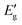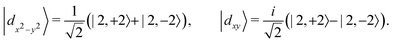Janus VAZ3H (A = Si, Ge; Z = N, P) single layers exhibiting valley polarization, magnetic anisotropy, and topological transition
Yang
Yang
 *ab,
Yanyang
Cao
ab,
Shao-Jie
Zhang
ab,
Luogang
Xie
*ab,
Yanyang
Cao
ab,
Shao-Jie
Zhang
ab,
Luogang
Xie
 ab and
Hongyan
Lu
ab and
Hongyan
Lu
 c
c
aSchool of Electronics and Information, Zhengzhou University of Light Industry, Zhengzhou, China. E-mail: yyphysics@zzuli.edu.cn
bHenan Key Laboratory of Information Functional Materials and Sensing Technology, Zhengzhou University of Light Industry, Zhengzhou, China
cSchool of Physics and Physical Engineering, Qufu Normal University, Qufu, China
First published on 27th November 2025
Abstract
Using first-principles calculations, we explore the electronic and topological properties of Janus VAZ3H single layers (A = Si, Ge; Z = N, P) that are dynamically and thermally stable. In the strain-free state, VSiN3H, VSiP3H, and VGeN3H demonstrate direct bandgap ferrovalley (FV) semiconducting properties, while VGeP3H displays an indirect bandgap. The easy magnetization axis varies among these materials, with VSiN3H and VGeN3H preferring in-plane magnetization, whereas VSiP3H and VGeP3H favor out-of-plane magnetization. Furthermore, the electronic structure analysis reveals valley polarization at the K and K′ points. When subjected to strain, these systems experience phase transitions, such as direct-to-indirect bandgap shift, the evolution from FV semiconducting to half-valley metal (HVM), and the emergence of a quantum anomalous Hall (QAH) phase within certain strain intervals. The QAH phase is identified by chiral edge states and quantized anomalous Hall conductivity (AHC), supported by an integer AHC plateau of 1e2/h and a Chern number of 1. These results highlight the tunability of VAZ3H SLs through strain engineering, providing a potential platform for valleytronic and topological applications.
1. Introduction
Recent progress in two-dimensional (2D) ferromagnetic materials and valley semiconductors has attracted significant attention for their potential in spintronic and valleytronic devices.1–5 These breakthroughs offer innovative avenues for controlling quantum properties and stimulate both theoretical research and practical applications.6–10 Notably, the emergence of ferrovalley (FV) materials represents a major milestone. By breaking time-reversal and spatial-inversion symmetries, FV materials naturally combine ferromagnetism with spontaneous valley polarization.11 By stabilizing valley characteristics through magnetic ordering, this unique combination facilitates efficient valley manipulation and opens avenues for investigating exotic quantum effects such as the anomalous valley Hall effect and the valley-polarized quantum anomalous Hall (QAH) effect.12–14 Consequently, FV materials present promising opportunities for developing robust, scalable valleytronic devices in next-generation quantum technologies.With advancements in 2D ferromagnetic and valley semiconductors, the exploration of FV materials has attracted growing attention. Notable examples include 2H-VSe2,11 LaBr2,15 2H-GdI2,16 Nb3I8,17 TiVI6,18 NbX2 (X = S, Se),19 2H-FeCl2,20 Cr2Se3,21 and VAgP2Se6.22 In addition, the development of Janus structures, which break inversion symmetry and provide additional degrees of freedom for tuning electronic and magnetic properties, has further expanded the FV material family. Compositional and structural innovations have led to promising candidates such as 2H-VSSe,23,24 VClBr,25 LaBrI,26 and GdClF.27 Despite progress, new FV materials remain essential. Existing compounds are promising but limited by stability, tunability, or integration. Novel compositions and structures are needed to enhance performance and uncover quantum phenomena from magnetic ordering and valley polarization.
Recent investigations into VA2Z4 (A = Si, Ge; Z = N, P) single layers (SLs) have shown that these materials are promising for valleytronic applications. Specifically, VSi2N4 is a ferromagnetic semiconductor with valley-specific physics and strong spin-valley coupling,28 and VSi2P4 displays correlation-driven magnetic, topological, and valley features.29,30 VGe2P4, meanwhile, exhibits quasi-half-valley metal behavior that facilitates electron–hole separation via tunable valley polarization.31 Motivated by these discoveries, we aim to explore innovative strategies, including the design of Janus structures, to further optimize the valley and topological functionalities of 2D materials.
Here, by performing first-principles calculations, we identify Janus SL VAZ3H (A = Si, Ge; Z = N, P) as 2D FV semiconductors. Our results indicate that VSiN3H, VSiP3H, and VGeN3H exhibit direct bandgap semiconducting properties, while VGeP3H is an indirect bandgap semiconductor. The easy magnetization axis differs among these materials, with VSiN3H and VGeN3H favoring in-plane magnetization, while VSiP3H and VGeP3H prefer out-of-plane magnetization. Owing to their intrinsic ferromagnetism (FM) and spin–orbit coupling (SOC), spontaneous valley polarization emerges at the K and K′ points. By applying strain, phase transitions occur from a FV state to a half-valley metal (HVM), and the QAH phase emerges within specific strain intervals, featuring chiral edge states and quantized Hall conductivity. These findings highlight the potential of SL VAZ3H materials for next-generation spintronic and valleytronic technologies.
2. Computational details
The calculations are performed using the Vienna Ab initio Simulation Package (VASP), based on density functional theory (DFT).32 Exchange–correlation interactions are treated with the generalized gradient approximation (GGA) using the Perdew–Burke–Ernzerhof (PBE) functional, and the projector augmented-wave (PAW) method is utilized to model the core-electron interactions.33 A plane-wave cutoff of 500 eV is employed, and the Brillouin zone is sampled within a 13 × 13 × 1 Monkhorst–Pack k-point mesh, ensuring convergence of the total energy within 1 meV per atom. Structural optimization continues until atomic forces are below 0.01 eV Å−1, and the total energy difference is smaller than 10−6 eV. To capture magnetic effects, spin-polarized calculations are employed, with SOC included as appropriate. The V atom is modeled with an effective Hubbard Ueff of 3.0 eV, consistent with previous works.14,29,34,35 To ensure accuracy in bandgap calculations, the HSE06 hybrid functional36 is tested, and van der Waals interactions are accounted for using the DFT-D3 method by Grimme. A vacuum of at least 20 Å is added to avoid spurious interactions between periodic images.To assess thermal stability, ab initio molecular dynamics (AIMD) simulations are carried out using a 4 × 4 × 1 supercell and a Nosé thermostat at 500 K,37 running for 6 ps with a time step of 1.5 fs in the canonical (NVT) ensemble. Additionally, density functional perturbation theory (DFPT) is employed to calculate the phonon dispersion, which helps assess the dynamical stability of the material.38 The Berry curvature is calculated using the maximally localized Wannier functions in Wannier90,39,40 while the edge states and Chern number are extracted and analyzed with the WannierTools package.41
To ensure reproducibility of the topological analysis, detailed computational settings are provided. The maximally localized Wannier functions (MLWFs) are constructed from the VASP wavefunctions using Wannier90, with the V-3d orbitals and Z-site p orbitals chosen as the initial projections. A total of 28 Wannier functions are generated. The disentanglement energy window ranged from −7 eV to 10 eV relative to the Fermi level, with a frozen window between −0.5 eV and +0.5 eV, ensuring accurate reproduction of the low-energy bands. The resulting MLWF Hamiltonian is imported into WannierTools for topological analysis. The Chern number is obtained by integrating the Berry curvature over the Brillouin zone using a 200 × 200 × 1 k-mesh, while the anomalous Hall conductivity is computed from the Berry curvature distribution via the Kubo formalism.
3. Results and discussion
As shown in Fig. 1, the crystal structure of the Janus VAZ3H SLs exhibits hexagonal lattice symmetry, with asymmetry introduced by substituting one Z layer with hydrogen atoms. This modification results in a six-layer atomic configuration, Z–A–Z–V–Z–H, while maintaining the overall hexagonal shape. This tailored structure not only optimizes the atomic arrangement but also fine-tunes the interlayer spacing, leading to a non-uniform electron density distribution at the interfaces. Although the asymmetry alters the properties of the material, the high-symmetry characteristics are still preserved, as shown in the Brillouin zone.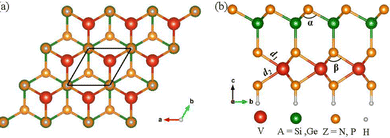 | ||
| Fig. 1 Side (a) and top (b) views of the crystal structures of SL VAZ3H (A = Si, Ge; Z = N, P), with dark solid lines marking the primitive unit cell. | ||
The structural parameters of VAZ3H SLs exhibit a systematic trend influenced by the constituent elements, as summarized in Table 1. The lattice constant (a) and layer thickness (h) increase when Si is replaced by Ge or N by P, consistent with the larger atomic radii of these elements. For example, VSiN3H has the smallest lattice constant of 2.89 Å, while VGeP3H has the largest at 3.53 Å. Similarly, the layer thickness increases from 5.60 Å in VSiN3H to 7.54 Å in VGeP3H. The bond lengths also follow a similar trend, with V–N and V–Si bonds being shorter than V–P and V–Ge bonds. For instance, in VSiN3H, the V–N bond length is 2.03 Å, whereas in VGeP3H, the V–P bond length increases to 2.44 Å. Furthermore, the bond angles α vary across different compositions, with α decreasing from 111.44° in VSiN3H to 99.17° in VGeP3H. In contrast, the β angle remains nearly constant across all compositions, fluctuating slightly between 90.70° and 93.57°.
 , with SOC within PBE (Eg), and with SOC within the HSE functional (EHSEg), valley polarization for the CBM (Cpol) and VBM (Vpol), and Berry curvature values at K and K′ (Ω(K), Ω (K′)
, with SOC within PBE (Eg), and with SOC within the HSE functional (EHSEg), valley polarization for the CBM (Cpol) and VBM (Vpol), and Berry curvature values at K and K′ (Ω(K), Ω (K′)
| a | h | d 1 | d 2 | α | β | C 11 | C 12 | ΔEAF | E MAE | E g | E HSEg | C pol/Vpol | Ω (K) | Ω (K′) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Å) | (Å) | (Å) | (Å) | (°) | (°) | (N m−1) | (N m−1) | (meV) | (μ eV) | (eV) | (eV) | (meV) | (Bohr2) | (Bohr2) | ||
| VSiN3H | 2.89 | 5.60 | 2.030 | 2.025 | 111.44 | 90.70 | 337.89 | 103.41 | 87.7 | −25.43 | 0.30 | 0.27 | 0.34 | −1.5/65.3 | −367.9 | 589.6 |
| VSiP3H | 3.46 | 7.41 | 2.412 | 2.421 | 100.94 | 91.72 | 126.86 | 34.66 | 30.9 | 4.20 | 0.22 | 0.17 | 0.58 | 85.5/−2.1 | 681.9 | −275.4 |
| VGeN3H | 2.98 | 5.82 | 2.057 | 2.056 | 108.15 | 92.58 | 289.62 | 96.77 | 59.0 | −2.01 | 0.17 | 0.13 | 0.62 | 68.4/−1.4 | 2164.6 | −907.4 |
| VGeP3H | 3.53 | 7.54 | 2.423 | 2.442 | 99.17 | 93.57 | 125.10 | 34.28 | 21.5 | 6.73 | 0.48 | 0.15 | 0.62 | 77.9/−1.6 | 50.3 | −32.2 |
The stability of VAZ3H structures is thoroughly evaluated through mechanical, thermal, and dynamical assessments. Since SL VGeZ3H yields results similar to SL VSiZ3 H, only the latter is presented in Fig. 2 for brevity. Mechanical stability is confirmed by the elastic constants in Table 1, which satisfy the Born–Huang criterion (C11 > |C12|), confirming the mechanical stability of the structure. Thermal stability is verified through AIMD simulations shown in Fig. 2(a) and (b), where the atomic structure remains intact for 6 ps at 500 K without significant distortion. Additionally, the phonon dispersion in Fig. 2(c) and (d) reveals no imaginary frequencies throughout the Brillouin zone, confirming dynamical stability and a well-maintained atomic configuration. These findings collectively support the structural stability of VAZ3H SLs, demonstrating their viability for experimental synthesis, much like the previously realized MoSi2N4 and MoAZ3H, as highlighted in ref. 42–44.
To further validate the thermal stability, additional AIMD simulations were performed for VSiN3H using a larger 5 × 5 × 1 supercell and a longer simulation time of 8 ps. The results show that the atomic structure remains intact, with no bond breaking or collapse, consistent with the original 6 ps simulations of the smaller supercell. These extended simulations confirm that VSiN3H can withstand finite-temperature fluctuations, providing additional theoretical support for the potential experimental realization of VAZ3H single layers. Based on the calculated stability, potential synthesis routes may include chemical vapor deposition (CVD) using suitable precursors for V, A-site, and Z-site elements, or exfoliation from bulk materials followed by surface functionalization to achieve the Janus structure. We emphasize that more extensive investigations, such as longer AIMD simulations, larger supercells, and thermodynamic convex hull analyses, will be necessary to fully assess the experimental synthesizability of these materials.
The electron localization function (ELF) analysis indicates that VAZ3H single layers exhibit similar bonding characteristics across different compositions. For clarity, the ELF distribution of VSiN3H is shown in Fig. 3(a). The V–N and Si–N bonds display strong covalent bonding with significant electron sharing between the atoms. The introduction of a hydrogen atom in the bottom layer slightly alters the electron distribution around the Si–N bonds, reflecting a subtle influence on the local electronic structure. Furthermore, the V–N bond shows partial ionic character due to the electronegativity difference between V and N, as evidenced by the lower ELF values. Owing to the Janus structure, bonding on both sides of the V atoms in VAZ3H single layers exhibits directional asymmetry, which in turn affects their chemical and electronic properties.
To gain deeper insight into the chemical origin of the bonding characteristics in VAZ3H single layers, Bader charge analysis was carried out for representative systems. In VSiN3H, the analysis reveals a complex redistribution of electrons among multiple atomic species. Each N atom gains approximately 0.9–1.7e, reflecting their high electronegativity and significant electron accumulation. Meanwhile, the Si atom also loses a substantial amount of charge, and V partially donates electrons as well, indicating that electron transfer is not solely from V to N but involves a collective redistribution among V, Si, and N atoms. This finding is consistent with the ELF analysis, where both V–N and Si–N bonds exhibit strong covalent character with pronounced electron sharing, while the Janus structure introduces directional asymmetry in bonding on either side of the V atoms. When N is replaced by P, the electron gain at the anion sites decreases to roughly 0.4e per P atom, indicating weaker covalency and a more localized electron distribution. In contrast, substituting Si with Ge slightly enhances electron accumulation on the A-site atom and moderately affects the V–Z hybridization. Overall, these results demonstrate that substitutions at both Z and A sites influence the charge redistribution and bonding characteristics in VAZ3H single layers, thereby shaping their overall electronic structure.
We now proceed to the magnetic properties of VAZ3H SLs. Since the V–X–V bond angle (β) in all VAZ3H SLs is close to 90°, it facilitates FM coupling according to the Goodenough–Kanamori–Anderson (GKA) rules.45–47 Using a 2 × 2 × 1 supercell, we investigated the magnetic properties through nonmagnetic (NM), FM, and stripy antiferromagnetic (AFM) configurations. The energy difference ΔEAF is defined as the energy of the AFM configuration minus that of the FM state. As seen in Table 1, ΔEAF decreases progressively as the β angle deviates further from 90°, indicating a gradual reduction in the energy difference between the FM and AFM states, with the FM state remaining the most stable configuration. For instance, in VSiN3H, the FM state is 347.8 meV lower in energy than the NM state and 87.7 meV lower than the stripy AFM state. The calculated magnetic moments, primarily localized on the V atoms, are approximately 1.0µB, reflecting the characteristic d1 electronic configuration of vanadium among these SLs.
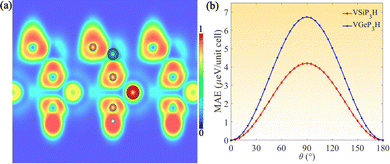
In line with the Mermin–Wagner theorem, a nonzero magnetic anisotropy energy (MAE) is crucial for sustaining long-range ferromagnetic ordering in two-dimensional magnets by counteracting the thermal agitation of magnons. To quantify this effect, the MAE is evaluated as a function of the polar angle θ with respect to the out-of-plane [001] axis,
| MAE = E(θ) − Emin, | (1) |
Having established the magnetic ground state, it is important to investigate the electronic properties of SL VAZ3H. As the HSE06 hybrid functional results exhibit the same overall trend as those obtained from PBE, the main difference lies in the magnitude of the band gaps (as shown in Table 1). This deviation mainly arises from the different treatments of exchange–correlation effects, where the inclusion of a fraction of exact exchange in HSE06 partially corrects the self-interaction error and leads to a larger band gap compared with PBE. Nevertheless, both methods yield consistent qualitative electronic features, so the PBE results are adopted for subsequent analysis due to their computational efficiency.
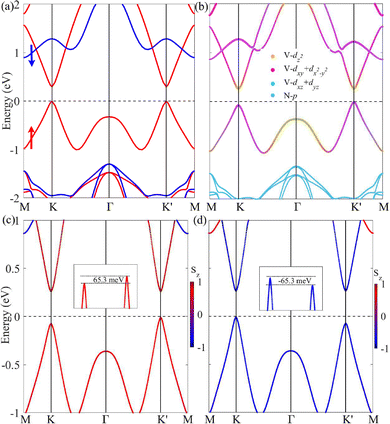
As shown in Fig. 4(a), VSiN3H possesses magnetic semiconducting properties, where the VBM and CBM are primarily composed of spin-up states. In the absence of SOC, the material exhibits a direct band gap of approximately 0.30 eV, while the band formed by the spin-down states (shown by the blue lines) has a band gap of around 2.20 eV. Remarkably, the K and K′ points in the Brillouin zone host both the VBM and CBM, creating two well-defined valleys.
Furthermore, the incorporation of SOC along with the intrinsic FM of the material leads to the spontaneous valley polarization in SL VSiN3H, as shown in Fig. 4(b). The energy difference between K and K′ in the valence and conduction bands can be defined as Vpol = EK′ − EK and Cpol = EK′ − EK, where EK′ and EK are the energies at the K′ and K points, respectively. Fig. 4(c) shows that VSiN3H exhibits a valley polarization (Vpol) of 65.3 meV in the valence band, while the valley polarization of CBM (Cpol) is negligible at −1.5 meV. By reversing the magnetic moment, the valley polarization at the K and K′ points is also inverted, leading to the K valley in the VBM having a higher energy compared to the K′ valley, as demonstrated in Fig. 4(d).
The discrepancy in valley polarization between the VBM and CBM is largely due to their differing orbital contributions and the effects of SOC. As shown in Fig. 4(b), for SL VSiN3H, the dx2−y2/dxy orbitals of V dominate the valleys in the valence band, while the dz2 orbital is the main contributor to the conduction band. The SOC Hamiltonian is here approximated by its axial component under the assumption of collinear spin polarization along the z′ direction,
ĤSOC ⋍ λŜz′![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z z |
![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z in the first-order approximation. The SOC-induced energy shift of a given state is therefore determined by its orbital magnetic quantum number m = 〈
z in the first-order approximation. The SOC-induced energy shift of a given state is therefore determined by its orbital magnetic quantum number m = 〈![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z〉.
z〉.
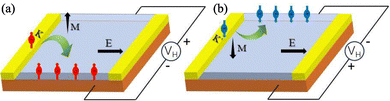
Although the real-space lobes of dxy and dx2−y2 differ, these two functions can be expressed as linear combinations of the spherical harmonics |L = 2, m = ±2〉,
The valley Bloch states at K and K′ are well approximated by chiral combinations that project onto |2, ∓2〉, hence they carry m = ∓2 and couple to ![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z with strength ±2. In this picture the leading SOC contribution to the valley splitting is
z with strength ±2. In this picture the leading SOC contribution to the valley splitting is
| ΔE = EK′ − EK = 4a, |
i.e. four times the effective coupling a. By contrast, the dz2 component corresponds to m = 0 and has negligible first-order shift under ![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z, which explains the much smaller SOC-induced splitting in the CBM.
z, which explains the much smaller SOC-induced splitting in the CBM.
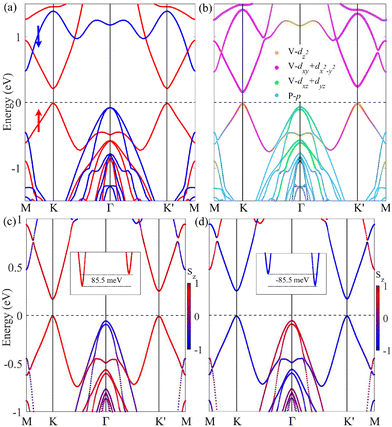
Building upon this understanding, a similar trend is observed in SL VSiP3H. As illustrated in Fig. 5, although SL VSiP3H retains a band structure similar to VSiN3H, its bandgap is significantly reduced. Moreover, the shifts in orbital contributions between the VBM and CBM lead to distinct valley polarization behaviors. At the CBM, the orbital contribution is primarily from the dx2−y2/dxy orbitals, which results in a pronounced valley polarization of approximately 85.5 meV. In contrast, the VBM is mainly influenced by the dz2 orbital, where valley polarization is negligible. This trend extends to other VAZ3H SLs, which share a comparable band structure with slight variations, as shown in Fig. 6. VGeN3H shares strong similarities with VSiN3H but features a further reduced bandgap of 0.46 eV and a CBM valley polarization of 68.4 meV. While VGeP3H preserves the CBM at the K/K′ valleys with a polarization of 77.9 meV, its VBM transitions to the Γ point, demonstrating an indirect bandgap feature.
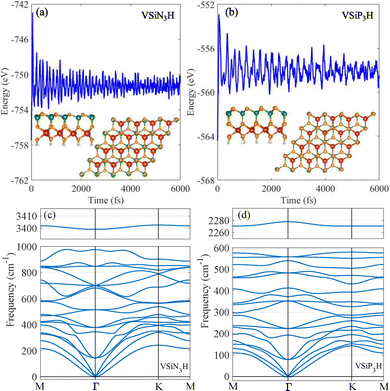
After recognizing the valley polarization in VAZ3H SLs, we further explore their valley-specific phenomena by calculating and analyzing the Berry curvature, with the results displayed in Fig. 7. As illustrated in Fig. 7(a), the Berry curvature exhibits two pronounced peaks of opposite signs at the K and K′ valleys, reflecting the broken inversion symmetry. For VSiN3H, the Berry curvature is −367.9 Bohr2 at the K point and 589.6 Bohr2 at K′. Meanwhile, in VSiP3H, a sign reversal occurs, shifting the valley contribution from the valence to the conduction band, with Berry curvature values of 681.9 Bohr2 at K and −275.4 Bohr2 at K′. Similar patterns are observed in VGeN3H and VGeP3H, with the values of their Berry curvature summarized in Table 1 for comparison.
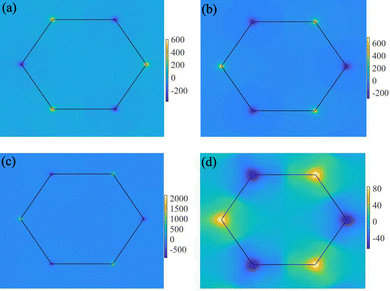 | ||
| Fig. 7 Contour map of Berry curvature for SL VAZ3H over the entire Brillouin zone. (a)–(d) display the corresponding results for SL VSiN3H, VSiP3H, VGeN3H, and VGeP3H, respectively. | ||
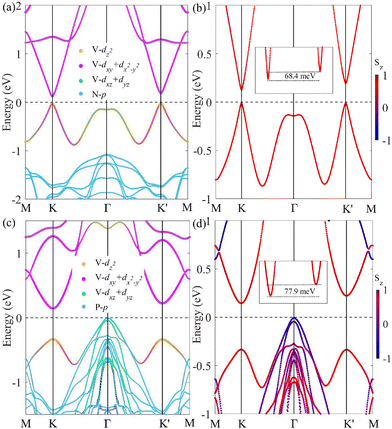
The nonzero Berry curvature (Ωz(k)) at the K and K′ valleys in VAZ3H generates an anomalous velocity v(k) in electronic carriers under an applied in-plane electric field E. This effect is described by the relation v(k) ∼ −E × Ωz(k).48 Due to valley polarization induced by intrinsic FM and SOC, carriers from different valleys exhibit distinct transport behaviors. Taking VSi3PH as an example, electron doping shifts the Fermi level between the K and K′ valleys in the conduction band, which induces an anomalous velocity in spin-up electrons from the K valley. Under an in-plane electric field, the electrons accumulate along the lower edge, giving rise to the anomalous valley Hall effect, as shown in Fig. 8(a). Upon magnetization reversal, as illustrated in Fig. 8(b), a comparable Fermi level shift directs spin-down electrons from the K′ valley to the upper edge. In addition to the anomalous valley Hall effect, this material features charge and spin Hall effects, enabling charge transport modulation via the valley degree of freedom. This highlights the potential of VAZ3H for valleytronic applications and their broader impact on spintronic technologies.
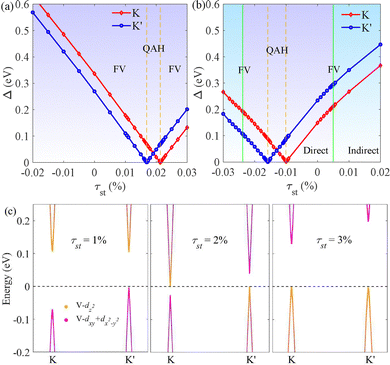
Strain engineering has emerged as a powerful tool for tuning electronic properties in 2D materials, enabling the modulation of band structures, transport characteristics, and even inducing novel quantum phases.49 To further understand how strain influences the valleytronic behavior and electronic structures of VAZ3H SLs, we focus on the effects of in-plane biaxial strain. The strain is defined as τst = (a − a0)/a0, where a and a0 are the lattice constants under strain and in the unstrained state, respectively. All four VAN3H SLs undergo a strain-induced evolution, beginning with the FV state and progressing through the HVM and QAH phases. As a result, we concentrate on VSiN3H and VSiP3H for a more detailed discussion.
The strain effects on SL VSiN3H are depicted in Fig. 9(a). As compressive strain increases, VSiN3H retains its FV state, with a gradual widening of the bandgap at the K and K′ valleys. When the strain reaches approximately −3.06%, the CBM shifts to the M point, leading to a transition into an indirect bandgap semiconductor while maintaining valley polarization at the VBM. Increasing tensile strain triggers a unique electronic transition. As tensile strain increases, the bandgaps at K and K′ decrease linearly. At 1.69% tensile strain, the bandgap at K′ closes while K retains a small gap, leading to HVM behavior. With further tensile strain, the gap at K′ reopens while the gap at K continues to shrink until it closes at 2.13%. Beyond this point, the bandgaps at both K and K′ gradually increase again, and the material returns to a direct bandgap FV semiconductor.
The energy band inversion occurs under strain, as is illustrated in Fig. 9(c). In the absence of strain or with a small strain of 1%, the VBM mainly originates from the dxy/dx2−y2 orbitals of the V atom, while the CBM originates from its dz2 orbital. During bandgap closure and reopening at K′, their orbital contributions switch. At 2% strain, the VBM and CBM at K are dominated by dxy + dx2−y2 and dz2 orbitals, respectively. As tensile strain increases like 3% strain, an energy band inversion occurs at K and K′, leading to a QAH phase in the 1.69–2.13% strain range, where SL VSiN3H exhibits a nontrivial topological phase.
The strain response of SL VSiP3H reveals some differences from that of VSiN3H. Under tensile strain, the bandgaps at K and K′ gradually increase. At a tensile strain of about 0.5%, the material transitions into an indirect bandgap semiconductor with the VBM shifting to the Γ point, while the valley of CBM remain preserved. Increasing compressive strain triggers the electronic transition. At −1.0% strain, the closure of the K-point bandgap results in a HVM state. As the strain intensifies beyond −1.0%, the K-point bandgap reopens, while K′ progressively narrows until it vanishes at −1.58%, defining the QAH phase. With further compression, the K′ bandgap reopens, and both valleys exhibit simultaneous bandgap expansion. By −2.38% strain, the CBM relocates to the M point, shifting the system to an indirect bandgap semiconductor.
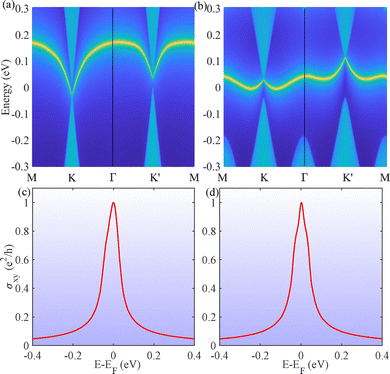
Both strained VSiN3H and VSiP3H demonstrate a chiral edge state in the QAH phase, with representative examples at τst = 1.9% for VSiN3H and τst = −1.3% for VSiP3H, as shown in Fig. 10(a) and (b) (highlighted in yellow). This edge state connects the valence and conduction bands, facilitating Bloch electron movement and resulting in Hall conductivity. Moreover, the quantized anomalous Hall conductivity σxy near the Fermi level can be obtained by integrating Ωz(k) over the first Brillouin zone, as described by 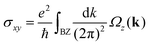 . Fig. 10(c) and (d) demonstrate that strained VSiN3H and VSiP3H display an integer AHC plateau of 1e2/h at the Fermi level, which aligns with a Chern number of 1, confirming their nontrivial QAH behavior.
. Fig. 10(c) and (d) demonstrate that strained VSiN3H and VSiP3H display an integer AHC plateau of 1e2/h at the Fermi level, which aligns with a Chern number of 1, confirming their nontrivial QAH behavior.
In addition to VSiN3H and VSiP3H, similar trends are observed in other VAZ3H SLs. For instance, VGeN3H and VGeP3H, while exhibiting distinct initial band structures, undergo phase transitions that closely resemble those of VSiP3H, highlighting the tunable nature of their electronic properties under strain. Under tensile strain, VGeN3H transitions into an indirect bandgap semiconductor beyond a critical strain while preserving its FV characteristics. Moreover, VGeP3H maintains FV characteristics in the CBM under tensile strain until it becomes metallic at 3%. Under compressive strain, the QAH phase emerges within −1.04% to −1.42% for VGeN3H and −4.11% to −3.82% for VGeP3H, during which the bandgaps at both K′ and K gradually shrink, close, and subsequently reopen. These findings highlight the tunability of the valleytronic and topological properties of VAZ3H through strain engineering.
4. Summary
This study explores the electronic and topological properties of VAZ3H SLs, including VSiN3H, VSiP3H, VGeN3H, and VGeP3H, with a focus on their valley properties and topological transitions under strain. Without strain, VSiN3H, VSiP3H, and VGeN3H exhibit direct bandgap FV semiconductor behavior, while VGeP3H displays an indirect bandgap. VSiN3H and VGeN3H have easy in-plane magnetization axis, whereas VSiP3H and VGeP3H prefer easy out-of-plane magnetization. The interplay between intrinsic FM and SOC in the d orbitals of the V atom leads to spontaneous valley polarization at the K and K′ points. This polarization is more evident at the VBM for VSiN3H and more apparent at the CBM for the other materials. Applying strain induces various phase transitions in these systems, including FV to HVM conversions, direct-to-indirect bandgap shifts, and the emergence of a QAH phase within specific strain intervals. In the QAH phase, chiral edge states connect the valence and conduction bands, resulting in quantum anomalous Hall conductivity, as confirmed by an integer AHC plateau of 1e2/h and a Chern number of 1. These findings underscore the combined role of intrinsic electronic properties and strain tuning in controlling the valleytronic and topological characteristics of VAZ3H SLs.Author contributions
Yang Yang and Yanyang Cao conceived the project and designed the research. Yanyang Cao and Shao-Jie Zhang performed the theoretical calculations and analyzed the results. Luogang Xie contributed to the interpretation of the findings and assisted in the literature review. Hong-Yan Lu supervised the project and provided critical insights into the theoretical models. Yang Yang wrote the manuscript with contributions from all authors. All authors reviewed and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. All relevant data, including computational details and numerical results, have been stored in a publicly accessible repository [if applicable, mention repository and accession details]. Additional information, such as input files for calculations and scripts used for analysis, can be provided upon request.Acknowledgements
The authors are grateful for support from the Natural Science Foundation of Henan Province (Grant No. 242300420265 and 252300421498), the Training Program for Young Backbone Teachers in Colleges and Universities of Henan Province (Grant No. 2024GGJS081), and the National Natural Science Foundation of China (Grant No. 12074213 and 12404088).References
- K. Ando, Science, 2006, 312, 1883–1885 CrossRef CAS PubMed.
- C. Huang, J. Feng, F. Wu, D. Ahmed, B. Huang, H. Xiang, K. Deng and E. Kan, J. Am. Chem. Soc., 2018, 140, 11519–11525 CrossRef CAS PubMed.
- A. Rycerz, J. Tworzydło and C. Beenakker, Nat. Phys., 2007, 3, 172–175 Search PubMed.
- J. R. Schaibley, H. Yu, G. Clark, P. Rivera, J. S. Ross, K. L. Seyler, W. Yao and X. Xu, Nat. Rev. Mater., 2016, 1, 1–15 Search PubMed.
- L. Tao and E. Y. Tsymbal, Phys. Rev. B, 2019, 100, 161110 CrossRef CAS.
- H.-Z. Lu, W. Yao, D. Xiao and S.-Q. Shen, Phys. Rev. Lett., 2013, 110, 016806 CrossRef PubMed.
- O. L. Sanchez, D. Ovchinnikov, S. Misra, A. Allain and A. Kis, Nano Lett., 2016, 16, 5792–5797 CrossRef CAS PubMed.
- W. Zhou, J. Chen, Z. Yang, J. Liu and F. Ouyang, Phys. Rev. B, 2019, 99, 075160 CrossRef.
- Z. Zhou, F. Yang, S. Wang, L. Wang, X. Wang, C. Wang, Y. Xie and Q. Liu, Front. Phys., 2022, 17, 1–14 Search PubMed.
- J. Wen, H. Wang, H. Chen, S. Deng and N. Xu, Chin. Phys. B, 2018, 27, 096101 CrossRef.
- W.-Y. Tong, S.-J. Gong, X. Wan and C.-G. Duan, Nat. Commun., 2016, 7, 13612 CrossRef CAS PubMed.
- H. Hu, W.-Y. Tong, Y.-H. Shen, X. Wan and C.-G. Duan, npj Comput. Mater., 2020, 6, 129 CrossRef CAS.
- H. Huan, Y. Xue, B. Zhao, G. Gao, H. Bao and Z. Yang, Phys. Rev. B, 2021, 104, 165427 CrossRef CAS.
- X. Zhou, R.-W. Zhang, Z. Zhang, W. Feng, Y. Mokrousov and Y. Yao, npj Comput. Mater., 2021, 7, 160 CrossRef.
- P. Zhao, Y. Ma, C. Lei, H. Wang, B. Huang and Y. Dai, Appl. Phys. Lett., 2019, 115, 261605 CrossRef.
- H.-X. Cheng, J. Zhou, W. Ji, Y.-N. Zhang and Y.-P. Feng, Phys. Rev. B, 2021, 103, 125121 CrossRef CAS.
- R. Peng, Y. Ma, X. Xu, Z. He, B. Huang and Y. Dai, Phys. Rev. B, 2020, 102, 035412 CrossRef CAS.
- W. Du, Y. Ma, R. Peng, H. Wang, B. Huang and Y. Dai, J. Mater. Chem. C, 2020, 8, 13220–13225 RSC.
- Y. Zang, Y. Ma, R. Peng, H. Wang, B. Huang and Y. Dai, Nano Res., 2021, 14, 834–839 CrossRef CAS.
- P. Zhao, Y. Dai, H. Wang, B. Huang and Y. Ma, ChemPhysMater, 2022, 1, 56–61 CrossRef CAS.
- Z. He, R. Peng, X. Feng, X. Xu, Y. Dai, B. Huang and Y. Ma, Phys. Rev. B, 2021, 104, 075105 CrossRef CAS.
- Z. Song, X. Sun, J. Zheng, F. Pan, Y. Hou, M.-H. Yung, J. Yang and J. Lu, Nanoscale, 2018, 10, 13986–13993 RSC.
- C. Zhang, Y. Nie, S. Sanvito and A. Du, Nano Lett., 2019, 19, 1366–1370 CrossRef CAS PubMed.
- C. Luo, X. Peng, J. Qu and J. Zhong, Phys. Rev. B, 2020, 101, 245416 CrossRef CAS.
- Y.-F. Zhao, Y.-H. Shen, H. Hu, W.-Y. Tong and C.-G. Duan, Phys. Rev. B, 2021, 103, 115124 CrossRef CAS.
- P. Jiang, L. Kang, Y.-L. Li, X. Zheng, Z. Zeng and S. Sanvito, Phys. Rev. B, 2021, 104, 035430 CrossRef CAS.
- S.-D. Guo, X.-S. Guo, X.-X. Cai and B.-G. Liu, Phys. Chem. Chem. Phys., 2022, 24, 715–723 RSC.
- Q. Cui, Y. Zhu, J. Liang, P. Cui and H. Yang, Phys. Rev. B, 2021, 103, 085421 CrossRef CAS.
- X. Feng, X. Xu, Z. He, R. Peng, Y. Dai, B. Huang and Y. Ma, Phys. Rev. B, 2021, 104, 075421 CrossRef CAS.
- S. Li, Q. Wang, C. Zhang, P. Guo and S. A. Yang, Phys. Rev. B, 2021, 104, 085149 CrossRef CAS.
- S.-D. Guo, Y.-L. Tao, H.-T. Guo, Z.-Y. Zhao, B. Wang, G. Wang and X. Wang, Phys. Rev. B, 2023, 107, 054414 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- P. Liu, S. Liu, M. Jia, H. Yin, G. Zhang, F. Ren, B. Wang and C. Liu, Appl. Phys. Lett., 2022, 121, 063103 CrossRef CAS.
- Y. Wang and Y. Ding, Appl. Phys. Lett., 2021, 119, 193101 CrossRef CAS.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- R. N. Barnett and U. Landman, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 2081 CrossRef CAS PubMed.
- S. Baroni, S. De Gironcoli, A. Dal Corso and P. Giannozzi, Rev. Mod. Phys., 2001, 73, 515 CrossRef CAS.
- A. A. Mostofi, J. R. Yates, Y.-S. Lee, I. Souza, D. Vanderbilt and N. Marzari, Comput. Phys. Commun., 2008, 178, 685–699 CrossRef CAS.
- A. A. Mostofi, J. R. Yates, G. Pizzi, Y.-S. Lee, I. Souza, D. Vanderbilt and N. Marzari, Comput. Phys. Commun., 2014, 185, 2309–2310 CrossRef CAS.
- Q. Wu, S. Zhang, H.-F. Song, M. Troyer and A. A. Soluyanov, Comput. Phys. Commun., 2018, 224, 405–416 CrossRef CAS.
- Y.-L. Hong, Z. Liu, L. Wang, T. Zhou, W. Ma, C. Xu, S. Feng, L. Chen, M.-L. Chen and D.-M. Sun, et al. , Science, 2020, 369, 670–674 CrossRef CAS PubMed.
- X. Cai, G. Chen, R. Li, W. Yu, X. Yang and Y. Jia, Phys. Chem. Chem. Phys., 2023, 25, 29594–29602 RSC.
- X. Cai, G. Chen, R. Li, Z. Pan and Y. Jia, J. Mater. Chem. C, 2024, 12, 4682–4689 RSC.
- J. B. Goodenough, Phys. Rev., 1955, 100, 564 CrossRef CAS.
- P. W. Anderson, Phys. Rev., 1959, 115, 2 CrossRef CAS.
- J. Kanamori, J. Appl. Phys., 1960, 31, S14–S23 CrossRef.
- D. Xiao, M.-C. Chang and Q. Niu, Rev. Mod. Phys., 2010, 82, 1959 CrossRef CAS.
- F. Guinea, M. I. Katsnelson and A. Geim, Nat. Phys., 2010, 6, 30–33 Search PubMed.
| This journal is © the Owner Societies 2026 |