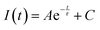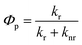Buchwald–Hartwig aminated pyrene-heterocycles with host–guest-enhanced NIR phosphorescence: DFT-guided design toward breast cancer imaging probes
Kaixuan
Hu†
ab,
Shufeng
Chen†
b,
Xinmin
Wang†
a,
Lingkai
Tang
 c,
Yan
Cheng
a,
Yuting
Song
a,
Hubing
Shi
a,
Jing
Jing
a,
Jianping
Hu
c,
Yan
Cheng
a,
Yuting
Song
a,
Hubing
Shi
a,
Jing
Jing
a,
Jianping
Hu
 *b and
Ting
Luo
*b and
Ting
Luo
 *a
*a
aBreast Health Medical Research Institute, West China Hospital, Sichuan University, Chengdu, China. E-mail: luotingwch@163.com
bKey Laboratory of Medicinal and Edible Plants Resources Development of Sichuan Education Department, School of Pharmacy, Chengdu University, Chengdu, China. E-mail: hjpcdu@163.com
cCollege of Chemistry and Life Science, Beijing University of Technology, Beijing, China
First published on 26th November 2025
Abstract
Organic near-infrared room-temperature phosphorescent (NIR-RTP) materials face fundamental challenges from the energy gap law, limiting emission efficiency of wavelengths >600 nm and long-lived triplet states. We report the rational design of Buchwald–Hartwig aminated pyrene-heterocycles embedded in benzophenone host matrices, achieving unprecedented NIR phosphorescence with ultralong lifetimes. The DFT-guided optimization reveals that the heteroatom-induced spin–orbit coupling modulation and host-mediated suppression of non-radiative transitions both overcome traditional efficiency bottlenecks. The O-pyrene/benzophenone (O-pyrene/BPO) system exhibits dual-peak emissions at 609/666 nm with lifetimes up to 275.56 ms—the reported highest value for organic NIR-RTP materials—while the S-pyrene/BPO demonstrates a 243.31 ms lifetime at 613 nm. Crucially, phenoxazine incorporation lowers the T1 energy level without compromising ISC efficiency, which is validated by SOC calculations. Host–guest confinement also restricts molecular vibrations. This work provides a research foundation for establishing the “heteroatom engineering-host rigidity” paradigm, helpful in the development of therapeutic and diagnostic imaging probes.
1 Introduction
In recent years, organic near-infrared room-temperature phosphorescent (NIR-RTP) materials have progressively become a focal point of research in various fields, including biological imaging, organic light-emitting diodes (OLEDs), sensing, and data encryption.1–4 Theoretically, compared with traditional fluorescent materials, pure organic room-temperature phosphorescent (RTP) materials can enable excitation of singlet excitons to undergo intersystem crossing (ISC), accompanied by the acquisition of triplet excited states with a prolonged lifetime. The organic NIR-RTP materials possess a unique advantage in biological imaging applications due to their ability to penetrate biological tissues more deeply, such as skin and blood.5–7 Factually, their sustained luminescence properties can partially mitigate ambient interference. Furthermore, organic RTP compounds generally exhibit relatively low toxicity and favorable biocompatibility, thus being beneficial for applications in tissue imaging, tumor diagnosis and drug tracking.8,9To date, most phosphorescent materials exhibit emission wavelengths below 580 nm, which limits their penetration capability in biological tissues.10 The synthesis of organic NIR-RTP materials with emission wavelengths >600 nm is exceptionally challenging due to the energy gap law. This law states that as the energy gap between the excited state and the ground state decreases, the rate constant for non-radiative transitions increases exponentially, thereby posing a significant challenge in developing efficient organic near-infrared emitters. Consequently, this limitation severely hampers the enhancement of the luminescent efficiency of RTP materials. In contrast to green and yellow phosphorescent materials, organic NIR-RTP materials exhibit an increased energy gap due to the lower energy of the triplet state, making the ISC process more difficult.
According to the luminescence mechanism of organic phosphorescence, achieving highly efficient and long-lived RTP materials relies on two critical strategies. Specifically, the first strategy is to facilitate the ISC process from the lowest excited singlet state (S1) to the triplet state (Tn), and the second one is to suppress non-radiative transitions from the lowest excited triplet state (T1) to the ground state (S0). To enhance phosphorescent performance, researchers have proposed various strategies and methods, such as the introduction of heavy atoms,11 heteroatoms12 and aromatic carbonyl groups13 to increase the spin–orbit coupling (SOC). Additionally, techniques like host–guest doping,14 crystallization15 and organic frameworks16 have been employed to inhibit non-radiative transitions of triplet excitons. Among all organic luminescent moieties, polycyclic aromatic hydrocarbons (PAHs) serve as significant core structures due to their unique tunable optical and electronic properties.17–19 For instance, pyrene is abundantly found in nature but notably lacks effective characteristics enhancing SOC or reducing the energy gap between the singlet and triplet states (i.e., ΔEST). Therefore, introducing external factors to optimize pyrene as an efficient RTP emitter is highly promising.
According to first-order perturbation theory, developing RTP materials with long-wavelength emissions and ultra-long lifetimes necessitates a reduction in the T1 energy level. Nevertheless, a lower T1 level brings up two major challenges for phosphorescent emissions. Firstly, a lower T1 energy level increases the ΔEST between the S1 and T1 states, which hinders the ISC of excitons. Another challenge is that a lower T1 level increases the likelihood of excitons being non-radiatively quenched and not conducive to phosphorescent lifetime and intensity.20 Adding halogen atoms to increase SOC is a typical traditional approach for enhancing the T1 energy level in organic emitters and achieving efficient RTP.21 However, heavy halogen substitution can sometimes result in excessively short RTP lifetimes that are not visually detectable. It is an undesirable characteristic for certain applications, such as anti-counterfeiting.22
To obtain bright RTP “afterglow” emissions (τ > 100 ms), lighter heteroatoms, such as O, N, S and P, have also been routinely incorporated into aromatic frameworks,23–25 as the ISC rate is more sensitive to smaller disturbances compared to RTP decay rates. Furthermore, based on the El-Sayed rule, the transition hybridization from 1(n, π*) to 3(π, π*) provides another crucial parameter for improving ISC and RTP performance.26 In this work, we selected pyrene as the core, then constructed ligands based on phenothiazine, phenoselenazine, and phenoxazine, which contain lighter heteroatoms, and investigated their potential enhancement effects on the RTP properties. The Ramakant Gavale research group27 designed and synthesized four mechanochromic materials (MFCs), namely BT-PTZ-1, BT-PTZ-2, BT-PTZ-3 and BT-PTZ-4, using Buchwald–Hartwig amination reactions. These materials are characterized by benzothiazole (BT) as the receptor fragment, and phenothiazine (PTZ) or triphenylamine (TPA) as different donors. Biological studies on these PTZ derivatives have revealed their potential therapeutic efficacy against benzo[α]pyrene (B[α]P)-induced carcinogenesis in A549 (lung) and HEK293 (kidney) cells. After treatment with BT-PTZ-2, upregulation of p53 and downregulation of β-catenin and pNF-κB were both observed, indicating significant anticancer activity. Additionally, DCFDA staining revealed that mitochondrial fission protein (DRP1) and oxidative stress were both downregulated after treatment with BT-PTZ-2. These findings strongly suggest that BT-PTZ-2 can inhibit the proliferation and survival of lung cancer cells, highlighting its potential as a promising anticancer drug. Most phosphorescent materials exhibit poor penetration in biological tissues due to their short emission spectra. This limitation results in effective imaging only within the superficial layers of biological systems.10,28,29 Furthermore, potentially constrained by the energy gap law, these red RTP materials often have very short phosphorescent lifetimes, which hinder their application in biological imaging.30,31 Therefore, for materials that already exhibit low T1 levels, enhancing the ISC capability of excitons and suppressing their non-radiative transitions are crucial for achieving ultra-long-lifetime near-infrared phosphorescence.
