Chirality amplification in semiconductors for advanced optoelectronics
Jaeyong
Ahn†
 ab,
Wonbin
Choi†
ab,
Wonbin
Choi†
 a,
Sang Hyuk
Lee†
a,
Sang Hyuk
Lee†
 a,
Jonghyun
Park
a,
Jonghyun
Park
 a,
Seoyoung
Kim
a,
Seoyoung
Kim
 a,
Inho
Song
a,
Inho
Song
 *c and
Joon Hak
Oh
*c and
Joon Hak
Oh
 *a
*a
aSchool of Chemical and Biological Engineering, Institute of Chemical Processes, Seoul National University, Seoul 08826, Republic of Korea. E-mail: joonhoh@snu.ac.kr
bDepartment of Chemical Engineering, Stanford University, Stanford, California 94305, USA
cDepartment of Chemical Engineering, Chung-Ang University (CAU), Seoul 06974, Republic of Korea. E-mail: ihsong@cau.ac.kr
First published on 26th November 2025
Abstract
Because circularly polarized light (CPL) uniquely carries spin-selective information, chiral optoelectronics offer a powerful platform for developing high-efficiency, spin-based optical devices and driving next-generation photonic technologies. Intrinsically chiral semiconductors can absorb or emit CPL through light–matter interactions, positioning them as highly attractive active materials for advanced optoelectronics. However, their weak chiroptical activities often hinder practical implementation. To address this challenge, researchers have explored a range of strategies aimed at enhancing chiroptical performance. Recent advances in molecular design, processing techniques, and device engineering have led to significant improvements in the chiroptical properties of these materials. This review summarizes recent progress in chirality amplification strategies for semiconductors in advanced optoelectronics. Intrinsically chiral semiconductors are classified into three groups: organic semiconductors, metal–organic materials, and chiral hybrid perovskites. Furthermore, strategies for enhancing chiroptical signal output in chiral optoelectronic devices are discussed, supported by relevant theoretical frameworks. These advancements establish a solid foundation for the development of high-performance chiral optoelectronic devices, paving the way for future innovations in photonic technology.
1. Introduction
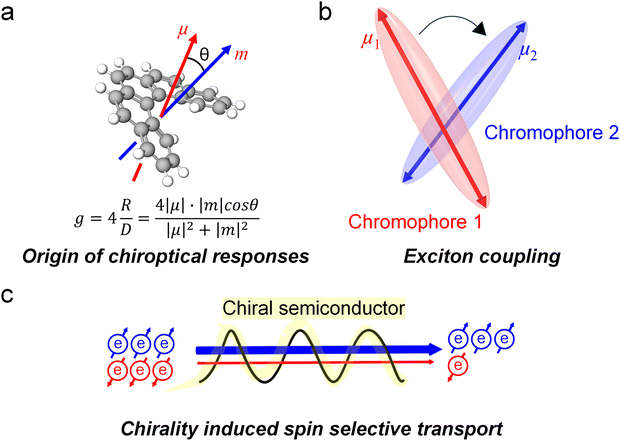
Chirality, a geometric asymmetry that makes objects non-superimposable on their mirror images, is ubiquitous–from the smallest neutrinos to the vastest galaxies–underscoring the fundamental role “handedness” plays in shaping the very fabric of our universe.1,2 It critically influences how systems interact with one another, whether molecules engaging with their environment, light interacting with materials, or fundamental particles participating in interactions.3–5 One notable example is thalidomide, in which one enantiomer acts as a tranquilizer, while its mirror image causes severe birth defects.6 Consequently, investigating chirality and its interactions is no trivial task, and many researchers have devoted significant effort to exploring this important topic.7Of particular relevance is circularly polarized light (CPL), an electromagnetic wave whose electric field rotates in either a left-handed or right-handed manner as it propagates, thereby exhibiting chirality determined by its direction of rotation.8,9 Owing to the unique helicity information carried by photons, CPL can be applied to a wide range of emerging optoelectronic fields, giving rise to chiral optoelectronics. This burgeoning field aims to develop advanced technologies in areas such as 3D displays, secure optical communications, biomolecular sensing, and even quantum computing, representing a pivotal platform for transformative innovations.10–12 Traditionally, CPL has been utilized by passing unpolarized light through optical elements, such as polarizers and wave plates. Most of the reported demonstrations to date still rely on such optical elements to generate or analyze CPL.13,14 However, this approach inevitably causes significant optical loss (over 50%) and increases system complexity due to the need for additional alignment and integration steps. As a result, direct emission or detection of CPL has become crucial for the development of chiral optoelectronics.
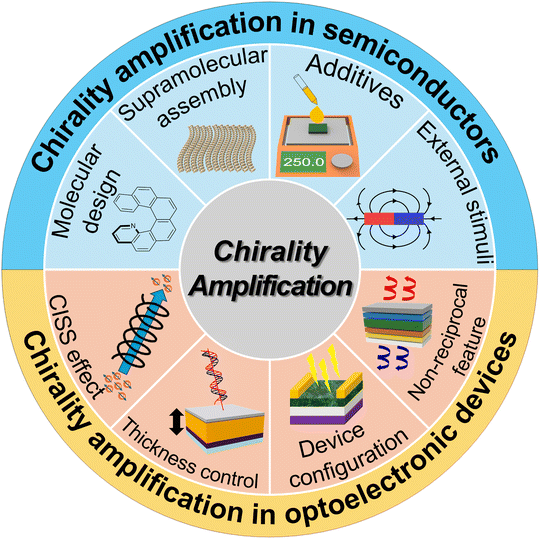
Chiral semiconductors offer intrinsic functionalities that cannot be achieved with achiral counterparts. Their helical molecular arrangement and asymmetric electronic structure enable handedness-dependent light–matter interactions, including selective absorption, emission, and charge transport governed by the chiral-induced spin selectivity (CISS) effect. These unique properties allow chiral materials to actively generate or discriminate circular polarization without external components, opening pathways to spin–photon coupling, enantioselective sensing, and polarization-encoded information processing. Therefore, chiral semiconducting materials hold strong potential for realizing compact, energy-efficient, and fully integrated chiral optoelectronic devices. Early research in this field frequently suffered from small chiroptical activities, and limited device performance. To address these challenges, recent efforts have focused on developing strategies for enhancing chiroptical properties of chiral semiconductors. Furthermore, researchers have begun elucidating amplification mechanisms when the intrinsically chiral semiconductors are integrated into CPL detection or emission devices, paving the way for practical, high-performance chiral optoelectronic technologies (Fig. 1).
 | ||
| Fig. 1 Schematic illustration of chirality amplification strategies for advanced chiral optoelectronics. | ||
Circular dichroism (CD) spectroscopy is one of the most powerful optical spectroscopic methods to analyze chiroptical properties of chiral materials. CD implies the difference between the absorption of left- and right-handed CPL. The output of CD is generally represented as ellipticity (in millidegree) for historical reasons.15 However, for optoelectronic applications, most chiral semiconductors are fabricated in to films as active layer in the device. It is important to note that the CD signal can also include contributions from linear birefringence and linear dichroism, which are unwanted terms arising from surface artifacts. To isolate the genuine circular dichroism (i.e., the circular term), it is necessary to measure the chiral film by rotating and flipping the sample. Circularly polarized luminescence spectroscopy, on the other hand, quantifies the difference in luminescence intensity between left- and right-handed CPL (LCPL and RCPL). To compare the chiroptical properties between the different samples irrespective of total absorption or emission intensities, dissymmetry factors (g) were established as follows.
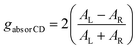 | (1) |
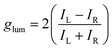 | (2) |
Theoretically, it was demonstrated that the gabs or glum of isolated chiral chromophores is related to the electronic and magnetic transition dipole moments of a given transition and defined as follows.15–17
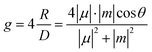 | (3) |
 | (4) |
Although the intrinsic chiroptical properties of materials are definitely crucial for chiral optoelectronic devices, several other factors can also influence their chiroptical output during device operation. For instance, when chiral semiconducting materials serve as channels for charge carrier transport in optoelectronic devices, charge transfer or transport with selective spin orientation occurs. This phenomenon is referred to as the CISS effect.18 Based on the Pauli exclusion principle, which stipulates that two electrons in the same spatial eigenstate must possess opposite spins, the spin orientation of an electron is inherently linked to molecular symmetry. Due to its spin, an electron has an associated magnetic moment, and an external magnetic field is typically required to split the energies of its two spin states. As an electron traverses a chiral molecule, it interacts with the molecule's chiral electrostatic potential. This chiral nature affects the electron's motion, potentially inducing spin–orbit coupling (SOC), where the electron's spin interacts with the molecule's orbital angular momentum (OAM). In the electron's rest frame, this motion generates a magnetic field due to the current. The electron's spin, associated with its magnetic dipole moment, then interacts with this effective magnetic field, leading to the splitting of the spin states that would otherwise be degenerated in the absence of a magnetic field. This interaction stabilizes one spin direction while destabilizing the other, resulting in a spin-dependent tunneling probability through the chiral system (Fig. 2c).19 The efficiency of converting the OAM of optically prepared excited states into specific spin states is determined by the strength of spin–orbit coupling in the chromophore of the chiral material.20 In this context, chiral organic–inorganic hybrid materials containing heavier metal atoms have been the subject of recent investigation for their enhanced capability to exploit the CISS effect. The enhanced SOC in these systems promotes the efficient conversion of orbital polarization, induced by the chiral structure, into spin polarization.
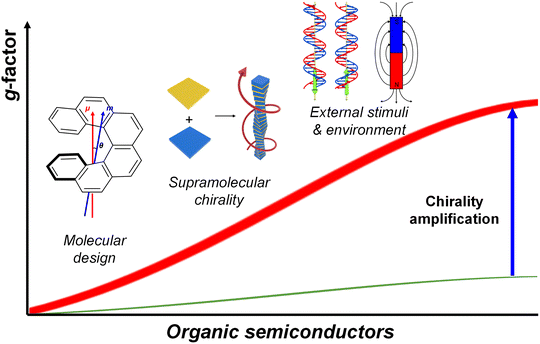
In this review, we concentrate on strategies to amplify the chirality for advanced optoelectronics. We first begin with the broad overview of chiral semiconductors and their strategies to amplify the intrinsic chirality of materials. These are categorized into organic semiconductors, organic–inorganic hybrid semiconductors, and chiral perovskites as a separate category, reflecting the emerging significance of chiral perovskite materials. Next, we systematically summarize the recent progress on amplification mechanisms in chiral optoelectronic devices that surpass the intrinsic chiroptical properties of materials. We hope this review will be of value to researchers in the field and inspire further exploration of this fascinating and important area.
2. Intrinsic chirality amplification in semiconductor materials
Chirality, a fundamental property of molecules and materials that lacks mirror symmetry, has gained significant attention in the field of semiconductor research due to its unique implications for optoelectronic devices. In particular, the ability to amplify intrinsic chirality within semiconductor materials holds promise for advancing a wide range of applications, from spintronics to bioimaging and circularly polarized light (CPL) detection. Recent developments have focused on leveraging the inherent properties of semiconductor materials to generate and amplify chirality across multiple hierarchical levels.This review aims to explore the mechanisms behind intrinsic chirality amplification in semiconductor materials, focusing on chiral organic semiconductors, metal–organic materials, and chiral perovskites. These materials are inherently chiral at the molecular level but can have their chirality strategically amplified through molecular design or fabrication processes such as supramolecular assembly, additive engineering and solvent engineering. The amplified values of chiroptical activity (gabs or glum) achieved through various amplification strategies for semiconductor materials are summarized in Table 1.
| Material | Amplified |gCD| or |glum| | Amplification method | Material classification | Ref. |
|---|---|---|---|---|
| Bridged triarylamine helicenes | 1.0 × 10−3 | Molecular design | Organic compound | 21 |
| π-extended PDI double-heterohelicene | 2.0 × 10−3 | 22 | ||
| pIDT6-(P/M)Hel11 | 1.4 × 10−3 | 23 | ||
| NDI-BN-NDI | 2.2 × 10−3 | 24 | ||
| (−)-(S)-Cz-Ax-CN | 4.8 × 10−3 | 25 | ||
| (P/M)-(12,8)-[4]CC | 1.7 × 10−2 | 26 | ||
| CPDI-Ph | 1.5 × 10−2 | Supramolecular chirality | 27 | |
| Doped ClCPDI-Ph | 1.0 × 10−3 | 28 | ||
| Fused ring non-fullerene acceptor BTP-4F | 2.6 × 10−2 | 29 | ||
| ITIC/R(S)5011/PBDBT | 1.5 × 10−2 | 30 | ||
| F8BT/aza[6]H | 0.5 | 31 | ||
| F8T2/aza[6]H | 0.2 | 32 | ||
| PCPDTTBTT/R(S)5011/PC70BM | 1.2 | 33 | ||
| DPP-T2F2 | 9.0 × 10−2 | 34 | ||
| PDOF | 1.3 × 10−3 | External stimuli | 35 | |
| PDOF | 1.4 × 10−2 | 36 | ||
| F6BT | 1.9 × 10−3 | 37 | ||
| [Eu(tfc)3(dpbp)]n | 1.7 × 10−2 | Ligand design | Metal organic complex | 38 |
| [Eu(hfa)3(B2QPO)] | 8.0 × 10−2 | 39 | ||
| [Eu0.5Ln0.5(+tfc)3dpbp]n (Ln = Gd and Sm) | 0.15 | 40 | ||
| Ln-BTC (Ln = Eu3+ and Tb3+) | 3.3 × 10 −3 | 41 | ||
| Zn(Phena-dpm)2 | 0.2 | Supramolecular chirality | 42 | |
| ZIF-8 →(R/S)-ZIF | 1.0 × 10−3 | 43 | ||
| γ-cyclodextrin MOF | 1.9 × 10−3 | 44 | ||
| UCMOF ⊃ DAECc | 6.8 × 10−2 | Host–guest interactions | 45 | |
| DSM@M-(−)-TbBTC | 2.5 × 10−3 | 46 | ||
| R/S-FMBA2PbI4 → R/S-ClMBA2PbI4 | 3.1 × 10−3 | Halogenation | Perovskites | 47 |
| R-1-NEA2PbI4 → R-2-NEA2PbI4 | 2.8 × 10−3 | Isomerization | 48 | |
| (S-4F-MBA)2PbI4 → (S-2F-MBA)2PbI4 | 1.7 × 10−3 | 49 | ||
| (R/S-mCPEA)2PbI4 → (R/S-oCPEA)PbI3 | 8.0 × 10−3 | 50 | ||
| [S-MePEA]2PbBr4 → [S-MePEA][C4A]PbBr4 | 2.5 × 10−4 | Organic cation mixing | 51 | |
| (S-oClPEA)PbI3 → (S-oClPEA)0.6HDA0.7PbI4 | 1.8 × 10−2 | 52 | ||
| (PEA)x(S-PRDA)2−xPbBr4 → (PEA)x(S-PRDA)2−xSn0.1Pb0.9Br4 | 1.5 × 10−2 | Inorganic cation mixing | 53 | |
| (R/S-MPA)2PbBr4 + Mn2+ doping | 3.0 × 10−4 | 54 | ||
| (R-MBA)2PbI2.8Br1.2 → (R-MBA)2PbI2Br2 | 1.5 × 10−3 | Cation molar ratio variation | 55 | |
| R/S-NEA2PbI4 → R/S-NEAPbI3 | 4.0 × 10−2 | 56 | ||
| (R-MBA)2PbI4 →(R-MBA)PbI3 | 7.9 × 10−4 | 57 | ||
| (R-MBA)2PbI2.7Br1.3 | 2.0 × 10−3 | External stimuli | 58 | |
| (R-MBA)2PbI4(0.7)Br4(0.3) + urea | 1.0 × 10−3 | Additive | 59 | |
| (R-MBA)2PbI4 | 5.8 × 10−3 | 60 | ||
| (R-MBA)2PbI4 | 5.7 × 10−4 | 61 | ||
| (S-MBA)PbI3 | 4.0 × 10−3 | Solvent engineering | 62 | |
| (R/S-MBA)2PbI4 | 6.9 × 10−3 | 63 |
2.1. Amplification of chirality in organic semiconductors
Organic semiconductors offer great potential because of their unique properties, such as flexibility, cost-effectiveness, lightweight, biocompatibility, and environmental sustainability.64–66 These materials are particularly versatile, as their properties, including electrical, optical, and even chiral characteristics can be finely tuned by tailoring molecular structures and intra- and intermolecular interactions.67–69 These attributes make organic semiconductors highly adaptable for use in modern electronic devices. Chirality in organic semiconductors can be introduced by incorporating asymmetric carbon centers or helical structures. However, most reported chiral organic semiconductors exhibit limited interaction with chiral substances, such as CPL, often showing g factors generally below the 10−2 level.70 This limitation has led to increasing interest in strategies to amplify chirality in organic semiconductors. In this section, we highlight representative chirality amplification strategies and their underlying mechanisms in organic semiconductors (Fig. 3).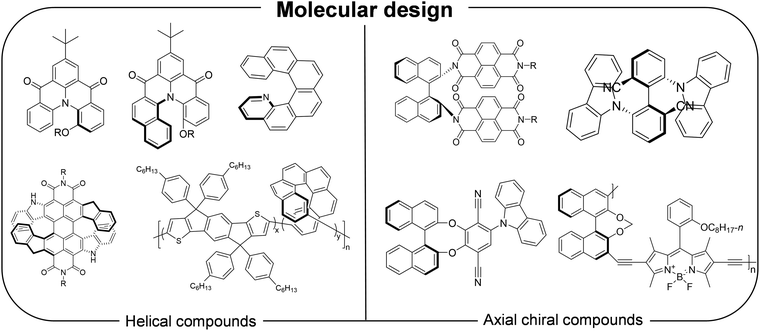 | ||
| Fig. 4 Chemical structures of chiral organic semiconductors exhibiting enhanced chiroptical properties achieved through rational molecular design. | ||
In subsequent studies, researchers have focused on improving the optoelectronic properties by modulating the frameworks and introducing heteroatom or functional groups.74 In 2022, our group reported π-extended perylene diimide (PDI) double-heterohelicenes that combine the properties of both PDIs and helicenes.22 From this molecule, the helicene structure imparts strong chiroptical properties, while the PDI's π-planar system ensures excellent charge transport characteristics. The resulting organic phototransistors (OPTs) exhibited a clear real-time photocurrent change under CPL irradiation (|gR| ∼ 0.01) at 730 nm, among with high photoresponsivity of 450 mA W−1. J. Crassous and coworkers developed chiral polymers containing helicene moieties.23,75 They investigated a series of polymers with both statistical and alternating carbo[6]helicene and indacenodithiophene (IDT) units.23 An increased helicene content led to stronger chiroptical responses. In addition, the authors observed chiroptical reponses in the absorption region of the IDT unit (|gabs| ∼ 10−3), confirming the transfer of the chiroptical response from the enantiopure helicene to the achiral IDT unit. In the fabricated organic photovoltaics (OPVs) using these chiral polymers, higher helicene content reduced efficiency, whereas enantiopure chiral polymers outperformed their racemic counterparts.
Axial chirality arises from the constrained rotation of substituents due to steric hinderance around a chiral axis and is mostly observed in atropisomeric biaryl derivatives. The large spatial separation between substituents is beneficial for acheiving high dissymmetry in molecules. In 2023, our group reported novel axially chiral n-type organic semiconductors.24 By intergrating binaphthyl group with naphthalene diimide moieties, the fabricated circular polarization (CP) photodetectors based on these materials possessed high CP selectivity along with efficient charge transport and responsivity to UV light. The resulting OPTs exhibited high electron mobilities up to 0.22 cm2 V−1 s−1, and a maximum detectivity of 3.9 × 1012, while clearly discriminating CPL handedness with a |gR| value of 0.05. Interestingly, the presence of a torsion angle between two building blocks forming axial chirality aligns well with the design principles of thermally activated delayed fluoresence (TADF) materials, which require a distorted distribution of electron donor and acceptor moieties to achieve a small energy gap between singlet and triplet excited states.76 Therefore, designing chiral TADF materials is a highly promising strategy for acheiving high performance in circularly polarized organic light emitting diodes (CP-OLEDs). In 2020, Li et al. reported novel stable enantiomers (−)-(S)-Cz-Ax-CN and (+)-(R)-Cz-Ax-CN by connecting two fluorophores lacking both chirality and TADF, thereby creating a chiral-emitting skeleton.25 CP-OLEDs based on these TADF materials achieved efficient blue emission with external quantum efficiencies up to 12.7% and intense |gEL| values of 1.4 × 10−2.
Recently, several chiral cyclic π-conjugated molecules with intensive chiroptical properties have been reported. Owing to their unique structures, the sum of electric transition dipole moments derived from individual chromophore components in the cyclic structure can be cancled out within the xy-plane, thereby increasing the relative contribution of the magnetic transition dipole moment and resulting in a high dissymmetry factor.77 In 2017, Sato et al. reported cyclic chrysene oligomers having three-dimensional, cylinder-shaped structures.26 Based on their CD spectra, these molecules exhibited extraordinarily large |gabs| values, exceeding 0.16. In addition, a high |glum| value of about 0.15 and a significantly high quantum yield (ϕPL = 0.8) were observed. The authors attributed these enhanced chiroptical properties to a unique magnetic transition dipole moment oriented along the cylinder axis, in contrast to the negligible magnetic transition dipole moment contributions typically seen in conventional organic molecules. Long and coworkers proposed boron- and nitrogen- embedded cylindrical molecules that, according to theoretical calculations, could achieve |glum| values as high as 0.56.78 They explained that these large dissymmetry values originated from a reduced electric transition dipole moment caused by short-range charge transfer between the N and B atoms.
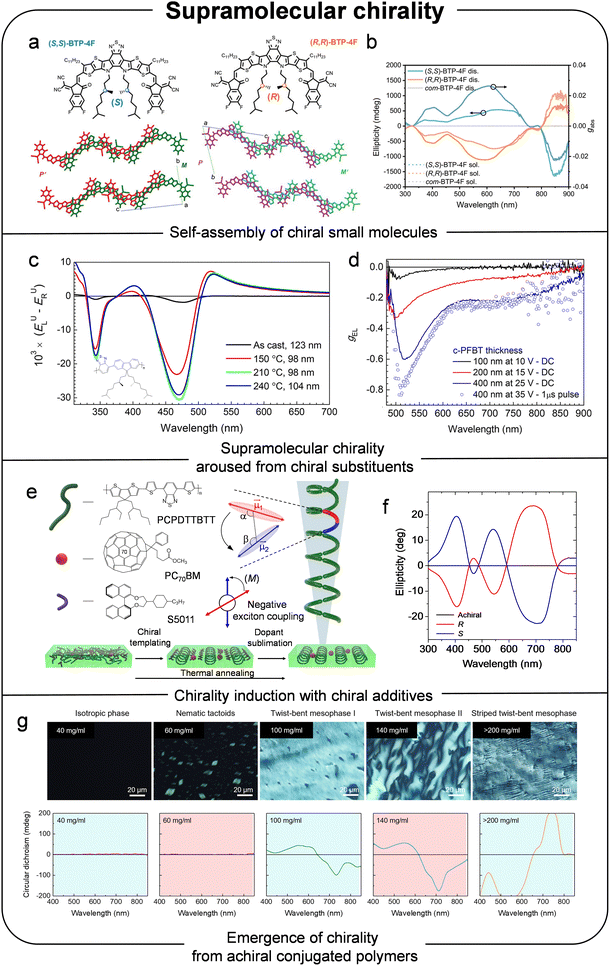 | ||
| Fig. 5 (a) Molecular structures and their supramolecular organizations of chiral non-fullerene electron acceptors. (b) CD and gabs spectra of chiral non-fullerene electron acceptor solutions and their self-assembled crystals. Reproduced with permission.29 Copyright 2023, Wiley-VCH. (c) Difference in extinction E of chirally substituted conjugated polymer for LCPL and RCPL before and after annealing at different temperature. (d) gEL of chirally substituted conjugated polymer based CP-OLEDs. Reproduced with permission.85 Copyright 2017, American Chemical Society. (e) Schematic of chiral transient templating generating supramolecular ordering of conjugated chromophores. (f) CD spectra of CPDT-based neat and R5011- or S5011- doped polymer films. Reproduced with permission.33 Copyright 2023, Nature Publishing Group. (g) Cross-polarized optical microscope images, and corresponding CD spectra of the PII-2T solutions. Reproduced with permission.88 Copyright 2022, Nature Publishing Group. | ||
Another representative approach to amplifying chirality in organic semiconductors is the use of additives that can induce chirality in these materials. This strategy offers distinct advantages in terms of universal applicability, enabling the use of already-developed, high-performance organic semiconductors. Wan et al. reported CPL photodetectors based on NFAs blended with chiral additives.30 They demonstrated that this method can universally amplify the chiroptical properties of various achiral NFAs with similar structures. For instance, ITIC, one of the representative NFAs, exhibited high optimal |gabs| value of 0.15 upon forming chiral assemblies by in mixed chloroform and chlorobenzene solvents with the chiral additive. Spectroscopic analyses and density functional theory calculations attributed the strong chiroptical activity to pronounced exciton coupling between chromophores in the NFA stacks. As a result, the fabricated near-infrared CPL photodetectors showed high photodetection performance, with average |gph| values of about 0.09.
In addition to small molecules, polymers which have chiral substituents can form supramolecular helical structures with amplified chirality. Among various types of polymers, chiral liquid crystalline semiconductors, such as chiral polyfluorene-based polymers, have shown notably large CD intensities.82–84 Thermal annealing is an effective means of inducing highly ordered helical arrangements in thermotropic liquid crystalline polymer chains, thereby generating supramolecular chirality in aggregates or assemblies. Such liquid crystalline ordering promotes long-range helical alignment and anisotropic dipole orientation, which are beneficial for achieving strong CPL emission in CP-OLEDs. In 2017, Nuzzo et al. reported a chirally substituted polyfluorene copolymer.85 The authors obtained amplified chiroptical properties upon annealing the film at 240 °C, whereas no circularly polarized electroluminescence was observed in the as-cast state. The formation of disordered, multidomain cholesteric liquid-crystalline phase after thermal annealing led to intense circular selective scattering and birefringence (Fig. 5c). As a result, a CP-OLED fabricated with this chiral polyfluorene copolymer achieved a high |gEL| value of about 0.8 (Fig. 5d). Alternatively, an achiral polyfluorene-based polymer can exhibit giant chiroptical properties when doped with chiral additives.31,86,87 In 2013, Yang et al. demonstrated an effective approach to fabricating CP-OLEDs using F8BT and 1-Aza[6]helicene.31 CD measurements revealed that the blended film exhibited a CD signal in the wavelength range associated with F8BT absorption, while pure F8BT showed no CD response, implying that the helicene dopants preorganized the polymer into chiral structures. The authors further confirmed the generality of their strategy by using [7]helicene as a chiral dopant, observing similar chiral properties. Their proof-of-concept devices with F8BT:1-Aza[6]helicene as the CP-active layer exhibited a |gEL| value of 0.2. In 2023, our group reported an advanced chirality induction and amplification technique, referred to as the chiral templating strategy using a 4,4-dialkyl-4H-cyclopenta[2,1-b:3,4-b′]dithiophene-2,6-diyl (CPDT)-based polymer and chiral dopants R/S5011.33 This method involves blending chiral dopants and inducing the chiral rearrangement of the polymers during thermal annealing (Fig. 5e). Under high-temperature thermal annealing, the chiral dopants promote multiscale reorganization of polymer chains into helical structures, and their simultaneous sublimation prevents any disruption to charge transport. Consequently, these helical polymers, which exhibit strong long-range interchromophore chiral communication, displayed amplified chiroptical properties compared to achiral polymer state before the chiral templating (Fig. 5f). The resulting thin films achieved a high |gabs| value of 1.2, enabling real-time spatiotemporal CPL detection and imaging, demonstrating significant potential for optical communication and quantum computing.
Recently, Diao group reported the emergence of chirality from achiral conjugated polymers.88 Because of its lyotropic liquid-crystalline nature, an isoindigo-bithiophene-based copolymer exhibited four different unknown mesophases, depending on the concentration of polymer solutions. Among these, three phases displayed chiral properties, and the authors confirmed that the probability of single-handedness increased with the polymer concentration. The mechanism underlying the helical confirmation of polymer was investigated through morphological and structural analyses combined with computational simulation. The flexible dihedral angle between thiophene–thiophene (T–T) units contributed to the helical conformation in single polymer chains. Although no local symmetry breaking occurs at the single-polymer level, weak asymmetry in dihedral angle distributions does exist. Consequently, as the concentration increased, these slight imbalances at the molecular level were amplified during the mutilmolecular assembly process, resulting in distinct chiral properties in the polymer films (Fig. 5g). As a follow-up study, the Diao group analyzed the effect of varying the torsional angle in T–T units by introducing methyl or fluorine substitutions into bithiophene moiety within DPP systems.34 Both substitutions significantly influenced the aggregation behavior of the polymers. The high torsion induced by methyl substitution led to short helical pitches, whereas the flexible T–T dihedrals in non-substituted DPP allowed the formation of two-dimensional crystalline sheet aggregates. Among these variants, the fluorine-substituted polymer achieved |gabs| values as high as 0.09, attributed to its relatively short helical pitch and exceptional long-range order due to more planar geometry induced by fluorine substitution.
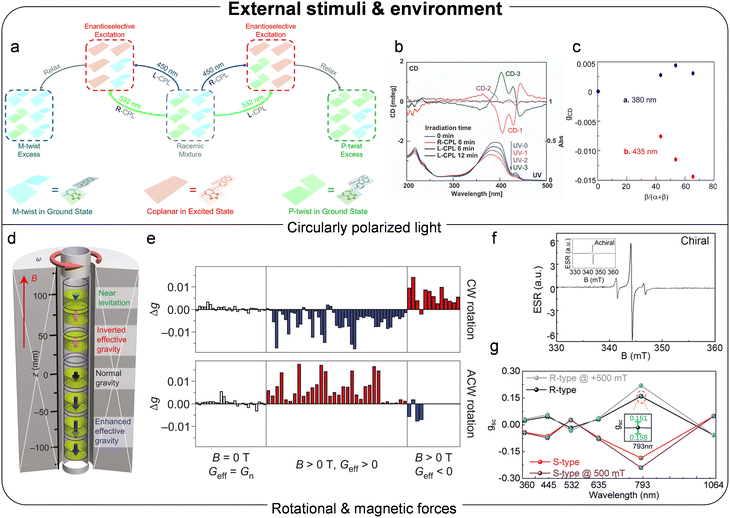 | ||
| Fig. 6 (a) Schematic illustration of chirality induction with CPL. Reproduced with permission.37 Copyright 2020, Royal Society of Chemistry. (b) CD and UV-vis spectra of PDOF films depending on the R or L-CPL irradiation time. Reproduced with permission.35 Copyright 2012, Royal Society of Chemistry. (c) The relationship between maximum gCD at 380 and 435 nm and β-phase content of PDOFs. Reproduced with permission.36 Copyright 2018, American Chemical Society. (d) The set-up for aggregating TPPS3 solution with magnetic forces and gravity. (e) Chirality parameter measured as a function of magnetic forces and effective gravity conditions. Reproduced with permission.90 Copyright 2012, Nature Publishing Group. (f) ESR spectra of chiral and achiral structure. (g) gsc of CP photodetectors with and without magnetic field. Reproduced with permission.91 Copyright 2023, Wiley-VCH. | ||
Micali et al. investigated the induction and amplification of chirality in J-aggregates of an achiral molecule, tris-(4- sulfonatophenyl)phenylporphyrin (TPPS3).90 The handedness of the helical supramolecular aggregates was controlled by applying rotational and magnetic forces during the self-assembly process (Fig. 6d). Rotating seven cylindrical vessels in clockwise and anticlockwise directions generated a chiral flow in the solution. However, additional magnetic forces were necessary to sufficiently align the aggregates and induce supramolecular chirality. When the angular momentum from the rotation was aligned in parallel with the effective gravity resulting from magnetic levitation forces, negative CD signals were observed, whereas an antiparallel configuration produced positive CD signals (Fig. 6e). The instantaneous chiral perturbation was transferred and amplified during supramolecular self-assembly, driven by broken time-reversal symmetry in a kinetically controlled system. In 2023, Hu et al. developed CP photodetectors with amplified dissymmetry and detectivity under a magnetic field.91 They formed chiral structures by blending PCDTPT with a chiral dopant, limonene, and fabricated photodiodes using a bulk heterojunction incorporting the chiral donor. The proposed mechanism for CPL detection was attributed to spin–orbit coupling in chiral materials. In such systems, circularly polarized photons transfer their spin angular momentum to the chiral orbital angular momentum of the material, leading to selective excitation of electrons with a specific spin orientation, which can manifest as characteristic changes in ESR spectra (Fig. 6f). Therefore, controlling spin relaxation in excited electrons can influence photocurrent dissymmetry. Because chirality-induced spin–orbit coupling led to a reduced binding energy under preferred CPL irradiation, singlet electron–hole pairs were effectively transferred to the triplet state under the magnetic field. The high dissociation rate of triplet electron–hole pairs then increased the current density of the devices. Consequently, applying an external magnetic field further enhanced the |gsc| of CP photodetectors from 0.18 to 0.23 (Fig. 6g).
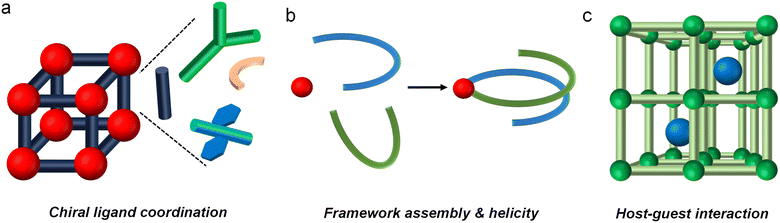
2.2. Amplification of chirality in metal–organic materials
Metal–organic materials (MOMs) are hybrid materials composed of metal centers and organic ligands, forming structures such as coordination complexes, metal–organic frameworks (MOFs), and coordination polymers. These materials exhibit remarkable structural and electronic tunability, enabling precise control over their properties for a wide range of applications. Among these, chiral MOMs stand out for their ability to incorporate and amplify chirality, making them particularly significant in chiroptical technologies such as circularly polarized luminescence. This unique capability has driven advancements in CPL-based devices, including displays, optoelectronic systems, and advanced sensors.Despite their potential, many MOMs still face challenges such as low photoluminescence quantum yields (PLQYs) and limited dissymmetry factors, which hinder their broader application in optical technologies. To address these limitations, researchers have developed various strategies to amplify chirality and improve optical performance (Fig. 7). In this section, we explore these approaches, highlighting their transformative impact on advancing MOM-based chiroptical materials.
In 2018, Hasegawa et al. synthesized a chiral 1D coordination polymer, [Eu(tfc)3(dpbp)]n where tfc (3-trifluoroacetyl camphorate) is a β-diketonate ligand and dpbp (4,4′-bis(diphenylphosphoryl)biphenyl) serves as a chiral bridging ligand.38 It demonstrated exceptionally strong CPL with a glum of 0.17 at 585 nm – the highest value reported to date for any coordination polymer or MOF (Fig. 8a and b). This high performance originates from the magnetically allowed 5D0 → 7F1 transition of Eu3+, which possesses a dominant magnetic-dipole character. According to eqn (3), glum depends on the interference term between the electric and magnetic transition dipole moments, and the strong chiral anisotropy at 585 nm arises from favorable μ–m coupling combined with the relatively small electric-dipole contribution inherent to magnetic dipole-allowed transitions. In contrast, the electric dipole-dominated 5D0 → 7F2 transition at 613 nm exhibited a glum of 0.015.
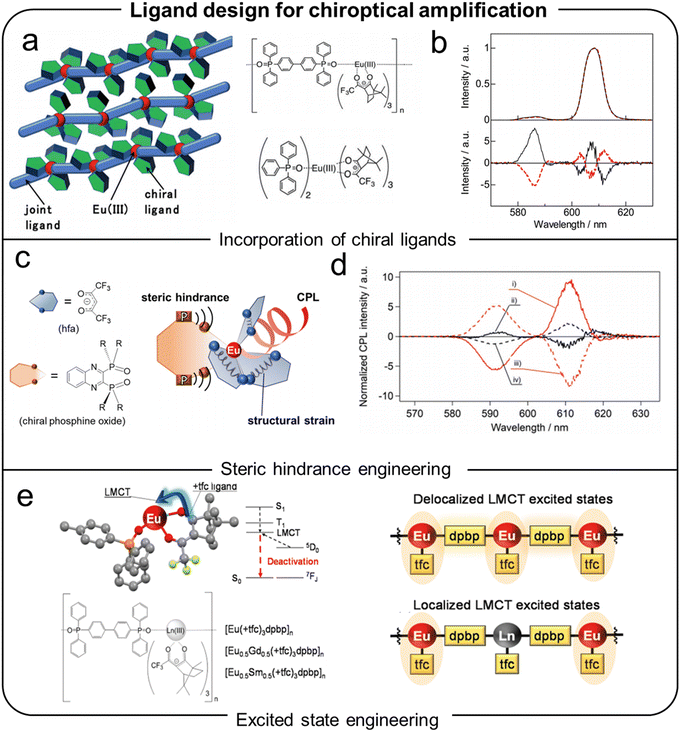 | ||
| Fig. 8 (a) Schematic image and chemical structures of Eu(III) coordination polymers. (b) Emission (upper figure) and CPL (lower figure) spectra of [Eu(+tfc)3(dpbp)]n (black lines) and [Eu(−tfc)3(dpbp)]n (red lines). Reproduced with permission.38 Copyright 2018, Royal Society of Chemistry. (c) Schematic of the electronic strain effect on Eu(III) complexes. (d) CPL spectra of [Eu(hfa)3((R,R)-B2QPO)] ((i), red solid line), [Eu(hfa)3((R)-B3QPO)] ((ii), black solid line), [Eu(hfa)3((S,S)-B2QPO)] ((iii), red dashed line) and [Eu(hfa)3((S)-B3QPO)] ((iv), black dashed line). Reproduced with permission.39 Copyright 2020, Royal Society of Chemistry. (e) Schematic of the LMCT in Eu(III) coordination polymers. Reproduced with permission.40 Copyright 2021, Royal Society of Chemistry. | ||
In addition, the tightly packed nature of the polymer minimized nonradiative decay pathways, achieving an overall PLQY of 30% and a PLQY of 57% for the 5D0 → 7F1 transition. Subsequently, Hasegawa and co-workers investigated the role of electronic strain in CPL amplification using the complex [Eu(hfa)3(B2QPO)] where hfa refers to hexafluoroacetylacetonate and B2QPO stands for 2,2′-biquinoline-4,4′-diylbis(diphenylphosphine oxide) (Fig. 8c and d).39 This study compared the chiroptical properties of complexes with different configurations of B2QPO ligands, including (R,R), (S,S), (R), and (S) forms. Among these, the (R,R)-configured complex exhibited the highest CPL activity, achieving a glum of 0.08 and a PLQY of 63%. The authors attributed this enhancement to the reduced electronic strain caused by the stereospecific alignment of the (R,R) ligands, which optimized the coordination geometry around the Eu3+ center. Furthermore, this study highlighted the role of ligand-to-metal charge transfer (LMCT) processes in enhancing CPL activity. By analyzing structural and electronic parameters, they demonstrated that the LMCT band in the (R,R)-configured complex contributed to efficient electronic communication between the ligands and the Eu3+ center, thus amplifying the magnetic dipole transition responsible for the observed CPL.
In another study, mixed coordination polymers incorporating Eu3+, Gd3+, and Sm3+ were used to modulate LMCT energy levels, leading to high luminescence properties (Fig. 8e).40 These mixed polymers achieved a glum of 0.15 and a quantum yield of 52%, demonstrating the potential of electronic structure adjustments to improve CPL performance in coordination polymers. Finally, the importance of ligand design is underscored by the work of Cheng et al., who synthesized binaphthyl-based Zn2+ 1D coordination polymers (R/S-P1) with a glum of 9 × 10−3, significantly higher than their monomeric counterparts (R/S-M1, glum of 2 × 10−3).92 This study highlights how the careful design of ligands can effectively amplify chirality and enhance CPL properties. The optimization of metal centers and ligand designs has demonstrated significant potential for amplifying CPL signals. These advancements highlight the importance of electronic interactions and structural asymmetry in designing MOMs with superior chiroptical properties.
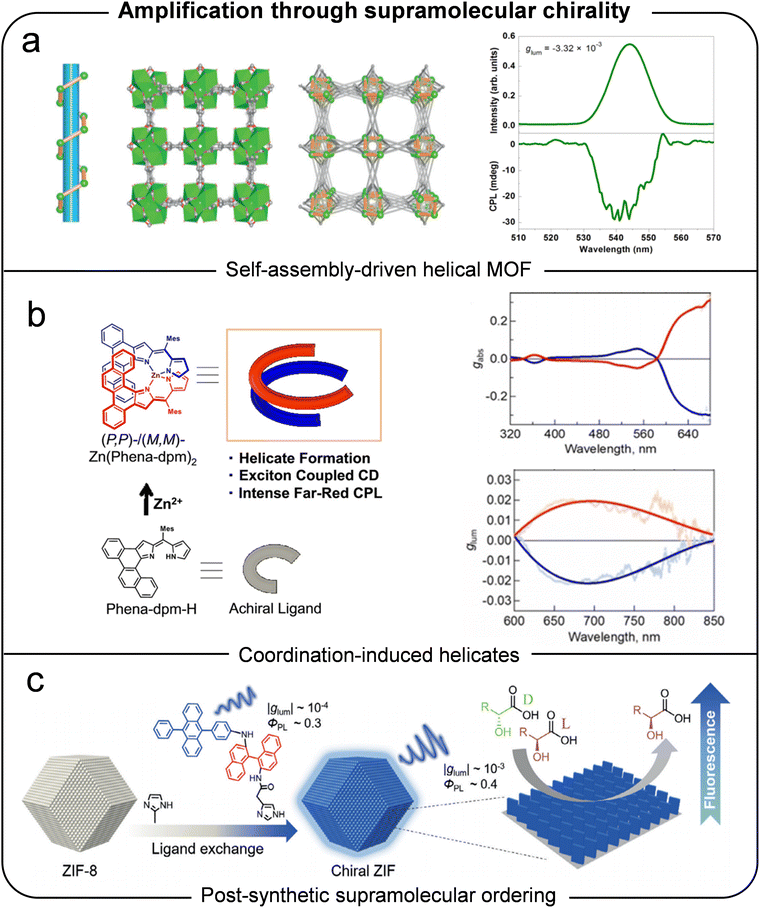 | ||
| Fig. 9 (a) Helical alignment in MOFs and angle-dependent polarized PL spectra. Reproduced with permission.41 Copyright 2017, Wiley-VCH. (b) Chiral homoleptic helicate organized by a pair of achiral ligands. Reproduced with permission.42 Copyright 2018, Wiley-VCH. (c) Chiral ZIFs demonstrating amplified CPL and enhanced fluorescence efficiency through ordered helical emitter alignment on ZIF-8. Reproduced with permission.43 Copyright 2019, Wiley-VCH. | ||
These findings collectively underscore the critical role of helical structures in amplifying chiroptical properties. By leveraging symmetry-breaking mechanisms and hierarchical organization, supramolecular self-assembly remains a cornerstone for achieving enhanced CPL performance in MOMs.
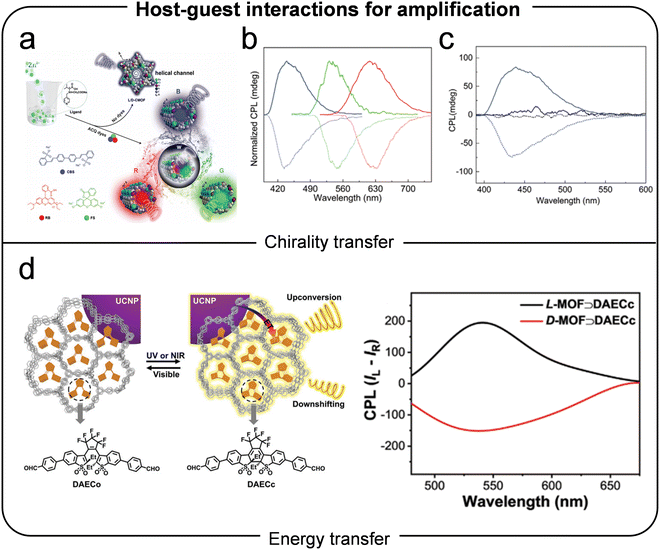 | ||
| Fig. 10 (a) Helical MOFs as hosts for achiral fluorophores, producing colorful CPL through host–guest interactions and enhanced quantum yield. (b) CPL spectra of chiral MOFs incorporating various achiral dyes, demonstrating differences in CPL signals based on dye type and interaction with the helical host. (c) Comparison of CPL spectra between chiral MOF ⊃ dye composites and mechanically mixed samples, highlighting the role of host–guest interactions in CPL amplification. Reproduced with permission.95 Copyright 2020, Wiley-VCH. (d) Upconverted circularly polarized luminescence in chiral MOFs. Reproduced with permission.45 Copyright 2021, Wiley-VCH. | ||
Thin films of MOFs have also emerged as effective platforms for CPL enhancement. In 2021, Zhai et al. developed surface-coordinated chiral MOFs encapsulated with lanthanide complexes such as Eu(acac)3 and Tb(acac)3.96 These thin films exhibited strong CPL signals due to efficient energy transfer between the lanthanide guests and the MOF framework. This work highlighted the potential of MOF thin films for tunable CPL applications. Expanding the application of ZIF-based host–guest systems, Zhao et al. introduced various emitters, including achiral dyes, quantum dots (QDs), and upconversion nanoparticles (UCNPs), into chiral ZIFs.97 By matching the size of the dyes with the ZIF cages, they induced CPL with glum values of ±1.2 × 10−3. Larger QDs, which could not fit into the ZIF cages, interacted with the external framework, resulting in CPL signals with inverted chirality. Additionally, they achieved upconverted CPL by incorporating UCNPs into chiral ZIFs, obtaining dissymmetry factors up to ±6.8 × 10−2.45 This dual-mode approach showcased the adaptability of ZIF-based systems for CPL-active materials (Fig. 10d). In another advancement, Zeng et al. encapsulated achiral stilbazolium dyes into lanthanide-based MOFs, achieving CPL signals with glum values of up to 10−3.46 The spatial confinement within the MOF channels facilitated efficient energy transfer from lanthanide centers to the dyes, optimizing their luminescent properties.
Together, these studies illustrate the transformative impact of host–guest interactions and energy transfer on the optical and functional properties of MOMs. By optimizing structural alignment, reducing energy losses, and enabling chirality transfer, researchers are advancing the development of high-performance materials for optoelectronic applications.
2.3. Chiral hybrid organic–inorganic perovskites
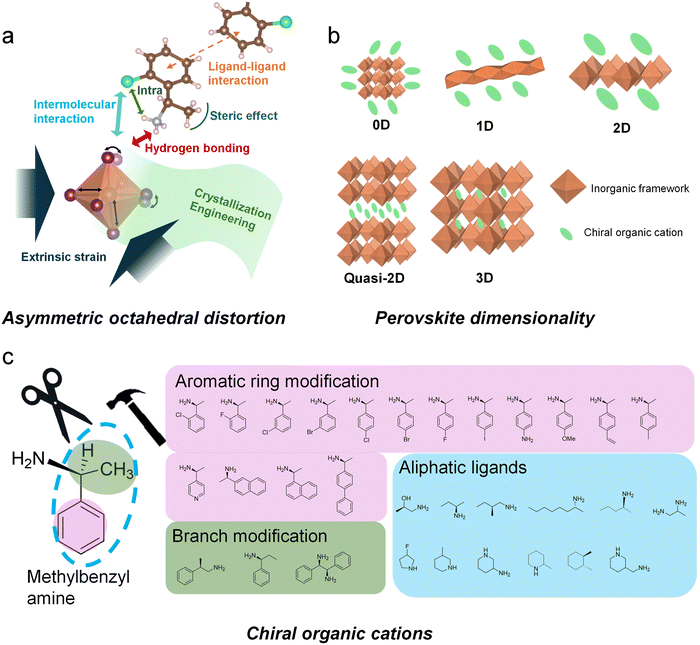
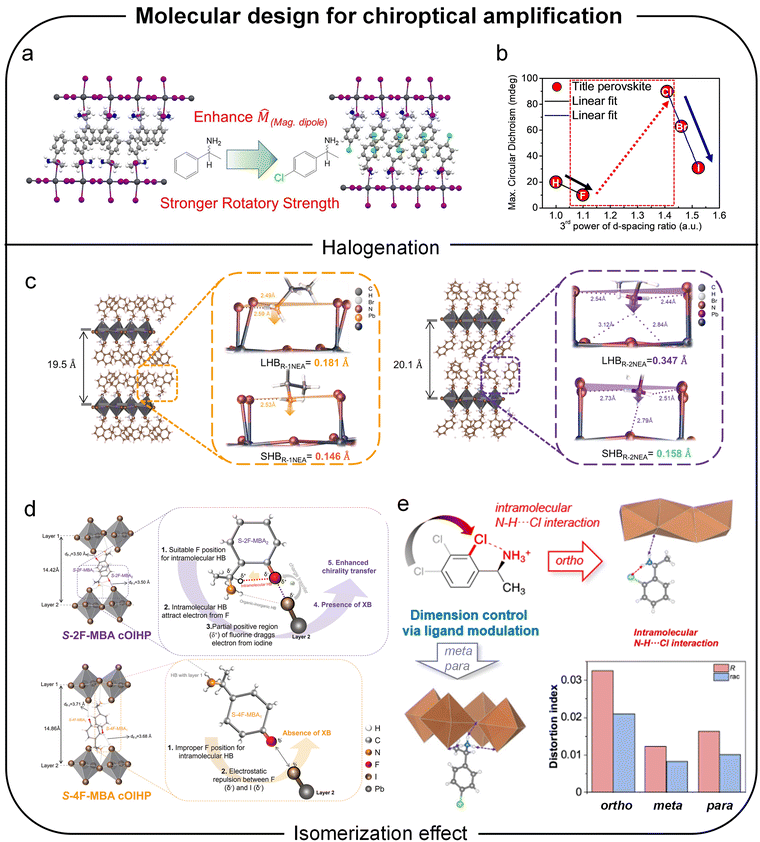
Over the past decade, hybrid organic–inorganic perovskites have emerged as pivotal materials in optoelectronics due to their exceptional properties: low trap densities and long carrier-diffusion lengths for high performance solar cells; near-unity PLQYs and tunable emission for efficient LEDs; high mobilities and dielectric constants for photodetectors; and large spin orbit coupling alongside long spin lifetimes for spintronic applications.98 Among these materials, chiral perovskites have attracted significant attention due to their unique advantages. While chiral perovskites are relatively new compared to other chiral nanomaterials, they stand out because of their ability to combine efficient light emission with notable chiroptical properties, such as significant circular polarization and high stability. The incorporation of chiral organic molecules enables the facile synthesis of chiral hybrid organic–inorganic perovskites, which have garnered significant attention as promising candidates for advancing spin-related and optoelectronic functionalities in chiral materials. These perovskites exhibit chiroptical activity through the inclusion of chiral organic molecules, which serve either as internal ligands or external surface modifiers of the perovskite crystal. This incorporation transfers chirality to the inorganic framework by inducing distorted, asymmetric crystal structures.99–101To elucidate the structural origins of chiroptical properties in perovskite, numerous studies have focused on synthesizing single crystals for crystallographic analysis, complemented by theoretical approaches.102–104 For instance, Kim et al. investigated the structural origin of chirality of chiral perovskites by attaching chiral cations to the surface of perovskite nanocrystals.105 Their findings revealed that centro-asymmetric distortions in the surface lattice extend up to five atomic unit cells deep into the perovskite nanocrystals, likely accounting for the observed chiroptical properties. The discovery that the optical chirality of perovskites arises from asymmetric hydrogen bonding between the chiral cations and the inorganic framework underscores the importance of several factors in determining hydrogen bonding geometry (Fig. 11a). These factors include the dimensionality of the perovskite structure (Fig. 11b), the molecular design of chiral cations (Fig. 11c), the perovskite composition, and the crystallization process. Together, these factors significantly influence the chirality of the perovskite crystal structure. In the following sections, we explore key factors and underlying mechanisms that have been reported to enhance the chiroptical properties of chiral hybrid organic–inorganic perovskites. These factors encompass the molecular engineering of chiral organic ligands or incorporation of chiral organic ligand mixtures, the tailored composition and morphology of the inorganic framework, and the meticulous control of interactions between organic and inorganic components during the crystallization process.
Among these studies, the comparative analysis of various molecular structures has identified several effective strategies and critical factors in molecular design. For instance, Lin et al. examined the influence of halogenation on organic cations by substituting the hydrogen atom at the para position of MBA (Fig. 12a).47 Their findings revealed that the strength of the magnetic transition dipole moment varied depending on the size of the halogen atoms. Specifically, an increase in d-spacing was found to diminish the magnetic transition dipole moment, while the incorporation of heavier halogen atoms facilitated halogen–halogen interactions (Fig. 12b). These opposing effects culminated in the highest chiroptical activity with chlorine substitution, suggesting that the combination of optimal angular momentum and d-spacing achieved the strongest magnetic transition dipole moment.
 | ||
| Fig. 12 (a) Enhancement in rotatory strength through halogenation of chiral ligand. (b) Penetration depth difference in chiral perovskites by chiral organic cation isomerization. Reproduced with permission.47 Copyright 2021, Wiley-VCH. (c) Enhanced chirality transfer via halogen bond depending on the halogenation site of chiral ligand. Reproduced with permission.48 Copyright 2023, Nature Publishing Group. (d) Dimensionality control of chiral perovskite by ligand modulation. Reproduced with permission.49 Copyright 2024, Wiley-VCH. (e) Chiroptical activity of halogenation on chiral ligand. Reproduced with permission.50 Copyright 2024, Elsevier. | ||
Another important consideration in molecular design for achieving higher chiroptical activity is the steric or isomeric effect. This effect influences chirality transfer by altering the hydrogen bonding interaction geometry between chiral organic cations and inorganic frameworks. Recent studies on chiral organic ligand isomers have extensively explored the impact of isomeric effects on the structural and optical properties of chiral perovskites. For example, Son et al. investigated the chiroptical activity and crystal structure of chiral perovskites incorporating two structural isomers: 1-(1-naphthyl)ethylamine and 1-(2-naphthyl)ethylamine.102 These isomers vary in the location of the ethylamine group attached to the naphthyl backbone (Fig. 12c). Because the amine group of the chiral ligands forms hydrogen bonds with the inorganic layers, shifting the position of this functional group alters the extent to which the NH3+ moiety penetrates into the inorganic framework. Deeper NH3+ into the inorganic layer induced a significant inversion asymmetric distortion in the chiral perovskite lattice, thereby enhancing chirality transfer from the chiral molecules to the inorganic framework.
Building on the comprehensive effects of substitution and isomerization, halogen substitution combined with variations in the position of substituents on aromatic rings has been extensively studied. Son et al. demonstrated that fluorinated isomerization of MBA, achieved by substituting a fluorine atom near the amine group, resulted in intramolecular hydrogen bonding with the methine hydrogen (Fig. 12d).49 This interaction further facilitated strong halogen–halogen interactions with the inorganic framework.
Compared to the para-substituted fluorine isomer or MBA without fluorine substitution–where only hydrogen bonding through the amine group occurs–the ortho-fluorinated isomer significantly enhanced chirality transfer. This enhancement was attributed to the synergistic effect of intermolecular hydrogen bonding and halogen–halogen interactions with the inorganic framework.
Similarly, our group investigated the effects of intramolecular interactions on chirality transfer by studying chlorinated isomers of MBA, varying the substitution positions of the chlorine atom at the ortho, meta, and para positions (Fig. 12e).50 Notably, the intramolecular interaction between the ammonium hydrogen and the chlorine atom at the ortho position induced geometric changes in hydrogen bonding. These changes caused a structural shift in the inorganic framework from a 2D layered structure to a 1D nanowire structure and increased the distortion index of the inorganic framework. As a result, the synergistic effect of reduced dimensionality and enhanced asymmetric hydrogen bonding enabled the ortho-chlorinated chiral cation to exhibit the highest chiroptical properties, followed by the para and meta isomers.
From a molecular design perspective, inducing more asymmetric hydrogen bonding with the inorganic framework through additional intra- or intermolecular interactions–achieved by functional group substitutions or structural isomerization–has proven to enhance the chiroptical activity of perovskites. These findings highlight the vast potential and possibilities for optimizing molecular designs to achieve superior chiroptical properties.
 | ||
| Fig. 13 (a) Chirality amplification through mixed ligand design in chiral perovskites. Reproduced with permission.51 Copyright 2021, American Chemical Society. (b) Structural transformation of perovskite by ligand mixing. Reproduced with permission.116 Copyright 2022, Wiley-VCH. (c) Schematic illustration of metal doping in chiral perovskites. Reproduced with permission.53 Copyright 2024, American Chemical Society. (d) Effect of metal doping in energy band splitting of chiral perovskites. Reproduced with permission.54 Copyright 2024, American Chemical Society. (e) Effect of dimensionality control of chiral perovskite on its chiral property. Reproduced with permission.56 Copyright 2020, The American Association for the Advancement of Science. (f) Effect of exciton–exciton distance on optical property of chiral perovskites. Reproduced with permission.57 Copyright 2022, American Chemical Society. | ||
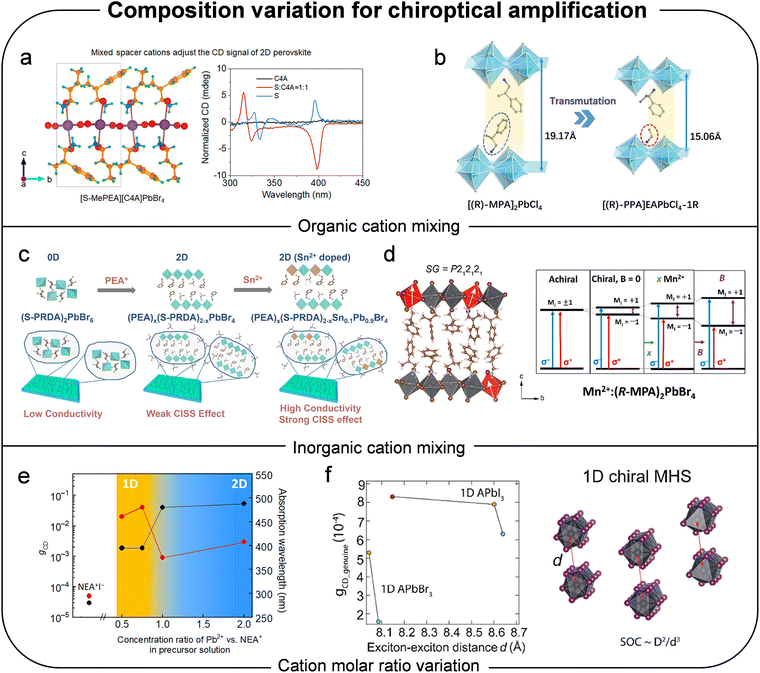
In addition to modifying the organic cation component, several groups have studied the effects of replacing the lead (Pb) with other metallic atoms such as Sn, Mn, In, Sb, Ag, Bi, Pd, Ge and Co.53,54,128–137 Feng et al. employed dimensional tuning and Sn2+ doping to optimize chiral perovskite layers, achieving an enhanced effect, higher hole mobility and better energy level alignment (Fig. 13c).53 Sn2+ doping increased structural distortion and induced p-type doping through partial oxidation of Sn2+. Similarly, Zhang et al. substituted a portion of Pb with Mn2+ as magnetic impurity dopants, anticipating that the chiral host lattice would facilitate parity- and spin-forbidden d–d transitions of Mn2+ through coupling with the symmetry-broken framework (Fig. 13d).54 They observed that the incorporation of chiral organic cations induced exciton-fine structure splitting up to 5.0 meV, which further increased upon Mn2+ doping. As the Mn2+ concentration rose, the optical activity of the chiral perovskite was enhanced due to efficient energy transfer from the chiral host to the Mn2+ dopants. Consequently, Mn2+ doping not only amplified the CD response of the host perovskite but also enabled circularly polarized luminescence originating from the Mn2+ excited-state transition (4T1 → 6A1).
By altering the molar ratio between two components–the organic cation and the inorganic counterpart, Ishii et al. demonstrated a dimensional transition from 2D to 1D, which enhanced chiroptical properties as the concentration of organic molecular content in precursor solution decreased (Fig. 13e).56 Decreasing the concentration ratio of Pb2+vs. NEA+ in precursor solution from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or below, the constructed chiral perovskite structure transformed from 2D nanosheet layered structure to 1D nanowires while exhibiting gabs of one order of magnitude higher. In a theoretical approach to understanding the relationship between dimensionality and chiroptical activity, Zhang et al. described how the chirality-induced in the chiral structures correlates with chiroptical activity.57gabs is primarily determined by the strength of chirality-induced SOC and exciton broadening, which can be considered as exciton–exciton distance in Fig. 13f. As dimensionality decreases from 2D to 1D, quantum confinement is significantly enhanced, leading to reduced exciton broadening. Consequently, the anisotropy factor generally increases with lower dimensionality. In addition, when comparing between lead bromide and lead iodide series with identical templating cations, although the center-to-center distance between adjacent excitons is similar, chiral lead bromide series exhibit higher exciton binding energy and, therefore, a smaller exciton dipole moment. Thus, the CD strength in chiral lead iodide series is greater than that of chiral lead bromide series (Fig. 13f). In addition to the theoretical approach to difference in chiroptical activity of lead iodide and lead bromide, Ahn et al. explored the effect of mixing halide anions on the chiroptical properties of perovskite and found that replacing 30% of iodine with bromine produced an optimal mixing ratio, exhibiting the highest chiral activity, likely due to the induced asymmetry by mixed components.55,58 An intriguing finding was that the wavelength of the optical property could be tuned over a wide range, from 375 nm to 495 nm, by incorporating NEA and bromide instead of MBA and iodide, respectively.
1 or below, the constructed chiral perovskite structure transformed from 2D nanosheet layered structure to 1D nanowires while exhibiting gabs of one order of magnitude higher. In a theoretical approach to understanding the relationship between dimensionality and chiroptical activity, Zhang et al. described how the chirality-induced in the chiral structures correlates with chiroptical activity.57gabs is primarily determined by the strength of chirality-induced SOC and exciton broadening, which can be considered as exciton–exciton distance in Fig. 13f. As dimensionality decreases from 2D to 1D, quantum confinement is significantly enhanced, leading to reduced exciton broadening. Consequently, the anisotropy factor generally increases with lower dimensionality. In addition, when comparing between lead bromide and lead iodide series with identical templating cations, although the center-to-center distance between adjacent excitons is similar, chiral lead bromide series exhibit higher exciton binding energy and, therefore, a smaller exciton dipole moment. Thus, the CD strength in chiral lead iodide series is greater than that of chiral lead bromide series (Fig. 13f). In addition to the theoretical approach to difference in chiroptical activity of lead iodide and lead bromide, Ahn et al. explored the effect of mixing halide anions on the chiroptical properties of perovskite and found that replacing 30% of iodine with bromine produced an optimal mixing ratio, exhibiting the highest chiral activity, likely due to the induced asymmetry by mixed components.55,58 An intriguing finding was that the wavelength of the optical property could be tuned over a wide range, from 375 nm to 495 nm, by incorporating NEA and bromide instead of MBA and iodide, respectively.
By varying the components in perovskites–organic molecules, inorganic atoms, halide anions and their ratios–researchers have reported that the intermolecular interactions comprising the chiral perovskite affect the chiral structure and its optical properties. This promises numeral approaches changing the specific configuration of each perovskite element - the organic cation ligand, metal ion and halide ion - as well as the overall arrangement of these components, remain crucial to be investigated to improve the chiroptical properties of the perovskite structure.
 | ||
| Fig. 14 (a) Effect of nano-confinement growth on chiral activity depending on its pore size. Reproduced with permission.58 Copyright 2022, Nature Publishing Group. (b) Effect of additive on optical activity of chiral perovskites. Reproduced with permission.59 Copyright 2022, American Chemical Society. (c) Schematic illustration of chiral dopant induced dynamic crystal reconstruction of chiral perovskites. Reproduced with permission.60 Copyright 2024, The American Association for the Advancement of Science. (d) Supramolecular assembly of chiral perovskites. Reproduced with permission.61 Copyright 2024, American Chemical Society. (e) Effect of dripping various antisolvent on optical activity of chiral perovskites. Reproduced with permission.62 Copyright 2024, Wiley-VCH. (f) Effect of various solvent on optical activity of chiral perovskites. Reproduced with permission.63 Copyright 2023, American Chemical Society. | ||
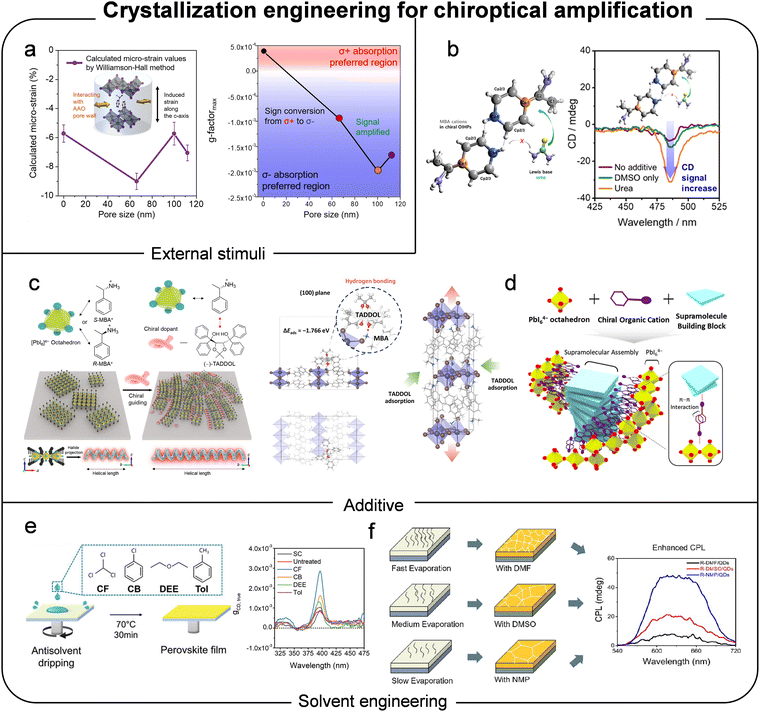
Moreover, to enhance the optoelectronic properties of perovskites in fields such as solar cells and LEDs, numerous strategies have been developed to improve the morphology of perovskite polycrystalline films by introducing additional molecules into precursor solutions. Notably, the application of these techniques has also been shown to enhance the chiroptical activity of chiral perovskite polycrystalline film. Lee et al. demonstrated that introducing external molecules, specifically nonvolatile Lewis bases, into the lattice of chiral perovskites offers an effective strategy to simultaneously enhance both chiroptical activity and charge transport (Fig. 14b).59 Within the chiral perovskite lattice, urea functions as a Lewis base oxygen donor and coordinates with divalent metal cations in the metal halide octahedral layers. This coordination induces a conformational redistribution of chiral organic molecules while maintaining the structural integrity of perovskite lattice. Theoretical simulations further revealed that the introduction of Lewis base urea strengthens interlayer connections within the chiral perovskite lattice, thereby enhancing both chiroptical activity and out-of-plane charge transport.
Our group reported that the incorporation of chiral dopants can drive dynamic long-range ordered crystallization in chiral perovskites, facilitated by strong interactions between the chiral dopants and chiral cations (Fig. 14c).60 This interaction facilitates the reorganization of the morphological and crystallographic properties of chiral perovskites through the additional interplay between chiral cations and chiral dopants. Notably, both homogeneous long-range chiral activity and compressive strain-induced lattice distortion caused by adsorption of chiral dopants enhanced the asymmetric behavior of chiral perovskites by more than tenfold. Similarly, Kim et al. demonstrated a co-assembly process involving the incorporation of supramolecular building blocks that interact with chiral organic cations, leading to the growth of chiral perovskites on supramolecular helical structures (Fig. 14d).61 These interactions between perovskites and chiral supramolecular structures promote crystal lattice distortion, thereby improving the chirality of chiral perovskites.
During the fabrication of polycrystalline films from perovskite precursor solutions, the interactions between solvents and solutes significantly influence the coordination among precursor elements, the formation of intermediate structures, and the solution's evaporation process – critical factors for crystal construction. In this context, Yang et al. demonstrated that introducing an antisolvent during the spin-coating process can effectively improve the chiroptical activities of polycrystalline thin films (Fig. 14e).62 A systematic study using various antisolvents revealed that the highly polar antisolvent chloroform forms hydrogen bonds with dimethyl sulfoxide molecules, which suppresses the formation of intermediate and secondary phases, accelerates the crystallization of chiral perovskites, and reduces iodine vacancy density in the films. The resulting decrease in iodine vacancies led amplification of the asymmetric distortion of the inorganic lattice, thereby strengthening the chiroptical response of the chiral perovskite material.
Zhu et al. reported that adopting a solvent modulation strategy with three different solvent reagents significantly improved the chirality of chiral perovskite films.63 They attributed the enhanced circularly polarized light luminescence to slower solvent evaporation, which increased the degree of lattice distortion (Fig. 14f). Similarly, Scalon et al. investigated the impact of processing conditions on the phase purity, microstructure, and chiroptical properties of chiral perovskites.139 They observed that less coordinating solvents, such as acetonitrile, accelerate film formation and induce anisotropic orientation of the perovskite phase, whereas highly coordinating solvents, such as dimethylformamide, promote the formation of larger crystal sizes with improved control over phase purity. Their study revealed that films prepared using acetonitrile exhibited a higher anisotropy factor–approximately one order of magnitude greater–compared to those prepared with dimethylformamide.
In conclusion, various physical and chemical interactions within perovskite films are critical for constructing asymmetric crystal structures that contribute to the chiroptical activity of chiral perovskites. Enhancing the interaction between chiral ligands and the inorganic framework through strategic engineering of chiral ligand design, combined with optimized composition and crystallization processes, will be pivotal for amplifying the chiroptical activity of perovskites. Such advancements will further their applications in chiral optoelectronic and spintronic devices.
3. Chirality amplification in devices
Chiral semiconducting materials with enhanced chiroptical properties offer significant potential for various advanced applications. These include spintronic devices, which exploit spin selectivity during charge generation and transport, and encrypted optical communication, where CPL can be selectively emitted or detected based on its handedness.140 Furthermore, such materials show promise for circularly polarized imaging of biomolecules and energy-efficient three-dimensional displays.33,164–166Interestingly, recent studies have demonstrated that the chiral properties of optoelectronic devices can be significantly amplified when integrating chiral semiconducting materials, as compared to the intrinsic chiral properties of the materials alone (Fig. 15a). Table 2 provides a summary of cases in which the chirality of materials was enhanced upon integration into devices. Numerous studies have revealed that chiral properties related to optoelectronic functions–such as the detection or emission of circularly polarized light–can be augmented through various factors (Fig. 15b). These studies have explored diverse amplification mechanisms, including harnessing CISS effect, fabricating single crystal devices, applying out-of-plane orientation and external bias, adopting phototransistor configurations, controlling the active layer thickness, and employing non-reciprocal features. Remarkably, by the fine-tuning, these parameters have resulted in amplification levels as high as 3684-fold have been reported.154 In a similar context of amplification development, the highest anisotropy factor achieved for a CPL detector was 1.97, with the theoretical maximum being 2.163 These findings underscore the synergistic importance of intrinsic material properties and device-driven mechanisms in enhancing chirality. They provide valuable insights and directions for future research, advancing the development of highly efficient chiral optoelectronic devices.
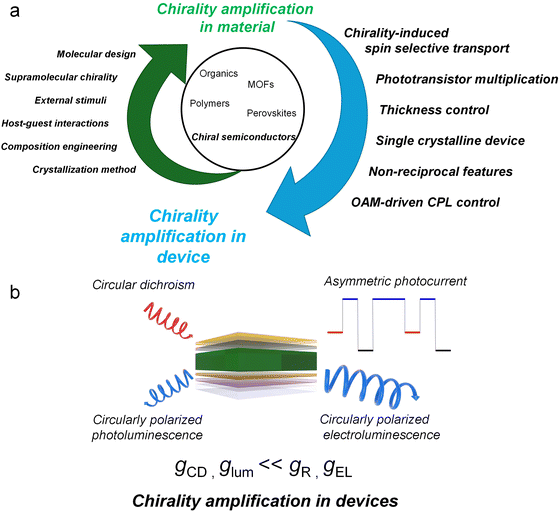 | ||
| Fig. 15 (a) Schematic illustration of chirality amplification strategies for semiconductors. (b) Schematic illustration of chirality amplification in devices. | ||
| Material | Intrinsic chirality (gCD or glum) | Device property (gR or gEL) | Amplified value (gR/gCD or gEL/glum) | Device structure | Operation condition | Ref. |
|---|---|---|---|---|---|---|
| CsEu((+)–hfbc)4 | 0.04 | 1.41 | ×35.25 | CP-OLED | — | 141 |
| F6BT | 1.87 × 10−3 | 4.16 × 10−2 | ×22 | — | 37 | |
| (R/S)-CPDCB | 5.8 × 10−4 | 3.9 × 10−3 | ×6.72 | — | 142 | |
| (R/S)-BN-AF | 0.02 | 0.091 | ×4.55 | 143 | ||
| Chiral MOF/[CdSe/ZnS]QD | achiral | 0.245 | — | CP-QLED | 1.3–5 V | 144 |
| R/S-MBA2PbI4/[CdSe/ZnS]QD | achiral | 0.016 | — | 1.9–8 V | 145 | |
| D/L-Eu(Tar)MOF/[CdSe/ZnS]QD | achiral | 0.44 | — | 1.2–4.8 V | 146 | |
| R/S-MBA2PbI4/[CsPbBr3]QD | achiral | 0.052 | — | — | 147 | |
| Coreshell [CsPbBr3] QD | achiral | 0.24 | — | — | 107 | |
| (R/S-MBA)2Pb0.9Sn0.1I4 | 1.6 × 10−3 | 0.44 | ×275 | Photodiode | Self-powered | 129 |
| (R/S-MBA)2Pbl2.8Br1.2 | 1 × 10−3 | 0.27 | ×270 | 59 | ||
| (R,S-MBA0.5nBA0.5)2PbI4 | 2.89 × 10−3 | 0.58 | ×200 | 118 | ||
| (R,S-MBA)2PbI4 | 6 × 10−3 | 1.16 | ×193 | 60 | ||
| (S-2F-MBA)2PbI4 | 1.68 × 10−3 | 0.288 | ×171 | 49 | ||
| DPP-6T/PC60BM | 0.02 | 0.17 | ×8.5 | 148 | ||
| R/S-MBA4Bi2Br10 | 0.1% | 30% | ×300 | 130 | ||
| 60% | ×600 | –1 V | ||||
| P3HT/R(S)-BN | 3.8 × 10−3 | 0.2 | ×52.6 | –1 V | 149 | |
| R/S-1,1-NEAPbI3 | 0.04 | 1.85 | ×46 | –0.5 V | 56 | |
| Polythiophene | 2 × 10−3 | 0.09 | ×45 | NA | 150 | |
| R/S-MBA2PbI4 | 0.3 × 10−3 | 0.07 | ×233 | Photo conductor | 5 V | 151 |
| R(S)-Cu(BDA) SURMOFs | 8 × 10−3 | 0.41 | ×51.25 | 5 V | 152 | |
| (R-BPEA)2PbI4/MAPbI3 | 0.9 × 10−3 | 0.13 | ×144 | 3 V | 153 | |
| (R/S-BPEA)EA6Pb4Cl15 | 7.6 × 10−5 | 0.28 | ×3684* | Single crystalline photo conductor | 10 V | 154 |
| Chiral COFs | 4.5 × 10−4 | 0.5 | ×1111 | 0.1 V | 155 | |
| R/S-MBA2PbI4 | 0.3 × 10−3 | 0.24 | ×800 | 5 V | 151 | |
| (R/S-PPA)(PA)PbBr4 | 0.74 × 10−3 | 0.5 | ×676 | 5 V | 156 | |
| R/S-PyEAPb2Br6 | 2.5 × 10−3 | 0.42 | ×168 | 5 V | 157 | |
| (R/S-3AMP)PbBr4 | 1.8 × 10−3 | 0.22 | ×122 | 5 V | 158 | |
| [R/S-BPEA]2FAPb2I7 | 0.6 × 10−3 | 0.376 | ×627 | Self-powered | 159 | |
| [(R/S)-ß-MPA]4AgBiI8 | 0.001 | 0.3 | ×300 | 120 | ||
| PPAEAPbBr4 | 2.5 × 10−3 | 0.42 | ×168 | 138 | ||
| (R/S-PPA)EAPbCl4 | 3.3 × 10−3 | 0.4 | ×121 | 116 | ||
| (anti,R)16,17-bis[60]PCBM | 6.3 × 10−4 | 1.27 | ×2016 | Photo transistor | — | 160 |
| NTPH-P/DPA crystal | 0.8 × 10−3 | 0.24 | ×300 | — | 161 | |
| Chiral dicyanostilbene | 4 × 10−3 | 0.83 | ×207.5 | — | 162 | |
| Doped ClCPDI-Ph | ∼10−3 | 0.13 | × ∼130 | — | 28 | |
| DPPPT/IGZO | 0.03 | 1.97* | ×65.7 | — | 163 | |
| Fused ring non-fullerene acceptor BTP-4F | 0.026 | 1.4 | ×53.8 | — | 29 | |
| NDI-BN-NDI | 2.2 × 10−3 | 0.05 | ×22.7 | — | 24 | |
| CPDI-Ph | 0.015 | 0.33 | ×22 | — | 27 | |
| π-extended PDI double-heterohelicene | 0.014 | 0.057 | ×4 | — | 22 |
3.1. Amplification through chirality-induced spin selective transport
For the application of chiral semiconducting materials in optoelectronic devices, the charge transport process following charge generation from asymmetric absorption is a critical factor. When charge carriers traverse the chiral semiconducting medium, spin selective charge transport occurs, a phenomenon known as the CISS effect.19 Furthermore, selective charge transfer with particular spin can take place at interfaces where charges are transferred from the chiral layer to adjacent layers. To maximize spin-selective charge transport, it is advantageous to extend spin lifetime, minimize spin relaxation, or increase charge transport speed. These goals can be achieved by applying an external voltage, utilizing single crystals with minimal defects, or employing optimized device architectures, such as diodes or transistors. Kim et al. introduced a chiral perovskite layer between the hole transporting layer and the light emitting layer, enabling selective injection of spin-polarized holes into achiral metal halide perovskite nanocrystals (Fig. 16a).147 By utilizing the CISS effect to generate spin-polarized carriers, they demonstrated a spin-LED, evidenced by circularly polarized luminescence from achiral perovskite nanocrystals. This result suggests that spin selective transport facilitated spin selective emission. In addition to emitting devices, Kim et al. also reported chirality amplification during device operation in CPL detecting devices (Fig. 16b).105 They fabricated phototransistors based on chiral perovskite nanocrystals and single wall carbon nanotube (SWCNT) heterojunctions, achieving an anisotropy factor of device operation (gPh) amplified by 2860 times compared to the optical property (gabs) (Table 2). To explain this amplification, they investigated the underlying mechanisms contributing to the significantly higher gPh observed in the phototransistors. Their analysis revealed that spin polarization of charge carriers, selectively excited by right- or left-handed CPL, is amplified as the carriers traverse multiple chiral perovskite nanocrystals within the films–an effect attributed to the CISS phenomenon (mechanism (2) in Fig. 16b). Additionally, spin selective charge transfer between the chiral perovskite nanocrystals and SWCNTs was identified as another contributing factor (mechanism (3) in Fig. 16b). The synergistic effect arises from the combination of spin-selective or spin-polarized charge transport within the device architecture and spin-selective absorption by the chiral semiconductor layer (mechanism (1) in Fig. 16b). Related to the spin-polarized charge transport, Maiti and Pal observed that a self-powered CPL detector exhibited a relatively low anisotropy factor (0.3), which was attributed to spin–flip events occurring during charge transport. However, when an external bias was applied, the anisotropy factor increased to 0.6, as the bias promoted an extended effective spin-diffusion distance (Fig. 16c).130 This finding suggests that the application of external voltage mitigates spin–flip events by enabling faster spin transport, allowing carriers to traverse the device before spin-flips occur. Interestingly, restricting spin-flipping through bias application did not improve CPL detection performance in 1D chiral perovskite devices, indicating that the dimensionality of the chiral perovskite layer is another significant factor for achieving efficient spin-selective transport.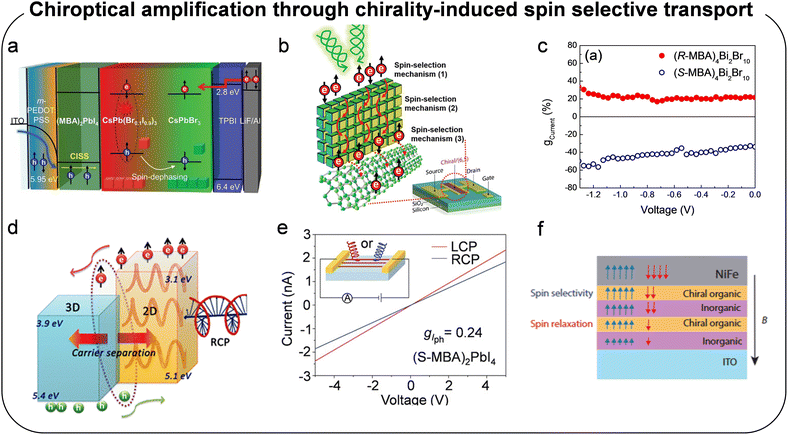 | ||
| Fig. 16 (a) Device architecture and CISS effect for spin-LED. Reproduced with permission.147 Copyright 2021, The American Association for the Advancement of Science. (b) Spin-selection mechanism 1 to 3 for heterojunction phototransistor. Reproduced with permission.105 Copyright 2022, Wiley-VCH. (c) Dependence of CPL detection property on applied voltage. Reproduced with permission.130 Copyright 2022, Wiley-VCH. (d) Schematic illustration of spin selective transport through out-of-plane in chiral perovskites. Reproduced with permission.167 Copyright 2019, The American Association for the Advancement of Science. (e) CPL detection property of chiral perovskite single crystal nanowire array. Reproduced with permission.151 Copyright 2021, Wiley-VCH. (f) Inner electric field formation in heterostructure for efficient CPL detection. Reproduced with permission.169 Copyright 2021, American Chemical Society. | ||
In subsequent studies, Zhang et al. proposed a heterostructure construction strategy to reduce the electron–hole recombination rate and establish a built-in electric field at the sharp interface of the as-grown heterostructure crystal (Fig. 16d).167 By integrating the concepts of charge transfer and chirality transfer, this approach effectively minimizes the recombination probability of photogenerated carriers while maintaining the chiroptical activity of chiral 2D perovskites. As a result, the CPL detector demonstrated significantly enhanced circular polarization sensitivity at zero bias, achieving an impressive anisotropy factor of up to 0.67, substantially higher than that of single-phase chiral 2D perovskite devices.
In addition to external bias or built-in electric field, single crystals might offer a promising alternative to polycrystalline thin films in optoelectronic devices, as they provide several advantages: long-range order, the absence of grain boundaries, reduced defect concentrations, enhanced and orientation-dependent carrier transport, a clearer structure–property relationship, and simplified device architectures.158 Liu et al. developed a direct CPL detector utilizing low-defect chiral single-crystal nanowire arrays, which demonstrated high photocurrent and polarization selectivity (Fig. 16e).151 Due to the high crystallinity and ordered crystallographic alignment of these nanowire arrays, the CPL detection performance was amplified more than threefold compared to CPL detectors based on polycrystalline thin films. This remarkable improvement culminated in a great enhancement of chiral properties (gph = 0.24) reaching an overall amplification of approximately 800 times relative to gabs (=0.0003) of the material.
Furthermore, the orientation of the photocurrent relative to the device geometry is a critical factor. Spin selectivity in charge transport remains limited when utilizing a lateral device geometry.168,169 Notably, the CISS effect predominantly arises from photocurrent directed along the asymmetric axis–specifically in the out-of-plane direction for 2D layered perovskites–as it passes through the chiral organic layer (Fig. 16f). Since most of these perovskites are highly oriented parallel to the substrates, achieving a high degree of spin-selective transport in a lateral geometry, which relies on in-plane transport, is inherently challenging. Conversely, vertical device orientation has been widely demonstrated as an effective amplification method, highlighting the advantages of out-of-plane transport for maximizing the CISS effect in chiral optoelectronic devices. These findings highlight the importance of optimizing both material properties and device architecture to maximize chiral optoelectronic performance. Furthermore, recent studies have not only enhanced the chiroptical performance of the active layer through the CISS effect, but have also utilized the chiral layer as a spin filter for spin-LED development (CP-QLED part in Table 2).144–146 This is an intriguing approach, as endowing chiroptical performance to achiral materials represents another significant area of research. In this review, however, we focus on amplifying the chiroptical activity of intrinsic chiral materials.
3.2. Impact of device type and design of chiral layer within operational mechanisms
In addition to the CISS effect, the device type and operational mechanisms play crucial roles in determining chiral optoelectronic performance. As summarized in Table 2, phototransistors have been extensively reported for their highly amplified gPh values compared to gabs when applied as chiral optoelectronic devices. For instance, Song et al. attributed the relatively larger gR compared to gabs to a synergetic effect arising from enhanced photocurrent differences due to photomultiplication phenomena induced by an applied gate bias, combined with spin-dependent carrier transport and collection effects governed by optical selection rules.22,170,171Shi et al. further explored the origins of the significantly larger gph compared to gabs in an organic phototransistor system.160 The authors proposed that the enhancement of gph relative to gabs arises from two cooperative device mechanisms: (1) the circularly polarized selective photogeneration of electrons in the channel, which increases the majority carrier density, and (2) the circularly polarized selective photogeneration of holes, which accumulate at the source electrode, thereby reducing the barrier to electron injection. Based on the two regimes of device behavior, for the same incident intensity of LCPL and RCPL, a greater number of photogenerated electrons is expected under illumination by the light with strongly absorbed handedness (SAH) compared to the weakly absorbed handedness (WAH). This phenomenon manifests as a greater photocurrent for the SAH in the photoconductive regime, as shown in Fig. 17a. Similarly, a greater number of photogenerated holes is expected for SAH, which accumulate at the source electrode. This accumulation reduces the threshold voltage (Vth) for the SAH compared to the WAH. Consequently, within a narrow range of applied gate voltages (VGS), the device remains in the on-state (VGS > Vth) for the SAH but transitions to the off-state (VGS < Vth) for the WAH. This behavior leads to a peak in gph near VGS = Vth for the devices under illumination, as observed in Fig. 17a. Lee et al. further advanced this amplification method by reporting the highest dissymmetry factor to date, reaching 1.97 where the theoretical maximum value is 2, through the adoption of an asymmetric photo-gating effect under SAH and WAH illumination in a chiroptical synaptic heterojunction phototransistor.163
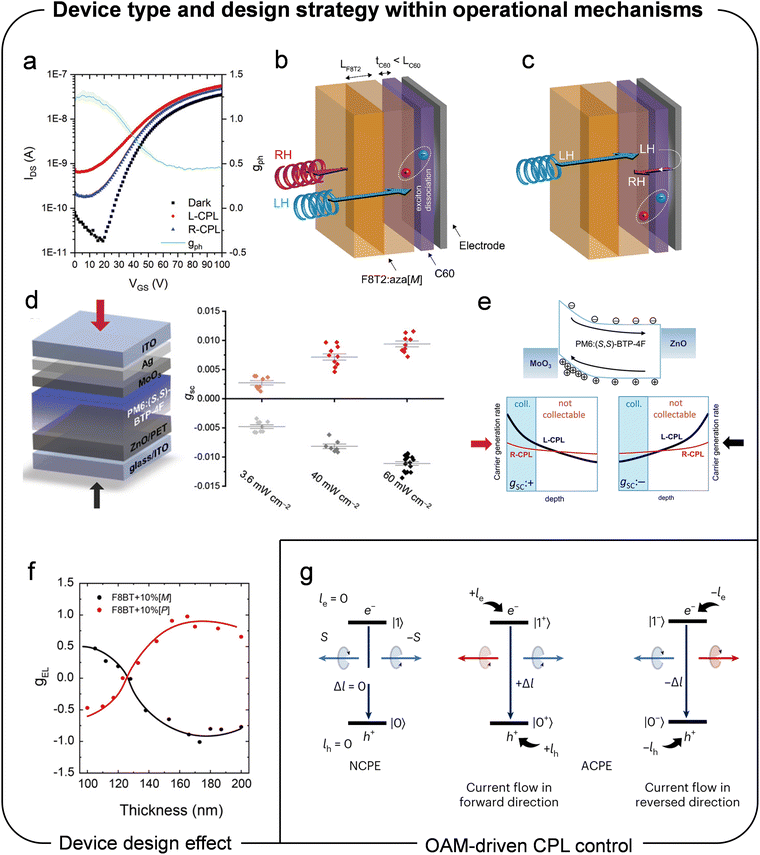 | ||
| Fig. 17 (a) Transfer curve under CPL illumination of chiral phototransistor. Reproduced with permission.160 Copyright 2021, Wiley-VCH. (b) Schematic illustration of CPL absorption at donor–acceptor layer interface. (c) Schematic illustration of transmission of CPL and reflection at electrode. Reproduced with permission.32 Copyright 2022, Wiley-VCH. (d) Effect of illumination direction and intensity on CPL detection. (e) Carrier generation rate depending on the depth in chiral active layer. Reproduced with permission.172 Copyright 2023, Wiley-VCH. (f) Chiroptical activity depending on the thickness of CP-OLED. Reproduced with permission.173 Copyright 2019, American Chemical Society. (g) Illustration of normal circular polarization emission (NCPE) and anomalous circular polarization emission (ACPE) from the excitation state to the ground state. Reproduced with permission.175 Copyright 2023, Nature Publishing Group. | ||
In addition to considerations of device architecture, recent studies have emphasized the critical role of the chiral semiconducting layer's thickness in the performance of CPL-detecting or emitting diodes with a vertically stacked configuration.86,148,161 Ward et al. investigated the effects of the film thickness of the chiral electron donor layer (tD) and the achiral electron acceptor layer (tA) on circularly polarized light detection performance in a bilayer organic photodiode.86 Their findings revealed that gph is highly sensitive to the thickness of both layers, with a trade-off existing between the external quantum efficiency and gph. As tD significantly exceeds the exciton diffusion length of chiral donor layer, most excitons generated beyond this length from the donor–acceptor (D–A) interface fail to dissociate before annihilation, thereby contributing minimally to the photocurrent. Conversely, gph increases with increasing tD and is consistently opposite in sign to gabs. This behavior arises from selective absorption of CPL handedness, where the intensity of light reaching the D–A interface is greater for WAH than SAH. As a result, an inversion of gph relative to gabs is observed (Fig. 17b and c). This “filter effect” becomes more pronounced with increasing tD, further enhancing gph. Additionally, reducing tA allows more light to transmit through the acceptor layer, which is then reflected by the back aluminum electrode. This reflection causes an inversion of CPL handedness, ultimately enhancing gph. By optimizing the thickness of both the chiral donor and achiral acceptor layer, the highest gph achieved was 0.85 under reverse bias (|gph| = 0.72 at zero bias), representing a significant enhancement from the initial value of 0.15.
In addition to the thickness of the chiral active layer, Zhu et al. revealed that gph can be regulated by the light intensity of the laser. With the increase of the intensity, the photocurrent increased by an order of magnitude, and the anisotropy factor of the device linearly increased with the light intensity.161 The results about the effect of light intensity and thickness of chiral active layer on gph factor indicate that the photocurrent is mainly generated from the photo-induced carriers in the chiral active layer. Moreover, Liu et al. investigated the circularly selective photoresponse of semi-transparent bulk-heterojunction (BHJ) organic photodiodes in the near-infrared range.172 They utilized a chiral non-fullerene acceptor in combination with an achiral π-conjugated polymer. By measuring the photoresponse under two opposite illumination directions on the same device, they observed opposite signs for gsc (Fig. 17d). Furthermore, the dissymmetry ratios varied in magnitude depending on the intensity of the illumination. A detailed analysis of the photocurrents indicated a space-charge limitation on carrier collection at higher illumination intensities. This behavior suggests that photocarriers generated across the bulk heterojunction can only be effectively collected from a thin active zone adjacent to the hole collecting contact, due to the lower mobility of holes compared to electrons. Consequently, only this limited zone of the BHJ layer contributes to the photocurrent. Experimental evidence supporting the limited active zone of BHJ layer was incorporated into a model that qualitatively explains both the sign inversion of gsc with illumination direction and the dependence of gsc on light intensity. As illustrated in Fig. 17e, only carriers generated in the thin zone next to the hole collecting MoO3 contact contribute to the photocurrent. If the absorption coefficient of the (S,S)-BHJ for L-CPL is higher than for R-CPL, then photons absorbed in this active zone from light entering via the adjacent hole-collecting contact will predominantly have L polarization. In contrast, for light entering through the electron-collecting contact, the majority of photons absorbed in the active zone will have R polarization, as most L-polarized photons are absorbed in the inactive region of the BHJ. This simple reasoning accounts for the inversion of the gsc sign upon changing the illumination direction. The reversal in CPL selectivity upon changing the illumination direction demonstrates that selectivity is not solely determined by a chirality-dependent material parameter but involves a convolution of multiple mechanistic steps. The interplay between selective absorption of left- and right-polarized photons and the selective collection of photogenerated carriers near the hole-collecting contact qualitatively explains the sign reversal and the magnitude of selectivity.
In addition to their role in CPL detecting devices, the thickness of the chiral layer also significantly influences the properties of CPL emitting devices. Wan et al. reported that the active layer thickness in circularly polarized light-emitting diodes (CP-PLEDs) affects the sign and magnitude of CPL due to an interplay between localized CPL emission from molecular chirality and its modulation through the chiral medium.173 The authors found that annealing above the glass transition temperature increased flexibility and twisted preferential handedness of polymer chains. This process induced circular scattering in the multidomain disordered cholesteric phase, resulting in an increase in gEL as the recombination zone moved deeper into the device. By modulating the active layer thickness from 110 nm to 160 nm, the anisotropy factor of CPL emission varied from +0.51 to −1.05, demonstrating doubly enhanced magnitude with opposite sign (Fig. 17f). This shift indicates that selective emissions can become negligible (nearly zero) under specific conditions. Such changes suggest a transition from molecularly localized CPL emission in thinner films to cholesteric multidomain scattering in thicker films. Additionally, circular birefringence from the chiral medium, which exhibits a wavelength dependence in the emission region, contributes to variations in gEL. This effect may arise from the established inverse-square relationship between outcoupled light intensity and refractive index in organic LEDs.
3.3. Nonreciprocal chiroptical features and OAM-driven CPL control in chiral media
One major issue affecting CP-OLEDs is the reflection from the back electrodes, which can reduce their dissymmetry. In conventional reciprocal CPL emission, CPL reflected from the back electrode compensates the handedness, thereby lowering the generated CPL dissymmetry. However, in a non-reciprocal context, where the optical response is not the same when the propagation direction is reversed, the CPL reflection from the backplane electrode can instead amplify the dissymmetry value. This non-reciprocal CPL emission phenomenon suggests a breaking time-reversal symmetry or the presence of conditions that cause the emitted CPL to behave differently depending on direction or polarization. In the solid state, the factors such as linear dichroism (LD), linear fluorescence anisotropy (f), and linear birefringence (LB) can influence the emitted CPL signals, as described by the following equation.174| CPL emission ≈ CPEint + (LB·f′ − LB′·f) | (5) |
In 2022, Wan et al. reported anomalous CPL emission in OLEDs utilizing the non-reciprocal features.175 Interestingly, the polymer films did not exhibit the linear anisotropies, but distinct non-reciprocal CPL emission appeared from the fabricated CP-OLEDs. Through their theoretical studies, the authors explained that the current flow through a chiral medium induces non-equilibrium orbital polarization in the charge carriers, which in turn facilitates the transfer of finite OAM from the charge carriers to the emitted photon spin, resulting in non-reciprocal CPL emission (Fig. 17g). Therefore, prototype devices with conventional and inverted device architectures exhibited opposite-handed CPL emission features, which are only observable in the electroluminescence. In addition, Qin et al. reported that fabricated helical polymeric nanowires with low absorption differences between left- and right-handed CPL exhibited significantly improved CPL detection performances.150 They explained this enhancement is attributed to the non-negligible chirality induced OAM, which resulted in the difference in binding energy of generated electron–hole pair through spin-orbital coupling depending on the CPL handedness.
4. Conclusion and outlook
Over the past few decades, substantial progress has been made in the development of chiral optoelectronic devices based on intrinsically chiral semiconductors. By harnessing their unique photophysical properties, these materials have emerged as promising candidates for high-performance, energy-efficient devices for next-generation photonic applications. However, despite these advances, several challenges remain that limit their practical implementation. The major issues that must be overcome to advance chiral optoelectronics toward real-world technologies are:(1) Performance trade-off: although the circular polarization selectivity of emitted and detected light has markedly improved through recent advances in chirality amplification strategies, most chiral optoelectronic devices still fall short of the performance levels required for industrial electronic applications. This limitation largely originates from the intrinsic trade-off between strong chiroptical activity and efficient charge transport, as increased molecular twisting often disrupts π-conjugation and charge transport.176 To overcome this issue, rational molecular design combined with optimized device architectures and fabrication processes will be essential to simultaneously achieve high circular polarization selectivity and robust electronic performance.
(2) Limited mechanism understanding: although numerous factors influencing CPL detection and emission have been explored, the overall origins of circular polarization selectivity in device operation remain unclear. In particular, how material chirality, charge–spin coupling, device architecture, and operational conditions collectively determine the chiroptical response has yet to be elucidated. Therefore, a clearer identification and quantitative evaluation of these contributing factors are required. To achieve this, comprehensive characterization techniques such as time-resolved spectroscopy, spin-resolved transport analysis, and polarization-dependent optical & electrical mapping, combined with theoretical modeling will be essential to elucidate the underlying working mechanisms and guide rational device design.
(3) Uniformity and scalability: chiral optoelectronic devices often exhibit significant variation in performance across different devices or even within different regions of the same thin film. This inconsistency arises mainly from the high sensitivity of chiral ordering to subtle variations in processing conditions such as solvent composition, evaporation rate, film thickness, and thermal or solvent annealing history which can lead to heterogeneous domain formation and local differences in circular polarization selectivity. To achieve reliable applications, it is essential to realize uniform and reproducible chiroptical thin films and device architectures by optimizing the fabrication processes. Emerging solution-based techniques, including blade coating and inkjet printing, offer a promising route toward scalable fabrication.177,178
(4) Stability: the operational stability of chiral semiconductors is often limited by thermal or photochemical, and ambient degradation of their molecular and supramolecular structures. To enhance the environmental and operational stability of chiral optoelectronic devices, various strategies including encapsulation, covalent crosslinking, and robust molecular designs should be further developed to suppress degradation and maintain long-term operation.
Nonetheless, ongoing research continues to yield more efficient and stable chiral semiconductors, along with optimized device architectures that address many of the challenges. These advances are bringing chiral optoelectronics closer to mainstream applications. The rapid progress in amplifying the chirality of semiconductors underscores the potential of chiral optoelectronics. Promising fields with the potential to be revolutionized by chiral optoelectronics include:
(1) Advanced display and imaging technologies: selective interaction with R-or L-CPL allows for enhanced encoding and decoding of optical information within a single communication channel. This capability supports the development of advanced display and imaging technologies, including 3D displays, virtual/augmented/extended reality (VR/AR/XR), and holographic systems.179,180 Moreover, CPL emitters can penetrate anti-glare filters with minimal losses, effectively doubling the brightness or efficiency of outdoor displays.181–183
(2) Chiral sensing and pharmaceutical applications: selective interactions with chiral molecules enable robust pharmaceutical quality control by facilitating enantiomeric discrimination184 and enantioselective synthesis,185 thereby helping prevent tragedies such as those caused by thalidomide. These interactions can also be applied to diagnostics via direct label-free chiral bioimaging,186 avoiding the detection efficiency losses associated with polarization filters and thus providing higher resolution and selectivity.
(3) Neuromorphic and logic devices: recently, there have been increasing reports of CPL-sensitive neuromorphic devices.187–189 Acting as biomimetic photoreceptors for adaptive CPL vision, these devices can extend the human visual recognition range offering additional and secure information transport. Furthermore, multiple-input logic gates using circularly polarized photons can consolidate complex logic circuits into a single gate, reducing the number of components.13 Multi-value logic that yields different output values based on polarization state is also feasible.190
(4) Quantum information and computing: chiral photons, which carry spin angular momentum, can serve as qubits operating at room temperature.191 Harnessing the quantum properties of entanglement, superposition, and interference enables advances in quantum communication, cryptography, and computation.11 Further development of chiral optoelectronic devices capable of handling single photons promises to revolutionize computational speed and information security.
(5) Spintronic applications: spintronic technologies based on the CISS effect are attracting growing interest. This approach allows for robust control over electron spin without the need for external magnetic fields, offering low-energy alternatives for spin-LEDs, spin-based logic circuits, and novel memory architectures.147,192
In this review, we have summarized recent advances in chirality amplification strategies aimed at enabling next-generation chiral optoelectronic devices. By consolidating the latest findings on chirality amplification strategies, we illustrate how systematic improvements in materials design, processing, device engineering, and other factors can drive this field toward practical application. In summary, continued efforts to elucidate the mechanisms behind chirality amplification, coupled with strategic advances in materials design, fabrication processes, and device integration, will be crucial to realizing the full potential of chiral optoelectronics. By systematically correlating chiroptical properties with fundamental material and device characteristics, along with developing reproducible and scalable techniques, the field is advancing toward next-generation technologies.
Author contributions
J. A., W. C., S. H. L., I. S., and J. H. O. jointly conceived and designed the overall theme of the review. J. A., W. C., and S. H. L. drafted the initial manuscript. J. P. and S. K. contributed to the literature survey and analysis. I. S. and J. H. O. critically revised and finalized the manuscript. J. H. O. supervised the project and secured funding support.Conflicts of interest
There are no conflicts to declare.Data availability
This article is a review of published literature and does not contain original data.Acknowledgements
This work was supported by the National Research Foundation (NRF) of Korea grant (2023R1A2C3007715, RS-2024-00398065, RS-2025-00558376). This work was also supported by the Technology development Program (RS-2025-25460624) funded by the Ministry of SMEs and Startups (MSS, Korea). The Institute of Engineering Research at Seoul National University provided research facilities for this work.Notes and references
- M. Sun, X. Wang, X. Guo, L. Xu, H. Kuang and C. Xu, Chem. Sci., 2022, 13, 3069–3081 RSC.
- M. Liu, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304–7397 CrossRef CAS PubMed.
- M. Z. Hasan, G. Chang, I. Belopolski, G. Bian, S.-Y. Xu and J.-X. Yin, Nat. Rev. Mater., 2021, 6, 784–803 CrossRef CAS.
- A. Lininger, G. Palermo, A. Guglielmelli, G. Nicoletta, M. Goel, M. Hinczewski and G. Strangi, Adv. Mater., 2023, 35, 2107325 CrossRef CAS.
- X. Niu, R. Zhao, S. Yan, Z. Pang, H. Li, X. Yang and K. Wang, Small, 2023, 19, 2303059 CrossRef CAS PubMed.
- N. Vargesson, Birth Defects Res., Part C, 2015, 105, 140–156 CrossRef CAS PubMed.
- K. P. Adamala, D. Agashe, Y. Belkaid, D. M. D. C. Bittencourt, Y. Cai, M. W. Chang, I. A. Chen, G. M. Church, V. S. Cooper and M. M. Davis, Science, 2024, 386, 1351–1353 CrossRef CAS PubMed.
- Y. Tang and A. E. Cohen, Phys. Rev. Lett., 2010, 104, 163901 CrossRef PubMed.
- K. Y. Bliokh, F. J. Rodríguez-Fortuño, F. Nori and A. V. Zayats, Nat. Photon., 2015, 9, 796–808 CrossRef CAS.
- J. F. Sherson, H. Krauter, R. K. Olsson, B. Julsgaard, K. Hammerer, I. Cirac and E. S. Polzik, Nature, 2006, 443, 557–560 CrossRef CAS PubMed.
- P. Lodahl, S. Mahmoodian, S. Stobbe, A. Rauschenbeutel, P. Schneeweiss, J. Volz, H. Pichler and P. Zoller, Nature, 2017, 541, 473–480 CrossRef CAS PubMed.
- J. Ahn, S. H. Lee, I. Song, P. Chidchob, Y. Kwon and J. H. Oh, Device, 2023, 1, 100176 CrossRef.
- Y. Zhang, Y. Wang, Y. Dai, X. Bai, X. Hu, L. Du, H. Hu, X. Yang, D. Li, Q. Dai, T. Hasan and Z. Sun, Sci. Adv., 2022, 8, eabq8246 CrossRef CAS PubMed.
- S. Richtberg and R. Girwidz, Phys. Teacher, 2017, 55, 406–408 CrossRef.
- N. Berova, L. Di Bari and G. Pescitelli, Chem. Soc. Rev., 2007, 36, 914–931 RSC.
- P. M. Blok and H. P. Dekkers, Chem. Phys. Lett., 1989, 161, 188–194 CrossRef CAS.
- J. L. Greenfield, J. Wade, J. R. Brandt, X. Shi, T. J. Penfold and M. J. Fuchter, Chem. Sci., 2021, 12, 8589–8602 RSC.
- B. P. Bloom, Y. Paltiel, R. Naaman and D. H. Waldeck, Chem. Rev., 2024, 124, 1950–1991 CrossRef CAS PubMed.
- R. Naaman, Y. Paltiel and D. H. Waldeck, Nat. Rev. Chem., 2019, 3, 250–260 CrossRef CAS.
- R. Naaman and D. H. Waldeck, J. Phys. Chem. Lett., 2012, 3, 2178–2187 CrossRef CAS PubMed.
- J. E. Field, G. Muller, J. P. Riehl and D. Venkataraman, J. Am. Chem. Soc., 2003, 125, 11808–11809 CrossRef CAS PubMed.
- L. Zhang, I. Song, J. Ahn, M. Han, M. Linares, M. Surin, H.-J. Zhang, J. H. Oh and J. Lin, Nat. Commun., 2021, 12, 142 CrossRef CAS PubMed.
- C. Gedeon, N. Del Rio, F. Furlan, A. Taddeucci, N. Vanthuyne, V. G. Gregoriou, M. J. Fuchter, G. Siligardi, N. Gasparini and J. Crassous, Adv. Mater., 2024, 36, 2314337 CrossRef CAS PubMed.
- Y. Kwon, J. Y. Jung, W. B. Lee and J. H. Oh, Adv. Sci., 2024, 11, 2308262 CrossRef CAS PubMed.
- M. Li, Y. F. Wang, D. Zhang, L. Duan and C. F. Chen, Angew. Chem., 2020, 132, 3528–3532 CrossRef.
- S. Sato, A. Yoshii, S. Takahashi, S. Furumi, M. Takeuchi and H. Isobe, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 13097–13101 CrossRef CAS PubMed.
- X. Shang, I. Song, H. Ohtsu, Y. H. Lee, T. Zhao, T. Kojima, J. H. Jung, M. Kawano and J. H. Oh, Adv. Mater., 2017, 29, 1605828 CrossRef PubMed.
- X. Shang, I. Song, J. H. Lee, W. Choi, J. Ahn, H. Ohtsu, J. C. Kim, J. Y. Koo, S. K. Kwak and J. H. Oh, ACS Nano, 2020, 14, 14146–14156 CrossRef CAS PubMed.
- L. Liu, Y. Yang, S. C. J. Meskers, Q. Wang, L. Zhang, C. Yang, J. Zhang, L. Zhu, Y. Zhang and Z. Wei, Adv. Mater., 2023, 35, 2304627 CrossRef CAS PubMed.
- L. Wan, R. Zhang, E. Cho, H. Li, V. Coropceanu, J.-L. Brédas and F. Gao, Nat. Photon., 2023, 17, 649–655 CrossRef CAS.
- Y. Yang, R. C. da Costa, D.-M. Smilgies, A. J. Campbell and M. J. Fuchter, Adv. Mater., 2013, 25, 2624–2628 CrossRef CAS PubMed.
- M. D. Ward, J. Wade, X. Shi, J. Nelson, A. J. Campbell and M. J. Fuchter, Adv. Opt. Mater., 2022, 10, 2101044 CrossRef CAS.
- I. Song, J. Ahn, H. Ahn, S. H. Lee, J. Mei, N. A. Kotov and J. H. Oh, Nature, 2023, 617, 92–99 CrossRef CAS PubMed.
- K. S. Park, X. Luo, J. J. Kwok, A. Khasbaatar, J. Mei and Y. Diao, ACS Cent. Sci., 2023, 9, 2096–2107 CrossRef CAS PubMed.
- Y. Wang, T. Sakamoto and T. Nakano, Chem. Commun., 2012, 48, 1871–1873 RSC.
- Y. Wang, T. Harada, L. Q. Phuong, Y. Kanemitsu and T. Nakano, Macromolecules, 2018, 51, 6865–6877 CrossRef CAS.
- J. Cheng, F. Ge, Y. Xiang, H. Zhang, Y. Kuai, P. Hou, D. Zhang, L. Qiu, Q. Zhang and G. Zou, J. Mater. Chem. C, 2020, 8, 6521–6527 RSC.
- Y. Hasegawa, Y. Miura, Y. Kitagawa, S. Wada, T. Nakanishi, K. Fushimi, T. Seki, H. Ito, T. Iwasa and T. Taketsugu, Chem. Commun., 2018, 54, 10695–10697 RSC.
- M. Tsurui, Y. Kitagawa, K. Fushimi, M. Gon, K. Tanaka and Y. Hasegawa, Dalton Trans., 2020, 49, 5352–5361 RSC.
- M. J. Islam, Y. Kitagawa, M. Tsurui and Y. Hasegawa, Dalton Trans., 2021, 50, 5433–5436 RSC.
- X. Yang, X. Lin, Y. Zhao, Y. S. Zhao and D. Yan, Angew. Chem., Int. Ed., 2017, 56, 7853–7857 CrossRef CAS PubMed.
- H. Ito, H. Sakai, Y. Okayasu, J. Yuasa, T. Mori and T. Hasobe, Chem. – Eur. J., 2018, 24, 16889–16894 CrossRef CAS PubMed.
- T. Zhao, J. Han, X. Jin, Y. Liu, M. Liu and P. Duan, Angew. Chem., Int. Ed., 2019, 58, 4978–4982 CrossRef CAS PubMed.
- L. Hu, K. Li, W. Shang, X. Zhu and M. Liu, Angew. Chem. Int. Ed., 2020, 59, 4953–4958 CrossRef CAS PubMed.
- T. Zhao, J. Han, Y. Shi, J. Zhou and P. Duan, Adv. Mater., 2021, 33, 2101797 CrossRef CAS PubMed.
- M. Zeng, A. Ren, W. Wu, Y. Zhao, C. Zhan and J. Yao, Chem. Sci., 2020, 11, 9154–9161 RSC.
- J. T. Lin, D. G. Chen, L. S. Yang, T. C. Lin, Y. H. Liu, Y. C. Chao, P. T. Chou and C. W. Chiu, Angew. Chem., Int. Ed., 2021, 60, 21434–21440 CrossRef CAS PubMed.
- J. Son, S. Ma, Y.-K. Jung, J. Tan, G. Jang, H. Lee, C. U. Lee, J. Lee, S. Moon, W. Jeong, A. Walsh and J. Moon, Nat. Commun., 2023, 14, 3124 CrossRef CAS PubMed.
- J. Son, G. Jang, S. Ma, H. Lee, C. U. Lee, S. Yang, J. Lee, S. Moon, W. Jeong and J. H. Park, Adv. Funct. Mater., 2025, 35, 2413041 CrossRef CAS.
- W. Choi, M. Kwak, J. Park, H. Kim, J. Kim, Y. Seo, D. Yoo and J. H. Oh, Chem. Eng. J., 2024, 498, 155504 CrossRef CAS.
- L. Yan, M. K. Jana, P. C. Sercel, D. B. Mitzi and W. You, J. Am. Chem. Soc., 2021, 143, 18114–18120 CrossRef CAS PubMed.
- H. Song, M. Kwak, W. Choi, D. Yoo and J. H. Oh, Adv. Opt. Mater., 2024, 12, 2401427 CrossRef CAS.
- L.-Z. Feng, Y.-H. Song, Z.-D. Li, B.-S. Zhu, Z.-Y. Ma, J.-N. Yang, Y.-C. Yin, J.-M. Hao, G.-J. Ding and Y.-R. Wang, Nano Lett., 2024, 24, 6084–6091 CrossRef CAS PubMed.
- Z. Zhang, W. Liang, J. Xue, X. Li, K. Wu and H. Lu, ACS Nano, 2024, 18, 5890–5897 CAS.
- J. Ahn, S. Ma, J.-Y. Kim, J. Kyhm, W. Yang, J. A. Lim, N. A. Kotov and J. Moon, J. Am. Chem. Soc., 2020, 142, 4206–4212 CrossRef CAS PubMed.
- A. Ishii and T. Miyasaka, Sci. Adv., 2020, 6, eabd3274 CrossRef CAS PubMed.
- Z. Zhang, Z. Wang, H. H.-Y. Sung, I. D. Williams, Z.-G. Yu and H. Lu, J. Am. Chem. Soc., 2022, 144, 22242–22250 CrossRef CAS PubMed.
- S. Ma, Y.-K. Jung, J. Ahn, J. Kyhm, J. Tan, H. Lee, G. Jang, C. U. Lee, A. Walsh and J. Moon, Nat. Commun., 2022, 13, 3259 CrossRef CAS PubMed.
- C. U. Lee, S. Ma, J. Ahn, J. Kyhm, J. Tan, H. Lee, G. Jang, Y. S. Park, J. Yun and J. Lee, J. Am. Chem. Soc., 2022, 144, 16020–16033 CrossRef CAS PubMed.
- H. Kim, W. Choi, Y. J. Kim, J. Kim, J. Ahn, I. Song, M. Kwak, J. Kim, J. Park, D. Yoo, J. Park, S. K. Kwak and J. H. Oh, Sci. Adv., 2024, 10, eado5942 CrossRef CAS PubMed.
- H. Kim, C. A. Figueroa Morales, S. Seong, Z. Hu and X. Gong, ACS Appl. Mater. Interfaces, 2024, 16, 16515–16521 CrossRef CAS PubMed.
- S. Yang, G. Jang, C. U. Lee, J. Son, J. Lee, W. Jeong, D. G. Roe, J. H. Cho and J. Moon, Adv. Funct. Mater., 2024, 34, 2310917 CrossRef CAS.
- H. Zhu, Q. Wang, K. Sun, W. Chen, J. Tang, J. Hao, Z. Wang, J. Sun, W. C. Choy and P. Muller-Buschbaum, ACS Appl. Mater. Interfaces, 2023, 15, 9978–9986 CrossRef CAS PubMed.
- J. Lee, A.-R. Han, H. Yu, T. J. Shin, C. Yang and J. H. Oh, J. Am. Chem. Soc., 2013, 135, 9540–9547 CrossRef CAS.
- H. Yu, Z. Bao and J. H. Oh, Adv. Funct. Mater., 2013, 23, 629–639 CrossRef CAS.
- M. Irimia-Vladu, Chem. Soc. Rev., 2014, 43, 588–610 RSC.
- X. Shang, I. Song, J. H. Lee, M. Han, J. C. Kim, H. Ohtsu, J. Ahn, S. K. Kwak and J. H. Oh, J. Mater. Chem. C, 2019, 7, 8688–8697 RSC.
- I. Song, J. Ahn, X. Shang and J. H. Oh, ACS Appl. Mater. Interfaces, 2020, 12, 49926–49934 CrossRef CAS PubMed.
- X. Shang, J. Ahn, J. H. Lee, J. C. Kim, H. Ohtsu, W. Choi, I. Song, S. K. Kwak and J. H. Oh, ACS Appl. Mater. Interfaces, 2021, 13, 12278–12285 CrossRef CAS.
- G. Albano, G. Pescitelli and L. Di Bari, Chem. Rev., 2020, 120, 10145–10243 CrossRef CAS PubMed.
- Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463–1535 CrossRef CAS PubMed.
- D. Zheng, L. Zheng, C. Yu, Y. Zhan, Y. Wang and H. Jiang, Org. Lett., 2019, 21, 2555–2559 CrossRef CAS PubMed.
- Y. Yang, R. C. Da Costa, M. J. Fuchter and A. J. Campbell, Nat. Photon., 2013, 7, 634–638 CrossRef CAS.
- Y. Zhang, S. Yu, B. Han, Y. Zhou, X. Zhang, X. Gao and Z. Tang, Matter, 2022, 5, 837–875 CrossRef CAS.
- K. Dhbaibi, C. Shen, M. Jean, N. Vanthuyne, T. Roisnel, M. Górecki, B. Jamoussi, L. Favereau and J. Crassous, Front. Chem., 2020, 8, 237 CrossRef CAS PubMed.
- S.-P. Wan, H.-Y. Lu, M. Li and C.-F. Chen, J. Photochem. Photobiol., C, 2022, 50, 100500 CrossRef CAS.
- M. Hasegawa, Y. Nojima and Y. Mazaki, ChemPhotoChem, 2021, 5, 1042–1058 CrossRef CAS.
- T. He, W. Zhao, M. Lin, B. Sun, Y. Chen, H.-L. Zhang and G. Long, J. Phys. Chem. Lett., 2024, 15, 9844–9851 CrossRef CAS PubMed.
- L. R. Sita and R. D. Bickerstaff, J. Am. Chem. Soc., 1989, 111, 6454–6456 CrossRef CAS.
- M. M. Green, J. W. Park, T. Sato, A. Teramoto, S. Lifson, R. L. Selinger and J. V. Selinger, Angew. Chem., Int. Ed., 1999, 38, 3138–3154 CrossRef PubMed.
- E. Yashima, K. Maeda and T. Nishimura, Chem. – Eur. J., 2004, 10, 42–51 Search PubMed.
- M. Oda, H. G. Nothofer, G. Lieser, U. Scherf, S. Meskers and D. Neher, Adv. Mater., 2000, 12, 362–365 CrossRef CAS.
- J. Gilot, R. Abbel, G. Lakhwani, E. Meijer, A. P. Schenning and S. C. Meskers, Adv. Mater., 2010, 22, E131–E134 Search PubMed.
- J. Wade, J. N. Hilfiker, J. R. Brandt, L. Liirò-Peluso, L. Wan, X. Shi, F. Salerno, S. T. Ryan, S. Schöche and O. Arteaga, Nat. Commun., 2020, 11, 6137 Search PubMed.
- D. Di Nuzzo, C. Kulkarni, B. Zhao, E. Smolinsky, F. Tassinari, S. C. Meskers, R. Naaman, E. Meijer and R. H. Friend, ACS Nano, 2017, 11, 12713–12722 CrossRef CAS PubMed.
- M. D. Ward, J. Wade, X. Shi, J. Nelson, A. J. Campbell and M. J. Fuchter, Adv. Opt. Mater., 2022, 10, 2101044 CrossRef CAS.
- L. Wan, J. Wade, X. Wang, A. J. Campbell and M. J. Fuchter, J. Mater. Chem. C, 2022, 10, 5168–5172 Search PubMed.
- K. S. Park, Z. Xue, B. B. Patel, H. An, J. J. Kwok, P. Kafle, Q. Chen, D. Shukla and Y. Diao, Nat. Commun., 2022, 13, 2738 Search PubMed.
- J. Cheng, F. Ge, C. Zhang, Y. Kuai, P. Hou, Y. Xiang, D. Zhang, L. Qiu, Q. Zhang and G. Zou, J. Mater. Chem. C, 2020, 8, 9271–9275 RSC.
- N. Micali, H. Engelkamp, P. Van Rhee, P. Christianen, L. M. Scolaro and J. Maan, Nat. Chem., 2012, 4, 201–207 CrossRef CAS PubMed.
- R. Hu, X. Lu, X. Hao and W. Qin, Adv. Mater., 2023, 35, 2211935 CrossRef CAS PubMed.
- Y. Wang, X. Li, L. Yang, W.-Y. Sun, C. Zhu and Y. Cheng, Mater. Chem. Front., 2018, 2, 554–558 Search PubMed.
- Y. Zhou, Y. Yao, Z. Cheng, T. Gao, H. Li and P. Yan, Inorg. Chem., 2020, 59, 12850–12857 Search PubMed.
- H. R. Fu, N. Wang, X. X. Wu, F. F. Li, Y. Zhao, L. F. Ma and M. Du, Adv. Opt. Mater., 2020, 8, 2000330 CrossRef CAS.
- C. Zhang, Z. P. Yan, X. Y. Dong, Z. Han, S. Li, T. Fu, Y. Y. Zhu, Y. X. Zheng, Y. Y. Niu and S. Q. Zang, Adv. Mater., 2020, 32, 2002914 CrossRef CAS PubMed.
- R. Zhai, Y. Xiao, Z. Gu and J. Zhang, Nano Res., 2022, 15, 1102–1108 CrossRef CAS.
- T. Zhao, J. Han, X. Jin, M. Zhou, Y. Liu, P. Duan and M. Liu, Research, 2020, 2020, 6452123 CAS.
- G. Long, R. Sabatini, M. I. Saidaminov, G. Lakhwani, A. Rasmita, X. Liu, E. H. Sargent and W. Gao, Nat. Rev. Mater., 2020, 5, 423–439 CrossRef.
- J. Ma, H. Wang and D. Li, Adv. Mater., 2021, 33, 2008785 CrossRef CAS PubMed.
- Y. Dong, Y. Zhang, X. Li, Y. Feng, H. Zhang and J. Xu, Small, 2019, 15, 1902237 CrossRef PubMed.
- S. Ma, J. Ahn and J. Moon, Adv. Mater., 2021, 33, 2005760 CrossRef CAS PubMed.
- J. Son, S. Ma, Y.-K. Jung, J. Tan, G. Jang, H. Lee, C. U. Lee, J. Lee, S. Moon and W. Jeong, Nat. Commun., 2023, 14, 3124 CrossRef CAS PubMed.
- A. Forde, A. C. Evans, W. Nie, S. Tretiak and A. J. Neukirch, J. Mater. Chem. C, 2023, 11, 12374–12383 RSC.
- M. K. Jana, R. Song, H. Liu, D. R. Khanal, S. M. Janke, R. Zhao, C. Liu, Z. Valy Vardeny, V. Blum and D. B. Mitzi, Nat. Commun., 2020, 11, 4699 CrossRef CAS PubMed.
- Y. H. Kim, R. Song, J. Hao, Y. Zhai, L. Yan, T. Moot, A. F. Palmstrom, R. Brunecky, W. You and J. J. Berry, Adv. Funct. Mater., 2022, 32, 2200454 CrossRef CAS.
- J. Ahn, E. Lee, J. Tan, W. Yang, B. Kim and J. Moon, Mater. Horiz., 2017, 4, 851–856 RSC.
- G. Jang, D. Y. Jo, S. Ma, J. Lee, J. Son, C. U. Lee, W. Jeong, S. Yang, J. H. Park and H. Yang, Adv. Mater., 2024, 36, 2309335 CrossRef CAS PubMed.
- J. Yao, Z. Wang, Y. Huang, J. Xue, D. Zhang, J. Chen, X. Chen, S.-C. Dong and H. Lu, J. Am. Chem. Soc., 2024, 146, 14157–14165 CrossRef CAS PubMed.
- S. Li, X. Xu, C. A. Kocoj, C. Zhou, Y. Li, D. Chen, J. A. Bennett, S. Liu, L. Quan and S. Sarker, Nat. Commun., 2024, 15, 2573 CrossRef CAS PubMed.
- T. Liu, W. Shi, W. Tang, Z. Liu, B. C. Schroeder, O. Fenwick and M. J. Fuchter, ACS Nano, 2022, 16, 2682–2689 CrossRef CAS PubMed.
- M. Li, F. Fang, X. Huang, G. Liu, Z. Lai, Z. Chen, J. Hong, Y. Chen, R.-J. Wei and G.-H. Ning, Chem. Mater., 2022, 34, 2955–2962 CrossRef CAS.
- C.-H. Yang, S.-B. Xiao, H. Xiao, L.-J. Xu and Z.-N. Chen, ACS Nano, 2023, 17, 7830–7836 CrossRef CAS PubMed.
- A. Pietropaolo, A. Mattoni, G. Pica, M. Fortino, G. Schifino and G. Grancini, Chem, 2022, 8, 1231–1253 CAS.
- T. Duan and Y. Zhou, Adv. Energy Mater., 2023, 13, 2200792 CrossRef CAS.
- W. Wu, L. Li, D. Li, Y. Yao, Z. Xu, X. Liu, M. Hong and J. Luo, Adv. Opt. Mater., 2022, 10, 2102678 CrossRef CAS.
- T. Zhu, H. Wu, C. Ji, X. Zhang, Y. Peng, Y. Yao, H. Ye, W. Weng, W. Lin and J. Luo, Adv. Opt. Mater., 2022, 10, 2200146 CrossRef CAS.
- W. Wu, X. Shang, Z. Xu, H. Ye, Y. Yao, X. Chen, M. Hong, J. Luo and L. Li, Adv. Sci., 2023, 10, 2206070 CrossRef CAS PubMed.
- X. Zhang, Y. Xu, A. N. Alphenaar, S. Ramakrishnan, Y. Zhang, A. J. Babatunde and Q. Yu, ACS Nano, 2024, 18, 14605–14616 CrossRef CAS PubMed.
- S. You, P. Yu, T. Zhu, Q. Guan, J. Wu, H. Dai, H. Zhong, Z.-K. Zhu and J. Luo, Mater. Horiz., 2023, 10, 5307–5312 RSC.
- D. Li, X. Liu, W. Wu, Y. Peng, S. Zhao, L. Li, M. Hong and J. Luo, Angew. Chem., 2021, 133, 8496–8499 CrossRef.
- Y. Zhao, M. Dong, J. Feng, J. Zhao, Y. Guo, Y. Fu, H. Gao, J. Yang, L. Jiang and Y. Wu, Adv. Opt. Mater., 2022, 10, 2102227 CrossRef CAS.
- J. Zhao, H. Huo, Y. Zhao, Y. Guo, M. Dong, Y. Fu, J. Zhang, Z. Gao and L. Kang, Chem. Mater., 2023, 35, 4347–4354 CrossRef CAS.
- N. Dehnhardt, M. Axt, J. Zimmermann, M. Yang, G. Mette and J. Heine, Chem. Mater., 2020, 32, 4801–4807 CrossRef CAS.
- Q. He, J. Feng, L. Du, Y. Shen, H. Chen, C. Yan, S. Kang, H. Wang, Y. Yin and L. Wan, Adv. Opt. Mater., 2024, 12, 2400682 CrossRef CAS.
- C. Coccia, M. Moroni, A. Treglia, M. Boiocchi, Y. Yang, C. Milanese, M. Morana, D. Capsoni, A. Porta and A. Petrozza, J. Am. Chem. Soc., 2024, 146, 24377–24388 CrossRef CAS.
- H. Lu, C. Xiao, R. Song, T. Li, A. E. Maughan, A. Levin, R. Brunecky, J. J. Berry, D. B. Mitzi and V. Blum, J. Am. Chem. Soc., 2020, 142, 13030–13040 CrossRef CAS PubMed.
- Y. Zheng, X. Han, P. Cheng, X. Jia, J. Xu and X.-H. Bu, J. Am. Chem. Soc., 2022, 144, 16471–16479 CrossRef CAS PubMed.
- Z. Li, C. Ji, Y. Fan, T. Zhu, S. You, J. Wu, R. Li, Z.-K. Zhu, P. Yu and X. Kuang, J. Am. Chem. Soc., 2023, 145, 25134–25142 CrossRef CAS PubMed.
- B. Yao, Q. Wei, Y. Yang, W. Zhou, X. Jiang, H. Wang, M. Ma, D. Yu, Y. Yang and Z. Ning, Nano Lett., 2023, 23, 1938–1945 CrossRef CAS PubMed.
- A. Maiti and A. J. Pal, Angew. Chem., 2022, 134, e202214161 CrossRef.
- L.-S. Li, Y.-H. Tan, W.-J. Wei, H.-Q. Gao, Y.-Z. Tang and X.-B. Han, ACS Appl. Mater. Interfaces, 2020, 13, 2044–2051 CrossRef PubMed.
- Y. Y. Tang, Y. Ai, W. Q. Liao, P. F. Li, Z. X. Wang and R. G. Xiong, Adv. Mater., 2019, 31, 1902163 CrossRef PubMed.
- Y. Lu, X. Yang, Y. Zhao, Z. He, W. Song, Z. Zhang, H. Niu, J. Liu, M. Li and Z. Miao, ACS Appl. Electron. Mater., 2024, 6, 6186–6193 CAS.
- Y. Liu, Y. Jiang, Z. Xu, L. Li, D. Zhang, W. Zheng, D. Liang, B. Zheng, H. Liu and X. Sun, Adv. Opt. Mater., 2022, 10, 2200183 CrossRef CAS.
- S. H. Nam, J. An, W. Jeong, J. G. Oh, J. M. Luther, M. C. Beard, T. H. Han, I.-H. Park and Y.-H. Kim, J. Am. Chem. Soc., 2024, 146, 15045–15052 CrossRef CAS PubMed.
- B. Sun, X.-F. Liu, X.-Y. Li, Y. Zhang, X. Shao, D. Yang and H.-L. Zhang, Chem. Mater., 2020, 32, 8914–8920 CrossRef CAS.
- L. Sui Li, W. Juan Wei, H. Qiang Gao, Y. Hui Tan and X. Bo Han, Chem. – Eur. J., 2021, 27, 9054–9059 CrossRef PubMed.
- T. Zhu, W. Weng, C. Ji, X. Zhang, H. Ye, Y. Yao, X. Li, J. Li, W. Lin and J. Luo, J. Am. Chem. Soc., 2022, 144, 18062–18068 CrossRef CAS PubMed.
- L. Scalon, J. Brunner, M. G. D. Guaita, R. Szostak, M. Albaladejo-Siguan, T. Kodalle, L. A. Guerrero-León, C. M. Sutter-Fella, C. C. Oliveira and Y. Vaynzof, Adv. Opt. Mater., 2024, 12, 2300776 CrossRef CAS.
- J. Lv, J. H. Han, G. Han, S. An, S. J. Kim, R. M. Kim, J. E. Ryu, R. Oh, H. Choi and I. H. Ha, Nat. Commun., 2024, 15, 8257 CrossRef CAS PubMed.
- F. Zinna, U. Giovanella and L. Di Bari, Adv. Mater., 2015, 27, 1791–1795 CrossRef CAS PubMed.
- S. Sun, J. Wang, L. Chen, R. Chen, J. Jin, C. Chen, S. Chen, G. Xie, C. Zheng and W. Huang, J. Mater. Chem. C, 2019, 7, 14511–14516 RSC.
- F. Song, Z. Xu, Q. Zhang, Z. Zhao, H. Zhang, W. Zhao, Z. Qiu, C. Qi, H. Zhang and H. H. Sung, Adv. Funct. Mater., 2018, 28, 1800051 CrossRef.
- M. Mustaqeem, P.-T. Chou, S. Kamal, N. Ahmad, J.-Y. Lin, Y.-J. Lu, X.-H. Lee, K.-H. Lin, K.-L. Lu and Y.-F. Chen, Adv. Funct. Mater., 2023, 33, 2213587 CrossRef CAS.
- Q. Wang, H. Zhu, Y. Tan, J. Hao, T. Ye, H. Tang, Z. Wang, J. Ma, J. Sun, T. Zhang, F. Zheng, W. Zhang, H. W. Choi, W. C. H. Choy, D. Wu, X. W. Sun and K. Wang, Adv. Mater., 2024, 36, 2305604 CrossRef CAS PubMed.
- M. Mustaqeem, Z.-B. Jin, W. C. Tsai, M. A. Gondal, P.-T. Chou, T.-H. Wu, K.-H. Lin, J. Zhang, Z.-G. Gu and Y.-F. Chen, Adv. Opt. Mater., 2025, 13, 2500060 CrossRef CAS.
- Y.-H. Kim, Y. Zhai, H. Lu, X. Pan, C. Xiao, E. A. Gaulding, S. P. Harvey, J. J. Berry, Z. V. Vardeny and J. M. Luther, Science, 2021, 371, 1129–1133 CrossRef CAS PubMed.
- L. Liu, Y. Yang, Y. Wang, M. A. Adil, Y. Zhao, J. Zhang, K. Chen, D. Deng, H. Zhang and K. Amin, ACS Mater. Lett., 2022, 4, 401–409 CrossRef CAS.
- H. Lee, W. M. Takele, J. I. Kwon, C. Choi, D. Kim, J. H. Cho, T. G. Habteyes and J. A. Lim, Adv. Funct. Mater., 2024, 34, 2409982 CrossRef CAS.
- Z. Wang, M. Gao, X. Hao and W. Qin, Appl. Phys. Lett., 2020, 116, 053301 CrossRef CAS.
- Z. Liu, C. Zhang, X. Liu, A. Ren, Z. Zhou, C. Qiao, Y. Guan, Y. Fan, F. Hu and Y. S. Zhao, Adv. Sci., 2021, 8, 2102065 CrossRef CAS PubMed.
- Y.-B. Tian, K. Tanaka, L.-M. Chang, C. Wöll, Z.-G. Gu and J. Zhang, Nano Lett., 2023, 23, 5794–5801 CrossRef CAS PubMed.
- L. Tao, W. Tang, M. Yan, L. Ding, J. Wei, L. Wang, L. Li, L. Li, D. Yang and Y. Fang, J. Mater. Chem. C, 2023, 11, 12392–12399 RSC.
- Q. Guan, T. Zhu, Z. K. Zhu, H. Ye, S. You, P. Xu, J. Wu, X. Niu, C. Zhang and X. Liu, Angew. Chem., Int. Ed., 2023, 62, e202307034 CrossRef CAS PubMed.
- Q. Gu, J. Zha, C. Chen, X. Wang, W. Yao, J. Liu, F. Kang, J. Yang, Y. Y. Li and D. Lei, Adv. Mater., 2024, 36, 2306414 CrossRef CAS PubMed.
- Z. K. Zhu, T. Zhu, S. You, P. Yu, J. Wu, Y. Zeng, Y. Jiang, X. Liu, L. Li and C. Ji, Adv. Sci., 2024, 11, 2307593 CrossRef CAS PubMed.
- J. Bai, H. Wang, J. Ma, Y. Zhao, H. Lu, Y. Zhang, S. Gull, T. Qiao, W. Qin and Y. Chen, J. Am. Chem. Soc., 2024, 146, 18771–18780 CrossRef CAS PubMed.
- C. C. Fan, X. B. Han, B. D. Liang, C. Shi, L. P. Miao, C. Y. Chai, C. D. Liu, Q. Ye and W. Zhang, Adv. Mater., 2022, 34, 2204119 CrossRef CAS PubMed.
- H. Ye, Y. Peng, M. Wei, X. Zhang, T. Zhu, Q. Guan, L. Li, S. Chen, X. Liu and J. Luo, Chem. Mater., 2023, 35, 6591–6597 CrossRef CAS.
- W. Shi, F. Salerno, M. D. Ward, A. Santana-Bonilla, J. Wade, X. Hou, T. Liu, T. J. S. Dennis, A. J. Campbell and K. E. Jelfs, Adv. Mater., 2021, 33, 2004115 CrossRef CAS PubMed.
- D. Zhu, W. Jiang, Z. Ma, J. Feng, X. Zhan, C. Lu, J. Liu, J. Liu, Y. Hu and D. Wang, Nat. Commun., 2022, 13, 3454 CrossRef CAS PubMed.
- Y. Xue, C. Zhang, T. Lv, L. Qiu and F. Wang, Angew. Chem., 2023, 135, e202300972 CrossRef.
- H. Lee, J. H. Hwang, S. H. Song, H. Han, S.-J. Han, B. L. Suh, K. Hur, J. Kyhm, J. Ahn, J. H. Cho, D. K. Hwang, E. Lee, C. Choi and J. A. Lim, Adv. Sci., 2023, 10, 2304039 CrossRef CAS PubMed.
- R. Hegedüs, G. Szél and G. Horváth, Vision Res., 2006, 46, 2786–2797 CrossRef PubMed.
- L. Ospina-Rozo, I. Medina, A. Hugall, K. J. Rankin, N. W. Roberts, A. Roberts, A. Mitchell, C. A. Reid, A. Moussalli and D. Stuart-Fox, Sci. Rep., 2024, 14, 29349 CrossRef CAS PubMed.
- P. Stachelek, L. MacKenzie, D. Parker and R. Pal, Nat. Commun., 2022, 13, 553 CrossRef CAS PubMed.
- X. Zhang, X. Liu, L. Li, C. Ji, Y. Yao and J. Luo, ACS Cent. Sci., 2021, 7, 1261–1268 CrossRef CAS PubMed.
- J. Wang, H. Lu, X. Pan, J. Xu, H. Liu, X. Liu, D. R. Khanal, M. F. Toney, M. C. Beard and Z. V. Vardeny, ACS Nano, 2020, 15, 588–595 CrossRef PubMed.
- H. Lu, J. Wang, C. Xiao, X. Pan, X. Chen, R. Brunecky, J. J. Berry, K. Zhu, M. C. Beard and Z. V. Vardeny, Sci. Adv., 2019, 5, eaay0571 CrossRef CAS PubMed.
- P. Odenthal, W. Talmadge, N. Gundlach, R. Wang, C. Zhang, D. Sun, Z.-G. Yu, Z. Valy Vardeny and Y. S. Li, Nat. Phys., 2017, 13, 894–899 Search PubMed.
- G. Long, C. Jiang, R. Sabatini, Z. Yang, M. Wei, L. N. Quan, Q. Liang, A. Rasmita, M. Askerka and G. Walters, Nat. Photon., 2018, 12, 528–533 CrossRef CAS.
- L. Liu, Z. Wei and S. C. Meskers, Adv. Mater., 2023, 35, 2209730 CrossRef CAS PubMed.
- L. Wan, J. Wade, F. Salerno, O. Arteaga, B. Laidlaw, X. Wang, T. Penfold, M. J. Fuchter and A. J. Campbell, ACS Nano, 2019, 13, 8099–8105 CrossRef CAS PubMed.
- G. Albano, G. Pescitelli and L. Di Bari, ChemNanoMat, 2022, 8, e202200219 CrossRef CAS.
- L. Wan, Y. Liu, M. J. Fuchter and B. Yan, Nat. Photon., 2023, 17, 193–199 CrossRef CAS.
- X. Shang, L. Wan, L. Wang, F. Gao and H. Li, J. Mater. Chem. C, 2022, 10, 2400–2410 RSC.
- Z. Zhan, J. An, Y. Wei, V. T. Tran and H. Du, Nanoscale, 2017, 9, 965–993 RSC.
- Y. Yang, L. Liu and Z. Wei, Acc. Mater. Res., 2024, 5, 329–346 CrossRef CAS.
- M. Zhang, Q. Guo, Z. Li, Y. Zhou, S. Zhao, Z. Tong, Y. Wang, G. Li, S. Jin, M. Zhu, T. Zhuang and S.-H. Yu, Sci. Adv., 2023, 9, eadi9944 CrossRef CAS PubMed.
- Q. Wang, E. Plum, Q. Yang, X. Zhang, Q. Xu, Y. Xu, J. Han and W. Zhang, Light: Sci. Appl., 2018, 7, 25 CrossRef PubMed.
- R. Singh, K. N. Narayanan Unni, A. Solanki and Deepak, Opt. Mater., 2012, 34, 716–723 CrossRef.
- J. R. Brandt, X. Wang, Y. Yang, A. J. Campbell and M. J. Fuchter, J. Am. Chem. Soc., 2016, 138, 9743–9746 CrossRef CAS PubMed.
- L. Frédéric, A. Desmarchelier, R. Plais, L. Lavnevich, G. Muller, C. Villafuerte, G. Clavier, E. Quesnel, B. Racine, S. Meunier-Della-Gatta, J.-P. Dognon, P. Thuéry, J. Crassous, L. Favereau and G. Pieters, Adv. Funct. Mater., 2020, 30, 2004838 CrossRef PubMed.
- X. Shang, I. Song, G. Y. Jung, W. Choi, H. Ohtsu, J. H. Lee, J. Y. Koo, B. Liu, J. Ahn, M. Kawano, S. K. Kwak and J. H. Oh, Nat. Commun., 2018, 9, 3933 CrossRef PubMed.
- T. Kawasaki, M. Sato, S. Ishiguro, T. Saito, Y. Morishita, I. Sato, H. Nishino, Y. Inoue and K. Soai, J. Am. Chem. Soc., 2005, 127, 3274–3275 CrossRef CAS PubMed.
- N. Ghosh, M. F. G. Wood, S.-H. Li, R. D. Weisel, B. C. Wilson, R.-K. Li and I. A. Vitkin, J. Biophotonics, 2009, 2, 145–156 CrossRef CAS PubMed.
- R. Lu, Y. Li, H. Song and J. Jiang, Adv. Funct. Mater., 2025, 35, 2423770 CrossRef CAS.
- B. Kim, J. Ahn, K. Gao, X. Shang and J. H. Oh, ACS Nano, 2025, 19, 28478–28490 CrossRef CAS PubMed.
- Q. Liu, Q. Wei, H. Ren, L. Zhou, Y. Zhou, P. Wang, C. Wang, J. Yin and M. Li, Nat. Commun., 2023, 14, 7179 CrossRef CAS PubMed.
- M. J. Han, M. Kim and V. V. Tsukruk, ACS Nano, 2022, 16, 13684–13694 CrossRef CAS PubMed.
- D. Chen, R. He, H. Cai, X. Liu and W. Gao, ACS Nano, 2021, 15, 1912–1916 CrossRef CAS.
- S. Dan, S. Paramanik and A. J. Pal, ACS Nano, 2024, 18, 14457–14468 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |