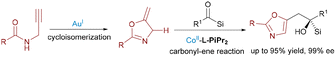Recent advances in asymmetric bimetallic catalysis
Fang
Wei†
 a,
Jialin
Qi†
b,
Xiangqing
Jia
a,
Jialin
Qi†
b,
Xiangqing
Jia
 *c and
Zhenghu
Xu
*c and
Zhenghu
Xu
 *c
*c
aJiangxi Provincial Key Laboratory of Organic Functional Molecules, Institute of Organic Chemistry, Jiangxi Science and Technology Normal University, Nanchang 330013, China
bCollege of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng 252000, China
cKey Lab for Colloid and Interface Chemistry of Education Ministry, School of Chemistry and Chemical Engineering, Shandong University, No. 27 South Shanda Road, Jinan 250100, China. E-mail: jiaxq@sdu.edu.cn; xuzh@sdu.edu.cn
First published on 19th November 2025
Abstract
Asymmetric bimetallic catalysis has emerged as a powerful and efficient approach for the development of novel enantioselective transformations. By employing two metal centers with complementary reactivity, bimetallic catalysts enable dual substrate activation, stabilize reactive intermediates, and facilitate unique transformations with high enantioselectivity. This review summarizes recent significant advances in the field, including three different reaction modes: dual metal Lewis acid catalysis, transition-metal/metal Lewis acid catalysis, and dual transition-metal catalysis. By exploring the latest breakthroughs and providing a comprehensive outlook on the promising potential of asymmetric bimetallic catalysis, we aim to inspire further progress in this rapidly evolving area and highlight future opportunities for expanding its applications.
1. Introduction
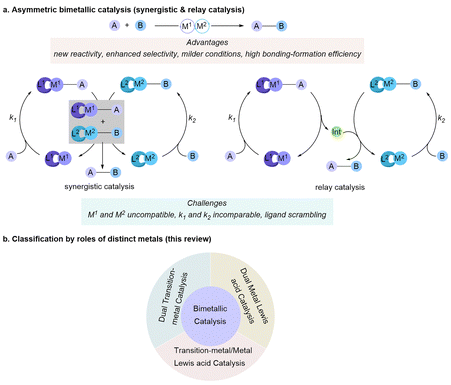
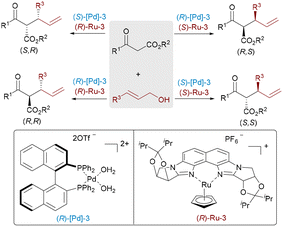
Asymmetric transition-metal catalysis has revolutionized modern synthetic chemistry by enabling the efficient and selective formation of chiral molecules essential for pharmaceuticals, agrochemicals, and materials science. By leveraging the unique electronic and steric properties of transition metals, these catalytic systems facilitate the formation of chiral molecules with remarkable efficiency and control. The ability to achieve high enantioselectivity through transition-metal catalysis has significantly impacted the development of novel synthetic methodologies and streamlined the production of complex organic molecules. In conventional monometallic catalytic systems, the single transition metal center with appropriate chiral ligands activates a substrate, and the other substrate reacts with the speciated activated complex or intermediate to promote the final product selectively. While impressive progress has been achieved using this approach over the years, numerous important asymmetric transformations still lack efficient catalytic methods. Recently, by mimicking enzyme catalysis in biosystems, a multicatalyst combination strategy,1 especially bimetallic asymmetric catalysis,2 has emerged as a powerful approach to achieve enhanced reactivity, selectivity, and novel reaction pathways that are often unattainable with monometallic systems. Particularly, one key advantage of bimetallic catalysis is that two chiral metal catalysts can independently control the stereochemical configurations of each stereocenter in the target products. This capability enables the stereodivergent synthesis of all stereoisomers from the same set of starting materials under identical reaction conditions, simply by altering the combination of chiral ligands.2h,m Asymmetric bimetallic catalysis encompasses synergistic and relay catalysis (Scheme 1a). Synergistic catalysis involves the activation of two substrates by distinct catalysts in concert to drive a bond-forming reaction.2b In contrast, relay catalysis operates through a cascade process, where two catalysts sequentially promote different bond-forming steps.2oFor successful bimetallic catalysis,3,4 each transition metal center selectively activates one substrate with comparable kinetic rates (k1 and k2) (Scheme 1a). If a significant disparity exists, the more active and longer-lived intermediate could either decompose or promote side reactions, ultimately reducing overall reaction efficiency. Furthermore, the compatibility of the two transition metals must be carefully considered, as redox interactions between two catalysts can lead to self-quenching, resulting in their deactivation. Additionally, transition metal catalysis usually relies on specific ligands to achieve optimal reactivity and selectivity. However, in a bimetallic catalytic system, potential ligand scrambling can disrupt the catalytic process. To address these challenges, a judicious selection of transition metal pairings and ligands is essential to ensure both efficiency and selectivity.
In 1996, the Sawamura and Ito group developed a bimetallic system consisting of a Pd catalyst and a Rh catalyst, coordinated with the same chiral ligand, for the enantioselective allylation of α-cyano esters.5 In 2004, the Jacobson group reported the highly enantioselective conjugate addition of cyanide to unsaturated imides, synergistically catalyzed by a chiral (Salen)Al complex and a chiral (Pybox)Er complex.6 However, it was not until the 2010s that the asymmetric bimetallic catalysis has garnered increasing attention, providing a powerful strategy for the development of asymmetric transformations.7
In this review, we present up-to-date promising examples which feature asymmetric bimetallic catalysis to enable access to enantioenriched molecules. Transition-metal photocatalysis8 and binuclear transition-metal catalysis,2a,9 which have been well overviewed by other reviews and accounts, will not be covered in this review. We will discuss the development of asymmetric bimetallic catalysis and the distinct roles played by different metals, including dual metal Lewis acid catalysts, transition-metal/metal Lewis acid catalysts, and dual transition-metal catalysts (Scheme 1b). Metal Lewis acids typically activate substrates through coordination, while the chemical valence generally remains constant throughout the reaction process. Transition metal catalysts typically undergo a cascade of oxidative addition and reductive elimination, during which the oxidation state may change throughout the catalytic cycle. In this review, a metal center that forms a metal–carbon bond with a reactant during the reaction process is referred to as a transition-metal catalyst. Otherwise, it is classified as a Lewis acid catalyst. Notably, we will emphasize catalyst pairings, the scope of the substrates, and the typical proposed catalytic mechanism, which plays a crucial role in facilitating the reactivity of both known and novel transformations.
2. Dual metal Lewis acid catalysis
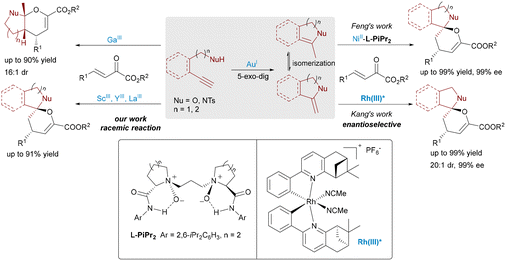
The combination of two distinct catalytic modes in a metal–metal relay system, which enables the simultaneous coordination and activation of different substrates by metal Lewis acids, has emerged as a powerful and innovative strategy in modern catalysis. As a late transition metal, gold has been extensively investigated in dual Lewis acid-catalyzed reactions, where it typically acts as a π-acid, effectively activating unsaturated systems—especially carbon–carbon triple bonds. The combination of π-acidic gold with a σ-type metal Lewis acid, such as an early transition metal, facilitates a dual activation strategy that simultaneously engages both substrates, paving the way for the development of diverse reactions and novel chemical transformations.A pioneering study from our group showcased the potential of Au/σ-Lewis acid relay catalysis in the synthesis of fused bicyclic aminals and spiroaminals.10 By pairing π-acidic gold catalysts with σ-type Lewis acids such as Sc(III), Ga(III), Y(III), and La(III), we achieved efficient intramolecular hydroamination followed by inverse-electron-demand hetero-Diels–Alder (IED-HDA) reactions (Scheme 2, left). Gold-catalyzed 5-exo-dig cyclization affords an exocyclic enamide, which may isomerize to a more stable methyl-substituted enamide under some conditions. Both intermediates can then undergo [4+2] cycloaddition with Lewis acid-activated unsaturated β-keto esters, generating fused bicyclic and spirocyclic products, respectively.
Building on this foundation, Feng et al. reported a significant advancement in enantioselective synthesis through the development of an asymmetric dual Lewis acid catalytic system (Scheme 2, right).11 They achieved highly stereocontrolled IED-HDA reactions by combining Au(I) with a Ni(II) complex coordinated by a chiral N,N′-dioxide ligand (Feng ligand),12 which represents a privileged class of modular and tunable organic ligands capable of coordinating with a wide range of metal centers to form highly efficient Lewis acid catalysts. Notably, this dual catalytic system effectively suppressed isomerization of the reactive enamide intermediate, selectively favoring spiro over fused products. In 2018, Kang's group demonstrated a dual Lewis acid catalytic system featuring achiral Au(I) and chiral Rh(III) in relay mode, enabling efficient asymmetric synthesis of spiroketals and spiroaminals.13
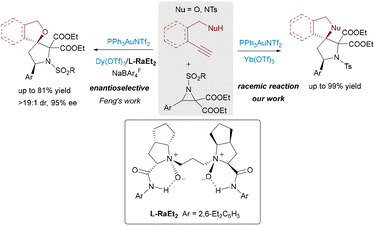
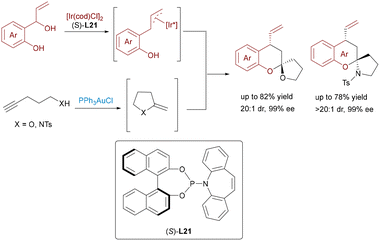
The progression from Au/σ-Lewis acid-catalyzed [4+2] cycloadditions yielding 5,6-spirocycles to [3+2] cycloadditions with aziridines, delivering versatile 5,5-spiro-N,O/N,N-heterocycles, underscores the remarkable versatility (Scheme 3).14 The strategic use of aziridines as masked 1,3-dipoles, activated by σ-Lewis acids via selective C–C bond cleavage, opens a distinct pathway previously inaccessible to other dipolarophiles. Furthermore, the integration of chiral N,N′-dioxide-lanthanide(III) complexes (e.g., Dy(III)) with achiral Au(I) catalysts successfully addresses the challenges in enantioselective control in this powerful cascade, enabling the asymmetric synthesis of complex spiroarchitectures prevalent in bioactive molecules. These developments highlight the potential of combining π-acidic gold with chiral σ-Lewis acids to unlock novel reaction manifolds and achieve precise stereochemical outcomes in the synthesis of intricate spirocyclic scaffolds.
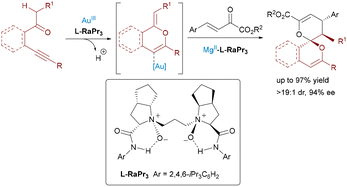
In 2019, Feng and colleagues extended the dual Lewis acid strategy to the synthesis of enantiomerically enriched 6,6-spiroketals using a relay Au(III)/Mg(II) system (Scheme 4).15 The reaction combined β-alkynyl ketones with β,γ-unsaturated α-ketoesters through a tandem cycloisomerization/inverse-electron-demand [4+2] cycloaddition. The achiral Au(III) catalyzed the initial 6-endo-dig oxo-cyclization to form a carbonyl ylide intermediate, while the chiral N,N′-dioxide–Mg(II) complex orchestrated the enantioselective [4+2] cycloaddition. Mechanistic studies revealed the critical role of the Feng ligand as a base, facilitating deprotonation to generate a reactive gold-bound alkene intermediate for stereocontrolled cyclization.
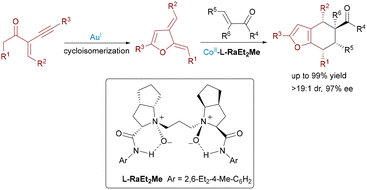
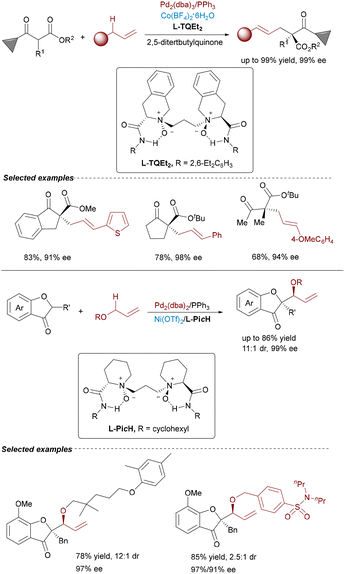
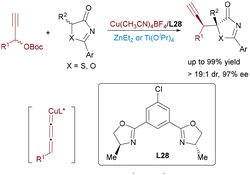
Further advancing the paradigm, Feng's group reported an Au(I)/Co(II) bimetallic system in 2020 for synthesizing chiral tetrahydrobenzofuran derivatives with four contiguous stereocenters (Scheme 5).16 The tandem reaction involved acyclic enynones and electron-deficient olefins, proceeding via gold-catalyzed cycloisomerization to generate furan-based ortho-quinodimethanes, followed by a Co(II)/N,N′-dioxide-mediated enantioselective Diels–Alder cycloaddition. In 2023, Feng leveraged Au(I)/Co(II) catalysis for enantioselective cycloisomerization of N-propargylamides followed by a carbonyl–ene reaction with acylsilanes (Scheme 6).17 This efficient cascade constructed valuable chiral 5-oxazoylmethyl α-silyl alcohols in excellent yields (up to 95%) and enantioselectivities (up to 99% ee) under mild conditions.
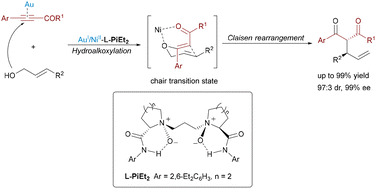
While earlier studies predominantly focused on cascade cyclization, gold-participating dual Lewis acid catalytic systems have also proven to be effective for constructing acyclic architectures through sequential non-cyclization steps. In 2017, Feng and co-workers demonstrated this versatility using an Au(I)/chiral N,N′-dioxide–Ni(II) relay system to enable an asymmetric tandem intermolecular hydroalkoxylation/Claisen rearrangement (Scheme 7).18 The reaction merged alkynyl esters and allylic alcohols under mild conditions, where Au(I) catalyzed the initial hydroalkoxylation to form allyl vinyl ether intermediates, while the chiral N,N′-dioxide–Ni(II) complex stereocontrolled the subsequent [3,3]-sigmatropic rearrangement. This strategy efficiently suppressed thermal background reactions and prevented epimerization, delivering acyclic α-allyl-β-keto esters with exceptional stereoselectivity (up to 99% ee, 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dr) and near-quantitative yields (up to 99%).
3 dr) and near-quantitative yields (up to 99%).
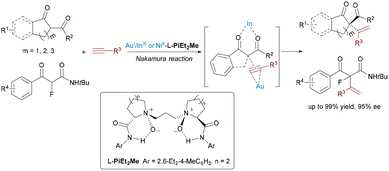
Expanding the sequential non-cyclization strategy in 2021, Feng's group achieved the first catalytic asymmetric Nakamura reaction via synergistic Au(I)/chiral N,N′-dioxide–In(III) or Ni(II) catalysis (Scheme 8).19 The system employed Au(I) to activate unactivated 1-alkynes and chiral In(III) or Ni(II) to generate enolates from 1,3-dicarbonyl compounds. Precise ligand tuning enabled enantiocontrol (up to 97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 e.r.) for tetra-substituted chiral centers. This breakthrough accommodated β-ketoesters, diketones, and even α-fluoro acyclic substrates, with trace water accelerating enolization.
2.5 e.r.) for tetra-substituted chiral centers. This breakthrough accommodated β-ketoesters, diketones, and even α-fluoro acyclic substrates, with trace water accelerating enolization.
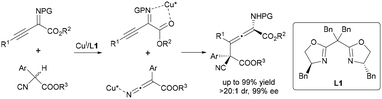
Zhang's breakthrough employs a single chiral Cu(I)-bis(oxazoline) catalyst to synthesize tetrasubstituted α-amino allenoates with vicinal all-carbon quaternary stereocenters (Scheme 9).20 This strategy employs a single chiral Cu(I)-BOX complex to dynamically activate two distinct substrates through a substrate-responsive mechanism: first, the Cu(I) center coordinates and polarizes the alkyne moiety of 1-alkynyl ketimines, enhancing the electrophilicity of the γ-position; subsequently, the same catalyst promotes deprotonation of α-substituted cyanoacetates to generate a reactive enolate intermediate. Crucially, the rigid chiral pocket of the BOX ligand enforces γ-regioselectivity (up to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) by steric repulsion, while simultaneously transmitting stereochemical information to achieve exceptional enantiocontrol (up to 99% ee). In contrast to traditional dual Lewis acid catalysis, which relies on intricate combinations of heterometallic or heteroleptic catalysts, this “dual-role” single-catalyst design significantly simplifies system complexity.
1 dr) by steric repulsion, while simultaneously transmitting stereochemical information to achieve exceptional enantiocontrol (up to 99% ee). In contrast to traditional dual Lewis acid catalysis, which relies on intricate combinations of heterometallic or heteroleptic catalysts, this “dual-role” single-catalyst design significantly simplifies system complexity.
3. Transition-metal/metal Lewis acid catalyst
Transition-metal-mediated catalysis has long been recognized as a central strategy for the formation of carbon–carbon and carbon–heteroatom bonds, while Lewis acid catalysis has been extensively utilized for substrate activation through coordination. Over the past few decades, the deliberate integration of these two catalytic modes – transition-metal catalysis combined with Lewis acid co-catalysis – has emerged as a powerful method for enhancing reactivity, selectivity, and overall catalytic efficiency in organic synthesis. A significant evolution in this field lies in the strategic utilization of transition metals themselves as Lewis acids capable of coordinating chiral ligands, thereby enabling exceptional levels of enantioselectivity and stereocontrol. This dual-catalysis paradigm continues to open new avenues in asymmetric synthesis and remains a vibrant area of investigation in modern organic chemistry.3.1. Pd/M catalytic system
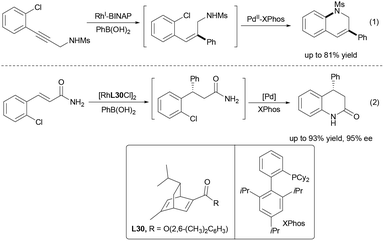
Palladium has emerged as one of the most versatile and widely used transition metals in catalysis, playing a pivotal role in modern synthetic chemistry.21 Its ability to adopt multiple oxidation states (0, +2, and +4) and compatibility with diverse ligands allow for precise control over reactivity and stereoselectivity. The development of dual catalytic systems that combine palladium with Lewis acids has further expanded the frontiers of catalytic design, offering levels of reactivity and selectivity that are often unattainable with either catalyst alone. Notably, the wide compatibility of palladium with various Lewis acids further expands the scope of accessible reactions, facilitating the efficient assembly of complex scaffolds.In 1996, Ito and Sawamura reported the first highly enantioselective transition-metal-catalyzed allylic alkylation to construct acyclic quaternary stereocenters using a Pd/Rh bimetallic system (Scheme 10).5 The π-allylpalladium complex activated the electrophile, while the chiral Rh(I) complex generated a chelated enolate and induced asymmetry. Mechanistic studies confirmed that rhodium was essential for enantioinduction and palladium was essential for electrophile activation, demonstrating the potential of synergistic bimetallic catalysis in asymmetric synthesis.
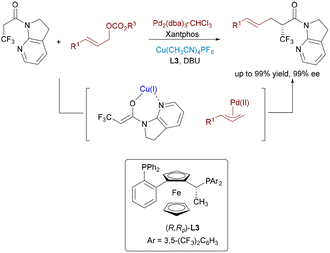
To further promote the progress of catalytic asymmetric α-allylation of amides, Shibasaki and Kumagai developed a Pd/Cu dual catalytic system in 2017 for the enantioselective α-allylation of α-CF3 amides (Scheme 11).22 The strategy integrates Cu(I)-mediated enolization with Pd(0)-catalyzed π-allyl formation, affording α-trifluoromethylated γ,δ-unsaturated amides in high enantiomeric excess. Each metal plays a distinct role, with the chiral Cu complex governing stereoselectivity. This method provides an efficient route to fluorinated chiral scaffolds valuable in pharmaceuticals and materials science.
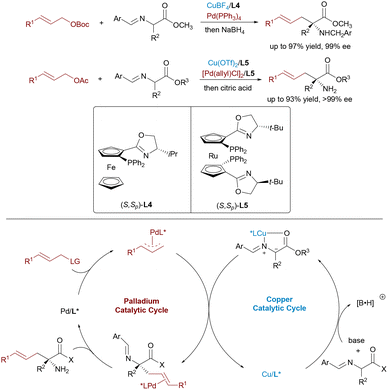
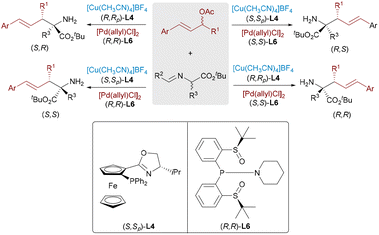
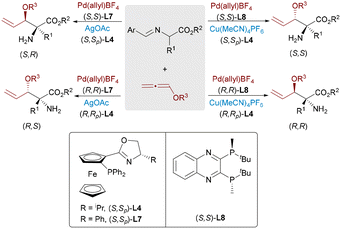
Optically active α,α-disubstituted α-amino acids (α,α-AAs) are key components of enzyme inhibitors and bioactive natural products. Nevertheless, the stereoselective synthesis of α,α-AAs remains challenging, particularly involving the construction of quaternary stereocenters. In 2017, Wang23 and Zhang24 independently reported dual catalytic systems featuring Pd and Cu complexes for the enantioselective allylic alkylation of aldimine esters (Scheme 12). In these systems, a chiral Cu(I)/Phosferrox complex facilitates the generation of nucleophilic azomethine ylides, while a Pd(0) catalyst activates allylic carbonates to form electrophilic π-allyl species. These synergistic systems afforded α-quaternary amino acids with excellent enantioselectivity and yield. Subsequently, Wang and Dang advanced a more sustainable variant by employing racemic allylic alcohols as electrophilic partners,25 using Et3B as a Lewis acid activator to promote the formation of π-allyl complexes. This strategy allowed direct access to α-AAs through regio- and stereodivergent Cu/Pd catalysis, thereby further expanding the synthetic utility of dual-metal systems in constructing densely functionalized chiral amine frameworks.
Dynamic kinetic asymmetric transformation (DyKAT)26 offers an efficient route for the conversion of racemic substrates into enantiomerically enriched products via simultaneous catalysis and racemization. In 2020, Zhang and Liao combined this strategy with Cu/Pd-catalyzed allylic alkylation of aldimine esters using racemic unsymmetrical 1,3-disubstituted allyl acetates.27 This approach delivered α,α-disubstituted α-amino acids bearing vicinal stereocenters with high enantioselectivities (up to >99% ee) and diastereoselectivities (up to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) (Scheme 13). Notably, all four stereoisomers of the products can be selectively obtained by independently tuning the chiral ligands on Pd and Cu, demonstrating exceptional stereodivergent potential of this bimetallic strategy.
1 dr) (Scheme 13). Notably, all four stereoisomers of the products can be selectively obtained by independently tuning the chiral ligands on Pd and Cu, demonstrating exceptional stereodivergent potential of this bimetallic strategy.
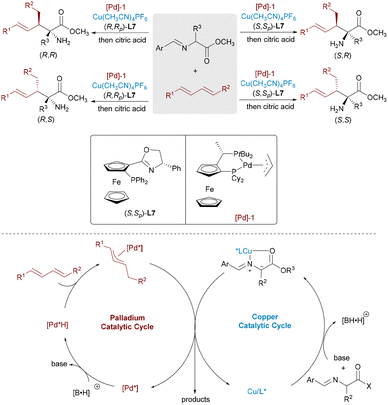
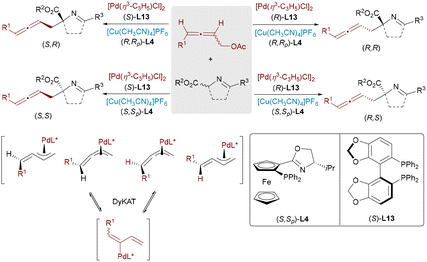
Transition-metal hydride (M-H) catalysts enable atom-economical C–C bond formation by adding to unsaturated hydrocarbons to generate π-allyl intermediates that subsequently couple with nucleophiles. Zi and co-workers developed a Pd/Cu system for the enantioselective coupling of 1,3-dienes with aldimine esters, forming amino acid derivatives bearing two adjacent stereocenters (Scheme 14).28 The Pd-H species inserts into the diene to form a π-allyl-Pd intermediate, while the Cu catalyst activates the nucleophile. Independent chiral control by the two metals allows selective access to all four stereoisomers. The He group applied this dual-metal strategy to 1,3-dienes and α-fluoroketones, achieving full stereocontrol over adjacent tertiary and quaternary fluorinated centers by fine-tuning ligand combinations.29 Zi and co-workers further demonstrated that alkoxyallenes could also undergo hydropalladation to furnish π-allyl intermediates, which coupled stereodivergently with aldimine esters under Pd/Cu or Pd/Ag catalysis (Scheme 15).30 All four β-hydroxy α-amino acid stereoisomers were obtained with excellent selectivity by adjusting the chirality of both catalysts. This approach not only facilitated access to complex chiral alcohols but also provided a flexible strategy for the stereodivergent synthesis of β-hydroxy α-amino acids and related bioactive scaffolds.
 | ||
| Scheme 15 Stereodivergent coupling of alkoxyallenes and aldimine esters by Pd/Cu and Pd/Ag catalysis. | ||
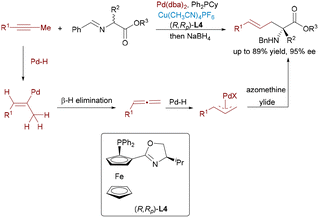
The Wang group described an innovative dual catalytic system that integrates chiral Cu(I) complexes with palladium nanoparticles to accomplish enantioselective allylic alkylation between alkynes and aldimine esters (Scheme 16).31 The Pd–H species, generated via protonation of Pd(0) followed by base-assisted deprotonation, undergoes hydropalladation of the alkyne to form a π-allyl-Pd intermediate. This species couples stereoselectively with the Cu-bound azomethine ylide, delivering the chiral product and regenerating both catalysts.
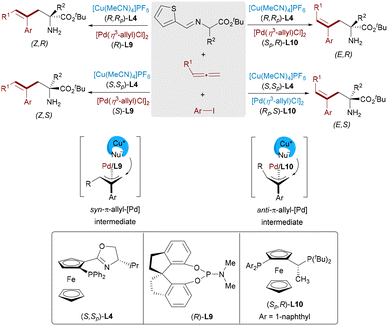
To simultaneously control both the stereochemistry at the chiral center and the geometry of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, Zhang and Ma designed a Pd/Cu bimetallic catalytic system for three-component coupling of aryl iodides, allenes, and aldimine esters (Scheme 17).32 By rational pairing of chiral ligands of two metal catalysts, all four stereoisomeric α-amino acids [(Z,R), (Z,S), (E,R), and (E,S)] showed outstanding selectivity. Mechanistic studies revealed that the Pd catalyst determines the E/Z configuration through selective formation of syn- or anti-π-allyl intermediates, whereas the Cu catalyst primarily governs the enantioselectivity. DFT calculations and experimental studies demonstrated that the E-selectivity arises from the thermodynamically favored anti-intermediate, whereas Z-isomers result from nucleophilic attack on the syn form.
C bond, Zhang and Ma designed a Pd/Cu bimetallic catalytic system for three-component coupling of aryl iodides, allenes, and aldimine esters (Scheme 17).32 By rational pairing of chiral ligands of two metal catalysts, all four stereoisomeric α-amino acids [(Z,R), (Z,S), (E,R), and (E,S)] showed outstanding selectivity. Mechanistic studies revealed that the Pd catalyst determines the E/Z configuration through selective formation of syn- or anti-π-allyl intermediates, whereas the Cu catalyst primarily governs the enantioselectivity. DFT calculations and experimental studies demonstrated that the E-selectivity arises from the thermodynamically favored anti-intermediate, whereas Z-isomers result from nucleophilic attack on the syn form.
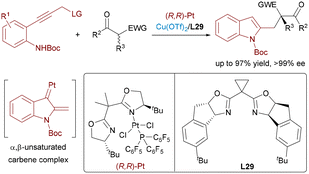
Conventional enantioselective allylic substitution reactions often rely on pre-activated electrophiles, necessitating additional synthetic manipulations. Feng and Liu overcame this limitation by developing a Pd/Co dual catalytic system for direct asymmetric allylic C–H alkylation of allylbenzenes and aliphatic olefins with β-ketoesters and oxindoles (Scheme 18, top).33 This approach combines achiral Pd(0) catalysis for C–H activation with a chiral N,N′-dioxide–Co(II) complex to achieve asymmetric induction. Notably, this design addresses the persistent challenges of limited chiral ligand availability and insufficient stereocontrol encountered in traditional Pd-catalyzed C–H alkylation. Under the optimized conditions, the protocol delivered a wide range of products in high yields (up to 99%) and excellent enantioselectivities (up to 99% ee), including biologically relevant compounds. Very recently, Feng, Wu and Liu developed a regiodivergent allylic C–H alkylation of allyl ethers with carbonyl nucleophiles, benzofuran-3(2H)-one, affording branched products with excellent regio- and stereoselectivity (Scheme 18, bottom).4b Feng and Liu also developed a solvent-controlled, enantioselective allylic C–H alkylation of 2,5-dihydrofuran via Pd(0)/Ni(II) synergistic catalysis. By tuning the reaction solvent, the authors could effectively modulate the regioselectivity and enantioinduction of the alkylation process.3p
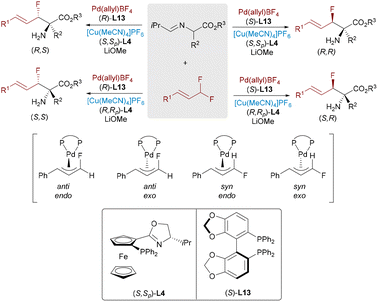
The incorporation of fluorinated stereocenters plays a crucial role in pharmaceutical and materials chemistry, as fluorine substitution is known to improve metabolic stability, bioavailability, and binding selectivity. In 2021, He and co-workers disclosed a Pd/Cu dual-catalytic system for the stereodivergent coupling of enynes and α-fluoroesters to access tertiary fluoride-tethered allenes bearing both central and axial chirality (Scheme 19).34 The protocol accommodates diverse substrates, including drug-derived enynes, and further allows for axial-to-central chirality transfer, leading to the formation of fluorinated hydrofuran scaffolds. Kinetic studies reveal time-dependent epimerization of the allene axis, while ligand permutations allow access to all four stereoisomers. This work significantly expands the realm of stereodivergent catalysis to include systems bearing both central and axial elements of chirality.
Fluorinated amino acids were achieved through the desymmetrization of geminal difluoromethylenes using an innovative Pd/Cu/Li ternary catalytic system (Scheme 20).35 The transformation involves initial C–F bond activation mediated by Pd and Li to generate π-allyl palladium intermediates, followed by a Pd/Cu-catalyzed allylic substitution with Schiff base-derived amino acid precursors. The fluorine atoms in styrene-substituted difluoroethylene exhibit minimal steric hindrance, leading to the formation of four structurally similar potential intermediates. The synergistic action of palladium and copper catalysts allows for their distinct recognition and selective transformation. In addition, DFT calculations reveal that F–Cu interactions play a pivotal role in controlling stereoselectivity. This strategy offers a significant advance in the field of stereodivergent C–F bond functionalization and opens new avenues for constructing fluorinated α-amino acid derivatives with high selectivity.
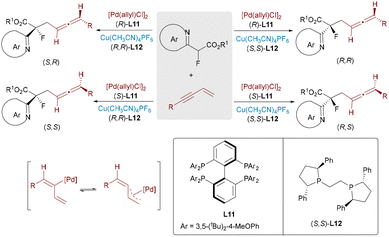
Allenes serve as versatile building blocks in organic synthesis due to their unique reactivity.36 The development of enantioselective routes to allene-bearing amino acids has become increasingly important for constructing chiral frameworks commonly encountered in natural products and pharmaceutical candidates. Wang, Ma, and Zhang developed a highly efficient Pd/Cu-catalyzed system for the stereoselective synthesis of allene-containing amino acid derivatives featuring central chirality.37 To further broaden substrate compatibility, Zhang and Ma later reported a stereodivergent allenylic alkylation strategy accommodating both aryl- and alkyl-substituted allenylic acetates (Scheme 21).38 By leveraging synergistic bimetallic catalysis, the dynamic kinetic asymmetric alkylation of racemized allyl ester was successfully achieved, establishing an effective approach to a challenging quaternary stereoscopic center with outstanding control (up to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and >99% ee). This success stems from the dynamic equilibrium between diastereomeric η3-butadienyl palladium intermediates, stabilized by strong Csp2–Pd bonding and weak π-coordination. The matched chiral environments provided by the Pd and Cu catalysts govern the stereochemical outcomes independently, allowing access to all four stereoisomeric products.
1 dr and >99% ee). This success stems from the dynamic equilibrium between diastereomeric η3-butadienyl palladium intermediates, stabilized by strong Csp2–Pd bonding and weak π-coordination. The matched chiral environments provided by the Pd and Cu catalysts govern the stereochemical outcomes independently, allowing access to all four stereoisomeric products.
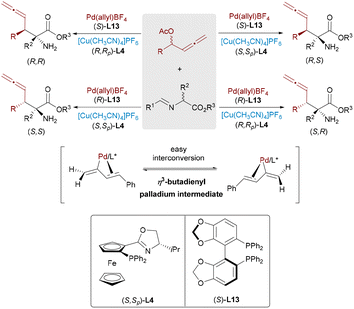
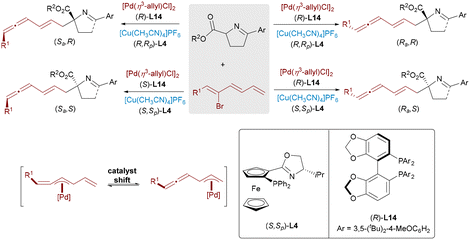
While methods for constructing single or adjacent stereocenters are well-established, the concurrent formation of nonadjacent stereocenters, particularly in acyclic systems, remains a formidable challenge. In 2021, Zhang and Ma presented a stereodivergent Pd/Cu-catalyzed allenylation strategy for synthesizing molecules bearing 1,3-nonadjacent axial and central chirality (Scheme 22).39 The catalytically active Pd(0) species, generated in situ from [Pd(η3-allyl)Cl]2, undergoes oxidative addition with racemic allenylic esters to form four diastereomeric α-alkynylidene π-allylpalladium intermediates. These intermediates rapidly interconvert via σ-(1,3-dien-2-yl)palladium species, establishing a dynamic equilibrium that allows for efficient kinetic resolution. The copper-activated azomethine ylide then couples with the π-allyl Pd complex in a stereoselective manner, delivering the chiral allene products with excellent enantio- and diastereoselectivities.
 | ||
| Scheme 22 Pd/Cu-catalyzed construction of 1,3-nonadjacent stereocenters with axial and central chirality. | ||
Building on their previous work, Zhang and Ma advanced a Pd/Cu synergistic catalytic system to tackle the challenging construction of 1,5-nonadjacent stereocenters—a field lacking established models for remote stereocontrol (Scheme 23).40 By employing a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond relay strategy, the authors achieved divergent synthesis of all four stereoisomers featuring both allenyl axial and central chirality. The process involves regioselective oxidative addition of Pd(0) to the terminal double bond of a triene substrate, forming a terminal η3-allyl palladium intermediate that undergoes a π-system migration to generate a less stable internal η3-allyl species. Nucleophilic attack at the terminal position then yields the desired products with high stereocontrol.
C bond relay strategy, the authors achieved divergent synthesis of all four stereoisomers featuring both allenyl axial and central chirality. The process involves regioselective oxidative addition of Pd(0) to the terminal double bond of a triene substrate, forming a terminal η3-allyl palladium intermediate that undergoes a π-system migration to generate a less stable internal η3-allyl species. Nucleophilic attack at the terminal position then yields the desired products with high stereocontrol.
The stereodivergent construction of remote 1,5 and 1,7-nonadjacent stereocenters remains a significant challenge, despite their prevalence in bioactive molecules. In 2024, Zi and colleagues developed a Pd/Cu-catalyzed 1,4-difunctionalization of 1,3-dienes via a Heck/Tsuji–Trost cascade, establishing a robust platform for constructing 1,5- and 1,7-nonadjacent stereocenters with excellent diastereoselectivities and enantioselectivities (Scheme 24, top).41 In the same year, Zhang and Huo introduced a dual Pd/Cu-catalyzed asymmetric Heck cascade process that furnished all four stereoisomers of compounds bearing two remote tetrasubstituted stereocenters (Scheme 24, bottom).42 These advances highlight the growing power of dual catalytic systems in addressing long-range stereochemical induction and accessing densely functionalized scaffolds.
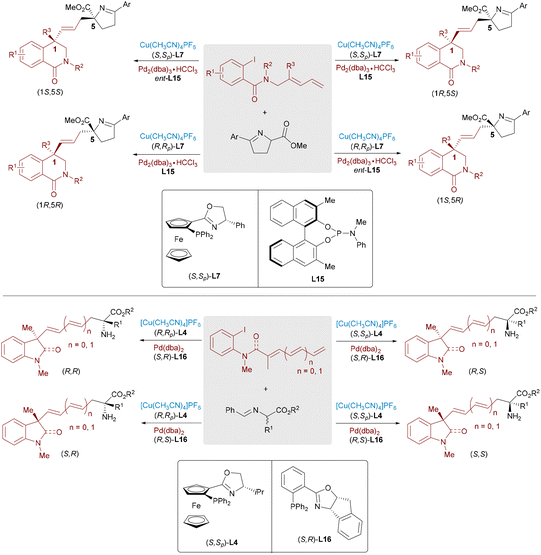
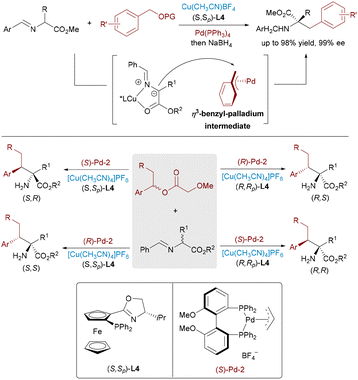
Significant advances have been achieved in metal-catalyzed asymmetric allylic substitutions via π-allyl-Pd intermediates, yet analogous transformations employing isoelectronic π-benzyl-Pd species remain underdeveloped,43 primarily due to unfavorable loss of aromaticity during intermediate formation. In 2022, a synergistic system merging chiral Lewis acids with achiral Pd catalysts enabled the asymmetric benzylation of imine esters, delivering α-benzylated α-amino acids via azomethine ylide addition to a π-benzyl-Pd complex (Scheme 25, top).44 Key to this success was the judicious choice of leaving groups (e.g., carbonates and phosphates) to stabilize reactive intermediates. In 2024, Zhang and Huo published pioneering work on the Pd/Cu dual-catalyzed stereodivergent benzylic substitution of racemic secondary benzyl esters (Scheme 25, bottom).45 Their strategy allowed the simultaneous construction of adjacent stereocenters, providing access to all four stereoisomeric α-benzylated amino acids with excellent diastereo- and enantioselectivity.
3.2. Ir/M catalytic system
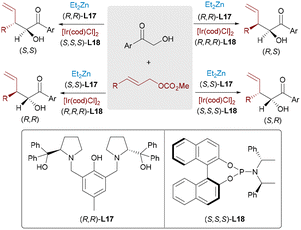
Transition metal-catalyzed asymmetric allylation is a significant transformation in organic synthesis. While palladium-based systems generally favor linear selectivity, iridium catalysts are known to promote the formation of branched products. In 2016, Hartwig and colleagues designed an Ir-catalyzed method for diastereo- and enantioselective allylic substitutions with acyclic α-alkoxy ketones (Scheme 26).46 In this transformation, chelation by a copper(I) cation governs the geometry of the unstabilized enolate in acyclic ketones. This method exhibited extensive substrate compatibility and features high yield and excellent stereoselectivity, albeit limited to a single stereoisomer. In the same year, the Zhang group first reported an Ir/Zn dual-catalytic system for the stereodivergent α-allylation of unprotected α-hydroxyketones (Scheme 27).47 The bidentate coordination of the zinc complex to both the carbonyl and hydroxyl groups rigidly locks the enolate geometry in a Z-configuration. This structural definition allows precise facial selectivity during nucleophilic attack on the Ir–π-allyl intermediate, delivering all four stereoisomers of the α-hydroxyl-γ,δ-unsaturated ketone with high enantioselectivity (up to >99% ee) and diastereoselectivity (up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).
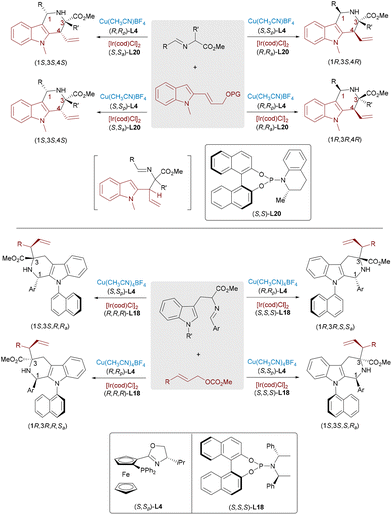
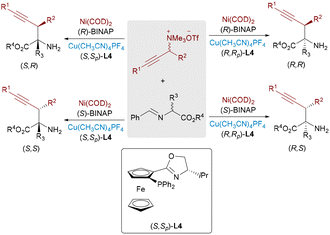
In 2018, the Zhang and Wang group independently reported significant advances in Ir/Cu bimetallic-catalyzed allylation reactions with aldimine esters (Scheme 28).48 By employing specific combinations of two chiral catalysts, all stereoisomers can be systematically obtained from the same starting materials while maintaining consistent reaction conditions. While mechanistically analogous to the established Pd/Cu-catalyzed allylic alkylation of aldimine esters, the present catalytic system demonstrates a distinct product profile by selectively generating branched-chain α,α-disubstituted α-amino acids.
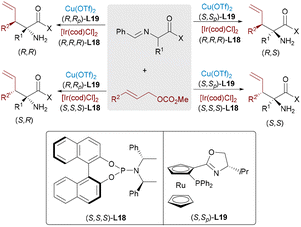
Due to the high nucleophilicity and basicity of free amines, protective group strategies are commonly employed in synthetic transformations to prevent catalyst deactivation or undesired side reactions. Challenging this convention, Zhang, Sun and Huo recently achieved the stereodivergent α-allylation reaction of unprotected primary amines using a ternary catalytic system involving aldehyde, copper, and iridium catalysts (Scheme 29).49 A catalytic amount of aldehyde was used to reversibly form an imine intermediate with the α-amino chromanone substrate, which was then activated by a chiral Cu complex to generate a nucleophilic azomethine ylide. This transient species underwent regio- and stereoselective addition to an Ir–π-allyl intermediate, affording α-allylated products bearing two adjacent stereocenters. The process efficiently furnished all four possible stereoisomers from the same starting material through ligand-controlled stereodivergence, without requiring amine protection.
In 2019, Hartwig et al. reported a catalytic Ir/Cu system for the stereodivergent construction of tertiary fluorides bearing vicinal stereocenters (Scheme 30),50 addressing a key challenge in the synthesis of fluorinated molecules for drug discovery. This strategy enabled independent control over both stereocenters to access all four isomers. More recently, the team successfully extended this catalytic strategy to couple two monofluorinated building blocks, achieving stereoselective synthesis of acyclic vicinal difluoride stereoisomers.51 These studies provide a versatile platform for exploring bioactive molecules containing challenging fluorinated stereochemical motifs. Hartwig and coworkers also successfully accomplished the stereodivergent allylic substitution of azaaryl acetamides and acetates using an Ir/Cu dual catalytic system.52
Tetrahydro-γ-carboline frameworks constitute a privileged class of indole alkaloids, extensively recognized for their prevalence as key structural elements in biologically active compounds and therapeutic agents. The Wang group developed a synergistic Ir/Cu-catalyzed asymmetric cascade reaction that combines allylic substitution with iso-Pictet–Spengler cyclization to construct tetrahydro-γ-carbolines bearing multiple stereocenters in a single catalytic process (Scheme 31, top).53 The Ir catalyst forms electrophilic π-allyl intermediates from indole-allyl carbonates, which couple with Cu-activated azomethine ylides, followed by stereospecific intramolecular cyclization. Similarly, tetrahydro-β-carbolines with up to four stereocenters, including a C–N axial unit, were accessed via Ir/Cu-catalyzed allylation and Brønsted acid-mediated Pictet–Spengler cyclization under mild conditions (Scheme 31, bottom).54
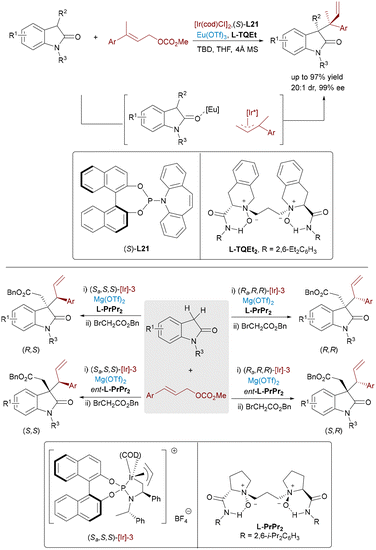
Enantiopure 3,3-disubstituted oxindoles represent privileged structural motifs that are widely distributed in bioactive natural products and therapeutic agents, driving significant interest in developing stereocontrolled synthetic methods. In 2021, Zhang et al. accomplished the asymmetric allylic alkylation of 3-substituted oxindoles through Ir/Cu dual catalysis,55 successfully constructing adjacent quaternary and tertiary stereocenters with high regioselectivity, enantioselectivity, and diastereoselectivity. The following year, Feng and co-workers developed an Ir/Eu dual catalytic system for the allylation of trisubstituted electrophiles and N-alkyl-substituted oxindoles (Scheme 32, top), achieving up to 99% ee and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
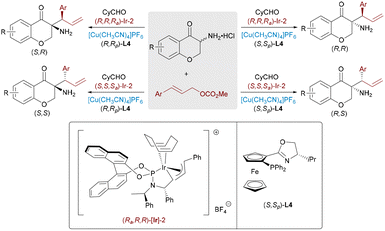
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr even with sterically demanding substrates.56 Notably, the chiral Eu complex enhances reactivity and stereocontrol, though diastereodivergence via ligand modulation remains elusive. To access all four stereoisomers of 3,3′-disubstituted oxindoles bearing contiguous stereocenters, Feng and co-workers devised a sequential Ir/Mg-catalyzed three-component cascade involving allylic alkylation followed by diastereoconvergent nucleophilic alkylation (Scheme 32, bottom).57 The chiral iridium complex generated an Ir–π-allyl intermediate from the allyl carbonate, which undergoes enantioselective allylation at the oxindole's C3 position. The resulting product then tautomerizes rapidly, allowing the chiral magnesium catalyst to coordinate the enolate and mediate diastereoconvergent C3′-alkylation with the electrophile.
1 dr even with sterically demanding substrates.56 Notably, the chiral Eu complex enhances reactivity and stereocontrol, though diastereodivergence via ligand modulation remains elusive. To access all four stereoisomers of 3,3′-disubstituted oxindoles bearing contiguous stereocenters, Feng and co-workers devised a sequential Ir/Mg-catalyzed three-component cascade involving allylic alkylation followed by diastereoconvergent nucleophilic alkylation (Scheme 32, bottom).57 The chiral iridium complex generated an Ir–π-allyl intermediate from the allyl carbonate, which undergoes enantioselective allylation at the oxindole's C3 position. The resulting product then tautomerizes rapidly, allowing the chiral magnesium catalyst to coordinate the enolate and mediate diastereoconvergent C3′-alkylation with the electrophile.
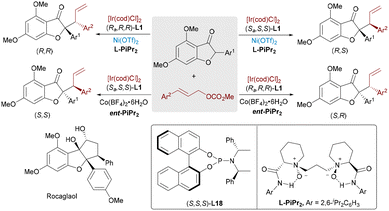
Flavaglines, a class of natural products bearing a densely functionalized cyclopenta[b]benzofuran core, display broad pharmacological activities, including anticancer, anti-inflammatory, and antiviral effects. In 2022, Feng and Liu demonstrated a synergistic dual-metal-catalyzed asymmetric allylic alkylation of benzofuran-3(2H)-one as a key step for the stereodivergent total synthesis of rocaglaol (Scheme 33).4a By combining a chiral N,N′-dioxide-Co or Ni Lewis acid with a chiral phosphoramidite/Ir(I) complex, all four stereoisomers of the α-allylated products were accessed with high diastereoselectivity and excellent enantiocontrol (up to >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 99% ee).
1 dr and 99% ee).
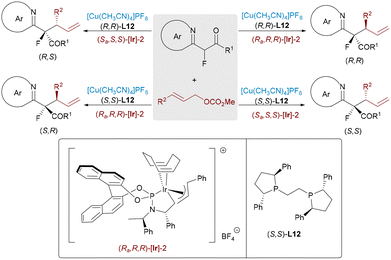
2-Acyl imidazoles have emerged as robust alternatives to traditional esters and amides in enantioselective catalysis due to their enhanced stability and reactivity. Their α-C–H bonds can be readily deprotonated under mild conditions, facilitated by chelation of the imidazole nitrogen and carbonyl oxygen to metal centers. Guo and co-workers developed a stereodivergent Ir/Ni dual-catalyzed allylic alkylation of 2-acylimidazoles (Scheme 34), affording α-allylated tertiary carbonyls with excellent regio-, diastereo-, and enantioselectivity.58 By tuning catalyst chirality and allylic leaving groups, all four stereoisomers could be selectively accessed.
Spiroketals are essential frameworks in numerous bioactive natural products and medicinal compounds, prompting continued interest in enantioselective strategies for their synthesis. In 2022, Deng and Yang depicted a novel Ir/Au dual-catalytic system for the enantioselective synthesis of spiroketals and spiroaminals through a cascade reaction between racemic 2-(1-hydroxyallyl)phenols and alkynols/alkynamides (Scheme 35).59 Au-catalyzed cycloisomerization forms reactive vinyl ether or enamide intermediates, which undergo enantioselective coupling with Ir–π-allyl species, followed by intramolecular spirocyclization via a hydrogen-bond-stabilized Zimmerman–Traxler transition state, furnishing spirocyclic products with excellent stereocontrol.
3.3. Ni/M catalytic system
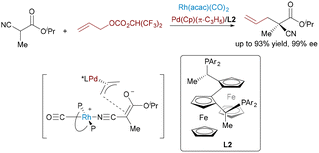
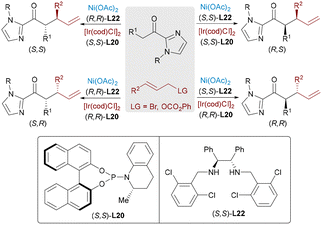
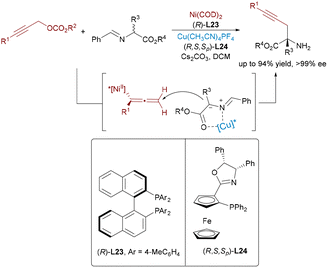
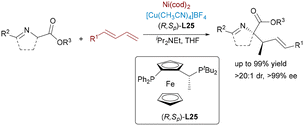
Nickel catalysis60 offers distinct advantages over other transition metals, notably its low electronegativity, flexible oxidation states (Ni0 to Ni4+), and propensity for single-electron pathways. These features enable nickel to excel in challenging transformations such as reductive couplings, cycloisomerizations, and C–O/C–N bond activations. Beyond its economic appeal as an earth-abundant and cost-efficient catalyst, nickel's true strength lies in its capacity to activate otherwise inert substrates and to mediate mechanistically unconventional pathways. Recent advances in Ni-based bimetallic systems have significantly expanded the scope of synergistic catalysis, enabling new modes of bond activation and selectivity control.In contrast to the well-developed asymmetric allylation, asymmetric propargylation61 remains significantly underexplored, due to the challenge of controlling multiple reactive sites. A novel Ni/Cu dual-catalyzed asymmetric propargylic alkylation of aldimine esters with propargylic carbonates bearing internal alkyne groups was described by the Guo group62 in 2020 (Scheme 36). A Ni(0) catalyst initially mediates the oxidative addition and decarboxylation of the propargylic carbonate substrate, generating an electrophilic allenylnickel(II) intermediate. The product was obtained by the nucleophilic attack of azomethine ylide species on the nickel-stable alkenyl intermediate. This bimetallic cooperation enables precise control over the regio- and enantioselective C–C bond formation, elegantly overcoming the inherent challenges of controlling multiple selectivities in propargylic transformations through its dual activation strategy. Exploiting the key benefits of this approach, the same group developed a dynamic kinetic asymmetric transformation of racemic propargylic ammonium salts (Scheme 37),63 granting access to all four stereoisomers of α-quaternary amino esters with excellent yields and stereoselectivity across diverse substrates.
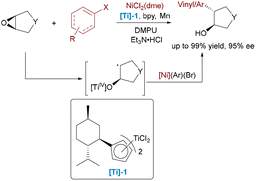
While epoxide ring-opening reactions have been well-established in organic synthesis, previous approaches struggled with enantioselective C–C bond formation using aryl nucleophiles, typically requiring harsh conditions or showing limited functional group tolerance. Weix and co-workers developed a Ni/Ti dual-catalytic system for enantioselective cross-coupling between aryl halides and epoxides, achieving trans-β-arylcycloalkanols in 57–99% yields and 78–95% ee under mild reductive conditions (Scheme 38).64 The chiral titanocene catalyst mediates the reductive ring-opening of the epoxide substrate, generating a β-titanoxy alkyl radical intermediate which subsequently couples with the aryl-nickel(II) species. Reductive elimination then delivers the enantioenriched product.
Asymmetric hydrofunctionalization of 1,3-dienes has been extensively studied with palladium and rhodium catalysts, while nickel-based systems remain less explored despite their promise in constructing chiral allylic products with vicinal stereocenters. A notable advance was achieved through a Ni/Cu dual catalytic system that enables the enantioselective coupling of 1,3-dienes with aldimine esters,65 furnishing a wide array of amino acid derivatives with high diastereo- and enantioselectivity (Scheme 39). The methodology demonstrated broad applicability across both cyclic and acyclic nucleophiles as well as structurally diverse diene substrates.
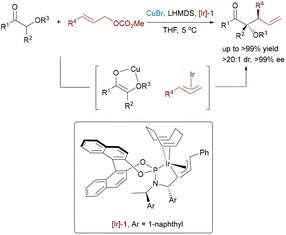
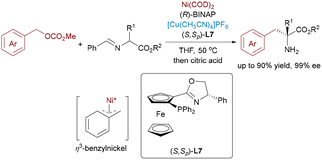
Despite the widespread application of η3-allylpalladium complexes in asymmetric transformations, their isoelectronic η3-benzylnickel counterparts remain significantly underdeveloped, primarily limited by the higher energy barrier associated with oxidative addition and dearomatization processes. In 2022, Zhang and coworkers achieved a breakthrough in asymmetric catalysis by developing a Ni/Cu bimetallic system for the benzylation of aldimine esters,66 constructing challenging α-quaternary amino acid derivatives with excellent enantioselectivity (Scheme 40). The transformation relies on a highly electrophilic η3-benzylnickel intermediate, which promotes efficient coupling under base-free conditions.
3.4. Ru/M catalytic system
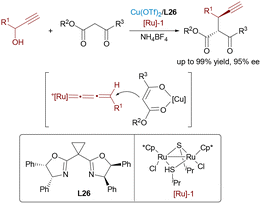
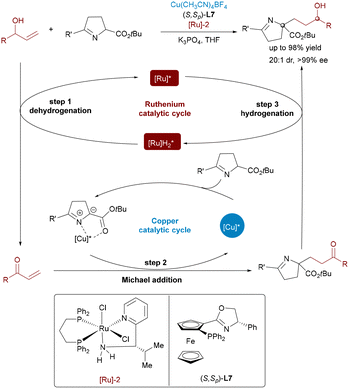
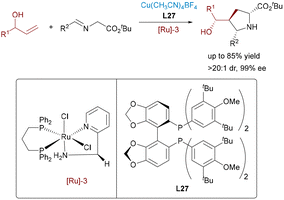
In 2012, Nishibayashi and colleagues completed a dual transition-metal catalytic approach using ruthenium and copper complexes for the enantioselective propargylic substitution of β-ketoesters with propargylic alcohols (Scheme 41).67 The ruthenium catalyst facilitates the formation of an electrophilic allenylidene intermediate from the propargylic alcohol, while the chiral copper complex activates the β-ketoester to generate a nucleophilic enolate. The synergistic activation enables highly enantioselective C–C bond formation, delivering propargylic substitution products with excellent yields and stereoselectivity. This work stands as an early and influential example of synergistic Ru/Cu catalysis via allenylidene intermediates.Borrowing-hydrogen catalysis provides an atom-economical approach by directly transforming simple alcohols into value-added compounds, circumventing the need for prefunctionalized reagents and minimizing byproduct formation.68 In 2022, Wang and co-workers presented an efficient approach to constructing 1,4-nonadjacent stereocenters through a synergistic Ru/Cu relay catalytic system, achieving comprehensive stereochemical control to access all four possible stereoisomers with outstanding enantioselectivity and diastereoselectivity (Scheme 42).69 Initially, the ruthenium catalyst dehydrogenates the alcohol substrate to form a reactive α,β-unsaturated carbonyl intermediate and metal-hydride species. This intermediate then undergoes an asymmetric Michael addition with a nucleophile metallated azomethine ylide, where a chiral copper catalyst controls the stereochemistry at this stage. The resulting enolate intermediate is subsequently reduced by the in situ formed ruthenium hydride, which exerts stereocontrol at the second chiral center. This dual catalysis provides an efficient route to densely functionalized δ-hydroxyesters from racemic allylic alcohols and ketimine esters, thereby expanding the utility of borrowing-hydrogen strategies in stereodivergent synthesis. In 2025, Wang and Dong developed a Cu/Ru relay catalytic system for the asymmetric synthesis of δ-hydroxy α-amino acids with two adjacent stereocenters from inert allylic alcohols and ketoimine esters, achieving high yields and stereoselectivity through a borrowing-hydrogen and Michael addition cascade.68f
The asymmetric 1,3-dipolar cycloaddition of inert allylic alcohols presents significant challenges, primarily due to their inherently low reactivity and the necessity for pre-activation. The Wang group addressed this challenge with a Ru/Cu relay-catalyzed 1,3-dipolar cycloaddition of inert allylic alcohols with aldimine esters (Scheme 43).70 The Ru catalyst oxidizes the alcohol to a transient α,β-unsaturated carbonyl intermediate, suppressing side reactions, while the Cu catalyst promotes asymmetric 1,3-dipolar cycloaddition with azomethine ylides, forming a chiral pyrrolidine framework with excellent diastereo- and enantioselectivity.
A key limitation of the asymmetric alkylation of β-keto esters is the difficulty in achieving high enantiopurity for mono-alkylated products, as the products readily racemize via keto–enol tautomerization under basic or acidic conditions. Kitamura and Tanaka developed a Ru/Pd catalyzed asymmetric allylation of unactivated β-keto esters with allyl alcohol dehydration, giving all possible diastereomers with high regio- and stereoselectivity (Scheme 44).71 The synergistic catalysis operates through a unique mechanism where the palladium complex generates an enolate while simultaneously producing a Brønsted acid that activates the ruthenium catalyst, creating a self-sustaining cycle that maintains nearly neutral conditions to prevent racemization. This synergistic catalytic system offers a compelling solution for enantioselective α-alkylation of β-keto esters,72 a fundamental transformation long hindered by the lability of the resulting stereocenters.
3.5. Rh/M catalytic system
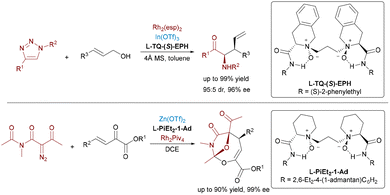
Over the past few decades, Rh-catalyzed transformations involving metal carbenes generated from diazo precursors have emerged as a powerful synthetic strategy, enabling diverse C–C and C–X bond formations through cyclopropanation, C–H insertion, and cycloaddition reactions.73 The Feng group has made remarkable advancements in developing N,N′-dioxide-metal complexes as efficient chiral Lewis acid catalysts.74 More recently, they have pioneered the innovative integration of Lewis acid catalysis with Rh-catalyzed carbene transfer processes, successfully establishing a series of unprecedented asymmetric transformations.Feng and colleagues pioneered a bimetallic Rh(II)/In(III) relay catalysis, achieving tandem insertion and asymmetric Claisen rearrangement of N-sulfonyl-1,2,3-triazoles with allyl alcohol esters (Scheme 45, top).75 The action between achiral Rh2(esp)2 and chiral N,N′-dioxide-In(III) complexes afforded β,γ-amino acid derivatives in excellent yields and with outstanding stereoselectivities. In addition, Feng et al. developed a Rh(II)/Zn(II) relay catalytic system to facilitate asymmetric [4+3] cycloadditions between β,γ-unsaturated α-ketoesters and diazoimides,76 enabling the synthesis of oxa-bridged oxazocines with high enantioselectivities (Scheme 45, bottom).
3.6. M/M catalytic system
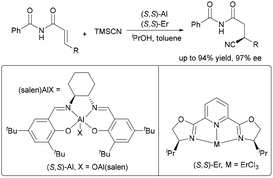
In 2004, Jacobsen and co-workers introduced a groundbreaking dual-metal catalytic system that integrates two distinct chiral metal complexes to accomplish highly enantioselective conjugate cyanation of α,β-unsaturated imides (Scheme 46).6 In this reaction system, a (salen)Al complex activates the unsaturated imide substrate, while a (pybox)lanthanide catalyst independently enhances the nucleophilicity of cyanide. This dual activation strategy significantly improved reaction efficiency and stereocontrol, delivering cyanide adducts in 80–94% yields and up to 97% ee.Despite considerable advances in the asymmetric propargyl substitution reaction, the development of copper-catalyzed variants remains underdeveloped. Niu and Zhang completed a highly stereoselective propargylation reaction of 5H-thiazol-4-ones and 5H-oxazol-4-ones using dual metal catalytic systems (Cu/Zn and Cu/Ti) (Scheme 47).77 Chiral copper–allenyl intermediates undergo stereocontrolled coupling with nucleophilic heterocycles, while distinct chiral ligands on each metal precisely guide the formation of vicinal stereocenters, affording products with excellent enantio- and diastereoselectivity.
Substituted indoles remain privileged structures in medicinal chemistry and synthesis, featuring prominently in bioactive compounds and serving as versatile synthetic intermediates. Platinum catalysis has emerged as a powerful tool for the efficient construction of indole scaffolds. In 2023, Guo et al. designed an efficient Pt/Cu dual-catalytic system for the asymmetric vinylogous addition reaction (Scheme 48),78 furnishing functionalized indoles with high enantioselectivity. The platinum center activates propargylic ether substrates through electrophilic alkyne coordination, generating α,β-unsaturated carbene intermediates. Meanwhile, the chiral copper complex coordinates with nucleophilic β-dicarbonyl compounds to form enolate species. The stereoselective nucleophilic addition to the platinum-carbene intermediate governs the formation of enantioenriched products.
4. Dual transition-metal catalysis
The strategic integration of two distinct transition-metal catalysts within a single reaction system represents a transformative approach for streamlining multistep transformations into efficient one-pot processes. This synergistic dual transition-metal catalysis leverages the complementary reactivity profiles of different metals to orchestrate complex reaction sequences, often enabling bond formations and functional group manipulations that are challenging or inaccessible with single-metal systems. By carefully pairing metals with orthogonal or sequentially compatible catalytic cycles—such as combining a metal adept at cross-coupling (e.g., Pd and Ir) with one excelling in redox processes (e.g., Cu and Rh)—researchers can achieve intricate cascade reactions with high levels of chemo-, regio-, and enantiocontrol. A critical aspect lies in ensuring compatibility between the metal centers and their ligands to prevent deleterious interactions like catalyst deactivation via transmetallation or redox quenching. Overcoming these challenges through judicious catalyst design unlocks the potential for novel disconnections and significantly enhances synthetic efficiency, making dual transition-metal catalysis a rapidly evolving frontier for developing powerful and atom-economical methodologies in asymmetric synthesis.Building upon the foundational principles of dual transition-metal catalysis, early advancements such as the Rh/Pd-catalyzed domino alkyne arylation/C–N cyclization reported by Lautens and coworkers in 2011 demonstrated the feasibility of combining orthogonal catalytic cycles for achiral dihydroquinoline synthesis (Scheme 49, eqn (1)).79a However, a pivotal breakthrough in achieving high enantioselectivity within such bimetallic systems emerged in 2014 with the development of an enantioselective variant (eqn (2)).79b This seminal work elegantly addressed the critical challenges inherent to its predecessor—specifically, the need for stereocontrol—by introducing a chiral diene ligand for the rhodium-catalyzed asymmetric conjugate arylation step. This chiral Rh complex was then paired with a palladium/XPhos system responsible for the subsequent intramolecular C–N coupling. The resulting sophisticated “two-catalyst-two-ligand” strategy achieved exceptional enantioselectivity (up to 95% ee) and high yields (93%) in the synthesis of dihydroquinolinones, exemplifying the power of ligand orthogonality and kinetic resolution within a dual-metal framework. Crucial to this success was the meticulous design ensuring compatibility: the selective coordination of the diene ligand to Rh enabled precise stereochemical induction without disrupting the Pd catalytic cycle, while the robust XPhos/Pd interaction effectively suppressed ligand crossover. Furthermore, the temporal separation of catalytic steps—rapid Rh-mediated arylation followed by slower Pd-driven cyclization—minimized interference from competing pathways, such as undesired Suzuki coupling involving the arylboronic acid and aryl bromide components. This landmark study provided a modular blueprint for designing effective dual transition-metal systems that integrate chiral and achiral ligands selectively.
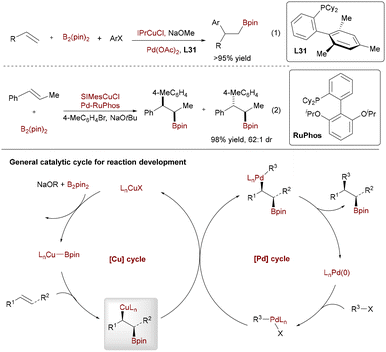
The Cu/Pd-catalyzed arylboration of alkenes has become a powerful strategy for constructing valuable organoboron compounds, enabling the simultaneous formation of C–C and C–B bonds.80a This approach leverages the complementary reactivity of Cu and Pd catalysts through transmetalation, where a key alkylcopper intermediate generated in the Cu cycle is transferred to the Pd cycle for further functionalization. Below, we outline the evolution of this methodology, from its inception to recent advances in asymmetric and complex substrate applications.
The foundation for Pd/Cu synergistic arylboration was established by the seminal, independent reports from Nakao and Brown in 2014, which demonstrated the arylboration of vinylarenes using aryl halides and B2(pin)2 (Scheme 50, eqn (1)).80b In this pioneering system, copper catalysis activates B2(pin)2 to generate a reactive NHC-Cu–Bpin species (NHC = N-heterocyclic carbene), enabling syn-borylcupration across the vinylarene to form a stable β-borylbenzylcopper intermediate. Palladium catalysis then facilitates oxidative addition of the aryl halide, transmetalation with the Cu-bound alkyl group, and reductive elimination to yield 1,2-diarylethylboronates with good regioselectivity and functional group tolerance. This breakthrough highlighted the potential of integrating Cu-mediated borylation with Pd-catalyzed cross-coupling in a dual transition-metal system.
Building on this foundation, Brown and coworkers significantly expanded the scope and control by developing a diastereodivergent arylboration strategy for 1,2-disubstituted alkenyl arenes (eqn (2)).80c Critically, by tuning the palladium catalyst system, they achieved stereoselective transmetalation from the stereodefined alkylcopper intermediate, allowing controlled access to either the syn- or anti-diastereomer of the 1,2-diaryl-1-borylalkane product with high diastereoselectivity.
Extending their diastereodivergent approach for racemic substrates, Brown and coworkers achieved a significant breakthrough by developing the enantioselective arylboration of internal alkenes (Scheme 51).80d This key advance employed a specifically designed chiral NHC-Cu catalyst, enabling the asymmetric synthesis of valuable 1,1-diarylalkanes bearing a synthetically versatile boronic ester group from substrates like cis-β-methyl styrene. When complexed with copper, this architecture created a well-defined chiral pocket essential for achieving high enantiocontrol during the critical syn-borylcupration step. When combined with Pd/RuPhos, it delivered products with excellent stereoselectivity. High diastereomeric ratios exceeding 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and enantiomeric ratios up to 96
1 and enantiomeric ratios up to 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were achieved across a diverse range of internal alkenylarenes, including challenging cyclic substrates such as indene and 1,2-dihydronaphthalene. The synthetic power of this method was further underscored by its application in a concise asymmetric synthesis of the biologically relevant glucocorticoid receptor partial agonist, where the boronic ester handle in the enantioenriched intermediate was leveraged for downstream transformations.
4 were achieved across a diverse range of internal alkenylarenes, including challenging cyclic substrates such as indene and 1,2-dihydronaphthalene. The synthetic power of this method was further underscored by its application in a concise asymmetric synthesis of the biologically relevant glucocorticoid receptor partial agonist, where the boronic ester handle in the enantioenriched intermediate was leveraged for downstream transformations.
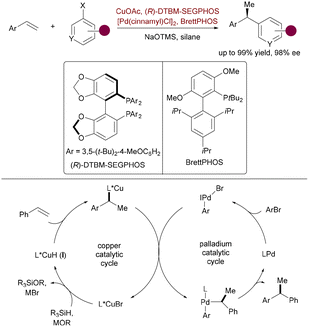
Further advancing the field, Pd/Cu synergistic catalysis was extended to the asymmetric functionalization of 1,3-enynes, achieving efficient synthesis of multisubstituted axially chiral allenes. Liao and coworkers reported a 1,4-arylboration strategy wherein Cu–Bpin addition to enynes generated chiral allenyl–Cu intermediates, which underwent PdCl2(dppf)-mediated transmetalation with aryl iodides to afford trisubstituted allenes with up to 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
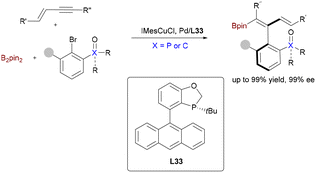
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er (Scheme 52).81a Central to this breakthrough was the design of a chiral sulfoxide phosphine (SOP) ligand, which, when paired with Cu(I), demonstrated exceptional stereocontrol in the catalytic cycle. This approach was generalized to 2-substituted enynes, yielding tetrasubstituted allenes—challenging yet valuable axially chiral scaffolds. Building on this foundation, Song and coworkers recently leveraged a related Cu/Pd-cocatalyzed alkynylboration strategy for the enantioselective synthesis of axially chiral 1,3-dienylboronates (Scheme 53).81b Key to this advancement was the implementation of TangPhos ligands L33, which facilitated chemo-, regio-, stereo-, and atroposelective arylboration of 1,3-enynes with B2pin2 and aryl bromides under mild conditions. This methodology significantly expands the utility of bimetallic catalysis to structurally intricate, functionally enriched unsaturated systems, delivering products with exceptional enantioselectivity (>99.5
3 er (Scheme 52).81a Central to this breakthrough was the design of a chiral sulfoxide phosphine (SOP) ligand, which, when paired with Cu(I), demonstrated exceptional stereocontrol in the catalytic cycle. This approach was generalized to 2-substituted enynes, yielding tetrasubstituted allenes—challenging yet valuable axially chiral scaffolds. Building on this foundation, Song and coworkers recently leveraged a related Cu/Pd-cocatalyzed alkynylboration strategy for the enantioselective synthesis of axially chiral 1,3-dienylboronates (Scheme 53).81b Key to this advancement was the implementation of TangPhos ligands L33, which facilitated chemo-, regio-, stereo-, and atroposelective arylboration of 1,3-enynes with B2pin2 and aryl bromides under mild conditions. This methodology significantly expands the utility of bimetallic catalysis to structurally intricate, functionally enriched unsaturated systems, delivering products with exceptional enantioselectivity (>99.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 er).
0.5 er).
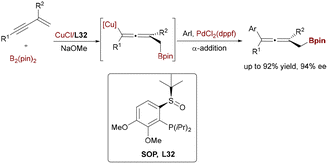 | ||
| Scheme 52 Cu/Pd-catalyzed enantioselective arylboration of 1,3-enynes for the synthesis of axially chiral allenes. | ||
 | ||
| Scheme 53 Cu/Pd-catalyzed enantioselective arylboration of 1-substituted enynes for the synthesis of axially chiral 1,3-dienylboronates. | ||
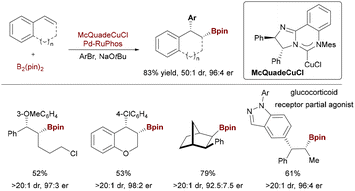
Expanding beyond enynes, Brown and coworkers demonstrated Cu/Pd-catalyzed arylboration of 1-silyl-1,3-cyclohexadienes (Scheme 54).82 Using a sterically demanding Mes-Pyridylidene ligand, this method achieved high trans-diastereoselectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) for polyfunctional cyclohexanes. While predominantly racemic, a single enantioselective variant (94
1 dr) for polyfunctional cyclohexanes. While predominantly racemic, a single enantioselective variant (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er) was realized with McQuadeCuCl, showcasing modular access to stereodefined alicyclic architectures. Copper/palladium synergistic catalysis enables enantioselective arylboration across diverse alkene substrates—including styrenes, internal alkenes, enynes, and dienes—delivering multifunctional chiral organoboron building blocks with high stereocontrol.
6 er) was realized with McQuadeCuCl, showcasing modular access to stereodefined alicyclic architectures. Copper/palladium synergistic catalysis enables enantioselective arylboration across diverse alkene substrates—including styrenes, internal alkenes, enynes, and dienes—delivering multifunctional chiral organoboron building blocks with high stereocontrol.
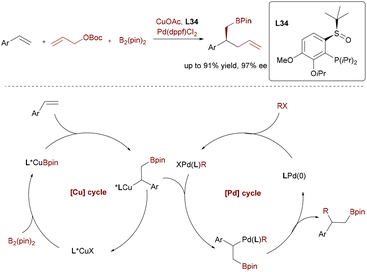
In a strategic departure from aryl electrophiles, Liao and colleagues pioneered Cu/Pd-catalyzed allylboration of styrenes using allyl carbonates (Scheme 55).83 This transformation leverages a chiral sulfoxide-phosphine L34 with Pd(dppf)Cl2 to achieve high enantioselectivity (up to 97% ee) and a broad substrate scope, including halogenated styrenes. As the first catalytic asymmetric alkene 1,2-allylboration, it provides direct access to enantioenriched β-allylboronic esters and enabled the synthesis of natural chiral alcohol.
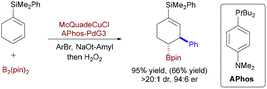
Buchwald and colleagues pioneered the first asymmetric CuH/Pd-catalyzed hydroarylation of vinylarenes (Scheme 56),84 diverging fundamentally from prior Cu-boryl strategies. Here, a chiral CuH species (generated from DTBM-SEGPHOS/Cu/silane) enantioselectively hydrocuprates styrenes to form benzylic-Cu intermediates, which undergo stereoretentive transmetalation with Pd–Ar complexes (from aryl bromide oxidative addition) and reductive elimination to deliver 1,1-diarylalkanes. This silane-driven hydrometallation mechanism replaces B2pin2-dependent borylcupration and avoids boronate byproducts, enabling up to 96% ee for β-substituted styrenes and heteroaryl bromides. It thus provides direct access to pharmaceutical scaffolds through an orthogonal, boron-free pathway.
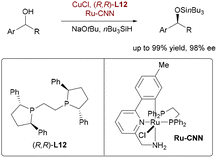
Parallelly, leveraging orthogonal bimetallic catalysis for alcohol functionalization, Oestreich and co-workers pioneered the first non-enzymatic dynamic kinetic resolution (DKR) enabling enantioselective silylation of racemic alcohols (Scheme 57).85 This strategy synergistically combines rapid alcohol racemization, catalyzed by a bifunctional ruthenium pincer complex, with an enantioselective dehydrogenative Si–O coupling mediated by a Cu–H catalyst system using nBu3SiH. Critically, the ruthenium catalyst enables efficient substrate racemization without promoting deleterious background reactions like silyl ether racemization or unselective coupling – challenges that incapacitated conventional ruthenium half-sandwich complexes. This orthogonal catalyst pairing delivered various acyclic and cyclic benzylic silyl ethers in near-quantitative yields and high enantioselectivity (up to 98% ee), providing efficient access to pharmaceutical precursors like duloxetine and rivastigmine, thereby demonstrating the power of orthogonal bimetallic design for complex chiral synthesis beyond alkene functionalization.
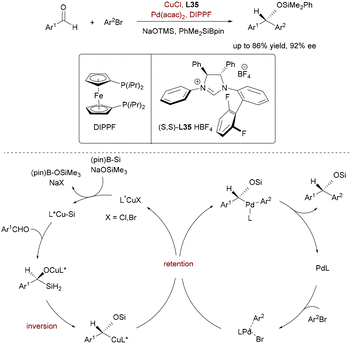
In 2019, Ohmiya's group pioneered a novel umpolung strategy that transforms aromatic aldehydes into chiral α-alkoxyalkyl anion equivalents via synergistic Pd/Cu catalysis (Scheme 58).86 While their earlier racemic system established catalytic generation of nucleophilic α-silyloxybenzylcopper(I) species from aldehydes and silylboronates, the asymmetric iteration achieved enantioselective Csp3–Csp2 coupling with aryl/allyl electrophiles. Key to success was a chiral N-heterocyclic carbene (NHC) ligand (L35) on copper, featuring fluorinated 2-(2,6-difluorophenyl)phenyl and 2-isopropylphenyl substituents. This ligand orchestrated enantioselective silylcupration of aldehydes (via C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O insertion) followed by stereoinvertive [1,2]-Brook rearrangement to form configurationally defined α-silyloxybenzylcopper intermediates. Subsequent stereoretentive transmetalation to Pd–aryl species and reductive elimination delivered silyl-protected chiral benzhydrols with broad functional tolerance (up to 92% ee). Mechanistic studies confirmed inversion at copper (Brook step) and retention at palladium (transmetalation), highlighting precise stereocontrol across two catalytic cycles. The system was extended to allylic carbonates, underscoring its versatility in accessing chiral alcohols through in situ anion generation.
O insertion) followed by stereoinvertive [1,2]-Brook rearrangement to form configurationally defined α-silyloxybenzylcopper intermediates. Subsequent stereoretentive transmetalation to Pd–aryl species and reductive elimination delivered silyl-protected chiral benzhydrols with broad functional tolerance (up to 92% ee). Mechanistic studies confirmed inversion at copper (Brook step) and retention at palladium (transmetalation), highlighting precise stereocontrol across two catalytic cycles. The system was extended to allylic carbonates, underscoring its versatility in accessing chiral alcohols through in situ anion generation.
 | ||
| Scheme 58 Cu/Pd-catalysis for transformation of aromatic aldehydes into silyl-protected chiral benzhydrols. | ||
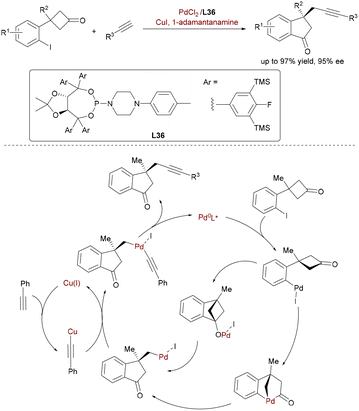
Diverging from carbonyl umpolung strategies, Xu and co-workers achieved enantioselective C(sp3)–C(sp) alkynylation via synergistic Pd/Cu-catalyzed C–C σ-bond activation/Sonogashira coupling (Scheme 59).87 This innovative tandem process involves Pd0-mediated oxidative addition into cyclobutanones' C(sp2)–C(sp3) bonds, followed by β-carbon elimination to generate σ-alkylpalladium species. Subsequent stereoselective transmetalation with copper acetylides (formed in situ from terminal alkynes and CuI) and reductive elimination yield chiral alkynylated indanones bearing all-carbon quaternary stereocenters (up to 97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 er). Key to controlling enantioselectivity across both C–C cleavage and C(sp3)–C(sp) bond formation was a novel TADDOL-derived phosphoramide ligand L36 sterically demanding fluorine/silicon-substituted aryl groups. This methodology expands bimetallic catalysis to strained ketone activation.
2.5 er). Key to controlling enantioselectivity across both C–C cleavage and C(sp3)–C(sp) bond formation was a novel TADDOL-derived phosphoramide ligand L36 sterically demanding fluorine/silicon-substituted aryl groups. This methodology expands bimetallic catalysis to strained ketone activation.
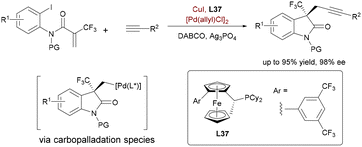
Further diversifying the alkynyl transmetalation manifold, Lu et al. demonstrated enantioselective Heck/Sonogashira tandem coupling for synthesizing oxindoles with trifluoromethylated quaternary stereocenters (Scheme 60).88a Unlike previous C–C activation or carbonyl umpolung strategies, this Pd/Cu-catalyzed process initiates with intramolecular Heck cyclization of o-iodoacrylanilides, generating alkylpalladium species that resist β-fluorine elimination. Subsequent stereoselective transmetalation with copper acetylides and reductive elimination delivers chiral 3-alkynyloxindoles. Critically, Josiphos-derived ligands (L37) enabled dual control over enantioselective C(sp2)–C(sp3) cyclization and C(sp3)–C(sp) bond formation. This methodology overcomes historical challenges in trifluoromethyl alkene Heck reactions, showcasing the versatility of bimetallic systems for complex heterocycle synthesis. In 2024, Yao demonstrated a synergistic Pd/Ag-catalyzed asymmetric diarylation leveraging aryl-silver species generated directly from simple arene C–H activation.88b This approach bypasses prefunctionalized aryl reagents, contrasting with Lu's Pd/Cu system, which employs alkynyl-copper transmetalation for Heck/Sonogashira sequences. Key to Yao's strategy is a single chiral bisphosphine ligand that orchestrates both Ag-mediated C–H cleavage and Pd-catalyzed enantioselective cyclization.
Niu and co-workers performed an iridium-catalyzed enantioselective allylation of bis[(pinacolato)boryl]methane, enabled by silver assistance (Scheme 61).89 Mechanistically, Ag3PO4 facilitates LiOtBu-mediated deborylative transmetallation to generate alkyl–silver species, which then undergoes stereoselective allylic substitution with Ir–π-allyl complexes. Chiral phosphoramidite ligands orchestrate enantiocontrol during C–C bond formation, delivering β-substituted homoallylic boronic esters as versatile chiral building blocks.
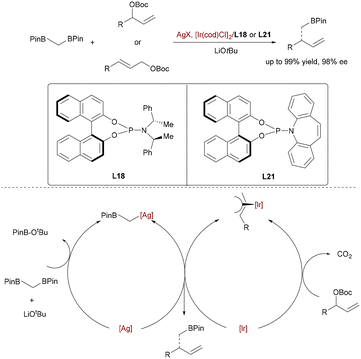 | ||
| Scheme 61 Ag/Ir-catalyzed allylation of bis-Bpin-methane for the synthesis of enantioenriched homoallylic organoboronic esters. | ||
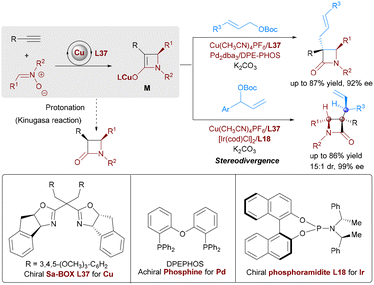
Synergistic bimetallic catalysis enables the concurrent generation and stereoselective coupling of transient organometallic intermediates, offering new powerful strategies for constructing complex chiral architectures. A landmark example is the Cu/Pd-catalyzed interrupted Kinugasa allylic alkylation (IKAA, Scheme 62).90 In this system, a Cu(I) catalyst promotes the cycloaddition of alkynes and nitrones to form a chiral enolate-copper intermediate M, while a Pd(0) catalyst activates allylic carbonates to generate a π-allylpalladium electrophile. The stereoselective coupling of these two intermediates—via nucleophilic attack of M on the electrophilic reagent—proceeds with high efficiency, delivering α-quaternary β-lactams with excellent enantioselectivity. Crucial to the success of this process is the synchronization of the two catalytic cycles and the deliberate ligand design: a chiral SA-BOX ligand L37 for Cu and an achiral Xantphos ligand for Pd, which together suppresses protonation of M and ensures precise stereochemical control. Complementing this, a Cu/Ir system achieves stereodivergent synthesis of β-lactams with adjacent tertiary/quaternary/tertiary stereocenters. By independently tuning chiral Cu and Ir catalysts, four stereoisomers are selectively accessed from identical substrates, demonstrating orthogonal control over multiple stereogenic centers.
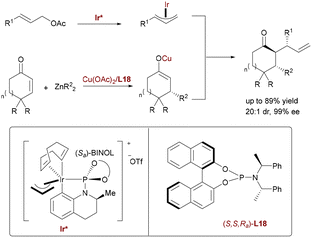
Extending synergistic bimetallic catalysis to non-adjacent stereocenters, You reported a Cu/Ir relay catalytic system for stereodivergent tandem asymmetric conjugate addition/allylic alkylation (Scheme 63).91 Copper catalysis (with chiral phosphoramidite ligand L18) generates enolates from cyclic enones and dialkylzinc, while iridium catalysis (using phosphoramidite) directs allylic substitution with cinnamyl acetates. This sequential nucleophile-electrophile pairing constructs 1,3-chiral centers in cyclohexanones with high stereoselectivity.
In 2021, Hu et al. developed a ternary Rh/Pd/CPA-catalyzed asymmetric three-component allylic alkylation of α-diazo carbonyls, alcohols, and allyl carbonates (Scheme 64).92 This strategy intercepts the transient Rh-associated enol (derived from an oxonium ylide) with an electrophilic π-allyl-Pd+·CPA− complex via an unprecedented SN1-type trapping pathway. The Xantphos ligand's large bite angle enhances Pd-allyl electrophilicity, suppressing competing O–H insertion, while the chiral phosphoric acid (CPA) controls enantioselectivity (up to 97% ee) through hydrogen bonding and ion-pairing interactions in the stereodetermining C–C bond formation.
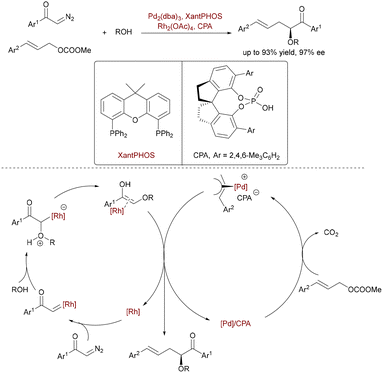 | ||
| Scheme 64 Rh/Pd/CPA-catalyzed asymmetric three-component allylic alkylation of α-diazo carbonyls, alcohols, and allyl carbonates. | ||
5. Conclusions and outlook
Asymmetric bimetallic catalysis has made significant strides since 2010, offering new avenues for controlling stereoselectivity. This strategy has expanded the toolbox of synthetic chemists, enabling previously inaccessible reaction paradigms, as demonstrated by a variety of enantioselective transformations discussed in this review. It is anticipated that this field will continue to increase the power of asymmetric catalysis.Although substantial progress has been made in the field of asymmetric bimetallic catalysis, it is still in its infancy with certain challenges yet to be addressed. Notably, the majority of asymmetric transformations presented herein are catalyzed by the Cu/Pd catalyst system. Thus, one of the remaining challenges is to develop new dual metal pairing types. Additionally, although a wide range of different nucleophiles were explored, the electrophiles are still limited to aryl halides or allylic precursors in the bimetallic catalyzed asymmetric transformations described up to date. Expanding the repertoire of electrophiles and developing new activation strategies are crucial for advancing this field. Beyond these limitations, in-depth mechanistic investigations are essential to provide a more comprehensive understanding of the enantioselective controlling parameters and the reaction mechanisms. Such insights are significant for refining existing catalysts and designing new bimetallic catalysts.
We anticipate that asymmetric bimetallic catalysis will evolve into a versatile strategy with broad applications in organic synthesis, pharmaceutical chemistry, and materials science.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
We are grateful for the financial support from National Natural Science Foundation of China (22471143 and 22402073), Jiangxi Provincial Key Laboratory of Organic Functional Molecules (No. 2024SSY05141), Natural Science Foundation of Shandong Province (No. ZR2023QB102), and the Taishan Scholar award of Shandong Province.References
- (a) T. L. Lohr and T. J. Marks, Nat. Chem., 2015, 7, 477–482 CrossRef CAS; (b) M. M. Lorion, K. Maindan, A. R. Kapdi and L. Ackermann, Chem. Soc. Rev., 2017, 46, 7399–7420 RSC; (c) L. K. G. Ackerman-Biegasiewicz, S. K. Kariofillis and D. J. Weix, J. Am. Chem. Soc., 2023, 145, 6596–6614 CrossRef CAS.
- (a) J. Park and S. Hong, Chem. Soc. Rev., 2012, 41, 6931–6943 RSC; (b) A. E. Allen and D. W. C. MacMillan, Chem. Sci., 2012, 3, 633–658 RSC; (c) J. Fu, X. Huo, B. Li and W. Zhang, Org. Biomol. Chem., 2017, 15, 9747–9759 RSC; (d) U. B. Kim, D. J. Jung, H. J. Jeon, K. Rathwell and S.-g. Lee, Chem. Rev., 2020, 120, 13382–13433 CrossRef CAS PubMed; (e) S. K. Dorn and M. K. Brown, ACS Catal., 2022, 12, 2058–2063 CrossRef CAS; (f) X. Huo, G. Li, X. Wang and W. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202210086 CrossRef CAS; (g) L. Wei and C.-J. Wang, Chem. Catal., 2023, 3, 100455 CAS; (h) L. Wei, C. Fu, Z.-F. Wang, H.-Y. Tao and C.-J. Wang, ACS Catal., 2024, 14, 3812–3844 CrossRef CAS; (i) J. Masson-Makdissi, L. Prieto, X. Abel-Snape and M. Lautens, Angew. Chem., Int. Ed., 2021, 60, 16932–16936 CrossRef CAS; (j) C. Fu, L. He, X. Chang, X. Cheng, Z.-F. Wang, Z. Zhang, V. A. Larionov, X.-Q. Dong and C.-J. Wang, Angew. Chem., Int. Ed., 2024, 63, e202315325 CrossRef CAS PubMed; (k) J. He, Z. Li, R. Li, X. Kou, D. Liu and W. Zhang, Adv. Sci., 2024, 11, 2400621 CrossRef CAS; (l) X. Lin, X. Mu, H. Cui, Q. Li, Z. Feng, Y. Liu, G. Li and C. Li, Nat. Commun., 2024, 15, 3683 CrossRef CAS PubMed For reviews on dual catalysis, see: ; (m) H. Sun, Y. Ma, G. Xiao and D. Kong, Trends Chem., 2024, 6, 684–701 CrossRef CAS; (n) Y. Wu, X. Huo and W. Zhang, Chem. Eur. J., 2020, 26, 4895–4916 CrossRef CAS PubMed; (o) D.-F. Chen, Z.-Y. Han, X.-L. Zhou and L.-Z. Gong, Acc. Chem. Res., 2014, 47, 2365–2377 CrossRef CAS.
- For selected examples on synergistic bimetallic catalysis, see: (a) L. Zhao, G. Li, R. He, P. Liu, F. Wang, X. Huo, M. Zhao and W. Zhang, Org. Biomol. Chem., 2021, 19, 1955–1959 RSC; (b) X. Huo, L. Zhao, Y. Luo, Y. Wu, Y. Sun, G. Li, T. Gridneva, J. Zhang, Y. Ye and W. Zhang, CCS Chem., 2022, 4, 1720–1731 CrossRef CAS; (c) Y. Liu, C. Li, R. Zhang, Z. Liu, Z. Han, Z. Wang and K. Ding, CCS Chem., 2024, 6, 2607–2618 CrossRef CAS; (d) C. Li, Y. Liu, Z. Han, Z. Wang and K. Ding, Chem. – Eur. J., 2025, 31, e202404209 CrossRef CAS; (e) C. Han, Y. Peng, G. Li, Q. Kong, X. Huo and W. Zhang, Nat. Commun., 2025, 16, 5467 CrossRef PubMed; (f) W. Chai, B. Guo, Q. Zhang and W. Zi, Chem. Catal., 2022, 2, 1428–1439 CAS; (g) M.-Q. Tang, Z.-J. Yang, A.-J. Han and Z.-T. He, Angew. Chem., Int. Ed., 2025, 64, e202413428 CrossRef CAS; (h) J. Zhang, W. Zhu, Z. Chen, Q. Zhang and C. Guo, J. Am. Chem. Soc., 2024, 146, 1522–1531 CrossRef CAS; (i) W. Zhu, C. Han, G. Yang, X. Huo and W. Zhang, J. Am. Chem. Soc., 2024, 146, 26121–26130 CrossRef CAS; (j) P. Li, Y. Zhang, Z. Liu, Q. Kong, L. Fu and X. Huo, Org. Lett., 2024, 26, 10356–10363 CrossRef CAS PubMed; (k) Y. Peng, X. Huo, Y. Luo, L. Wu and W. Zhang, Angew. Chem., Int. Ed., 2021, 60, 24941–24949 CrossRef CAS; (l) L. Xiao, L. Wei and C.-J. Wang, Angew. Chem., Int. Ed., 2021, 60, 24930–24940 CrossRef CAS PubMed; (m) K. Tian, X. Chang, L. Xiao, X.-Q. Dong and C.-J. Wang, Fundam. Res., 2024, 4, 77–85 CrossRef CAS; (n) Z. He, L. Peng and C. Guo, Nat. Synth., 2022, 1, 393–400 CrossRef CAS; (o) X. Chang, J. Zhang, X. Cheng, X. Lv and C. Guo, Adv. Sci., 2024, 11, 2406764 CrossRef CAS; (p) F. Wu, H. Wang, Z. Wu, Y. Liu and X. Feng, J. Am. Chem. Soc., 2025, 147, 16237–16247 CrossRef CAS.
- For the application of bimetallic synergistic asymmetric catalysis, see: (a) Y. Xu, H. Wang, Z. Yang, Y. Zhou, Y. Liu and X. Feng, Chem, 2022, 8, 2011–2022 CrossRef CAS; (b) H. Wang, L. Song, J. Huang, F. Wu, Z. Yang, Y. Liu, Y.-D. Wu and X. Feng, Angew. Chem., Int. Ed., 2025, 64, e202500125 CrossRef CAS.
- M. Sawamura, M. Sudoh and Y. Ito, J. Am. Chem. Soc., 1996, 118, 3309–3310 CrossRef CAS.
- G. M. Sammis, H. Danjo and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 9928–9929 CrossRef CAS.
- C. C. Malakar, L. Dell'Amico and W. Zhang, Eur. J. Org. Chem., 2023, e202201114 CrossRef CAS.
- (a) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS PubMed; (b) J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev. Chem., 2017, 1, 0052 CrossRef CAS; (c) A. Hossain, A. Bhattacharyya and O. Reiser, Science, 2019, 364, eaav9713 CrossRef PubMed; (d) H.-H. Zhang, H. Chen, C. Zhu and S. Yu, Sci. China Chem., 2020, 63, 637–647 CrossRef CAS; (e) A. Y. Chan, I. B. Perry, N. B. Bissonnette, B. F. Buksh, G. A. Edwards, L. I. Frye, O. L. Garry, M. N. Lavagnino, B. X. Li, Y. Liang, E. Mao, A. Millet, J. V. Oakley, N. L. Reed, H. A. Sakai, C. P. Seath and D. W. C. MacMillan, Chem. Rev., 2022, 122, 1485–1542 CrossRef CAS PubMed.
- E. K. van den Beuken and B. L. Feringa, Tetrahedron, 1998, 54, 12985–13011 CrossRef CAS.
- (a) X. Wang, Z. Yao, S. Dong, F. Wei, H. Wang and Z. Xu, Org. Lett., 2013, 15, 2234–2237 CrossRef CAS PubMed; (b) X. Wang, S. Dong, Z. Yao, L. Feng, P. Daka, H. Wang and Z. Xu, Org. Lett., 2014, 16, 22–25 CrossRef CAS PubMed; (c) S. Zhang, Z. Xu, J. Jia, C.-H. Tung and Z. Xu, Chem. Commun., 2014, 50, 12084–12087 RSC; (d) B. Wang, M. Liang, J. Tang, Y. Deng, J. Zhao, H. Sun, C.-H. Tung, J. Jia and Z. Xu, Org. Lett., 2016, 18, 4614–4617 CrossRef CAS PubMed.
- J. Li, L. Lin, B. Hu, X. Lian, G. Wang, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2016, 55, 6075–6078 CrossRef CAS PubMed.
- (a) W. Cao, X. Liu and X. Feng, Acc. Chem. Res., 2025, 58, 2496–2510 CrossRef CAS; (b) S. Dong, X. Liu and X. Feng, Acc. Chem. Res., 2022, 55, 415–428 CrossRef CAS; (c) X. Liu, H. Zheng, Y. Xia, L. Lin and X. Feng, Acc. Chem. Res., 2017, 50, 2621–2631 CrossRef CAS PubMed; (d) X. Liu, L. Lin and X. Feng, Acc. Chem. Res., 2011, 44, 574–587 CrossRef CAS PubMed.
- J. Gong, Q. Wan and Q. Kang, Adv. Synth. Catal., 2018, 360, 4031–4036 CrossRef CAS.
- P. Dong, L. Chen, Z. Yang, S. Dong and X. Feng, Org. Chem. Front., 2021, 8, 6874–6880 RSC.
- S. Ge, W. Cao, T. Kang, B. Hu, H. Zhang, Z. Su, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2019, 58, 4017–4021 CrossRef CAS.
- S. Ge, Y. Zhang, Z. Tan, D. Li, S. Dong, X. Liu and X. Feng, Org. Lett., 2020, 22, 3551–3556 CrossRef CAS PubMed.
- X. Sang, Y. Mo, S. Li, X. Liu, W. Cao and X. Feng, Chem. Sci., 2023, 14, 8315–8320 RSC.
- J. Li, L. Lin, B. Hu, P. Zhou, T. Huang, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2017, 56, 885–888 CrossRef CAS.
- X. Hu, X. Tang, X. Zhang, L. Lin and X. Feng, Nat. Commun., 2021, 12, 3012 CrossRef PubMed.
- C. Sheng, Z. Ling, J. Xiao, K. Yang, F. Xie, S. Ma and W. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202305680 CrossRef.
- (a) S. Shi, S. P. Nolan and M. Szostak, Acc. Chem. Res., 2018, 51, 2589–2599 CrossRef CAS PubMed; (b) A. Biffis, P. Centomo, A. D. Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249–2295 CrossRef; (c) P. Ruiz-Castillo and S. L. Buchwald, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS PubMed; (d) H. Li, C. C. C. J. Seechurn and T. J. Colacot, ACS Catal., 2012, 2, 1147–1164 CrossRef CAS.
- A. Saito, N. Kumagai and M. Shibasaki, Angew. Chem., Int. Ed., 2017, 56, 5551–5555 CrossRef CAS.
- L. Wei, S.-M. Xu, Q. Zhu, C. Che and C.-J. Wang, Angew. Chem., Int. Ed., 2017, 56, 12312–12316 CrossRef CAS PubMed.
- X. Huo, R. He, J. Fu, J. Zhang, G. Yang and W. Zhang, J. Am. Chem. Soc., 2017, 139, 9819–9822 CrossRef CAS PubMed.
- L. Xiao, X. Chang, H. Xu, Q. Xiong, Y. Dang and C.-J. Wang, Angew. Chem., Int. Ed., 2022, 61, e202212948 CrossRef CAS.
- (a) V. Bhat, E. R. Welin, X. Guo and B. M. Stoltz, Chem. Rev., 2017, 117, 4528–4561 CrossRef CAS PubMed; (b) J. Steinreiber, K. Faber and H. Griengl, Chem. Eur. J., 2008, 14, 8060–8072 CrossRef CAS.
- R. He, X. Huo, L. Zhao, F. Wang, L. Jiang, J. Liao and W. Zhang, J. Am. Chem. Soc., 2020, 142, 8097–8103 CrossRef CAS PubMed.
- Q. Zhang, H. Yu, L. Shen, T. Tang, D. Dong, W. Chai and W. Zi, J. Am. Chem. Soc., 2019, 141, 14554–14559 CrossRef CAS.
- Q.-Y. Liao, C. Ma, Y.-C. Wang, S.-Q. Yang, J.-S. Ma and Z.-T. He, Chin. Chem. Lett., 2023, 34, 10837 Search PubMed.
- M. Zhu, Q. Zhang and W. Zi, Angew. Chem., Int. Ed., 2021, 60, 6545–6552 CrossRef CAS PubMed.
- Y. Liu, H. Chen and X. Wang, J. Am. Chem. Soc., 2024, 146, 28427–28436 CrossRef CAS.
- P. Li, E. Zheng, G. Li, Y. Luo, X. Huo, S. Ma and W. Zhang, Science, 2024, 385, 972–979 CrossRef CAS PubMed.
- H. Wang, Y. Xu, F. Zhang, Y. Liu and X. Feng, Angew. Chem., Int. Ed., 2022, 61, e202115715 CrossRef CAS.
- S.-Q. Yang, Y.-F. Wang, W.-C. Zhao, G.-Q. Lin and Z.-T. He, J. Am. Chem. Soc., 2021, 143, 7285–7291 CrossRef CAS PubMed.
- Y. Luo, Y. Ma, G. Li, X. Huo and W. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202313838 CrossRef CAS PubMed.
- (a) L. Liu, R. M. Ward and J. M. Schomaker, Chem. Rev., 2019, 119, 12422–12490 CrossRef CAS PubMed; (b) S. Kitagaki, F. Inagaki and C. Mukai, Chem. Soc. Rev., 2014, 43, 2956–2978 RSC; (c) L. Gan, X. Wan, Y. Pang, Y. Zou, Y.-H. Deng and Z. Shao, Org. Chem. Front, 2025, 12, 1001–1064 RSC; (d) B. Yang, Y. Qiu and J.-E. Bäckvall, Acc. Chem. Res., 2018, 51, 1520–1531 CrossRef CAS.
- (a) H.-C. Liu, Y.-Z. Hu, Z.-F. Wang, H.-Y. Tao and C.-J. Wang, Chem. – Eur. J., 2019, 25, 8681–8685 CrossRef CAS PubMed; (b) J. Xiao, H. Xu, X. Huo, W. Zhang and S. Ma, Chin. J. Chem., 2021, 39, 1958–1964 CrossRef CAS.
- L. Zhao, Y. Luo, J. Xiao, X. Huo, S. Ma and W. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202218146 CrossRef CAS PubMed.
- J. Zhang, X. Huo, J. Xiao, L. Zhao, S. Ma and W. Zhang, J. Am. Chem. Soc., 2021, 143, 12622–12632 CrossRef CAS PubMed.
- J. Zhang, Y. Luo, E. Zheng, X. Huo, S. Ma and W. Zhang, J. Am. Chem. Soc., 2024, 146, 9241–9251 CrossRef CAS PubMed.
- H. Wang, R. Zhang and W. Zi, Angew. Chem., Int. Ed., 2024, 63, e202402843 CrossRef CAS PubMed.
- P. Li, Z. Liu, X. Huo and W. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202407498 CrossRef CAS PubMed.
- (a) B. M. Trost and L. C. Czabaniuk, Angew. Chem., Int. Ed., 2014, 53, 2826–2851 CrossRef CAS; (b) B. Liégault, J.-L. Renaud and C. Bruneau, Chem. Soc. Rev., 2008, 37, 290–299 RSC.
- X. Chang, J.-D. Ran, X.-T. Liu and C.-J. Wang, Org. Lett., 2022, 24, 2573–2578 CrossRef CAS.
- Y. Peng, G. Li, C. Han, Y. Luo, X. Huo and W. Zhang, CCS Chem., 2024, 6, 2452–2464 CrossRef CAS.
- X. Jiang, W. Chen and J. F. Hartwig, Angew. Chem., Int. Ed., 2016, 55, 5819–5823 CrossRef CAS.
- X. Huo, R. He, X. Zhang and W. Zhang, J. Am. Chem. Soc., 2016, 138, 11093–11096 CrossRef CAS.
- (a) X. Huo, J. Zhang, J. Fu, R. He and W. Zhang, J. Am. Chem. Soc., 2018, 140, 2080–2084 CrossRef CAS PubMed; (b) L. Wei, Q. Zhu, S.-M. Xu, X. Chang and C.-J. Wang, J. Am. Chem. Soc., 2018, 140, 1508–1513 CrossRef CAS.
- Z. Liu, P. Li, H. Wang, J. Zhang, X. Huo, Z.-L. Sun and W. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202508335 CrossRef CAS.
- Z.-T. He, X. Jiang and J. F. Hartwig, J. Am. Chem. Soc., 2019, 141, 13066–13073 CrossRef CAS PubMed.
- Y. Qiu, V. C. J. X. Thomas, T. Fantoni, R. Chen, X. Jiang, Z.-T. He, T. W. Butcher, D. K. Nomura and J. F. Hartwig, Chem, 2024, 10, 3709–3721 CAS.
- X. Jiang, P. Boehm and J. F. Hartwig, J. Am. Chem. Soc., 2018, 140, 1239–1242 CrossRef CAS PubMed.
- S.-M. Xu, L. Wei, C. Shen, L. Xiao, H.-Y. Tao and C.-J. Wang, Nat. Commun., 2019, 10, 5553 CrossRef PubMed.
- T. Chen, Q. Xiong, H. Xu, L. Xiao, Z.-F. Wang, X. Chang, Y. Dang, X.-Q. Dong and C.-J. Wang, J. Am. Chem. Soc., 2024, 146, 29928–29942 CrossRef CAS PubMed.
- T. Wang, Y. Peng, G. Li, Y. Luo, Y. Ye, X. Huo and W. Zhang, Chem. – Eur. J., 2021, 27, 10255–10260 CrossRef CAS PubMed.
- W. Wang, F. Zhang, Y. Liu and X. Feng, Angew. Chem., Int. Ed., 2022, 61, e202208837 CrossRef CAS PubMed.
- B. Qing, Z. Yang, Z. Wu, Z. Zhang, Y. Zhou, X. Yan, Y. Liu and X. Feng, J. Am. Chem. Soc., 2025, 147, 7729–7740 CrossRef CAS.
- R. Lu, Q. Zhang and C. Guo, Nat. Commun., 2023, 14, 8118 CrossRef CAS.
- W.-L. Yang, X.-Y. Shang, X. Luo and W.-P. Deng, Angew. Chem., Int. Ed., 2022, 61, e202203661 CrossRef CAS PubMed.
- (a) J. J. Monteith and S. A. L. Rousseaux, Acc. Chem. Res., 2023, 56, 3581–3594 CrossRef CAS; (b) Y. Wang, Y. He and S. Zhu, Acc. Chem. Res., 2023, 56, 3475–3491 CrossRef CAS PubMed; (c) Y. Li and G. Yin, Acc. Chem. Res., 2023, 56, 3246–3259 CrossRef CAS; (d) B. C. Lee, C.-F. Liu, L. Q. H. Lin, K. Z. Yap, N. Song, C. H. M. Ko, P. H. Chan and M. J. Koh, Chem. Soc. Rev., 2023, 52, 2946–2991 RSC; (e) Q. Pan, Y. Ping and W. Kong, Acc. Chem. Res., 2023, 56, 515–535 CrossRef CAS; (f) S. Zhu, X. Zhao, H. Li and L. Chu, Chem. Soc. Rev., 2021, 50, 10836–10856 RSC; (g) D. P. Satoute, G. N. Vaidya, S. K. Lokhande, S. D. Shinde, S. M. Bhujbal, D. R. Chatterjee, P. Rana, A. Venkatesh, M. Nagpure and D. Kumar, Green Chem., 2021, 23, 6273–6300 RSC; (h) A. D. Marchese, T. Adrianov and M. Lautens, Angew. Chem., Int. Ed., 2021, 60, 16750–16762 CrossRef CAS; (i) W. Xue, X. Jia, X. Wang, X. Tao, Z. Yin and H. Gong, Chem. Soc. Rev., 2021, 50, 4162–4184 RSC.
- (a) F. Tong, D. Hu, C. Zhang, J.-Q. Zhang and H. Ren, Org. Chem. Front., 2024, 11, 1843–1857 RSC; (b) C.-H. Ding and X.-L. Hou, Chem. Rev., 2011, 111, 1914–1937 CrossRef CAS.
- L. Peng, Z. He, X. Xu and C. Guo, Angew. Chem., Int. Ed., 2020, 59, 14270–14274 CrossRef CAS PubMed.
- R. Geng, L. Peng and C. Guo, Chin. Chem. Lett., 2024, 35, 109433 CrossRef CAS.
- Y. Zhao and D. J. Weix, J. Am. Chem. Soc., 2015, 137, 3237–3240 CrossRef CAS PubMed.
- J. Xia, T. Hirai, S. Katayama, H. Nagae, W. Zhang and K. Mashima, ACS Catal., 2021, 11, 6643–6655 CrossRef CAS.
- Y. Peng, C. Han, Y. Luo, G. Li, X. Huo and W. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202203448 CrossRef CAS PubMed.
- M. Ikeda, Y. Miyake and Y. Nishibayashi, Chem. – Eur. J., 2012, 18, 3321–3328 CrossRef CAS PubMed.
- (a) Y. Gao, G. Hong, B.-M. Yang and Y. Zhao, Chem. Soc. Rev., 2023, 52, 5541–5562 RSC; (b) A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410–1459 CrossRef CAS PubMed; (c) A. Alexandridis and A. Quintard, ChemCatChem, 2024, 16, e202400902 CrossRef CAS; (d) W. Chen, M. Sohail, Y. Veeranna, Y. Yang, A. A. Bengali, H.-C. Zhou and S. T. Madrahimov, ACS appl. Mater. Interfaces, 2025, 17, 17775–17782 CrossRef CAS PubMed; (e) Z.-X. Liu, Y.-D. Gao and L.-C. Yang, JACS Au, 2024, 4, 877–892 CrossRef CAS PubMed; (f) Q. Xiong, B.-B. Chen, X.-Q. Dong and C.-J. Wang, J. Am. Chem. Soc., 2025, 147, 26102–26108 CrossRef CAS; (g) C. Fu, L. He, X. Chang, X. Cheng, Z.-F. Wang, Z. Zhang, V. A. Larionov, X.-Q. Dong and C.-J. Wang, Angew. Chem., Int. Ed., 2024, 63, e202315325 CrossRef CAS.
- X. Chang, X. Cheng, X.-T. Liu, C. Fu, W.-Y. Wang and C.-J. Wang, Angew. Chem., Int. Ed., 2022, 61, e202206517 CrossRef CAS.
- X. Cheng, C. Fu, B.-B. Chen, X. Chang, X.-Q. Dong and C.-J. Wang, J. Am. Chem. Soc., 2025, 147, 5014–5024 CrossRef CAS.
- T. P. Le, S. Tanaka, M. Yoshimura, K. Sato and M. Kitamura, Nat. Commun., 2022, 13, 5876 CrossRef CAS PubMed.
- X. Liang, Q.-H. Ding, J.-T. Yang, H.-F. Yang, Y. Deng, L. Shi, K. Wei and Y.-R. Yang, Nat. Commun., 2024, 15, 10812 CrossRef CAS.
- (a) X. Zhang, P. Sivaguru, Y. Pan, N. Wang., W. Zhang and X. Bi, Chem. Rev., 2025, 125, 1049–1190 CrossRef PubMed; (b) Y. He, Z. Huang, K. Wu, J. Ma, Y.-G. Zhou and Z. Yu, Chem. Soc. Rev., 2022, 51, 2759–2852 RSC; (c) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981–10080 CrossRef CAS; (d) S. Dong, X. Liu and X. Feng, Acc. Chem. Res., 2022, 55, 415–428 CrossRef CAS.
- (a) X. Liu, H. Zheng, Y. Xia, L. Lin and X. Feng, Acc. Chem. Res., 2017, 50, 2621–2631 CrossRef CAS; (b) X. Liu, L. Lin and X. Feng, Acc. Chem. Res., 2011, 44, 574–587 CrossRef CAS.
- Y. Chen, S. Dong, X. Xu, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2018, 57, 16554–16558 CrossRef CAS.
- C. Xu, K. Wang, D. Li, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2018, 58, 18438–18442 CrossRef.
- G. M. Sammis, H. Danjo and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 9928–9929 CrossRef CAS.
- Q. Hu and C. Guo, Angew. Chem., Int. Ed., 2023, 62, e202305638 CrossRef CAS PubMed.
- (a) J. Panteleev, L. Zhang and M. Lautens, Angew. Chem., Int. Ed., 2011, 50, 9089–9092 CrossRef CAS; (b) L. Zhang, Z. Qureshi, L. Sonaglia and M. Lautens, Angew. Chem., Int. Ed., 2014, 53, 13850–13853 CrossRef CAS PubMed.
- (a) S. K. Dorn and M. K. Brown, ACS Catal., 2022, 12, 2058–2063 CrossRef CAS; (b) K. Semba and Y. Nakao, J. Am. Chem. Soc., 2014, 136, 7567–7570 CrossRef CAS; (c) K. M. Logan, K. B. Smith and M. K. Brown, Angew. Chem., Int. Ed., 2015, 54, 5228–5231 CrossRef CAS PubMed; (d) K. M. Logan and M. K. Brown, Angew. Chem., Int. Ed., 2017, 56, 851–855 CrossRef CAS.
- (a) Y. Liao, X. Yin, X. Wang, W. Yu, D. Fang, L. Hu, M. Wang and J. Liao, Angew. Chem., Int. Ed., 2020, 59, 1176–1180 CrossRef CAS PubMed; (b) W. Li, H. Chen, Y. Zheng, Y. Lu, J. Xie, S. Chen, Y. Lan and Q. Song, ACS Catal., 2024, 14, 11318–11331 CrossRef CAS.
- P. F. Crook, A. R. Lear, S. Das and M. K. Brown, Chem. Sci., 2023, 14, 10467–10470 RSC.
- T. Jia, P. Cao, B. Wang, Y. Lou, X. Yin, M. Wang and J. Liao, J. Am. Chem. Soc., 2015, 137, 13760–13763 CrossRef CAS PubMed.
- S. D. Friis, M. T. Pirnot and S. L. Buchwald, J. Am. Chem. Soc., 2016, 138, 8372–8375 CrossRef CAS.
- J. Seliger and M. Oestreich, Angew. Chem., Int. Ed., 2021, 60, 247–251 CrossRef CAS PubMed.
- (a) M. Takeda, K. Yabushita, S. Yasuda and H. Ohmiya, Chem. Commun., 2018, 54, 6776–6779 RSC; (b) K. Yabushita, A. Yuasa, K. Nagao and H. Ohmiya, J. Am. Chem. Soc., 2019, 141, 113–117 CrossRef CAS.
- F.-N. Sun, W.-C. Yang, X.-B. Chen, Y.-L. Sun, J. Cao, Z. Xu and L.-W. Xu, Chem. Sci., 2019, 10, 7579–7583 RSC.
- (a) X. Bai, C. Wu, S. Ge and Y. Lu, Angew. Chem., Int. Ed., 2020, 59, 2764–2768 CrossRef CAS; (b) J. Yao, C. Zhao, L. Shao, X. Huo and X. Wang, Sci. China Chem., 2024, 67, 2710–2718 CrossRef CAS.
- M. Zhan, R.-Z. Li, Z.-D. Mou, C.-G. Cao, J. Liu, Y.-W. Chen and D. Niu, ACS Catal., 2016, 6, 3381–3386 CrossRef CAS.
- (a) J. Qi, F. Wei, S. Huang, C.-H. Tung and Z. Xu, Angew. Chem., Int. Ed., 2021, 60, 4561–4565 CrossRef CAS PubMed; (b) J. Qi, F. Wei, C.-H. Tung and Z. Xu, Angew. Chem., Int. Ed., 2021, 60, 13814–13818 CrossRef CAS; (c) J. Qi, C.-H. Tung and Z. Xu, Trends Chem., 2021, 3, 984–985 CrossRef CAS; (d) J. Qi, T. Song, Z. Yang and S. Sun, ACS Catal., 2023, 13, 2555–2564 CrossRef CAS.
- J.-H. Xie, Y.-M. Hou, Z. Feng and S.-L. You, Angew. Chem., Int. Ed., 2023, 62, e202216396 CrossRef CAS.
- Z. Kang, W. Chang, X. Tian, X. Fu, W. Zhao, X. Xu, Y. Liang and W. Hu, J. Am. Chem. Soc., 2021, 143, 20818–20827 CrossRef CAS.
Footnote |
| † F. W. and J. Q. contributed equally to this review. |
| This journal is © The Royal Society of Chemistry 2026 |