Stepwise single-crystal-to-single-crystal phase transition in copper-based coordination polymers triggered by solvent release
Massimo
Guelfi
 *ab,
Marco
Taddei
*ab,
Marco
Taddei
 ab and
Giulio
Bresciani
ab and
Giulio
Bresciani
 *ab
*ab
aDipartimento di Chimica e Chimica Industriale, Università di Pisa, Via G. Moruzzi 13, I-56124 Pisa, Italy. E-mail: massimo.guelfi@unipi.it; giulio.bresciani@unipi.it
bCentro per l'Integrazione della Strumentazione scientifica dell'Università di Pisa (C.I.S.U.P.), Università di Pisa, Pisa, Italy
First published on 24th November 2025
Abstract
A new copper-based coordination polymer, [Cu(bib)3(MeOH)2](BF4)2 (bib = 1,4-bis(imidazol-1-yl)benzene), was isolated and structurally characterized by single-crystal X-ray diffraction. This novel phase, herein denoted as UdP-7·2MeOHα, undergoes a series of single-crystal-to-single-crystal (SC–SC) transformations upon thermal treatment, yielding three new crystalline phases: UdP-7·2MeOHβ, UdP-7·MeOH, and UdP-7. Each transition is associated with discrete structural rearrangements driven by the progressive loss of coordinated methanol and supramolecular reorganization, leading from a 1D coordination polymer to an extended 2D layered architecture. While the α-to-β transition is fully reversible and maintains most of the structural features, the subsequent transformations involve significant shifts in Cu–N interactions and chain proximity, culminating in the formation of a new layered framework in UdP-7 [Cu(bib)3](BF4)2. This study highlights the rich structural adaptability of bib-based copper polymers and provides a detailed crystallographic insight into their temperature-induced evolution.
1. Introduction
Coordination polymers (CPs) and metal–organic frameworks (MOFs)1–6 have emerged as a major class of crystalline materials owing to their vast structural diversity, tunable porosity, and wide range of potential applications, including gas storage and separation,7 heterogeneous catalysis,8 luminescence9 and drug delivery.10,11 The design of these materials relies on the combination of metal nodes and organic linkers, where both the connectivity and the dimensionality of the resulting frameworks can be finely modulated by judicious selection of synthetic parameters such as the metal ion, the organic ligand, the counterions, and the solvent system employed.12Among these factors, ligand choice has been identified as one of the most critical aspects in dictating the topology and dimensionality of CPs and MOFs.13–15 In particular, N-donor ligands, especially those bearing imidazole, pyridine, or triazole functionalities, have been widely investigated for their ability to form extended coordination networks with diverse topologies while maintaining good chemical and thermal stability.16–19 Within this family of ligands, 1,4-bis(imidazol-1-yl)benzene (bib) has proven to be especially versatile: its extended geometry enables bridging multiple metal centers in predictable orientations, thereby supporting the formation of one-, two-, and even three-dimensional architectures with diverse functional properties. Moreover, frameworks constructed using this neutral N-donor ligand are not solely influenced by the ligand geometry; the nature of the counter anions (e.g., BF4−, PF6−, NO3−, SO42−, SiF62−),20–25 the identity of the metal center (e.g., Cu2+, Co2+, Zn2+),26–30 and the solvent molecules incorporated during crystallization can also exert a profound influence on the resulting framework dimensionality, topology, and flexibility.15,18,27,31,32
Dimensional transformations in CPs and MOFs, wherein the connectivity shifts between 1D chains, 2D layers, or 3D frameworks, represent a particularly compelling facet of reticular chemistry. Such transitions can dramatically alter key material properties, including porosity, flexibility, optical response, and sorption capacity, and are typically induced by external stimuli such as solvent exchange, vapor exposure or guest uptake/release upon thermal treatment.33–35 Rogovoy et al. reported a rapid and reversible 1D-to-2D transformation in an Ag(I)-based MOF triggered by organic solvent vapors, accompanied by distinct changes in phosphorescence that enabled real-time monitoring of the process.33 Similarly, Choe and co-workers demonstrated the conversion of a 1D Cu(II) CP into a 2D MOF thin film via a vapor-phase route, emphasizing the role of controlled nucleation and growth in achieving dimensionality tuning,34 while Vizuet et al. described the ligand-modulated transformation of a low-dimensional Cu(II) CP into higher-dimensional MOFs.35
Beyond solvent- or vapor-driven phenomena, temperature has emerged as a crucial stimulus for driving dimensional transformations. Thermal activation can induce desolvation, ligand reorientation, or subtle rearrangements of metal–ligand bond lengths and angles, thereby facilitating connectivity changes that would be difficult to achieve under purely chemical stimuli.35,36 Castillo-Blas et al. recently reviewed thermally activated structural transitions in MOFs, categorizing them into high- and low-temperature regimes and highlighting their mechanistic diversity, ranging from simple lattice solvent removal to cooperative framework rearrangements.36 Schneemann et al. extensively discussed “breathing” phenomena in flexible frameworks such as MIL-53, where thermal modulation of pore size and framework topology arises from cooperative distortions of both inorganic nodes and organic linkers.14 These studies collectively demonstrate that temperature-induced transformations can unlock unique dynamic behaviors and enable the deliberate design of responsive frameworks.
Within this broader context, single-crystal-to-single-crystal (SC–SC) transformations hold particular significance as they enable direct observation of structural rearrangements at the atomic level while preserving long-range crystallinity. Such transformations, generally triggered by temperature, pressure, light, or guest exchange, provide unparalleled insights into framework flexibility, dynamic response, and adaptive behavior.12,14 Thermally induced SC–SC transformations, in particular, have proven especially valuable for understanding solvent removal, framework breathing, and topology changes, and can involve extensive reconstructions of metal coordination environments and ligand connectivity, occasionally producing new phases with different dimensionalities. Stepwise thermally driven SC–SC transitions, where multiple crystalline intermediates can be trapped and structurally characterized, remain relatively rare but are exceptionally informative for disentangling cooperative versus discrete molecular rearrangements.37–43
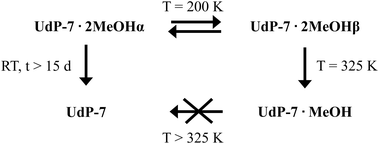
Copper-based CPs featuring bib ligands represent a fertile ground for exploring such dynamic behaviors. However, multi-step thermal transitions involving successive structural reorganizations across several crystalline phases, particularly those implicating both coordinated and lattice solvents, remain uncommon. In this work, we report the isolation and stepwise thermal evolution of a new Cu–bib coordination polymer, initially identified as an unintentional by-product during the crystallization of the known UdP-2 phase.44,45 The new phase, designated UdP-7·2MeOHα, undergoes a cascade of SC–SC transformations upon controlled heating, giving rise to three distinct crystalline phases: UdP-7·2MeOHβ, UdP-7·MeOH, and UdP-7. These transitions involve sequential solvent loss, supramolecular rearrangements, and coordination reorganizations, culminating in the formation of an extended 2D layered architecture. This study thus provides a rare example of a thermally triggered multi-step dimensional transformation fully monitored by single-crystal X-ray diffraction.
2. Results and discussion
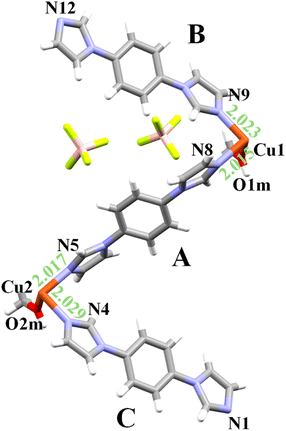
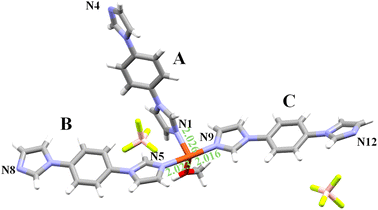
During attempts to crystallize the known UdP-2 (ref. 44 and 45) (i.e., [Cu(bib)2(BF4)2]) phase via the slow diffusion of a methanolic solution of bib into an aqueous solution of Cu(BF4)2, a second, morphologically distinct crystalline material was obtained. Deep-blue crystals, clearly distinguishable from the dominant purple phase of UdP-2 (Fig. S1), were isolated and subjected to single-crystal X-ray diffraction (SCXRD) analysis, revealing a new CP. This previously unreported phase, of formula [Cu(bib)3(MeOH)2](BF4)2, has been designated as UdP-7·2MeOHα, and its structure and thermal behavior are described in detail below.2.1 Structural features of UdP-7·2MeOHα
UdP-7·2MeOHα has formula [Cu(bib)3(MeOH)2](BF4)2 and crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with the following unit cell parameters: a = 9.9469(4) Å, b = 15.0114(6) Å, c = 15.5126(6) Å, α = 71.776(1)°, β = 88.392(1)°, γ = 81.732(2)°, V = 2176.8(2) Å3, with Z = 2 (for further details, see Table 1). The asymmetric unit comprises two copper atoms (Cu1 and Cu2) both lying on an inversion center: Cu1 is positioned at the center of the ac face, and Cu2 at the center of the unit cell. Cu1 and Cu2 are bridged by one bib ligand (denoted as ligand A) and, additionally, two bib ligands (ligands B and C) are coordinated to Cu1 and Cu2, respectively. The coordination sphere of Cu is completed by a terminal MeOH ligand bound orthogonally to the plane defined by the bib ligands. Two free BF4− anions residing in the void space between Cu–bib chains warrant electroneutrality (Fig. 1). In summary, every Cu atom coordinates: two terminal bib ligands, two bridging bib ligands (shared with two different Cu atoms) and two MeOH molecules. The +2 charge, given by the Cu atom, is balanced by two uncoordinated tetrafluoroborate anions.
, with the following unit cell parameters: a = 9.9469(4) Å, b = 15.0114(6) Å, c = 15.5126(6) Å, α = 71.776(1)°, β = 88.392(1)°, γ = 81.732(2)°, V = 2176.8(2) Å3, with Z = 2 (for further details, see Table 1). The asymmetric unit comprises two copper atoms (Cu1 and Cu2) both lying on an inversion center: Cu1 is positioned at the center of the ac face, and Cu2 at the center of the unit cell. Cu1 and Cu2 are bridged by one bib ligand (denoted as ligand A) and, additionally, two bib ligands (ligands B and C) are coordinated to Cu1 and Cu2, respectively. The coordination sphere of Cu is completed by a terminal MeOH ligand bound orthogonally to the plane defined by the bib ligands. Two free BF4− anions residing in the void space between Cu–bib chains warrant electroneutrality (Fig. 1). In summary, every Cu atom coordinates: two terminal bib ligands, two bridging bib ligands (shared with two different Cu atoms) and two MeOH molecules. The +2 charge, given by the Cu atom, is balanced by two uncoordinated tetrafluoroborate anions.
| CCDC ID | 2490389 | 2490391 | 2490393 | 2490395 |
|---|---|---|---|---|
| Compound | UdP-7·2MeOHα | UdP-7·2MeOHβ | UdP-7·MeOH | UdP-7 |
| Formula | C38H38B2CuF8N12O2 | C38H38B2CuF8N12O2 | C37H34B2CuF8N12O | C36H30B2CuF8N12 |
| FW, g mol−1 | 931.96 | 931.96 | 899.92 | 867.88 |
| T, K | 100(2) | 200(2) | 100(2) | 301(2) |
| λ, Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a, Å | 9.9469(4) | 7.5279(3) | 7.7119(7) | 7.7551(4) |
| b, Å | 15.0114(6) | 10.0007(4) | 9.8289(9) | 10.0259(5) |
| c, Å | 15.5126(6) | 15.0093(5) | 27.118(3) | 12.7358(6) |
| α, ° | 71.776(1) | 87.693(1) | 94.875(4) | 84.026(2) |
| β, ° | 88.392(1) | 80.356(1) | 94.077(4) | 84.231(2) |
| γ, ° | 81.732(2) | 82.071(1) | 97.335(3) | 84.570(2) |
| Cell volume, Å3 | 2176.8(2) | 1103.18(7) | 2024.3(3) | 976.32(8) |
| Z | 2 | 1 | 2 | 1 |
| Density calculated, g cm−3 | 1.422 | 1.403 | 1.476 | 1.476 |
| Absorption coefficient, mm−1 | 0.585 | 0.577 | 0.624 | 0.643 |
| F(000) | 954 | 477 | 918 | 441 |
| Crystal size, mm | 0.158 × 0.120 × 0.072 | 0.158 × 0.120 × 0.072 | 0.158 × 0.120 × 0.072 | 0.200 × 0.105 × 0.089 |
| θ limits, ° | 2.07 to 26.39 | 2.06 to 26.41 | 2.10 to 25.02 | 2.05 to 27.95 |
| Reflection collected | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 893 893 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241 241 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 868 868 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 379 379 |
| Independent reflection | 8889 [R(int) = 0.0376] | 4527 [R(int) = 0.0356] | 6339 [R(int) = 0.0395] | 4680 [R(int) = 0.0520] |
| Data/restraints/parameters | 8889/0/573 | 4527/20/294 | 6339/0/567 | 4680/20/269 |
| Goodness of fit on F2 | 1.071 | 1.048 | 1.212 | 1.067 |
| R 1 (I > 2σ(I)) | 0.0413 | 0.0561 | 0.0917 | 0.0640 |
| wR2 (I > 2σ(I)) | 0.1190 | 0.1663 | 0.2098 | 0.1902 |
| R 1 (all data) | 0.0471 | 0.0574 | 0.0944 | 0.0759 |
| wR2 (all data) | 0.1247 | 0.1676 | 0.2108 | 0.2030 |
| Largest diff. peak and hole, e Å−3 | 1.366 and −0.349 | 1.804 and −1.104 | 1.419 and −1.021 | 0.731 and −0.768 |
Both metal centers adopt distorted octahedral geometries, elongated along the O1m–Cu1–O1m and O2m–Cu2–O2m axes, respectively. The axial Cu1–O1m and Cu2–O2m bond lengths are 2.419 Å and 2.382 Å, while the equatorial Cu–N bonds fall in the range of 2.015–2.029 Å (for further analysis of coordination geometry, polyhedral distortion and unit cell, see Tables S1–S4).
The Cu1–N8–N5–Cu2 torsion angle along the bridging ligand is nearly linear at −179.86° (Table S5). The resulting architecture is a one-dimensional (1D) coordination polymer (Table S2) that propagates along the direction defined by bib ligand A. The pendant bib-B and bib-C ligands, extending into the interchain space, adopt a stacked arrangement with an interplanar separation of 3.913 Å (measured by taking into account the distance between the centroid of ligand B and of ligand C, see Table S6). As a result, the 1D chains are packed together by stacking interactions between ligand B and ligand C (Table S7). In addition to π–π stacking, the supramolecular architecture of UdP-7·2MeOH is further stabilized by several hydrogen-bond interactions involving the coordinated methanol molecules and the imidazole nitrogen atoms of the pendant ligands. Specifically, N12 and N1 (from ligands B and C, respectively) form hydrogen bonds with the hydroxyl hydrogens H2M and H1M of the coordinated methanol molecules. These intermolecular contacts, though weak, contribute to linking adjacent chains and reinforcing the overall packing stability of the one-dimensional polymer (see Table S8). Further descriptive figures and parameters of the UdP-7·2MeOHα structure are collected in Tables S1–S8.
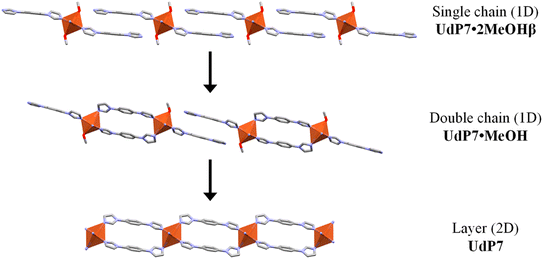
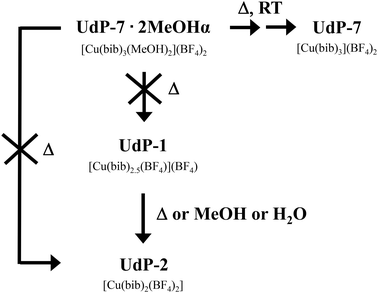
As previously reported,44,45UdP-2 ([Cu(bib)2(BF4)2]) crystallizes as a 2D layered framework in which each coppers center is coordinated by two bib ligands (four imidazole donors) and two BF4− anions, whereas UdP-1 ([Cu(bib)2.5(BF4)](BF4)) adopts a 3D interpenetrated structure in which the copper atoms are bound to 2.5 bib ligands (five imidazole donors) and one BF4− anion, with a second BF4− anion located in the framework cavities. Remarkably, UdP-1 readily converts into UdP-2 upon removal of 0.5 equiv. of the bib ligand from the framework. Considering these structural relationships, we sought to assess whether UdP-7·2MeOHα could undergo analogous transformations upon the stepwise loss of its two coordinated methanol molecules and 0.5 or 1 equiv. of bib. To this end, we performed a single-crystal-to-single-crystal (SC–SC) thermal transformation study over the temperature range 100–325 K on the herein reported material (see Experimental for further information). This study revealed three additional crystalline phases (Scheme 1), none of which corresponded to UdP-1 or UdP-2. A detailed structural description of these newly formed phases is provided below.
2.2 UdP-7·2MeOHα to UdP-7·2MeOHβ transition
Upon heating a specimen of UdP-7·2MeOHα from 100 K to 200 K, a reversible phase transformation occurs, leading to a new phase denoted as UdP-7·2MeOHβ. At this temperature, the material retains the chemical formula (i.e., [Cu(bib)3(MeOH)2](BF4)2) and the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , but with modified unit cell parameters: a = 7.5279(3) Å, b = 10.0007(4) Å, c = 15.0093(5) Å, α = 87.693(1)°, β = 80.356(1)°, γ = 82.071(1)°, V = 1103.18(7) Å3, and Z = 1 (see Table 1 for additional crystallographic details).
, but with modified unit cell parameters: a = 7.5279(3) Å, b = 10.0007(4) Å, c = 15.0093(5) Å, α = 87.693(1)°, β = 80.356(1)°, γ = 82.071(1)°, V = 1103.18(7) Å3, and Z = 1 (see Table 1 for additional crystallographic details).
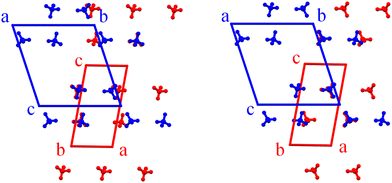
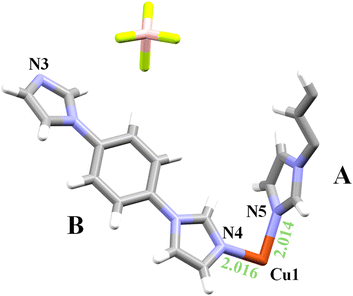
In this case, the asymmetric unit (Fig. 2) comprises one copper atom located on an inversion center at the center of the ab face of the unit cell, half of a bridging bib ligand (designated as ligand A), whose center of mass lies on an inversion center and connects two Cu atoms along the 1D polymeric chain (Table S2), one terminal bib ligand (ligand B), and one coordinated methanol molecule. The structure is further completed by one disordered BF4− anion, which was modeled over two positions (see Experimental part for details of the occupancy), based on electron density maps derived from the observed structure factors (Fo) by Fourier synthesis46 (Fig. S2). As already stated, the overall formula remains unchanged respect to the previously described phase, i.e. [Cu(bib)3(MeOH)2](BF4)2).
The structural parameters of the UdP-7·2MeOHβ phase deviate only slightly from those of the UdP-7·2MeOHα phase, with the minor differences summarized in Tables S1–S7. The two structures are virtually superimposable with respect to all framework atoms (Fig. S3), except for the BF4− anions. In fact, the 1D chains propagate along the ligand A in both phases and the single chains are packed together by stacking interaction between terminal ligands (ligand B–ligand C in the α phase; ligand B–ligand B in the β phase). In the β phase, the supramolecular stabilization through O–H⋯N interactions is preserved respect to the α phase, albeit represented by a reduced number of crystallographically independent contacts (Table S8). In UdP-7·2MeOHβ, the positional disorder of BF4− anions results in only partial overlap with those in UdP-7·2MeOHα, and this occurs only when each of the two alternative disordered positions is considered independently (Fig. 3). Ultimately, this phase transition can be ascribed mainly to the increased positional disorder of the BF4− anions induced by the higher temperature, without any changes in local or extended coordination.
2.3 UdP-7·2MeOHβ to UdP-7·MeOH transition
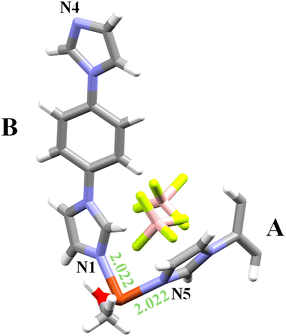
A specimen of UdP-7·2MeOHβ was heated with a Cryostream (see Experimental for further information) up to 325 K. At this temperature, the loss of one methanol molecule from the coordination sphere resulted in the formation of a new crystalline phase of formula [Cu(bib)3(MeOH)](BF4)2), herein referred to as UdP-7·MeOH (Fig. 4). This phase was solved in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with the following unit cell parameters: a = 7.7119(7) Å, b = 9.8289(9) Å, c = 27.118(3) Å, α = 94.875(4)°, β = 94.077(4)°, γ = 97.335(3)°, V = 2024.3(3) Å3, and Z = 2 (see Table 1 for additional crystallographic details).
with the following unit cell parameters: a = 7.7119(7) Å, b = 9.8289(9) Å, c = 27.118(3) Å, α = 94.875(4)°, β = 94.077(4)°, γ = 97.335(3)°, V = 2024.3(3) Å3, and Z = 2 (see Table 1 for additional crystallographic details).
The asymmetric unit of UdP-7·MeOH (Fig. 4) consists of a single copper atom (Cu1) coordinated by three bib ligands (ligand A, ligand B and ligand C) and one methanol molecule. The electroneutrality is reached by the presence of two uncoordinated BF4− anions, giving the overall minimal formula of [Cu(bib)3(MeOH)](BF4)2.
The overall structure (Table S2) can be described taking into consideration the ligand coordination motif. Ligand A is a bridging ligand that coordinates Cu1 through nitrogen atom N1 and extends along the polymeric chain by binding to an adjacent Cu1 atom via N4. Ligand B is a terminal ligand that binds exclusively through nitrogen atom N5. Ligand C is a bridging ligand and coordinates Cu1 through N9 and bridges to another Cu1 atom belonging to a parallel chain via an interaction that involves nitrogen atom N12 (see below). This bridging results in the formation of a one-dimensional double-chain polymer (Fig. 5, see also Table S2 for the view along the a-axis). These double 1D chains are packed together by stacking interaction between terminal ligands B (Table S6). Upon the loss of one methanol molecule, only part of the hydrogen-bond network observed in the previous phase is preserved (Table S8). Specifically, ligand B (through nitrogen atom N8) forms a hydrogen bond with the hydroxyl hydrogen H9 of the remaining coordinated methanol molecule. Thus, in contrast to the UdP-1 to UdP-2 interconversion,44,45 heating did not result in the coordination of BF4− anions.
During the transition from UdP-7·2MeOHβ [Cu(bib)3(MeOH)2](BF4)2) to UdP-7·MeOH [Cu(bib)3(MeOH)](BF4)2), the coordination vacancy obtained on the copper center upon the departure of a methanol molecule leads to a significant rearrangement in the framework, bringing two adjacent 1D chains closer together. On the side where the chains remain separated (i.e. ligand B in UdP-7·MeOH), the non-bonding Cu–N distance slightly decreases from 4.365 Å (Cu1–N4 in UdP-7·2MeOHβ) to 4.269 Å (Cu1–N8 in UdP-7·MeOH). On the opposite side, where the chains become connected (i.e. ligand C in UdP-7·MeOH), the Cu–N distance decreases markedly from 4.365 Å (Cu1–N4 in UdP-7·2MeOHβ) to 2.855 Å (Cu1–N12 in UdP-7·MeOH). In parallel, the interchain Cu⋯Cu distances are significantly shortened (see Table S7), consistent with the approach of the two 1D motifs during this transformation. Moreover, the loss of one methanol molecule and the subsequent contraction of the interchain separation leads ligand B to a substantial conformational change. On the side where the chains approach each other, the torsion angle of ligand B shifts from a pseudo-anti arrangement (ca. −142°) in UdP-7·2MeOHβ to a pseudo-syn conformation (ca. −62°) in UdP-7·MeOH (Table S5).
Although the Cu1–N12 distance falls within the commonly accepted range for Cu–N coordination bonds (Fig. S4) and is slightly shorter than the sum of the van der Waals radii of the two atoms (Cu = 1.4 Å and N = 1.55 Å),47 the nature of this interaction appears to be borderline. PLATON coordination analysis48 indicates only a marginal contribution of N12 (Fig. S5) to the coordination sphere of Cu1, and the software suggests a coordination number of five rather than six. This is consistent with the significantly bent Cu1–N12 axis (ca. 132°, Fig. S6) compared to the nearly linear geometry expected for a fully coordinated axial ligand. Moreover, N12 also engages in a close contact with the hydrogen atom H1 of a neighboring imidazole ring (Table S8), which may contribute to stabilizing this interaction. Overall, these observations suggest that the Cu1–N12 contact represents a weak or incipient coordination interaction rather than a fully developed coordination bond. Additional geometric parameters are summarized in Tables S1–S7.
2.4 UdP-7·2MeOHα to UdP-7 transition
Upon continued heating of a UdP-7·MeOH specimen above 325 K, as illustrated in Scheme 1, the thermal stress, despite a slow heating rate (see Experimental section), proved to be excessive. This led to a gradual loss of crystallinity, making the original single crystal unsuitable for further SCXRD analysis. To investigate the final stage of this transformation, a new crystallization batch of UdP-7·2MeOHα was left exposed to air at room temperature (see Experimental section). After 15 days, the complete loss of coordinated methanol molecules led to obtaining a new crystalline phase of formula [Cu(bib)3](BF4)2, hereafter referred to as UdP-7. This phase crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with unit cell parameters: a = 7.7551(4) Å, b = 10.0259(5) Å, c = 12.7358(6) Å, α = 84.026(2)°, β = 84.231(2)°, γ = 84.570(2)°, V = 976.32(8) Å3, and Z = 1 (see Table 1 for additional crystallographic details, Tables S1–S7 for additional structural features). Unfortunately, all the attempts to isolate the intermediate UdP-7·MeOH ([Cu(bib)3(MeOH)](BF4)2) phase via this method were unsuccessful.
, with unit cell parameters: a = 7.7551(4) Å, b = 10.0259(5) Å, c = 12.7358(6) Å, α = 84.026(2)°, β = 84.231(2)°, γ = 84.570(2)°, V = 976.32(8) Å3, and Z = 1 (see Table 1 for additional crystallographic details, Tables S1–S7 for additional structural features). Unfortunately, all the attempts to isolate the intermediate UdP-7·MeOH ([Cu(bib)3(MeOH)](BF4)2) phase via this method were unsuccessful.
The asymmetric unit of UdP-7 (Fig. 6) contains half a copper atom (Cu1 lying on an inversion center), half of a bib ligand (ligand A) whose center of mass lies on an inversion center, a complete bib ligand (ligand B), and one BF4− anion, giving the above-mentioned minimal formula [Cu(bib)3](BF4)2.
The loss of both methanol molecules allows adjacent 1D chains to approach from both sides ultimately resulting in the formation of an extended 2D layered structure (Fig. 5 and Table S2). In place of Cu–methanol bond, two new Cu–N interactions were formed. In particular, Cu–N distances significantly decrease from UdP-7·MeOH (2.855 Å on the interacting side and 4.269 Å on the non-interacting side) to UdP-7 (2.757 Å on both sides), while the corresponding coordination angle slightly increases from 132° (UdP-7·MeOH) to 136° (UdP-7) (Fig. S7). At the same time, the torsional angle of the bridging ligand becomes more syn-like (ca. 52°), further reflecting the enhanced proximity between the chains (Table S5). As already stated for the Cu1–N12 interaction in UdP-7·MeOH, although the apical Cu1–N3 contacts in UdP-7 fall within the range expected for coordination bonds, their nature remains somewhat borderline. PLATON coordination analysis indicates only a minor contribution of N3 to the coordination sphere, consistent with a slightly distorted geometry around the copper atom (Fig. S5).
To verify the reversibility of the SC–SC transformation, several crystals of UdP-7 were exposed to methanol vapors for two weeks. However, no methanol incorporation was detected. Other solvents (e.g., acetonitrile and water) were also tested but yielded the same negative result.
Several attempts were made to isolate one of the phases presented in this work in the form of a bulk microcrystalline powder, but unfortunately none of these gave satisfactory results. Moreover, grinding fresh prepared crystals of UdP-7·2MeOH led to the fast loss of methanol molecules with formation of UdP-7 in mixture with an unidentified phase (Fig. S7). The isolation of a sufficient quantity of UdP-7·MeOH crystals for grinding and further PXRD analysis proved unsuccessful, owing to the intrinsic difficulty in distinguishing the different phases macroscopically.
2.5 Divergent thermal behavior: UdP-7·2MeOHα/UdP-7vs.UdP-1/UdP-2
Contrary to initial expectations, heating or air exposure of UdP-7·2MeOHα ([Cu(bib)3(MeOH)2](BF4)2) crystals does not lead to the formation of either of the closely related phases UdP-1 ([Cu(bib)2.5(BF4)](BF4)) or UdP-2 ([Cu(bib)2(BF4)2]), despite UdP-7·2MeOHα being a by-product observed during the synthesis of UdP-2, and UdP-1 representing a plausible structural derivative (see Scheme 2). In contrast, as previously reported,44,45 direct interconversion between UdP-1 and UdP-2 can be achieved either by heating or by soaking in methanol or water, highlighting a different transformation pathway within this family of copper–bib phases (see Scheme 2).While it remains uncertain whether this transformation series represents the final chapter in the structural evolution of these intriguing materials, the discovery of such an unusual cascade highlights the remarkable organizational possibilities of this deceptively simple metal–ligand–anion system. In this regard, our findings lend further support to the insightful words of G. R. Desiraju:49 “The essence of supramolecular chemistry is that the structure and properties of the higher-level entities (supermolecules, crystals) cannot be predicted directly or immediately from those of the lower-level entities (molecules)”.
3. Conclusion
The structural analysis of the novel coordination polymer of formula [Cu(bib)3(MeOH)2](BF4)2 (UdP-7·2MeOHα) and its thermally induced phase transformations has unveiled a sequence of solid-state transitions involving discrete and cooperative rearrangements of the coordination environment. Starting from a 1D polymeric structure, the stepwise removal of methanol molecules, either through controlled heating or air exposure, drives the system through distinct crystalline phases of formula [Cu(bib)3(MeOH)2](BF4)2 (UdP-7·2MeOHβ) and [Cu(bib)3(MeOH)](BF4)2 (UdP-7·MeOH), ultimately leading to the formation of a 2D layered network of formula [Cu(bib)3](BF4)2 (UdP-7). Each transformation preserves crystallinity to a remarkable extent, allowing detailed SCXRD characterization and demonstrating noteworthy single-crystal-to-single-crystal behavior.In particular, the transition from UdP-7·2MeOHβ to UdP-7·MeOH is accompanied by a structural rearrangement that brings two polymeric chains into proximity, enabling new Cu–N interactions that define a double-chain motif. Above 325 K, the SC–SC process could no longer be monitored due to the loss of crystallinity. The transformation into UdP-7 was achieved by exposing the crystals to air for a long time. This final phase results in full layer formation via bridging bib ligands, with shortened Cu–N contacts. Except for the initial reversible α↔β transformation, the methanol loss leading from UdP-7·2MeOH to UdP-7 transition seems to be irreversible.
All these findings not only expand the structural landscape of copper–bib materials but also underscore the potential of solvent-responsive frameworks for stimuli-responsive crystalline materials.
4. Experimental
4.1 Materials and methods
All reagents and solvents were purchased from Alfa Aesar, Merck, Strem, FluoroChem, or TCI Chemicals and were used as received without further purification. Glassware for ligand synthesis was dried at 140 °C, evacuated to 10−2 mbar, and backfilled with nitrogen before use. Ligand synthesis was performed under a nitrogen atmosphere using standard Schlenk techniques, according to literature procedures.22,44Powder X-ray diffraction (PXRD) patterns were recorded over the 2θ range 5–35° on a Rigaku MiniFlex 600C diffractometer using Cu Kα radiation (λ = 1.54056 Å). The X-ray tube operated at 40 kV and 15 mA.
4.2 Crystallization of [Cu(bib)3(MeOH)2](BF4)2 (UdP-7·2MeOHα)
An aqueous solution of Cu(BF4)2·6H2O (20 mg, 0.06 mmol) in 6 mL water was placed in a 15 mm × 160 mm screw-cap test tube. A 6 mL layer of MeOH/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) was carefully added, followed by a third layer consisting of a methanolic solution of bib (25 mg, 0.12 mmol in 6 mL MeOH). The tube was sealed and stored at 4 °C for four weeks. Blue plate-like crystals of UdP-7·2MeOH were separated from violet crystals of UdP-2 and collected for analysis.
5 v/v) was carefully added, followed by a third layer consisting of a methanolic solution of bib (25 mg, 0.12 mmol in 6 mL MeOH). The tube was sealed and stored at 4 °C for four weeks. Blue plate-like crystals of UdP-7·2MeOH were separated from violet crystals of UdP-2 and collected for analysis.
4.3 Single-crystal X-ray diffraction
Single-crystal X-ray diffraction (SCXRD) data were collected on a Bruker D8 Venture diffractometer equipped with a Mo Kα microfocus source (λ = 0.71073 Å) and a Photon II 2D detector. Crystals were mounted on MiTeGen loops of appropriate size and coated with Cargille Immersion Oil (type NVH-658), suitable for measurements across the full temperature range employed. Data were acquired at temperatures between 100 K and 325 K using an Oxford Cryostream mounted on the diffractometer and N2 as the cooling gas. Unit-cell parameters were determined every 25 K, and full data collections were performed only when a phase transition was detected. Key experimental parameters are summarized in Table 1.Unit-cell determination and initial refinement were carried out using APEX4.50 Data integration and reduction were performed with SAINT51 and XPREP,52 and absorption corrections were applied using SADABS.53 Structures were solved via intrinsic phasing with ShelXT54 and refined by full-matrix least-squares methods.
All non-hydrogen atoms were refined anisotropically. The fluorine atoms of the BF4− anions in UdP-7·2MeOHβ, UdP-7·MeOH, and UdP-7 were highly disordered; their electron densities were modeled over multiple positions. The occupancies of the disordered BF4− groups were freely refined using the FVAR instruction (for UdP-7·2MeOHβ, the refined value of 0.583 indicates that the two alternative positions are nearly equally populated). The EADP constraint was applied to minimize excessive anisotropy in selected fluorine atoms, while DFIX and DANG restraints were used to optimize the BF4− geometry. Hydrogen atoms were placed in calculated positions and refined using a riding model.
Crystallographic data for UdP-7·2MeOHα, UdP-7·2MeOHβ, UdP-7·MeOH, and UdP-7 have been deposited at the Cambridge Crystallographic Data Centre under the deposition numbers 2490389, 2490391, 2490393, and 2490395, respectively.
4.4 Reversible transition between α and β phases in UdP-7·2MeOH [Cu(bib)3(MeOH)2](BF4)2
A specimen of UdP-7·2MeOH, obtained using the method described above, was mounted and cooled to 100 K with an Oxford Cryostream mounted on the diffractometer. After collecting the data required for the crystallographic determination of UdP-7·2MeOHα, the mounted specimen was heated to 125 K and the temperature was held for 5 minutes. Subsequently, 180 frames (1 s per °) were collected to determine the unit cell. The same procedure was repeated on the same specimen every 25 K until 200 K, where a new phase (UdP-7·2MeOHβ) was detected. Data collection at 200 K allowed the determination of the UdP-7·2MeOHβ structure. Cooling the specimen back to 100 K and determining the unit cell using the same procedure confirmed the reversible transition to UdP-7·2MeOHα. The unit cell determination at 200 K and 100 K was repeated twice on the same specimen to assess the complete reversibility of the phase transition.4.5 Phase transition from UdP-7·2MeOHβ phase ([Cu(bib)3(MeOH)2](BF4)2) to UdP-7·MeOH phase ([Cu(bib)3(MeOH)](BF4)2)
A specimen of UdP-7·2MeOH, obtained using the method described above, was mounted and cooled to 200 K with an Oxford Cryostream mounted on the diffractometer. A total of 180 frames (1 s per °) were collected to determine the unit cell, confirming the formation of the UdP-7·2MeOHβ phase. The mounted specimen was then heated to 225 K, and the temperature was held for 5 minutes. Subsequently, 180 frames were collected to determine the unit cell. The same procedure was repeated on the same specimen every 25 K up to 325 K, where a new phase (UdP-7·MeOH) was detected. The crystal was then cooled back to 100 K, and the unit cell was determined again, confirming the persistence of the UdP-7·MeOH phase. Data collection at 100 K allowed the structure of UdP-7·MeOH to be determined.Heating the mounted crystal above 325 K resulted in a complete loss of crystallinity. Several attempts were made on different specimens, but prolonged heating did not lead to the formation of any additional crystalline phases.
4.6 Synthesis of UdP-7 ([Cu(bib)3](BF4)2) from UdP-7·2MeOH phase ([Cu(bib)3(MeOH)2](BF4)2)
A new batch of UdP-7·2MeOH crystals, obtained as described above, was separated from the mother liquor and washed with 3 mL of methanol. The crystals were then placed in a Petri dish and left to dry in air at room temperature for two weeks. Although no visible change in appearance was observed, several specimens were mounted, and single-crystal X-ray diffraction analysis at room temperature revealed that the crystals had fully transformed into a new phase (UdP-7). Data collection on the selected specimen allowed to determine the UdP-7 structure.Author contributions
G. B. and M. T. conceived and designed the study. G. B. and M. G. carried out experimental data collection. M. G. wrote the original draft. All authors contributed to the writing and revision of the manuscript and approved the final version.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data supporting this article such as, PXRD patterns, structural information and picture of crystals can be found in the supplementary information (SI) as .PDF file.Crystallographic data for all the compound has been deposited at the CCDC (2490389 UdP-7·2MeOHα, 2490391 UdP-7·2MeOHβ, 2490393 UdP-7·MeOH and 2490395 UdP-7) and contain the supplementary crystallographic data for this paper. CIF files can also be found attached as part of the SI.55a–d
Supplementary information is available. See DOI: https://doi.org/10.1039/d5ce00919g.
Acknowledgements
The authors thank the Italian MUR for the provision of funding through the Project PRIN 2020 doMino (ref. 2020P9KBKZ).References
- T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef.
- C. Janiak and J. K. Vieth, New J. Chem., 2010, 34, 2366 RSC.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS.
- R. Robson, Chem. Rec., 2024, 24, e202400038 CrossRef CAS.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC.
- X. Cui, K. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong, Y. Han, B. Li, Q. Ren, M. J. Zaworotko and B. Chen, Science, 2016, 353, 141–144 CrossRef CAS.
- A. Vikal, R. Maurya, P. Patel, S. R. Paliwal, R. K. Narang, G. D. Gupta and B. D. Kurmi, Appl. Mater. Today, 2024, 41, 102443 CrossRef.
- D. S. R. Khafaga, M. T. El-Morsy, H. Faried, A. H. Diab, S. Shehab, A. M. Saleh and G. A. M. Ali, RSC Adv., 2024, 14, 30201–30229 RSC.
- G. Férey, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- H. Kajiro, A. Kondo, K. Kaneko and H. Kanoh, IJMS, 2010, 11, 3803–3845 CrossRef CAS PubMed.
- S.-S. Chen, CrystEngComm, 2016, 18, 6543–6565 RSC.
- M. Eddaoudi, D. F. Sava, J. F. Eubank, K. Adil and V. Guillerm, Chem. Soc. Rev., 2015, 44, 228–249 RSC.
- B. Manna, A. V. Desai and S. K. Ghosh, Dalton Trans., 2016, 45, 4060–4072 RSC.
- X. Liu, H. Wang, C. Liu, J. Chen, Z. Zhou, S. Deng and J. Wang, Chem Bio Eng., 2024, 1, 469–487 CrossRef CAS.
- B.-Q. Song, Q.-Y. Yang, S.-Q. Wang, M. Vandichel, A. Kumar, C. Crowley, N. Kumar, C.-H. Deng, V. GasconPerez, M. Lusi, H. Wu, W. Zhou and M. J. Zaworotko, J. Am. Chem. Soc., 2020, 142, 6896–6901 CrossRef CAS PubMed.
- M. Guelfi, M. Taddei and G. Bresciani, J. Chem. Crystallogr., 2025, 55, 198–205 CrossRef CAS.
- G. Bresciani, M. Guelfi, M. Dosa, V. Guiotto, V. Crocellà, M. Lessi and M. Taddei, CrystEngComm, 2025, 27, 4071–4080 RSC.
- Z.-X. Li, K.-Y. Zou, X. Zhang, T. Han and Y. Yang, Inorg. Chem., 2016, 55, 6552–6562 CrossRef CAS.
- H.-H. Duan, C.-H. Bai, J.-Y. Li, Y. Yang, B.-L. Yang, X.-F. Gou, M.-L. Yue and Z.-X. Li, Inorg. Chem., 2019, 58, 2856–2864 CrossRef CAS PubMed.
- Y. H. Andaloussi, D. Sensharma, A. A. Bezrukov, D. C. Castell, T. He, S. Darwish and M. J. Zaworotko, Cryst. Growth Des., 2024, 24, 2573–2579 CrossRef CAS.
- P.-C. Wang, F.-L. Meng, C. Liu, K.-Y. Zou and Z.-X. Li, Inorg. Chem. Commun., 2012, 17, 95–98 CrossRef CAS.
- G. Ye, K.-Y. Zou, Y. Yang, J.-J. Wang, X.-F. Gou and Z.-X. Li, J. Solid State Chem., 2015, 225, 31–40 CrossRef CAS.
- W.-J. Sun, C.-Y. Ke, Q.-Z. Zhang and X.-L. Zhang, J. Inorg. Organomet. Polym., 2017, 27, 186–193 CrossRef CAS.
- Z.-X. Li, Y.-F. Zeng, H. Ma and X.-H. Bu, Chem. Commun., 2010, 46, 8540 RSC.
- K. Zou, J. Zhao, C. Liu, Z. Wang and Z. Li, Eur. J. Inorg. Chem., 2013, 2013, 293–298 CrossRef CAS.
- K. Shen, M. Zhang and H. Zheng, CrystEngComm, 2015, 17, 981–991 RSC.
- C.-P. Li and M. Du, Chem. Commun., 2011, 47, 5958–5972 RSC.
- M. I. Rogovoy, M. I. Rakhmanova, E. H. Sadykov, G. M. Carignan, I. Yu. Bagryanskaya, J. Li and A. V. Artem'ev, Chem. Commun., 2023, 59, 11413–11416 RSC.
- M. Choe, S. S. Park and H. C. Choi, Inorg. Chem., 2024, 63, 22662–22666 CrossRef CAS.
- J. P. Vizuet, T. S. Howlett, A. L. Lewis, Z. D. Chroust, G. T. McCandless and K. J. Balkus, Inorg. Chem., 2019, 58, 5031–5041 CrossRef CAS.
- C. Castillo-Blas, A. M. Chester, D. A. Keen and T. D. Bennett, Chem. Soc. Rev., 2024, 53, 3606–3629 RSC.
- A. Chaudhary, A. Mohammad and S. M. Mobin, Cryst. Growth Des., 2017, 17, 2893–2910 CrossRef CAS.
- Y. Li, B. Zhao, J.-P. Xue, J. Xie, Z.-S. Yao and J. Tao, Nat. Commun., 2011, 12, 6908 CrossRef.
- T. Seki, K. Sakurada, M. Muromoto and H. Ito, Chem. Sci., 2015, 6, 1491–1497 RSC.
- Y. Zheng, X. Jia, K. Li, J. Xu and X. Bu, Adv. Energy Mater., 2022, 12, 2100324 CrossRef CAS.
- F. Hu, X. Bi, X. Chen, Q. Pan and Y. Zhao, Chem. Lett., 2021, 50, 1015–1029 CrossRef CAS.
- X. Wei, S. de Beer and L. J. Barbour, Cryst. Growth Des., 2023, 23, 6258–6262 CrossRef CAS.
- J. Li, P. Huang, X.-R. Wu, J. Tao, R.-B. Huang and L.-S. Zheng, Chem. Sci., 2013, 4, 3232 RSC.
- G. Bresciani, M. Guelfi, M. Dosa, V. Guiotto, V. Crocellà, M. Lessi and M. Taddei, Cryst. Growth Des., 2024, 24, 8999–9010 CrossRef CAS.
- G. Bresciani, M. Guelfi, M. Dosa, V. Guiotto, V. Crocellà, M. Lessi and M. Taddei, Cryst. Growth Des., 2025, 25, 462–464 CrossRef CAS.
- C. Koschnick, M. W. Terban, R. Frison, M. Etter, F. A. Böhm, D. M. Proserpio, S. Krause, R. E. Dinnebier, S. Canossa and B. V. Lotsch, J. Am. Chem. Soc., 2023, 145, 10051–10060 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 8342–8356 CrossRef CAS.
- Bruker, APEX4 V2021.10–0, Bruker AXS Inc., Madison, Wisconsin, USA, 2021 Search PubMed.
- Bruker, SAINT v8.30A, Bruker AXS Inc., Madison, Wisconsin, USA, 2012 Search PubMed.
- Bruker, XPREP V2014/2, Bruker AXS Inc., Madison, Wisconsin, USA, 2014 Search PubMed.
- Bruker, SADABS V2016/2, Bruker AXS Inc., Madison, Wisconsin, USA, 2016 Search PubMed.
- G. M. Sheldrick, SHELXL-2019/1, Bruker AXS Inc., Madison, Wisconsin, USA, 2019 Search PubMed.
- (a) CCDC 2490389: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plg4x; (b) CCDC 2490391: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plg6z; (c) CCDC 2490393: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plg81; (d) CCDC 2490395: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2plgb3.
| This journal is © The Royal Society of Chemistry 2026 |