Constrained carbon bonding inside fullerene cages
Yaoxiao
Zhao
 a,
Mengyang
Li
a,
Mengyang
Li
 *c,
Wangqiang
Shen
*c,
Wangqiang
Shen
 b,
Kun
Guo
b,
Kun
Guo
 b,
Lipiao
Bao
b,
Lipiao
Bao
 *b and
Xing
Lu
*b and
Xing
Lu
 *b
*b
aSchool of Materials and Chemical Engineering, Xi’an Technological University, Xi’an 710032, P. R. China
bState Key Laboratory of Material Processing and Die & Mould Technology, College of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, P. R. China. E-mail: baol@hust.edu.cn; lux@hust.edu.cn
cSchool of Physics, Xidian University, Xi’an 710071, P. R. China. E-mail: limengyang@xidian.edu.cn
First published on 24th November 2025
Abstract
Carbon is an incredibly versatile element and can form bonds via sp, sp2, and sp3 hybridization, forming diverse structures, which are responsible for the vast complexity and diversity of chemistry and biology. Therefore, understanding carbon bonding is crucial for comprehending the fundamental principles of natural science. Beyond conventional chemistry, carbon bonding confined inside carbon cages can adopt unusual and seemingly unpredictable bond states. Within these spatially restricted environments, encapsulated carbon atoms can bond with multiple nonmetal atoms (e.g., H, C, N, and O) and a variety of metal atoms (e.g., Sc, V, Ti, and Dy), forming otherwise unstable clusters with different bonding models and oxidation states of carbon. This leads to unprecedented bonding situations, including multiple and multicenter carbon–metal bonds, covalent carbon–metal bonds, superatomic states, and pronounced donation bonds (e.g. C2 → metal atoms). These bonding situations enrich the carbon bonding models beyond traditional organic chemistry. This review provides a comprehensive summary of the recent findings regarding constrained carbon bonding with varying numbers of carbon atoms inside carbon cages. It will encompass crucial aspects of this special constrained carbon bonding such as the dispersion of negative charge on the carbon cage, reduction of Coulomb repulsion, maximization of coordinated metal ions, and determination of optimal configurations for metal atoms within the carbon cages. Accordingly, new carbon bonding could be identified in carbon cages, which holds significant implications in the development of innovative carbon-based compounds. Additionally, the current challenges faced and future developments anticipated from the aspect of confined carbon bonding inside carbon cages will be discussed to provide deeper insights into the intricacies of carbon bonding. Through this comprehensive exploration, we hope to advance knowledge in this exciting area of carbon chemistry.
I. Introduction
Carbon bonding lies at the core of modern chemistry and plays a fundamental role in forming a vast array of compounds that are essential for life and materials science.1–4 This versatility arises from the unique electronic structure of carbon and its ability to hybridize orbitals into sp, sp2, or sp3 configurations, enabling the formation of diverse bonds. For instance, in diamond, the carbon atoms adopt sp3 hybridization and arrange themselves into a three-dimensional lattice, imparting exceptional hardness and durability.5,6 In contrast, graphene is composed of a two-dimensional sheet of sp2-hybridized carbon atoms arranged in a hexagonal lattice, endowing it with remarkable strength, electrical conductivity, and flexibility.7,8Beyond diamond and graphene, fullerenes represent a structurally distinct third allotrope of carbon. Fullerenes are composed of hollow, cage-like architectures built entirely of carbon atoms, which can encapsulate a wide range of atoms and clusters within their confined interiors.9,10 These endohedral structures enable the stabilization and isolation of metal atoms as well as highly reactive or otherwise unstable species. Of particular interest is the emergence of constrained carbon bonding motifs, including C, CH, CN, CNH, C2, C2H, C3, and C3N, in these spatially confined environments. In these cases, the encapsulated carbon atoms or units form unprecedented bonding interactions, such as multiple bonds between a single carbon atom and lanthanide ion, representing bonding scenarios not accessible under conventional molecular conditions.11
Over the past two decades, several reviews have addressed the structures, stability, electronic structures, and interactions in constrained host–guest systems based on carbon cages.12–16 For example, in 2013, a detailed, consistent overview of carbon cages as robust containers focused on different aspects of endohedral fullerenes, primarily based on the development of this field after 2000, thereby supplying a significant understanding of the interplay between carbon cages and their encaged species.17 There are also several reviews focusing either on the historical debate regarding whether the two carbon atoms are located on or inside fullerene cages, or on the stabilities, geometries, and electronic structures of the MxC2n chemical formula.18–21 However, the role and nature of constrained carbon bonding, particularly from the perspective of the encapsulated carbon bonds themselves, remain largely unaddressed. This gap is particularly striking considering recent achievements. Beyond the well-studied two-carbon clusters, a variety of constrained carbon units containing one or three carbon atoms have now been captured within carbon cages.11,22–27 These constrained carbon species display unusual carbon bonding motifs and oxidation states that deviate significantly from traditional carbon bonding frameworks, offering a fertile platform for the discovery of new carbon chemical principles.
To provide a comprehensive overview of this emerging class of constrained carbon bonding, we summarize comprehensive examples of encapsulated carbon units within carbon cages (Table 1). These examples are categorized by the number of carbon atoms, their bonding types (μ1, μ2, μ3, or μ4), and the associated carbon cages. Notably, these constrained carbon species exhibit diverse bonding motifs with encapsulated metal atoms, including 2-center 2-electron (2c–2e), 3-center 2-electron (3c–2e), and superatomic bonding configurations. This classification serves as the foundation for the detailed discussions in Sections II–IV, where each class of constrained carbon units is explored in depth. These discussions are further enriched by theoretical insights into the characteristics of carbon bonding. Although significant progress has been made, the findings in this area remain scattered throughout the literature. Understanding these unique, spatially constrained bonding environments is critical not only for advancing fundamental carbon chemistry, but also for unlocking new possibilities in materials design and cluster chemistry. This review aims to integrate recent advances, identify unifying carbon bonding concepts, highlight emerging trends, and offer forward-looking perspectives on the unique phenomenon of constrained carbon bonding in carbon cages.
| Bonding formats | Moleculesa | Oxidation statesb | Carbon bonding features | Ref. | |
|---|---|---|---|---|---|
| a The molecules containing the corresponding constrained carbon bonding. b Unless otherwise noted, oxidation states are assigned to the encapsulated carbon units. All carbon atoms comply with the eight-electron rule within the examined cluster. | |||||
| Bonding pattern of one carbon atom | μ1-C bonding | MCN@C2n (2n = 76, 82, and 84 for M = Y and Tb; 2n = 76 and 82 for M = Lu; 2n = 82 and 84 for M = Dy; and 2n = 82 for M = U) | −1 | Tuning oxidation states and diverse bonding patterns; modulating the configurations of inner cluster; fulfilling maximum coordination | 37 and 41–43 |
| μ2-C bonding | U2C@C2n (2n = 60, 68, 72, 78, 80, and 88); UCCe@C2n (2n = 72 and 78); ThCTi@C82; M2TiC@C80 (M = Lu, Dy, Sc, and Tb); Sc2UC@C80; and Sc3CH@C80 | −4 | Building multicenter carbon bonds; leading to the multiple bonds between carbon and metal atoms; providing an anchoring point | 22, 23, 25, 31, 32, 49–54 and 58 | |
| μ3-C bonding | Sc3CN@C2n (2n = 68, 78 and 80) | −3 | Planar quinary cluster; double bond feature between constrained carbon and nitrogen atoms | 38–40 and 59–61 | |
| USc2CN@C80 | −7 | Trifoliate configuration; leading to an additional multicenter triple-bond character and donation bonding | 26 | ||
| CeTi2CN@C80 | −5 | Strong coordination interaction; governing conformations; first Ce-based single-molecule magnet | 24 | ||
| CeSc2C@C80 | −4 | Formation of Ce![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C likely triple bond characters C likely triple bond characters |
11 | ||
| μ4-C bonding | Sc4C@C80 | −4 | Tetrahedral Sc4 cluster; resulting in a formal charge state of +2.5 for scandium atom | 62 | |
| Sc4CNH@C80 | −5 | Rare multilayer nesting structure and multiple bonding types; new oxidation state of (CN)5− | 63 | ||
| Bonding pattern of two carbon atoms | μ1-C2 bonding | ThC2@C82 | −2 | Substantial donation bonding from C2 to Th; three-center bonding | 70 |
| μ2-C2 bonding | M2C2@C2n (2n = 68, 72, 74, 80–88 for M = Sc; 2n = 82–92, 100, 108 for M = Y; 2n = 78 and 80 for M = U; 2n = 90–104 for M = La; 2n = 80–92 for M = Er; 2n = 76–80 and 84–92 for M = Lu; 2n = 84 for M = Gd; and 2n = 88 for M = Dy) | −2 | Modulating the chemical properties of cages; reducing electron transfer; mitigating Coulomb repulsion; modulating coupling constants; limited σ-donation and a greater degree of π-donation; fulfilling the metal coordination; improving the efficiency of energy transfer; widening the HOMO–LUMO gap | 27, 71, 85–99, 102–109, 119 and 121–131 | |
| Ti2C2@C2n (2n = 78 and 82) | −2/−4 | Modulating the electronic states; balancing the covalent and ionic interactions | 74 and 112–118 | ||
| μ3-C2 bonding | M3C2@C2n (2n = 80 and 82 for M = Sc and 2n = 88 for M = Lu) | −3 | Unique bonding; spherical charge distribution; dative bond with the dπ of metal; underscoring the complex interplay between carbide and cage | 76 and 134–138 | |
| M3C2@C2n (2n = 80 for Dy and 2n = 80–86 for Er) | −2 | Constructing a 3c–1e metal bond | 139 and 140 | ||
| M2TiC2@C80 (M = Dy, Lu, and Sc) | −4 | Tuning the electronic structure, geometries, and magnetism | 51 and 141 | ||
| CeTi2C2@C80 | −6 | Coordinating vertically to the CeTi2 plane | 24 | ||
| MSc2C2@C80 (M = V and U) | −4 | Unique oxidation states of V4+ and U6+; facilitating the C1 implantation | 142 and 143 | ||
| μ4-C2 bonding | Sc4C2@C80 | −6 | A stable closed-shell electronic configuration | 63 and 144–147 | |
| Sc4C2H@C80 | −6 | Leading to one unique Sc2+ cation | 80 | ||
| Bonding pattern of three carbon atom | μ3-C2/μ3-CN bonding | Sc3(C2)(CN)@C80 | −2/−1 | Unique oxidation states of constrained carbon units; stabilizing the molecule with 7 constrained atoms | 152 |
| μ3-C3 bonding | Ti3C3@C2n (2n = 80/82) | −1 | A superatomic state; a closed-shell electronic configuration of 1S21P61D10; a unique double-butterfly Ti3C3 cluster; the coexistence of characteristics of Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double and Ti–C single bond C double and Ti–C single bond |
151, 153 and 154 | |
| μ4-C3 bonding | Sc4C3@C80 | −6 | High aromatic character of the constrained C3 | 62 | |
II. Bonding patterns of one carbon atom
A single carbon atom generally obeys the octet rule by forming four covalent bonds with nonmetal atoms. When bonded to only two or three nonmetal atoms, it may exist as a reactive intermediate such as a radical or carbene.28–30 In contrast to these classical species, recent discoveries have demonstrated the stabilization of isolated carbon atoms coordinated exclusively by metal centers within spatially confined fullerene environments.The emergence of constrained carbon bonding within CXMy@C2n systems represents a significant departure from classical concepts of single-carbon (1C) stabilization. In these complexes, a single carbon atom is encapsulated within a carbon cage (C2n) and coordinated predominantly by metal atoms (M) in varying number (y), with X either representing an additional nonmetal atom or remaining vacant. Recently, a single carbon atom confined within a carbon cage has been shown to form a strong carbon–lanthanide bond, characterized primarily by a formal Ce![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond. This achievement represents a rare example of a lanthanide–carbon multiple bond, whose isolation and stabilization remain a longstanding challenge in carbon chemistry.11 Representative examples, such as YCN, U2C, Dy2TiC, DyYTiC, TiLu2C, and Sc3CH, encompass a wide range of carbon–metal bond species spanning group III, IV, and V elements, as well as the lanthanide and actinide series.
C triple bond. This achievement represents a rare example of a lanthanide–carbon multiple bond, whose isolation and stabilization remain a longstanding challenge in carbon chemistry.11 Representative examples, such as YCN, U2C, Dy2TiC, DyYTiC, TiLu2C, and Sc3CH, encompass a wide range of carbon–metal bond species spanning group III, IV, and V elements, as well as the lanthanide and actinide series.
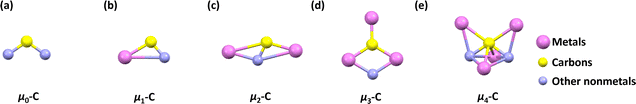
These findings reveal a distinct carbon bonding paradigm in which the carbon atom does not achieve stabilization through conventional covalent bonds with nonmetals, but rather through direct interactions with multiple metal centers. For example, in addition to covalent two-center two-electron (2c–2e) U–C bonds, the U2C unit consistently features two three-center two-electron (3c–2e) carbon bonds in U2C@C2n (2n = 60, 68, 72, 78, 80, 88, 96, and 104). These multicenter bonds involving the carbon atom enrich the bonding models in traditional organometallic chemistry.31,32 Clearly, these metal–carbon coordination networks endow the encapsulated carbon atom with electronic environments markedly different from that in traditional organic or organometallic systems. The high electron density and multimetallic bonding interactions confer exceptional stabilization to the otherwise highly reactive, isolated carbon species. These 1C-centered clusters not only challenge established paradigms in carbon chemistry but also open avenues for designing new classes of carbon-based materials with tailored electronic, magnetic, and catalytic properties. The work demonstrates that carbon, traditionally viewed as reliant on covalent bonding with nonmetals, can exhibit remarkable stability when embedded in purely metallic frameworks, providing a suitable confined environment. This insight paves the way for a deeper understanding of the bonding versatility of carbon and its potential roles in unconventional material architectures. In detail, we categorize these constrained 1C carbon bonds within fullerene cages into four types based on the number of interacting metal atoms, as shown in Fig. 1.
1 CXM@C2n
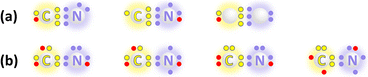
Since Smalley proposed in 1985 that fullerenes, composed entirely of carbon atoms, can encapsulate atoms, ions, or clusters, they have been recognized as unique hosts capable of stabilizing new clusters that would otherwise be unstable in their isolated forms.9,10,33–36 This interesting property leads to the formation of constrained carbon bonds within carbon cages, where the carbon atoms not only construct the carbon cage framework but also participate in the formation of unique carbon–metal bonding. Numerous examples of these carbon cage-based host–guest molecules containing constrained carbon bonding have been documented. In 2013, Yang et al. reported an unprecedented stable monometallic carbon unit encapsulated in a carbon cage, YCN@C82. They proposed an electronic configuration of Y3+(CN)−@(C82)2−,37 highlighting that the (CN)− moiety, typically found in traditional metal cyanide compounds, differs significantly from the (CN)3− unit observed in the previously reported (Sc3+)3(CN)3−@(C80)6− (Fig. 2).38–40 This stabilization of a monometallic cation together with a confined (CN)− unit opens new avenues for carbon bond stabilization using a single-metal cation within a carbon cage. In this constrained setting, the (CN)− fragment exhibits radical or nitrogen carbene characters, forming a triple bond that can be effectively stabilized by coordination to a single metal center. However, the more reduced (CN)3−, featuring a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ti double bond, could interact simultaneously with multiple metals. These confinement-induced variations reveal how the same chemical formula can adopt distinct electronic configurations under different coordination and charge conditions within carbon cages.
Ti double bond, could interact simultaneously with multiple metals. These confinement-induced variations reveal how the same chemical formula can adopt distinct electronic configurations under different coordination and charge conditions within carbon cages.