In recent years, there has been increasing interest in the application of host–guest doping strategies to develop ultra-long-lifetime near-infrared RTP materials.14,32 This approach leverages the ability of host molecules to inhibit non-radiative transitions of guest molecules within the host–guest system.33,34 Additionally, energy exchange between the host and guest molecules can facilitate efficient excitation energy transfer to the guest ones.32 The Si group35 proposed a fused cyclic structure that effectively lowers the triplet state energy level, resulting in an extended phosphorescent emission wavelength. Due to the rigid structure, the fused cyclic design also exhibits a high molar extinction coefficient and luminous efficiency. Recently, a new class of host materials was developed to stabilize the triplet excitons generated by the fused cyclic molecules. The maximum RTP wavelength of the doped materials reaches up to 635 nm with a lifetime of 9.35 ms. Additionally, water-dispersible nanoparticles have been successfully fabricated for in vivo time-resolved bioimaging, effectively eliminating background fluorescence interference from biological tissues. Similarly, Dai et al.36 reported the development of red-emitting RTP materials obtained through the combination of the fused ring effect and host–guest interactions. The guest molecules with fused rings exhibit low triplet state energy levels, which enable long-wavelength emissions, while the host molecules bridge the significant energy gap between the triplet and singlet states of the guest molecules, facilitating efficient ISC processes. Due to enhanced conjugation and restricted intramolecular motion, the host–guest materials with the fused ring configuration demonstrate red phosphorescent lifetimes of up to 274 ms. Well-dispersed phosphorescent nanoparticles, with uniform sizes of approximately 200 nm, exhibit excellent biocompatibility and low cytotoxicity.
Zhao et al.37 have linked mannose to the near-infrared dye (NIR) IR 780 via disulfide bonds to obtain the mannose-IR 780 conjugate (i.e., MR 780), which subsequently self-assembles into near-infrared nanoparticles. When selectively targeting M2 tumor-associated macrophages (TAMs) that highly express CD 206 on their surface, the disulfide bonds are cleaved by the elevated levels of glutathione in the microenvironment, resulting in restored luminescence. It enables near-infrared molecular imaging of TAMs, which provides a diagnostic tool for lymph node metastasis in mouse tumors. Axillary lymph node metastasis is generally considered as one of the most critical prognostic factors in the treatment of early-stage breast cancer (BC). However, ultrasound and MRI can detect only approximately 10% of early BC lymph node micrometastasis, making its early identification crucial. He's group38 utilized 1,1-bis(18-hydroxy)-3,3,3,3-tetramethylindole tricarbonyl iodide (DiR) as a near-infrared probe, embedding it in a photothermal nanoparticle drug (DPN). After exposure to an 808 nm laser, DPN exhibited an average particle size of 24.5 ± 4.1 nm, with a photothermal efficiency of 100% under optimal conditions. DPN demonstrated superior heat generation in both in vitro and in vivo settings compared to free DiR. Furthermore, DPN combined with NIR irradiation markedly inhibited the proliferation and migration of metastatic 4T1 BC cells. DPN was found to be accumulated significantly within tumor tissues and deeply penetrated into these tissues. According to the in vivo photothermal therapy (PTT), DPN could promote tumor cell growth, while the combination with NIR irradiation completely suppressed BC metastasis. Thus, the ability of DPN to penetrate deep into tumors presents a promising strategy for advancing PTT applications in BC progression and metastasis. Zhang and colleagues39 introduced a novel method that employs genetically engineered reaction components to selectively detect estrogen receptor (ER) and progesterone receptor (PR) positive tumors. They found that the dual ER/PR reaction components are the most sensitive to steroid receptors in BC. A luminescent protein system was then constructed using cationic polymer carriers to create a reaction component, which allows for selective imaging of ER/PR positive BC in mouse models under near-infrared laser excitation. This non-invasive imaging technique enables high-resolution detection without causing animal morbidity or severe allergic reactions. Overall, the system reported by Zhang was composed of steroid receptor reaction components and near-infrared proteins, providing a practical approach to identify biomarkers and advance cancer diagnosis and treatment.
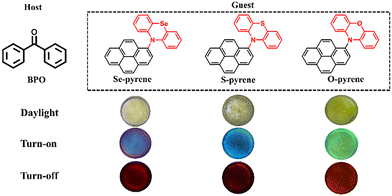
In this work, the coupling complexes of pyrene with phenoselenazine, phenothiazine and phenoxazine, respectively, were first synthesized via Buchwald–Hartwig amination reactions (see Fig. 1). The structures of three synthesized compounds (i.e., Se-pyrene, S-pyrene and O-pyrene) were then characterized using nuclear magnetic resonance (NMR) spectroscopy and were incorporated into the host material benzophenone (BPO) through a host–guest doping method. Next, the phosphorescent spectra of the doped solids were measured to obtain their phosphorescent emission wavelengths and lifetimes, fully confirming their classification as organic room-temperature phosphorescent materials. Finally, density functional theory (DFT) calculations were conducted to investigate the potential mechanism of their near-infrared long phosphorescence at the electronic level.
2 Methods
2.1 Buchwald–Hartwig amination
1-Bromopyrene (0.5 mM, 1.0 equiv.), sodium tert-butoxide (1.0 mM, 2.0 equiv.), tert-butylphosphonium tetrafluoroborate (15 mg), and tris(dibenzylideneacetone)dipalladium (Pd2(dba)3, 0.03 mM, 6%) were mixed respectively with 10H-phenoxazine, phenothiazine or phenoselenazine (0.5 mM, 1.0 equiv.) in toluene (3.0 mL) to obtain three similar reaction mixtures (see Scheme S1). After evacuation and nitrogen purging to ensure an inert atmosphere, the reaction was carried out in a microwave synthesizer at 110 °C for 1.0 h. The progress was monitored by thin-layer chromatography (TLC). Upon completion, the mixture was cooled to room temperature, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude products were purified by silica–gel column chromatography using a gradient of ethyl acetate and n-hexane (<20%) to afford the pure solid products: Se-pyrene (brown), S-pyrene (pale yellow), and O-pyrene (bright yellow). All compounds were characterized by NMR spectroscopy (see Fig. S1–S6).2.2 Preparation of host–guest doped crystals
To evaluate whether the synthesized phenyl-substituted derivatives (i.e., Se-pyrene, S-pyrene and O-pyrene) exhibit room-temperature phosphorescence (RTP) characteristics, the solid-matrix RTP measurements were conducted. Each pyrene derivative bearing different heterocyclic substituents was doped into benzophenone (BPO) at a host–guest molar ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, following previously reported host–guest RTP systems.14,40,41 The mixture was melted using a thermostatic heater at a specific temperature, then cooled and allowed to stand in an ice-water bath for 30 minutes to obtain single crystals of the doped compound (i.e., Se-pyrene/BPO, S-pyrene/BPO and O-pyrene/BPO). Three single crystals were subsequently examined under 365 nm ultraviolet irradiation to determine the presence of phosphorescent characteristics.
1, following previously reported host–guest RTP systems.14,40,41 The mixture was melted using a thermostatic heater at a specific temperature, then cooled and allowed to stand in an ice-water bath for 30 minutes to obtain single crystals of the doped compound (i.e., Se-pyrene/BPO, S-pyrene/BPO and O-pyrene/BPO). Three single crystals were subsequently examined under 365 nm ultraviolet irradiation to determine the presence of phosphorescent characteristics.
2.3 Preparation and characterization of nanomaterials
Although most reported room-temperature organic phosphorescent systems are based on millimeter-sized bulk crystals, the inability to form stable suspensions in biocompatible media severely limits their applications in the life sciences. To overcome this limitation, researchers have developed various nanosizing strategies. In this work, nanoparticles (NPs) were prepared using a top-down approach. Specifically, 5 mg of host–guest doped crystals (i.e., Se-pyrene/BPO, S-pyrene/BPO and O-pyrene/BPO) were respectively added to 3 mL of an aqueous solution of PEG-b-PPG-b-PEG (F127) (5 mg mL−1), and then probe-sonicated in an ice bath for 20 minutes. A uniform milky-white suspension was obtained after ultrasonic treatment, which was then filtered through a 0.45 µm polyvinylidene fluoride (PVDF) membrane to remove large aggregates. Moreover, the morphology and size of the resulting NPs were characterized using transmission electron microscopy (TEM; FEI Tecnai G2 F30) at an accelerating voltage of 300 kV. Here, three samples were dispersed in pure water for imaging.2.4 Cellular experiments
HEK293T cells were cultured in low-glucose (5.5 mmol L−1) DMEM supplemented with 10% fetal bovine serum. The cells were seeded into 100 mm culture dishes and maintained in a controlled CO2 incubator at 37 °C with a 5% CO2 atmosphere. Cell proliferation dynamics were monitored every 12 hours using an inverted microscope, and the culture medium was replaced every 24 hours. When the confluency reached approximately 85%, trypsinization was performed for subculturing at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio. Given that rapid proliferation and overconfluence could result in cell death, daily observation of cell density was conducted. During the subculturing, the culture medium was removed, and the cells were rinsed once with 2 mL phosphate-buffered saline (PBS). Subsequently, 2 mL of 0.25% trypsin-EDTA solution was added for digestion. After approximately 1 minute, when the cells could be gently detached using a pipette tip, the trypsin was aspirated, and digestion was then terminated by adding 2 mL of DMEM containing 10% FBS. The cells were gently resuspended and evenly distributed into four 10 cm culture dishes, with each containing 10 mL fresh culture medium. Finally, the dishes were returned to the incubator for continued cultivation.
4 ratio. Given that rapid proliferation and overconfluence could result in cell death, daily observation of cell density was conducted. During the subculturing, the culture medium was removed, and the cells were rinsed once with 2 mL phosphate-buffered saline (PBS). Subsequently, 2 mL of 0.25% trypsin-EDTA solution was added for digestion. After approximately 1 minute, when the cells could be gently detached using a pipette tip, the trypsin was aspirated, and digestion was then terminated by adding 2 mL of DMEM containing 10% FBS. The cells were gently resuspended and evenly distributed into four 10 cm culture dishes, with each containing 10 mL fresh culture medium. Finally, the dishes were returned to the incubator for continued cultivation.
The biocompatibility of Se-pyrene/BPO-NPs, S-pyrene/BPO-NPs, and O-pyrene/BPO-NPs was evaluated using HEK293T cells via the MTT assay. Briefly, 100 µL of a cell suspension with a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well was seeded into a 96-well plate and incubated for 24 hours at 37 °C under 5% CO2. Subsequently, 10 µL of nanoparticle solutions at various concentrations (i.e., 20, 40, 80, 160, and 320 µg mL−1) was respectively added to the wells. After incubation, the culture medium was removed and replaced with 100 µL fresh medium containing MTT (0.5 mg mL−1). The cells were further cultured in the dark at 37 °C for 4 hours. The MTT-containing medium was then aspirated, and 100 µL DMSO was added to each well to dissolve the formazan crystals. The absorbance at 570 nm was measured and recorded.
000 cells per well was seeded into a 96-well plate and incubated for 24 hours at 37 °C under 5% CO2. Subsequently, 10 µL of nanoparticle solutions at various concentrations (i.e., 20, 40, 80, 160, and 320 µg mL−1) was respectively added to the wells. After incubation, the culture medium was removed and replaced with 100 µL fresh medium containing MTT (0.5 mg mL−1). The cells were further cultured in the dark at 37 °C for 4 hours. The MTT-containing medium was then aspirated, and 100 µL DMSO was added to each well to dissolve the formazan crystals. The absorbance at 570 nm was measured and recorded.
The primary experimental reagents and instruments used are listed as follows: HEK293T cells (Procell Life Science & Technology Co., Ltd); bovine embryonic serum (Gibico Company); low-glucose DMEM (Cytiva Company); high-glucose DMEM (Wuhan Promeso Life Science Technology Co., Ltd.); 0.25% pancreatic ferment (Shanghai Biyun Tian Company); CCK-8 (Shanghai Biyun Tian Company); a benchtop (Thermo Scientific Company); cell culture plates (Corning Company); a high speed freezing centrifuge (Thermo Scientific Company); an ultra-pure water purifier (Millipore Company); a carbon dioxide constant-temperature incubator (Heal Force Company); an inverted microscope (Chongqing Dahua Optoelectronics Technology Co., Ltd); 96-well plates (Shanghai Biyun Tian Company); and ELISA (Gene Company Limited).
2.5 Theoretical calculations
To further investigate the intriguing RTP properties exhibited by the Se-pyrene/BPO, S-pyrene/BPO and O-pyrene/BPO systems, comparative theoretical calculations were performed on the energy level structure, intersystem crossings (ISC), internal transitions, and radiative decay. In time-dependent density functional theory (TD-DFT) calculations, the use of hybrid functionals, such as B3LYP, can partially reduce self-interaction errors. Compared to the conventional B3LYP functional, the novel range-separated ωB97X-D functional shows superior performance in calculating electronic excited states.42 In this work, ground-state structures were optimized at the ωB97X-D/6-31G(d,p) level, with electron transitions in both vacuum and solvent environments calculated and compared using TD-DFT.43–45 All computations were carried out using the Gaussian 16 software package.46 To evaluate the intersystem crossings, the spin–orbit coupling (SOC) matrix elements between S1 and Tn states for various geometries were calculated at the ωB97X-D3/def2-TZVP(-f) level employing the spin–orbit mean field (SOMF) approach embedded in ORCA 5.0.3.47 Solvent effects were evaluated using the PCM model (dichloromethane). Additionally, the excitation energies and SOC values calculated using the PCM model (dichloromethane) exhibit only minor deviations (<0.1 eV) from the gas-phase results (Tables S4–S9). This consistency indicates that solvent polarization has a negligible effect on the molecular electronic structure.2.6 Phosphorescence lifetime fitting
To accurately analyze the measured decay data, Origin software was used for data fitting. First, the experimentally obtained phosphorescence decay signals, represented as intensity (I) versus time (t), both were imported into Origin as two-dimensional coordinates. Here, various fitting models were employed, including single-exponential decay, double-exponential decay, and other relevant models. During the fitting in Origin, the Nonlinear Curve Fit tool was utilized with the ExpDec3 function, and initial parameters were adjusted based on the experimental data trends. The decay function is generally expressed as:where I(t) represents the phosphorescence intensity, and A is the initial intensity; τ denotes the phosphorescence lifetime, and C corresponds to the background signal. To ensure the reliability of the fitting results, the least squares method (LSM) was applied to optimize the parameters, yielding the best-fit function. Specifically, the parameters were rigorously evaluated using the standard error (SE) and the coefficient of determination (R2) as metrics. Generally, an R2 value greater than 0.95 is considered indicative of an excellent fit.
2.7 Structural relaxation and phosphorescence calculations
To investigate the structural relaxation between the ground and triplet excited states, the root-mean-square deviation (RMSD) between the optimized S0 and T1 geometries of Se-pyrene, S-pyrene, and O-pyrene was calculated using the Discovery Studio 2019 software package. The RMSD was computed as:where N is the number of atoms in the molecule, and
 and
and  represent the coordinates of atom i in the S0 and T1 states, respectively. The phosphorescence quantum yield (Φp) for each system was then calculated based on the radiative (kr) and non-radiative (knr) rate constants using the equation:
represent the coordinates of atom i in the S0 and T1 states, respectively. The phosphorescence quantum yield (Φp) for each system was then calculated based on the radiative (kr) and non-radiative (knr) rate constants using the equation:Here, kr and knr were derived from the calculated excitation energies, spin–orbit coupling (SOC) matrix elements, and relevant transition probabilities using standard theoretical models. All calculations were performed using Gaussian16 at the optimized geometries. These methods provide a quantitative basis for evaluating structural relaxation, non-radiative decay, and phosphorescence efficiency in the three systems.
3 Results
3.1 Phosphorescence performance
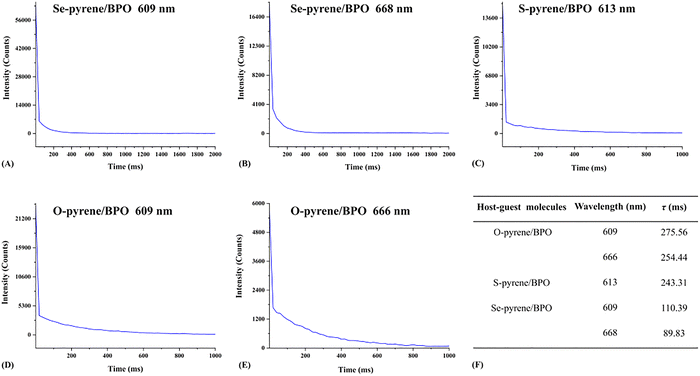 | ||
| Fig. 4 Decay curves of phosphorescent emission intensity over time (A)–(E), along with the fitted phosphorescence lifetimes (F). | ||
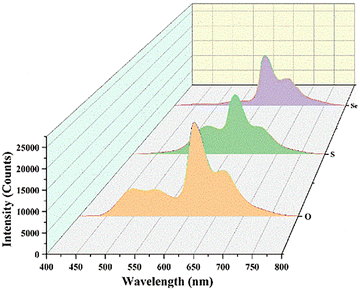
The three host–guest doped molecules exhibit distinct phosphorescence lifetimes. For Se-pyrene/BPO, the lifetimes at 609 and 668 nm were 110.39 and 89.83 ms, respectively. The S-pyrene/BPO showed a lifetime of 243.31 ms at 613 nm, while the O-pyrene/BPO exhibited lifetimes of 275.56 and 254.44 ms at 609 and 666 nm, respectively. Notably, the phosphorescence lifetimes of both S-pyrene/BPO and O-pyrene/BPO were more than twice those of Se-pyrene/BPO. Among them, O-pyrene/BPO demonstrated the longest lifetime at 609 nm, displaying a dual-peak emission profile. It indicates that O-pyrene/BPO not only offers an improved detection window for room-temperature delayed phosphorescence but also holds great potential as an excellent near-infrared room-temperature phosphorescent material. Recent studies further support our findings. For instance, Welscher et al.48 reported that phenoxazine conjugated to a pyrene core exhibits a pronounced red shift in fluorescence emission with a maximum emission at 562 nm, compared with a series of violet-blue emissive molecules.
3.2 Morphology and cellular experiments of nanoparticles
Fig. 5 shows the dispersion of the Se-pyrene/BPO, S-pyrene/BPO and O-pyrene/BPO nanoparticles (NPs) in aqueous solutions. All three NP aqueous solutions exhibited near-infrared phosphorescence, confirming the successful incorporation of host–guest materials. In the transmission electron microscope (TEM) images, all NPs adopted nearly spherical shapes, with Se-pyrene/BPO-NPs displaying somewhat an irregular morphology. Statistical analysis indicated the average diameters of approximately 100 nm for Se-pyrene/BPO-NPs, 150 nm for S-pyrene/BPO-NPs, and 200 nm for O-pyrene/BPO-NPs. Combined with previous phosphorescence spectroscopy and lifetime analyses, the S-pyrene/BPO-NPs and O-pyrene/BPO-NPs systems have larger nanoparticle diameters, promoting the persistence of phosphorescence emission to some extent. For the Se-pyrene/BPO-NPs system, the smaller-sized nanoparticles may make it difficult for the host matrix to effectively encapsulate the guest molecules, which results in poor luminescence effects in subsequent real cell experiments.49,50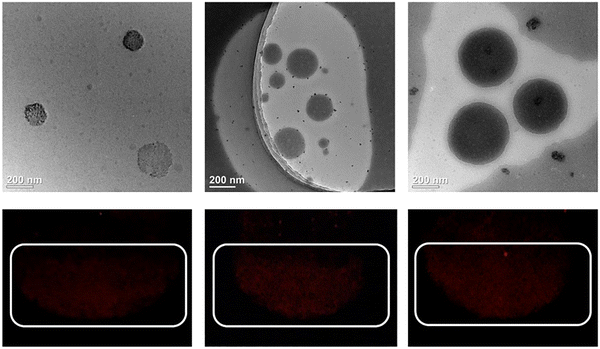 | ||
| Fig. 5 TEM and nanoparticle phosphorescence images of Se-pyrene/BPO-NPs (left), S-pyrene/BPO-NPs (center) and O-pyrene/BPO-NPs (right). | ||
It should be noted that, in this study, the aqueous-phase characterization of nanoparticles—including hydrodynamic diameter (DLS), polydispersity index (PDI), ζ-potential, and phosphorescence quantum yield/lifetime (ΦP/τ)—was not performed, as the primary focus of the work is on the solid-state photophysical behavior and the mechanistic understanding of the host–guest NIR-RTP system. These parameters are important for bioimaging applications and will be systematically investigated in future studies targeting biological uses.
To ensure the safety of the prepared nanoparticles, a preliminary cytotoxicity test was also conducted (Fig. 6). In the designed blank control and a set of concentration gradient tests, the optical density (OD) values of the Se-pyrene/BPO, S-pyrene/BPO and O-pyrene/BPO nanoparticles remained relatively stable with the increase in concentrations. The correlation analysis indicated that there was no statistically significant difference in OD values compared with the blank control group, clarifying that the concentration range used in this work did not show obvious toxic effects on cells. In addition, there was no significant dose-dependent inhibitory relationship between cell activity and concentrations for the three materials. The above results fully demonstrate that the three nanocomposite systems (i.e., Se-pyrene/BPO, S-pyrene/BPO and O-pyrene/BPO) with pyrene as the skeleton have good biocompatibility and high cellular safety. It has laid a solid foundation for the subsequent research on more complex in vivo biological effects and therapeutic applications.51 According to the results of the MTT assay, the cell survival rates of the above three systems did not change significantly within a wide concentration range of 0 to 320 µg mL−1 (Fig. 6).
 | ||
| Fig. 6 MTT assay results for the Se-pyrene/BPO-NPS (A), S-pyrene/BPO-NPS (B) and O-pyrene/BPO-NPS systems (C). | ||
3.3 Structure optimization
To investigate the structural differences and changes in pyrene upon introducing different ligands (phenylseleniumazine, phenylthiazole, and phenyloxazine), we analyzed the ground-state (i.e., S0) geometries for the Se-pyrene, S-pyrene and O-pyrene systems. Since the first excited state of the triplet (T1) is often involved in the photoinactivation process, the geometric structures of the T1 state, in addition to those of the S0 state, were also examined. Here, the excited-state structures were optimized using the ωB97X-D functional, with the key bond lengths and angles both being summarized in Fig. 7. Comparison between the ground and excited states reveals no notable differences in the N–C bond lengths or C–N–C bond angles across the three systems (i.e., Se-pyrene, S-pyrene and O-pyrene). It indicates that the binding stability of all three ligands to the pyrene core is comparable. There are minor variations observed in the bond lengths between the heteroatoms and carbon. Specifically, the Se–C3 and S–C3 bonds are considerably longer than the O–C3 bond, suggesting that the introduction of phenoxazine may slightly influence the molecular structure.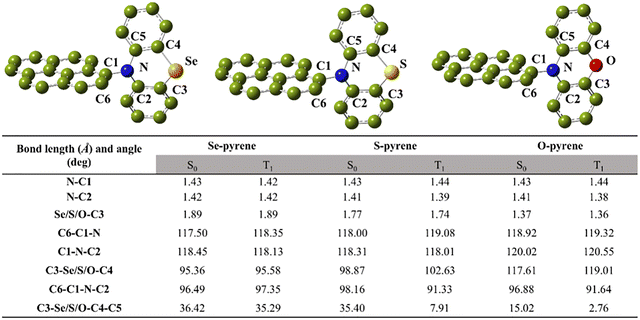 | ||
| Fig. 7 Structure data of the S0 and T1 states for the Se-pyrene, S-pyrene and O-pyrene systems at the ωB97X-D/6-31G(d,p) theoretical level. | ||
Upon transition from the S0 to T1 states, certain changes occur in the dihedral angles C6–C1–N–C2 and C3–X–C4–C5 (where X = Se, S, or O). Among these, the most pronounced change is observed for C3–S–C4–C5 in S-pyrene with a difference of 27.49 deg. It indicates substantial structural distortion during the transition. The marked deformation between the S0 and T1 structures enhances vibronic coupling (i.e., the Franck–Condon factor), thereby having a high probability of promoting non-radiative decay. Consequently, S-pyrene is likely to exhibit a higher temperature-independent non-radiative rate constant in phosphorescence emission compared to the Se-pyrene and O-pyrene systems.
3.4 Hole–electron distribution
To further elucidate the transition characteristics from the S0 to T1 states, we carried out the hole–electron analysis (Fig. 8). In this method, the electronic excitation processes are conceptualized as transitions from holes to electrons. It offers an intuitive visualization of the origins and destinations of electrons involved in the excitation. A clear spatial distinction is observed: electrons are primarily localized on the pyrene backbone, while holes are predominantly situated on the introduced ligands. This pronounced separation between holes and electrons indicates the charge-transfer characteristic and electronic dissociation across the entire system. It is worth mentioning that the significant degree of electron–hole separation further implies favorable electron transport properties in the excited state. | ||
| Fig. 8 Hole–electron distributions for Se-pyrene, S-pyrene and O-pyrene, where the green and blue colors, respectively, represent the electron and hole. | ||
3.5 Singlet and triplet energy levels
Given that the solvent effect exerts only a negligible influence on the triplet energy levels of the investigated systems (Fig. S7 and Tables S1–S6), the following discussion is based on the gas-phase results. Fig. 9 presents the excitation energies of Sn and Tn (n = 1–5) states computed at the TD-ωB97X-D3/def2-TZVP(-f) theoretical level for Se-, S-, and O-pyrene. Both Se-pyrene and S-pyrene exhibit similar energy distributions for the T5, T4, T3, and T1 states, while the T2 state differs markedly. In S-pyrene, the T2 state lies lower in energy and is more strongly coupled to S1via spin–orbit coupling (SOC), which facilitates an efficient S1 → T2 intersystem crossing (ISC). The subsequent internal conversion (T2 → T1) efficiently populates the lowest triplet state, leading to enhanced phosphorescence. This is consistent with their observed phosphorescence spectra, in which the variation in emission behavior between S-pyrene and Se-pyrene primarily originates from differences in their T2-state energetics and ISC pathways. In contrast, for O-pyrene, the T2 level is higher in energy and exhibits weaker SOC with S1, making direct S1 → T1 ISC the dominant relaxation route. The phenoxazine moiety effectively stabilizes the T1 state, lowering its energy and yielding red-shifted, long-lived phosphorescence (∼275.56 ms). Thus, the distinct photophysical characteristics of the three systems arise from their different S1–T2–T1 energy alignments and SOC-mediated ISC mechanisms rather than from a simple difference in triplet energy levels. | ||
| Fig. 9 Calculated excitation energies for Sn and Tn (n = 1, 2, 3, 4, and 5) states at the TD-ωB97X-D3/def2-TZVP(-f) theoretical level. | ||
3.6 Frontier molecular orbitals
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels were calculated to explore how the electronic structure affects the optical properties of Se-pyrene, S-pyrene, and O-pyrene. Fig. 10 presents the frontier molecular orbital distributions and the corresponding energy gaps (ΔE). The results reveal a moderate intramolecular charge transfer (ICT) characteristic upon excitation. The HOMO is primarily localized on the phenoselenazine, phenothiazine, and phenoxazine moieties, whereas the LUMO is mainly distributed over the pyrene core. This spatial separation between HOMO and LUMO confirms the presence of ICT, which likely contributes to the red-shift of the pyrene emission. The calculated energy gap values follow the order: O-pyrene (6.45 eV) < S-pyrene (6.70 eV) < Se-pyrene (6.77 eV). In general, a larger energy gap corresponds to lower molecular reactivity and higher structural stability, since a higher excitation energy is required to promote electrons. Based on the optimized T1-state structures, the emission spectra of all complexes were further simulated using the TDDFT method. It should be noted that the SOC effect was not considered in the frontier orbital calculations. Consequently, the oscillator intensity of phosphorescent emission is zero for these systems. | ||
| Fig. 10 Calculated energy levels of frontier molecular orbitals for the Se-pyrene, S-pyrene and O-pyrene systems. | ||
| Systems | λ (nm) | Configuration | Emission color |
|---|---|---|---|
| Se-pyrene | 609, 666 | HOMO → LUMO (73.8%) | Red |
| HOMO → LUMO+2 (12.4%) | |||
| S-pyrene | 613 | HOMO → LUMO (89.0%) | Red |
| O-pyrene | 609, 668 | HOMO → LUMO (80.7%) | Red |
| HOMO → LUMO+2 (8.1%) |
Notably, in Se-pyrene and O-pyrene, which exhibit bimodal emission, additional orbital transitions (i.e., HOMO to LUMO+2) also contribute significantly. When such secondary transitions participate, the excited population may access alternative excited or vibronic levels that typically possess weaker oscillator strengths, enhanced structural relaxation, and slower radiative decay, thereby facilitating non-radiative pathways and altering the overall emission lifetimes. Furthermore, variations in the energy gap between these states and the lowest triplet or ground state can modify internal conversion and intersystem crossing rates, collectively influencing the observed lifetime behavior.
This interpretation aligns with recent theoretical insights. Ma et al.52 demonstrated that electron–phonon coupling can substantially affect phosphorescence lifetimes, where secondary orbital contributions (i.e., HOMO–2 to LUMO) enhance coupling strength and reduce transition intensity. Similarly, Guerrini et al.53 reported that when multiple close-lying excited states coexist, vibronic coupling between these states induces energy redistribution and competition among decay channels, ultimately impacting both the lifetime and quantum yield. These studies provide valuable theoretical support and further substantiate our interpretation of the emission characteristics of Se- and O-pyrene systems.
3.7 Spin–orbit coupling
For the forward intersystem crossing (ISC) processes, the spin–orbit coupling (SOC) values between Sn and various Tn states were calculated for different molecular geometries (i.e., S0, S1 and T1) via the spin–orbit mean field (SOMF) method implemented in ORCA 5.0.3 at the ωB97X-D3/def2-tzvp(-f) theory level. In the calculation process of the SOC matrix element, it was also found that the solvation effect exhibits a relatively limited influence (see Table 2 and Fig. S7–S9). As summarized in Table 2, most ISC pathways possess SOC values exceeding 1 cm−1, with some transition states even reaching values greater than 10 cm−1. It indicates a strong propensity for the forward ISC processes in these systems. In fact, the non-radiative rate constants are closely related to the matrix elements between S0 and Tn states, where the stronger SOC effects facilitate ISC from the excited state.| Transition types | Se-pyrene | S-pyrene | O-pyrene | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | T1 | S0 | S1 | T1 | S0 | S1 | T1 | |
| |〈T1|HSOC|S0〉| | 0.010 | 0.000 | 0.010 | 0.021 | 0.010 | 0.010 | 0.010 | 0.121 | 0.021 |
| |〈T2|HSOC|S0〉| | 158.0 | 28.79 | 155.0 | 25.93 | 4.924 | 5.064 | 0.336 | 0.319 | 0.253 |
| |〈T3|HSOC|S0〉| | 10.39 | 7.437 | 0.222 | 3.119 | 0.632 | 0.553 | 0.501 | 1.548 | 1.510 |
| |〈T4|HSOC|S0〉| | 0.861 | 5.679 | 12.13 | 0.220 | 1.399 | 1.380 | 0.090 | 0.191 | 0.061 |
| |〈T5|HSOC|S0〉| | 0.300 | 0.270 | 1.140 | 0.000 | 0.021 | 0.021 | 0.930 | 0.378 | 0.249 |
| |〈T1|HSOC|S1〉| | 1.403 | 1.696 | 0.000 | 0.430 | 0.376 | 0.360 | 0.807 | 0.951 | 0.968 |
| |〈T2|HSOC|S1〉| | 3.551 | 0.480 | 0.497 | 0.260 | 0.559 | 0.599 | 0.901 | 1.013 | 0.960 |
| |〈T3|HSOC|S1〉| | 34.00 | 0.230 | 0.599 | 3.873 | 0.400 | 0.390 | 0.539 | 0.249 | 0.230 |
| |〈T4|HSOC|S1〉| | 1.720 | 3.459 | 0.159 | 0.327 | 0.331 | 0.300 | 0.151 | 0.175 | 0.184 |
| |〈T5|HSOC|S1〉 | 0.785 | 0.260 | 0.021 | 0.191 | 0.158 | 0.173 | 0.029 | 0.578 | 0.568 |
Specifically, Se-pyrene shows the highest SOC value of 158.0 cm−1 between the S0 and T2 states, making ISC particularly favorable with a high conversion rate. It enables relaxation to the T2 state, followed by phosphorescence emission from the ground state (S0). A similar behavior was found in S-pyrene, whose SOC value between S0 and T2 is only 25.93 cm−1. It indicates a weaker ISC effect and a significantly lower ISC rate compared to Se-pyrene. The exceptionally large SOC in Se-pyrene (e.g., 158 cm−1) accelerates ISC but can also enhance competing non-radiative channels (kIC/knr), which rationalizes its relatively short phosphorescence lifetime compared with S-pyrene and O-pyrene.54–56 In contrast, O-pyrene presents a more favorable balance between SOC and triplet energy: its moderate SOC is sufficient to promote ISC without excessively increasing non-radiative loss, while the lowered T1 and host-mediated rigidity stabilize the triplet and suppress knr.
As discussed in the previous section, a wider HOMO–LUMO energy gap generally indicates greater molecular stability and lower chemical reactivity, since higher excitation energy reduces the likelihood of undesirable chemical reactions. However, this energy gap primarily determines excitation and emission energies rather than directly affecting the phosphorescence lifetime. The long-lived RTP behavior observed for O-pyrene thus arises mainly from suppressed non-radiative decay (knr) and enhanced triplet-state stabilization, rather than from the HOMO–LUMO gap per se. Overall, it is the synergistic combination of appropriate SOC strength, triplet stabilization, and host–guest confinement that accounts for the superior room-temperature phosphorescence performance of O-pyrene.
3.8 Structural relaxation and correlation with phosphorescence behavior
To further understand the factors governing phosphorescence efficiency, we analyzed the structural relaxation of the three systems (Se-pyrene, S-pyrene, and O-pyrene) based on the root-mean-square deviation (RMSD) between the S0 and T1 states. As observed from Fig. 11, both O-pyrene and Se-pyrene exhibit smaller RMSD values compared to S-pyrene, indicating limited structural reorganization upon excitation and lower barriers for the triplet-singlet transition. Such structural rigidity suppresses non-radiative decay (knr) and supports the enhanced phosphorescence efficiency of O-pyrene. Although Se-pyrene shows the smallest RMSD value among the three systems, its exceptionally large SOC facilitates very efficient intersystem crossing (ISC), which also enhances competing non-radiative channels, resulting in a lower Φp than that of O-pyrene. | ||
| Fig. 11 Overlay of the S0 and T1 geometries of Se-, S-, and O-pyrenes, together with their calculated radiative (kr) and non-radiative (knr) rate constants and phosphorescence quantum yields (Φp). | ||
These observations are consistent with the conclusions drawn from our previous bond length and bond angle analyses, which revealed that O-pyrene possesses shorter bonds and smaller bond angle variations, leading to limited geometric distortion and reduced non-radiative decay. The moderate SOC strength of O-pyrene enables efficient ISC without excessively promoting non-radiative pathways, further contributing to its superior phosphorescence performance.
Furthermore, the frontier molecular orbital analysis shows that O-pyrene possesses the smallest HOMO–LUMO gap among the three systems. While this gap primarily determines excitation and emission energies rather than the phosphorescence lifetime, its combination with structural rigidity and appropriate SOC strength stabilizes the triplet state and enhances room-temperature phosphorescence (RTP) efficiency, contributing to its high Φp and long phosphorescence lifetime.
4 Conclusion
In this work, luminescent properties were investigated for a group of organic room-temperature phosphorescent materials by combining organic synthesis with computational simulation methods. Firstly, three novel pyrene derivatives (i.e., Se-pyrene, S-pyrene, and O-pyrene) were successfully synthesized via Buchwald–Hartwig amination of phenoselenazine, phenothiazine, and phenoxazine, respectively, onto a bromopyrene core. These compounds served as guest molecules chelated into BPO host matrices to fabricate the doped solid-state materials. Under 365 nm irradiation at room temperature, all three doped materials emitted pronounced red delayed phosphorescence, demonstrating their potential as efficient room-temperature phosphorescence (RTP) materials. Detailed photophysical studies successfully revealed distinct emission peaks and varying phosphorescence lifetimes among the samples, with O-pyrene and S-pyrene both showing notably longer lifetimes than Se-pyrene, which suggests differences in their excited-state dynamics.The systematic quantum mechanical calculations provided deep insights into the underlying mechanisms governing these photophysical behaviors. According to DFT analyses, although Se-pyrene and S-pyrene share similar triplet energy level distributions, the lower energy of the T2 state in S-pyrene favors phosphorescence emission by facilitating the ISC process. With the introduction of the phenoxazine moiety, O-pyrene exhibits an even more dramatically reduced T1 state energy, which correlates well with the observed long-wavelength phosphorescence and extended lifetime. In the calculation of frontier molecular orbitals, the HOMO–LUMO energy gaps followed the order O-pyrene < S-pyrene < Se-pyrene, with the former showing enhanced stability yet favorable photophysical properties. In addition, the high SOC constant of Se-pyrene leads to excessive non-radiative decay, lowering the phosphorescence quantum yield and lifetime. Conversely, O-pyrene balances a lower energy gap with moderate SOC values, optimizing its ISC efficiency. The host matrix further stabilizes the guest conformations, restricting molecular motions and facilitating efficient ISC processes. In summary, this experiment-simulation synergistic approach not only investigates the RTP properties of organic luminophores but also offers valuable guidelines for the development of novel, high-performance phosphorescent materials for optoelectronic applications.
Author contributions
Kaixuan Hu: resources, writing original draft. Shufeng Chen and Xinmin Wang: conceptualization, resources. Lingkai Tang: review, editing. Yan Cheng: visualization. Yuting Song: data mining. Hubing Shi and Jing Jing: project administration, supervision. Jianping Hu: project administration, supervision, methodology. Ting Luo: funding acquisition.Conflicts of interest
The authors declare no conflict of interest, financial or otherwise.Data availability
All data generated or analyzed during this study are included in this published article.The data supporting this article have been included as part of the supplementary information (SI), which contains: (1) general procedures for the synthesis of the target compounds, and (2) theoretical calculations. Supplementary information is available. See DOI: https://doi.org/10.1039/d5cp03599f.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFA1207300 [2022YFA1207303]); National Natural Science Foundation of China (82172634); 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD23028); National Guidance Fund on Developing Local Science and Technology for Sichuan Province (2023ZYD0167, China); and Sichuan Science and Technology Program (2025ZDZX0012).References
- L. Xiao and H. Fu, Enhanced room-temperature phosphorescence through intermolecular halogen/hydrogen bonding, Chem. – Eur. J., 2019, 25(3), 714–723 CrossRef CAS PubMed.
- Z. Guan, Z. Tang, J. Deng, Y. Zheng, H. Li and X. Liu, Multi-Color Pure Organic Room Temperature Phosphorescent Materials with Long Lifetime and High Efficiency, Adv. Funct. Mater., 2024, 34(7), 2310198 CrossRef CAS.
- M. Dong, A. Lv, X. Zou, N. Gan, C. Peng and M. Ding, et al., Polymorphism-Dependent Organic Room Temperature Phosphorescent Scintillation for X-Ray Imaging, Adv. Mater., 2024, 36(18), 2310663 CrossRef CAS PubMed.
- J.-H. Hu, R.-X. Hou, C. Liu, Y.-P. Wang, Y.-F. Liu and Y. Huang, et al., Ultra-long room temperature phosphorescent materials based on tetraphenylethylene derivatives for anti-counterfeiting and encryption applications, Chem. Eng. J., 2024, 491, 152177 CrossRef CAS.
- W. Zhao, Z. He and B. Z. Tang, Room-temperature phosphorescence from organic aggregates. Nature Reviews, Materials, 2020, 5(12), 869–885 CAS.
- Q. Peng, H. Ma and Z. Shuai, Theory of long-lived room-temperature phosphorescence in organic aggregates, Acc. Chem. Res., 2020, 54(4), 940–949 CrossRef PubMed.
- X. Wang, H. Shi, H. Ma, W. Ye, L. Song and J. Zan, et al., Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence, Nat. Photonics, 2021, 15(3), 187–192 CrossRef CAS.
- Y. Wang, H. Gao, J. Yang, M. Fang, D. Ding and B. Z. Tang, et al., High performance of simple organic phosphorescence host–guest materials and their application in time-resolved bioimaging, Adv. Mater., 2021, 33(18), 2007811 CrossRef CAS PubMed.
- H. Gao, Z. Gao, D. Jiao, J. Zhang, X. Li and Q. Tang, et al., Boosting room temperature phosphorescence performance by alkyl modification for intravital orthotopic lung tumor imaging, Small, 2021, 17(22), 2005449 CrossRef CAS PubMed.
- J. Ren, Y. Wang, Y. Tian, Z. Liu, X. Xiao and J. Yang, et al., Force-Induced Turn-On Persistent Room-Temperature Phosphorescence in Purely Organic Luminogen, Angew. Chem., Int. Ed., 2021, 133(22), 12443–12448 CrossRef.
- H. Ma, H. Yu, Q. Peng, Z. An, D. Wang and Z. Shuai, Hydrogen bonding-induced morphology dependence of long-lived organic room-temperature phosphorescence: a computational study, J. Phys. Chem. Lett., 2019, 10(21), 6948–6954 CrossRef CAS PubMed.
- X. Jia, C. Shao, X. Bai, Q. Zhou, B. Wu and L. Wang, et al., Photoexcitation-controlled self-recoverable molecular aggregation for flicker phosphorescence, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(11), 4816–4821 CrossRef CAS PubMed.
- Y. Gong, G. Chen, Q. Peng, W. Z. Yuan, Y. Xie and S. Li, et al., Achieving Persistent Room Temperature Phosphorescence and Remarkable Mechanochromism from Pure Organic Luminogens, Adv. Mater., 2015, 27(40), 6195–6201 CrossRef CAS PubMed.
- F. Xiao, H. Gao, Y. Lei, W. Dai, M. Liu and X. Zheng, et al., Guest–host doped strategy for constructing ultralong-lifetime near-infrared organic phosphorescence materials for bioimaging, Nat. Commun., 2022, 13(1), 186 CrossRef CAS PubMed.
- O. Bolton, K. Lee, H.-J. Kim, K. Y. Lin and J. Kim, Activating efficient phosphorescence from purely organic materials by crystal design, Nat. Chem., 2011, 3(3), 205–210 CrossRef CAS PubMed.
- L. Bian, H. Shi, X. Wang, K. Ling, H. Ma and M. Li, et al., Simultaneously Enhancing Efficiency and Lifetime of Ultralong Organic Phosphorescence Materials by Molecular Self-Assembly, J. Am. Chem. Soc., 2018, 140(34), 10734–10739, DOI:10.1021/jacs.8b03867.
- X. Zeng, L. Wang, H. Dai, T. Huang, M. Du and D. Wang, et al., Orbital symmetry engineering in fused polycyclic heteroaromatics toward extremely narrowband green emissions with an FWHM of 13 nm, Adv. Mater., 2023, 35(22), 2211316 CrossRef CAS PubMed.
- H. L. Lee, S. O. Jeon, I. Kim, S. C. Kim, J. Lim and J. Kim, et al., Multiple-Resonance Extension and Spin-Vibronic-Coupling-Based Narrowband Blue Organic Fluorescence Emitters with Over 30% Quantum Efficiency, Adv. Mater., 2022, 34(33), 2202464 CrossRef CAS PubMed.
- X. Zeng, X. Wang, Y. Zhang, G. Meng, J. Wei and Z. Liu, et al., Nitrogen-embedded multi-resonance heteroaromatics with prolonged homogeneous hexatomic rings, Angew. Chem., Int. Ed., 2022, 134(14), e202117181 CrossRef.
- H. Zhu, I. Badia-Dominguez, B. Shi, Q. Li, P. Wei and H. Xing, et al., Cyclization-promoted ultralong low-temperature phosphorescence via boosting intersystem crossing, J. Am. Chem. Soc., 2021, 143(4), 2164–2169 CrossRef CAS PubMed.
- T. Zhu, T. Yang, Q. Zhang and W. Z. Yuan, Clustering and halogen effects enabled red/near-infrared room temperature phosphorescence from aliphatic cyclic imides, Nat. Commun., 2022, 13(1), 2658 CrossRef CAS PubMed.
- S. Guo, W. Dai, X. Chen, Y. Lei, J. Shi and B. Tong, et al., Recent progress in pure organic room temperature phosphorescence of small molecular host–guest systems, ACS Mater. Lett., 2021, 3(4), 379–397 CrossRef CAS.
- J. Yang, M. Fang and Z. Li, Stimulus-responsive room temperature phosphorescence in purely organic luminogens, InfoMat, 2020, 2(5), 791–806 CrossRef CAS.
- L. Gu, H. Shi, L. Bian, M. Gu, K. Ling and X. Wang, et al., Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal, Nat. Photonics, 2019, 13(6), 406–411 CrossRef CAS.
- Y. Zhang, X. Chen, J. Xu, Q. Zhang, L. Gao and Z. Wang, et al., Cross-linked polyphosphazene nanospheres boosting long-lived organic room-temperature phosphorescence, J. Am. Chem. Soc., 2022, 144(13), 6107–6117 CrossRef CAS PubMed.
- H. Chen, Y. Sun, M. Liu, F. Li, Q. Peng and H. Huang, Modulating Triplet Excited States of Organic Semiconductors via Tuning Molecular Conformation for Dual-ratiometric Thermometers, Angew. Chem., Int. Ed., 2023, 135(20), e202302629 CrossRef.
- R. Gavale, S. Singh, A. Ekbote, H. C. Jha and R. Misra, Stimuli-responsive benzothiazole-phenothiazine derivatives: mechanochromism, AIE, acid sensing, and anticancer efficacy in benzo[a]pyrene-induced cancer models, J. Mater. Chem. B, 2025, 13(8), 2834–2854, 10.1039/D4TB02408G.
- Y. Zhang, L. Gao, X. Zheng, Z. Wang, C. Yang and H. Tang, et al., Ultraviolet irradiation-responsive dynamic ultralong organic phosphorescence in polymeric systems, Nat. Commun., 2021, 12(1), 2297 CrossRef CAS PubMed.
- X. Luo, B. Tian, Y. Zhai, H. Guo, S. Liu and J. Li, et al., Room-temperature phosphorescent materials derived from natural resources, Nat. Rev. Chem., 2023, 7(11), 800–812 CrossRef PubMed.
- T. Ono, K. Kimura, M. Ihara, Y. Yamanaka, M. Sasaki and H. Mori, et al., Room-Temperature Phosphorescence Emitters Exhibiting Red to Near-Infrared Emission Derived from Intermolecular Charge-Transfer Triplet States of Naphthalenediimide− Halobenzoate Triad Molecules, Chem. – Eur. J., 2021, 27(37), 9535–9541 CrossRef CAS PubMed.
- Y. Katsurada, S. Hirata, K. Totani, T. Watanabe and M. Vacha, Photoreversible on–off recording of persistent room-temperature phosphorescence, Adv. Opt. Mater., 2015, 3(12), 1726–1737 CrossRef CAS.
- Y. Lei, J. Yang, W. Dai, Y. Lan, J. Yang and X. Zheng, et al., Efficient and organic host–guest room-temperature phosphorescence: tunable triplet–singlet crossing and theoretical calculations for molecular packing, Chem. Sci., 2021, 12(19), 6518–6525 RSC.
- D. Wang, Y. Xie, X. Wu, Y. Lei, Y. Zhou and Z. Cai, et al., Excitation-dependent triplet–singlet intensity from organic host–guest materials: tunable color, white-light emission, and room-temperature phosphorescence, J. Phys. Chem. Lett., 2021, 12(7), 1814–1821 CrossRef CAS PubMed.
- B. Chen, W. Huang, X. Nie, F. Liao, H. Miao and X. Zhang, et al., An organic host–guest system producing room-temperature phosphorescence at the parts-per-billion level, Angew. Chem., Int. Ed., 2021, 60(31), 16970–16973 CrossRef CAS PubMed.
- Y. Si, Y. Zhao, W. Dai, S. Cui, P. Sun and J. Shi, et al., Organic Host-Guest Materials with Bright Red Room-Temperature Phosphorescence for Persistent Bioimaging, Chin. J. Chem., 2023, 41(13), 1575–1582 CrossRef CAS.
- W. Dai, Y. Zhang, X. Wu, S. Guo, J. Ma and J. Shi, et al., Red-emissive organic room-temperature phosphorescence material for time-resolved luminescence bioimaging, CCS Chem., 2022, 4(8), 2550–2559 CrossRef CAS.
- D. Zhao, M. Xu, S. Yang, H. Ma, H. Li and R. Wu, et al., Specific diagnosis of lymph node micrometastasis in breast cancer by targeting activatable near-infrared fluorescence imaging, Biomaterials, 2022, 282, 121388 CrossRef CAS PubMed.
- X. He, X. Bao, H. Cao, Z. Zhang, Q. Yin and W. Gu, et al., Tumor-Penetrating Nanotherapeutics Loading a Near-Infrared Probe Inhibit Growth and Metastasis of Breast Cancer, Adv. Funct. Mater., 2015, 25(19), 2831–2839 CrossRef CAS.
- G. Zhang, M. Dong, X. Yao, Y. Xia, H. Yu and Y. Zhou, et al., Advancing breast cancer diagnosis with a near-infrared fluorescence imaging smart sensor for estrogen/progesterone receptor detection, Sci. Rep., 2023, 13(1), 21086 CrossRef CAS PubMed.
- S. Mao, Y. Zhao, Y. Hong, L. Ma and Y. Wang, Tunable Thermally Activated Delayed Fluorescence and Room-Temperature Phosphorescence Emissions, White and Red Long Afterglow Driven by Host–Guest Doping and Substituent Electronic Effects, ACS Appl. Mater. Interfaces, 2025, 17(42), 58669–58680 CAS.
- J. Cao, M. Zhang, M. Singh, Z. An, L. Ji and H. Shi, et al., Highly efficient heavy atom free room temperature phosphorescence by host-guest doping, Front. Chem., 2021, 9, 781294 CrossRef CAS PubMed.
- J. P. Mancinelli, W.-Y. Kong, W. Guo, D. J. Tantillo and S. M. Wilkerson-Hill, Correction to “Borane-Catalyzed C–F Bond Functionalization of gem-Difluorocyclopropenes Enables the Synthesis of Orphaned Cyclopropanes”, J. Am. Chem. Soc., 2023, 145(44), 24434–24435 CrossRef CAS PubMed.
- J. Autschbach, T. Ziegler, S. J. van Gisbergen and E. J. Baerends, Chiroptical properties from time-dependent density functional theory. I. Circular dichroism spectra of organic molecules. The, J. Chem. Phys., 2002, 116(16), 6930–6940 CrossRef CAS.
- T. Helgaker and P. Jo/rgensen, An electronic Hamiltonian for origin independent calculations of magnetic properties, J. Chem. Phys., 1991, 95(4), 2595–2601 CrossRef CAS.
- K. L. Bak, P. Jo/rgensen, T. Helgaker and K. Ruud, Jensen HJrA. Gauge-origin independent multiconfigurational self-consistent-field theory for vibrational circular dichroism, J. Chem. Phys., 1993, 98(11), 8873–8887 CrossRef CAS.
- M. J. Frisch, Gaussian 09, revision A. 01, 2009 Search PubMed.
- F. Neese, F. Wennmohs, U. Becker and C. Riplinger, The ORCA quantum chemistry program package, J. Chem. Phys., 2020, 152(22), 224108, DOI:10.1063/5.0004608.
- P. J. Welscher, D. Straub, F. Stümpges, A. L. Respondek, B. Esser and A. J. Kuehne, Electron Donor-Functionalized Pyrenes with Amplified Spontaneous Emission for Violet–Blue Electroluminescent Devices Beyond the Spin Statistical Limit, Adv. Funct. Mater., 2025, 35(11), 2417129 CrossRef CAS.
- X. Zhen, Y. Tao, Z. An, P. Chen, C. Xu and R. Chen, et al., Ultralong phosphorescence of water-soluble organic nanoparticles for in vivo afterglow imaging, Adv. Mater., 2017, 29(33), 1606665 CrossRef PubMed.
- Q. Dang, Y. Jiang, J. Wang, J. Wang, Q. Zhang and M. Zhang, et al., Room-temperature phosphorescence resonance energy transfer for construction of near-infrared afterglow imaging agents, Adv. Mater., 2020, 32(52), 2006752 CrossRef PubMed.
- K. G. Ozdemir, H. Yılmaz and S. Yılmaz, In vitro evaluation of cytotoxicity of soft lining materials on L929 cells by MTT assay, J. Biomed. Mater. Res., Part B, 2009, 90(1), 82–86 CrossRef PubMed.
- L. Ma, M. Cong, S. Sun and X. Ma, Manipulating room-temperature phosphorescence by electron–phonon coupling, Chem. Sci., 2025, 16(19), 8282–8290 RSC.
- M. Guerrini, J. Krumland and C. Cocchi, Vibronic dynamics from real-time time-dependent density-functional theory coupled to the Ehrenfest scheme: the example of p-coumaric acid, Theor. Chem. Acc., 2023, 142(11), 110 Search PubMed.
- L. Tang, J. Gao, Y. Luo, Y. Cheng, L. Liu and D. Zheng, et al., Regulation of internal reorganization energy to change the non-radiative channel in the Ir(III) complex: the role of N atoms, New J. Chem., 2023, 47(32), 15076–15088 RSC.
- P. C. Teeuwen, Z. Melissari, M. O. Senge and R. M. Williams, Metal coordination effects on the photophysics of dipyrrinato photosensitizers, Molecules, 2022, 27(20), 6967 CrossRef CAS PubMed.
- N. Ibrayev, E. Seliverstova, R. Valiev, A. Aymagambetova and D. Sundholm, The effect of heavy atoms on the deactivation of electronic excited states of dye molecules near the surface of metal nanoparticles, Phys. Chem. Chem. Phys., 2024, 26(40), 25986–25993 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © the Owner Societies 2026 |