Edge sites on TiO2 are photocatalytic active site
Longxia
Wu
ab,
Zongfang
Wu
*c,
Zhengming
Wang
 a,
Hong
Xu
a,
Peng
Chai
a,
Junjie
Shi
a and
Weixin
Huang
a,
Hong
Xu
a,
Peng
Chai
a,
Junjie
Shi
a and
Weixin
Huang
 *a
*a
aState Key Laboratory of Precision and Intelligent Chemistry, iChEM, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Department of Chemical Physics, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: huangwx@ustc.edu.cn
bSchool of Materials Science and Engineering, Hefei Institute of Technology, Hefei 230601, China
cHefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: wzfang@ustc.edu.cn
First published on 4th November 2025
Abstract
Although TiO2 has been widely used as an efficient photocatalyst, the photocatalytic active sites remain ambiguous. Using rutile TiO2(110) surfaces with well-defined defects, we herein unambiguously identify that edge sites on TiO2 surfaces with interstitial Ti3+ defects are photocatalytically active. On the oxidized TiO2(110) surface with TiO2 islands, chemisorbed CO2 and CO are photo-inactive; on the TiO2(110) surface with surface bridging oxygen vacancies and bulk interstitial Ti3+ defects, CO2 and CO chemisorbed at the vacancy sites become photoactive; on the TiO2(110) surface with TiO2 islands and interstitial Ti3+ defects, CO2 and CO chemisorbed at the edge sites of TiO2 islands are also photoactive. CO chemisorbed at the surface oxygen vacancies shows the highest photo-induced desorption probability, while CO2 chemisorbed at the edge sites of TiO2 islands with interstitial Ti3+ exhibits the highest photo-induced desorption probability. Considering their abundance on powder TiO2 photocatalysts, the edge sites are among the photocatalytic active sites contributing to TiO2 photocatalysis.
Introduction
Photocatalysis has been increasingly demonstrated to be capable of reshaping chemistry in a green and sustainable manner.1–7 TiO2 as an earth-abundant, stable, and environmentally-friendly photocatalyst has been extensively studied since the seminal report of UV light-induced water splitting on the TiO2 electrode,8 but the photocatalytic active sites remain ambiguous. This has consequently inspired numerous fundamental studies of TiO2 photocatalysis using various single-crystal TiO2 model catalysts to identify photocatalytic active sites and elucidate the underlying reaction mechanisms.9–16 The results show that the active site on TiO2 photocatalysts varies with the photocatalyzed reactions.17–30 Photogenerated holes at point defect sites mediate the desorption of CO and CO2, such as surface bridging oxygen vacancies (VO), hydroxyl groups (OH), and Ti5c sites near Ti interstitials (Tiint) on various rutile TiO2 surfaces,17,19,20 while the interstitial Ti3+ site in the rutile TiO2(001) subsurface also serves as an active site.17 CO2 chemisorbed at the double oxygen vacancies on the rutile TiO2(011)-(2 × 1) surface exhibits photoreactivity.17 Molecular O2 adsorbed on the TiO2 surface acquires excess negative charges at the terminal Ti atoms (Ti5c on TiO2(110) and Ti4c on anatase TiO2(001)-(1 × 4) surfaces) and at VO sites to undergo photodesorption and photodissociation, respectively.17,18,21,31 Methanol undergoes the photogenerated hole-mediated oxidation reaction via the methoxy species at Ti5c sites on various TiO2 surfaces,22–24,26,28 as well as Ti4c sites on anatase TiO2(001)-(1 × 4) surfaces.27 Meanwhile, methoxy species at the Ti5c site on the reconstructed rutile TiO2(001)-(1 × 1) surface also exhibited photoreduction reactivity.29 Edge sites are the most common and abundant intrinsic defect on powder catalysts.32–36 Step edges were found as charge-trapping centers with increased reactivity towards O2 adsorption on the anatase TiO2(101) surface32 and as active sites for water and alcohol dissociation on the rutile TiO2(110) surface.34–36 Herein, using the rutile TiO2(110) surface with well-defined defects including surface bridging oxygen vacancies, bulk interstitial Ti3+ sites, and TiO2 islands, we successfully identify the edge sites of TiO2 islands as a novel type of photocatalytic active sites, suggesting that the widely-existing edge sites are the probable photocatalytic active sites on powder TiO2 photocatalysts.Results and discussion
The rutile TiO2(110) surface is well-known to exhibit a variety of reconstructions and defect structures depending on the sample treatments, as illustrated in Fig. 1.16,37 Under ultra-high vacuum (UHV) conditions, VO defects are easily generated on the TiO2(110) surface during sputtering and annealing, which is often accompanied by the formation of Tiint defects in the subsurface or bulk.9,13,16 Upon subsequent oxidation treatments, Tiint species migrate to the surface and react with oxygen to form TiO2 islands.38–41 Sputtering and annealing in UHV at 600–800 K produce TiO2(110)-(1 × 1) terraces with step bunches, while annealing at 800–900 K forms TiO2(110)-(1 × 1) flat terraces with single steps. Annealing at 900–1100 K leads to the formation of double-ridge structures on the terraces, arising from the partially reduced oxide.42 Further annealing at 1150 K reconstructs the original TiO2(110)-(1 × 1) surface into a TiO2(110)-(1 × 2) structure.42,43 Additionally, flat (1 × 1)-terminated TiO2(110) surfaces, when annealed in oxygen at 470–660 K, develop irregular networks of rosettes, Ti2O3 strands, and small (1 × 1) islands. These rough surfaces can be flattened into regular (1×1) terraces by annealing above 830 K, either in oxygen or under UHV conditions.44–47 | ||
| Fig. 1 Schematic illustration of defect structures of the TiO2(110)-(1 × 1) surface with various sample treatments. | ||
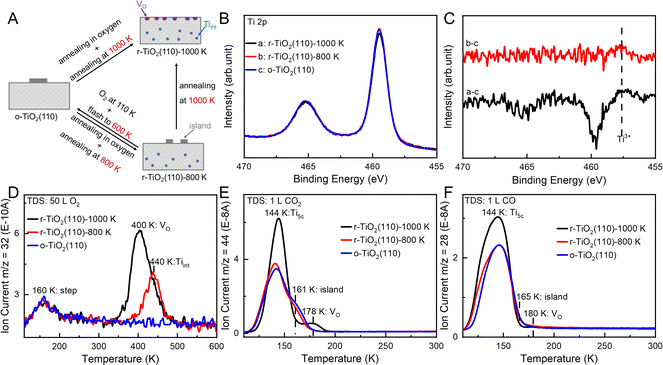
A new TiO2(110) single crystal was initially cleaned by argon-ion bombardment, resulting in argon embedded in the sample.48 Repeated cycles of annealing in oxygen and vacuum annealing effectively removed the residual argon (Fig. S1). The cleaned sample was then used for the subsequent experiments. Three different TiO2(110) surfaces with various types of defects were prepared through different treatments, as illustrated in Fig. 2A. The clean TiO2(110) surface was first annealed in oxygen at 700 K for 30 min, followed by vacuum annealing at either 800 K or 1000 K for 10 min. These surfaces are referred to r-TiO2(110)-800 K and r-TiO2(110)-1000 K, respectively. The r-TiO2(110)-800 K surface was fully oxidized by exposure to 50 L of O2 at 110 K, followed by a flash to 600 K, and is denoted as o-TiO2(110). This process can be referred to as O2 oxidation treatment. Fig. 2B and C show the X-ray photoelectron spectroscopy (XPS) of Ti 2p spectra for these three surfaces and the corresponding Ti 2p difference spectra. The characteristic Ti3+ 2p3/2 feature at 457.7 eV verifies the existence of defects on both r-TiO2(110)-800 K and r-TiO2(110)-1000 K surfaces (Fig. S2). Additionally, Ti3+ species were regenerated at temperatures above 600 K, as indicated by the Ti 2p XPS spectra during O2 exposure and annealing (Fig. S3).
Adsorption of O2, CO2, and CO molecules at 110 K was studied to probe the structures of various r-TiO2(110) surfaces. In the O2 temperature-programmed desorption (TPD) spectra (Fig. 2D), a major desorption peak at 400 K with a shoulder at 440 K and a minor desorption peak at 160 K appeared for r-TiO2(110)-1000 K, two desorption peaks at 440 and 160 K for r-TiO2(110)-800 K, and only one desorption peak at 160 K for o-TiO2(110). The high-temperature O2 desorption peaks were previously attributed to molecularly adsorbed O2 species at Vo49–51 or Tiint sites.38–40 We carried out repeated O2-TPD experiments on r-TiO2(110)-1000 K (Fig. 3A), during which the r-TiO2(110)-1000 K surface gradually converted to the r-TiO2(110)-800 K surface via the reaction of O2 with Tiint to form TiO2 islands.38–40 It can be seen that the O2 desorption peak at 440 K grew at the expense of that at 400 K while the O2 desorption peak at 160 K barely changed. Thus, the O2 desorption peaks at 400 and 440 K arise from molecularly adsorbed O2 species at VO and Tiint sites, respectively. Previous studies proposed that O2 chemisorbed at Ti5c sites near Tiint species in the near-surface region on reduced TiO2(110) surfaces18 or at step sites31,32 gave low-temperature desorption peaks. As discussed below, the O2 desorption peaks at 160 K observed herein arise from molecularly adsorbed O2 species at step sites on the surface.
In the CO2 TPD spectra (Fig. 2E and S4), at 110 K (black line in Fig. 2E), a major desorption peak at 144 K and another tiny one at 178 K appeared for r-TiO2(110)-1000 K, corresponding to CO2 adsorbed at Ti5c and VO sites,52–54 respectively. For both r-TiO2(110)-800 K and o-TiO2(110), the major desorption peak of CO2 adsorbed at Ti5c sites remained but the desorption peak of CO2 adsorbed at VO sites disappeared, meanwhile, a new shoulder desorption peak at 161 K emerged. Fig. 3B shows the TPD spectra following CO2 adsorption on the r-TiO2(110)-1000 K surface subjected to repeated O2 TPD experiments. As the r-TiO2(110)-1000 K surface gradually converted to the r-TiO2(110)-800 K surface, both CO2 desorption peaks at 144 and 178 K attenuated, and the peak at 178 K eventually disappeared, while the shoulder feature at 161 K emerged and grew and thus could be assigned to CO2 chemisorbed at the edge sites of TiO2 islands.
In the CO TPD spectra (Fig. 2F and S5), a major broad desorption peak at 144 K and another tiny one at 180 K appeared for r-TiO2(110)-1000 K, corresponding to CO adsorbed at Ti5c and VO sites,55 respectively. Similar to the case of CO2 adsorption, the desorption feature of CO at Ti5c sites weakened for r-TiO2(110)-800 K and o-TiO2(110) and that at VO sites disappeared, while a new shoulder feature emerged, corresponding to CO chemisorbed at the edge sites of TiO2 islands.
Thus, as probed by O2, CO2, and CO adsorption, the defects primarily are VO and Tiint on the r-TiO2(110)-1000 K surface, Tiint and TiO2 islands on the r-TiO2(110)-800 K surface, and TiO2 islands on the o-TiO2(110) surface. It is worth noting that the O2 and CO2 TPD spectra barely changed with repeated exposures of 50 L O2 on the r-TiO2(110)-800 K surface (Fig. S6), indicating that the Tiint defects on the r-TiO2(110)-800 K surface cannot be oxidized by O2 adsorption at 110 K followed by heating to a temperature higher than 600 K but they can by O2 oxidation treatment. Meanwhile, both CO2 and CO desorption peaks at Ti5c sites on the r-TiO2(110)-800 K and o-TiO2(110) surfaces are smaller than those on the r-TiO2(110)-1000 K surface, indicating that the Ti5c density on TiO2 islands should be less than on the underlying r-TiO2(110) surface. Probed by O2 adsorption (Fig. S7), both r-TiO2(110)-1000 K and r-TiO2(110)-800 K surfaces can be reproducibly prepared, and so can the o-TiO2(110) surface.
Photochemistry of O2, CO2, and CO molecules chemisorbed on various r-TiO2(110) surfaces was then examined. As shown in Fig. 4A, upon 20 min UV light illumination, the desorption feature of molecular O2 absorbed at VO or Tiint sites on r-TiO2(110)-1000 K and r-TiO2(110)-800 K significantly weakened, suggesting the occurrence of photoinduced O2(a) dissociation or desorption, in line with previous findings,18–21,56–64 however, the minor desorption feature at 160 K remained unaffected. The O2 adsorbed at Ti5c sites near Tiint defects in the near-surface region on the reduced TiO2(110) surfaces was reported to be photoactive,18 thus the low-temperature desorption feature at 160 K probably originates from O2 chemisorbed at step sites.
As shown in Fig. 4B and C and S8 and S9, UV light illumination barely changed the CO2 and CO TPD profiles from the o-TiO2(110) surface, indicating that CO2 and CO chemisorbed at Ti5c sites and edge sites of TiO2 islands on the oxidized TiO2(110) surface are photoinactive. However, weakening of desorption peaks of CO2 and CO chemisorbed at Ti5c sites on r-TiO2(110)-1000 K and r-TiO2(110)-800 K were clearly observed upon UV light illumination while other desorption features remained the same. Since CO2 and CO chemisorbed at Ti5c sites are photoinactive, the observed apparent decrease of their low-temperature desorption peaks is analogous to the photo-induced desorption of CO reported previously.17,19,20 The “low-temperature depletion” phenomenon describes the process wherein strongly chemisorbed CO2 and CO species undergo photo-induced desorption under UV light illumination, with the resulting vacant sites subsequently occupied by weakly-chemisorbed CO2 and CO species via surface diffusion. Such a “low-temperature depletion” phenomenon leads to the unchanged desorption structures of strongly-chemisorbed CO2 and CO species and decreased desorption peaks of weakly-chemisorbed CO2 and CO species in the TPD spectra following UV light illumination. Therefore, CO2 and CO chemisorbed at VO sites and edge sites of TiO2 islands with Tiint defects are photoactive. As shown in Fig. 5, CO2 chemisorbed at the edge sites of TiO2 islands with Tiint defects and CO at VO sites exhibit the highest photo-induced desorption probability, meanwhile, CO2 and CO chemisorbed at the edge sites of TiO2 islands with Tiint defects show similar photo-induced desorption probabilities. Herein, the photodesorption probability incorporates multiple factors, including direct photodesorption and thermal/photostimulated diffusion. These observations demonstrate site-specific photocatalysis.65–68
The above results have important implications for powder TiO2 photocatalysis. On one hand, TiO2 powder photocatalyst generally exhibits edge sites, VO and Tiint defects, among which the edge site is significantly more abundant than the VO and Tiint defects, therefore, the edge site is probably the dominant photocatalytic active site on the TiO2 powder photocatalyst. On the other hand, the edge site of the TiO2 powder photocatalyst generally exhibits stronger adsorption ability than the terrace site, and the photoinactive adsorbed species on the terrace site can contribute to the photocatalytic reaction by surface migration to the vacant photocatalytic active edge site. Notably, while our study with single-crystal model catalysts unambiguously demonstrates the edge sites as one type of photocatalytic active sites, real powder TiO2 photocatalysts expose various types of crystal facets and defects whose photocatalytic activity need to be studied in order to comprehensively understand the apparent photocatalytic performance.
Conclusions
In summary, we have successfully used surface chemistry and photochemistry of O2, CO2, and CO to probe the photocatalytic activity of the oxidized TiO2(110) surface with TiO2 islands, TiO2(110) surface with surface bridging oxygen vacancies and bulk interstitial Ti3+ defects, and TiO2(110) surface with TiO2 islands and interstitial Ti3+ defects. CO2 and CO chemisorbed at the Ti5c site are photoinactive, while CO2 and CO chemisorbed at the surface bridging the oxygen vacancy site and edge site of TiO2 islands with bulk interstitial Ti3+ defects are photoactive. These results for the first time unambiguously identify the abundant edge sites on TiO2 powder photocatalysts as the photocatalytic active site, which greatly deepens the fundamental understanding of TiO2 photocatalysis and provides executable guide for the structural design of efficient TiO2 photocatalysts.Author contributions
L. W. performed the experiments; Z. W., Z. W., H. X., P. C., and J. S. assisted the experiments; L. W., Z. W., and W. H. wrote and revised the manuscript. W. H. and Z. W. acquired the funding. W. H. supervised all aspects of the work. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cy01201e.Acknowledgements
We appreciate the support from the National Key R&D Program of the Ministry of Science and Technology of China (2021YFA1502804), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0450102), the National Natural Science Foundation of China (22250710677, 52261135636, 22202191, 22472160), the Changjiang Scholars Program of the Ministry of Education of China, and the USTC Research Funds of the Double First-Class Initiative (YD9990002026). This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China.Notes and references
- Y. Wang, A. Vogel, M. Sachs, R. S. Sprick, L. Wilbraham, S. J. A. Moniz, R. Godin, M. A. Zwijnenburg, J. R. Durrant, A. I. Cooper and J. Tang, Nat. Energy, 2019, 4, 746–760 CrossRef CAS.
- X. Lang, X. Chen and J. Zhao, Chem. Soc. Rev., 2013, 43, 473–486 RSC.
- Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han and C. Li, Chem. Rev., 2014, 114, 9987–10043 CrossRef CAS PubMed.
- X. Li, C. Wang and J. Tang, Nat. Rev. Mater., 2022, 7, 617–632 CrossRef CAS.
- G. Liao, G. Ding, B. Yang and C. Li, Precis. Chem., 2024, 2, 49–56 CrossRef CAS.
- Y. Wu, M. Wu, Y. Sun and Y. Xie, Sci. China:Chem., 2024, 67, 2434–2447 CrossRef CAS.
- P. Babu, H. Park and J. Y. Park, Surfactant Sci. Technol., 2023, 1, 29 CrossRef.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- U. Diebold, Surf. Sci. Rep., 2003, 48, 53–229 CrossRef CAS.
- M. A. Henderson and I. Lyubinetsky, Chem. Rev., 2013, 113, 4428–4455 CrossRef CAS PubMed.
- C. L. Pang, R. Lindsay and G. Thornton, Chem. Rev., 2013, 113, 3887–3948 CrossRef CAS.
- Q. Guo, C. Zhou, Z. Ma, Z. Ren, H. Fan and X. Yang, Chem. Soc. Rev., 2016, 45, 3701–3730 RSC.
- W.-J. Yin, B. Wen, C. Zhou, A. Selloni and L.-M. Liu, Surf. Sci. Rep., 2018, 73, 58–82 CrossRef CAS.
- S. Chen, F. Xiong and W. Huang, Surf. Sci. Rep., 2019, 74, 100471 CrossRef CAS.
- C. A. Walenta, M. Tschurl and U. Heiz, J. Phys.:Condens. Matter, 2019, 31, 473002 CrossRef CAS.
- L. Wu, C. Fu and W. Huang, Phys. Chem. Chem. Phys., 2020, 22, 9875 RSC.
- L. Wu, Z. Wang, F. Xiong, G. Sun, P. Chai, Z. Zhang, H. Xu, C. Fu and W. Huang, J. Chem. Phys., 2020, 152, 044702 CrossRef CAS.
- F. Xiong, L.-L. Yin, F. Li, Z. Wu, Z. Wang, G. Sun, H. Xu, P. Chai, X.-Q. Gong and W. Huang, J. Phys. Chem. C, 2019, 123, 24558–24565 CrossRef CAS.
- R. Mu, A. Dahal, Z.-T. Wang, Z. Dohnálek, G. A. Kimmel, N. G. Petrik and I. Lyubinetsky, J. Phys. Chem. Lett., 2017, 8, 4565–4572 CrossRef CAS.
- N. G. Petrik, R. Mu, A. Dahal, Z. Wang, I. Lyubinetsky and G. A. Kimmel, J. Phys. Chem. C, 2018, 122, 15382–15389 CrossRef CAS.
- Z.-T. Wang, N. A. Deskins and I. Lyubinetsky, J. Phys. Chem. Lett., 2012, 3, 102–106 CrossRef CAS PubMed.
- K. R. Phillips, S. C. Jensen, M. Baron, S.-C. Li and C. M. Friend, J. Am. Chem. Soc., 2013, 135, 574–577 CrossRef CAS PubMed.
- Q. Guo, C. Xu, W. Yang, Z. Ren, Z. Ma, D. Dai, T. K. Minton and X. Yang, J. Phys. Chem. C, 2013, 117, 5293–5300 CrossRef CAS.
- Q. Yuan, Z. Wu, Y. Jin, L. Xu, F. Xiong, Y. Ma and W. Huang, J. Am. Chem. Soc., 2013, 135, 5212–5219 CrossRef CAS PubMed.
- C. Xu, W. Yang, Q. Guo, D. Dai, M. Chen and X. Yang, J. Am. Chem. Soc., 2014, 136, 602–605 CrossRef CAS PubMed.
- Z. Wang, Q. Yuan, Z. Zhang, F. Xiong, G. Sun, H. Xu, P. Chai and W. Huang, J. Phys. Chem. C, 2019, 123, 31073–31081 CrossRef CAS.
- F. Xiong, L.-L. Yin, Z. Wang, Y. Jin, G. Sun, X.-Q. Gong and W. Huang, J. Phys. Chem. C, 2017, 121, 9991–9999 CrossRef CAS.
- L. Wu, Z. Wu, Z. Wang, H. Xu, P. Chai and W. Huang, J. Phys. Chem. Lett., 2022, 13, 2614–2618 CrossRef CAS.
- L. Wu, Z. Wu, F. Li, Z. Wang, Z. Zhang, X.-Q. Gong and W. Huang, J. Phys. Chem. Lett., 2024, 15, 8481–8486 CrossRef CAS.
- W. Wang, L. Wu, C. Fu, H. Xu, Z. Wu and W. Huang, Chin. J. Chem. Phys., 2024, 37, 614–618 CrossRef CAS.
- M. Setvin, J. Hulva, G. S. Parkinson, M. Schmid and U. Diebold, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E2556 CrossRef CAS.
- M. Setvin, X. Hao, B. Daniel, J. Pavelec, Z. Novotny, G. S. Parkinson, M. Schmid, G. Kresse, C. Franchini and U. Diebold, Angew. Chem., Int. Ed., 2014, 53, 4714–4716 CrossRef CAS PubMed.
- X.-Q. Gong, A. Selloni, M. Batzill and U. Diebold, Nat. Mater., 2006, 5, 665–670 CrossRef CAS PubMed.
- U. Martinez, L. B. Vilhelmsen, H. H. Kristoffersen, J. Stausholm-Møller and B. Hammer, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 205434 CrossRef.
- U. Martinez, J. Ø. Hansen, E. Lira, H. H. Kristoffersen, P. Huo, R. Bechstein, E. Lægsgaard, F. Besenbacher, B. Hammer and S. Wendt, Phys. Rev. Lett., 2012, 109, 155501 CrossRef CAS PubMed.
- H. H. Kristoffersen, J. Ø. Hansen, U. Martinez, Y. Y. Wei, J. Matthiesen, R. Streber, R. Bechstein, E. Lægsgaard, F. Besenbacher, B. Hammer and S. Wendt, Phys. Rev. Lett., 2013, 110, 146101 CrossRef CAS.
- F. Polo-Garzon, Z. Bao, X. Zhang, W. Huang and Z. Wu, ACS Catal., 2019, 9, 5692–5707 CrossRef CAS.
- S. Wendt, P. T. Sprunger, E. Lira, G. K. H. Madsen, Z. Li, J. Ø. Hansen, J. Matthiesen, A. Blekinge-Rasmussen, E. Lægsgaard, B. Hammer and F. Besenbacher, Science, 2008, 320, 1755 CrossRef CAS.
- E. Lira, S. Wendt, P. Huo, J. O. Hansen, R. Streber, S. Porsgaard, Y. Wei, R. Bechstein, E. Laegsgaard and F. Besenbacher, J. Am. Chem. Soc., 2011, 133, 6529–6532 CrossRef CAS.
- E. Lira, J. Ø. Hansen, P. Huo, R. Bechstein, P. Galliker, E. Lægsgaard, B. Hammer, S. Wendt and F. Besenbacher, Surf. Sci., 2010, 604, 1945–1960 CrossRef CAS.
- Z. Zhang, J. Lee, J. T. Yates, R. Bechstein, E. Lira, J. Ø. Hansen, S. Wendt and F. Besenbacher, J. Phys. Chem. C, 2010, 114, 3059–3062 CrossRef CAS.
- H. Onishi and Y. Iwasawa, Surf. Sci., 1994, 313, L783–L789 CrossRef CAS.
- M. Blanco-Rey, J. Abad, C. Rogero, J. Mendez, M. F. Lopez, J. A. Martin-Gago and P. L. de Andres, Phys. Rev. Lett., 2006, 96, 055502 CrossRef CAS.
- M. Li, W. Hebenstreit and U. Diebold, Surf. Sci., 1998, 414, L951–L956 CrossRef CAS.
- M. Li, W. Hebenstreit, U. Diebold, M. A. Henderson and D. R. Jennison, Faraday Discuss., 1999, 114, 245–258 RSC.