Following this breakthrough, encapsulating a single carbon atom in a carbon cage with one metal atom emerged as a distinctive branch of carbon chemistry, characterized by monometallic cyanide clusterfullerenes (CYCFs). Thus, it is meaningful to study the carbon–metal bonds and the structure–property relationships in these CYCF models. To date, a series of metallic carbon clusters have been encapsulated in carbon cages, leading to the formation of unique carbon–metal bonds in MCN@C76 (M = Y, Tb, and Lu),41,42 MCN@C84 (M = Y, Tb, and Dy),43 and MCN@C82 (M = Y, Tb, Lu, Dy, and U)37,42,44–46 (Fig. 3). Most studies focus on the influence of the fullerene cage and the metal atom on the configuration and bonding mode of the inner MCN cluster. For example, the triangular DyCN cluster is found to be tuneable in three DyCN@C82 isomers, as revealed by single-crystal X-ray diffraction (XRD) analyses, owing to the strong metal–cage interactions.45 Variations of up to 20° in the Tb–C–N bond angle in TbCN@C82 isomers further underscore the geometry-dependent properties of the entrapped metal cluster.44 Subsequently, Yang, Xie, and co-workers studied the MCN@C84 series (M = Y, Dy, and Tb), revealing that both the ionic radius of the metal cation and the fullerene isomer significantly influence the metal–cage interactions.43 These findings highlight effective strategies for manipulating the carbon–metal interactions constrained within carbon cages.
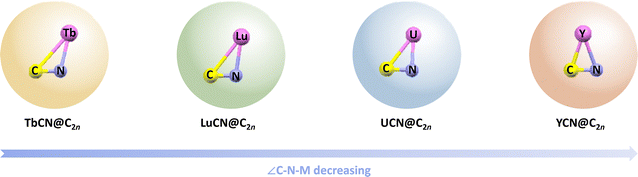 | ||
| Fig. 3 Geometrical models of monometallic cyanide clusterfullerenes MCN@C2n (M = Y, Tb, Lu, and U). There is a decrease in the bond angle of ∠C–N–M with a change in the encaged metal atoms. | ||
Recently, researchers have increasingly focused on the role of nonmetal atoms within the inner carbon clusters constrained in CYCF models. The (CN)− ligand, a prevalent species in coordination chemistry, is known for its strong coordination ability, which stabilizes different metals in various oxidation states and adopts diverse bonding patterns.47 Chen, Autschbach, and co-workers revealed that the CN ligand adopts a novel η2 coordination format in the successfully synthesized UCN@C82. The bonding analysis demonstrated significant donation bonding between the (CN)− fragment and the uranium atom, while examination of its electronic features indicated that the uranium atom donates α-spin electron to the carbon cage. As a result, the U spin population is reduced to less than 3, partially compensating for the donation bonding from the (CN)− fragment in terms of the total electron count in the uranium atom.46 Lu, Jin, and co-workers identified a negative charge on the carbon atom in four novel Lu-based CYCFs, and observed that the nitrogen atom consistently resides closer to the metal atom to optimize the electrostatic interactions. They also highlighted the importance of Lu–cage coordination in regulating the cluster configuration, as interactions between the carbon or nitrogen atoms and the encapsulated Lu center play a crucial role, occasionally even giving rise to a terminal carbon atom within the constrained cluster (Fig. 4). Specifically, when the lutetium atom bonds with both internal nitrogen and carbon atoms, it tends toward the maximum coordination, resulting in weaker Lu–cage interactions and a triangular rather than linear LuCN cluster configuration.42
2 CXM2@C2n
Unlike the previously studied μ2-oxygen–metal bonds constrained within fullerene cages,48 μ2-carbon-bridging metal bonds in CXM2@C2n were only isolated recently for the first time, comprising a single carbon atom bridging two metal centers within a fullerene cage and representing an unprecedented form of constrained carbon bonding and endohedral configuration. This achievement also highlights the uniqueness of constrained multicenter carbon bonding, which is rare in conventional organometallic compounds. The inner carbon atom, with an oxidation state of −4, of the U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U unit was successfully constrained in the diuranium carbide cluster fullerene U2C@C80 by Echegoyen, Chen, and co-workers. The carbon atom forms double bonds with each uranium atom, leading to a high oxidation state of +5 for both uranium atoms.49 Later, Echegoyen et al. reported a series of U2C@C2n (2n = 72, 78, and 80),31 providing valuable insights for further exploring the confined carbon bonding inside carbon cages. In 2019, Jin et al. studied the constrained carbon bonding characteristics in U2C@C2n (2n = 60, 68, 72, 78, 80, 88, 96, and 104) using density functional theory (DFT) calculations. They notably found that in addition to covalent two-center two-electron (2c–2e) U–C bonds, the constrained U2C unit consistently features two three-center two-electron (3c–2e) carbon bonds (Fig. 5).
U unit was successfully constrained in the diuranium carbide cluster fullerene U2C@C80 by Echegoyen, Chen, and co-workers. The carbon atom forms double bonds with each uranium atom, leading to a high oxidation state of +5 for both uranium atoms.49 Later, Echegoyen et al. reported a series of U2C@C2n (2n = 72, 78, and 80),31 providing valuable insights for further exploring the confined carbon bonding inside carbon cages. In 2019, Jin et al. studied the constrained carbon bonding characteristics in U2C@C2n (2n = 60, 68, 72, 78, 80, 88, 96, and 104) using density functional theory (DFT) calculations. They notably found that in addition to covalent two-center two-electron (2c–2e) U–C bonds, the constrained U2C unit consistently features two three-center two-electron (3c–2e) carbon bonds (Fig. 5).
 | ||
| Fig. 5 Localized molecular orbitals (LMOs) of (a) U2C@C80 and (b) U2C@C104. These LMOs represent the constrained carbon–metal bonding patterns (U: blue and C: gray). Reprinted from ref. 32 with permission from the American Chemical Society. Copyright © 2019. | ||
Furthermore, the U–C–U bond angle within the cluster increases from 96.9° in C60 to 180.0° in C104, suggesting a cage-size-dependent cluster configuration. However, additional evidence suggests that the geometry of the U2C unit is also affected by the charge transferred from the cluster to the outer cage, with 6-electron transfer favouring a bent configuration and 4-electron transfer favouring a linear one. Changes in the bond angle closely correlate with the charge state and hybridization state of the internally constrained carbon atom. As the bond angle increases, the central carbon atom becomes more negatively charged, reflecting an increased population of both 2s and 2p electrons. This shift results in increased s-character of the carbon atom, indicating greater involvement of the 2s orbital in the U–C bond and giving rise to distinct hybridization states.32 These findings provide direct insights into the electronic factors governing constrained carbon–metal bonding.
Carbon–metal double bonds show evident covalent characteristics within confined carbon cages by forming a μ2-bridged carbide cluster. Recently, researchers successfully encapsulated uranium-based carbide-bridged bimetallic UCCe units within C72 and C78 carbon cages, which are characterized through single-crystal XRD and various spectroscopic analyses. These studies revealed that limited π-overlap leads to significant charge accumulation on the carbide atom, with partial electron density donated into a uranium 5f/6d-hybrid orbital. The central carbon atom is fully ordered in both UCCe@C72 and UCCe@C78, providing an anchoring point. Accordingly, the electronic configurations of the inner carbon unit can be formally represented as (UCCe)6+ in UCCe@C72 and UCCe@C78, where the metal atoms attain their highest formal oxidation states (+6 for U and +4 for Ce), connected by a bridging carbon atom with four bonding electron pairs (formally C4−). Insights into the bonding within endohedral bimetallic carbide UCCe clusters have been provided by natural localized molecular orbitals (NLMOs), which indicate that the four electron pairs on the carbon atom form four optimized LMOs, two σz and two πx/πy pairs, with the UCCe unit aligned along the z-axis (Fig. 6).22 Additionally, Chen et al. reported the successful synthesis of a carbon–thorium double bond [Th![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ti] constrained in a fullerene carbon cage, ThCTi@C82, which was characterized by single-crystal X-ray diffraction as a bent μ2-bridged carbide cluster. The Th–C bond has the shortest distance reported to date in any isolable compound, which is 2.123(18) Å. Th
Ti] constrained in a fullerene carbon cage, ThCTi@C82, which was characterized by single-crystal X-ray diffraction as a bent μ2-bridged carbide cluster. The Th–C bond has the shortest distance reported to date in any isolable compound, which is 2.123(18) Å. Th![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ti adopts an unexpected nonlinear configuration with a bond angle of 133.0(10)°. Combined experimental and theoretical investigations revealed that the Th
Ti adopts an unexpected nonlinear configuration with a bond angle of 133.0(10)°. Combined experimental and theoretical investigations revealed that the Th![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is polarized toward the bridged carbon and exhibits significantly greater covalency than previously reported Th–C bonds in organometallic complexes.23 These results collectively underscore the exceptional role of carbon cages in enabling the formation of otherwise elusive carbon–metal bonding motifs through spatial and electronic confinement.
C bond is polarized toward the bridged carbon and exhibits significantly greater covalency than previously reported Th–C bonds in organometallic complexes.23 These results collectively underscore the exceptional role of carbon cages in enabling the formation of otherwise elusive carbon–metal bonding motifs through spatial and electronic confinement.
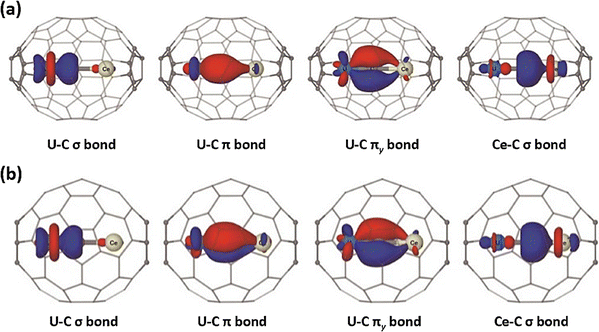 | ||
| Fig. 6 Optimized natural localized molecular orbitals (NLMOs) of the encaged UCCe moiety and constrained carbon–metal bonding patterns in (a) UCCe@C72 and (b) UCCe@C78. Reprinted from ref. 22 with permission from the American Chemical Society. Copyright © 2023. | ||
3 CXM3@C2n
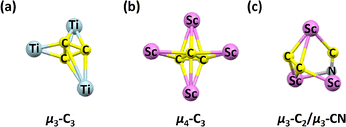
The confinement of three metal atoms and one carbon atom within carbon cages leads to the formation of a μ3-carbido ligand in endohedral metallofullerenes (EMFs), which may include hydrocarbon, cyanides, or carbide units. The μ3-carbido ligand provides a unique opportunity to explore multiple bonds between carbon and metal atoms, while also stabilizing metal cations in various oxidation states, an important pursuit in carbon chemistry. Typically, a single bond exists between the inner metal atom and a central nonmetal atom in EMFs. However, the diverse oxidation states in these systems enable the formation of TiLu2C@C80, featuring a μ3-carbido ligand and representing the first EMF with a constrained multiple carbon bond between metal and central carbon atoms (Fig. 7).50 Subsequently, carbon–titanium bonds were also confirmed in metal carbide clusterfullerenes (CCFs), including Dy2TiC@C80 (no crystal structure),51 Sc2TiC@C80,52 TiTb2C@C80,53 and CeTi2CN@C80.24 The first mixed actinide–scandium cluster fullerene, Sc2UC@C80, was characterized by single-crystal X-ray diffraction, showing that the uranium atom in Sc2UC exists in the +4 oxidation state. This oxidation state is essential for stabilizing the overall molecule and is influenced by the specific cluster composition.54 | ||
| Fig. 7 Schematic of the multiple bonds and electronic distribution in TiLu2C@C80 with a μ3-carbido ligand. | ||
Crystallographic studies reveal singly bonded CN ligands coordinating vertically to the CeTi2 trimetallic plane within the constrained cluster, differing significantly from the bat-ray conformations reported in all homonuclear trimetallic fullerenes. These bonding characteristics result in variable electronic structures, such as Ce3+(Ti2)8+(CN)5−@(C80)6−, with fixed tetravalent Ti4+ and trivalent Ce3+, as confirmed by X-ray absorption spectroscopy, magnetometry, and theoretical studies. The conformations of trimetallic carbonitride clusters are precisely dictated by the bonding nature of the CN ligand, which enables strong coordination with the encapsulated metals. Following this general principle, a strong ligand field is achieved at Ce3+ in the presence of a singly bonded CN ligand in CeTi2CN@C80, giving rise to the first cerium-based single-molecule magnet with open magnetic hysteresis at 2 K.24 For example, a multiple carbon bond [Ce![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSc2] between a lanthanide ion and a carbon atom constrained inside a carbon cage was recently reported, representing an unprecedented bonding scenario in carbon chemistry.11 Lanthanide–carbon multiple bonds are difficult to form in traditional coordination and organometallic chemistry.55 X-ray crystallography, spectroscopic analyses, and quantum chemical calculations reveal a Ce–C bond distance of 1.969(7) Å, which is close to the sum of Pyykkö's triple-bond covalent radii of 1.91 Å, suggesting the assignment of a formal Ce
CSc2] between a lanthanide ion and a carbon atom constrained inside a carbon cage was recently reported, representing an unprecedented bonding scenario in carbon chemistry.11 Lanthanide–carbon multiple bonds are difficult to form in traditional coordination and organometallic chemistry.55 X-ray crystallography, spectroscopic analyses, and quantum chemical calculations reveal a Ce–C bond distance of 1.969(7) Å, which is close to the sum of Pyykkö's triple-bond covalent radii of 1.91 Å, suggesting the assignment of a formal Ce![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond. This bonding primarily arises from the stronger affinity between carbon and cerium compared to scandium in the encapsulated cluster. These findings reveal that lanthanides can form stable multiple carbon bonds, even though the isolation of lanthanide–carbon double or triple bonds remains challenging in traditional coordination and organometallic systems.
C triple bond. This bonding primarily arises from the stronger affinity between carbon and cerium compared to scandium in the encapsulated cluster. These findings reveal that lanthanides can form stable multiple carbon bonds, even though the isolation of lanthanide–carbon double or triple bonds remains challenging in traditional coordination and organometallic systems.
Encapsulation of hydrocarbon clusters is currently the only known way to stabilize hydrogen atoms within fullerene cages, aside from diatomic molecules such as H2O and NH3.56,57 Notably, the first five-atom hydrocarbon cluster encapsulated in a fullerene, Sc3CH@C80, was characterized by Dunsch and co-workers using spectroscopy and DFT calculations.58 This finding was later corroborated by Popov et al. through 13C NMR spectroscopy.25 They discovered that Sc3N@C80 and Sc3CH@C80 exhibit nearly identical frontier molecular orbital (FMO) distributions, as shown in Fig. 8, which is attributed to the isoelectronic nature of CH and N. In this case, the inner carbon atom or the constrained carbon bond plays a crucial role in stabilizing the EMFs due to its versatile hybridization states.
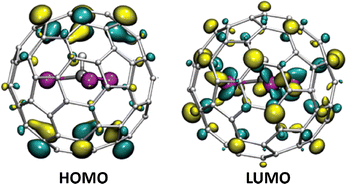 | ||
| Fig. 8 Frontier molecular orbitals (FMO) of Sc3CH@C80 showing the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). Reprinted from ref. 25 with permission from the Royal Society of Chemistry. Copyright © 2016. | ||
The stabilization of the (CN)3− and (CN)− bonds with metal cations opens new pathways for the design of carbon bonds within fullerene cages. In 2010, the unprecedented (CN)3− trianion was discovered in as-synthesized Sc3CN@C80, as characterized by Wang and co-workers. Unlike the pyramid M3CH clusters constrained within fullerenes, the Sc3CN moiety is the first planar five-atom cluster identified inside a fullerene cage.59 Following the isolation of Sc3CN@C80, a similar planar Sc3CN structure and (CN)3− bonding motif were also revealed in Sc3CN@C78.60 This was achieved using the arc-discharge method, suggesting that these planar clusters are key templates in the design of EMFs. A C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond is evident in Sc3CN@C2n (2n = 68, 78 and 80), as demonstrated in the theoretical study by Jin, Chen, Lu, and co-workers.61 Similar to Sc3N@C80, Sc3CN transfers six electrons to the fullerene cage, along with an additional carbon atom. Direct crystallography of M3CN was presented in the new actinide-rare earth mixed metallic cluster fullerene USc2CN@C80 with a unique trifoliate configuration. The bonding study indicated that USc2CN contains C
N double bond is evident in Sc3CN@C2n (2n = 68, 78 and 80), as demonstrated in the theoretical study by Jin, Chen, Lu, and co-workers.61 Similar to Sc3N@C80, Sc3CN transfers six electrons to the fullerene cage, along with an additional carbon atom. Direct crystallography of M3CN was presented in the new actinide-rare earth mixed metallic cluster fullerene USc2CN@C80 with a unique trifoliate configuration. The bonding study indicated that USc2CN contains C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonding motif (Fig. 9), with multicenter triple-bond characters and pronounced donation bonding. Its electronic structure was determined to be U5+(Sc2)6+N3−C4−@(C80)4−, indicating that the carbon and nitrogen atoms achieve filled octet, and thus are unable to bond with each other.26
N bonding motif (Fig. 9), with multicenter triple-bond characters and pronounced donation bonding. Its electronic structure was determined to be U5+(Sc2)6+N3−C4−@(C80)4−, indicating that the carbon and nitrogen atoms achieve filled octet, and thus are unable to bond with each other.26
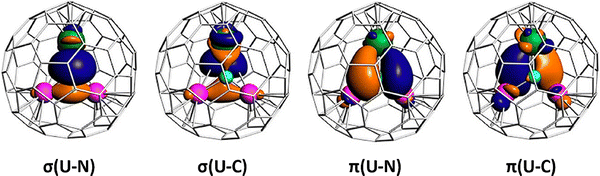 | ||
| Fig. 9 NLMOs of USc2CN@C80. These orbitals are obtained from the natural bond orbital (NBO) calculations and show the constrained bonding formats. (U: dark green; Sc: purple; N: cyan; and C: gray). Reprinted from ref. 26 with permission from the American Chemical Society. Copyright © 2023. | ||
4 CXM4@C2n
In theory, a carbon atom can form four bonds, but bonding with four metal atoms has traditionally remained unachievable. Due to the confinement effect of the carbon cage, a central μ4-C atom bonded to a tetrahedral Sc4 cluster has been stabilized in Sc4C@C80 (Fig. 10). This structure, prepared and characterized through both experimental and theoretical methods, represents the first example of a carbon atom bonded to four metal atoms.62 Electronic structure analysis revealed that the fullerene cage carries a −6 charge, while the central carbon atom holds a −4 charge. This suggests that the combined charge on the scandium atoms does not exceed +10. Analysis of the frontier molecular orbitals of Sc4C@C80 shows that the HOMO is primarily localized on the scandium atoms and evenly distributed among them. As a result, each scandium atom has a formal oxidation state of +2.5.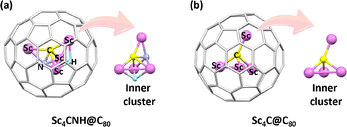 | ||
| Fig. 10 Geometries of (a) Sc4CNH@C80 and (b) Sc4C@C80 including their corresponding inner moieties with the constrained μ4-carbon center. | ||
A second tetrahedron Sc4 cluster supported by CN and H ligands has been stabilized within the fullerene cage, forming Sc4CNH@C80. This metallofullerene exhibits a rare multilayered nested structure (Fig. 10), making it one of the most complex endohedral assemblies reported to date. It features multiple bonding types, including an unprecedented metal–hydride bond within a fullerene cage. Theoretical calculations reveal that the encapsulated cluster contains a unique μ4-(CN)5− anion, showcasing the versatility of carbon and a novel oxidation state for the CN moiety.63 However, despite these advancements, no crystal structure has yet confirmed the encapsulation of four metal atoms and one carbon atom within a single fullerene cage. The discovery of the unique μ4-(CN)5− anion expands the known carbon bonding motifs and provides a foundation for designing new carbon-based compounds. These systems not only expand the known boundaries of carbon chemistry but also establish new principles for understanding carbon–metal bonding under extreme confinement.
III. Bonding pattern of two carbon atoms
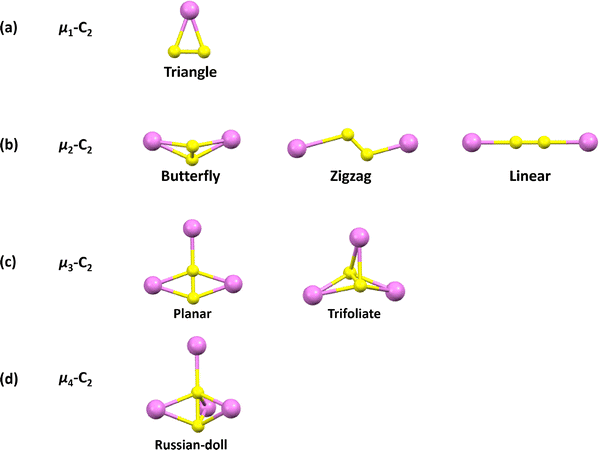
The incorporation of two carbon atoms (2C) within carbon cages represents one of the most prevalent and distinctive bonding motifs. In these systems, the constrained carbon atoms remain distinct from those in the structural backbone of the cage, forming unconventional encapsulated units, which give rise to unique C2 motifs. The dicarbon C2, the smallest diatomic species containing a carbon–carbon bond, is an intriguing species owing to its fundamental yet elusive nature. Unlike diatomic species such as F2, O2 and N2, C2 has been observed only indirectly under extreme conditions,64–66 typically in the gas phase, and remains highly reactive and transient under ambient conditions. In conventional chemistry, C2 has been stabilized in ligand-bound complexes of the type L → C2 ← L or L → C2,65,67 but the bonding situation of the central C2 in these motifs differs markedly from that of free C2. Recent advances have enabled the identification of numerous C2-containing endohedral clusters, including Sc4C2H, Sc4C2, Dy3C2, Dy2TiC2, Y2C2, VSc2C2, Sc2/3C2, ErYC2, and ThC2 in various conformations (Fig. 11). These compounds feature Cq−2 (q = 2, 3, 4, 5, and 6) anions and exhibit diverse carbon–metal bonding modes.68,69 For example, combined theoretical and experimental studies revealed pronounced donation bonding from (C2)2− to Th4+ (i.e., C2 → Th), along with three-center bonding characteristics involving the inner constrained ThC2 unit in the formal electronic state of Th4+(C2)2−@(C82)2−.70 These achievements provide unprecedented opportunities to study the behavior of the C2 unit under spatial confinement and within a well-defined coordination environment, thereby opening new avenues for exploring unconventional main-group bonding motifs and previously inaccessible carbon–metal bonding modes.One of the most remarkable features of encapsulated C2 units is the contraction of their carbon–carbon bond length relative to that of typical triple bonds. While free C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds generally have a bond length of around 1.20 Å, the C–C bonds constrained within carbon cages can range from 1.00 to 1.23 Å.71,72 This contraction arises from the highly constrained electronic environment imposed by the carbon cage and strong interactions with surrounding metal atoms, resulting in bonding characteristics that differ significantly from those of isolated molecules. A deeper understanding of these novel carbon–carbon bonding modes enables the detailed interpretation of structure–property relationships in endohedral carbon clusters, including variations in bond lengths, charge distributions, and frontier molecular orbitals, which collectively govern their stability and reactivity. Moreover, this suggests a new dimension in carbon chemistry, wherein spatial confinement and metal–carbon coordination can be exploited to engineer novel carbon bonding motifs. Thus, a systematic investigation of the carbon–nonmetal and carbon–metal interactions within carbon cages will not only enrich our understanding of fundamental carbon bonding principles but also guide the rational design of advanced carbon-based materials.
C bonds generally have a bond length of around 1.20 Å, the C–C bonds constrained within carbon cages can range from 1.00 to 1.23 Å.71,72 This contraction arises from the highly constrained electronic environment imposed by the carbon cage and strong interactions with surrounding metal atoms, resulting in bonding characteristics that differ significantly from those of isolated molecules. A deeper understanding of these novel carbon–carbon bonding modes enables the detailed interpretation of structure–property relationships in endohedral carbon clusters, including variations in bond lengths, charge distributions, and frontier molecular orbitals, which collectively govern their stability and reactivity. Moreover, this suggests a new dimension in carbon chemistry, wherein spatial confinement and metal–carbon coordination can be exploited to engineer novel carbon bonding motifs. Thus, a systematic investigation of the carbon–nonmetal and carbon–metal interactions within carbon cages will not only enrich our understanding of fundamental carbon bonding principles but also guide the rational design of advanced carbon-based materials.
1 C2XM@C2n
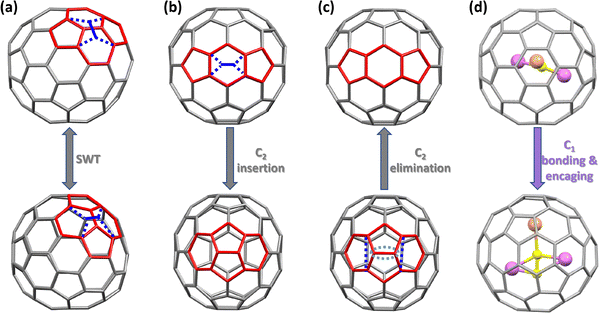
To date, constrained μ1-C2 units remain elusive in carbon cages because most MC2n molecules exist as M@C2n rather than MC2@C2n−2, despite the wide variety of carbide cluster fullerenes (CCFs) reported with μ2-, μ3-, and μ4-C2 motifs entrapping two to four metal atoms.27,73–80 The first terminal μ1-C2 unit was stabilized in 2022 via coordination to a thorium atom in ThC2@C82, the first thorium-containing single-metal CCF, in which the encapsulated cluster adopts an isosceles triangular configuration.70 In this molecule, a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C fragment binds directly to the Th centre, forming a terminal μ1-C2 ligand, as confirmed by mass spectrometry, single-crystal XRD, UV-vis-NIR spectroscopy, and theoretical calculations.70 Computational studies support a formal electronic structure of Th4+(C2)2−@(C82)2−, revealing substantial donation from (C2)2− to Th4+ (C2 → Th), as shown in Fig. 12. The structure of ThC2@C82 establishes a terminal μ1-C2–metal bonding as a viable motif for endohedral fullerenes and suggests that MC2@C2n−2 should also be considered among the possible isomers for the stoichiometric MC2n composition, commonly assigned as M@C2n. The existence of μ1-C2–metal motifs is further supported by the observation of simple metal carbides such as ScC2, YC2, and AlC2 in the vapour above solid carbides or metal–graphite systems.81–84 However, investigations on their structural and electronic properties have thus far been largely theoretical. Stabilising μ1-C2–metal clusters inside carbon cages remains a significant synthetic challenge.
C fragment binds directly to the Th centre, forming a terminal μ1-C2 ligand, as confirmed by mass spectrometry, single-crystal XRD, UV-vis-NIR spectroscopy, and theoretical calculations.70 Computational studies support a formal electronic structure of Th4+(C2)2−@(C82)2−, revealing substantial donation from (C2)2− to Th4+ (C2 → Th), as shown in Fig. 12. The structure of ThC2@C82 establishes a terminal μ1-C2–metal bonding as a viable motif for endohedral fullerenes and suggests that MC2@C2n−2 should also be considered among the possible isomers for the stoichiometric MC2n composition, commonly assigned as M@C2n. The existence of μ1-C2–metal motifs is further supported by the observation of simple metal carbides such as ScC2, YC2, and AlC2 in the vapour above solid carbides or metal–graphite systems.81–84 However, investigations on their structural and electronic properties have thus far been largely theoretical. Stabilising μ1-C2–metal clusters inside carbon cages remains a significant synthetic challenge.
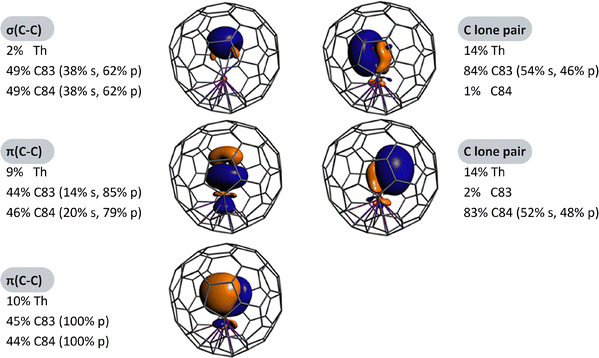 | ||
| Fig. 12 NLMOs of singlet ThC2@C80 and atomic orbital weight compositions. Reprinted from ref. 70 with permission from the Royal Society of Chemistry. Copyright © 2022. | ||
2 C2XM2@C2n
In 2001, the first constrained μ2-C2–metal bonding in an endohedral metallofullerene (EMF) was identified through the isolation and characterization of Sc2C2@C84, using the 13C NMR spectroscopy and synchrotron X-ray powder diffraction techniques.85 This finding marked the emergence of systematic studies on CCFs and opened opportunities to understand the constrained μ2-C2 carbon bonding and M2C2n molecules. The single 13C NMR signal at δ = 92 ppm corresponds to an sp-hybridized carbon atom in Sc2C2, suggesting that the Sc2C2 cluster undergoes rapid rocking along the Sc–Sc axis between two mutually orthogonal orientations. Subsequent studies revealed a planar C2 rotor within crystalline Sc2C2@C84, described as a quantum gyroscope with a cardanic suspension. This configuration reflects substantial Coulomb interactions together with covalent overlap between the dicarbon unit and the scandium centers.86 The μ2-C2 carbon bonding, exhibiting both covalent and ionic characteristics, has been examined experimentally and theoretically in a series of scandium-based CCFs, including Sc2C2@C2n (2n = 68, 72, 74, and 80 ≤ 2n ≤ 88).87–94 As shown in Fig. 13, systematic variations are observed in the Sc–C2–Sc dihedral angles as a function of cage size. Larger dihedral angles are found in C68 and C70, where the constrained carbon clusters are oriented along the long axis of the cage. These findings highlight the pronounced confinement effect exerted by the fullerene framework on the geometry of the encapsulated carbon clusters. Re-investigations revealed that the structure originally assigned as Sc2@C84 was in fact Sc2C2@C82, exhibiting a μ2-C2 carbon–metal bonding motif,88 as confirmed by single-crystal XRD analysis, carbene reactions, DFT calculations, and analysis of the 13C NMR chemical shifts of the C2 unit.89,95,96 This bonding pattern was further corroborated by the characterization of a carbene adduct of Sc2C2@C80via X-ray crystallographic analysis by Akasaka, et al.90 Lu and co-workers later demonstrated that incorporation of a C2 unit balances the interactions between the metal atoms, and between the fullerene cage and the encapsulated cluster, yielding a bent configuration in Sc2C2@C82.97 These findings underpin the dominant covalent characteristics of the bonding between the dicarbon unit and the metal centers.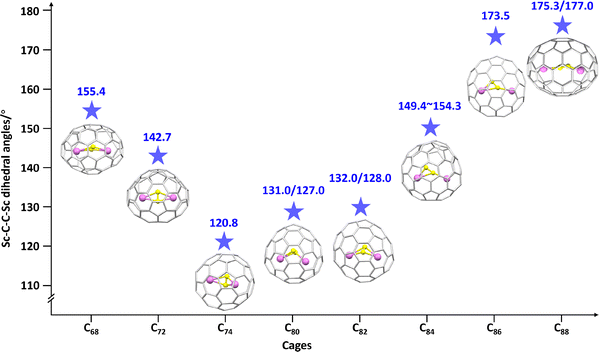 | ||
| Fig. 13 Variation in Sc–C–C–Sc dihedral angle in Sc2C2@C2n (2n = 68, 72, 74, 80, 82, 84, 86, and 88) as a function of fullerene cage size. Encaged carbon atom: yellow and scandium atom: violet. | ||
The significant shielding effect of the C2 unit on metal nuclei was elucidated by Wang et al. in the synthesized Sc2C2@C72 molecule, characterized by single-crystal XRD and NMR spectroscopy. This observation underscores how endohedral nonmetallic elements, such as C2 units, significantly influence the electronic structures of cluster fullerenes and the interactions between clusters and cages, including constrained carbon bonding.92 Lu et al. further demonstrated that endohedral carbide clusters create highly reactive regions critical for modulating the chemical properties of the fullerene cage.98 Spectroscopic studies by Wang et al. revealed a unique temperature-dependent C2-dominated wagging vibration in Sc2C2@C82, affecting the cage motion.99 Furthermore, NMR investigations indicated no cross-peak at δ = 253.2 ppm for the C2 unit, suggesting its electronic separation from the fullerene cage.95
The first example of metal–carbon bonding constrained within a non-IPR (isolated pentagon rule) carbon cage was reported in 2006 with the isolation and characterization of Sc2C2@C68. The formal charge state of (Sc2C2)4+@(C68)4− indicates a transfer of four electrons from the encapsulated Sc2C2 cluster to the C68 cage, resulting in a closed-shell electronic configuration.87 The attachment of an addend to the fullerene cage can significantly alter both the cage structure and the cluster conformation compared to pristine EMFs.100 Notably, chemical modification can induce significant framework rearrangements in fullerenes cages. For example, the IPR-obeying D2–C76 cage undergoes a transformation to a non-IPR isomer upon chlorination.101
Akasaka and co-workers obtained X-ray crystallographic structures of a series of unfunctionalized carbide cluster EMFs, Sc2C2@C80, Sc2C2@C82, and Sc2C2@C84, co-crystallized with cobalt-based octaethylporphinate. The Sc2C2 cluster adopts a planar structure, as evidenced by increasing the Sc–Sc distances and Sc–C2–Sc dihedral angles with larger cage sizes (Fig. 14), indicating variations in the strength of the constrained carbon–metal bonds.90 However, the C–C distances within the carbide cluster remain constant, suggesting its stabilizing effect on the entire cluster.91 In 2015, Balch, Olmstead, Echegoyen, and co-workers synthesized, isolated, and characterized a new CCF, Sc2C2@C86, which features a notably planar but twisted arrangement of the inner Sc2C2 unit, giving rise to an irregular overall geometry.93 Additionally, the first heptagon-containing endohedral carbide fullerene, Sc2C2@Cs(hept)–C88, was synthesized and isolated, featuring a zigzag Sc2C2 inner structure.102
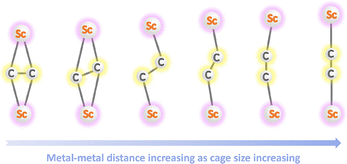 | ||
| Fig. 14 Influence of fullerene cage size on the elongation of metal–metal distances within encapsulated Sc2C2 clusters. | ||
The first example of a constrained μ2-C2 carbon–yttrium bonding in a carbon cage was elucidated with the isolation of Y2C2@C82.103 Shinohara et al. proposed that highly excited Y2@C84 preferentially evaporates a C2 unit, either outward or inward, stabilizing the fullerene and forming Y2@C82 and Y2C2@C82, respectively.104 Entrapment of the C2 radical appears to slightly reduce electron transfer to the C82 cage. Later, maximum entropy method (MEM) charge density distributions showed that the C2 unit resides within a pentagonal dodecahedron formed by Y2 ions, likely mitigating the Coulomb repulsion between Y metallic ions, in Y2C2@C82, which was determined using the maximum entropy method (MEM)/Rietveld and synchrotron X-ray powder diffraction.105 In 2018, Yang et al. presented the first unambiguous structural determination of a yttrium-based CCF, specifically its trifluoromethyl derivatives, Y2C2@C82(CF3)16, with confirmed μ2-C2 carbon–yttrium bonding.27 Recently, we reported a unique cage elongation along the cycloparaphenylene belt, induced by direct C2 insertion within a C82 cage through crystallographic analysis of Y2C2@C82 and its comparative counterpart Y2@C82.106 These results provide crystallographic evidence for the endohedral C2-induced cage elongation and an in-depth understanding of the ambidextrous deformability of fullerene cages.
Subsequently, Dorn et al. explored the constrained carbon–yttrium bonding in a family of yttrium-based CCFs, including Y2C2@C82, Y2C2@C92, and Y2C2@C100, with bent constrained units (Fig. 15) utilizing high-field 13C NMR and DFT calculations, and the results highlight the role of the endohedral carbide atom in the yttrium–carbide scalar coupling constants.107 Crystallographic studies reveal that Y2C2@C108 (Fig. 15), the largest metallofullerene characterized to date, assumes a carbide form and functions as a good electron donor rather than an electron acceptor.108 Lu et al. also confirmed that a C2 unit is constrained in the CCFs Y2C2@C2n (2n = 86, 88, 90, and 92) (Fig. 15), which was identified by UV-vis-NIR spectroscopy and X-ray crystallography, demonstrating that the inserted C2 unit readily accepts valence electrons from metal atoms to form the constrained carbon–yttrium bonding in carbon cages.109 Clearly, the inner Y2C2 units adopt a more bent geometry in relatively smaller cages (e.g., Y2C2@C82), whereas they become more linear in larger cages (e.g., Y2C2@C108).
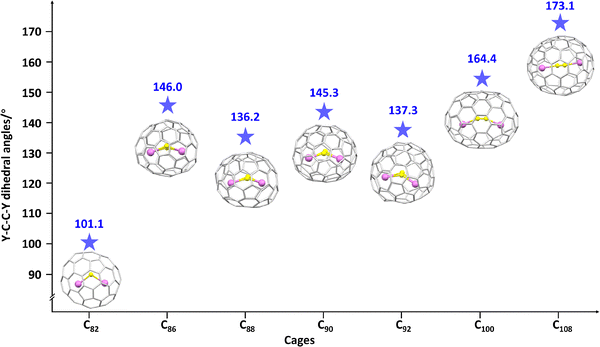 | ||
| Fig. 15 Changes in Y–C–C–Y dihedral angle in Y2C2@C2n (2n = 82, 86, 88, 90, 92, 100, and 108) across different fullerene cages. Encaged carbon atom: yellow and yttrium atom: violet. | ||
Although carbon–titanium (multiple) bonds have been achieved in organometallic and coordinated chemistry,110,111 it is excited to find a constrained carbon–titanium bond with slight distortion in a carbon cage in which the C2 unit is characterized as a bonding bridge. In 2001, Ti2C2n molecules were characterized as Ti2@C80 and Ti2@C84 using electron energy loss spectroscopy and 13C NMR studies.112,113 However, theoretical calculations indicated that Ti2C2@C78 is the energetically preferred isomer with a constrained carbon–titanium bond in the carbon cage, which aligns with high-energy transmission electron microscopy findings.114 Employing DFT calculations, Lu et al. demonstrated a titanium carbide cluster in the Ti2C80 molecule, specifically characterized as endohedral metallofullerene Ti2C2@C78. This electronic structure can be conceptualized as (Ti2)8+(C2)2−@(C78)6− with a stable closed-shell electronic configuration, indicating covalent dative bonding between the Ti4+ cations and (C78)6− cage, along with ionic Ti4+–acetylide interactions.115 High-resolution transmission electron microscopy revealed two configurations of Ti2C2 within the C78 cage (Fig. 16), highlighting the flexibility of the C2 bonds.116 Theoretical studies indicated that encapsulating a C2 unit within a Ti2@C78 molecule is exothermic, as it reduces the Coulomb repulsion between the positively charged titanium atoms in Ti2C2@C78, despite the formation of heteronuclear bonds between the C2 unit and the titanium centers. This encapsulation modulates the electronic states via the significant hybridization of the 3d orbitals of titanium atoms and the 2p orbitals of carbon atoms in both the cage and the encapsulated C2 unit.117
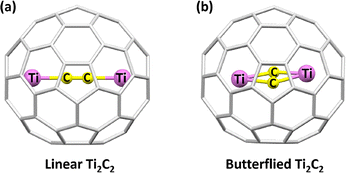 | ||
| Fig. 16 Two structural configurations of Ti2C2 inside the C78 fullerene cage: (a) linear and (b) butterfly arrangement. | ||
Constrained carbon–titanium bonding shows that the coexistence of covalent and ionic bonding characteristics stabilizes the inner carbon unit in different oxidation sates. Zhao et al. advanced the understanding of titanium-based CCFs by presenting a thermodynamically stable singlet structure of (Ti2C2)4+@(C82)4− using DFT calculations and thermodynamic analyses. Quantum theory of atoms in molecules (QTAIM) studies revealed that titanium–carbon bonding within Ti2C2 exhibits a unique balance of covalent and ionic interactions, which contrasts with the largely ionic nature of the μ2-C2–scandium bonding within fullerene carbon cages.74 These distinct bonding behaviours emphasize the adaptability of titanium–carbon interactions in fullerene environments. In 2020, Lu et al. revealed that Ti2C2 adopts different charged states in two unsupported titanium-based CCFs, namely Ti2C2@C78 and Ti2C2@C82. The oxidation states of Ti2C2 are +6 and +4 in Ti2C2@C78 and Ti2C2@C82, respectively, relying on the host cage environment.118 Crystallographic analysis showed that the combination of the charge states and resulting electrostatic potential distribution influences the electronic and geometric configurations of the cluster. This variability in charge states marks a sharp distinction from lanthanide-based Ln2C2 clusters, which consistently maintain a +4 charge state regardless of cage size or shape. This difference is likely driven by the versatile electronic structures of the C2 unit, allowing titanium-based clusters to adapt their electronic configurations according to the specific fullerene carbon cage, and thus suggesting tuneable electronic properties in CCFs.
Strong carbon–metal bonding always leads to the axial compression of carbon cages and reduced metal–metal distances. For example, the X-ray crystallographic structure of an La2C2-containing EMF, La2C2@C100, reveals that the characteristic structural flexibility inherent to carbon nanotubes is preserved within nanotubular fullerenes, even under internal strain (Fig. 17). The embedded C2 unit plays a crucial role in reducing the Coulombic repulsions between the two La ions by partially neutralizing their positive charges, leading to a reduced La–La distance within the carbon cage. Theoretical results suggest the coexistence of covalent and ionic interactions between the metal ions and constrained carbon atoms in lanthanum-based CCFs. The axial compression of the carbon cage arises from the stronger bonding interactions between the lanthanum ions and C2 unit, which behaves like a molecular spring, thereby contracting the surrounding carbon atoms of the carbon cage.119,120 The presence of the C2 unit plays an essential role in stabilizing giant fullerene cages by mitigating the repulsive forces between La ions, which is revealed in the further characterization of two giant fullerenes, La2C2@C102 and La2C2@C104, through X-ray crystallography.121 This series of lanthanum carbide fullerenes, particularly La2C2@C2n (2n = 90–104) (Fig. 17), appears to favour the carbide form over the di-EMF structure La2@C2n (2n = 92–106). This preference is due to the ability of the C2 unit to fulfill the coordination requirements of the La3+ ions, ensuring strong metal–cage interactions. The constrained carbon atoms also accept partial charges from the metal ions, thereby reducing the surface charge density on the fullerene carbon cage. These findings were further confirmed by the X-ray crystallographic study of additional La2C2@C2n (2n = 90–98) structures, reinforcing the role of the C2 unit in stabilizing and optimizing the charge distribution within these complex cages. This charge redistribution by the C2 unit ensures both structural stability and enhances the metal–cage interaction.122
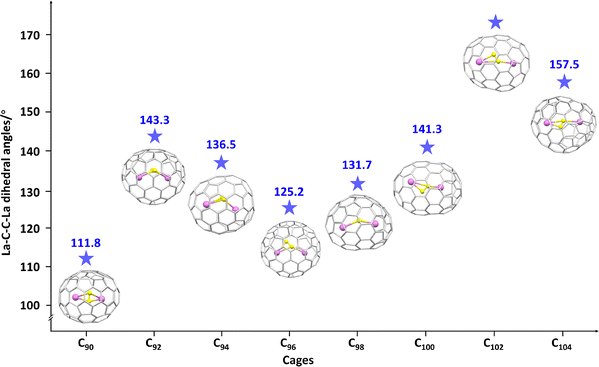 | ||
| Fig. 17 Changes in La–C–C–La dihedral angle in La2C2@C2n (2n = 90–104) with different fullerene cage sizes. Encaged carbon atom: yellow and lanthanum atom: violet. | ||
Shinohara et al. explained that the enhanced photoluminescence (PL) intensity of Er2C2@C82 is due to the encapsulated C2 unit improving the efficiency of energy transfer from the C82 cage to the Er3+ ions compared to that of Er2@C82 when measured in CS2 solution. High-performance liquid chromatography (HPLC) data suggested a slight negative charge on the encapsulated C2 unit, which was indicated by the slightly lower charge density on the carbon cage in Er2C2@C82 than in Er2@C82. Absorption spectral comparisons suggested that the encaged C2 unit widens the HOMO–LUMO gap (Fig. 18) of the C82 carbon cage but does not contribute directly to the f–f transition of the Er3+ ions.123 Furthermore, they noted that the encapsulated C2 unit mediated the intramolecular magnetic interactions in Er2C2@C82 between the Er ions, which were relatively weak with only slightly direct exchange and super-exchange interactions.124 Balch, Olmstead, and co-workers revealed that the internal 2Er3+/(C2)2− group donates four electrons to stabilize the C92 cage in the isolated Er2C2@C92, which was characterized by X-ray crystallographic studies. Furthermore, with the insertion of the C2 unit, the erbium–erbium distance increases in carbon cage.125 Lu et al. isolated and structurally determined six isomers of erbium-containing CCFs as Er2C2@C2n (80 ≤ 2n ≤ 88) via single-crystal XRD. Interestingly, the first oxidation potentials of these erbium-containing CCFs were shifted positively compared to dimetallofullerenes with the same cage but different inner clusters, indicating that erbium-containing CCFs are weaker electron donors, possibly due to the changes in orbital derived from C2 insertion.126 Additionally, X-ray crystallographic analyses of four Er2C2@C90 isomers revealed that the erbium–erbium distance increases along the major axis of the cage, while the bond length of the encapsulated C2 unit decreases accordingly. This inverse relationship suggests that the C2 unit functions as a molecular spring, modulating the metal–cage interactions and adapting to variations in cage size and shape, while maintaining structural stability.127
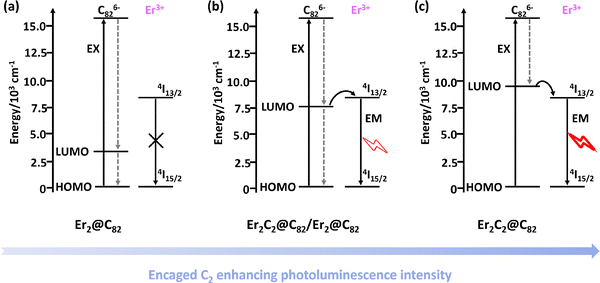 | ||
| Fig. 18 Schematic of the energy dynamics possibly occurring in (a) Er2@C82, (b) Er2C2@C82 (majority) or Er2@C82, and (c) Er2C2@C82. Reprinted from ref. 126 with permission from the American Chemical Society. Copyright © 2007. | ||
Bridged lutetium–carbon bonding is rare in organometallic compounds. However, bridged lutetium–carbon bonding has been constrained in a series of lutetium-containing CCFs, denoted as Lu2C2n (2n = 76, 78, 80, 84, 86, 88, and 90), which have been isolated and structurally characterized in experiment and theory. The experimental and theoretical results revealed that cage expansion leads to elongation of the lutetium–lutetium distance due to the enhanced lutetium–cage interactions. Additionally, the insertion of a C2 unit between the lutetium atoms, leading to the formation of CCFs such as Lu2C2@C2n (2n = 86 and 88), increases the stability by partially taking over the charges from the metal atoms.128 Studies on Lu2C2@C2n (2n = 88, 90, and 92) reveal that an additional C2 unit can be inserted, coordinating with the lutetium atoms to further stabilize the entire molecule.129
Similarly, carbon–metal bonds also act as a ligand field and bonding bridge in carbon cages, which improve the transformation of carbon cages and enhance their magnetic interactions. For instance, the structure of Gd2C2@C84 was elucidated through single-crystal XRD analysis, revealing that the response of the fullerene cage to different endohedral units depends on their size and charge. These variations can induce transformations in fullerene cages, with metal carbide clusters stabilizing the C84 fullerene cage or converting it to C82 (Fig. 19), whereas trimetallic nitrogen clusters tend to induce the conversion of C82 and C80.130 Studies on the Dy⋯Dy superexchange interactions in Dy2C2@C88, which was synthesized and characterized using single-crystal XRD, vibrational spectroscopy, and DFT calculations, revealed that the acetylide group (C2)2− bridge supports ferromagnetic coupling of the magnetic moments of the Dy ions, while the oxide ion O2− bridge in Dy2O@C88 prefers antiferromagnetic coupling. This highlights how the non-metal (C2)2− unit influences the single-ion magnetic anisotropy of the Dy ions, impacting the ligand field as a bonding bridge between Dy ions, and thereby modulating the strength of the superexchange interactions.131
 | ||
| Fig. 19 Transformation map of fullerene cages. This transformation is initiated from a missing fullerene C84, stabilized by encaging a metal carbide cluster, to C80 and the fullerenes in brackets represent the transition state and intermediate. Reprinted from ref. 130 with permission from Springer Nature Limited. Copyright © 2013. | ||
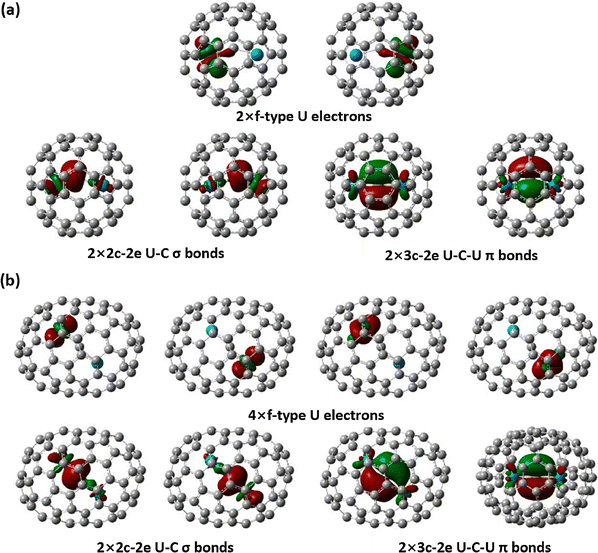
The study on the interactions of actinide atoms and nonmetals, including constrained carbon–actinide bonding, has been advanced by the synthesis and characterization of actinide cluster fullerenes, particularly U2C2@C80 and U2C2@C78, which were characterized using mass spectrometric methods, single-crystal XRD, DFT and multireference wave function calculations. The encapsulated U2C2 represents the first example of constrained μ2-C2–uranium bonding in a carbon cage, forming a distinctive butterfly-shaped geometry in which two uranium centers are bridged by a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C unit. Experimental and quantum-chemical analyses indicate that each uranium atom in the U2C2 cluster assumes a formal oxidation state of +4. Notably, each uranium center transfers three electrons to the C2n carbon cage and one electron to the encapsulated C2 unit, amounting to a net donation of six electrons to the carbon cage. This finding has been supported by Mulliken spin population analysis, which shows two unpaired electrons per uranium atomic center. In the U2C2 unit, the C
C unit. Experimental and quantum-chemical analyses indicate that each uranium atom in the U2C2 cluster assumes a formal oxidation state of +4. Notably, each uranium center transfers three electrons to the C2n carbon cage and one electron to the encapsulated C2 unit, amounting to a net donation of six electrons to the carbon cage. This finding has been supported by Mulliken spin population analysis, which shows two unpaired electrons per uranium atomic center. In the U2C2 unit, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond is somewhat weaker than that in a typical acetylene molecule (HC
C triple bond is somewhat weaker than that in a typical acetylene molecule (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), and the U–C bonds exhibit a primarily ionic nature with a reduced degree of covalency. The U–C bonding is facilitated by limited σ-donation and a great degree of π-donation from the (C2)2− unit to the 5f and 6d orbitals of the uranium centers (Fig. 20). This type of donation carbon bonding (C2 → uranium) enhances the stability of the U2C2 unit within the carbon cages.71
CH), and the U–C bonds exhibit a primarily ionic nature with a reduced degree of covalency. The U–C bonding is facilitated by limited σ-donation and a great degree of π-donation from the (C2)2− unit to the 5f and 6d orbitals of the uranium centers (Fig. 20). This type of donation carbon bonding (C2 → uranium) enhances the stability of the U2C2 unit within the carbon cages.71
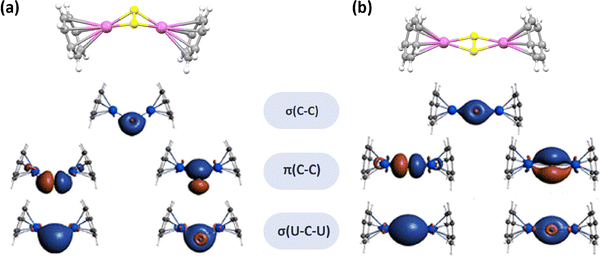 | ||
| Fig. 20 NLMOs of the well-replicated U2C2@C80 model of (a) constraining the ligand coordinates and (b) using C2v symmetry (encaged carbon atom: yellow and uranium atom: violet). The ground states for these molecules are in quintet and the NLMOs are derived from natural bond orbital calculations and mapped with the isosurfaces (±0.03 a.u.). Reprinted from ref. 71 with permission from the American Chemical Society. Copyright © 2019. | ||
3 C2XM3@C2n
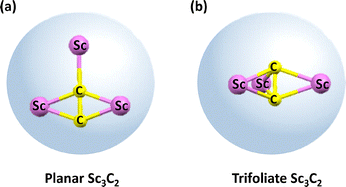
Constrained μ3-C2 carbon–metal bonding is identified in EMF M3C2@C2n, significantly expanding the metallic carbide family with unique oxidation sates of carbon and the types of constrained carbon bonding. One of the earliest characterized EMFs, Sc3C82, was initially described as Sc3@C82 through MEM/Rietveld analysis and DFT calculations in 2005.132,133 This molecule was later revisited and identified as Sc3C2@C82 containing constrained carbon bonding by using 13C NMR spectroscopy, which was later confirmed through chemical functionalization with adamantylidene carbene (Ad). The X-ray crystallographic structure of the resultant Sc3C2@C82(Ad) cycloadduct revealed an unusual trianion (C2)3− unit coordinated by three Sc3+ cations.134 The structure of Sc3C82 was further confirmed later by Shinohara et al. at the spring-8 synchrotron facility, where high-resolution data provided insights into the constrained Sc–Sc interatomic distances within the C80 cage. This study identified a spherical charge distribution coming from the constrained C2 unit, positioned centrally within the triangle of three scandium atoms.135 First-principles molecular dynamics (MD) simulations further demonstrated the dynamic nature of the Sc3C2 moiety, showing its reorientation along the equatorial six-membered ring belt of the fullerene carbon cage and “flipping” motion of the C2 unit across the Sc3 plane.136Additionally, constrained carbon bonding can also tune the configurations of encaged metallic clusters. In 2014, Lu et al. revealed the presence of both “planar” and computationally predicted “trifoliate” configurations of the Sc3C2 cluster in Sc3C2@C80 and its single-bonded derivative by X-ray crystallographic studies (Fig. 21).76,137 Two configurations of encapsulated Sc3C2 cluster were tuned by the constrained carbon bonding formats in the carbon cage. This observation marked the first crystallographic evidence of the trifoliate configuration in this cluster. Investigations of Sc3C2@C80/Ni(OEP) cocrystals showed only the planar Sc3C2 form in pristine Sc3C2@C80, with the unpaired electron localized within the Sc3C2 cluster, thus leaving the fullerene carbon cage electronically unaffected.76 Lu and Tan's DFT studies on the electronic structures and redox properties of Sc3C2@C80 revealed that this EMF exhibits the unusual valence state (Sc3+)3(C2)3−@(C80)6− with a unique oxidation state of the constrained carbon unit. The carbide group in this configuration exhibits a trianionic state (C2)3−, isoelectronic with nitric oxide NO, where its singly occupied π* orbital forms a dative bond with the dπ orbitals of the surrounding three scandium cations.137
There is one unpaired electron stabilized in the inner constrained metal–carbon bonding. The advancement in isolating and characterizing CCFs has led to the discovery of trimetallic carbide systems for transition metals and lanthanide metals, including Lu3C2@C88 and various dysprosium- and erbium-based compounds. For instance, a planar lutetium-based trimetallic carbide cluster has been constructed in Lu3C2@C88, which was successfully prepared and characterized through Raman spectroscopy and DFT calculations. In a comparative analysis with the closed-shell EMF Lu3N@C88, both internal moieties supply the C88 cage with six electrons. However, Lu3C2@C88 possesses an unpaired electron localized on the internal (Lu3C2)6+ moiety, highlighting the role of (C2)3− in the electronic structure of EMFs.138 The valence state of (Lu3C2)6+ primarily originates from the covalent dative bonding between the atomic orbitals of the lutetium cations and the π* orbital of the constrained C2 moiety.
The discovery of multi-centre carbon–metal bonds (3c–1e) within carbon cages enriches the understanding of lanthanide–carbon interactions. The flexible charge state for the constrained C2 unit in lanthanide-based CCFs has led to the unprecedented observation of a 3c–1e Dy–Dy–Dy bond, which was not previously reported in lanthanide chemistry. This groundbreaking finding enriches the bonding theory of lanthanides, highlighting that the unique electronic adaptability of constrained C2 units can enable novel multi-centre metallic bonding configurations. Additionally, (C2)2− has been identified in the new dysprosium-based trimetallic CCF Dy3C2@C80, determined by single-crystal XRD, where the encapsulated Dy3C2 cluster adopts a bat-ray configuration with the acetylide unit C2 elevating above the Dy3 plane.139 In 2022, the crystallographic characterization of the unprecedented erbium-based trimetallic CCF, Er3C2@C80, was reported. DFT calculations indicated that the Er3 plane constructs a 3c–1e Er–Er–Er bond (Fig. 22), where the C2-lifted bat-ray configuration of Er3C2 facilitates single-electron localization among the three metal atoms.140
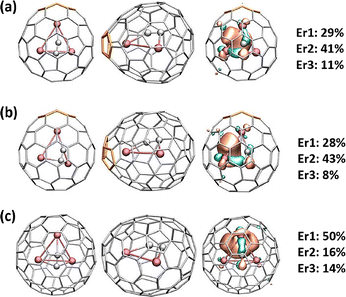 | ||
| Fig. 22 Geometries (top and side view) and single occupied molecular orbitals (SOMOs) of (a) Er3C2@C82, (b) Er3C2@C84, and (c) Er3C2@C86 (erbium atoms: red and carbon atoms: gray). The pentagon adjacency (PA) is highlighted in orange. The numbers represent the contributions of different metal atoms to the molecular orbitals. Reprinted from ref. 140 with permission from the American Chemical Society. Copyright © 2022. | ||
Different metal atoms and carbon units can be confined in one carbon cage at the same time, leading to the formation of multitype constrained carbon–metal bonding. To achieve this aim, the application of methane as a reactive gas can significantly enhance the selectivity of the arc-discharge synthesis of mixed-metal M–Ti CCFs (M = Y, Nd, Gd, Dy, Er, and Lu). A new cluster, featuring an endohedral constrained acetylide unit, in fullerenes, namely M2TiC2@C80 (M = Dy and Lu), has been discovered to exhibit single-molecule magnet (SMM) behaviour, and the presence of a C2 unit in the endohedral cluster has been shown to adversely affect these properties. DFT calculations revealed that the M2TiC2 cluster can adopt two conformations with similar energies. In the most stable conformation, the C2 unit is perpendicular to the M2Ti plane and exhibits μ2-coordination with all metal atoms. In contrast, the other conformation (9 kJ mol−1 higher in energy) has the C2 unit tilted out of the M2Ti plane, displaying μ2-coordination with Ti and one lanthanide, and μ1-coordination with another lanthanide, suggesting that C2 may undergo fluxional motion at room temperature.51 A similar case was identified in the mixed-metal CCF Sc2TiC2@C80 with NMR spectroscopy by Popov et al., where the formal charge and bond distribution in the C2 unit resemble (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)4− that of C4− in Sc2TiC@C80.141 Very recently, Yang et al. synthesized and isolated a novel lanthanide-transition metal heteronuclear EMF, namely, CeTi2C2@C80, which was revealed as a singly bonded C2 ligands coordinating vertically to the CeTi2 trimetallic plane within the cluster by X-ray crystallographic studies, drastically different from the bat-ray conformations reported for homonuclear trimetallic cluster fullerenes.24 DFT calculations further verify the electronic structures and bonding nature of CeTi2C2@C80. Localized molecular orbital (LMO) analysis of this compound indicates four single bonding orbitals based on each nonmetal atom in the C2 unit (Fig. 23). One C–C σ bond, three metal–carbon bonds including one Ce–C and two Ti–C bonds can be clearly assigned for each endohedral carbon atom, leading to a (C2)6− ligand, which necessitates three tetravalent metal ions to form a +6 charged cluster. This remarkable bonding format highlights the close relevance of C2 units governing the cluster conformation in clusterfullerenes.
C)4− that of C4− in Sc2TiC@C80.141 Very recently, Yang et al. synthesized and isolated a novel lanthanide-transition metal heteronuclear EMF, namely, CeTi2C2@C80, which was revealed as a singly bonded C2 ligands coordinating vertically to the CeTi2 trimetallic plane within the cluster by X-ray crystallographic studies, drastically different from the bat-ray conformations reported for homonuclear trimetallic cluster fullerenes.24 DFT calculations further verify the electronic structures and bonding nature of CeTi2C2@C80. Localized molecular orbital (LMO) analysis of this compound indicates four single bonding orbitals based on each nonmetal atom in the C2 unit (Fig. 23). One C–C σ bond, three metal–carbon bonds including one Ce–C and two Ti–C bonds can be clearly assigned for each endohedral carbon atom, leading to a (C2)6− ligand, which necessitates three tetravalent metal ions to form a +6 charged cluster. This remarkable bonding format highlights the close relevance of C2 units governing the cluster conformation in clusterfullerenes.
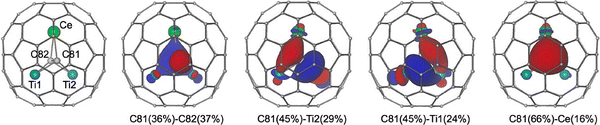 | ||
| Fig. 23 Cluster-based LMOs of CeTi2C2@C80. The C81 atom has four bonding orbitals, and an additional 4f orbital on the cerium atom is identified in CeTi2C2@C80 (left). Reprinted from ref. 24 with permission from the American Chemical Society. Copyright © 2025. | ||
Clearly, the C2 unit plays a significant role in the electronic structure, geometries, dynamics, and SMM characteristics of the inner clusters in CCFs. However, the exact formation mechanism of these units remains unclear, which is crucial for further understanding their roles and enabling their controllable and high-yield synthesis. Recently, Yang et al. proposed a C1 implantation mechanism, suggesting that a carbon atom can infiltrate the carbon wall and enter the fullerene cage, facilitating the transition from VSc2C to VSc2C2 (Fig. 24) based on the successful synthesis of two representative V-Sc CCFs. This C1 implantation mechanism is supported by theoretical calculations, showing an energy barrier of 3.95 eV, which is positioned between the well-known C2 insertion (approximately 2 eV) and Stone–Wales transformation (approximately 7 eV). Furthermore, VSc2C2@C80 represents a crystallographically characterized transition metal-based trimetallic CCF, where its (C2)4− unit leads to the unique oxidation state of V4+, and the single electron on vanadium in VSc2C@C80 facilitates C1 implantation.142
The chemistry of f-block metal–carbon multiple bonds is still underdeveloped compared to the well-established carbene complexes of d-block transition metals. Recently, the new actinide-rare earth mixed metallic CCF USc2C2@C80 was successfully synthesized and characterized by single-crystal X-ray diffraction and DFT calculations, exhibiting a trifoliate configuration in which the C2 unit is nearly vertically inserted into the plane defined by the uranium and two scandium atoms. Combined experimental and theoretical studies revealed that USc2C2 includes constrained C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonding motifs alongside additional multicentral triple-bond characteristics and pronounced donation bonding. Its electronic structure is determined to be U6+(Sc2)6+(C4−)2@(C80)4−, which indicates a filled octet for the constrained carbon atoms. Notably, this represents the first example of uranium in a +6 oxidation state within trimetallic fullerenes due to the incorporation of a C2 unit.143
C bonding motifs alongside additional multicentral triple-bond characteristics and pronounced donation bonding. Its electronic structure is determined to be U6+(Sc2)6+(C4−)2@(C80)4−, which indicates a filled octet for the constrained carbon atoms. Notably, this represents the first example of uranium in a +6 oxidation state within trimetallic fullerenes due to the incorporation of a C2 unit.143
4 C2XM4@C2n
μ4-C2 carbon bonding, although rarely observed in organometallic chemistry, has been both theoretically predicted and experimentally achieved in carbon cage systems. A notable example in this field is the prediction and subsequent characterization of an unprecedented (C2)6−, entrapped in the endofullerene (Sc3+)4(C2)6−@(C80)6− achieving a stable closed-shell electronic configuration, which is thermodynamically and kinetically more favourable than the simple Sc4@C82 structure. DFT calculations revealed that two fully occupied π* orbitals of the (μ4-C2)6− anion are substantially stabilized by the formation of two covalent dative bonds with the dπ orbitals of the four surrounding Sc3+ cations. This finding provides a rational structural model for the elusive Sc4C82 molecule.144 In 2009, Wang, Lu, and co-workers reported the first synthesis, isolation, and characterization of this elusive compound. They confirmed a unique Russian-doll-type structure for Sc4C2@C80, unlike the other endohedral fullerenes encountered to date, using 13C NMR and DFT calculations.145 A subsequent study demonstrated the separation and characterization of Sc4C2@C80 through radio frequency induction furnace, off-line mass spectrometry, electronic absorption spectra, and retention time analyses.146 First-principles DFT calculations indicated that the structural and electronic stability of the Russian-doll Sc4C2@C80 is derived from a quantum registry effect. Moreover, the high icosahedral symmetry of the C80 cage plays a crucial role in facilitating the significant charge transfer of six electrons, contributing to its stable closed-shell electronic structure.147 By 2020, the predicted Russian-doll-type geometry of Sc4C2@C80 was characterized by Raman and mass spectroscopy by Wang et al.63Another EMF, Sc4C2H@C80, was synthesized using the arc-discharge method and isolated through multi-step HPLC. The retention time of Sc4C2H@C80 is similar to that of Sc4C2@C80, and its structure was elucidated through spectroscopic characterization in conjunction with DFT calculations. Specifically, four scandium atoms arranged in a tetrahedron configuration encasing the C2 unit were constrained in a C80 carbon cage. The hydrogen atom bonded the C2 unit forms a hydrocarbon moiety, which is similar to that observed in Sc3CH@C80. Detailed analyses of Kohn–Sham molecular orbitals (Fig. 25) indicated that Sc4C2H@C80 has a valence state of (Sc4C2H)6+@(C80)6−, where the (Sc4C2H)6+ cluster consists of one Sc2+ cation, three Sc3+ cations, a (C2)6− anion, and an H+ cation.80 A later theoretical study identified a new Sc4C2H@C80 isomer featuring μ3-H coordinated to three scandium centers and exhibiting a significantly larger SOMO–LUMO gap. Further multicenter bond analyses revealed the presence of three-center one-electron, three-center two-electron, and even four-center two-electron bonds.148 These findings underscore the crucial influence of the hydrogen position on the electronic structure, bonding characteristics, and relative stability of these constrained endohedral units.
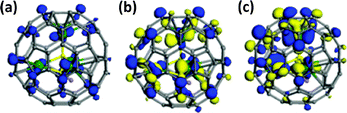 | ||
| Fig. 25 (a) Spin density distributions (blue parts); (b) SOMO; and (c) LUMO of Sc4C2H@C80. (H: pink, Sc: green, Ccarbide: yellow, and Ccage: gray). Reprinted from ref. 80 with permission from the Royal Society of Chemistry. Copyright © 2014. | ||
IV. Bonding pattern of three carbon atoms
Earlier theoretical simulations suggested that up to 17 carbon atoms with complex constrained carbon bonds can be accommodated within the C60 cavity, while still resembling its original spherical structure.149 Another study indicated that the C60 molecule can stabilize endohedral carbon clusters containing up to 9 carbon atoms.150 The encapsulation of three carbon atoms (3C) represents the current upper limit of carbon atom inclusion within carbon cages. These unique 3C-containing clusters provide a valuable platform to explore unconventional carbon bonding under spatial confinement. For example, the observation of the first cyclopropane without any organic substituents was achieved with the formation of multitype constrained carbon–metal bonding, including localized 2c–2e Ti–C/C–C bonds and two 3c–2e Ti–C–Ti bonds. These constrained carbon–metal bonding models led to the superatomic state characteristics with a perfect closed-shell electronic configuration of 1S21P61D10 and the well-known jellium model of an encaged Ti3C3 unit.151 Here, we examine the nature of constrained three-carbon bonding motifs in carbon cages, focusing on their geometries, electronic structures, and coordination modes, based on representative examples (Fig. 26) such as Ti3C3, Sc4C3, and Sc3(μ3-C2)(μ3-CN). This coordination reflects the remarkable adaptability of carbon bonding under confinement and highlights the capacity of carbon cages to stabilize highly unusual bonding motifs, which are rarely accessible in free molecular systems. Understanding these systems not only enriches our knowledge of versatile carbon bonding but also opens new pathways for designing advanced carbon-based materials with novel structural and electronic features.1 C3XM3@C2n
Different carbon bonding formats are constrained within one carbon cage, in combination with the stabilization of the carbon–metal interaction. In 2014, Wang and co-workers successfully revealed a combination of constrained (μ3-C2)2− and (μ3-CN)− carbon–metal bonding in a new EMF, Sc3(C2)(CN)@C80, which was prepared and isolated by using the arc-discharge method and HPLC. Subsequent experimental 13C NMR spectra and theoretical studies confirmed that the endohedral (μ3-C2)2− and (μ3-CN)− moieties are positioned on either side of the triangular Sc3 unit. These components work together to construct and stabilize the unique molecule represented as (Sc3+)3(C2)2−(CN)−@(C80)6−.152 Unfortunately, single-crystal growth was unsuccessful due to insufficient sample availability.The constrained μ3-C3 carbon bonding, i.e., multicentral carbon–metal bonding, stabilizes the inner (Ti3C3)6+ unit with a superatomic state. The successful synthesis of the unique Ti3C3@C80, characterized by single-crystal XRD, marked a significant achievement in the entrapment of a μ3-C3 cluster within a fullerene carbon cage. The μ3-C3 unit adopts an unprecedented cyclopropane-like structure, which coordinates with the three titanium atoms in an unexpected manner, with the triangular μ3-C3 unit oriented nearly perpendicular to the Ti3 plane. This intercalation of a cyclopropane-like μ3-C3 unit within the titanium layer has been unambiguously confirmed. Theoretical results indicated that the Ti3C3 cluster transfers six electrons to the C80 cage, resulting in each titanium atom with a positive charge slightly above +2, while the C3 unit carries an approximate charge of −1.153 This represents the first observation of cyclopropane coordination in a reported organometallic complex. Further theoretical calculations revealed that the (Ti3C3)6+ unit of the recently synthesized Ti3C3@C80 exhibits a superatomic state (Fig. 27), characterized by a perfect closed-shell electronic configuration of 1S21P61D10, which is consistent with the well-known jellium model. This “trapped superatom” exhibits significant aromaticity and hyperconjugation interactions, which has never been reported for other clusterfullerenes. In addition to localized 2c–2e Ti–C/C–C bonds, it also features two 3c–2e Ti–C–Ti bonds. Moreover, the ring strain of the cyclopropane-like C3 core is effectively alleviated upon coordination with the titanium cations.151 This bonding scenario leads to an unusual stabilization of the carbon cluster, facilitated by strong Ti–C interactions and back-donation into the π* orbitals of the carbon framework. Very recently, Chen et al. revealed a unique double-butterfly Ti3C3 cluster encapsulated within a C82 carbon cage with one titanium atom aligned with the symmetry plane of the carbon cage, distinct from that observed in Ti3C3@C80.154 Further crystallographic insights revealed that the Ti–C bond lengths within the Ti3C3 cluster show the coexistence of characteristics of Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds and Ti–C single bonds in Ti3C3@C82. The constrained Ti3C3 configuration represents a novel Ti–C coordination mode, which has never been reported in organometallic complexes or EMF compounds.
C double bonds and Ti–C single bonds in Ti3C3@C82. The constrained Ti3C3 configuration represents a novel Ti–C coordination mode, which has never been reported in organometallic complexes or EMF compounds.
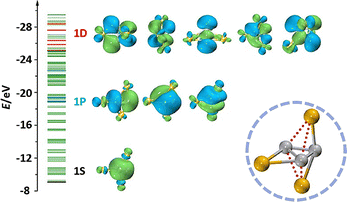 | ||
| Fig. 27 Atom-like superatomic state orbitals (1S21P61D10) of the inner (Ti3C3)6+ moiety in Ti3C3@C80. The occupied molecular orbitals with the energy levels in green for the C80 cage are not shown for clarity (C: gray and Ti: golden). Reprinted from ref. 151 with permission from the American Chemical Society. Copyright © 2020. | ||
2 C3XM4@C2n
The arc-discharge synthesis of Sc-based EMFs in the presence of methane led to the formation of a new metallofullerene, Sc4C83, but chromatographic separation of this EMF proved challenging due to its insufficient stability. The initial structural conjecture centred on Sc–C clusters consisting of four scandium atoms and three carbon atoms encapsulated within an icosahedral C80 cage. DFT calculations were conducted to further investigate the reasonable molecular structures of Sc4C83. The results indicated that the most stable configuration is Sc4C3@C80, where the triangular μ4-C3 moiety is coordinated to the scandium atoms in both η3 and η2 modes. The inner C3 cluster in Sc4C3@C2n provides a highly symmetric electronic environment that supports bond contraction and enhanced electronic coupling. The HOMO–LUMO gap of Sc4C3@C80 is significantly high, exceeding that of Sc4C2@C80, which suggests that Sc4C3@C80 is a stable endohedral fullerene in chemical kinetics. In this structure, all the scandium atoms are in a trivalent state, while the C3 cluster carries a formal charge of −6. Additionally, based on the magnetic criterion of aromaticity, the C3 cluster in Sc4C3@C80 exhibits high aromatic characters.62 It is expected that the X-ray crystallographic structure of Sc4C3@C80 will be achieved in the future, allowing further studies on the roles of confined carbon atoms in EMFs.V. Summary and outlook
This review presents a comprehensive overview of carbon bonding confined in carbon cages. Carbon atoms not only form the structural framework of the cages but also create inner carbon clusters with diverse oxidation states ranging from −1 to −7. This complexity leads to unprecedented carbon–metal bonding interactions, including the challenging multiple carbon–metal bonds, multicenter carbon–metal bonds, carbon–metal covalent bonds, superatomic state carbon bonds, and pronounced donation carbon bonding, enriching the carbon bonding models in traditional organometallic chemistry. This review highlights the significant advancements in understanding key aspects of constrained carbon bonding such as the dispersion of negative charge, reduction of Coulomb repulsion, coordination with metal ions, enhancement of single-molecule magnetic properties, shifts in NMR spectra, and optimal positioning of atoms within the carbon cage. Collectively, these insights into constrained carbon bonding unravel potential pathways for developing innovative carbon-based compounds.Looking ahead, expanding our understanding of constrained carbon bonding is essential for realizing the full potential of EMFs. Beyond their intrinsic chemical interest, EMFs represent promising building blocks for quantum materials, molecular electronics, and catalysis, where tunable charge transfer and spin interactions could be harnessed for device and energy applications.155–163 Future progress will depend on developing selective and controllable encapsulation strategies, enabling precise tuning of cage size, cluster composition, and metal coordination. Furthermore, machine learning and AI-based prediction frameworks offer exciting opportunities to accelerate the discovery of stable EMF structures and bonding motifs,164–167 including constrained one or multiple carbons with unconventional connectivities, hypervalent states, or mixed-metal cooperative bonding, through data-driven exploration. By bridging fundamental chemistry with emerging technologies, the study of carbon bonding under confinement not only deepens our understanding of intrinsic versatility of carbon but also paves the way for the rational design of novel carbon-based materials with tailored electronic, magnetic, and catalytic properties. Sustained interdisciplinary efforts in this area are poised to transform both the conceptual and practical boundaries of carbon chemistry.
Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the conclusions of this study are available within the review article.Acknowledgements
This work was supported by NSFC (No. 22201086, 22301088, 22471084, 92261204, and 22431005), Shaanxi Science and Technology Foundation (No. 2024JC-YBQN-0997 and 2024JC-YBQN-0146), Hubei Provincial Science and Technology Innovation Team Project [2022], Basic and Applied Basic Research Foundation of Guangdong Province (No. I024480008), Start-up Foundation of Xidian University (126022036), Shaanxi Province Postdoctoral Science Foundation (No. 30102230001), Shaanxi Provincial Department of Education Special Research Program (24JK0480), Xi'an Youth Talent Support Program (0959202513128), and the National 111 Center.References
- E. S. Nielsen, Nature, 1946, 158, 594–596 CrossRef CAS PubMed.
- J. F. Dean, G. Coxon, Y. Zheng, J. Bishop, M. H. Garnett, D. Bastviken, V. Galy, R. G. M. Spencer, S. E. Tank, E. T. Tipper, J. E. Vonk, M. B. Wallin, L. Zhang, C. D. Evans and R. G. Hilton, Nature, 2025, 642, 105–111 CrossRef CAS PubMed.
- X. Zhang, X. Lei, X. Jia, T. Sun, J. Luo, S. Xu, L. Li, D. Yan, Y. Shao, Z. Yong, Y. Zhang, X. Wu, E. Gao, M. Jian and J. Zhang, Science, 2024, 384, 1318–1323 CrossRef CAS.
- J. B. Roque, Y. Kuroda, L. T. Göttemann and R. Sarpong, Science, 2018, 361, 171–174 CrossRef CAS PubMed.
- J. Gabler, L. Schafer and H. Westermann, Diamond Relat. Mater., 2000, 9, 921–924 CrossRef CAS.
- R. B. Kaner, J. J. Gilman and S. H. Tolbert, Science, 2005, 308, 1268–1269 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- S. V. Morozov, K. S. Novoselov, M. I. Katsnelson, F. Schedin, D. C. Elias, J. A. Jaszczak and A. K. Geim, Phys. Rev. Lett., 2008, 100, 016602 CrossRef CAS.
- H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- J. R. Heath, S. C. O’Brien, Q. Zhang, Y. Liu, R. F. Curl, F. K. Tittel and R. E. Smalley, J. Am. Chem. Soc., 1985, 107, 7779–7780 CrossRef CAS.
- H. Jiang, J. Zhao, Q. Meng, X. K. Zhao, M. Guo, H. S. Hu, J. Li and N. Chen, Nat. Chem., 2025, 17, 1364–1370 CrossRef CAS.
- X. Lu, L. Feng, T. Akasaka and S. Nagase, Chem. Soc. Rev., 2012, 41, 7723–7760 RSC.
- M. Yamada, T. Akasaka and S. Nagase, Acc. Chem. Res., 2010, 43, 92–102 CrossRef CAS PubMed.
