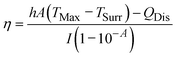Multifunctional nanomaterials for dental photo-theranostics
Yujia
Shi
 a,
Xiaolin
Sun
a,
Xiaolin
Sun
 a,
Jiao
Fang
a,
Jiao
Fang
 a,
Chunyan
Li
a,
Chunyan
Li
 b,
Biao
Dong
b,
Biao
Dong
 c,
Manlin
Qi
c,
Manlin
Qi
 *a and
Lin
Wang
*a and
Lin
Wang
 *a
*a
aDepartment of Oral Implantology, Jilin Provincial Key Laboratory of Sciences and Technology for Stomatology Nanoengineering, School and Hospital of Stomatology, Jilin University, Changchun 130021, China
bDepartment of Prosthodontics, Jilin Provincial Key Laboratory of Tooth Development and Bone Remodelling, School and Hospital of Stomatology, Jilin University, Changchun 130021, China
cState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, China
First published on 14th November 2025
Abstract
Recent studies highlight the significant promise of nanomaterial-mediated diagnostic and therapeutic strategies for managing dental diseases. Among these, photo-responsive technologies have emerged as non-invasive, targeted, and spatiotemporally controllable modalities capable of delivering efficient and site-specific interventions. The oral cavity's inherent accessibility to external light sources makes it an ideal environment for light-triggered therapeutic strategies, enabling precise control over treatment activation while minimising systemic exposure and side effects. When activated by specific wavelengths of light, photo-responsive nanomaterials trigger physicochemical reactions that can modulate the local microenvironment or visualise early-stage lesions with high precision. Advances in materials science and nanotechnology have enabled the rational design of diverse light-activated nanomaterials, including inorganic nanoparticles, organic photosensitisers, and hybrid nanocomposites, tailored for dental applications. This review provides a comprehensive overview of representative light-responsive nanomaterials with therapeutic and/or diagnostic functionality in the oral context. We investigate their mechanisms of action under light stimulation, analyse their performance relative to conventional and non-photoactivated treatments, and appraise their translational potential. In addition, we explore the current challenges facing the clinical implementation of light-activated nanomedicine in dentistry, including biocompatibility, penetration depth, and complex oral microenvironments. Finally, we offer recommendations on the design principles and treatment strategies for next-generation photo-theranostic platforms, aiming to inspire innovative approaches to dental disease management by integrating nanotechnology and photomedicine.
Key learning points(1) Introduction to the advantages of photo-theranostics and the distinct oral ecosystem.(2) The diverse light-activated nanomaterials are employed in dental therapy. (3) A systematic review of other light-based diagnostics in dentistry. (4) Key design factors for nanomaterials aimed at oral microenvironments. (5) The current challenges and future perspectives in this field. |
1. Introduction
Phototheranostics trace their origins to foundational discoveries in the late 19th century.1–3 In 1896, Niels Ryberg Finsen demonstrated the therapeutic potential of red and blue light for treating cutaneous tuberculosis, establishing a basis for modern light-based medical interventions. Subsequent advancements in laser technology and optical engineering enabled precise control over key light parameters, including wavelength, intensity, and exposure duration, thereby facilitating non-invasive, spatially controlled, and multifunctional biomedical applications.4 These developments are underpinned by photophysical mechanisms such as light-induced electron excitation, intersystem crossing (ISC), and thermal dissipation, collectively enabling energy conversion processes essential for imaging and therapy.5,6 Based on this foundation, phototheranostics have evolved over the past decade into a central pillar of precision medicine by uniting real-time diagnostics with targeted therapeutic modalities in a single, light-activated platform. In this review, the term “theranostics” is used broadly to encompass diagnostic and/or therapeutic applications, unless otherwise specified.Nanomaterials have emerged as key enablers in phototheranostics owing to their tuneable physicochemical properties and versatile biological functionalities. In contrast to conventional therapeutic agents, nanostructures exhibit unique capabilities such as targeted surface functionalisation, controlled drug release via porous architectures, and size-dependent tissue penetration, all of which are particularly advantageous for precision medicine. Photo-responsive nanoparticles (NPs) can convert light energy into reactive oxygen species (ROS), thermal energy, electrons, or fluorescent signals, facilitating a range of applications including photodynamic therapy (PDT), photothermal therapy (PTT), photoelectric conversion, and diagnostic imaging. Representative materials include noble metal NPs (e.g., gold and silver), inorganic semiconductors, metal–organic frameworks (MOFs), and hybrid organic–inorganic nanocomposites.7–11 Through rational design of energy dissipation pathways and modulation of electronic structures, these nanomaterials can be engineered to enhance ROS production, optimise photothermal conversion efficiency, and exploit phenomena such as aggregation-induced emission (AIE).12 Such strategies not only elevate theranostic efficacy but also offer additional benefits, including minimised drug resistance, broad-spectrum antimicrobial activity, and rapid, high-sensitivity diagnostics, positioning photo-responsive nanoplatforms as promising alternatives to conventional treatment modalities.
Building on its success in fields such as oncology and inflammatory disease management, nanomedicine has increasingly expanded into dentistry, where photo-induced diagnostic and therapeutic modalities are being specifically tailored to address oral health challenges. In this context, phototheranostics has progressed beyond its initial antimicrobial applications to encompass a broader range of functions, including disease detection, tooth whitening, and light-activated restorative materials. These strategies have been employed to treat diverse oral pathologies, ranging from microbial infections and hypersensitivity reactions to hard and soft tissue defects, with particular emphasis on biofilm-associated infections as a key theranostic target.13–16 Oral biofilms are complex microbial communities that contribute not only to local diseases such as dental caries, endodontic infections, and periodontitis. Still, they are also implicated in systemic conditions including cardiovascular disease, diabetes, and immune dysregulation. The structural, metabolic, and compositional diversity of these microbial consortia makes them highly resistant to eradication using conventional mono-mechanistic treatments.17–19 Current clinical interventions, which primarily involve antibiotic administration and mechanical debridement, often yield suboptimal outcomes due to limited drug penetration, rising antimicrobial resistance, and the anatomical complexity of the oral environment.20–25 Inaccessible microstructures, such as root canals, periodontal pockets, and alveolar bone lacunae, further complicate therapeutic delivery and efficacy. These deep-seated, structurally complex occult lesions further hinder the early diagnostic detection of diseases. These challenges underscore the urgent need for advanced, multifunctional nanotherapeutic platforms capable of overcoming physical and biological barriers to achieve precise, localised, and effective diagnosis and treatment within the oral cavity.
To overcome the unique challenges posed by the oral microenvironment, nanomaterial-based phototheranostics provides highly adaptable and targeted solutions. By fine-tuning structural, chemical, and surface properties, researchers have engineered nanoplatforms with enhanced antimicrobial efficacy, stimuli-responsive drug delivery, selective interactions with oral metabolites, and the mechanical flexibility needed to penetrate complex and confined oral niches. Multifunctional nanocomposites capable of integrating multiple therapeutic modalities, such as PDT and photothermal PTT, offer synergistic treatment effects that are particularly effective against biofilm-associated and treatment-resistant infections. Simultaneously, the integration of oral wearable devices with diagnostic strategies leveraging intrinsic oral gases or biomolecules has opened novel avenues for disease detection. Notably, near-infrared (NIR)-responsive nanomaterials have been developed to achieve deeper tissue penetration and localised activation. These systems leverage rational design principles, including plasmonic tuning, photosensitiser optimisation, and bandgap engineering to tailor light absorption and conversion properties, thereby enhancing therapeutic performance in otherwise inaccessible oral sites.
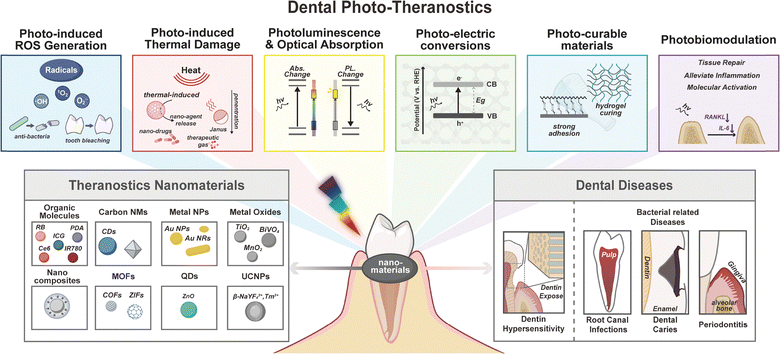
This review provides a comprehensive overview of recent advances in phototheranostics for dental diseases, as illustrated in Fig. 1. We begin by introducing the fundamental photonic principles, core concepts in materials science, and key aspects of oral biology that underpin the development of light-activated nanomedicines. Subsequently, we systematically classify phototheranostic strategies based on material composition and structural characteristics, with a focus on their biological applications. Emphasis is placed on the photophysical and photochemical properties of these materials, as well as critical design considerations for their performance within the unique oral microenvironment. We also compare light-triggered systems with alternative stimuli-responsive nanotechnologies, highlighting their respective advantages, limitations, and potential complementarities. Finally, we outline the current challenges and future directions for the clinical translation of oral phototheranostics. By integrating insights from nanotechnology, photomedicine, and dental science, this review aims to establish a conceptual and practical framework to guide future research and advance clinical applications of phototheranostic nanomaterials in oral healthcare.
2. Fundamental mechanisms and biological interfaces
Over the past several decades, light-activated nanotechnologies have attracted significant attention across diverse disciplines, ranging from biomedicine to energy conversion. These systems exploit light as a spatiotemporally controllable, non-invasive, and renewable external stimulus, enabling precise modulation of targeted biological and chemical processes. In dentistry, the anatomical accessibility of oral tissues, particularly the thin gingiva and mucosa, facilitates direct light irradiation of pathological sites, often resulting in more visible and immediate therapeutic effects compared to deeply seated lesions such as solid tumours. Furthermore, nanomaterials can be locally administered via the tooth surface, gingival sulcus, or periodontal pockets, circumventing challenges associated with systemic drug delivery, including metabolic degradation, off-target effects, and subtherapeutic concentrations at the target site. This localised approach enhances treatment precision and clinical feasibility, offering a more streamlined and patient-friendly therapeutic pathway. In this section, we systematically examine: (1) the fundamental principles of light relevant to phototheranostics; (2) the physicochemical mechanisms governing photo-induced therapeutic efficacy; (3) the essential material properties required for effective photoactivation; and (4) the oral-biological considerations necessary for the successful implementation of these technologies in dental theranostic applications.2.1 The light-relevant background
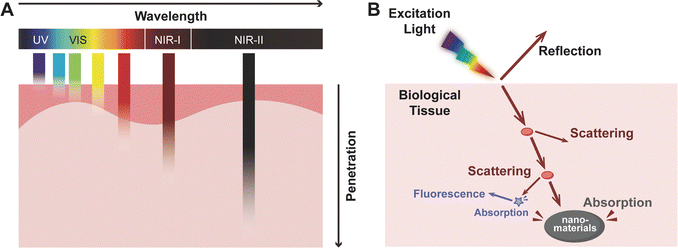
In therapeutic applications, achieving high specificity and selectivity is paramount. Light-activated strategies offer a relatively safe and precisely controllable means for material delivery, as well as targeted cell ablation, thereby minimising off-target effects and enhancing therapeutic efficacy.26,27 The selection of appropriate light wavelengths is critical for both safety and efficacy; in particular, ultraviolet (UV) light below 400 nm is generally avoided due to its mutagenic potential and its ability to induce apoptosis through DNA damage. Blue light, initially employed in dermatology for the treatment of conditions such as psoriasis, has demonstrated the ability to stimulate tissue repair at low irradiation doses. In dentistry, blue light (typically 400–500 nm) is widely used in clinical settings for photopolymerization of resin-based restorative materials. However, concerns regarding potential retinal damage have led to ongoing debate about the broader therapeutic use of blue light wavelengths. By contrast, red and NIR light are well-established for both diagnostic and therapeutic purposes, including the promotion of wound healing and the modulation of inflammatory responses. These wavelengths enable deeper tissue penetration and are commonly employed in the treatment of internal lesions (Fig. 2A). Studies have shown that red and NIR light can penetrate biological tissues to depths of approximately 1–3 mm (in NIR-I, 700–900 nm) and 5–20 mm (in NIR-II, 1000–1700 nm), with deeper penetration achievable at higher intensities.28–30 However, light propagation in biological tissues is inherently complex due to significant scattering, absorption, and autofluorescence (Fig. 2B). Water, a major constituent of blood and soft tissue, exhibits strong absorption bands near 970 nm, 1200 nm, 1450 nm, and above 1800 nm. Excitation near these wavelengths can lead to unwanted thermal effects. Therefore, optimal excitation typically occurs in the so-called “biological transparency window” (650–1350 nm). In this region, tissue absorption is minimised through spectral avoidance of dominant chromophore bands, and scattering intensity decreases significantly with increasing wavelength according to fundamental light-tissue interaction principles, and autofluorescence is substantially reduced. As tissue autofluorescence declines sharply beyond 1300 nm, wavelengths such as 808 nm and 1064 nm, emitted by diode and Nd:YAG lasers, respectively, have become standard in biomedical applications, including those in dentistry. These wavelengths strike a favourable balance between penetration depth, minimal tissue heating, and low background interference, making them ideal for both diagnostic imaging and phototherapeutic interventions in oral tissues.Phototherapy has shown notable promise in biological regulation, leveraging specific light wavelengths to activate photo-reactions. For instance, pathogens such as Porphyromonas gingivalis (P. gingivalis, the key pathogen in periodontitis) accumulate endogenous porphyrins via haem metabolism, enabling bactericidal effects upon blue light activation. However, this intrinsic mechanism is ineffective against pathogens lacking endogenous photosensitisers, such as Streptococcus mutans (S. mutans, the key pathogen in dental caries).31 To address this critical gap, the integration of exogenous photosensitising nanomaterials emerges as a transformative strategy. Engineered nanoplatforms not only serve as precision carriers for photosensitisers but also enable spatiotemporally controlled activation and multifunctional synergy, enhancing light energy utilisation, improving biofilm penetration, and minimising off-target cytotoxicity. By bridging the gap between photonic energy delivery and microbial specificity, advanced nanomaterials are poised to redefine phototherapeutic paradigms in oral healthcare, driving innovations toward high-efficiency, precision-targeted antimicrobial interventions.
2.2 The physicochemical mechanisms of nanomaterials
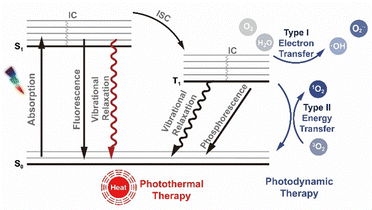
Conversional photosensitisers faced limitations of poor water solubility, aggregation, non-specific biodistribution and low photostability, thereby calling for the development of nanoagents, such as novel photosensitive molecules, photosensitiser-loaded nanomaterials and nanomaterials with photoconversion abilities. Among all, organic molecules and inorganic semiconductor materials have demonstrated significant potential in phototherapeutic applications owing to their tuneable photophysical and photochemical properties, excellent biocompatibility, high biosafety, and ease of processing and functionalisation. These attributes position them as attractive candidates for developing nanomaterial-based photo-theranostic platforms across a broad range of biomedical applications. This subsection explores the photophysical processes of optical agents for further molecular design and nanoengineering approaches. By precisely manipulating the structural and electronic characteristics of nanomaterials, researchers can optimise the conversion of absorbed light energy into ROS for PDT or thermal energy for PTT.32,33 These photo-induced mechanisms are central to achieving effective and targeted treatment of diseases. It is important to note that for certain organic–inorganic composite materials, the underlying physicochemical mechanisms can be highly complex and require case-specific analysis. For example, hybrid systems that integrate organic photosensitisers within MOFs or nanocages to enhance light-harvesting capabilities necessitate special consideration of their photophysical mechanisms.Particularly, during triplet-state relaxation, energy transfer to molecular oxygen generates cytotoxic ROS for PDT applications through two primary mechanisms. The type I pathway involves electron transfer between photosensitisers and molecular oxygen, generating superoxide anions (O2˙−) that undergo aqueous protonation to form ˙OH and secondary ROS via radical chain reactions. These reactive species predominantly inflict localised oxidative damage proximal to photosensitiser localisation. In contrast, the type II pathway directly produces 1O2 through energy transfer, enabling mitochondrial targeting and subsequent initiation of apoptosis. Critically, the therapeutic outcomes depend on ROS spatiotemporal dynamics. The half-life of PDT-generated ROS exhibits medium-dependent variability: 1O2 persists for 15–30 µs with diffusion ranges of 100–300 nm, while ˙OH radicals remain stable for nanoseconds, limiting their diffusion to <50 nm.35–38 This disparity highlights the importance of strategically modulating competing relaxation pathways (radiative, non-radiative, and triplet-state) to optimise energy partitioning for specific therapeutic objectives.
Despite these mechanistic advantages, conventional organic photosensitisers suffer from the aggregation-caused quenching (ACQ) effects due to π–π stacking or excimer formation in aggregated states. This phenomenon significantly diminishes both fluorescence emission and ROS production efficiency. To address these limitations, AIE luminogens (AIEgens) have been developed. Featuring twisted multi-rotor molecular architectures, AIE-active compounds suppress non-radiative decay pathways through restricted intramolecular rotation, simultaneously enhancing fluorescence quantum yield and ROS generation in aggregated states.39 Importantly, the photophysical behaviour of these materials adheres to the same excited-state relaxation principles governing conventional small-molecule photosensitisers, while effectively overcoming ACQ-related drawbacks.
Beyond their mechanistic advantages, small-molecule photosensitisers have been widely applied in both preclinical and clinical contexts. For instance, porphyrin derivatives such as photofrin have been clinically approved for PDT in oncology, while methylene blue and toluidine blue O have been employed in antimicrobial PDT for periodontal and endodontic infections. Similarly, indocyanine green (ICG), a cyanine dye, has been extensively used for NIR theranostics, despite its inherent photo-instability. In the dental field, small-molecule dyes are increasingly applied in caries detection, biofilm disruption, and adjunctive periodontal therapy, underscoring their translational potential. These representative cases highlight the importance of linking the photophysical mechanisms of small-molecule dyes to tangible therapeutic and diagnostic outcomes.
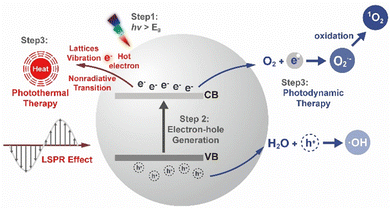
In parallel, the photothermal effect in semiconductor nanostructures occurs through multiple mechanisms. Primarily, non-radiative recombination of photoexcited electron–hole pairs leads to the generation of lattice vibrations (phonons) that convert photon energy into heat. Furthermore, interfacial energy gradients produce high-energy “hot electrons” that dissipate thermal energy via lattice collisions. Additionally, composite structures incorporating metallic components exhibit enhanced photothermal conversion efficiency through localised surface plasmon resonance (LSPR) effects.
Notably, many semiconductor materials are capable of concurrently mediating both photodynamic and photothermal effects, enabling synergistic therapeutic strategies. For instance, photothermally induced thermal gradients may facilitate electron transitions within photosensitisers, thereby amplifying ROS production. Concurrently, structural defects (e.g., anion/cation vacancies) in engineered nanomaterials act as catalytic centres, facilitating surface-mediated ROS production. This multimodal energy conversion mechanism, integrating thermal activation, electronic transitions, and defect catalysis, significantly improves therapeutic outcomes through complementary action pathways.
Nevertheless, practical implementation faces three key challenges. First, prolonged illumination induces phase transitions in metal oxide semiconductors, compromising structural integrity. Second, nanoparticle agglomeration in physiological media reduces bioavailability and therapeutic consistency. Third, achieving optimal balance between light absorption efficiency, charge separation dynamics, and thermal dissipation in oral environments remains technically demanding. Addressing these limitations requires rational design of heterojunction architectures with stabilised interfaces and controlled defect engineering to advance clinical translation of semiconductor-based photo-nanotherapeutics.
2.3 Essential characteristics of nanomaterials for photo-theranostics
Building on the mechanistic insights discussed above, the development of effective photo-theranostic agents hinges not only on light-induced processes but also on a set of essential physicochemical characteristics that govern their clinical applicability. The cornerstone of photo-theranostics is the photosensitive nanomaterials, functional agents capable of strong wavelength-specific absorption and efficient photochemical energy conversion.36,41,42First of all, the biosafety evaluation of these nanomaterials must be rigorously established as a fundamental prerequisite for clinical translation. This requires systematic preclinical characterisation, encompassing thorough analyses of clearance kinetics, biodistribution patterns, and long-term biocompatibility to reconcile therapeutic efficacy with patient safety. To circumvent unintended biosystem responses, such as macrophage-mediated phagocytosis, strategic material engineering should focus on modulating particle dimensions, surface charge distribution, and the implementation of biomimetic coatings or biodegradable matrices. Furthermore, in the physiologically complex oral environment, particular emphasis must be placed on assessing nanomaterial interactions with mineralised dental tissues and the dynamic periodontal interface, ensuring minimal iatrogenic compromise while maintaining therapeutic functionality.
Secondly, the operational longevity of light-activated nanomaterials in oral applications critically depends not only on their photostability under irradiation but also on their product shelf life during storage and transportation. For instance, many organic photosensitisers and nanoparticle formulations are prone to aggregation, hydrolysis, or oxidation when stored over time, which can significantly compromise their clinical utility before application. Therefore, strategies such as lyophilisation, encapsulation in protective carriers, or incorporation of stabilising excipients should be considered in parallel with photostability and thermal resistance to ensure both pre-use and in-use longevity. This requirement extends to PTT agents, where thermal stability under cyclical heating–cooling regimes is equally paramount. Notably, existing commercially available photosensitisers such as ICG inherently suffer from rapid photodegradation, severely limiting their clinical utility. To address these limitations, structural modifications, including surface functionalisation or encapsulation within protective matrices, combined with “afterglow” strategies, could significantly reduce light exposure duration while maintaining therapeutic efficacy.
Concurrently, the theranostic efficacy of these nanomaterials necessitates rigorous quantification to establish clinically relevant performance benchmarks. Here, we systematically evaluate analytical and computational methodologies for assessing nanomaterial performance. Our analysis prioritises ROS generation and photothermal conversion efficiency, two pivotal determinants of photoactivated therapeutic efficacy.
ROS production is typically quantified via fluorometric assays employing probe-specific detection: dichlorodihydrofluorescein diacetate (DCFH-DA) for total ROS, singlet oxygen sensor green (SOSG), and 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) for 1O2. Specific probes include hydroxyphenyl fluorescein (HPE) for ˙OH, dihydroethidium (DHE) for O2˙−, and anthracene moieties (Si-DMA) for 1O2. Complementary techniques include electron paramagnetic resonance spectroscopy and enzymatic activity assays. Quantification is achieved through absorbance/fluorescence calibration curves or normalisation against reference photosensitisers with established ROS quantum yields.
For photothermal conversion efficiency (η), the standardised methodology involves analysing temperature decay kinetics post-irradiation, as described by:30
These performance metrics provide the foundation for rational material optimisation, guiding the development of next-generation nanoplatforms with enhanced precision and therapeutic efficacy.
2.4 The oral biology
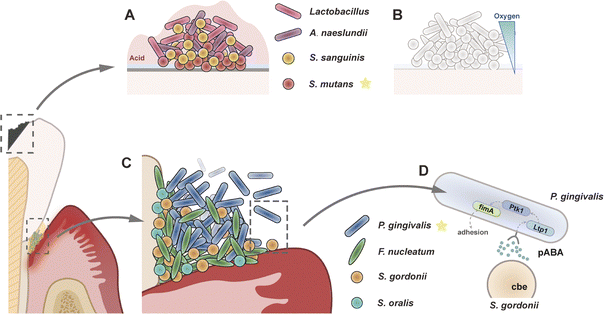
The development of oral nanomedicine for infectious dental diseases, particularly periodontitis and caries, has seen significant advances through the engineering of microenvironment-responsive nanomaterials.43,44 Effective design of these therapeutic agents demands a multiscale understanding of oral tissue complexity. Anatomically, the oral cavity encompasses mineralised structures (teeth, alveolar bone) and soft tissues (mucosa, gingiva), all continuously interfaced with biological fluids like saliva and gingival fluid. At cellular and molecular scales, these microenvironments host diverse populations of microbial communities, host cells, collagen fibres, and biomineralised phases like hydroxyapatite. Therefore, a detailed understanding of these biological components is essential for the rational design of responsive nanomaterials that can effectively operate within such dynamic oral niches. This section explores the key features of oral tissues and their associated microenvironments.Oral biofilms demonstrate site-specific structural and functional differentiation. Early colonisers such as Streptococcus gordonii (S. gordonii), Streptococcus oralis (S. oralis), Streptococcus sanguinis (S. sanguinis) and Actinomyces naeslundii (A. naeslundii) lay the foundation of dental biofilms and shape subsequent microbial succession. As colonisation progresses, intermediate “bridging” taxa—notably Fusobacterium nucleatum (F. nucleatum) and Veillonella spp., together with Capnocytophaga spp.—promote co-aggregation and oxygen depletion, facilitating recruitment of late, obligate anaerobes. Representative late colonisers include P. gingivalis, Tannerella forsythia, Treponema denticola, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans. Moreover, tooth-associated oral biofilms can be categorised into supragingival biofilms (formed on exposed enamel surfaces, as shown in Fig. 5A) and subgingival biofilms (located below the gumline and within the periodontal pocket or sulci). The subgingival environment is nutrient-rich and characterised by anaerobic conditions within the periodontal pockets (Fig. 5B).46 The bacteria in this region are rarely subjected to saliva washing or mastication forces, and predominantly comprise anaerobes and short bacilli. This environmental specialisation necessitates complex cultivation methodologies, particularly for anaerobic species.
Multiple environmental factors govern the survival and growth of these bacterial communities. Bacterial survival relates to physicochemical factors, host innate characteristics, and bacterial attributes.47 Physicochemical factors include temperature (approximately 37 °C), oxygen tension, pH, and nutrient availability. Most bacteria require oxygen or carbon dioxide for growth, with energy substrates primarily derived from glucose produced by biological oxidation. However, certain anaerobic species utilise alternative electron acceptors such as lactate or nitrate. As plaque accumulates and matures, oxygen diffusion is hindered, creating deeper anaerobic zones that facilitate distinct metabolic profiles. Supragingival communities metabolise dietary carbohydrates, generating acidic byproducts (pH 4.5–5.5) that drive enamel demineralisation. Conversely, subgingival biofilms utilise host-derived nutrients (e.g., hemin) under alkaline conditions (pH 7.4–7.8), with plaque maturation creating oxygen-deprived zones favouring anaerobic respiration via lactate/nitrate reduction.
Ecological dysbiosis, defined as the disruption of microbial homeostasis, is central to the pathogenesis of oral diseases. Homeostatic disruption enables pathogen overgrowth, exemplified by S. mutans (tooth decay) and P. gingivalis (periodontal tissue destruction and immune dysregulation).48–50 Mechanistically, S. mutans generates acidic microenvironments, causing demineralisation of dental tissues. Meanwhile, P. gingivalis expresses a range of virulence factors, including proteolytic enzymes (gingipains) and adhesins (e.g., HagB), enabling erythrocyte lysis and haem acquisition.51 These virulence factors participate in host tissue degradation and immune modulation, necessitating pathogen-specific therapeutic targeting. Additional taxa are also tightly linked to disease initiation and progression. As dysbiosis deepens and acidogenic selection intensifies, strongly acidogenic taxa. In cariogenic plaque, acidogenic/aciduric taxa such as S. mutans and Streptococcus sobrinus (S. sobrinus), Lactobacillus spp. frequently dominates in the mature biofilm, drives enamel demineralisation and accelerates caries progression. By contrast, within the endodontic niche, Enterococcus faecalis (E. faecalis) emerges as a major pathogen in persistent root-canal infections, distinguished by exceptional tolerance to high pH and resistance to antimicrobial agents.
Beyond individual bacterial characteristics, the formation of complex bacterial communities presents additional therapeutic challenges. Coaggregation also represents a pivotal step in biofilm formation, facilitating colonisation and exacerbating infection by pathogenic bacteria within the oral cavity. The extracellular polymeric substances (EPS) that form the biofilm matrix act as a physical barrier, limiting the penetration of antimicrobial agents and shielding resident bacteria from host immune defences.
Conventional antibiotic therapies often fail to penetrate these dense matrices, and drug resistance further compromises efficacy. Some bacteria serve as bridging species during biofilm formation, constructing complex multispecies aggregates. For example, F. nucleatum coaggregates with bacteria from over 11 genera, including P. gingivalis and S. gordonii, facilitating the development of complex multispecies biofilms (Fig. 5C). This architectural complexity necessitates multifunctional interventions. F. nucleatum utilises distinct adhesins to coordinate biofilm assembly via two main mechanisms. During primary colonisation, RadD binds streptococcal adhesin SpaP, while Aid1 and CmpA participate in this attachment phase. The secondary stage involves RadD collaborating with Fap2 and FomA to recruit late-arriving pathogens such as P. gingivalis and additional bacterial colonisers. Likewise, the S. gordonii, the initial colonising bacterium, binds to the saliva components through its surface adhesion proteins, such as SspA/B. And the P. gingivalis relies on the colonisation layer formed by S. gordonii to establish a symbiotic biofilm, with its fimbriae recognising and binding to SspA/B on the surface of S. gordonii.
At the molecular level, coaggregation is regulated by a specific signalling pathway. One example involves S. gordonii-secreted Cbe enzyme, which detects 4-aminobenzoate (pABA), a metabolic product of S. gordonii. This interaction activates the tyrosine phosphatase Ltp1 in P. gingivalis, which in turn upregulates fimA expression, enhancing fimbriae production and promoting biofilm maturation (Fig. 5D). Such interspecies molecular dialogues represent promising targets for disrupting pathogenic biofilm development.
Altogether, the structural, metabolic, and signalling complexity of oral biofilms necessitates multifunctional nanomaterials that can target microbial virulence, penetrate protective EPS matrices, and adapt to the unique conditions within supragingival and subgingival environments.
Oral hard tissues, including enamel, dentin, and alveolar bone, exhibit complex hierarchical architectures with high mineralisation. Dental enamel is primarily composed of hydroxyapatite crystals, Ca10(PO4)6(OH)2, secreted by ameloblasts, containing less than 1% organic components such as residual proteins and matrix metalloproteinase-20 (MMP-20). However, during enamel development, stoichiometric perfection of hydroxyapatite is not always achieved, which may lead to structural defects and increased vulnerability to conditions such as enamel hypomineralisation or dental caries. Preventive strategies, including fluoride treatment and agents that promote remineralisation, have been reported to stabilise crystal formation and reduce such risks. Complementary measures, such as biofilm pH modulation (e.g., arginine) and microstructural sealing/protection (resin infiltration or sealants), reduce acid challenge and shield porous enamel.
In contrast, dentin harbours 40-fold higher organic content in a deeper layer, primarily collagen fibrils, rendering its antimicrobial treatment more challenging than that of enamel caries. The dentinal tubules, with diameters ranging from 0.5 to 2.5 µm, traverse the dentine from the dentin-enamel junction to the pulp chamber, forming a conduit for external stimuli such as acids or bacteria. These tubules contain odontoblastic processes and sparse nerve fibres, contributing to dentine sensitivity when exposed. To achieve effective intratubular delivery, nanomaterials must be tailored with diameters smaller than the tubule lumen. Surface erosion caused by acid exposure or mechanical abrasion can lead to the unsealing of dentinal tubules, permitting external stimuli to trigger neural activation and hypersensitivity, commonly referred to as dentin hypersensitivity.
Beyond structural vulnerability, oral hard tissues exhibit limited regenerative capacity due to intrinsic biological constraints. Enamel self-repair mechanisms are irreversibly lost following the apoptosis of ameloblasts after tooth eruption, as these cells lack regenerative potential. Dentin regeneration faces dual limitations: insufficient odontoblast populations and fibrotic transformation of the pulp microenvironment following injury, which impede reparative dentinogenesis.
In addition to dentin and enamel, alveolar bone repair is also significantly constrained under pathological conditions, such as infection or metabolic dysregulation, and is compromised by disrupted osteoblast–osteoclast equilibrium, impaired vascular regeneration, and abnormal extracellular matrix (ECM) mineralisation. This multifaceted failure is exemplified in periodontitis-mediated bone loss, where lipopolysaccharide (LPS)-activated TLR4 signalling initiates a self-perpetuating inflammatory cascade. Sustained M1 macrophage polarisation drives excessive release of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) and matrix metalloproteinases (MMP-8/9), collectively amplifying osteoclastic activity and hindering physiological bone remodelling. These factors exacerbate bone resorption through various pathways, making the physiological regeneration of periodontal hard tissues challenging.
Finally, the biochemical properties and dynamic behaviour of oral biological fluids critically influence the stability, distribution, and therapeutic performance of nanomaterials. Saliva, a Ca2+-rich fluid containing mucins, antimicrobial proteins (lactoferrin), and microbial flora, may induce nanoparticle aggregation or functional deactivation. Similarly, gingival crevicular fluid, enriched with neutrophils and host defence peptides, can neutralise periodontal pathogens but also compromise nanocarrier stability. Neutrophil extracellular traps (NETs), DNA–protein complexes released during NETosis, accumulate in periodontal and periapical lesions.52,53 Composed of neutrophil elastase and histones, NETs exhibit dual roles: antimicrobial activity via oxidative damage and promotion of pathological calcification and sustained inflammation. In chronic periodontitis, excessive NET formation correlates with impaired tissue regeneration and calculus mineralisation, highlighting their therapeutic relevance. In addition to the fluids, the oral cavity contains numerous metabolically generated gaseous molecules. Periodontal pockets contain substantial volatile sulphur compounds (VSCs), particularly at sites of oral pathology such as caries and periodontitis, where anaerobic bacteria metabolise sulphur-containing amino acids, including methionine and cysteine, releasing VSCs such as H2S and methanethiol. These volatile substances are considered primary contributors to halitosis, while potentially interacting with theranostic nanomaterials.
3. Design of nanomaterials for photo-induced applications
Given the multifaceted challenges posed by oral biological interfaces, particularly those involving hard tissues and fluidic environments, there is a pressing need for precision-targeted, stimuli-responsive antimicrobial strategies. Conventional oral antibiotics, exemplified by β-lactams, exert bactericidal activity by inhibiting peptidoglycan cross-linking. However, their clinical utility is constrained by multiple pharmacological barriers, including susceptibility to gastric acid degradation, short plasma half-lives, and risks of severe hypersensitivity reactions. Topical antimicrobial formulations encounter distinct challenges: rapid clearance by salivary flow compromises retention efficacy, while poor penetration into periodontal pockets limits therapeutic reach. These limitations are compounded by additional issues such as tooth discolouration and decreased patient compliance due to frequent dosing requirements. Although tetracyclines possess broad-spectrum antimicrobial activity, their effectiveness is reduced by insufficient biofilm penetration and pH-sensitive instability in acidic microenvironments. More critically, the non-selective nature of conventional antimicrobials disrupts oral microbiome homeostasis, creating dysbiosis conditions that promote disease recurrence and drive antimicrobial resistance through selective evolutionary pressure.In light of these limitations, photo-induced antimicrobial strategies have garnered increasing interest, offering spatially and temporally controlled activation that addresses the need for precision targeting within complex oral environments. Advanced nanomaterials address conventional therapeutic shortcomings through the synergistic integration of non-structural optimisation, surface engineering, and photophysical tailoring. By precisely modulating optical characteristics, including absorption spectra, exciton dynamics, and quantum yield, these systems enable spatiotemporally controlled therapeutic actions and diagnostic capabilities.
The following section systematically examines photo-excitable nanomaterials based on their functional properties, including ROS generation, photothermal conversion, photoelectric transduction, photobiomodulation effects, photo-curable kinetics, and optical properties relevant for diagnostics, and evaluates their applications in oral healthcare management.
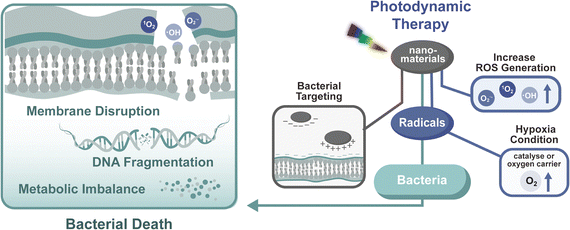
3.1 Photo-induced reactive oxygen species (ROS) generation
Among the spectrum of light-activated antimicrobial mechanisms, ROS generation has emerged as a primary modality, offering a compelling alternative to conventional therapies. In particular, it addresses the therapeutic limitations outlined above by enabling spatiotemporal control, reduced systemic toxicity, and enhanced efficacy in complex oral environments. Antimicrobial photodynamic therapy (aPDT) harnesses ROS produced by light-activated nanomaterials to induce broad-spectrum bactericidal effects with minimal side effects. In contrast to traditional antibiotics, which face challenges such as bacterial resistance, poor biofilm penetration, and systemic adverse effects, aPDT enables microbial eradication by compromising membrane integrity, inflicting oxidative damage to nucleic acids, and interrupting essential metabolic pathways (Fig. 6).The oral cavity's distinctive microenvironment, characterised by moisture, microbial diversity, and anaerobic conditions, imposes specific design requirements for aPDT systems. Effective oral nanotherapeutics must demonstrate pH tolerance, hypoxic functionality, and optimal biocompatibility. Notably, contemporary research on nanomaterials for combating oral pathogens has been predominantly focused on P. gingivalis, with comparatively fewer studies addressing other clinically important species such as S. gordonii and S. oralis, which require further research.54,55
Quantitative performance of light-responsive strategies against oral biofilms. Across recent oral-relevant models, aPDT typically achieves approximately 1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reductions in viable counts depending on the target organism, photosensitiser, formulation and irradiation parameters. For reader orientation, 1, 2, 3 and 4
log10 reductions in viable counts depending on the target organism, photosensitiser, formulation and irradiation parameters. For reader orientation, 1, 2, 3 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 correspond to ∼90%, 99%, 99.9% and 99.99% reductions, respectively. Crucially, photodynamic efficacy depends on ROS quantum yield and lifetime, necessitating targeted pathogen proximity through membrane-anchoring modifications, surface charge optimisation, and hypoxia condition management. Enhanced ROS generation can be achieved through strategic manipulation of ISC processes and spin–orbit coupling (SOC) effects. Furthermore, synergistic antimicrobial effects may arise from combining exogenous ROS production with endogenous oxidative stress pathways in biological systems. Subsequent discussion explores practical implementations of photoinduced ROS generation strategies. In this section, we discuss the ROS-generating nanomaterials through the utilisation of excited-state engineering, and their applications in dental bactericidal and bleaching.
log10 correspond to ∼90%, 99%, 99.9% and 99.99% reductions, respectively. Crucially, photodynamic efficacy depends on ROS quantum yield and lifetime, necessitating targeted pathogen proximity through membrane-anchoring modifications, surface charge optimisation, and hypoxia condition management. Enhanced ROS generation can be achieved through strategic manipulation of ISC processes and spin–orbit coupling (SOC) effects. Furthermore, synergistic antimicrobial effects may arise from combining exogenous ROS production with endogenous oxidative stress pathways in biological systems. Subsequent discussion explores practical implementations of photoinduced ROS generation strategies. In this section, we discuss the ROS-generating nanomaterials through the utilisation of excited-state engineering, and their applications in dental bactericidal and bleaching.
Moreover, the CeO2@Ce6 nanocomposite functions through 630 nm red light excitation of Ce6, generating ROS with bactericidal effects.58 This mechanism effectively disrupts bacterial biofilms, including both mono-species and multi-species biofilms, and inhibits the metabolism and virulence factor expression of periodontal pathogens, such as P. gingivalis and F. nucleatum. Simultaneously, the antioxidant enzyme-mimetic activity of CeO2 facilitates the elimination of residual ROS, continuing to scavenge these species post-irradiation, thereby preventing excessive oxidative stress that could otherwise trigger inflammatory responses.
Apart from overcoming the inherent limitations of photosensitisers, refining aPDT performance through bacterial-targeted material delivery and enhanced ROS generation achieves superior therapeutic outcomes. Among all, the selection of light wavelength ranges, irradiation duration, light intensity, and material dosage constitutes critical parameters that must be considered when designing aPDT strategy. To optimise aPDT efficacy, the interaction distance between the photosensitising materials and bacterial cells must be considered. Given the limited diffusion distance of ROS, spatial proximity between the site of ROS generation and bacteria is a crucial factor.
The most structural design exploits the negatively charged bacterial membrane by engineering positively charged materials to enhance bacterial targeting specificity. However, given that bacteria in different infectious diseases possess unique structures and metabolic products, designing materials with surface chemistry that specifically targets certain bacterial structures or products can also facilitate the accumulation of materials around bacteria. MPP-Ce6 NPs can generate PDT effects under 660 nm light source excitation, efficiently eliminating S. mutans, S. sobrinus, and S. sanguinis (Fig. 7A).59 Due to the acidic environment of dental caries, this material achieves responsive targeted delivery of Ce6 through designed amphiphilic polymer MPP decomposition under acidic conditions, increasing local photosensitiser concentration and enhancing ROS yield. The nanoscale size (54 nm) and positive surface charge (+19.6 mV) of MPP-Ce6 facilitate adsorption to negatively charged bacterial biofilms, improving penetration compared to Ce6 alone. Similarly, the Ce6@PDN-SAP nanocomposite was also designed by modifying Ce6 with salivary acquired peptide (SAP) and enhancing bacterial targeting through wet adhesion, which mimics salivary acquired pellicle and binds to calcium ions on tooth surfaces via phosphorylated serine residues.60 When activated by 665 nm laser irradiation, Ce6 generates ROS, inhibiting cariogenic bacteria and preventing dental caries.
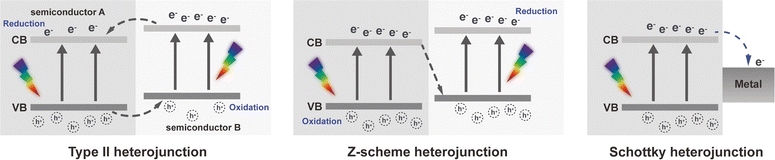
 | ||
| Fig. 7 The enhancing strategies of aPDT in bacterial targeting and ROS production. (A) The bio-responsive release and interaction of MPP-Ce6 with cariogenic biofilm. The UV-vis spectra, zeta potentials of MPP, MPP-Ce6, and free-Ce6. And the release profiles of Ce6 from MPP-Ce6 at different pH values. Reproduced with permission from ref. 59 Copyright 2022 KeAi Communications Co., Ltd. (B) The membrane-anchoring ability of HASeV. And SEM images of P. gingivalis and F. nucleatum treated with HASeV and QASeV, with or without light irradiation for 5 min (scale bars = 5 µm). Reproduced with permission from ref. 62 Copyright 2024 Elsevier Publishing. (C) The purine-based Ir(III) photosensitisers and their efficient ROS production mechanism. And the representative colony formation images of P. gingivalis with different concentrations of Ir–PCF under dark or light irradiation (405 nm). Reproduced with permission from ref. 67 Copyright 2024 Wiley-VCH GmbH. | ||
The organic photosensitisers, such as RB, are often anionic, whose surface charge properties and poor biofilm permeability limit their aPDT efficacy. To address these issues, surface engineering for managing the charge and anchoring is used. For instance, the quaternary ammonium groups are cationic groups that facilitate the absorption of photosensitisers onto the bacteria through electrostatic interaction. Meanwhile, such groups also prevent the molecular aggregation, thereby sustaining single-particle formation. In HQRB-SS-Dex, the nano-composite is coated with quaternary ammonium groups and dextran moieties. As a result, the surface charge of RB reversed and thus promotes bacterial adhesion, while dextran moieties enhance biofilm penetration. This enables closer contact between RB and target pathogens, thereby significantly improving its aPDT performance in the treatment of periodontitis.61 Meanwhile, to achieve better bacteria aiming effects, the surface engineering further incorporates a membrane anchoring strategy. The hydrophobic anchoring effect can be enhanced by functionalising materials (HASeV) with hexyl chains (C6H13), which bind to the hydrophobic core of membranes through van der Waals forces and hydrophobic interactions (Fig. 7B).62 This enables membrane penetration through hydrophobic insertion into bacterial membranes, which shows higher aPDT results than the electrostatic interaction of quaternary ammonium groups. Following insertion, bacterial membrane architecture becomes distorted, disrupting phospholipid alignment and increasing membrane permeability, which induces leakage of intracellular components such as nucleic acids and proteins and augments the bactericidal efficacy of PDT against periodontitis-associated bacteria.
In the complex biofilms, the bacteria use surface adhesins to recognise and bind to other bacteria, which is the design basis for bacterium-targeted delivery.63,64 Through surface encapsulation with the specific membrane vesicles, nanomaterials can masquerade as bacteria and infiltrate the biofilm formation of homologous bacterial strains, enabling high-concentration accumulation at biofilm sites. For instance, the nanomaterials are coated with bio-mimic vesicles, i.e. the bacterial outer membrane of F. nucleatum with RadD, Aid1 and CmpA, which exhibit similar adhesive properties as the F. nucleatum. The bio-mimic nanomaterials could efficiently infiltrate into periodontal biofilms, targeted pathogens, and maximise the photo-therapeutic effects.65 Moreover, the supramolecular photosensitiser, which consists of a selenoviologen cyclophane (SeVB) host encapsulating a fimbriae-binding heptapeptide (PQGPPQF), can specifically recognise the P. gingivalis FimA fimbriae through hydrogen bonding and hydrophobic interactions. This targeted anchoring markedly increases local ROS accumulation upon light irradiation and thereby achieves selective photodynamic eradication of P. gingivalis.66
Beyond targeting strategies, modulating the photophysical mechanisms of nanomaterials to achieve efficient ROS generation represents another viable approach. Ir(III) complex photosensitisers with 300–550 nm absorption bands can be excited under 405 nm blue light to generate ROS and achieve 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 bacterial reduction in both P. gingivalis and E. faecalis (Fig. 7C).67 Compared to N∧N ligands, C∧N ligands accelerate the ISC process. The donor–acceptor (D–A) strength formed between C∧N ligands and Ir further promotes ISC. Ir(III)'s strong SOC heavy atom effect significantly enhances ROS yield, generating substantial ROS within 5 seconds of irradiation, directly destroying bacterial structures and eliminating biofilms (P. gingivalis and E. faecalis). The positively charged material can bind to negatively charged bacterial cell membranes, enhancing local drug concentration. Its ability to generate ROS via the type I pathway also avoids the oxygen dependency issue of the type II pathway. In addition to improving ROS yield through material modifications, photo-induced ROS yield can be increased through complementary approaches. Designing ROS-triggered drug release can create a positive feedback loop for continuous bactericidal effects and promote endogenous bacterial ROS generation. Furthermore, some materials are designed to utilise local microenvironmental factors in dental plaque, such as pH changes or hypoxia, to further enhance ROS yield. Pillararene-embedded covalent organic frameworks loaded with thioacetal can be excited by 440 nm blue light to generate ROS targeting bacteria, while simultaneously triggering the release of antibacterial cinnamaldehyde, further disrupting bacterial respiratory chains and promoting endogenous bacterial ROS generation for sustained bactericidal effects.68
log10 bacterial reduction in both P. gingivalis and E. faecalis (Fig. 7C).67 Compared to N∧N ligands, C∧N ligands accelerate the ISC process. The donor–acceptor (D–A) strength formed between C∧N ligands and Ir further promotes ISC. Ir(III)'s strong SOC heavy atom effect significantly enhances ROS yield, generating substantial ROS within 5 seconds of irradiation, directly destroying bacterial structures and eliminating biofilms (P. gingivalis and E. faecalis). The positively charged material can bind to negatively charged bacterial cell membranes, enhancing local drug concentration. Its ability to generate ROS via the type I pathway also avoids the oxygen dependency issue of the type II pathway. In addition to improving ROS yield through material modifications, photo-induced ROS yield can be increased through complementary approaches. Designing ROS-triggered drug release can create a positive feedback loop for continuous bactericidal effects and promote endogenous bacterial ROS generation. Furthermore, some materials are designed to utilise local microenvironmental factors in dental plaque, such as pH changes or hypoxia, to further enhance ROS yield. Pillararene-embedded covalent organic frameworks loaded with thioacetal can be excited by 440 nm blue light to generate ROS targeting bacteria, while simultaneously triggering the release of antibacterial cinnamaldehyde, further disrupting bacterial respiratory chains and promoting endogenous bacterial ROS generation for sustained bactericidal effects.68
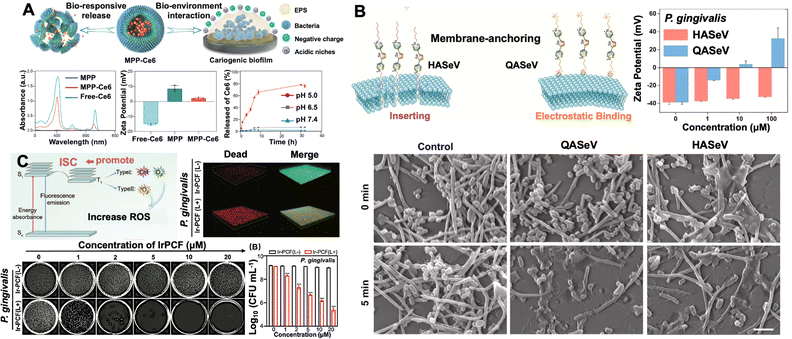
The synergistic integration of bacterial targeting, dual intra/extracellular ROS generation, and iron porphyrin metabolic interference demonstrates potent antibacterial efficacy against periodontitis pathogens. For instance, certain nanomaterials mimic erythrocyte structures to exploit P. gingivalis' haem acquisition dependency. This keystone pathogen lacks autonomous iron/porphyrin synthesis, relying instead on erythrocyte adhesion and lysis to obtain haem, an essential cofactor for growth, protease virulence expression, and biofilm formation.69 Gallium protoporphyrin IX chloride (Ga(PPIX)Cl) exploits bacterial haem uptake mechanisms by mimicking iron protoporphyrin. Following internalisation, it disrupts essential iron-dependent cellular functions and simultaneously creates an antioxidant deficit by failing to fulfil iron's protective roles, ultimately potentiating bacterial sensitivity to ROS. This targeting mechanism is operationalised through gallium porphyrin-loaded erythrocyte membrane biomimetic nanovesicles (GLR nanovesicles) (Fig. 8).70 Surface-exposed erythrocyte membrane proteins mediate specific binding to P. gingivalis haemagglutinins, achieving both pathogen-selective adhesion and targeted delivery. The nanovesicles exploit the bacterium's native porphyrin uptake machinery to induce iron metabolism dysregulation, compounded by gallium's structural substitution of iron. This substitution enhances blue light absorption through increased extinction coefficients and improved electron transfer kinetics, enabling efficient extracellular ROS generation under photoirradiation. Crucially, intracellular gallium porphyrin penetration was confirmed via flow cytometry, revealing concurrent oxidative stress through endogenous ROS production. DCFH assays further verified extracellular ROS generation, establishing a dual-action oxidative assault across cellular compartments.71
 | ||
| Fig. 8 The antibacterial mechanism of GLR nanovesicles. (A) Illustration of erythrocyte-mimicking nanovesicle targeting P. gingivalis through metabolism disorder and aPDT. (B) High-resolution confocal fluorescence microscopy of P. gingivalis (labelled SYTO 9)-containing biofilm (labelled Hoechst 33342), incubated with GLR (labelled Cy-5.5). (C) Detection of the extracellular and intracellular ROS. Reproduced with permission from ref. 70 Copyright 2024 American Chemical Society. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 colony forming unit (CFU) reductions for both P. gingivalis and F. nucleatum, whereas without light, the nano-platform yielded <3
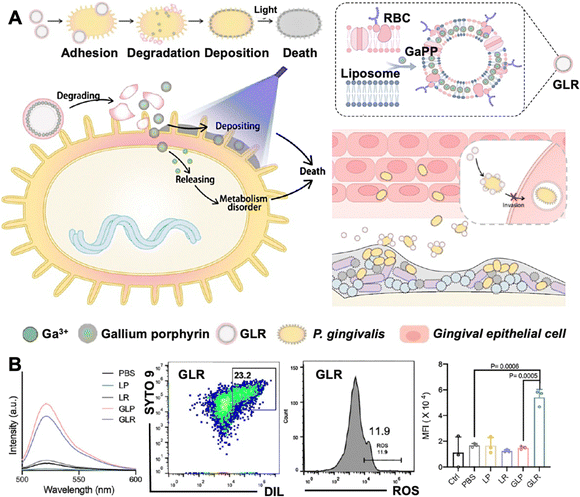
log10 colony forming unit (CFU) reductions for both P. gingivalis and F. nucleatum, whereas without light, the nano-platform yielded <3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reduction. Pt nanozyme-loaded MOF materials generate oxygen through catalysis, with elevated local oxygen concentrations directly inhibiting anaerobic bacterial growth.74 Additionally, a dual oxygen-supply strategy can achieve more efficient oxygen generation in hypoxic conditions. In the CeCyan–Cu5.4O nanocomposite, cyanobacteria have been employed for continuous oxygen production through photosynthesis under light irradiation. Meanwhile, the Cu5.4O component catalyses H2O2, generating sufficient oxygen for Ce6's aPDT performance under 660 nm excitation. Meanwhile, the close-range combination of cyanobacteria and Ce6 could promote the photonic energy transformation of molecular oxygen into free radicals, thereby increasing the ROS production while reducing the dosage of Ce6 (Fig. 9A).75
log10 reduction. Pt nanozyme-loaded MOF materials generate oxygen through catalysis, with elevated local oxygen concentrations directly inhibiting anaerobic bacterial growth.74 Additionally, a dual oxygen-supply strategy can achieve more efficient oxygen generation in hypoxic conditions. In the CeCyan–Cu5.4O nanocomposite, cyanobacteria have been employed for continuous oxygen production through photosynthesis under light irradiation. Meanwhile, the Cu5.4O component catalyses H2O2, generating sufficient oxygen for Ce6's aPDT performance under 660 nm excitation. Meanwhile, the close-range combination of cyanobacteria and Ce6 could promote the photonic energy transformation of molecular oxygen into free radicals, thereby increasing the ROS production while reducing the dosage of Ce6 (Fig. 9A).75
 | ||
| Fig. 9 Oxygen production and photocatalytic modification for hypoxic conditions. (A) The synthesis of CeCyan–Cu5.4O and its oxygenation ability. Calculating the change in P. gingivalis bacterial count by the dilute plate count method after treatment with different methods. The various components in each group were free Ce6 at 5 µg mL−1, Ce6 in CeCyan at 5 µg mL−1, cyan at 1 × 108 CFU per mL, and Cu5.4O at 365 ng mL−1 (L means irradiated under a 660 nm laser at 200 mW cm−2, 2 min) ****P < 0.0001. Reproduced with permission from ref. 75 Copyright 2022 KeAi Communications Co., Ltd. (B) Photo-induced redox imbalance and the ultrafast time-resolved spectroscopy of TBSMSPy+. The confocal laser scanning microscopy images and colocalization analysis of P. gingivalis (stained with TBSMSPy+ in green, DAPI in red, respectively). Absorbance changes of NADH at 339 nm after photocatalytic oxidation by 3 µM TBSMP and TBSMSPy+ with Fe3+ in deoxygenated DMSO solution. Quantitative analysis of NADH level in bacterial suspension treated with TBSMP and TBSMSPy+ at laser irradiation and without irradiation. Reproduced with permission from ref. 79 Copyright 2024 Springer Nature. | ||
Beyond enzyme-mimicking activities, redox cycling represents an alternative catalytic strategy for oxygen generation. This cycling involves the reversible interconversion between different oxidation states of transition metal ions, enabling continuous catalytic reactions through repeated oxidation-reduction processes. SnS2-based nanomaterials address hypoxic environments through redox cycling between Sn2+/Sn4+ states, catalysing the decomposition of H2O2 to generate molecular oxygen and thereby alleviating local hypoxia.76 Carrying hydrolysis components is also an alternative strategy for anaerobic environments. An oxygen-self-supplying Ce6/CaO2/ZIF-8@PEI nanoplatform—in which CaO2 cores hydrolyse in the aqueous/acidic bacterial microenvironment to generate O2 (via H2O2 intermediates), elevates local O2 to enhance 660![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm-activated Ce6 production of 1O2, thereby effectively eradicating E. faecalis planktonic cells and mature root-canal biofilms.77 Alternatively, oxygen-carrying materials can be incorporated to overcome hypoxic limitations by pre-carrying oxygen during synthesis and providing sustained oxygen release during aPDT. Guanidine and galactose-modified nano-photosensitiser utilise copolymerised perfluoropolyether as an oxygen carrier to release oxygen in hypoxic biofilm microenvironments.78 Guanidine confers positive charges to NPs, enabling penetration of negatively charged biofilm EPS through electrostatic interactions. Under 532 nm laser irradiation, the BODIPY-I generates 1O2 for PDT applications against periodontitis.
nm-activated Ce6 production of 1O2, thereby effectively eradicating E. faecalis planktonic cells and mature root-canal biofilms.77 Alternatively, oxygen-carrying materials can be incorporated to overcome hypoxic limitations by pre-carrying oxygen during synthesis and providing sustained oxygen release during aPDT. Guanidine and galactose-modified nano-photosensitiser utilise copolymerised perfluoropolyether as an oxygen carrier to release oxygen in hypoxic biofilm microenvironments.78 Guanidine confers positive charges to NPs, enabling penetration of negatively charged biofilm EPS through electrostatic interactions. Under 532 nm laser irradiation, the BODIPY-I generates 1O2 for PDT applications against periodontitis.
Beyond conventional aPDT approaches utilising ROS and free radicals, recent research has demonstrated potent antimicrobial efficacy through photocatalytic disruption of bacterial redox homeostasis (NADH/NAD+).80,81 Utilising this electron respiratory chain can potentially circumvent the hypoxia limitations associated with aPDT. TBSMSPy+ exhibits a remarkable mechanism whereby it binds to bacterial DNA and directly catalyses NADH oxidation through multi-step electron transfer upon photoexcitation, without the dependency of oxygen (Fig. 9B).79 This process interferes with normal electron transport chain function, inhibits ATP synthesis, and blocks energy supply for DNA replication, ultimately causing bacterial cell death. Femtosecond transient absorption spectroscopy conducted on this material revealed distinct HOMO–LUMO separation, suggesting a multistage intramolecular electron transfer process. This observation substantiates that TBSMSPy+ generates free radicals that promote photo-induced redox imbalance processes. Notably, as this mechanism operates directly through electron transfer without requiring oxygen participation, it effectively addresses challenges associated with hypoxic environments.
In a different strategy, “afterglow” enables sustained PDT by prolonging ROS generation through ISC pathways, thereby maintaining therapeutic efficacy even after cessation of light irradiation. Researchers proposed carbon dots (CDs)-embedded silica NPs (CDs@SiO2) for “afterglow” PDT, enabling non-destructive tooth whitening (Fig. 10).85 PCDs@SiO2 materials efficiently generate ROS under 370 nm violet light excitation with long afterglow effects. The afterglow principle involves energy transfer to the T1 state through ISC, with slow energy release from the T1 state after light source deactivation, creating afterglow and extending ROS half-life (PCDs@SiO2 afterglow lifetime reaches 1.36 seconds). After photo-excitation, it continuously releases ROS to oxidise and decompose deep enamel stains, with 95% stain degradation efficiency, which effectively reduces treatment time and light exposure requirements.
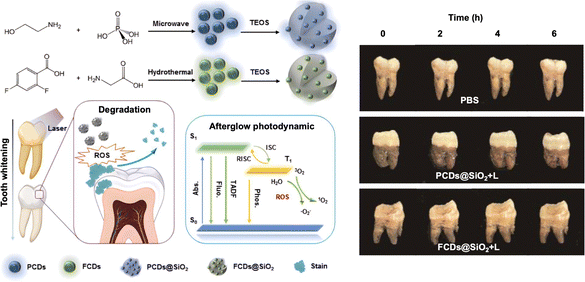 | ||
| Fig. 10 The “afterglow” photodynamic teeth whitening based on CDs@SiO2. Reproduced with permission from ref. 85 Copyright 2023 American Chemical Society. | ||
Conventional blue light-activated dental whitening techniques predominantly depend on H2O2 decomposition to generate ROS for pigment oxidation. Emerging nanomaterial-mediated approaches demonstrate superior enamel preservation through alternative oxidative pathways while concurrently delivering anticaries prophylaxis, highlighting their clinical promise. Nevertheless, the necessity for extended irradiation periods in these next-generation systems remains a key limitation requiring resolution before widespread clinical adoption.
In summary, PDT strategies exhibit distinct clinical merits through non-invasive implementation, circumvention of persistent antimicrobial resistance development, and capacity for synergistic therapeutic outcomes. However, translational challenges persist regarding insufficient biocompatibility data for photosensitiser nanomaterials, undefined long-term biosafety profiles, and reliance on costly narrowband near-infrared illumination systems. Comparatively, alternative antimicrobial strategies employing catalytic nanozymes or ROS-generating chemical cascades demonstrate operational advantages through illumination independence and occasional environmental responsiveness (e.g. pH-triggered agent release). Despite these practical benefits, such systems inherently lack the spatial precision and deep periodontal tissue targeting achievable through photosensitiser activation, representing a fundamental limitation in managing complex oral pathologies. While each therapeutic modality exhibits distinct strengths and inherent constraints, concerted research efforts remain imperative to systematically address current limitations and optimise clinical applicability.
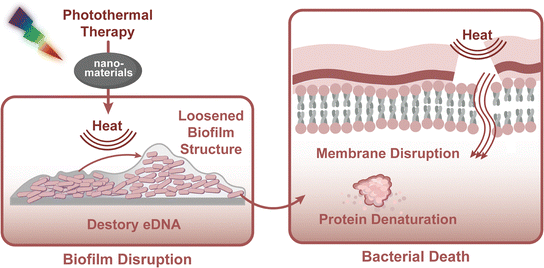
3.2 Photo-induced thermal damage
PTT employs light-responsive nanomaterials to generate localised hyperthermia, achieving rapid and confined temperature elevation that physically disrupts pathogenic structures without inducing antimicrobial resistance.86,87 Upon irradiation, nanoagents such as gold nanorods (GNRs), carbon-based architectures, and polydopamine (PDA) NPs convert light energy into heat, irreversibly degrading bacterial membranes and biofilm matrices.88 Modern advancements have optimised these systems through enhanced photostability and energy conversion efficiency, broadening their therapeutic scope in dental applications. Because oral tissues have finite and tissue-specific thermal tolerances, even modest temperature rises can precipitate injury—e.g., small intrapulpal increases are associated with irreversible pulp damage. Peri-implant and alveolar bone are particularly susceptible to temperatures around 47–50 °C, where exposure for a certain duration can lead to damage to the surrounding bone. Therefore, meticulous temperature control is fundamental during PTT. In practice, a ‘mild hyperthermia’ window around 42–45 °C is often targeted to limit collateral effects; however, such sub-ablative temperatures may be inadequate to eradicate biofilm-embedded or drug-resistant bacteria. To avoid inadvertent overheating of surrounding oral structures from direct thermal ablation, and to reconcile efficacy with safety, other therapeutic strategies have been incorporated. The generated heat synergistically potentiates drug release, amplifies enzymatic nano-activity, and propels nanostructures into biofilms via engineered designs like Janus configurations. This modality addresses diverse oral pathologies, including root canal infections and dentin hypersensitivity, while enabling real-time treatment monitoring through thermal imaging. To overcome historical limitations of hydrophilicity and photodegradation, material innovations now employ morphological engineering (e.g., aspect ratio modulation) and spectral tuning strategies, with doping techniques shifting absorption peaks into the NIR-II window for deeper tissue penetration. Collectively, PTT's multifaceted action, spanning structural disruption of pathogens, enhanced therapeutic delivery, and quantifiable outcomes, positions it as a transformative strategy in precision dentistry. Further optimisation remains critical to address residual biocompatibility and clinical standardisation challenges.Photothermal therapeutic strategies in dentistry employ diverse nanomaterials tailored for specific clinical requirements. Organic photothermal agents such as ICG demonstrate clinical utility due to their strong NIR absorption and biofilm elimination. Meanwhile, gold-based nanostructures, particularly GNRs, leverage plasmonic effects to achieve efficient photothermal conversion, establishing them as cornerstone materials in dental PTT applications. To address the inherent limitations of single-component systems, advanced nanocomposites integrate multiple functional constituents, synergistically enhancing photothermal performance through optimised energy transfer and structural engineering. Recent developments have further expanded the therapeutic scope of these materials, with rationally designed platforms combining photothermal activity with antibacterial or drug-delivery functionalities for multimodal treatment of complex oral diseases.
The nanoagent ICG is a water-soluble anionic photosensitiser that generates heat under 808 nm irradiation, whose aggregation tendency and photobleaching limit its phototherapeutic efficacy. In ICG-based nanocomposites, the molecular modifications employing DPPC and sPDMA significantly optimised ICG formulations by addressing inherent aggregation tendencies, while demonstrating enhanced photostability and superior temperature elevation kinetics compared to unmodified ICG (Fig. 12A).89,90 Similarly, to enhance the photothermal conversion efficiency, calixarene (GC5AF5) loads ZnPcS4 through hydrophobic interactions and electrostatic interactions, forming stable GFZ NPs.91 Charge transfer occurs between GC5AF5's electron-rich cavities and ZnPcS4, suppressing fluorescence emission and promoting excited-state energy release as thermal energy. Under 660 nm light excitation, disrupting S. mutans bacterial cell membranes and releasing ATP, achieving 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reduction of bacterial counts, while maintaining stable performance under multiple laser irradiations with anti-photobleaching capabilities. However, the dense EPS of biofilms inevitably hinder the penetration of photothermal agents, thereby compromising their bactericidal efficiency. To overcome this limitation, Janus-structured nanomaterials featuring asymmetric surface properties have been engineered into nanomotors capable of actively disrupting biofilm matrices through mechanical propulsion.92 J-CeM@Au nanomotors exemplify this strategy, generating localised hyperthermia under 808 nm laser irradiation (Fig. 12B). The resultant thermal gradient induces directional liquid thermophoresis (Soret effect), propelling these nanostructures into deeper biofilm regions while simultaneously enhancing bactericidal efficacy through combined photothermal and mechanical disruption.93
log10 reduction of bacterial counts, while maintaining stable performance under multiple laser irradiations with anti-photobleaching capabilities. However, the dense EPS of biofilms inevitably hinder the penetration of photothermal agents, thereby compromising their bactericidal efficiency. To overcome this limitation, Janus-structured nanomaterials featuring asymmetric surface properties have been engineered into nanomotors capable of actively disrupting biofilm matrices through mechanical propulsion.92 J-CeM@Au nanomotors exemplify this strategy, generating localised hyperthermia under 808 nm laser irradiation (Fig. 12B). The resultant thermal gradient induces directional liquid thermophoresis (Soret effect), propelling these nanostructures into deeper biofilm regions while simultaneously enhancing bactericidal efficacy through combined photothermal and mechanical disruption.93
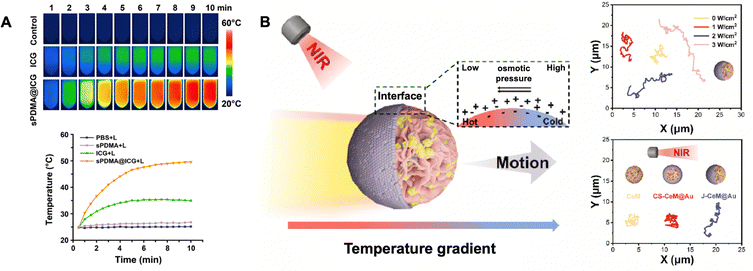 | ||
| Fig. 12 (A) Infrared thermal images and temperature changes of bacterial suspensions incubated with PBS, free ICG and sPDMA@ICG NPs, followed by laser irradiation. Reproduced with permission from ref. 90 Copyright 2021 Springer Nature. (B) The motion behaviour of J-CeM@Au nanomotors under NIR irradiation. Reproduced with permission from ref. 93 Copyright 2024 KeAi Communications Co., Ltd. | ||
Drug delivery materials commonly employed should possess characteristic structural features such as porosity. For example, Au nanocage structures can open their three-dimensional framework under photothermal effects, thereby releasing the loaded resveratrol.94 PS-NP@(C+I) surface-coated with PEG shells, shed in caries weakly acidic (pH 6.5) environments, releasing the nanoagent IR780, which generates heat under 808 nm laser irradiation.95
Apart from drug delivery, the controlled-release ions also demonstrate therapeutic effects. Dual polymer-functionalised melanin–silver NPs (P/D–MNP–Ag) activate photothermal effects under 808 nm irradiation, accelerating Ag+ ions release from the nanocomposite for efficient periodontitis treatment.96 Photothermal materials can also be engineered to deliver Ca2+, as demonstrated by Mg–MOF@PDA@CaP composites. These systems eradicate biofilms and release Ca2+ under acidic conditions (pH < 5.5), promoting remineralisation and repair of dental hard tissues such as enamel and dentin.97 In CuS/MnS@MnO2 materials, the nanocomposite releases Mn2+ from MnS under 808 nm irradiation, restoring microbial balance.98 Also, PDA-coated Prussian blue nanozyme platforms (PPM NPs) combine NIR-activated PTT with local minocycline delivery and intrinsic nanozyme ROS-scavenging to enhance antibacterial activity against S. sanguinis.99
Following photothermal-induced release, these nanoagents can additionally participate in immunomodulatory processes, thereby enhancing therapeutic efficacy. NETs persist in chronic infection foci (such as periodontitis), impeding inflammation resolution and causing tissue damage and bone destruction, which is also the primary cause of dental calculus formation. To reduce NET damage to inflamed tissues, black phosphorus (BP) loaded with DNase I, while coated with elastin (E) on the periphery of the nano-capsule, forms the composite nanomaterial E-TA-BP@D.100 Elastin (E) targets neutrophil elastase, whilst the BP in composite materials partially decomposes under 808 nm irradiation, leading to DNase I release. The DNase I degrades NET DNA scaffolds and promotes macrophage polarisation toward M2 phenotypes, reducing inflammation. Simultaneously, BP exerts PTT and PDT effects for multi-mechanism synergistic action. To expand the range of immunomodulatory actions, MOF-based nanocomposites have been employed. For instance, Prussian blue-loaded baicalein NPs (MPB-BA) convert light to heat, achieving local temperatures of approximately 53.7 °C to control baicalein release.101 The Prussian blue converts light energy to thermal energy (local temperatures reaching 53.7 °C), controlling the composite release of baicalein. Western blot (reduced p-IKKβ, p-P65 protein levels) and qRT-PCR (downregulated IL-1β, TNF-α) analyses demonstrate downregulation of NF-κB pathway key proteins and downstream pro-inflammatory factors, confirming photothermal effects can inhibit IKKβ/P65 phosphorylation, blocking NF-κB activation. Additionally, immunofluorescence showing enhanced p-Nrf2 nuclear accumulation indicates photothermal activation of Nrf2, forming anti-inflammatory-antioxidant synergistic effects. To ensure stable delivery of small-molecule immunomodulators, dimethyl fumarate (DMF) has been incorporated into yolk–shell nanozymes (Au@CeO2–DMF). The hollow CeO2 shell encapsulates DMF and prevents premature degradation, while the gold core generates heat under 635 nm irradiation (photothermal conversion efficiency ∼57.9%), resulting in thermal expansion and stimulus-responsive release of DMF.102 The released DMF activates the NRF2 pathway, inhibits NLRP3 inflammasome assembly, and enhances antioxidant enzyme expression (superoxide dismutase, catalase, glutathione peroxidase), thereby suppressing oxidative stress and promoting immune repair. Simultaneously, thermal stimulation enhances Ce3+/Ce4+ conversion in the CeO2 shell, further enhancing its catalytic activity.
Moreover, thermal stimulation could trigger the controlled release of therapeutic gas molecules. Nitric oxide (NO) exhibits both antibacterial and immunomodulatory functions.103 The GNRs@mSiO2–SNO/ICG composite, when excited at 808 nm, exhibits bactericidal effects against various bacterial species within multi-species biofilms (including P. gingivalis, F. nucleatum, and S. gordonii), resulting in disruption of the three-dimensional biofilm architecture (Fig. 13A).104 In nanocomposite gas therapy, S-nitrosothiols (SNO) were loaded and subsequently released NO under thermal stimulation. NO exerts inflammation inhibition by suppressing the assembly of pro-inflammatory factors and the NLRP3 inflammasome. Moreover, L-Arg is a natural amino acid that can serve as a NO donor.105 In NO nanocomposites (Ag2S@ZIF-90/Arg/ICG), ZIF-90 utilises its inherent porous structure and the Schiff base reaction between its surface aldehyde groups (–CHO) and the amino groups (–NH2) of Arg to achieve high drug loading capacity for Arg (Fig. 13B).106 It can generate heat through Ag2S structures and ROS through ICG structures under 808 nm irradiation, whilst Arg releases NO under heat and ROS, achieving multi-effect synergistic antibacterial action. NO can activate phosphodiesterase (PDE), reduce the second messenger c-di-AMP pathway, disrupt biofilm integrity, and enhance the antibacterial efficacy of the composite material. The Ag2S@ZIF-90/Arg/ICG has achieved approximately a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reduction in biofilm in vitro. Similarly, Prussian blue nanozymes (SPBzyme) generate heat under NIR irradiation while accelerating NO release.107 These nanozymes possess multiple enzymatic activities, such as catalase and superoxide dismutase, effectively reducing ROS levels at inflammation sites. Light-stimulated NO production from sodium nitroprusside can modulate osteoblast differentiation, achieving immunomodulation and hard tissue repair. Meanwhile, carbon monoxide (CO) is also a therapeutic gas molecule, demonstrating multiple modulatory properties.108 Ti–DOPA–CO generates photothermal effects under 808 nm, inducing Fe3(CO)12 release of high-concentration CO (Fig. 13C).109 High concentrations of CO can compromise bacterial cell membranes, inhibit ATP synthesis and cellular respiration, with particularly significant effects against periodontitis-associated anaerobic bacteria. Concurrently, without light irradiation, Ti–DOPA–CO can produce low doses of CO, which activate the haem oxygenase-1 (HO-1) pathway, inducing macrophage polarisation from the pro-inflammatory M1 phenotype to the regeneration-promoting M2 phenotype, suppressing mitogen-activated protein kinase (MAPK) and NF-κB signalling pathways, and reducing pro-inflammatory gene transcription.
log10 reduction in biofilm in vitro. Similarly, Prussian blue nanozymes (SPBzyme) generate heat under NIR irradiation while accelerating NO release.107 These nanozymes possess multiple enzymatic activities, such as catalase and superoxide dismutase, effectively reducing ROS levels at inflammation sites. Light-stimulated NO production from sodium nitroprusside can modulate osteoblast differentiation, achieving immunomodulation and hard tissue repair. Meanwhile, carbon monoxide (CO) is also a therapeutic gas molecule, demonstrating multiple modulatory properties.108 Ti–DOPA–CO generates photothermal effects under 808 nm, inducing Fe3(CO)12 release of high-concentration CO (Fig. 13C).109 High concentrations of CO can compromise bacterial cell membranes, inhibit ATP synthesis and cellular respiration, with particularly significant effects against periodontitis-associated anaerobic bacteria. Concurrently, without light irradiation, Ti–DOPA–CO can produce low doses of CO, which activate the haem oxygenase-1 (HO-1) pathway, inducing macrophage polarisation from the pro-inflammatory M1 phenotype to the regeneration-promoting M2 phenotype, suppressing mitogen-activated protein kinase (MAPK) and NF-κB signalling pathways, and reducing pro-inflammatory gene transcription.
 | ||
| Fig. 13 The thermal-stimulated controlled gas releases and the following immunomodulation. (A) The NO generation of GNRs@mSiO2–SNO/ICG under an 808 nm laser. Transmission electron microscopy (TEM) photomicrographs of S. gordonii, F. nucleatum, and P. gingivalis after being treated with NPs (green, blue and red represent S. gordonii, F. nucleatum, and P. gingivalis, respectively). A schematic illustration of the possible mechanism of NPs to inhibit inflammation. Reproduced with permission from ref. 104 Copyright 2022 Elsevier Publishing. (B) The NO-guided c-di-AMP degradation and biofilm dispersion of Ag2S@ZIF-90/Arg/ICG. Reproduced with permission from ref. 106 Copyright 2023 Ivyspring International Publishing. (C) Ti–DOPA–CO implant for peri-implant soft-tissue integration enhancement through CO-mediated immunomodulation and antibiosis under light irradiation. Reproduced with permission from ref. 109 Copyright 2024 KeAi Communications Co., Ltd. | ||
PTT induces bacterial heat shock response via localised hyperthermia, promoting the expression of heat shock proteins (HSPs). These HSPs assist bacteria in repairing thermally damaged proteins and enhance their thermotolerance, thereby compromising the bactericidal efficacy of PTT. To counter this, under 1064 nm excitation, Cu3P@PAH@Lox locally generates high temperatures that accelerate the Fenton-like reaction of chemodynamic therapy (CDT) to produce ˙OH radicals.110 Simultaneously, CDT downregulates the expression of bacterial HSPs, reducing bacterial tolerance to high temperatures and enhancing the PTT effect. The thermal effect disrupts the enzymatic activity of Cbe in S. gordonii, blocking the Cbe-Ltp1-Ptk1-fimA signalling axis, which reduces P. gingivalis adhesion and co-aggregation, thereby inhibiting biofilm formation at its source. Similarly, LuBi2Te3 can also generate photothermal effects when excited at 1064 nm.111
Spatially axial boron-coordinated single-atom nanozymes (Fe–B/N–C SAzymes) can induce localised high temperatures under 1064 nm laser irradiation, thermally promoting enzyme-mimetic activity.112 Introduction of axial boron ligands adjusts the local coordination environment of planar Fe–N4 active centres, optimising electronic distribution and enhancing enzymatic catalytic performance. And the efficient periodontitis treatment is achieved through multi-enzyme-mimetic synergy (oxidase, peroxidase, catalase, etc.) and photothermal effects. Similarly, sulfonated lignin forms metal phenolic networks, uniformly dispersing ultra-small Pd NPs in layered structures to form MPN–Pd with broadband absorption (650–1000 nm).113 Through synergistic oxidase-like activity and photothermal effects, it demonstrates efficient, safe advantages in oral polymicrobial biofilm infection treatment. Under 808 nm NIR light excitation, temperatures reach 45.3 °C with a photothermal conversion efficiency of approximately 42%. The high temperature disrupts membrane structures and promotes oxidase-like activity, and inhibits biofilm growth against E. faecalis with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 bacterial reduction. Interestingly, these Pd NPs also effectively eradicate polymicrobial biofilms by leveraging the higher sensitivity of bacteria to ROS and of fungi to heat and induced a 5
log10 bacterial reduction. Interestingly, these Pd NPs also effectively eradicate polymicrobial biofilms by leveraging the higher sensitivity of bacteria to ROS and of fungi to heat and induced a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reduction in Candida albicans CFU enumeration. The MN-SF/CuS nanocomposite demonstrates precise periodontal tissue targeting capability. Through encapsulation within a hyaluronic acid methacrylate microneedle array architecture, this therapeutic system maintains optimal functionality within the complex microenvironment of periodontal tissues. Localised heating significantly enhances the peroxidase-mimetic catalytic activity inherent in the SF/CuS nanozymes, thereby accelerating substrate turnover rates through photoactivated redox cycling.114
log10 reduction in Candida albicans CFU enumeration. The MN-SF/CuS nanocomposite demonstrates precise periodontal tissue targeting capability. Through encapsulation within a hyaluronic acid methacrylate microneedle array architecture, this therapeutic system maintains optimal functionality within the complex microenvironment of periodontal tissues. Localised heating significantly enhances the peroxidase-mimetic catalytic activity inherent in the SF/CuS nanozymes, thereby accelerating substrate turnover rates through photoactivated redox cycling.114
 | ||
| Fig. 14 Infrared thermal images of PBDT-DIID NPs at different concentrations under 808 nm laser irradiation, along with representative SEM images of the sample tooth's root surface from various groups (scale bars: 10 µm). Reproduced with permission from ref. 115 Copyright 2022 Wiley-VCH GmbH. | ||
Conventional therapies for dentin hypersensitivity, including desensitising agents, light-cured materials, and lasers, face limitations such as poor material retention and pulp thermal injury. Effective interventions require nanomaterials smaller than dentinal tubule apertures, as confirmed by field emission SEM and dynamic light scattering analyses. NDTB NPs, engineered with a donor–π–acceptor (D–π–A) molecular architecture, have higher photostability than ICG, achieving efficient dentin treatment.117 Under 808 nm irradiation, surface mineral melting rapidly occludes tubule openings (86% sealing efficacy vs. 57% for lasers alone), reducing stimulus transmission (Fig. 15). Mechanistically, twisted intramolecular charge transfer effects suppress radiative decay, maximising heat generation via non-radiative dissipation. In addition, dual-action sealing of dentinal tubules can be achieved using BP nanosheets through photothermally mediated mechanisms.118 Under 808 nm laser irradiation, photothermal melting reduces tubule diameter via surface mineral liquefaction, physically sealing exposed openings. Simultaneously, phosphate groups released from BP interact with endogenous Ca2+ ions within dentinal fluid, precipitating hydroxyapatite (HAp)-like mineralised layers that reinforce occlusion stability. This synergistic strategy, combining immediate physical sealing with long-term biomineralisation, demonstrates superior efficacy to standalone laser therapies, offering durable desensitisation while minimising iatrogenic complications.
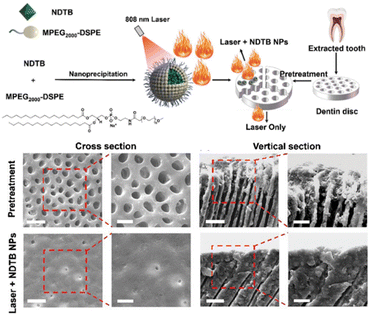 | ||
| Fig. 15 Cross-section and vertical-section SEM images of dentin discs with NDTB NPs before and after pretreatment (scale bars: 10 µm). Reproduced with permission from ref. 117 Copyright 2021 The Royal Society of Chemistry. | ||
Moreover, in other fields, PTT is widely applied to more diseases. Pt–Au@C–P.g.–MM exemplifies systemic therapeutic potential. By encapsulating oxygen-generating Pt NPs within P. gingivalis-targeting macrophage membranes, this platform penetrates the blood–brain barrier under NIR irradiation. Localised photothermal ablation of neuroinvasive P. gingivalis mitigates glial activation and neuroinflammation, offering a targeted approach to curbing pathogen-induced neuronal damage.119 In oncology, NanoLock and PLTs-GNRs exploit NIR-triggered hyperthermia to disrupt mitochondrial integrity in oral squamous cell carcinomas.120–122 These systems enable precise deep-tissue thermotherapy, circumventing collateral damage through tumour-selective energy deposition, a paradigm shift from conventional surgical resection.
3.3 Photoelectric conversion of semiconductor nanocomposites
Photoelectric conversion, exemplified by solar energy harvesting and photocatalytic systems for clean fuel production, holds transformative potential in medical therapeutics, particularly for oral disease management. Central to this technology is the ability of semiconducting nanomaterials to generate electrical signals or drive electron transfer under light irradiation, a process governed by photoexcited charge carrier generation and separation. When irradiated with photons exceeding the material's bandgap energy, VB electrons transition to the CB, creating electron–hole pairs. Strategic heterojunction engineering or surface modifications enable directional carrier migration, yielding measurable photocurrents or voltages with therapeutic utility.In biomedical applications, researchers have focused on transition metal oxides and sulphides (e.g., Ti, Zn, Cu) and their multi-metallic composites, which exhibit tuneable photocatalytic activity.123,124 For instance, I-MoO3−x nanoribbons demonstrate enhanced NIR absorption and ROS production through structural optimisations, including shortened dimensions, oxygen vacancy introduction, and expanded interlayer spacing. These modifications amplify oxidative damage to pathogens while maintaining material stability, offering a targeted approach for oral biofilm disruption.125 Likewise, Au–RE/TiO2 demonstrates antibacterial activity and the promotion of osseointegration through photo-electric conversion under 980 nm irradiation.126
The subgingival depth of periodontitis lesions (several millimetres) necessitates phototherapeutic agents responsive to longer wavelengths with superior tissue penetration. Bandgap modulation in nanocomposites enables red shifts of absorption. Through strontium (Sr) doped ZnO and PDA encapsulation to form a Sr–ZnO@PDA core–shell structure, the band gap of ZnO is synergistically narrowed from 3.37 eV to 1.78 eV, thereby achieving a red shift in the absorption spectrum and enabling the excitation wavelength to shift from UV light towards the biologically safe yellow light region.127 Similarly, the Fe2O3@Cu–TCPP nanocomposite exhibits enhanced visible light absorption through atomic layer deposition surface engineering. The Fe2O3 modification increases the absorption ability of Cu–TCPP at 660 nm. This improved light-harvesting ability, combined with the intrinsic porphyrin framework's capacity for 1O2 generation, enables effective 660 nm light-driven PDT for periodontal pathogen eradication.128 Meanwhile, the up-conversion NPs (UCNPs) materials are primarily excited in the NIR spectrum.129 Researchers utilise up-conversion mechanisms with 808/980 nm light sources to convert emissions to UV light, enabling PDT effects even after material delivery to deep tissues.130,131 β-NaYF4:Yb3+,Tm3+ UCNPs have been confirmed to activate TiO2 through up-conversion, generating electron–hole pairs that react with water and oxygen to produce ROS.
Building on the principles of photogenerated carrier dynamics, heterojunction engineering offers a strategic approach to optimise interfacial electronic structures. Unlike homojunctions formed by identical semiconductors, heterojunctions arise at the interface between materials with distinct band structures, creating band bending and energy level realignment that enables efficient carrier separation. Key interfacial engineering approaches include type II systems with staggered band alignment and Schottky junctions with optimised metal–semiconductor contacts. These approaches, often combined with defect engineering strategies, collectively enhance photoelectric conversion efficiency through improved charge carrier dynamics (Fig. 16).
The photo-related antibacterial mechanisms of type II heterojunctions are characterised by staggered band alignment, which enables spatial separation of photogenerated electrons and holes, reducing recombination and supporting multimodal antibacterial effects. In ZnO/black TiO2−x nanosystem, Ti–Zn covalent bonding induces interfacial charge redistribution, facilitating enhanced charge transfer (Fig. 17A).132 This process reduces the composite bandgap to 2.04 eV, extending light absorption into the NIR region (808 nm). The oxygen vacancies enhance photothermal conversion, achieving surface temperatures of 56.7 °C to thermally inactivate pathogens. Meanwhile, accelerated charge transfer generates photocurrents that disrupt bacterial electron transport chains and impair ATP synthesis. These photocurrents induce intracellular ROS production within bacteria, amplifying oxidative stress and increasing membrane permeability, ultimately leading to bacterial death. Similarly, in violet phosphorus (VP)/Ti3C2 MXene heterojunctions, where P–Ti chemical bonds act as electron transport bridges, facilitating the transfer of photogenerated electrons under visible light irradiation (Fig. 17B).133 The built-in electric field promotes carrier separation, while Ti3C2's high conductivity enhances electron migration efficiency. The spatially separated photogenerated electrons (accumulated on Ti3C2 surfaces) and holes (accumulated on VP surfaces) are captured by the adsorbed O2 and H2O, catalytically generating ˙OH and 1O2. Additionally, Ti3C2' strong absorption and efficient hot electron-to-photon energy conversion, which lead to significant photothermal effects, complement the aPDT with localised heat.
 | ||
| Fig. 17 The photoelectric conversion mechanism of heterojunction structures. (A) Photoelectric and photothermal performance of ZnO/black TiO2−x heterojunction nanofilms under 808 nm NIR excitation. Reproduced with permission from ref. 132 Copyright 2024 American Chemical Society. (B) The electron transfer channel at the VP/Ti3C2 heterojunction interface. And the reaction mechanism diagram of photocatalytic production of ROS from the VP/Ti3C2 heterojunction. Reproduced with permission from ref. 133 Copyright 2024 American Chemical Society. (C) The photocatalytic mechanism of the charge transfer for ROS evolution over the Bi2S3/Cu–TCPP under 635 nm light irradiation. Reproduced with permission from ref. 134 Copyright 2023 Wiley-VCH GmbH. | ||
Z-scheme heterojunctions demonstrate enhanced redox capabilities compared to individual components by preserving stronger oxidation/reduction potentials through directional charge recombination. This structural configuration concurrently extends the light absorption range of nanocomposites, particularly into the red spectrum. As exemplified by the Bi2S3/Cu–TCPP system, interfacial electronic interactions induce a distinct red shift in absorption relative to pristine Cu–TCPP, enabling efficient utilisation of 635 nm laser irradiation (Fig. 17C).134 The optimised charge dynamics yield a 5-fold increase in ROS generation compared to individual components, demonstrating superior therapeutic outcomes in periodontitis management through oxidative damage to pathogenic biofilms. These systems collectively illustrate how Z-scheme heterojunctions overcome spectral limitations through band structure engineering while enabling multifunctional therapeutic modalities. The preserved high-potential carriers and extended light responsiveness address critical challenges in deep-tissue antimicrobial applications, particularly in complex anatomical environments where light penetration and biofilm eradication require coordinated physical-chemical strategies.
As a key structure in interfacial engineering, Schottky junctions exemplify tailored charge dynamics at the metal–semiconductor interfaces. These structures arise from metal–semiconductor interfaces, which selectively drive electrons or holes across the boundary. The h-GNRs@CeO2@PDS nanoplatform leverages a plasmonic metal–semiconductor Schottky structure wherein one end of GNRs generates heat via the photothermal effect, whilst the other end, encapsulated with CeO2, produces a free radical storm.135 This partial encapsulation avoids the light absorption constraints of fully shielded architectures. GNRs absorb 808 nm light to generate hot electrons, which are transferred to the semiconductor's CB through the Schottky heterojunction, activating the enzymatic activity of CeO2. This catalyses the production of sulphate radicals (with a lifetime of 30–40 µs), enabling long-distance diffusion and deep antibacterial action.136 Moreover, B-TiO2/Ag nanorobots utilise black-TiO2 (B-TiO2) to produce ROS under UV/blue light irradiation. The asymmetric Ag coating configuration on the nanotube surface results in rotational motion. This autonomous movement enhances contact with biofilms and facilitates improved ROS distribution and penetration for enhanced antibacterial efficacy.137
SSN–Ag@Pt–GLM can penetrate deep into dentinal tubules through autonomous movement, primarily relying on the Pt layer catalysing H2O2 to generate bubbles, propelling forward through recoil force.138 Under 405 nm blue light irradiation, the GLM on the surface polymerises, forming a tight structure from inside to outside, effectively sealing exposed tubules.
Building on the synergy between photogenerated carriers and catalytic activity, researchers have developed EV@ZIF nano-hybrids through in situ biomineralisation of photo-responsive ZIF layers onto extracellular vesicle (EV) surfaces. Under light irradiation, these nano-hybrids generate electron–hole pairs, transferring photoelectrons from ZIF semiconductors to EVs.139 This electron transfer accelerates intravascular NADP+ reduction to NADPH, providing essential electron donors for the integrated iNOS enzymes. The resulting cascade amplifies L-arginine conversion to NO, enabling precise photo-controlled NO release that effectively disrupts bacterial metabolism and destabilises biofilms through enhanced oxidative stress. Building upon this foundation, 3D biomimetic photoelectric optoelectronic scaffolds employ 808 nm NIR light to stimulate silicon microstructures, converting photonic signals into precise electrical cues.140 These photoelectronic signals effectively modulate membrane potentials and intracellular calcium ion dynamics in human bone marrow mesenchymal stem cells (hBMSCs), driving osteoblast differentiation and promoting osteogenic differentiation. Engineered with photolithography-etched micro-columnar and nanopyramid micropillar and nanopyramid architectures, these scaffolds achieve enhanced light absorption and photoelectric conversion efficiency (operating at <1 W cm−2 energy density with <1.5 °C temperature rise), ensuring tissue-safe operation while maintaining biological compatibility.
Semiconductor-based nanotechnologies have emerged as transformative tools in oral biomedicine, leveraging their unique photo-electronic properties to enable precise, minimally invasive interventions. From photocatalytic platforms for biofilm disruption to biohybrid systems for targeted drug delivery, these materials offer spatiotemporal control over therapeutic processes while maintaining biocompatibility. Standardising treatments and ensuring long-term safety remain challenges, but advances in biodegradable semiconductors and multimodal systems are advancing lab innovations toward clinical use.
3.4 Photo-biomodulation effects
Photo-biomodulation employs a specific wavelength of light (primarily low-intensity light) to non-thermally and non-invasively modulate cellular functions. Currently, it is commonly used for chronic pain relief, skin wound healing, accelerating wound healing, treating chronic inflammation, and improving certain neurological conditions.141,142 In dentistry, recent studies have explored the applications of blue to NIR light wavelengths for therapeutic purposes, including anti-bacterium, anti-inflammation, and also regeneration. Additionally, combining photo-biomodulation with other materials (such as NPs and biomaterials) can enhance therapeutic efficacy.To tackle bacterial infections around dental implants, wireless blue light-emitting diodes (LEDs) encapsulated in polypara-xylene-C coatings have been designed for 360° irradiation across implant surfaces (Fig. 18).143 When activated by 410 nm blue light, these LED devices exploit endogenous bacterial porphyrins to generate intracellular ROS, selectively eliminating pathogens like P. gingivalis and methicillin-resistant Staphylococcus aureus (MRSA). This strategy suppresses biofilm formation without requiring external photosensitisers. Pure 405 nm blue light further demonstrates dual antibacterial mechanisms, combining porphyrin-mediated photodynamic effects with gene expression modulation and also shows significant biocompatibility.144
 | ||
| Fig. 18 The treatment procedure of wireless-powered blue light implant for peri-implantitis. And the peri-implant infection, bacterial sampling, tissue HE, and giemsa staining. Reproduced with permission from ref. 143 Copyright 2023 Wiley-VCH GmbH. | ||
With the ability to modulate cellular expression, in the red-NIR light spectrum, the combined 650 nm and 910 nm wavelengths have been employed to regulate mandibular osteoblast differentiation.145,146 These wavelengths inhibit pathological matrix remodelling while exerting anti-inflammatory and anti-osteoclastic effects, including the downregulation of pro-inflammatory cytokines (such as IL-6, IL-1β) and osteoclast-activating factor RANKL, thereby reducing bone resorption.
Meanwhile, researchers have developed biodegradable optical fibres for bone regeneration, harnessing 520 nm green light to deliver photobiomodulation effects. Under specific parameters (25 mW cm−2 intensity, 188-second single exposure, administered every other day over 5 sessions, with a penetration depth of about 5 mm), these fibres activate the ERK/RUNX2 pathway, offering a minimally invasive and degradable alternative to traditional rigid materials.147 This approach enables sustained biological regulation while addressing issues such as poor tissue compatibility. Notably, green light could activate TRPV1 channels, enhancing calcium signalling for calcification-related therapy.
In summary, future advancements in treating deep oral infections may focus on biodegradable optical materials and integrated photobiomodulation systems, which hold promise for tissue regeneration in diverse biomedical contexts. However, challenges persist in the photo-biomodulation domain, such as incomplete mechanistic understanding, non-standardised protocols, unverified long-term safety, dependence on specialised equipment, operator skill requirements, and ongoing light source optimisation.
3.5 Photo-curable nanomaterials
Commonly used resins and other light-curable materials in the oral cavity fill carious tissues, but their function is limited to restoring oral hard tissue morphology and masticatory function without concurrent biomodulations. However, next-generation light-curable materials, designed according to various oral disease characteristics through multiple material integration, enable nanomaterials to exert antibacterial or anti-inflammatory effects whilst curing.148 Specifically, hydrogel-based nanomaterials offer significant advantages for applications in oral medicine, particularly due to their ability to adapt to the challenging moist environment of the oral cavity. Their inherent hydrophilicity and three-dimensional porous structure enable stable, long-term retention on mucosal surfaces or dental tissues, resisting displacement by saliva or mechanical forces. This durability supports sustained therapeutic effects without frequent reapplication. Meanwhile, another key benefit lies in their versatile drug-loading capacity. Therapeutic nanoagents, such as antimicrobials, anti-inflammatory compounds, or remineralising agents, can be effectively incorporated. The materials' stimuli-responsive properties allow targeted drug delivery at infection sites or carious lesions, enhancing treatment precision while minimising systemic side effects. Furthermore, their tuneable mechanical properties and biocompatibility facilitate comfortable integration with oral soft tissues, reducing irritation risks. The combination of prolonged mucosal adhesion and controlled drug release mechanisms positions these nanomaterials as promising candidates for managing chronic oral conditions like periodontal disease and other infections.In the complex microenvironment of periodontal tissues, effective hydrogel curing demands robust adhesion and minimal volumetric expansion. To address these challenges, researchers have engineered alginate-based, light-curable hydrogels tailored for moist oral conditions. These materials polymerise within two minutes under 365 nm UV light, overcoming traditional limitations of poor adhesion and infection susceptibility through a dual-network design that ensures strong wet adhesion and inherent antibacterial properties (Fig. 19A).149 Functioning as both physical barriers (retaining integrity for about 120 hours) and therapeutic platforms, they arrest periodontitis progression while promoting tissue repair. Further enhancements include chlorhexidine acetate encapsulation for targeted elimination of P. gingivalis and Staphylococcus aureus (S. aureus), combined with epigallocatechin gallate to scavenge ROS in inflamed tissues.150 In diabetic periodontitis models, such hydrogels significantly reduce alveolar bone loss and stimulate collagen regeneration, demonstrating clinical potential. To mitigate oxidative damage during repair, lysosome-inspired TiO2−x–alginate (TA) composites generate ROS, while alginate encapsulation confines oxidative activity to infected areas, sparing healthy tissues, and finally achieving implant decontamination (Fig. 19B).151
 | ||
| Fig. 19 The photo-curable nanomaterials in the dental field. (A) The illustration of commercially available periodontitis hydrogel and light-curing hydrogel for periodontitis treatment. Reproduced with permission from ref. 149 Copyright 2025 Wiley-VCH GmbH. (B) The preparation process and application form of NIR-activated alginate complex for implant decontamination, and subsequently biological benefits of relieved inflammation and enhanced re-osseointegration. Reproduced with permission from ref. 151 Copyright 2024 Wiley-VCH GmbH. | ||
Parallel advancements focus on injectable thermosensitive systems. For instance, CH–BPNs–NBP hydrogels deliver black phosphorus nanosheets (BPNs) and DL-3-n-butylphthalide via local injection, achieving synergistic ROS clearance and vascular protection at inflammatory sites.152 These gels undergo phase transitions under 808 nm near-infrared irradiation, enhancing periodontal retention and therapeutic precision.
Beyond soft tissue applications, light-activated materials are revolutionising hard tissue regeneration. Thiol-alkyne triazine-trione polymers, for example, cure within 10 seconds under 385 nm blue-violet light, forming highly crosslinked 3D networks adaptable to complex anatomical shapes (e.g., bone fixation plates, screws).153,154 Similarly, triacrylate-based systems rapidly solidify under 365 nm light, supporting stem cell differentiation and dentine regeneration.155
Hydrogel nanomaterials have emerged as versatile platforms, enabling targeted antimicrobial therapy, tissue regeneration, and minimally invasive drug delivery through their tuneable biocompatibility and injectability. While challenges such as stability in dynamic oral environments and long-term biosafety require further optimisation, advances in stimuli-responsive and nanocomposite hydrogels drive their transition toward clinical applications.
3.6 Photo-induced diagnostic applications
Common oral conditions like periodontitis and dental caries are typically occult, slow-progressing diseases. Without timely detection and treatment, they affect oral health, with severe cases causing tooth loss and even threatening systemic health. Therefore, early monitoring, particularly for occult lesions, is challenging but crucial. Alterations in photoluminescence (transition from non-luminescent to luminescent states) or modifications in the wavelength of reflected/projected light perceived by the human eye (chromatic variations resulting from changes in material absorption properties) can provide intuitive visual manifestations of disease occurrence. These photophysical transformations possess substantial value for disease diagnostics and clinical assessment.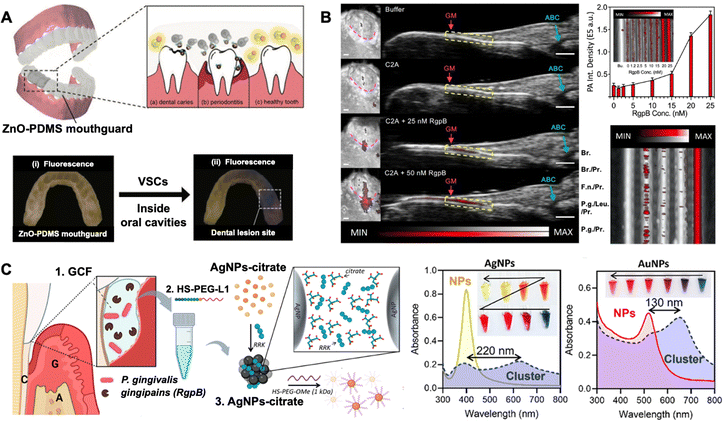 | ||
| Fig. 20 The photo-relevant diagnostic applications in the dental field. (A) Fluorescence images of a ZnO–PDMS mouthguard were applied to visualise dental lesion sites before and after being worn by a patient with dental caries to locate the lesion sites. Reproduced with permission from ref. 156 Copyright 2020 Wiley-VCH GmbH. (B) Bioimaging of RgpB-activated C2A probe in the gingival sulci of porcine jaws. Reproduced with permission from ref. 159 Copyright 2022 Wiley-VCH GmbH. (C) The assembly of Ag NPs–citrate is done with the addition of the tripeptide RRK, and then its dissociation with HS–PEG–OMe. And the colourimetric signal generation of Ag NPs: UV-vis spectra of Ag NPs and Au NPs before and after dissociation in monodispersed NPs with HS–PEG–OMe. Reproduced with permission from ref. 163 Copyright 2023 American Chemical Society. | ||
In another case, Cy5.5 dye connected through peptide segments (APRIK) forms intramolecular dimers that specifically recognise P. gingivalis-secreted gingipain protease RgpB (Fig. 20B).159 After APRIK cleavage, dye fluorescence and photoacoustic signals intensify. Under 680 nm excitation light irradiation, real-time fluorescence and photoacoustic dual-modal imaging are achieved with high sensitivity for early diagnosis of periodontitis lesion sites. AuNRs@RgpB utilise NIR laser-induced photoacoustic response that is activatable via RgpB-mediated peptide cleavage, whereby proteolytic dissociation restores plasmon resonance and amplifies photoacoustic signals, thus realising enzyme-specific imaging of P. gingivalis infections.160 The AIEgens MeOTpy binds to S. mutans and forms aggregates when excited at 488 nm, subsequently emitting strong fluorescence at 650 nm, thus facilitating rapid localisation of carious lesions.161 The positively charged 4-methylpyridinium moiety interacts electrostatically with negatively charged components (such as peptidoglycan) on the S. mutans surface, conferring selective recognition. Additionally, the hydrophobic nature of the pyridinium group assists in membrane insertion, strengthening the binding affinity with the S. mutans cell membrane.
In addition to detecting infectious diseases, nanomaterials are also utilised for tumour detection. GNRs conjugated with epidermal growth factor receptor (EGFR) specifically bind to cancer cells with high EGFR expression.162 Under 650 nm wavelength light source excitation, real-time imaging is achieved using GNR scattering signals with 1 mm resolution, superior to the spatial limitations of traditional frozen sections and magnetic resonance imaging. This provides an efficient, precise solution for tumour margin detection, with advantages including real-time imaging, high resolution, and specificity, overcoming traditional method limitations.
4. Conclusions and perspectives
Recent research demonstrates the transformative potential of photo-responsive nanomaterials in advancing dental healthcare through multifunctional theranostic capabilities. NIR-activated nanomaterials for periodontitis management have emerged as a particularly active research frontier, leveraging deep tissue penetration and spatiotemporal control. Additionally, nanocomposites engineered from organic molecules and metallic NPs (particularly gold) have been widely implemented to enhance therapeutic interventions for various dental diseases while facilitating early-stage diagnosis. These engineered nanomaterials, designed to absorb photonic energy and subsequently transduce it through PDT, PTT, and other modalities, have effectively addressed the limitations inherent in conventional clinical techniques. This review examines the research progress over the past decade regarding rational design strategies for photosensitive nanomaterials, focusing on materials engineering approaches and underlying photo-physicochemical mechanisms. Nevertheless, these material functionalisation strategies encounter substantial challenges regarding mechanistic elucidation and clinical translation. We therefore propose feasible conceptual frameworks for photo-theranostics in oral healthcare from multiple perspectives, encompassing material design principles, optimisation of theranostic mechanisms, and technological advancements in light source instrumentation.(1) Simplify the structural design of nanomaterials:
To enhance the efficacy of nanomaterials, particle design continues to focus on multifunctional nanoplatforms with a “heterogeneous composite system” solution. However, such designs often involve complex synthetic procedures, unpredictable nucleation and growth dynamics, and potential interactions with biomacromolecules such as proteins in vivo. These factors increase the risk of generating undesirable by-products and complicate the assessment of therapeutic function, particularly for spatiotemporally controlled systems activated by external stimuli like light. In such cases, it is often challenging to determine whether the materials function as intended in vivo. For example, with layered encapsulation structures, it remains difficult to ascertain whether materials can sequentially target biological tissues and release therapeutic substances as designed in the oral microenvironment. Therefore, future material design should focus on minimising the diversity of material components through precise atomic-scale nanostructures. Alternatively, modulating the photophysical energy conversion pathways of nanomaterials could enhance the efficiency of both PDT and PTT within a single nanoagent. Critically, such streamlined material configurations could significantly facilitate biosafety evaluation and preclinical validation processes, which are critical steps in overcoming the translational barriers currently hindering clinical adoption of sophisticated nanomedicines.
(2) Absorption wavelength selection for nanomaterials:
Currently, most NIR-responsive nanomaterials in dentistry are concentrated in the 808 nm range. Compared to the NIR-I window, the NIR-II window offers enhanced tissue penetration depth, reduced photon scattering, and lower biological tissue absorption. Therefore, engineering nanomaterials with enhanced absorption ability in the NIR-II window (about 1000–1300 nm) is essential, particularly for managing deep-seated pathogens such as periapical inflammation. Since NIR-II responsive nanomaterials with high quantum yield frequently incorporate QDs (such as Ag2S), perovskites, single-walled carbon nanotubes and analogous nano-architectures, rigorous evaluation of biocompatibility parameters during material design is imperative. Therefore, designing nanomaterials with colloidal stability in aqueous solution, superior biocompatibility, and efficient NIR-II absorption capabilities is of paramount importance for advancing photo-therapeutic modality.
(3) Challenges of aPDT in periodontitis:
For aPDT, two primary challenges exist. Firstly, the ROS generated has a limited transmission distance, and biofilms or EPS matrix form protective barriers around pathogenic bacteria. To address this, the lethal effects of nanomaterials must occur near the bacteria. More efficiently, nanomaterials could be designed with an ultra-small size to penetrate the membrane pores of pathogenic bacteria (such as noble metal nanoclusters and other ultrasmall NPs), or target organelles such as mitochondria, thereby achieving bactericidal effects. Secondly, most aPDT approaches have focused on eradicating preexisting planktonic bacteria or biofilms, achieving remarkable efficacy. However, contemporary medical paradigms emphasise that “prevention supersedes treatment.” Consequently, there is a critical need to develop nano-engineered systems specifically designed for the prophylaxis of bacterial infectious diseases, thereby preventing quantitative imbalances of opportunistic pathogens or the aggregation of pathogenic bacterial biofilms.
(4) The physical removal mechanism for persistent structures:
The advantages of aPDT and PTT lie in their capacity to eliminate bacterial biofilms located in anatomically complex and deeper regions surrounding the tooth structure. However, when confronting recalcitrant biofilm architectures, physical forces demonstrate superior efficacy. Therefore, applying these forces through the nanostructures, such as those generated by brush- or knife-like structures, demonstrates significant bacterium-eliminating efficacy. For instance, magnetically controlled iron oxide NPs are applied for biofilm elimination.164 These NPs self-assemble into reconfigurable brush-like superstructures that adjust their morphology to accommodate complex topographies such as dental fissures.165 These structures generate multi-axial shear forces that physically dislodge biofilms. Similarly, the nano-knife mechanism employs vertically aligned graphene sheets with sharp nano-edges that penetrate bacterial cell membranes, causing cytoplasmic leakage and subsequent bacterial death.166 Combining the advantages of photo-active and magnetic-response, in Fe3O4@PEI/BiVO4 microrobots, the BiVO4 activates H2O2 decomposition into ROS under 395 nm excitation. Fe3O4 displays rolling microswarm under a magnetic field, inducing localised shear forces that mechanically disassemble biofilm matrices.167 This mechanical force also simultaneously ensures spatially uniform ROS penetration. The integrated magnetic propulsion system synergises photocatalytic action with physical biofilm disruption within multiple-species microgrooves. These physical removal approaches demonstrate superior efficacy and circumvent antimicrobial resistance issues. Future research should integrate physical removal mechanisms with photo-therapeutics to enhance bactericidal efficacy.
(5) Improving the portability of light device:
Various light sources, including lasers, xenon lamps, illumination torches, and dental curing lights, differ significantly in light intensity and energy density. However, photo-theranostics currently rely predominantly on high-power laser systems. These photonic devices pose challenges related to portability, high maintenance costs, and complex operational procedures during treatment. Therefore, investigating the feasibility of wearable, miniaturised photonic devices, such as luminescent dental braces, photoactivated periodontal probes, or specialised phototherapeutic instruments, merits thorough exploration to achieve enhanced precision and portability in localised photo-theranostic applications within the oral cavity. Furthermore, the rational implementation of photo-theranostic protocols requires establishing standardised irradiation parameters, specifically light intensity and exposure duration, to ensure compliance with clinically safe thresholds while maintaining therapeutic efficacy.
(6) Optimisation of theranostic protocols:
Engineering nanomaterials that respond to multiple light wavelengths shows promise for achieving synergistic effects within a single material. For instance, in bioimaging, employing different wavelengths of light can enhance resolution differentiation.168 Furthermore, beyond the therapeutic effects generated by the photo-stimulation of the materials themselves, integration with photobiomodulation effects could lead to synergistic therapeutic outcomes under multiple light irradiations. Therefore, designing nanomedicine strategies based on various light sources to achieve multifunctionality holds potential for improving photo-theranostic efficiency.169 However, it cannot be denied that multi-wavelength light complicates diagnostic and therapeutic procedures, making the exploration of therapeutic mechanisms more intricate. The balance between the complexity of material structure design and light source selection requires further research for proper evaluation.
(7) Design of integrated nanomaterials for theranostics:
Diagnosis and treatment have consistently progressed in tandem, with equal importance attributed to both domains. Employing theranostic nanoagents in the dental field would prove invaluable for the detection of occult oral diseases and prevention of their progression to more severe conditions, exemplified by the diagnosis and treatment of incipient periodontitis, dental caries and oral tumours, which ultimately contribute to improved oral health outcomes. However, the integrated nanoplatforms remain inadequately explored in dental research. Current integrated research mainly focuses on combining phototherapeutics with NIR bioimaging, which lacks early detection or high sensitivity detection ability. Nanomaterials (such as Au nanoclusters and QDs) show NIR absorption and photoluminescence properties, demonstrate theranostic properties in other diseases such as rheumatoid arthritis and tumours, thereby are excellent material candidates in the dental field.170 Meanwhile, implementing diagnostic-therapeutic integration through the design of new optical probes (or engineering on existing probes such as ICG) would significantly enhance theranostic efficacy for various pathologies.
(8) Challenges in translating nanomaterials to clinical applications:
In order to further link the chemical mechanisms reviewed with current clinical applications, we summarise the commercially available photosensitisers used in the field of oral biology (Table 1). These products utilise the mechanisms discussed earlier, providing practical solutions for clinical settings. By presenting this table, we aim to clearly establish the connection between fundamental chemical concepts and their real-world applications.
| Product | Active component | Light source/wavelength | Mechanism (PDT/PTT) | Indications |
|---|---|---|---|---|
| HELBO® | Phenothiazinium dye | Diode laser 660 nm (± 10 nm) | PDT | Periodontology and endodontics. |
| FotoSan® | Toluidine blue O | High-power LED 620–640 nm (peak ∼630 nm) | PDT | Adjunctive disinfection for periodontal pockets and endodontics. |
| Periowave™ | Methylene blue | Calibrated red diode (∼635 nm) | PDT | Periodontal disease, endodontics and peri-implantitis. |
| PACT® 500 | Toluidine blue | Red laser 650 nm | PDT | Periodontitis, peri-implantitis, endodontics and soft-tissue infections. |
| Perio green® | ICG | Diode laser 808 nm | PTT | Periodontitis and peri-implantitis. |
| EmunDo® | ICG-based chromophore | Diode laser 810 nm | PTT | Periodontics, peri-implantitis and oral mucositis. |
Although these photosensitiser products have been applied in clinical practice, the use of nanomaterials in oral biology still faces a range of challenges. Despite the enormous potential of nanomaterials, their translation from the laboratory to clinical practice remains hindered by several obstacles. Key issues include concerns regarding biocompatibility, potential toxicity, and the feasibility of scaling production. In addition, stringent regulatory requirements and the need for long-term clinical trials often delay the clinical adoption of novel nanomaterial-based therapies. Addressing these challenges is crucial for the successful integration of nanomaterials into routine clinical treatment.
In brief, photo-theranostics has emerged as a transformative paradigm in dental nanomedicine. The integration of nanotechnology with photo-theranostics has catalysed remarkable advances, particularly through the development of NIR-responsive theranostic nano-agents. This review examines the multifaceted functions of light-activated nanomaterials across the therapeutics-diagnostics continuum in the dental field. Certainly, the design strategies for nanomaterials should not be confined to the discrete performance classifications delineated herein, but rather embrace multifunctional synergistic approaches that transcend conventional categorisation. Despite the myriad complex and challenging processes that warrant further systematic investigation, we posit that the future trajectory of photo-theranostics in oral healthcare holds exceptional promise for revolutionising clinical practice and patient outcomes.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was supported by the National Science Foundation of China (82571148, 82170998, 62205122, 82301832), Jilin Provincial Science and Technology Department's Young Talent Training Program (20250602067RC), Science and Technology Project of Jilin Province Financial Department (JCSZ2025678-4, -11, -15, -19), Interdisciplinary Innovation Team Project of Norman Bethune Health Science Department Jilin University (2025JBGS04).Notes and references
- G. Feng, G.-Q. Zhang and D. Ding, Chem. Soc. Rev., 2020, 49, 8179–8234 RSC.
- Y. Wang, K. Ma, M. Kang, D. Yan, N. Niu, S. Yan, P. Sun, L. Zhang, L. Sun, D. Wang, H. Tan and B. Z. Tang, Chem. Soc. Rev., 2024, 53, 12014–12042 RSC.
- W. Hu, P. N. Prasad and W. Huang, Acc. Chem. Res., 2021, 54, 697–706 CrossRef CAS.
- J. Shi, Y. Fan, Q. Zhang, Y. Huang and M. Yang, Adv. Mater., 2025, 2501623 CrossRef CAS.
- K. Mizutani, A. Aoki, D. Coluzzi, R. Yukna, C. Wang, V. Pavlic and Y. Izumi, Periodontol. 2000, 2016, 71, 185–212 CrossRef.
- Q. Yu, C. Wang, X. Zhang, H. Chen, M. X. Wu and M. Lu, ACS Nano, 2024, 18, 14085–14122 CrossRef CAS PubMed.
- Q. Yang, X. Sun, Q. Ding, M. Qi, C. Liu, T. Li, F. Shi, L. Wang, C. Li and J. S. Kim, Natl. Sci. Rev., 2024, 11, nwae225 CrossRef CAS.
- Y. Huang, J. Li, Z. Yu, J. Li, K. Liang and Y. Deng, Adv. Mater., 2024, 36, 2414111 CrossRef CAS.
- X. Zhou, Z. Wang, Y. K. Chan, Y. Yang, Z. Jiao, L. Li, J. Li, K. Liang and Y. Deng, Adv. Funct. Mater., 2022, 32, 2109469 CrossRef CAS.
- C. Liu, T. Tian, Y. Shi, M. Li, L. Hong, J. Zhou, J. Liu, Y. Zhong, X. Wang, Z. Wang, X. Bai, L. Wang, C. Li and Z. Wu, Aggregate, 2025, 6, e666 CrossRef CAS.
- C. Song, D. Huang, C. Zhao and Y. Zhao, Adv. Sci., 2022, 9, 2202829 CrossRef CAS.
- H.-C. Flemming, E. D. Van Hullebusch, B. J. Little, T. R. Neu, P. H. Nielsen, T. Seviour, P. Stoodley, J. Wingender and S. Wuertz, Nat. Rev. Microbiol., 2025, 23, 87–105 CrossRef CAS.
- B. J. Kunath, C. De Rudder, C. C. Laczny, E. Letellier and P. Wilmes, Nat. Rev. Microbiol., 2024, 22, 791–805 CrossRef CAS PubMed.
- T. Turocy and J. M. Crawford, Nat. Rev. Microbiol., 2025, 23, 106–121 CrossRef CAS.
- J. Tao, Y. Sun, G. Wang, J. Sun, S. Dong and J. Ding, Bioact. Mater., 2025, 48, 474–492 CAS.
- W. Choi and J. Hong, Biomaterials, 2026, 325, 123610 CrossRef CAS.
- B. Jia, B. Zhang, J. Li, J. Qin, Y. Huang, M. Huang, Y. Ming, J. Jiang, R. Chen, Y. Xiao and J. Du, Chem. Soc. Rev., 2024, 53, 3273–3301 RSC.
- S. Zhang, N. Kong, Z. Wang, Y. Zhang, C. Ni, L. Li, H. Wang, M. Yang, W. Yang and F. Yan, Chem. Soc. Rev., 2024, 53, 3656–3686 RSC.
- D. S. W. Benoit, K. R. Sims and D. Fraser, ACS Nano, 2019, 13, 4869–4875 CrossRef CAS.
- Z. Yin, Y. Liu, A. Anniwaer, Y. You, J. Guo, Y. Tang, L. Fu, L. Yi and C. Huang, Adv. Mater., 2023, 2305633 Search PubMed.
- H. Wu, Y. Li, L. Shi, Y. Liu and J. Shen, Adv. Healthcare Mater., 2025, 14, 2403206 CrossRef CAS PubMed.
- X. Chen, N. Huang, D. Wang, M. Zhang, X. Deng, F. Guo, B. Yi, C. Yuan and Q. Zhou, ACS Nano, 2024, 18, 14312–14326 CrossRef CAS PubMed.
- Y. Xie, S. Xiao, L. Huang, J. Guo, M. Bai, Y. Gao, H. Zhou, L. Qiu, C. Cheng and X. Han, ACS Nano, 2023, 17, 15097–15112 CrossRef CAS.
- Y. Huang, Y. Liu, N. K. Pandey, S. Shah, A. Simon-Soro, J. C. Hsu, Z. Ren, Z. Xiang, D. Kim, T. Ito, M. J. Oh, C. Buckley, F. Alawi, Y. Li, P. J. M. Smeets, S. Boyer, X. Zhao, D. Joester, D. T. Zero, D. P. Cormode and H. Koo, Nat. Commun., 2023, 14, 6087 CrossRef CAS.
- C. Su, M. Zhu, Y. Guo, J. Sun, M. Liu, Y. Ma, Y. Xu, Y. Bai, X. Che and N. Zhang, Mater. Today Bio, 2025, 33, 101969 CrossRef CAS.
- M. Piksa, C. Lian, I. C. Samuel, K. J. Pawlik, I. D. W. Samuel and K. Matczyszyn, Chem. Soc. Rev., 2023, 52, 1697–1722 RSC.
- J. C. Scaiano, Chem. Soc. Rev., 2023, 52, 6330–6343 RSC.
- Y. Chen, S. Wang and F. Zhang, Nat. Rev. Bioeng., 2023, 1, 60–78 CrossRef CAS.
- E. L. Schmidt, Z. Ou, E. Ximendes, H. Cui, C. H. C. Keck, D. Jaque and G. Hong, Nat. Rev. Methods Primers, 2024, 4, 23 CrossRef CAS.
- Y. Kuwasaki, K. Suzuki, G. Yu, S. Yamamoto, T. Otabe, Y. Kakihara, M. Nishiwaki, K. Miyake, K. Fushimi, R. Bekdash, Y. Shimizu, R. Narikawa, T. Nakajima, M. Yazawa and M. Sato, Nat. Biotechnol., 2022, 40, 1672–1679 CrossRef CAS PubMed.
- V. V. Tuchin, E. A. Genina, E. S. Tuchina, A. V. Svetlakova and Y. I. Svenskaya, Adv. Drug Delivery Rev., 2022, 180, 114037 CrossRef CAS PubMed.
- C. Xu and K. Pu, Chem. Soc. Rev., 2021, 50, 1111–1137 RSC.
- J. Chen, Z. Ye, F. Yang and Y. Yin, Small Sci., 2021, 1, 2000055 CrossRef CAS PubMed.
- X. Pang, D. Li, J. Zhu, J. Cheng and G. Liu, Nano-Micro Lett., 2020, 12, 144 CrossRef CAS PubMed.
- N. K. Bennett, M. Lee, A. L. Orr and K. Nakamura, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2307904121 CrossRef CAS.
- E. F. F. Silva, C. Serpa, J. M. Dąbrowski, C. J. P. Monteiro, S. J. Formosinho, G. Stochel, K. Urbanska, S. Simões, M. M. Pereira and L. G. Arnaut, Chem. – Eur. J., 2010, 16, 9273–9286 CrossRef CAS PubMed.
- S. J. Dixon and B. R. Stockwell, Nat. Chem. Biol., 2014, 10, 9–17 CrossRef CAS.
- X. Li, J. Gao, C. Wu, C. Wang, R. Zhang, J. He, Z. J. Xia, N. Joshi, J. M. Karp and R. Kuai, Sci. Adv., 2024, 10, eadl0479 CrossRef CAS PubMed.
- Q. Ding, L. Ding, C. Xiang, C. Li, E. Kim, C. Yoon, H. Wang, M. Gu, P. Zhang, L. Wang, B. Z. Tang and J. S. Kim, Angew. Chem., Int. Ed., 2025, e202506505 CAS.
- M. Hasanzadeh Kafshgari and W. H. Goldmann, Nano-Micro Lett., 2020, 12, 22 CrossRef.
- M. Lan, S. Zhao, W. Liu, C. Lee, W. Zhang and P. Wang, Adv. Healthcare Mater., 2019, 8, 1900132 CrossRef.
- M. Li, Y. Xu, Z. Pu, T. Xiong, H. Huang, S. Long, S. Son, L. Yu, N. Singh, Y. Tong, J. L. Sessler, X. Peng and J. S. Kim, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2210504119 CrossRef CAS PubMed.
- P. E. Kolenbrander, R. J. Palmer, S. Periasamy and N. S. Jakubovics, Nat. Rev. Microbiol., 2010, 8, 471–480 CrossRef CAS.
- C. Zhou, Q. Tang, S. Yang, G. Zhao, T. Wang, Z. Chen, Y. Zhang, P. Tan and X. Ma, Responsive Mater., 2025, e20240043 CrossRef CAS.
- R. J. Lamont, H. Koo and G. Hajishengallis, Nat. Rev. Microbiol., 2018, 16, 745–759 Search PubMed.
- Z. Lu and J. A. Imlay, Nat. Rev. Microbiol., 2021, 19, 774–785 CrossRef CAS PubMed.
- H. Koo, R. N. Allan, R. P. Howlin, P. Stoodley and L. Hall-Stoodley, Nat. Rev. Microbiol., 2017, 15, 740–755 CrossRef CAS.
- R. P. Darveau, Nat. Rev. Microbiol., 2010, 8, 481–490 CrossRef CAS PubMed.
- S. H. L. Martins, A. B. Novaes, M. Taba, D. B. Palioto, M. R. Messora, D. M. Reino and S. L. S. Souza, J. Clin. Periodontol., 2017, 44, 717–728 CrossRef CAS.
- W. Xu, G. Huang, Z. Yang, Z. Deng, C. Zhou, J.-A. Li, M.-D. Li, T. Hu, B. Z. Tang and D. L. Phillips, Nat. Commun., 2024, 15, 2561 CrossRef CAS PubMed.
- D. Mizgalska, T. Goulas, A. Rodríguez-Banqueri, F. Veillard, M. Madej, E. Małecka, K. Szczesniak, M. Ksiazek, M. Widziołek, T. Guevara, U. Eckhard, M. Solà, J. Potempa and F. X. Gomis-Rüth, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2103573118 CrossRef CAS.
- Y. Wei, G. Dang, Z. Ren, M. Wan, C. Wang, H. Li, T. Zhang, F. R. Tay and L. Niu, npj Biofilms Microbiomes, 2024, 10, 56 CrossRef CAS PubMed.
- M. Wan, K. Jiao, Y. Zhu, Q. Wan, Y. Zhang, L. Niu, C. Lei, J. Song, W. Lu, H. Liu, Z. Ren, F. Tay and L. Niu, Nat. Biomed. Eng., 2024, 8, 1177–1190 CrossRef CAS PubMed.
- Y. Tian, Y. Song, J. Liu, S. Lan, B. Chen, Y. Li and J. Han, Colloids Surf., A, 2025, 708, 135988 CrossRef CAS.
- C. C. Tonon, B. Panariello, M. Chorilli, D. M. P. Spolidorio and S. Duarte, Photodiagn. Photodyn. Ther., 2022, 40, 103150 CrossRef CAS.
- Y. Liu, S. Xu, Q. Lyu, Y. Huang and W. Wang, Aggregate, 2024, 5, e443 CrossRef CAS.
- Z. Li, W. Pan, E. Shi, L. Bai, H. Liu, C. Li, Y. Wang, J. Deng and Y. Wang, ACS Biomater. Sci. Eng., 2021, 7, 772–786 CrossRef CAS PubMed.
- Y. Sun, X. Sun, X. Li, W. Li, C. Li, Y. Zhou, L. Wang and B. Dong, Biomaterials, 2021, 268, 120614 CrossRef CAS PubMed.
- D. Liu, X. Ma, Y. Ji, R. Chen, S. Zhou, H. Yao, Z. Zhang, M. Ye, Z. Xu and M. Du, Bioact. Mater., 2022, 14, 1–14 CAS.
- J. Shi, X. Qi, Y. Ran, Q. Zhou, Y. Ding, L. Li, Y. Zeng, D. Qiu, Z. Cai, X. Cai and Y. Pan, Bioact. Mater., 2025, 47, 212–228 CAS.
- Y. Qian, Y. Sun, L. Zhang, Y. Zhu, N. Li, F. Dong, C. Xu, N. An, H. Chen, Y. Sun, B. Yu, Y. Wang and F. Xu, Adv. Funct. Mater., 2024, 34, 2310636 CrossRef CAS.
- R. Ding, X. Liu, X. Zhao, Q. Sun, Y. Cheng, A. Li, D. Pei and G. He, Biomaterials, 2024, 307, 122536 CrossRef CAS.
- Q. Cao, X. Xiao, C. Tao, R. Shi, R. Lv, R. Guo, X. Li, B. Sui, X. Liu and J. Liu, Biomater. Sci., 2023, 11, 5680–5693 RSC.
- L. Zhang, D. Zhang, C. Liu, B. Tang, Y. Cui, D. Guo, M. Duan, Y. Tu, H. Zheng, X. Ning, Y. Liu, H. Chen, M. Huang, Z. Niu, Y. Zhao, X. Liu and J. Xie, Adv. Sci., 2024, 11, 2400882 CrossRef CAS.
- M. Qi, Q. Ding, Y. Shi, K. Wang, J. Liu, J. Zhou, W. Zhang, C. Liu, S. Liang, B. Dong, J. S. Kim and L. Wang, Biomaterials, 2026, 324, 123487 CrossRef CAS PubMed.
- R. Ding, Y. Li, Y. Zhang, Q. Sun, A. Li, K. Zhou, D. Pei and G. He, Aggregate, 2025, e70159 CrossRef.
- R. Ding, X. Liu, W. Zhang, X. Chen, S. Chen, X. Yu, Z. Zhao and K. Li, Adv. Funct. Mater., 2024, 34, 2405499 CrossRef CAS.
- S. Liang, M.-H. Li, M.-L. Qi, H. Hui, H.-P. Zhang, J. Zhou, L. Wang and Y.-W. Yang, Nano Lett., 2024, 24, 13708–13717 CrossRef CAS.
- W. Sun, J. Sun, Q. Ding, M. Qi, J. Zhou, Y. Shi, J. Liu, M. Won, X. Sun, X. Bai, B. Dong, J. S. Kim and L. Wang, Angew. Chem., 2024, 136, e202319690 CrossRef.
- Y. Tang, Y. Qi, Y. Chen, Y.-Q. Wang, C. Zhang, Y. Sun, C. Huang and X.-Z. Zhang, ACS Nano, 2024, 18, 21077–21090 CrossRef CAS.
- Y. Liu, Z. Yin, H. Wu, R. Li, J. Li, C. Wang, L. Yi, M. Zeng, C. Huang and L. Fu, Cell Biomater., 2025, 1, 100009 CrossRef.
- Q. Yao, J. Fan, S. Long, X. Zhao, H. Li, J. Du, K. Shao and X. Peng, Chem, 2022, 8, 197–209 CAS.
- X. Sun, J. Sun, Y. Sun, C. Li, J. Fang, T. Zhang, Y. Wan, L. Xu, Y. Zhou, L. Wang and B. Dong, Adv. Funct. Mater., 2021, 31, 2101040 CrossRef CAS.
- F. Dong, Y. Liu, H. Dong, X. Sun, B. Xu, Q. Wu, X. Xu, Y. Jiang, H. Liu and Y. Wang, Nano Today, 2024, 56, 102280 CrossRef CAS.
- B. Wang, L. Zhou, Y. Guo, H. Guo, Y. Zhong, X. Huang, Y. Ge, Q. Wang, X. Chu, Y. Jin, K. Lan, M. Yang and J. Qu, Bioact. Mater., 2022, 12, 314–326 CAS.
- J. Gong, S. Wang, J. Liu, Y. Zhang, J. Li, H. Yang, K. Liang and Y. Deng, Chem. Eng. J., 2024, 498, 155083 CrossRef CAS.
- J. Chen, H. Zhang, T. Zhao, Y. Yu, J. Song, Y. Zhao, H. Alshawwa, X. Zou and Z. Zhang, Adv. Healthcare Mater., 2024, 13, 2302926 CrossRef CAS PubMed.
- W. Wang, Y. Hu, Z. Chen, L. Yu, S. Huang, Y. Zhang, J. Li, Y. Xue, A. Li, Y. Wang, Z. Wu and X. Zhang, Adv. Funct. Mater., 2023, 33, 2300474 CrossRef CAS.
- K. Zhou, L. Du, R. Ding, L. Xu, S. Shi, S. Wang, Z. Wang, G. Zhang, G. He, Z. Zhao and B. Z. Tang, Nat. Commun., 2024, 15, 10551 CrossRef CAS.
- H. Huang, S. Banerjee, K. Qiu, P. Zhang, O. Blacque, T. Malcomson, M. J. Paterson, G. J. Clarkson, M. Staniforth, V. G. Stavros, G. Gasser, H. Chao and P. J. Sadler, Nat. Chem., 2019, 11, 1041–1048 CrossRef CAS PubMed.
- J. Tan, H. Zhang, Y. Liu, Z. Hou, D. Wang, J. Zhou, Y. Cao, S. Qian, B. Zheng, J. Nie, Y. Cui, Y. Du, K. Huang, S. Yang, D. Chen and X. Liu, Sci. Adv., 2025, 11, eadt3159 CrossRef CAS.
- J. Gao, J. Wang, X. Yue, Y. Zhou, M. Wang, Y. Sun, Q. Zhang, Z. Gao, G.-Q. Zhang, J. Shen and D. Ding, ACS Appl. Nano Mater., 2022, 5, 5944–5951 CrossRef CAS.
- H. Zhang, Y. Zhu, Y. Li, X. Qi, J. Yang, H. Qi, Q. Li, Y. Ma, Y. Zhang, X. Zhang and L. Zhang, Adv. Funct. Mater., 2021, 31, 2104799 CrossRef CAS.
- M. Gu, S. Jiang, X. Xu, M. Wu, C. Chen, Y. Yuan, Q. Chen, Y. Sun, L. Chen, C. Shen, P. Guo, S. Liu, E. Zhao, S. Chen and S. Chen, Adv. Sci., 2022, 9, 2106071 CrossRef CAS PubMed.
- H. Liu, Y. Chen, L. Mo, F. Long, Y. Wang, Z. Guo, H. Chen, C. Hu and Z. Liu, ACS Nano, 2023, 17, 21195–21205 CrossRef.
- C. Song, X. Wu, Y. Wang, J. Wang and Y. Zhao, Small, 2024, 20, 2310444 CrossRef CAS.
- Z. Xiu, Y. Zhu, X. Li, X. Jiang, Y. Feng, L. He, C. Li, R. Cai and G. Tao, Theranostics, 2025, 15, 3750–3780 CrossRef CAS PubMed.
- S. Pan, Y. Lan, Y. Yang, Y. Zhang, S. Yu, T. Wang, Y. Zhu, S. Yang, X. Yuan, X. Gao and J. Song, ACS Nano, 2025, 19, 23919–23944 CrossRef CAS PubMed.
- L. Xiao, M. Feng, C. Chen, Q. Xiao, Y. Cui and Y. Zhang, Adv. Mater., 2023, 2304982 Search PubMed.
- E. Shi, L. Bai, L. Mao, H. Wang, X. Yang, Y. Wang, M. Zhang, C. Li and Y. Wang, J. Nanobiotechnol., 2021, 19, 413 CrossRef CAS PubMed.
- Y. Zhang, Z.-T. Jiang, Y. Wang, H.-Y. Wang, S. Hong, W. Li, D.-S. Guo and X. Zhang, ACS Nano, 2024, 18, 27340–27357 CrossRef CAS PubMed.
- K. Villa and M. Pumera, Chem. Soc. Rev., 2019, 48, 4966–4978 RSC.
- X. Bai, W. Peng, Y. Tang, Z. Wang, J. Guo, F. Song, H. Yang and C. Huang, Bioact. Mater., 2024, 41, 271–292 CAS.
- M. Han, K. Tang and Z. Chen, Chem. Eng. J., 2025, 504, 158774 CrossRef CAS.
- Y. Yu, Y. Zhang, Y. Cheng, Y. Wang, Z. Chen, H. Sun, X. Wei, Z. Ma, J. Li, Y. Bai, Z. Wu and X. Zhang, Bioact. Mater., 2022, 13, 269–285 CAS.
- B. Cao, J. Zhang, Y. Ma, Y. Wang, Y. Li, R. Wang, D. Cao, Y. Yang and R. Zhang, Small, 2024, 20, 2400771 CrossRef CAS PubMed.
- Q. Li, J. Liu, H. Liu, Y. Sun, Y. Xu, K. Wang, W. Huang, L. Liao and X. Wang, Bioact. Mater., 2023, 29, 72–84 CAS.
- Q. Chen, M. Qi, F. Shi, C. Liu, Y. Shi, Y. Sun, X. Bai, L. Wang, X. Sun, B. Dong and C. Li, Adv. Healthcare Mater., 2023, 12, 2300313 CrossRef CAS PubMed.
- P. Wang, L. Wang, Y. Zhan, Y. Liu, Z. Chen, J. Xu, J. Guo, J. Luo, J. Wei, F. Tong and Z. Li, Chem. Eng. J., 2023, 463, 142293 CrossRef CAS.
- S. Tao, Y. Yang, C. Wu, J. Yang, Z. Wang, F. Zhou, K. Liang, Y. Deng, J. Li and J. Li, Adv. Sci., 2025, 12, 2411274 CrossRef CAS.
- Y. Tian, Y. Li, J. Liu, Y. Lin, J. Jiao, B. Chen, W. Wang, S. Wu and C. Li, Bioact. Mater., 2022, 9, 428–445 CAS.
- T. Li, M. Shu, C. Zhu, Q. Liu, Y. Li, R. Wang, L. Chen, W. Shi, Z. Sun, Z. Hou, B. Fang and L. Xia, Adv. Sci., 2025, 12, 2413891 CrossRef CAS.
- H. Shi, H. Yang, C. Wu, S. Wang, S. He, L. Chen, Y. K. Chan, S. Lai, K. Liang and Y. Deng, Biomaterials, 2025, 321, 123334 CrossRef CAS PubMed.
- M. Qi, X. Ren, W. Li, Y. Sun, X. Sun, C. Li, S. Yu, L. Xu, Y. Zhou, S. Song, B. Dong and L. Wang, Nano Today, 2022, 43, 101447 CrossRef CAS.
- H. Yao, E. S. Turali Emre, Y. Fan, J. Wang, F. Liu and J. Wei, Bioact. Mater., 2025, 48, 200–216 CAS.
- X. Wu, M. Qi, C. Liu, Q. Yang, S. Li, F. Shi, X. Sun, L. Wang, C. Li and B. Dong, Theranostics, 2023, 13, 2350–2367 CrossRef CAS PubMed.
- Z. Li, X. Fan, Y. Liu, M. Yue, T. Wu, X. Wang, W. Jiang and K. Fan, Adv. Mater., 2025, 37, 2409840 CrossRef CAS.
- J. Li, M. Fan, Z. Jiao, Y. K. Chan, L. Cheng, J. Li, Y. Deng and K. Liang, Nano Today, 2024, 59, 102541 CrossRef CAS.
- M. Zhou, G. Li, J. Yu, Q. Zhou, K. Wang, J. Kang, T. Wang, P. Li and H. Wei, Bioact. Mater., 2024, 40, 318–333 CAS.
- J. Lin, J. Fang, J. Zhou, M. Qi, Y. Shi, C. Li, X. Sun, B. Dong and L. Wang, Acta Biomater., 2024, 187, 396–408 CrossRef CAS.
- X. Dai, Y. Liu, F. Meng, Q. Li, F. Wu, J. Yuan, H. Chen, H. Lv, Y. Zhou and Y. Chang, Acta Biomater., 2023, 171, 519–531 CrossRef CAS PubMed.
- W. Liu, E. Shi, H. Wu, Y. Liang, M. Chen, H. Zhang, R. Zhang, X. Li, Y. Wang and L. Zhang, Adv. Funct. Mater., 2024, 34, 2403386 CrossRef CAS.
- L. Chen, M. Peng, H. Li, J. Zhou, W. He, R. Hu, F. Ye, Y. Li, L. Shi and Y. Liu, Adv. Mater., 2024, 36, 2306376 CrossRef CAS.
- H. Chen, N. Yu, J. Wang, S. Zhang, L. Cao, M. Zhou, Z. Xu, S. Lin, S. Yin, X. Jiang and M. Zhu, Nano Today, 2024, 56, 102297 CrossRef CAS.
- X. Duan, Q. Zhang, Y. Jiang, X. Wu, X. Yue, Y. Geng, J. Shen and D. Ding, Adv. Mater., 2022, 34, 2200179 CrossRef CAS PubMed.
- Y. Zhou, D. Li, X. Yue, Y. Shi, C. Li, Y. Wang, Y. Chen, Q. Liu, D. Ding, D. Wang and J. Shen, Adv. Healthcare Mater., 2024, 13, 2401434 CrossRef CAS.
- H. Gao, L. Zhang, X. Lian, Y. Wang, S. Jiang, G. Wang, X. Dai, H. Zou and D. Ding, Mater. Chem. Front., 2021, 5, 3388–3395 RSC.
- Q. Wang, G. Wang, X. Li, D. Li, C. Zhang and J. Ding, Adv. Sci., 2025, 12, 2412561 CrossRef CAS.
- Y. Zhang, K. Liu, Q. Sun, Y. Qi, F. Li, X. Su, M. Song, R. Lv, H. Sui, Y. Shi and L. Zhao, ACS Nano, 2025, 19, 16448–16468 CrossRef CAS PubMed.
- Y. Xu, X. Zhang, A. Zhou, C. Cheng, K. Chen, X. Zhou, G. Zhang, L. Ding, X. Wu, H. Ge, H. Wu and X. Ning, Adv. Mater., 2023, 35, 2207384 CrossRef CAS.
- L. Rao, L. Bu, L. Ma, W. Wang, H. Liu, D. Wan, J. Liu, A. Li, S. Guo, L. Zhang, W. Zhang, X. Zhao, Z. Sun and W. Liu, Angew. Chem., Int. Ed., 2018, 130, 998–1003 CrossRef.
- E. Shi, T. Shan, H. Wang, L. Mao, Y. Liang, M. Cao, Q. Wu, C. Li, Y. Wang and Y. Wang, Small, 2023, 19, 2304014 CrossRef CAS.
- C. Wang, S. Wu, C. Mao, L. Jin, H. Liu, Y. Zheng, C. Liang, S. Zhu, Z. Li, H. Jiang and X. Liu, Adv. Sci., 2025, e03186 CrossRef CAS.
- J. Zhu, L. He, X. Xu, H. Wu, J. Li, B. Yan, Y. Deng, Y. Gao and K. Liang, J. Dent. Res., 2025, 104(10), 1095–1104 CrossRef CAS.
- B. Li, D. Chu, H. Cui, Z. Li, Z. Zhou, C. Tan and J. Li, SmartMat, 2023, 4, e1243 CrossRef CAS.
- Y. Tang, K. Wang, B. Wu, K. Yao, S. Feng, X. Zhou and L. Xiang, Adv. Mater., 2024, 36, 2307756 CrossRef CAS.
- Y. Mu, Y. Wang, L. Huang, Z. Weng, T. Zhong, S. Yu, Y. Wen, Y. Xu and X. Wang, Bioact. Mater., 2025, 47, 403–416 CAS.
- J. Li, S. Song, J. Meng, L. Tan, X. Liu, Y. Zheng, Z. Li, K. W. K. Yeung, Z. Cui, Y. Liang, S. Zhu, X. Zhang and S. Wu, J. Am. Chem. Soc., 2021, 143, 15427–15439 CrossRef CAS PubMed.
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Chem. Soc. Rev., 2015, 44, 1449–1478 RSC.
- M. Qi, X. Li, X. Sun, C. Li, F. R. Tay, M. D. Weir, B. Dong, Y. Zhou, L. Wang and H. H. K. Xu, Dent. Mater., 2019, 35, 1665–1681 CrossRef CAS.
- D. Hu, C. Zhang, C. Sun, H. Bai, J. Xie, Y. Gu, M. Li, J. Jiang, A. Le, J. Qiu and X. Wang, Nano Res., 2023, 16, 7199–7215 CrossRef CAS.
- B. Chen, W. Wang, M. Hu, Y. Liang, N. Wang, C. Li and Y. Li, ACS Nano, 2024, 18, 24968–24983 CrossRef CAS.
- Q. Wu, T. Deng, J. Cheng, Y. Yang, Y. Wang, Y. Xiao and Y. Zhang, ACS Nano, 2024, 18, 11988–12009 CrossRef CAS.
- Q. Kong, M. Qi, W. Li, Y. Shi, J. Su, S. Xiao, J. Sun, X. Bai, B. Dong and L. Wang, Small, 2023, 19, 2302547 CrossRef CAS.
- S. Li, Q. Ding, L. Zhang, F. Shi, C. Liu, T. Li, Y. Shi, M. Qi, L. Wang, B. Dong, S. Song, J. Sun, J. S. Kim and C. Li, J. Controlled Release, 2024, 370, 600–613 CrossRef CAS PubMed.
- Z. Wang, Y. Huang, S. He, M. Li, J. Gong, L. Cheng, J. Li, Y. Deng and K. Liang, ACS Nano, 2025, 19, 18804–18823 CrossRef CAS PubMed.
- M. Ussia, M. Urso, S. Kment, T. Fialova, K. Klima, K. Dolezelikova and M. Pumera, Small, 2022, 18, 2200708 CrossRef CAS.
- Q. Zhang, Y. Zhang, W. Wu, Y. Wu, Z. Jiang and N. Hu, J. Mater. Chem. B, 2025, 13, 1643–1652 RSC.
- S. Wan, W. Liu, Q. Wu, K. Wang, Y. Li, P. Huang, Y. Wu, Y. Mu, Y. Fan, J. Tao, J. Yao, F. Peng, Y. Zou, L. Wang, Z. Yuan and X. Ding, ACS Nano, 2024, 18, 32899–32909 CrossRef CAS.
- H. Wang, J. Tian, Y. Jiang, S. Liu, J. Zheng, N. Li, G. Wang, F. Dong, J. Chen, Y. Xie, Y. Huang, X. Cai, X. Wang, W. Xiong, H. Qi, L. Yin, Y. Wang and X. Sheng, Sci. Adv., 2023, 9, eabq7750 CrossRef CAS.
- O. Karatum, M.-J. Gwak, J. Hyun, A. Onal, G. R. Koirala, T. Kim and S. Nizamoglu, Chem. Soc. Rev., 2023, 52, 3326–3352 RSC.
- Q. Lu, Y. Sun, Z. Liang, Y. Zhang, Z. Wang and Q. Mei, ACS Nano, 2024, 18, 14123–14144 CrossRef CAS.
- L. Zhang, Y. Li, L. Yuan, Q. Zhang, Y. Yan, F. Dong, J. Tang and Y. Wang, Adv. Sci., 2023, 10, 2203472 CrossRef CAS.
- L. Yuan, Y. Wang, Y. Zong, F. Dong, L. Zhang, G. Wang, H. Dong and Y. Wang, J. Photochem. Photobiol., B, 2023, 241, 112670 CrossRef CAS PubMed.
- B. Palmisano, A. D. Vecchio, A. Passaretti, A. Stefano, G. Miracolo, G. Farinacci, A. Corsi, M. Riminucci, U. Romeo and A. Cicconetti, Lasers Med. Sci., 2024, 39, 247 CrossRef.
- Y. He, J. Leng, K. Li, K. Xu, C. Lin, Z. Yuan, R. Zhang, D. Wang, B. Tao, T. J. Huang and K. Cai, Biomaterials, 2021, 278, 121164 CrossRef CAS.
- Y. Jiang, W. Qi, Q. Zhang, H. Liu, J. Zhang, N. Du, R. Nazempour, Y. Su, R. Fu, K. Zhang, P. Lyu, F. Dong, L. Yin, X. Sheng and Y. Wang, Small Methods, 2020, 4, 1900879 CrossRef CAS.
- Q. Cui, L. Yu, X. Song, Y. Wei, Y. Zou and X. Wu, Adv. Funct. Mater., 2025, 2420551 CrossRef CAS.
- H. Chen, Z. Zhao, R. Zhang, G. Zhang, X. Liang, C. Xu, Y. Sun, Y. Li, C. Boyer and F. Xu, Adv. Mater., 2025, 37, 2413373 CrossRef CAS.
- X. Ge, J. Hu, X. Qi, Y. Shi, X. Chen, Y. Xiang, H. Xu, Y. Li, Y. Zhang, J. Shen and H. Deng, Adv. Mater., 2025, 37, 2412240 CrossRef CAS.
- X. Hu, L. Xie, Z. Xu, F. Huo, G. Shu, K. He, L. Fan, R. Zhang, S. Liu, Y. Mai, M. He, L. Liao, W. Tang and W. Tian, Adv. Funct. Mater., 2024, 34, 2314034 CrossRef CAS.
- Y. Wang, Y. Yuan, R. Wang, T. Wang, F. Guo, Y. Bian, T. Wang, Q. Ma, H. Yuan, Y. Du, J. Jin, H. Jiang, F. Han, J. Jiang, Y. Pan, L. Wang and F. Wu, Adv. Healthcare Mater., 2024, 13, 2400533 CrossRef CAS.
- M. Arseneault, V. Granskog, S. Khosravi, I. M. Heckler, P. Mesa-Antunez, D. Hult, Y. Zhang and M. Malkoch, Adv. Mater., 2018, 30, 1804966 CrossRef PubMed.
- V. Granskog, S. García-Gallego, J. Von Kieseritzky, J. Rosendahl, P. Stenlund, Y. Zhang, S. Petronis, B. Lyvén, M. Arner, J. Håkansson and M. Malkoch, Adv. Funct. Mater., 2018, 28, 1800372 CrossRef.
- K. H. Vining, J. C. Scherba, A. M. Bever, M. R. Alexander, A. D. Celiz and D. J. Mooney, Adv. Mater., 2018, 30, 1704486 CrossRef PubMed.
- X. Li, C. Luo, Q. Fu, C. Zhou, M. Ruelas, Y. Wang, J. He, Y. Wang, Y. S. Zhang and J. Zhou, Adv. Mater., 2020, 32, 2000060 CrossRef CAS PubMed.
- J. Xue, H. Dong, L. Ji, Y. Wang and J. Zhang, ACS Appl. Nano Mater., 2023, 6, 14431–14438 CrossRef CAS.
- X. Guo, Y. Zhao, X. Xu, Z. Wu, L. Xiao, X. Ma, Z. Lei, Y. Zhao, C. Zhang, L. Song and M. Yin, Sens. Actuators, B, 2026, 446, 138642 CrossRef CAS.
- C. Moore, Y. Cheng, N. Tjokro, B. Zhang, M. Kerr, M. Hayati, K. C. J. Chang, N. Shah, C. Chen and J. V. Jokerst, Angew. Chem., 2022, 134, e202201843 CrossRef.
- M. Retout, V. Lepeintre, L. Amer, W. Yim and J. V. Jokerst, ACS Nano, 2025, 19, 12041–12052 CrossRef CAS PubMed.
- Y. Zhang, W. Wang, X. Yang, X. An, X. Liu, W. Zhao, L. Zhu, T. Wang, Y. Wang, Y. Chen, J. Feng, J. Shao, X. Zhou, B. Z. Tang, S. Ge and J. Li, Aggregate, 2025, e733 CrossRef CAS.
- R. Ankri, A. Ashkenazy, Y. Milstein, Y. Brami, A. Olshinka, N. Goldenberg-Cohen, A. Popovtzer, D. Fixler and A. Hirshberg, ACS Nano, 2016, 10, 2349–2356 CrossRef CAS.
- M. Retout, L. Amer, W. Yim, M. N. Creyer, B. Lam, D. F. Trujillo, J. Potempa, A. J. O’Donoghue, C. Chen and J. V. Jokerst, ACS Nano, 2023, 17, 17308–17319 CrossRef CAS PubMed.
- A. A. Balhaddad, Y. Xia, Y. Lan, L. Mokeem, M. S. Ibrahim, M. D. Weir, H. H. K. Xu and M. A. S. Melo, ACS Nano, 2021, 15, 19888–19904 CrossRef CAS.
- M. J. Oh, A. Babeer, Y. Liu, Z. Ren, J. Wu, D. A. Issadore, K. J. Stebe, D. Lee, E. Steager and H. Koo, ACS Nano, 2022, 16, 11998–12012 CrossRef CAS PubMed.
- M. Xing, W. Qian, K. Ye, H. Zhang, J. Feng, X. Liu and J. Qiu, Biomaterials, 2025, 320, 123251 CrossRef CAS PubMed.
- C. C. Mayorga-Martinez, J. Zelenka, K. Klima, P. Mayorga-Burrezo, L. Hoang, T. Ruml and M. Pumera, ACS Nano, 2022, 16, 8694–8703 CrossRef CAS.
- E. D. Cosco, A. L. Spearman, S. Ramakrishnan, J. G. P. Lingg, M. Saccomano, M. Pengshung, B. A. Arús, K. C. Y. Wong, S. Glasl, V. Ntziachristos, M. Warmer, R. R. McLaughlin, O. T. Bruns and E. M. Sletten, Nat. Chem., 2020, 12, 1123–1130 CrossRef CAS.
- Q. Ding, J. Liu, Y. Wang, J. Kim, Z. Huang, Y. Lee, H. Zhou, P. Zhang, J. L. Sessler and J. S. Kim, Proc. Natl. Acad. Sci. U. S. A., 2025, 122, e2503574122 CrossRef.
- A. Baghdasaryan and H. Dai, Chem. Rev., 2025, 125, 5195–5227 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |