Promoting low-temperature oxidative dehydrogenation of propane through oxide–support interaction regulation
Wenjie
Li
 a,
Hong
Wang
e,
Zhandong
Wang
ae,
Heng
Cao
a,
Hong
Wang
e,
Zhandong
Wang
ae,
Heng
Cao
 *a and
Jun
Bao
*a and
Jun
Bao
 *abcd
*abcd
aNational Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, Anhui 230026, China. E-mail: hengcao@ustc.edu.cn; baoj@ustc.edu.cn
bState Key Laboratory of Precision and Intelligent Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, China
ciChEM (Collaborative Innovation Center of Chemistry for Energy Materials), University of Science and Technology of China, Hefei, Anhui 230026, China
dAnhui Industrial Innovation Research Institute of Advanced Optoelectronic Materials and Systems, Hefei, Anhui 230031, China
eState Key Laboratory of Fire Science, University of Science and Technology of China, Hefei, Anhui 230026, China
First published on 10th November 2025
Abstract
Oxide–support interaction (OSI) plays a significant role in governing the catalytic performance of metal oxides and their supports. However, the influence of OSI strength on activity and selectivity remains poorly understood. Here, we achieved a tunable OSI strength in a NiO/SiO2 catalyst, thereby tailoring the catalytic performance of low-temperature oxidative dehydrogenation of propane (ODHP). A moderate OSI establishes a delicate balance between geometric and electronic effects, enabling the exposure of highly selective active sites and promoting efficient propane activation. As a result, the NiO/SiO2 catalyst with moderate OSI exhibited a C3H6 formation rate of 112 molC3H6 molNi−1 h−1 with a selectivity of 64% at 280 °C. Mechanistic insights from in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and in situ synchrotron vacuum ultraviolet photoionization mass spectrometry (SVUV-PIMS) revealed that a moderate OSI effectively suppresses undesired side reactions of direct C3H8 over-oxidation and C3H6 secondary oxidation.
1 Introduction
Propene (C3H6), a major platform chemical, is increasingly produced through propane (C3H8) dehydrogenation.1 This trend is driven by the large-scale exploitation of shale gas, which has greatly increased C3H8 supply.2 Among various propane-to-propene technologies, ODHP stands out as a promising alternative to the conventional non-oxidative dehydrogenation route.3 The introduction of molecular oxygen (O2) not only overcomes thermodynamic limitations but also turns the reaction from highly endothermic to mildly exothermic, significantly reducing energy requirements. Furthermore, the presence of O2 suppresses coke formation, thereby enhancing catalyst stability and enabling long-term operation.4NiO-based catalysts exhibit remarkable activity for low-temperature ODHP, but their strong oxidation propensity often results in undesired deep oxidation.5 To improve C3H6 selectivity, most research efforts have focused on incorporating “dopants” such as Nb and Zr to form bimetallic oxide solid solutions.6 However, in the absence of a support that provides effective anchoring and dispersion, this approach offers limited control over nanoparticle size, thereby restricting the exposure of surface active sites.
A more flexible alternative strategy is to manipulate the interaction between active components and support, which has proven highly effective in heterogeneous catalysis.7–10 Such an interaction typically influences catalytic performance through synergistic geometric and electronic effects.11 Geometrically, it stabilizes small nanoparticles and promotes the exposure of low-coordination sites, whose distinct local structure strongly influences adsorption and activation of reactants. Electronically, charge transfer across the interface modifies the electronic states of the active sites, which in turn affects their orbital hybridization and charge transfer efficiency with reactants. These changes ultimately govern the breaking and making of chemical bonds, thus dictating the reaction activity and selectivity.
Notably, the strength of the interaction strongly depends on the nature of the support.12 Irreducible oxides such as SiO2, due to their strong oxygen affinity, can form particularly strong interactions with oxides like NiO.13 In this work, we systematically regulated the OSI strength of the NiO/SiO2 catalysts by tuning the interfacial bonding modes between the nickel precursor and the silica support. NiO/SiO2 with a moderate OSI exhibited optimal ODHP performance, which was attributed to balanced geometric and electronic effects. At this circumstance, NiO nanoparticles (NPs) expose low-coordination sites, promoting C3H8 activation while suppressing direct C3H8 over-oxidation. When the OSI strength is further increased, excessive electron transfer from NiO to SiO2 hinders C3H8 activation while simultaneously strengthening C3H6 adsorption, which readily leads to undesired secondary oxidation. These findings provide mechanistic insight into the role of OSI strength in governing ODHP performance, thus affording guidelines for designing efficient catalysts for low-temperature ODHP.
2 Experimental section
2.1 Chemical reagents
The silica (SiO2, surface area = 300 m2 g−1, S861488) support was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. Nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O), citric acid monohydrate (C6H8O7·H2O), and ammonia solution (25–28 wt%) were purchased from Sinopharm Chemical Reagent Co., Ltd in Shanghai. All of the above chemicals were analytically pure and used directly during the preparation process of the samples. All aqueous solutions were prepared using deionized (DI) water with a resistivity of 18.25 MΩ cm−1.2.2 Preparation of catalysts
Preparation of NiO/SiO2-WI by wet impregnation (WI): 793 mg of Ni(NO3)2·6H2O was dissolved in 10 mL of DI water to form a uniform solution by stirring. Meanwhile, 1 g of SiO2 was dispersed in 50 mL of DI water and stirred until uniform. The nickel solution was slowly dripped into the SiO2 suspension, and after mixing, the solvent was evaporated in an 80 °C oil bath. The obtained precursor was dried in an oven at 110 °C for 12 h, then calcined in a muffle furnace at a heating rate of 5 °C min−1 to 400 °C for 4 h, and subsequently cooled naturally to room temperature. The loadings of Ni on SiO2 were 13.3 wt%, determined by inductively coupled plasma–atomic emission spectrometry (ICP-AES) measurements.Preparation of NiO/SiO2-CA by citrate-assisted (CA) impregnation: the preparation of this catalyst followed the same procedure as that of NiO/SiO2-WI, except that 574 mg of C6H8O7·H2O was added to the nickel solution. The loading of Ni on SiO2 was 13.7 wt%, determined by ICP-AES measurements.
Preparation of NiO/SiO2-SEA by strong electrostatic adsorption (SEA): 500 mg of SiO2 was dispersed in 50 mL of DI water at room temperature. The pH of the solution was adjusted to around 11 by adding 1 mL of ammonium solution (25–28 wt%). The solution was stirred for 10 min to ensure a negatively charged SiO2 surface. Subsequently, 400 mg of Ni(NO3)2·6H2O was dissolved in 1 mL of DI water. Vacuum filtration was used to collect samples, which were dried at 70 °C for 12 h, followed by calcination in a muffle furnace at a heating rate of 5 °C min−1 to 400 °C for 4 h. The loading of Ni on SiO2 was 13.5 wt%, determined by ICP-AES measurements.
2.3 Characterization, experimental procedures, and analytical methods
Detailed characterization methods, experimental procedures, and analytical methods can be found in the SI.3 Results and discussion
3.1 Regulation OSI in NiO/SiO2
In supported catalysts, the precursor–support bonding mode determines the interaction strength, thereby governing precursor mobility and aggregation during calcination and ultimately influencing dispersion and particle size.14 Conventional wet impregnation relies on weak van der Waals force bonding between the precursor and support, providing limited anchoring. The citrate-assisted strategy enhances this interaction by employing citric acid as a “molecular bridge” that forms stronger coordination bonds with both the precursor and support.15 In contrast, the strong electrostatic adsorption approach strengthens the interaction further through directed electrostatic forces between the precursor and specific surface sites, enabling precise atomic-scale separation.16 Accordingly, the NiO/SiO2 catalysts prepared by these three methods (denoted as NiO/SiO2-WI, NiO/SiO2-CA, and NiO/SiO2-SEA) were designed to exhibit progressively stronger OSI as illustrated in Fig. 1a.Since the NiO/SiO2 catalysts prepared by these three methods contained a similar Ni loading (∼13.5 wt%) as measured by ICP-AES, a stronger OSI generally enhances the dispersion of NiO NPs, which in turn results in smaller particle sizes.17 X-ray diffraction (XRD) patterns exhibited broader NiO diffraction peaks for NiO/SiO2-CA than those for NiO/SiO2-WI, while these characteristic NiO peaks were absent in the pattern for NiO/SiO2-SEA, revealing a marked decreasing trend in the crystallite size of NiO NPs (Fig. S1). Transmission electron microscopy (TEM) and energy dispersive X-ray (EDX) elemental mapping images demonstrated that NiO NPs of NiO/SiO2-CA and NiO/SiO2-SEA were dispersed homogeneously on the entire SiO2, while severe aggregation occurred in NiO/SiO2-WI (Fig. S2 and S3). The corresponding size distribution histogram showed that the mean sizes of NiO NPs are 3.6 nm for NiO/SiO2-CA and 2.3 nm for NiO/SiO2-SEA, both featuring narrow size distributions (Fig. S2). In contrast, NiO/SiO2-WI exhibited a much larger TEM-measured mean size of 94.7 nm along with a broad distribution, significantly larger than the XRD-estimated value (20.7 nm), indicating agglomeration of primary NPs (Fig. S4).
A smaller contact angle at the interface between active component and support signifies a stronger interfacial interaction.18,19 High-resolution transmission electron microscopy (HRTEM) images showed that in NiO/SiO2-WI, NiO NPs partially detach from the SiO2 surface, indicating a weak OSI (Fig. 1b and S5). NiO/SiO2-CA and NiO/SiO2-SEA displayed mean contact angles of 58° and 43°, respectively (Fig. 1c, d and S6–S8). NiO NPs on NiO/SiO2-SEA exhibited the smallest contact angle and particle size, confirming that NiO/SiO2 prepared by the SEA method exhibited the strongest OSI. Furthermore, the main peak in the H2 temperature-programmed reduction (H2-TPR) profiles shifted progressively to higher temperatures, appearing at 363 °C for NiO/SiO2-WI, 513 °C for NiO/SiO2-CA, and 563 °C for NiO/SiO2-SEA (Fig. S9). These results clearly demonstrate that the OSI strength follows the trend: NiO/SiO2-WI < NiO/SiO2-CA < NiO/SiO2-SEA.
3.2 Physicochemical properties of NiO/SiO2 with different OSI strengths
The X-ray photoelectron spectroscopy (XPS) results for Ni 2p showed clear differences in the electronic structure of the NiO/SiO2 catalysts (Fig. 2a and S10). Specifically, the Ni 2p3/2 spectra of NiO/SiO2-WI exhibited characteristic multiplet splitting with peaks at 853.6 and 855.5 eV, consistent with bulk NiO.20 In contrast, NiO/SiO2-SEA showed only a broad peak centered at 856.1 eV, with features close to NiSiO3 but 0.6 eV lower in binding energy, which can be attributed to the formation of abundant NiO–SiO2 interfaces.21 Notably, NiO/SiO2-CA with a moderate OSI exhibited an intermediate electronic state, with spectral features lying between those of the other two catalysts. The O 1s and Si 1s binding energies associated with the Si–O bonds in the SiO2 support were 0.3 eV and 0.4 eV lower in NiO/SiO2-SEA than in NiO/SiO2-WI, respectively, while NiO/SiO2-CA showed intermediate values (Fig. 2b). These results indicate that a stronger OSI promotes more pronounced electron transfer from NiO NPs to SiO2 support. | ||
| Fig. 2 Electronic and coordination structure characterization. Ni 2p (a), O 1s and Si 1s (b) XPS results of the NiO/SiO2 catalysts. a.u., arbitrary units. To ensure clear visibility, the XPS results in Fig. 2a and b were appropriately adjusted, and the spectra were offset vertically. (c) sXAS spectra at the O K-edge of the NiO/SiO2 catalysts. XANES (d) and EXAFS (e) spectra at the Ni K-edge of the NiO/SiO2 catalysts. The red squares indicate the stepwise magnifications of the local sites. (f) Raman spectra of the NiO/SiO2 catalysts and SiO2 support. (g–i) WT for the k3-weighted EXAFS signal of the Ni K-edge of NiO/SiO2-WI (g), NiO/SiO2-CA (h) and NiO/SiO2-SEA (i). | ||
To obtain detailed electronic structure information at the surface, we performed soft X-ray absorption spectroscopy (sXAS) of the NiO/SiO2 catalysts with the sample stage rotated at 50° relative to the incident beam. As shown in Fig. 2c, the O K-edge X-ray absorption spectra exhibited features from both NiO NPs and SiO2 support. Specifically, the spectra of NiO/SiO2-CA and NiO/SiO2-WI exhibited NiO-like features, while NiO/SiO2-SEA possessed a SiO2-like feature. Peaks A, B, and C correspond to the Ni–O hybridization in NiO6 octahedra, while peaks D and E correspond to Si–O hybridization.22–25 The gradual weakening of the Ni–O hybridization peak, partial in NiO/SiO2-CA and complete in NiO/SiO2-SEA, indicated that stronger OSI promotes the NiO NPs transition from local structural disorder to overall disorder, aligning with the O 1s XPS results (Fig. S11).26 Since sXAS acquired in total electron yield mode reflects the density of states of orbitals involved in electron transition, electron transfer from NiO NPs to SiO2 would fill the unoccupied antibonding orbitals of Si–O, causing attenuation of the hybridization peak.27 Based on the changes in its intensity, electron transfer was slightly enhanced in NiO/SiO2-CA and strongly enhanced in NiO/SiO2-SEA compared with NiO/SiO2-WI. This trend was further supported by the extent of the positive pre-edge peak shift in the Ni K-edge X-ray absorption near-edge structure (XANES) spectra (Fig. 2d). These results demonstrate that a moderate OSI induces limited electron transfer from NiO NPs to SiO2, whereas a strong OSI drives substantial transfer, accompanied by increased local disorder in the NiO NPs.
The intensity ratio of the double-peak features in the Ni L3 region serves as a fingerprint for the oxidation states of Ni.28,29 Despite substantial electron transfer occurring in NiO/SiO2-SEA, Ni2+ remained the dominant species, indicating that the electron transfer primarily alters the coordination structure rather than the valence state of Ni (Fig. S12).30 The local coordination environment was further investigated by extended X-ray absorption fine structure (EXAFS) spectroscopy. The corresponding fitting results are provided in Fig. S13 and Table S1. As shown in Fig. 2e, the Ni–O coordination in the first shell in the NiO/SiO2 catalysts remained essentially unchanged, but the oscillation in the second shell changed significantly. Specifically, the Ni–O–Ni bond was dominant in both NiO/SiO2-WI and NiO/SiO2-CA, with coordination numbers of 12.2 and 10.7, respectively. This decrease in coordination number can be attributed to the disappearance of the top oxygen as the NiO NPs shrink below 4 nm, resulting in a structural transition from octahedral NiO6 (bulk) to pyramidal NiO5 (surface).31 For NiO/SiO2-SEA, the Ni–O–Ni bond disappeared, and a Ni–O–Si bond with a coordination number of 4.7 appeared, attributed to the close contact between NiO NPs and SiO2 support through a strong OSI. The structure of NiO/SiO2 with different OSI strengths was further investigated via Raman spectroscopy (Fig. 2f). For NiO/SiO2-WI and NiO/SiO2-CA, the peak at around 503 cm−l was assigned to the first-order longitudinal optical phonon mode of the Ni–O–Ni bond.32 For NiO/SiO2-SEA, a new peak at 671 cm−1 appeared, which could originate from the vibration of the Ni–O–Si bond. The wavelet transformed (WT) simulation images of the k3-weighted EXAFS for Ni K-edge displayed the visualized radial distance resolutions in the K space. From Fig. 2g–i, the gradual weakening of oscillation in the second shell, relative to the first shell, provided further evidence for the disorder of NiO NPs induced by the stronger OSI, consistent with the XRD results (Fig. S1). In summary, the stronger OSI drives electron transfer from NiO NPs to the SiO2 support and induces local structural disorder within the NiO NPs. Notably, a strong OSI further promotes the formation of Ni–O–Si interfacial bonds, thereby effectively modulating the electronic and coordination structures of the catalyst.
3.3 Catalytic performance towards ODHP
The catalytic performance of the NiO/SiO2 catalysts in the ODHP reaction was evaluated at temperatures between 280 and 360 °C with a gas hourly space velocity (GHSV) of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml gcat−1 h−1. As shown in Fig. 3a and b and Table S2, NiO/SiO2-CA with a moderate OSI exhibited the highest activity and C3H6 selectivity across the entire temperature range. The most notable performance difference was observed at 280 °C, where NiO/SiO2-CA achieved a C3H6 selectivity of 64%, 4.3 times and 1.6 times those of NiO/SiO2-WI and NiO/SiO2-SEA, respectively (Fig. 3c). Meanwhile, the C3H6 production rate reached 112 molC3H6 molNi−1 h−1, exceeding those of NiO/SiO2-WI and NiO/SiO2-SEA by factors of 12.4 and 6.2. Interestingly, although the activity showed similar temperature dependence across all catalysts, the C3H6 selectivity trends differed markedly. This divergence suggests that the structural change of the active sites induced by OSI strength likely altered the catalytic reaction mechanism. Furthermore, comparison with representative catalysts operating below 400 °C demonstrates the excellent low-temperature ODHP performance of NiO/SiO2-CA (Fig. 3d).
000 ml gcat−1 h−1. As shown in Fig. 3a and b and Table S2, NiO/SiO2-CA with a moderate OSI exhibited the highest activity and C3H6 selectivity across the entire temperature range. The most notable performance difference was observed at 280 °C, where NiO/SiO2-CA achieved a C3H6 selectivity of 64%, 4.3 times and 1.6 times those of NiO/SiO2-WI and NiO/SiO2-SEA, respectively (Fig. 3c). Meanwhile, the C3H6 production rate reached 112 molC3H6 molNi−1 h−1, exceeding those of NiO/SiO2-WI and NiO/SiO2-SEA by factors of 12.4 and 6.2. Interestingly, although the activity showed similar temperature dependence across all catalysts, the C3H6 selectivity trends differed markedly. This divergence suggests that the structural change of the active sites induced by OSI strength likely altered the catalytic reaction mechanism. Furthermore, comparison with representative catalysts operating below 400 °C demonstrates the excellent low-temperature ODHP performance of NiO/SiO2-CA (Fig. 3d).
 | ||
Fig. 3 Catalytic performance for the ODHP reaction. C3H8 consumption rates (a) and C3H6 selectivities (b) over the NiO/SiO2 catalysts at temperatures ranging from 280 to 360 °C with a GHSV of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml gcat−1 h−1. (c) C3H6 selectivity and C3H6 productivity rate at 280 °C with a GHSV of 12 000 ml gcat−1 h−1. (c) C3H6 selectivity and C3H6 productivity rate at 280 °C with a GHSV of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml gcat−1 h−1. (d) C3H6 selectivity plotted against C3H8 conversion for ODHP at temperatures ≤ 400 °C, comparing previously reported data from representative catalysts to NiO/SiO2-CA.6,33–40 000 ml gcat−1 h−1. (d) C3H6 selectivity plotted against C3H8 conversion for ODHP at temperatures ≤ 400 °C, comparing previously reported data from representative catalysts to NiO/SiO2-CA.6,33–40 | ||
3.4 Reaction mechanism study
As illustrated in Fig. 4a, the activation of C3H8 on metal oxides typically follows two sequential dehydrogenation pathways.41 In the hetero-carbon route, CH2C*HCH3 (* denotes the adsorption on the catalyst surface) is formed and readily desorbs as the desired product, C3H6. In contrast, the other homo-carbon pathway produces CH3C*CH3, which will rapidly react with lattice oxygen to form CH3CO*CH3. This intermediate is then prone to further oxidation, either on the surface or in the gas phase.42,43 In addition to the direct C3H8 over-oxidation (path I), C3H6 with its reactive C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is highly susceptible to secondary oxidation (path II).
C bond is highly susceptible to secondary oxidation (path II).
The interaction between C3H8 and lattice oxygen was examined using C3H8 temperature-programmed surface reaction (C3H8 TPSR) experiments. NiO/SiO2-CA exhibited the lowest initial activation temperature (410 °C), compared with NiO/SiO2-WI (442 °C) and NiO/SiO2-SEA (468 °C, Fig. 4b), indicating that the NiO/SiO2 catalysts with a moderate OSI (low-coordination sites with moderate electron density) activate C3H8 more effectively than those with a weak OSI (high-coordination) or strong OSI (electron-deficient). In addition, NiO/SiO2-WI produced CO2 much faster than NiO/SiO2-SEA and NiO/SiO2-CA, which can be attributed to the rapid reaction of C3H8 with lattice oxygen through path I. Once the lattice oxygen was depleted, the reduced NiO catalyzed the cracking reaction of C3H8, resulting in methane formation (Fig. S14).
In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was employed to investigate the re-adsorption of C3H6 on the catalyst surface. For NiO/SiO2-WI, bands at 1609 cm−1 (symmetric stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and 1492 cm−1 (scissoring vibration of CH2) were observed after the purge process, corresponding to the chemical adsorption of C3H6 at 300 °C (Fig. 4b).44,45 For NiO/SiO2-SEA, the bands at 1420 cm−1 and 1373 cm−1 were assigned to the symmetric stretching vibrations of C–O in carbonate and formate species, respectively, while the broad band at 1550 cm−1 was attributed to overlapping asymmetric stretching.46 The presence of formate was further confirmed by the characteristic C–H stretching bands at 2948 and 2888 cm−1 (Fig. S15).47 For NiO/SiO2-CA, the spectra showed features intermediate between the two catalysts, reflecting C3H6 adsorbed on the bulk NiO NPs and at the NiO–SiO2 interface, respectively. In short, the stronger OSI promotes electron transfer from NiO NPs to the SiO2 support, making active sites more electron-deficient. This strengthens the interaction with the electron-rich C
C) and 1492 cm−1 (scissoring vibration of CH2) were observed after the purge process, corresponding to the chemical adsorption of C3H6 at 300 °C (Fig. 4b).44,45 For NiO/SiO2-SEA, the bands at 1420 cm−1 and 1373 cm−1 were assigned to the symmetric stretching vibrations of C–O in carbonate and formate species, respectively, while the broad band at 1550 cm−1 was attributed to overlapping asymmetric stretching.46 The presence of formate was further confirmed by the characteristic C–H stretching bands at 2948 and 2888 cm−1 (Fig. S15).47 For NiO/SiO2-CA, the spectra showed features intermediate between the two catalysts, reflecting C3H6 adsorbed on the bulk NiO NPs and at the NiO–SiO2 interface, respectively. In short, the stronger OSI promotes electron transfer from NiO NPs to the SiO2 support, making active sites more electron-deficient. This strengthens the interaction with the electron-rich C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, favoring over-oxidation through path II and reducing C3H6 selectivity.
C bond, favoring over-oxidation through path II and reducing C3H6 selectivity.
To elucidate the reaction pathways governing C3H6 selectivity during ODHP over the NiO/SiO2 catalysts, desorbed gas-phase intermediates were monitored by synchrotron vacuum ultraviolet photoionization mass spectrometry (SVUV-PIMS). As shown in Fig. 4e and S16, several key intermediates were detected, including formaldehyde, acrolein, and acetone, with distinct patterns emerging for each catalyst that reflect different dominant over-oxidation routes. For NiO/SiO2-WI, relatively strong signals of acetone and formaldehyde appeared as early as 280 °C with a low C3H6 level, indicating that over-oxidation proceeds mainly via the direct C3H8 over-oxidation. For NiO/SiO2-SEA, a significant increase in acrolein intensity was observed at 320 °C, coinciding with C3H6 formation and accompanied by ethylene evolution, suggesting that over-oxidation occurs via secondary oxidation of C3H6, accompanied by C–C bond cleavage (Fig. S17 and S18 and Table S2). This assignment was further supported by C3H6-O2 TPSR experiments, which confirmed that acrolein and acetone originate from the C3H6 secondary oxidation in NiO/SiO2-SEA (Fig. S18). In contrast, NiO/SiO2-CA achieves a favorable balance, showing a relatively low degree of over-oxidation while generating a substantial amount of C3H6. This suggests that the NiO/SiO2-CA catalyst effectively suppresses over-oxidation pathways, including both direct C3H8 over-oxidation and C3H6 secondary oxidation, which results in the highest C3H6 selectivity.
Based on the experimental results, we propose a possible reaction mechanism for C3H8 oxidation over NiO/SiO2 with different OSI strengths, as depicted in Scheme 1. Under a weak OSI, NiO NPs tend to agglomerate into large particles, and electron transfer to SiO2 is negligible. In this regime, the geometric effect dominates: the number of active sites decreases and more low-activity high-coordination sites are exposed, which favor the C3H8 homo-carbon dehydrogenation to acetone and drives its subsequent over-oxidation. Under a strong OSI, NiO NPs remain small and provide more active sites. However, excessive electron transfer to the support makes the electronic effect dominant: C3H8 activation is weakened, while stronger C3H6 adsorption directly facilitates its secondary oxidation. The highly selective active sites in NiO/SiO2-CA are characterized with a particle size below 4 nm, which exposes more surface NiO5 species with low-coordination. This configuration may keep the surface lattice oxygen atoms farther apart, thereby reducing the likelihood of homo-carbon dehydrogenation and suppressing direct C3H8 oxidation. Meanwhile, the moderate electron transfer weakens the adsorption and subsequent conversion of C3H6, thus minimizing its secondary oxidation.
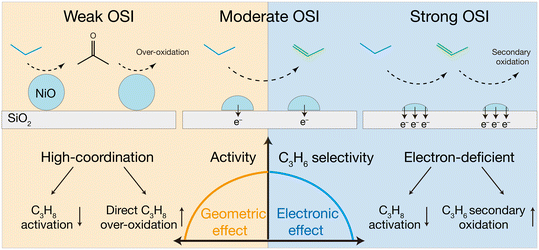 | ||
| Scheme 1 Schematic illustration of the ODHP reaction catalyzed by the NiO/SiO2 catalysts with different OSI strengths. | ||
4 Conclusion
In summary, the OSI strength of the NiO/SiO2 catalysts was tuned by altering the interfacial bonding modes between the nickel precursor and the support. Structural characterization reveals that a stronger OSI induces smaller NiO NPs and enhances electron transfer from NiO NPs to SiO2 support. Catalytic performance demonstrates that the NiO/SiO2 catalyst with moderate OSI exhibited the highest activity and C3H6 selectivity. In situ investigations (TPSR, DRIFTS, and SVUV-PIMS) further identified two distinct over-oxidation pathways governed by OSI strength: a weak OSI highlights geometric effects, favoring direct C3H8 over-oxidation, while a strong OSI amplifies electronic effects, facilitating C3H6 secondary oxidation. These insights reveal the key role of OSI modulation in balancing geometric and electronic effects for selective oxidation catalysis and provide valuable guidance for the development of more efficient catalysts.Author contributions
W. Li: conceptualization, methodology, investigation, validation, visualization, data curation, writing – original draft. H. Wang: investigation, validation. Z. Wang: investigation, validation. H. Cao: supervision, writing – review & editing, funding acquisition. J. Bao: supervision, writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
All data needed to support the conclusions in the paper are presented in the manuscript and/or the supporting information (SI) including the supplementary experimental section, Fig. S1–S18, Tables S1 and S2, and the corresponding discussion. Supplementary information is available. See DOI: https://doi.org/10.1039/d5cy01239b.Acknowledgements
This work was supported by the National Natural Science Foundation of China (12241502), the Fundamental Research Funds for the Central Universities (20720220010), the National Key Research and Development Program of China (2019YFA0405602) and the Anhui Provincial Natural Science Foundation (2408085QB049). This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China. We thank the BL09U (https://cstr.cn/31131.02.HLS.AMP) and BL12B (https://cstr.cn/31131.02.HLS.XMCD.b) of the National Synchrotron Radiation Laboratory (NSRL) for providing technical support and assistance in data collection and analysis. We thank the BL11B (https://cstr.cn/31124.02.SSRF.BL11B) of Shanghai Synchrotron Radiation Facility (SSRF) for the assistance on XAS measurements.References
- S. Chen, X. Chang, G. Sun, T. Zhang, Y. Xu, Y. Wang, C. Pei and J. Gong, Chem. Soc. Rev., 2021, 50, 3315–3354 RSC.
- Y. Dai, X. Gao, Q. Wang, X. Wan, C. Zhou and Y. Yang, Chem. Soc. Rev., 2021, 50, 5590–5630 RSC.
- H. Liu, S. Sun, D. Li and Y. Lei, Chem. Commun., 2024, 60, 7535–7554 RSC.
- Y. Chai, Y. Zhou, S. Lin, X. Wang and J. Lin, ACS Catal., 2024, 14, 2502–2521 CrossRef CAS.
- J. H. Carter, T. Bere, J. R. Pitchers, D. G. Hewes, B. D. Vandegehuchte, C. J. Kiely, S. H. Taylor and G. J. Hutchings, Green Chem., 2021, 23, 9747–9799 RSC.
- D. Delgado, R. Sanchis, B. Solsona, P. Concepción and J. M. López Nieto, Top. Catal., 2020, 63, 1731–1742 CrossRef CAS.
- C. Hernández Mejía, T. W. van Deelen and K. P. de Jong, Nat. Commun., 2018, 9, 4459 CrossRef.
- P. Wu, S. Tan, J. Moon, Z. Yan, V. Fung, N. Li, S.-Z. Yang, Y. Cheng, C. W. Abney, Z. Wu, A. Savara, A. M. Momen, D. Jiang, D. Su, H. Li, W. Zhu, S. Dai and H. Zhu, Nat. Commun., 2020, 11, 3042 CrossRef CAS PubMed.
- T. W. van Deelen, C. Hernández Mejía and K. P. de Jong, Nat. Catal., 2019, 2, 955–970 CrossRef CAS.
- X. Liu, M.-H. Liu, Y.-C. Luo, C.-Y. Mou, S. D. Lin, H. Cheng, J.-M. Chen, J.-F. Lee and T.-S. Lin, J. Am. Chem. Soc., 2012, 134, 10251–10258 CrossRef CAS.
- H. Wang, X.-K. Gu, X. Zheng, H. Pan, J. Zhu, S. Chen, L. Cao, W.-X. Li and J. Lu, Sci. Adv., 2019, 5, eaat6413 CrossRef.
- J. Yu, X. Yang, Y. Jia, Z.-Q. Wang, W. Li, Y. Jiang, S. Dai and W. Zhan, Nat. Commun., 2024, 15, 10229 CrossRef CAS PubMed.
- C. Dong, R. Li, Z. Qu, Y. Fan, J. Wang, X. Du, C. Liu, X. Feng, Y. Ning, R. Mu, Q. Fu and X. Bao, J. Am. Chem. Soc., 2025, 147, 13210–13219 CrossRef CAS PubMed.
- B. A. T. Mehrabadi, S. Eskandari, U. Khan, R. D. White and J. R. Regalbuto, in Advances in Catalysis, Elsevier, 2017, vol. 61, pp. 1–35 Search PubMed.
- J. Zhang, T. Wang, C. Shi, L. Pan, X. Zhang, C. Peng and J.-J. Zou, Chem. Eng. J., 2023, 470, 144197 CrossRef CAS.
- X. Li, X. I. Pereira-Hernández, Y. Chen, J. Xu, J. Zhao, C.-W. Pao, C.-Y. Fang, J. Zeng, Y. Wang, B. C. Gates and J. Liu, Nature, 2022, 611, 284–288 CrossRef CAS.
- A. Parastaev, V. Muravev, E. Huertas Osta, A. J. F. van Hoof, T. F. Kimpel, N. Kosinov and E. J. M. Hensen, Nat. Catal., 2020, 3, 526–533 CrossRef CAS.
- T. Zhou, X. Li, J. Zhao, L. Luo, Y. Wang, Z. Xiao, S. Hu, R. Wang, Z. Zhao, C. Liu, W. Wu, H. Li, Z. Zhang, L. Zhao, H. Yan and J. Zeng, Nat. Mater., 2025, 24, 891–899 CrossRef CAS PubMed.
- P. Yin, S. Hu, K. Qian, Z. Wei, L.-L. Zhang, Y. Lin, W. Huang, H. Xiong, W.-X. Li and H.-W. Liang, Nat. Commun., 2021, 12, 4865 CrossRef CAS PubMed.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson, R. St and C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- Y. H. Ikuhara, T. Saito, S. Takahashi, Y. Sasaki and T. Hirayama, J. Am. Ceram. Soc., 2012, 95, 524–529 CrossRef CAS.
- F. Frati, M. O. J. Y. Hunault and F. M. F. De Groot, Chem. Rev., 2020, 120, 4056–4110 CrossRef CAS PubMed.
- S. Xu, W. Wang, H. Li, Y. Gao, Y. Min, P. Liu, X. Zheng, D. Liu, J. Chen, H. Yu, X. Zhou and Y. Wu, Adv. Mater., 2025, 37, 2500371 CrossRef CAS PubMed.
- W. Katayama, T. Tamura, Y. Nishino and T. Hirose, Comput. Mater. Sci., 2021, 196, 110555 CrossRef CAS.
- L. Soriano, A. Gutiérrez, I. Preda, S. Palacín, J. M. Sanz, M. Abbate, J. F. Trigo, A. Vollmer and P. R. Bressler, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 193402 CrossRef.
- X. Zhou, W. Mao, Y. Cui, H. Zhang, Q. Liu, K. Nie, X. Xu, Y. Jiang, N. Chen and J. Chen, Adv. Funct. Mater., 2023, 33, 2303416 CrossRef CAS.
- X. Sun, X. Chen, C. Fu, Q. Yu, X.-S. Zheng, F. Fang, Y. Liu, J. Zhu, W. Zhang and W. Huang, Nat. Commun., 2022, 13, 6677 CrossRef CAS.
- H. Wang, S. M. Butorin, A. T. Young and J. Guo, J. Phys. Chem. C, 2013, 117, 24767–24772 CrossRef CAS.
- X. Liu, D. Wang, G. Liu, V. Srinivasan, Z. Liu, Z. Hussain and W. Yang, Nat. Commun., 2013, 4, 2568 CrossRef PubMed.
- G. J. Colpas, M. J. Maroney, C. Bagyinka, M. Kumar, W. S. Willis, S. L. Suib, P. K. Mascharak and N. Baidya, Inorg. Chem., 1991, 30, 920–928 CrossRef CAS.
- L. Soriano, I. Preda, A. Gutiérrez, S. Palacín, M. Abbate and A. Vollmer, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 233417 CrossRef.
- A. Sunny and K. Balasubramanian, J. Phys. Chem. C, 2020, 124, 12636–12644 CrossRef CAS.
- M. G. Álvarez, A. Urdă, V. Rives, S. R. G. Carrazán, C. Martín, D. Tichit and I.-C. Marcu, C. R. Chim., 2017, 21, 210–220 CrossRef.
- X. Li, T. Lunkenbein, V. Pfeifer, M. Jastak, P. K. Nielsen, F. Girgsdies, A. Knop-Gericke, F. Rosowski, R. Schlögl and A. Trunschke, Angew. Chem., Int. Ed., 2016, 55, 4092–4096 CrossRef CAS PubMed.
- B. Frank, J. Zhang, R. Blume, R. Schlögl and D. S. Su, Angew. Chem., Int. Ed., 2009, 48, 6913–6917 CrossRef CAS PubMed.
- J. Liu, M. Hao, C. Chen, K. Du, Q. Zhou, S. Zou, L. Xiao and J. Fan, Appl. Surf. Sci., 2020, 528, 147025 CrossRef CAS.
- A. Christodoulakis, M. Machli, A. A. Lemonidou and S. Boghosian, J. Catal., 2004, 222, 293–306 CrossRef CAS.
- R. You, X. Zhang, L. Luo, Y. Pan, H. Pan, J. Yang, L. Wu, X. Zheng, Y. Jin and W. Huang, J. Catal., 2017, 348, 189–199 CrossRef CAS.
- L. Wang, C. Ao, Y. Zhai, B. Feng, J. Duan, S. Qian, W. Zhao, L. Zhang and F. Liu, Inorg. Chem. Commun., 2020, 112, 107725 CrossRef CAS.
- Z. Li, A. W. Peters, A. E. Platero-Prats, J. Liu, C.-W. Kung, H. Noh, M. R. DeStefano, N. M. Schweitzer, K. W. Chapman, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2017, 139, 15251–15258 CrossRef CAS PubMed.
- G.-L. Dai, Z.-H. Li, J. Lu, W.-N. Wang and K.-N. Fan, J. Phys. Chem. C, 2012, 116, 807–817 CrossRef CAS.
- C. Jiang, H. Song, G. Sun, X. Chang, S. Zhen, S. Wu, Z. Zhao and J. Gong, Angew. Chem., Int. Ed., 2022, 61, e202206758 CrossRef CAS PubMed.
- C. Xiong, S. Chen, P. Yang, S. Zha, Z.-J. Zhao and J. Gong, ACS Catal., 2019, 9, 5816–5827 CrossRef CAS.
- Q. Hua, T. Cao, X.-K. Gu, J. Lu, Z. Jiang, X. Pan, L. Luo, W.-X. Li and W. Huang, Angew. Chem., Int. Ed., 2014, 53, 4856–4861 CrossRef CAS.
- W. Xiong, X.-K. Gu, Z. Zhang, P. Chai, Y. Zang, Z. Yu, D. Li, H. Zhang, Z. Liu and W. Huang, Nat. Commun., 2021, 12, 5921 CrossRef CAS PubMed.
- Y. Wang, W. Zheng, F. Chen and X. Zhan, Appl. Catal., A, 2008, 351, 75–81 CrossRef CAS.
- Y. Wang, S. Kattel, W. Gao, K. Li, P. Liu, J. G. Chen and H. Wang, Nat. Commun., 2019, 10, 1166 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |


