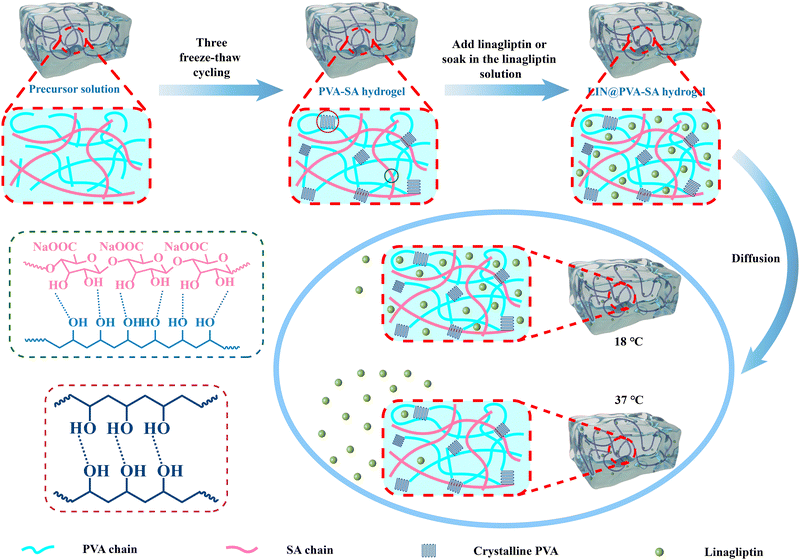
Mechanically robust PVA/SA semi-IPN hydrogels for highly effective temperature-triggered linagliptin delivery
Yue
Wang
ab,
Guineng
Li
b,
Yeying
Li
b,
Mutian
Yao
b,
Qiaobo
Wang
b,
Liang
Peng
*a and
Hua
Gu
 *bc
*bc
aSchool of Pharmacy, Jiangxi Science and Technology Normal University, Nanchang, 330013, China. E-mail: 1020100997@jxstnu.edu.cn
bJiangxi Provincial Key Laboratory of Flexible Electronics, Flexible Electronics Innovation Institute, Jiangxi Science and Technology Normal University, Nanchang, 330013, China. E-mail: guhua1254@163.com
cInstitute of Energy Materials and Nanotechnology, Nanchang Jiaotong Institute, Nanchang, 330100, P.R. China
First published on 19th November 2025
Abstract
Hydrogels with excellent mechanical properties and high drug-delivery capacity are highly advantageous for wound healing applications. However, reconciling mechanical properties with drug-delivery performance remains challenging. Herein, a semi-interpenetrating polymer network (semi-IPN) hydrogel composed of poly(vinyl alcohol) (PVA) and sodium alginate (SA) was fabricated via freeze–thaw cycling. Through component optimization, this hydrogel achieved a unique combination of desirable properties, including softness (Young's modulus of 32.5 kPa), high stretchability (fracture strain of 283.9%), and high water content (96.46%). Linagliptin (LIN), a DPP-4 inhibitor crucial for diabetic wound repair, was loaded into the system with an effective loading efficiency of 89.25%. The hydrogel system demonstrated temperature-responsive release kinetics: minimal release occurred at low temperatures, while rapid, sustained release was achieved at physiological temperature, reaching a cumulative release efficiency of up to 85.17%, which was highly beneficial for the storage and application of drug-loaded hydrogels. This study presents a hydrogel platform with effectively integrated material robustness, temperature-triggered drug delivery and high-efficiency loading and release of LIN, which showcases significant potential as a novel therapeutic material for diabetic wound healing.
Introduction
Chronic wounds, particularly in diabetic patients, are exacerbated by inflammation, vascular dysfunction, and impaired healing, often leading to severe complications such as amputations.3–5 Clinical treatment methods usually rely mainly on drug therapy. However, improper dosage of the drugs can often lead to serious complications.8 Therefore, it is necessary to develop a controllable drug administration method to eliminate the harm caused by excessive drug administration. Hydrogels are polymer materials with a three-dimensional network structure, which is very easy to be endowed with excellent mechanical, optical, and electrical properties, as well as the ability to be tailored across multiple scales.12 Due to their versatility, hydrogels are widely used in various fields such as bioelectronics,15 water purification,16–20 strain sensors,21–23 electrochromic devices,24 and soft robotics.22,25 Moreover, hydrogels are promising drug delivery systems because of their high-water content, excellent biocompatibility, tunable physicochemical properties, and effective drug-loading capacity, which together enhance therapeutic outcomes.26–28 They enable sustained and controlled drug release, improving drug utilization, while also offering high flexibility and environmental adaptability to support stable drug delivery. In wound healing applications, hydrogels help maintain a moist environment that promotes cell migration and proliferation, accelerating tissue repair.29,30 Nevertheless, reconciling robust mechanical properties with efficient drug loading and controlled release persists as a critical challenge.In recent years, microenvironment-responsive drug-release hydrogels, such as those responsive to reactive oxygen species (ROS), pH variations, and specific enzymes, have been widely developed for application in intelligent drug delivery systems.31–34 However, the complexity of drug release mechanisms, the dynamic activity of the microenvironment, variability in substrate concentration, and the presence of false positive signals often results in incomplete or uncontrolled (burst) drug release, thereby prolonging the healing process of chronic wounds.35–37 Furthermore, these responsive drug delivery systems are susceptible to oxidation, degradation, and inactivation during storage, leading to unnecessary drug waste and economic losses. Developing a storage-stable and highly efficient drug delivery system for wound treatment in humans remains a significant challenge.
Polyvinyl alcohol (PVA) is a polar polymer characterized by excellent water solubility, inherent non-toxicity, and favorable mechanical properties. Through chemical or physical crosslinking, PVA can form hydrogels that are extensively utilized as matrix materials in wound dressing applications.19,38–40 Sodium alginate (SA), a naturally derived polyanionic, linear carbohydrate biopolymer extracted from seaweed, exhibits excellent biocompatibility, non-toxicity, and strong hydrophilic properties.41,42 Both PVA and SA are considered promising materials for hydrogel design. PVA contributes structural integrity through physical crosslinking, whereas SA enhances biocompatibility and hydrophilicity. The integration of PVA and SA results in the formation of a semi-interpenetrating network (semi-IPN) structure, which facilitates efficient drug encapsulation and enables temperature-responsive drug release.43
In addition, linagliptin (LIN), as a DPP-4 inhibitor, is widely used in the treatment of type 2 diabetes, where it works by enhancing insulin secretion and lowering blood glucose levels.44–47 LIN exerts its pharmacological effects while exhibiting absolute safety.6,9,48 However, LIN faces several challenges during treatment as an oral drug, such as incomplete absorption in the gastrointestinal tract, which can make it difficult to achieve adequate drug concentrations.49,50 Furthermore, traditional oral administration methods struggle to provide sustained and efficient drug release at the local site of diabetic wounds. Therefore, applying LIN in wound dressings for local drug delivery can more directly promote wound healing while avoiding systemic side effects.46,49–51
Therefore, in this study, we developed a three-dimensional semi-IPN SA-PVA hydrogel through a simple freeze-thawing cycle approach, following the regulation and optimization of the solid content of PVA. Among the prepared hydrogels, the one with a solid content of 90.9 wt% PVA (SA–PVA90.9) exhibited the best overall performance, characterized by superior mechanical properties (maximum stress: 84.5 kPa; maximum strain: 283.9%), a high water content of 96.46%, and an ideal swelling rate of 2275%. When immersed in a LIN solution, the optimized PVA–SA90.9% hydrogel demonstrated an exceptionally high drug-loading capacity. Notably, the drug-loaded hydrogel exhibited minimal release at 18 °C, while showing rapid, sustained release at human body temperature (37 °C), indicating excellent storage stability and temperature responsiveness. The aim of this study was to investigate the influence of different concentrations of PVA and SA on the structural and functional properties of the hydrogel, aiming to provide a novel material support for the treatment of diabetic wounds.
Materials and methods
Materials
Polyvinyl alcohol (PVA-124, 99.0% alcoholysis, Mr ≈ 195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), sodium alginate (SA), and dimethyl sulphoxide (DMSO, 99%) were purchased from Aladdin (Shanghai, China). Phosphate buffered saline (PBS, pH = 7.4) was purchased from Solarbio (Beijing, China). Linagliptin (LIN, ≥99%) was purchased from Shanghai Sekerui Biotechnology Co., Ltd. Acidic electrolyzed oxidizing water (EOW) was purchased from Panike (Shenzhen, China) and used as received. All other chemicals were of analytical grade and used without additional purification.
000), sodium alginate (SA), and dimethyl sulphoxide (DMSO, 99%) were purchased from Aladdin (Shanghai, China). Phosphate buffered saline (PBS, pH = 7.4) was purchased from Solarbio (Beijing, China). Linagliptin (LIN, ≥99%) was purchased from Shanghai Sekerui Biotechnology Co., Ltd. Acidic electrolyzed oxidizing water (EOW) was purchased from Panike (Shenzhen, China) and used as received. All other chemicals were of analytical grade and used without additional purification.
Preparation of SA–PVA composite hydrogels
4 g of SA powder was added to 196 mL of deionized water and stirred until completely dissolved at room temperature to obtain a 2 wt% SA solution. PVA-124 powder was dissolved in deionized water at 90 °C to prepare PVA solutions of different mass concentrations (10, 12.5, 15, 17.5, and 20 wt%). Different concentrations of PVA solution (20 g) were mixed with 2 wt% SA solution (20 g) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio by vortex mixing. After degassing, sticky SA–PVA mixtures with different PVA concentrations were obtained, namely 83.3, 86.2, 88.2, 89.7, and 91.9 wt%. Finally, the mixtures were poured into molds and subjected to three cycles of freezing and thawing (i.e., frozen at −20 °C for 8 h and thawed at 25 °C for 4 h) to form mechanically stable SA–PVA.
1 mass ratio by vortex mixing. After degassing, sticky SA–PVA mixtures with different PVA concentrations were obtained, namely 83.3, 86.2, 88.2, 89.7, and 91.9 wt%. Finally, the mixtures were poured into molds and subjected to three cycles of freezing and thawing (i.e., frozen at −20 °C for 8 h and thawed at 25 °C for 4 h) to form mechanically stable SA–PVA.
Preparation of the SA–PVA@LIN system
The LIN was dissolved in dimethyl sulfoxide (DMSO) (10 mg mL−1) to prepare a storage solution. The SA–PVA composite hydrogel solution was prepared as described above, and LIN was then added to the solution at concentrations of 50, 100, 200 and 400 µg mL−1. After thorough mixing, the SA–PVA@GLIN was obtained through three freeze–thaw cycles. In addition, the SA–PVA@SLIN hydrogel system was developed by equilibrating PVA–SA hydrogels in a LIN solution. The drug loading hydrogel obtained through the soaking method was referred to as SA–PVA@SLIN. It should be noted that during the initial stage of mechanical property research, since the mechanism of drug delivery was not being investigated, we uniformly prepared the hydrogel dressings using the immersion method.Characterization
Water content
The initial weight of SA–PVA composite hydrogels with different solid contents was calculated, and then they were dried in a vacuum freeze dryer for 24 h before weighing again. Three measurements for each sample were carried out and the average result was taken. The water content (Wc) of the samples was calculated using formula (S1).Swelling test
The swelling behavior was evaluated by immersing the hydrogel in excess PBS solution (pH = 2.8, 6.2, 7.0, 8.2, 10.8). The freeze-dried hydrogels were soaked in a large volume of PBS solution, and swollen samples were taken out at fixed time intervals until swelling equilibrium was reached. Each sample was tested three times, and the average value was taken. The swelling ratio (ESR) of the hydrogel was calculated using formula (S2).Mechanical test
To address the propensity of hydrogels to dehydrate and subsequently undergo structural collapse and alterations in mechanical properties, an underwater tensile testing apparatus (ZQ-990L with 5 N load cell, Zhiqu Precision Instrument) was utilized to evaluate the mechanical properties of dumbbell-shaped SA–PVA specimens. The width of all dumbbell-shaped hydrogels was 2 mm, the thickness was 2.5 mm, and the gauge length was 20 mm. The stretching rate was set to be 100 mm min−1, and the samples were stretched until fracture to obtain the stress–strain curves. Each sample was tested 10 times, and the average value was taken. The Young's modulus was calculated from the slope of the curve in the strain range of 5–15%. The toughness was calculated from the area under the stress–strain curve. The elongation at break was determined from the strain value. Additionally, a total of 1000 stretching cycles were performed on the 90.9 wt% SA–PVA (SA–PVA90.9) in the 0–100% strain range.Drug release
Gel method: SA–PVA@GLIN with different solid contents and drug concentrations was fixed between two mesh disks of an intelligent dissolution tester (ZRS-8GD, Tianda Tianfa). Then, 500 mL of dissolution medium (PBS, pH = 7.4) was added, and the temperature was maintained at 18 ± 0.5 °C, 25 ± 0.5 °C and 37 ± 0.5 °C with a constant rotation speed of 100 rpm. Samples were taken at fixed intervals (0.5, 1, 2, 3, 6, 12, 24, 36, 48, 60 h), and 5 mL of dissolution solution was collected from each dissolution cup, to which an equal volume of PBS solution is added. The absorbance of all samples was then measured at 293 nm. The drug release at each time point was calculated based on the standard curve, and the drug release curve was plotted. The drug release efficiency (DRE) was calculated from the concentration of the drug in SA–PVA@LIN and the amount of drug release by formula (S3).Soaking method: SA–PVA with different solid contents of PVA (83.3, 86.2, 88.2, 89.7, 90.9 wt%) was soaked in 20 mL of a standard LIN solution (50, 100, 200 and 400 µg mL−1) for 24 h to verify the drug loading efficiency of the hydrogel. SA–PVA@SLIN was suspended in PBS at 37 °C for 24 h to release LIN. The releasing solution was diluted to 20 mL with deionized water, and the absorbance of the sample was measured at 293 nm. Based on the absorbance–concentration calibration curve, the absorbance was converted into concentration, and the drug loading efficiency (DLE) was calculated using formula (S4).
Statistical analyses
Origin 2024 software was used for statistical analysis, and the results were expressed as mean ± standard deviation (SD), and the results of significant differences were as follows: P-values were calculated using Student's t-test; *P < 0.05, **P < 0.01, ***P < 0.001.Results and discussion
Design and fabrication of SA–PVA
A physically crosslinked network is constructed via a freeze–thaw cycle method, as illustrated in Fig. 1. Between SA and PVA, a loose hydrogel structure is formed through hydrogen bonding. When this hydrogel is frozen, the water within it will form ice crystals, which promotes the aggregation of SA and PVA chains and the formation of more hydrogen bonds to stabilize the gel system.52,53 Upon thawing, the melted ice crystals release water, leaving a stable three-dimensional framework maintained by hydrogen bonds and physical entanglements. With repeated freeze–thaw cycles, the PVA and SA chains further aggregate and re-arrange to form the gel system with a more stable structure. Finally, the resulting hydrogel, rich in hydroxyl groups, exhibits excellent water absorption and swelling properties.30,54Furthermore, the linear SA, rich in a large amount of carboxyl groups, forms hydrogen bonds with the hydroxyl groups in PVA, and simultaneously penetrates through the network voids of PVA to form a physical entanglement structure, thereby forming a semi-IPN structure.41,55,56 By adjusting the density of the crosslinking points and the number of hydrogen bonds in the semi-IPN, macroscopic control of the mechanical properties can be achieved.43,57–59 In addition, LIN is a water-soluble molecule, which can easily be encapsulated within the three-dimensional hydrogel system. LIN is continuously released from this three-dimensional network via diffusion. By providing an appropriate dose of linagliptin over time, it may directly or indirectly induce keratinocytes to undergo epithelial–mesenchymal transition, thereby promoting keratinocyte migration and improving diabetic wound healing.51,60
Characterization of SA–PVA
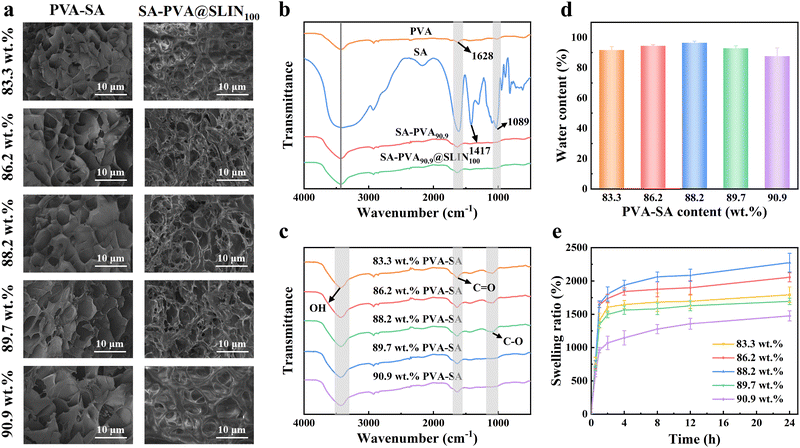
Fig. 2a shows the SEM images of the surfaces of the SA–PVA and SA–PVA@SLIN100 hydrogels with different PVA formulations. SA–PVA90.9 features a distinct porous structure with abundant continuous pores, forming a semi-interpenetrating polymer network (semi-IPN) architecture. In contrast, the SA–PVA90.9@SLIN100 drug-loaded hydrogels possess a highly porous three-dimensional network with more densely packed pores. Pore size and morphology vary with increasing PVA content, leading to a structural transition from a compact to a more porous configuration. This phenomenon can be attributed to the formation of the semi-IPN structure, which allows both components to retain their intrinsic chemical structures. Specifically, SA is not chemically crosslinked but physically entangled within the PVA network – SA chains diffuse into the PVA matrix as linear chains to form the semi-IPN structure.55 Furthermore, SA may interfere with the crosslinking sites between the PVA molecular chains, thereby restricting the effective physical crosslinking scope of PVA.61,62In Fig. 2b, all the characteristic peaks of PVA and SA are observed. For instance, the peak at 3417 cm−1 is attributed to the O–H stretching vibration of the hydroxyl group in PVA, the peak at 1628 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of SA, the peak at 1417 cm−1 is associated with the bending vibration of O–H, and the peak at 1087 cm−1 is attributed to the C–O stretching vibration. FT-IR analyses are performed on PVA, SA, SA–PVA90.9, and SA–PVA90.9@SLIN100 to investigate the interactions among the constituent components. The intensities of the O–H and C–O peaks primarily reflect the extent of hydrogen bonding; when PVA is incorporated into SA, no significant shift in peak positions is observed, suggesting the absence of hydrogen bond formation. However, the slight shift in the O–H peak from 3431 cm−1 to 3437 cm−1, along with the shift in the C–O peak from 1087 cm−1 to 1089 cm−1, indicates the formation of a synergistic hydrogen bonding system between SA and PVA. As the PVA content increases in the hydrogel, the intensities of the peaks at 1628 cm−1 and 1087 cm−1 decrease, which is attributed to the reduction in the number of free C
O stretching vibration of SA, the peak at 1417 cm−1 is associated with the bending vibration of O–H, and the peak at 1087 cm−1 is attributed to the C–O stretching vibration. FT-IR analyses are performed on PVA, SA, SA–PVA90.9, and SA–PVA90.9@SLIN100 to investigate the interactions among the constituent components. The intensities of the O–H and C–O peaks primarily reflect the extent of hydrogen bonding; when PVA is incorporated into SA, no significant shift in peak positions is observed, suggesting the absence of hydrogen bond formation. However, the slight shift in the O–H peak from 3431 cm−1 to 3437 cm−1, along with the shift in the C–O peak from 1087 cm−1 to 1089 cm−1, indicates the formation of a synergistic hydrogen bonding system between SA and PVA. As the PVA content increases in the hydrogel, the intensities of the peaks at 1628 cm−1 and 1087 cm−1 decrease, which is attributed to the reduction in the number of free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (Fig. 2c).
O groups (Fig. 2c).
Water content and swelling ratio of SA–PVA
Regardless of the PVA content, the water content of all SA–PVA hydrogels remains around 90% (Fig. 2d). This high water content is crucial for biomedical applications, particularly in wound care and tissue engineering, as it helps create a moist environment, promotes tissue healing, and aids in drug delivery.63–65 Additionally, the swelling rate of different types of SA–PVA composite hydrogels is demonstrated in Fig. 2e. During the initial swelling stage, the hydrogel's swelling rate increases rapidly as water molecules penetrate the network and interact with internal macromolecules. This is followed by a slower rise in swelling degree, which stabilizes by approximately 12 h. For SA–PVA with PVA contents of 83.3 wt%, 86.2 wt%, 88.2 wt%, 89.7 wt%, and 90.9 wt%, the maximum swelling degrees are approximately 1792%, 2055%, 2275%, 1728%, and 1476%, respectively, showcasing that the swelling rate first increases and then decreases upon increasing PVA content, which is mainly due to the hydroxyl groups in the PVA chains. The hydrogen bonds forming between hydroxyl groups and H2O endow the hydrogel with strong water-absorption capacity. However, higher PVA content reduces the hydrogel's inherent water content, limiting the enhancement of osmotic pressure. This, in turn, restricts the hydrogel's ability to absorb water molecules, leading to a subsequent decrease in swelling rate.66,67In addition, the swelling behavior of SA–PVA is also dictated by the ionization state of the alginate fraction. In neutral to weakly alkaline environments (pH = 7–8), carboxylate moieties (–COO−) along the alginate backbone undergo deprotonation, inducing robust electrostatic repulsion that drives polymer network expansion and yields maximal swelling capacity.68,69 Under acidic conditions (pH = 2.8–6), these moieties are protonated to form carboxyl groups (–COOH), abrogating electrostatic repulsion while facilitating intermolecular hydrogen bonding, thereby inducing polymer network shrinkage. In strongly alkaline solutions (pH = 9–10.8), the high ionic strength may screen electrostatic repulsions, and excess ions can cause an “anti-polyelectrolyte” effect, also resulting in reduced swelling (Fig. S1).
Mechanical properties
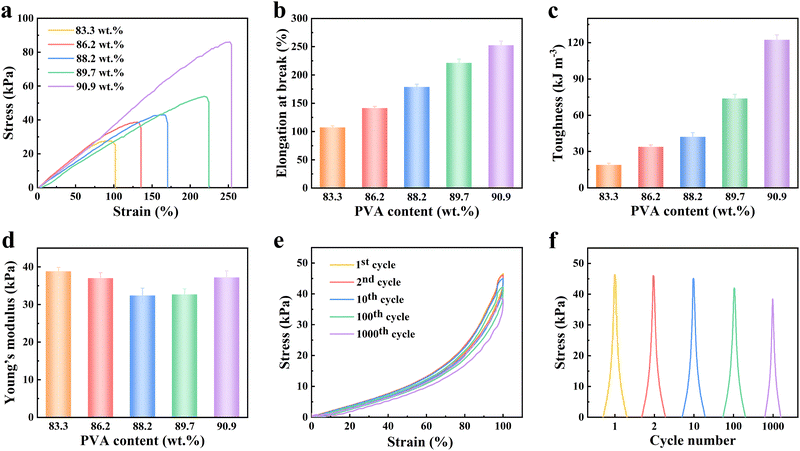
Hydrogel dressings for biomedical applications are required to possess excellent stretchability, flexibility, and structural stability. The designed hydrogels should facilitate drug release regulation, promote wound healing, enhance patient comfort, and be biocompatible with human skin. To evaluate the mechanical properties of SA–PVA, tensile and cyclic tensile tests were performed. Comparative analysis of SA–PVA with different component ratios reveals that as the PVA solid content increases from 83.3 wt% to 90.9 wt%, the maximum stress (27.6–86.1 kPa), elongation at break (102.0–253.9%), and toughness (18.9–122.3 kJ m−3) all show a continuous increasing trend (Fig. 3a–c). In contrast, the Young's modulus (32.4–38.8 kPa) first decreases and then increases with rising PVA content (Fig. 3d). Notably, this combination of superior tensile performance and tunable flexibility is highly favorable for maintaining conformability and compatibility between the hydrogel dressing and human skin (typical skin strain: 6–60%; ideal Young's modulus range for dressings: 5–2600 kPa). These mechanical characteristics thus establish a robust foundation for the biomedical application of the SA–PVA dressing (Fig. S1).70,71 This phenomenon may be attributed to the significant increase in the network density of PVA as the monomer content increases, thereby ensuring the stability of the physical network during stretching. Moreover, at a strain of 100%, continuous stress–strain cyclic stretching demonstrates that the 90.9 wt% SA–PVA has good mechanical stability (Fig. 3e), maintaining 83% stability in maximum stress (46.6–38.6 kPa) after 1000 cyclic stretches (Fig. 3f). Dynamic mechanical analysis (DMA) confirmed the viscoelastic properties of the hydrogel, demonstrating its solid-like behavior (G′ > G″) and mechanical stability (Fig. S2). In conclusion, SA–PVA hydrogels not only exhibit excellent mechanical properties in terms of stretchability in coordination with body movements, but also possess effective drug release capabilities, making them a promising solution with significant potential for enhancing the comfort and effectiveness of diabetic wound healing treatments.Drug release analysis
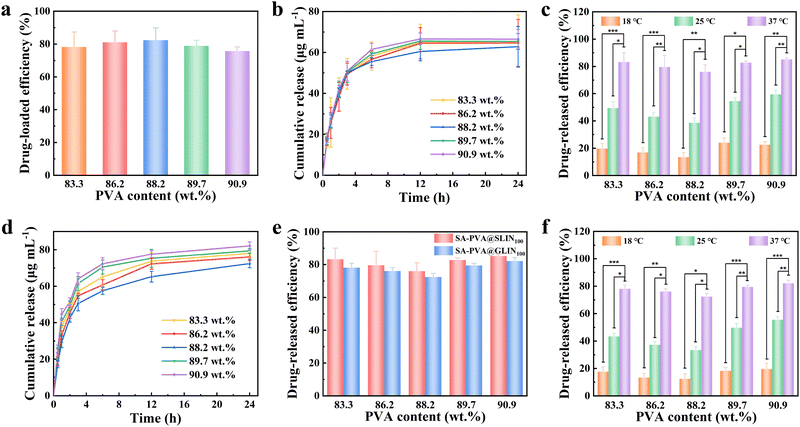
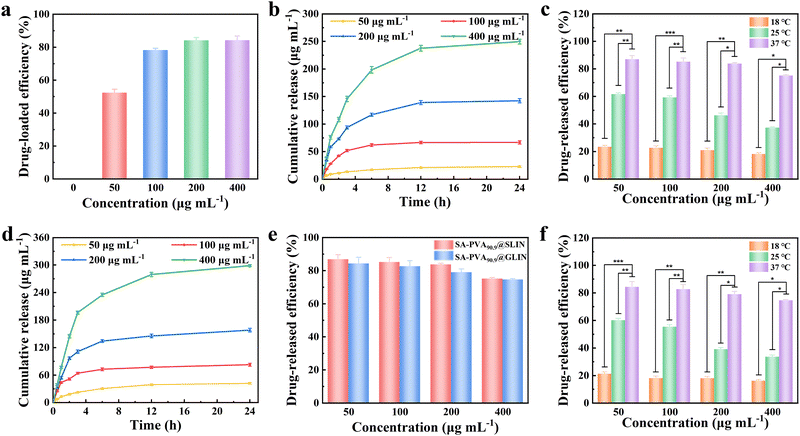
The concentration of LIN solution is plotted on the X-axis, and the UV absorption intensity is plotted on the Y-axis. The fitted absorbance–concentration standard curve is manifested in Fig. S4a, with the equation: y = 0.0094x + 0.021, R2 = 0.9993.PVA hydrogels exhibit a sparsely physically cross-linked network, enabling drugs to diffuse readily through the pores. Additionally, their strong hydrophilic nature induces significant network swelling upon water absorption, expanding the pore structure and further enhancing drug diffusion. Therefore, PVA-based hydrogels generally exhibit high drug release efficiency; however, since LIN is a non-hydrophilic drug, its release efficiency is relatively low (Table 1). The SA–PVA@GLIN and SA–PVA@SLIN systems are quantitatively assessed for drug release over time, along with the drug loading capacity of SA–PVA@SLIN. Under a drug concentration of 100 µg mL−1, as the solid content of PVA in the hydrogel increases, the drug loading efficiency initially increases and then decreases (78.31–83.34%) (Fig. 4a and Table S1). The trend is similar to the principle governing the swelling rate. However, the drug release performance first decreases and then increases with increasing PVA content, meaning that the drug release amount (63.32–82.13 µg mL−1) and drug release efficiency (72.46–85.17%) both exhibit a trend of first decreasing and then increasing (Fig. 4b–f and Tables S1 and S2). This may be because when PVA and SA form a semi-IPN structure, the increase in PVA leads to more crosslinking points in the network, which restricts the free movement of polymer chains, thus limiting the drug release to some extent.43,59,72 However, when the water content of the hydrogel is relatively low, it generates a stronger osmotic pressure, further promoting drug release, which results in an increased drug release amount as the water content decreases in SA–PVA with 89.7 wt% and 90.9 wt% PVA. By comparing the drug release efficiency of SA–PVA@GLIN100 and SA–PVA@SLIN100 at different PVA contents, it is found that SA–PVA@SLIN100 has a significantly higher release efficiency than SA–PVA@GLIN100 (Fig. 4e and Tables S1 and S2). LIN is a poorly water-soluble drug and tends to form a gel easily when mixed with the SA–PVA precursor solution. Therefore, SA–PVA@SLIN100 provides more flexibility in drug loading and release.
When the hydrogel solid content was fixed, the water content remained constant. As the drug concentration increases, both the drug loading efficiency (52.42–89.25%) and the drug release amount (22.91–298.65 µg mL−1) exhibited an increasing trend (Fig. 5a, b and d and Tables S3 and S4). A concentration of 400 µg mL−1 demonstrated the highest drug loading efficiency (Fig. 5a and Table S3), with rapid release of LIN in the first 12 hours, followed by a slower, sustained release phase that maintains a stable concentration within 24 hours (Fig. 5b and d and Table S4). The release mechanism was governed by concentration gradient-driven diffusion, enabling prolonged LIN release at the target site.73 Similarly, when comparing the drug release efficiency of SA–PVA90.9@SLIN and SA–PVA90.9@GLIN at different LIN concentrations, SA–PVA90.9@SLIN consistently showed significantly higher release efficiency than SA–PVA90.9@GLIN; however, both exhibited a decreasing trend with increasing concentration (Fig. 5e and Table S4). Generally, higher initial drug concentrations result in greater drug loading within the hydrogel. Consequently, higher drug concentrations might enhance the loading capacity, leading to improved early-stage release efficiency. However, at elevated concentrations, intermolecular interactions (e.g., hydrogen bonding, van der Waals forces) might intensify, and drug molecules might occupy hydrogel pores, reducing free volume and subsequently decreasing the diffusion rate of the drug. Collectively, these findings indicated that SA–PVA demonstrated excellent drug loading and release performance for LIN (Table 2). Ultimately, based on the optimization of the mechanical properties, drug loading and drug release capabilities, SA–PVA90.9@SLIN100 is deemed to be the most promising system, which not only has good skin compatibility but also demonstrates efficient drug delivery capabilities, and shows great potential for application in hydrogel wound healing.
It is particularly noteworthy that both SA–PVA90.9@SLIN100 and SA–PVA90.9@GLIN100 consistently exhibit strong thermosensitivity, that is the drug-release ability is controllable at different temperatures. When the temperature rises to 37 °C, LIN in PVA90.9@SLIN100 releases rapidly with a high efficiency of up to 85.17%. However, when the temperature drops to 18 °C, the network of the hydrogel successfully returned to the contracted and low-release state, with the drug release efficiency being less than 20% (Fig. 4c, f and 5c, f and Fig. S5–S8). This is because at lower temperatures, the SA–PVA90.9 network contracts, narrowing the diffusion channels for LIN molecules and physically entrapping the drug within the network. However, at elevated temperatures, phase separation occurs throughout the gel, generating larger micropores that offer lower diffusion resistance.72,74,75 Meanwhile, the cyclic heating–cooling experiments confirmed its robust reversibility without damaging the structure or function of the hydrogel (Fig. S4b and c).
Conclusions
A semi-IPN hydrogel composed of PVA and SA was successfully fabricated through freeze–thaw cycling. The optimized hydrogel of SA–PVA90.8 exhibited remarkable mechanical properties (tensile strength: 84.5 kPa, fracture strain: 283.9%, Young's modulus: 32.5 kPa) and high-water content (96.46%), fulfilling the requirements for flexible wound dressings. Efficient LIN loading (89.25%) and thermally triggered release (85.17% cumulative efficiency at 37 °C) demonstrated its capability for intelligent drug-delivery ability. This system overcomes the limitations of oral administration, enabling sustained local release at the wound site under normal human body temperature and allowing low-temperature preservation. The combination of mechanical robustness and stimuli-responsive functionality positions the PVA/SA hydrogel as a promising platform for diabetic wound therapy.Author contributions
Y. W.: writing – conceptualization, data curation, software, formal analysis, and original draft. G. L., L. P., Y. L., M. Y. and Q. W.: data curation and formal analysis. L. P. and H. G.: resources, conceptualization, supervision, data curation, writing – review and editing, and funding acquisition. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Data availability
All data generated or analyzed during this study are included in this published article.The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: Fig. S1. The swelling rate of hydrogels at different pH values. Fig. S2. Dynamic frequency-sweep measurements of SA–PVA. Fig. S3. Adhesion performance and compression performance of SA–PVA. (a) and (b) Photo showing adhesive effects of SA–PVA adhering to various substrates (heart, liver, lung, kidney, spleen, rubber, glass, PI, PET and Ti); (c) compression performance of SA–PVA; (d) applications of hydrogels in different skin regions. Fig. S4. Standard curve of LIN. Fig. S5. The cumulative LIN release of SA–PVA@GLIN100 at various temperatures: (a) 83.3 wt%, (b) 86.2 wt%, (c) 88.2 wt%, (d) 89.7 wt%, and (e) 90.9 wt%. Fig. S6. The cumulative LIN release of SA–PVA90.9@GLIN at various temperatures: (a) 50 µg mL−1, (b) 100 µg mL−1, (c) 200 µg mL−1, and (d) 400 µg mL−1. Fig. S7. The cumulative LIN release of SA–PVA@SLIN100 at various temperatures: (a) 83.3 wt%, (b) 86.2 wt%, (c) 88.2 wt%, (d) 89.7 wt%, and (e) 90.9 wt%. Fig. S8. The cumulative LIN release of SA–PVA90.9@SLIN at various temperatures: (a) 50 µg mL−1, (b) 100 µg mL−1, (c) 200 µg mL−1, and (d) 400 µg mL−1. Table S1. SA–PVA@SLIN100 at different PVA solid contents. Table S2. SA–PVA@GLIN100 at different PVA solid contents. Table S3. SA–PVA90.9@SLIN at different LIN concentrations. Table S4. SA–PVA90.9@SLIN at different LIN concentrations. See DOI: https://doi.org/10.1039/d5cp03379a.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22308049 and 82360763), the Jiangxi Provincial Key Laboratory of Flexible Electronics (20242BCC32010), the China Postdoctoral Science Foundation (2023M740486), Ningbo Natural Science Foundation (2024J096), and the Graduate School-level Innovation Fund of Jiangxi Science and Technology Normal University (YC2024-X55). The authors express their heartfelt gratitude to Prof. Baoyang Lu and Caicai Jiao (Jiangxi Science and Technology Normal University) for valuable discussions and extend their thanks to Manting Song, Yu Lin, Gen Li, Juan Teng, Changchen Gong and Ziyang Qiu for the invaluable assistance rendered throughout the course of this research.Notes and references
- J. Liu, S. Guo, S. Hong, J. Piao and M. Piao, Curr. Drug Delivery, 2024, 21, 1537–1547 CrossRef CAS PubMed.
- S. Hua, H. Ma, X. Li, H. Yang and A. Wang, Int. J. Biol. Macromol., 2010, 46, 517–523 CrossRef CAS PubMed.
- H. Bhardwaj, S. Khute, R. Sahu and R. K. Jangde, Curr. Drug Targets, 2023, 24, 1239–1259 CrossRef CAS PubMed.
- R. Lobmann, G. Schultz and H. Lehnert, Diabetes Care, 2005, 28, 337–442 CrossRef PubMed.
- V. Ramachandran, T. Mohanasundaram, D. Karunakaran, M. Gunasekaran and R. Tiwari, Curr. Diabetes Rev., 2023, 19, 127–139 Search PubMed.
- W. Zhao, L. Qiang, C. Zhang, S. Li, Y. Liu, C. Wang, X. Ma, J. Wang and Y. Bao, ACS Appl. Mater. Interfaces, 2024, 16, 50175–50187 CrossRef CAS PubMed.
- M. Figueroa-Pizano, I. Vélaz, F. Peñas, P. Zavala-Rivera, A. Rosas-Durazo, A. Maldonado-Arce and M. Martínez-Barbosa, Carbohydr. Polym., 2018, 195, 476–485 CrossRef CAS PubMed.
- Q. Wang, W. Zheng, J. Wang, C. Jiao, D. Gao, J. Liu and B. Lu, ACS Appl. Mater. Interfaces, 2025, 17, 30747–30758 CrossRef CAS PubMed.
- G. Xu, K. Liu, X. Chen, Y. Lin, C. Yu, X. Nie, W. He, N. Karin and Y. Luan, Nanoscale Horiz., 2024, 9, 295–304 RSC.
- S. Khan and N. Ranjha, Polym. Bull., 2014, 71, 2133–2158 CrossRef CAS.
- J. Varshousaz and N. Koopaie, Iran. Polym. J., 2002, 11, 123–131 Search PubMed.
- Y. S. Zhang and A. Khademhosseini, Science, 2017, 356, eaaf3627, DOI:10.1126/science.aaf3627.
- P. Yang, X. Chen, Y. Qin, L. Yu, G. Ge, W. Yin, W. Zhang, W. Li, W. Li and W. Xia, Biomaterials, 2025, 320, 123273 CrossRef CAS PubMed.
- L. Liu, Q. Zhu, Q. Zhang, Z. Dong, C. Chen, G. Zhao and P. Ma, J. Ind. Text., 2024, 54, 15280837241293156, DOI:10.1177/15280837241293156.
- J. Yu, R. Wan, F. Tian, J. Cao, W. Wang, Q. Liu, H. Yang, J. Liu, X. Liu, T. Lin, J. Xu and B. Lu, Small, 2024, 20, 2308778 CrossRef CAS PubMed.
- Y. Gong, Z. Xu, J. Wu, J. Zhong and D. Ma, Appl. Surf. Sci., 2024, 657, 159790 CrossRef CAS.
- J. Qiu, X. Xu, Z. Li, Y. Hu, G. Liu, X. Lv, J. Xu and B. Lu, Nano Energy, 2024, 130, 110057 CrossRef CAS.
- Z. Li, Z. Qiu, J. Xu and B. Lu, Chem. Eng. J., 2025, 169656 CrossRef CAS.
- X. Xu, Q. Zhao, Q. Liu, J. Qiu, J. Li, W. Zheng, J. Cao, L. Wang, W. Wang, S. Yuan, A. Fu, H. Yang, C. Wang, J. Xu and B. Lu, Desalination, 2024, 577, 117400 CrossRef CAS.
- Q. Zhao, J. Liu, Z. Wu, X. Xu, H. Ma, J. Hou, Q. Xu, R. Yang, K. Zhang, M. Zhang, H. Yang, W. Peng, X. Liu, C. Zhang, J. Xu and B. Lu, Chem. Eng. J., 2023, 442, 136284 CrossRef.
- G. Li, C. Li, G. Li, D. Yu, Z. Song, H. Wang, X. Liu, H. Liu and W. Liu, Small, 2022, 18, 2101518 CrossRef CAS PubMed.
- Z. Zhang, G. Chen, Y. Xue, Q. Duan, X. Liang, T. Lin, Z. Wu, Y. Tan, Q. Zhao, W. Zheng, L. Wang, F. Wang, X. Luo, J. Xu, J. Liu and B. Lu, Adv. Funct. Mater., 2023, 33, 2305705 CrossRef CAS.
- L. Wang, W. Wang, R. Wan, M. Yao, W. Chen, L. Zhang, J. Xu, X. Liu and B. Lu, Soft Sci., 2025, 5, 2 CAS.
- X. Luo, R. Wan, Z. Zhang, M. Song, L. Yan, J. Xu, H. Yang and B. Lu, Adv. Sci., 2024, 11, 2404679 CrossRef CAS PubMed.
- H. Li, J. Cao, R. Wan, V. R. Feig, C. M. Tringides, J. Xu, H. Yuk and B. Lu, Adv. Mater., 2025, 37, 2415151 CrossRef CAS PubMed.
- M. Dattilo, F. Patitucci, S. Prete, O. I. Parisi and F. Puoci, J. Funct. Biomater., 2023, 14, 55 CrossRef CAS PubMed.
- S. Jacob, A. B. Nair, J. Shah, N. Sreeharsha, S. Gupta and P. Shinu, Pharmaceutics, 2021, 13, 357 CrossRef CAS PubMed.
- Z. Sun, C. Song, C. Wang, Y. Hu and J. Wu, Mol. Pharmaceutics, 2019, 17, 373–391 Search PubMed.
- F. Huang, X. Lu, Y. Yang, Y. Yang, Y. Li, L. Kuai, B. Li, H. Dong and J. Shi, Adv. Sci., 2023, 10, 2203308 CrossRef CAS PubMed.
- H. Liu, C. Wang, C. Li, Y. Qin, Z. Wang, F. Yang, Z. Li and J. Wang, RSC Adv., 2018, 8, 7533–7549 RSC.
- P. Gupta, K. Vermani and S. Garg, Drug Discovery Today, 2002, 7, 569–579 CrossRef CAS PubMed.
- J. Liu, B. Jia, Z. Li and W. Li, Front. Bioeng. Biotechnol., 2023, 11, 1115603 CrossRef PubMed.
- M. Sobczak, Int. J. Mol. Sci., 2022, 23, 4421 CrossRef CAS PubMed.
- S. Zhang, G. Ge, Y. Qin, W. Li, J. Dong, J. Mei, R. Ma, X. Zhang, J. Bai and C. Zhu, Mater. Today Bio, 2023, 18, 100508 CrossRef CAS PubMed.
- S. Fuchs, A. U. Ernst, L.-H. Wang, K. Shariati, X. Wang, Q. Liu and M. Ma, Chem. Rev., 2020, 121, 11458–11526 CrossRef PubMed.
- Z. Hao, X. Li, R. Zhang and L. Zhang, Adv. Healthcare Mater., 2024, 13, 2400513 CrossRef CAS PubMed.
- Q. Sun, Z. Wang, B. Liu, F. He, S. Gai, P. Yang, D. Yang, C. Li and J. Lin, Coord. Chem. Rev., 2022, 451, 214267 CrossRef CAS.
- R. Murmu, D. Roy, H. Sutar, P. Senapati and S. Mohapatra, J. Polym. Mater., 2022, 39, 89–109 CrossRef CAS.
- X. Xu, Q. Sun, A. Xu and X. Guo, J. Polym. Mater., 2022, 39, 167–181 CrossRef CAS.
- W. Zheng, L. Wang, H. Jiao, Z. Wu, Q. Zhao, T. Lin, H. Ma, Z. Zhang, X. Xu, J. Cao, J. Zhong, J. Xu and B. Lu, Chem. Eng. J., 2023, 455, 140553 CrossRef CAS.
- I. Sterin, A. Tverdokhlebova, E. Katz and O. Smutok, ACS Appl. Mater. Interfaces, 2024, 16, 28222–28229 CrossRef CAS PubMed.
- T. Wang, W. Yi, Y. Zhang, H. Wu, H. Fan, J. Zhao and S. Wang, Colloids Surf., B, 2023, 222, 113096 CrossRef CAS PubMed.
- X. Kuang, M. O. Arıcan, T. Zhou, X. Zhao and Y. S. Zhang, Acc. Mater. Res., 2022, 4, 101–114 CrossRef.
- J. Doupis, Drug Des., Dev. Ther., 2014, 431–446, DOI:10.2147/dddt.s59523.
- M. Long, L. Cai, W. Li, L. Zhang, S. Guo, R. Zhang, Y. Zheng, X. Liu, M. Wang and X. Zhou, Diabetes, 2016, 65, 780–793 CrossRef CAS PubMed.
- M. Long, L. Cai, W. Li, L. Zhang, S. Guo, R. Zhang, Y. Zheng, X. Liu, M. Wang and X. Zhou, Diabetes, 2018, 67, 518–531 CrossRef CAS PubMed.
- L. B. Pucar, E. P. Pugel, D. Detel and J. Varljen, Wound Repair Regen., 2017, 25, 25–40 CrossRef PubMed.
- P. Yang, X. Chen, Y. Qin, L. Yu, G. R. Ge, W. L. Yin, W. Zhang, W. M. Li, W. H. Li, W. Y. Xia, Z. B. Wu, F. Ding, J. X. Bai, F. W. Meng and D. C. Geng, Biomaterials, 2025, 320, 123273 CrossRef CAS PubMed.
- M. Cao and Z. Bloomgarden, J. Diabetes, 2020, 12, 844–847 CrossRef PubMed.
- T. Karve and A. Banga, Int. J. Pharm., 2024, 654, 123992 CrossRef CAS PubMed.
- R. Marfella, F. Sasso, M. Rizzo, P. Paolisso, M. Barbieri, V. Padovano, O. Carbonara, P. Gualdiero, P. Petronella and F. Ferraraccio, J. Diabetes Res., 2012, 2012, 892706 Search PubMed.
- W. Yu, J. Chen, Q. Gao, Y. Guo, S. Zhang, Y. Pan, B. Nie, X. Zhang, L. Jiang and J. Qiu, Chem. Eng. J., 2025, 519, 164695 CrossRef CAS.
- Y. Zheng, H. Huang, J. Yu, Z. Hu and Y. Wang, Chem. Eng. J., 2023, 454, 140054 CrossRef CAS.
- P. Kaur, V. Gondil and S. Chhibber, Int. J. Pharm., 2019, 572, 118779 CrossRef CAS PubMed.
- Y. Jiang, Y. Hou, J. Fang, W. Liu, Y. Zhao, T. Huang, J. Cui, Y. Yang and Z. Zhou, Int. J. Polym. Anal. Charact., 2019, 24, 132–141 CrossRef CAS.
- M. Saraiva, M. d S. Campelo, J. Neto, M. d C. Gonzaga, M. Bastos, S. d A. Soares, N. Ricardo, G. Cerqueira, R. d C. Leitão and M. Ribeiro, Int. J. Biol. Macromol., 2023, 244, 125278 CrossRef CAS PubMed.
- M. Afshar, G. Dini, S. Vaezifar, M. Mehdikhani and B. Movahedi, J. Drug Delivery Sci. Technol., 2020, 56, 101530 CrossRef CAS.
- K. Zhang, Q. Feng, Z. Fang, L. Gu and L. Bian, Chem. Rev., 2021, 121, 11149–11193 CrossRef CAS PubMed.
- X. Zhao, X. Chen, H. Yuk, S. Lin, X. Liu and G. Parada, Chem. Rev., 2021, 121, 4309–4372 CrossRef CAS PubMed.
- N. Ta, Y. Li, C. Schuyler, M. Virella and Y. Huang, Atherosclerosis, 2010, 213, 429–435 CrossRef CAS PubMed.
- P. Candry, B. Godfrey, Z. Wang and F. Sabba, Sci. Rep., 2022, 12, 20822 CrossRef CAS PubMed.
- Z. Lin, Y. Gao, L. Chen, W. Wang and T. Chen, J. Environ. Chem. Eng., 2025, 117584, DOI:10.1016/j.jece.2025.117584.
- Y. Huang, T. Dong, X. Zhang, S. Xu, X. Song, Z. Wang, M. Qin, L. Deng and Y. Li, J. Polym. Mater., 2025, 42 DOI:10.32604/jpm.2025.060699.
- M. Liao, Y. Zhao, Y. Pan, J. Pan, Q. Yao, S. Zhang, H. Zhao, Y. Hu, W. Zheng and W. Zhou, J. Mater. Sci., 2023, 58, 5756–5772 CrossRef CAS.
- W. Zhang, Y. Wu, H. Chen, Y. Gao, L. Zhou, J. Wan, Y. Li, M. Tang, Y. Peng and B. Wang, Sep. Purif. Technol., 2024, 336, 126261 CrossRef CAS.
- H. Liu, J. Gu, Y. Liu, L. Yang, A. Li, J. Yu, L. Wang and X. Qin, Langmuir, 2023, 39, 13641–13648 CrossRef CAS PubMed.
- J. Ren, J. Li, Z. Xu, Z. Du and F. Cheng, J. Environ. Chem. Eng., 2021, 9, 105370 CrossRef CAS.
- L. Gu, X. Sun, J. Pan, D. Liu, L. Huang, Y. Yu, B. Yu and H. Cong, Biomaterials, 2025, 123432 CrossRef CAS PubMed.
- J. Xu, W. Song, L. Ren, N. Wu, R. Zeng, S. Wang, Z. Wang and Q. Zhang, Int. J. Biol. Macromol., 2024, 263, 130282 CrossRef CAS PubMed.
- H. Joodaki and M. Panzer, Proc. Inst. Mech. Eng., Part H, 2018, 232, 323–343 CrossRef PubMed.
- S. Liu, Y. Rao, H. Jang, P. Tan and N. Lu, Matter, 2022, 5, 1104–1136 CrossRef CAS.
- J. Li and D. Mooney, Nat. Rev. Mater., 2016, 1, 1–17 CrossRef PubMed.
- A. Dravid, B. Raos, Z. Aqrawe, S. Parittotokkaporn, S. O’Carroll and D. Svirskis, Front. Chem., 2019, 7, 638 CrossRef CAS PubMed.
- A. C. Marques and M. Vale, Materials, 2021, 14, 4247 CrossRef CAS PubMed.
- K. Nakanishi and N. Tanaka, Acc. Chem. Res., 2007, 40, 863–873 CrossRef CAS PubMed.
- G. Cheng, S. Wang, W. Li, X. Liu and Q. Huang, Ind. Crops Prod., 2024, 220, 119234 CrossRef CAS.
| This journal is © the Owner Societies 2026 |