- S. Yang, F. Liu, C. Chen, M. Jiao and T. Wei, Chem. Commun., 2011, 43, 11822–11839 RSC.
- A. A. Popov, S. M. Avdoshenko, A. M. Pendás and L. Dunsch, Chem. Commun., 2012, 48, 8031–8050 RSC.
- M. Rudolf, S. Wolfrum, D. M. Guldi, L. Feng, T. Tsuchiya, T. Akasaka and L. Echegoyen, Chem. – Eur. J., 2012, 18, 5136–5148 CrossRef CAS PubMed.
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
- X. Lu, T. Akasaka and S. Nagase, Acc. Chem. Res., 2013, 46, 1627–1635 CrossRef CAS PubMed.
- A. Rodriguez-Fortea, A. L. Balch and J. M. Poblet, Chem. Soc. Rev., 2011, 40, 3551–3563 RSC.
- P. Jin, C. Tang and Z. Chen, Coord. Chem. Rev., 2014, 270–271, 89–111 CrossRef CAS.
- J. He, M. Li, W. Zhang and X. Zhao, Chem. Rec., 2022, 22, e202200148 CrossRef CAS PubMed.
- Y. R. Yao, J. Zhao, Q. Meng, H. S. Hu, M. Guo, Y. Yan, J. Zhuang, S. Yang, S. Fortier, L. Echegoyen, W. H. E. Schwarz, J. Li and N. Chen, J. Am. Chem. Soc., 2023, 145, 25440–25449 CrossRef CAS.
- Z. Cao, X. Yu, Y. R. Yao, J. Autschbach and N. Chen, J. Am. Chem. Soc., 2025, 147, 3584–3592 CrossRef CAS PubMed.
- Z. Jiang, Z. Hu, Y. R. Yao, X. Han, J. Xin and S. Yang, J. Am. Chem. Soc., 2025, 147, 8939–8947 CrossRef CAS PubMed.
- K. Junghans, M. Rosenkranz and A. A. Popov, Chem. Commun., 2016, 52, 6561–6564 RSC.
- H. Jiang, X. Yu, M. Guo, Y.-R. Yao, Q. Meng, L. Echegoyen, J. Autschbach and N. Chen, J. Am. Chem. Soc., 2023, 145, 5645–5654 CrossRef CAS.
- F. Jin, N. B. Tamm, S. I. Troyanov and S. Yang, J. Am. Chem. Soc., 2018, 140, 3496–3499 CrossRef CAS PubMed.
- K. Kato and A. Osuka, Angew. Chem., Int. Ed., 2019, 58, 8978–8986 CrossRef CAS.
- S. Roy, K. C. Mondal and H. W. Roesky, Acc. Chem. Res., 2016, 49, 357–369 CrossRef CAS PubMed.
- R. Lai and Q. Cui, J. Am. Chem. Soc., 2022, 144, 20739–20751 CrossRef CAS.
- L. Echegoyen, N. Chen, S. Fortier, W. Cai, J. Murillo and M. Gomez, Meet. Abstr., 2018, MA2018-01, 791 CrossRef.
- Y. Li, L. Yang, Z. Li, Q. Hou, L. Li and P. Jin, Inorg. Chem., 2019, 58, 10648–10655 CrossRef CAS PubMed.
- L. Bao, P. Yu, C. Pan, W. Shen and X. Lu, Chem. Sci., 2019, 10, 2153–2158 RSC.
- P. Yu, H. Mei, S. Hu, C. Pan, W. Shen, P. Yu, K. Guo, Y. Xie, T. Akasaka, L. Bao and X. Lu, Chin. J. Chem., 2023, 41, 1915–1920 CrossRef CAS.
- X. Lu, L. Bao, T. Akasaka and S. Nagase, Chem. Commun., 2014, 50, 14701–14715 RSC.
- L. Bao, M. Chen, C. Pan, T. Yamaguchi, T. Kato, M. M. Olmstead, A. L. Balch, T. Akasaka and X. Lu, Angew. Chem., Int. Ed., 2016, 55, 4242–4246 CrossRef CAS.
- S. Yang, C. Chen, F. Liu, Y. Xie, F. Li, M. Jiao, M. Suzuki, T. Wei, S. Wang, Z. Chen, X. Lu and T. Akasaka, Sci. Rep., 2013, 3, 1487 CrossRef PubMed.
- K. J. Harris and R. E. Wasylishen, Inorg. Chem., 2009, 48, 2316–2332 CrossRef CAS PubMed.
- T. S. Wang, L. Feng, J. Y. Wu, W. Xu, J. F. Xiang, K. Tan, Y. H. Ma, J. P. Zheng, L. Jiang, X. Lu, C. Y. Shu and C. R. Wang, J. Am. Chem. Soc., 2010, 132, 16362–16364 CrossRef CAS.
- P. Jin, Z. Zhou, C. Hao, Z. X. Gao, K. Tan, X. Lu and Z. F. Chen, Phys. Chem. Chem. Phys., 2010, 12, 12442–12449 RSC.
- F. Liu, S. Wang, C. L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerstrom, F. Jin, S. Y. Xie, A. A. Popov, T. Greber and S. Yang, Angew. Chem., Int. Ed., 2017, 56, 1830–1834 CrossRef CAS.
- W. Shen, Z. Hu, P. Yu, Z. Wei, P. Jin, Z. Shi and X. Lu, Inorg. Chem. Front., 2020, 7, 4563–4571 RSC.
- R. Guan, M. Chen, J. Xin, X. M. Xie, F. Jin, Q. Zhang, S. Y. Xie and S. Yang, J. Am. Chem. Soc., 2021, 143, 8078–8085 CrossRef CAS PubMed.
- F. Liu, C. L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerstrom, S. Wang, Y. Z. Tan, J. Tao, S. Y. Xie, A. A. Popov, T. Greber and S. Yang, J. Am. Chem. Soc., 2016, 138, 14764–14771 CrossRef CAS PubMed.
- J. Xin, F. Jin, R. Guan, M. Chen, X.-M. Xie, Q. Zhang, S.-Y. Xie and S. Yang, Inorg. Chem. Front., 2021, 8, 1719–1726 RSC.
- Q. Meng, L. Abella, W. Yang, Y. R. Yao, X. Liu, J. Zhuang, X. Li, L. Echegoyen, J. Autschbach and N. Chen, J. Am. Chem. Soc., 2021, 143, 16226–16234 CrossRef CAS PubMed.
- J. C. Berthet, P. Thuéry and M. Ephritikhine, Dalton Trans., 2015, 44, 7727–7742 RSC.
- L. Feng, Y. Hao, A. Liu and Z. Slanina, Acc. Chem. Res., 2019, 52, 1802–1811 CrossRef CAS PubMed.
- X. Zhang, W. Li, L. Feng, X. Chen, A. Hansen, S. Grimme, S. Fortier, D. C. Sergentu, T. J. Duignan, J. Autschbach, S. Wang, Y. Wang, G. Velkos, A. A. Popov, N. Aghdassi, S. Duhm, X. Li, J. Li, L. Echegoyen, W. H. E. Schwarz and N. Chen, Nat. Commun., 2018, 9, 2753 CrossRef PubMed.
- A. L. Svitova, K. B. Ghiassi, C. Schlesier, K. Junghans, Y. Zhang, M. M. Olmstead, A. L. Balch, L. Dunsch and A. A. Popov, Nat. Commun., 2014, 5, 3568 CrossRef CAS.
- K. Junghans, C. Schlesier, A. Kostanyan, N. A. Samoylova, Q. Deng, M. Rosenkranz, S. Schiemenz, R. Westerstrom, T. Greber, B. Buchner and A. A. Popov, Angew. Chem., Int. Ed., 2015, 54, 13411–13415 CrossRef CAS.
- K. Junghans, K. B. Ghiassi, N. A. Samoylova, Q. M. Deng, M. Rosenkranz, M. M. Olmstead, A. L. Balch and A. A. Popov, Chem. – Eur. J., 2016, 22, 13098–13107 CrossRef CAS PubMed.
- F. Liu, F. Jin, S. Wang, A. A. Popov and S. Yang, Inorg. Chim. Acta, 2017, 468, 203–208 CrossRef CAS.
- X. Li, Y.-R. Yao, W. Yang, J. Zhuang, L. Echegoyen and N. Chen, Chem. Commun., 2020, 56, 3867–3870 RSC.
- Q. Zhuo, H. Zhang, Y. Hua, H. Kang, X. Zhou, X. Lin, Z. Chen, J. Lin, K. Zhuo and H. Xia, Sci. Adv., 2018, 4, eaat0336 CrossRef.
- S. Kaneko, Y. Hashikawa, S. Fujii, Y. Murata and M. Kiguchi, ChemPhysChem, 2017, 18, 1229–1233 CrossRef CAS PubMed.
- Z. Slanina, F. Uhlík, L. Adamowicz and S. Nagase, Mol. Simul., 2005, 31, 801–806 CrossRef CAS.
- M. Krause, F. Ziegs, A. A. Popov and L. Dunsch, ChemPhysChem, 2007, 8, 537–540 CrossRef CAS PubMed.
- T.-S. Wang, L. Feng, J.-Y. Wu, W. Xu, J.-F. Xiang, K. Tan, Y.-H. Ma, J.-P. Zheng, L. Jiang and X. Lu, J. Am. Chem. Soc., 2010, 132, 16362–16364 CrossRef CAS PubMed.
- J. Wu, T. Wang, Y. Ma, L. Jiang, C. Shu and C. Wang, J. Phys. Chem. C, 2011, 115, 23755–23759 CrossRef CAS.
- P. Jin, Z. Zhou, C. Hao, Z. Gao, K. Tan, X. Lu and Z. Chen, Phys. Chem. Chem. Phys., 2010, 12, 12442–12449 RSC.
- Q. Deng, K. Junghans and A. A. Popov, Theor. Chem. Acc., 2015, 134, 10 Search PubMed.
- C. Zhao, K. Tan, M. Nie, Y. Lu, J. Zhang, C. Wang, X. Lu and T. Wang, Inorg. Chem., 2020, 59, 8284–8290 CrossRef CAS PubMed.
- T. F. Leung, D. Jiang, M. C. Wu, D. Xiao, W. M. Ching, G. P. A. Yap, T. Yang, L. Zhao, T. G. Ong and G. Frenking, Nat. Chem., 2021, 13, 89–93 CrossRef CAS PubMed.
- M. Hermann and G. Frenking, Chem. – Eur. J., 2016, 22, 4100–4108 CrossRef CAS PubMed.
- T. W. Schmidt, Acc. Chem. Res., 2021, 54, 481–489 CrossRef CAS.
- M. C. Wu, Y. F. Liang, T. Jurca, G. P. A. Yap, T. F. Leung and T. G. Ong, J. Am. Chem. Soc., 2022, 144, 12996–13005 CrossRef CAS PubMed.
- R. B. King and J. Organomet, Chem, 1997, 536–537, 7–15 Search PubMed.
- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced inorganic chemistry, Wiley-Interscience, New York, 6th edn, 1999, p. 211 Search PubMed.
- Y. Shen, X. Yu, Q. Meng, Y.-R. Yao, J. Autschbach and N. Chen, Chem. Sci., 2022, 13, 12980–12986 RSC.
- J. Zhuang, L. Abella, D. C. Sergentu, Y. R. Yao, M. Jin, W. Yang, X. Zhang, X. Li, D. Zhang, Y. Zhao, X. Li, S. Wang, L. Echegoyen, J. Autschbach and N. Chen, J. Am. Chem. Soc., 2019, 141, 20249–20260 CrossRef CAS PubMed.
- F. Liu, T. Wei, S. Wang, J. Guan, X. Lu and S. Yang, Fullerenes, Nanotubes Carbon Nanostruct., 2014, 22, 215–226 CrossRef CAS.
- Z. Ma, B. Wang, L. Ou, Y. Zhang, X. Zhang and Z. Zhou, Nanotechnology, 2016, 27, 415203 CrossRef.
- Y. X. Zhao, M. Y. Li, R. S. Zhao, P. Zhao, K. Yuan, Q. Z. Li and X. Zhao, J. Phys. Chem. C, 2018, 122, 13148–13155 CrossRef CAS.
- Y. Iiduka, T. Wakahara, T. Nakahodo, T. Tsuchiya, A. Sakuraba, Y. Maeda, T. Akasaka, K. Yoza, E. Horn, T. Kato, M. T. H. Liu, N. Mizorogi, K. Kobayashi and S. Nagase, J. Am. Chem. Soc., 2005, 127, 12500–12501 CrossRef CAS PubMed.
- H. Y. Fang, H. L. Cong, M. Suzuki, L. P. Bao, B. Yu, Y. P. Xie, N. Mizorogi, M. M. Olmstead, A. L. Balch, S. Nagase, T. Akasaka and X. Lu, J. Am. Chem. Soc., 2014, 136, 10534–10540 CrossRef CAS.
- Z. Liu, B. W. Dong, H. B. Meng, M. X. Xu, T. S. Wang, B. W. Wang, C. R. Wang, S. D. Jiang and S. Gao, Chem. Sci., 2018, 9, 457–462 RSC.
- T.-S. Wang, N. Chen, J.-F. Xiang, B. Li, J.-Y. Wu, W. Xu, L. Jiang, K. Tan, C.-Y. Shu, X. Lu and C.-R. Wang, J. Am. Chem. Soc., 2009, 131, 16646–16647 CrossRef CAS PubMed.
- M. Nie, H. Meng, C. Zhao, Y. Lu, J. Zhang, L. Feng, C. Wang and T. Wang, Chem. Commun., 2020, 56, 10879–10882 RSC.
- Y. Feng, T. Wang, J. Wu, Z. Zhang, L. Jiang, H. Han and C. Wang, Chem. Commun., 2014, 50, 12166–12168 RSC.
- J. Min, D. T. Halfen and L. M. Ziurys, Chem. Phys. Lett., 2014, 609, 70–75 CrossRef CAS.
- D. T. Halfen, J. Min and L. M. Ziurys, Chem. Phys. Lett., 2013, 555, 31–37 Search PubMed.
- J. Yang, R. H. Judge and D. J. Clouthier, J. Chem. Phys., 2011, 135, 124302 CrossRef PubMed.
- M. A. Burton, Q. Cheng, D. T. Halfen, J. H. Lane, N. J. DeYonker and L. M. Ziurys, J. Chem. Phys., 2020, 153, 034304 CrossRef CAS.
- C. R. Wang, T. Kai, T. Tomiyama, T. Yoshida, Y. Kobayashi, E. Nishibori, M. Takata, M. Sakata and H. Shinohara, Angew. Chem., Int. Ed., 2001, 40, 397–399 CrossRef CAS PubMed.
- M. Krause, M. Hulman, H. Kuzmany, O. Dubay, G. Kresse, K. Vietze, G. Seifert, C. Wang and H. Shinohara, Phys. Rev. Lett., 2004, 93, 137403 CrossRef CAS.
- Z. Q. Shi, X. Wu, C. R. Wang, X. Lu and H. Shinohara, Angew. Chem., Int. Ed., 2006, 45, 2107–2111 CrossRef CAS PubMed.
- Y. Iiduka, T. Wakahara, K. Nakajima, T. Tsuchiya, T. Nakahodo, Y. Maeda, T. Akasaka, N. Mizorogi and S. Nagase, Chem. Commun., 2006, 2057–2059 RSC.
- Y. Iiduka, T. Wakahara, K. Nakajima, T. Nakahodo, T. Tsuchiya, Y. Maeda, T. Akasaka, K. Yoza, M. T. H. Liu, N. Mizorogi and S. Nagase, Angew. Chem., Int. Ed., 2007, 119, 5658–5660 CrossRef.
- H. Kurihara, X. Lu, Y. Iiduka, N. Mizorogi, Z. Slanina, T. Tsuchiya, T. Akasaka and S. Nagase, J. Am. Chem. Soc., 2011, 133, 2382–2385 CrossRef CAS.
- H. Kurihara, X. Lu, Y. Iiduka, H. Nikawa, M. Hachiya, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, Inorg. Chem., 2012, 51, 746–750 CrossRef CAS.
- Y. Feng, T. Wang, J. Wu, L. Feng, J. Xiang, Y. Ma, Z. Zhang, L. Jiang, C. Shu and C. Wang, Nanoscale, 2013, 5, 6704–6707 RSC.
- C. H. Chen, K. B. Ghiassi, M. R. Ceron, M. A. Guerrero-Ayala, L. Echegoyen, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2015, 137, 10116–10119 CrossRef CAS PubMed.
- Y. Wang, Q. Tang, L. Feng and N. Chen, Inorg. Chem., 2017, 56, 1974–1980 CrossRef CAS.
- Y. Yamazaki, K. Nakajima, T. Wakahara, T. Tsuchiya, M. O. Ishitsuka, Y. Maeda, T. Akasaka, M. Waelchli, N. Mizorogi and S. Nagase, Angew. Chem., Int. Ed., 2008, 47, 7905–7908 CrossRef CAS.
- X. Lu, K. Nakajima, Y. Iiduka, H. Nikawa, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, J. Am. Chem. Soc., 2011, 133, 19553–19558 CrossRef CAS PubMed.
- X. Lu, K. Nakajima, Y. Iiduka, H. Nikawa, T. Tsuchiya, N. Mizorogi, Z. Slanina, S. Nagase and T. Akasaka, Angew. Chem., Int. Ed., 2012, 51, 5889–5892 CrossRef CAS PubMed.
- W. Cai, M. Chen, L. Bao, Y. Xie, T. Akasaka and X. Lu, Chem. – Eur. J., 2015, 21, 3449–3454 CrossRef CAS PubMed.
- B. Wu, T. Wang, Z. Zhang, L. Jiang and C. Wang, Chem. Commun., 2018, 54, 775–777 RSC.
- X. Lu, T. Akasaka and S. Nagase, Chem. Commun., 2011, 47, 5942–5957 RSC.
- I. N. Ioffe, A. A. Goryunkov, N. B. Tamm, L. N. Sidorov, E. Kemnitz and S. I. Troyanov, Angew. Chem., Int. Ed., 2009, 48, 5904–5907 CrossRef CAS PubMed.
- C. H. Chen, L. Abella, M. R. Ceron, M. A. Guerrero-Ayala, A. Rodriguez-Fortea, M. M. Olmstead, X. B. Powers, A. L. Balch, J. M. Poblet and L. Echegoyen, J. Am. Chem. Soc., 2016, 138, 13030–13037 CrossRef CAS.
- T. Inoue, T. Tomiyama, T. Sugai and H. Shinohara, Chem. Phys. Lett., 2003, 382, 226–231 CrossRef CAS.
- T. Inoue, T. Tomiyama, T. Sugai, T. Okazaki, T. Suematsu, N. Fujii, H. Utsumi, K. Nojima and H. Shinohara, J. Phys. Chem. B, 2004, 108, 7573–7579 CrossRef CAS.
- E. Nishibori, S. Narioka, M. Takata, M. Sakata, T. Inoue and H. Shinohara, ChemPhysChem, 2006, 7, 345–348 CrossRef CAS.
- S. Jia, Z. Hu, X. Shao, C. Huang, J. Zhang, K. Guo, C. Pan, W. Chen, L. Bao and X. Lu, Inorg. Chem., 2025, 64, 16572–16577 CrossRef CAS PubMed.
- J. Zhang, T. Fuhrer, W. Fu, J. Ge, D. W. Bearden, J. Dallas, J. Duchamp, K. Walker, H. Champion, H. Azurmendi, K. Harich and H. C. Dorn, J. Am. Chem. Soc., 2012, 134, 8487–8493 CrossRef CAS.
- C. Pan, L. Bao, X. Yu, H. Fang, Y. Xie, T. Akasaka and X. Lu, ACS Nano, 2018, 12, 2065–2069 CrossRef CAS PubMed.
- C. Pan, W. Shen, L. Yang, L. Bao, Z. Wei, P. Jin, H. Fang, Y. Xie, T. Akasaka and X. Lu, Chem. Sci., 2019, 10, 4707–4713 RSC.
- J. A. Flores, V. N. Cavaliere, D. Buck, B. Pintér, G. Chen, M. G. Crestani, M. H. Baik and D. J. Mindiola, Chem. Sci., 2011, 2, 1457–1462 RSC.
- T. Kurogi, J. Won, B. Park, O. S. Trofymchuk, P. J. Carroll, M. H. Baik and D. J. Mindiola, Chem. Sci., 2018, 9, 3376–3385 RSC.
- B. Cao, M. Hasegawa, K. Okada, T. Tomiyama, T. Okazaki, K. Suenaga and H. Shinohara, J. Am. Chem. Soc., 2001, 123, 9679–9680 CrossRef CAS PubMed.
- B. Cao, K. Suenaga, T. Okazaki and H. Shinohara, J. Phys. Chem. B, 2002, 106, 9295–9298 CrossRef CAS.
- T. Yumura, Y. Sato, K. Suenaga and S. Iijima, J. Phys. Chem. B, 2005, 109, 20251–20255 CrossRef CAS.
- K. Tan and X. Lu, Chem. Commun., 2005, 4444–4446 RSC.
- Y. Sato, T. Yumura, K. Suenaga, H. Moribe, D. Nishide, M. Ishida, H. Shinohara and S. Iijima, Phys. Rev. B:Condens. Matter Mater. Phys., 2006, 73, 193401 CrossRef.
- M. Otani, S. Okada and A. Oshiyama, Chem. Phys. Lett., 2007, 438, 274–278 CrossRef CAS.
- P. Yu, L. Bao, L. Yang, D. Hao, P. Jin, W. Shen, H. Fang, T. Akasaka and X. Lu, Inorg. Chem., 2020, 59, 9416–9423 CrossRef CAS PubMed.
- W. Cai, L. Bao, S. Zhao, Y. Xie, T. Akasaka and X. Lu, J. Am. Chem. Soc., 2015, 137, 10292–10296 CrossRef CAS.
- Z. Slanina, F. Uhlík, T. Akasaka, X. Lu and L. Adamowicz, Inorganics, 2024, 12, 196 CrossRef CAS.
- W. Cai, F. F. Li, L. Bao, Y. Xie and X. Lu, J. Am. Chem. Soc., 2016, 138, 6670–6675 CrossRef CAS PubMed.
- S. Zhao, P. Zhao, W. Cai, L. Bao, M. Chen, Y. Xie, X. Zhao and X. Lu, J. Am. Chem. Soc., 2017, 139, 4724–4728 CrossRef CAS.
- Y. Ito, T. Okazaki, S. Okubo, M. Akachi, Y. Ohno, T. Mizutani, T. Nakamura, R. Kitaura, T. Sugai and H. Shinohara, ACS Nano, 2007, 1, 456–462 CrossRef CAS PubMed.
- H. Okimoto, R. Kitaura, T. Nakamura, Y. Ito, Y. Kitamura, T. Akachi, D. Ogawa, N. Imazu, Y. Kato, Y. Asada, T. Sugai, H. Osawa, T. Matsushita, T. Muro and H. Shinohara, J. Phys. Chem. C, 2008, 112, 6103–6109 CrossRef CAS.
- S. Stevenson, K. R. Tepper, C. M. Davison, X. B. Powers, M. M. Olmstead and A. L. Balch, Chem. – Eur. J., 2018, 24, 13479–13484 CrossRef CAS.
- S. F. Hu, P. Zhao, W. Q. Shen, M. Ehara, Y. P. Xie, T. Akasaka and X. Lu, Inorg. Chem., 2020, 59, 1940–1946 CrossRef CAS PubMed.
- S. Hu, W. Shen, P. Zhao, T. Xu, Z. Slanina, M. Ehara, X. Zhao, Y. Xie, T. Akasaka and X. Lu, Nanoscale, 2019, 11, 17319–17326 RSC.
- W. Shen, L. Bao, S. Hu, L. Yang, P. Jin, Y. Xie, T. Akasaka and X. Lu, Chem. Sci., 2019, 10, 829–836 RSC.
- W. Shen, L. Bao, P. Yu, L. Yang, B. Li, P. Yu, P. Jin and X. Lu, Carbon, 2020, 164, 157–163 CrossRef CAS.
- J. Zhang, F. L. Bowles, D. W. Bearden, W. K. Ray, T. Fuhrer, Y. Ye, C. Dixon, K. Harich, R. F. Helm, M. M. Olmstead, A. L. Balch and H. C. Dorn, Nat. Chem., 2013, 5, 880–885 CrossRef CAS PubMed.
- W. Yang, G. Velkos, S. Sudarkova, B. Büchner, S. M. Avdoshenko, F. Liu, A. A. Popov and N. Chen, Inorg. Chem. Front., 2022, 9, 5805–5819 RSC.
- E. Nishibori, K. Iwata, M. Sakata, M. Takata, H. Tanaka, H. Kato and H. Shinohara, Phys. Rev. B:Condens. Matter Mater. Phys., 2004, 69, 113412 CrossRef.
- K. Kobayashi and S. Nagase, Chem. Phys. Lett., 1999, 313, 45–51 CrossRef CAS.
- Y. Iiduka, T. Wakahara, T. Nakahodo, T. Tsuchiya, A. Sakuraba, Y. Maeda, T. Akasaka, K. Yoza, E. Horn, T. Kato, M. T. H. Liu, N. Mizorogi, K. Kobayashi and S. Nagase, J. Am. Chem. Soc., 2005, 127, 12500–12501 CrossRef CAS.
- E. Nishibori, I. Terauchi, M. Sakata, M. Takata, Y. Ito, T. Sugai and H. Shinohara, J. Phys. Chem. B, 2006, 110, 19215–19219 CrossRef CAS.
- S. Taubert, M. Straka, T. O. Pennanen, D. Sundholm and J. Vaara, Phys. Chem. Chem. Phys., 2008, 10, 7158–7168 RSC.
- K. Tan and X. Lu, J. Phys. Chem. A, 2006, 110, 1171–1176 CrossRef CAS.
- W. Xu, T.-S. Wang, J.-Y. Wu, Y.-H. Ma, J.-P. Zheng, H. Li, B. Wang, L. Jiang, C.-Y. Shu and C.-R. Wang, J. Phys. Chem. C, 2011, 115, 402–405 CrossRef CAS.
- F. Jin, J. Xin, R. Guan, X. M. Xie, M. Chen, Q. Zhang, A. A. Popov, S. Y. Xie and S. Yang, Chem. Sci., 2021, 12, 6890–6895 RSC.
- S. Hu, P. Zhao, B. Li, P. Yu, L. Yang, M. Ehara, P. Jin, T. Akasaka and X. Lu, Inorg. Chem., 2022, 61, 11277–11283 CrossRef CAS.
- K. Junghans, K. B. Ghiassi, N. A. Samoylova, Q. Deng, M. Rosenkranz, M. M. Olmstead, A. L. Balch and A. A. Popov, Chem. – Eur. J., 2016, 22, 13098–13107 CrossRef CAS PubMed.
- R. Guan, Z. C. Chen, J. Huang, H. R. Tian, J. Xin, S. W. Ying, M. Chen, Q. Zhang, Q. Li, S. Y. Xie, L. S. Zheng and S. Yang, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2202563119 CrossRef CAS.
- H. Jiang, X. Yu, M. Guo, Y. R. Yao, Q. Meng, L. Echegoyen, J. Autschbach and N. Chen, J. Am. Chem. Soc., 2023, 145, 5645–5654 CrossRef CAS PubMed.
- K. Tan, X. Lu and C.-R. Wang, J. Phys. Chem. B, 2006, 110, 11098–11102 CrossRef CAS.
- T.-S. Wang, N. Chen, J.-F. Xiang, B. Li, J.-Y. Wu, W. Xu, L. Jiang, K. Tan, C.-Y. Shu, X. Lu and C.-R. Wang, J. Am. Chem. Soc., 2009, 131, 16646–16647 CrossRef CAS PubMed.
- E. Krokos, J. Phys. Chem. C, 2010, 114, 7626–7630 CrossRef CAS.
- C. B. Kah, J. Nathaniel, K. Suggs and X.-Q. Wang, J. Phys. Chem. C, 2010, 114, 13017–13019 CrossRef CAS.
- B. Li, L. Lou and P. Jin, Phys. Chem. Chem. Phys., 2023, 25, 2451–2461 RSC.
- R. Astala, M. Kaukonen, R. M. Nieminen, G. Jungnickel and T. Frauenheim, Phys. Rev. B:Condens. Matter Mater. Phys., 2002, 65, 245423 CrossRef.
- R. Devi and R. Kumar, Mod. Phys. Lett. B, 2011, 24, 1255–1266 CrossRef.
- D. Hao, L. Yang, B. Li, Q. Hou, L. Li and P. Jin, J. Phys. Chem. A, 2020, 124, 2694–2699 CrossRef CAS.
- T. Wang, J. Wu and Y. Feng, Dalton Trans., 2014, 43, 16270–16274 RSC.
- P. Yu, W. Shen, L. Bao, C. Pan, Z. Slanina and X. Lu, Chem. Sci., 2019, 10, 10925–10930 RSC.
- Z. He, Z. Cao, Y. R. Yao and N. Chen, Chem. Commun., 2025, 61, 9432–9435 RSC.
- X. Han, Z. Jiang, Z. Hu, Y. R. Yao, A. Ullah, A. Gaita-Ariño, W. Yan, Y. Lu and S. Yang, Angew. Chem., Int. Ed., 2025, 64, e202502228 CrossRef CAS PubMed.
- Z. Hu and S. Yang, Chem. Soc. Rev., 2024, 53, 2863–2897 RSC.
- W. Yang, M. F. D. S. Barbosa, N. Israel, M. Rosenkranz, F. Liu, S. W. Avdoshenko and A. A. Popov, J. Am. Chem. Soc., 2025, 147, 33812–33827 CrossRef CAS.
- W. Li, Z. Zhang, C. Wang and T. Wang, CCS Chem., 2025 DOI:10.31635/ccschem.025.202506054.
- M. Zhen, Y. Xu, C. Wang and C. Bai, Adv. Funct. Mater., 2024, 34, 2409319 CrossRef CAS.
- J. Zheng, L. Huang, C. H. Cui, Z. C. Chen, X. F. Liu, X. Duan, X. Y. Cao, T. Z. Yang, H. Zhu, K. Shi, P. Du, S. W. Ying, C. F. Zhu, Y. G. Yao, G. C. Guo, Y. Yuan, S. Y. Xie and L. S. Zheng, Science, 2022, 376, 288–292 CrossRef CAS.
- C. Huang, Y. Yang, M. Li, X. Qi, C. Pan, K. Guo, L. Bao and X. Lu, Adv. Mater., 2024, 36, 2306244 CrossRef CAS PubMed.
- C. Huang, R. Sun, L. Bao, X. Tian, C. Pan, M. Li, W. Shen, K. Guo, B. Wang, X. Lu and S. Gao, Nat. Commun., 2023, 14, 8443 CrossRef CAS.
- N. Li, K. Guo, S. Lu, L. Bao, Z. Xu and X. Lu, Chem. Commun., 2024, 60, 11964–11967 RSC.
- Y. Han, M. Li, M. Ehara and X. Zhao, Phys. Chem. Chem. Phys., 2025, 27, 9767–9773 RSC.
- Y. Zhao, Z. Hu, P. Chuai, H. Jin, S. Yang, J. Su and Z. Shi, J. Am. Chem. Soc., 2024, 146, 17003–17008 CrossRef CAS.
- P. Chuai, Z. Hu, Y. Yao, Z. Jiang, A. Ullah, Y. Zhao, W. Cheng, M. Chen, E. Coronado, S. Yang and Z. Shi, Nat. Chem., 2025, 17, 1053–1057 CrossRef CAS.
- X. Q. Guo, P. Yu, L. P. Zhou, S. J. Hu, X. F. Duan, L. X. Cai, L. Bao, X. Lu and Q. F. Sun, Nat. Syn., 2025, 4, 359–369 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |