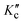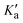Open Access Article
Open Access ArticleIsotopic chirality and high-resolution gigahertz and terahertz spectroscopy of trans-2,3-dideutero-oxirane (tc-CHDCHDO)
Ziqiu
Chen
 *ab,
Sieghard
Albert
*ab,
Sieghard
Albert
 b,
Karen
Keppler
b,
Karen
Keppler
 b,
Gunther
Wichmann
b,
Gunther
Wichmann
 b,
Martin
Quack
b,
Martin
Quack
 *b,
Volker
Schurig
c and
Oliver
Trapp
*b,
Volker
Schurig
c and
Oliver
Trapp
 d
d
aCollege of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China. E-mail: chenziqiu@lzu.edu.cn
bDepartment of Chemistry and Applied Biosciences, ETH Zurich, CH-8093 Zurich, Switzerland. E-mail: martin@quack.ch
cInstitute of Organic Chemistry, University of Tübingen, Tübingen, Germany
dDepartment of Chemistry, Ludwig-Maximilians-University, Munich, Germany
First published on 9th December 2025
Abstract
We report the observation and assignment of the rotational spectra of the isotopically chiral molecule, trans-2,3-dideutero-oxirane (tc-CHDCHDO) measured with the Zurich gigahertz (GHz) spectrometer (64 to 500 GHz and Δν/ν = 10−11) and with our highest resolution Fourier transform infrared spectrometer at the Swiss synchrotron light source (SLS) (best instrumental resolution Δṽ = 0.00053 cm−1) in the terahertz range (far infrared from 25 to 80 cm−1). The combined GHz and THz spectra were analysed using an accurate effective Hamiltonian providing newly determined rotational parameters, which are crucial for the prediction of trans-2,3-dideutero-oxirane transitions to be observed by astrophysical spectroscopy. The recent detection of singly deuterated oxirane in the protostar IRAS 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293–2422 B based on our previous predictions highlights the potential for the detection of doubly deuterated oxiranes including trans-2,3-dideutero-oxirane. These offer insights into deuterium fractionation in space and the formation mechanisms of complex and in particular chiral molecules. This study is also related to the new isotope effect in isotopically chiral molecules characterised by the parity violation energy differences of the ground states of the enantiomers and to biomolecular homochirality. In addition, we report the analysis of the pure rotational spectra of cis-2,3-dideutero-oxirane also present in our experimental spectra in the GHz range.
293–2422 B based on our previous predictions highlights the potential for the detection of doubly deuterated oxiranes including trans-2,3-dideutero-oxirane. These offer insights into deuterium fractionation in space and the formation mechanisms of complex and in particular chiral molecules. This study is also related to the new isotope effect in isotopically chiral molecules characterised by the parity violation energy differences of the ground states of the enantiomers and to biomolecular homochirality. In addition, we report the analysis of the pure rotational spectra of cis-2,3-dideutero-oxirane also present in our experimental spectra in the GHz range.
1. Introduction
Chiral symmetry breaking1 in molecules that are chiral only by isotopic substitution results in several important effects ranging from quantum structure to anharmonic vibrational quantum dynamics and finally parity violation, which introduces a fundamentally new isotope effect.2–10 Indeed, in such “isotopically chiral molecules”, a small parity violating energy difference ΔpvE between the ground states of the enantiomers is predicted, which arises through the weak nuclear interaction from the different weak nuclear charge of the isotopes with different numbers of neutrons.2–7 While calculated to be very small, on the order of feV to aeV depending on the molecule, this could, in principle, be measured in special experiments,2,11,12 although until now no successful experiment has been reported (see the reviews in ref. 9 and 10). On the other hand, when neglecting the parity violating weak nuclear force, which is the common assumption in current quantum chemical theory, ΔpvE would be exactly zero by symmetry.A further interesting aspect of isotopically chiral molecules is related to astrophysical spectroscopy: the detection of an achiral “parent molecule” can lead to the detection of a corresponding isotopically chiral molecule. In this context we have initiated some time ago a spectroscopic project on isotopically chiral oxirane derivatives, given the well-established astrophysical detection of the achiral oxirane molecule (ethylene epoxide, cyclo-C2H4O).13–18 Starting with high resolution GHz and THz (FTIR) spectroscopy of monodeutero-oxirane,19 our prediction of some relevant spectral lines in the range 300 to 400 GHz led, indeed, to the astrophysical detection of this molecule in IRAS 16293-2422B using ALMA (the Atacama Large Millimetre/submillimetre Array),20 see also ref. 21 for further spectroscopic results.
Chiral molecules are of particular astrophysical interest, because the detection of some systematic extraterrestrial homochirality would be a very strong indication for extraterrestrial life, as there is no known chemical mechanism except the biochemistry of life which would generate a consistent global homochirality.22,23 While various techniques for analysis are possible by sending missions within the solar system,24 only a spectroscopic approach (using circular dichroism or high resolution vibrational circular dichroism, for instance22,23) would be able to go beyond the solar system, say, by the spectroscopy of exoplanetary atmospheres.25–27 While chirally sensitive astrophysical spectroscopy in this context is not to be expected in the nearest future, the spectroscopic detection of chiral molecules beyond the solar system prepares a path for such future studies. So far, only two chiral molecules have been detected by astrophysical spectroscopy in the interstellar medium (ISM), monodeutero-oxirane as already mentioned19–21 and methyloxirane.28 Clearly more effort is needed towards identifying further chiral molecules for such observations.
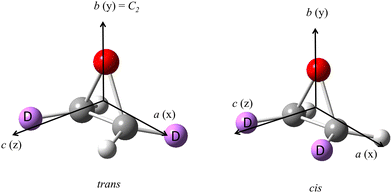
In the present publication we report our results on the isotopically chiral trans-2,3-dideutero-oxirane as a further chiral oxirane isotopomer likely to be observed in a way analogous to monodeutero-oxirane. Trans-2,3-dideutero-oxirane (or trans-[2,3-2H2] oxirane, trans-cyclo-CHDCHDO, short-hand used here as tc-CHDCHDO, see Fig. 1) has been a prototypical chiral compound for experimental and theoretical studies of vibrational circular dichroism for some time,29–38 and it has also been used more recently as an example for the direct determination of absolute configuration by Coulomb explosion imaging (CEI)39–42 (see also ref. 43 for an alternative technique used for the chiral prototype molecule CHFClBr43 as compared to the “classic” approach using X-ray crystallography44,45). However, so far only very little high resolution spectroscopic work exists for tc-CHDCHDO. Indeed, there seems to be only the singular early microwave spectroscopy of the various oxirane isotopomers by Hirose,46,47 which reported 21 rotational transitions for tc-CHDCHDO with the overall aim of a structure determination from the rotational constants of several isotopomers. Here, we shall report the first extensive study of rotational transitions in the GHz range (64 to 500 GHz) and the THz range by synchrotron-based Fourier transform infrared (FTIR) spectroscopy (0.75 to 2.4 THz), thus covering most of the rotational spectrum with a remaining gap only from 500 to 750 GHz. The analyses of more than 4000 assigned line positions provide accurate rotational parameters for the vibrational ground state and a very accurate basis for the prediction of lines for the astrophysical detection of tc-CHDCHDO among other possible applications.
In a broader context, our spectroscopic results on monodeutero- and dideutero-oxirane should also be seen in relation to deuterium fractionation in astrochemistry. By these astrochemical processes deuterium is preferentially incorporated into a variety of molecules, the detection of which can then offer insight into these processes, which occur in particular in the cold and dense regions of interstellar space.48–54 Despite the low cosmic abundance of deuterium with a ratio estimated to be about [D]/[H] ≈ 10−5, deuterium enriched molecules have been detected in various astronomical environments51,52 with [D]/[H] ratios as high as 50% in low mass prestellar cores53 and about 15% in protostars.54 Such observations are crucial for refining models for chemical evolution in space, and in this context studies of deuterium fractionation in isotopically chiral molecules containing deuterium are also of greatest interest.
The undeuterated parent species oxirane (c-C2H4O) is an isomer of acetaldehyde (CH3CHO) which is more stable by about 1 eV or 100 kJ mol−1. The high-resolution spectroscopy of acetaldehyde55 enabled its detection in the interstellar medium in the past.56 The monodeutero isotopomer CH3CDO has also been detected in the ISM,57 and interestingly dideuteroacetaldehyde CHD2CHO has recently been detected as well,58 and tentatively also CD2CH2O59 as isomers of tc-CHDCHDO. Clearly, the isotopomer CD3CHO, which we studied some time ago in a different context,60–62 would also be a candidate for future astrophysical observations. We might mention here also the isomers vinyl alcohol (CH2CHOH) and methylhydroxycarbene (CH3COH) and their isotopomers as further interesting candidates for a detection by astrophysical spectroscopy.
To complete this introduction, we should refer to the very extensive spectroscopic work which exists on normal achiral oxirane, quite in contrast to its isotopically chiral isotopomers. There are studies by microwave spectroscopy,46,47,63,64 submillimetre wave spectroscopy,65,66 and synchrotron-based FTIR spectroscopy.67,68 As a result, the rotational parameters of the vibrational ground state of C2H4O are extremely well established. The rovibrationally resolved infrared spectra of oxirane were also analysed in the range from 600 to 3500 cm−1.68–73 For comparison, extensive ab initio results are available as well, which include also results on deutero isotopomers.21,38,74
The present paper is organised as follows. In Section 2, we provide an overview over the experiments (synthesis, Section 2.1, GHz experiments Section 2.2, THz (FTIR) experiments Section 2.3). In Section 3, we briefly provide the theoretical background for the analysis (effective Hamiltonian Section 3.1, symmetry considerations for the analysis, Section 3.2). Section 4 discusses the assignment procedures and Section 5 provides results and discussion with the concluding Section 6 providing a summary of the main results and conclusions including an outlook. Some preliminary results of our work were presented in ref. 75 and 76. Because our sample of tc-CHDCHDO contained the achiral cis-CHDCHDO as an impurity, we were able to assign more than 1000 lines in the spectrum to this species, and we report also the rotational parameters resulting from this analysis.
2. Experimental
2.1 Synthesis of di-deuterated oxirane
In brief, the synthesis of trans-c-C2H2D2O is described by the scheme below. The procedures can be presented as follows (Scheme 1):GC-MS (EI): m/z = 28.04 [M]+, 26.06 [M–D]+
GC-MS (EI): m/z = 30.15 [M]+, 29.10 [M–H]+, 28.12 [M–D]+, 27.12 [M–H–D]+
1H-NMR (500.13 MHz, CDCl3): δ = 3.88–3.92 (m, 1H, 1-H), 3.50–3.54 (m, 1H, 2-H), 2.69 (bs, 1H, OH).
13C-NMR (125.77 MHz, CDCl3): δ = 62.29 (t, J = 22.6 Hz), δ = 35.28 (t, J = 22.6 Hz).
1H-NMR (500.13 MHz, CDCl3, −10 °C): δ = 2.70–2.71 (m, 2H, 1-H/2-H).
13C-NMR (125.77 MHz, CDCl3, −10 °C): δ = 40.7 (t, J = 23.9 Hz).
GC-MS (EI): m/z = 46.13 [M]+, 44.08 [M–D]+, 30.10 [M–O]+, 29.09 [M–O–H]+.
The reaction product contained mainly tc-CHDCHDO as racemate, with cis-CHDCHDO as an impurity. The identities of the substances were obvious from the high resolution spectra (see also ref. 19 and 21 for further aspects of the synthesis).
2.2 GHz measurements
The rotational spectra of trans-2,3-dideutero-oxirane in the GHz region were recorded using our Zurich GHz spectrometer,80 with some modifications which will be described elsewhere in more detail.94 In this setup, the GHz radiation is phase modulated and sent through a 2.5 m long gas cell. Subsequently, the second harmonic is detected by a RF lock-in amplifier resulting in a derivative line shape from the absorption and dispersion of the gas sample. The high sensitivity of this setup has been demonstrated in our high-resolution spectroscopic studies of dithiine,81 deuterated phenols82 and monodeutero-oxirane.19,21Trans-2,3-dideutero-oxirane was allowed to flow from the sample holder to the 2.5 m glass cell at optimised pressures between 9 and 30 µbar and room temperature. The survey spectra were initially recorded in the 95–105 GHz range in 500 MHz segments with a typical effective linewidth (FWHM, full-width at half-maximum) of ∼200 kHz observed at 100 GHz. Based on the rotational constants reported by Hirose,47 transitions with decent intensities were assigned readily, leading to more accurate predicted line positions. Subsequently, longer integration times were applied to individual weaker transitions by selecting a narrow bandwidth (∼5 MHz) for the best signal-to-noise (S/N) ratio. Finally, the measurement of individual lines was extended to 64 GHz (lower limit) and 500 GHz (upper limit) to obtain better predictions through an improved effective Hamiltonian with additional transitions.
The relative frequency uncertainty of the GHz spectrometer is only Δν/ν = 10−11 and therefore the effective resolution and frequency accuracy is determined by Doppler and possible collisional effects as well as by the procedures used to extract peak positions of the lines (here also with the aid of PGOPHER83,84). The accuracy of an isolated line position has been tested with carbonylsulfide (OCS) and reaches at least 30 kHz or better. Through the derivative detection method, however, the maxima of two lines separated by more than one full-width at half-maximum (FWHM) can still be shifted in the same order as the FWHM. Also, lines with stronger absorbance can have a deviation between line shape maximum and line position by more than 30 kHz, which is not always taken into account by the parameter extraction. Therefore, the frequency uncertainty for some lines might be as large as 200 kHz even though the root mean square deviation dRMS of the fit indicates an average precision of about 7 kHz. Therefore, these exceptional uncertainties have no significant effect on the analysis with the effective Hamiltonian. To minimise uncertainties, transitions in Table S3 have been remeasured separately at low pressures <2 µbar.
2.3 THz measurements
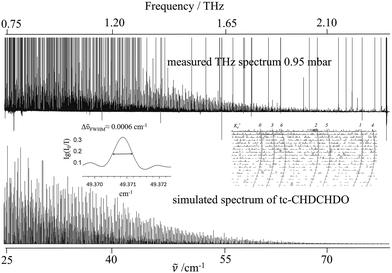
The far infrared beamline at the Swiss light source (SLS) was equipped with the ETH-SLS Bruker IFS 125 HR (extended) spectrometer, which was built as a prototype for our group in 2009 and is capable of measuring rotationally-resolved vibrational spectra in the THz range with an unapodized instrumental resolution of 0.00053 cm−1 (16 MHz), the highest for an FTIR spectrometer of this type worldwide.9,85–87 The combination of the high resolution of this prototype spectrometer with the high sensitivity enabled by the bright synchrotron radiation provides a powerful tool to measure and analyse the pure rotational transitions in the traditionally difficult THz region for FTIR spectroscopy. This capability was recently demonstrated by our successful study of the THz spectrum of monodeutero-oxirane.19The pure rotational spectra in the range from 25 to 80 cm−1 (0.75–2.40 THz) were measured with a maximum optical path difference dMOPD of 11.7 m. A 6 µm Mylar beamsplitter, an aperture of 3.15 mm and a helium-cooled Si bolometer were used for the measurements. The scanner velocity was 30 kHz. The measurements of the spectra started with a sample with a vapour pressure of 0.95 mbar with 140 interferograms collected. Further measurements were then conducted at two reduced pressures (166 and 140 scans, respectively), so that the line positions of strong but saturated transitions at the starting pressure were also accurately recorded. Fig. 2 shows an overview of the measured THz spectra of trans-2,3-dideutero-oxirane.
All measured line positions were calibrated using residual H2O vapour lines between 36 and 100 cm−1 as compared to wavenumbers reported in the HITRAN database.88 Based on a simple linear fit, the correction for the calibrated wavenumber ṽcalibrated = 0.999999 × ṽobserved + 0.000148 cm−1 is small enough to be taken as constant in this range. In general, the uncertainties of the recorded line positions can be estimated to be less than 0.0001 cm−1. The wavenumber tables in the supplementary information (SI) give the calibrated values. The typical line widths ΔṽFWHM (full width at half-maximum, FWHM) were observed to be less than ΔṽFWHM = 0.0006 cm−1, indicating that the resolution is instrument limited given the Doppler width of about 0.00009 cm−1 at 50 cm−1 and small pressure effects only. An example of an isolated line is shown in Fig. 2 as an insert (middle left).
3. Theoretical background for the analysis
3.1 Effective Hamiltonian
The analyses of the pure rotational spectra in the GHz and THz regions were carried out using Watson's A-reduced effective Hamiltonian89–93 in the Ir representation, which is used here including up to octic centrifugal distortion constants: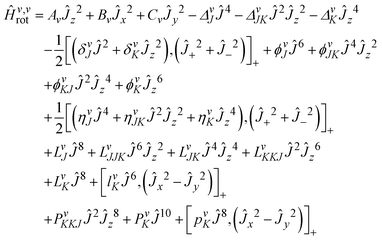 | (1) |
 we have the angular momentum operators:
we have the angular momentum operators:| Ĵ2 = Ĵx2 + Ĵy2 + Ĵz2 | (2) |
| Ĵ± = Ĵx ± iĴy | (3) |
3.2 Symmetry considerations
Trans-2,3-dideutero-oxirane is a highly asymmetric rotor of C2 symmetry with an asymmetry parameter . Its permanent electric dipole moment is expected to lie along the C2-symmetry (b)-axis allowing transitions obeying b-type selection rules (eo ↔ oe and ee ↔ oo, e for even and o for odd values of the quantum numbers Ka and Kc)90,91 with μb estimated to be similar to that of normal oxirane of 1.88 ± 0.01 D.63,64 The principal axis systems for trans-2,3-dideutero-oxirane and cis-2,3-dideutero-oxirane are shown in Fig. 1, and Table 1 includes the character table for the point group C2 and the molecular symmetry group MS2 relevant for tc-CHDCHDO.
. Its permanent electric dipole moment is expected to lie along the C2-symmetry (b)-axis allowing transitions obeying b-type selection rules (eo ↔ oe and ee ↔ oo, e for even and o for odd values of the quantum numbers Ka and Kc)90,91 with μb estimated to be similar to that of normal oxirane of 1.88 ± 0.01 D.63,64 The principal axis systems for trans-2,3-dideutero-oxirane and cis-2,3-dideutero-oxirane are shown in Fig. 1, and Table 1 includes the character table for the point group C2 and the molecular symmetry group MS2 relevant for tc-CHDCHDO.
| C 2 | M S2 | E | C 2 | K a K c | μ | g |
|---|---|---|---|---|---|---|
| E | (αβ) | |||||
| a (αβ) is the simultaneous exchange of the CHD groups, g is the nuclear spin statistical weight and μx, μy, μz are the electric dipole moment components. | ||||||
| A | A | 1 | 1 | ee, oo | μ y | 15 |
| B | B | 1 | −1 | eo, oe | μ x , μz | 21 |
The nuclear spin statistical weights g of trans-2,3-dideutero-oxirane arise from the nuclear spin functions of two protons (IH = 1/2) and two deuterons (ID = 1). For the main 12C and 16O species, the nuclear spin is zero for C and O. In total, there are Π(2Ii + 1) = (2IH + 1) (2IH + 1) (2ID + 1) (2ID + 1) = 36 nuclear spin functions. Because of the generalised Pauli principle8 as applied to all particles involved here, the complete internal wave function of the molecule must be antisymmetric (or B in Table 1). This leads to the nuclear spin statistical weights g for the rotational levels described by the combination KaKc in the totally symmetric vibrational and electronic ground state as given in Table 1. Nuclear spin symmetry (A or B) is an approximately conserved property for the radiative transitions in our experiments, where we have not observed any transitions connecting the two nuclear spin isomers (A or B). As the rovibronic A and B levels are statistically equally abundant for this C2-symmetric molecule,95 the B isomer has the higher equilibrium concentration at room temperature, given the higher nuclear spin statistical weight 21 and, by convention called the ortho-isomer. The other isomer (A) with weight 15 is called the para-isomer, by the usual convention. Parity is not a good quantum number for tc-CHDCHDO, because the very high barrier for inversion at the C-atoms leads to a complete suppression of inversion tunnelling: the small ΔpvE is many orders of magnitude larger than the hypothetical tunnelling splitting.8–10 For further discussions of these symmetry aspects, see ref. 8–10 and 96.
4. Assignment of the pure rotational spectra in the ground state
The rotational spectra of trans-2,3-dideutero-oxirane in the GHz and THz ranges were first assigned and fitted independently, followed by a global analysis of all transitions measured in both ranges. As a result, a common set of spectroscopic parameters for the ground state was obtained.The assignment of the GHz spectra was assisted by a simulation of the spectrum using the previously reported rotational constants in ref. 46 and 47, which were based on the analysis of 21 transitions with Jmax = 8 and Kc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) max = 4. In our work, a total of 1413 b-type transitions were assigned using the PGOPHER program83,84 in the range of 64–500 GHz with Jmax = 51 and Kc
max = 4. In our work, a total of 1413 b-type transitions were assigned using the PGOPHER program83,84 in the range of 64–500 GHz with Jmax = 51 and Kc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) max = 34. The newly assigned transitions were introduced into a least squares analysis and the resulting spectroscopic parameters are given in Table 2. In these analyses we used the programs WANG92 and SPFIT,93 giving essentially identical results. The root-mean-square deviation drms of the fit is less than 8 kHz, with the typical linewidths (FWHM) of about 200 kHz in the GHz spectra. The rotational constants A, B and C are in good agreement with those previously determined by microwave spectroscopy.47 Many higher order parameters are determined here for the first time.
max = 34. The newly assigned transitions were introduced into a least squares analysis and the resulting spectroscopic parameters are given in Table 2. In these analyses we used the programs WANG92 and SPFIT,93 giving essentially identical results. The root-mean-square deviation drms of the fit is less than 8 kHz, with the typical linewidths (FWHM) of about 200 kHz in the GHz spectra. The rotational constants A, B and C are in good agreement with those previously determined by microwave spectroscopy.47 Many higher order parameters are determined here for the first time.
| Trans-2,3-dideutero-oxirane | Cis-2,3-dideutero-oxirane | |||||
|---|---|---|---|---|---|---|
| MW previousa | GHz (SP) | GHz (LP) | MW previousa | GHz (SP) | GHz (LP) | |
| a Ref. 47. | ||||||
| A/MHz | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943.19 943.19 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943.193860(59) 943.193860(59) |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943.194100(58) 943.194100(58) |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700.41 700.41 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700.415590(82) 700.415590(82) |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700.415740(78) 700.415740(78) |
| B/MHz | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198.47 198.47 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198.503910(58) 198.503910(58) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198.503910(53) 198.503910(53) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318.39 318.39 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318.511470(84) 318.511470(84) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318.511380(78) 318.511380(78) |
| C/MHz | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 585.27 585.27 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 585.291410(63) 585.291410(63) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 585.291390(57) 585.291390(57) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650.08 650.08 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650.101900(90) 650.101900(90) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650.101810(83) 650.101810(83) |
| Δ J /kHz | — | 13.94040(20) | 13.94050(18) | — | 14.63690(42) | 14.63650(39) |
| Δ K /kHz | — | 24.423800(77) | 24.42600(14) | — | 24.16270(11) | 24.16540(22) |
| Δ JK /kHz | — | 14.379500(82) | 14.37910(11) | — | 12.31080(14) | 12.31010(22) |
| δ j /kHz | — | 3.568150(12) | 3.568160(11) | — | 3.846750(31) | 3.846760(28) |
| δ k /kHz | — | 9.739950(56) | 9.740000(52) | — | 9.002370(90) | 9.002100(87) |
| ϕ J /mHz | — | 6.00(19) | 6.00(18) | — | 9.70(61) | 9.00(57) |
| ϕ JK /mHz | — | 255.320(99) | 254.80(10) | — | 250.20(14) | 249.10(20) |
| ϕ KJ /mHz | — | −1174.10(18) | −1173.30(20) | — | −1177.50(25) | −1177.40(54) |
| ϕ K /mHz | — | 1086.01(11) | 1088.80(21) | — | 1087.50(17) | 1092.80(50) |
| η J /mHz | — | 0.9530(73) | 0.9560(67) | — | 2.590(20) | 2.600(18) |
| η JK /mHz | — | 108.490(56) | 108.500(51) | — | 111.270(92) | 110.960(90) |
| η K /mHz | — | −120.170(84) | −120.000(77) | — | −130.20(11) | −130.10(10) |
| L JK /Hz | — | — | 0.870(90) | — | — | 2.90(48) |
| L KKJ /Hz | — | — | −2.30(15) | — | — | −5.30(47) |
| d rms/kHz | — | 7.380 | 6.710 | — | 6.573 | 5.986 |
| N data | 21 | 1577 | 1577 | 22 | 1121 | 1121 |
The assignment of the THz spectrum was more challenging as transitions due to other species, likely by-products of the synthesis such as monodeutero-oxirane and normal oxirane, were present in the observed spectrum (see below). To ensure an unambiguous assignment, a cautious procedure was carried out: (1) a Loomis–Wood diagram91,97,98 was constructed so that the regularly occurring patterns in several series were evident. The right insert in Fig. 2 shows progressions of transitions with common 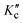 values; (2) only isolated transitions in these series with strong intensities (Kc ≤ 8 with 20 ≤ J ≤ 30) were assigned initially; (3) these preliminary line positions were added to the line list together with the lines assigned in the GHz range, which are 70 times more heavily weighted; (4) thereafter further transitions were assigned and fitted, the simulated spectrum was refined, leading to the assignment of further transitions (Kc ≥ 8 with J ≥ 30). This iterative process was continued until all stronger transitions had been accounted for. An additional 2556 rotational transitions were assigned in the range of 0.75–2.4 THz (25 to 80 cm−1). The range of quantum numbers of assigned transitions extends from J = 17 to 65 with 0 ≤ Kc ≤ 65.
values; (2) only isolated transitions in these series with strong intensities (Kc ≤ 8 with 20 ≤ J ≤ 30) were assigned initially; (3) these preliminary line positions were added to the line list together with the lines assigned in the GHz range, which are 70 times more heavily weighted; (4) thereafter further transitions were assigned and fitted, the simulated spectrum was refined, leading to the assignment of further transitions (Kc ≥ 8 with J ≥ 30). This iterative process was continued until all stronger transitions had been accounted for. An additional 2556 rotational transitions were assigned in the range of 0.75–2.4 THz (25 to 80 cm−1). The range of quantum numbers of assigned transitions extends from J = 17 to 65 with 0 ≤ Kc ≤ 65.
Ultimately, 4133 assigned line positions in the THz and GHz range and 21 lines reported by ref. 47 were co-fitted to obtain a common set of ground state spectroscopic parameters of tc-CHDCHDO. It turned out by inspection of the MW data from ref. 46 and 47 and the resulting fits that these had much larger uncertainties than most of the GHz lines reported in this study. Therefore, while presenting all known line data in Tables S1 and S2 in the SI, in the final best compromise fit we excluded some of the low frequency data (indicated by an asterisk * in the supplementary tables) in order to obtain a best estimate for the effective Hamiltonian parameters given in Tables 2 and 3. However, the results of a fit including all data are also given in the SI (Table S4). The differences between the parameters from the two fits are not large. In the global fit, the line frequencies assigned in the THz and GHz regions were weighted based on the linewidths of 0.2 MHz and 14 MHz, respectively, such that the greater precision of the GHz measurement is reflected in a ratio of roughly 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Table 2 compares also the result obtained with a small parameter set (SP) excluding octic parameters, and a larger parameter set, including some of the octic parameters (LJK and LKKJ). It appears that the fit with the smaller parameter set is adequate for the present analysis. Table 3 presents the results from the global fit.
1. Table 2 compares also the result obtained with a small parameter set (SP) excluding octic parameters, and a larger parameter set, including some of the octic parameters (LJK and LKKJ). It appears that the fit with the smaller parameter set is adequate for the present analysis. Table 3 presents the results from the global fit.
| Cis-2,3-dideutero-oxirane (GHz, SP) | Monodeutero-oxrianea (THz, GHz and mw) | Trans-2,3-dideutero-oxirane (GHz and THz, SP) | Trans-2,3-dideutero-oxirane (GHz and THz, LP) | |
|---|---|---|---|---|
| a Ref. 21, Table 4 SP. | ||||
| A/MHz | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700.415590(82) 700.415590(82) |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 252.64570(15) 252.64570(15) |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943.19366(12) 943.19366(12) |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943.19415(14) 943.19415(14) |
| B/MHz | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318.511470(84) 318.511470(84) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 905.52270(20) 905.52270(20) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198.50392(10) 198.50392(10) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198.50396(10) 198.50396(10) |
| C/MHz | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650.101900(90) 650.101900(90) |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327.58370(16) 327.58370(16) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 585.29181(11) 585.29181(11) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 585.29173(11) 585.29173(11) |
| Δ J /kHz | 14.63690(42) | 17.04117(26) | 13.94023(19) | 13.94027(19) |
| Δ K /kHz | 24.16270(11) | 25.45675(24) | 24.42401(22) | 24.42699(30) |
| Δ JK /kHz | 12.31080(14) | 17.25473(26) | 14.37793(21) | 14.37796(33) |
| δ j /kHz | 3.846750(31) | 4.741796(42) | 3.567874(34) | 3.567959(33) |
| δ k /kHz | 9.002370(90) | 13.23277(11) | 9.74110(15) | 9.74086(15) |
| ϕ J /mHz | 9.70(61) | 11.697(95) | 4.330(57) | 4.460(56) |
| ϕ JK /mHz | 250.20(14) | 138.67(17) | 253.41(25) | 253.34(30) |
| ϕ KJ /mHz | −1177.50(25) | −829.84(22) | −1171.00(50) | −1170.70(55) |
| ϕ K /mHz | 1087.50(17) | 797.41(14) | 1083.95(30) | 1087.90(49) |
| η J /mHz | 2.590(20) | 4.602(15) | 0.784(18) | 0.832(18) |
| η JK /mHz | 111.270(92) | 62.056(62) | 109.65(14) | 109.37(14) |
| η K /mHz | −130.20(11) | 33.301(74) | −121.47(23) | −120.99(22) |
| L JK /Hz | — | — | — | 0.95(24) |
| L KKJ /Hz | — | — | — | −2.60(28) |
| d rms/kHz | 6.242 | 2679.7 | 1498.5 | 1467.4 |
| N data | 1121 | 3370 | 4133 | 4133 |
5. Results and discussion
Pure rotational transitions were measured and assigned in the GHz range from 64 to 500 GHz, allowing a much broader coverage of energy levels than in the previous microwave study.47 This allowed for an accurate determination of the quartic (ΔJ, ΔK, ΔJK, δj, δk), sextic (ϕK, ϕJ, ϕJK, ϕKJ, ηJ, ηK, ηJK), and some octic (LJK, LKKJ) centrifugal distortion parameters of the ground state of tc-CHDCHDO, which were not previously available, in addition to the now much more accurate rotational constants. The root-mean-square deviations of the fit of about 7 kHz is significantly smaller than one-tenth of the observed linewidths of ∼200 kHz, indicating that the effective Hamiltonian used here provides an accurate description of the observed spectral features in this range. As a result, the simulation based on the spectroscopic parameters in Table 2 reproduces the experimental spectra very well. Such comparisons are shown in Fig. 3–5.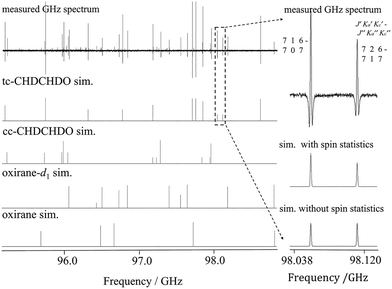 | ||
Fig. 3 A comparison of observed and simulated rotational spectra in the GHz region. From top to bottom: (1) experimental spectrum with pressure = 25 µbar and temperature = 294 K; (2) simulation of trans-2,3-dideutero-oxirane (tc-CHDCHDO); (3) simulation of cis-2,3-dideutero-oxirane (cc-CHDCHDO); (4) simulation of monodeutero-oxirane (oxirane-d1) using spectroscopic parameters from ref. 21; (5) simulation of normal oxirane using spectroscopic parameters from ref. 67 and 68. The right panel shows an enlarged section with the simulation of the spectra of tc-CHDCHDO with (middle trace) and without nuclear spin statistics (lower trace) with quantum numbers defined as 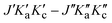 . . | ||
 | ||
| Fig. 5 A comparison of observed and simulated rotational spectra in the GHz region. From top to bottom: (1) experimental spectrum, pressure = 25 µbar and temperature = 294 K; (2) simulation of cis-2,3-dideutero-oxirane (cc-CHDCHDO); (3) simulation of monodeutero-oxirane (C2H3DO) using spectroscopic parameters from ref. 21; (4) simulation of trans-2,3-dideutero-oxirane (tc-CHDCHDO). Some lines of oxirane-d1 and cc-CHDCHDO are shown magnified in the inserts with their assignments (see also Fig. 3). | ||
For the analysis of the FTIR spectra consisting of transitions with higher J and Kc values in the THz range, further higher order parameters were used in the fit as shown in Table 3. The three measurements of THz spectra at different pressures allowed the recording of a large number of transitions with different intensities. A total of 2556 assigned transitions in the range from J = 17 to 65 with 0 ≤ Kc ≤ 65 provided energy levels to accurately determine higher order parameters, to which the high Kc lines are extremely sensitive. The transitions assigned in the GHz and THz range were finally fitted simultaneously. The drms of this global fit is less than 1.5 MHz. Again, the fit with the small parameter set seems to be adequate also in comparison with a fit including some octic constants (LP). The low drms is further evidence of the excellent representation of the observed spectra by the effective Hamiltonian used. Fig. 4 shows the comparison between the observed THz spectrum and the simulation based on the spectroscopic parameters given in Table 3.
In addition to the lines assigned to tc-CHDCHDO, additional lines were observed in the GHz spectrum as shown in Fig. 3. Many of these transitions could be attributed to cis-2,3-dideutero-oxirane (1121 new transitions were assigned, see Table 2 for the resulting spectroscopic parameters), monodeutero-oxirane21 and normal oxirane67,68,70 as by-products of the synthesis firmly established as based on the available high-resolution data. In the THz region, the spectral overlap is much more pronounced, and it is evident from Fig. 4 that no more than half of the observed lines are due to trans-2,3-dideutero-oxirane, while the remainder are due to other compounds such as monodeutero-oxirane, cis-2,3-dideutero-oxirane and normal oxirane. Therefore, we introduced a routine that adds only strong, isolated lines in a series in the Loomis–Wood diagram to the line list while co-fitting these transitions with the GHz lines, which are weighted 70 times more heavily in the preliminary assignment, so that accidental misassignments arising from overlapping lines arising from other species can be avoided. As the Hamiltonian model was improved after each iteration, we were gradually able to assign almost all predicted lines between 25 to 80 cm−1, except for overlapped lines. Tables 2 and 3 also summarise the results for cis-2,3-dideutero-oxirane, which could be obtained from the “impurity” lines in a similar fashion.
It was possible to observe the intensity alternation in the spectra due to the nuclear spin statistical weights (Table 1). Fig. 3 and 4 show examples of a comparison of tc-CHDCHDO transitions with simulations with (right panel, upper trace) and without (right panel, lower trace) nuclear spin statistical weights in both the GHz and THz regions. Even for such a dense spectrum, the intensity alternation due to the different nuclear spin statistical weights for the nuclear spin isomers (A and B) is obvious, and confirms the assignments. Moreover, such intensity profiles play a crucial role in distinguishing this molecule from the other deuterated species in the sample, especially when dense spectra are observed. Interestingly, the presence of impurities in our sample illustrated the power of high-resolution spectroscopy in unambiguously identifying molecules in such a mixture. More specifically, it provides the possibility to distinguish the spectra of oxirane and its isotopomers, which would be a likely scenario in field and astrophysical observations. Fig. 5 shows an enlarged section from Fig. 3, where the inserts show details of the weaker lines as assigned to mondeutero-oxirane and cis-dideutero-oxirane, which are clearly separated from the dominant tc-CHDCHDO lines.
The laboratory spectroscopy of both cis- and trans-2,3-dideutero-oxirane presented in this study provides important data that can significantly help in its possible detection in the ISM. The accurate determination of the rotational parameters allows for a precise prediction of the spectral line positions that can be targeted in astronomical observations. Given the recent discovery of mono-deutero-oxirane in the low-mass protostar IRAS 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293–2422 B,20 it is plausible that 2,3-dideutero-oxirane could also be detected in similar environments. The key to its detectability lies in its large dipole moment, which plays a crucial role in its radio astronomical signature. Table 4 provides a small selection of lines, which can be predicted to be possibly useful for such a detection and Table S3 in the SI provides a more extended list. In any case, the very accurate parameters of the effective Hamiltonian derived here allow for an accurate prediction of rotational transitions in the complete spectral range covered by our results from the low GHz up to 2 THz. Fig. 6 shows a further detailed section of the spectra from the range 330 to 360 GHz, which has been important for the detection of CD2CH2O59 and CHD2CHO58 as isomers of tc-CHDCHDO.
293–2422 B,20 it is plausible that 2,3-dideutero-oxirane could also be detected in similar environments. The key to its detectability lies in its large dipole moment, which plays a crucial role in its radio astronomical signature. Table 4 provides a small selection of lines, which can be predicted to be possibly useful for such a detection and Table S3 in the SI provides a more extended list. In any case, the very accurate parameters of the effective Hamiltonian derived here allow for an accurate prediction of rotational transitions in the complete spectral range covered by our results from the low GHz up to 2 THz. Fig. 6 shows a further detailed section of the spectra from the range 330 to 360 GHz, which has been important for the detection of CD2CH2O59 and CHD2CHO58 as isomers of tc-CHDCHDO.
| J′ | J″ | Predicted frequency | Observed frequency | E″/(h MHz) | ||||
|---|---|---|---|---|---|---|---|---|
| 7 | 3 | 4 | 6 | 2 | 5 | 335![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 574.637 574.637 |
335![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 574.643 574.643 |
656![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 997.870 997.870 |
| 12 | 2 | 10 | 11 | 3 | 9 | 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 189.481 189.481 |
340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 189.480 189.480 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046 046![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732.696 732.696 |
| 12 | 3 | 10 | 11 | 2 | 9 | 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 197.669 197.669 |
340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 197.673 197.673 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046 046![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 725.981 725.981 |
| 8 | 7 | 2 | 7 | 6 | 1 | 345![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710.989 710.989 |
345![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710.989 710.989 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 529.094 529.094 |
| 8 | 7 | 1 | 7 | 6 | 2 | 346![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 055.873 055.873 |
346![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 055.872 055.872 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260.690 260.690 |
| 13 | 1 | 12 | 12 | 2 | 11 | 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030.212 030.212 |
350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030.222 030.222 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 228 228![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 872.264 872.264 |
| 13 | 2 | 12 | 12 | 1 | 11 | 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030.245 030.245 |
350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030.222 030.222 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 228 228![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 872.234 872.234 |
| 7 | 5 | 3 | 6 | 2 | 4 | 351![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902.080 902.080 |
351![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902.086 902.086 |
719![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670.023 670.023 |
| 14 | 0 | 14 | 13 | 1 | 13 | 359![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 933.472 933.472 |
359![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 933.474 933.474 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389 389![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 201.061 201.061 |
| 14 | 1 | 14 | 13 | 0 | 13 | 359![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 933.472 933.472 |
359![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 933.474 933.474 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389 389![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 201.061 201.061 |
| 7 | 2 | 5 | 6 | 1 | 6 | 362![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046.505 046.505 |
362![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046.512 046.512 |
574![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 102.527 102.527 |
| 8 | 4 | 4 | 7 | 3 | 5 | 363![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 509.178 509.178 |
363![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 509.175 509.175 |
937![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 797.007 797.007 |
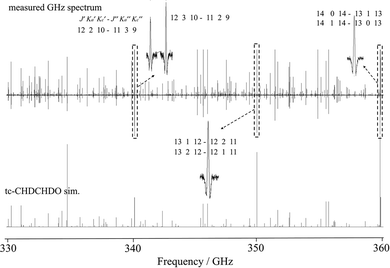 | ||
Fig. 6 A comparison of the observed spectrum at 30 µbar and simulated rotational spectra of trans-2,3-dideutero-oxirane (tc-CHDCHDO) in the range of 330 to 360 GHz at 294 K. Inserts show some of the transitions (quantum numbers defined as 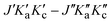 ) measured at reduced pressures below 2 µbar, which are among promising candidates for astrophysical searches listed in Table 4 and Table S3. ) measured at reduced pressures below 2 µbar, which are among promising candidates for astrophysical searches listed in Table 4 and Table S3. | ||
6. Conclusions and outlook
The combination of highest resolution synchrotron based FTIR spectroscopy9,85–87 in the difficult THz range with high precision GHz spectroscopy in our laboratory80–82 enabled us here to obtain an accurate effective rotational Hamiltonian for the chiral trans-2,3-dideutero-oxirane and its achiral isotopomer cis-2,3-dideutero-oxirane in the vibrational ground state. Our analysis of the rotational spectra of these molecules covered a wide frequency range from about 60 GHz to more than 2 THz. The most obvious application of these results concerns astrophysical spectroscopy, where our results may lead to a detection of these molecules in the interstellar medium (ISM), given the very accurate rotational line results and predictions, the large dipole moments of these species and the previous detection of oxirane and monodeutero-oxirane.13–21 From such observations one can expect important information on the processes of deuterium fractionation in the formation of isotopically chiral molecules, in particular, of which so far only one example, monodeutero-oxirane,19–21 has been detected in the ISM. Indeed, only two chiral molecules overall have so far been detected by astrophysical spectroscopy, with one additional example methyloxirane,28,99 thus there is a great gap in our knowledge of the prevalence of chiral molecules in space outside the Earth. Our present work aims at helping to close this gap.However, this is by no means the only possible application of our results. Apart from the obvious use for improving the structural information on the important prototype molecule oxirane (C2H4O), even of industrial relevance, tc-CHDCHDO is also an interesting candidate for the study of vibrational quantum dynamics of highly excited states by high resolution infrared spectroscopy,60,61,91,100 for which the present accurate ground state parameters provide a starting point. Indeed, this chiral molecule has two “isolated CH-chromophores” separated by a C–C bond, and the quantum dynamics of vibrational energy transfer across bonds101–103 in this kind of molecules is of great interest, with little knowledge available so far for such chiral systems. When combined with the study of time-dependent vibrational circular dichroism, it would presumably provide the first example of this kind hypothesized a longer time ago already.3,101,104 One should furthermore consider the interesting expected differences in vibrational energy flow with respect to the isomer CHD2–CHO58 with two isolated CH-chromophores involving a slightly different chemical environment with an aldehydic CH-chromophore.60–62 The extension of our work on tc-CHDCHDO to high resolution vibrational–rotational spectroscopy is in progress, similar to monodeutero-oxirane21 and has also possible astrophysical applications with the recent successful launch of the James Webb telescope.105–108
Isotopically chiral molecules such as tc-CHDCHDO are also of obvious interest in relation to the fundamental physics of parity violation and tunnelling in chiral molecules.2,9,10C2-symmetric molecules theoretically studied so far include HOOH,109,110 HSSH,111 where tunnelling dominates, but also ClSSCl96,112 and 1,2-dithiine,81 for example, where parity violation dominates and tunnelling is completely suppressed in the ground state. tc-CHDCHDO clearly is expected to fall in the latter class of molecules where chirality is completely dominated by parity violation, but a more quantitative theoretical study would be of interest. We should emphasise, though, that because of the qualitatively expected,10 although so far not quantitatively calculated very small value of ΔpvE for tc-CHDCHDO (<1 aeV), this molecule would not be a priority choice for measurements of ΔpvE. In this context 1,2-dithiine (C4H4S4),81 with a predicted ΔpvE ≈ 1.3 feV or among substituted oxiranes fluoro-oxirane (21.1 aeV)113–115 or cyano-oxirane (12.4 aeV)99,116 would be more favourable candidates.
Returning to the perhaps most inspiring question of possible investigations on extraterrestrial homochirality in relation to the origin of life, accurate spectroscopic results on chiral molecules like the present one provide an early step towards such studies.22,117 In contrast to other “chemical-analytical” approaches,24 astrophysical spectroscopy is not limited to the solar system, one can, indeed, reach far out to other stars and galaxies.117,118 While the complexity of life in relation to biomolecular homochirality presumably requires biopolymers such as proteins and DNA, not easily detected by gas phase spectroscopy in astrophysics, small chiral molecules can possibly be used as “tracers” for homochirality to be studied by chirally sensitive high resolution spectroscopy (circular dichroism, vibrational circular dichroism, Raman optical activity…, with observations on satellites to be planned for the future). While results along these lines are not expected in the nearest future, they could address some of the most fundamental open questions in science concerning the existence and prevalence of extraterrestrial life in the first place. This would be followed by the further open question, whether the given selection of homochirality (if any) is “de facto” (by chance, resulting in a statistical distribution over many different homochiralities) or “de lege” (by necessity, resulting in a preference of one, such as ours with L-aminoacids and D-sugars).2,22,119–121 With high resolution spectroscopic results on chiral molecules such as presented here, one has a very early step towards answering such questions, although clearly very many further steps will be required.
Conflicts of interest
There are no conflicts to declare.Data availability
Relevant data including lists of all transitions assigned are provided in the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cp03143e.Acknowledgements
We are indebted to Philippe Lerch for help in the earlier experiments. Help from and discussions with Carine Manca Tanner, Georg Seyfang and Jürgen Stohner and continuous support from Frédéric Merkt are gratefully acknowledged. This research was funded by ETH Zürich, in particular the LPC and the IMPS, the Swiss National Science Foundation (40-B2-0218759/Bridge), an ERC Advanced Grant as well as the COST projects MOLIM and COSY and the Fundamental Research Funds for Chinese Central Universities (No. lzujbky-2018-k08, lzujbky-2019-65 and lzujbky-2019-ct05). The research leading to these results has in particular also received funding from the European Union's seventh Framework Program (FP7/2007-2013) ERC grant agreement No. 290925. With deep gratitude we dedicate this publication to Jürgen Troe on the occasion of his 85th birthday 2025. He was involved in the foundation of PCCP and is a member of its honorary board, and he also has made fundamental contributions to the understandings of interstellar chemistry (see ref. 122).References
- E. Babaev, D. Kharzeev, M. Larsson, A. Molochkov and V. Zhaunerchyk, Eds., Chiral Matter, Proceedings of the Nobel Symposium 167, Lidingö (Stockholm) 28 June to 2 July 2021, pp. 209–268; ed. E. Babaev, D. Kharzeev, M. Larsson, A. Molochkov, V. Zhaunerchyk, World Scientific Publishing Co, Singapore, 2023.
- M. Quack, Angew. Chem., 1989, 101, 588 CrossRef CAS; M. Quack, Angew. Chem., Int. Ed. Engl., 1989, 28, 571 CrossRef.
- A. Beil, D. Luckhaus, R. Marquardt and M. Quack, Faraday Discuss., 1994, 99, 49 RSC.
- R. Berger, M. Quack, A. Sieben and M. Willeke, Helv. Chim. Acta, 2003, 86, 4048 CrossRef CAS.
- R. Berger, G. Laubender, M. Quack, A. Sieben, J. Stohner and M. Willeke, Angew. Chem., 2005, 117, 3689 CrossRef CAS; R. Berger, G. Laubender, M. Quack, A. Sieben, J. Stohner and M. Willeke, Angew. Chem., Int. Ed., 2005, 44, 3623 CrossRef PubMed.
- M. Hippler and M. Quack, Isotope Selective Infrared Spectroscopy and Intramolecular Dynamics, in Isotope Effects in Chemistry and Biology, Part III Isotope Effects in Chemical Dynamics, ed. A. Kohen and H.-H. Limbach, Marcel Dekker Inc., New York, 2005, pp. 305–359 Search PubMed.
- M. Hippler, E. Miloglyadov, M. Quack and G. Seyfang, Mass and Isotope Selective Infrared Spectroscopy, in Handbook of High-Resolution Spectroscopy, ed. M. Quack and F. Merkt, Wiley, Chichester, 2011, vol. 2, ch. 28, pp. 1069–1118, ISBN-13:978-0-470-06653-9 Search PubMed.
- M. Quack, Fundamental Symmetries and Symmetry Violations from High Resolution Spectroscopy, in Handbook of High-Resolution Spectroscopy, ed. M. Quack and F. Merkt, Wiley, Chichester, 2011, vol. 1, ch. 18, pp. 659–722, ISBN-13:978-0-470-06653-9 Search PubMed.
- M. Quack, G. Seyfang and G. Wichmann, Parity Violation in Chiral Molecules: From Theory, towards Spectroscopic Experiment and the Evolution of Biomolecular Homochirality, in ‘Chiral Matter’, Proceedings of the Nobel Symposium 167, Lidingö (Stockholm) 28 June to 2 July 2021, ed. E. Babaev, D. Kharzeev, M. Larsson, A. Molochkov and V. Zhaunerchyk, World Scientific Publishing Co, Singapore, 2023, pp. 209–268 (after lecture M. Quack).
- M. Quack, G. Seyfang and G. Wichmann, Chem. Sci., 2022, 13, 10598 RSC.
- M. Quack, Chem. Phys. Lett., 1986, 132, 147 CrossRef CAS.
- P. Dietiker, E. Miloglyadov, M. Quack, A. Schneider and G. Seyfang, J. Chem. Phys., 2015, 143, 244305 CrossRef CAS PubMed.
- J. E. Dickens, W. M. Irvine, M. Ohishi, M. Ikeda, S. Ishikawa, A. Nummelin and A. Hjalmarson, Astrophys. J., 1997, 489, 753 CrossRef CAS.
- A. Nummelin, J. E. Dickens, P. Bergman, A. Hjalmarson, W. M. Irvine, M. Ikeda and M. Ohishi, Astron. Astrophys., 1998, 337, 275 CAS.
- M. Ikeda, M. Ohishi, A. Nummelin, J. E. Dickens, P. Bergman, A. Hjalmarson, W. M. Irvine and S. Ishikawa, Astrophys. J., 2001, 560, 792 CrossRef CAS.
- M. A. Requena-Torres, J. Martín-Pintado, S. Martín and M. R. Morris, Astrophys. J., 2008, 672, 352 CrossRef CAS.
- J. K. Jørgensen, M. H. D. van der Wiel, A. Coutens, J. M. Lykke, H. S. P. Müller, E. F. van Dishoeck, H. Calcutt, P. Bjerkeli, T. L. Bourke, M. N. Drozdovskaya, C. Favre, E. C. Fayolle, R. T. Garrod, S. K. Jacobsen, K. I. Öberg, M. V. Persson and S. F. Wampfler, Astron. Astrophys., 2016, 595, A117 CrossRef.
- J. M. Lykke, A. Coutens, J. K. Jørgensen, M. H. D. van der Wiel, R. T. Garrod, H. S. P. Müller, P. Bjerkeli, T. L. Bourke, H. Calcutt, M. N. Drozdovskaya, C. Favre, E. C. Fayolle, S. K. Jacobsen, K. I. Öberg, M. V. Persson, E. F. van Dishoeck and S. F. Wampfler, Astron. Astrophys., 2017, 597, A53 CrossRef.
- S. Albert, Z. Chen, K. Keppler, P. Lerch, M. Quack, V. Schurig and O. Trapp, Phys. Chem. Chem. Phys., 2019, 20, 3669 RSC.
- H. S. P. Müller, J. K. Jorgensen, J.-C. Guillemin, F. Lewen and S. Schlemmer, Mon. Not. R. Astron. Soc., 2023, 518(1), 185 CrossRef.
- S. Albert, Z. Chen, K. Keppler, G. Wichmann, M. Quack, V. Schurig and O. Trapp, Phys. Chem. Chem. Phys., 2025, 27, 14240 RSC.
- M. Quack, Adv. Chem. Phys., 2015, 157, 249 Search PubMed.
- M. Quack, Faraday Discuss., 2011, 150, 533 RSC.
- A. C. Evans, C. Meinert, J. H. Bredehoft, C. Giri, N. C. Jones, S. V. Hoffmann and U. J. Meierhenrich, Top. Curr. Chem., in Differentiation of Enantiomers II, ed. V. Schurig, notably section 4, The Rosetta mission and the COSAC experiment, Springer-Verlag, Berlin, Heidelberg, 2013, vol. 341, pp. 271–300 Search PubMed.
- X. Bonfils, X. Delfosse, S. Udry, T. Forveille, M. Mayor, C. Perrier, F. Bouchy, M. Gillon, C. Lovis, F. Pepe, D. Queloz, N. C. Santos, D. Ségransan and J.-L. Bertaux, Astron. Astrophys., 2013, 549, A109 CrossRef.
- N. Madhusudhan, Annu. Rev. Astron. Astrophys., 2019, 57, 617 CrossRef.
- S. Rukdee, Sci. Rep., 2024, 14, 27356 CrossRef CAS.
- Y. Ellinger, F. Pauzat, A. Markovits, A. Allaire and J.-C. Guillemin, Astron. Astrophys., 2020, 633, A49 CrossRef CAS.
- T. B. Freedman, M. G. Paterlini, N.-S. Lee, L. A. Nafie, J. M. Schwab and T. Ray, J. Am. Chem. Soc., 1987, 109, 4727 CrossRef CAS.
- T. B. Freedman, K. M. Spencer, N. Ragunathan, L. A. Nafie, J. A. Moore and J. M. Schwab, Can. J. Chem., 1991, 69, 1619 CrossRef CAS.
- K. J. Jalkanen, P. J. Stephens, R. D. Amos and N. C. Handy, J. Am. Chem. Soc., 1988, 110, 2012 CrossRef CAS.
- R. Dutler and A. Rauk, J. Am. Chem. Soc., 1989, 111, 6957 CrossRef CAS.
- P. J. Stephens, K. J. Jalkanen and R. W. Kawiecki, J. Am. Chem. Soc., 1990, 112, 6518 CrossRef CAS.
- P. L. Polavarapu and P. K. Bose, J. Chem. Phys., 1990, 93, 7524 CrossRef CAS.
- P. J. Stephens, K. J. Jalkanen, F. J. Devlin and C. F. Chabalowski, J. Phys. Chem., 1993, 97, 6107 CrossRef CAS.
- K. L. Bak, P. Jørgensen, T. Helgaker and K. Ruud, Faraday Discuss., 1994, 99, 121 RSC (see also discussion M. Quack pp. 98–99, 200–201, K. L. Bak pp. 201 herein).
- K. L. Bak, O. Bludský and P. Jørgensen, J. Chem. Phys., 1995, 103, 10548 CrossRef CAS.
- V. Barone, M. Biczysko, J. Bloino and C. Puzzarini, J. Chem. Phys., 2014, 141, 034107 CrossRef PubMed.
- P. Herwig, K. Zawatzky, M. Grieser, O. Heber, B. Jordon-Thaden, C. Krantz, O. Novotný, R. Repnow, V. Schurig, D. Schwalm, Z. Vager, A. Wolf, O. Trapp and H. Kreckel, Science, 2013, 342, 1084 CrossRef CAS.
- P. Herwig, K. Zawatzky, D. Schwalm, M. Grieser, O. Heber, B. Jordon-Thaden, C. Krantz, O. Novotný, R. Repnow, V. Schurig, Z. Vager, A. Wolf, O. Trapp and H. Kreckel, Phys. Rev. A:At., Mol., Opt. Phys., 2014, 90, 052503 CrossRef.
- K. Zawatzky, P. Herwig, M. Grieser, O. Heber, B. Jordon-Thaden, C. Krantz, O. Novotný, R. Repnow, V. Schurig, D. Schwalm, Z. Vager, A. Wolf, H. Kreckel and O. Trapp, Chem. – Eur. J., 2014, 20, 5555 CrossRef CAS.
- O. Trapp and K. Zawatzky, Isr. J. Chem., 2016, 56, 1082 CrossRef CAS.
- M. Pitzer, M. Kunitski, A. S. Johnson, T. Jahnke, H. Sann, F. Sturm, L. P. H. Schmidt, H. Schmidt-Böcking, R. Dörner, J. Stohner, J. Kiedrowski, M. Reggelin, S. Marquardt, A. Schießer, R. Berger and M. S. Schöffler, Science, 2013, 341, 1096 CrossRef CAS.
- J. M. Bijvoet, A. F. Peerdeman and A. J. van Bommel, Nature, 1951, 168, 271 CrossRef CAS.
- J. D. Dunitz, X-ray Analysis and the Structure of Organic Molecules, Cornell University Press, Ithaca and London, 1979 Search PubMed.
- C. Hirose, Astrophys. J., 1974, 189, L145 CrossRef CAS.
- C. Hirose, Bull. Chem. Soc. Jpn., 1974, 47, 1311 CrossRef CAS.
- E. Herbst, Deuterium Fractionation in Interstellar Space, ACS Symposium Series, 1992, vol. 502, ch. 22, pp. 358–368 Search PubMed.
- A. G. C. Tielens, Rev. Mod. Phys., 2013, 85, 1021 CrossRef CAS.
- J. L. Linsky, Space Sci. Rev., 2003, 106, 49 CrossRef CAS.
- C. Ceccarelli, Planet. Space Sci., 2002, 50, 1267 CrossRef CAS.
- E. Roueff and M. Gerin, Space Sci. Rev., 2003, 106, 61 CrossRef CAS.
- B. Parise, C. Ceccarelli, A. G. G. M. Tielens, E. Herbst, B. Lefloch, E. Caux, A. Castets, I. Mukhopadhyay, L. Pagani and L. Loinard, Astron. Astrophys., 2002, 393, L49 CrossRef CAS.
- P. Bergman, B. Parise, R. Liseau and B. Larsson, Astron. Astrophys., 2011, 527, 39 CrossRef.
- A. Bauder and H. H. Günthard, J. Mol. Spectrosc., 1976, 60, 290 CrossRef CAS.
- W. Gilmore, M. Morris, D. R. Johnson, F. J. Lovas, B. Zuckerman and B. E. Turner, Astrophys. J., 1976, 204, 43 CrossRef CAS.
- J. K. Jørgensen, H. S. P. Müller, H. Calcutt, A. Coutens, M. N. Drozdovskaya, K. I. Öberg, M. V. Persson, V. Taquet, E. F. van Dishoeck and S. F. Wampfler, Astron. Astrophys., 2018, 620, A170 CrossRef.
- J. Ferrer Asensio, S. Spezzano, L. H. Coudert, V. Lattanzi, C. P. Endres, J. K. Jørgensen and P. Caselli, Astron. Astrophys., 2023, 670, A177 CrossRef.
- H. S. P. Müller, J. K. Jorgensen, J.-C. Guillemin, F. Lewen and S. Schlemmer, J. Mol. Spectrosc., 2023, 394, 111777 CrossRef.
- A. Amrein, H. Hollenstein, M. Quack, R. Zenobi, J. Segall and R. N. Zare, J. Chem. Phys., 1989, 90, 3944 CrossRef CAS.
- M. Quack and J. Stohner, J. Phys. Chem., 1993, 97, 12574 CrossRef CAS.
- T. K. Ha, M. Quack and J. Stohner, Mol. Phys., 2002, 100, 1797 CrossRef CAS.
- G. Cunningham Jr., A. W. Boyd, R. J. Myers, W. D. Gwinn and W. I. Le Van, J. Chem. Phys., 1951, 19, 676 CrossRef.
- R. A. Creswell and R. H. Schwendeman, Chem. Phys. Lett., 1974, 27, 521 CrossRef CAS.
- L. Pan, S. Albert, K. V. L. N. Sastry, E. Herbst and F. C. De Lucia, Astrophys. J., 1998, 499, 517 CrossRef.
- D. T. Petkie, T. M. Goyette, R. P. A. Bettens, S. P. Belov, S. Albert, P. Helminger and F. C. De Lucia, Rev. Sci. Instrum., 1997, 68, 1675 CrossRef CAS.
- C. Medcraft, C. D. Thompson, E. G. Robertson, D. R. T. Appadoo and D. McNaughton, Astrophys. J., 2012, 753, 18 CrossRef.
- S. Albert, K. Keppler Albert and M. Quack, in Proc. 22nd Colloquium on High Resolution Molecular Spectroscopy, Dijon, France 29 August – 2 September, 2011, H12, The high resolution FTIR spectrum of oxirane (ethyleneoxide, C2H4O): Rovibrational analysis of the A1 fundamental ν5.
- D. K. Russel and R. Wesendrup, J. Mol. Spectrosc., 2003, 217, 59 CrossRef.
- F. Kwabia Tchana, J.-M. Flaud, W. J. Lafferty and M. Ngom, Mol. Phys., 2014, 112, 1633 CrossRef CAS.
- J.-M. Flaud, W. J. Lafferty, F. Kwabia Tchana, A. Perrin and X. Landsheere, J. Mol. Spectrosc., 2012, 271, 38 CrossRef CAS.
- W. J. Lafferty, J. M. Flaud, F. Kwabia Tchana and J. M. Fernandez, Mol. Phys., 2013, 111, 1983 CrossRef CAS.
- F. Kwabia Tchana, M. Ngom, A. Perrin, J.-M. Flaud, W. J. Lafferty, S. A. Ndiaye and El. A. Ngom, J. Mol. Spectrosc., 2013, 292, 1 CrossRef CAS.
- C. Puzzarini, M. Biczysko, J. Bloino and V. Barone, Astrophys. J., 2014, 785, 107 CrossRef.
- Z. Chen, S. Albert, K. Keppler, M. Quack, V. Schurig and O. Trapp, Proc. 2021 Int. Symposium on Molecular Spectroscopy, Urbana, IL, USA, paper WJ01, 2021.
- Z. Chen, S. Albert, K. Keppler, M. Quack, V. Schurig and O. Trapp, International Workshop on Molecular Quantum Dynamics and Kinetics “Molecules in Motion” MOLIM2018, p35 – Academy of Athens, Athens, Greece, October 8 – 10, 2018, ed. F. Merkt, M. Quack, I. Thanopulos and C. G. Vayenas, Athens, 2018 (PCCP Prize paper) Search PubMed.
- P. P. Nicholas and R. T. Carroll, J. Org. Chem., 1968, 33, 2345 CrossRef CAS.
- W. Traube and W. Passarge, Chem. Ber., 1916, 49, 1692 CrossRef CAS.
- C. P. Casey and L. J. Smith, Organometallics, 1989, 8, 2288 CrossRef CAS.
- M. Suter and M. Quack, Appl. Opt., 2015, 54, 4417 CrossRef CAS PubMed.
- S. Albert, I. Bolotova, Z. Chen, C. Fabri, L. Horny, M. Quack, G. Seyfang and D. Zindel, Phys. Chem. Chem. Phys., 2016, 18, 21976 RSC.
- S. Albert, Z. Chen, C. Fabri, P. Lerch, R. Prentner and M. Quack, Mol. Phys., 2016, 114, 2751–2768 CrossRef CAS.
- C. M. Western, PGOPHER version 9.1, University of Bristol Research Data Repository, 2016 DOI:10.5523/bris.1nz94wvrfzzdo1d67et0t4v4nc.
- C. M. Western, Introduction to Modeling High-resolution Spectra, in Handbook of High Resolution Spectroscopy, ed. M. Quack and F. Merkt, Wiley, John Wiley & Sons, Ltd, 2011, vol. 3, ch. 1, pp. 1415–1435 Search PubMed.
- S. Albert, K. Keppler Albert, P. Lerch and M. Quack, Faraday Discuss., 2011, vol. 150, p. 71 Search PubMed.
- S. Albert, K. Keppler Albert and M. Quack, High Resolution Fourier Transform Infrared Spectroscopy, in Handbook of High Resolution Spectroscopy, ed. M. Quack and F. Merkt, Wiley, Ltd, 2011, vol. 2, ch. 26, pp. 965–1019 Search PubMed.
- S. Albert, K. Keppler, P. Lerch, M. Quack and A. Wokaun, J. Mol. Spectrosc., 2015, 315, 92 CrossRef CAS.
- L. S. Rothman, I. E. Gordon, A. Barbe, D. Chris Benner, P. F. Bernath, M. Birk, V. Boudon, L. R. Brown, A. Campargue, J.-P. Champion, K. Chance, L. H. Coudert, V. Danaj, V. M. Devi, S. Fally, J.-M. Flaud, R. R. Gamache, A. Goldmanm, D. Jacquemart, I. Kleiner, N. Lacome, W. J. Lafferty, J.-Y. Mandin, S. T. Massie, S. N. Mikhailenko, C. E. Miller, N. Moazzen-Ahmadi, O. V. Naumenko, A. V. Nikitin, J. Orphal, V. I. Perevalov, A. Perrin, A. Predoi-Cross, C. P. Rinsland, M. Rotger, M. Šimecková, M. A. H. Smith, K. Sung, S. A. Tashkun, J. Tennyson, R. A. Toth, A. C. Vandaele and J. Vander Auwera, J. Quant. Spectrosc. Radiat. Transfer, 2009, 110, 533 CrossRef CAS.
- J. K. G. Watson, Aspects of quartic and sextic centrifugal effects on rotational energy levels, in Vibrational Spectra and Structure, ed. J. Durig, Elsevier, Amsterdam, 1977, vol. 6, pp. 1–89 Search PubMed.
- A. Bauder, Fundamentals of Rotational Spectroscopy, in Handbook of High Resolution Spectroscopy, ed. M. Quack and F. Merkt, Wiley, John Wiley & Sons, Ltd, 2011, vol. 1, ch. 2, pp. 57–116 Search PubMed.
- S. Albert, K. Keppler Albert, H. Hollenstein, C. Manca Tanner and M. Quack, Fundamentals of Rotation-Vibration Spectra, in Handbok of High Resolution Spectroscopy, ed. M. Quack and F. Merkt, Wiley & Sons, Chichester, 2011, vol. 2, ch. 3, pp. 117–173 Search PubMed.
- D. Luckhaus and M. Quack, Mol. Phys., 1989, 68, 745 CrossRef CAS.
- H. M. Pickett, J. Mol. Spectrosc., 1991, 148, 371 CrossRef CAS.
- G. Wichmann, S. Albert, P. Lerch, K. Keppler and M. Quack, Mol. Phys., 2025, e2593929, DOI:10.1080/00268976.2025.2593929.
- M. Quack, J. Chem. Phys., 1985, 82, 3277 CrossRef CAS.
- G. Wichmann, G. Seyfang and M. Quack, Mol. Phys., 2021, 119, e1959073 CrossRef.
- F. W. Loomis and R. W. Wood, Phys. Rev., 1928, 32, 223 CrossRef CAS.
- B. P. Winnewisser, J. Reinstädtler, K. M. T. Yamada and J. Behrend, J. Mol. Spectrosc., 1989, 136, 12–16 CrossRef CAS.
- S. Albert, P. Lerch, K. Keppler and M. Quack, THz Spectroscopy of cyano-oxirane (c-C2H3OCN) and methyl oxirane (c-C2H3OCH3) with synchrotron light, in Proc. 20th Symposium on Atomic, Cluster and Surface Physics 2016 (SASP 2016), Davos, Switzerland, 7 to 12 February 2016”, pp. 165–168, ed. J. Stohner and Ch. Yeretzian, Innsbruck University Press (IUP), Innsbruck, 2016.
- M. Quack, Bunsen-Mag., 2022, 24, 238 Search PubMed.
- J. Pochert and M. Quack, Mol. Phys., 1998, 95, 1055 CrossRef CAS.
- A. Kushnarenko, V. Krylov, E. Miloglyadov, M. Quack and G. Seyfang, Intramolecular Vibrational Energy Redistribution Measured by Femtosecond Pump–Probe Experiments in a Hollow Waveguide, in “Ultrafast Phenomena XVI”, Proc. 16th Int. Conf. on Ultrafast Phenomena, Stresa, Italia, June 2008, pages. 349–351 (2009), ed. P. Corkum, S. De Silvestri, K. Nelson, E. Riedle and R. Schoenlein, Springer Series in Chemical Physics, Berlin, 2009.
- A. Kushnarenko, E. Miloglyadov, M. Quack and G. Seyfang, Phys. Chem. Chem. Phys., 2018, 20, 10949 RSC.
- R. Marquardt and M. Quack, Z. Phys. D:At., Mol. Clusters, 1996, 36, 229 CrossRef CAS.
- P. Jakobsen, P. Ferruit, C. Alves de Oliveira, S. Arribas, G. Bagnasco, R. Barho, T. L. Beck, S. Birkmann, T. Böker, A. J. Bunker, S. Charlot, P. de Jong, G. de Marchi, R. Ehrenwinkler, M. Falcolini, R. Fels, M. Franx, D. Franz, M. Funke, G. Giardino, X. Gnata, W. Holota, K. Honnen, P. L. Jensen, M. Jentsch, T. Johnson, D. Jollet, H. Karl, G. Kling, J. Köhler, M.-G. Kolm, N. Kumari, M. E. Lander, R. Lemke, M. López-Caniego, N. Lützgendorf, R. Maiolino, E. Manjavacas, A. Marston, M. Maschmann, R. Maurer, B. Messerschmidt, S. H. Moseley, P. Mosner, D. B. Mott, J. Muzerolle, N. Pirzkal, J.-F. Pittet, A. Plitzke, W. Posselt, B. Rapp, B. J. Rauscher, T. Rawle, H.-W. Rix, A. Rödel, P. Rumler, E. Sabbi, J.-C. Salvignol, T. Schmid, M. Sirianni, C. Smith, P. Strada, M. te Plate, J. Valenti, T. Wettemann, T. Wiehe, M. Wiesmayer, C. J. Willott, R. Wright, P. Zeidler and C. Zincke, Astron. Astrophys., 2022, 661, A80 CrossRef CAS.
- P. Ferruit, P. Jakobsen, G. Giardino, T. Rawle, C. Alves de Oliveira, S. Arribas, T. L. Beck, S. Birkmann, T. Böker, A. J. Bunker, S. Charlot, G. de Marchi, M. Franx, A. Henry, D. Karakla, S. A. Kassin, N. Kumari, M. López-Caniego, N. Lützgendorf, R. Maiolino, E. Manjavacas, A. Marston, S. H. Moseley, J. Muzerolle, N. Pirzkal, B. Rauscher, H.-W. Rix, E. Sabbi, M. Sirianni, M. te Plate, J. Valenti, C. J. Willott and P. Zeidler, Astron. Astrophys., 2022, 661, A81 CrossRef CAS.
- T. Böker, S. Arribas, N. Lützgendorf, C. Alves de Oliveira, T. L. Beck, S. Birkmann, A. J. Bunker, S. Charlot, G. de Marchi, P. Ferruit, G. Giardino, P. Jakobsen, N. Kumari, M. López-Caniego, R. Maiolino, E. Manjavacas, A. Marston, S. H. Moseley, J. Muzerolle, P. Ogle, N. Pirzkal, B. Rauscher, T. Rawle, H.-W. Rix, E. Sabbi, B. Sargent, M. Sirianni, M. te Plate, J. Valenti, C. J. Willott and P. Zeidler, Astron. Astrophys., 2022, 661, A82 CrossRef.
- S. M. Birkmann, P. Ferruit, G. Giardino, L. D. Nielsen, A. García Muñoz, S. Kendrew, B. J. Rauscher, T. L. Beck, C. Keyes, J. A. Valenti, P. Jakobsen, B. Dorner, C. Alves de Oliveira, S. Arribas, T. Böker, A. J. Bunker, S. Charlot, G. de Marchi, N. Kumari, M. López-Caniego, N. Lützgendorf, R. Maiolino, E. Manjavacas, A. Marston, S. H. Moseley, N. Pirzkal, C. Proffitt, T. Rawle, H.-W. Rix, M. te Plate, E. Sabbi, M. Sirianni, C. J. Willott and P. Zeidler, Astron. Astrophys., 2022, 661, A83 CrossRef CAS.
- A. Bakasov, T. K. Ha and M. Quack, J. Chem. Phys., 1998, 109, 7263 CrossRef CAS.
- B. Fehrensen, D. Luckhaus and M. Quack, Chem. Phys., 2007, 338, 90 CrossRef CAS.
- M. Gottselig, D. Luckhaus, M. Quack, J. Stohner and M. Willeke, Helv. Chim. Acta, 2001, 84, 1846 CrossRef CAS.
- R. Berger, M. Gottselig, M. Quack and M. Willeke, Angew. Chem., 2001, 113, 4342 CrossRef CAS; R. Berger, M. Gottselig, M. Quack and M. Willeke, Angew. Chem., Int. Ed., 2001, 40, 4195 CrossRef PubMed.
- H. Hollenstein, D. Luckhaus, J. Pochert, M. Quack and G. Seyfang, Angew. Chem., Int. Ed. Engl., 1997, 36, 140 CrossRef CAS.
- R. Berger, M. Quack and J. Stohner, Angew. Chem., Int. Ed., 2001, 40, 1667 CrossRef CAS.
- F. Hobi, R. Berger and J. Stohner, Mol. Phys., 2013, 111, 2345 CrossRef CAS.
- R. Berger, M. Quack and G. Tschumper, Helv. Chim. Acta, 2000, 83, 1919 CrossRef CAS.
- M. Quack, Chirality and Molecular Signatures of Life: Detecting and Communicating Life in the Universe, in 15th Brioni Conference on Deep Space Communication, Navigation and Propulsion, Brioni August 2022.
- M. Guélin and J. Cernicharo, Front. Astron. Space Sci., 2022, 9, 787567 CrossRef.
- J. Monod, Le Hasard et la Nécessité, Éditions du Seuil, Paris, 1970 Search PubMed.
- S. F. Mason, Chemical Evolution, Clarendon Press, Oxford, 1991 Search PubMed.
- M. Eigen, Steps towards life, Oxford University Press, Oxford, 1992 (German original: Stufen zum Leben, with Ruthild Winkler-Oswatitsch, translation by Paul Woolley) Search PubMed.
- J. Troe, Bunsen-Mag., 2016, 18, 228 Search PubMed (lecture on the occasion of receiving the Otto-Hahn-Preis, Paulskirche Frankfurt 24 November 2015).
| This journal is © the Owner Societies 2026 |