Advances in oxide thermoelectric materials: strategies, applications and beyond
Qing
Wang†
 a,
Zhifang
Zhou†
a,
Zhifang
Zhou†
 b,
Chang
Liu†
b,
Chang
Liu†
 a,
Yunpeng
Zheng
a,
Yunpeng
Zheng
 c,
Zongmo
Shi
c,
Zongmo
Shi
 ad,
Bin
Wei
ad,
Bin
Wei
 e,
Wenyu
Zhang
e,
Wenyu
Zhang
 a,
Ce-Wen
Nan
a,
Ce-Wen
Nan
 a and
Yuan-Hua
Lin
a and
Yuan-Hua
Lin
 *a
*a
aState Key Laboratory of New Ceramics Materials, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, P. R. China. E-mail: linyh@mail.tsinghua.edu.cn
bState Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, P. R. China
cKey Laboratory of Eco-materials Advanced Technology, College of Materials Science and Engineering, Fuzhou University, Fuzhou 350108, P. R. China
dCollege of Materials Science and Engineering, Xi'an University of Architecture and Technology, Xi'an, 710055, P. R. China
eSchool of Materials Science and Engineering, Henan Polytechnic University, Jiaozuo 454000, P. R. China
First published on 26th November 2025
Abstract
Oxide thermoelectric materials have emerged as promising candidates for sustainable energy applications owing to their inherent thermal stability, environmental benignity, elemental abundance, and low cost. This review comprehensively summarizes the recent advances in oxide thermoelectrics, covering synthesis methodologies for bulk and thin-film oxides as well as state-of-the-art advances in thermoelectric performance. Particular emphasis is placed on multiple optimization strategies aimed at carrier-phonon decoupling in oxides (such as high entropy design, texturization, homo-structure construction, and symmetry modulation) and emerging applications based on oxide thermoelectrics (including the photothermoelectric effect, and transverse thermoelectric effect), distinguished from conventional thermoelectric energy conversion. These coupled functionalities open new avenues for multi-modal energy harvesting and intelligent device integration. Finally, we highlight critical challenges and unresolved issues that need to be addressed in future research and practical applications in oxide thermoelectrics.
1. Introduction
The urgent demand for clean energy has driven the development of energy storage/conversion materials, including lithium batteries, solar cells, dielectric energy storage materials, and thermoelectric materials.1–10 With the capability of recovering waste heat into electricity and precise temperature control based on the Seebeck and Peltier effects, thermoelectric materials, showing the advantages of noiselessness, high reliability, and long working life, have been widely investigated for decades.11–18 As Fig. 1a shows, the number of publications focused on thermoelectric materials is increasing year by year, which is sourced from the Web of Science Core Collection database. Even so, thermoelectric materials and devices currently face limited practical adoption due to the challenges in efficiency, cost, and scalability. Currently, plastic inorganic thermoelectric materials, flexible thermoelectric materials used in wearable electronics, thermoelectric interface materials for devices, and thermoelectric performance improvements of different materials are the main research topics in the thermoelectric field.3,13,16,19–22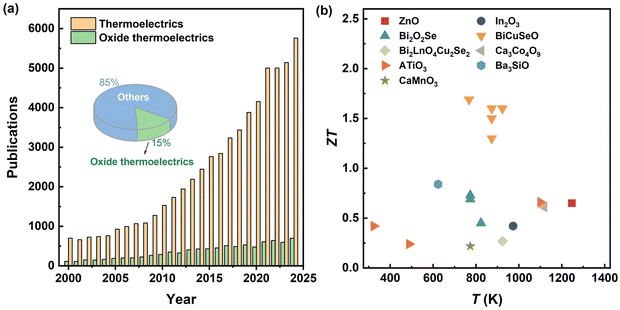 | ||
| Fig. 1 (a) Annual publications related to thermoelectrics and oxide thermoelectrics from 2000 to 2024 based on the database of Web of Science Core Collection; the inset shows the percentage of oxide thermoelectrics. (b) ZT values of different kinds of oxide thermoelectric materials, including ZnO,37 In2O3,38 Bi2O2Se,39–41 BiCuSeO,11,33–36 Bi2LaO4Cu2Se2,42 Ca3Co4O9,43 CaMnO3,44 ATiO3,45–47 and Ba3SiO.48 | ||
The efficiency of thermoelectric devices is highly relevant to the thermoelectric properties of materials, which are generally evaluated by a dimensionless figure of merit, ZT = S2σT/κ.23 Here, S, σ, κ, and T are the Seebeck coefficient, electrical conductivity, thermal conductivity, and absolute temperature. Thus, a large power factor (PF = S2σ) and a low κ are conducive to excellent thermoelectric performance at a given temperature. Until now, high-performance alloys like SnSe, Cu2Se, GeTe, and PbTe have achieved high ZT values over 2.0 and even 3.0.12,24–30 However, the poor antioxidant ability and reliability at high temperatures of most alloys still limit their practical applications.
Oxide thermoelectrics with good service stability represent a promising alternative to conventional alloys, particularly in the high-temperature range.31,32 Furthermore, they usually contain nontoxic and naturally abundant elements. Thus, oxide thermoelectrics are one of the most important parts of thermoelectric materials. However, publications on oxide thermoelectrics (sourced from the Web of Science Core Collection database) account for merely ∼10% of the total publications in the thermoelectric field, as presented in Fig. 1a. This is mainly attributed to the poor thermoelectric performance of oxide thermoelectrics originating from the inherent trade-off between their high lattice thermal conductivity and poor electrical properties. Although BiCuSeO, one of the p-type layered oxyselenides, exhibits high ZT values over 1.5,11,33–36 most of the oxide thermoelectrics possess low ZT values lower than 1.0 and even less than 0.5.29
Oxide thermoelectric materials can be primarily categorized into three groups: transparent conductive oxides (e.g., ZnO and In2O3),37,38 perovskite-structured oxides (e.g., SrTiO3 and CaMnO3),44,47 and layered oxides (e.g., NaxCo2O4, Ca3Co4O9, BiCuSeO, and Bi2O2Se).34,39,43,49 Researchers have faced challenges in enhancing the thermoelectric properties of oxide thermoelectrics over the past few decades. Recent progress regarding their maximum ZT values is reflected in Fig. 1b. Although most oxide thermoelectrics demonstrate limited thermoelectric performance, the multifunctionality of these oxides makes them valuable for diverse applications. For instance, ZnO, a versatile semiconductor applicable in piezoelectric, catalytic, or solar cell fields, holds more potential when considered as a thermoelectric material.50,51 Additionally, Bi2O2Se, a promising novel quasi-2D material, has exhibited the highest ZT value among n-type oxide thermoelectrics.52 Consequently, two key issues are emphasized concerning oxide thermoelectrics, including optimizing thermoelectric performance and leveraging the thermoelectric effect of oxides in other applications.
In this review, we systematically summarize the latest advances in oxide thermoelectric materials, focusing on key developments, performance-enhancement strategies, and emerging applications, as illustrated in Fig. 2. First, we comprehensively outline the synthesis methods for oxide thermoelectric bulk materials and films, followed by an update on recent progress in major oxide thermoelectrics, including transparent conducting oxides, perovskite oxides, layered cobalt oxides, and layered oxychalcogenides. Then, the effective strategies for thermoelectric performance optimization, like doping, texturization, entropy engineering, and homo-structure construction, are emphasized. Additionally, emerging applications based on the thermoelectric effects in oxides, such as the photothermoelectric effect, transverse thermoelectric effect, and thermo-electrocatalysis, are presented and discussed. Finally, the current challenges and future perspectives are highlighted to guide future research directions for the continued advancement of oxide thermoelectrics.
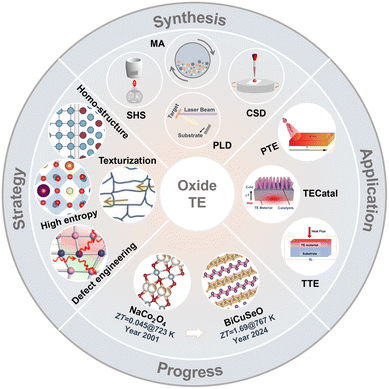 | ||
| Fig. 2 Overview of oxide thermoelectric (TE) materials. Four aspects are presented, including synthesis methods (e.g., self-propagating high-temperature synthesis (SHS), mechanical alloying (MA), pulsed laser deposition (PLD), and chemical solution deposition (CSD)), progress in thermoelectric performance of oxides (e.g., ZT from 0.045 for NaCo2O453 to 1.69 for BiCuSeO), effective strategies used for performance enhancement of oxide thermoelectrics (e.g., defect engineering, reproduced with permission;36 Copyright 2024 The Authors; texturization, reproduced with permission;54 Copyright 2024 Elsevier Ltd; high entropy design, reproduced with permission;47 Copyright 2024 The Authors; and homo-structure construction), and emerging applications (e.g., photothermoelectric (PTE), reproduced with permission.55 Copyright 2019 Wiley-VCH; transverse thermoelectric (TTE); and thermoelectrocatalysis (TECatal), reproduced with permission.56 Copyright 2024 The Authors). | ||
2. Synthesis of oxide thermoelectric materials
The choice of an appropriate synthesis method for oxide thermoelectric materials is inherently driven by their specific structural and chemical requirements. Therefore, selecting the optimal synthesis strategy necessitates a comprehensive evaluation of factors such as thermoelectric performance, material compatibility, process scalability, and sustainability. This careful consideration is essential for the efficient development of high-performance oxide thermoelectrics. The subsequent sections will provide an overview of the synthesis methods for oxide thermoelectric materials, focusing on both bulk materials and thin films.2.1 Oxide bulks
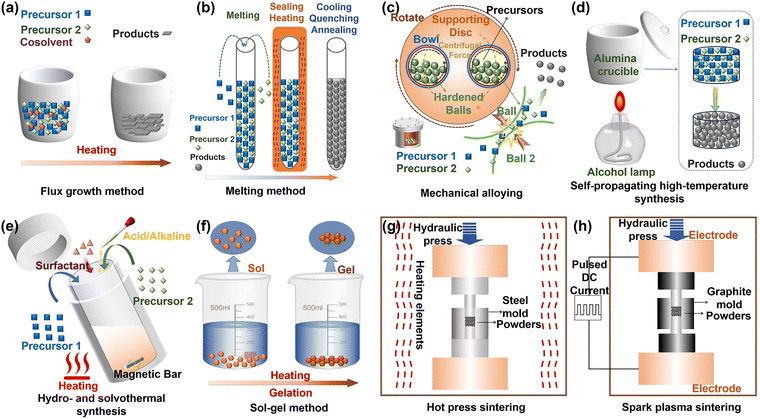
Generally, in oxide thermoelectric materials, the thermoelectric performance of single crystals is superior to that of polycrystals. As illustrated in Fig. 3a, the flux growth method is a widely used technique for preparing oxide single crystals, in which a flux dissolves the raw materials at a temperature below the material's melting point, and a supersaturated solution is formed through cooling or evaporation, thereby promoting the crystallization of single crystals. The flux growth method has been widely applied to oxide compounds, such as NaxCoO2,57 Ca3CoO9,58 and (ZnO)5In2O3.59 This method offers low-temperature growth, high-quality crystals, and simple equipment, but it is limited by small crystal sizes, long growth times, and potential impurity contamination.The synthesis of polycrystalline oxide thermoelectric materials generally involves two steps: the preparation of pre-sintered powders and the subsequent sintering of compact bulk ceramic samples suitable for testing various properties. The synthesis of pre-sintered powders of oxide thermoelectric materials is typically achieved through the melting method,60 mechanical alloying,40 self-propagating high-temperature synthesis,11 sol–gel method, and hydro and solvothermal synthesis. In the first step, metal oxides, metal, or metal salts are mixed in the desired stoichiometric ratios and subjected to high temperatures to form homogeneous precursors with the targeted chemical composition. These precursors can be processed into powders for the next sintering step. The melting method, a typical solid-state reaction, is effective for producing high-purity crystalline phases but is often energy-intensive due to prolonged high-temperature treatments. Fig. 3b depicts a standard melting process where high-purity precursors are heated above their melting point within an evacuated and sealed quartz tube, maintained at high temperature for a specific duration, and then slowly cooled or quenched to room temperature, resulting in the formation of new compounds. Pb-doped BiCuSeO61 and Bi2O2−2xTe2xSe41 are prepared using the melting method. Mechanical alloying is a technique for producing solid alloys or solid solutions via high-speed ball milling or grinding, as depicted in Fig. 3c. This method has garnered considerable attention due to its advantages, such as short processing times, low energy requirements, and straightforward execution. La-doped SrTiO3,62 Bi1−2xCaxPbxCuSeO,63 and Bi2O2Se40 powders were synthesized via mechanical alloying. Self-propagating high-temperature synthesis (SHS) is a technique that employs exothermic reactions to generate sufficient heat, enabling the reaction to sustain and propagate without external heat sources, as illustrated in Fig. 3d. In this process, a mixture of powdered reactants is initially ignited with a small energy input. Once ignited, the exothermic reaction becomes self-sustaining, producing enough heat to maintain the high temperatures necessary for subsequent chemical reactions, ultimately resulting in the formation of the desired product. SHS is widely employed for synthesizing ceramics, intermetallics, and composite materials due to its high efficiency, rapid processing, and ability to produce complex compounds with relatively low energy consumption. Specifically, notable examples synthesized using SHS include Ca1.24Co1.62O3.86,64 Bi1−xPbxCuSeO,65 and Bi0.96Pb0.04CuSe0.95Te0.05O.66 In contrast to conventional solid-state routes, methods like mechanical alloying and SHS often enhance energy efficiency by minimizing net external energy inputs and reducing overall reaction time, making them more sustainable alternatives. As illustrated in Fig. 3e, hydro and solvothermal synthesis is a solution-based reaction process capable of synthesizing nanomaterials and has been successfully applied to the preparation of oxide thermoelectric materials. This method offers advantages such as controllable crystal size, uniform distribution, and ideal crystal morphology at lower temperatures. For example, Nb–La co-doped SrTiO3 nanopowders,46 ultra-thin BiCuSeO nanosheets,67 and ZnO nanosheets68 have been successfully synthesized using this method. The sol–gel method typically utilizes inorganic salts or organic alkoxides as precursors. Through a sequence of chemical reactions, such as hydrolysis, polymerization, nucleation, and growth, these liquid precursors are transformed into a sol, which then evolves into a gel-like network structure. Following drying and heat treatment, the desired material is obtained, as depicted in Fig. 3f. For instance, Michael et al.69 employed the sol–gel technique to synthesize high-porosity Ca3Co4O9 thermoelectric ceramics.
The synthesized compound powders must undergo a densification process via sintering to produce compact bulk ceramic samples, which are essential for testing their various thermoelectric properties. Common sintering methods include hot pressing (HP), spark plasma sintering (SPS), or conventional sintering, where heat and pressure are applied to achieve a compact, mechanically stable bulk material. During these processes, careful control of temperature, pressure, and processing time is crucial to ensure the optimal material density and to avoid the formation of undesired phases or defects that could negatively impact thermoelectric performance. As illustrated in Fig. 3g and h, the primary distinction between SPS and HP lies in their heating methods. SPS utilizes pulsed current to rapidly heat the material and induce plasma effects, enabling low-temperature densification within short processing times, and uses pulsed current to rapidly heat the material, enabling low-temperature densification with shorter processing times, thus offering better energy efficiency and a lower environmental impact, whereas HP requires higher temperatures and longer sintering times, making it less energy-efficient and more environmentally demanding. SPS is more widely applied in the sintering of oxide thermoelectric materials, such as ZnO,70 SrTiO3,71 Ca3Co4O9,64 BiCuSeO,11 and Bi2O2Se,39–41 and new layered oxyselenides.42
2.2 Oxide films
The preparation methods for thermoelectric oxide films can be divided into physical and chemical methods. Physical methods mainly include pulsed laser deposition (PLD) and magnetron sputtering. Methods like chemical vapor deposition (CVD), atomic layer deposition (ALD), and chemical solution deposition (CSD) are categorized as chemical methods. Each preparation method possesses distinct advantages and limitations. Thus, it is essential to tailor the selection based on the specific characteristics of the target products.Pulsed laser deposition utilizes high-energy pulsed laser interactions with a target material to fabricate high-quality thin films, enabling precise control over the composition and microstructure of thin films.72 The details about PLD are shown in Fig. 4a.72 PLD has been adopted in many thermoelectric oxide thin films, such as Ga-doped ZnO (GZO) thin films,76 GZO-based multilayer-structured thin films,77 high entropy SrTiO3 thin films,47 and SrTiO3-based superlattices.78 Although PLD allows precise control of preparation parameters, it cannot be used to fabricate highly scalable products since the homogeneity is limited in small regions.74 Besides, magnetron sputtering is also a widely used physical deposition method for uniform inorganic thin films with high performance on variable substrates,73 which has been used in most thin films of alloys such as Bi2Te3,79 Cu2Se,80 Ag2Se,81 and SnSe.82 The preparation process to grow thin films by using magnetron sputtering under two conditions is illustrated in Fig. 4b.73 It can also be used to prepare oxide thin films like ZnO thin films.83,84
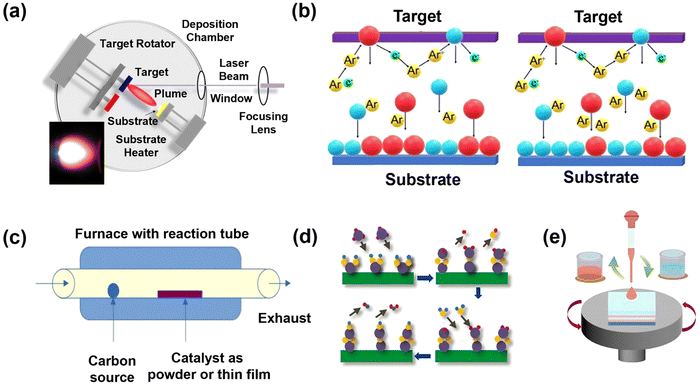 | ||
| Fig. 4 Preparation methods for thermoelectric oxide films: (a) pulsed laser deposition (PLD), reproduced with permission.72 Copyright 2020 Elsevier B.V.; (b) magnetron sputtering, reproduced with permission.73 Copyright 2023 The Authors; (c) chemical vapor deposition (CVD), reproduced with permission.74 Copyright 2024 The Authors; (d) atomic layer deposition (ALD), reproduced with permission.75 Copyright 2021 Elsevier Ltd.; and (e) chemical solution deposition (CSD), reproduced with permission.75 Copyright 2021 Elsevier Ltd. | ||
Chemical vapor deposition (CVD) is a kind of chemical method to fabricate thin films where the gas molecules decompose or react on the surface of substrates to create the desired deposition and obtain the thin films,85 as shown in Fig. 4c.74 CVD demonstrates strong industrial adaptability for mass production, delivering economically viable solutions with reliable coating uniformity, yet faces limitations in achieving nanoscale precision for complex oxides.74 It has been successfully used to synthesize ZnO-based thin films.86 Similarly, thin films prepared by atomic layer deposition (ALD) also need precursors. However, the different precursors are alternatively introduced on the surface of the substrate,87 as illustrated in Fig. 4d.75 It has been used to grow Al-doped SnO2 thin films and undoped ZnO thin films with a relatively good thermoelectric performance.88 Chemical solution deposition (CSD), which is also called the sol–gel method, has been widely used in high-performance dielectric energy storage oxide films.8,89–92 The gel precursors are uniformly deposited onto substrates via spin-coating, as shown in Fig. 4e,75 followed by multi-stage thermal processing (e.g., stepwise drying, pyrolysis, and crystallization) to achieve structurally integrated thin films.93 Thermoelectric oxide thin films, such as ZnO and La0.95Sr0.05CoO3 films, can also be prepared by CSD.93,94 Compared to vapor deposition techniques, the advantage of CSD is that it does not require expensive vacuum steps. However, the annealing temperatures involved in the thermal processing are too high for most polymer substrates, thus limiting substrate selection.95 When choosing suitable methods, the advantages and limitations mentioned above should be considered first. Then, the sustainability of each method is also important. The CSD and ALD methods demonstrate significant green potential in terms of material utilization efficiency and low-temperature processing, while the high energy consumption of PLD, magnetron sputtering and CVD represents critical sustainability limitations.
3. Progresses for oxide thermoelectric materials
Table 1 lists the thermoelectric performance of state-of-the-art oxide thermoelectric materials, including transparent conducting oxides, perovskite oxides, layered cobalt oxides, and layered oxychalcogenides. The followings briefly summarize the progress for oxide thermoelectric materials.| Materials | Classification | Type | ZT max | T (K) | σ (S cm−1) | S (µV K−1) | PF (µW m−1 K−2) | κ (W m−1 K−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Zn0.98Al0.02O | ZnO | n | 0.30 | 1273 | 394 | −180 | 1264 | 5.36 | 103 |
| Zn0.985Ga0.015O | ZnO | n | 0.25 | 1273 | 1610 | −88 | 1250 | 6.37 | 104 |
| Zn0.96Al0.02Ga0.02O | ZnO | n | 0.65 | 1247 | 350 | −256 | 2293 | 4.41 | 37 |
| IGZO film | ZnO | n | 0.02 | 383 | 1079 | −29 | 89 | 1.80 | 110 |
| GZO-3 nm ZnO–GZO film | ZnO | n | — | 623 | 594 | −85 | 434 | — | 77 |
| 10 nm ZnO/GZO film | ZnO | n | — | 623 | 1367 | −57 | 449 | — | 116 |
| ZnO(sub)–GZO film | ZnO | n | — | 373 | 1035 | −62 | 391 | — | 119 |
| In1.88V0.12O3 | In2O3 | n | 0.42 | 973 | 388 | −141 | 771 | 1.79 | 38 |
| In1.92(Zn,Ce)0.08O3 | In2O3 | n | 0.40 | 1050 | 367 | −150 | 826 | 2.20 | 126 |
| In1.92Ce0.08O3 | In2O3 | n | 0.40 | 1173 | 186 | −189 | 664 | 1.90 | 127 |
| In1.9Ga0.1O3 | In2O3 | n | 0.37 | 973 | 383 | −164 | 103 | 2.67 | 129 |
| Bi2O2Se0.985Cl0.015 | Bi2O2Se | n | 0.23 | 823 | 198 | −111 | 244 | 0.87 | 206 |
| Bi1.98La0.02O2Se | Bi2O2Se | n | 0.35 | 823 | 59 | −249 | 365 | 0.85 | 208 |
| Bi1.90Ta0.10O2Se | Bi2O2Se | n | 0.36 | 773 | 94 | −201 | 380 | 0.82 | 205 |
| Bi2O2Se-1.0 mol% CuI | Bi2O2Se | n | 0.45 | 823 | 169 | −157 | 417 | 0.76 | 40 |
| Bi1.98Sb0.02O2Se | Bi2O2Se | n | 0.59 | 773 | 192 | −169 | 548 | 0.71 | 211 |
| Bi2O1.96Te0.04Se | Bi2O2Se | n | 0.69 | 773 | 160 | −211 | 713 | 0.80 | 41 |
| Bi2O2Se-0.5 mol% graphite | Bi2O2Se | n | 0.73 | 773 | 126 | −217 | 593 | 0.63 | 39 |
| Bi0.96Pb0.04CuSe0.95Te0.05O | BiCuSeO | p | 1.2 | 873 | 101 | 244 | 603 | 0.44 | 66 |
| Bi0.86Pb0.14CuSeO | BiCuSeO | p | 1.3 | 873 | 382 | 172 | 1140 | 0.77 | 34 |
| Bi0.88Ca0.06Pb0.06CuSeO | BiCuSeO | p | 1.5 | 873 | 162 | 230 | 863 | 0.50 | 33 |
| Bi0.94Pb0.06CuSe1.01O0.99 | BiCuSeO | p | 1.6 | 873 | 180 | 246 | 1089 | 0.60 | 35 |
| Bi0.88Pb0.06Ca0.06CuSeO | BiCuSeO | p | 1.6 | 923 | 202 | 204 | 835 | 0.48 | 11 |
| −0.10 wt% graphene | |||||||||
| Bi0.96Pb0.04CuSeO (2 GPa) | BiCuSeO | p | 1.69 | 767 | 132 | 220 | 639 | 0.29 | 36 |
| Bi2DyO4Cu2Se2 | Bi2LnO4Cu2Se2 | p | 0.27 | 923 | 329 | 96 | 303 | 1.05 | 42 |
| Sr2FeO3CuSe | Sr2MO2Cu2Se2 | p | 0.04 | 923 | 3.5 | 335 | 38 | 1.18 | 225 |
| Ca0.87Ag0.1Dy0.03MnO3 | CaMnO3 | n | 0.22 | 773 | 61.7 | −193 | 340 | 1.74 | 44 |
| Ca0.98Dy0.2MnO3 | CaMnO3 | n | 0.15 | 973 | 184 | −373 | 382 | 2.48 | 406 |
| CaMnO3–O2 exsolution | CaMnO3 | p | 0.11 | 1173 | 45 | 115 | 600 | 0.56 | 407 |
| Hexagonal NaxCoO2 | NaxCoO2 | p | — | 1073 | 160 | 235 | 880 | — | 184 |
| Ca3Co4O9-66 nm | Ca3Co4O9 | p | 0.5 | 973 | 92.5 | 193 | 248 | 0.49 | 408 |
| Ca3Co4−xO9+δ ceramic nanoribbons | Ca3Co4O9 | p | 0.30 | 1073 | 118 | 201 | 475 | 1.40 | 189 |
| Sandwich-type Ca3Co4O9 composites | Ca3Co4O9 | p | 0.49 | 1073 | 370 | 139 | 490 | 0.99 | 195 |
| Ca2.7Ag0.3Co4O9+δ/Ag-10% | Ca3Co4O9 | p | 0.61 | 1118 | 136 | 237 | 432 | 1.41 | 43 |
| (Ca0.35Sr0.2Ba0.15Na0.2Bi0.1)3Co4O9 | Ca3Co4O9 | p | 0.3 | 973 | 89 | 174 | 270 | 0.87 | 409 |
| (Ca0.2Sr0.2Ba0.2Pb0.2La0.2)TiO3 | ATiO3 | n | 0.24 | 488 | 212(693 K) | −228(693 K) | 1100(693 K) | 1.25 | 47 |
| (Ca0.2Sr0.2Ba0.2Pb0.2La0.2)TiO3 | ATiO3 | n | 0.20 | 873 | 45 | −225 | ∼295 | 1.20 | 154 |
| Sr0.9La0.1Ti0.9Nb0.1O3 | ATiO3 | n | 0.66 | 1100 | 202 | −288 | 1675 | 2.80 | 46 |
| La0.067Sr0.9TiO3 + 0.6G | ATiO3 | n | 0.42 | 323 | 1067 | −123 | 1614 | 1.80 | 45 |
| SrTi0.9Nb0.1O3 | ATiO3 | n | 0.40 | 1100 | 144 | −280 | 1129 | 3.13 | 146 |
| Sr0.92La0.08TiO3 | ATiO3 | n | 0.37 | 1405 | 200 | −233 | 1086 | 3.0 | 145 |
| 20 at% Ce-doped CaTiO3 | ATiO3 | n | 0.41 | 1031 | 287 | −178 | 910 | 2.32 | 166 |
| Sr0.9La0.1TiO3 + 20 wt% Ti | ATiO3 | n | 0.50 | 1073 | 59 | −321 | 608 | 1.3 | 267 |
| La0.6K0.4TiO3 | ATiO3 | n | 0.19 | 860 | 162 | −178 | 513 | 2.37 | 167 |
| SrTiO2.932H0.068 | ATiO3 | n | 0.22 | 657 | 367 | −196 | 1408 | 4.34 | 147 |
| Sr0.4Ba0.5La0.1Ti0.9Nb0.1O3−δ | ATiO3 | n | 0.15 | 973 | ∼150 | −160 | ∼400 | ∼3.5 | 410 |
| Sr(Ti0.2Fe0.2Mo0.2Nb0.2Cr0.2)O3 | ATiO3 | n | 0.1 | 1073 | 77 | −94 | 68 | 0.71 | 282 |
| 5 at% Sr& Sb co-doped BaSnO3 | BaSnO3 | n | 0.22 | 579 | 5620 | −68 | 2599 | 6.94 | 173 |
| Ba3SiO | Ba3SiO | p | 0.84 | 623 | 37 | 507 | 900 | 0.66 | 48 |
| (LaNdPrSmEu)0.95Sr0.05CoO3 | (La,Sr)CoO3 | p | 0.23 | 350 | 68 | 271 | 499 | 0.75 | 281 |
| (Sr0.2Ba0.2Li0.2K0.2Na0.2)Nb2O6 | (Ba,Sr)Nb2O6 | n | 0.23 | 1150 | 24 | −370 | 324 | ∼0.8 | 411 |
3.1 Transparent conducting oxides
Transparent conducting oxides such as ZnO and In2O3 have been widely investigated as thermoelectric materials. Their transparency contributes to broadening applications for thermoelectric oxides in the field of cooling for chips or converting waste heat from flat panel displays.96 Here, ZnO and In2O3 will be briefly introduced.Donor doping is usually adopted to increase the carrier concentration of ZnO. Elements such as Al, Ga, In, Sb, and Sn have been investigated,102 in which Al and Ga show better doping efficiency. Tsubota et al. used the solid-state sintering method to realize Al-doped ZnO (AZO), resulting in largely improved carrier concentration from 5.2 × 1017 cm−3 to 7.2 × 1019 cm−3. Although the Seebeck coefficient decreased, the power factor increased to 1400 µW m−1 K−2 at 1273 K due to the enhanced electrical conductivity. Meanwhile, with the thermal conductivity remaining almost unchanged, the ZT value of AZO reached 0.3.103 Similar to AZO, Ga-doped ZnO (GZO) has also been seen as a promising thermoelectric oxide. Jung et al. prepared GZO ceramics via the spark plasma sintering method, varying the Ga doping amounts of 0.5%, 1%, 1.5%, and 2%. When doped with 2% Ga, the carrier concentration reached 3.9 × 1020 cm−3, indicating Ga is an effective donor.104 Generally, the carrier concentration will be continuously increased with the increasing doping amounts. However, elements like Al and Ga have certain solubility limits in ZnO, restricting the improvement of thermoelectric performance. To solve this problem, co-doping with two elements, such as Al–Mg, Al–Ga, Sb–Sn, Al–Ti, Al–Ni, and Ga–In, was adopted to increase solubility and reduce lattice thermal conductivity, as the mass and strain fluctuations would be caused by elements with various masses and sizes.37,105–111 Ohtaki et al. co-doped ZnO with Al and Ga, achieving a power factor of about 2400 µW m−1 K−2 near 1150 K for the Zn0.96Al0.02Ga0.02O ceramic sample. With a thermal conductivity below 5.0 W m−1 K−1 at 1247 K, the ZT value reached 0.65, the highest reported value for the ZnO materials.37
Although the maximum ZT value at high temperatures of ZnO has been largely improved by doping, its near-room temperature thermoelectric performance is still limited by the high thermal conductivity.37 Therefore, reducing thermal conductivity is also an important issue that needs to be focused on. Koumoto et al. formed nano-laminated (ZnO)mIn2O3 by mixing ZnO and In2O3 in a certain ratio. Due to the strengthened phonon scattering from the layered structure, the thermal conductivity was decreased to ∼3.0 W m−1 K−1 in the temperature range between 500 K and 1100 K.112 Nanostructuring can also increase the number of interfaces that hinder phonon transport and reduce the lattice thermal conductivity. Jood et al. synthesized nano-sized AZO bulk materials using the microwave hydrothermal method and realized a low thermal conductivity of ∼1.5 W m−1 K−1. However, electrical transport properties were restricted to only 0.01 S cm−1.113 Thus, decoupling carrier-phonon transport is crucial for further optimizing ZT values for ZnO.
Since low dimensionality may change the phonon transport mode and bring stronger phonon scatterings, resulting in lower lattice thermal conductivity, recent years have seen intensified research efforts toward ZnO thermoelectric thin films.76,77,110,111,114–119 Phan et al. prepared ZnO, GZO, and IGZO (In–Ga co-doping ZnO) films with a thickness of ∼1100 nm by magnetron sputtering, and the thermal conductivities of the samples were measured by time-domain thermoreflectivity (TDTR) with the results of 4.7 W m−1 K−1, 2.5 W m−1 K−1, and 1.8 W m−1 K−1 at 383 K, respectively.110 With a relatively low thermal conductivity, improving electrical transport properties is more important for ZnO thin films. Zhou et al. prepared high-quality GZO thin films on single-crystal sapphire substrates using the PLD technique. By tuning the growth temperature during the deposition process, its power factor reached 333 µW m−1 K−2 at 623 K.76 To synergistically optimize the Seebeck coefficient and electrical conductivity, GZO–ZnO–GZO sandwich-structured thin films were designed and successfully prepared.77 Combined with defect engineering, GZO–ZnO–GZO thin films showed an improved power factor of 434 µW m−1 K−2 at 623 K.77 Based on the sandwich structure, a multi-ZnO–GZO interfacial-structured film or so-called sandwich-like structured film was further designed (Fig. 5a), and a reducing atmosphere heat treatment was also applied to obtain a power factor of up to 439 µW m−1 K−2 at 623 K.118 Considering the large lattice mismatch (∼18%) and thermal expansion (∼39%) between sapphire substrates and GZO thin films, Zhou et al. inserted a homogenous ZnO buffer layer between them, as shown in Fig. 5b, effectively enhancing the carrier mobility of GZO thin films.116 GZO thin film with a 10 nm thick ZnO buffer layer possessed a power factor of 449 µW m−1 K−2 at 623 K.116 Furthermore, to improve the thermoelectric performance at near-room-temperature ranges, GZO thin films were directly deposited on ZnO single-crystal substrates to modify the substrate-film interface,119 which has been illustrated in Fig. 5c. With a largely improved carrier mobility, the outstanding near-room-temperature power factor values of 333 µW m−1 K−2 at 300 K and 391 µW m−1 K−2 at 373 K were realized on homogenously grown GZO thin films, which is competitive among the state-of-the-art ZnO-based thermoelectric thin films and other thermoelectric oxides,119 as demonstrated in Fig. 5d.
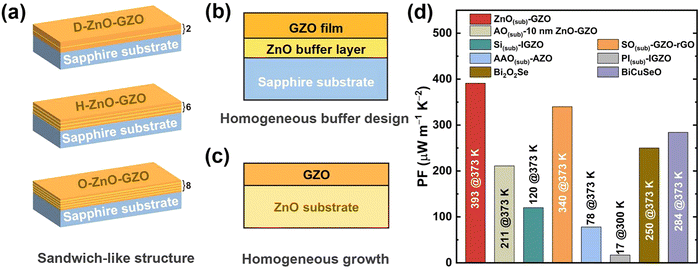 | ||
| Fig. 5 Schematic illustration of (a) sandwich-like structure (D, H and O stand for 2, 6 and 8 ZnO–GZO interfaces), reproduced with permission.77 Copyright 2022 The American Ceramic Society; (b) homogeneous buffer design, reproduced with permission.77 Copyright 2021 The American Ceramic Society; and (c) homogeneous growth, reproduced with permission.77 Copyright 2024 AIP Publishing. (d) Comparison on near-room-temperature PF values of state-of-the-art ZnO-based thin films grown on different substrates including ZnO(sub)–GZO, AO (Al2O3)(sub)-10 nm ZnO–GZO, Si(sub)–IGZO, AAO (anodic alumina)(sub)–AZO, SO (SiO2)(sub)–GZO–rGO, and PI (polyimide)(sub)–IGZO, as well as layered oxygen-containing materials including BiCuSeO and Bi2O2Se, reproduced with permission.77 Copyright 2024 AIP Publishing. | ||
Doping is the most widely used method to enhance the electrical conductivity of In2O3. Dopants such as Ge, Ce, Zn, Co, Ti, Zr, Sn, Ta, Lu, Nb, and V have been investigated.38,123–129 For example, by doping with pentavalent V elements on the trivalent In sites, the carrier concentration was improved from 1.17 × 1018 cm−3 to 4.28 × 1019 cm−3, and the room-temperature electrical conductivity was increased from 0.5 S cm−1 to 95 S cm−1. Meanwhile, the mass fluctuations caused by V doping led to a large reduction of lattice thermal conductivity. Consequently, an outstanding ZT value of ∼0.42 at 973 K was realized.38
To simultaneously improve the electrical and thermal properties, proper doping combined with nanostructuring has been adopted in In2O3 thermoelectric materials. Lan et al. decreased the grain size of Zn/Ce co-doped In2O3 ceramics from ∼2 µm to 50 nm by using co-precipitation and the spark plasma sintering method, realizing a significant reduction in lattice thermal conductivity.126 The total thermal conductivity was decreased from 13.2 W m−1 K−1 for the 2 µm-grained sample to 7.9 W m−1 K−1 for the 50 nm-grained ceramic sample at room temperature. At high temperatures, the thermal conductivity was 2.2 W m−1 K−1 for the 50 nm-grained ceramic sample. Finally, its ZT value reached 0.4 at 1050 K.126 Similarly, Liu et al. also controlled the grain sizes at the nanoscale but doped with only Ce elements for In2O3.127 Due to the solubility limit of Ce, a secondary phase, CeO2, existed as nanoclusters, which could be scattering sources to hinder phonon transport. Thus, due to the grain size reduction and nano-cluster formation, the thermal conductivity further declined to 7.6 W m−1 K−1 at room temperature and 1.9 W m−1 K−1 at high temperatures.
Besides aliovalent doping, isovalent doping was also used in In2O3. Liu et al. found that the solubility of Ga is ∼10 at%, which is much higher than other dopants like Ge and Ce.129 By substituting with Ga, the bandgap of In2O3 was narrowed, which is beneficial for optimizing carrier concentration and electrical transport properties. The largest electrical conductivity reached ∼600 S cm−1 for Ga-doped In2O3 ceramics, which is about ten times larger than that of undoped In2O3. The thermal conductivity was also limited to a relatively low level due to the heavier doping by Ga. Ultimately, the ZT value of 0.37 was realized at 973 K.129
To conclude, there remains substantial scope for enhancing the thermoelectric performance of In2O3-based ceramics. This area holds significant potential for further exploration and optimization in future research.
3.2 Perovskite oxides
Perovskite oxides are considered as prototypes of multiple research fields, including thermoelectrics.29 The common points of perovskite thermoelectric oxides, where their transport properties originate from, could be listed as follows. Firstly, as oxides, the strong metal-oxygen ionic bonds lead to comparatively high lattice thermal conductivity, a large bandgap over 2 eV, reduced carrier mobility and apparent brittleness, which is common in oxides.97,130,131 Secondly, the ABO3 structure consists of an A metal cation framework and BO6 octahedrons, providing the opportunity to relatively tune the electrical and thermal transport behaviors independently.47 Specifically, the A–O vibrations decide the acoustic branches and low-frequency optical branches in the phonon dispersion, mainly affecting thermal transport.132 While BO6 octahedrons dominate the conduction band minimum and valence band maximum, directly influencing the carrier transport.133 Thirdly, most B metals are transition metals, which means that the d orbitals would play an important role in electrical transport. The d orbitals have the merits of multiple band valleys and flat dispersion, causing a large density of states and effective mass.134 As a result, the perovskite thermoelectric oxides are the most heavily doped semiconductors with comparatively good electrical performance.135 Considering the above, the perovskite thermoelectric oxides share the advantages of high performance tunability, high degree of degeneracy, and large effective mass, while enduring the disadvantages of large lattice thermal conductivity and low carrier mobility.136a. Perovskite titanates. Perovskite titanates have received considerable attention. Ti4+ in ATiO3, where A is a divalent cation like Sr, Ca, Ba, Pb(II), has empty 3d orbitals, so undoped ATiO3 is an insulator. During n-type doping, electrons fill the t2g orbitals at the bottom of the conduction band, making it conductive. SrTiO3 is the most classical thermoelectric perovskite oxide with an indirect wide bandgap of ∼3.2 eV.133,137 Due to its high cubic symmetry (Fm
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), SrTiO3 has a good electrical performance, especially its high density of states effective mass resulting from the degree of degeneracy of 6 (Fig. 6).138 For acquiring high electrical performance, different doping elements were used at A sites, Ti sites and together, mainly aiming to increase the electron concentration. In terms of single dopants, lanthanide elements and Y were widely used to dope SrTiO3 at the Sr site,139–143 among which La doping is the most common one.144 For instance, by combining the reduction process to create oxygen vacancies and La doping together, Sr0.92La0.08TiO3 was fabricated which displayed an improved ZT value of 0.37 at l045 K.145 On the other hand, Nb is an effective dopant at Ti sites to provide electrons to the 3d orbitals of Ti, and according to the work by Zhang et al.,146ZT reached 0.40 at 1100 K in 10–15 mol% Nb-doped samples. Furthermore, dual doping was also proved effective, and in La/Nb co-doped Sr0.9La0.1Ti0.9Nb0.1O3, a much improved ZT value of 0.6 at 1100 K was achieved by Wang et al.46 Furthermore, it is rare but novel that He et al. used topochemical reactions to obtain SrTiO3−xHx by fast synthesis in a sealed capsule with CaH2. The H doping increased the carrier concentration and strongly scattered phonons by a large mass difference, thus realizing a ZT value of 0.22 at 657 K.147 Overall, heavy doping could optimize the carrier concentration to reach a high PF value, while the high thermal conductivity remains a challenge.
m), SrTiO3 has a good electrical performance, especially its high density of states effective mass resulting from the degree of degeneracy of 6 (Fig. 6).138 For acquiring high electrical performance, different doping elements were used at A sites, Ti sites and together, mainly aiming to increase the electron concentration. In terms of single dopants, lanthanide elements and Y were widely used to dope SrTiO3 at the Sr site,139–143 among which La doping is the most common one.144 For instance, by combining the reduction process to create oxygen vacancies and La doping together, Sr0.92La0.08TiO3 was fabricated which displayed an improved ZT value of 0.37 at l045 K.145 On the other hand, Nb is an effective dopant at Ti sites to provide electrons to the 3d orbitals of Ti, and according to the work by Zhang et al.,146ZT reached 0.40 at 1100 K in 10–15 mol% Nb-doped samples. Furthermore, dual doping was also proved effective, and in La/Nb co-doped Sr0.9La0.1Ti0.9Nb0.1O3, a much improved ZT value of 0.6 at 1100 K was achieved by Wang et al.46 Furthermore, it is rare but novel that He et al. used topochemical reactions to obtain SrTiO3−xHx by fast synthesis in a sealed capsule with CaH2. The H doping increased the carrier concentration and strongly scattered phonons by a large mass difference, thus realizing a ZT value of 0.22 at 657 K.147 Overall, heavy doping could optimize the carrier concentration to reach a high PF value, while the high thermal conductivity remains a challenge.
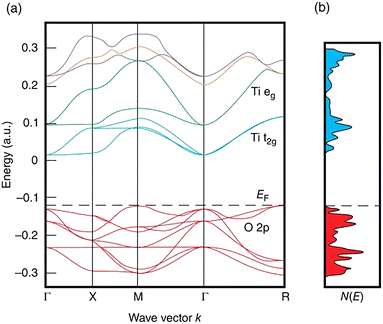 | ||
| Fig. 6 (a) The (approximate) band structure of cubic SrTiO3; (b) density of states N(E) corresponding to (a), reproduced with permission.136 Copyright 2016 John Wiley & Sons, Ltd. | ||
Facing high lattice thermal conductivity, an all-scale scattering strategy was applied.66 Grain-fining could effectively reduce the lattice thermal conductivity of SrTiO3, for which the lattice thermal conductivity of single crystals is ∼11.0 W m−1 K−1 at room temperature.148,149 After suppressing the grain size to 55 nm, the lattice thermal conductivity was halved by Wang et al.148 Nano second phases could also scatter phonons strongly, which was proved by Pr2O3 and Y2O3 stabilized ZrO2 (YSZ) composited SrTiO3.150,151 For the lattice scale, it was found that A-site vacancies and aliovalent doping could strongly scatter phonons, leading to glass-like thermal transport behaviors. Popuri et al. introduced 27% vacancies at A sites of La-doped SrTiO3 to obtain Sr0.2La0.53TiO3, which successfully achieved amorphous-like thermal transport behaviors in the ceramics with room temperature thermal conductivity of ∼2.0 W m−1 K−1.132,152 Further increasing the density of defects by a high entropy strategy would introduce large mass, valence, and size variations. Benefiting from the high density of point defects by multiple elements and the high density of dislocations stabilized by entropy effects,153 Zheng et al. and Zhang et al. designed A-site and B-site high entropy SrTiO3-based perovskite ceramics and thin films,47,154–159 effectively suppressing the lattice thermal conductivity to 1.0–2.0 W m−1 K−1, which might be the lower limit of perovskite oxides.
Apart from cubic SrTiO3, other perovskite titanates also show prospects for thermoelectric performance. Khan et al. conducted the first principles calculation on SrTiO3, CaTiO3, and BaTiO3 and found that though SrTiO3 performs better in electrical transport, CaTiO3 and BaTiO3 remain competitive in ZT due to lower thermal conductivity.160 DFT calculations were also performed on V-doped BaTiO3 by Shenoy et al.161 Yamamoto et al. measured the thermoelectric properties of the (CaSrBa)TiO3 solid solution and drew a phase diagram of the thermoelectric performance, showing that the perovskite solid solution is potential in thermoelectrics,162 which could give a clue to high entropy design.47 However, due to the lower symmetry caused by Ti displacement, though the thermal conductivity was suppressed, the Ba introduction would deteriorate the electrical conductivity.163 Compared to BaTiO3, CaTiO3-based ceramics with tilted TiO6 octahedrons show better thermoelectric promise. It is reported that the Nb-doped CaTiO3 showed a ZT value of 0.28 at 1030 K,164 and Eu0.1Ca0.9Ti0.9Nb0.1O3 displayed a ZT value of 0.42 at 1077 K, comparable to SrTiO3-based thermoelectrics.165 Interestingly, Li et al. reported that a self-assembly homogeneous superlattice structure formed in variable-valence Ce-doped CaTiO3. Ce selectively occupied the Ca site and Ti site, resulting in an ordered superlattice structure. Due to the superlattice structure, ZT was enhanced to 0.41 at 1031 K, with lattice thermal conductivity significantly reduced to 1.82 W m−1 K−1.166
Choosing equal molar +1 and +3 valence metal cations to substitute +2 valence cations could bring about significant charge and size fluctuation, including glass-like thermal transport properties. Daniels et al. have done a series of studies on Nb-doped (La,Na)TiO3 and (La,K)TiO3, concluding that A-site charge and size fluctuation have a significant effect on thermal transport and could easily realize glass-like thermal transport behaviors, thus reaching a ZT value of ∼0.2 at 800–900 K.166,167 Therefore, (Ln0.5A0.5)TiO3-based titanates are also promising due to their low thermal conductivity.
b. Perovskite manganate. The Ruddlesden–Popper (RP) phase of the CaMnO3 compound has been extensively studied, and the single crystal bulk has a peak ZT value of 1.0, which combines with the large Seebeck coefficient of −550 µV K−1. Due to Mn4+ occupying the oxygen octahedron, the electrical properties of CaMnO3 were determined by the O 2p and Mn 3d states. The indirect band gap is ∼0.7 eV. Therefore, there is an urgent need to discuss a broad selection of process-thermoelectric performance combinations for CaMnO3 ceramics. Lei168et al. reported that the (La, Sr) dual-doped Ca-sites can effectively relax the distortion of the Mn–O6 octahedron and introduce extra electrons. It can be seen that the oxygen defect concentration is attributed to Mn–O6 octahedron tilts due to the tremendous difference in effective mass between the substituted ions and Ca-site ions.
In addition, plate-like CaMnO3 microcrystals and the effect of the sintering atmosphere on CaMnO3 ceramics have been systematically studied by Shi et al.169,170Fig. 7a shows that the plate-like CaMnO3 seeds are successfully prepared through topochemical conversion technology. The effect of Ca/Mn ratios on the morphologies was explored by the two-step molten salt method. It has been noted that Mn4+ ions in β-MnO2 migrated to the surface of the Ca3Mn2O7 precursor during dissolution–precipitation, resulting in a high concentration gradient on the reaction interface. Then the reconstruction of Mn–O and Ca–O bonds was promoted and Ca2+ ions entered the concern-shared Mn–O octahedron. As a result, the plate-like CaMnO3 template seeds were formed due to the inheritance of the precursor morphology. Ca0.87Ag0.1Dy0.03MnO3 ceramics were sintered in different atmospheres (Ar, air, and O2).44 The multiscale defects, including grain boundaries, oxygen defects, and Ag nanoprecipitations, were observed and formed carrier-trapping acceptor states, thereby increasing the charge carrier density. Atomic-scale oxygen defects corresponded well to the oxygen vacancies in the perovskite structure, which were studied by scanning transmission electron microscopy-annular bright field (STEM-ABF) and integrated differential phase contrast (iDPC) STEM results, shown in Fig. 7b and c. The highly distorted nanostructures can strongly scatter the short- and mid-wavelength phonons. Meanwhile, the thermoelectric properties of Ca0.87Ag0.1Dy0.03MnO3 are presented in Fig. 7d. It can be seen that the ZT values increased significantly and then decreased.
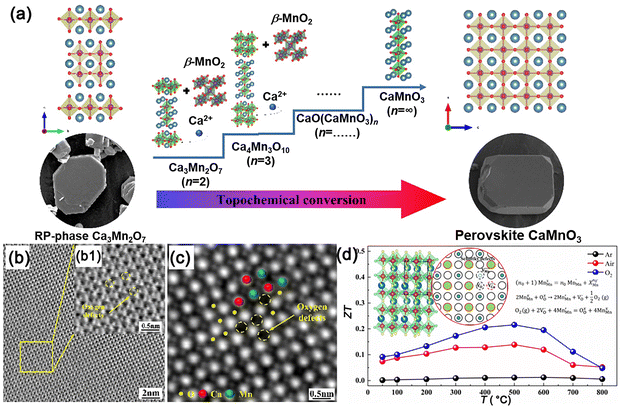 | ||
| Fig. 7 (a) Schematic mechanism of the RP-phase CaMnO3 through topochemical conversion reaction, reproduced with permission.169 Copyright 2024 American Chemical Society; (b) ABF-STEM image of CaMnO3 ceramics; (c) iDPC-STEM image of CaMnO3 ceramics; (d) ZT values of CaMnO3 ceramics, reproduced with permission.44 Copyright 2022 American Chemical Society. | ||
c. Perovskite stannates. BaSnO3-based perovskite stannates are transparent conductors with a wide band gap and high electron mobility. Different from other perovskite thermoelectric oxides, the CBM of perovskite stannate consists of the s orbital of Sn and p orbitals of O, which, compared to d orbitals of transition metals, show dispersive energy bands and high carrier mobility. For maintaining high mobility, most research on BaSnO3 and SrSnO3 was on thin films of high quality grown by PLD. The La-doped BaSnO3 thin films displayed carrier hall mobility of 50–100 cm2 V−1 s−1 after annealing, which is rather high in thermoelectric oxides limited by strong ionized ionic bonds.171,172 In ceramics, 5% Sr and Sb co-doped BaSnO3 displayed a promising ZT value of 0.22.173 However, due to the dispersive bands and high electrical conductivity, the disadvantages of BaSnO3-based perovskites are the low Seebeck coefficient (absolute value lower than 125 µV K−1 when carrier concentration around 1019–1021 cm-3) and the high thermal conductivity (thermal diffusivity over 5 mm2 s−1), which need to be further improved.
a. Perovskite cobalt oxides. The development of advanced energy harvesters relies on the coupling of both n-type and p-type thermoelectrics with matched high performance. Among research efforts of probing possible strong candidates of p-type thermoelectric oxides, cobalt oxides have been proven to realize a promising figure of merit, for example, (La,Sr)CoO3. Since Co is a transition metal element, cobalt oxides present a high Seebeck coefficient and power factor, likewise in other transition metal oxides applied in thermoelectrics. However, degeneracy of Co due to the correlation between Hund's rule coupling and crystal field results in strong coupling of thermal and electrical behaviors. Co is a notable element and other structures beyond perovskites have displayed impressive p-type thermoelectric behaviors. For example, as for p-type oxide thermoelectrics, AxCoO2 (A could be Na, Ba, Ca, Sr, etc.), with the rigid CoO2 layer and mobile Ax layer alternately stacked along the c-axis, has exhibited a relatively large power factor174 and a rather high ZT among the thermoelectric oxides (∼1.2 at 800 K, single crystal57). Unlike cobalt oxides, which seem to have higher ZT in the near room temperature range, AxCoO2 could be a very promising material for use in high temperatures.
In perovskite thermoelectric oxides, metal ions participating in bonding with O provide orbitals for electrons and holes to transport in n-type and p-type thermoelectric oxides, dominating the electrical properties in thermoelectric oxides. Lanthanide cobalates show deteriorated electrical behaviors because of thermally induced cobalt spin transitions, which can result in a significantly lower lattice thermal conductivity than the widely studied SrTiO3.175 Besides, the complex spin state can affect the Seebeck coefficient. An example is stabilized focusing on La1−xSrxMnO3. The sign of the Seebeck coefficient can be changed around the insulator-metal phase boundary of La1−xSrMnO3, both for its paramagnetic phase and ferromagnetic phase, depending on the doping ratio.176 As an element rich in magnetic responses, this might also apply to perovskite cobalt oxides. Perovskite cobalt oxides can have complex structures of oxygen vacancy distribution (from oxygen vacancy order to disorder) and this has been proven to be possible to realize a wide-range continuous tuning of the thermal conductivity via room-temperature ion-gel gating.177 This indicates the possibility of further controlling the distribution of oxygen vacancies and thus improving thermoelectric properties.
Rare earth cobalt oxides LnCoO3 (Ln = a rare earth metal) have been proven to be competitive in the near room-temperature thermoelectric application range. LnCoO3 has a fairly high Seebeck coefficient at room temperature. SmCoO3 investigated by Dudnikov et al.178 attains a maximum Seebeck coefficient value of ∼1000 µV K−1 at ∼300 K. The fastest increase in the thermoelectric power factor corresponds to the anomalies caused by the spin transition of Co3+ ions and the dielectric-metal transition. Substitutions of Sr2+ ions for Gd3+ ions in Gd1−xSrxCoO3−δ (x = 0.8 and 0.9) could lead to the single-phase disordered nonstoichiometric cubic perovskites and superstructures with ordered Sr2+/Gd3+ ions and anion vacancies. The disordered Gd0.2Sr0.8CoO3−δ showed the highest ZT ∼ 0.057 at 284 K among this series.179
b. Inverse perovskites. As a variant of perovskites, the inverse perovskites are attracting increasing attention.180 He et al. reported inverse perovskite Ba3BO (B = Si, and Ge) as promising p-type thermoelectric oxides. Ba3BO consists of OBa6 octahedrons, which form a soft framework due to the long O-Ba bonds and B4- frameworks. The soft bonds and the distorted OBa6 lead to the low lattice thermal conductivity of 1.0–0.4 W m−1 K−1 at 300–600 K (Fig. 8). The −4 valence Si, Ge provide dispersive VBM and multiple valleys, contributing to high PF. The ZT of this novel material could be 0.65 at 623 K and is predicted to achieve 2.14 for Ba3SiO at 600 K after tuning the hole concentration by doping, which needs to be accomplished by efforts on fabrication.
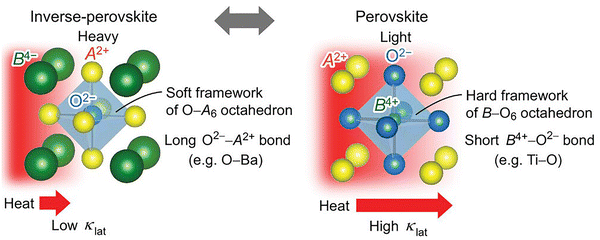 | ||
| Fig. 8 Schematic illustration of crystal structures and phonon transport in inverse-perovskite A3BO (left) and normal perovskite ABO3 (right). The normal perovskite structure of ABO3 (e.g., SrTiO3) is built with the hard framework of B–O6 octahedron with short B–O bonds, providing a high-density packing structure of the light element O2-ions. In contrast, the inverse-perovskite structure of A3BO (e.g., Ba3BO (B = Si and Ge)) is constructed from the soft framework of O–A6 octahedron with long O–A bonds, providing the high-density packing structure of heavy A2+ ions. The lattice thermal conductivity of normal perovskite ABO3 is usually high, while the largely contrasting structure characteristics are expected to lead to a large reduction of lattice thermal conductivity in inverse-perovskite A3BO, reproduced with permission.48 Copyright 2023 The Authors. | ||
3.3 Layered cobalt oxides
Layered cobalt-based oxide bulks (Ca3Co4O9, NaxCoO2, and Bi–Sr–Co–O) have attracted widespread attention at high-temperature applications (beyond 1073 K) due to the thermal stability, the acid/alkali corrosion resistance, the low cost of raw materials, and the environmental friendliness. The electron and phonon transports of these oxides are well manipulated by the complex crystal structure and chemical bonding strength, which are inferior to ZT values. In addition, high-quality layered cobalt oxide thin films such as Na3/4CoO2, Ca1/3CoO2, Sr1/3CoO2, Ba1/3CoO2, and Ca3Co4O9, which exhibit good air stability and outstanding thermoelectric performance, deserve attention.181 For example, high-quality epitaxial Ba1/3CoO2 films achieve a reliable ZT value of ∼0.55 at 873 K in air, demonstrating potential for high-temperature thermoelectric applications.174There are several methods to prepare the Ca3Co4O9 ceramics, such as solid-state reaction, molten salt method, flash firing method, spark plasma sintering, hot pressing, pulsed laser deposition, and tape casting. The plate-like Ca3Co4O9 particles can be obtained by the molten salt method, which presents the obvious anisotropy.183,184 The anisotropic Ca3Co4O9 as template seeds play a key role in the grain orientation and degree of weaving of the Ca3Co4O9 ceramics to form the anisotropic properties. Shi et al.185 have synthesized the plate-like Ca3Co4O9 seeds with different amounts of molten salt and calcinated sintering. The formation mechanism of plate-like Ca3Co4O9 seeds can be dominated by the dissolution/precipitation process (Ostwald ripening mechanism). The optimized morphology of Ca3Co4O9 template seeds was obtained when the weight ratio of salt to oxides is 1.0, which exhibited 7.92 µm in diameter and an aspect ratio of 7.01. Ca3Co4O9 thin films with a thickness of 100 nm were prepared by Yong et al.182 and a size of 10 × 5 mm2 was deposited on 5° vicinal cut LaAlO3(001) single crystal substrates as sensing materials by PLD.
In addition, several modulation engineering effects are complicated effects on the Seebeck coefficient, electrical conductivity, and thermal conductivity of Ca3Co4O9 ceramics. The transport and interaction of carriers and phonons are determined by point defects and stacking faults engineering, non-stoichiometric engineering, grain boundary engineering, nanostructure engineering, and entropy engineering.186–190 In addition to controlling Ca vacancies,191 much research has focused on the element substitution of Ca-sites, such as La, Ag, Ba, and Tb, as well as Mn, Ti, and Zr elements introduced into the Co-sites. In particular, due to the special interface of the secondary phase and the nanostructure effect, Ca3Co4O9 easily forms a three-dimensional conductive network structure, and the conductivity of the grain boundary and phase boundary increases. The nano-compositing phase included metallic nanoparticles (Ag, Ni, and Co), carbon-based nanomaterials (rGO and CNTs), and non-oxide semiconductors (SiC and MoS2). Fig. 9 presents the point defects and the schematic diagram of structural defects for Ca3Co4O9 ceramics. The results show that the introduction of the conductive phase increases the sintering temperature of Ca3Co4O9 matrix composites, and the construction of the conductive network increases the multi-scale carrier transport performance between neighboring Ca3Co4O9 grains.192–196
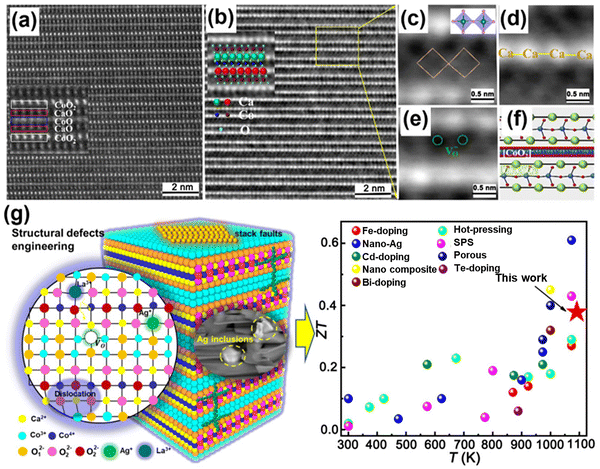 | ||
| Fig. 9 (a) Scanning transmission electron microscopy–high-angle annular dark field image of Ca3Co4O9-based ceramics and the corresponding (b) integrated differential phase contrast (iDPC) image, presenting the crystal structure of Ca3Co4O9, reproduced with permission.195 Copyright 2024 Royal Society of Chemistry; (c)–(f) magnified views of point defects in the iDPC image, showing Ca-site defects, oxygen vacancies, and the layered atomic arrangement, reproduced with permission.195 Copyright 2024 Royal Society of Chemistry; (g) schematic diagram of structural defects and ZT values for Ca3Co4O9-based ceramics, with the ZT value being noteworthy among numerous research works, reproduced with permission.196 Copyright 2020 American Chemical Society. | ||
The Pb cation doping has been largely investigated in the [Bi0.87SrO2]2[CoO2]1.82 phase, and the replacement of Sr for Ca, confirming the intergrowth of additional layers in the rock-salt block.198–200 The excellent Seebeck value of 165 µV K−1 was achieved at 300 K, and the power factor reached the value of 165 µW m−1 K−2.
3.4 Layered oxychalcogenides
Layered oxychalcogenides have recently emerged as one of the most promising and rapidly evolving materials systems for thermoelectric applications due to their unique structural and electronic characteristics. These materials typically feature a mixed-anion architecture composed of alternating conductive chalcogenide and insulating oxide layers, which enables favorable anisotropic charge transport and phonon scattering. This natural layered architecture enables a desirable combination of properties and helps to decouple electrical and thermal transport, allowing for high Seebeck coefficients alongside relatively low thermal conductivity.201,202 The chalcogenide layers, with their pronounced covalent bonding, support high charge carrier mobility, while the oxide layers, dominated by ionic interactions, effectively suppress lattice thermal conductivity.203 Representative systems within this class include Bi2O2Se, BiCuSeO, and other complex layered oxychalcogenides, which exemplify the tunability and versatility of this material platform for optimizing thermoelectric performance.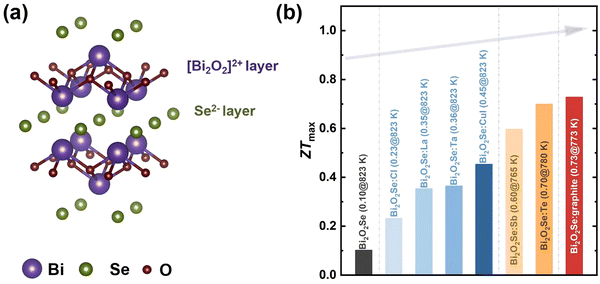 | ||
| Fig. 10 (a) Crystal structure of Bi2O2Se; (b) progress in the maximum ZT value (ZTmax) of Bi2O2Se-based thermoelectric materials including pristine Bi2O2Se,205 Cl-doped Bi2O2Se,206 La-doped Bi2O2Se,208 Ta-doped Bi2O2Se,205 CuI-doped Bi2O2Se,40 Sb-doped Bi2O2Se by shear exfoliation,211 Te-doped Bi2O2Se by shear exfoliation, and Bi2O2Se-graphite composites.39 | ||
The first step in improving the thermoelectric performance of Bi2O2Se is to increase its carrier concentration. Effective doping with elements like Cl, La, Ta, Te, and CuI has largely increased the carrier concentration of Bi2O2Se.40,205–208 For example, the carrier concentration could reach 1.4 × 1020 cm−3 when doped with 1.5 mol% CuI using the mechanical alloying method, and the electrical conductivity was improved from 3 S cm−1 to 296 S cm−1. Ultimately, an enhanced ZT value of 0.45 at 823 K was obtained.40 To further decrease the lattice thermal conductivity, texturing using a shear exfoliation method has been adopted for Bi2O2Se.41,211 An extremely low lattice thermal conductivity of ∼0.57 W m−1 K−1 was realized, resulting in an outstanding ZT value of 0.69 at 773 K for Te-doped Bi2O2Se.41 Using the same method, graphite was successfully composited with Bi2O2Se. The electrical and thermal transport performances were simultaneously optimized, where the power factor reached 600 µW m−1 K−2 and the lattice thermal conductivity declined to 0.47 W m−1 K−1. As a result, a record-high ZT value of 0.73 at 773 K was achieved.39 The progress in the maximum ZT values (ZTmax) of Bi2O2Se-based thermoelectric materials that were optimized by using strategies including doping, texturing, and compositing is collected and shown in Fig. 10b.
The bismuth-based isostructural analog of these oxysulfide compounds, BiCuChO (Ch = S, Se), was first developed in the 1990s with a high Seebeck coefficient at room temperature (∼600 µV K−1). Hiramatsu et al.217 later showed by DFT calculations that the band gap (Eg) of BiCuChO (Ch = S, Se, Te) is significantly lower than that of LaCuChO, thus exhibiting significantly higher conductivity. In addition, the sharper density of states (DOS) near the Fermi energy level (EF) results in a higher Seebeck coefficient for BiCuChO than for LaCuChO. Subsequently, in 2010, Zhao et al. demonstrated that Sr-doped p-type Bi1−xSrxCuSeO could achieve a high ZT of 0.76 at 873 K,218 followed by a record high ZT of ∼1.4 at 923 K in the woven Bi0.875Ba0.125CuSeO.219 This has triggered a new wave of interest in the scientific community in bismuth-based oxysulfide compounds as promising thermoelectric materials. In a recent report, Yin et al.36 generated dense dislocations in ceramic oxides by ultrahigh-pressure sintering, which greatly suppressed phonon transport and thus reduced the lattice thermal conductivity of the structures, culminating in a record ZT value of 1.69 at 767 K for Bi0.96Pb0.04CuSeO. The progress in the ZTmax values of BiCuSeO-based thermoelectric materials is collected and shown in Fig. 11a.
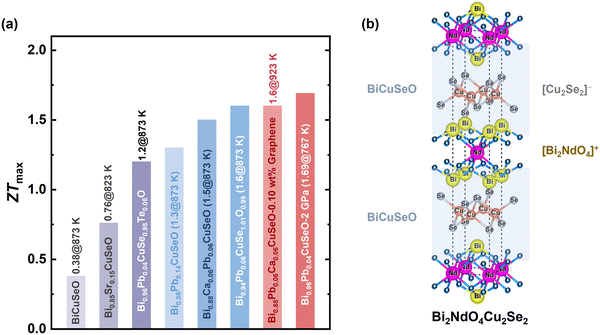 | ||
| Fig. 11 (a) Progress in the ZTmax value of BiCuSeO-based thermoelectric materials including pristine BiCuSeO,218 Bi0.85Sr0.15CuSeO,218 Bi0.96Pb0.04CuSe0.95Te0.05O,66 Bi0.86Pb0.14CuSeO,34 Bi0.88Ca0.06Pb0.06CuSeO,33 Bi0.94Pb0.06CuSe1.01O0.99,35 Bi0.88Pb0.06Ca0.06CuSeO–0.10 wt% graphene,11 and Bi0.96Pb0.04CuSeO prepared under high pressure of 2 GPa.36 (b) Crystal structure of Bi2NdO4Cu2Se2, reproduced with permission.42 Copyright 2022 Wiley VCH GmbH. | ||
Similar developments exist for Sr2MO2Cu2Se2 (M = Mn, Co, and Zn): the electrical properties of Sr2FeO3CuS and Sr2FeO3CuSe were first reported in 2014 at room temperature (Seebeck of 270 and 350 µV K−1, respectively, at room temperature),222 but only the current reported the thermoelectric properties of the layered oxotelluride Sr2CuO2Cu2Te2 (ZT of 0.045 at 770 K)223 and the low power factor of Sr2CoO2Cu2Se2 at room temperature,224 whereas studies on the high-temperature thermoelectric properties of similar layered selenium oxides, in particular, the thermal conductivities, have not been addressed. It was not until 2022 that Yang et al.225 reported for the first time the electrical and thermal properties of layered selenide oxides Sr2MO2Cu2Se2 (M = Co, Ni, Zn) and Sr2FeO3CuSe over a wide temperature range. Meanwhile, Ai et al.226 designed a new oxygen-containing compound La2Bi4Cu2O6Se4 through a high entropy strategy, where the unique intercalation strategy leads to four energy bands convergence and multiple light-heavy bands combination characteristics at the top of the valence bands, which significantly enhances the power factor of the p-type doped material, whereas the weak chemical bonding characteristics of the Cu_3d–Se_4p coupling drive the phonon softening, which decreases the lattice thermal conductivity, and ultimately, the ZT values as high as 2.3 at 700 K are estimated. Yang et al.225 used a combination of SHS and SPS to synthesize Sr2FeO3CuSe, Sr2NiO2Cu2Se2, and Sr2ZnO2Cu2, which have inherently low thermal conductivity due to their high degree of irreconcilability and deformable interfaces, with Sr2NiO2Cu2Se2 having a ZT value of 0.07 at 923 K. This combination of multiple wide selection of elements at multiple positions provides flexibility in the tuning of physical properties and allows for optimization of thermoelectric properties through doping and other strategies. These compounds have high Seebeck coefficients and low thermal conductivity in the medium to high temperature range.
4. Strategies to improve the thermoelectric performance of oxides
The thermoelectric performance of oxide materials is fundamentally constrained by their intrinsic electrical and thermal transport properties. To overcome these limitations, various advanced strategies have been developed to enhance the ZT values of oxide thermoelectrics. These strategies primarily focus on decoupling electrical and thermal transport, a critical factor for optimizing thermoelectric efficiency. As illustrated in Fig. 12, the strategies encompass multiple levels of material design, including modifications at the atomic, cell, interface, and symmetry levels. Key strategies include defect engineering, high entropy design, texturization, and homo-structures construction, all aimed at enhancing electrical transport and minimizing thermal conductivity. Additional approaches, such as carrier-phonon decoupling techniques, including orientation modulation, compositing effects, and symmetry modulation, further contribute to improving thermoelectric performance. By effectively combining these strategies, significant advancements in the thermoelectric efficiency of oxide materials can be achieved, paving the way for new applications in energy harvesting and waste heat recovery.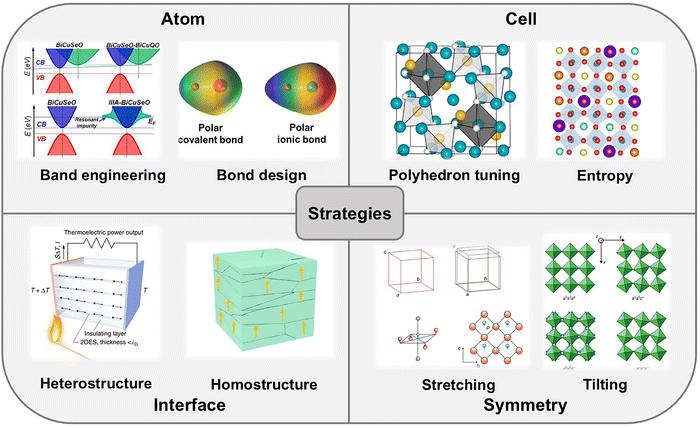 | ||
| Fig. 12 Comprehensive overview of strategies to improve the thermoelectric performance of oxides, including modifications at the atomic (reproduced with permission.30 Copyright 2019 Elsevier Ltd), cell (reproduced with permission.47,227 Copyright 2024 The Authors and 2022 American Chemical Society), interface (reproduced with permission.29,228 Copyright 2018 The Authors and 2023 The American Ceramic Society) and symmetry levels (reproduced with permission.136 Copyright 2016 John Wiley & Sons, Ltd). | ||
4.1 Defect engineering
Defect engineering is a powerful approach for optimizing thermoelectric performance by strategically manipulating charge and heat transport.229,230 Point defects (e.g., dopants and vacancies) directly modulate the carrier concentration, whereas extended defects (e.g., dislocations and nanoprecipitates) selectively scatter phonons across diverse frequency regimes to suppress lattice thermal conductivity. Critically, such defects can also reshape electronic band structures, enhance valley degeneracy, or induce resonant levels to decouple the traditional trade-off between the Seebeck coefficient and electrical conductivity. However, the uncontrolled formation of defects often leads to counterproductive effects such as carrier mobility degradation from ionized impurity scattering or chemical instability under operational conditions. To overcome these limitations, targeted doping is expected to orchestrate synergistic interactions between electronic, phononic, and structural degrees of freedom. The ensuing sections dissect how defect strategies differentially address these challenges to elevate ZT.a. Mono-doping. The easiest way to apply the doping strategy is to choose a single element to realize mono-doping. The first principle of mono-doping is to maintain a high degree of charge balance. For example, to maintain the charge balance in BiCuSeO, Bi in BiCuSeO has a valence of +3, Cu has a valence of +1, and Se and O have a valence of −1. Therefore, elements with valence states ≤3 can be selected as p-type dopants on Bi sites. Trivalent elements (Al,231 Sm,232,233 and La234), divalent elements (Ca,235,236 Mg,237,238 and Pb239–241), and monovalent elements (Ag242 and Na243) have been widely investigated in doping studies on Bi sites. For example, the substitution of Pb2+ for Bi3+ increases the density of states (DOS) due to the lone-pair electron delocalization effect in its 6s orbital, while decreasing the energy gap (Eg), resulting in a significant enhancement of the electrical conductivity (σ) while maintaining a stable Seebeck coefficient (S). This effect maintains the ZT values of Pb-doped BiCuSeO at a high level (0.91–0.95).239,240 Sm doping, on the other hand, triggers significant changes in the energy band structure. Kang et al.244 showed that the doping of Sm not only inhibits the growth of grains but also reduces the lattice thermal conductivity by increasing the number of grain boundaries. When the doping amount of Sm is x = 0.025, the band gap increases to bring the light and heavy energy bands closer together, thereby optimizing the electrical transport properties. The ZT value of Bi0.975Sm0.025CuSeO increased by 37% to 0.65 at 923 K compared with that of the pure BiCuSeO sample.
For mono-doping, different sites may result in different changes in band structures and thus different thermoelectric performances. The substitution of the homologous elements S and Te at Se sites can significantly tune the energy band structure of BiCuSeO. The structure–property mechanism is quite different from doping at the Bi sites. The conductive layer of the BiCuSeO system is dominated by ionic bonding, which produces strong electron-phonon scattering, resulting in a low carrier mobility. The introduction of the low-electronegativity Te in the Se site improved the electronegativity of the BiCuSeO system. The covalent bonding distribution in the conducting layer can effectively enhance the carrier mobility of the sample and thus improve electrical transport.30
In mono-doping, it should be noted that hetero-element doping and homo-element doping are different. Liu et al.245 found that replacing Se with Te reduced the bandgap to 0.4 eV, which significantly enhanced the electrical properties of the material, with a maximum ZT value of 0.71 at 923 K. Similarly, Sb doping enhanced the carrier concentration and conductivity by shortening the Cu/Bs–Se bond lengths, and enhancing the hybridization of the Cu and Se orbitals. The ZT value of Bi0.92Sb0.08CuSeO was significantly enhanced when the doping amount was 0.08.246
For Bi2O2Se, heterovalent element doping mainly uses elements with higher chemical valences to replace the Bi or Se sites to introduce additional electrons into Bi2O2Se, thus increasing the carrier concentration and optimizing the electrical properties. For example, Tan et al.206 obtained a ZT value three times better than that of the original structure by doping the Se sites with Cl, resulting in a significant increase in the carrier concentration and conductivity of the structure. In addition to the doping of Se sites, similar optimization effects were also achieved for Bi sites. In another work, Tan et al.205 doped Ta into Bi sites, which led to a significant increase in the structural carrier concentration (by approximately four orders of magnitude) accompanied by higher carrier mobility, and at the same time Ta doping induced a hierarchical microstructure and lowered the thermal conductivity of the crystal lattice, which resulted in an enhancement of the structural ZT value by a factor of approximately 3.5.
In addition, there is another way to consider a mono-doping strategy, focusing on the influence of covalent-ionic bonding. Regarding covalent element doping, Tan's team208 achieved a significant increase in the carrier concentration and conductivity using La. This is because the electronegativity of Bi is much higher than that of La, and La doping triggers isoelectronic hole trapping, which excites the electrons and narrows the energy band bandgap, which ultimately leads to an increase in the ZT value of the structure by a factor of about 4.5 at 873 K. Pan et al.247 found that S doping helps Bi to be a more stable and more efficient structure by doping S at the O-site. It was found that S doping helps in the sintering and grain growth of Bi2O2Se; with an increase in the doping concentration, the structural band gap decreases and the electrical properties are optimized, which in turn leads to an exponential increase in the ZT value of the structure at 793 K owing to the inherently low thermal conductivity of Bi2O2Se.
On the thermal side, the lattice thermal conductivity is usually affected by atomic bonding incongruities and external crystal defects, which affect the low lattice thermal conductivity of the structure, whereas doping equally improves the thermal properties of the structure. For example, doping of Pb into BiCuSeO leads to the formation of nanostructures, where the lattice thermal conductivity decreases gradually with an increase in the Pb content, which in turn improves the ZT value of the material.61
b. Dual-doping. Dual-doping has emerged as a superior defect-engineering strategy for thermoelectric oxides, effectively addressing the inherent limitations of mono-doping, such as carrier overcompensation or saturation.248–250 In terms of electrical properties, dual-doping allows for more precise tuning of carrier concentration and improved carrier mobility by balancing donor and acceptor dopants and minimizing lattice distortions. From a band structure perspective, it enables targeted modification of the band gap, band degeneracy, and effective mass, thereby reshaping the density of states near the Fermi level. Regarding thermal transport, dual-doping introduces multiscale hierarchical defects, including point defects, dislocations, and nanoscale precipitates, which facilitate broadband phonon scattering and significantly reduce thermal conductivity. Moreover, dopant pairs contribute to lattice stabilization through charge compensation and suppression of elemental diffusion, resulting in improved thermal and chemical stability for practical device integration. Since the Seebeck coefficient is considered to be the entropy flux per unit charge of a material, it can be improved by increasing the entropy, whereas doping magnetic ions at Bi and Cu sites for BiCuSeO can introduce rotational entropy to effectively increase the Seebeck coefficient of the material and thus optimize the ZT value.251–253 For example, Wen et al.252 used Ba and magnetic Ni ions as co-dopants at Bi and Cu sites, respectively, to optimize the thermoelectric properties of BiCuSeO, which resulted in a lower thermal conductivity (0.54 W m−1 K−1), and a high ZT value of 0.97 at 923 K for the double-doped sample due to double-atom point-defect scattering. In addition, Tang's team251 combined the light element Li and magnetic ion Mn co-dopants and found that the electronic spin grouping of magnetic ion Mn is simple and can generate spin entropy, and the light element introduces spin entropy to enhance the PF, which leads to a larger Seebeck coefficient as well as the PF, and at the same time, the nano-precipitation and diatomic point-defect scattering enhances the phonon scattering, which leads to a lower lattice thermal conductivity. This ultimately leads to a ZT as high as 0.9 at 873 K for the doped sample, while the thermal conductivity can be reduced to 0.51 W m−1 K−1.
Double doping synergistically optimizes the electrical and acoustic transport properties by introducing more lattice defects and vacancies. Yin et al.254 introduced the co-doping of Pb and Yb at the Bi site by using ball milling and high-temperature and high-pressure sintering techniques. Because the average ionic radii of Pb2+ and Yb2+ are larger than that of Bi3+, their doping leads to changes in the lattice parameters and introduces holes in the conductive layer, which attenuates the interlayer Coulombic interactions. In addition, the co-doping of Pb and Yb leads to lattice distortions and the formation of strong strain fields, further optimizing the electron and phonon transport properties. At 850 K, the ZT value of Bi0.88Pb0.06Yb0.06CuSeO reaches 1.2, which is a significant performance enhancement.
The introduction of oxygen vacancies in layered oxysulfide systems is an effective paradigm to improve the thermoelectric properties by reducing the thermal conductivity through phonon scattering and electrical resistivity through vacancy binding to cavities and has been widely investigated in various layered oxide systems such as BiCuSeO, BiCuTeO, and Bi2O2Se. And Das et al.259 used a furnace solid-phase reaction method to prepare oxygen vacancy-containing BiCuSeO samples. Although oxygen vacancies systematically reduced the Seebeck coefficient, higher ZT values could not be obtained for the samples with vacancies because of their higher thermal conductivity than the pristine samples. However, the opposite results were obtained for BiCuTeO. Chang et al.260 optimized the carrier concentration and mobility by adjusting the oxygen content in BiCuTeO to improve the power factor and also affected the grain size and hence phonon scattering by varying the oxygen content, leading to a decrease in the lattice thermal conductivity, which ultimately improved the ZT value of BiCuTeO. In addition, Wu et al.261 used annealing to modulate the oxygen vacancies in 2D Bi2O2Se to effectively regulate the concentration of oxygen vacancies, which significantly improved the Seebeck coefficient and the power factor of the structure. All these studies indicate that oxygen vacancy engineering is an effective means to modulate the thermoelectric properties of layered oxygen-sulfur compounds. Oxygen vacancies are found to strongly affect thermoelectric properties in DFT calculations of donor-doped CaMnO3−δ.262 Tensile strain can theoretically reduce the formation energy, which is consistent with the established concept of chemical expansion whereby oxygen deficiency increases the molar volume of oxides.263 Li et al.264 studied the synergetic effect of mono-doping and oxygen vacancies in a CaMnO3 system; the latter was generated by substituting Ca2+ with La3+. Coupled with the decreased thermal conductivity resulting from the generated point defects and nano-precipitates to scatter phonons, the desired ZT value of 0.4 at 1011 K for bulk Ca0.8La0.2TiO3 was achieved, which was 470% higher than that of pristine bulk CaTiO3.
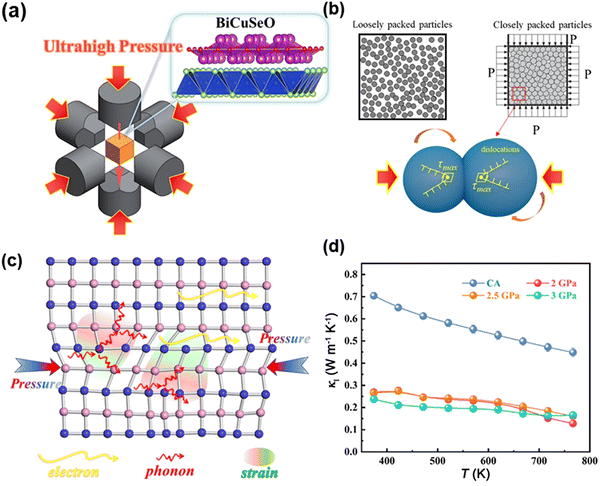 | ||
| Fig. 13 (a) Schematic diagram of the UHP process. (b) Schematic illustration of the high-density dislocations generated during the UHP process. (c) Scattering mechanisms of phonons. (d) Lattice thermal conductivity of Bi0.96Pb0.04CuSeO ceramics prepared under different conditions, reproduced with permission.36 Copyright 2024 The Authors. | ||
4.2 Texturization
Texturization of oxide thermoelectric ceramics is an example of structure design and tailoring at the grain scale, which can significantly improve the physical properties of thermoelectric materials, such as electrical and thermal properties.265 Focused on the anisotropic properties of the textured oxide ceramics with grain preferred orientation growth, the basic theory of shape control and performance control has been developed and enriched. Thereby, there is a huge effect of texturization on their morphology and transport properties.Textured Sr0.9La0.1TiO3-based ceramics are successfully fabricated by the reactive template grain growth method, which has been reported.266 Plate-like Sr3Ti2O7 template seeds are used as templates, in which Sr0.9La0.1TiO3/20 wt%Ti is used as a matrix. The results show that grains grow with a preferred orientation along (h 0 0) and a maximum ZT of 0.26 at 1073 K is achieved.267 This work provides a strategy of microstructural design for thermoelectric oxides to regulate thermoelectric properties via texture engineering.
The high ZT of 1.4 in the textured Bi0.875Ba0.125CuSeO is obtained by a hot-forging process. The carrier mobility was significantly increased along the direction perpendicular to the pressing direction, leading to an increment in the electrical conductivity.54,219,268 Based on the outstanding performance of textured BiCuSeO-based materials, a 7-pair oxide thermoelectric device was successfully built, which is the first BiCuSeO-based thermoelectric device (Fig. 14). The maximum conversion efficiency of 2.8% exhibits at the temperature difference of 510 K (Fig. 14c). This work indicates that the texturization is an effective approach for enhancing the thermoelectric performance of oxide materials. Fig. 14d shows the multi-scale parallel and texture interface evolution of Ca3Co4O9 ceramics. The vacancy-linked defects at the lattice of textured grains were formed, which acted as phonon scattering centers in the middle-temperature region. In addition, the grain boundary and parallel textured interface in the “brick-wall” structure played a dominant role at low temperatures. It can be seen that the texturization process promoted the enhancement of whole-scale phonon scattering.
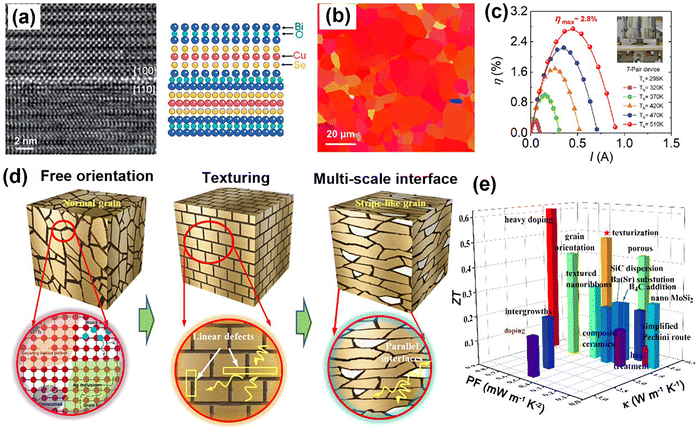 | ||
| Fig. 14 (a) TEM results of BiCuSeO, and the atomic configuration was presented, reproduced with permission.268 Copyright 2014 RSC Publishing; (b) EBSD for high textured BiCuSeO, (c) thermoelectric conversion efficiency of textured BiCuSeO-leg, the large efficiency presented, reproduced with permission.54 Copyright 2024 Elsevier Ltd; (d) texturization process of Ca3Co4O9, including epitaxial growth and multiscale interface, reproduced with permission.194 Copyright 2024 Elsevier B.V.; (e) a comparison of ZT values for Ca3Co4O9. | ||
Ultimately, the large ZT value of 0.493 is obtained at 1073 K for sandwich-type silver/Ca3Co4O9 composites.269 The multilayer co-fired strategy is adopted to build textured composites, and the complex and enriched point defects tuned at the textured interface are realized. It can be seen from Fig. 14e that the non-oxides SiC and MoSi2 additives deteriorate the electrical conductivity and then the power factor decreases. By comparison, the texturization technology is very conducive to the electrical transportation of Ca3Co4O9. In particular, the Ca3Co4O9-based textured composites lead to rational interface-enriched defects. The results show that the metal-semiconductor interface between silver and lamellae-shaped matrix is smooth along the parallel orientation direction. It is an elevated fundamental understanding of the crucial effect of the textured interface to enhance the thermoelectric performance of thermoelectric oxides, which synergistically modulates charge carriers and phonon transport properties.
Besides, ZnO, CaMnO3, Bi2O2Se, and Bi2O2Te oxyselenides are all promising for improving thermoelectric performance through the texturization regulation, which offers potential implications in medium- and high-temperature areas.
4.3 High entropy
Several strategies, for example, band alignment,270 quantum effect,271 orientation engineering,272 and compositing effect,25,273 have been adopted to improve the thermoelectric performance of oxide candidates. Entropy engineering, first reported in 2015,274 has been proven to be a promising and flexible strategy to boost high ZT.275–278Attempts to synthesize high entropy oxides still remain in a primary stage, while more progress has been reported in other thermoelectric materials such as chalcogenides.26,279 The entropy stabilization effect can afford heavy doping and complex element substitution to form single-phase thermoelectric materials. Additionally, the entropy stabilization effect can stabilize a relatively large amount of strain caused by the mass and size fluctuation of multiple elements and thus allow the coexistence of multiple defects such as oxygen vacancies, edge dislocations, in-phase rotations of octahedra, and antiparallel cation displacements.155 Considering the structure-property relations brought by the high entropy effect, the lattice distortion effect can strongly suppress the thermal conductivity, and the sluggish diffusion effect can control the grain boundary, which might help realize fine grains. Besides, the cocktail effect can bring performance optimization with elements doped together instead of a single dopant.
High entropy oxides are mostly designed as A-site entropy engineering of perovskite oxides. High entropy doping of the A-site of n-type SrTiO3 as high-quality single crystalline thin films has realized significant scattering on heat transport (resulting in thermal conductivity close to the amorphous limit), indirectly affecting the tolerance factor and thus improving mobility. By introducing the element size fluctuation, mass fluctuation, and strong strain through entropy design, the high-density dislocation was stabilized, and the phonon scattering was enhanced. With the decrease of Ti displacement as the configuration entropy increased, the weighted mobility of the sample increased. As a result, a high level of key indicator for electron-phonon decoupling (μW/κL ∼ 5.2 × 103 cm3 K J−1 V−1 for (Sr0.2Ba0.2Ca0.2Pb0.2La0.2)TiO3 thin film) has validated the feasibility of applying the high entropy strategy to improve thermoelectric properties, as shown in Fig. 15a–c.47 New n-type high entropy oxides, such as tungsten bronze47 and wolframite oxides,280 have also been explored. (Sr0.2Ba0.2Li0.2K0.2Na0.2)Nb2O6 synthesized by Subhra could realize a maximum ZT value of ∼0.23 at 1150 K.47 For p-type thermoelectric oxides, Kumar et al.281 reported a maximum figure of merit (ZT) of 0.23 ± 0.02 at 350 K is obtained for (LaNdPrSmEu)0.95Sr0.05CoO3, as shown in Fig. 15d. For other attempts of high entropy design in the B-site of SrTiO3-based ceramics, the reduction of lattice thermal conductivity is combined with a severe deterioration of electrical conductivity.155,282
 | ||
| Fig. 15 (a)–(c) An entropy design case focuses on the (Sr0.2Ba0.2Ca0.2Pb0.2La0.2)TiO3 thin film, reproduced with permission.47 Copyright 2024 The Authors. (a) The schematic of a series structure design based on high entropy design, followed by (b) the correlation between the tolerance factor tobs and μW, which displayed the effect of tuning the element's average radius. As a result, the entropy design contributed to (c) the improved ZT. (d) Entropy design to boost ZT performance of La, SrCoO3-based ceramics, reproduced with permission.281 Copyright 2022 The Authors; (e) Correlation of thermal conductivity with size disorder parameter of all 22 single-phase pyrochlores, indicating the nonmonotonic variation between thermal conductivity and entropy, reproduced with permission.284 Copyright 2020 Elsevier Ltd. | ||
Elements still matter in deciding the properties. The 4f orbitals of Pr/Tb dopants in high entropy (Zr0.2La0.2Tb0.2Sm0.2Y0.2)O2−δ could reduce the band gap to 2.0 eV.283 However, a direct relationship between entropy and a certain property has not been established yet. Take thermal property as an example. Size disorder factor, as an indirect indicator of high entropy effect, does not fit well in a linear relationship with thermal conductivity, in the case shown by Wright et al.,284 as shown in Fig. 15e. The lattice thermal conductivity does not necessarily decrease with increasing entropy. Furthermore, high entropy does not always bring the best properties. Attempts at middle entropy can also give rather nice results, and a combination of several strategies has started to become useful. Eu and Nb co-doped Eu0.1Ca0.9Ti0.8Nb0.2O3 ceramics designed via entropy engineering and recrystallization strategies are reported to reach a high ZT value of 0.42 at 1077 K, which is among the best reported CaTiO3-based materials.165 Systematic and fundamental research to correlate the entropy design and the corresponding properties is strongly warranted. How the disorder from certain cation sites to the overall system can result in certain structure characteristics from the atomic level to mesoscale and thus cause unique properties, remains unpredictable.
In general, the lattice thermal conductivity reduction from the high entropy lattice distortion effect is common in literature, and the mediocre PF of ∼250 to 500 µW m−1 K−2 limited the ZT to the range of 0.1 to 0.3. The electrical mobility deteriorates in high entropy oxides when reducing the phonon mean free path. When applying the high entropy strategy, the design of the element is quite important. Studies up till now could not monotonically relate all properties to increasing entropy.
4.4 Homo-structures
Designing homo-structures is beneficial to constructing coherent interfaces, inducing energy filtering effects, forming a two-dimensional electron gas, and so on, which has the potential to decouple electron-phonon transport properties and achieve better thermoelectric performance.228,285,286 To realize the above modulation effects, methods like in situ compositing, modulation doping, and sandwich/superlattice structure design are widely used.25,77,78,287,288 The following will introduce four strategies related to homo-structures that have been adopted in thermoelectric oxides, including coherent-interface construction, modulation doping, energy filtering effects, and two-dimensional-electron-gas formation.Coherent interfaces, illustrated in Fig. 16a, can scatter phonons while maintaining charge transport, leading to low thermal conductivity and high carrier mobility simultaneously.289 By using in situ compositing methods, coherent interfaces may be formed.25,287 Zhou et al. used self-propagating high-temperature synthesis combined with the spark plasma sintering (SHS-SPS) method to prepare Cu2Se–BPCCSO (Pb, Ca co-doped BiCuSeO) composites.25 Interestingly, the additional Cu-Se layer and Cu layer were coherently intercalated in the BPCCSO lattice. Since the lattice structure of BiCuSeO consists of (Bi2O2)2+ layers and (Cu2Se2)2− layers, such phenomena could be interpreted and may be expanded to other similar materials, where it is possible for two different phases to form homo-structures. Here, the lattice thermal conductivity of Cu2Se–BPCCSO was lower than Cu2Se or BPCCSO before compositing. At the same time, the carrier mobility was slightly improved after in situ composting.
 | ||
| Fig. 16 Schematic illustrations showing (a) coherent interface; (b) modulation doping, reproduced with permission.288 Copyright 2014 American Chemical Society; (c) energy filtering effect, reproduced with permission.290 Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; (d) two-dimensional electron gas (2DEG), reproduced with permission.291 Copyright 2020 The Chinese Ceramic Society. | ||
To simultaneously improve the carrier concentration and mobility, modulation doping is an effective strategy, which has been applied in many thermoelectric materials such as BiCuSeO, SiGe, and Bi6Cu2Se4O6.288,292,293 Different from conventional doping, modulation-doped samples are composites mixed with undoped and heavily doped phases, as shown in Fig. 16b.288 Due to the difference in Fermi levels, the carriers in heavily doped phases can spontaneously move to undoped phases with less scattering, which is conducive to carrier mobility enhancement.289 For example, the carrier mobility of modulation-doped Bi0.875Ba0.125CuSeO that was mixed by BiCuSeO and Bi0.75Ba0.25CuSeO was twice as much as that of uniformly doped Bi0.875Ba0.125CuSeO, when the carrier concentration was almost the same.288 Modulation doping can be used in many other thermoelectric materials to further improve carrier mobility, but long-term stability, especially under high-temperature environments, should also be considered.
Energy filtering effects are beneficial to improving the Seebeck coefficients, which may occur at interfaces between matrix and precipitate or interfaces of two semiconductors with different bandgaps.286 A potential barrier will be formed at the interface, which filters out low-energy carriers while selectively allowing high-energy ones to pass through, as illustrated in Fig. 16c.290 This mechanism enhances the average energy of carriers participating in electrical transport, leading to an increased density of states and a higher Seebeck coefficient.294 Such phenomena have been successfully proven in ZnO-based thin films.77,118 For example, the electrical conductivity can be largely improved from 135 S cm−1 to 466 S cm−1 after vacuum annealing due to the increased carrier concentration. However, the increased carrier concentration has detrimental effects on the Seebeck coefficients. To balance the correlation between electrical conductivity and the Seebeck coefficient, Zhou et al. designed GZO–ZnO–GZO sandwich structures.77 A series of high-quality GZO–ZnO–GZO thin films were prepared by using the PLD technique. Attributed to the energy filtering effects that occurred at the GZO–ZnO interfaces, the effective density of states was improved from ∼0.9 m0 to 1.0 m0, and the Seebeck coefficient was optimized during the entire measuring temperature range. Based on the sandwich structure design and defect engineering, a ∼30% improvement in power factor was realized in GZO–ZnO–GZO-based thin films.
Forming a two-dimensional electron gas (2DEG) has also been demonstrated as an effective strategy for enhancing thermoelectric performance. 2DEG refers to electrons confined to move within a two-dimensional plane while their transport in the perpendicular direction is limited. Such 2DEG systems typically emerge at interfaces between materials with either polar discontinuity or modulation doping.295–297 As Fig. 16d illustrates, when two semiconductors with different Fermi levels come into contact, carriers migrate from the material with higher Fermi levels to the one with lower Fermi levels. This carrier redistribution causes upward and downward shifts of the respective Fermi levels until equilibrium is achieved, resulting in band bending and spatial confinement of electrons within the 2D interfacial region.291 Ohta et al. fabricated SrTiO3/SrTi0.8Nb0.2O3/SrTiO3 superlattice thin films on LaAlO3 substrates, observing that when the thickness of SrTi0.8Nb0.2O3 layer was reduced below 1.56 nm, the absolute Seebeck coefficient increased dramatically from 100 µV K−1 to 480 µV K−1.78 Similarly, an ultrahigh Seebeck coefficient of 850 µV K−1 was achieved in TiO2/SrTiO3 heterostructure films, with comparative experiments confirming the dominant role of interfacial 2DEG.78 Furthermore, Chen et al. demonstrated that lattice polarization and interfacial polarization could synergistically induce 2DEG, enabling SrTi0.8Nb0.2O3 thin films directly grown on SrTiO3 substrates to attain a ZT value of 1.6.298
4.5 Other strategies for carrier-phonon decoupling
The key point to improving the thermoelectric performance is to decouple the electrical and thermal transport,23 which means differentiating the scattering source of carriers and phonons.299 Since the lattice thermal conductivity is high in oxides, it is common to apply methods to scatter phonons. However, the carriers could be scattered simultaneously, limiting the property enhancement of thermoelectric oxides. A representative study demonstrates the formation of phonon-glass electron-crystal behavior in La0.5Na0.5Ti1−xNbxO3 thermoelectric oxides, where there is a very weak coupling of phonon transport to the electronic power factor.300 Additionally, several strategies have been developed to synergistically improve the electrical and thermal transport in thermoelectric oxides, including interface engineering from extrinsic and mesoscopic aspects like orientation modulation and compositing design, and symmetry engineering from intrinsic and lattice aspects like symmetry engineering, which will be reviewed below. | ||
Fig. 17 Grain boundary scattering. (a) Schematic diagram of ionized impurity scattering (IIS) and grain boundary scattering (GBS), reproduced with permission.302 Copyright 2022 RSC Publishing; (b) the schematic explaining how the vertical grain boundaries affect the n, md*, and µ simultaneously, reproduced with permission.272 Copyright 2023 Wiley-VCH GmbH; (c) the electrical conductivity of strontium titanate and magnesium antimonide both suffer from the effect of grain boundaries, reproduced with permission.309 Copyright 2020 The Authors; (d) and (e) the lower oxygen vacancy concentrations 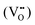 in the (d) grain boundary regions result in free carrier (n) depletion at (e) grain boundaries. Thus, the grain boundaries become resistive. Graphene promotes the formation of oxygen vacancies in the vicinity of grain boundaries adjacent to the graphene, resulting in localized increases in carrier concentration, reproduced with permission.309 Copyright 2020 The Authors. in the (d) grain boundary regions result in free carrier (n) depletion at (e) grain boundaries. Thus, the grain boundaries become resistive. Graphene promotes the formation of oxygen vacancies in the vicinity of grain boundaries adjacent to the graphene, resulting in localized increases in carrier concentration, reproduced with permission.309 Copyright 2020 The Authors. | ||
Commonly, a large grain misorientation angle would lead to dense defects due to the lattice mismatch.303 The dense defects around the grain boundary would induce charge accumulation and energy barriers, scattering carriers. Taking SrTiO3 as an example, in donor-doped SrTiO3, the Sr vacancies would be rich at grain boundaries, forming a negatively charged barrier to prevent electrons from passing through.304–306 Therefore, minimizing the orientation mismatch could improve carrier conduction. For ceramics, texturing could tune the orientation of grains. When the materials are layered structured, like Bi-containing layered compounds, the sintering under pressure would induce a textured structure.42,225 Using tape-casting combined with templated grain growth methods, Shi et al. acquired Ca3Co4O9 with highly oriented grains, achieving a PF of 640 µW m−1 K−2, 3 times the PF of unoriented samples.307 Zhang et al. added Sr3Ti2O7 nanoplates as seeds when fabricating La-doped SrTiO3, successfully suppressing the thermal conductivity while maintaining good PF.308 For thin films, film growth of high quality is important, and the orientation of the substrates would decide the surface energy and growth modes, hence influencing the morphology of thin films. By trying different substrates of different orientations, Zheng et al. found that when growing on the LSAT(001) and (110) single-crystal substrates, the La-doped SrTiO3 prefers to apply a layer-by-layer growth mode, reducing the density of grain boundaries and the misorientation angles, and causing single-crystal-like electrical transport. While the (111)-oriented growth leads to island growth modes, bringing about significant grain boundary scattering at room temperature (Fig. 17b).
On the one hand, the electrical conductivity could be improved. Firstly, the introduced phases are often conducting materials, and increasing the quantity of conducting materials could improve the electrical conductivity. Dixit et al. introduced MXene into Nb-doped SrTiO3 and realized 1851% enhancement in electrical conductivity.310 ZnO of high mobility and electrical conductivity was also composited with SrTiO3.311 Secondly, the introduced materials like carbon-based materials or metals could reduce the grain boundary to create oxygen vacancies, which could help lower the energy barrier caused by metal vacancies312 in n-type oxides, resulting in single-crystal-like electron transport behaviors309,313 (Fig. 17c–e). Metal Ti in SrTiO3,267,314,315 reduced graphene oxide in Nb-doped SrTiO3,316 graphene in La-doped SrTiO3,45 graphite in La, Nb co-doped SrTiO3,312in situ grown carbon nanotube on ZnO,317 Fe2O3-functionized graphene in Nb-doped SrTiO3,318 multiwalled carbon nanotubes in ZnO,319 and graphite nanosheets in exfoliated Bi2O2Se39 were extensively reported in the field. Thirdly, the modulation doping could provide second phases with low carrier concentration and simultaneously high mobility, which is also a method to improve mobility, and has been demonstrated in BiCuSeO.288
On the other hand, the Seebeck coefficient could be enhanced. The energy filtering effect is widely applied in many kinds of thermoelectric composites. When carriers transport across interfaces with energy barriers, low-energy carriers would be scattered, with high-energy carriers moving through. As a result, the Seebeck coefficient would be enhanced.320–322 Lin et al. proposed a two-phase model, taking into account the thermal resistance and related additional Seebeck coefficient of grain boundary phases, well explaining the grain boundary phase effect on the Seebeck coefficient.323
It is noteworthy that recently, thermoelectric oxides have been used as second phases in alloy-based thermoelectrics. Zhou et al. constructed Cu2Se–BiCuSeO–graphene three-phase polycrystal ceramics, improving the stability of Cu2Se and simultaneously reaching ZT ∼ 2.8 at 1000 K.25 By incorporating TiO2−n into the Mg3(Sb,Bi)2-based matrix, the grain boundary phases are in situ engineered, yielding a superior ZT exceeding 2.0 at 798 K.324
In perovskite thermoelectric oxides, there is a factor that primarily guides the modulation of symmetry, which is called the tolerance factor  .136 The closer t is to 1, the more symmetrical the material is. There are different kinds of BO6 distortion, including cation displacement, BO6 tilting, and BO6 rotation, which could lower the effective mass and mobility. They could be inferred from CaTiO3 and BaTiO3-based ceramics163 (Fig. 18). According to the tolerance factor, by tuning the average radius of A sites and B sites, the symmetry would be modulated. Wang et al. reported that in (SrO)(SrTiO3)2, Ln-doping and increase of temperature could both restore the cubic symmetry of octahedron-tilted (SrO)(SrTiO3)2, with Ti–O–Ti angle changing from 177 degrees to ∼180 degrees, hence increasing effective mass from 3m0 to 7.5 m0 at 1000 K86 (Fig. 19a and b). The cation displacement would cause d orbital overlapping, leading to localization of carriers. Zheng et al. introduced entropy engineering to the A site of SrTiO3. Apart from minimized lattice thermal conductivity, through A-site size tuning when engineering entropy, the displacement of Ti was reduced, thus recovering the carrier mobility (Fig. 19c and d). Since the symmetry modulation is often realized by forming a different solid solution that naturally strongly scatters phonons, the symmetry modulation is regarded as an ideal strategy for decoupling electrical and thermal transport.
.136 The closer t is to 1, the more symmetrical the material is. There are different kinds of BO6 distortion, including cation displacement, BO6 tilting, and BO6 rotation, which could lower the effective mass and mobility. They could be inferred from CaTiO3 and BaTiO3-based ceramics163 (Fig. 18). According to the tolerance factor, by tuning the average radius of A sites and B sites, the symmetry would be modulated. Wang et al. reported that in (SrO)(SrTiO3)2, Ln-doping and increase of temperature could both restore the cubic symmetry of octahedron-tilted (SrO)(SrTiO3)2, with Ti–O–Ti angle changing from 177 degrees to ∼180 degrees, hence increasing effective mass from 3m0 to 7.5 m0 at 1000 K86 (Fig. 19a and b). The cation displacement would cause d orbital overlapping, leading to localization of carriers. Zheng et al. introduced entropy engineering to the A site of SrTiO3. Apart from minimized lattice thermal conductivity, through A-site size tuning when engineering entropy, the displacement of Ti was reduced, thus recovering the carrier mobility (Fig. 19c and d). Since the symmetry modulation is often realized by forming a different solid solution that naturally strongly scatters phonons, the symmetry modulation is regarded as an ideal strategy for decoupling electrical and thermal transport.
 | ||
| Fig. 18 Distortion of BO6 octahedron. (a)–(c) Cation displacement in a BO6 octahedron: cation displacement along (a) the tetrad axis, (b) the triad axis, and (c) the diad axis. (d)–(f) Tilt axes of an octahedron: (d) one of three tetrad axes; (e) one of six diad axes; (f) one of four triad axes, reproduced with permission.136 Copyright 2016 John Wiley & Sons, Ltd. | ||
 | ||
| Fig. 19 Tuning thermoelectric properties of perovskite oxides by modulating symmetry. (a) Temperature dependence of electrical conductivity σ, Hall mobility μH, carrier concentration nw, Seebeck coefficient S, DOS effective mass md* and scattering factor r of 5 at% Sm-doped SrO(SrTiO3) (SSSTO) and 5 at% Nb-doped SrO(SrTiO3) (SSTNO), reproduced with permission.325 Copyright 2007 AIP Publishing; (b) variation of the O(3)–Ti–O(3) bond angle with temperature for SSSTO, reproduced with permission.325 Copyright 2007 AIP Publishing; (c) schematic of the possible mechanism of electron scattering and localization in Ti-displaced TiO6 octahedrons. The C point marked is the center of the TiO6 octahedron, and the length of Ti displacement is the length of C–Ti, reproduced with permission.47 Copyright 2024 The Authors; (d) the relation between room temperature weighted mobility (μW) and average normalized Ti displacement (dTi) of SBLTO, SBCLTO, and SBCPLTO with increased entropy. The displacement was normalized by the ratio dTi = length(C–Ti)/length(C–Ob) in (c). The error bar is the standard deviation δd. The blue and red arrows are the guides to show the trends of weighted mobility and displacement, reproduced with permission.47 Copyright 2024 The Authors. | ||
5. Emerging applications of oxide thermoelectrics
Emerging applications based on oxide thermoelectrics are gaining increasing attention due to the unique combination of material properties and functional versatility. Oxide-based thermoelectric materials offer several advantages, including low cost, simple fabrication, high thermal stability, excellent resistance to high temperatures and oxidation, non-toxicity, and reliable long-term performance. These features position oxide thermoelectrics not only as viable candidates for conventional uses such as power generation and cooling, but also as key enablers of a new class of emerging technologies that leverage thermoelectric effects in innovative and cross-disciplinary contexts. In particular, oxide thermoelectrics are being explored in rapidly developing areas such as the photothermoelectric effect, transverse thermoelectric devices, thermoelectrocatalysis, and multifunctional sensing. These emerging applications demonstrate the adaptability of oxide materials and open new pathways toward next-generation energy conversion, intelligent sensing, and integrated electronic–thermal systems.5.1 Photothermoelectric effect
The photothermoelectric (PET) effect refers to the generation of an electrical voltage or current in a material when it is exposed to light, resulting in a localized temperature gradient that drives the thermoelectric effect.55,326 As Fig. 20a exhibits, this phenomenon combines photothermal conversion and thermoelectric conversion, where absorbed light energy is converted into heat, creating a temperature gradient, which is then harnessed to generate electrical energy. The photothermoelectric effect was first discovered by Tauc327 in 1955 and subsequently investigated further in conventional semiconductors.328,329 The photothermoelectric effect has potential applications in fields such as solar energy harvesting, photodetectors, energy-efficient optoelectronic devices330 and infrared in-sensor computing.325 Compared to traditional photodetectors, PTE photodetectors eliminate the need for external bias and electronic transitions, thereby reducing noise, overcoming bandgap limitations, and enabling a broader spectral detection range. Due to their high thermal stability, non-toxicity, low cost, and improved mechanical properties, oxide thermoelectric materials are regarded as outstanding candidates for photothermoelectric applications. These materials exhibit remarkable photothermoelectric performance across a wide spectral range, with notable examples including ZnO,331,332 PbO,333 SrTiO3,334 and BiCuSeO.335 As summarized in Table 2, oxide-based PTE detectors offer broadband spectral responses along with diverse performance characteristics, such as high responsivity and rapid response times, exemplified by materials like SrTiO3, LaAlO3/SrTiO3, Co-doped BiCuSeO, and ZnO.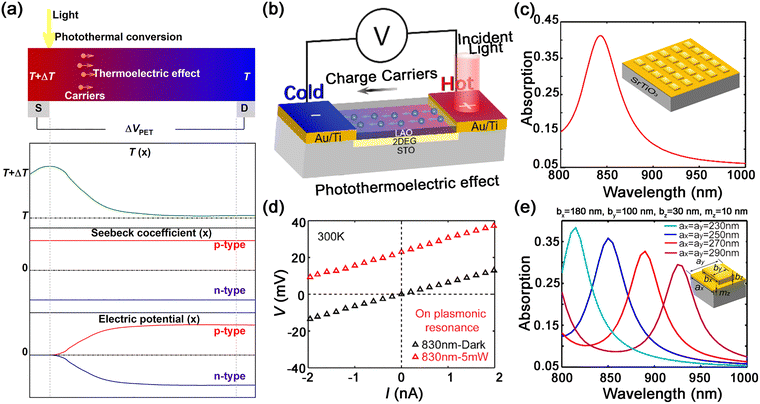 | ||
| Fig. 20 (a) Illustration of the PTE (photothermoelectric) conversion processes, including both p-type and n-type materials. (b) Schematic of infrared PTE voltage generation by 2DEG channel. (c) Absorption spectrum of the nanocuboid array used in the experiment. (d) I–V curves at room temperature with the 830 nm laser beam focused on the patterned electrode in the presence of the 2DEG channel. (e) Absorption spectra of nanocuboid arrays at nearinfrared wavelengths with varying periodicity values, reproduced with permission.336 Copyright 2019 American Chemical Society. | ||
| Active material | Spectral range | Responsivity | Response time | Ref. |
|---|---|---|---|---|
| SrTiO3 | 0.325–10.67 µm | 1.18 V W−1 at 10.57 µm | 1.5 s | 334 |
| LaAlO3/SrTiO3 | 830 nm | 4.4 V W−1 at 830 nm | — | 336 |
| 4% Co:BiCuSeO | 444–1600 nm | 0.48 V W−1 at 808 nm | 195 ms | 335 |
| ZnO | 365–760 nm | 1.56 × 10−4 A W−1 at 365 nm | — | 331 |
| AZO | 200–2200 nm | 1.5 mV W−1 under solar radiation | — | 412 |
High-sensitivity PTE sensors require materials with a large Seebeck coefficient, high photon absorption capacity, and low thermal conductivity.55 As a traditional perovskite-type oxide thermoelectric material, the reduced SrTiO3-based PTE photodetector demonstrates outstanding photothermal performance, with a sensitivity of up to 1.18 V W−1 and a broadband spectral response ranging from 325 nm to 10.67 µm. This exceptional performance is attributed to its high Seebeck coefficient (∼1037 µW K−1) and phonon-enhanced photoresponse in the long-wavelength infrared region.334 One key strategy to enhance PET responsivity is by increasing the Seebeck coefficient. For example, slight Co3+ doping in BiCuSeO single crystals effectively modulates the Seebeck coefficients, significantly improving the performance of BiCuSeO photodetectors across the visible to infrared spectral regions, achieving an excellent responsivity of 0.48 V W−1 at 808 nm. Another effective approach is increasing the temperature gradient, which further enhances PET responsivity. The ZnO device demonstrates a coupling enhancement of photothermoelectric conversion, with a 67.2% increase in output current and a 10.9% increase in output voltage at 227.4 mW cm−2 light illumination and a 12.5 K temperature gradient.
The formation of a 2DEG significantly enhances the Seebeck coefficient, thereby improving the responsivity of the PTE effect. Additionally, by leveraging surface plasmon resonance, antenna effects, and phonon absorption to enhance light absorption, the responsivity of PTE detectors can be further optimized.55 As demonstrated in Fig. 20b–e, substantial infrared photothermoelectric voltage generation at LaAlO3/SrTiO3 (LAO/STO) interfaces is achieved by exploiting the giant Seebeck coefficient of the interfacial 2DEG and the strong absorption of low-energy photons facilitated by plasmonic metasurface structures, yielding a room-temperature responsivity of 4.4 V W−1.336 Here, integrating plasmonic metasurface structures (nanocuboid arrays) on top of metal contacts significantly improves infrared light absorption, boosting the PTE voltage by more than 20 times compared to plain metal contacts, due to the enhanced absorption near plasmonic resonances. Notably, the light absorption efficiency of the nanocuboid array varies with frequency, and its design parameters can be tuned to significantly enhance optical absorption across a range of wavelengths, enabling broadband and programmable spectral responses. For instance, adjusting the separation between adjacent nanocuboids from 230 nm to 290 nm shifts the resonant absorption wavelength from 815 nm to 935 nm. This work presents a novel pathway for efficient and programmable light sensing and optoelectronic applications by combining the large Seebeck coefficient of the 2DEG at the LAO/STO oxide interface with plasmonic metasurfaces, enabling high-performance photothermoelectric effects across a wide range of infrared wavelengths.
Oxide thermoelectric materials, especially those exhibiting the PTE effect, are garnering significant attention for their promising applications in energy harvesting, photodetectors, and advanced optoelectronic devices. These materials offer advantages such as high thermal stability, non-toxicity, and low cost, making them ideal for large-scale applications. Notably, the optimization of PTE performance through strategies such as Seebeck coefficient enhancement, temperature gradient control, and the use of plasmonic metasurfaces has paved the way for more efficient devices. Moreover, with breakthroughs in multifunctional interfaces and the integration of machine learning algorithms to accelerate high-performance material screening and multi-dimensional structural design, oxide-based PTE systems hold transformative potential. The future of these materials lies in their ability to not only achieve high sensitivity and broadband responses but also to integrate seamlessly with intelligent systems for precise optical positioning,337,338 energy-efficient infrared sensing, real-time photothermal detection, image sensing, and infrared in-sensor computing.325 This evolution could lead to the development of compact, self-powered devices, which are already being explored for in-sensor convolutional computing, offering great promise for next-generation applications in smart imaging and real-time data processing.
5.2 Transverse thermoelectric effect
The transverse thermoelectric (TTE) effect refers to a distinct thermoelectric phenomenon in which a longitudinal temperature gradient is converted into a transverse voltage within materials exhibiting anisotropic Seebeck coefficients. In this effect, the thermoelectric properties of the material exhibit direction-dependent behavior, meaning that a temperature gradient in one direction induces an electrical voltage in a perpendicular direction. The transverse thermoelectric effect, when driven by optical radiation as a heat source, is commonly referred to as the light-induced transverse thermoelectric effect (LITT effect). The LITT effect was first observed by Chang et al. in 1990 in YBa2Cu3O7−δ thin films with a c-axis inclined growth.339 Subsequently, Lengfellner et al. proposed a model of a series connection of thermocouples, suggesting that the layered inclined structure of the YBa2Cu3O7−δ films naturally forms a thermocouple array.340 This structure generates a significant transverse voltage under the influence of a temperature gradient. They further developed a theoretical formula to describe the transverse voltage in the LITT effect. As illustrated in Fig. 21, when a film with an inclined crystal structure is exposed to light or a heat source, a temperature gradient (ΔTz) forms along its thickness. This results in a voltage signal (Ux) appearing on the film surface in the projection direction of the induced crystal plane. The generated voltage (Ux) is explained using an atomic layer thermopile model341 and can be quantitatively expressed as:| Ux = sin(2θ)l|Sab − Sc|ΔTz/2d |
| TTE material | θ (°) | Voltage (V) | Response time (ns) | ΔS (µV K−1) | Energy (mJ) | Ref. |
|---|---|---|---|---|---|---|
| SrTiO3 | 15 | 0.052 | 80 | — | 0.15 | 351 |
| SrTiO3/SrTi1−xNbxO3 | 10 | 0.744 | 200 | −11.8 | 3.94 | 347 |
| La1−xAxMnO3 (A = Ca, Sr, Pb) | 5–20 | 0.017–2.23 | 82–500 | 0.14–4.58 | 0.13–1.8 | 355–360 |
| La1−xSrxCoO3 | 5–10 | 0.15–0.99 | 16–77 | 0.03 | 0.24–0.81 | 361–363 |
| AxCoO2 (A = Co, Na) | 10–59 | 0.45–63 | 10–100 | 35–50 | 1–1.5 | 346, 364 and 365 |
| Ca3Co4O9 | 3–10 | 0.23–6.4 | 100–700 | 33.4 | 1 | 366 and 367 |
| Bi2Sr2Co2Oy | 10 | 3.35 | 200 | — | 5 | 368 |
| Bi1−xAxCuSeO (A = Ba, Pb, La) | 10 | 4.5–31.4 | 68.8–208 | 3 to 20 | 3–15 | 343 and 369–371 |
The transverse thermoelectric voltage is strongly influenced by both geometric parameters and thermoelectric transport properties. It has been reported that optimizing the transverse thermoelectric performance can be achieved by adjusting both the inclination angle and thickness of the film.349,353,372 For example, the TTE performance of the YBa2Cu3O7−δ film was optimized by regulating the film thickness and inclination angle (∼18°, 200 nm), with the response time decreasing monotonically with the thickness.373 Doping can effectively modulate thermoelectric transport properties, thereby enhancing TTE performance. For instance, doping BiCuSeO with Ba343 or La374 increases electrical conductivity and enhances the absorption coefficient (γ), resulting in a higher ΔTz under irradiation, which subsequently improves the TTE voltage.
Additionally, the construction of superlattice structures provides a promising strategy to optimize the transverse thermoelectric performance. As shown in Fig. 22a–c, artificial SrTiO3/SrTi1−xNbxO3 (STO/NSTO) multilayer films were epitaxially grown on 10° off-cut (001)-oriented LaAlO3 single crystal substrates using a pulsed laser. Remarkably, the unique transverse thermoelectric voltage signal of STO/NSTO reached up to 0.744 V (Fig. 22d and e), which is several hundred times higher than that of bulk Nb-doped SrTiO3375 (∼3.0 mV) single crystals with the same inclined orientation. Layered constrained structures with inclined crystal orientations play a crucial role in inducing anisotropic transport properties both in-plane and out-of-plane, thereby increasing ΔS. The enhanced anisotropy is attributed to the distinct electron transport properties in the ab plane versus the c-axis direction, which stem from the confinement of electrons between the two STO insulating layers. The multilayered NSTO structure not only improves photothermal conversion efficiency but also allows for fine-tuning of the response time by adjusting the NSTO layer thickness and the number of thermal scattering interfaces.347
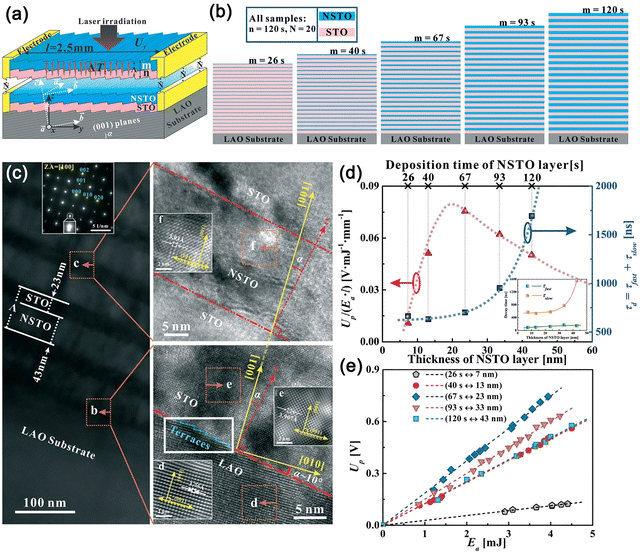 | ||
| Fig. 22 (a) The schematic cross-sectional view of the STO/NSTO multilayer films with an inclined orientation. (b) Sketch for the formula of STO/NSTO multilayer films. (c) Cross-sectional TEM studies in the zy-plane showing the orientation relationship and epitaxial behavior of the STO/NSTO multilayer films on a 10° off-cut (001)-oriented LaAlO3 substrate. (d) Typical time dependence of the TTE voltage signals of a STO/NSTO multilayer film with a thickness of NSTO layer (tNSTO) ≈ 23 nm at Ea = 3.94 mJ along the x direction and y direction. (e) The tNSTO dependence of the magnitudes of Up/(Ea·l) and response time. The dashed lines are used to guide the trend. The inset shows the relationship between the two decay time components and tNSTO, reproduced with permission.347 Copyright 2014 Royal Society of Chemistry. | ||
The LITT effect, using light as the heat source, is a photo-thermal-electric conversion process that involves both photo-thermal and thermoelectric conversion. By incorporating a photothermal layer on the film surface, the photo-thermal conversion efficiency is enhanced, thereby improving the overall LITT performance. For instance, introducing a graphite layer as a light-absorbing material onto c-axis inclined Bi2Sr2Co2Oy thin films leads to a notable enhancement of the transverse thermoelectric effect, attributed to the increased light absorption in the graphite layer.376 Additionally, BiCuSeO thin films combined with ultra-thin AuNPs layers further enhance photothermal conversion efficiency through localized surface plasmon resonance, resulting in a lateral voltage response that is 2.8 times greater than that of conventional structures.371
The optimization of TTE performance in oxide materials has made significant progress through strategies such as geometric structure parameter tuning, element doping, multilayered structure construction, and photo-thermal layer design. These approaches have led to notable improvements in TTE voltage generation and response times, thus expanding the potential applications of the TTE effect in areas like waste heat recovery, photodetectors, and thermal sensing systems. Further advancements in the transverse thermoelectric effect can be achieved not only through the strategies outlined above but also by integrating modern semiconductor technologies to enhance the performance of TTE devices. For instance, the development of miniaturized devices based on the TTE effect can be realized by combining it with advanced techniques such as lithography. This integration not only facilitates device miniaturization but also contributes to improved performance, thereby broadening the potential for their application in compact, high-performance systems, including integrated sensors and energy-harvesting devices. The synergy between material design, fabrication techniques, and device optimization holds great promise for the future of transverse thermoelectric devices, opening up new avenues for their practical implementation and commercial viability.
5.3 Thermoelectrocatalysis
Thermoelectrocatalysis (TECatal) has recently emerged as a promising and innovative approach that integrates the thermoelectric effect with surface catalysis processes.56 This concept leverages thermoelectric (TE) materials as both catalysts and catalyst promoters, facilitating a wide range of applications. TECatal operates by converting various forms of heat—whether arising from relatively small temperature gradients in natural environments, industrial processes, or everyday activities—into chemical energy through the interaction of TECatal materials.The converted energy can then drive a broad spectrum of catalytic reactions,377 enabling impactful applications in diverse fields such as environmental gas mitigation, energy storage, and the photoreduction of nuclear wastewater.378 Notably, the Seebeck voltage generated by TE materials has been found to significantly enhance catalytic activity, boosting reaction rates by several orders of magnitude.379,380 This phenomenon, driven by the thermoelectric effect, holds considerable promise for advancing catalytic processes and unlocking new avenues for efficient and sustainable technologies.
To guide the search for optimal thermoelectric (TE) materials for thermoelectrocatalysis, Huang et al. proposed a new parameter, β, representing the Seebeck voltage per unit of heat loss as a figure of merit (FOM) for thermoelectrocatalysis applications. The expression for β is given by: β = V/Q = SΔT/κΔT = S/κ. This definition gives positive values for the p-type TEs and negative values for the n-type TEs, and the higher the magnitude of the |β|, the higher the capability of the TE material for thermoelectrocatalysis according to the observed rate equation Ln(r/r0) = −rSΔT/kBTh.381 Generally speaking, heat loss is inversely proportional to the thermal conductivity of the TE, and for many practical application scenarios, the temperature gradient ΔT is dependent on the thermal conductivity of the TE materials, not an independent variable. As this FOM involves the Seebeck coefficient S and thermal conductivity κ only, its optimization should be different and easier than for the energy conversion dimensionless figure of merit ZT. The calculated β of some oxides69,268,382–387 are shown in Table 4. Compared with other thermoelectric oxides, the β values of BiCuSeO-based oxyselenides are higher. Combined with its easy preparation, low cost, and ability to operate at moderately high temperatures (up to 723 K), BiCuSeO (BCSO) has been widely applied in thermoelectrocatalysis.378–380,388
| Material | Dopant | κ (W m−1 K−1) | S (µV K−1) | σ (s cm−1) | ZT | PF (µW cm−1 K−2) | β (µV m W−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| BiCuSeO | 0.32 | 425 | 13.5 | 0.45 | 2.5 | 1328 | 268 | |
| BiCu1−xSeO | Cu | 0.5 | 275 | 50 | 0.81 | 3.8 | 550 | 382 |
| Bi0.975Cu0.975SeO | 0.36 | 300 | 4700 | 0.6 | 2.88 | 833 | 383 | |
| CaCoO3 | Tb | 1.57 | 320 | 112 | 0.73 | 11.5 | 204 | 384 |
| NaCoO3 | K, Sr, Y, Nd, Yd | 1.6 | 160 | 285 | 0.5 | 7.3 | 100 | 385 |
| ZnO | Al | 2 | −145 | 262 | 0.34 | 5.5 | −72 | 386 |
| SrTiO3 | Gd | 3 | −275 | 144 | 0.37 | 10.9 | −92 | 386 |
| CaMnO3 | Dy, Ho, Er, Yd | 1.7 | −155 | 146 | 0.2 | 3.5 | −91 | 387 |
| In2O3 | Ge, Mn, Zn | 10 | −180 | 111 | 0.08 | 3.6 | −18 | 69 |
BiCuSeO as a catalyst support and promoter has been applied in the reverse water-gas shift (RWGS) reaction380 and ethylene oxidation reaction.379 Here, BiCuSeO is employed as a thermoelectric material to modify the effective work function of catalyst particles, resulting in a substantial enhancement of catalytic activity. As illustrated in Fig. 23a, when a temperature gradient is applied across the BCSO material, holes on the hot side diffuse from the hot side to the cold side, generating an internal electric field. Upon reaching thermal equilibrium, the Fermi level on the hot side (eF,h) becomes higher than that on the cold side (eF,c), resulting in a potential difference (ΔεF = εF,h − εF,c = −eV), where V represents the Seebeck voltage induced by the temperature gradient. Fig. 23b depicts a schematic of a single-chamber reactor employed for the thermoelectrocatalytic RWGS reaction, where the reaction chamber, positioned above a hot plate, establishes a significant temperature gradient between the chamber bottom and the sample surface, inducing strong vertical convection that facilitates the efficient delivery of reactants and removal of products. As shown in Fig. 23c–e, it was indeed observed in experiments that the catalytic activity of metallic particles supported on thermoelectric materials, as indicated by CO2 conversion, was significantly enhanced by the Seebeck voltage generated by a temperature difference. Additionally, as depicted in Fig. 23f, there is a linear relationship between the logarithm of the catalytic activity (Ln(X)) and −eV/kBTh, which can be interpreted as the ratio of the additional electrochemical energy (−eV) induced by the thermoelectric effect to the thermal energy (kBT) of an electron, which is also in thermoelectrocatalytic ethylene oxidation reaction.
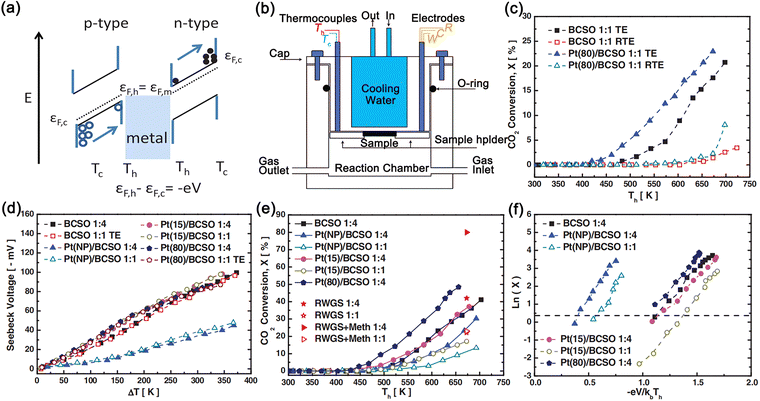 | ||
Fig. 23 (a) Energy bands of p-type and n-type thermoelectric materials in contact with metals under a temperature gradient. (b) Schematic diagram of a single-chamber reactor combining the thermoelectric effect with catalytic chemical reactions for CO2 hydrogenation (c) CO2 conversion (X) for Pt(80)/BCSO and bare BCSO with an inlet gas ratio of CO2/H2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, showing that for the same sample at the same temperature (Th), the conversion under a large temperature gradient (TE) is at least 10 times higher than under reduced temperature gradient (RTE) conditions. (d) Measured Seebeck voltages as a function of the temperature difference across the sample thickness. (e) CO2 conversion rate X increased with temperature Th. (f) A linear relationship exists between Ln(X) and −eV/kBTh, reproduced with permission.380 Copyright 2017 The Authors. 1, showing that for the same sample at the same temperature (Th), the conversion under a large temperature gradient (TE) is at least 10 times higher than under reduced temperature gradient (RTE) conditions. (d) Measured Seebeck voltages as a function of the temperature difference across the sample thickness. (e) CO2 conversion rate X increased with temperature Th. (f) A linear relationship exists between Ln(X) and −eV/kBTh, reproduced with permission.380 Copyright 2017 The Authors. | ||
Thermoelectrocatalysis represents a groundbreaking approach in catalytic science, with its fundamental mechanisms still in their early stages of exploration. This emerging field shows great promise across various catalytic applications, particularly in energy, environmental, and biomedical technologies that leverage small temperature gradients. The integration of thermoelectrocatalysis with these technologies offers a transformative opportunity to combine waste heat recovery with green catalytic processes, driving sustainable innovations. Expanding the material portfolio for thermoelectrocatalysis, broadening the scope of catalytic reactions it can support, and enhancing our understanding of its fundamental mechanisms, especially the interplay between thermal, electrical, and chemical factors, are essential for optimizing efficiency, stability, and scalability. These advancements will position thermoelectrocatalysis as a pivotal technology in the development of sustainable energy solutions.
6. Summary and outlook
6.1 Summary
Oxide thermoelectrics are a promising choice for environmentally and economically friendly thermoelectric devices, particularly for high-temperature applications. Over time, they have evolved into a substantial family of materials with diverse structures, such as perovskites and layered oxides. Depending on the sample scale, typical oxide candidates for thermoelectrics can be synthesized using a variety of methods, including sintering, melting, and thermal techniques for bulk materials, layer deposition and solution deposition for thin films, and general methods for complex systems. Among oxide thermoelectrics, ZnO, In2O3, SrTiO3, CaMnO3, and Bi2O2Se are promising n-type candidates, with Bi2O2Se layered oxyselenides demonstrating particularly high potential for achieving superior ZT. For p-type oxides, layered cobalt oxides and BiCuSeO are the most competitive candidates. However, the thermoelectric performance match between p- and n-type oxides still falls short of expectations, as many n-type materials require further optimization. Various strategies, such as defect engineering, texturization, high entropy design, and the construction of homo-structures, have been explored to enhance the thermoelectric properties of oxides.Beyond material synthesis and performance enhancement, oxide thermoelectrics have the potential to be applied in advanced devices and integrated for multi-signal transfer and output. The photothermoelectric effect and thermoelectrocatalysis open possibilities for collecting, utilizing, and converting multiple energy signals. Given that oxides are classic materials with applications in various functional fields (e.g., ferroelectrics, catalysis, electrochemical transport, and magnetism), they offer exciting opportunities for developing unique future thermometric devices. The transverse thermoelectric effect is another distinct phenomenon that generates thermoelectric currents in the vertical direction relative to the thermal flux. However, further exploration is needed to decouple electron-phonon interactions and improve other properties, such as mechanical properties, for practical applications. Integration with other functional effects remains an area for further research.
6.2 Outlook
Oxide thermoelectrics have been shown to hold promising potential for high-temperature thermoelectric applications and have been extensively studied to fully utilize their unique and diverse functionalities. However, there is still much more to investigate and optimize. First of all, compared with other star materials such as half Heusler alloys,389 lead tellurides12 and tin selenides,24 further optimization of thermoelectric performance is still necessary, as the highest ZT reported for most oxides remains below 1.0, including the average ZT over the high-temperature range. The key to improving thermoelectric performance is achieving a PF by realizing the decoupling of phonons and electrons. Advanced structure design, such as artificial superlattices, might be helpful to decouple electrical and thermal transport. N- and p-type materials can be achieved either by exploiting the interface polarity of superlattices or by using epitaxial strain to shift orbital-dependent transport resonances across the Fermi level.390 By engineering the coherence of phonons via the design of complex oxide heterostructures, thermal conductivity can be tuned by changing the superlattice period,391 which is useful for future thermoelectric devices. Targeted at thermoelectric device design and fabrication, oxide thermoelectrics need to play their unique strengths, both based on the environmental friendliness to cooperate with other functional composites and the abundant experience of property tunability in low-dimensional form. For example, for oxide thin films, the special engineering methods based on orientation, strain and interface modulation should be further utilized and novel thermoelectric responses might be revealed under certain boundary conditions, just like some topological behaviors appearing on the surface or interface.392,393Secondly, correlating thermoelectric properties with others is appealing and can broaden the applications of oxide thermoelectrics and fully take advantage of the intrinsic characteristics of oxides. A general characteristic of next generation smart devices is flexibility or plasticity. Other thermoelectric materials, such as traditional Bi2Te3 and Mg3Bi2 bulk materials11,19 have been shown to exhibit plasticity, and the novel ductile material Ag2(S,Se,Te) further broadens the research scope of plastic inorganic semiconductors.394 Looking in the region of oxides, oxides are versatile in their atomic order under an external field (such as polarization order driving under a certain voltage) and complex oxide interfaces are widely regarded as the source of new physical phenomena.395,396 With the diverse functionalities that oxides can exhibit, it opens up opportunities for coupling different effects to realize novel functionalities. Strategies to magnetically tune their thermoelectric properties have begun to be investigated.397 Other attempts to relate multiple effects have launched some basic prototype devices. Compositing oxide thermoelectrics with piezoelectric materials could realize mechanical-thermal-electrical couplings and concurrently harvest mechanical/thermal energies.398
Furthermore, for practical applications in the silicon-based semiconductor industry, the design and fabrication of oxide thermoelectric devices, particularly for cooling and sensing, should be taken into serious consideration. Oxide thermoelectrics can be prepared as freestanding thin films to undergo unique strain conditions and be integrated into silicon-based chips for real-world deployment. While the development of thermoelectric devices at the micro- and even nano-scale remains technically challenging, such miniaturization holds great promise for industrial applications. Most existing efforts have focused on group IVB element-based material systems and MEMS-compatible device architectures.399,400
Last but not least, in the exploration of new types of oxide thermoelectrics, artificial intelligence (AI) frameworks and techniques may lead to a paradigm shift in accelerating the discovery and design of novel thermoelectric materials. Several machine learning architectures and algorithms, such as extreme gradient boosting regressor (XGBR)401 and neural network potential (NNP),402 have been employed to develop previously unexplored material groups based on collected datasets. Various material performance descriptors and dataset selection methods have also been proposed, playing a crucial role in constructing a widely applicable approach to discover and predict new thermoelectrics.403 For oxides, AI-driven material design has already been explored in certain fields. For instance, in entropy engineering, AI-assisted material design is particularly beneficial for accurately mapping high entropy composition spaces.404 However, in the field of oxide thermoelectrics, the use of AI tools for material design and screening remains underdeveloped. A major bottleneck is the lack of a sufficient amount of high-quality, standardized experimental data, particularly regarding microstructure and defect information. Insights from other common thermoelectric materials suggest that typical performance factors primarily focus on two properties: power factor and lattice thermal conductivity.405 Electrical properties are more challenging to characterize, which may also apply to oxide thermoelectrics. Further research on oxide thermoelectrics in conjunction with AI is strongly warranted.
Author contributions
Qing Wang: conceptualization, data curation, formal analysis, investigation, validation, visualization, writing-original draft, and writing-review & editing; Zhifang Zhou: conceptualization, data curation, formal analysis, investigation, validation, visualization, writing-original draft, and writing-review & editing; Chang Liu: conceptualization, data curation, formal analysis, investigation, validation, visualization, writing-original draft, and writing-review & editing; Yunpeng Zheng: conceptualization, formal analysis, validation, and writing-original draft; Zongmo Shi: validation and writing-original draft; Bin Wei: validation, writing-original draft; Wenyu Zhang: writing-review & editing; Ce-Wen Nan: writing-review & editing; Yuan-Hua Lin: conceptualization, funding acquisition, project administration, resources, supervision, validation, and writing-review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was financially supported by the Basic Science Center Project of the National Natural Science Foundation of China (No. 52388201), National Natural Science Foundation of China (No. 52202113, 52272089, 12304040, 52502134 and 52502327), China Postdoctoral Science Foundation (No. 2024M761648), Fuzhou University Testing Fund of precious apparatus (No. 2025T003) and funding (No. XRC-25036-0180-511573), Guangdong Basic and Applied Basic Research Foundation (No. 2023A1515110762), the Natural Science Foundation of Fujian Province of China (2025J08029), Lifting Program for Young Talents of Shaanxi Science and Technology Association (No. 20220422), State Key Laboratory of New Ceramic Materials Tsinghua University (No. KF202511, KF202525) and State Key Laboratory of Powder Metallurgy, Central South University, China.References
- P. Chen, Y. Xiao, S. Li, X. Jia, D. Luo, W. Zhang, H. J. Snaith, Q. Gong and R. Zhu, Chem. Rev., 2024, 124, 10623–10700 CrossRef CAS PubMed.
- Q. Jiang and K. Zhu, Nat. Rev. Mater., 2024, 9, 399–419 CrossRef CAS.
- X.-L. Shi, L. Wang, W. Lyu, T. Cao, W. Chen, B. Hu and Z.-G. Chen, Chem. Soc. Rev., 2024, 53, 9254–9305 RSC.
- J. Wang, J. Ma, Z. Zhuang, Z. Liang, K. Jia, G. Ji, G. Zhou and H.-M. Cheng, Chem. Rev., 2024, 124, 2839–2887 CrossRef CAS PubMed.
- T. Wei, J. Zou, X. Zhou, M. Song, Y. Zhang, C. Nan, Y. Lin and D. Zhang, Nat. Commun., 2025, 16, 807 CrossRef CAS.
- F. Wu, J. Maier and Y. Yu, Chem. Soc. Rev., 2020, 49, 1569–1614 RSC.
- Q. Yan and M. G. Kanatzidis, Nat. Mater., 2022, 21, 503–513 CrossRef CAS PubMed.
- B. Yang, Y. Liu, R.-J. Jiang, S. Lan, S.-Z. Liu, Z. Zhou, L. Dou, M. Zhang, H. Huang, L.-Q. Chen, Y.-L. Zhu, S. Zhang, X.-L. Ma, C.-W. Nan and Y.-H. Lin, Nature, 2025, 637, 1104–1110 CrossRef CAS.
- M. Zhang, S. Lan, B. B. Yang, H. Pan, Y. Q. Liu, Q. H. Zhang, J. L. Qi, D. Chen, H. Su, D. Yi, Y. Y. Yang, R. Wei, H. D. Cai, H. J. Han, L. Gu, C.-W. Nan and Y.-H. Lin, Science, 2024, 384, 185–189 CrossRef CAS PubMed.
- Z. Zhou, R. Wei, X. Zhou, Y. Liu, D. Zhang and Y.-H. Lin, Acc. Mater. Res., 2024, 5, 1571–1582 CrossRef CAS.
- Z. Zhou, J. Guo, Y. Zheng, Y. Yang, B. Yang, D. Li, W. Zhang, B. Wei, C. Liu, J. L. Lan, C. W. Nan and Y. H. Lin, Small Methods, 2024, 8, 2301619 CrossRef CAS.
- B. Jia, D. Wu, L. Xie, W. Wang, T. Yu, S. Li, Y. Wang, Y. Xu, B. Jiang, Z. Chen, Y. Weng and J. He, Science, 2024, 384, 81–86 CrossRef CAS.
- S. Liu, S. Bai, Y. Wen, J. Lou, Y. Jiang, Y. Zhu, D. Liu, Y. Li, H. Shi, S. Liu, L. Wang, J. Zheng, Z. Zhao, Y. Qin, Z. Liu, X. Gao, B. Qin, C. Chang, C. Chang and L.-D. Zhao, Science, 2025, 387, 202–208 CrossRef CAS.
- Y. Qin, B. Qin, T. Hong, X. Zhang, D. Wang, D. Liu, Z.-Y. Wang, L. Su, S. Wang, X. Gao, Z.-H. Ge and L.-D. Zhao, Science, 2024, 383, 1204–1209 CrossRef CAS PubMed.
- Q. Sun, C. Du and G. Chen, Prog. Mater. Sci., 2025, 149, 101402 CrossRef CAS.
- L. Xie, L. Yin, Y. Yu, G. Peng, S. Song, P. Ying, S. Cai, Y. Sun, W. Shi, H. Wu, N. Qu, F. Guo, W. Cai, H. Wu, Q. Zhang, K. Nielsch, Z. Ren, Z. Liu and J. Sui, Science, 2023, 382, 921–928 CrossRef CAS.
- S. Xu, S. Horta, A. Lawal, K. Maji, M. Lorion and M. Ibanez, Science, 2025, 387, 845–850 CrossRef CAS PubMed.
- P. Yu, M. Li, W. Lv, Z. Liu, S. Yu, Z. Zhou, J.-L. Lan, Y. Yu, X. Yang and Y.-H. Lin, Small, 2025, 21, 2412632 CrossRef CAS PubMed.
- P. Zhao, W. Xue, Y. Zhang, S. Zhi, X. Ma, J. Qiu, T. Zhang, S. Ye, H. Mu, J. Cheng, X. Wang, S. Hou, L. Zhao, G. Xie, F. Cao, X. Liu, J. Mao, Y. Fu, Y. Wang and Q. Zhang, Nature, 2024, 631, 777–782 CrossRef CAS.
- P. Qiu, T. Deng, L. Chen and X. Shi, Joule, 2024, 8, 622–634 CrossRef CAS.
- W. Zuo, H. Chen, Z. Yu, Y. Fu, X. Ai, Y. Cheng, M. Jiang, S. Wan, Z. Fu, R. Liu, G. Cheng, R. Xu, L. Wang, F. Xu, Q. Zhang, D. Makarov and W. Jiang, Nat. Mater., 2025, 24, 735–742 CrossRef CAS.
- T. Deng, Z. Gao, Z. Li, P. Qiu, Z. Li, X. Yuan, C. Ming, T.-R. Wei, L. Chen and X. Shi, Science, 2024, 386, 1112–1117 CrossRef CAS PubMed.
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105–114 CrossRef CAS PubMed.
- C. Zhou, Y. K. Lee, Y. Yu, S. Byun, Z.-Z. Luo, H. Lee, B. Ge, Y.-L. Lee, X. Chen, J. Y. Lee, O. Cojocaru-Mirédin, H. Chang, J. Im, S.-P. Cho, M. Wuttig, V. P. Dravid, M. G. Kanatzidis and I. Chung, Nat. Mater., 2021, 20, 1378–1384 CrossRef CAS.
- Z. Zhou, Y. Huang, B. Wei, Y. Yang, D. Yu, Y. Zheng, D. He, W. Zhang, M. Zou, J.-L. Lan, J. He, C.-W. Nan and Y.-H. Lin, Nat. Commun., 2023, 14, 2410 CrossRef CAS PubMed.
- B. Jiang, W. Wang, S. Liu, Y. Wang, C. Wang, Y. Chen, L. Xie, M. Huang and J. He, Science, 2022, 377, 208–213 CrossRef CAS.
- B. Qin, M. G. Kanatzidis and L.-D. Zhao, Science, 2024, 386, 285 CrossRef PubMed.
- P. Ying, Q. Jian, Y. Gong, T. Song, Y. Yang, Y. Geng, J. Huang, R. Sun, C. Chen, T. Shen, Y. Li, W. Dou, C. Liang, Y. Liu, D. Xiang, T. Feng, X. Fei, Y. Zhang, K. Song, Y. Zhang, H. Wu and G. Tang, Nat. Commun., 2025, 16, 3305 CrossRef CAS.
- B. Ge, R. Li, G. Wang, M. Zhu and C. Zhou, J. Am. Ceram. Soc., 2024, 107, 1985–1995 CrossRef CAS.
- G.-K. Ren, J.-L. Lan, L.-D. Zhao, C. Liu, H. Yuan, Y. Shi, Z. Zhou and Y.-H. Lin, Mater. Today, 2019, 29, 68–85 CrossRef CAS.
- C. Liu, S. Li, Y. Zheng, M. Xu, H. Su, X. Miao, Y. Liu, Z. Zhou, J. Qi, B. Yang, D. Chen, C.-W. Nan and Y.-H. Lin, Prog. Mater. Sci., 2025, 148, 101385 CrossRef CAS.
- Z. Zhao, X. Zhang and L.-D. Zhao, Matter, 2023, 6, 3274–3295 CrossRef CAS.
- Y. Liu, L.-D. Zhao, Y. Zhu, Y. Liu, F. Li, M. Yu, D.-B. Liu, W. Xu, Y.-H. Lin and C.-W. Nan, Adv. Energy Mater., 2016, 6, 1502423 CrossRef.
- G.-K. Ren, S. Wang, Z. Zhou, X. Li, J. Yang, W. Zhang, Y.-H. Lin, J. Yang and C.-W. Nan, Nat. Commun., 2019, 10, 2814 CrossRef PubMed.
- Y. Gu, X.-L. Shi, L. Pan, W.-D. Liu, Q. Sun, X. Tang, L.-Z. Kou, Q.-F. Liu, Y.-F. Wang and Z.-G. Chen, Adv. Funct. Mater., 2021, 31, 2101289 CrossRef CAS.
- Z. Yin, H. Zhang, Y. Wang, Y. Wu, Y. Xing, X. Wang, X. Fang, Y. Yu and X. Guo, Adv. Energy Mater., 2025, 15, 2403174 CrossRef CAS.
- M. Ohtaki, K. Araki and K. Yamamoto, J. Electron. Mater., 2009, 38, 1234–1238 CrossRef CAS.
- A. Ahmad, M. Hussain, Z. Zhou, R. Liu, Y.-H. Lin and C.-W. Nan, ACS Appl. Energy Mater., 2020, 3, 1552–1558 CrossRef CAS.
- L. Pan, X.-L. Shi, C. Song, W.-D. Liu, Q. Sun, C. Lu, Q. Liu, Y. Wang and Z.-G. Chen, Adv. Funct. Mater., 2022, 32, 2202927 CrossRef CAS.
- Z. Zhou, R. Liu, Y. Yang, Y. Zheng, B. Wei, W. Zhang, M. Zou, J. Han, Y. Liu, J. Lan, C.-W. Nan and Y.-H. Lin, Scr. Mater., 2023, 225, 115163 CrossRef CAS.
- L. Pan, W.-D. Liu, J.-Y. Zhang, X.-L. Shi, H. Gao, Q.-F. Liu, X. Shen, C. Lu, Y.-F. Wang and Z.-G. Chen, Nano Energy, 2020, 69, 104394 CrossRef CAS.
- Y. Yang, J. Han, Z. Zhou, M. Zou, Y. Xu, Y. Zheng, C.-W. Nan and Y.-H. Lin, Adv. Funct. Mater., 2022, 32, 2113164 CrossRef CAS.
- N. Ngo Van, N. Pryds, S. Linderoth and M. Ohtaki, Adv. Mater., 2011, 23, 2484–2490 CrossRef.
- Z. Shi, S. Tong, J. Wei, Y. Guo, Y. Zhang, L. Wang and J. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 32166–32175 CrossRef CAS.
- Y. Lin, C. Norman, D. Srivastava, F. Azough, L. Wang, M. Robbins, K. Simpson, R. Freer and I. A. Kinloch, ACS Appl. Mater. Interfaces, 2015, 7, 15898–15908 CrossRef CAS PubMed.
- J. Wang, B.-Y. Zhang, H.-J. Kang, Y. Li, X. Yaer, J.-F. Li, Q. Tan, S. Zhang, G.-H. Fan, C.-Y. Liu, L. Miao, D. Nan, T.-M. Wang and L.-D. Zhao, Nano Energy, 2017, 35, 387–395 CrossRef CAS.
- Y. P. Zheng, Q. H. Zhang, C. J. Shi, Z. F. Zhou, Y. Lu, J. Han, H. T. Chen, Y. P. Ma, Y. J. Zhang, C. P. Lin, W. Xu, W. G. Ma, Q. Li, Y. Y. Yang, B. Wei, B. B. Yang, M. C. Zou, W. Y. Zhang, C. Liu, L. Y. Dou, D. L. Yang, J. L. Lan, D. Yi, X. Zhang, L. Gu, C. W. Nan and Y. H. Lin, Nat. Commun., 2024, 15, 7650 CrossRef CAS PubMed.
- X. He, S. Kimura, T. Katase, T. Tadano, S. Matsuishi, M. Minohara, H. Hiramatsu, H. Kumigashira, H. Hosono and T. Kamiya, Adv. Sci., 2024, 11, 2307058 CrossRef CAS PubMed.
- K. Kruppa, T. Hennig, G. E. Cano, J. Moeckelmann and A. Feldhoff, J. Am. Ceram. Soc., 2024, 107, 7951–7965 CrossRef CAS.
- S. Kumar, H. H. Singh and N. Khare, Energy Convers. Manage., 2019, 198, 111783 CrossRef CAS.
- Y. K. Mishra and R. Adelung, Mater. Today, 2018, 21, 631–651 CrossRef CAS.
- X. Ding, M. Li, P. Chen, Y. Zhao, M. Zhao, H. Leng, Y. Wang, S. Ali, F. Raziq, X. Wu, H. Xiao, X. Zu, Q. Wang, A. Vinu, J. Yi and L. Qiao, Matter, 2022, 5, 4274–4314 CrossRef CAS.
- K. Kurosaki, H. Muta, M. Uno and S. Yamanaka, J. Alloys Compd., 2001, 315, 234–236 CrossRef CAS.
- Z. Zhao, J. Zheng, Y. Li, S. Wang, S. Liu, S. Zhan, L. Wang, X. Zhang and L.-D. Zhao, Nano Energy, 2024, 126, 109649 CrossRef CAS.
- X. Lu, L. Sun, P. Jiang and X. Bao, Adv. Mater., 2019, 31, 1902044 CrossRef CAS.
- Y. Zhang, S. Li, J. Zhang, L.-D. Zhao, Y. Lin, W. Liu and F. Rosei, Natl. Sci. Rev., 2024, 11, nwae036 CrossRef CAS PubMed.
- K. Fujita, T. Mochida and K. Nakamura, Jpn. J. Appl. Phys., 2001, 40, 4644 CrossRef CAS.
- M. Shikano and R. Funahashi, Appl. Phys. Lett., 2003, 82, 1851–1853 CrossRef CAS.
- O. V. Malochkin, W. S. Seo and K. Koumoto, Jpn. J. Appl. Phys., 2004, 43, 194 CrossRef.
- Z. Yin, H. Zhang, Y. Wang, Y. Wu, Y. Xing, L. Deng, P. He and X. Guo, Small, 2023, 19, 2304430 CrossRef CAS.
- J. L. Lan, Y. C. Liu, B. Zhan, Y. H. Lin, B. Zhang, X. Yuan, W. Zhang, W. Xu and C. W. Nan, Adv. Mater., 2013, 25, 5086–5090 CrossRef CAS PubMed.
- D. Liu, Y. Zhang, H. Kang, J. Li, Z. Chen and T. Wang, J. Eur. Ceram. Soc., 2018, 38, 807–811 CrossRef CAS.
- B. Feng, G. Li, Z. Pan, H. Xiaoming, L. Peihai, H. Zhu, L. Yawei and X. A. Fan, J. Alloys Compd., 2018, 754, 131–138 CrossRef CAS.
- S. Lin and J. Selig, J. Alloys Compd., 2010, 503, 402–409 CrossRef CAS.
- W. X. Tang, W. Q. Ma, P. L. Yu, Z. F. Zhou, J. L. Lan, Y. H. Lin and X. P. Yang, Mater. Today Phys., 2022, 28, 100898 CrossRef CAS.
- G.-K. Ren, S.-Y. Wang, Y.-C. Zhu, K. J. Ventura, X. Tan, W. Xu, Y.-H. Lin, J. Yang and C.-W. Nan, Energy Environ. Sci., 2017, 10, 1590–1599 RSC.
- M. Samanta, S. N. Guin and K. Biswas, Inorg. Chem. Front., 2017, 4, 84–90 RSC.
- J. George, M. Aiswarya, V. K. Mythri, S. Sathiyamoorthy, S. Paulraj, V. Kathirvel, M. Maaza, A. Majumdar and P. Veluswamy, Ceram. Int., 2022, 48, 28874–28880 CrossRef CAS.
- M. Bittner, N. Kanas, R. Hinterding, F. Steinbach, J. Räthel, M. Schrade, K. Wiik, M.-A. Einarsrud and A. Feldhoff, J. Power Sources, 2019, 410, 143–151 CrossRef.
- A. Jeong, M. Ohtaki and B. K. Jang, J. Ceram. Soc. Jpn., 2022, 130, 889–894 CrossRef CAS.
- S. He, M. F. Guo, Z. K. Dan, S. Lan, W. B. Ren, L. Zhou, Y. Wang, Y. H. Liang, Y. P. Zheng, J. Y. Pan and Y. Shen, Sci. Bull., 2021, 66, 1080–1090 CrossRef CAS PubMed.
- L. W. Martin, Y. H. Chu and R. Ramesh, Mater. Sci. Eng., R, 2010, 68, 111–133 CrossRef.
- B. X. Hu, X. L. Shi, T. Y. Cao, M. Li, W. Y. Chen, W. D. Liu, W. Lyu, T. Tesfamichael and Z. G. Chen, Small Sci., 2025, 5, 2300061 CrossRef CAS PubMed.
- T. R. Bhandari, R. P. Bhattarai and R. Adhikari, J. Mater. Sci., 2024, 59, 20204–20220 CrossRef CAS.
- Z. H. Wu, S. Zhang, Z. K. Liu, E. R. Mu and Z. Y. Hu, Nano Energy, 2022, 91, 106692 CrossRef CAS.
- Z. F. Zhou, G. K. Ren, X. Tan, R. Liu, C. Liu, Y. H. Lin and C. W. Nan, J. Mater. Chem. A, 2018, 6, 24128–24135 RSC.
- Z. F. Zhou, Y. S. Xu, M. C. Zou, C. Liu, J. L. Lan, Y. H. Lin and C. W. Nan, J. Am. Ceram. Soc., 2021, 104, 1370–1378 CrossRef CAS.
- H. Ohta, S. Kim, Y. Mune, T. Mizoguchi, K. Nomura, S. Ohta, T. Nomura, Y. Nakanishi, Y. Ikuhara, M. Hirano, H. Hosono and K. Koumoto, Nat. Mater., 2007, 6, 129–134 CrossRef CAS PubMed.
- Z.-H. Zheng, X.-L. Shi, D.-W. Ao, W.-D. Liu, M. Li, L.-Z. Kou, Y.-X. Chen, F. Li, M. Wei, G.-X. Liang, P. Fan, G. Q. Lu and Z.-G. Chen, Nat. Sustainability, 2023, 6, 180–191 CrossRef.
- P. Fan, X.-l Huang, T.-b Chen, F. Li, Y.-x Chen, B. Jabar, S. Chen, H.-l Ma, G.-x Liang, J.-t Luo, X.-h Zhang and Z.-h Zheng, Chem. Eng. J., 2021, 410, 128444 CrossRef CAS.
- Y. Lei, R. Qi, M. Chen, H. Chen, C. Xing, F. Sui, L. Gu, W. He, Y. Zhang, T. Baba, T. Baba, H. Lin, T. Mori, K. Koumoto, Y. Lin and Z. Zheng, Adv. Mater., 2022, 34, 2104786 CrossRef CAS PubMed.
- L. Song, J. Zhang and B. B. Iversen, J. Mater. Chem. A, 2019, 7, 17981–17986 RSC.
- P. Fan, Y.-z Li, Z.-h Zheng, Q.-y Lin, J.-t Luo, G.-x Liang, M.-q Zhang and M.-c Chen, Appl. Surf. Sci., 2013, 284, 145–149 CrossRef CAS.
- A. Tuan Thanh Pham, P. Thanh Ngoc Vo, H. Kieu Thi Ta, N. Kim Pham, H. Thi Lai, H. Nhu Thi Tran, V. Cao Tran, T. Le Hoang Doan, S. Park and T. Bach Phan, Mater. Sci. Eng., B, 2020, 261, 114712 CrossRef CAS.
- S. M. Gates, Chem. Rev., 1996, 96, 1519–1532 Search PubMed.
- J. L. Yang, S. J. An, W. I. Park, G. C. Yi and W. Choi, Adv. Mater., 2004, 16, 1661–1664 CrossRef CAS.
- S. M. George, Chem. Rev., 2010, 110, 111–131 CrossRef CAS PubMed.
- S. H. Lee, J. M. Choi, J. H. Lim, J. Park and J. S. Park, Ceram. Int., 2018, 44, 1978–1983 CrossRef CAS.
- B. B. Yang, Q. H. Zhang, H. B. Huang, H. Pan, W. X. Zhu, F. Q. Meng, S. Lan, Y. Q. Liu, B. Wei, Y. Q. Liu, L. T. Yang, L. Gu, L. Q. Chen, C. W. Nan and Y. H. Lin, Nat. Energy, 2023, 8, 956–964 CrossRef CAS.
- B. B. Yang, Y. Zhang, H. Pan, W. L. Si, Q. H. Zhang, Z. H. Shen, Y. Yu, S. Lan, F. Q. Meng, Y. Q. Liu, H. B. Huang, J. Q. He, L. Gu, S. J. Zhang, L. Q. Chen, J. Zhu, C. W. Nan and Y. H. Lin, Nat. Mater., 2022, 21, 1074–1080 CrossRef CAS PubMed.
- B. B. Yang, Y. Q. Liu, C. Z. Gong, S. Lan, Z. F. Zhou, X. B. Zhu, C. W. Nan and Y. H. Lin, Adv. Funct. Mater., 2024, 34, 2409344 CrossRef CAS.
- Z. F. Zhou, Y. Q. Liu, S. Lan, B. B. Yang, C. W. Nan and Y. H. Lin, Scr. Mater., 2024, 243, 115968 CrossRef CAS.
- L. Znaidi, Mater. Sci. Eng., B, 2010, 174, 18–30 CrossRef CAS.
- L. Zhang, P. Li, K. Huang, Z. Tang, G. H. Liu and Y. B. Li, Mater. Lett., 2011, 65, 1696–1698 CrossRef CAS.
- M. Napari, T. N. Huq, R. L. Z. Hoye and J. L. MacManus-Driscoll, InfoMat, 2021, 3, 536–576 CrossRef CAS.
- E. Fortunato, P. Barquinha and R. Martins, Adv. Mater., 2012, 24, 2945–2986 CrossRef CAS.
- Y.-H. Lin, J. Lan and C. Nan, Oxide Thermoelectric Materials: From Basic Principles to Applications, Wiley, Hoboken, NJ, 2019 Search PubMed.
- T. Olorunyolemi, A. Birnboim, Y. Carmel, O. C. Wilson, I. K. Lloyd, S. Smith and R. Campbell, J. Am. Ceram. Soc., 2002, 85, 1249–1253 Search PubMed.
- K. P. Ong, D. J. Singh and P. Wu, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 115110 CrossRef.
- J. D. Albrecht, P. P. Ruden, S. Limpijumnong, W. R. L. Lambrecht and K. F. Brennan, J. Appl. Phys., 1999, 86, 6864–6867 CrossRef CAS.
- Ü. Özgür, Y. I. Alivov, C. Liu, A. Teke, M. A. Reshchikov, S. Doğan, V. Avrutin, S.-J. Cho and H. Morkoç, J. Appl. Phys., 2005, 98, 041301 CrossRef.
- S. D. N. Luu, D. Tuan Anh and P. Thang Bach, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2019, 10, 023001 CAS.
- T. Tsubota, M. Ohtaki, K. Eguchi and H. Arai, J. Mater. Chem., 1997, 7, 85–90 RSC.
- J. Kwang-Hee, L. Kyu Hyoung, S. Won-Seon and C. Soon-Mok, Appl. Phys. Lett., 2012, 100, 253902 CrossRef.
- T. Tsubota, M. Ohtaki, K. Eguchi and H. Arai, J. Mater. Chem., 1998, 8, 409–412 RSC.
- H. Yamaguchi, Y. Chonan, M. Oda, T. Komiyama, T. Aoyama and S. Sugiyama, J. Electron. Mater., 2011, 40, 723–727 CrossRef CAS.
- K. Park, K. Y. Ko, W. S. Seo, W. S. Cho, J. G. Kim and J. Y. Kim, J. Eur. Ceram. Soc., 2007, 27, 813–817 CrossRef CAS.
- K. Park and J. K. Seong, J. Alloys Compd., 2008, 464, 1–5 CrossRef CAS.
- D. K. Seo, S. Shin, H. H. Cho, B. H. Kong, D. M. Whang and H. K. Cho, Acta Mater., 2011, 59, 6743–6750 CrossRef CAS.
- N. H. T. Nguyen, T. H. Nguyen, Y. R. Liu, M. Arninzare, A. T. T. Pham, S. Cho, D. P. Wong, K. H. Chen, T. Seetawan, N. K. Pham, H. K. T. Ta, V. C. Tran and T. B. Phan, ACS Appl. Mater. Interfaces, 2016, 8, 33916–33923 CrossRef PubMed.
- A. T. T. Pham, H. K. T. Ta, Y. R. Liu, M. Aminzare, D. P. Wong, T. H. Nguyen, N. K. Pham, T. B. N. Le, T. Seetawan, H. Ju, S. Cho, K. H. Chen, V. C. Tran and T. B. Phan, J. Alloys Compd., 2018, 747, 156–165 CrossRef CAS.
- H. Ohta, W. S. Seo and K. Koumoto, J. Am. Ceram. Soc., 1996, 79, 2193–2196 CrossRef CAS.
- P. Jood, R. J. Mehta, Y. L. Zhang, G. Peleckis, X. L. Wang, R. W. Siegel, T. Borca-Tasciuc, S. X. Dou and G. Ramanath, Nano Lett., 2011, 11, 4337–4342 CrossRef CAS PubMed.
- A. T. T. Pham, O. K. T. Le, D. Van Hoang, T. H. Nguyen, K. H. Chen, S. Park, T. B. Phan and V. C. Tran, Acta Mater., 2022, 241, 118415 CrossRef CAS.
- A. Vora-ud, A. T. T. Pham, D. C. Truong, S. Thoawankeaw, H. T. Lai, T. B. N. Le, N. M. Q. Tran, M. Insawang, P. Muthitamongkol, M. Horprathum, M. Kumar, S. Park, G. J. Snyder, T. Seetawan and T. B. Phan, Chem. Eng. J., 2023, 465, 142954 CrossRef CAS.
- Z. F. Zhou, M. C. Zou, Y. S. Xu, J. L. Lan, C. Liu, A. Ahmad, Y. H. Lin and C. W. Nan, J. Am. Ceram. Soc., 2021, 104, 3992–3999 CrossRef CAS.
- Z. F. Zhou, Y. P. Zheng, Y. Y. Yang, W. Y. Zhang, M. C. Zou, C. W. Nan and Y. H. Lin, Crystals, 2022, 12, 1351 CrossRef CAS.
- Z. Zhou, Y. Zheng, Y. Yang, W. Zhang, B. Wei, M. Zou, J. Lan, C.-W. Nan and Y.-H. Lin, J. Am. Ceram. Soc., 2023, 106, 2911–2917 CrossRef CAS.
- Z. Zhou, Y. Zheng, Y. Yang, C. Liu, B. Wei, W. Zhang, J.-L. Lan, C.-W. Nan and Y.-H. Lin, Appl. Phys. Lett., 2024, 124, 013903 CrossRef CAS.
- P. D. C. King, T. D. Veal, F. Fuchs, C. Y. Wang, D. J. Payne, A. Bourlange, H. Zhang, G. R. Bell, V. Cimalla, O. Ambacher, R. G. Egdell, F. Bechstedt and C. F. McConville, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 205211 CrossRef.
- G. Korotcenkov, V. Brinzari and B. K. Cho, Solid State Sci., 2016, 52, 141–148 CrossRef CAS.
- L. Xu, B. Fauque, Z. Zhu, Z. Galazka, K. Irmscher and K. Behnia, Phys. Rev. Mater., 2021, 5, 014603 CrossRef CAS.
- D. Berardan, E. Guilmeau, A. Maignan and B. Raveau, Solid State Commun., 2008, 146, 97–101 CrossRef CAS.
- E. Guilmeau, D. Berardan, C. Simon, A. Maignan, B. Raveau, D. O. Ovono and F. Delorme, J. Appl. Phys., 2009, 106, 053715 CrossRef.
- Y. Liu, Y.-H. Lin, J. Lan, W. Xu, B.-P. Zhang, C.-W. Nan and H. Zhu, J. Am. Ceram. Soc., 2010, 93, 2938–2941 CrossRef CAS.
- J. Lan, Y.-H. Lin, Y. Liu, S. Xu and C.-W. Nan, J. Am. Ceram. Soc., 2012, 95, 2465–2469 CrossRef CAS.
- Y. Liu, Y.-H. Lin, W. Xu, B. Cheng, J. Lan, D. Chen, H. Zhu and C.-W. Nan, J. Am. Ceram. Soc., 2012, 95, 2568–2572 CrossRef CAS.
- B. Zhu, T. Zhang, R. Tian, T. T. Tan, R. Donelson and S. Li, J. Alloys Compd., 2015, 644, 119–123 CrossRef CAS.
- Y. Liu, W. Xu, D.-B. Liu, M. Yu, Y.-H. Lin and C.-W. Nan, Phys. Chem. Chem. Phys., 2015, 17, 11229–11233 RSC.
- K. Koumoto, Y. Wang, R. Zhang, A. Kosuga and R. Funahashi, Annu. Rev. Mater. Res., 2010, 40, 363–394 CrossRef CAS.
- R. Freer, D. Ekren, T. Ghosh, K. Biswas, P. Qiu, S. Wan, L. Chen, S. Han, C. Fu, T. Zhu, A. K. M. Ashiquzzaman Shawon, A. Zevalkink, K. Imasato, G. J. Snyder, M. Ozen, K. Saglik, U. Aydemir, R. Cardoso-Gil, E. Svanidze, R. Funahashi, A. V. Powell, S. Mukherjee, S. Tippireddy, P. Vaqueiro, F. Gascoin, T. Kyratsi, P. Sauerschnig and T. Mori, J. Phys. Energy, 2022, 4, 022002 CrossRef CAS.
- S. R. Popuri, R. Decourt, J. A. McNulty, M. Pollet, A. D. Fortes, F. D. Morrison, M. S. Senn and J. W. G. Bos, J. Phys. Chem. C, 2019, 123, 5198–5208 CrossRef CAS.
- M. T. Dylla, S. D. Kang and G. J. Snyder, Angew. Chem., Int. Ed., 2019, 58, 5503–5512 CrossRef CAS.
- S. Walia, S. Balendhran, H. Nili, S. Zhuiykov, G. Rosengarten, Q. H. Wang, M. Bhaskaran, S. Sriram, M. S. Strano and K. Kalantar-zadeh, Prog. Mater. Sci., 2013, 58, 1443–1489 CrossRef CAS.
- S. Han, S. Dai, J. Ma, Q. Ren, C. Hu, Z. Gao, M. Duc Le, D. Sheptyakov, P. Miao, S. Torii, T. Kamiyama, C. Felser, J. Yang, C. Fu and T. Zhu, Nat. Phys., 2023, 19, 1649–1657 Search PubMed.
- R. J. D. Tilley, Perovskites: Structure-Property Relationships, John Wiley & Sons, 2016 Search PubMed.
- M. Takizawa, K. Maekawa, H. Wadati, T. Yoshida, A. Fujimori, H. Kumigashira and M. Oshima, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 113103 CrossRef.
- A. Sarantopoulos, E. Ferreiro-Vila, V. Pardo, C. Magén, M. H. Aguirre and F. Rivadulla, Phys. Rev. Lett., 2015, 115, 166801 CrossRef CAS PubMed.
- Y. Cui, J. R. Salvador, J. Yang, H. Wang, G. Amow and H. Kleinke, J. Electron. Mater., 2009, 38, 1002–1007 CrossRef CAS.
- J. Liu, C. L. Wang, W. B. Su, H. C. Wang, J. C. Li, J. L. Zhang and L. M. Mei, J. Alloys Compd., 2010, 492, L54–L56 CrossRef CAS.
- C. Chen, T. Zhang, R. Donelson, T. T. Tan and S. Li, J. Alloys Compd., 2015, 629, 49–54 CrossRef CAS.
- J. Liu, C. L. Wang, Y. Li, W. B. Su, Y. H. Zhu, J. C. Li and L. M. Mei, J. Appl. Phys., 2013, 114, 223714 CrossRef.
- H. Muta, K. Kurosaki and S. Yamanaka, J. Alloys Compd., 2005, 392, 306–309 CrossRef CAS.
- T. Maekawa, K. Kurosaki, H. Muta, M. Uno and S. Yamanaka, J. Alloys Compd., 2005, 387, 56–59 CrossRef CAS.
- A. Kikuchi, N. Okinaka and T. Akiyama, Scr. Mater., 2010, 63, 407–410 CrossRef CAS.
- B. Zhang, J. Wang, T. Zou, S. Zhang, X. Yaer, N. Ding, C. Liu, L. Miao, Y. Lia and Y. Wu, J. Mater. Chem. C, 2015, 3, 11406–11411 RSC.
- X. He, S. Nomoto, T. Komatsu, T. Katase, T. Tadano, S. Kitani, H. Yoshida, T. Yamamoto, H. Mizoguchi, K. Ide, H. Hiramatsu, H. Kawaji, H. Hosono and T. Kamiya, Adv. Funct. Mater., 2023, 33, 2213144 CrossRef CAS.
- Y. Wang, K. Fujinami, R. Zhang, C. Wan, N. Wang, Y. Ba and K. Koumoto, Appl. Phys. Express, 2010, 3, 031101 CrossRef.
- M. T. Buscaglia, F. Maglia, U. Anselmi-Tamburini, D. Marré, I. Pallecchi, A. Ianculescu, G. Canu, M. Viviani, M. Fabrizio and V. Buscaglia, J. Eur. Ceram. Soc., 2014, 34, 307–316 CrossRef CAS.
- N. Wang, H. Chen, H. He, W. Norimatsu, M. Kusunoki and K. Koumoto, Sci. Rep., 2013, 3, 3449 CrossRef.
- A. Mehdizadeh Dehkordi, S. Bhattacharya, T. Darroudi, J. W. Graff, U. Schwingenschlögl, H. N. Alshareef and T. M. Tritt, Chem. Mater., 2014, 26, 2478–2485 CrossRef CAS.
- S. R. Popuri, A. J. M. Scott, R. A. Downie, M. A. Hall, E. Suard, R. Decourt, M. Pollet and J. W. G. Bos, RSC Adv., 2014, 4, 33720–33723 RSC.
- Y. Han, X. Liu, Q. Zhang, M. Huang, Y. Li, W. Pan, P.-A. Zong, L. Li, Z. Yang, Y. Feng, P. Zhang and C. Wan, Nat. Commun., 2022, 13, 2871 CrossRef CAS.
- Y. Zheng, M. Zou, W. Zhang, D. Yi, J. Lan, C.-W. Nan and Y.-H. Lin, J. Adv. Ceram., 2021, 10, 377–384 CrossRef CAS.
- Z. Lou, P. Zhang, J. Zhu, L. Gong, J. Xu, Q. Chen, M. J. Reece, H. Yan and F. Gao, J. Eur. Ceram. Soc., 2022, 42, 3480–3488 CrossRef CAS.
- P. Zhang, L. Gong, Z. Lou, J. Xu, S. Cao, J. Zhu, H. Yan and F. Gao, J. Alloys Compd., 2022, 898, 162858 CrossRef CAS.
- P. Zhang, L. Gong, X. Xu, Z. Lou, Z. Wei, P. Chen, Z. Wu, J. Xu and F. Gao, Chem. Eng. J., 2023, 472, 144974 CrossRef CAS.
- P. Zhang, Z. Lou, L. Gong, J. Xu, Q. Chen, M. J. Reece, H. Yan, Z. Dashevsky and F. Gao, J. Alloys Compd., 2023, 937, 168366 CrossRef CAS.
- P. Cao, J. Yao, Y. Sun, A. I. Klyndyuk, Z. Li, A. Abbas, N. S. Krasutskaya, W. Su, C. Wang and H. Wang, J. Eur. Ceram. Soc., 2025, 45, 117290 CrossRef CAS.
- A. Z. Khan, J. M. Flitcroft and J. M. Skelton, Mater. Adv., 2024, 5, 652–664 RSC.
- U. S. Shenoy and D. K. Bhat, Mater. Today Chem., 2020, 18, 100384 CrossRef CAS.
- M. Yamamoto, H. Ohta and K. Koumoto, Appl. Phys. Lett., 2007, 90, 2475878 Search PubMed.
- H. Muta, K. Kurosaki and S. Yamanaka, J. Alloys Compd., 2004, 368, 22–24 CrossRef CAS.
- J. Li, Q. Jiang, Z. Tian, H. Kang, Z. Chen, E. Guo, Z. Cao and T. Wang, ACS Appl. Energy Mater., 2023, 6, 8053–8062 CrossRef CAS.
- B. Fang, J. Li, Y. Fu, J. Zhao, M. Du, H. Kang and J. Wang, Chem. Eng. J., 2025, 509, 161480 CrossRef CAS.
- J. Li, Y. Wang, X. Jiang, Z. Tian, H. Kang, Z. Chen, E. Guo, Z. Cao and T. Wang, Mater. Horiz., 2023, 10, 454–465 RSC.
- L. M. Daniels, S. Ling, S. N. Savvin, M. J. Pitcher, M. S. Dyer, J. B. Claridge, B. Slater, F. Corà, J. Alaria and M. J. Rosseinsky, J. Mater. Chem. A, 2018, 6, 15640–15652 RSC.
- T. Liu, J. Chen, M. Li, G. Han, C. Liu, D. Zhou, J. Zou, Z.-G. Chen and L. Yang, Chem. Eng. J., 2021, 408, 127364 CrossRef CAS.
- Y. Liu, Z. Shi, J. Zhang, C. Chen, Y. Zhang, L. Li, Q. Chen, Q. Zhang and F. Xing, Inorg. Chem., 2024, 63, 4628–4635 CrossRef CAS PubMed.
- Y. Zhang, S. Tong, S. Cao, F. Xing, J. Zhang and Z. Shi, Ceram. Int., 2023, 49, 7089–7093 CrossRef CAS.
- H. J. Cho, T. Onozato, M. Wei, A. Sanchela and H. Ohta, APL Mater., 2018, 7, 022507 CrossRef.
- Y. Ozaki, D. Kan and Y. Shimakawa, J. Appl. Phys., 2017, 121, 215304 CrossRef.
- P. Rajasekaran, M. Arivanandhan, N. Sato, Y. Kumaki, T. Mori, Y. Hayakawa, K. Hayakawa, Y. Kubota, R. Jayavel and M. Shimomura, J. Alloys Compd., 2022, 894, 162335 CrossRef CAS.
- X. Zhang, Y. Zhang, L. Wu, A. Tsuruta, M. Mikami, H. J. Cho and H. Ohta, ACS Appl. Mater. Interfaces, 2022, 14, 33355–33360 CrossRef CAS.
- U. Deepika Shanubhogue, A. Pal, A. Rao, S. Chattopadhyay, A. M. Ashok and N. Davis, J. Alloys Compd., 2023, 941, 168987 CrossRef CAS.
- A. Asamitsu, Y. Moritomo and Y. Tokura, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 53, R2952–R2955 CrossRef CAS.
- Y. Zhang, W. M. Postiglione, R. Xie, C. Zhang, H. Zhou, V. Chaturvedi, K. Heltemes, H. Zhou, T. Feng, C. Leighton and X. Wang, Nat. Commun., 2023, 14, 2626 CrossRef CAS PubMed.
- V. A. Dudnikov, A. S. Fedorov, Y. S. Orlov, L. A. Solovyov, S. N. Vereshchagin, S. Y. Gavrilkin, A. Y. Tsvetkov, M. V. Gorev, S. V. Novikov and S. G. Ovchinnikov, Ceram. Int., 2020, 46, 17987–17991 CrossRef CAS.
- V. A. Dudnikov, Y. S. Orlov, N. V. Kazak, A. S. Fedorov, L. A. Solov'yov, S. N. Vereshchagin, A. T. Burkov, S. V. Novikov, S. Y. Gavrilkin and S. G. Ovchinnikov, Ceram. Int., 2018, 44, 10299–10305 CrossRef CAS.
- H. Huang, Z. Chen, Y. Yang, Y. Lin, Y. Fan, X. Wu, Y. Ji, Y. Wang, C. Yang, Y. Yu and Z. Zou, Adv. Funct. Mater., 2025, 35, 2414298 CrossRef CAS.
- Y. Zhang and H. Ohta, NPG Asia Mater., 2023, 15, 67 CrossRef.
- H. Chen, Y. Huang, L. Yu, Z. Li, G. Wang, B. Dai and Y. Wang, Appl. Phys. Lett., 2024, 124, 013905 CrossRef CAS.
- Z. Shi, Z. Han, W. Huang, Y. Zhang, J. Wei, X. Zhang, C. Chen and J. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 43617–43625 CrossRef CAS PubMed.
- Z. Shi, Z. Han, F. Xing, Y. Zhang, J. Xu, C. Chen, J. Zhang, H. Yuan and X. Zhang, J. Am. Ceram. Soc., 2024, 107, 6130–6137 CrossRef CAS.
- Z. Shi, J. Xu, J. Zhu, Y. Zhang, T. Gao, M. Qin, H. Sun, G. Dong and F. Gao, Ceram. Int., 2019, 45, 1977–1983 CrossRef CAS.
- Y. Wang, Y. Sui, J. Cheng, X. Wang, W. Su, X. Liu and H. J. Fan, J. Phys. Chem. C, 2010, 114, 5174–5181 CrossRef CAS.
- H. Amaveda, M. Mora, O. J. Dura, M. A. Torres, M. A. Madre, S. Marinel and A. Sotelo, J. Eur. Ceram. Soc., 2021, 41, 402–408 CrossRef CAS.
- C. Yang, H. Wu, H. Song, X. Wang, S. Chen, X. Xu, L. Chen, Z. Zhao, L. Yu and B. Liu, J. Alloys Compd., 2023, 940, 168802 CrossRef CAS.
- I. I. Maor, K. Kruppa, A. Rozencweig, A. Sterzer, F. Steinbach, V. Beilin, B. Breidenstein, G. E. Shter, M. Mann-Lahav, A. Feldhoff and G. S. Grader, Adv. Funct. Mater., 2023, 33, 2304464 CrossRef CAS.
- Y.-N. Li, P. Wu, S. Zhang, Y. Pei, J. Yang, S. Chen and L. Wang, Ceram. Int., 2022, 48, 33967–33975 CrossRef CAS.
- Z. Han, J. Zhang, H. Ma, F. Xing, Y. Qi, J. Wei, G. He, Y. Zhang, Y. Xin, Q. Wang and Z. Shi, ACS Appl. Mater. Interfaces, 2025, 17, 5114–5123 CrossRef PubMed.
- J. Yu, M. Nelo, X. Liu, S. Shao, B. Wang, S. J. Haigh, H. Jantunen and R. Freer, J. Eur. Ceram. Soc., 2022, 42, 3920–3928 CrossRef CAS.
- Y. Zheng, X. Zhang, W. Xie, N. Ge, Y. Ren, X. Wei and B. Dai, J. Electron. Mater., 2022, 51, 4938–4943 CrossRef CAS.
- Z. Shi, Z. Han, J. Wei, C. Chen, Y. Zhang, H. Yuan, F. Song, J. Zhang, G. He and X. Li, Appl. Surf. Sci., 2024, 661, 160046 CrossRef CAS.
- Z. Shi, Z. Han, W. Huang, J. Xu, Y. Liu, Y. Zhang, C. Chen, J. Wei, G. He and J. Zhang, J. Mater. Chem. A, 2024, 12, 21288–21300 RSC.
- Z. Shi, C. Zhang, T. Su, J. Xu, J. Zhu, H. Chen, T. Gao, M. Qin, P. Zhang, Y. Zhang, H. Yan and F. Gao, ACS Appl. Mater. Interfaces, 2020, 12, 21623–21632 CrossRef CAS PubMed.
- M. M. Mallick and S. Vitta, J. Electron. Mater., 2018, 47, 3230–3237 CrossRef CAS.
- N. M. Ferreira, M. A. Madre, M. A. Torres, A. Davarpanah, V. Amaral, F. M. Costa and A. Sotelo, Mater. Res. Bull., 2020, 130, 110933 CrossRef CAS.
- K. Rubesova, T. Hlasek, V. Jakes, S. Huber, J. Hejtmanek and D. Sedmidubsky, J. Eur. Ceram. Soc., 2015, 35, 525–531 CrossRef CAS.
- O. Jankovsky and D. Sedmidubsky, J. Eur. Ceram. Soc., 2018, 38, 131–135 CrossRef.
- B. Wei, J. Li, Y. Yang, W. Li, Z. Zhou, Y. Zheng, W. Zhang, Y. Chai, Z. Chang, C.-W. Nan and Y.-H. Lin, npj Comput. Mater., 2023, 9, 160 CrossRef CAS.
- B. Wei, W. Li, Y. Yang, J. Li, Y. Zheng, W. Zhang, Z. Zhou, C. Lin, Z. Chang, X. Jiang, C.-W. Nan and Y.-H. Lin, Acta Mater., 2025, 286, 120699 CrossRef CAS.
- S. D. N. Luu and P. Vaqueiro, J. Materiomics, 2016, 2, 131–140 CrossRef.
- R. Liu, J.-L. Lan, X. Tan, Y.-C. Liu, G.-K. Ren, C. Liu, Z.-F. Zhou, C.-W. Nan and Y.-H. Lin, J. Eur. Ceram. Soc., 2018, 38, 2742–2746 CrossRef CAS.
- X. Tan, Y. Liu, R. Liu, Z. Zhou, C. Liu, J.-L. Lan, Q. Zhang, Y.-H. Lin and C.-W. Nan, Adv. Energy Mater., 2019, 9, 1900354 CrossRef.
- X. Tan, J.-l Lan, G. Ren, Y. Liu, Y.-H. Lin and C.-W. Nan, J. Am. Ceram. Soc., 2017, 100, 1494–1501 CrossRef CAS.
- X. Tan, Y. Liu, K. Hu, G. Ren, Y. Li, R. Liu, Y.-H. Lin, J.-L. Lan and C.-W. Nan, J. Am. Ceram. Soc., 2018, 101, 326–333 CrossRef CAS.
- X. Tan, J.-L. Lan, K. Hu, B. Xu, Y. Liu, P. Zhang, X.-Z. Cao, Y. Zhu, W. Xu, Y.-H. Lin and C.-W. Nan, J. Am. Ceram. Soc., 2018, 101, 4634–4644 CrossRef CAS.
- A. Novitskii, M. Y. Toriyama, I. Serhiienko, T. Mori, G. J. Snyder and P. Gorai, Adv. Funct. Mater., 2025, 35, 2416509 CrossRef CAS.
- Z. Zhou, W. Zhang, Y. Zheng, Y. Yang, B. Wei, C. Liu, J.-L. Lan, C.-W. Nan and Y.-H. Lin, Mater. Today Phys., 2023, 39, 101292 CrossRef CAS.
- N. Yang, L. Pan, C. Chen and Y. Wang, J. Alloys Compd., 2021, 858, 157748 CrossRef CAS.
- B. Wei, X. Zhang, W. Li, J. Li, Y. Li, Q. Gao, J. Hong, C.-W. Nan and Y.-H. Lin, Appl. Phys. Rev., 2025, 12, 011324 CAS.
- M. Palazzi and S. Jaulmes, Acta Crystallogr., Sect. B, 1981, 37, 1337–1339 CrossRef.
- K. Ishikawa, S. Kinoshita, Y. Suzuki, S. Matsuura, T. Nakanishi, M. Aizawa and Y. Suzuki, J. Electrochem. Soc., 1991, 138, 1166 CrossRef CAS.
- H. Hiramatsu, K. Ueda, H. Ohta, M. Hirano, M. Kikuchi, H. Yanagi, T. Kamiya and H. Hosono, Appl. Phys. Lett., 2007, 91, 012104 CrossRef.
- M. Yasukawa, K. Ueda and H. Hosono, J. Appl. Phys., 2004, 95, 3594–3597 CrossRef CAS.
- H. Hiramatsu, H. Yanagi, T. Kamiya, K. Ueda, M. Hirano and H. Hosono, Chem. Mater., 2008, 20, 326–334 CrossRef CAS.
- L. D. Zhao, D. Berardan, Y. L. Pei, C. Byl, L. Pinsard-Gaudart and N. Dragoe, Appl. Phys. Lett., 2010, 97, 092118 CrossRef.
- J. Sui, J. Li, J. He, Y.-L. Pei, D. Berardan, H. Wu, N. Dragoe, W. Cai and L.-D. Zhao, Energy Environ. Sci., 2013, 6, 2916–2920 RSC.
- J. S. O. Evans, E. B. Brogden, A. L. Thompson and R. L. Cordiner, Chem. Commun., 2002, 912–913 RSC.
- S. Tan, C. Gao, C. Wang, Q. Jing, T. Zhou, G. Yin, M. Sun, F. Xing, R. Cao and Y. Sun, R. Soc. Open Sci., 2020, 7, 201078 CrossRef CAS.
- D. Berthebaud, O. I. Lebedev, D. Pelloquin and A. Maignan, Solid State Sci., 2014, 36, 94–100 CrossRef CAS.
- D. Song, G. Guélou, T. Mori, M. Ochi, K. Kuroki, H. Fujihisa, Y. Gotoh, Y. Iwasa, H. Eisaki and H. Ogino, J. Mater. Chem. C, 2018, 6, 12260–12266 RSC.
- T. L. Chou, O. Mustonen, T. S. Tripathi and M. Karppinen, J. Phys.: Condens. Matter, 2016, 28, 035802 CrossRef CAS.
- Y. Yang, Z. Zhou, B. Wei, J. Liu, J.-L. Lan, M. Zou, Y. Xu, Y. Zheng, C.-W. Nan and Y.-H. Lin, J. Am. Ceram. Soc., 2023, 106, 2918–2929 CrossRef CAS.
- P. Ai, H. Wang, S. Tang, T. Yan, S. Bai, D. Wan, W. Guo, P. Zhang and T. Zheng, J. Energy Chem., 2025, 107, 376–385 CrossRef CAS.
- G. H. J. Johnstone, M. U. González-Rivas, K. M. Taddei, R. Sutarto, G. A. Sawatzky, R. J. Green, M. Oudah and A. M. Hallas, J. Am. Chem. Soc., 2022, 144, 20590–20600 CrossRef CAS PubMed.
- Y. Zhang, B. Feng, H. Hayashi, C.-P. Chang, Y.-M. Sheu, I. Tanaka, Y. Ikuhara and H. Ohta, Nat. Commun., 2018, 9, 2224 CrossRef.
- Z. Li, C. Xiao, H. Zhu and Y. Xie, J. Am. Chem. Soc., 2016, 138, 14810–14819 CrossRef CAS PubMed.
- C. Wu, X.-L. Shi, L. Wang, W. Lyu, P. Yuan, L. Cheng, Z.-G. Chen and X. Yao, ACS Nano, 2024, 18, 31660–31712 CrossRef CAS.
- F. Xu, A. Li, Y. Rao, Z. Huang and S. Wei, Trans. Indian Inst. Met., 2021, 74, 2367–2377 CrossRef CAS.
- A. Novitskii, I. Serhiienko, S. Novikov, K. Kuskov, D. Pankratova, T. Sviridova, A. Voronin, A. Bogach, E. Skryleva, Y. Parkhomenko, A. Burkov, T. Mori and V. Khovaylo, J. Alloys Compd., 2022, 912, 165208 CrossRef CAS.
- B. Feng, X. Jiang, Z. Pan, L. Hu, X. Hu, P. Liu, Y. Ren, G. Li, Y. Li and X. A. Fan, Mater. Des., 2020, 185, 108263 CrossRef CAS.
- Y. Liu, J. Ding, B. Xu, J. Lan, Y. Zheng, B. Zhan, B. Zhang, Y. Lin and C. Nan, Appl. Phys. Lett., 2015, 106, 233903 CrossRef.
- F. Li, T.-R. Wei, F. Kang and J.-F. Li, J. Mater. Chem. A, 2013, 1, 11942–11949 RSC.
- Y.-L. Pei, J. He, J.-F. Li, F. Li, Q. Liu, W. Pan, C. Barreteau, D. Berardan, N. Dragoe and L.-D. Zhao, NPG Asia Mater., 2013, 5, e47 CrossRef CAS.
- J. Li, J. Sui, C. Barreteau, D. Berardan, N. Dragoe, W. Cai, Y. Pei and L.-D. Zhao, J. Alloys Compd., 2013, 551, 649–653 CrossRef CAS.
- J.-L. Lan, B. Zhan, Y.-C. Liu, B. Zheng, Y. Liu, Y.-H. Lin and C.-W. Nan, Appl. Phys. Lett., 2013, 102, 123905 CrossRef.
- G.-K. Ren, J.-l Lan, S. Butt, K. J. Ventura, Y.-H. Lin and C.-W. Nan, RSC Adv., 2015, 5, 69878–69885 RSC.
- Y. C. Liu, J. L. Lan, B. Zhan, J. X. Ding, Y. Liu, Y. H. Lin, B. P. Zhang and C. W. Nan, J. Am. Ceram. Soc., 2013, 96, 2710–2713 CrossRef CAS.
- D. Yang, X. Su, Y. Yan, T. Hu, H. Xie, J. He, C. Uher, M. G. Kanatzidis and X. Tang, Chem. Mater., 2016, 28, 4628–4640 CrossRef CAS.
- Y.-c Liu, Y.-h Zheng, B. Zhan, K. Chen, S. Butt, B. Zhang and Y.-h Lin, J. Eur. Ceram. Soc., 2015, 35, 845–849 CrossRef CAS.
- J. Li, J. Sui, Y. Pei, X. Meng, D. Berardan, N. Dragoe, W. Cai and L.-D. Zhao, J. Mater. Chem. A, 2014, 2, 4903–4906 RSC.
- H. Kang, X. Zhang, Y. Wang, J. Li, D. Liu, Z. Chen, E. Guo, X. Jiang and T. Wang, Mater. Res. Bull., 2020, 126, 110841 CrossRef CAS.
- Y. Liu, J. Lan, W. Xu, Y. Liu, Y.-L. Pei, B. Cheng, D.-B. Liu, Y.-H. Lin and L.-D. Zhao, Chem. Commun., 2013, 49, 8075–8077 RSC.
- B. Feng, G. Li, Y. Hou, C. Zhang, C. Jiang, J. Hu, Q. Xiang, Y. Li, Z. He and X. A. Fan, J. Alloys Compd., 2017, 712, 386–393 CrossRef CAS.
- L. Pan, Z. Zhao, N. Yang, W. Xing, J. Zhang, Y. Liu, C. Chen, D. Li and Y. Wang, J. Eur. Ceram. Soc., 2020, 40, 5543–5548 CrossRef CAS.
- A. Ahmad, M. Hussain and Y. H. Lin, Mater. Res. Express, 2019, 6, 105507 CrossRef CAS.
- P. Amirkhizi, M. A. Madre, O. J. Dura, M. A. Torres, A. Sotelo, A. V. Kovalevsky and S. Rasekh, Ceram. Int., 2025, 51, 9421–9428 CrossRef CAS.
- U. Hira, S. S. Ali, S. Latif, N. Pryds and F. Sher, ACS Omega, 2022, 7, 6579–6590 CrossRef CAS.
- J. Tang, R. Xu, J. Zhang, D. Li, W. Zhou, X. Li, Z. Wang, F. Xu, G. Tang and G. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 15543–15551 CrossRef CAS.
- Q. Wen, C. Chang, L. Pan, X. Li, T. Yang, H. Guo, Z. Wang, J. Zhang, F. Xu, Z. Zhang and G. Tang, J. Mater. Chem. A, 2017, 5, 13392–13399 RSC.
- L. Wang, M. Wang and D. Zhao, J. Alloys Compd., 2009, 471, 519–523 CrossRef CAS.
- Z. Yin, Z. Liu, Y. Yu, C. Zhang, P. Chen, J. Zhao, P. He and X. Guo, ACS Appl. Mater. Interfaces, 2021, 13, 57638–57645 CrossRef CAS.
- J. F. Schooley, W. R. Hosler and M. L. Cohen, Phys. Rev. Lett., 1964, 12, 474–479 CrossRef CAS.
- O. N. Tufte and P. W. Chapman, Phys. Rev., 1967, 155, 796–802 CrossRef CAS.
- Y. Kozuka, Y. Hikita, C. Bell and H. Y. Hwang, Appl. Phys. Lett., 2010, 97, 012107 CrossRef.
- A. T. T. Pham, T. B. Tran, T. T. T. Phan, V. Q. Doan, U. T. T. Doan, H. T. Lai, N. M. Q. Tran, T. D. T. Ung, P. D. Doan, H. B. T. Thu, V. C. Tran and T. B. Phan, Ceram. Int., 2024, 50, 3511–3518 CrossRef CAS.
- S. Das, A. Ramakrishnan, K.-H. Chen, D. K. Misra and R. C. Mallik, J. Phys. D: Appl. Phys., 2018, 51, 035501 CrossRef.
- H. C. Chang, T. H. Chen, R. Sankar, Y. J. Yang, L. C. Chen and K. H. Chen, Mater. Today Phys., 2020, 15, 100248 CrossRef.
- Z. Wu, Y. Wang, G. Liu, X. Yang, T. Wei, H. Zhang, J. Zhou and J. Zhu, Mater. Today Energy, 2021, 21, 100810 CrossRef CAS.
- O. V. Merkulov, I. V. Shamsutov, M. A. Ryzhkov, B. V. Politov, I. V. Baklanova, E. V. Chulkov and V. P. Zhukov, J. Solid State Chem., 2023, 326, 124231 CrossRef CAS.
- U. Aschauer, R. Pfenninger, S. M. Selbach, T. Grande and N. A. Spaldin, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 054111 CrossRef.
- X. Ding, M. Li, P. Chen, Y. Zhao, M. Zhao, H. Leng, Y. Wang, S. Ali, F. Raziq, X. Wu, H. Xiao, X. Zu, Q. Wang, A. Vinu, J. Yi and L. Qiao, Matter, 2022, 5, 4274–4314 CrossRef CAS.
- R. Prasad and S. D. Bhame, Mater. Renewable Sustainable Energy, 2020, 9, 3 CrossRef.
- M. Qin, F. Gao, J. Cizek, S. Yang, X. Fan, L. Zhao, J. Xu, G. Dong, M. Reece and H. Yan, Acta Mater., 2019, 164, 76–89 CrossRef CAS.
- M. Qin, Z. Lou, P. Zhang, Z. Shi, J. Xu, Y. Chen and F. Gao, ACS Appl. Mater. Interfaces, 2020, 12, 53899–53909 CrossRef CAS.
- L.-D. Zhao, J. He, D. Berardan, Y. Lin, J.-F. Li, C.-W. Nan and N. Dragoe, Energy Environ. Sci., 2014, 7, 2900–2924 RSC.
- Z. Shi, Y. Liu, J. Wei, Y. Zhang, D. Zhao, J. Zhang, F. Xing, C. Chen and Z. Han, Ceram. Int., 2024, 50, 27331–27338 CrossRef CAS.
- Y. Liu, X. Zhang, P. Nan, B. Zou, Q. Zhang, Y. Hou, S. Li, Y. Gong, Q. Liu, B. Ge, O. Cojocaru-Mirédin, Y. Yu, Y. Zhang, G. Chen, M. Wuttig and G. Tang, Adv. Funct. Mater., 2022, 32, 2209980 CrossRef CAS.
- Y. Zhu, W. Wang, B. Liang, W. Liu, T. Zhou, B. Meng, H. Liu, W. Gao, Y. Yang, C. Niu, C. Zheng, Z. An, S. Wu, W. Liu, Y. Zhang, C. Yuan, Y. Zhu, L. Yin and J. Shen, J. Mater. Chem. C, 2025, 13, 2279–2285 RSC.
- Y. Zheng, H. Chen, Z. Zhou, Y. Yang, M. Zou, W. Zhang, B. Wei, J. Cai, J.-L. Lan, D. Yi, C.-W. Nan and Y.-H. Lin, Adv. Funct. Mater., 2023, 33, 2301815 CrossRef CAS.
- S. Fan, Y. Jin, X. Ai, S. Gu, E. Song, Q. Zhang, L. Wang and W. Jiang, Small, 2025, 21, 2411022 CrossRef CAS.
- C. M. Rost, E. Sachet, T. Borman, A. Moballegh, E. C. Dickey, D. Hou, J. L. Jones, S. Curtarolo and J.-P. Maria, Nat. Commun., 2015, 6, 8485 CrossRef CAS PubMed.
- P. Ai, S. Tang, S. Bai, D. Wan, W. Guo, P. Zhang, T. Zheng, H. Wang and T. Yan, J. Energy Chem., 2025, 107, 376–385 CrossRef CAS.
- S. S. Jana, R. Banerjee and T. Maiti, J. Mater. Chem. A, 2025, 13, 27050–27068 RSC.
- H. Yu, S. Gao, H. Ma and X. Jia, Ceram. Int., 2025, 51, 46135–46141 CrossRef CAS.
- R.-C. Chen, X.-L. Shi, H.-J. Kang, E.-Y. Guo, Z.-N. Chen, Y.-P. Lu, T.-M. Wang and Z.-G. Chen, Mater. Today, 2025, 90, 815–837 CrossRef CAS.
- B. Jiang, Y. Yu, J. Cui, X. Liu, L. Xie, J. Liao, Q. Zhang, Y. Huang, S. Ning, B. Jia, B. Zhu, S. Bai, L. Chen, S. J. Pennycook and J. He, Science, 2021, 371, 830–834 CrossRef CAS PubMed.
- A. Kumar, A. Moll, M. N. Mouhamadsiradjoudine, F. Brisset, D. Berardan and N. Dragoe, Phys. Status Solidi RRL, 2024, 18, 2300372 CrossRef CAS.
- A. Kumar, D. Dragoe, D. Berardan and N. Dragoe, J. Materiomics, 2023, 9, 191–196 CrossRef.
- R. Banerjee, S. Chatterjee, M. Ranjan, T. Bhattacharya, S. Mukherjee, S. S. Jana, A. Dwivedi and T. Maiti, ACS Sustainable Chem. Eng., 2020, 8, 17022–17032 CrossRef CAS.
- A. Sarkar, B. Eggert, L. Velasco, X. Mu, J. Lill, K. Ollefs, S. S. Bhattacharya, H. Wende, R. Kruk, R. A. Brand and H. Hahn, APL Mater., 2020, 8, 051111 CrossRef CAS.
- A. J. Wright, Q. Wang, S.-T. Ko, K. M. Chung, R. Chen and J. Luo, Scr. Mater., 2020, 181, 76–81 CrossRef CAS.
- K. Biswas, J. He, Q. Zhang, G. Wang, C. Uher, V. P. Dravid and M. G. Kanatzidis, Nat. Chem., 2011, 3, 160–166 CrossRef CAS.
- C. Gayner and Y. Amouyal, Adv. Funct. Mater., 2020, 30, 1901789 CrossRef CAS.
- S. Wang, L. Zhang, T. Hong, L. Su, Y. Wen, B. Qin, Y. Xiao, Y. Wang, H. Shi, J. Zheng, Y. Qiu and L.-D. Zhao, Adv. Funct. Mater., 2024, 34, 2310335 CrossRef CAS.
- Y.-L. Pei, H. Wu, D. Wu, F. Zheng and J. He, J. Am. Chem. Soc., 2014, 136, 13902–13908 CrossRef CAS.
- G. Tan, L.-D. Zhao and M. G. Kanatzidis, Chem. Rev., 2016, 116, 12123–12149 CrossRef CAS PubMed.
- M. Tan, W.-D. Liu, X.-L. Shi, H. Gao, H. Li, C. Li, X.-B. Liu, Y. Deng and Z.-G. Chen, Small Methods, 2019, 3, 201900582 CrossRef.
- X. Chen, Z. Zhou, Y.-H. Lin and C. Nan, J. Materiomics, 2020, 6, 494–512 CrossRef.
- B. Yu, M. Zebarjadi, H. Wang, K. Lukas, H. Wang, D. Wang, C. Opeil, M. Dresselhaus, G. Chen and Z. Ren, Nano Lett., 2012, 12, 2077–2082 CrossRef CAS PubMed.
- J. Zheng, S. Wang, Z. Zhao, X. Gao, T. Hong and L.-D. Zhao, Adv. Funct. Mater., 2023, 33, 202300447 Search PubMed.
- A. Mehdizadeh Dehkordi, M. Zebarjadi, J. He and T. M. Tritt, Mater. Sci. Eng., R, 2015, 97, 1–22 CrossRef.
- H. Y. Hwang, Science, 2006, 313, 1895–1896 CrossRef CAS PubMed.
- T. L. Kim and H. W. Jang, Curr. Appl. Phys., 2017, 17, 626–639 CrossRef.
- S. Stemmer and S. J. Allen, Annu. Rev. Mater. Res., 2014, 44, 151–171 CrossRef CAS.
- J. Chen, H. Chen, F. Hao, X. Ke, N. Chen, T. Yajima, Y. Jiang, X. Shi, K. Zhou, M. Dobeli, T. Zhang, B. Ge, H. Dong, H. Zeng, W. Wu and L. Chen, ACS Energy Lett., 2017, 2, 915–921 CrossRef CAS.
- V. Taneja, S. Das, K. Dolui, T. Ghosh, A. Bhui, U. Bhat, D. K. Kedia, K. Pal, R. Datta and K. Biswas, Adv. Mater., 2024, 36, 2307058 CrossRef CAS PubMed.
- L. M. Daniels, S. N. Savvin, M. J. Pitcher, M. S. Dyer, J. B. Claridge, S. Ling, B. Slater, F. Corà, J. Alaria and M. J. Rosseinsky, Energy Environ. Sci., 2017, 10, 1917–1922 RSC.
- E. Isotta, S. Jiang, G. Moller, A. Zevalkink, G. J. Snyder and O. Balogun, Adv. Mater., 2023, 35, 2302777 CrossRef CAS.
- C. Hu, K. Xia, C. Fu, X. Zhao and T. Zhu, Energy Environ. Sci., 2022, 15, 1406–1422 RSC.
- R. Wu, Y. Yu, S. Jia, C. Zhou, O. Cojocaru-Mirédin and M. Wuttig, Nat. Commun., 2023, 14, 719 CrossRef CAS PubMed.
- P. Balaya, J. Jamnik, J. Fleig and J. Maier, Appl. Phys. Lett., 2006, 88, 062109 CrossRef.
- J. Fleig and J. Maier, Phys. Chem. Chem. Phys., 1999, 1, 3315–3320 RSC.
- R. Merkle and J. Maier, Phys. Chem. Chem. Phys., 2002, 4, 4140–4148 RSC.
- Z. Shi, T. Su, P. Zhang, Z. Lou, M. Qin, T. Gao, J. Xu, J. Zhu and F. Gao, J. Mater. Chem. A, 2020, 8, 19561–19572 RSC.
- P. Zhang, M. Qin, Z. Lou, S. Cao, L. Gong, J. Xu, M. J. Reece, H. Yan, Z. Dashevsky and F. Gao, J. Eur. Ceram. Soc., 2022, 42, 7017–7026 CrossRef CAS.
- Y. Lin, M. T. Dylla, J. J. Kuo, J. P. Male, I. A. Kinloch, R. Freer and G. J. Snyder, Adv. Funct. Mater., 2020, 30, 1910079 CrossRef CAS.
- P. Dixit, S. S. Jana and T. Maiti, Small, 2023, 19, 2206710 CrossRef CAS.
- L. Zhang, Y.-Q. Du, J.-B. Li and J. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 52712–52718 CrossRef CAS.
- S. S. Jana and T. Maiti, ACS Appl. Mater. Interfaces, 2022, 14, 14174–14181 CrossRef CAS.
- M. T. Dylla, J. J. Kuo, I. Witting and G. J. Snyder, Adv. Mater. Interfaces, 2019, 6, 1900222 CrossRef.
- Z. Lou, P. Zhang, P. Chen, Z. Wei, J. Gou, J. Xu, C. Gong and F. Gao, J. Am. Ceram. Soc., 2024, 107, 4717–4728 CrossRef CAS.
- Z. Lou, Z. Wei, J. Gou, J. Xu, C. Gong and F. Gao, ACS Appl. Energy Mater., 2024, 7, 12119–12130 CrossRef CAS.
- C. Wu, J. Li, Y. Fan, J. Xing, H. Gu, Z. Zhou, X. Lu, Q. Zhang, L. Wang and W. Jiang, J. Alloys Compd., 2019, 786, 884–893 CrossRef CAS.
- S. J. Fan, T. T. Sun, M. Jiang, S. J. Gu, L. J. Wang, H. X. Yan and W. Jiang, J. Adv. Ceram., 2022, 11, 1932–1943 CrossRef CAS.
- S. S. Jana, D. Chatterjee and T. Maiti, ACS Appl. Mater. Interfaces, 2023, 15, 48246–48254 CrossRef CAS PubMed.
- W. H. Nam, B. B. Kim, Y. S. Lim, K. S. Dae, W.-S. Seo, H.-H. Park and J. Y. Lee, Nanoscale, 2017, 9, 12941–12948 RSC.
- C. Kim, J. Cho, T. Kim and D. H. Lopez, J. Mater. Chem. A, 2022, 10, 13780–13792 RSC.
- P. Pichanusakorn and P. Bandaru, Mater. Sci. Eng., R, 2010, 67, 19–63 CrossRef.
- W. Yan, X. Nie, S. Ke, Y. Hu, X. Ai, W. Zhu, W. Zhao and Q. Zhang, Adv. Funct. Mater., 2022, 32, 2209739 CrossRef CAS.
- Y. Lin, M. Wood, K. Imasato, J. J. Kuo, D. Lam, A. N. Mortazavi, T. J. Slade, S. A. Hodge, K. Xi, M. G. Kanatzidis, D. R. Clarke, M. C. Hersam and G. J. Snyder, Energy Environ. Sci., 2020, 13, 4114–4121 RSC.
- J.-W. Li, H. Gao, Z. Han, J. Yu, H.-L. Zhuang, L. Chen, H. Li, Y. Jiang, Z. Wang, Q. Zheng and J.-F. Li, Adv. Mater., 2025, 37, 2503665 CrossRef CAS.
- Y. Wang, K. H. Lee, H. Hyuga, H. Kita, K. Inaba, H. Ohta and K. Koumoto, Appl. Phys. Lett., 2007, 91, 242102 CrossRef.
- J. Liao, H. Shao, Y. Zhang, Y. Yan, J. Zeng, C. Lan, B. Gao, D. Chen, Q. Quan, P. Xie, Y. Meng and J. C. Ho, Adv. Mater., 2025, 37, 2419653 CrossRef CAS PubMed.
- J. Tauc, Czech. J. Phys., 1955, 5, 528–535 CrossRef.
- J. G. Harper, H. E. Matthews and R. H. Bube, J. Appl. Phys., 1970, 41, 3182–3184 CrossRef CAS.
- H. B. Kwok and R. H. Bube, J. Appl. Phys., 1973, 44, 138–144 CrossRef CAS.
- W. Dai, W. Liu, J. Yang, C. Xu, A. Alabastri, C. Liu, P. Nordlander, Z. Guan and H. Xu, Light: Sci. Appl., 2020, 9, 120 CrossRef CAS PubMed.
- K. Zhang, B. Ouyang, Y. Wang, Y. Xia and Y. Yang, ACS Appl. Energy Mater., 2019, 2, 7647–7654 CrossRef CAS.
- C. Bianchi, A. Marques and I. Ferreira, Adv. Mater. Technol., 2023, 8, 2300133 CrossRef CAS.
- P. S. Mondal, R. Okazaki, H. Taniguchi and I. Terasaki, J. Appl. Phys., 2013, 114, 173710 CrossRef.
- X. Lu, P. Jiang and X. Bao, Nat. Commun., 2019, 10, 138 CrossRef PubMed.
- F. Wang, Y. Lv, Y. Xu, L. Cao, L. Chen, C. Zhang, S. Yao, J. Xu, J. Zhou and Y. Chen, Opt. Express, 2022, 30, 8356 CrossRef CAS.
- W. Dai, Y. Liang, M. Yang, D. Schrecongost, P. Gajurel, H. Lee, J.-W. Lee, J. Chen, C.-B. Eom and C. Cen, Nano Lett., 2019, 19, 7149–7154 CrossRef CAS.
- M. Qiu, W. Zheng, J. Chen, Z. Cheng, L. Wang and Q. Wang, Appl. Phys. Lett., 2024, 124, 261104 CrossRef CAS.
- Y. Zhong, H. Fang, Y. Ran and H. Zhu, InfoMat, 2023, 5, e12384 CrossRef CAS.
- C. L. Chang, A. Kleinhammes, W. G. Moulton and L. R. Testardi, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 11564–11567 CrossRef CAS PubMed.
- H. Lengfellner, S. Zeuner, W. Prettl and K. F. Renk, Europhys. Lett., 1994, 25, 375–378 CrossRef CAS.
- H. Lengfellner, G. Kremb, A. Schnellbögl, J. Betz, K. F. Renk and W. Prettl, Appl. Phys. Lett., 1992, 60, 501–503 CrossRef CAS.
- A. A. S. Ibrahim, A. Bochmann, R. Löhnert and J. Töpfer, Adv. Funct. Mater., 2024, 35, 2413166 CrossRef.
- J. Wu, G. Yan, M. Chen, Y. Xue, L. Gao, J. Wang and S. Wang, Appl. Surf. Sci., 2021, 570, 121254 Search PubMed.
- Y. Sun, H. Wu, L. Yu, H. Sun, P. Zhang, X. Zhang, B. Dai and Y. Wang, Sensors, 2022, 22, 4867 CrossRef CAS.
- W. Brozio, F. Wolf, C. Feratl, O. Kus and K. F. Renk, IEEE, 1997, 768 CAS.
- K. Takahashi, T. Kanno, A. Sakai, H. Adachi and Y. Yamada, Appl. Phys. Lett., 2010, 97, 021906 CrossRef.
- Y. Qin, T. Zhao, B. Wang, P. Zhang and J. Yang, CrystEngComm, 2014, 16, 5345–5351 RSC.
- H. Chen, Y. Huang, L. Yu, Z. Li, G. Wang, B. Dai and Y. Wang, Appl. Phys. Lett., 2024, 124, 013905 CrossRef CAS.
- Y. Song, Z. Li, H. Li, S. Tang, G. Mu, L. Xu, W. Peng, D. Shen, Y. Chen, X. Xie and M. Jiang, Nanotechnology, 2020, 31, 165704 CrossRef CAS PubMed.
- T. Kanno, K. Takahashi, A. Sakai, H. Tamaki, H. Kusada and Y. Yamada, J. Electron. Mater., 2014, 43, 2072 CrossRef CAS.
- K. Zhao, K. J. Jin, Y. Huang, S. Zhao, H. Lu, M. He, Z. Chen, Y. Zhou and G. Yang, Appl. Phys. Lett., 2006, 89, 7433 Search PubMed.
- X. Chen, B. Tao, R. Zhao, K. Yang, Y. Xia, Z. Li, T. Xie, Y. Zhong and T. Zhang, Appl. Phys. Lett., 2022, 121, 204102 CrossRef CAS.
- K. Takahashi, A. Sakai, T. Kanno and H. Adachi, Appl. Phys. Lett., 2009, 95, 105 Search PubMed.
- L. D. Zhao, D. Berardan, Y. L. Pei, C. Byl and N. Dragoe, Appl. Phys. Lett., 2010, 97, 092118 CrossRef.
- X. M. Li, K. Zhao, H. Ni, S. Q. Zhao, W. F. Xiang, Z. Q. Lu, Z. J. Yue, F. Wang, Y.-C. Kong and H. K. Wong, Appl. Phys. Lett., 2010, 97, 044104 CrossRef.
- X. Liu, Y.-z Yan, Q.-m Chen, H. Zhang and X.-p Yin, Appl. Phys. A: Mater. Sci. Process., 2014, 115, 1371–1374 CrossRef CAS.
- P. X. Zhang, C. Wang, S. L. Tan, H. Zhang and H. U. Habermeier, J. Cryst. Growth, 2008, 310, 2732–2737 CrossRef CAS.
- J. Ma, M. Theingi, H. Zhang, Q. Chen and X. Liu, Appl. Phys. A: Mater. Sci. Process., 2014, 114, 1075–1078 CrossRef CAS.
- X. Liu, X. Yin, Q. Chen, H. Zhang and M. Cao, Mater. Sci. Eng., B, 2014, 185, 105–108 CrossRef CAS.
- P. X. Zhang, C. Wang, G. Y. Zhang, L. Yu, W. K. Lee and H. U. Habermeier, Opt. Laser Technol., 2004, 36, 341–343 CrossRef CAS.
- Y. Wang, L. D. Yu, B. Jiang and P. Zhang, J. Appl. Phys., 2011, 110, 123111 CrossRef.
- Y. Wang, L. Yu and P. X. Zhang, Opt. Laser Technol., 2011, 43, 1462–1465 CrossRef CAS.
- L. Yu, Y. Wang, P. Zhang and H.-U. Habermeier, Phys. Status Solidi RRL, 2013, 7, 180–183 CrossRef CAS.
- K. Takahashi, T. Kanno, A. Sakai, H. Adachi and Y. Yamada, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 115107 CrossRef.
- G. W. Yan, L. Yu, Y. Wang, H. Zhang, P. X. Zhang and H.-U. Habermeier, J. Appl. Phys., 2011, 110, 10310 Search PubMed.
- S. Song, L. Yu, J. Hu, A. Liu and Y. Zhong, Appl. Phys. A: Mater. Sci. Process., 2017, 123, 595 CrossRef.
- S.-F. Wang, M.-J. Chen, S.-R. Zhao, J.-C. Chen, L.-P. He, W. Yu, J.-L. Wang and G.-S. Fu, Chin. Phys. B, 2010, 19, 107201 CrossRef.
- S. Wang, Z. Bai, G. Yan, H. Zhang, J. Wang, W. Yu and G. Fu, Opt. Express, 2013, 21, 18336–18343 CrossRef.
- L. Wang, G. Yan, G. Dong, S. Qiao, G. Fu and S. Wang, Opt. Mater. Express, 2016, 6, 2537–2544 CrossRef CAS.
- G. Yan, L. Wang, S. Qiao, X. Wu, S. Wang and G. Fu, Opt. Mater. Express, 2016, 6, 558–565 CrossRef CAS.
- W. Yu, G. Yan, Y. Xue, Y. Zhang, J. Wang, G. Fu and S. Wang, Nanoscale Res. Lett., 2019, 14, 151254 Search PubMed.
- Y. Qin, T. Zhao, H. H. Zhang and B. Wang, Appl. Phys. Lett., 2013, 102, 253901 CrossRef.
- X. Chen, B. Tao, R. Zhao, K. Yang, Y. Xia, Q. Wang, Z. Li and T. Xie, IEEE Sens. J., 2023, 23, 27053–27058 CAS.
- M. Chen, X. Wang, X. Ning, Z. Chen, Y. Zhen, C. Yue, G. Yan, L. Fang and S. Wang, Appl. Surf. Sci., 2024, 655, 159579 CrossRef CAS.
- N. Zhou, K. Zhao, H. Liu, Z. Lu, H. Zhao, L. Tian, W. Liu and S. Zhao, J. Appl. Phys., 2009, 105, 083110 CrossRef.
- G. Y. Yan, H. L. Zhang, Z. L. Bai, S. F. Wang and J. L. Wang, Chin. Phys. Lett., 2013, 30, 046801 CrossRef.
- T. Sharifi, X. Zhang, G. Costin, S. Yazdi, C. F. Woellner, Y. Liu, C. S. Tiwary and P. Ajayan, Nano Lett., 2017, 17, 7908–7913 CrossRef CAS PubMed.
- J. Wu, K. Chen, M. J. Reece and Z. Huang, Energy Technol., 2024, 12, 2400973 CrossRef CAS.
- A. Achour, J. Liu, P. Peng, C. Shaw and Z. Huang, ACS Catal., 2018, 8, 10164–10172 CrossRef CAS.
- A. Achour, K. Chen, M. J. Reece and Z. Huang, Adv. Energy Mater., 2017, 8, 1701430 CrossRef.
- C. G. Vayenas, S. Bebelis and S. Ladas, Nature, 1990, 343, 625–627 CrossRef CAS.
- Y. Liu, L.-D. Zhao, Y. Liu, J. Lan, W. Xu, F. Li, B.-P. Zhang, D. Berardan, N. Dragoe, Y.-H. Lin, C.-W. Nan, J.-F. Li and H. Zhu, J. Am. Chem. Soc., 2011, 133, 20112–20115 CrossRef CAS PubMed.
- Z. Li, C. Xiao, S. Fan, Y. Deng, W. Zhang, B. Ye and Y. Xie, J. Am. Chem. Soc., 2015, 137, 6587–6593 CrossRef CAS PubMed.
- S. Saini, H. S. Yaddanapudi, K. Tian, Y. Yin, D. Magginetti and A. Tiwari, Sci. Rep., 2017, 7, 44621 CrossRef CAS PubMed.
- T. Nagira, M. Ito, S. Katsuyama, K. Majima and H. Nagai, J. Alloys Compd., 2003, 348, 263–269 CrossRef CAS.
- L. Li, Y. Liu, X. Qin, D. Li, J. Zhang, C. Song and L. Wang, J. Alloys Compd., 2014, 588, 562–567 CrossRef CAS.
- Y. Wang, Y. Sui and W. Su, J. Appl. Phys., 2008, 104, 093703 CrossRef.
- Y. Xu, J. Han, Y. Luo, Y. Liu, J. Ding, Z. Zhou, C. Liu, M. Zou, J. Lan, C. W. Nan and Y. Lin, Adv. Funct. Mater., 2021, 31, 2105001 CrossRef CAS.
- Y. Zhang, G. Peng, S. Li, H. Wu, K. Chen, J. Wang, Z. Zhao, T. Lyu, Y. Yu, C. Zhang, Y. Zhang, C. Ma, S. Guo, X. Ding, J. Sun, F. Liu and L. Hu, Nat. Commun., 2024, 15, 5978 CrossRef CAS PubMed.
- B. Geisler, P. Yordanov, M. E. Gruner, B. Keimer and R. Pentcheva, Phys. Status Solidi B, 2022, 259, 2100270 CrossRef CAS.
- I. H. Choi, S. G. Jeong, D.-G. Jeong, A. Seo, W. S. Choi and J. S. Lee, Adv. Sci., 2025, 12, 2407382 CrossRef CAS PubMed.
- G. Pennelli, Beilstein J. Nanotechnol., 2014, 5, 1268–1284 CrossRef PubMed.
- G. Pennelli, E. Dimaggio and A. Masci, Materials, 2021, 14, 5305 CrossRef CAS PubMed.
- H. Chen, C. Shao, S. Huang, Z. Gao, H. Huang, Z. Pan, K. Zhao, P. Qiu, T.-R. Wei and X. Shi, Adv. Energy Mater., 2024, 14, 2303473 CrossRef CAS.
- P. Yu, Y.-H. Chu and R. Ramesh, Mater. Today, 2012, 15, 320–327 CrossRef CAS.
- Z. Huang, Ariando, X. Renshaw Wang, A. Rusydi, J. Chen, H. Yang and T. Venkatesan, Adv. Mater., 2018, 30, 1802439 CrossRef PubMed.
- D. P. Dubey and R. Chatterjee, Mater. Res. Express, 2024, 11, 072002 CrossRef CAS.
- Y. Zhou, S. Zhang, X. Xu, W. Liu, S. Zhang, G. Li and J. He, Nano Energy, 2020, 69, 104397 CrossRef CAS.
- A. K. Yadav, C. T. Nelson, S. L. Hsu, Z. Hong, J. D. Clarkson, C. M. Schlepütz, A. R. Damodaran, P. Shafer, E. Arenholz, L. R. Dedon, D. Chen, A. Vishwanath, A. M. Minor, L. Q. Chen, J. F. Scott, L. W. Martin and R. Ramesh, Nature, 2016, 530, 198–201 CrossRef CAS PubMed.
- W.-R. Geng, X. Guo, Y.-L. Zhu, D. Ma, Y.-L. Tang, Y.-J. Wang, Y. Wu, Z. Hong and X.-L. Ma, Nat. Commun., 2025, 16, 2804 CrossRef CAS PubMed.
- G. S. Na and H. Chang, npj Comput. Mater., 2022, 8, 214 CrossRef.
- M. Qin, X. Zhang, J. Zhu, Y. Yang, Z. Ti, Y. Shen, X. Wang, X. Liu and Y. Zhang, J. Mater. Chem. A, 2023, 11, 10612–10627 RSC.
- C.-L. Fu, M. Cheng, N. T. Hung, E. Rha, Z. Chen, R. Okabe, D. C. Carrizales, M. Mandal, Y. Cheng and M. Li, Adv. Mater., 2025, 37, 2505642 CrossRef CAS PubMed.
- J. T. Sivak, S. S. I. Almishal, M. K. Caucci, Y. Tan, D. Srikanth, J. Petruska, M. Furst, L.-Q. Chen, C. M. Rost, J.-P. Maria and S. B. Sinnott, Phys. Rev. Lett., 2025, 134, 216101 CrossRef CAS PubMed.
- T. Deng, P. Qiu, T. Yin, Z. Li, J. Yang, T. Wei and X. Shi, Adv. Mater., 2024, 36, 2311278 CrossRef CAS PubMed.
- B. Zhan, J. Lan, Y. Liu, Y. Lin, Y. Shen and C. Nan, J. Mater. Sci. Technol., 2014, 30, 821–825 CrossRef CAS.
- N. Kanas, B. A. D. Williamson, F. Steinbach, R. Hinterding, M.-A. Einarsrud, S. M. Selbach, A. Feldhoff and K. Wuek, ACS Appl. Energy Mater., 2022, 5, 12396–12407 CrossRef CAS.
- W. Wang, Y. Liu, Y. Xue, Z. Yin, W. Lee, Z.-G. Chen, L. Yang, K. Koumoto, J. Yang, W. Li and S. Li, Acta Mater., 2023, 260, 119347 CrossRef CAS.
- C. Yang, H. Wu, H. Song, X. Wang, S. Chen, X. Xu, L. Chen, Z. Zhao, L. Yu and B. Liu, J. Alloys Compd., 2023, 940, 168802 CrossRef CAS.
- J. Yao, T. Chen, H. Wang, M. Khan, C. Tan, Y. Sun, W. Su, H. Wang and C. Wang, J. Mater. Chem. A, 2022, 10, 24561–24572 RSC.
- S. S. Jana and T. Maiti, Mater. Horiz., 2023, 10, 1848–1855 RSC.
- C. Bianchi, B. M. M. Faustino, A. Marques and I. Ferreira, Adv. Mater. Technol., 2024, 10, 2400706 CrossRef.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |