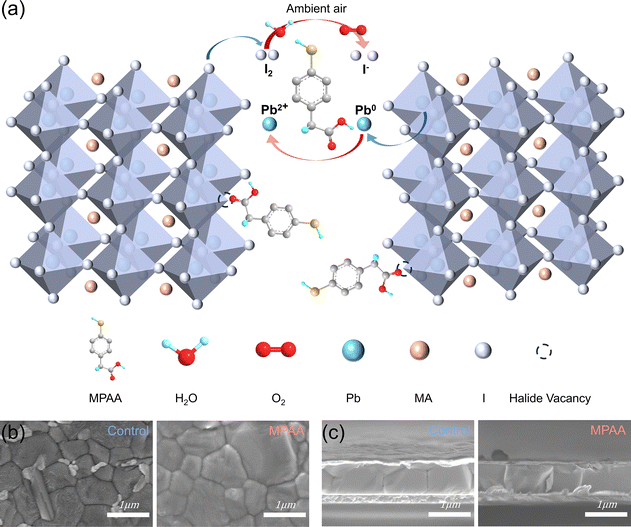
(I2 + I−) ratio through XPS peak fitting is challenging. Therefore, the oxidation state of iodide ions was indirectly monitored by analyzing changes in the theoretical stoichiometric ratio of I/Pb. The I/Pb atomic ratio of the control film was estimated to be 2.00, which falls short of the theoretical value of 2.7. This suggests that iodide ions were oxidized to form volatile I2 and iodine vacancy defects.27 In contrast, the I/Pb atomic ratio in the MPAA-doped film increased to 2.61, indicating that MPAA effectively suppresses the oxidation of iodide ions and the formation of iodine vacancies. As shown in Fig. 1g, the binding energy of the I 3d orbital in the MPAA-doped film exhibits a slight shift compared to that in the control film, suggesting that the interaction between MPAA and Pb2+ increases the electron cloud density around iodine, thereby reducing the binding energy. These results confirm that MPAA can simultaneously reduce I2 and oxidized Pb0 in perovskite films while being regenerated, thereby suppressing halide segregation and significantly improving the lattice integrity and structural stability of the film. Fig. 2a illustrates the interaction mechanism between MPAA and the perovskite.
Subsequently, the effect of MPAA on the morphology of perovskite films was investigated using scanning electron microscopy (SEM) and atomic force microscopy (AFM). The SEM images in Fig. 2b compare the surface morphology of the control film and the MPAA-doped film. The MPAA-doped film shows significantly larger grain sizes than the control film; its surface lead iodide content is also reduced in comparison with that of the control film.28 This suggests a decrease in the number of grain boundaries in the MPAA-doped film, which not only lowers the defect density but also effectively suppresses carrier recombination caused by defects.29 The AFM results shown in Fig. S10 indicate that the root mean square roughness of the MPAA-doped film decreases from 16.0 nm to 10.6 nm compared to the control film. MPAA is present on the crystal planes and at the grain boundaries, filling the tiny gaps between adjacent grains and thereby reducing the roughness. The reduced surface roughness implies fewer surface defects in the MPAA-doped film, thereby improving its contact with the electron transport layer and ultimately enhancing device performance.30 In addition, while cross-sectional SEM images (Fig. 2c) show that the crystal growth orientation of the MPAA-doped film is visually similar to that of the control film, our XRD analysis results indicate that the crystal orientation of the MPAA-doped thin film has been improved. This suggests that MPAA facilitates the formation of larger grains and more orderly perovskite crystal growth, thereby improving the overall film quality.
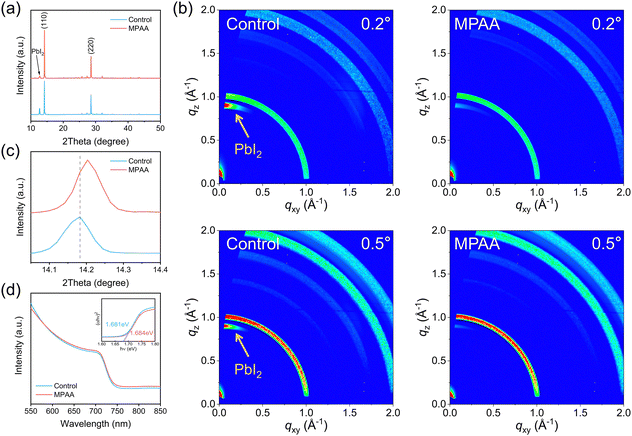
XRD measurements confirmed that the crystallinity of perovskite films was enhanced by the incorporation of MPAA. All XRD patterns exhibited two prominent diffraction peaks (Fig. 3a) at 2θ values of 14.18° and 28.55°, corresponding to the (110) and (220) crystal planes of the perovskite, respectively. Notably, in the MPAA-doped film, the intensities of the (110) and (220) diffraction peaks were significantly increased, and the full width at half maximum was markedly reduced (see Fig. S11 for details). In addition, the intensity of the lead iodide diffraction peak was relatively low, indicating that MPAA doping improved the crystallinity of the perovskite, leading to an increase in the grain size, which is consistent with the SEM results.31,32 This conclusion was further supported by grazing incidence wide-angle X-ray scattering (GIWAXS) measurements. As shown in Fig. 3b, the crystallinity of the MPAA-doped film was significantly enhanced. In addition, the diffraction signal of lead iodide in the MPAA-doped film is weaker than that in the control film, which is consistent with the XRD results. A slight rightward shift of the diffraction peak at 14.18° was also observed in the MPAA-doped film (Fig. 3c), indicating a decrease in the lattice constant of the MPAA-doped perovskite. This suggests that the bandgap of the MPAA-doped film may be larger.14 Subsequent UV-vis absorption measurements (Fig. 3d) confirmed that MPAA doping not only slightly increased the bandgap but also slightly enhanced the light absorption capability of the MPAA-doped film, the calculated bandgap of which was 1.684 eV.
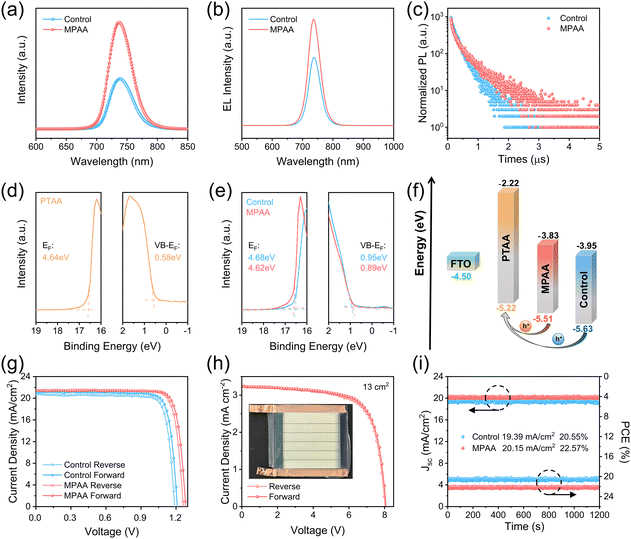
Carrier dynamics of both the control film and the MPAA-doped film on glass substrates were investigated using steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) measurements. As shown in Fig. 4a, PL quenching in the MPAA-doped film is significantly less pronounced than that in the control film, indicating a lower density of defects and reduced non-radiative recombination losses. This confirms the effectiveness of MPAA in defect passivation.33,34 Furthermore, photoluminescence quantum yield (PLQY) measurements (Fig. S12) reveal that the PLQY of the MPAA-doped perovskite film is 2.18%, which is 1.65 fold higher than that of the control film (1.32%). Electroluminescence (EL) measurements were also performed on both devices. As shown in Fig. 4b, the EL emission intensity of the MPAA-doped device is markedly higher than that of the control device, suggesting a significant reduction in non-radiative recombination pathways.35 The TRPL decay curves of the two types of films (Fig. 4c) were fitted using a biexponential decay equation (eqn (S1)), with the results summarized in Table S1. With the addition of MPAA, the average carrier lifetime (tavg) increased significantly from 261 ns to 436 ns (eqn (S2)). This notable increase in lifetime indicates the effective suppression of non-radiative recombination by MPAA.
An in-depth investigation was conducted on the perovskite phase separation phenomenon induced by halide oxidation. To examine the effect of MPAA on halide segregation, PL spectra of perovskite films were analyzed under various illumination durations. The control film showed a gradual decrease in emission intensity accompanied by a pronounced redshift of the peak position under prolonged illumination (Fig. S13). The PL intensity of the MPAA-doped film remained stable without any redshift (Fig. S14), attributable to the reversible conversion mechanism between thiol groups and disulfide bonds, which enables MPAA to regenerate while simultaneously eliminating I2 and Pb0.36 As a result, the PL intensity of the doped film remains stable during the aging process without any redshift, demonstrating that MPAA effectively suppresses phase separation under illumination. Meanwhile, we prepared perovskite thin films with bandgaps of 1.8 eV and 1.9 eV, respectively, and conducted PL experiments on these perovskite films under different illumination durations to detect the phase separation phenomenon. As shown in Fig. S15 and S16, we were delighted to find that the introduction of MPAA exhibited excellent phase separation suppression effects in both the 1.8 eV and 1.9 eV WBG perovskite systems. This discovery strongly demonstrates the cross bandgap applicability of our proposed redox-mediated strategy, injecting new impetus and providing robust support for the research and development of all perovskite tandem solar cells.
The energy band structure of the devices was investigated using ultraviolet photoelectron spectroscopy (UPS) (Fig. 4d and e). The work functions (WF) of each film were calculated by analyzing the secondary electron cutoff peak in the UPS spectra: 4.64 eV for PTAA, 4.68 eV for the control film, and 4.62 eV for the MPAA-doped film. The corresponding valence band maximum (VBM) values were calculated to be −5.22 eV, −5.63 eV, and −5.51 eV, respectively. Notably, the addition of MPAA leads to a decrease in the WF of the perovskite film, resulting in an energy band bending effect that favors hole transport (Fig. S17). Furthermore, by combining the UPS data with the bandgap data obtained from UV-vis spectroscopy, an energy band alignment diagram was constructed. As shown in Fig. 4f, the VBM energy level difference between the MPAA-doped film and the PTAA film is reduced. This decreased contact barrier not only significantly improves the efficiency of hole extraction and transport between the perovskite and PTAA but also effectively suppresses charge accumulation at the interface, allowing the device performance to reach its optimal level.
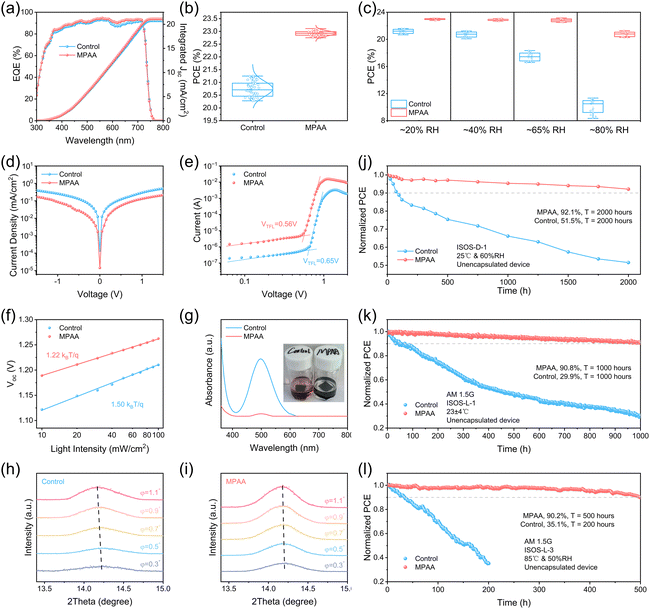
To verify the impact of MPAA on photovoltaics performance, inverted wide-bandgap (WBG) perovskite solar cells (PSCs) (Fig. S18) were fabricated in this study using the blade coating method. All active layers were fabricated via this method (Fig. S19). As shown in Fig. S20, the optimal MPAA concentration for achieving the highest power conversion efficiency (PCE) was determined. Fig. 4g illustrates the photocurrent density (J)–voltage (V) characteristic curves of the best-performing control device and MPAA-doped device, with relevant photovoltaic parameters detailed in Table 1. To our knowledge, this represents the first instance of fully blade-coated WBG PSCs being fabricated. Furthermore, the champion MPAA-doped device, fabricated in ambient air at 65% relative humidity (RH), achieved a PCE of 23.16%, setting a new record not only for WBG (≥1.68 eV) PSCs fabricated in ambient air, but also for those fabricated by blade coating. Table S2 summarizes the PCE data of WBG PSCs fabricated under ambient air, and Table S3 lists the PCE data of WBG PSCs fabricated by blade coating. More noteworthy is that we successfully fabricated WBG perovskite mini-modules (an active area of 13 cm2) under the above conditions using the same process, achieving a peak power conversion efficiency of 18.46% (Fig. 4h), with relevant photovoltaic parameters detailed in Table S4. This result fully demonstrates the feasibility for large-scale production. In addition, the hysteresis index (HI) of the MPAA-doped device was calculated to be only 1.93% according to eqn (S3), which is significantly lower than the control device's 3.61%. Under AM 1.5 G constant illumination, the steady-state power output tracking results (Fig. 4i) show that the steady-state PCE values of the control device and the MPAA-doped device are 20.55% and 22.57%, respectively, which verifies the reliability of the J–V characteristic curves. The external quantum efficiency (EQE) spectra of the devices were analyzed (Fig. 5a), and the integrated short-circuit current density of the MPAA-doped device and the control device was found to be 21.11 mA cm−2 and 20.72 mA cm−2, respectively, consistent with the J–V test results. Fig. 5b presents the performance statistics of 50 cells for both the control device and the MPAA-doped device, demonstrating improved reproducibility of the MPAA-doped devices.
| Devices | J sc [mA cm−2] | V oc [V] | FF [%] | PCE [%] | HI [%] | |
|---|---|---|---|---|---|---|
| Control | Reverse | 20.77 | 1.19 | 83.20 | 20.52 | 3.61 |
| Forward | 21.17 | 1.21 | 82.97 | 21.26 | ||
| MPAA-doped | Reverse | 21.44 | 1.26 | 83.91 | 22.72 | 1.93 |
| Forward | 21.43 | 1.28 | 84.25 | 23.16 |
It was surprisingly found that MPAA can extend the shelf life of the perovskite precursor solution to over 100 days. Devices fabricated with the perovskite precursor solution aged in ambient air at room temperature for 100 days were compared to those fabricated with freshly prepared perovskite precursor solution. Experimental data (Fig. S21) show that the PCE of the devices fabricated using the 100-day-aged perovskite precursor solution decreased significantly. However, the devices fabricated with MPAA added to the aged solution still retained 98.27% of the highest PCE (see Table S5 for specific photovoltaic parameters). This highlights the great potential of MPAA doping in the large-scale production of PSCs, laying a solid foundation for perovskite commercialization.
To verify the applicability of MPAA to fabricate WBG PSCs in high-humidity ambient air, the hydrophobic properties of perovskite films were first tested (Fig. S22). The results showed that the static water contact angle of the MPAA-doped film reached 84.8°, significantly higher than the control film's 63.9°. This difference confirms that the hydrophobic benzene ring structure in the MPAA molecule can form a dense protective layer on the surface and grain boundaries of the perovskite film, effectively improving the film's hydrophobicity. This characteristic can significantly inhibit the erosion of the perovskite by environmental moisture during the device fabricate process, thus providing a key guarantee for process stability in high-humidity ambient air. Based on these findings, a series of devices were further fabricated in ambient air with different humidities (20%–80% RH), and their PCE (Fig. 5c) was tested. It is worth noting that the MPAA-doped devices fabricated under 65% RH exhibit optimal performance, and even those fabricated under an extreme humidity of 80% RH can still retain 91.84% of the highest PCE. These data fully demonstrate that the MPAA doping strategy effectively eliminates the dependence on low-humidity environments during the fabrication of perovskite devices, enabling the fabrication of efficient and stable WBG PSCs in high-humidity ambient air.
To further elucidate the mechanism of performance enhancement in MPAA-doped devices, the charge transfer and carrier recombination mechanisms inside the device were investigated in depth. The analysis of the dark J–V curves (Fig. 5d) reveals that the MPAA-doped device exhibits a lower saturation current density, indicating that MPAA doping can effectively passivate shallow defect energy levels in the perovskite film and suppress non-radiative recombination processes. Moreover, space charge limited current (SCLC) measurements were performed on the devices, as shown in Fig. 5e, with a schematic diagram of the hole-only device structure shown in Fig. S23. The defect state density (ntrap) of the perovskite film can then be calculated according to eqn (S4). Based on the dark J–V curves of the hole-only devices, the trap-filled limit voltages (VTFL) for the control and MPAA-doped devices were estimated to be 0.65 V and 0.56 V, respectively. The ntrap values were 2.28 × 1015 cm−3 for the control device and 1.96 × 1015 cm−3 for the MPAA-doped device, respectively, indicating a significant reduction in defect state density with the addition of MPAA. The trap-induced recombination process was also evaluated by the open-circuit voltage (Voc) dependence on light intensity (Fig. 5f).37 Based on calculations using eqn (S5), the slopes of the curves for the control and MPAA-doped devices were found to be 1.50kBT/q and 1.22kBT/q, respectively, suggesting that trap-assisted recombination was suppressed in the MPAA-doped device.38,39
The increase in built-in potential in the MPAA-doped device was also observed in the Mott–Schottky (M–S) measurement (Fig. S24). Furthermore, the M–S curve of the MPAA-doped device exhibited a steeper slope, suggesting a lower carrier density at the interface, which reduced charge accumulation and suppressed carrier recombination.40 Electronic impedance spectroscopy (EIS) of the devices was further analyzed in a dark environment, and the series resistance (Rs) and recombination resistance (Rrec) were measured (see Table S6 for details). As shown in Fig. S25, the Rrec of the MPAA-doped device (16.29 kΩ) was significantly higher than that of the control device (4.63 kΩ), indicating that MPAA effectively suppressed non-radiative recombination.41 In summary, MPAA not only passivates the defects in the perovskite film but also significantly reduces non-radiative recombination, thereby improving the overall performance of the device.
The stability issue of the perovskite in high-humidity ambient air severely restricts its commercialization process. To systematically evaluate the long-term moisture resistance of the MPAA-doped film, the films were subjected to a 15-day aging test in ambient air with 60% RH. XRD analysis results (Fig. S26) show that the control film exhibits significant lead iodide characteristic diffraction peaks, indicating that the water-oxygen environment induces phase transition and structural degradation of the film. In contrast, only weak lead iodide diffraction signals were observed in the MPAA-doped film, demonstrating that MPAA can effectively prevent the erosion of the perovskite by environmental water and oxygen. Furthermore, photo-induced degradation is also a key factor in the instability of PSCs, and the I2 generated within the perovskite under illumination exacerbates this phenomenon.42,43 To extract I2 generated during light soaking, the control film and the MPAA-doped film were immersed in toluene after continuous illumination under the same solar light intensity for 24 hours. The absorption spectra of the two toluene solutions were then measured (Fig. 5g). The results showed that the control film turned purple after immersion in toluene, exhibiting a significant absorption peak at 500 nm, which indicates the presence of I2. In contrast, the MPAA-doped film remained colorless, and the absorption peak was significantly reduced, suggesting that it effectively inhibited I2 generation, thereby suppressing photo-induced degradation.
In addition, residual stress in the perovskite can lead to lattice distortion, resulting in additional defects and recombination centers, which in turn affects device performance and stability.24 The change in the 14.2° diffraction peak of the perovskite (110) crystal plane was analyzed using grazing incidence X-ray diffraction (GIXRD). As shown in Fig. 5h and i, a significant reduction in tensile strain was observed in the MPAA-doped film. This indicates that doping with MPAA effectively alleviates lattice distortion, thereby improving the crystal quality and intrinsic stability of the perovskite film. These findings suggest that MPAA not only prevents moisture-induced degradation of the perovskite film, but also dynamically repairs defects and degradation related to I2 and Pb0 within the perovskite film, demonstrating its potential to effectively address the stability issues of PSCs in practical applications.
Therefore, the long-term stability of the MPAA-doped PSC was evaluated based on the International summit on organic photovoltaic stability (ISOS) guidelines, specifically under ISOS-D (dark storage) and ISOS-L (light exposure) testing protocols. As shown in Fig. 5j, the ISOS-D-1 protocol was adopted, which involves dark storage under conditions of 60% RH and controlled ambient temperature of 25 °C. Under these conditions, the unencapsulated MPAA-doped device exhibited enhanced stability, retaining 92.1% of its initial PCE after 2000 hours. In contrast, the control device retained only 51.5% of its initial PCE. Additionally, in accordance with the ISOS-L-1 protocol, the operational stability of the devices was assessed by maximum power point (MPP) tracking under an AM 1.5 G light in air at 25 °C. As shown in Fig. 5k, the MPAA-doped device demonstrated significantly improved operational durability, retaining 90.8% of its initial PCE after 1000 hours, whereas the control device retained only 29.9% of its initial PCE. Moreover, the more stringent ISOS-L-3 protocol was adopted to perform MPP tracking under conditions of 50% RH and heating at 85 °C. As shown in Fig. 5l, the MPAA-doped device demonstrated significantly enhanced photo-thermal stability, retaining 90.2% of its initial PCE after 500 hours of aging, whereas the control device had almost completely degraded after 200 hours. This improvement in stability is attributed to the reversible transformation between thiol groups and disulfide bonds, which enables continuous defect passivation by simultaneously eliminating I2 and Pb0, thereby effectively enhancing device stability.
Conclusions
We have developed an innovative strategy that simultaneously enables breakthroughs in the optoelectronic performance and long-term operational stability of WBG PSCs. Employing the redox mediator MPAA enables simultaneous regeneration by reducing I2 and oxidizing Pb0, effectively suppressing phase separation in WBG perovskites. Additionally, the hydrophobic benzene ring structure forms a moisture barrier during the fabrication process, allowing the fabrication of perovskite films with low defect density and high structural stability even under high-humidity ambient air. The single-junction device fabricated entirely via blade coating demonstrates outstanding performance, achieving a maximum PCE of 23.16%. This achievement not only sets a new PCE record for WBG PSCs fabricated in ambient air, but also represents the highest PCE reported for WBG PSCs fabricated via blade coating. More notably, the feasibility of this technology for large-scale production was successfully validated, with fabricated WBG perovskite mini-modules (an active area of 13 cm2) achieving a PCE of 18.46%, fully demonstrating its potential for industrial applications. Most importantly, the MPAA-doped device exhibited outstanding performance under maximum power point tracking: after 1000 hours of aging under the ISOS-L-1 protocol; the MPAA-doped device retained 90.8% of its initial PCE; under the more stringent ISOS-L-3 protocol, it retained 90.2% of its initial PCE after 500 hours, while the control device nearly completely failed. This level of stability ranks among the best for PSCs, effectively overcoming a major bottleneck in their path to commercialization. This significant advance provides a comprehensive solution for the large-scale production of high-efficiency, high-stability PSCs. It is applicable not only to large-area single-junction devices but also extendable to tandem device architectures, significantly accelerating the commercialization of perovskite photovoltaic technology.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information contains experimental details and supporting figures. See DOI: https://doi.org/10.1039/d5ee05252a.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 62274075 to X. F. Wang). The 2D GIWAXS measurement was performed at SPring-8 at BL19B2 with the approval of the JASRI, proposal no. 2024B1697, 2024A1567 and 2024B1916.References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS
.
- N. Pellet, P. Gao, G. Gregori, T.-Y. Yang, M. K. Nazeeruddin, J. Maier and M. Graetzel, Angew. Chem., Int. Ed., 2014, 53, 3151–3157 CrossRef CAS PubMed
.
- J.-W. Lee, D.-J. Seol, A.-N. Cho and N.-G. Park, Adv. Mater., 2014, 26, 4991–4998 CrossRef CAS
.
- J. Jeong, M. Kim, J. Seo, H. Lu, P. Ahlawat, A. Mishra, Y. Yang, M. A. Hope, F. T. Eickemeyer, M. Kim, Y. J. Yoon, I. W. Choi, B. P. Darwich, S. J. Choi, Y. Jo, J. H. Lee, B. Walker, S. M. Zakeeruddin, L. Emsley, U. Rothlisberger, A. Hagfeldt, D. S. Kim, M. Graetzel and J. Y. Kim, Nature, 2021, 592, 381–385 CrossRef CAS PubMed
.
- M. Kim, G.-H. Kim, T. K. Lee, I. W. Choi, H. W. Choi, Y. Jo, Y. J. Yoon, J. W. Kim, J. Lee, D. Huh, H. Lee, S. K. Kwak, J. Y. Kim and D. S. Kim, Joule, 2019, 3, 2179–2192 CrossRef CAS
.
- J. Zhou, L. Tan, Y. Liu, H. Li, X. Liu, M. Li, S. Wang, Y. Zhang, C. Jiang, R. Hua, W. Tress, S. Meloni and C. Yi, Joule, 2024, 8, 1691–1706 CrossRef CAS
.
- S. Mariotti, E. Koehnen, F. Scheler, K. Sveinbjoernsson, L. Zimmermann, M. Piot, F. Yang, B. Li, J. Warby, A. Musiienko, D. Menzel, F. Lang, S. Kessler, I. Levine, D. Mantione, A. Al-Ashouri, M. S. Haertel, K. Xu, A. Cruz, J. Kurpiers, P. Wagner, H. Koebler, J. Li, A. Magomedov, D. Mecerreyes, E. Unger, A. Abate, M. Stolterfoht, B. Stannowski, R. Schlatmann, L. Korte and S. Albrecht, Science, 2023, 381, 63–69 CrossRef CAS PubMed
.
- C. Li, Y. Chen, Y. Li, Z. Zhang, J. Yang, Y. Wang, L. Gong, Z. Yuan, L. Liang, S. Liu, Y. Zhu, C. Lian, M. Haider, T. Guo, X. Xu, D. Li, E. Bi and P. Gao, Angew. Chem., Int. Ed., 2025, 64, e202502730 CrossRef CAS PubMed
.
- Y. Zhou, E. L. Wong, G. Folpini, M. Zanolini, J. Jimenez-Lopez, A. Treglia and A. Petrozza, Adv. Funct. Mater., 2024, 34, 2406317 CrossRef CAS
.
- R. A. Kerner, Z. Xu, B. W. Larson and B. P. Rand, Joule, 2021, 5, 2273–2295 CrossRef CAS
.
- Z. Xu, R. A. Kerner, S. P. Harvey, K. Zhu, J. J. Berry and B. P. Rand, ACS Energy Lett., 2023, 8, 513–520 CrossRef CAS
.
- Z. Li, A. Sun, C. Tian, R. Zhuang, Y. Zheng, X. Wu, B. Ouyang, J. Du, J. Cai, J. Chen, T. Xue, R. Li, T. Cen, Y. Zhao, K. Zhao, Q. Chen and C.-C. Chen, ACS Energy Lett., 2024, 9, 5471–5482 CrossRef CAS
.
- P. S. Mathew, G. F. Samu, C. Janaky and P. V. Kamat, ACS Energy Lett., 2020, 5, 1872–1880 CrossRef CAS
.
- Y. Song, Z. Liu, X. Cai, H. Ge, X. Liu, X. Wang, A. Li, T. Miyasaka, N. Shibayama and X.-F. Wang, Adv. Energy Mater., 2025, 15, 2500650 CrossRef CAS
.
- L. Wang, H. Zhou, J. Hu, B. Huang, M. Sun, B. Dong, G. Zheng, Y. Huang, Y. Chen, L. Li, Z. Xu, N. Li, Z. Liu, Q. Chen, L.-D. Sun and C.-H. Yan, Science, 2019, 363, 265–270 CrossRef CAS PubMed
.
- K. X. Steirer, P. Schulz, G. Teeter, V. Stevanovic, M. Yang, K. Zhu and J. J. Berry, ACS Energy Lett., 2016, 1, 360–366 CrossRef CAS
.
- A. J. Barker, A. Sadhanala, F. Deschler, M. Gandini, S. P. Senanayak, P. M. Pearce, E. Mosconi, A. J. Pearson, Y. Wu, A. R. S. Kandada, T. Leijtens, F. De Angelis, S. E. Dutton, A. Petrozza and R. H. Friend, ACS Energy Lett., 2017, 2, 1416–1424 CrossRef CAS
.
- S. Draguta, O. Sharia, S. J. Yoon, M. C. Brennan, Y. V. Morozov, J. M. Manser, P. V. Kamat, W. F. Schneider and M. Kuno, Nat. Commun., 2017, 8, 200 CrossRef PubMed
.
- G. F. Samu, A. Balog, F. De Angelis, D. Meggiolaro, P. V. Kamat and C. Janaky, J. Am. Chem. Soc., 2019, 141, 10812–10820 CrossRef CAS
.
- L. A. Frolova, S. Y. Luchkin, Y. Lekina, L. G. Gutsev, S. A. Tsarev, I. S. Zhidkov, E. Z. Kurmaev, Z. X. Shen, K. J. Stevenson, S. M. Aldoshin and P. A. Troshin, Adv. Energy Mater., 2021, 11, 2002934 CrossRef CAS
.
- F. Cao, S. Zhan, X. Dai, F. Cheng, W. Li, Q. Feng, X. Huang, J. Yin, J. Li, N. Zheng and B. Wu, J. Am. Chem. Soc., 2024, 146, 11782–11791 CrossRef CAS
.
- M. Li, H. Li, Q. Zhuang, D. He, B. Liu, C. Chen, B. Zhang, T. Pauporte, Z. Zang and J. Chen, Angew. Chem., Int. Ed., 2022, 61, e202206914 CrossRef CAS PubMed
.
- Z. Ni, C. Bao, Y. Liu, Q. Jiang, W.-Q. Wu, S. Chen, X. Dai, B. Chen, B. Hartweg, Z. Yu, Z. Holman and J. Huang, Science, 2020, 367, 1352–1358 CrossRef CAS PubMed
.
- X. Cai, X. Wang, Y. Song, H. Ge, A. Li, Q. Zhao, Z. Liu, N. Shibayama, Y. Zheng and X.-F. Wang, Chem. Eng. J., 2025, 509, 161388 CrossRef CAS
.
- H.-S. Duan, H. Zhou, Q. Chen, P. Sun, S. Luo, T.-B. Song, B. Bob and Y. Yang, Phys. Chem. Chem. Phys., 2015, 17, 112–116 RSC
.
- G. Sadoughi, D. E. Starr, E. Handick, S. D. Stranks, M. Gorgoi, R. G. Wilks, M. Baer and H. J. Snaith, ACS Appl. Mater. Interfaces, 2015, 7, 13440–13444 CrossRef CAS
.
- L. Wang, H. Zhou, J. Hu, B. Huang, M. Sun, B. Dong, G. Zheng, Y. Huang, Y. Chen, L. Li, Z. Xu, N. Li, Z. Liu, Q. Chen, L.-D. Sun and C.-H. Yan, Science, 2019, 363, 265–270 CrossRef CAS PubMed
.
- T. J. Jacobsson, J.-P. Correa-Baena, E. H. Anaraki, B. Philippe, S. D. Stranks, M. E. F. Bouduban, W. Tress, K. Schenk, J. Teuscher, J.-E. Moser, H. Rensmo and A. Hagfeldt, J. Am. Chem. Soc., 2016, 138, 10331–10343 CrossRef CAS PubMed
.
- H. Ge, X. Wang, X. Cai, Y. Song, H. Xu, A. Li and X.-F. Wang, ACS Photonics, 2025, 12, 3537–3549 CrossRef CAS
.
- J. Carrillo, A. Guerrero, S. Rahimnejad, O. Almora, I. Zarazua, E. Mas-Marza, J. Bisquert and G. Garcia-Belmonte, Adv. Energy Mater., 2016, 6, 1502246 CrossRef
.
- W. Zhang, L. He, D. Tang and X. Li, Sol. RRL, 2020, 4, 2000376 CrossRef CAS
.
- H. Zheng, W. Wu, H. Xu, F. Zheng, G. Liu, X. Pan and Q. Chen, Adv. Funct. Mater., 2020, 30, 2000034 CrossRef CAS
.
- Z. Li, M. Wu, L. Yang, K. Guo, Y. Duan, Y. Li, K. He, Y. Xing, Z. Zhang, H. Zhou, D. Xu, J. Wang, H. Zou, D. Li and Z. Liu, Adv. Funct. Mater., 2023, 33, 2212606 CrossRef CAS
.
- Y. Du, J. Wu, X. Zhang, Q. Zhu, M. Zhang, X. Liu, Y. Zou, S. Wang and W. Sun, J. Energy Chem., 2021, 52, 84–91 CrossRef CAS
.
- Y. Liu, H. Li, J. Wei, W. Feng, B. Tu, W. Peng, Z. Chen, L. Zeng, Y. Mai and F. Guo, Nano Energy, 2025, 141, 111093 CrossRef CAS
.
- S. Wu, Y. Yan, J. Yin, K. Jiang, F. Li, Z. Zeng, S.-W. Tsang and A. K. Y. Jen, Nat. Energy, 2024, 9, 411–421 CrossRef CAS
.
- C. Zhu, X. Niu, Y. Fu, N. Li, C. Hu, Y. Chen, X. He, G. Na, P. Liu, H. Zai, Y. Ge, Y. Lu, X. Ke, Y. Bai, S. Yang, P. Chen, Y. Li, M. Sui, L. Zhang, H. Zhou and Q. Chen, Nat. Commun., 2019, 10, 815 CrossRef CAS
.
- J. Yang, C. Liu, C. Cai, X. Hu, Z. Huang, X. Duan, X. Meng, Z. Yuan, L. Tan and Y. Chen, Adv. Energy Mater., 2019, 9, 1900198 CrossRef
.
- C. Li, A. Wang, L. Xie, X. Deng, K. Liao, J.-a Yang, T. Li and F. Hao, J. Mater. Chem. A, 2019, 7, 24150–24163 RSC
.
- N. Li, S. Tao, Y. Chen, X. Niu, C. K. Onwudinanti, C. Hu, Z. Qiu, Z. Xu, G. Zheng, L. Wang, Y. Zhang, L. Li, H. Liu, Y. Lun, J. Hong, X. Wang, Y. Liu, H. Xie, Y. Gao, Y. Bai, S. Yang, G. Brocks, Q. Chen and H. Zhou, Nat. Energy, 2019, 4, 408–415 CrossRef CAS
.
- E. Ghahremanirad, O. Almora, S. Suresh, A. A. Drew, T. H. Chowdhury and A. R. Uhl, Adv. Energy Mater., 2023, 13, 2204370 CrossRef CAS
.
- G. Y. Kim, A. Senocrate, T.-Y. Yang, G. Gregori, M. Gratzel and J. Maier, Nat. Mater., 2018, 17, 445–449 CrossRef CAS
.
- S. Wang, Y. Jiang, E. J. Juarez-Perez, L. K. Ono and Y. Qi, Nat. Energy, 2017, 2, 16195 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2026 |