- M. Li, W. Hebenstreit, L. Gross, U. Diebold, M. A. Henderson, D. R. Jennison, P. A. Schultz and M. P. Sears, Surf. Sci., 1999, 437, 173–190 CrossRef CAS.
- M. Li, W. Hebenstreit and U. Diebold, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 4926–4933 CrossRef CAS.
- L. Mohrhusen, J. Kräuter, M. Willms and K. Al-Shamery, J. Phys. Chem. C, 2019, 123, 20434–20442 CrossRef CAS.
- S. Tan, Y. Ji, Y. Zhao, A. Zhao, B. Wang, J. Yang and J. G. Hou, J. Am. Chem. Soc., 2011, 133, 2002–2009 CrossRef CAS.
- M. A. Henderson, W. S. Epling, C. L. Perkins, C. H. F. Peden and U. Diebold, J. Phys. Chem. B, 1999, 103, 5328–5337 CrossRef CAS.
- G. A. Kimmel and N. G. Petrik, Phys. Rev. Lett., 2008, 100, 196102 CrossRef.
- T. L. Thompson, O. Diwald and J. T. Yates, J. Phys. Chem. B, 2003, 107, 11700–11704 CrossRef CAS.
- X. Lin, Y. Yoon, N. G. Petrik, Z. Li, Z.-T. Wang, V.-A. Glezakou, B. D. Kay, I. Lyubinetsky, G. A. Kimmel, R. Rousseau and Z. Dohnálek, J. Phys. Chem. C, 2012, 116, 26322–26334 CrossRef CAS.
- Z. Wu, H. Wang, J. Shi, Z. Li and W. Huang, J. Phys. Chem. C, 2024, 128, 21702–21709 CrossRef CAS.
- M. Kunat, F. Traeger, D. Silber, H. Qiu, Y. Wang, A. C. van Veen, C. Wöll, P. M. Kowalski, B. Meyer, C. Hättig and D. Marx, J. Chem. Phys., 2009, 130, 144703 CrossRef CAS.
- I. Sokolović, M. Reticcioli, M. Čalkovský, M. Wagner, M. Schmid, C. Franchini, U. Diebold and M. Setvín, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 14827–14837 CrossRef PubMed.
- G. Lu, A. Linsebigler and J. T. Yates, J. Chem. Phys., 1995, 102, 4657–4662 CrossRef CAS.
- C. N. Rusu and J. T. Yates, Langmuir, 1997, 13, 4311–4316 CrossRef CAS.
- C. L. Perkins and M. A. Henderson, J. Phys. Chem. B, 2001, 105, 3856–3863 CrossRef CAS.
- T. L. Thompson and J. T. Yates, J. Phys. Chem. B, 2006, 110, 7431–7435 CrossRef CAS.
- D. Sporleder, D. P. Wilson and M. G. White, J. Phys. Chem. C, 2009, 113, 13180–13191 CrossRef CAS.
- N. G. Petrik and G. A. Kimmel, J. Phys. Chem. Lett., 2010, 1, 1758–1762 CrossRef CAS.
- Z. Zhang and J. T. Yates, J. Phys. Chem. C, 2010, 114, 3098–3101 CrossRef CAS.
- N. G. Petrik and G. A. Kimmel, J. Phys. Chem. Lett., 2011, 2, 2790–2796 CrossRef CAS.
- C. Fu, F. Li, J. Zhang, D. Li, K. Qian, Y. Liu, J. Tang, F. Fan, Q. Zhang, X.-Q. Gong and W. Huang, Angew. Chem., Int. Ed., 2021, 60, 6160–6169 CrossRef CAS PubMed.
- C. Fu, F. Li, J. Yang, J. Xie, Y. Zhang, X. Sun, X. Zheng, Y. Liu, J. Zhu, J. Tang, X.-Q. Gong and W. Huang, ACS Catal., 2022, 12, 6457–6463 CrossRef CAS.
- X. Sun, X. Chen, C. Fu, Q. Yu, X.-S. Zheng, F. Fang, Y. Liu, J. Zhu, W. Zhang and W. Huang, Nat. Commun., 2022, 13, 6677 CrossRef CAS PubMed.
- C. Fu, F. Li, Z. Wu, F. Xiong, J. Zhu, X.-Q. Gong and W. Huang, J. Phys. Chem. Lett., 2023, 14, 8916–8921 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |