Supramolecular dyes: advancing precision medicine through molecular engineering
Jun
Li†
 b,
Yuling
Xu†
f,
Yida
Pang†
e,
Fang
Zhao†
e,
Wenjun
Zhang
b,
Chonglu
Li
b,
Yuling
Xu†
f,
Yida
Pang†
e,
Fang
Zhao†
e,
Wenjun
Zhang
b,
Chonglu
Li
 *c,
Honglin
Jin
*c,
Honglin
Jin
 *a,
Chao
Yuan
*d,
Suhua
Wang
*a,
Chao
Yuan
*d,
Suhua
Wang
 *d and
Yao
Sun
*agh
*d and
Yao
Sun
*agh
aCollege of Biomedicine and Health, Huazhong Agricultural University, Wuhan, 430070, China. E-mail: sunyaogbasp@mail.hzau.edu.cn; jin@mail.hzau.edu.cn
bCollege of Chemistry, Huazhong Agricultural University, Wuhan, 430070, China
cHubei Province Key Laboratory of Occupational Hazard Identification and Control, School of Medicine, School of Public Health, Wuhan University of Science and Technology, Wuhan 430065, China. E-mail: lichonglu@wust.edu.cn
dGuangdong Provincial Key Laboratory for Green Agricultural Production and Intelligent Equipment, School of Environmental Science and Engineering, Guangdong University of Petrochemical Technology, Maoming 525000, China. E-mail: yuanchao@gdupt.edu.cn; wangsh@gdupt.edu.cn
eNational Key Laboratory of Green Pesticide, College of Chemistry, Central China Normal University, Wuhan 430079, China
fDepartment of Chemistry, College of Science, Korea University, Seoul 02841, Korea
gNational Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, 430070, China
hHubei Jiangxia Laboratory, Wuhan 430200, China
First published on 28th November 2025
Abstract
Precision medicine is aimed at achieving a more personalized approach tailored to individual characteristics and urgently requires the development of precise diagnostic and therapeutic methods. Small-molecule dyes play indispensable roles in medical imaging and surgery procedures, attracting significant attention regarding disease diagnosis and therapy. However, their widespread utilization for accurate tumor localization and long-term intraoperative imaging remains hindered by their inherent limitations, including tedious synthesis protocols, poor photostability, susceptibility to fluorescence quenching in physiological environments, and rapid systemic clearance. Supramolecular dyes, defined as small-molecule dye-based assemblies, usually present unique and superior photophysical properties, including tunable optical properties, enhanced photodynamic and photothermal performance, improved photostability and optimized anti-quenching capability, collectively enabling high-precision optical diagnosis and therapy. Despite remarkable progress in supramolecular dyes, a systemic review summarizing their applications in precision biomedicine remains lacking. In this review, we systematically summarize the recent advances on the development of supramolecular dyes across three key self-assembly systems: supramolecular coordination complexes (SCCs) systems, host–guest systems (including cyclodextrin, cucurbit[n]urils (CB [n]s), calixarenes and pillararenes), and enzyme instructed self-assembly (EISA) systems. Moreover, we highlight current challenges and future perspectives to accelerate their translation from fundamental research to clinical applications.
 Wenjun Zhang | Wenjun Zhang is currently a Master's student at Huazhong Agricultural University, working on a project of small-molecule based phototherapy. |
Key learning points(1) The basic concept of supramolecular dyes.(2) Definition of the relationship between small-molecule dyes and supramolecular dyes. (3) The advantages of supramolecular dyes in biomedicine. (4) The classic types of supramolecular dyes in precision medicine. (5) The new perspectives in the future development of supramolecular dyes for precision medicine. |
1. Introduction
Since the COVID-19 pandemic, public health has come to the forefront of global attention.1,2 Concurrently, various diseases such as cancer, cardiovascular disorders, diabetes, and chronic respiratory diseases continue to seriously impact global health, accounting for the majority of deaths and seriously affecting human health around the world.3–6 Therefore, precision medicine, an initiative launched by former U.S. President Barack Obama in 2015, is aimed at advancing a personalized healthcare approach based on individual characteristics.7 Similarly, “Healthy China,” which was launched by the Chinese government, plans to enhance population health outcomes and reduce the proportion of out-of-pocket health expenditure to approximately 25% by 2030.8,9 To achieve precision medicine, it is essential to develop precise diagnostic and therapeutic methods that are significant to improve the cure rates.Small-molecule dyes play an indispensable role in biomedicine, attracting significant attention regarding disease diagnosis and therapy.10–15 They possess a plethora of advantages, including feasible synthesis and structural modification, favorable optical properties, rapid metabolism and excellent biosafety.16–24 Fluorescein and rhodamine were first discovered in the 19th century, laying the groundwork for the development of their derivatives as powerful imaging tools in the fields of chemical biology and biochemistry.25,26 The 21st century witnessed an explosive growth in small-molecule dye innovation for biomedicine.27,28 Indocyanine green (ICG) and methylene blue (MB) have been clinically approved for tumor imaging and surgical guidance. However, their widespread utilization is still hampered by limitations such as poor photostability, susceptibility to fluorescence quenching, and rapid systemic clearance, which hinder accurate tumor localization and long-term intraoperative imaging.29–31 Traditional approaches to optimizing optical properties primarily rely on tedious organic synthesis.
Thus, developing efficient chemical strategies to address these challenges holds significant importance for advancing their clinical applications. In this context, supramolecular chemistry has emerged as a particularly promising approach.32,33 Celebrated by two Nobel Prizes (1987 and 2016), this discipline enables the construction of macromolecular systems through non-covalent interactions such as hydrogen bonding, coordination, π–π stacking, hydrophobic interactions or electrostatic interactions.34 Supramolecular dyes, defined as assemblies formed by small-molecule dyes, typically exhibit unique optical properties including tunable optical properties, enhanced photodynamic and photothermal properties, improved photo-stability and optimized anti-quenching capability. These features provide precise spatiotemporal control over their photophysical and therapeutic functions. Consequently, supramolecular dyes have been extensively employed in diverse biological applications, including chemo-sensors, optical imaging, and phototherapy.35
Recently, a variety of host–guest systems have been developed to construct supramolecular dyes, aiming to overcome the inherent limitations of traditional small-molecule dyes. For example, supramolecular coordination complexes (SCCs), assembled through metal–ligand coordination bonds, have demonstrated superior photostability, excellent anti-quenching capabilities and enhanced chemo-phototherapeutic efficacy.36 Incorporation of cyclodextrins (CDs) has significantly improved water solubility, chemical stability, and biocompatibility, thereby elevating the biological applicability of the small-molecule dyes.37 Host–guest interactions with cucurbit[n]urils (CB [n]s, where n = 5–8, 10, and 13–15) not only stabilize the dyes but also modulate their optical properties, further expanding their functional applications.38 Additionally, conjugation of small-molecule dyes with calixarenes and pillararenes (macrocyclic structures comprising phenolic or aromatic units linked by methylene bridges) leads to improved water solubility and strong binding affinity toward diverse guests.39 Meanwhile, enzyme-mediated in situ self-assembly offers distinct advantages, including high spatiotemporal resolution, enhanced accumulation at target sites, improved stability, and minimal off-target effects. This approach enables precise visualization of pathological processes such as tumor progression, inflammation, and apoptosis.40,41 These innovative strategies have opened new opportunities for advancing precision medicine.
Despite significant progress in supramolecular dyes, a systemic review of their applications in precision biomedicine remains scarce. In this review, we systematically summarize recent advances on the design strategies of supramolecular dyes including SCCs systems, host–guest systems (cyclodextrin, CB [n]s, calixarenes and pillararenes), and enzyme instructed self-assembly (EISA) of small-molecule systems (Scheme 1). We further highlight their applications in molecular recognition, bioimaging and therapy. More importantly, this review not only points out the current challenges in the development of supramolecular dyes, but also provides future perspectives to bridge the gap between fundamental research and clinical translation.
2. Supramolecular dyes based on supramolecular coordination complexes (SCCs) systems for precision medicine
Due to the directional and reversible coordination between metal ions and ligands, discrete SCCs with definite dimensions and shapes can be precisely designed and constructed through self-assembly. SCCs that incorporate small-molecule dyes assemble through metal–ligand coordination combined with supramolecular non-covalent forces and exhibit unique characteristics, including inherent ring strain, positive charge, tunable cavity size, and suppressed aggregation tendency. The assembly of supramolecular metal–ligand coordination complexes is guided by complementary building blocks, where coordination bonds provide energies intermediate between strong covalent bonds and weak noncovalent interactions.42–44 By incorporating functional small-molecule dyes into the rigid scaffold of SCCs, their photophysical properties, especially the transition between ground and excited states, could be effectively modulated. For instance, employing long-wavelength dyes as building blocks allows the construction of near-infrared emission/absorption supramolecular dyes.45 Leveraging aggregation-induced emission (AIE) characteristics further facilitates the development of supramolecular systems with superior luminescence performance in both solution and solid states. The rigid framework of these SCCs effectively restricts the intramolecular dyes’ motion, leading to significantly enhanced luminescence efficiency and reducing the aggregation caused quenching (ACQ) effect. Furthermore, in SCC systems, the incorporation of heavy metal atoms strongly promotes intersystem crossing (ISC) in dyes.46–48 Through systematic research and precise engineering of SCCs, these supramolecular dyes have shown broad application potential in fluorescence sensing, photocatalysis, and biomedicine.2.1 SCCs optimized the optical properties of small-molecule dyes
BODIPY dyes exhibit significant advantages in biological applications. Characterized by their exceptional optical properties, structural versatility and superior biocompatibility, these dyes demonstrate a high fluorescence quantum yield and good photo-stability, making them suitable for long-term dynamic imaging and complex experimental environments. The integration of BODIPY into SCCs has overcome a critical limitation of conventional Pt-based chemotherapeutic SCCs, a lack of intrinsic fluorescence, thereby enabling fluorescence imaging-guided cancer therapy. Leveraging these advantages, Timothy R. Cook et al. reasonably designed and synthesized two Pt(II)-based SCCs 1 and 2,49 employing BODIPY as the fluorescent skeleton with excellent NIR emission for biological imaging. In terms of structural design, the introduction of ethyl spacer groups altered the steric distance to Pt(II), which could alleviate the heavy atom effect on the luminescence intensity (Fig. 1(a)). Spectral analysis revealed that 1 and 2 displayed similar absorption bands at 556 nm, corresponding to the π–π* transition of the BODIPY moiety. Upon excitation at 365 nm, both SCCs 1 and 2 exhibited strong fluorescence with emission at ∼577 nm, characteristic of the BODIPY dye (Fig. 1(b)). Notably, SCCs 1 and 2 maintained good fluorescence performance in different solvents (Fig. 1(c)). The fluorescence quantum yields (ΦF) of the BODIPY ligand and SCCs 1 and 2 were determined using Rhodamine B as a reference. The results verified that compared to the prior BODIPY ligand (53.1% in DCM), SCCs 1 (57.9% in DCM) and SCCs 2 (69.0% in DCM) have much higher ΦF.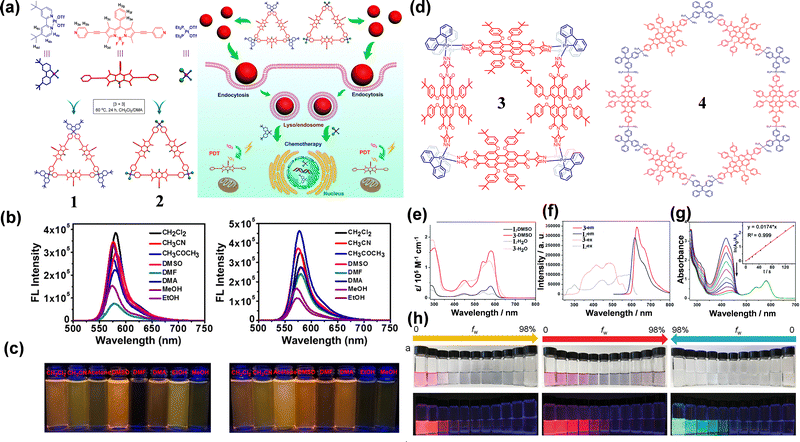 | ||
| Fig. 1 (a) The structure of SCCs 1 and 2 and their anticancer mechanism; (b) the fluorescence intensity spectra of SCCs 1 and 2 in different solvents; and (c) the fluorescence imaging pictures of SCCs 1 and 2 in different solvents under laser irradiation; reproduced with permission from ref. 49. Copyright © 2018, American Chemical Society. (d) The structure of SCCs 3 and 4; the absorption spectra (e) and fluorescence spectra (f) of SCCs 3; and (g) plot illustrating the 1O2 generation efficiency of SCCs 3; reproduced with permission from ref. 50. Copyright © 2020, Royal Society of Chemistry. (h) The fluorescence imaging pictures of (left) PPy, (middle) 4, and (right) TPE-Pt with different water fractions; reproduced with permission from ref. 51. Copyright © 2022, Wiley-VCH. | ||
Perylene tetracarboxydiimide (PDI) derivatives exhibit good photostability, high fluorescence quantum yield, and large Stokes shifts, making them promising candidates for the construction of SCCs-based dyes. However, their large π conjugated planes are prone to cause π–π stacking, which reduces solubility, promotes self-aggregation, and ultimately leads to fluorescence and ROS quenching. Faced with this issue, Sun et al.50 developed a highly stable SCCs 3 through a spontaneous deprotonation self-assembly process, where NIR perylene diimide ligands were assembled with a 2,2-bipyridine palladium [BpyPd(NO3)2] unit (Fig. 1(d)). The resulting SCCs-based dye exhibited excellent chemical stability and efficient 1O2 generation capacity. In the NIR region, SCCs 3 demonstrated a distinct red emission band, with the maximum emission wavelengths concentrated at 617 nm and 627 nm (Fig. 1(e) and (f)). Compared with the ligand, the photoluminescence rate of SCCs 3 decreased by 50.7%, which could be attributed to the aggregation of SCC causing luminescence quenching. To solve this issue, Mao et al.51 designed a hexagonal SCCs 4 by coordinating a bis-Pt(II) precursor (TPE-Pt) with a long-wavelength PDI-derived dye (PPy) (Fig. 1(d)). The incorporation of propeller-shaped TPE units and cationic pyridine groups effectively disrupted π–π stacking through steric hindrance and electrostatic repulsion, respectively. Consequently, SCCs 4 exhibited significantly enhanced fluorescence intensity and quantum yield relative to the free PPy dye (Fig. 1(h)). Further analysis confirmed the short distance between the intramolecular donor and acceptor and the spectral overlap led to the Förster resonance energy transfer (FRET) (with an energy transfer efficiency of 68.4%), thereby altering the luminescence lifetime of PPy dye.
The detection and imaging of hypoxia in living cells plays a pivotal role not only in precise cancer diagnosis, but also in therapeutic efficacy evaluation. While nitroreductase responsive fluorescent probes offer high selectivity and synthetic accessibility, their irreversible signal makes it difficult to dynamically monitor the oxygen concentration. Conversely, phosphorescent probes enable oxygen-dependent intensity modulation, yet conventional systems relying on single phosphorescent dyes suffer from interference by probe concentration and biological microenvironment variations. To overcome this issue, Stang and Huang et al.52 ingeniously designed a dual-emissive SCCs 5 with blue fluorescence and red phosphorescence under hypoxia conditions (Fig. 2(a)). The UV/visible spectrum verified that SCCs formation induced bathochromic shifts in both the Soret band and Q band, effectively suppressing π–π stacking while enabling dual-dye excitation through enhanced absorption overlap (Fig. 2(b)). Upon 390 nm excitation, the acetone solution of SCCs 5 exhibited intense blue fluorescence (420–480 nm) accompanied by weak red phosphorescence (630–740 nm) (Fig. 2(c)). Remarkably, nitrogen purging triggered a 48-fold red phosphorescence enhancement with merely 0.3-fold blue fluorescence increase, establishing a robust dual-emission system (Fig. 2(c)). To further verify the phosphorescent characteristics, the lifetime of the emission at 660 nm was measured and determined to be 30.1 µs. Notably, nanoparticle-encapsulated SCCs 5 maintained oxygen-responsive behavior, demonstrating progressively intensified red phosphorescence under hypoxic conditions. In vitro studies validated the linear correlation between the phosphorescence to fluorescence ratio and oxygen concentration gradients. Similarly, in vivo investigations further highlighted SCCs 5 with superior tumor accumulation ability, prolonged circulating half-life, imaging manifestations, and treatment performance.
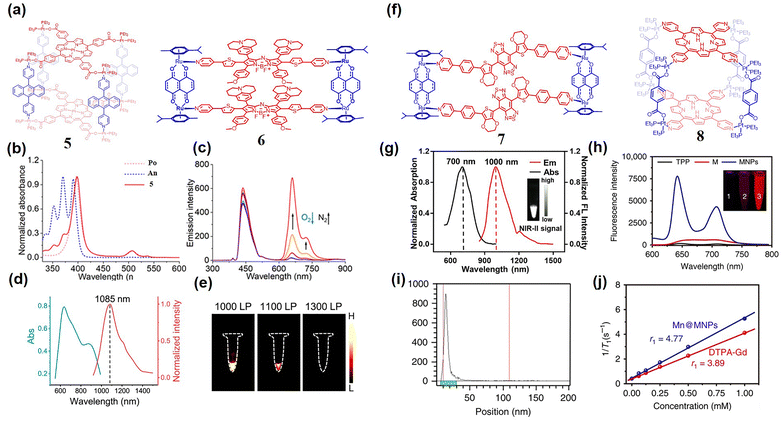 | ||
| Fig. 2 (a) The structure of SCCs 5 and 6. (b) The absorption spectrum of SCCs, An (anthracene-based ligand) and Po (porphyrin-based ligand). (c) The emission behavior of SCCs 5 under 390 nm when purged with nitrogen bubbles, reproduced with permission from ref. 52. Copyright © 2020, Wiley-VCH. (d) The absorption and emission spectra of SCCs 6. (e) The fluorescence imaging in different filter films of SCCs 6 under 808 nm laser irradiation; reproduced with permission from ref. 53. Copyright © 2022, Springer Nature. (f) The structure of SCCs 7 and 8. (g) The absorption and fluorescence spectra of SCCs 7 in DMSO; reproduced with permission from ref. 54. Copyright © 2022, Wiley-VCH. (h) The fluorescence spectrum and imaging picture (inset picture) of SCCs 8 under laser irradiation. (i) TLC chromatogram of SCCs 8 after incubation in mouse serum for 24 hours. (j) The relationship graph between T1–1 of SCC 8 and the concentration of Mn (or Gd); reproduced with permission from ref. 55. Copyright © 2018, Springer Nature. | ||
Small molecule dyes with emission wavelengths located in the NIR-II (1.0–1.7 µm) biological imaging window offer distinct advantages of minimized photon scattering, reduced tissue autofluorescence, enhanced tissue penetration depth, and superior spatiotemporal resolution. Capitalizing on these merits, Sun et al.53 pioneered the design and synthesis of NIR-II emission rectangular SCCs 6 through [2+2] self-assembly between 180° pyridine-modified azo-BODIPY and 0° semi-intercalated Ru(II)-based receptors (Fig. 2(a)). SCCs 6 exhibited a broad absorption band (560–980 nm, with a molar extinction coefficient of 4.67 × 104 M−1 cm−1) in DCM, a peak absorption wavelength of 874 nm, and NIR-II emission at 1085 nm (Fig. 2(d) and (e)). In DCM, the relative fluorescence quantum yield of SCCs 6 was 0.084% (using IR-26 as a reference), enabling excellent tissue penetration (>6 mm within 1% lipids). The presence of heavy atomic Ru enhanced singlet–triplet orbital coupling efficiency, yielding both singlet oxygen yield (ΦΔ = 0.14) and notable photothermal conversion efficiency (30.9%). Hindlimb vascular imaging revealed SCCs 6's exceptional NIR-II capability: arteries and veins were clearly distinguishable against background tissue, achieving a high signal-to-background ratio (SBR = 13.6) and prolonged blood retention. Similarly, a donor–receptor–donor NIR-II fluorescent small-molecule dye featuring a benzodithiadiazole core was assembled with semi-sandwich Ru(II)-based receptors to form rectangular SCCs 7 (Fig. 2(f)).54 SCCs 7 displayed a significant ILCT absorption band (derived from the azo-BODIPY ligand) at 600–900 nm and emission at 1000 nm (300 nm Stokes shift; ΦF = 0.94% in DMSO) (Fig. 2(g)). Under 808 nm laser irradiation, SCCs 7 demonstrated exceptional photothermal stability (confirmed through cycling tests) with 17.6% photothermal conversion efficiency. SCCs 7 enabled high-contrast lymphatic vessel imaging in the NIR-II region (808 nm excitation; SBR = 4.7), resolving blood vessels as narrow as 281 µm. Following intratumoral injection in A549 tumor models, the fluorescence signal of the tumor peaked at 12 hours with the SBR of tumor/normal tissue of approximately 25, confirming its excellent in vivo imaging performance.
For high-precision tumor imaging, multimodal imaging approaches often yield superior diagnostic and therapeutic outcomes. Positron emission tomography (PET) offers exceptional sensitivity and unlimited tissue penetration, while magnetic resonance imaging (MRI) provides high-spatial resolution. By leveraging the complementary advantages of these imaging modalities, Chen et al. developed porphyrin-based SCCs 8 chelated with Cu2+ and Mn2+ through a multicomponent ligand-driven self-assembly strategy (Fig. 2(f)).55 In the mixture of DCM/MeOH solvent (V/V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the UV/Vis spectrum revealed that the free porphyrin ligand displayed characteristic absorption features: a Soret band at 445 nm and four Q bands at 519, 555, 586 and 640 nm. Upon porphyrin ligand assembly into the coplanar structure of SCCs 8, systematic bathochromic shifts were observed, with the Soret band shifting to 450 nm, and Q bands to 522, 557, 596 and 652 nm respectively (Fig. 2(h)). This phenomenon indicated strong electronic coupling between the macrocyclic π systems. The incorporation of heavy atoms (Pt) significantly promoted the 1O2 generation, with SCCs 8 exhibiting a remarkable 1O2 quantum yield of 0.44 in water, which was approximately 110-fold higher than that of the porphyrin ligand. In vitro studies demonstrated that 8 enabled αvβ3 integrin receptor targeting capability, accumulated in lysosomes and displayed exceptional ROS production under 671 nm laser irradiation. Beyond fluorescence imaging, 8 also served as an MRI or PET contrast agent after chelating Mn2+ or 64Cu ions (Fig. 2(i) and (j)).
1), the UV/Vis spectrum revealed that the free porphyrin ligand displayed characteristic absorption features: a Soret band at 445 nm and four Q bands at 519, 555, 586 and 640 nm. Upon porphyrin ligand assembly into the coplanar structure of SCCs 8, systematic bathochromic shifts were observed, with the Soret band shifting to 450 nm, and Q bands to 522, 557, 596 and 652 nm respectively (Fig. 2(h)). This phenomenon indicated strong electronic coupling between the macrocyclic π systems. The incorporation of heavy atoms (Pt) significantly promoted the 1O2 generation, with SCCs 8 exhibiting a remarkable 1O2 quantum yield of 0.44 in water, which was approximately 110-fold higher than that of the porphyrin ligand. In vitro studies demonstrated that 8 enabled αvβ3 integrin receptor targeting capability, accumulated in lysosomes and displayed exceptional ROS production under 671 nm laser irradiation. Beyond fluorescence imaging, 8 also served as an MRI or PET contrast agent after chelating Mn2+ or 64Cu ions (Fig. 2(i) and (j)).
2.2 SCCs enhance the therapeutic outcomes of small-molecule dyes
SCCs have emerged as versatile platforms in biomedicine, demonstrating remarkable potential for integrated cancer therapy and bioimaging.56–58 Unlike conventional mononuclear platinum drugs, these SCCs feature well-defined cavity structures through self-assembly. This distinctive architecture not only facilitates efficient incorporation of metallic chemo-therapeutic components but also enables theranostics integration through judicious selection of fluorescent dyes (e.g., for PDT/PTT applications). For instance, Yang et al. developed an SCCs 959 capable of reversibly modulating 1O2 generation (Fig. 3(a)). By incorporating both porphyrin and diene moieties, the resulting SCCs 9 exploited the superior photoisomerization properties of dienes to function as a molecular switch for precise 1O2 generation. The open-loop O-NPs exhibited obvious phototoxicity to tumors upon irradiation, enabling spatiotemporal control of PDT in vivo. Yin and Stang et al.60 innovatively used a phenylthiophenol-modified BODIPY dye, which was self-assembled with Pt(II)-based receptors at 60 °C to form triangular SCCs 10 (Fig. 3(b)). The triangular SCCs 10 demonstrated NIR FL imaging performance and enhanced chemo-PDT synergistic therapeutic effects in U87 tumor-bearing mice.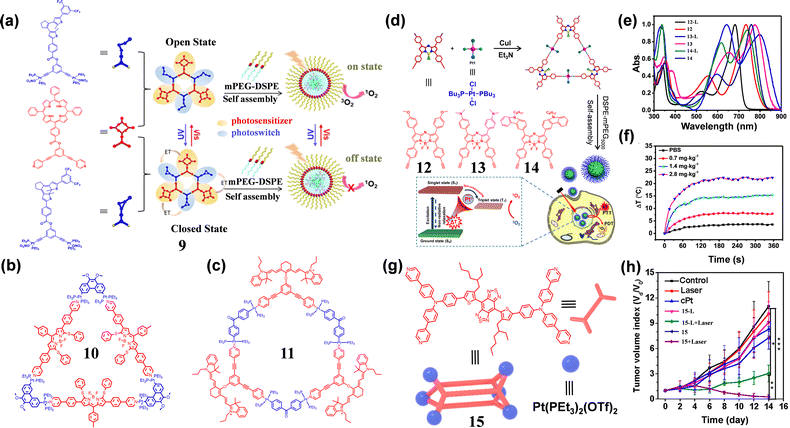 | ||
| Fig. 3 (a) The structure of SCCs 9 and its 1O2 release mechanism; reproduced with permission from ref. 59. Copyright © 2019, American Chemical Society. (b) and (c) The structures of SCCs 10 and 11; (d) the structures of SCCs 12, 13 and 14 and their mechanism against tumor; (e) the absorption spectra of SCCs 12–14 and their ligands; (f) the corresponding temperature elevation at the tumor caused by SCCs 14 under 785 nm laser irradiation; reproduced with permission from ref. 62. Copyright © 2020, American Chemical Society. (g) The structure of SCCs 15; (h) the tumor inhibition capacity of SCCs 15 under different treatments; reproduced with permission from ref. 63. Copyright © 2022, American Chemical Society. | ||
The inherent lipophilic cationic character of cyanine dyes confers exceptional mitochondria targeting capability, making them particularly effective for phototherapy applications. Therefore, Xu et al.61 developed hexagonal SCCs 11 through [3+3] coordination between 120° pyridine-modified cyanine dyes and 120° Pt(II) acceptors (Fig. 3(c)). To improve biological compatibility, SCCs 11 was encapsulated with amphiphilic DSPE-mPEG, yielding 11 NPs with maximum NIR absorption at 780 nm. In vitro tests showed that the photothermal conversion efficiency of 11 NPs was 8.05%, indicating their enhanced PTT potential. Under 808 nm laser irradiation, 11 NPs effectively produced ROS and heat, indicating their PDT/PTT dual-modal phototherapy abilities.
The development of SCCs with high stability and strong NIR absorption is critical for achieving deep tissue therapeutic performance in cancer treatment. Based on this principle, Zhao et al.62 rationally designed a series of triangular SCCs (12, 13 and 14) based on 180° platinum receptors and 60° Azo-BODIPY dye, which exhibited efficient light conversion properties for phototherapy (Fig. 3(d)). SCCs 12–14 demonstrated enhanced stability and intense NIR absorption, with absorption maxima at 735 nm (ε = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1), 775 nm (ε = 67
300 M−1 cm−1), 775 nm (ε = 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1) and 760 nm (ε = 66
200 M−1 cm−1) and 760 nm (ε = 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 L mol−1 cm−1) (Fig. 3(e)). Using DPBF as an indicator, the researchers confirmed the superior ROS generation capability of these SCCs 12–14. The rigid conjugated planes facilitated enhanced non-radiative decay, promoting efficient photothermal conversion. Notably, SCCs 14 demonstrated a strong synergistic effect between PTT and PDT under NIR irradiation (Fig. 3(f)). In a complementary study, Tang et al.63 rationally assembled a tetrapyridine-modified benzothiadiazole dye with the 90° Pt(II) receptor to obtain the SCCs 15 (Fig. 3(g)), which exhibited dual absorption peaks at 368 nm (π–π* transition) and 675 nm (CT band), and NIR-II fluorescence emission at 993 nm in THF. Furthermore, SCCs 15 also exhibited AIE characteristics, with a 36-fold fluorescence enhancement and increased quantum yield in toluene/DMSO (99
900 L mol−1 cm−1) (Fig. 3(e)). Using DPBF as an indicator, the researchers confirmed the superior ROS generation capability of these SCCs 12–14. The rigid conjugated planes facilitated enhanced non-radiative decay, promoting efficient photothermal conversion. Notably, SCCs 14 demonstrated a strong synergistic effect between PTT and PDT under NIR irradiation (Fig. 3(f)). In a complementary study, Tang et al.63 rationally assembled a tetrapyridine-modified benzothiadiazole dye with the 90° Pt(II) receptor to obtain the SCCs 15 (Fig. 3(g)), which exhibited dual absorption peaks at 368 nm (π–π* transition) and 675 nm (CT band), and NIR-II fluorescence emission at 993 nm in THF. Furthermore, SCCs 15 also exhibited AIE characteristics, with a 36-fold fluorescence enhancement and increased quantum yield in toluene/DMSO (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Moreover, SCCs 15 displayed a high photothermal conversion efficiency (39.3%) and significantly improved ROS generation compared to that of benzothiadiazole dye. In vivo experiments have also shown that 15 could effectively destroy tumor tissues with minimal systemic toxicity (Fig. 3(h)). Another innovative example64 was the [3+3] self-assembly of pyridine-modified 180° azo-BODIPY dye and 60° binuclear Pt(II) receptors, yielding triangular SCCs 16 (Fig. 4(a)). The maximum absorption of SCCs 16 in a DMSO solution was 895 nm (ε = 1.37 × 104 M−1 cm−1), and the maximum emission was 1115 nm. Under 808 nm laser irradiation, SCCs 16 generated a large number of superoxide anions, which was beneficial for overcoming the hypoxic microenvironment of tumors (Fig. 4(b)).
1). Moreover, SCCs 15 displayed a high photothermal conversion efficiency (39.3%) and significantly improved ROS generation compared to that of benzothiadiazole dye. In vivo experiments have also shown that 15 could effectively destroy tumor tissues with minimal systemic toxicity (Fig. 3(h)). Another innovative example64 was the [3+3] self-assembly of pyridine-modified 180° azo-BODIPY dye and 60° binuclear Pt(II) receptors, yielding triangular SCCs 16 (Fig. 4(a)). The maximum absorption of SCCs 16 in a DMSO solution was 895 nm (ε = 1.37 × 104 M−1 cm−1), and the maximum emission was 1115 nm. Under 808 nm laser irradiation, SCCs 16 generated a large number of superoxide anions, which was beneficial for overcoming the hypoxic microenvironment of tumors (Fig. 4(b)).
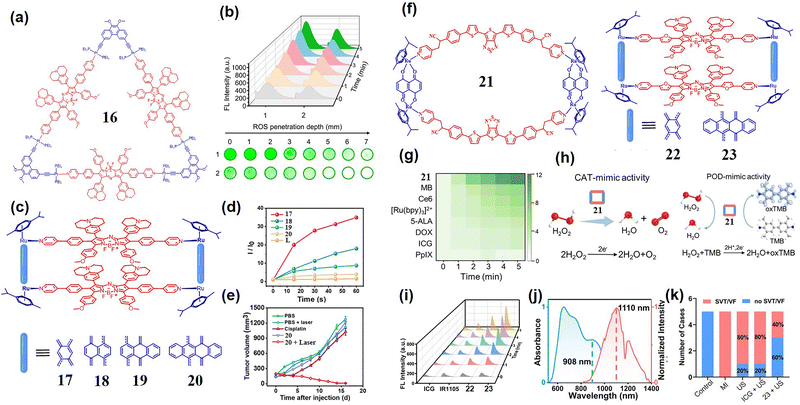 | ||
| Fig. 4 (a) The structure of SCCs 16; (b) the ROS generation capacity of SCCs 16 under 808 laser irradiation; reproduced with permission from ref. 64. Copyright © 2024, Wiley-VCH. (c) The structure of SCCs 17–20; (d) the ROS production capacity of SCCs 17–20 under 808 nm laser irradiation; (e) the tumor inhibition capacity of different treatments; reproduced with permission from ref. 65. Copyright © 2022, Royal Society of Chemistry. (f) The structure of SCCs 21, 22 and 23; (g) the ROS generation capacity of SCCs 21 and different commercial sonosensitizers; (h) the CAT-mimic activity (left) and POD-mimic activity (right) of SCCs 21; reproduced with permission from ref. 66. Copyright © 2023, Wiley-VCH. (i) The ROS generation capacity of SCCs 22 and SCCs 23; (j) the absorption and fluorescence spectra of SCCs 23 in DMSO; (k) the modulation of the occurrence rate of the VAs by US-mediated SCCs 23; reproduced with permission from ref. 67. Copyright © 2025, Wiley-VCH. | ||
Investigating the structure–activity relationship of photosensitizers is the primary choice for developing highly efficient photosensitizers. Recently, Stang and Sun et al. systematically investigated how π-conjugation extension in semi-core Ru(II) receptors influenced the photodynamic performance of SCCs 17–20.65 Based on this, they successfully screened out SCCs 20 with the lowest dark toxicity and the highest ROS generation ability and PI values (∼146) (Fig. 4(c)). All four SCCs 17–20, assembled from 180° azo-BODIPY dyes and Ru(II) receptors with progressively extended π-conjugation, displayed dual broad absorption bands at ∼650 and 830 nm. Under 808 nm laser irradiation, SCCs 17–20 exhibited the maximum emission at 1108, 1050, 1093, and 1085 nm, and their emission wavelengths were all tailing to 1400 nm, ideal for deep-tissue NIR-II imaging. Compared with the ROS generation ability of ligands (1.5-fold increase), SCCs 17–20 showed successively enhanced ROS generation ability under 808 nm laser irradiation (4–31-fold enhancement) (Fig. 4(d)). Notably, the species of ROS produced by SCCs 17–20 were mainly superoxide anions, which were beneficial for hypoxic tumor treatment. Among the SCCs, SCCs 20 exhibited the highest O2˙− yield (3.0-fold) exceeding that of 17 (1.2-fold), 18 (1.5-fold), and 19 (2.6-fold), attributable to its large and electron-rich receptor structure. Under light irradiation, 20 showed the strongest cytotoxicity against A549 cells (IC50 = 3.6 µM) and maintained high phototoxicity even under hypoxic conditions. The in vivo anti-tumor effect indicated that the tumor inhibition rate of SCCs 20 on A549 tumor-bearing nude mice was as high as 95%, and there were no obvious side effects during the treatment process (Fig. 4(e)).
The NIR-I fluorescence imaging-guided SDT offers distinct advantages to precisely locate lesions through real-time fluorescence imaging, combined with the high targeting specificity of sonosensitizers. Under ultrasound (US) activation, this approach enabled precise disease modulation in deep tissues while minimizing damage to surrounding healthy tissues. Sun et al.66 designed SCCs 21, which reduced the HOMO–LUMO energy gap through π conjugation expansion (Fig. 4(f)). This innovative design demonstrated significantly enhanced ROS generation efficiency under US activation compared to traditional sonosensitizers (e.g., PpIX and Ce6) (Fig. 4(g)), while simultaneously enabling NIR imaging-guided therapy. Photophysical characterization revealed that SCCs 21 exhibited a significant ILCT absorption band at 550–800 nm, with an emission at 880 nm (Stokes shift 230 nm). SCCs 21 operated through multiple therapeutic mechanisms, including simulating CAT activity to alleviate hypoxia in the biofilm microenvironment (BME), and increased the total ROS levels via peroxidase (POD)-like enzymatic activity (Fig. 4(h)). In a related study, Sun et al.67 developed rectangular SCCs 22 and 23 through self-assembly of an Azo-BODIPY dye based sonosensitizer with semi-sandwich Ru(II) receptors (Fig. 4(f)). Structural optimization through molecular engineering, particularly benzene ring extension in SCCs 23, yielded remarkable sonodynamic performance with a 1O2 yield (ΦΔ) of 0.88 (Fig. 4(i)). Spectral analysis revealed a charge transfer absorption peak of the Azo-BODIPY ligand at 650–950 nm in DMSO, with 23 emitting 1110 nm NIR-II fluorescence under 808 nm excitation (Fig. 4(j)), suggesting potential for deep tissue imaging. In vivo experiments confirmed that 23-mediated US modulation effectively suppressed sympathetic nerve activity and inflammatory responses, consequently reducing ventricular arrhythmia-related death (Fig. 4(k)).
2.3 SCCs assisted the bioimaging performance of small-molecule dyes
Recently, SCCs have garnered significant attention in biomedical applications, especially in the bioimaging field.68–71 Their molecular architectures can be strategically engineered to either incorporate luminescent metal centers (e.g., ruthenium, iridium, or lanthanides) or to encapsulate customized small-molecule dyes within their cavities, enabling them to serve as contrast agents or optical probes. This design flexibility enables the development of optical imaging agents with exceptional signal intensity, superior photostability, and tunable emission wavelengths, which are crucial characteristics for both in vitro and in vivo imaging. Furthermore, their well-defined size and dimensions, chemical stability under physiological conditions, and capacity for surface modification facilitate targeted imaging of cells, tissues, or even specific biomolecules.72 These combined attributes established SCCs as a versatile and customizable platform for developing next-generation bioimaging tools.42Although lanthanide-based dyes exhibit unique superiority in both fluorescence imaging (FI) and MRI, their poor aqueous solubility and stability have limited their biological applicability. To address this challenge, Sun et al.73 developed a series of supramolecular lanthanide dye-based SCCs, leveraging tridentate chelation to enhance water solubility and stability. The modular design of these supramolecular dyes allowed precise tuning of the photophysical properties through systematic variation of lanthanide ions and ligand structures, thereby optimizing their performance for FL/MRI dual-modal imaging (Fig. 5(a)). The resulting improvements in physiological stability and solubility not only extended imaging durations but also minimized cytotoxicity. These advances established supramolecular lanthanide dyes as highly promising candidates for next-generation diagnostic tools, with potential applications in targeted imaging and theranostics.
 | ||
| Fig. 5 (a) The lanthanide dye-based SCCs for FL/MRI dual-modal imaging. Reprinted with permission from ref. 73. Copyright 2020, American Chemical Society; (b) CLSM images of 4T1 cells stained with Syto 9 (green, staining nucleus) and MNPs (blue and red) under normoxic (top) or hypoxic (bottom) conditions; (c) in vivo fluorescent images of different sized tumor-bearing mice after the injection of MNPs. Reprinted with permission from ref. 52. Copyright 2020, Wiley-VCH; (d) holographic images viewed at different angles upon illumination with white light; rewritable fluorescent images and photochromic images are viewed upon illumination with UV or natural light. Reprinted with permission from ref. 74. Copyright 2024, Wiley-VCH. | ||
Currently, the emission intensity of most mono-phosphorescent dyes is highly susceptible to variations in probe concentration and biological conditions. Faced with this issue, Stang et al.52 developed a dual-emissive, supramolecular phosphorescent dye-based SCCs for hypoxia imaging and targeted chemotherapy in malignant tumors. Unlike conventional mono-emissive hypoxia probes, this SCC exhibited reversible, oxygen-sensitive phosphorescence and enabled precise hypoxia detection through ratiometric quantification of red-to-blue emission, significantly enhancing reliability. The 4T1 cell tests further suggested that the blue and red emissions were located identically, demonstrating the SCC's dual-emissive imaging capability under the hypoxia conditions (Fig. 5(b)). Further in vivo investigations revealed that the SCC facilitated fluorescence imaging-guided chemotherapy with high precision, leading to improved therapeutic outcomes (Fig. 5(c)). By integrating diagnostic imaging with therapeutic delivery, this versatile platform advances precision oncology and holds promise for broader applications in oxygen-dependent disease management.
Owing to integrating a diverse array of functional motifs, supramolecular dyes also provide a modular platform for responsive materials.46 For instance, Xu et al.74 designed a light-activated, liquid-crystalline SCCs via precision coordination-driven self-assembly, which exhibited dynamic structural and emissive properties. By leveraging its intrinsic mesogenic order, luminescence, and a rapid photocyclization pathway, a photochromic spiropyran unit was integrated to construct a spatially tunable FRET matrix. This breakthrough enabled the first demonstration of a liquid-crystalline SCCs capable of orthogonal photopatterning across three discrete modalities: fluorescence, holography, and photochromism, providing new insights into the design of programmable molecular dyes (Fig. 5(d)).
To address the inherent non-emissive characteristics, suboptimal tumor-specific accumulation, and inevitable off-target cytotoxicity of platinum-based drugs, Mao et al. constructed a NIR emissive supramolecular dye-based SCC constructed from perylene bisimide (PPy) and tetraphenylethylene-based Pt(II) complexes (TPE-Pt). This NIR emissive SCCs was subsequently encapsulated into glutathione (GSH)-responsive nanoparticles (MNPs), enabling in vivo NIR imaging-guided cancer radio-chemotherapy. In this engineered nanoplatform, PPy served dual functions as both a FRET acceptor and a NIR dye, enabling real-time, noninvasive tracking of nanoparticle delivery and biodistribution via in vivo and ex vivo high-performance fluorescence imaging (Fig. 6(a)). This synergistic integration of diagnostic imaging and therapeutic intervention highlighted the promise of supramolecular nanomedicine in treating multidrug-resistant malignancies.51 Benefiting from the advantages of the NIR-I bio-window over that of visible light, Xu et al. recently demonstrated a NIR cyanine dye-based SCC for in vivo fluorescence imaging guided precise chemo/photo trimodal cancer therapy, further expanding the applications of supramolecular systems in precision medicine.75
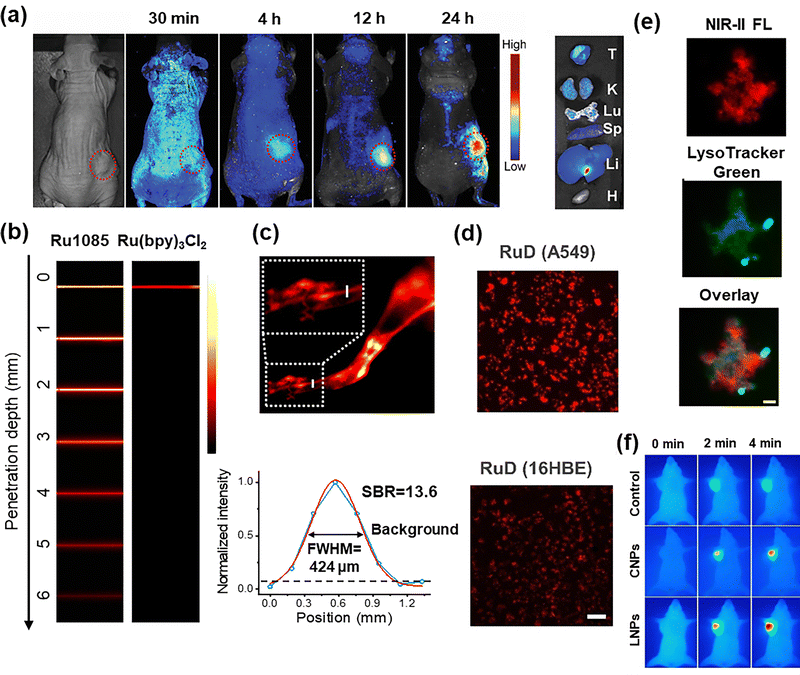 | ||
| Fig. 6 (a) Time-dependent fluorescence imaging of a mouse bearing an A2780CIS tumor; ex vivo fluorescence imaging of the main organs harvested from the mouse at 24 h post i.v. injection of MNPs. T: tumor, K: kidney, Lu: lung, Sp: spleen, Li: liver, and H: heart. Reprinted with permission from ref. 51. Copyright 2022, Wiley-VCH; (b) fluorescence images of Ru1085 and Ru(bpy)3Cl2 encapsulated in capillaries and immersed at different depths in 1% intralipid; (c) NIR-II fluorescence images of hindlimb vessels after intravenous injection of Ru1085 NPs, and NIR-II fluorescence intensity profiles (blue line) and Gaussian fit (red line) along the white full line. Reprinted with permission from ref. 53. Copyright 2022, Springer Nature; (d) fluorescence images of A549 cells after incubation with RuD (20 µM, 1% DMSO, v%) for 12 h. Scale bar, 100 µm; (e) demonstration of colocalization of RuD (10 µM, 1% DMSO, v%) in A549 cells (PCC = 0.70). Scale bar, 10 µm. Reprinted with permission from ref. 80. Copyright 2022, Wiley-VCH; (f) thermal images of MDA-MB-231 tumor-bearing mice under continuous 808 nm laser irradiation for different times. The laser irradiation was conducted at 12 h post-injection of PBS, CNPs, and LNPs. Reprinted with permission from ref. 63. Copyright 2022, American Chemical Society. | ||
The transition to fluorescence emission in the NIR-II bio-window significantly reduced autofluorescence from endogenous biomolecules, thereby improving the signal-to-noise ratio.76–78 However, the extended π-conjugated system of small-molecule dyes tends to aggregate through π–π stacking interactions, compromising their fluorescence performance for bioimaging. To address these limitations, Sun et al.53 presented the concept of “Molecular Dye Coordination Confinement” strategy and developed the first case of NIR-II supramolecular BODIPY dye based on a Ru(II) SCCs. This approach leveraged metal-to-BODIPY dye coordination interactions to simultaneously suppress both intramolecular motion and intermolecular π–π stacking, endowing the supramolecular BODIPY dye with exceptional fluorescence properties in the NIR-II bio-window. With emission beyond 1000 nm, this supramolecular dye achieved deep tissue penetration (up to 6 mm, Fig. 6(b)), high spatial resolution (424 µm) and an outstanding signal-to-background ratio (13.6) in hindlimb vessel imaging, demonstrating minimal interference from autofluorescence/scattering (Fig. 6(c)). More importantly, based on this universal Molecular Dye Coordination Confinement strategy, Sun et al. subsequently developed a series of NIR-II fluorescent supramolecular dyes by combining BODIPY/donor–acceptor–donor dyes with Ru(II)/Pt(II) metals.64,65
Although the NIR-II fluorescent supramolecular dye-based SCCs have attracted increasing attention in biomedicine, their potential clinical translation is heavily hindered by the potential toxicity from metal ions.79 To overcome this hurdle, Sun et al.80 introduced a Multidimensional Molecular Extension approach for supramolecule dye design, creating a series of NIR-II emissive SCCs with enhanced steric bulk and bending angles. Remarkably, the RuD with the maximal steric hindrance exhibited negligible cytotoxicity (IC50 > 700.00 µm), attributed to minimized interactions between the biomolecules and RuD. NIR-II cellular imaging revealed significantly higher uptake in tumor cells (A549) compared to normal cells (16HBE) (Fig. 6(d)), highlighting the tumor cell selective targeting capability of RuD. Subcellular localization studies demonstrated predominant lysosomal accumulation (PCC = 0.7) (Fig. 6(e)) with minor mitochondria distribution (PCC = 0.57), as further confirmed by ICP-MS analysis. Meanwhile, Tang and Wang reported a supramolecular AIE dye-based SCCs (C-DTTP) exhibiting both NIR-II fluorescence emission and excellent photothermal conversion efficiency, which was successfully employed for in vivo thermal imaging precisely guided PTT on MDA-MB-231 tumor-bearing mice (Fig. 6(f)).63
Bacterial infection poses a significant threat to public health, driving substantial interest in developing supramolecular dyes for pathogen diagnosis and treatment. Moving from the tumor-centric bioimaging advancements enabled by supramolecular dye-based SCCs, researchers have recently explored these supramolecular dyes in bacterial management. These innovative supramolecular dyes demonstrated remarkable potential for in vitro bacterial imaging, in vivo infection visualization and therapeutic intervention.81–83 A notable example was the work by Sun et al.,84 who developed a NIR-II emissive supramolecular BODIPY dye (1) based on a quadrilateral Ru metallacycle, for bacterial bioimaging and therapy. In vitro NIR-II fluorescence imaging revealed a striking difference in uptake, with S. aureus exhibiting over 2-fold higher fluorescence intensity than E. coli (Fig. 7(a) and (b)). The differential uptake was quantitatively confirmed through inductively coupled plasma mass spectrometry (ICP-MS) analysis, showing excellent correlation with NIR-II fluorescence imaging results (Fig. 7(c)).
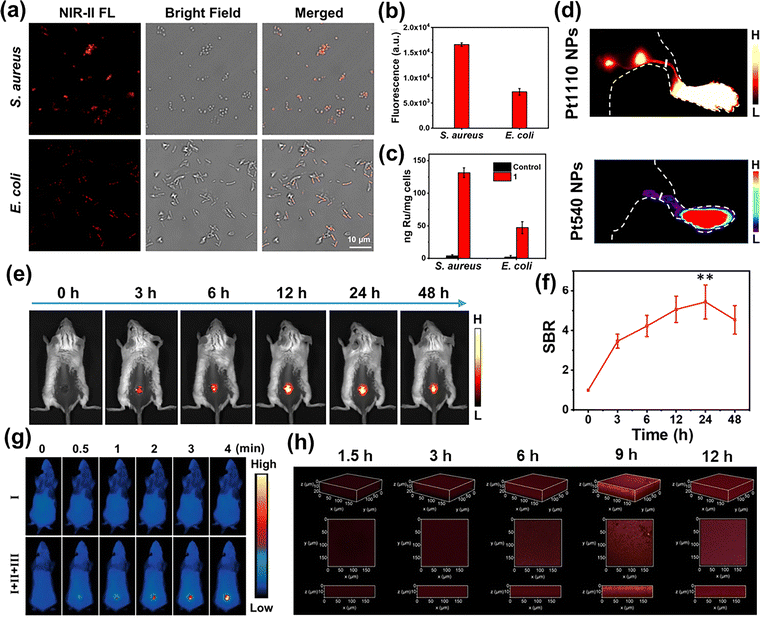 | ||
Fig. 7 (a) FL images of Staphylococcus aureus and Escherichia coli after being incubated with 1 for 2 h; (b) fluorescent intensity of S. aureus and E. coli; (c) inductively coupled plasma mass spectrometry result of the Ru amount after S. aureus and E. coli incubation with 1 for 2 h. Reprinted with permission from ref. 84. Copyright 2022, Proceedings of the National Academy of Sciences of the United States of America; (d) fluorescence images of lymphatic systems after injection of Pt1110 NPs and Pt540 NPs; (e) NIR-II fluorescence images of a wound infection mouse model after injection of Pt1110 NPs; (f) the analysis of SBR in (e). Reprinted with permission from ref. 85. Copyright 2023, Springer Nature; (g) infrared thermal images of the wound sites of rats under 660 nm light irradiation (0.5 W cm−2). Reprinted with permission from ref. 88. Copyright 2024, Proceedings of the National Academy of Sciences of the United States of America; (h) 3D CLSM images of MDR E. coli biofilms after incubation with Ru-A3-TTD (10 µM, 1% DMSO, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) %) for different times. Reprinted with permission from ref. 66. Copyright 2024, Wiley-VCH. %) for different times. Reprinted with permission from ref. 66. Copyright 2024, Wiley-VCH. | ||
To enhance deep-tissue infection imaging capabilities, Sun et al.85 subsequently designed Pt1100, a NIR-II absorption/emission supramolecular BODIPY dye-based Pt SCCs activatable by 980 nm laser excitation. Encapsulation into DSPE-PEG5000 yielded Pt1100 NPs with optimal biosafety and solubility. Comparative studies demonstrated Pt1100 NPs’ superior performance, showing significantly higher SBR and spatial resolution than those of visible-controlled Pt540 NPs in lymphatic system imaging (Fig. 7(d)). These advantages translated effectively to in vivo applications, where Pt1100 NPs achieved high-contrast (SBR = 4.5) NIR-II imaging of S. aureus-infected wounds (Fig. 7(e) and (f)), enabling precise imaging-guided sterilization in a wound infection mouse model.
Despite these promising pathogen diagnosis and treatment features, practical implementation of nanoscale SCCs faces challenges in processability and transferability.86,87 Addressing this limitation, Li and Sun et al.88 recently presented centimeter-scale supramolecular AIE dye-based SCCs for bacterial sensing and wound healing. Real-time thermal imaging noninvasively monitored temperature changes in S. aureus infected wounds during treatment, providing critical guidance for safe and efficient PTT treatment (Fig. 7(g)). The growing concern regarding microbial biofilm-associated infections has prompted innovative solutions. For instance, Sun et al.66 recently developed a NIR supramolecular dye-based Ru-SCC (Ru-A3-TTD) with exceptional bacterial selectivity and biofilm penetration capabilities. Three-dimensional (3D) confocal fluorescence imaging revealed time-dependent fluorescence enhancement of Ru-A3-TTD in MDR E. coli biofilms, outperforming small-molecule dye ligands (Fig. 7(h)). The positive charges and adequate nanosizes of the supramolecular dye-based SCCs facilitated superior biofilm accumulation and penetration, offering a transformative approach to combating infectious diseases and marking a significant advancement in precision bioimaging and therapy.89
3. Supramolecular dyes based on cyclodextrin systems for precision medicine
Cyclodextrins (CDs) are cyclic oligosaccharides consisting of glucose units linked by α-1,4-glycosidic bonds, forming a unique cyclic structure with a hydrophobic inner cavity and a hydrophilic outer surface. Firstly discovered in the late 19th century as starch degradation by-products, their molecular encapsulation capability was not fully recognized until the mid-20th century.90–92 Natural CDs (α-, β- and γ-CDs containing 6–8 glucose units respectively) exhibit limitations in solubility and toxicity. These drawbacks have spurred the development of chemically modified derivatives (such as hydroxypropyl-β-CD and sulfobutyl ether-β-CD), which offer enhanced biocompatibility and functionality. The hydrophobicity-driven, multi-interaction cooperative recognition process enables the guest encapsulation and stabilization of cyclodextrins.90,91 The ability of CDs to form host–host complexes with hydrophobic molecules has underpinned their biomedical advantages, driving significant progress in CD-based supramolecular assemblies for fluorescence imaging, drug delivery, and phototherapy. These engineering systems synergistically improved the key parameters of guest molecules (e.g., small-molecule dyes), including fluorescence intensity, solubility, stability, and bioavailability.93–95 For example, leveraging non-covalent bond interactions such as hydrogen bonding and hydrophilic/hydrophobic interactions formed between guest molecules and CDs, Fu et al.96 successfully assembled a series of visible-range (350–750 nm) small-molecule dyes with CDs in aqueous solutions (Fig. 8(a)). This approach effectively suppressed non-radiative decay and shielded the guest dyes from water-induced quenching (Fig. 8(b)), yielding small, highly bright supramolecular nanoparticles (SNAs) with exceptional photostability and biocompatibility. These SNAs have been successfully applied in stimulated emission depletion (STED) super-resolution imaging (Fig. 8(c)). Moreover, the intrinsic non-immunogenicity and enzymatic biodegradability of CDs addressed critical biocompatibility challenges minimizing toxic and side effects, which was a key consideration for optimizing drug delivery patterns. A notable example is the modification of an Ir(III) polypyridine complex with hydrophobic adamane substituents, enabling high-affinity binding (Ka = 1 × 106 M−1) to the CD cavity in the biological medium (Fig. 8(d)).97 This host–guest complex (CDV) effectively reduced the cytotoxicity of the Ir(III) complex while improving its solubility and cellular uptake. The resulting CDV selectively localized in mitochondria, where they emitted strong phosphorescence that enabled high-contrast mitochondrial imaging.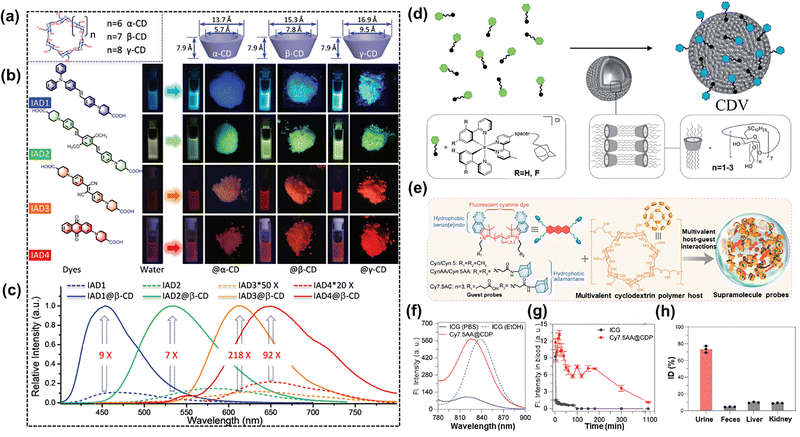 | ||
| Fig. 8 (a) The different types of CDs including α-CD, β-CD and γ-CD; (b) the different dye-CD system-based luminescence behavior in water; (c) the luminescence spectrum of different dye-CD systems; reproduced with permission from ref. 96. Copyright © 2021, Wiley-VCH. (d) Scheme illustrating the assembly behavior of an Ir(III)-based derivative and β-CD; reproduced with permission from ref. 97. Copyright © 2018, Royal Society of Chemistry. (e) Scheme illustrating the assembly of Cy7.55AA dyes with a multivalent CD polymer host; (f) absorption spectra of compounds Cy7.5AA@CDP in EtOH and PBS solutions; (g) intensity of fluorescence images of mouse blood samples at different times after administration of Cy7.5AA@CDP; (h) HPLC characterization of renal clearance in the Cy7.5AA@CDP study; reproduced with permission from ref. 98. Copyright © 2024, American Association for the Advancement of Science. | ||
3.1 Cyclodextrin improved the optical properties of small-molecule dyes
Despite its widespread clinical use, ICG faces rapid biological metabolism and poor photostability. Additionally, intravenously injected imaging agents are often rapidly cleared by the mononuclear phagocytic system (MPS), resulting in unintended accumulation in non-targeted tissues. To address these challenges, Zhang et al.98 developed a supramolecular dye (CD-1) by modifying an anthocyanin dye with multiple adamantane substituents, enabling strong multivalent host–guest interactions with CD (Fig. 8(e)). The binding constant between the anthocyanin dye and CD reached 107 M−1, shielding the anthocyanin dye from the influence of the external environment. Unlike conventional small-molecule cyanine dyes, which suffered from aggregation induced quenching (Fig. 8(f)), CD-1 exhibited superior fluorescence performance in PBS, achieving a fluorescence quantum yield of 15.5%, far exceeding that of ICG (2.0%). A key advantage of CD-1 is its unique clearance mechanism. With approximately 400 nm size, CD-1 underwent kidney clearance primarily via tubular secretion. This property extended its blood circulation time, yielding an elimination half-life exceeding 210 minutes (Fig. 8(g)). Quantitative analysis revealed that 76.4 ± 9.3% ID of CD-1 was renally cleared within 24 hours, a dramatic improvement over the clearance rate of ICG (1.8 ± 0.6% ID) (Fig. 8(h)). Therefore, CD-1 can significantly shorten the traditional clinical detection time (66 hours) and allow for earlier intervention.Activation-type fluorescence imaging demonstrated superior performance over direct imaging through intelligent probe design. These responsive probes enable real-time monitoring of biological processes with excellent spatiotemporal resolution. Pu et al.99 developed a NIR fluorescence-activated supramolecular dye (SARS-CyCD) for detecting SARS-CoV-2 protease activity in live mice (Fig. 9(a)). SARS-CyCD consisted of a hemicyanine dye as the NIRF signal module, a protease-specific peptide substrate and a CD unit facilitating renal clearance. The peptide substrate of SARS-CyCD was specifically cleaved by SARS-CoV-2 main protease (Mpro), triggering NIRF signal activation while releasing the fluorescent CyCD fragments for renal clearance (Fig. 9(b)). Upon specific enzymatic hydrolysis by SARS-CoV-2 Mpro, SARS-CyCD exhibited a distinct spectral shift: the absorption peak at 625 nm vanished, accompanied by a 50-fold fluorescence enhancement at 710 nm. Enzyme kinetics analysis revealed high catalytic efficiency (Km = 11.5 µM, kcat = 0.014 s−1, catalytic efficiency = 12.2 × 106 M−1 s−1), with exclusive selectivity for SARS-CoV-2. No significant fluorescence response was observed with other enzymes, confirming its high specificity. After tracheal injection, the 24-hour renal clearance rate of SARS-CyCD (59 ± 5.0%) was lower than that of CyCD (79 ± 1.5%), attributed to the enhanced hydrophilicity of CyCD (Fig. 9(c)).
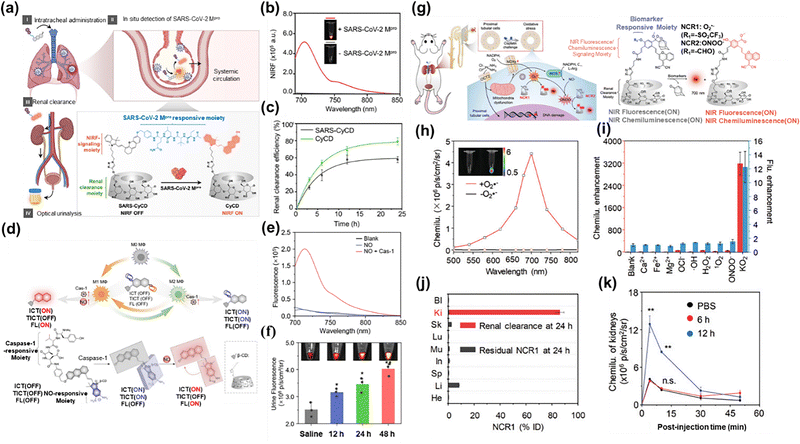 | ||
| Fig. 9 (a) Scheme illustrating the structure of SARS-CyCD; (b) the response ability of SARS-CyCS to SARS-CoV-2 in solution; (c) lung clearance efficiency determined for SARS-CyCD measured using the fluorescence measurement method; reproduced with permission from ref. 99. Copyright © 2021, American Chemical Society. (d) The chemical structure of DMRPNOCas and its fluorescence activation mechanism during the polarization process of M1 macrophages; (e) the response ability of DMRPNOC to NO and CAS-1 in solution; (f) the fluorescence intensity of urine in mice 6 hours after injection of DMRPNOCas was detected by fluorescence imaging; reproduced with permission from ref. 100. Copyright © 2025, American Chemical Society. (g) The chemical structures of NCR1 and NCR2 and the schematic diagrams of chemiluminescence activation of O2˙− and ONOO− during the detection of AKI progression; (h) the response ability of NCR1 to O2˙− in solution; (i) the selectivity of NCR1 for the chemiluminescence activation of O2˙−; (j) HPLC analysis of NCR1 excreted into urine by the kidneys 24 hours after injection; (k) chemiluminescence imaging at different time points after cisplatin treatment with NCR1; reproduced with permission from ref. 101. Copyright © 2020, Wiley-VCH. | ||
Double-lock-activated probes represent a significant advancement in molecular detection, requiring the simultaneous presence of two specific activation conditions (e.g., dual enzymes or a pH+ reduction environment) to trigger fluorescence. This dual-activation mechanism offers superior specificity compared to single-activated probes through several key advantages, including significantly reduced interference from non-target factors, particularly valuable for distinguishing multiple biomarkers in complex biological environments. A notable example is the double-locking macrophage-specific renal clearance supramolecular dye (DMRPNOCas) developed by Pu et al.100 for dynamic monitoring of lung macrophages during influenza A virus (IAV) infection (Fig. 9(d)). DMRPNOCas required co-activation by two M1 macrophage biomarkers (caspase-1 and NO). DMRPNOCas exhibited remarkable activation by caspase-1 (Km = 107.6 µM, kcat = 0.146 min−1) and NO, triggering an absorption peak bathochromic shift to 700 nm with 9-fold fluorescence enhancement at 720 nm (Fig. 9(e)). This probe displayed 12-fold the specific signal for M1 macrophages than that of other immune cells. The structure incorporation of CD conferred excellent renal clearance properties, with urine recovery rates of 58.63 ± 8.6 at 6 hours and 84.6 ± 3.6% at 24 hours post injection (p.i.) (Fig. 9(f)).
Compared with fluorescent probes that require real-time external excitation, chemiluminescent probes generate light through intrinsic chemical reactions, offering distinct advantages such as ultra-low background interference and deep tissue penetration. However, traditional chemiluminescent probes suffer from limitations including poor thermal stability and low emission brightness. To address these challenges, activatable probes based on the Schaap dioxacyclobutane scaffold have been developed, enabling selective detection of enzymes and small molecules via an inhibitor-activation mechanism. Nevertheless, their high uptake by the mononuclear phagocytic system resulted in prolonged hepatic accumulation and delayed clearance. To address these issues, Pu et al.101 designed two CD-modified NIR chemiluminescence reporters (NCR1/NCR2) with high renal clearance efficiency, allowing real-time imaging of ROS and RNS in the kidneys (Fig. 9(g)). Under reaction with O2˙− and ONOO−, the fluorescence signal of the NCR1/NCR2 probe was enhanced by 12.2-/10.9-fold at 700 nm, the chemiluminescence intensity was increased by 3180-/2990-fold, and the quantum yield reached 2.3% (Fig. 9(h)). The high specificity of NCR1/NCR2 exhibited no response to other active molecules (Fig. 9(i)). Owing to its nanomolar sensitivity and efficient renal clearance (Fig. 9(j)), NCR1 could not only detect subtle upregulation of endogenous O2˙− in cells, but also enable non-invasive monitoring of renal O2˙− dynamics under nephrotoxic exposure (Fig. 9(k)).
3.2 Cyclodextrins enhanced the phototherapeutic capability of small-molecule dyes
The distinctive architecture enables CDs to encapsulate various hydrophobic small-molecule dyes through non-covalent interactions, including hydrophobic effects, van der Waals forces, and hydrogen bonding,102–105 which enable advanced functionalities such as targeted delivery and controlled release. For instance, Tian et al. have developed a supramolecular peptide point (Spd) delivery system based on DCM7-β-CD, which forms approximately 100 nm particles through self-assembly with 1-bromonaphthalene-modified peptides (P1–P4).106 Spd-1 to Spd-3 demonstrated significantly improved cellular delivery efficiency of fluorescent peptides (P1–P3) and enabled effective caspase-3 imaging, while Spd-4 enhanced the antibacterial efficacy of the antimicrobial peptide P4 against both Escherichia coli and Staphylococcus aureus, effectively addressing the delivery challenges of peptide therapeutics. CDs could also be directly incorporated into the molecular framework of small-molecule dyes to enhance their photophysical properties. For instance, Liu et al.107 developed supramolecular multivalent nanoparticles (NPs) composed of pyrene derivatives (APA2), aluminum sulfonated phthalocyanine (PcS), and folic acid-modified β-cyclodextrin (FA-CD). In this system, CD not only facilitated the formation of highly ordered structures, but also prevented porphyrin aggregation, thereby promoting more efficient singlet oxygen generation. Notably, APA2 and PcS demonstrated precise mitochondria and lysosome targeting, respectively, enabling dual organelle localization under NIR imaging.In PDT, the spatiotemporal-controlled release of ROS is achieved through precise modulation of light parameters (wavelength, intensity, and duration) and targeted photosensitizer delivery (e.g., nanocarriers and antibody conjugates).108,109 This approach enabled selective ROS generation while minimizing oxidative damage to normal tissues. However, existing systems lack reversible regulatory mechanisms for dynamic ROS control. To address this limitation, Liu et al.110 developed a novel CD-based supramolecular assembly in aqueous solution using a host–guest recognition strategy, which comprised three key components: (1) ethylene bridged CD as a photochromic switch; (2) alkyl polypyridyl ruthenium as a photosensitizer; and (3) β-CD grafted hyaluronic acid (HA-CD) as a targeting scaffold (Fig. 10(a)). OF-1 exhibited exceptional reversible photochromic properties in aqueous solution. Upon 254 nm UV irradiation (18 s), it underwent a ring-closing reaction, characterized by a redshift absorption (from 285 nm to 593 nm) with an isosbestic point at 320 nm. This transition is fully reversible under visible light (>490 nm), restoring the initial state. The supramolecular assembly system maintained excellent photoresponsivity: the conversion of OF-1 to CF-1 was completed within 38 s under UV irradiation (solution color: light yellow to blue), while it can be fully restored after 60 s under visible irradiation (Fig. 10(b) and (c)). Notably, the supramolecular assembly system maintained a highly efficient FRET process (93.7% efficiency, ON/OFF ratio: 15.7) and controllable 1O2 generation (quantum yield: 0.93) in water (Fig. 10(d)). In vitro cytotoxicity tests confirmed the therapeutic potential of this platform. Under 450 nm light irradiation, OF NPs (10 mM) induced significant cytotoxicity in A549 cancer cells, achieving 88% cell death, which was a 4.4-fold increase compared to CF NPs (Fig. 10(e)). This work established a reversible, light-regulated ROS generation system, offering a promising strategy for precision PDT.
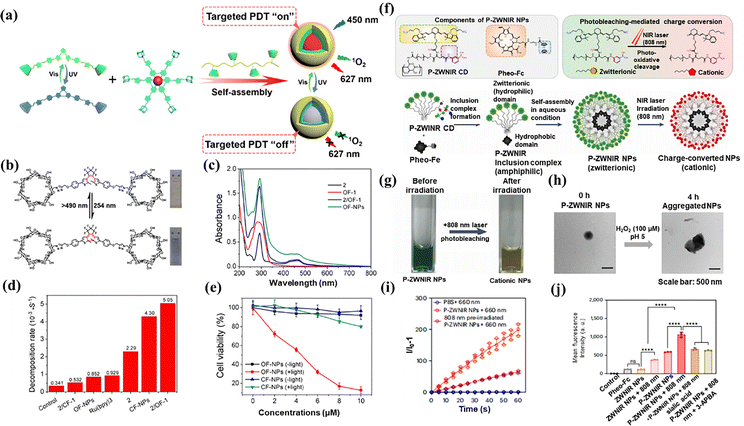 | ||
| Fig. 10 (a) Schematic diagram of the assembly of diarylethylene-bridged cyclodextrin 1 and adamantane polypyridine ruthenium photosensitizer; (b) the chromatogram and color changes of sample no. 1 under alternating ultraviolet and visible light irradiation; (c) the changes in the absorption spectra of nanoparticles in aqueous solutions; (d) the decomposition rate of ABDA was determined in water to measure ROS in different treatment groups; (e) the cell viability of A549 cancer cells after different treatment groups; reproduced with permission from ref. 110. Copyright © 2020, American Chemical Society. (f) Schematic diagram of the assembly progress of P-ZWNIR CD; (g) the photobleaching phenomenon of P-ZWNIR CD after irradiation at 808 nm; (h) the particle size change of nanoparticles after irradiation at 808 nm for 4 hours; (i) the generation of ROS in different treatment groups; (j) the cell uptake conditions of different treatment groups; reproduced with permission from ref. 111. Copyright © 2024, Springer Nature. | ||
The elevated interstitial pressure and dense extracellular matrix characteristic of solid tumors severely hinder the penetration of nanoparticles. While charge-converting nanoparticles have shown potential to enhance tumor penetration via transendocytosis, current systems face three critical bottlenecks: (1) off-target activation of pH-response materials; (2) insufficient charge conversion kinetics; and (3) heterogeneous conversion resulting from uneven distribution of tumor microenvironmental stimuli. Hence, Kim et al.111 ingeniously developed an inclusion complex featuring β-CD derivatives conjugated with small-molecule dye IR783 and a photosensitizer. This design capitalized on the photobleaching propensity of IR783 under light exposure to create efficient charge-converting nanoparticles (Fig. 10(f)). Remarkably, upon 808 nm laser irradiation, the nanoparticle solution exhibited a visible color transition from green to yellow at 5 minutes (Fig. 10(g)), accompanied by an increase in zeta potential. Small-molecule photosensitizers were further coupled to ferrocene, which were then loaded into the nanoparticles via the strong host–guest interactions with CD cavities. This integration endowed the nanoparticles with combined photodynamic/chemodynamic therapeutic capabilities (Fig. 10(h)). Following 808 nm pre-irradiation and subsequent 660 nm laser activation, the nanoparticles demonstrated efficient cellular uptake in HT-29 cells and significantly inhibited the growth of cancer cells (Fig. 10(i) and (j)). In vivo investigations verified that a single dual-laser treatment completely eradicated clinically relevant rectal tumors without systemic toxicity, demonstrating outstanding therapeutic efficacy.
3.3 Cyclodextrin system-based small-molecule dyes for biological applications
To improve the performance of molecular probes, the means of multivalent interactions have been widely used for enhancing the stability and targeting capability through synergistic host–guest binding.98,112 Zhang et al.98 constructed a supramolecular dye (Cy7.5AA@CDP) dependent on the multivalent host–guest interactions between cyanine module and β-CD. Such multivalent host–guest interactions critically elevated the binding stability and fluorescence efficiency while evading MPS clearance (Fig. 11(a)). This cooperative combination of in vivo stability, outstanding fluorescent performance, and optimized biodistribution resulted in an exceptionally high SBR for fluorescence imaging. The researchers applied this supramolecular dye for real-time monitoring of cisplatin-induced acute kidney injury (AKI) via whole-body longitudinal fluorescence imaging. In the saline-treated group, the bladder signal gradually rose, plateauing at around 2 h post-injection. In contrast, the cisplatin-treated group exhibited sustained FL signal accumulation in the kidneys, with minimal bladder fluorescence, yielding a kidney-to-bladder fluorescence intensity ratio of 5.51 (Fig. 11(b) and (c)). Complementary urinalysis further confirmed reduced urinary clearance of the supramolecular dye (Cy7.5AA@CDP) in cisplatin-injured kidneys (Fig. 11(d)), consistent with FL imaging results. Beyond AKI imaging, Cy7.5AA@CDP demonstrated exceptional tumor targeting performance, reaching peak NIR-II fluorescence at 24 h with an SBR of 6.4, significantly outperforming the ICG group. In image-guided surgery, the probe enabled precise tumor resection under NIR-II fluorescence guidance at 48 h, clearly delineating subcutaneous tumor margins while avoiding injury to normal tissues (Fig. 11(e)). Furthermore, in an orthotopic breast cancer model, the probe achieved an SBR exceeding 7.0 within 3 to 6 h, highlighting its potential for deep-tissue surgical navigation (Fig. 11(f)).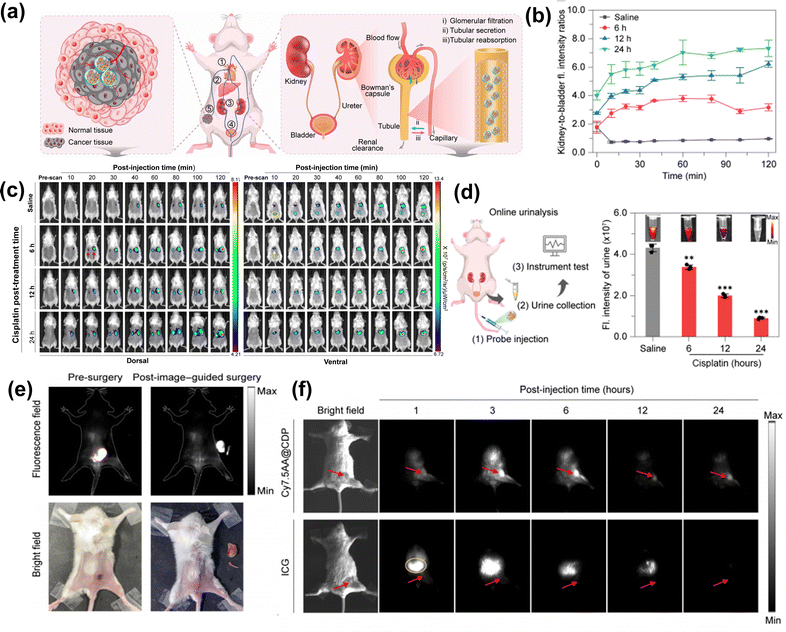 | ||
| Fig. 11 (a) Schematic diagram of the renal clearance mechanisms. Reproduced with permission from ref. 98. Copyright 2024, Science. (b) The fluorescence intensity ratios of Cy7.5AA@CDP for kidney-to-bladder in mice post-treatment with saline or cisplatin. (c) In vivo fluorescence images of mice treated with saline or cisplatin, followed by injection of Cy7.5AA@CDP. (d) Left: Schematic diagram of the online urinalysis, right: fluorescence images and their corresponding intensities from mouse urine samples treated with cisplatin or saline. (e) The resection of subcutaneous CT-26 tumor guided by the NIR-II fluorescence of Cy7.5AA@CDP. (f) The NIR-II fluorescence images of mice with orthotopic breast cancer using Cy7.5AA@CDP or ICG. Red arrows indicate the tumor site. | ||
Capitalizing on the renal-clearance properties of CDs, Pu et al.99 developed a series of supramolecular dyes for biomarker imaging and urinalysis. Among them, preactivated NIR probe SARS-CyCD was used for in vivo imaging and urinalysis of SARS-CoV-2. In healthy mice, SARS-CyCD remained metabolically stable, but upon Mpro activation, it underwent rapid cleavage and renal excretion. In Mpro-positive mice, intense lung NIR signals were observed within 60 min post i.t. injection, significantly surpassing control levels (Fig. 12(a)). Concurrently, bladder fluorescence emerged due to renal clearance, with NIR signals at 3 h post-injection being 3-fold higher than those of control groups (Fig. 12(b) and (c)).
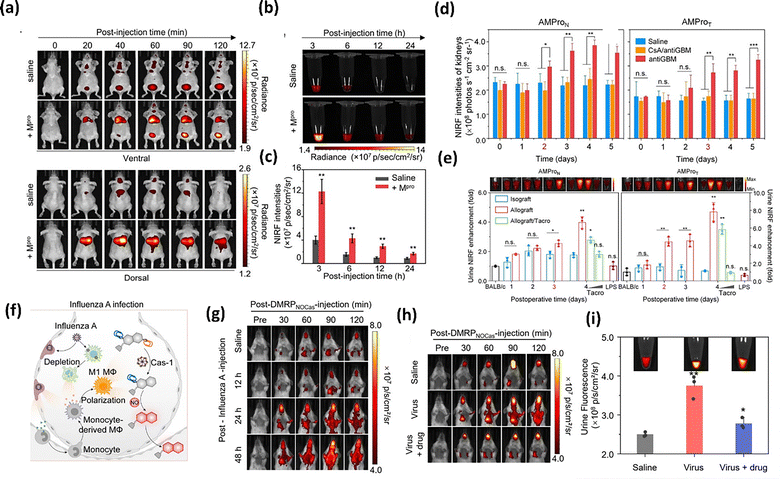 | ||
| Fig. 12 (a) NIR FL images at different time points after i.t. injection of SARS-CoV-2 Mpro and SARS-CyCD into living mice. Reproduced with permission from ref. 99. Copyright 2021, American Chemical Society. (b) Fluorescence images and (c) intensities of excreted SARS-CyCD or CyCD in the mouse urine samples collected at different time points post i.t. injection. (d) NIR FL intensities of kidneys in living mice t = 60 min after i.v. injection of AMProN and AMProT at the different drug post-treatment time points (1, 2, 3, 4 or 5 days). Reproduced with permission from ref. 113. Copyright 2023, Wiley-VCH. (e) Relative NIR FL in mouse urine samples after 6 h injection of AMProN and AMProT into different treatment groups. (f) Schematic illustration of changes in the process of IVA infection. (g) Real-time imaging of mouse breast at different IVA infection time points after intravenous injection of DMRPNOCas. Reproduced with permission from ref. 100. Copyright 2025, American Chemical Society. (h) Real-time imaging of mouse breast with different treatments after intravenous injection of DMRPNOCas. (i) Fluorescence imaging and intensity of mouse urine samples collected after i.t. injection of DMRPNOCas for 6 h. | ||
Subsequently, they designed two additional CD-based supramolecular dyes AMProN and AMProT for early detection of acute renal allograft rejection (ARAR).113 These supramolecular dyes exhibited high renal clearance efficiency, enabling urinary biomarker monitoring. AMProN and AMProT specifically responded to neutrophil elastases, granzyme B and glutamyl transpeptidase (GGT). In the anti-GBM-induced murine nephritis model, AMPro fluorescence remained stable on day 1 but progressively increased from day 2 to 5, correlating with urinary NIR trends (Fig. 12(d)). Notably, AMProN and AMProT effectively distinguished isogeneic from allogeneic transplant mice, with statistically significant differences at time points of 3 and 2 postoperative days (POD), respectively (Fig. 12(e)). In the tacrolimus (TacL) treatment group, the NIR fluorescence signals of AMProN significantly declined, suggesting its potential to monitor immunosuppressive therapy. Importantly, AMProN showed no significant signal in the LPS-induced inflammation model, confirming its specificity for renal transplantation, while histological injuries failed. Compared to histopathology, AMProT-based urinalysis detected ARAR one day earlier, offering superior sensitivity among diagnostic methods.
Furthermore, they proposed a dual-locked CD-based renal-clearable supramolecular dye (DMRPNOCas) for dynamic monitoring of pulmonary macrophage polarization (Fig. 12(f)).100 DMRPNOCas was activated only in the coexistence of caspase 1 and NO, minimizing false positive signals. In IAV-infected mice, lung NIR signals peak at 48 h post-infection (1.5-fold higher than the saline group), reflecting M1 macrophage polarization (Fig. 12(g)). Moreover, DMRPNOCas effectively tracked the progression of oseltamivir treatment on pulmonary macrophage polarization, where the fluorescence intensities in the lungs, bladder, and urine were several-fold lower than those in the control group (Fig. 12(h) and (i)), consistent with reduced M1 polarization confirmed by immunofluorescence staining and flow cytometry.
Traditional CL biosensors exhibit poor water solubility and high MPS uptake, for which they continually employed the CD moiety to construct two high renal clearance NIR CL probes bypassing MPS, NCR1 and NCR2, for real-time imaging of ROS and RNS in acute kidney injury (Fig. 13(a)).101 Leveraging the superior tissue-penetration depth and sensitivity of the CL reporter, NCRs possess an exceptional SBR of up to 35.1 at a tissue depth of 2 cm. In cisplatin-induced AKI mouse models, CL imaging outperformed corresponding FL imaging. At 6 h post-cisplatin treatment, neither NCR1 nor NCR2 showed detectable CL signals. However, by 12 h, NCR1 exhibited clear CL signals in the kidneys, while NCR2 signals only became detectable at 24 h. These findings, consistent with urine analysis results, suggested the upregulation of O2˙− ahead of the ONOO− during the drug-induced AKI (Fig. 13(b)–(d)). Comparative analysis revealed that NCR-based CL imaging detected pathological changes 60 h earlier than H&E staining and 36 h earlier than immunofluorescence staining (Fig. 13(e)).
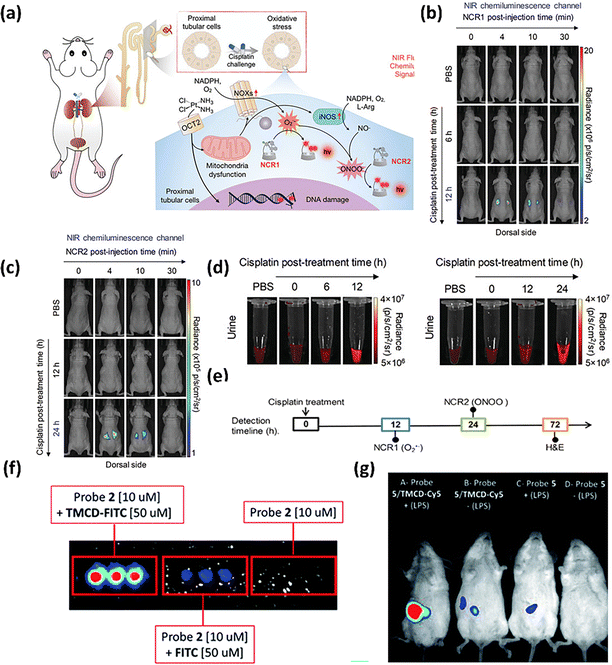 | ||
| Fig. 13 (a) Schematic illustration of the variation of O2˙− and ONOO− detected by NCR1 and NCR2 during AKI. Reproduced with permission from ref. 101. Copyright 2020, Wiley-VCH. (b) NIR chemiluminescence images of living mice acquired at different time points post i.t. injection of (a) NCR1 or (c) NCR2 at different cisplatin post-treatment timepoints. (d) NIR fluorescence images of excreted NCR1 (left) and NCR2 (right) in urine samples from living mice at different cisplatin post-injection times. (e) Detection time points for cisplatin-induced AKI of chemiluminescence imaging versus the histological method. (f) Chemiluminescence imaging of HEK-293-LacZ cells incubated with different groups. Reproduced with permission from ref. 114. Copyright 2019, Royal Society of Chemistry. (g) In vivo images of endogenous hydrogen peroxide in LPS-induced inflammatory mouse models using probe 5 and TMCD–Cy5. | ||
The encapsulation of CL dyes with CDs has been proved to be essential for enhancing CL intensity, enabling more accurate and sensitive detection. Shabat et al.114 developed a supramolecular CL dye that significantly improved aqueous-phase luminescence efficiency by assembling adamantyl-dioxetane with trimethylated β-CD (TMCD). This supramolecular system worked effectively in bio-sensing. Probe 2/TMCD-FITC demonstrated strong CL emission signals for endogenous β-galactosidase imaging in HEK-293-LacZ cells, around 195-fold relative to the probe alone (Fig. 13(f)). Furthermore, when substituting FITC with Cy5 dye, the supramolecular system achieved NIR in vivo imaging with a strong contrast CL signal in an LPS-induced inflammatory mouse model (Fig. 13(g)), confirming the platform's versatility.
4. Supramolecular dyes based on cucurbit[n]uril systems for precision medicine
Cucurbit[n]uril (CB [n]), firstly synthesized by Freeman et al. in 1981, consists of glycoside urea units linked by methylene bridges.115 The carbonyl groups at the portal of CB [n] facilitate charge–dipole interactions and hydrogen bonding with guest molecules, enabling CB [n] to preferentially bind to positively charged guests. Owing to its unique hydrophobic cavities and external hydrophilic features, CB [n] has demonstrated great potential in modulating the optical properties of small-molecule dyes via host–guest interactions.38 These interactions could significantly influence quantum yields, room temperature phosphorescence (RTP), photodynamic/photothermal efficiencies, as well as toxicity and photostability. This section highlights recent progress of CB [n]-based supramolecular dyes for biomedicine applications.4.1 CB [n] regulates the optical properties of small-molecule dyes
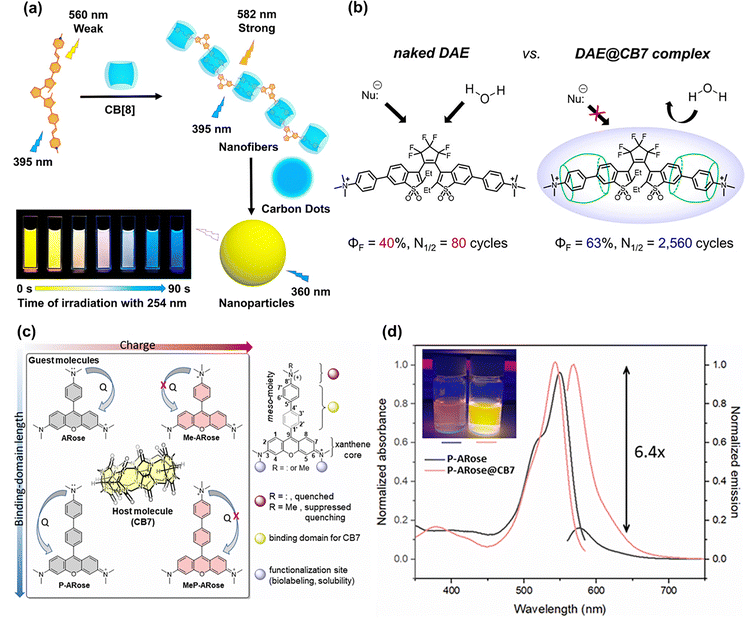
Due to its negatively charged hydrophobic cavity, CB [n] could readily assemble with cationic small-molecule dyes containing ammonium or pyridinium groups. Diarylethenes (DAEs), as a classic of photo-switchable dyes, exhibit reversible fluorescence switching upon light irradiation, making them valuable for bioimaging, protein tracking, and super-resolution microscopy.116 To enhance their luminescent properties, Liu et al.117 developed a styrylpyridinium-modified DAE derivative that formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 supramolecular complex with CB [8]. Upon irradiation at 395 nm, this supramolecular complex displayed significantly enhanced fluorescence compared to free DAE. Furthermore, the cationic supramolecular nanofibers derived from this system could interact with anionic carbon dots, yielding broad-spectrum fluorescence emission ranging from yellow to blue (Fig. 14(a)).
2 supramolecular complex with CB [8]. Upon irradiation at 395 nm, this supramolecular complex displayed significantly enhanced fluorescence compared to free DAE. Furthermore, the cationic supramolecular nanofibers derived from this system could interact with anionic carbon dots, yielding broad-spectrum fluorescence emission ranging from yellow to blue (Fig. 14(a)).
 | ||
| Fig. 14 The design of CB [n] based supramolecular dyes. (a) The supramolecular nanofibers employing a styrylpyridinium-modified DAE derivative assembled with CB [8] and carbon dots. Reproduced with permission from ref. 117. Copyright © 2019, American Chemical Society. (b) Trimethyl ammonium modified DAE derivative assembled with CB [7]. Reproduced with permission from ref. 118. Copyright © 2022, American Chemical Society. (c) Design of four rosamine-based supramolecular systems; (d) absorption and emission spectra of P-Arose and P-Arose@CB 7. Reproduced with permission from ref. 119. Copyright © 2025, American Chemical Society. | ||
In a related study, Hell and co-workers designed a supramolecular system based on a fluorescent DAE and cucurbit[7]uril (CB7) (DAE@CB7).118 The DAE dye contained two trimethylammonium groups, enabling encapsulation by CB [7] to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex (Fig. 14(b)). The fluorescence quantum yield increased from 0.40 to 0.63 upon CB [7] complexation. Under UV and visible light irradiation, the photoswitching could be sustained 2560 times in aqueous solution before half-bleaching occurs, far exceeding the 80 cycles achievable with free DAE (Fig. 14(b)). More importantly, when DAE@CB7 conjugated with functional groups (maleimide and N-hydroxysuccinimidyl (NHS) ester), the DAE@CB7 complex enabled successful confocal and super-resolution imaging of antibodies. Very recently, Hell and co-workers reported rosamine-based supramolecular systems for super-resolution imaging applications.119 Four rosamine-based small-molecule dyes (ARose, P-ARose, Me-ARose, and MeP-ARose) were rationally designed and synthesized (Fig. 14(c)). ARose and P-ARose showed weak fluorescence intensity due to the thermal deactivation of the arylpyrylium excited state and/or a twisted intramolecular charge transfer (TICT) state. After interaction with CB [7], the fluorescence intensity was increased 6.4-fold (Fig. 14(d)). Further modification of P-ARose with a functional group of HaloTag ligand or an NHS reactive group made it suitable for super-resolution imaging in live-cell and immunofluorescence labelling.
2 complex (Fig. 14(b)). The fluorescence quantum yield increased from 0.40 to 0.63 upon CB [7] complexation. Under UV and visible light irradiation, the photoswitching could be sustained 2560 times in aqueous solution before half-bleaching occurs, far exceeding the 80 cycles achievable with free DAE (Fig. 14(b)). More importantly, when DAE@CB7 conjugated with functional groups (maleimide and N-hydroxysuccinimidyl (NHS) ester), the DAE@CB7 complex enabled successful confocal and super-resolution imaging of antibodies. Very recently, Hell and co-workers reported rosamine-based supramolecular systems for super-resolution imaging applications.119 Four rosamine-based small-molecule dyes (ARose, P-ARose, Me-ARose, and MeP-ARose) were rationally designed and synthesized (Fig. 14(c)). ARose and P-ARose showed weak fluorescence intensity due to the thermal deactivation of the arylpyrylium excited state and/or a twisted intramolecular charge transfer (TICT) state. After interaction with CB [7], the fluorescence intensity was increased 6.4-fold (Fig. 14(d)). Further modification of P-ARose with a functional group of HaloTag ligand or an NHS reactive group made it suitable for super-resolution imaging in live-cell and immunofluorescence labelling.
Besides fluorescence, phosphorescence represents another type of photoluminescence mechanism involving the ISC process to a triplet state.120 Compared to fluorescence, phosphorescence offers distinct advantages for bioimaging, including increased emission lifetimes, reduced background interference, and enhanced temporal resolution.121 However, practical applications of small-molecule phosphorescent dyes require low temperatures or oxygen-free conditions to suppress non-radiative decay pathways. Furthermore, the susceptibility of triplet states to oxygen quenching presents additional challenges for biological applications.
CB [n] has emerged as a powerful supermolecule enabling room-temperature phosphorescence (RTP) by providing a rigid and confined microenvironment that restricts molecular motion and minimizes oxygen quenching. The host–guest complexation not only suppressed non-radiative decay but also enhanced ISC efficiency, facilitating effective population of the triplet state.122 These properties have led to the successful development of various supramolecular RTP probes for bioimaging. In 2021, Liu et al.123 reported a photooxidation-driven RTP for lysosome imaging (Fig. 15(a)). The anthracene-conjugated bromophenylpyridinium guest exhibited only weak fluorescence at 502 nm. However, upon complexation with CB [8], the resulting linear supramolecular assembly demonstrated significantly enhanced fluorescence intensity with a red-shifted emission at 613 nm (Fig. 15(b)). Subsequent UV irradiation induced photo-oxidization of the anthryl group to anthraquinone, transforming the assembly into a homoternary inclusion complex that exhibited strong RTP at 529 nm in aqueous solution. Xiao and Wang et al.124 recently developed a Zn2+ responsive supramolecular RTP probe for live cell imaging (Fig. 15(c)). The RTP probe was constructed through host–guest assembly of 1-(3-(bis(pyridin-2-ylmethyl)amino)propyl)-4-(4-bromophenyl)-pyridin-1-ium bromide (BP-DPA) with CB [8]. The cationic pyridinium moiety facilitated encapsulation within CB [8], yielding BP-DPA@CB [8] with enhanced phosphorescence at 505 nm. Upon Zn2+ binding to the DPA moiety, the phosphorescence intensity displayed remarkable improvements, the lifetime extended from 0.48 to 1.25 ms, and the quantum yield improved from 1.75% to 6.25% (Fig. 15(d)).
 | ||
| Fig. 15 Construction of CB [n] based RTP probes for cell imaging. (a) Design of a photooxidation-driven RTP for lysosome imaging; (b) the fluorescence intensity of photooxidation-driven RTP after the addition of CB [8]; reproduced with permission from ref. 123. Copyright © 2021, American Chemical Society. (c) Zn2+ responsive supramolecular RTP probe and its fluorescence enhancement after addition of Zn2+ (d). Reproduced with permission from ref. 124. Copyright © 2025, American Chemical Society. (e) Construction of a PRET system by a guest molecule alkyl-bridged methoxy–tetraphenylethylene–phenylpyridine derivative, cucurbit[n]uril (n = 7, 8) and β-cyclodextrin modified hyaluronic acid. Reproduced with permission from ref. 125. Copyright © 2024, Nature Publishing Group. | ||
Pure organic phosphorescence resonance energy transfer (PRET) has emerged as an efficient method for achieving long-wavelength and long-lifetime delayed fluorescence with tunable afterglow emission. In 2024, Liu et al.125 developed a supramolecular complex (TPE-DPY/CB [7]/CB [8]) that displayed strong RTP at 540 nm with an extended lifetime from 29.09 µs to 80.64 µs (Fig. 15(e)). Furthermore, secondary assembly with β-cyclodextrin modified hyaluronic acid (HA) activated an intramolecular PRET process, generating a NIR delayed fluorescence at 700 nm, which was ultimately applied to mitochondrial targeted NIR imaging for cancer cells.
Small-molecule dyes are widely employed for molecular recognition in vivo; however, their utility still suffers from strong background signals arising from unbound or nonspecific labeling. Developing an efficient strategy to address the above issues is still a challenge. Agasti et al.126 developed a supramolecular guest exchange strategy utilizing CB [7] as a host for bioorthogonal imaging (Fig. 16(a)). In this system, the fluorescence of CB [7]-conjugated dye could be effectively quenched through complexation with various azo-derivatives. Remarkably, fluorescence could be quickly restored upon addition of adamantylamine (ADA) or its conjugated targeting agents due to their competitive assembling with CB [7]. This approach enabled high-contrast super-resolution imaging and multiplexed visualization of metabolically tagged mycobacteria under no-wash conditions.
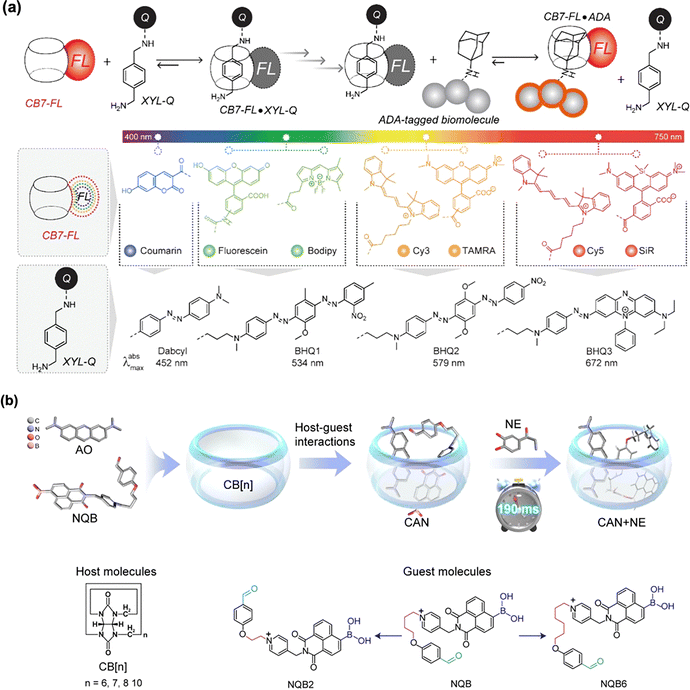 | ||
| Fig. 16 CB [n] based supramolecular systems for molecular recognition. (a) A supramolecular guest exchange strategy employing CB [7] as a host for bioorthogonal imaging; reproduced with permission from ref. 126. Copyright © 2024, The Authors. Published by the American Chemical Society. (b) A CB [n] based supramolecular fluorescent probe for the imaging and quantification of NE. Reproduced with permission from ref. 127. Copyright © 2025, American Chemical Society. | ||
Tian et al.127 developed a supramolecular dye for norepinephrine (NE) fluorescent imaging and quantification. The host complex CB [n] could encapsulate two guest dyes: (1) a naphthalimide-derived dye (NQB) containing boric acid moieties and aldehyde groups and (2) acridine orange (AO) which was employed as an internal reference (525 nm). Upon addition of NE, the fluorescence intensity at 455 nm decreased with concentration dependence (Fig. 16(b)). This dual-emission supramolecular dye was successfully implemented for NE imaging in various models including neuronal cytomembranes, brain tissues, and zebrafish.
4.2 CB [n] regulates the therapeutic properties of small-molecule dyes
As a versatile supramolecular host, CB [n] not only modulates the optical properties of organic dyes but also plays a pivotal role in regulating the PDT effect of small-molecule photosensitizers via host–guest interactions. Unlike conventional PSs, CB [n] involved PS offers distinct advantages for PDT by enabling spatiotemporal control of ROS generation. This approach minimized off-target toxicity and photodamage to healthy tissues while circumventing the tedious and complex organic synthesis.38In 2016, Zhang and Sun reported a CB [8]-regulated PDT system using toluidine blue dye (TB) for simultaneous tumor imaging and therapy (Fig. 17(a)).128 The TB dye was coupled with a biotin group for endowing tumor targeting ability, which was further assembled with CB [8] to form a stable 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 supramolecular complex (2TB-B @ CB [8]) (Fig. 17(b)). The dimerization within the CB [8] cavity effectively quenched both fluorescence and photodynamic activity. However, upon encountering intracellular triggers in cancer cells, the complex dissociated to restore the native fluorescence and photodynamic activity of TB-biotin, enabling tumor-selective therapy. Recently, Zhang et al.129 designed a novel cyanine dye, Naph-α-TCy5, which exhibited efficient J-aggregation and remarkable 1O2 generation in its native state (Fig. 17(c)). Upon host–guest interaction with CB [7], the resulting Naph-α-TCy5-CB [7] complex disrupted the J-aggregates, converting the active PS into a “caged” form with completely quenched 1O2 production. The “caged” PS could be activated by spermine in cancer cells through competitive host–guest complexations to form its native J-aggregation state of Naph-α-TCy5, which could generate efficient 1O2 to selectively kill tumor cells (Fig. 17(d)). Besides modulating photodynamic activity, CB [n] based supramolecular complexes could promote PS self-degraded post-PDT, significantly enhancing biosafety. In 2021, Zhang et al.130 developed a CB [7] caged BODIPY-based PS (BDP2IPh-CB [7]) (Fig. 17(e)). Compared to the guest molecule (BDP2IPh), the supramolecular complex not only displayed enhanced 1O2 production under light irradiation with prolonged triplet lifetime, but also showed ROS-triggered self-degradation capability, which reduced PDT side effects without compromising therapeutic efficiency (Fig. 17(f)). Tang et al.131 employed a stereoisomeric engineering strategy to develop AIE PSs for antifungal PDT. The (Z)- and (E)-TPE-EPy isomers (Fig. 17(g)) exhibited distinct photophysical properties and ROS generation abilities. Importantly, supramolecular assembly with CB [8] further enhanced both fluorescence and ROS generation by restricting intramolecular motion and improving ISC (Fig. 17(h) and (i)). These assemblies exhibited reduced dark toxicity while maintaining high phototoxicity.
1 supramolecular complex (2TB-B @ CB [8]) (Fig. 17(b)). The dimerization within the CB [8] cavity effectively quenched both fluorescence and photodynamic activity. However, upon encountering intracellular triggers in cancer cells, the complex dissociated to restore the native fluorescence and photodynamic activity of TB-biotin, enabling tumor-selective therapy. Recently, Zhang et al.129 designed a novel cyanine dye, Naph-α-TCy5, which exhibited efficient J-aggregation and remarkable 1O2 generation in its native state (Fig. 17(c)). Upon host–guest interaction with CB [7], the resulting Naph-α-TCy5-CB [7] complex disrupted the J-aggregates, converting the active PS into a “caged” form with completely quenched 1O2 production. The “caged” PS could be activated by spermine in cancer cells through competitive host–guest complexations to form its native J-aggregation state of Naph-α-TCy5, which could generate efficient 1O2 to selectively kill tumor cells (Fig. 17(d)). Besides modulating photodynamic activity, CB [n] based supramolecular complexes could promote PS self-degraded post-PDT, significantly enhancing biosafety. In 2021, Zhang et al.130 developed a CB [7] caged BODIPY-based PS (BDP2IPh-CB [7]) (Fig. 17(e)). Compared to the guest molecule (BDP2IPh), the supramolecular complex not only displayed enhanced 1O2 production under light irradiation with prolonged triplet lifetime, but also showed ROS-triggered self-degradation capability, which reduced PDT side effects without compromising therapeutic efficiency (Fig. 17(f)). Tang et al.131 employed a stereoisomeric engineering strategy to develop AIE PSs for antifungal PDT. The (Z)- and (E)-TPE-EPy isomers (Fig. 17(g)) exhibited distinct photophysical properties and ROS generation abilities. Importantly, supramolecular assembly with CB [8] further enhanced both fluorescence and ROS generation by restricting intramolecular motion and improving ISC (Fig. 17(h) and (i)). These assemblies exhibited reduced dark toxicity while maintaining high phototoxicity.
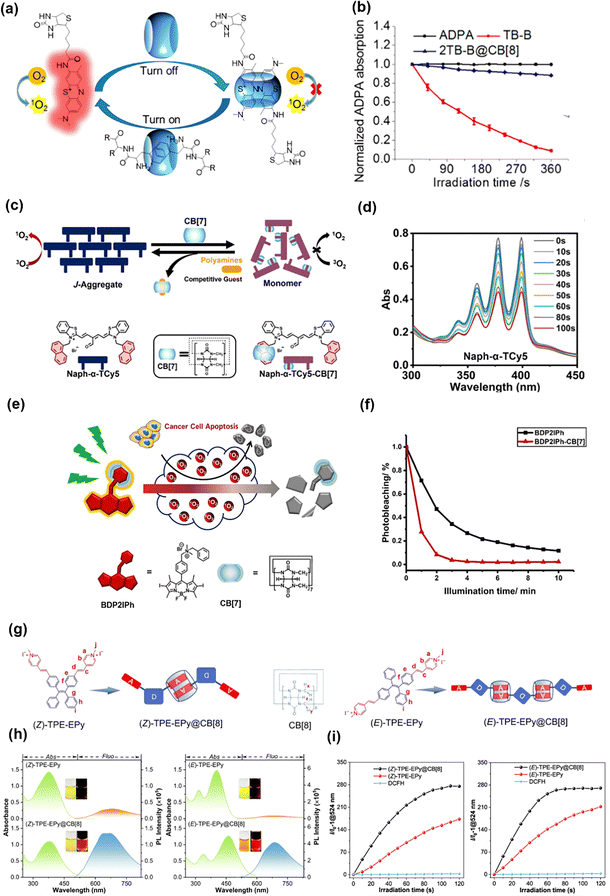 | ||
| Fig. 17 Construction of CB [n] based supramolecular dyes with PDT properties. (a) CB [8] regulated PDT of Toluidine blue (TB) for tumor imaging and therapy; (b) normalized absorbance decay of ADPA, TB-B and 2TB-B@ CB [8] with light irradiation; reproduced with permission from ref. 128. Copyright © 2016, American Chemical Society. (c) CB [7] modulated J-aggregation of cyanine dye and its PDT properties; (d) UV-vis absorption spectra recording 9,10-anthracenediyl-bis(methylene)-dimalonic acid (ABDA) over Naph-α-TCy5 under light irradiation at different times; reproduced with permission from ref. 129. Copyright © 2025, Chinese Chemical Society. (e) CB [n] based supramolecular complex accelerating the PS “self-degraded” after PDT treatment; (f) photostability of BDP2IPh and BDP2IPh-CB [7] after illumination at different times; reproduced with permission from ref. 130. Copyright © 2020, Wiley-VCH GmbH. (g) The chemical structures and assembling modes of (Z)-TPE-EPy (a) and (E)-TPE-EPy; (h) the UV and fluorescence spectra of (Z)-TPE-EPy, (Z)-TPE-EPy@CB [8], (E)-TPE-EPy and (E)-TPE-EPy@CB [8]; (i) the ROS production of (Z)-TPE-EPy, (Z)-TPE-EPy@CB [8], (E)-TPE-EPy and (E)-TPE-EPy@CB [8]. Reproduced with permission from ref. 131. Copyright © 2022, Nature Publishing Group. | ||
NIR-II photothermal agents have garnered great attention for cancer treatment and bacterial infection therapy due to their superior tissue penetration and enhanced radiation tolerance compared to visible/NIR-I agents. Naphthalene diimides (NDIs) have been widely employed in supramolecular photochemistry owing to their π-electron-deficient planar structure. However, conventional NDI radical anions often suffer from attenuation caused by their limited conjugated systems. To address the issue, Zhang et al.132 developed a supramolecular NDI anion system using CB [7] as a host to stabilize the NDI radical anions (Fig. 18(a)). The designed NDI derivative featured two positively charged quaternary ammonium salts that improve the water solubility and increase the binding affinity to negatively charged bacterial surfaces. The resulting NDI-2CB [7] demonstrated remarkable photothermal conversion efficiency (η = 66.9%) upon reduction under hypoxia environments. Upon 1064 nm laser irradiation for 15 min, the solution temperature increased from 33.7 °C to 54.5 °C (Fig. 18(b)), achieving over 99% inhibition efficiency against E. coli (Fig. 18(c) and (d)). In 2025, the same group reported a thiocarbonyl modified NDI derivative (SNDI). This strategic modification elevated the reduction potential of SNDI,133 resulting in faster bacterial response (within 3 s under hypoxia) while maintaining excellent photothermal conversion efficiency (η = 59.7%) (Fig. 18(e)). The in situ therapeutic synthesis from biocompatible molecules has emerged as a promising strategy to minimize off-target side effects in cancer treatment. Wang et al.134 developed a supramolecular complex based on CB [7] and perylene diimide derivative (PDI) (Fig. 18(f)). The CB [7] host effectively stabilized PDI radical anions under a hypoxia environment, while enabling in situ polymerization with the biocompatible 2-hydroxyethyl methacrylate (HEMA) monomer for tumor ablation (Fig. 18(g)).
 | ||
| Fig. 18 (a) Naphthalene diimide (NDI) based supramolecular complex employing CB [7] as a host for photothermal antibacterial therapy; (b) thermal images of the medium with E. coli and NDI-2CB [7] before and after light irradiation; (c) CFU ratio of E. coli incubated with/without NDI-2CB [7]; (d) plate coating results after different treatments; reproduced with permission from ref. 132. Copyright © 2023, Wiley-VCH GmbH. (e) Thiocarbonyl group substituted NDI supramolecular complex for photothermal antibacterial therapy; reproduced with permission from ref. 133. Copyright © 2025, Wiley-VCH GmbH. (f) The in situ polymerization of a PDI based supramolecular complex for tumor ablation; (g) SEM images of PDI + HEMA and PDI + 2CB [7] + HEMA in a normal or hypoxic environment. Reproduced with permission from ref. 134. Copyright © 2025, The Authors. Published by the American Chemical Society. | ||
Compared to conventional fluorescence imaging, photoacoustic (PA) imaging offers superior tissue penetration and spatial resolution. However, many small-molecule dyes exhibit typically weak PA signals. Zhao and coworkers constructed two supramolecular dyes using dixanthene-based dyes (DXP and DXBTZ) as guest molecules and CB [8] as a host (Fig. 19(a)).135 The resulting nanoparticles (Fig. 19(d)) displayed significant fluorescence quenching, but enhanced PA intensity and PTT properties (Fig. 19(b) and (c)), and the photothermal temperature of the supramolecular complex (DPX + CB [8]) could reach nearly 60 °C after about 500 s of irradiation.
 | ||
| Fig. 19 (a) Construction of CB [8] based PA contrast dyes based on host–guest interactions; (b) the FL decay after assembling CB [8] with DXP; (c) photothermal conversion behavior of DXP before and after assembling with CB [8]; (d) DLS properties of the supramolecular complex (DXP + CB [8]). Reproduced with permission from ref. 135. Copyright © 2023, Nature Publishing Group. | ||
Supramolecular organic photosensitizers based on CB [n]-mediated self-assembly offer several notable advantages, including enhanced water solubility, photostability, and biocompatibility, as well as improved fluorescence or PA signals and ROS generation. Moreover, CB [n]-based assemblies typically demonstrate superior targeting capabilities and reduced dark toxicity. However, challenges remain: the bulky macrocyclic structure of CB [n]s could improve cellular uptake, and competitive biological interactions could compromise the stability of supramolecular complexes under physiological conditions.
4.3 CB [n] based supramolecular dyes for bioimaging applications
Rotello et al.136 designed a bioorthogonal catalytic nanozyme, NP_Ru_CB [7], utilizing CB [7] as a “gating molecule” to mimic enzymatic allosteric regulation. This approach enabled precise catalyst delivery and activity control in cellular environments. NP_Ru_CB [7] demonstrated remarkable versatility for targeted cellular imaging and therapeutic applications. In HeLa cells, NP_Ru_CB [7] effectively controlled the activation of the pro-fluorophore bis-allyloxycarbonyl rhodamine 110. While NP_Ru-treated cells showed significant fluorescent signals in lysosomes, the NP_Ru_CB [7] group remained non-fluorescent, confirming successful catalytic inhibition by CB [7] (Fig. 20(a)). Notably, subsequent ADA addition restored fluorescence with enhanced intensity compared to the unblocked group, likely due to CB [7]-mediated nanoparticle protection. This supramolecular regulation strategy enabled spatiotemporally precise live-cell labeling and detection. | ||
| Fig. 20 (a) Confocal microscopy images of HeLa cells supramolecularly regulated by intracellular chemical reactions. Reproduced with permission from ref. 136. Copyright 2015, Nature Publishing Group. (b) Confocal microscopy images of HeLa cells, left: TBP-CB [8] complex, middle: bright field, and right: merge. Reproduced with permission from ref. 137. Copyright 2020, Wiley-VCH. (c) Confocal microscopy images of A549 cells costained with ANPY⊂CB [8] and Hoechst and AQPY⊂CB [8] and Lysotracker Blue. Reproduced with permission from ref. 123. Copyright 2021, American Chemical Society. (d) Confocal microscopy images and merged images of HeLa cells in the presence of TPE-DPY/CB [7]/CB [8] @HACD, Hoechst and Mito-Tracker Green. Reproduced with permission from ref. 125. Copyright 2024, Nature Publishing Group. (e) Confocal versus RESOLFT images of microtubules on fixed CV1 cells stained with secondary antibodies labeled with DAE@CB [7] complex and corresponding line profiles along indicated lines. Reproduced with permission from ref. 118. Copyright 2022, American Chemical Society. (f) Illumination microscopy (SIM) fluorogenic imaging in live intestine samples of Drosophila melanogaster. Scale bar: 10 µm. Reproduced with permission from ref. 97. Copyright 2024, American Chemical Society. (g) Illustration of the guest-modified trehalose-based metabolic labeling and SIM fluorogenic imaging of metabolically labeled mycobacteria. | ||
Besides CB [7], CB [8] has also been employed to construct supramolecular dyes for cellular imaging. Tian et al.137 designed a structure-restricted dimer, TBP-CB [8], by encapsulating a heavy-atom-modified triazine derivative (TBP) within the CB [8] cavity. This spatial confinement stabilized the charge-transfer triplet state, enabling yellow RTP emission in aqueous solution, which is advantageous for cellular-level imaging. In HeLa cell imaging, TBP-CB [8] exhibited distinct yellow RTP, demonstrating how macrocyclic host–guest chemistry could be leveraged to design novel luminescent materials with enhanced photophysical properties for bioimaging (Fig. 20(b)).
Different supramolecular architectures exhibit unique subcellular organelle-targeting capabilities. Liu et al.123 engineered a dynamic nano-assembly based on CB [8] and an anthracene-conjugated bromophenylpyridinium salt (ANPY), achieving dual-organelle targeting without covalent modification. Under UV excitation, this system underwent a photooxidation-driven transformation from red fluorescent supramolecular polymers (ANPY⊂CB [8]) to green phosphorescent homo-ternary complexes (AQPY⊂CB [8]). CB [8] encapsulation not only enhanced fluorescence by stabilizing charge-transfer interactions but also promoted intersystem crossing, enabling RTP emission for more stable and long-term bioimaging. In cellular imaging, ANPY⊂CB [8] specifically localized to the nucleus, exhibiting a high colocalization coefficient of 0.94 with Hoechst 33342 (Fig. 20(c)). Compared to an unencapsulated guest molecule, this linear nanostructure demonstrated significantly reduced cytotoxicity. Meanwhile, the photooxidation product, AQPY⊂CB [8], selectively accumulated in lysosomes, showing strong colocalization (0.90) with LysoTracker (Fig. 20(c)). This organelle-specific switching without covalent modification fully highlighted the unique value of supramolecular assembly in spatiotemporal precision control and modular design. The platform's low cytotoxicity and high photostability provided a theoretical foundation for dynamic subcellular tracking in living cells. Furthermore, leveraging the high-affinity and macrocyclic confinement of CB and β-CD, they developed a PRET system, achieving a large Stokes Shift (367 nm) and long-lived NIR photoluminescence (700 nm).125 The aggregate was successfully used for cancer cell imaging, demonstrating strong mitochondria colocalization in HeLa and 293T cells (Fig. 20(d)). More importantly, it showed selective uptake by cancer cells over normal cells, while maintaining high cell viability.
In addition to regulating the phosphorescent properties for confocal imaging, CB [n] derivatives have been successfully employed in super-resolution cellular imaging owing to their ability to protect switchable groups from environmental quenching through molecular encapsulation.138 Hell et al.118 reported a supramolecular system comprising CB [7] and fluorescent diarylethene, which exhibited a 23% enhancement in fluorescence quantum yield and demonstrated dramatically improved cycling stability, increasing from 80 to 2560 cycles compared to its monomer. On the foundation of increased brightness and fatigue resistance, the system was evaluated for RESOLFT microscopy applications, where it achieved spatial resolution beyond the diffraction limit (70–90 nm) (Fig. 20(e)). This performance enabled clear resolution of adjacent filaments that were indistinguishable in conventional confocal imaging.
Agasti et al.126 further developed a general bioorthogonal probe platform based on CB [7] host–guest chemistry. This system utilized competitive guest binding to achieve fluorogenic turn-on upon interaction with targeted biomarkers, enabling high-contrast visualization. Apart from no-wash live-cell imaging, it was also used for labeling and imaging actin filaments in the thoracic muscle of Drosophila melanogaster, providing specific and distinct visualization of a two-dimensional (2D) actin pattern. Notably, this fluorescent probe demonstrated exceptional performance across various biological targets, including cell membranes, microtubule networks inside live HeLa cells, or Drosophila intestinal tissue microtubules, all while maintaining minimal background fluorescence and negligible nonspecific signal (Fig. 20(f)). More importantly, this system could specifically label Mycobacterium bacteria through metabolic incorporation, demonstrating behaviour consistent with high-contrast metabolic labeling (Fig. 20(g)).
CB [n]-based host–guest fluorescence probes have emerged as powerful biosensing tools due to their multifunctionality, adjustable features, and exceptional selectivity.139 Tian et al.127 developed a CB [n]-based supramolecular fluorescent chemosensor CAN for real-time imaging and quantification of NE dynamics. It is worth noting that the confinement effect and conformational control within the CB [n] cavity enhanced both selectivity and reaction rates, achieving an impressive 190 ms temporal resolution. In larval zebrafish imaging, electrical stimulation induced a measurable F455/F525 ratio decrease within 7.2, correlating with NE concentration changes, while the PBS group exhibited minimal fluctuations (Fig. 21(a) and (b)). This fluorescence probe effectively mapped NE dynamics across brain regions, revealing elevated levels in stressed mice compared to controls (Fig. 21(c)). Notably, such a system allowed quantification analysis of NE significantly increasing in 26 brain areas under stress conditions, particularly in the amygdala, thalamus, hypothalamus, and hippocampus, implicating these regions in stress response pathways. This state-of-the-art supramolecular imaging platform represented a superior approach for accurate, selective and real-time NE quantification.
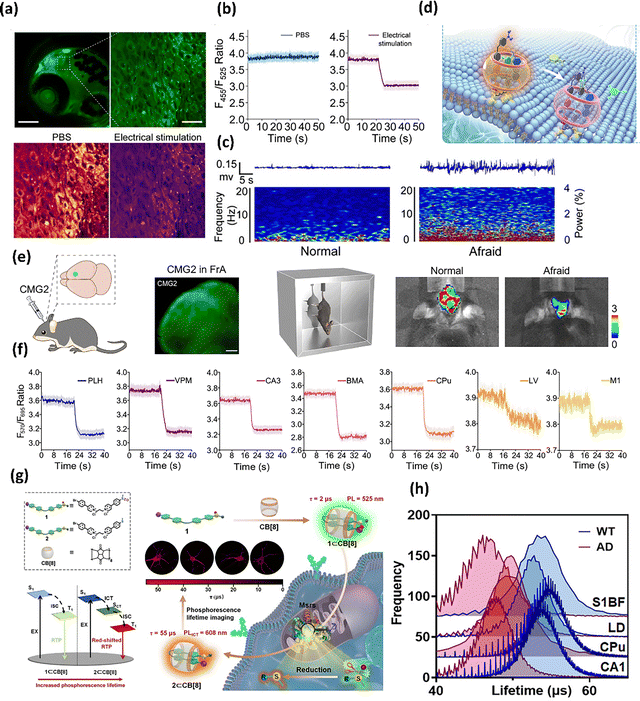 | ||
| Fig. 21 (a) Fluorescence images of zebrafish treated with CAN following electrical stimulation. Scale bars: 130 and 10 µm. Reproduced with permission from ref. 127. Copyright 2025, American Chemical Society. (b) Fluorescence changes of CAN in zebrafish after PBS treatment (blue line) or electrical stimulation (purple line). (c) Electrophysiological signal recording in normal mice and afraid mice. (d) Qualitative fluorescence imaging and real-time quantification of EP in the cell membrane. Reproduced with permission from ref. 140. Copyright 2025, Nature Publishing Group. (e) Left: Schematic diagram depicting probe injection and fluorescence imaging in vivo; example image showing CMG2 in FrA in a coronal brain slice, scale bar: 500 µm; right: schematic illustration of tail suspension experiments. In vivo imaging of normal and afraid mouse brains treated with CMG2. (f) Fluorescence signal ratio changes in seven representative brains. (g) Schematic illustration of the supramolecular phosphorescent sensor for mitochondria-specific Msrs detection via phosphorescence lifetime imaging. Reproduced with permission from ref. 141. Copyright 2025, American Chemical Society. (h) Phosphorescence lifetime distribution curves of probe 1⊂CB [8] in cornu ammonis region 1 (CA1) of the hippocampus, caudate putamen (CPu), laterodorsal thalamic nucleus (LD), and primary somatosensory cortex, barrel field (S1BF) of both Alzheimer's disease (AD) model and wild-type (WT) mouse brains. | ||
Building upon the same strategy, they designed an analogous supramolecular dye for epinephrine (EP) in the brain.140 Through self-assembly with a tailored guest molecule, the resulting CMG2 complex enabled rapid (∼240 ms response time) and highly sensitive EP detection. Moreover, CMG2 exhibited strong membrane anchoring, likely due to the electrostatic interaction between its cationic pyridinium moiety and cytomembranes, while its hydrophobic alkyl chain facilitated stable membrane embedding (Fig. 21(d)). CMG2 demonstrated robust real-time EP monitoring in brain tissues and living specimens. The ratio of F570/F695 in the CA1 region of brain tissues decreased by around 0.2 within 6.6 s after electrical stimulation. In the model of zebrafish, the value declined sharply by 0.94 within 7.8 s. CMG2 also performed well in living mice (Fig. 21(e)), successfully tracking EP dynamics across 26 brain regions in freely tail hanging mice. Notably, EP levels increased significantly in the amygdala, thalamus, hypothalamus, hippocampus and striatum, showing a slight elevation in the cerebral cortex, and remained nearly unchanged (<2.8%) in the other 16 brain regions (Fig. 21(f)).
They further engineered a supramolecular probe⊂CB [8] for the detection of mitochondria-targeted methionine sulfoxide reductases (Msrs), which was related to oxidative stress-associated disorders, such as Alzheimer's disease (AD) (Fig. 21(g)).141 The rigid cavity of CB [8] suppressed nonradiative decay, enabling dual-channel ratiometric phosphorescence (525 nm to 608 nm) and lifetime imaging (2 µs to 55 µs). In Aβ1–42 treated neurons, the phosphorescence lifetime dropped from 53 µs to 28 µs after 20 h of incubation, accompanied by the diminished emission intensity, while the PBS group exhibited minimal change. Acute brain slices from AD model mice exhibited a pronounced phosphorescence lifetime reduction in both the CA1 and S1BF regions compared to WT mice, with the CA1 region showing the most dramatic decline (Fig. 21(h)). This indicated impaired Msrs activity, suggesting diminished neuroprotective antioxidant capacity that could exacerbate oxidative damage and accelerate AD progression.
5. Supramolecular dyes based on calixarene/pillararene systems for precision medicine
Like other supramolecular host–guest systems, calixarenes (calix [n] arenes) adopted a cup-like, non-symmetric structure formed through phenol–formaldehyde condensation. Their cavities can be readily functionalized with sulfonate, carboxylate, ammonium, or phosphate groups, enhancing water solubility and biocompatibility while enabling selective binding of biomolecules and small-molecule dyes.142 In contrast, (pillararenes (pillar [n]) arenes) displayed a rigid, symmetrical, pillar-shaped framework composed of para-linked aromatic rings. Pillararenes and calixarenes encapsulate guest molecules within hydrophobic cavities, where the hydrophobic effect reduces the system's free energy. In calixarenes, phenolic hydroxyls or introduced substituents form hydrogen bonds or ion pairs with the guest, whereas pillararenes rely on end-group functionalization to generate electrostatic interactions. Aromatic guests further enhance binding through π–π stacking and van der Waals forces. Calixarenes possess conformational flexibility that adapts to guest size, while pillararenes require precise spatial complementarity. Guest entry displaces cavity water, and the associated entropy gain drives complexation. Both hosts thus share a hydrophobicity-driven, multi-interaction recognition mechanism, but their distinct architectures confer different modes of molecular recognition. Their electron-rich cavities also facilitated strong host–guest interactions, making them ideal for molecular recognition and phototherapeutic applications.143,144 In this section, we summarize the representative works referring to calix[n]arene and pillar [n] arene based supramolecular dyes for bio-medicinal applications.5.1 Calixarenes and pillararenes regulate the optical properties of small-molecule dyes
Obtaining fluorescent nanoparticles (NPs) with both superior brightness and protein size (3–7 nm) is a key challenge for biological applications. Klymchenko's group developed protein-sized fluorescent NPs based on calixarene micelles.145 Cyanine (Cy) 3 and 5 containing azide groups and hydrophilic PEG chains were cross-linked to the calixarene amphiphile CX8TP via a Cu-catalyzed click reaction, producing the fluorescent CX/Cy3L NPs and CX/Cy5L NPs (Fig. 22(a)). The dialyzed cross-linked micelles had a hydrodynamic diameter of 7 nm. The absorption data suggested the cross-linking yields of 59 and 49% for Cy3L and Cy5L dyes, respectively (Fig. 22(b)). Compared with free dyes Cy3L and Cy5L, the crosslinked micelles CX/Cy3L NPs and CX/Cy5L NPs showed a significant increase in the short-wavelength shoulder and red-shifted emission bands (Fig. 22(c)). The CX/Cy3L NPs showed lower fluorescence quantum yields than free dye Cy3L due to the ACQ effect, while the fluorescence intensity significantly increased in glycerol, with a QY of 15% (Fig. 22(d) and (e)). The strong FRET signal was observed at the different ratios of donor (Cy3L)/acceptor (Cy5L) (Fig. 22(f)), which was further successfully applied in cell imaging.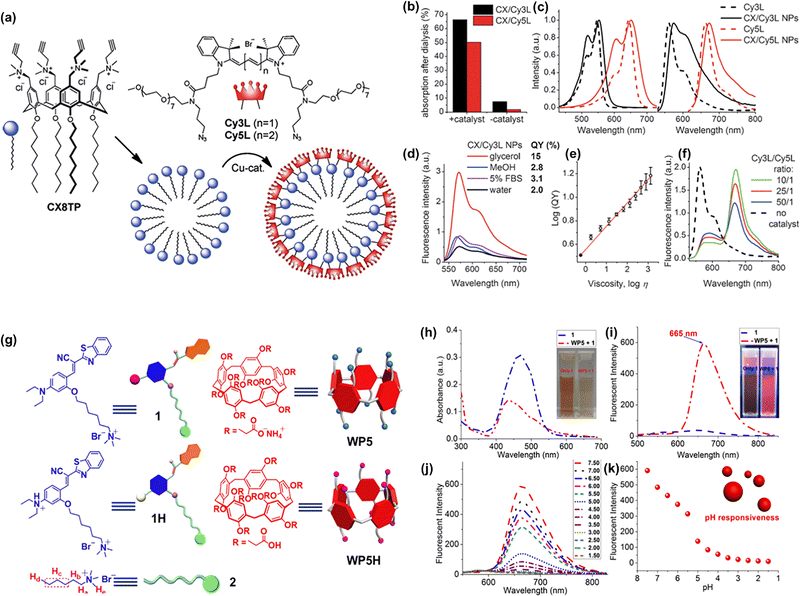 | ||
| Fig. 22 (a) Illustration of calixarene micelles with cyanine corona. Reproduced with permission from ref. 145. Copyright 2016, Wiley-VCH. (b) Peak absorbance ratio (after vs. before dialysis) of dye cross-linkers and CX8TP (CX) micelles with and without the Cu catalyst. (c) Absorption and fluorescence spectra in water of free cross-linker dyes and the corresponding cross-linked micelles after dialysis. (d) Fluorescence spectra of Cy3L cross-linked micelles in different media. (e) Dependence of fluorescence quantum yield (QY) on viscosity. (f) Fluorescence spectra of FRET micelles prepared at different Cy3L (donor) to Cy5L (acceptor) ratios in water. Reproduced with permission from ref. 145. Copyright 2016, Wiley-VCH. (g) The structures of the small-molecule dye (compound 1) and supramolecular host WP5. (h) Absorbance spectra of compound 1 in aqueous phosphate-buffered saline buffer solution (APBSBS) without and in the presence of WP5. (i) Fluorescence spectral response of 1 upon addition of 1.0 equiv. of WP5 (λex = 470 nm). (j) Fluorescence spectra of pH dependence of WP5⊃1 in APBSBS. (k) pH dependence of the fluorescence intensity of WP5⊃1 in APBSBS at 665 nm. Reproduced with permission from ref. 146. Copyright 2016, American Chemical Society. | ||
To enhance the NIR fluorescence of small-molecule dyes, Huang's group reported pillararene-based NIR NPs (WP5⊃1), which were assembled by compound 1 and WP5 (Fig. 22(g)).146 Compound 1 showed very weak fluorescence in water solution due to the π–π stacking interactions under UV irradiation. The formation of WP5⊃1 resulted in significant fluorescence enhancement due to the host–guest complexation-enhanced aggregation (Fig. 22(h) and (i)). Moreover, WP5⊃1 is pH-biodegradable which could collapse after treatment with an acid (Fig. 22(j) and (k)), indicating its good biosafety.
Sonodynamic therapy (SDT) has emerged as one of the non-invasive therapeutic methods activating ROS under ultrasound (US). However, many organic sonosensitizers suffer from some limitations including poor solubility and chemical stability in blood circulation. To address the above issues, Tang et al. reported an interfacial engineering strategy that modified the organic sonosensitizers (TPA-OS) on the surface of inorganic material CP5@CeOx which was assembled by carboxyl-pillar[5]arene (CP5) with oxygen vacancies (VO) of CeOx, producing the intelligent sonosensitizer TPA–OS⊂CP5@CeOx (Fig. 23(a)).147 The EPR (electron paramagnetic resonance) and ROS production experiments demonstrated that TPA–OS⊂CP5@CeOx displayed the highest ˙OH generation compared with CP5@CeOx, CeOx, TPA-OS, and TPA-OS⊂CP5, indicating its best sonodynamic efficiency (Fig. 23(b)–(d)). It is worth noting that lowering the pH (pH 5) would weaken the host–guest interaction, resulting in delivery of TPA–OS from CP5@CeOx in a tumor or lysosome environment.
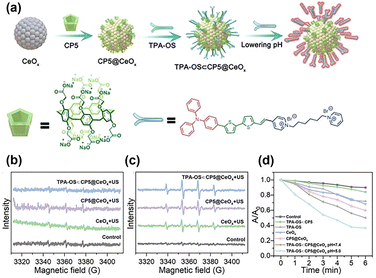 | ||
| Fig. 23 (a) Illustration for the preparation of TPA–OS⊂CP5@CeOx. EPR spectra for the generation of 1O2 (b) and ˙OH (c). (d) The ROS generation under US irradiation (DPBF was used as the indicator). Reproduced with permission from ref. 147. Copyright 2025, Wiley-VCH. | ||
The undesired dark cytotoxicity of small-molecule dyes strongly hinder their applications in clinical translation. To address this issue, Tang and Ding et al.148 developed a host–guest strategy via electrostatic interaction of pyridinium-functionalized tetraphenyl-ethylene (TPE-PHO) and water-soluble calixarene (SC4A). The host–guest complexation suppressed intramolecular motion, promoting radiative decay and significantly reducing dark cytotoxicity while maintaining bioimaging capability. Competitive displacement by 4,4′-benzidine dihydrochloride (BZD) restored the activity of TPE-PHO (Fig. 24(a)), enabling controlled tumor therapy in murine models. In a related study, Ding et al. encapsulated pyridinium-decorated AIEgens with calix [5] arene, effectively inhibiting the ISC pathway and thermal deactivation. This led to a significant fluorescence enhancement, facilitating precise image-guided cancer surgery.149 Notably, the supramolecular complex 1-loaded S-AIE dots exhibited quenched PDT activity but significantly brighter fluorescence compared to the control group (1-loaded DSPE-PEG-AIE dots) (Fig. 24(b) and (c)). In 2022, Yao et al.150 constructed a supramolecular complex (DPTTIC NPs) by combining an A–D–A small-molecule dye with an amphiphilic pillararene. DPTTIC NPs displayed both type I and II PDT effects alongside PTT, achieving a maximum temperature of 44 °C that would not cause damage to normal tissues (Fig. 24(d)). In addition, DPTTIC NPs showed minimal dark cytotoxicity even at 300 µg mL−1, underscoring their good biocompatibility. Yang et al.151 exploited pillar [5] arene to modulate the photosensitizer behavior. The type II photosensitizer BODIPY-G, which typically generates 1O2, was converted into a type I system (O2˙−) upon binding to bispillar [5] arene (Fig. 24(e)).
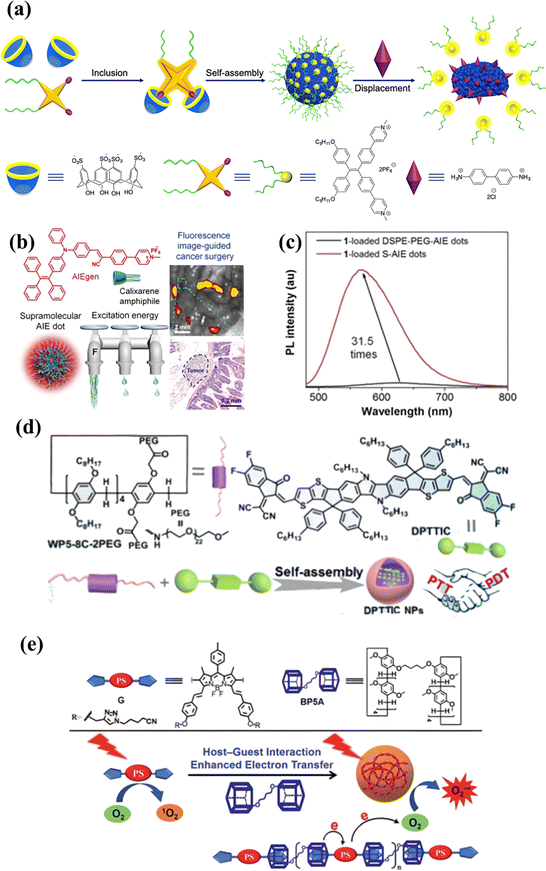 | ||
| Fig. 24 (a) Design of the SC4A and AIEgen based supramolecular complex for precise phototheranostics; reproduced with permission from ref. 148. Copyright © 2020, American Chemical Society. (b) Design of a supramolecular AIE dot with calixarene and its application in fluorescence imaging guided tumor surgery; (c) fluorescence intensity of DSPE-PEG encapsulated AIE dots (black line) and supramolecular AIE dot with calixarene (red line); reproduced with permission from ref. 149. Copyright © 2020, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Temperature enhancement curves of different concentrations. (d) The construction of WP5-8C-2PEG and DPTTIC based supramolecular dyes. Reproduced with permission from ref. 150. Copyright © 2022, Royal Society of Chemistry. (e) The design of bispillar [5] arene and BODIPY based supramolecular complex for converting the photodynamic agents. Reproduced with permission from ref. 151. Copyright © 2022, Royal Society of Chemistry. | ||
5.2 Calixarene/pillararene based small-molecule dyes for bioimaging and phototherapy applications
Calixarene and pillar [n] arene based supramolecular dyes exhibit excellent optical properties that enable superior performance in bioimaging and phototherapy. Neurotransmitters play crucial roles in regulating physiological processes, especially in neuronal events and brain diseases (Fig. 25(a)). However, the simultaneous detection of multiple neurotransmitters remains challenging. Tian et al.152 developed a supramolecular dye (CN-DFP5) featuring a dual-functionalized fluorescent pillar [5] arene derivative with borate-naphthalene and aldehyde-coumarin recognition moieties. The boric-acid moiety at the naphthalene site could selectively bind to catechol-type neurotransmitters via reversible condensation, while the aldehyde-functionalized coumarin unit could react with monoamine neurotransmitters to form the imine bonds (Fig. 25(b) and (c)). The electron-rich pillar [5] arene cavity further enhanced the binding affinity through electrostatic interactions. CN-DFP5 enabled simultaneous, ratiometric imaging of seven neurotransmitters, including dopamine (DA), norepinephrine (NE), epinephrine (Ep), acetylcholine (Ach), serotonin (5-HT), histamine (HA) and glutamate (Glu) under two-photon excitation (Fig. 25(d)). Very recently, Tian's group reported a supramolecular fluorescence probe for visualization of glutamate (Glu) dynamics in living systems.153 The engineered sensor TympG2 displayed high sensitivity and selectivity for the detection of Glu. Further structural modification on the cationic moiety of TympG2 enabled the locations of Glu in multiple cellular organelles, including the plasma membrane, cytoplasm, mitochondria, and lysosomes (Fig. 25(e)). Two-photon imaging of brain tissue slices under a hypoxia environment revealed that subregions such as SB1F, CA1, and LD exhibited more pronounced Glu responses than CPU. Moreover, the authors established a multiplexed fiber-optic fluorescence spectroscopy platform for monitoring Glu in freely behaving subjects. The networks of Glu content across 24 brain regions were successfully obtained. The results revealed that the levels of Glu in the cortex, hippocampus, and thalamic regions were obviously increased in response to hypoxic stimulation.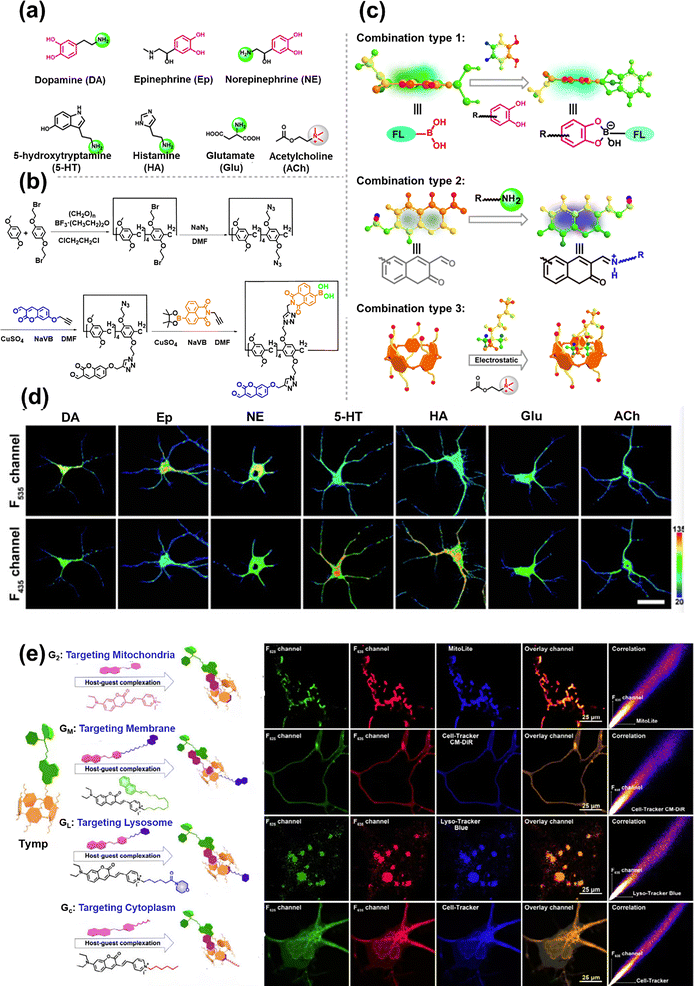 | ||
| Fig. 25 (a) Seven neurotransmitters to be tested. (b) Synthetic route of coumarin–naphthyl pillar [5] arene (CN-DFP5). (c) Interaction patterns of the host molecule with three types of neurotransmitters. (d) Two-photon fluorescence microscopy imaging of neurons co-incubated with CN-DFP5. Reproduced with permission from ref. 152. Copyright © 2022, American Chemical Society. (e) Design and confocal imaging of four host–guest molecules targeting mitochondria, membrane, lysosomes, and cytosol, respectively. Copyright with permission from ref. 153. Copyright © 2025, American Chemical Society. | ||
The supramolecular dye TPE-PHO⊂SC4A developed by Tang and Ding et al.148 demonstrated preferential accumulation at tumor sites through the enhanced permeability and retention (EPR) effect. Fluorescence signals in tumor tissue reached the maximum at 5 h treatment, while the fluorescence signals in normal organs diminished during the same period, highlighting its tumor-specific imaging capability (Fig. 26(a)). Moreover, this supramolecular complex displayed good antitumor activity in 4T1 tumor bearing mouse models, and the tumor growth was significantly inhibited after treatment with TPE-PHO⊂SC4A and BZD under light irradiation. Another supramolecular dye, PEGylated calix [5] arene-based S-AIE dots, reported by Ding et al.149 displayed enhanced fluorescence intensity through inhibiting the ISC process following host–guest interactions. The S-AIE dots exhibited good EPR effects, achieving specific tumor accumulation with an exceptionally high SBR of 48.5 ± 5.6. Tumor-specific accumulation was further confirmed using a D-luciferin bioluminescent probe, through colocalization analysis (Fig. 26(b)). Guo and Ding et al.154 pioneered a biomarker displacement strategy via host–guest interactions. This system employed PS-AlPcS4 assembled with calix [5] arene pentadodecyl ether (GC5A-12C), which completely quenched fluorescence until displacement by adenosine triphosphate (ATP), a tumor biomarker. Upon intertumoral injection, the supramolecular dye demonstrated selective fluorescence activation in ATP-overexpressed tumor regions, with minimal background signal in normal skin or major organs (liver, spleen, lungs, and kidneys) (Fig. 26(c)). Moreover, the AlPcS4/GC5A-12C system also exhibited excellent PDT efficacy and biocompatibility under 660 nm laser irradiation, significantly suppressing tumor growth.
 | ||
| Fig. 26 (a) The fluorescence imaging of different organs in a mouse model after treatment with TPE-PHO⊂SC4A; reproduced with permission from ref. 148. Copyright © 2020, American Chemical Society. (b) The fluorescence and bioluminescence imaging of S-AIE dots and D-luciferin in mouse models; reproduced with permission from ref. 149. Copyright © 2020, Wiley-VCH. (c) Fluorescence imaging of the 4T1 tumor-bearing nude mice at 1, 4, 6, 8, and 24 h after intravenous injection of AlPcS4/GC5A-12C and free AlPcS4. Reproduced with permission from ref. 154. Copyright © 2018, American Chemical Society. (d) The photographs of MRSA infected sites on mice within 9-day post-treatment with various treatments. Reproduced with permission from ref. 155. Copyright 2022, Wiley-VCH. | ||
Besides cancer, bacterial infection is another kind of the biggest health problems that pose a great threat to human life. One of the current challenges for developing antibacterial agents is improving the permeability into the biofilm barrier. Zhang and Tian's group developed a supramolecular photosensitizer platform based on CP5 and tetrafluorophenyl porphyrin functionalized with a quaternary ammonium group (TFPP-QA).155 The organic photosensitizer can be released from the supramolecular complex (TFPP-QA/CP5) under an acidic microenvironment of the bacterial infection site, enabling the enhanced PDT effect. The phototherapeutic effect on Gram-positive bacteria (MRSA) infected mice demonstrated that the TFPP-QA/CP5 treated group displayed almost complete healing effect at day 9 upon laser irradiation (Fig. 26(d)), indicating the superiority of the supramolecular strategy.
6. Supramolecular dyes based on enzyme-instructed self-assembly (EISA) systems for precision medicine
Beyond conventional supramolecular dyes, enzyme-instructed self-assembly (EISA)-based systems, constructed via intermolecular π–π stacking and Phe–Phe hydrophobic interactions, have attracted increasing attention in recent years.156–158 These EISA dyes rely on the enzymatic transformation of small-molecule precursors into self-assembled nanostructures in situ at tumor sites.159 Key enzymes such as alkaline phosphatase (ALP), deacetylases, and tyrosinase have been shown to be closely associated with tumor growth and metabolism, making them ideal triggers for the self-assembly process.160,161Compared to conventional nanomaterials, small-molecule dyes exhibit excellent biocompatibility and strong tumor penetration, enabling them to reach deep into tumor tissues.162 However, their inherent lack of targeting specificity often leads to poor accumulation at tumor sites, short retention times, and potential side effects on healthy tissues.163,164 To address these limitations, researchers have leveraged the abnormal expression or elevated activity of tumor-associated enzymes to promote the in situ aggregation of small molecules into large self-assembled nanostructures. This not only facilitated targeted delivery, accumulation, and prolonged retention in tumor tissues but also minimized off-target toxicity.165
In terms of cancer therapy, EISA enabled the organelle-specific assembly of small molecules, leading to the dysfunction of key cellular organelles and ultimately inducing tumor cell death.166,167 For imaging applications, EISA dramatically amplified signal intensity, thereby enabling high-contrast visualization of tumors with exceptional specificity.168,169 Consequently, EISA-based supramolecular dyes represented a powerful platform for precise cancer theranostics.
6.1 EISA regulates the optical properties of small-molecule dyes
The integration of diverse imaging moieties enables the construction of various EISA optical imaging probes,170–174 primarily encompassing fluorescence imaging, photoacoustic (PA) imaging, and Raman imaging modalities.In 2010, Rao and Liang pioneered an enzyme-triggered bioorthogonal reaction based on the specific condensation between 6-hydroxy-2-cyanobenzothiazole (CBT) and D-cysteine (D-Cys), enabling the in situ self-assembly of small-molecule dyes at the cellular level.170 Building upon this strategy, Rao and Ye subsequently extended the reaction to in vivo applications, successfully achieving self-assembly within living organisms and markedly enhancing imaging performance. They later demonstrated a NIR fluorescent EISA probe (C-SNAF),171 capable of visualizing caspase-3/7 activity, an apoptosis-related signal, through in vivo imaging (Fig. 27(a)). The probe was composed of three functional components: (1) a bioorthogonal reaction pair consisting of 2-cyano-6-hydroxyquinoline (CHQ) and a D-cysteine (D-Cys) residue based on an aminofluorescein scaffold; (2) an N-terminal D-Cys residue masked by a caspase-3/7-cleavable peptide substrate (DEVD) and a GSH-responsive disulfide linkage; and (3) a NIR dye (Cy5.5) for fluorescence imaging (FLI). In aqueous solution, C-SNAF remained as a small molecule. Upon exposure to active caspase-3/7 and intracellular GSH, sequential cleavage of the DEVD peptide and reduction of the disulfide bond released free N-terminal D-Cys. This liberated D-Cys subsequently underwent an intramolecular cyclization reaction with CHQ to form the macrocyclic compound C-SNAF-cycle. The resulting hydrophobic and conformationally rigid macrocycles spontaneously underwent supramolecular self-assembly into larger nanostructures with an average size of 174 ± 44 nm. Compared to the parent small-molecule probe C-SNAF, these nanoaggregates exhibited significantly prolonged retention at tumor sites. Given the positive correlation between caspase-3/7 activity and the extent of apoptosis, the in situ formation of fluorescent nanoassemblies at tumor sites enabled real-time monitoring of tumor apoptosis following chemotherapy, as reflected by changes in fluorescence intensity. Ye et al.172 innovatively integrated the CHQ/D-Cy bioorthogonal reaction pair with a triazole-linked radioactive 18F-labeled fragment into the heptamethine cyanine backbone, successfully constructing a NIR macrocyclic probe, [18F]-IR780-1 (Fig. 27(b)). This probe underwent bioorthogonal-triggered macrocyclization and subsequent self-assembly and could precisely form nanoparticles with dual-modal imaging capabilities for both photoacoustic and PET imaging. Notably, IR780-1 exhibited a typical ACQ effect upon self-assembling into nanoparticles, and its fluorescence intensity at 813 nm decreased approximately 5.7-fold, while the PA signal at 790 nm increased by 1.4-fold (Fig. 27(c) and (d)). The dual-modal probe [18F]-IR780-1 demonstrated specific activation by caspase-3 and GSH, undergoing intramolecular macrocyclization followed by self-assembly to form functionalized nanoparticles. This unique molecular design not only significantly prolonged the probe's retention time and accumulation efficiency at the target site but also achieved dual enhancement of PAI and PET signals, providing a novel tool for precision molecular imaging.
 | ||
| Fig. 27 (a) Illustration of C-SNAF-enabled in vivo imaging for monitoring caspase-3/7 activity in tumor xenograft models. Adapted with permission from ref. 171, Copyright 2014, Nature Publishing Group. (b) Chemical structure of the dual-modal PAI/PET probe [18F]-IR780-1, highlighting its caspase-3/GSH-triggered macrocyclization and subsequent self-assembly. (c) FL and (d) PA spectra of IR780-1 before (black) and after (red) Casp-3 activation; the inset shows NIR fluorescence images in the presence and in the absence of Casp-3. Reproduced from ref. 172, Copyright 2022, Wiley-VCH. (e) Caspase-1-mediated dual aggregation behavior of QMT-CBT compared to the single aggregation pattern of its control counterpart QMT-CBT-Ctrl. (f) Fluorescence spectra of QMT-CBT (red) and QMT-CBT-Ctrl (blue), with (solid lines) or without (dashed lines) Casp-1 incubation. (g) Bio-TEM images of PC12 cells treated with Aβ and incubated with QMT-CBT for 1 hour. Adapted from ref. 173, Copyright 2023, American Chemical Society. (h) CTSB-responsive CBT-Cys click chemistry for single-cell SHINERS imaging of cathepsin B activity. (i) Raman (top) and SHINERS (bottom) spectra of Yne-CBT in PBS with Au@SiO2 nanoparticles. (j) SHINERS spectra of Yne-CBT following incubation with TCEP or CTSB, recorded after adding Au@SiO2 NPs. Reproduced from ref. 174, Copyright 2024, American Chemical Society. | ||
Recent advances have combined EISA with the AIE strategy to develop highly sensitive in vivo imaging probes. A notable example was the NIR fluorescent probe QMT-CBT, recently developed by Liang's team, which has employed a unique dual-AIE mechanism for sensitive in vivo imaging of caspase-1 (Fig. 27(e)).173 Experimental data showed that QMT-CBT exhibited a remarkable fluorescence enhancement effect after incubation with caspase-1, with fluorescence intensity increasing approximately 15.7-fold, while only a 7.7-fold enhancement was observed in the control group (Fig. 27(f)). At the cellular level, the probe was selectively activated by intracellular caspase-1, forming fluorescently enhanced QMT-NPs (Fig. 27(g)), thereby achieving sensitive detection of inflammation-related caspase-1 activity. The mechanism involves two key steps: first, under the synergistic action of caspase-1 and GSH, the CBT-Cys click reaction converted QMT-CBT into cyclic dimers (QMT-dimers), completing the initial aggregation. Subsequently, these dimers further assembled into AIE-active nanoparticles (QMT-NPs) with NIR emission characteristics, ultimately achieving significant signal amplification. This design strategy provided a new approach for highly sensitive imaging of brain diseases. Liang et al. further designed a self-calibrating Raman probe Yne-CBT for quantitative spatially resolved imaging of cathepsin B (CTSB) activity in living cells (Fig. 27(h)).174 The Yne-CBT probe comprised three functional components: a Val-Cit peptide sequence serving as a CTSB-specific substrate; a CBT moiety and cysteine residue to initiate a CBT–Cys click reaction; and an alkyne group (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) on a propargylglycine (Pra) residue acting as an internal reference standard. Upon cellular uptake, the disulfide bond in Yne-CBT was reduced by intracellular GSH, followed by CTSB-mediated cleavage of the Val-Cit linker, exposing a D-cysteine residue that triggers an intramolecular CBT-Cys click reaction. This process resulted in the formation of a cyclic dimer, Yne-CBT-Dimer. During this transformation, the nitrile group (C
C) on a propargylglycine (Pra) residue acting as an internal reference standard. Upon cellular uptake, the disulfide bond in Yne-CBT was reduced by intracellular GSH, followed by CTSB-mediated cleavage of the Val-Cit linker, exposing a D-cysteine residue that triggers an intramolecular CBT-Cys click reaction. This process resulted in the formation of a cyclic dimer, Yne-CBT-Dimer. During this transformation, the nitrile group (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) is consumed, leading to a decrease in its Raman signal, whereas the alkyne group (C
N) is consumed, leading to a decrease in its Raman signal, whereas the alkyne group (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) retained a stable Raman signal at ∼2120 cm−1, serving as an internal reference (Fig. 27(i) and (j)). Both the C
C) retained a stable Raman signal at ∼2120 cm−1, serving as an internal reference (Fig. 27(i) and (j)). Both the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and C
N and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C Raman signals were significantly enhanced using a core–shell nanostructure of gold-coated silica nanoparticles (Au@SiO2 NPs). The relative Raman intensity ratio between C
C Raman signals were significantly enhanced using a core–shell nanostructure of gold-coated silica nanoparticles (Au@SiO2 NPs). The relative Raman intensity ratio between C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and C
N and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C showed a linear correlation with CTSB activity, with a limit of detection (LOD) as low as 61.4 U L−1. Through the application of an EISA strategy, the probe exhibited prolonged intracellular retention. By integrating self-calibration with shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS), this approach enabled precise quantitative imaging of enzymatic activity in living cells. This strategy represented the first successful mapping of intracellular enzyme activity at the single-cell level, revealing pronounced heterogeneity in CTSB activity among individual live cells.
C showed a linear correlation with CTSB activity, with a limit of detection (LOD) as low as 61.4 U L−1. Through the application of an EISA strategy, the probe exhibited prolonged intracellular retention. By integrating self-calibration with shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS), this approach enabled precise quantitative imaging of enzymatic activity in living cells. This strategy represented the first successful mapping of intracellular enzyme activity at the single-cell level, revealing pronounced heterogeneity in CTSB activity among individual live cells.
In addition to the widely explored bioorthogonal cyclization-assisted EISA strategies, a non-bioorthogonal EISA approach has also been developed. Yang et al.175 designed a novel class of small-molecule probes, NBD-FFFGKsuccG, along with a control compound, Fmoc-FFFGKsuccG (Fig. 28(a)). By introducing a succinyl modification on the lysine residue, the researchers significantly improved the aqueous solubility of the compounds. Upon specific desuccinylation catalyzed by the enzyme SIRT5, probe 2 underwent a hydrophobicity switch, triggering EISA both in vitro and in live cells to form nanofibrous structures. This in situ self-assembly not only induced aggregation-induced emission enhancement (AIEE) of the NBD fluorophore, but also enabled targeted retention and signal amplification at tumor sites. Consequently, this work established, for the first time, a fluorescence-based strategy for real-time imaging of SIRT5 enzymatic activity within living cells (Fig. 28(b)). Li et al.176 developed a novel tumor imaging probe, M1, based on a biorthogonal EISA strategy (Fig. 28(c)). This probe achieved highly sensitive and specific imaging of pancreatic tumors through its sophisticated multifunctional design. M1 consisted of five key functional components: a long-circulating mPEG2000 segment, a Gly–Pro–Ala enzyme-responsive regulatory unit, a self-assembling peptide sequence (Lys–Leu–Val–Phe–Phe–Gly–Cys–Gly), an Arg–Gly–Asp targeting motif, and the near-infrared fluorophore IR783. This nanoprobe exhibited unique nucleation-growth kinetics, and its dynamic assembly process could be characterized by 8-anilino-1-naphthalenesulfonic acid (ANS) staining curves. As a hydrophobic microenvironment-sensitive probe, the fluorescence changes of ANS revealed the assembly mechanism of M1: during the initial oligomerization stage, enhanced hydrophobicity leads to increased ANS fluorescence intensity, whereas further molecular elongation and stacking into higher-order nanofibers resulted in a blue shift and quenching of ANS fluorescence due to the formation of β-sheet structures. FITC-labeled comparative experiments demonstrated that M1 exhibited significant retention advantages on the cell membrane, whereas the control probe M2 showed weaker retention (Fig. 28(d)). This difference could stem from the secondary assembly process altering the dynamic equilibrium between targeting ligands and receptors, driving the system toward a more stable assembled state. Notably, the rapid in situ dynamic assembly of the probe around the cell membrane effectively promoted the formation and long-term retention of nanofiber structures. In summary, this design strategy endowed the M1 probe with both highly efficient active targeting capability and excellent in vivo circulation stability. Ye et al.177 developed an innovative orthogonal dual pretargeting strategy by ingeniously integrating EISA, inverse electron-demand Diels–Alder (IEDDA) click chemistry, and the biotin–streptavidin (SA) affinity system, enabling high-resolution NIR FL and magnetic resonance (MR) dual-modal imaging of tumors (Fig. 28(e)). The small-molecule probe P-Cy-TCO&Bio was designed with dual tumor-targeting functionality through biotin and TCO groups. Leveraging the biotin-mediated targeting pathway, the probe preferentially accumulated in subcutaneous tumor tissues and underwent in situ self-assembly, triggered by alkaline phosphatase (ALP) overexpressed on tumor cell membranes, to form biotinylated TCO-modified nanoparticles (Cy-TCO&Bio NPs). The resulting nanoparticles featured a dense biotin surface display, enabling multivalent interactions with biotin receptors on tumor cells and substantially enhancing intratumoral retention. Notably, the surface biotin groups facilitated highly efficient capture of streptavidin (SA), thereby achieving precise pretargeted localization of imaging agents. This strategy significantly enhanced the longitudinal relaxivity (r1) and promoted the synergistic enrichment of Tz-Gd and SA-780 at the tumor site, collectively yielding intensified NIR fluorescence signals (Fig. 28(f)), superior MR contrast, and a prolonged imaging time window. Consequently, this platform enabled high-sensitivity and high-specificity dual-modal molecular imaging in HeLa tumor models.
 | ||
| Fig. 28 (a) Chemical structures of peptide precursors (1 and 2) and their SIRT5-responsive self-assembling products (3 and 4). (b) Intracellular nanofiber formation triggered by SIRT5 in HeLa cells. Adapted from ref. 175, Copyright 2022, American Chemical Society. (c) Design of the BIVA (bioactivated in vivo assembly) system. (d) Activation mechanism involving targeted delivery and subsequent aggregation-induced retention (AIR). From ref. 176, Copyright 2022, Nature Publishing Group. (e) Molecular structure of P-Cy-TCO&Bio and overview of a dual-pretargeting approach integrating ALP-induced assembly, IEDDA ligation, and biotin–streptavidin interaction. (f) Images and dual-channel fluorescence (mCy and IR780) of treated HeLa and HUVEC cell pellets. Adapted from ref. 177, Copyright 2024, American Chemical Society. | ||
6.2 EISA regulates the therapeutic properties of small-molecule dyes
EISA probes not only facilitated the in situ accumulation and prolonged retention of imaging moieties at tumor sites, thereby enabling the visualization of diverse enzymatic activities in vivo, but also offered a versatile platform for theranostic integration. By incorporating therapeutic modules into EISA-based optical imaging probes, it is possible to achieve image-guided, on-demand therapy, along with real-time monitoring of therapeutic responses at the site of action. To date, a wide array of EISA-enabled theranostic probes have been developed, combining various imaging modalities with distinct treatment strategies,178–181 including chemotherapy, phototherapy such as PDT, SDT, and multimodal combination therapies.To achieve efficient delivery and prolonged retention of doxorubicin (DOX) at tumor sites, Zhao et al.178 developed a tumor-selective cascade-activated self-retention system (TCASS) featuring caspase-3-responsive EISA probes for dual-modal imaging and chemotherapy (Fig. 29(a)). The modular probe design comprised: (1) an XIAP-targeting peptide (AVPIAQK), (2) a caspase-3/7-cleavable linker (DEVD), (3) a self-assembling β-sheet-forming peptide (KLVFFAECG), and (4) a functional payload (Cy for imaging or DOX for therapy). In vitro studies using XIAP-high H460 lung cancer cells and XIAP-low 293T kidney cells confirmed probe specificity. Western blot verified elevated XIAP expression in H460 cells (Fig. 29(b) and (c)). Molecule 1 exhibited sustained (>48 h) fluorescence in H460 cells but minimal signal in 293T cells, with a 5-fold intensity difference (Fig. 29(d)). Bio-TEM/EDS revealed iodine-tagged nanofiber formation exclusively in Molecule 1-treated H460 cells (Fig. 29(e) and (f)), confirming XIAP-dependent targeting and self-assembly. In vivo, TCASS probes accumulated in tumors via AVPIAQK-mediated targeting. Caspase-3/7 activation triggered DEVD cleavage, inducing β-sheet nanofiber formation that retained therapeutics locally, demonstrating a smart strategy for tumor-selective drug delivery and retention. To enhance the accumulation efficiency of anticancer drugs at tumor sites, Ye et al.179 innovatively proposed a cascade in situ self-assembly/disassembly strategy based on an enzyme/reduction dual-responsive triggering mechanism, achieving precise targeted delivery and controllable release of antitumor drugs (Fig. 29(g)). This study breaks through the conventional design concepts of nanomedicines by ingeniously constructing an alkaline phosphatase (ALP)-responsive small-molecule Pt(II) prodrug (P-CyPt). Upon ALP triggering in the tumor microenvironment, the prodrug underwent in situ self-assembly, significantly enhancing drug accumulation at the tumor site. Subsequently, GSH-mediated nanoparticle dissociation enabled burst release of the drug within tumor cells. Studies demonstrated that PtIVNPs exhibited excellent stability under physiological conditions (CMC ≈ 0.3 µM), whereas in the presence of 5–10 mM GSH, CyPt was completely reduced to Cy-COOH within 60 min. ICP-OES quantitative analysis confirmed a near 100% release rate of CDDP under these conditions, while DLS revealed a gradual reduction in nanoparticle size until complete dissociation. This process was accompanied by distinct spectroscopic changes, including enhanced near-infrared absorption at 700 nm and the disappearance of a shoulder peak at 750 nm.
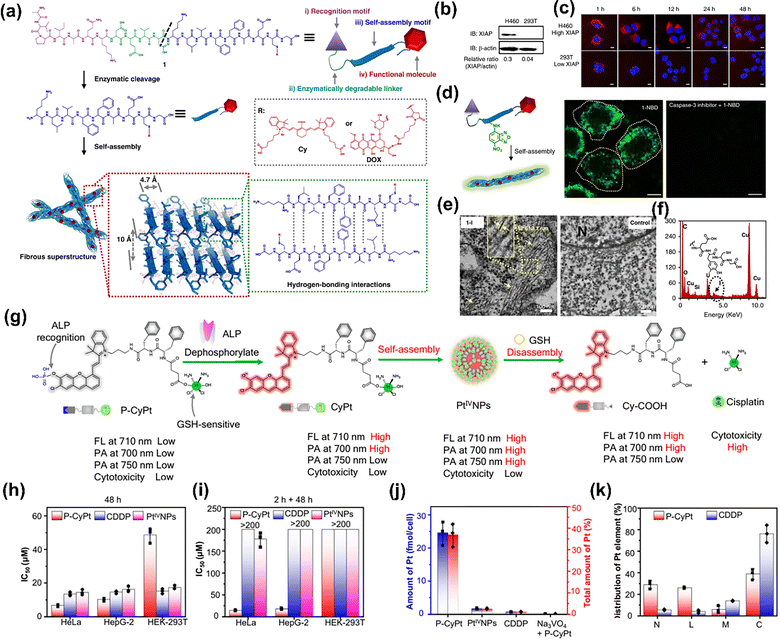 | ||
| Fig. 29 (a) Illustration of a multifunctional peptide construct composed of four modules: AVPIAQK (targeting sequence), DEVD (enzyme-sensitive linker), KLVFFAECG (assembly domain), and either a cyanine dye or doxorubicin as the effector. (b) XIAP protein expression in H460 and 293T cells detected by western blot. (c) Confocal images of H460 cells incubated with compound 1, following optional pretreatment with an energy transport inhibitor. (d) Schematic showing NBD fluorophore activation, alongside imaging of H460 cells after 1 h exposure to 1-NBD. (e) Transmission electron microscopy of cells with or without I-1 application (12 h), revealing fibrillar aggregates near nuclei (marked with arrows). (f) Energy-dispersive X-ray spectroscopy confirming iodine localization in intracellular nanostructures. Scale: 10 µm. Adapted from ref. 178, Copyright 2019, Nature Publishing Group. (g) Structural depiction of P-CyPt and its sequential activation process: ALP-induced dephosphorylation drives PtIVNP formation (activating NIR/PA signals), followed by GSH-triggered disintegration and payload release (signal off). (h) and (i) Half-maximal inhibitory concentrations of P-CyPt, PtIVNPs, and cisplatin in HeLa, HepG2, and HEK-293T cells under two conditions: (h) direct 48 h drug exposure and (i) 2 h pulse treatment plus 48 h recovery. (j) Intracellular Pt levels and retention efficiency measured via ICP-OES. (k) Distribution of Pt across subcellular compartments post-treatment with P-CyPt or cisplatin. From ref. 179, Copyright 2023, Nature Publishing Group. | ||
Concurrently, fluorescence intensity at 710 nm increased by 4.5-fold, and the photoacoustic signal at 750 nm attenuated by 6-fold, providing multiple monitoring indicators for drug release. In vitro antitumor experiments showed that P-CyPt exhibited significantly lower IC50 values against HeLa and HepG2 cells (14.56 ± 1.24 µM and 18.12 ± 1.78 µM, respectively) compared to normal HEK-293T cells, while demonstrating superior tumor-killing efficacy over conventional CDDP and pre-synthesized PtIVNPs (IC50 > 150 µM) (Fig. 29(h) and (i)). Cellular uptake assays confirmed that HeLa cells treated with P-CyPt exhibited significantly higher Pt(II) accumulation, with preferential distribution in target sites such as the nucleus and mitochondria, thereby enhancing antitumor effects (Fig. 29(j) and (k)). This system integrated activatable NIR fluorescence and dual-channel photoacoustic imaging enabled not only high-sensitivity and high-resolution tumor imaging but also real-time visualization of the entire drug delivery and release process in vivo. It provided a novel theranostic platform for precision medicine, offering promising potential for clinical translation.
To enhance the singlet oxygen (1O2) yield of photosensitizers, Liang et al.180 rationally designed a peptide–porphyrin conjugate, Ac–Asp–Glu–Val–Asp–Asp-4-(10,15,20-triphenyl-21H,23H-porphyrin-5-yl) aniline (Ac-DEVDD-TPP) (Fig. 30(a)). This bifunctional molecule integrated two synergistic modules: (1) the hydrophilic Ac-DEVDD peptide segment, which served dual purposes by incorporating a caspase-3-specific recognition sequence (DEVD) while improving the aqueous solubility of the hydrophobic porphyrin core; and (2) the 10,15,20-triphenylporphyrin (TPP) module, which functioned as the PDT active center with an extended π-conjugated system that facilitated subsequent nanofiber self-assembly. The designed system operated through an enzyme-responsive activation mechanism. Upon caspase-3-mediated cleavage, the hydrophilic Ac-DEVDD-TPP precursor was converted into the hydrophobic product D-TPP. This transformation triggered the spontaneous self-assembly of D-TPP into highly ordered porphyrin nanofibers in the perimitochondrial region. Remarkably, under 660 nm laser irradiation, these nanofibers demonstrated significantly enhanced 1O2 generation compared to their precursor forms. Specifically, the SOSG fluorescence intensity of D-TPP nanofibers was 1.4-fold higher than that of hydrophilic porphyrin derivatives (TCPP, Ac-DEVDD-TPP, and Ac-DEDVD-TPP) (Fig. 30(b)). This improvement was attributed to the fibrous nanostructure, which promoted intermolecular interactions and enhanced the intersystem crossing process, thereby boosting the photodynamic efficiency. Notably, the system exhibited a self-reinforcing positive feedback loop: newly activated caspase-3 further cleaved additional Ac-DEVDD-TPP precursors, leading to continuous nanofiber formation and sustained 1O2 production. This “cleavage-self-assembly-ROS generation” cascade not only amplified apoptotic signals but also established a long-lasting therapeutic effect (Fig. 30(c) and (d)). The combination of enzymatic activation and nanostructural reorganization provided a promising strategy to overcome the limitations of conventional photosensitizers, offering enhanced specificity and efficacy in PDT.
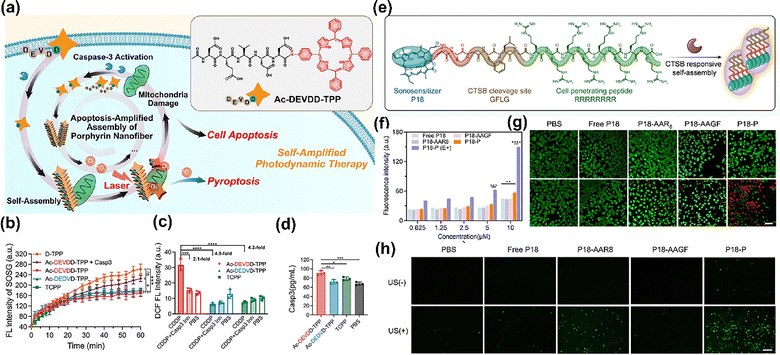 | ||
| Fig. 30 (a) Schematic of the Ac-DEVDD-TPP-mediated self-amplified photodynamic therapy, enhancing apoptosis and pyroptosis via a porphyrin nanofiber assembly. (b) FL intensities of 25 nM SOSG treated with 10 µM D-TPP, TCPP, Ac-DEDVD-TPP, or Ac-DEVDD-TPP (with/without Casp3 pre-incubation) under 660 nm irradiation (4 mW cm−2, 60 min). (c) Quantitative analysis of DCF fluorescence intensity from (b). (d) Caspase-3 levels in CDDP-pretreated SCC7 cells exposed to test compounds (10 µM) or PBS, followed by laser irradiation. Reproduced with permission from ref. 180. Copyright 2023, American Chemical Society. (e) Design of a sonotheranostic p18-p conjugate enabling CTSB-responsive self-assembly for activity monitoring and enhanced SDT. (f) Viability of HepG2 cells treated with peptide–purpurin conjugates ± ultrasound. (g) Live/dead staining of HepG2 cells incubated with conjugates ± ultrasound. Scale: 150 µm. (h) DCF fluorescence in HepG2 cells exposed to conjugates ± ultrasound. Scale: 40 µm. Reproduced with permission from ref. 181. Copyright 2024, American Chemical Society. | ||
Compared with PDT, SDT exhibited superior tissue penetration depth, enabling more effective treatment of deep-seated lesions. The strategic combination of SDT and EISA can further enhance tumor-targeted accumulation and retention of sonosensitizers, thereby significantly improving therapeutic efficacy. For example, Liu et al.181 designed and synthesized a cathepsin B (CTSB) triggered self-assembled probe (P18-P) by introducing porphyrin into a peptide scaffold (Fig. 30(e)). This probe mainly consisted of four parts: (1) a cell-penetrating peptide (RRRRRRRR) using enhanced cell uptake of P18-P; (2) CTSB-cleavable enzyme-responsive linker peptide (GFLG); (3) a self-assembly regulatory motif (AA) that modulated the hydrophobic–hydrophilic balance to form discrete nanostructures; and (4) the porphyrin-based sonosensitizer purpurin 18 (P18). Under physiological conditions, P18-P exhibited a hydrophilic disordered structure, enabling free diffusion and penetration through tumor cell membranes. Upon cleavage by CTSB, P18-P underwent self-assembly to form J-aggregated nanofibers (P18-Ps), significantly enhancing CTSB-specific photoacoustic imaging performance. Meanwhile, the P18-P group incubated with CTSB and activated by ultrasound displayed the highest DCF fluorescence signal compared to the control group, indicating that CTSB-triggered peptide self-assembly effectively enhances ROS generation. Cell experiments demonstrated that the ultrasound-activated P18-P group generated substantial ROS, leading to extensive cancer cell death (Fig. 30(f)–(h)). In short, the overexpression of CTSB triggered the in situ self-assembly of the P18-P probe, significantly improving the accumulation and retention of the sonosensitizer at the tumor site, thereby enabling tumor photoacoustic imaging and enhancing the efficacy of SDT.
6.3 EISA system based small-molecule dyes for biological applications
Recent advancements in EISA strategies have provided novel avenues for precise cancer therapy by enabling stimuli-responsive intracellular nanostructure formation and prolonged drug retention. These approaches leverage tumor-specific enzymatic activities and the unique intracellular microenvironment to initiate spatially confined assembly processes, effectively amplifying therapeutic efficacy while minimizing systemic toxicity. Bulte et al.182 developed a novel antitumor agent, Olsa-RVRR, by conjugating the anticancer drug olsalazine (Olsa) to the cell-penetrating peptide RVRR. Upon cellular uptake, Olsa-RVRR underwent furin-mediated cleavage near the Golgi apparatus in furin-expressing cancer cells (e.g., HCT116) (Fig. 31(a)). Subsequently, intracellular GSH triggered the release of D-cysteine, enabling a bioorthogonal CBT-Cys click reaction to form a cyclic Olsa-containing structure. This process was followed by π–π stacking-driven self-assembly into olsalazine nanoparticles (OlsaNPs). Super-resolution fluorescence imaging via three-dimensional structured illumination microscopy (3D-SIM) revealed significantly stronger green fluorescence signals in HCT116 cells compared to LoVo cells, confirming furin-dependent nanoparticle aggregation. This furin-triggered in situ EISA mechanism substantially prolonged intracellular drug retention, resulting in a 20-fold and 5.2-fold increase in cytotoxicity against HCT116 cells in vitro and in vivo, respectively. Notably, in an HCT116 xenograft model, Olsa-RVRR exhibited a 6.5-fold enhancement in antitumor efficacy compared to unconjugated Olsa, demonstrating its therapeutic potential for furin-overexpressing cancers (Fig. 31(b) and (c)).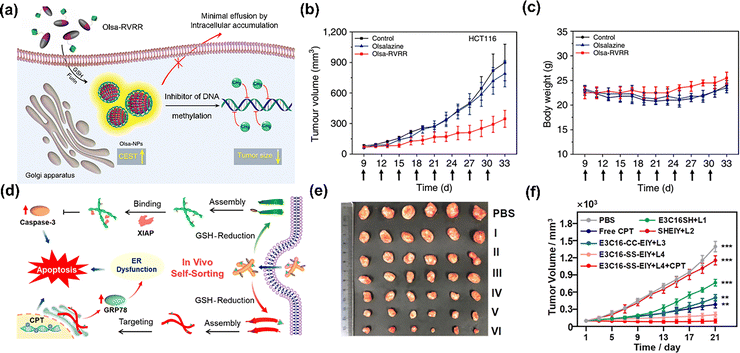 | ||
| Fig. 31 (a) Once internalized by cells with high furin expression (e.g., HCT116), Olsa-RVRR undergoes GSH-mediated reduction followed by furin-catalyzed cleavage near the Golgi apparatus, generating its active fragment. (b) Comparative evaluation of the anti-tumor efficacy of Olsa and Olsa-RVRR in HCT116 tumor models. (c) Temporal changes in mouse body weight throughout the study period. Reproduced with permission from ref. 182. Copyright 2019, Nature Publishing Group. (d) Diagrammatic representation of in vivo peptide self-sorting via in situ assembly evolution for synergistic cancer treatment. (e) Representative tumor tissue images extracted from mice treated intravenously with PBS (control), SHEIY + L2 (i), E3C16SH + L1 (ii), E3C16-CC-EIY + L3 (iii), CPT (iv), E3C16-SS-EIY + L4 (v), and E3C16-SS-EIY + L4 + CPT (vi) at day 21. (f) Tumor growth progression curves in mice following intravenous administration of the tested compounds. Reproduced with permission from ref. 183. Copyright 2024, American Chemical Society. | ||
Building upon the concept of EISA, Yu et al.183 reported an in situ EISA-mediated self-sorting peptide probe (E3C16-SS-EIY) for combined cancer therapy (Fig. 31(d)). This peptide consisted of two peptide chains, E3C16SH and SHEIY, connected via a disulfide bond. Initially, it self-assembled into nanorods, which subsequently dissociated into E3C16SH and SHEIY upon GSH stimulation, triggering a self-sorting process that leads to the formation of nanobelts. To achieve therapeutic effects, a ligand L4 was designed by incorporating AVPIAQK (a ligand targeting the endoplasmic reticulum (ER) anti-apoptotic protein XIAP) and p-toluenesulfonamide (Ts, an ER-targeting moiety) into the E3C16-SS-EIY framework. The self-sorted nanobelts formed by E3C16-SS-EIY + L4 (an assembly of L4 and E3C16-SS-EIY) effectively inhibited XIAP and induced aggregation around the ER, thereby upregulating the expression of caspase-3 and glucose-regulated protein 78 (GRP78). This process induced ER dysfunction and promoted cell death. In vivo studies demonstrated that the self-sorted assembly significantly suppressed tumor growth in tumor-bearing mice while exhibiting excellent biosafety (Fig. 31(e) and (f)).
To further enhance therapeutic outcomes by integrating multiple functional modalities, Liang et al.184 reported a trident molecule, Nap-CPT-HCQ-Yp, combining phosphotyrosine, camptothecin (CPT), and hydroxychloroquine (HCQ) (Fig. 32(a)). This system underwent EISA to enhance chemo-immunotherapy efficacy. Sequentially processed by ALP and CES enzymes, Nap-CPT-HCQ-Yp underwent morphological evolution from nanobrush structures through nanoparticles to nanofibers (Nap-Y), facilitating the timed release of CPT and HCQ. Scratch wound healing and transwell assays showed that Nap-CPT-HCQ-Yp markedly restricted tumor cell migration and invasion, resulting from the on-site assembly of nanofibers within metastatic cells. Moreover, the HCQ moiety inhibited autophagy, synergistically enhancing the chemotherapeutic efficacy of CPT. Remarkably, treatment of 4T1 cells with Nap-CPT-HCQ-Yp induced immunogenic cell death (ICD), as evidenced by upregulated calreticulin (CRT) exposure, HMGB1 release, and ATP secretion. When combined with checkpoint blockade therapy, Nap-CPT-HCQ-Yp elicited robust systemic antitumor immunity, leading to significant suppression of both primary and distant tumors in a murine breast cancer model (Fig. 32(b)–(d)).
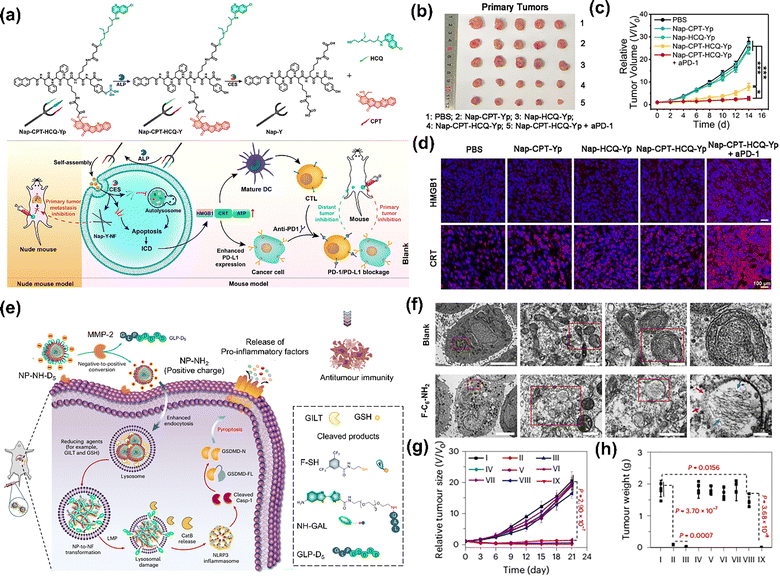 | ||
| Fig. 32 (a) Schematic of the trident-shaped molecule Nap-CPT-HCQ-Yp, which exhibits dynamic structural transitions to spatially inhibit tumor metastasis. (b) Primary tumor images from mice after intratumoral (i.t.) injection with PBS (control), Nap-CPT-Yp, Nap-HCQ-Yp, Nap-CPT-HCQ-Yp, and Nap-CPT-HCQ-Yp + three intraperitoneal (i.p.) doses of aPD-1. Mice were euthanized after 14 days. (c) Tumor volume growth kinetics for the treatment groups in (b). (d) Immunofluorescence analysis of HMGB1 and CRT expression in tumor tissues. Scale bar: 100 µm. Reproduced with permission from ref. 184. Copyright 2022, American Chemical Society. (e) Mechanism of NP-NH-D5 facilitating targeted delivery of F-C6–NH2 to tumor cells for immunotherapy. (f) Bio-TEM images of HeLa cells untreated or treated with F-C6–NH2. (g) Relative tumor volume changes (V/V0) in mice across different treatment regimens. (h) Mean tumor weights at day 21 post-treatment. Reproduced with permission from ref. 185. Copyright 2025, Nature Publishing Group. | ||
Pushing the frontier of EISA toward pyroptosis-mediated immunotherapy, Ye et al.185 reported a small-molecule non-peptide amphiphilic compound (F-C6–NH2) capable of self-assembling into nanofibers within lysosomes (Fig. 32(e)). The high rigidity of the nanofibers and the primary amine groups induced lysosomal membrane permeabilization (LMP) and lysosomal damage through strong mechanical forces and the “proton sponge effect”, thereby triggering gasdermin D (GSDMD)-mediated pyroptosis in tumor cells and subsequently activating ICD (Fig. 32(f)). To enhance tumor selectivity, a derivative containing five negatively charged glutamate residues (D5), F-SS-NH-GALGLP-D5, was further synthesized. Unlike F-C6–NH2, F-SS-NH-GALGLP-D5 could be cleaved by extracellular matrix metalloproteinase-2 (MMP-2) and reduced by GSH. Through co-assembly of F-C6–NH2 and F-SS-NH-GALGLP-D5, negatively charged surface nanoparticles (NP-NH-D5) were fabricated, which underwent a cascade response to MMP-2 and GSH, enabling a “nanoparticle-to-nanofiber” transformation. In tumor tissues, NP-NH-D5 was proteolytically converted by MMP-2 into positively charged NP-NH2, facilitating cellular internalization and lysosomal accumulation. The abundant reductants in lysosomes further cleaved the disulfide bond in NP-NH2, releasing fragmented F-SH and NH-GAL. The remaining F-C6–NH2 could be reassembled into rigid nanofibers within lysosomes, causing lysosomal damage and inducing pyroptosis. This process promoted the secretion of pro-inflammatory cytokines, elicited a robust ICD response, and reversed the immunosuppressive tumor microenvironment, thereby enhancing antitumor immunotherapy, in a 4T1 orthotopic breast cancer model. Hence, NP-NH-D5 not only effectively eradicated primary tumors and suppressed metastasis and recurrence but also significantly prolonged survival without apparent systemic toxicity (Fig. 32(g) and (h)). More importantly, in advanced 4T1 lung metastasis and aggressive orthotopic Pan02 pancreatic cancer models, NP-NH-D5 markedly potentiated the therapeutic efficacy of the PD-L1 checkpoint blockade.
7. Conclusion and perspective
The early diagnosis and treatment of diseases are paramount for achieving precision medicine objectives. Supramolecular dyes have emerged as superior alternatives to traditional small-molecule dyes, offering tunable photophysical properties, enhanced photo-diagnostic and phototherapy efficiency, improved photostability/biocompatibility, optimized anti-quenching capability, and superior targeting abilities. These attributes have enabled their widespread application in bioimaging, molecular recognition and phototherapy. Notably, supramolecular dyes could be facilely constructed via host–guest interaction in solutions, circumventing the need for tedious synthesis and isolation procedures. Thus, this review systemically summarizes recent progress in the development of supramolecular dyes including SCC systems, cyclodextrin systems, CB [n] systems, calixarene and pillararene systems, and EISA systems. The advantages and limitations of these supramolecular platforms are briefly summarized in Table 1.| Supramolecular platforms | Advantages | Limitations | Applications |
|---|---|---|---|
| SCCs | 1. Adjustable photophysical properties | 1. Poor water solubility | 1. Phototherapy |
| 2. Synergistic therapeutic effect | 2. Multistep synthesis | 2. Chemotherapy | |
| 3. Tunable cavity size | 3. Drug delivery | ||
| 4. Suppressed aggregation tendency | 4. Optical imaging | ||
| CDs | 1. Improving water solubility | 1. ACQ effect | 1. Molecular recognition |
| 2. Enhanced biocompatibility | 2. Limited chemical tunability | 2. Drug delivery | |
| 3. Enhanced dyes’ performance in aqueous media | 3. Optical imaging | ||
| 4. Phototherapy | |||
| CB [n]s | 1. Regulation of dyes’ optical properties | 1. Size and shape restrictions | 1. Molecular recognition |
| 2. Enhanced PDT/PTT effect | 2. Competitive binding in biological media | 2. Phototherapy | |
| 3. Host–guest interaction for biosensing | 3. Poor water solubility of host molecules | 3. Optical imaging | |
| Calixarenes/pillararenes | 1. Regulation of dyes’ optical properties | 1. Synthetic complexity | 1. Molecular recognition |
| 2. Host–guest interaction for biosensing | 2. Host–guest binding limitations | 2. Phototherapy | |
| 3. Regulation of PDT effect | 3. pH sensitivity | 3. Optical imaging | |
| 4. Drug delivery | |||
| EISA | 1. Enzyme-responsive | 1. Synthetic complexity | 1. Molecular recognition |
| 2. In situ self-assembly | 2. Difficulty in scale-up | 2. Phototherapy | |
| 3. Minimal side effects | 3. Optical imaging | ||
| 4. Signal amplification | 4. Drug delivery |
To help undergraduate students get a better understanding of this article, the key technologies, basic principles and bio-applications are shown in Table 2.
| Techniques | Basic principles | Applications in biomedicine |
|---|---|---|
| Fluorescence imaging | Molecules absorb light and emit fluorescence when returning from the excited state to the ground state. | Tumor imaging, cellular imaging, tissue imaging |
| Photoacoustic imaging | Short laser pulses induce thermoelastic expansion in the sample, generating ultrasonic waves. | Tumor imaging, cellular imaging, tissue imaging |
| PDT | Photosensitizers are excited by specific light wavelengths to produce ROS that induce cell death. | Cancer treatment, antibacterial therapy |
| PTT | Photosensitizers absorb near-infrared (NIR) light and convert photon energy into localized heat, causing thermal ablation of diseased tissue. | Cancer treatment, antibacterial therapy |
| SDT | Ultrasound activates sonosensitizers to generate ROS, enabling treatment in deep tissues | Cancer treatment, antibacterial therapy |
| Self-assembly | Molecules spontaneously organize into ordered nanostructures via non-covalent interactions such as hydrogen bonding, π–π stacking, and hydrophobic forces | Construction of supramolecular dyes or nanotherapeutic agents/drugs |
During the past decades, the discipline of supramolecular chemistry has developed rapidly. As one of its new branches, earlier work based on supramolecular dyes mainly focused on improving the optical properties, including fluorescence quantum yields, fluorescence lifetime, and photostability. Moreover, most of the recent studies have achieved further applications in bioimaging and phototherapy in vivo. Future efforts should prioritize the following directions to further advance the field:
(1) Multimodal imaging integration. Supramolecular dyes could be engineered for multifunctional imaging by combining fluorescence with complementary imaging modalities (e.g., MRI, PA, infrared (IR), positron emission tomography (PET), or computed tomography (CT)).186 Such hybrid systems could overcome the shortcomings of single optical imaging and improve diagnostic accuracy while reducing false signals. Besides, co-assembling dyes with multiple emission channels could enable multichannel and multicolor imaging, facilitating target tracking in complex biological environments with minimized background interference.
(2) Stimuli-responsive systems for targeted activation. Lesion sites (e.g., tumor and inflamed tissues) often exhibit regulated biomarkers (pH, bio-thiols, ROS, and enzymes). Stimuli-responsive supramolecular dyes can selectively activate optical signals at target sites, enhancing signal-to-noise ratios and targeting precision.187 However, a critical challenge lies in maintaining post-activation stability, as most of the supramolecular dyes rely on non-covalent interactions (hydrogen bonding, π–π stacking, and coordination hydrophobic or electrostatic interactions) that may be disrupted by stimuli (e.g., GSH and ROS). Strategies to improve stability include optimizing host–guest affinity, encapsulating dyes in protective matrices, and incorporating supramolecular dyes into crosslinked networks.
(3) Size-tunable nanoparticles for enhanced biocompatibility and pharmacokinetics. The nano-size should be regulated to improve the biocompatibility and metabolism in vivo. Nanoprobes with optimized sizes are to be efficiently cleared through renal pathways, minimizing long-term toxicity and ensuring biosafety. Moreover, nanoscale adjustment can modulate cellular uptake, toxicity and intracellular distribution, further affecting diagnostic or therapeutic performance. Supramolecular engineering bridges the gap between small-molecule dyes and nanostructures, enabling dual-directional control over biodistribution and activation. For instance, tuning host–guest interactions within a supramolecular system can dynamically adjust assembly size, surface charge, and lesion-specific responsiveness, ensuring stability during therapy but controlled disassembly post-treatment. This strategy not only enhances the therapeutic efficiency but also minimizes off-target accumulation and toxicity.
(4) AI-driven design and screening. Artificial Intelligence (AI)-assisted design and screening of supramolecular dyes can accelerate their development and applications in clinical translation.188,189 By comprehensive consideration and precise prediction of key parameters such as photophysical properties, host–guest affinities, and biocompatibility, setting up the database of supramolecular dyes, the AI-assisted design will improve translational potential and pave the way for personalized diagnostics and targeted therapies in precision medicine.
(5) Clinical translation: challenges and opportunities. Facilitating the supramolecular dyes to clinical translation should comprehensively consider the following key points including biocompatibility, targeting specificity, stability in vivo, reproducible large-scale synthesis, metabolism, and compliance with regulatory standards to ensure safety and effectiveness in biomedical use.190 For instance, small molecule dyes such as ICG and MB are clinically used, but also suffer from significant limitations, including poor photostability, rapid clearance, non-specific biodistribution, and ACQ effect. Assembling of ICG within β-cyclodextrin cavities could not only improve its aqueous solubility, but also reduce the ACQ effect and cytotoxicity. In addition, there are many endogenous biomolecules such as GSH, ROS and enzymes in vivo, which may displace guest dyes or destabilize coordination cages. Scaling up self-assembly is a great challenge because small changes in concentration or environment may disrupt the thermodynamic equilibria and kinetic pathways, leading to poor reproducibility and structural heterogeneity. Potential immunogenicity is another major concern before clinical translation. The supramolecular dyes could be recognized by the immune system and trigger innate immune responses, creating risks of inflammation, hypersensitivity, etc. It is essential to reduce the above risks through systematic immunotoxicological evaluation and regulatory considerations.
Abbreviations
| ICG | Indocyanine green |
| MB | Methylene blue |
| SCCs | Supramolecular coordination complexes |
| CDs | Cyclodextrins |
| CB [n] | Cucurbit[n]urils |
| EISA | Enzyme instructed self-assembly |
| AIE | Aggregation-induced emission |
| ISC | Intersystem crossing |
| SBR | Signal-to-background ratio |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
| SDT | Sonodynamic therapy |
| US | Ultrasound |
| NIR | Near-infrared region |
| MRI | Magnetic resonance imaging |
| FI | Fluorescence imaging |
| GSH | Glutathione |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| 3D | Three-dimensional |
| STED | Stimulated emission depletion |
| CDV | Host–guest complex |
| MPS | Mononuclear phagocytic system |
| RTP | Room temperature phosphorescence |
| DAEs | Diarylethenes |
| TPA | Triphenylamine |
| HA | Hyaluronic acid |
| PRET | Phosphorescence resonance energy transfer |
| FRET | Förster resonance energy transfer |
| NDI | Naphthalene diimides |
| PDI | Perylene diimides |
| ACQ | Aggregation-caused quenching |
| PET | Positron emission tomography |
| LOD | Limit of detection |
| IEDDA | Inverse electron-demand Diels–Alder |
| ALP | Alkaline phosphatase |
| TCO | trans-cyclooctene |
| DOX | Doxorubicin |
| ER | Endoplasmic reticulum |
| AI | Artificial Intelligence |
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Acknowledgements
We thank the National Natural Science Foundation of China (22374055, 22022404, 22204055, U21A20290 and 22276041), the National Natural Science Foundation of Hubei Province (2022CFA033), the Fundamental Research Funds for the Central Universities (program no. 2662025SYPY005), and the Guangdong Basic and Applied Basic Research Foundation (2023A1515030008).References
- M. Brülhart, V. Klotzbücher, R. Lalive and S. K. Reich, Mental health concerns during the COVID-19 pandemic as revealed by helpline calls, Nature, 2021, 600, 121–126 CrossRef PubMed.
- L. O. Gostin and S. Wetter, Fix the backlash against public health, Science, 2023, 379, 1277 CrossRef.
- M. Sharma, M. S. Akhter, S. Roy and R. Srejon, Future issues in global health: challenges and conundrums, Int. J. Environ. Res. Public Health, 2025, 22, 325 CrossRef PubMed.
- F. Shahbazi, S. Moslehi, Z. Mirzaei and Y. Mohammadi, The effect of addressing the top 10 global causes of death on life expectancy in 2019: a global and regional analysis, Int. Health, 2025, 1–10 Search PubMed.
- S. Machado, I. Kyriopoulos, E. J. Orav and I. Papanicolas, Association between wealth and mortality in the United States and Europe, N. Engl. J. Med., 2025, 392, 1310–1319 CrossRef.
- M. Zhu, W. Jin, W. He and L. Zhang, The incidence, mortality and disease burden of cardiovascular diseases in China: a comparative study with the United States and Japan based on the GBD 2019 time trend analysis, Front. Cardiovasc. Med., 2024, 11, 1–12 Search PubMed.
- B. A. Aguado, J. C. Grim, A. M. Rosales, J. J. Watson-Capps and K. S. Anseth, Engineering precision biomaterials for personalized medicine, Sci. Transl. Med., 2018, 10, eaam8645 CrossRef PubMed.
- H.-Y. Li and R.-X. Zhang, Analysis of the structure and trend prediction of China's total health expenditure, Front. Public Health, 2024, 12, 1425716 CrossRef.
- W. Fu, S. Zhao, Y. Zhang, P. Chai and J. Goss, Research in health policy making in China: out-of-pocket payments in healthy China 2030, Brit. Med. J., 2018, 360, k234 CrossRef.
- K. Yan, Z. Hu, P. Yu, Z. He, Y. Chen, J. Chen, H. Sun, S. Wang and F. Zhang., Ultra-photostable small-molecule dyes facilitate near-infrared biophotonics, Nat. Commun., 2024, 15, 2593 CrossRef CAS.
- J. Zhang, W. Wang, J. Shao, J. Chen and X. Dong., Small molecular cyanine dyes for phototheranostics, Coord. Chem. Rev., 2024, 516, 215986 CrossRef CAS.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS.
- J. Yin, L. Huang, L. Wu, J. Li, T. D. James and W. Lin, Small molecule based fluorescent chemosensors for imaging the microenvironment within specific cellular regions, Chem. Soc. Rev., 2021, 50, 12098–12150 RSC.
- L. Zhang, Z. Wang, X. Zhang, Y. Zhou, A. Ji, H. Lou, X. Liu, H. Chen and Z. Cheng, Development of mitochondria-targeted small-molecule dyes for myocardial PET and fluorescence bimodal imaging, J. Med. Chem., 2022, 65, 497–506 CrossRef PubMed.
- W. Zhou, L. Yin, X. Zhang, T. Liang, Z. Guo, Y. Liu, C. Xie and Q. Fan, Recent advances in small molecule dye-based nanotheranostics for NIR-II photoacoustic imaging-guided cancer therapy, Front. Bioeng. Biotechnol., 2022, 10, 1002006 CrossRef.
- Y. Huang, Y. Zhang, F. Hou, Y. Wen and C. Yin, Design strategy and bioimaging of small organic molecule multicolor fluorescent probes, Sci. China. Chem., 2020, 63, 1742–1755 CrossRef CAS.
- Y. Li, H. Zhu, X. Wang, Y. Cui, L. Gu, X. Hou, M. Guan, J. Wu, Y. Xiao, X. Xiong, X. Meng and X. Hong, Small-molecule fluorophores for near-infrared IIb imaging and image-guided therapy of vascular diseases, CCS Chem., 2022, 4, 3735–3750 CrossRef CAS.
- F.-Y. Zhu, L.-J. Mei, R. Tian, C. Li, Y.-L. Wang, S.-L. Xiang, M.-Q. Zhu and B. Z. Tang, Recent advances in super-resolution optical imaging based on aggregation-induced emission, Chem. Soc. Rev., 2024, 53, 3350–3383 RSC.
- J. Yuan, H. Yang, W. Huang, S. Liu, H. Zhang, X. Zhang and X. Peng, Design strategies and applications of cyanine dyes in phototherapy, Chem. Soc. Rev., 2025, 54, 341–366 RSC.
- J. Zhu, L. Zhao, W. An and Q. Miao, Recent advances and design strategies for organic afterglow agents to enhance autofluorescence-free imaging performance, Chem. Soc. Rev., 2025, 54, 1429–1452 RSC.
- S. Zeng, X. Liu, Y. S. Kafuti, H. Kim, J. Wang, X. Peng, H. Li and J. Yoon, Fluorescent dyes based on rhodamine derivatives for bioimaging and therapeutics: recent progress, challenges, and prospects, Chem. Soc. Rev., 2023, 52, 5607–5651 RSC.
- Y. Jiang and K. Pu, Molecular probes for autofluorescence-free optical imaging, Chem. Rev., 2021, 12, 13086–13131 CrossRef.
- J. Zhang, L. Ning, J. Huang, C. Zhang and K. Pu, Activatable molecular agents for cancer theranostics, Chem. Sci., 2020, 11, 618–630 RSC.
- J. Zhan, Y. Cai, P. Cheng, L. Zhang and K. Pu, Body fluid diagnostics using activatable optical probes, Chem. Soc. Rev., 2025, 54, 3906–3929 RSC.
- H. Zheng, X.-Q. Zhan, Q.-N. Bian and X.-J. Zhang, Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors, Chem. Commun., 2013, 49, 429–447 RSC.
- E. Noelting and K. Dziewonsky, Zur Kenntniss der Rhodamine, Ber. Dtsch. Chem. Ges., 1905, 38, 3516 CrossRef CAS.
- Y. Kuriki, T. Yoshioka, M. Kamiya, T. Komatsu, H. Takamaru, K. Fujita, H. Iwaki, A. Nanjo, Y. Akagi, K. Takeshita, H. Hino, R. Hino, R. Kojima, T. Ueno, K. Hanaoka, S. Abe, Y. Saito, J. Nakajima and Y. Urano, Development of a fluorescent probe library enabling efficient screening of tumour-imaging probes based on discovery of biomarker enzymatic activities, Chem. Sci., 2022, 13, 4474–4481 RSC.
- Y.-X. Ye, J.-C. Pan, H.-C. Wang, X.-T. Zhang, H.-L. Zhu and X.-H. Liu, Advances in small-molecules fluorescent probes for the study of apoptosis, Chem. Soc. Rev., 2024, 53, 9133–9189 RSC.
- L. v Manen, H. J. M. Handgraaf, M. Diana, J. Dijkstra, T. Ishizawa, A. L. Vahrmeijer and J. S. D. Mieog., A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery, J. Surg. Oncol., 2018, 118, 283–300 CrossRef.
- K. Jiang, B. Luo, Z. Hou, C. Li, H. Cai, J. Tang and G. Yao, Application of an indocyanine green surgical fluorescence imaging system in sentinel lymph node biopsy of acral malignant melanoma, Ann. Transl. Med., 2021, 9, 1456 CrossRef PubMed.
- Q.-H. Yang and X.-J. Zhang, Indocyanine green combined with methylene blue versus methylene blue alone for sentinel lymph node biopsy in breast cancer: a retrospective study, BMC Surgery, 2023, 23, 133 CrossRef CAS.
- Q. Wu, Q. Lei, H.-C. Zhong, T.-B. Ren, Y. Sun, X.-B. Zhang and L. Yuan, Fluorophore-based host–guest assembly complexes for imaging and therapy, Chem. Commun., 2023, 59, 3024–3029 RSC.
- J. Li, J. Wang, H. Li, N. Song, D. Wang and B. Z. Tang, Supramolecular materials based on AIE luminogens (AIEgens): construction and applications, Chem. Soc. Rev., 2020, 49, 1144–1172 RSC.
- J. Zhou, L. Rao, G. Yu, T. R. Cook, X. Chen and F. Huang, Supramolecular cancer nanotheranostics, Chem. Soc. Rev., 2021, 50, 2839–2891 RSC.
- H. Nie, Z. Wei, X.-L. Ni and Y. Liu, Assembly and applications of macrocyclic-confinement-derived supramolecular organic luminescent emissions from cucurbiturils, Chem. Rev., 2022, 122, 9032–9077 CrossRef CAS.
- Y. Li, F. Huang, P. J. Stang and S. Yin, Supramolecular Coordination Complexes for Synergistic Cancer Therapy, Acc. Chem. Res., 2024, 57, 1174–1187 CrossRef CAS.
- H. B. Jin, L. Yang, M. J. R. Ahonen and M. H. Schoenfisch, Nitric oxide-releasing cyclodextrins, J. Am. Chem. Soc., 2018, 140, 14178–14184 CrossRef CAS.
- D. Wu, J. Wang, X. Du, Y. Cao, K. Ping and D. Liu, Cucurbit [8] uril-based supramolecular theranostics, J. Nanobiotechnol., 2024, 22, 235 CrossRef.
- G. Yu and X. Chen, Host-guest chemistry in supramolecular theranostics, Theranostics, 2019, 9, 3041–3074 CrossRef CAS PubMed.
- M. Yan and J. Zhou, Pillararene-based supramolecular polymers for cancer therapy, Molecules, 2023, 28, 1470 CrossRef CAS PubMed.
- Y. Li, H. Zhou, J. Chen, S. A. Shahzad and C. Yu, Controlled self-assembly of small molecule probes and the related applications in bioanalysis, Biosens. Bioelectron., 2016, 76, 38–53 CrossRef CAS PubMed.
- D. Xu, Y. Li, S. Yin and F. Huang, Strategies to address key challenges of metallacycle/metallacage-based supramolecular coordination complexes in biomedical applications, Chem. Soc. Rev., 2024, 53, 3167–3204 RSC.
- W. Gao, H. Zhang and G. Jin, Supramolecular catalysis based on discrete heterometallic coordination-driven metallacycles and metallacages, Coord. Chem. Rev., 2019, 386, 69–84 CrossRef CAS.
- H. Sepehrpour, W. Fu, Y. Sun and P. Stang, Biomedically relevant self-assembled metallacycles and metallacages, J. Am. Chem. Soc., 2019, 141, 14005–14020 CrossRef CAS PubMed.
- G. Gupta, Y. Sun, A. Das, P. Stang and C. Lee, BODIPY based metal-organic macrocycles and frameworks: Recent therapeutic developments, Coord. Chem. Rev., 2022, 452, 214308 CrossRef CAS PubMed.
- T. Wei, Y. Xu, Y. Fan, J. Li, M. Qiu, X. Xiong, X. Li and Y. Sun, Biomedical applications of Pt(II) metallacycle/metallacage-based agents: From mono-chemotherapy to versatile imaging contrasts and theranostic platforms, Coord. Chem. Rev., 2021, 443, 214017 CrossRef.
- L. Xu, Y. Wang, L. Chen and H. Yang, Construction of multiferrocenyl metallacycles and metallacages via coordination-driven self-assembly: from structure to functions, Chem. Soc. Rev., 2015, 44, 2148–2167 RSC.
- W. Wang, Y. Wang and H. Yang, Supramolecular transformations within discrete coordination-driven supramolecular architectures, Chem. Soc. Rev., 2016, 45, 2656–2693 RSC.
- J. Zhou, Y. Zhang, G. Yu, M. Crawley, C. Fulong, A. Friedman, S. Sengupta, J. Sun, Q. Li, F. Huang and T. Cook, Highly emissive self-assembled BODIPY-platinum supramolecular triangles, J. Am. Chem. Soc., 2018, 140, 7730–7736 CrossRef CAS PubMed.
- L. He, L. Cai, M. Li, G. Zhang, L. Zhou, T. Chen, M. Lin and Q. Sun, Designing a highly stable coordination-driven metallacycle for imaging-guided photodynamic cancer theranostics, Chem. Sci., 2020, 11, 7940–7949 RSC.
- Y. Ding, Z. Tong, L. Jin, B. Ye, J. Zhou, Z. Sun, H. Yang, L. Hong, F. Huang, W. Wang and Z. Mao, An NIR discrete metallacycle constructed from perylene bisimide and tetraphenylethylene fluorophores for imaging-guided cancer radio-chemotherapy, Adv. Mater., 2022, 34, e2106388 CrossRef.
- H. Zhu, Q. Li, B. Shi, F. Ge, Y. Liu, Z. Mao, H. Zhu, S. Wang, G. Yu, F. Huang and P. Stang, Dual-emissive platinum(II) metallacage with a sensitive oxygen response for imaging of hypoxia and imaging-guided chemotherapy, Angew. Chem., Int. Ed., 2020, 59, 20208–20214 CrossRef CAS PubMed.
- Y. Xu, C. Li, S. Lu, Z. Wang, S. Liu, X. Yu, X. Li and Y. Sun, Construction of emissive ruthenium(II) metallacycle over 1000
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm wavelength for in vivo biomedical applications, Nat. Commun., 2022, 13, 2009 CrossRef CAS.
nm wavelength for in vivo biomedical applications, Nat. Commun., 2022, 13, 2009 CrossRef CAS. - Y. Fan, C. Li, S. Bai, X. Ma, J. Yang, X. Guan and Y. Sun, NIR-II emissive Ru(II) metallacycle assisting fluorescence imaging and cancer therapy., Small, 2022, 18, e2201625 CrossRef.
- G. Yu, S. Yu, M. Saha, J. Zhou, T. Cook, B. Yung, J. Chen, Z. Mao, F. Zhang, Z. Zhou, Y. Liu, L. Shao, S. Wang, C. Gao, F. Huang, P. Stang and X. Chen, A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy, Nat. Commun., 2018, 9, 4335 CrossRef.
- T. Cook, V. Vajpayee, M. Lee, P. Stang and K. Chi, Biomedical and biochemical applications of self-assembled metallacycles and metallacages, Acc. Chem. Res., 2013, 46, 2464–2474 CrossRef CAS.
- J. Li and T. Chen, Transition metal complexes as photosensitizers for integrated cancer theranostic applications, Coord. Chem. Rev., 2020, 418, 213355 CrossRef CAS.
- X. Wang and Z. Guo, Targeting and delivery of platinum-based anticancer drugs, Chem. Soc. Rev., 2013, 42, 202–224 RSC.
- Y. Qin, L. Chen, F. Dong, S. Jiang, G. Yin, X. Li, Y. Tian and H. Yang, Light-controlled generation of singlet oxygen within a discrete dual-stage metallacycle for cancer therapy, J. Am. Chem. Soc., 2019, 141, 8943–8950 CrossRef CAS.
- X. Lin, F. Chen, X. Yu, H. Wang, H. Qiu, Y. Li, S. Yin and P. Stang, Phenylthiol-BODIPY-based supramolecular metallacycles for synergistic tumor chemo-photodynamic therapy, Proc. Natl. Acad. Sci. U. S. A., 2022, 11, e2203994119 CrossRef PubMed.
- B. Huang, X. Ling, G. Yang, J. Tian, Y. Li, Y. Zhu, X. Li, G. Yin, W. Zheng, L. Xu and W. Zhang, A near-infrared organoplatinum(II) metallacycle conjugated with heptamethine cyanine for trimodal cancer therapy, CCS Chem., 2022, 4, 2090–2101 CrossRef CAS.
- G. Li, X. Zhang, W. Zhao, W. Zhao, F. Li, K. Xiao, Q. Yu, S. Liu and Q. Zhao, Stable and well-organized near-infrared platinum(II)–acetylide-based metallacycles-mediated cancer phototherapy, ACS Appl. Mater. Interfaces, 2020, 12, 20180–20190 CrossRef CAS PubMed.
- Y. Qin, X. Chen, Y. Gui, H. Wang, B. Tang and D. Wang, Self-assembled metallacage with second near-infrared aggregation-induced emission for enhanced multimodal theranostics, J. Am. Chem. Soc., 2022, 144, 12825–12833 CrossRef CAS PubMed.
- C. Li, L. Tu, Y. Xu, M. Li, J. Du, P. Stang, Y. Sun and Y. Sun, A NIR-light-activated and lysosomal-targeted Pt(II) metallacycle for highly potent evoking of immunogenic cell death that potentiates cancer immunotherapy of deep-seated Tumors, Angew. Chem., Int. Ed., 2024, 63, e202406392 CrossRef CAS PubMed.
- C. Li, L. Tu, J. Yang, C. Liu, Y. Xu, J. Li, W. Tuo, B. Olenyuk, Y. Sun, P. Stang and Y. Sun, Acceptor engineering of metallacycles with high phototoxicity indices for safe and effective photodynamic therapy, Chem. Sci., 2023, 14, 2901–2909 RSC.
- Y. Xu, Y. Pang, L. Luo, A. Sharma, J. Yang, C. Li, S. Liu, J. Zhan and Y. Sun, De novo designed Ru(II) metallacycle as a microenvironment-adaptive sonosensitizer and sonocatalyst for multidrug-resistant biofilms eradication, Angew. Chem., Int. Ed., 2024, 63, e202319966 CrossRef CAS PubMed.
- Y. Pang, Q. Li, J. Wang, S. Wang, A. Sharma, Y. Xu, H. Hu, J. Li, S. Liu and Y. Sun, An ultrasound-activated supramolecular modulator enhancing autophagy to prevent ventricular arrhythmias post-myocardial infarction, Angew. Chem., Int. Ed., 2024, 64, e202415802 CrossRef.
- B. Li, T. He, Y. Q. Fan, X. C. Yuan, H. Y. Qiu and S. C. Yin, Recent developments in the construction of metallacycle/metallacage-cored supramolecular polymers via hierarchical self-assembly, Chem. Commun., 2019, 55, 8036–8059 RSC.
- M. E. Carnes, M. S. Collins and D. W. Johnson, Transmetalation of self-assembled, supramolecular complexes, Chem. Soc. Rev., 2014, 43, 1825–1834 RSC.
- Y. Xu, Y. Dou, Q. Li, H. Ye, Y. Li, S. Qiu, X. Xiong, J. Li and Y. Sun, When near-infrared fluorescence meets supramolecular coordination complexes: Challenges and opportunities of metallacycles/metallacages in precision biomedicine, Coord. Chem. Rev., 2023, 493, 215320 CrossRef CAS.
- Y. Pang, C. Li, H. Deng and Y. Sun, Recent advances in luminescent metallacycles/metallacages for biomedical imaging and cancer therapy, Dalton Trans., 2022, 51, 16428–16438 RSC.
- C. Li, Y. Pang, Y. Xu, M. Lu, L. Tu, Q. Li, A. Sharma, Z. Guo, X. Li and Y. Sun, Near-infrared metal agents assisting precision medicine: from strategic design to bioimaging and therapeutic applications, Chem. Soc. Rev., 2023, 52, 4392–4442 RSC.
- Z. Wang, L. He, B. Liu, L. Zhou, L. Cai, S. Hu, X. Li, Z. Li, T. Chen, X. Li and Q. Sun, Coordination-assembled water-soluble anionic lanthanide organic polyhedral for luminescent labeling and magnetic resonance imaging, J. Am. Chem. Soc, 2020, 142, 16409–16419 CrossRef CAS PubMed.
- Y. Hu, X. Hao, D. Wang, Z. Zhang, H. Sun, X. Xu, X. Xie, X. Shi, H. Peng, H. Yang and L. Xu, Light-responsive supramolecular liquid-crystalline metallacycle for orthogonal multimode photopatterning, Angew. Chem., Int. Ed., 2024, 63, e202315061 CrossRef CAS PubMed.
- W. Dou, H. Yang and L. Xu, Fluorescent metallacycles via coordination-driven self-assembly: preparation, regulation, and applications, Acc. Chem. Res., 2025, 58, 1151–1167 CrossRef CAS.
- F. Wang, Y. Zhong, O. Bruns, Y. Liang and H. Dai, In vivo NIR-II fluorescence imaging for biology and medicine, Nat. Photonics, 2024, 18, 535–547 CrossRef CAS.
- Z. Lei and F. Zhang, Molecular engineering of NIR-II fluorophores for improved biomedical detection, Angew. Chem., Int. Ed., 2021, 60, 16294–16308 CrossRef CAS PubMed.
- C. Li, Y. Xu, L. Tu, M. Choi, Y. Fan, X. Chen, J. L. Sessler, J. S. Kim and Y. Sun, Rationally designed Ru(II)-metallacycle chemo-phototheranostic that emits beyond 1000 nm, Chem. Sci., 2022, 13, 6541–6549 RSC.
- Z. Zhang, H. Ye, F. Cai and Y. Sun, Recent advances on the construction of long-wavelength emissive supramolecular coordination complexes for photo-diagnosis and therapy, Dalton Trans., 2023, 52, 15193–15202 RSC.
- L. Tu, X. Xiong, J. H. Kim, Q. Li, L. Mei, J. Li, S. Liu, J. S. Kim and Y. Sun, Engineered metallacycle-based supramolecular photosensitizers for effective photodynamic therapy, Angew. Chem., Int. Ed., 2023, 62, e202301560 CrossRef CAS PubMed.
- P. Jeyakkumar, K. Du, R. Zhang, X. Tian, Q. Liu, J. Jiao, H. Jiang, X. Yu and X.-Y. Hu, Pillararene-based AIE-active metallacycle for efficient eradication of antibiotic-resistant bacteria, Sci. China: Chem., 2025 DOI:10.1007/s11426-025-2890-2.
- Q. Li, H. Ye, F. Zhao, Y. Li, Z. Zhang, Q. Yan and Y. Sun, Recent advances in combating bacterial infections via well-designed metallacycles/metallacages, Dalton Trans., 2024, 53, 3434–3444 RSC.
- Y. Xu, W. Tuo, L. Yang, Y. Sun, C. Li, X. Chen, W. Yang, G. Yang, P. J. Stang and Y. Sun, Design of a metallacycle-based supramolecular photosensitizer for in vivo image-guided photodynamic inactivation of bacteria, Angew. Chem., Int. Ed., 2022, 61, e202110048 CrossRef CAS PubMed.
- Y. Xu, C. Li, X. Ma, W. Tuo, L. Tu, X. Li, Y. Sun, P. J. Stang and Y. Sun, Long wavelength-emissive Ru(II) metallacycle-based photosensitizer assisting in vivo bacterial diagnosis and antibacterial treatment, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2209904119 CrossRef CAS PubMed.
- Y. Xu, C. Li, J. An, X. Ma, J. Yang, L. Luo, Y. Deng, J. S. Kim and Y. Sun, Construction of a 980 nm laser-activated Pt(II) metallacycle nanosystem for efficient and safe photo-induced bacteria sterilization, Sci. China: Chem., 2023, 66, 155–163 CrossRef CAS.
- C. Li, J. Zhan and B. Dong., An NIR-II-emissive Ru(II) metallacycle for efficient and safe immunotherapy, Sci. China: Chem., 2025, 68, 5–7 CrossRef CAS.
- L. Tu, C. Li, C. Liu, S. Bai, J. Yang, X. Zhang, L. Xu, X. Xiong and Y. Sun, Rationally designed Ru(II) metallacycles with tunable imidazole ligands for synergistical chemo-phototherapy of cancer, Chem. Commun., 2022, 58, 9068–9071 RSC.
- W. Li, X. Wang, L. Liu, J. Xiao, X. Wang, Y. Ye, Z. Wang, M. Zhu, Y. Sun, P. J. Stang and Y. Sun, Supramolecular coordination platinum metallacycle-based multilevel wound dressing for bacteria sensing and wound healing, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2318391121 CrossRef CAS.
- S. Tian, W. Huang, J. Hu, H. Wang, Z. Zhang, L. Xu, J. Li and Y. Sun., Exploring the frontiers of plant health: Harnessing NIR fluorescence and surface-enhanced Raman scattering modalities for innovative detection, Chin. Chem. Lett., 2025, 36, 110336 CrossRef CAS.
- W. Lai, A. Rogach and W. Wong, Chemistry and engineering of cyclodextrins for molecular imaging, Chem. Soc. Rev., 2017, 46, 6379–6419 RSC.
- S. Dummert, H. Saini, M. Hussain, K. Yadava, K. Jayaramulu, A. Casini and R. Fischer, Cyclodextrin metal–organic frameworks and derivatives: recent developments and applications, Chem. Soc. Rev., 2022, 51, 5175–5213 RSC.
- Y. Liang, E. Li, K. Wang, Z. Guan, H. He, L. Zhang, H. Zhou, F. Huang and Y. Fang, Organo-macrocycle-containing hierarchical metal–organic frameworks and cages: design, structures, and applications, Chem. Soc. Rev., 2022, 51, 8378–8405 RSC.
- W. Zhou, W. Lin, Y. Chen and Y. Liu, Supramolecular assembly confined purely organic room temperature phosphorescence and its biological imaging, Chem. Sci., 2022, 13, 7976–7989 RSC.
- S. Hu, L. Huang, L. Zhou, T. Wu, S. Zhao and L. Zhang, Single-excitation triple-emission down-/up-conversion nanoassemblies for tumor microenvironment-enhanced ratiometric NIR-II fluorescence imaging and chemo-/photodynamic combination therapy, Anal. Chem., 2023, 95, 3830–3839 CrossRef CAS.
- W. Chen, P. Chen, G. Zhang, G. Xing, Y. Feng, Y. Yang and L. Chen, Macrocycle-derived hierarchical porous organic polymers: synthesis and applications, Chem. Soc. Rev., 2021, 50, 11684–11714 RSC.
- Z. Man, Z. Lv, Z. Xu, J. Yao and H. Fu, Strategic engineering of sub-5 nm Dyes@CDs nanoassemblies platform for super resolution imaging, Adv. Funct. Mater., 2021, 31, 2106516 CrossRef CAS.
- F. Schibilla, A. Holthenrich, B. Song, A. Linard Matos, D. Grill, D. Rota Martir, V. Gerke, E. Zysman-Colman and B. Ravoo, Phosphorescent cationic iridium(III) complexes dynamically bound to cyclodextrin vesicles: applications in live cell imaging, Chem. Sci., 2018, 9, 7822–7828 RSC.
- Q. Wu, Z. Zhou, L. Xu, H. Zhong, B. Xiong, T. Ren, Z. Li, L. Yuan and X. Zhang, Multivalent supramolecular fluorescent probes for accurate disease imaging, Sci. Adv., 2024, 10, eadp8719 CrossRef CAS.
- S. Liew, Z. Zeng, P. Cheng, S. He, C. Zhang and K. Pu, Renal-clearable molecular probe for near-infrared fluorescence imaging and urinalysis of SARS-CoV-2, J. Am. Chem. Soc., 2021, 143, 18827–18831 CrossRef CAS PubMed.
- Y. Hu, J. Liu, M. Xu and K. Pu, Dual-locked fluorescence probe for monitoring the dynamic transition of pulmonary macrophages, J. Am. Chem. Soc, 2025, 147, 7148–7157 CrossRef CAS PubMed.
- J. Huang, J. Huang, P. Cheng, Y. Jiang and K. Pu, Near-infrared chemiluminescent reporters for in vivo imaging of reactive oxygen and nitrogen species in kidneys, Adv. Funct. Mater., 2020, 30, 2003628 CrossRef CAS.
- M. Baek, D. Nguyen, D. Kim, S. Yoo, S. Lee, J. Lee and D. Kim, Tailoring renal-clearable zwitterionic cyclodextrin for colorectal cancer-selective drug delivery, Nat. Nanotechnol., 2023, 18, 945–956 CrossRef CAS PubMed.
- H. Mousazadeh, Y. Pilehvar-Soltanahmadi, M. Dadashpour and N. Zarghami, Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy, J. Controlled Release, 2021, 330, 1046–1070 CrossRef CAS PubMed.
- Y. Ishitsuka, T. Irie and M. Matsuo, Cyclodextrins applied to the treatment of lysosomal storage disorders, Adv. Drug Delivery Rev., 2022, 191, 114617 CrossRef CAS.
- V. Sehgal, S. Pandey and P. Singh, Prospects of charged cyclodextrins in biomedical applications, Carbohydr. Polym., 2024, 323, 121348 CrossRef CAS PubMed.
- J. Jiao, G. Wang, X. Hu, Y. Zang, S. Maisonneuve, A. Sedgwick, J. Sessler, J. Xie, J. Li, X. He and H. Tian, Cyclodextrin-based peptide self-assemblies (Spds) that enhance peptide-based fluorescence imaging and antimicrobial efficacy, J. Am. Chem. Soc., 2020, 142, 1925–1932 CrossRef CAS.
- X. Dai, M. Huo, B. Zhang, Z. Liu and Y. Liu, Folic acid-modified cyclodextrin multivalent supramolecular assembly for photodynamic therapy, Biomacromolecules, 2022, 23, 3549–3559 CrossRef CAS.
- G. Yu, X. Zhao, J. Zhou, Z. Mao, X. Huang, Z. Wang, B. Hua, Y. Liu, F. Zhang, Z. He, O. Jacobson, C. Gao, W. Wang, C. Yu, X. Zhu, F. Huang and X. Chen, Supramolecular polymer-based nanomedicine: high therapeutic performance and negligible long-term immunotoxicity, J. Am. Chem. Soc, 2018, 140, 8005–8019 CrossRef CAS.
- G. Yang, L. Hao, Q. Cao, H. Zhang, J. Yang, L. Ji and Z. Mao, Three-in-one self-assembled nanocarrier for dual-drug delivery, two-photon imaging, and chemo-photodynamic synergistic therapy, ACS Appl. Mater. Interfaces, 2018, 10, 28301–28313 CrossRef CAS.
- X. Dai, X. Dong, Z. Liu, G. Liu and Y. Liu, Controllable singlet oxygen generation in water based on cyclodextrin secondary assembly for targeted photodynamic therapy, Biomacromolecules, 2020, 21, 5369–5379 CrossRef CAS.
- D. Nguyen, M. Baek, S. Lee, D. Kim, S. Yoo, J. Lee and D. Kim, Photobleaching-mediated charge-convertible cyclodextrin nanoparticles achieve deep tumour penetration for rectal cancer theranostics, Nat. Nanotechnol., 2024, 19, 1723–1734 CrossRef CAS.
- E. M. Peck, P. M. Battles, D. R. Rice, F. M. Roland, K. A. Norquest and B. D. Smith, Pre-assembly of near-infrared fluorescent multivalent molecular probes for biological imaging, Bioconjugate Chem., 2016, 27, 1400–1410 CrossRef CAS PubMed.
- P. Cheng, R. Wang, S. He, P. Yan, H. Huang, J. Chen, J. Shen and K. Pu, Artificial urinary biomarkers for early diagnosis of acute renal allograft rejection, Angew. Chem., Int. Ed., 2023, 62, e202306539 CrossRef CAS.
- S. Gnaim, A. Scomparin, A. Eldar-Boock, C. R. Bauer, R. Satchi-Fainaro and D. Shabat, Light emission enhancement by supramolecular complexation of chemiluminescence probes designed for bioimaging, Chem. Sci., 2019, 10, 2945–2955 RSC.
- W. A. Freeman, W. L. Mock and N.-Y. Shih, Cucurbituril, J. Am. Chem. Soc., 1981, 103, 7367–7368 CrossRef CAS.
- T. Fukaminato, Single-molecule fluorescence photoswitching: Design and synthesis of photoswitchable fluorescent molecules, J. Photochem. Photobiol., C, 2011, 12, 177–208 CrossRef CAS.
- H. Wu, Y. Chen, X. Dai, P. Li, J. F. Stoddart and Y. Liu, In situ photoconversion of multicolor luminescence and pure white light emission based on carbon dot-supported supramolecular assembly, J. Am. Chem. Soc., 2019, 141, 6583–6591 CrossRef CAS PubMed.
- D. Kim, A. Aktalay, N. Jensen, K. Uno, M. L. Bossi, V. N. Belov and S. W. Hell, Supramolecular complex of photochromic diarylethene and cucurbit [7] uril: fluorescent photoswitching system for biolabeling and imaging, J. Am. Chem. Soc., 2022, 144, 14235–14247 CrossRef CAS.
- D. Kolarski, M. L. Bossi, R. Lincoln, J. C. Fuentes-Monteverde, V. N. Belov and S. W. Hell, Supramolecular complexation of quenched rosamines with cucurbit [7] uril: fluorescence turn-on effect for super-resolution imaging, J. Am. Chem. Soc, 2025, 147, 28893–28902 CrossRef CAS.
- Z. Yin, Z. Wu and B. Liu, Recent advances in impurity-induced room-temperature phosphorescence, Adv. Mater., 2025, 2506549 CrossRef.
- Y. Li, Z. Wu, Z. Huang, C. Yin, H. Tian and X. Ma, Activatable red/near-infrared aqueous organic phosphorescence probes for improved time-resolved bioimaging, Natl. Sci. Rev., 2025, 12, nwae383 CrossRef CAS PubMed.
- D. V. Berdnikova and E. Y. Chernikova, Fluorescence and phosphorescence energy transfer in cucurbituril-based supramolecular systems, ChemPhotoChem, 2024, 8, e202300140 CrossRef CAS.
- H.-J. Hu, Q. Zhou, X. Dai, F.-F. Shen, Y.-M. Zhang, X. Xu and Y. Liu, Photooxidation-driven purely organic room-temperature phosphorescent lysosome-targeted imaging, J. Am. Chem. Soc., 2021, 143, 13887–13894 CrossRef.
- Y. Xia, Q. Bai, Y. Jiang, Q. Li, D. Wang and X. Xiao, Supramolecular self-assembly and metal−ligand-enhanced organic room-temperature phosphorescence for live cell imaging, ACS Appl. Mater. Interfaces, 2025, 17, 22375–22383 CrossRef CAS.
- X. Zhou, X. Bai, F. Shang, H.-Y. Zhang, L.-H. Wang, X. Xu and Y. Liu, Supramolecular assembly activated single molecule phosphorescence resonance energy transfer for near-infrared targeted cell imaging, Nat. Commun., 2024, 15, 4787 CrossRef CAS PubMed.
- R. Sasmal, A. Som, P. Kumari, R. V. Nair, S. Show, N. S. Barge, M. Pahwa, N. D. Saha, S. Rao, S. Vasu, R. Agarwal and S. S. Agasti, Supramolecular guest exchange in cucurbit [7] uril for bioorthogonal fluorogenic imaging across the visible spectrum, ACS Cent. Sci., 2024, 10, 1945–1959 CrossRef CAS PubMed.
- Y. Zhao, Y. Mei, J. Sun and Y. Tian, A supramolecular fluorescent chemosensor enabling specific and rapid quantification of norepinephrine dynamics, J. Am. Chem. Soc., 2025, 147, 5025–5034 CrossRef CAS PubMed.
- X.-Q. Wang, Q. Lei, J.-Y. Zhu, W.-J. Wang, Q. Cheng, F. Gao, Y.-X. Sun and X.-Z. Zhang, Cucurbit [8] uril regulated activatable supramolecular photosensitizer for targeted cancer imaging and photodynamic therapy, ACS Appl. Mater. Interfaces, 2016, 8, 22892–22899 CrossRef CAS.
- H. Ma, W. Xu, X. Tang, Y. Kang, J.-F. Xu and X. Zhang, A supramolecularly activatable photosensitizer: controllable cyanine J-aggregation for efficient photodynamic therapy, CCS Chem., 2025, 7, 832–842 CrossRef CAS.
- B. Yuan, H. Wu, H. Wang, B. Tang, J.-F. Xu and X. Zhang, A self-degradable supramolecular photosensitizer with high photodynamic therapeutic efficiency and improved safety, Angew. Chem., Int. Ed., 2021, 60, 706–710 CrossRef CAS.
- W. Zhu, Y. Li, S. Guo, W.-J. Guo, T. Peng, H. Li, B. Liu, H.-Q. Peng and B. Z. Tang, Stereoisomeric engineering of aggregation induced emission photosensitizers towards fungal killing, Nat. Commun., 2022, 13, 7046 CrossRef CAS PubMed.
- H. Hu, Y.-Y. Zhang, H. Ma, Y. Yang, S. Mei, J. Li, J.-F. Xu and X. Zhang, A supramolecular naphthalene diimide radical anion with efficient NIR-II photothermal conversion for E. coli-responsive photothermal therapy, Angew. Chem., Int. Ed., 2023, 62, e202308513 CrossRef CAS.
- H. Ma, Y. Kang, W. Xu, Y. Shen, H. Yu, H. Hu, X. Tang, J.-F. Xu and X. Zhang., An immediate bacterial-responsive supramolecular thio-naphthalene diimide: a real-time NIR-II photothermal anti-bacterial, Angew. Chem., Int. Ed., 2025, 64, e20250569 Search PubMed.
- M. Tang, Z. Yang, X. Tang, H. Ma, B. Xie, J.-F. Xu, C. Gao, D. Bardelang and R. Wang, Hypoxia-initiated supramolecular free radicals induce intracellular polymerization for precision tumor therapy, J. Am. Chem. Soc, 2025, 147, 3488–3499 CrossRef CAS.
- Y. Wu, L. Sun, X. Chen, J. Liu, J. Ouyang, X. Zhang, Y. Guo, Y. Chen, W. Yuan, D. Wang, T. He, F. Zeng, H. Chen, S. Wu and Y. Zhao, Cucurbit [8] uril-based water-dispersible assemblies with enhanced optoacoustic performance for multispectral optoacoustic imaging, Nat. Commun., 2023, 14, 3918 CrossRef CAS.
- G. Tonga, Y. Jeong, B. Duncan, B. R. Rydberg, M. Rotello, K. A. Nelson, C. R. Bertozzi and V. M. Rotello, Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts, Nat. Chem., 2015, 7, 597–603 CrossRef CAS PubMed.
- J. Wang, Z. Huang, X. Ma and H. Tian, Visible-Light-Excited Room-Temperature Phosphorescence in Water by Cucurbituril-Mediated Supramolecular Assembly, Angew. Chem., Int. Ed., 2020, 59, 9928–9933 CrossRef CAS PubMed.
- X. C. Hu, F. B. Liu, X. Z. Zhang, Z. Y. Zhao and S. M. Liu, Expected and unexpected photoreactions of 9-(10-) substituted anthracene derivatives in cucurbit [n] uril hosts, Chem. Sci., 2020, 11, 4779–4785 RSC.
- Y. H. Zhang, X. L. Luo, H. J. Li, S. G. Sun and Y. Q. Xu, Host-Guest supramolecular Cubes: A versatile engineering system of squaraine and Cuburbit [8] uril for selective fluorescence imaging and enhanced photodynamic therapy in tumor cells, Chem. Eng. J., 2024, 488, 150979 CrossRef CAS.
- Y. Zhao, Y. Mei, Z. Liu, J. Sun and Y. Tian, Molecularly engineered supramolecular fluorescent chemodosimeter for measuring epinephrine dynamics, Nat. Commun., 2025, 16, 1848 CrossRef CAS.
- X. Wang, C. Chen, Y. Tian and Q.-W. Zhang, Dual-Channel phosphorescence ratiometry and phosphorescence lifetime imaging of mitochondria-specific methionine sulfoxide reductase activity, J. Am. Chem. Soc., 2025, 147, 17994–18002 CrossRef CAS.
- S. B. Nimse and T. Kim, Biological applications of functionalized calixarenes, Chem. Soc. Rev., 2013, 42, 366–386 RSC.
- H. Zhang, Z. Liu and Y. Zhao, Pillararene-based self-assembled amphiphiles, Chem. Soc. Rev., 2018, 47, 5491–5528 RSC.
- W.-C. Geng, J. L. Sessler and D.-S. Guo, Supramolecular prodrugs based on host-guest interactions, Chem. Soc. Rev., 2020, 49, 2303–2315 RSC.
- I. Shulow, R. V. Rodik, Y. Arntz, A. Reish, V. I. Kalchenko and A. S. Klymchenko, Protein-sized bright fluorogenic nanoparticles based on cross linked calixarene micelles with cyanine corona, Angew. Chem., Int. Ed., 2016, 55, 15884–15888 CrossRef.
- B. Shi, K. Jie, Y. Zhou, J. Zhou, D. Xia and F. Huang, Nanoparticles with near-infrared emission enhanced by pillararene based molecular recognition in water, J. Am. Chem. Soc., 2016, 138, 80–83 CrossRef CAS.
- C. Wang, Y. Guo, G. Tan, W. Kang, W. Guo, N. Song and Y. Tang, Interfacial engineering of pillararene-modified ceria nanoparticles for regulable enhanced sonodynamic therapy, Aggregate, 2025, 6, e70062 CrossRef CAS.
- H.-T. Feng, Y. Li, X. Duan, X. Wang, C. Qi, J. W. Y. Lam, D. Ding and B. Z. Tang, Substitution activated precise phototheranostics through supramolecular assembly of AIEgen and calixarene, J. Am. Chem. Soc., 2020, 142, 15966–15974 CrossRef CAS.
- C. Chen, X. Ni, H.-W. Tian, Q. Liu, D.-S. Guo and D. Ding, Calixarene-based supramolecular AIE dots with highly inhibited nonradiative decay and intersystem crossing for ultrasensitive fluorescence image-guided cancer surgery, Angew. Chem., Int. Ed., 2020, 59, 1008–10012 Search PubMed.
- B. Lu, Z. Zhang, Y. Huang, Y. Zhang, J. Wang, Y. Ding, Y. Wang and Y. Yao, A nanoplatform for mild-temperature photothermal and type I & II photodynamic therapy in the NIR-II biowindow, Chem. Commun., 2022, 58, 10353–10356 RSC.
- K.-X. Teng, L.-Y. Niu and Q.-Z. Yang, A host–guest strategy for converting the photodynamic agents from a singlet oxygen generator to a superoxide radical generator, Chem. Sci., 2022, 13, 5951–5956 RSC.
- Y. Mei, Q.-W. Zhang, Q. Gu, Z. Liu, X. He and Y. Tian, Pillar [5] arene-based fluorescent sensor array for biosensing of intracellular multi-neurotransmitters through host−guest recognitions, J. Am. Chem. Soc., 2022, 144, 2351–2359 CrossRef CAS.
- Y. Mei, J. Sun, Z. Liu, Y. Zhao, Q. Zhang and Y. Tian, An engineered supramolecular fluorescent chemosensor for multiscale visualization of glutamate dynamics in living systems, J. Am. Chem. Soc., 2025, 147, 34034–34044 CrossRef CAS PubMed.
- J. Gao, J. Li, W.-C. Geng, F.-Y. Chen, X. Duan, Z. Zheng, D. Ding and D.-S. Guo, Biomarker displacement activation: a general host–guest strategy for targeted phototheranostics in vivo, J. Am. Chem. Soc., 2018, 140, 4945–4953 CrossRef CAS.
- L. Xia, J. Tian, T. Yue, H. Cao, J. Chu, H. Cai and W. Zhang, Pillar [5] arene-based acid-triggered supramolecular porphyrin photosensitizer for combating bacterial infections and biofilm dispersion, Adv. Healthcare Mater., 2022, 11, 2102015 CrossRef CAS PubMed.
- K. R. Deng, Z. S. Luo, L. Tan and Z. W. Quan, Self-assembly of anisotropic nanoparticles into functional superstructures, Chem. Soc. Rev., 2020, 49, 6002–6038 RSC.
- J. Gao, J. Zhan and Z. M. Yang, Enzyme-instructed self-assembly (EISA) and hydrogelation of peptides, Adv. Mater., 2020, 32, 1805798 CrossRef CAS.
- M. Jenkyn-Bedford, M. L. Jones, Y. Baris, K. P. M. Labib, G. Cannone, J. T. P. Yeeles and T. D. Deegan, A conserved mechanism for regulating replisome disassembly in eukaryotes, Nature, 2021, 600, 743–747 CrossRef CAS.
- H. J. He, W. Y. Tan, J. Q. Guo, M. H. Yi, A. N. Shy and B. Xu, Enzymatic noncovalent synthesis, Chem. Rev., 2020, 120, 9994–10078 CrossRef CAS.
- K. D. Judd, D. M. de Oliveira, A. S. Urbina and D. Ben-Amotz, Influence of H+, OH− and salts on hydrophobic self-assembly, Chem. Sci., 2024, 15, 6378–6384 RSC.
- S. Bera, S. Basu, B. Jana and P. Dastidar, Real-time observation of macroscopic helical morphologies under optical microscope: a curious case of π−π stacking driven molecular self-assembly of an organic gelator devoid of hydrogen bonding, Angew. Chem., Int. Ed., 2023, 62, e202216447 CrossRef CAS PubMed.
- Y. Q. Wang, Y. X. Hu and D. J. Ye, Activatable multimodal probes for in vivo imaging and theranostics, Angew. Chem., Int. Ed., 2022, 61, e202209512 CrossRef CAS.
- X. X. Sun, X. H. Yang, Y. Chen, J. Sun, Z. G. He, S. W. Zhang and C. Luo, In situ self-assembled nanomedicines for cancer treatment, Chem. Eng. J., 2023, 466, 143365 CrossRef CAS.
- J. Kim, S. Lee, Y. Kim, M. Choi, I. Lee, E. Kim, C. G. Yoon, K. Pu, H. M. Kang and J. S. Kim, In situ self-assembly for cancer therapy and imaging, Nat. Rev. Mater., 2023, 8, 710–725 CrossRef.
- M. Sun, C. Y. Wang, M. C. Lv, Z. Fan and J. Z. Du, Intracellular self-assembly of peptides to induce apoptosis against drug-resistant melanoma, J. Am. Chem. Soc., 2022, 144, 7337–7345 CrossRef CAS.
- D. Y. Hou, D. B. Cheng, N. Y. Zhang, Z. J. Wang, X. J. Hu, X. Li, M. Y. Lv, X. P. Li, L. R. Jian, J. P. Ma, T. L. Sun, Z. Y. Qiao, W. H. Xu and H. Wang, In vivo assembly enhanced binding effect augments tumor specific ferroptosis therapy, Nat. Commun., 2024, 15, 454 CrossRef CAS PubMed.
- E. Froimchuk, S. T. Carey, C. Edwards and C. M. Jewell, Self-assembly as a molecular strategy to improve immunotherapy, Acc. Chem. Res., 2020, 53, 2534–2545 CrossRef CAS PubMed.
- H. W. Liu, L. L. Chen, C. Y. Xu, Z. Li, H. Y. Zhang, X. B. Zhang and W. H. Tan, Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging, Chem. Soc. Rev., 2018, 47, 7140–7180 RSC.
- Z. Li, P. Z. Liang, T. B. Ren, L. Yuan and X. B. Zhang, Orderly self-assembly of organic fluorophores for sensing and imaging, Angew. Chem., Int. Ed., 2023, 62, e202305742 CrossRef CAS.
- G. L. Liang, H. J. Ren and J. H. Rao, Biocompatible condensation reaction for controlled assembly of nanostructures in living cells, Nat. Chem., 2010, 2, 54–60 CrossRef CAS.
- D. J. Ye, A. J. Shuhendler, L. N. Cui, L. Tong, S. S. Tee, G. Tikhomirov, D. W. Felsher and J. H. Rao, Bioorthogonal cyclization mediated in situ self-assembly of small-molecule probes for imaging caspase activity in vivo, Nat. Chem., 2014, 6, 519–526 CrossRef CAS PubMed.
- Y. Q. Wang, H. Bai, Y. X. Miao, J. H. Weng, Z. Huang, J. Y. Fu, Y. Zhang, J. G. Lin and D. J. Ye, Tailoring a near-infrared macrocyclization scaffold allows the control of in situ self-assembly for photoacoustic/PET bimodal imaging, Angew. Chem., Int. Ed., 2022, 61, e202200369 CrossRef CAS.
- L. L. Xu, H. Gao, W. J. Zhan, Y. Deng, X. Y. Liu, Q. C. Jiang, X. B. Sun, J. J. Xu and G. L. Liang, Dual aggregations of a near-infrared aggregation-induced emission luminogen for enhanced imaging of Alzheimer's disease, J. Am. Chem. Soc., 2023, 145, 27748–27756 CrossRef CAS PubMed.
- R. Wang, L. Zhou, Y. Y. Yang, F. R. Zhao, X. B. Sun, X. Y. Liu, Z. Zou and G. L. Liang, Spatially quantitative imaging of enzyme activity in a living cell, J. Am. Chem. Soc., 2024, 146, 34870–34877 CrossRef CAS.
- L. Yang, R. Peltier, M. M. Zhang, D. Song, H. Huang, G. C. Chen, Y. Chen, F. H. Zhou, Q. Hao, L. M. Bian, M. L. He, Z. K. Wang, Y. Hu and H. Y. Sun, Desuccinylation-triggered peptide self-assembly: live cell imaging of SIRT5 activity and mitochondrial activity modulation, J. Am. Chem. Soc., 2020, 142, 18150–18159 CrossRef CAS PubMed.
- H. Ren, X. Z. Zeng, X. X. Zhao, D. Y. Hou, H. D. Yao, M. Yaseen, L. N. Zhao, W. H. Xu, H. Wang and L. L. Li, A bioactivated in vivo assembly nanotechnology fabricated NIR probe for small pancreatic tumor intraoperative imaging, Nat. Commun., 2022, 13, 418 CrossRef CAS PubMed.
- J. H. Weng, Z. Huang, Y. L. Liu, X. D. Wen, Y. X. Miao, J. J. Xu and D. J. Ye, Controlled in situ self-assembly of biotinylated trans-cyclooctene nanoparticles for orthogonal dual-pretargeted near-infrared fluorescence and magnetic resonance imaging, J. Am. Chem. Soc., 2024, 146, 13163–13175 CrossRef CAS.
- H. W. An, L. L. Li, Y. Wang, Z. Q. Wang, D. Y. Hou, Y. X. Lin, S. L. Qiao, M. D. Wang, C. Yang, Y. Cong, Y. Ma, X. X. Zhao, Q. Cai, W. T. Chen, C. Q. Lu, W. H. Xu, H. Wang and Y. L. Zhao, A tumour-selective cascade activatable self-detained system for drug delivery and cancer Imaging, Nat. Commun., 2019, 10, 4861 CrossRef.
- X. D. Wen, R. Zhang, Y. X. Hu, L. Y. Wu, H. Bai, D. F. Song, Y. F. Wang, R. B. An, J. H. Weng, S. R. Zhang, R. Wang, L. Qiu, J. G. Lin, G. D. Gao, H. Liu, Z. J. Guo and D. J. Ye, Controlled sequential in situ self-assembly and disassembly of a fluorogenic cisplatin prodrug for cancer theranostics, Nat. Commun., 2023, 14, 800 CrossRef CAS.
- X. Liu, W. J. Zhan, G. Gao, Q. C. Jiang, X. P. Zhang, H. B. Zhang, X. B. Sun, W. Han, F. G. Wu and G. L. Liang, Apoptosis-amplified assembly of porphyrin nanofiber enhances photodynamic therapy of oral tumor, J. Am. Chem. Soc., 2023, 145, 7918–7930 CrossRef CAS PubMed.
- Q. X. Dai, L. S. Xie, E. Ren and G. Liu, Cathepsin B Responsive peptide−purpurin conjugates assembly initiated in situ self-aggregation for cancer sonotheranostics, Nano Lett., 2024, 24, 950–957 CrossRef CAS PubMed.
- Y. Yuan, J. Zhang, X. L. Qi, S. G. Li, G. S. Liu, S. Siddhanta, I. Barman, X. L. Song, M. T. McMahon and J. W. M. Bulte, Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy, Nat. Mater., 2019, 18, 1376–1383 CrossRef CAS.
- X. Liu, F. Tian, Z. Y. Zhang, J. Z. Liu, S. Y. Wang, R. C. Guo, B. B. Hu, H. Wang, H. Zhu, A. A. Liu, L. Q. Shi and Z. L. Yu, In vivo self-sorting of peptides via in situ assembly evolution, J. Am. Chem. Soc., 2024, 146, 24177–24187 CrossRef CAS.
- G. Gao, Y. W. Jiang, W. J. Zhan, X. Y. Liu, R. Q. Tang, X. B. Sun, Y. Deng, L. L. Xu and G. L. Liang, Trident molecule with nanobrush–nanoparticle–nanofiber transition property spatially suppresses tumor metastasis, J. Am. Chem. Soc., 2022, 144, 11897–11910 CrossRef CAS.
- J. Y. Zhang, Y. X. Hu, X. D. Wen, Z. Y. Yang, Z. Y. Wang, Z. Y. Feng, H. Bai, Q. Xue, Y. X. Miao, T. Tian, P. Zheng, J. J. Zhang, J. Li, L. Qiu, J. J. Xu and D. J. Ye, Tandem-controlled lysosomal assembly of nanofibres induces pyroptosis for cancer immunotherapy, Nat. Nanotechnol., 2025, 20, 563–574 CrossRef CAS.
- Z. Li, E. Sarikhani, S. Prayotamornkul, D. P. Meganathan, Z. Jahed and L. Shi, Multimodal imaging unveils the impact of nanotopography on cellular metabolic activities, Chem. Biomed. Imaging, 2024, 2, 825–834 CrossRef CAS PubMed.
- M. Sitara, W. Zhang, H. Gao, J. Li and J. Tian, Recent progress in molecular probes for imaging of acute kidney injury, Chem. Biomed. Imaging, 2024, 2, 526–541 CrossRef CAS PubMed.
- J.-X. Cheng, T.-Y. Chen and P. Chen, The evolution of sub-diffraction chemical imaging from nanoscale to AI, Chem. Biomed. Imaging, 2024, 2, 731–732 CrossRef CAS PubMed.
- Y. C. Wang, P. F. Song, H. Q. Zhou, P. W. Wang, Y. Li, Z. Y. Shao, L. Wang, Y. You, Z. H. Lei, J. H. Yu and C. Li, An AI-assisted fluorescence microscopic system for screening mitophagy inducers by simultaneous analysis of mitophagic intermediates, Nat. Commun., 2025, 16, 5179 CrossRef CAS PubMed.
- C. Li, S. Zhou, J. Chen and X. Jiang, Fluorescence imaging of inflammation with optical probes, Chem. Biomed. Imaging, 2023, 1, 495–508 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |