Boosting alkaline hydrogen evolution via cobalt functionalization of organic–inorganic hybrid germanoniobate electrocatalysts
Xin-Rong
Jin
,
Jin-Yang
Li
,
Yong-Jiang
Wang
,
Yan-Qiong
Sun
 *,
Xin-Xiong
Li
*,
Xin-Xiong
Li
 and
Shou-Tian
Zheng
and
Shou-Tian
Zheng
 *
*
College of Chemistry, Fuzhou University, Fuzhou, Fujian 350108, China. E-mail: sunyq@fzu.edu.cn; stzheng@fzu.edu.cn
First published on 24th November 2025
Abstract
Two organic–inorganic hybrid germanoniobates with analogous supramolecular frameworks H3Na2(H2O)4[CoIII(en)3]6(CoIII(OH)2(H2O)2)[CoII(en)(H2O)Ge4(OH)2Nb16O54]2·32H2O ({Co9(Ge4Nb16)2}, en = ethylenediamine) and Na4(H2O)10(H2en)5 [Ge4(OH)2Nb16O54]·15H2O ({Ge4Nb16}), have been successfully built from {Ge4(OH)2Nb16O54} polyoxoanions functionalized with either the Co–amine complex or en organic ligand. Compound {Co9(Ge4Nb16)2} represents the first example of a germanoniobate incorporating both CoII and CoIII complexes. Both compounds form three-dimensional supramolecular structures with an unusual eight-connected hex-type topology. Electrochemical experiments demonstrate that the Co-containing {Co9(Ge4Nb16)2} exhibits significantly enhanced hydrogen evolution reaction (HER) activity under strongly alkaline conditions compared to the Co-free analogue {Ge4Nb16}. This finding highlights the crucial role of cobalt in promoting electrocatalytic performance. This work provides molecular-level insights into how deliberate structural modification of polyoxometalates (POMs) modulates their electrocatalytic HER activity.
Introduction
The global energy crisis has intensified the urgency of accelerating the transition to green energy. Renewable energy and associated energy storage technologies play a pivotal role in addressing these issues.1,2 Recent studies have indicated a rapid increase in the installed capacity of wind power and photovoltaic energy sources around the world, particularly in China. However, the inherent intermittency and volatility of these sources complicate grid stability, highlighting the demand for large-scale, long-duration energy storage solutions. In this context, hydrogen – particularly “green” hydrogen produced from renewable energy sources – is increasingly regarded as a strategic energy carrier for long-term energy storage and as a cross-sector clean fuel.3–5 However, to date, the vast majority of hydrogen is still derived from fossil fuels (“grey” hydrogen), a process that releases substantial CO2 and worsens climate change. Therefore, the advancement of green hydrogen technologies and reduction of their costs are essential to the realization of a sustainable energy future.Electrolysis of water is considered as one of the most promising pathways for green hydrogen production.6,7 However, the widespread adoption of this technology is hindered by the high cost of precious metal platinum group electrocatalysts.8–17 Therefore, the development of low-cost, high-performance non-precious metal catalysts is essential for large-scale commercialization.18–22 Among non-noble catalysts, cobalt (Co) has garnered considerable interest due to its intrinsic activity and natural abundance. It has been widely employed as an electrocatalyst for the hydrogen evolution reaction (HER) in the energy conversion and storage devices. However, the practical application of Co-based catalysts is often limited by their intrinsically low electrical conductivity and small specific surface area.23–27 Additionally, cobalt-based compounds are often water-soluble and exhibit poor stability in aqueous environments. In alkaline media, they are prone to hydrolysis and precipitation, which adversely affects their catalytic performance and durability during the HER.
Polyoxometalates (POMs) possess tunable chemical compositions and structures, enabling precise adjustment of their electrocatalytic properties.28–34 Polyoxoniobates (PONbs), an important subclass of POMs, exhibit promising electrocatalytic properties because they facilitate multi-electron transfer and exhibit intriguing electrochemical redox behaviour. Furthermore, they demonstrate broad pH stability and retain their structural integrity even under highly alkaline conditions.35–46 Keggin-type heteropolyoxoniobates (HPONbs) and their derivatives (containing Ge, P, Si, and Te) offer abundant vacant sites for coordinating transition metals, thereby boosting their electrocatalytic activity.47–56 For instance, the Zheng group synthesized five Keggin-type HPONb derivatives by incorporating different transition metals into the GeNb12 cluster. These compounds not only exhibit stable HER activity under highly alkaline conditions, but also serve as ideal models for probing the active sites and the impact of structural modification on the electrocatalytic HER at the atomic level.57 Similarly, Chen et al. reported two new organic–inorganic hybrid Co-containing HPONbs based on the [Ti2Nb8O28]8− anion.58 Among these, the Co3–Ti2Nb8 sample with higher Co content demonstrated excellent HER activity and exceptional durability in alkaline media. These findings confirm that incorporating catalytically active cobalt species into the HPONb structures is an effective strategy for fine-tuning their electronic and catalytic properties. The resulting Co-containing HPONb hybrids exhibit good stability, tunability and efficient HER performance in a strongly alkaline medium. Despite the growing interest in Co-containing HPONbs as HER catalysts, the electrocatalytic application of Co-functionalized germanoniobates remains unexplored.
Herein, we report the first cobalt-complex-functionalized germanoniobate H3Na2(H2O)4[CoIII(en)3]6(CoIII(OH)2(H2O)2)[CoII(en)(H2O)Ge4(OH)2Nb16O54]2·32H2O ({Co9(Ge4Nb16)2}, en = ethylene diamine). The structure is built from centrosymmetric dimers {CoIII(OH)2(H2O)2[CoII(en)(H2O)Ge4(OH)2Nb16O54]2} ({Co3Ge8Nb32}) and [CoIII(en)3] complexes. This compound represents the first example of germanoniobate integrating both CoII and CoIII complexes. Electrochemical experiments show that it has high electrocatalytic activity for the HER under alkaline conditions. To elucidate the role of cobalt as the active center, a cobalt-free analogue, Na4(H2O)10(H2en)5[Ge4(OH)2Nb16O54]·15H2O ({Ge4Nb16}) was synthesized with H2en as counterions. Compared to the high activity of the cobalt-containing {Co9(Ge4Nb16)2}, {Ge4Nb16} exhibits much lower electrocatalytic HER activity under the same conditions. This definitive result indicates that the cobalt centers are essential for the catalytic activity, thereby validating the strategy of grafting redox-active cobalt complexes onto the polyoxoniobate platform to generate efficient alkaline HER electrocatalysts.
Experimental section
Materials and methods
The K7HNb6O19·13H2O precursor was synthesized according to the reported literature method59 and confirmed by IR spectroscopy. All other chemicals were commercially obtained and used without further purification. The Na2CO3/NaHCO3 buffer solution (pH = 10.5) was prepared by dissolving Na2CO3 (5.96 g, 0.125 mol) and NaHCO3 (0.84 g, 0.01 mol) in 250.00 mL deionized water under constant stirring.Powder X-ray diffraction (PXRD) patterns were obtained on a Rigaku Ultima IV diffractometer using Cu-Kα radiation (λ = 1.54056 Å). FT-IR spectra were recorded from KBr pellets on a Nicolet IS 50 FT-IR spectrometer in the range 400–4000 cm−1. Thermogravimetric analyses (TGA) were carried out on a Mettler Toledo TGA/SDTA 851e analyzer under an N2-flow atmosphere with a heating rate of 10 °C min−1 from 30 to 800 °C. Ultraviolet-visible (UV-vis) spectra were measured on a SHIMADZU UV-2600 UV-visible spectrophotometer from 200 to 800 nm using BaSO4 as a blank. X-ray photoelectron spectroscopy (XPS) was conducted with a ThermoFisher ESCALAB250 X-ray photoelectron spectrometer using Al Kα radiation (150 W).
Syntheses of compounds
X-ray single-crystal structure determination
Crystals of compounds {Co9(Ge4Nb16)2} and {Ge4Nb16} were mounted on a Bruker APEX II diffractometer at 175 K with Mo-Kα radiation (λ = 0.71073 Å). The structures of {Co9(Ge4Nb16)2} and {Ge4Nb16} were solved by the direct method and refined by full matrix least squares based on F2 using SHELX-2016 software. Crystallographic data and structure refinements for compounds {Co9(Ge4Nb16)2} and {Ge4Nb16} are summarized in Table S1. CCDC 2489848 and 2489858 contain supplementary crystallographic data for {Co9(Ge4Nb16)2} and {Ge4Nb16}, respectively.Results and discussion
Crystal structures of {Co9(Ge4Nb16)2} and {Ge4Nb16}
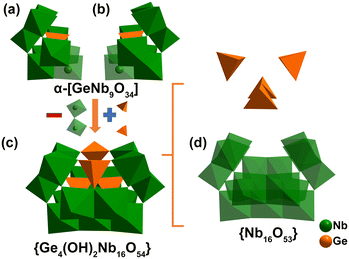
Single crystal X-ray diffraction (SCXRD) analysis reveals that both the {Co9(Ge4Nb16)2} and {Ge4Nb16} compounds crystallize in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) group and contain germanoniobate clusters {Ge4(OH)2Nb16O54} (Fig. S2, Table S1). The cage-like {Ge4(OH)2Nb16O54} cluster can be formally derived from the classical Keggin-type α-[GeNb12O40]16− anionic cluster.60,61 Specifically, removing an edge-sharing {Nb3O13} unit from a saturated Keggin α-[GeNb12O40]16− unit results in a trivacant Keggin-type heteropolyoxoniobate A-α-[GeNb9O34]19− ({GeNb9}) fragment (Fig. 1a and b). Two such trivacant {GeNb9} units are connected by two shared {NbO6} octahedra, and two [GeO3(OH)] tetrahedra, forming a {Ge4(OH)2Nb16O54} cluster (Fig. 1c).
group and contain germanoniobate clusters {Ge4(OH)2Nb16O54} (Fig. S2, Table S1). The cage-like {Ge4(OH)2Nb16O54} cluster can be formally derived from the classical Keggin-type α-[GeNb12O40]16− anionic cluster.60,61 Specifically, removing an edge-sharing {Nb3O13} unit from a saturated Keggin α-[GeNb12O40]16− unit results in a trivacant Keggin-type heteropolyoxoniobate A-α-[GeNb9O34]19− ({GeNb9}) fragment (Fig. 1a and b). Two such trivacant {GeNb9} units are connected by two shared {NbO6} octahedra, and two [GeO3(OH)] tetrahedra, forming a {Ge4(OH)2Nb16O54} cluster (Fig. 1c).
Alternatively, the structure of {Ge4(OH)2Nb16O54} may be described as a nest-like {Nb16O53} cluster, encapsulating a Ge2O7 dimer and two [GeO3(OH)] tetrahedra (Fig. 1d).
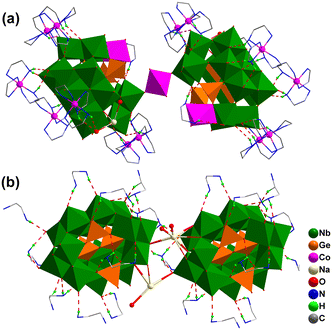
The compound {Co9(Ge4Nb16)2} consists of a dimeric cluster {(CoIII(OH)2(H2O)2)[CoII(en)(H2O)Ge4(OH)2Nb16O54]2} ({Co3(Ge4Nb16)2}) and six [CoIII(en)3]3+ and two [Na(H2O)2]+ counterions. As illustrated in Fig. 2a, the centrosymmetric {Co3(Ge4Nb16)2} dimer is constructed from two {CoII(en)(H2O)Ge4(OH)2Nb16O54} ({CoGe4Nb16}) subunits bridged by a [CoIII(OH)2(H2O)2]− unit. Within each {CoGe4Nb16} subunit, a [CoII(en)(H2O)]2+ moiety is anchored within the lacunary site of the {Ge4(OH)2Nb16O54} cluster (Fig. S3). The Co2+ ion in {CoGe4Nb16} subunit adopts a distorted octahedral geometry, coordinated by three μ3-O atoms from the {Ge4(OH)2Nb16O54} cluster, two N atoms from an en ligand and one water molecule (Co–O: 2.080–2.212 Å; Co–N: 2.108–2.140 Å). This configuration of the {CoGe4Nb16} cluster is considered favourable for enhancing catalytic activity. The central Co3+ ion in the [CoIII(OH)2(H2O)2]− bridge is also six-coordinated with six oxygen atoms: two μ4-O atoms from the {Ge4Nb16} cluster, two OH− and two water molecules, forming a distorted octahedron. The corresponding Co–O bond lengths fall in the range of 1.855(1) to 2.377(1) Å (Fig. S4 and S5).
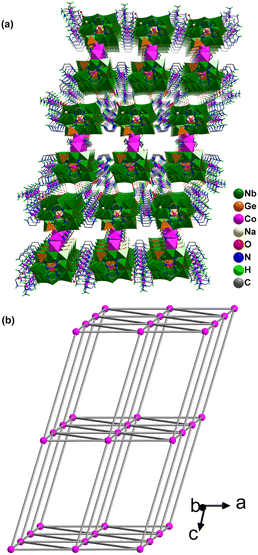
In this dimeric assembly, two [Na(H2O)2]+ cations cap on the {Ge4(OH)2Nb16O54} cluster, while sixteen [CoIII(en)3]3+ fragments are distributed freely around it. These [Na(H2O)2]+ and [CoIII(en)3]3+ units not only serve to balance the overall charge, but also act as hydrogen-bond donors (Fig. 2a). Through supramolecular interactions, specially N–H⋯O and O–H⋯O hydrogen bonds (N⋯O distances: 2.851(1)–3.371(2) Å), the {Co3(Ge4Nb16)2} dimers, the [CoIII(en)3]3+ complexes and the [Na(H2O)2]+ units are interconnected to form a 3D supramolecular framework. This framework features nanoscale cylindrical channels of size 12 × 15 nm2 propagating along the b-axis (Fig. 3a). Topologically, each {Co3(Ge4Nb16)2} dimer can be regarded as an 8-connected node linked by the [CoIII(en)3]3+ units. Consequently, the overall supramolecular network of {Co9(Ge4Nb16)2} exhibits a hex-type topology with the point symbol (36·418·53·6) (Fig. 3b and S4b).
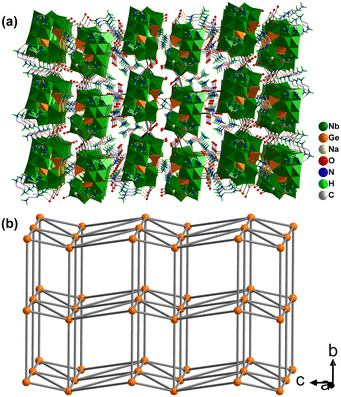
Unlike {Co9(Ge4Nb16)2}, the compound {Ge4Nb16} is an organic–inorganic hybrid germanoniobate. Its asymmetric structural unit consists of one {Ge4(OH)2Nb16O54} polyoxoanion, four Na+ ions and five protonated ethylenediamine (H2en) cations (Fig. S6). Each {Ge4(OH)2Nb16O54} cluster is surrounded by eleven H2en cations (Fig. 2b). These clusters are interconnected by two Na+ ions via Nb–O–Na bonds, forming a one-dimensional linear chain along the b-axis (Fig. S7). Owing to the presence of H2en cations in {Ge4Nb16}, extensive N–H⋯O hydrogen bonding (2.70–3.324 Å) between the chains and the H2en cations cross-links each chain to six neighbours, affording an eight-connected 3D supramolecular framework (Fig. 4a). This 3D framework exhibits an eight-connected hex-type topology wherein each {Ge4(OH)2Nb16O54} cluster serves as an eight-connected node and Na+, and five H2en cations act as linkers (Fig. 4b and S7).
The bond-valence sum (BVS) calculations confirm that all Ge and Nb atoms in the {Co9(Ge4Nb16)2} and {Ge4Nb16} compounds are in the oxidation states of +4 and +5, respectively (Tables S2 and S3). Notably, the Co atoms in {Co9(Ge4Nb16)2} are found in two valence states, +2 and +3 (Table S2). The valence states of the Nb and Co atoms were further verified by X-ray photoelectron spectroscopy (XPS) (Fig. S9). In both compounds, the Ge–O bond lengths range from 1.722 to 1.780 Å, while the Nb–O bond lengths vary from 1.752 to 2.438 Å.
Powder X-ray diffraction (PXRD) (Fig. S10) and IR (Fig. S1) spectroscopy confirms the phase purity of both compounds. Thermogravimetric analysis (TGA) (Fig. S11) indicates the presence of approximately 32 and 15 water molecules in compounds {Co9(Ge4Nb16)2} and {Ge4Nb16}, respectively. Additionally, the UV-vis absorption spectra (Fig. S12) were recorded for both compounds in the 200–800 nm range. The results reveal similar UV-visible absorption bands between 200 and 300 nm for the two compounds, which are assigned to the O → Nb charge transfer transition (OMCT). Furthermore, compound {Co9(Ge4Nb16)2} exhibits additional absorption peaks in the 300–700 nm region, which are attributed to the d–d transition of the cobalt ions.
Electrocatalytic properties
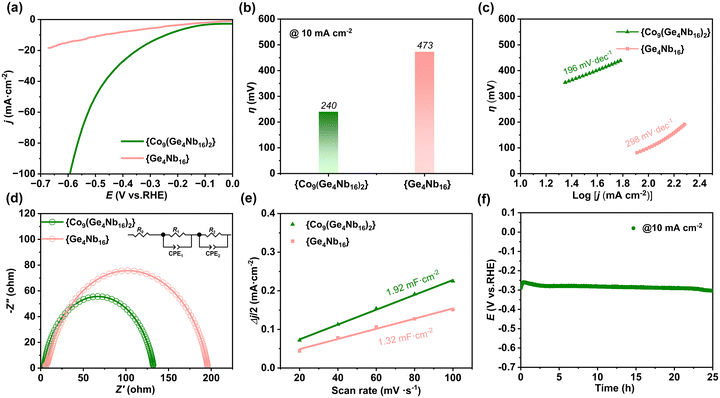
Polyoxometalates (POMs) have significant potential in electrocatalysis due to their well-defined molecular structures and highly electronegative surfaces. Moreover, the incorporation of the transition metal cobalt atom has been shown to effectively modulate the electronic structure of POMs, thereby enhancing their hydrogen evolution reaction (HER) activity. The HER activity of germanoniobates {Co9(Ge4Nb16)2} and {Ge4Nb16} was evaluated in a 1.0 M KOH aqueous solution (pH = 13.9, 25 °C) using a standard three-electrode system at room temperature. A Hg/HgO electrode, a graphite rod, and {Co9(Ge4Nb16)2} or {Ge4Nb16}-modified carbon cloth were employed as the reference, counter, and working electrodes, respectively. Linear sweep voltammetry (LSV) was employed to record the polarization curves of all catalysts. As depicted in Fig. 5, the compound {Co9(Ge4Nb16)2} exhibits significantly enhanced electrocatalytic activity for the HER. At a current density of 10 mA cm−2, {Co9(Ge4Nb16)2} exhibits an overpotential of 240 mV, which is approximately 233 mV lower than that of the Co-free analogue {Ge4Nb16} (473 mV) under identical conditions (Fig. 5a and b). The overpotential of {Co9(Ge4Nb16)2} is lower than that of the most reported Co-based HER catalysts (Table S4).Additionally, the Tafel slope was derived from the LSV curves in order to investigate the kinetics of the HER process. As shown in Fig. 5c, compound {Co9(Ge4Nb16)2} exhibits a Tafel slope of 196 mV dec−1, which is lower than that of {Ge4Nb16} (298 mV dec−1). The Tafel slope reflects the sensitivity of the reaction rate to changes in overpotential: a smaller value indicates more favourable kinetics and a higher intrinsic activity for the HER. The catalytic activity of {Co9(Ge4Nb16)2} is higher than that of {Ge4Nb16}. Considering that {Co9(Ge4Nb16)2} contains cobalt atoms, it can provide active sites. This indicates that the Co-based PONbs exhibit an excellent catalytic effect.
Electrochemical impedance spectroscopy (EIS) was employed to investigate the interfacial kinetics and charge transfer properties of the catalytic systems. Consequently, EIS measurements were performed on the two catalysts, {Co9(Ge4Nb16)2} and {Ge4Nb16}, in a 1.0 M aqueous KOH solution. The Nyquist plots revealed a charge transfer resistance (Rct) of 127 Ω for {Co9(Ge4Nb16)2} and 190 Ω for {Ge4Nb16} (Fig. 5d), which correlated directly with the HER kinetic rate. The significantly lower Rct value of the Co-containing {Co9(Ge4Nb16)2} indicates enhanced charge transfer efficiency and more favourable HER kinetics relative to the Co-free {Ge4Nb16} catalyst. These results suggest that the incorporation of cobalt atoms markedly facilitates interfacial electron transfer, thereby improving the electrocatalytic performance of the material.
The electrochemical active surface area (ECSA) is a key parameter that reflects the apparent electrocatalytic activity. It is estimated from the electrochemical double-layer capacitance (Cdl). Cdl is proportional to the number of accessible active sites. Cdl values were derived from cyclic voltammetry (CV) scans performed in the non-Faraday region. As shown in Fig. 5e the Cdl value for the Co-containing {Co9(Ge4Nb16)2} was found to be 1.92 mF cm−2, which is higher than that of the Co-free analogue {Ge4Nb16} (1.32 mF cm−2). To enable direct comparison, the current densities were normalized with respect to the ECSA, to correct for differences in catalyst mass loading on the carbon cloth (Fig. S14). These results suggest that {Co9(Ge4Nb16)2} possesses a larger electrochemically active surface area, likely due to the exposure of more catalytic sites, facilitated by the incorporation of Co atoms. These Co atoms appear to play a crucial role in improving the HER activity of the polyoxoniobate-based catalyst.
To assess the operational stability of the electrocatalyst, chronopotentiometric tests were conducted on {Co9(Ge4Nb16)2} at a fixed current density of 10 mA cm−1. As depicted in Fig. 5f, the electrode potential remained almost unchanged over a continuous 24-hour period, indicating outstanding durability of the catalyst. In contrast, the {Ge4Nb16} catalyst exhibited a significant overpotential shift after 12 hours of testing (Fig. S15). Furthermore, post-catalysis characterization via PXRD and FTIR spectroscopy (Fig. S16–S19) confirmed that the structural and compositional integrity of the catalyst was well preserved after the long-term stability test. This demonstrates its robust chemical and structural stability under electrocatalytic conditions.
Conclusions
In this work, two novel inorganic–organic hybrid germanoniobates with analogous supramolecular frameworks are constructed from identical {Ge4(OH)2Nb16O54} polyoxoanions, combined with either Co–amine complexes or en organic ligands. The Co-containing {Co9(Ge4Nb16)2} exhibits significantly enhanced HER activity under highly alkaline conditions compared to the Co-free analogue {Co9(Ge4Nb16)2}. These results clearly indicate that the incorporation of Co atoms markedly boosts the HER activity under alkaline conditions. This study provides molecular-level insights into how structural modifications of polyoxometalates (POMs) influence electrocatalytic HER behavior, and offers a model system for further theoretical investigations on structure–property correlations. Furthermore, it proposes a promising strategy for designing highly active and cost-effective HER electrocatalysts.Author contributions
Yan-Qiong Sun, Shou-Tian Zheng and Xin-Xiong Li designed and led the project, revised the manuscript and acquired fundings. Xin-Rong Jin performed material characterization and wrote the original draft. Jin-Yang Li and Yong-Jiang Wang performed the crystal synthesis and crystal structure determination.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI).Supplementary information: the SI provides experimental details, single-crystal X-ray diffraction data, additional structural figures; additional characterizations such as PXRD patterns, IR spectra, UV-vis absorption spectrums. See DOI: https://doi.org/10.1039/d5ce00954e.
CCDC 2489848 {Co9(Ge4Nb16)2} and 2489858 {Ge4Nb16} contain the supplementary crystallographic data for this paper.62a,b
Acknowledgements
Yan-Qiong Sun, Shou-Tian Zheng and Xin-Xiong Li gratefully give thanks to the financial support from the National Natural Science Foundations of China (No. 21971040, 21971039, and 21773029) and the Natural Science Fund of Fujian Province (No. 2024J01234).Notes and references
- N. Ade, A. Alsuhaibani, M. M. El-Halwagi, H. Goyette and B. Wilhite, Int. J. Hydrogen Energy, 2022, 47, 6404–6414 CrossRef CAS.
- Y. Wang, R. Wang, K. Tanaka, P. Ciais, J. Penuelas, Y. Balkanski, J. Sardans, D. Hauglustaine, W. Liu, X. Xing, J. Li, S. Xu, Y. Xiong, R. Yang, J. Cao, J. Chen, L. Wang, X. Tang and R. Zhang, Nature, 2023, 619, 761–767 CrossRef CAS PubMed.
- G. Glenk and S. Reichelstein, Nat. Energy, 2019, 4, 216–222 CrossRef CAS.
- T. Gu, R. Sa, L. Zhang, D.-S. Li and R. Wang, Appl. Catal., B, 2021, 296, 120360 CrossRef CAS.
- P. P. Katti, B. M. Praveen, B. K. Devendra, S. Varadaraj and J. R. N. Kumar, Hybrid Adv., 2024, 7, 100296 CrossRef.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- Y. Liu, J. Zhang, Y. Li, Q. Qian, Z. Li, Y. Zhu and G. Zhang, Nat. Commun., 2020, 11, 1853 CrossRef CAS PubMed.
- M. A. Naseeb, M. Murtaza, K. Farooq, W. A. Shah and A. Waseem, Nanoscale Adv., 2025, 7, 5300–5312 RSC.
- J.-T. Ren, L. Chen, C.-C. Weng, G.-G. Yuan and Z.-Y. Yuan, ACS Appl. Mater. Interfaces, 2018, 10, 33276–33286 CrossRef CAS PubMed.
- Z. Wang, X. Tan, Z. Ye, S. Chen, G. Li, Q. Wang and S. Yuan, Green Chem., 2024, 26, 9569–9598 RSC.
- Y. J. Tang, M. R. Gao, C. H. Liu, S. L. Li, H. L. Jiang, Y. Q. Lan, M. Han and S. H. Yu, Angew. Chem., 2015, 127, 13120–13124 CrossRef.
- W. Zhang, S. Zhu, R. Luque, S. Han, L. Hu and G. Xu, Chem. Soc. Rev., 2016, 45, 715–752 RSC.
- X. Liang, Z. Zhang, Z. Wang, M. Hu, D. Cheng, Y. Jiang, H. Ren, F. Shen, S. Yang, X. Yang, W. Jiang, X. Shi, Z. Ma and K. Zhou, Energy Environ. Sci., 2025, 18, 4302–4311 RSC.
- F. Zhang, S. Hong, R. Qiao, W.-H. Huang, Z. Tang, J. Tang, C.-W. Pao, M.-H. Yeh, J. Dai, Y. Chen, J. Lu, Z. Hu, F. Gong, Y. Zhu and H. Wang, ACS Nano, 2025, 19, 11176–11186 CrossRef CAS.
- J. Cao, D. Zhang, B. Ren, P. Song and W. Xu, Chin. Chem. Lett., 2024, 35, 109863 CrossRef CAS.
- G. Gao, G. Zhao, G. Zhu, B. Sun, Z. Sun, S. Li and Y.-Q. Lan, Chin. Chem. Lett., 2025, 36, 109557 CrossRef CAS.
- X. Zhu, L. Chen, Y. Liu and Z. Tang, Polyoxometalates, 2023, 2, 9140031 CrossRef.
- L. Yan, Y. Xu, P. Chen, S. Zhang, H. Jiang, L. Yang, Y. Wang, L. Zhang, J. Shen, X. Zhao and L. Wang, Adv. Mater., 2020, 32, 2003313 CrossRef CAS.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- S. Min, G. Yang, Y. Jiao, J. Wang, Y. Liu, Z. Chen, H. Yan and H. Fu, Adv. Funct. Mater., 2025, e14250 CrossRef.
- Z.-Y. Tian, X.-Q. Han, J. Du, Z.-B. Li, Y.-Y. Ma and Z.-G. Han, ACS Appl. Mater. Interfaces, 2023, 15, 11853–11865 CrossRef CAS.
- Q. Wang, Z.-Y. Tian, W.-J. Cui, N. Hu, S.-M. Zhang, Y.-Y. Ma and Z.-G. Han, Int. J. Hydrogen Energy, 2022, 47, 12629–12641 CrossRef CAS.
- L. Qin, Q.-M. Zheng, J.-L. Liu, M.-D. Zhang, M.-X. Zhang and H.-G. Zheng, Mater. Chem. Front., 2021, 5, 7833–7842 RSC.
- C. Zhang, Y. Liu, J. Wang, W. Li, Y. Wang, G. Qin and Z. Lv, Appl. Surf. Sci., 2022, 595, 153532 CrossRef CAS.
- S. Li, P. Ren, C. Yang, X. Liu, Z. Yin, W. Li, H. Yang, J. Li, X. Wang, Y. Wang, R. Cao, L. Lin, S. Yao, X. Wen and D. Ma, Sci. Bull., 2018, 63, 1358–1363 CrossRef CAS PubMed.
- L. Zhao, S. Liu, L. Wei, H. He, B. Jiang, Z. Zhan, J. Wang, X. Li and W. Gou, Catal. Lett., 2024, 154, 5294–5302 CrossRef.
- Y. Yang, J. Hao, C. Lyu, J. Zheng, Y. Yuan, C. Wang, K. Li, H. Y. Yang and X. Pang, Adv. Funct. Mater., 2025, 2507225 CrossRef CAS.
- B. Fabre, C. Falaise and E. Cadot, ACS Catal., 2022, 12, 12055–12091 CrossRef CAS.
- Y. An, L. Wang, W. Jiang, X. Lv, G. Yuan, X. Hang and H. Pang, Polyoxometalates, 2023, 2, 9140030 CrossRef.
- N. Hu, J. Du, Y.-Y. Ma, W.-J. Cui, B.-R. Yu, Z.-G. Han and Y.-G. Li, Appl. Surf. Sci., 2021, 540, 148306 CrossRef CAS.
- Y. Guan, W. Tang, Y. Zhao, S. Li, L. Wang, M. Tian, Y. Li and F. Chai, Polyoxometalates, 2025, 4, 9140098 CrossRef.
- J. C. Liu, J. F. Wang, Q. Han, P. Shangguan, L. L. Liu, L. J. Chen, J. W. Zhao, C. Streb and Y. F. Song, Angew. Chem., Int. Ed., 2021, 60, 11153–11157 CrossRef CAS PubMed.
- N. Song, M. Lu, J. Liu, M. Lin, P. Shangguan, J. Wang, B. Shi and J. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202319700 CrossRef CAS PubMed.
- H. Zhang, W.-L. Zhao, H. Li, Q. Zhuang, Z. Sun, D. Cui, X. Chen, A. Guo, X. Ji, S. An, W. Chen and Y.-F. Song, Polyoxometalates, 2022, 1, 9140011 CrossRef.
- D. H. Li, X. Y. Zhang, J. Q. Lv, P. W. Cai, Y. Q. Sun, C. Sun and S. T. Zheng, Angew. Chem., Int. Ed., 2023, 62, e202312706 CrossRef CAS PubMed.
- Y. Liu, J. Li, D. Sun, L. Men, B. Sun, X. Li, Q. An, F. Liu and Z. Su, New J. Chem., 2022, 46, 178–184 RSC.
- W. Luo, J. Hu, H. Diao, B. Schwarz, C. Streb and Y. F. Song, Angew. Chem., Int. Ed., 2017, 56, 4941–4944 CrossRef CAS PubMed.
- D.-H. Li, L. Jia, Y.-X. Liu, C. Sun, X.-X. Li, P.-W. Cai, Y.-Q. Sun and S.-T. Zheng, Inorg. Chem. Front., 2024, 11, 7049–7057 RSC.
- L. Wu, F. Hu, R.-Z. Sun, W.-J. Xia, Q.-X. Zeng, P.-W. Cai, S.-T. Zheng and X.-X. Li, J. Solid State Chem., 2025, 345, 125224 CrossRef CAS.
- D.-H. Li, N. Shi, Y.-J. Wang, P.-W. Cai, Y.-Q. Sun and S.-T. Zheng, Inorg. Chem. Front., 2023, 10, 4789–4796 RSC.
- Z.-H. Chen, X.-Y. Zhang, D.-H. Li, X.-X. Li, Y.-Q. Sun, C. Sun and S.-T. Zheng, CrystEngComm, 2024, 26, 5840–5844 RSC.
- S.-S. Wang and G.-Y. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed.
- N. Li, J. Liu, J. J. Liu, L. Z. Dong, S. L. Li, B. X. Dong, Y. H. Kan and Y. Q. Lan, Angew. Chem., 2019, 131, 17420–17424 CrossRef.
- Z.-Y. Liu, Y.-D. Lin, Y. Hao, H.-N. Chen, Z.-W. Guo, X.-X. Li and S.-T. Zheng, Tungsten, 2022, 4, 81–98 CrossRef.
- H. Shen, Y. Huang, P. Zhao, R. Gao, Z. Wei, X. Gao, M. Liao, W. Dai, X. Liu, D. Sui, J. Liu, S. Zhu and Y. Wei, Adv. Funct. Mater., 2025, e14862 CrossRef.
- Z.-K. Zhu, Y.-Y. Lin, R.-D. Lai, X.-X. Li, Y.-Q. Sun and S.-T. Zheng, Chin. Chem. Lett., 2023, 34, 107773 CrossRef CAS.
- C. Sun, J. P. Chen, Y. L. Wu, Y. Y. Li, X. X. Li, P. W. Cai, C. Streb and S. T. Zheng, Angew. Chem., Int. Ed., 2025, 64, e202506533 CrossRef CAS PubMed.
- Z. Liang, T. Li, L. Zhang, L. Zheng, W. Jia and Q. Mao, Inorg. Chem. Commun., 2020, 116, 107895 CrossRef CAS.
- F. Steffler and R. L. A. Haiduke, J. Mol. Struct., 2021, 1246, 131156 CrossRef CAS.
- J. Dong, J. Hu, Y. Chi, Z. Lin, B. Zou, S. Yang, C. L. Hill and C. Hu, Angew. Chem., Int. Ed., 2017, 56, 4473–4477 CrossRef CAS PubMed.
- Z. Yang, J. Shang, Y. Yang, P. Ma, J. Niu and J. Wang, Dalton Trans., 2021, 50, 7610–7620 RSC.
- Z.-W. Guo, Y.-H. Yan, Y. Chen, Y.-Y. Li, X.-X. Li, C. Sun and S.-T. Zheng, Nat. Commun., 2025, 16, 6379 CrossRef CAS PubMed.
- Y. Hou, M. Nyman and M. A. Rodriguez, Angew. Chem., Int. Ed., 2011, 50, 12514–12517 CrossRef CAS PubMed.
- Z. Zhang, Q. Lin, D. Kurunthu, T. Wu, F. Zuo, S.-T. Zheng, C. J. Bardeen, X. Bu and P. Feng, J. Am. Chem. Soc., 2011, 133, 6934–6937 CrossRef CAS PubMed.
- M. Nyman, F. Bonhomme, M. A. Rodriguez, B. R. Cherry, J. L. Krumhansl, T. M. Nenoff and A. M. Sattler, Science, 2002, 297, 996–998 CrossRef CAS PubMed.
- Z. Liang, Y. He, Y. Qiao, P. Ma, J. Niu and J. Wang, Inorg. Chem., 2020, 59, 7895–7899 CrossRef CAS PubMed.
- R.-Z. Sun, J.-X. Chen, S.-L. Huang, W.-T. Weng, H.-P. Wu, P.-W. Cai, Z.-H. Wen and S.-T. Zheng, CCS Chem., 2025, 7, 1216–1226 CrossRef CAS.
- C.-X. Chen, S.-L. Duan, X.-Y. Zhang, R.-Z. Sun, P.-W. Cai, C. Sun and S.-T. Zheng, Dalton Trans., 2025, 54, 3591–3596 RSC.
- M. Filowitz, R. K. C. Ho, W. G. Klemperer and W. Shum, Inorg. Chem., 1979, 18, 93–103 CrossRef CAS.
- Rishabh, M. Rani, U. Shanker, B. Singh Kaith and M. Sillanpää, Inorg. Chem. Commun., 2024, 159, 111878 CrossRef CAS.
- Y.-J. Wang, L. Yu, X.-X. Li, Y.-Q. Sun and S.-T. Zheng, Inorg. Chem., 2024, 63, 1388–1394 CrossRef CAS PubMed.
- (a) CCDC 2489848: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pkwpv; (b) CCDC 2489858: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pkx06.
| This journal is © The Royal Society of Chemistry 2026 |