Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Size-controlled synthesis of monodisperse zinc gallium oxide particles via coprecipitation under hydrothermal conditions using trisodium citrate
Fumiyuki
Shiba
 *,
Naoki
Koyama
and
Yusuke
Okawa
*,
Naoki
Koyama
and
Yusuke
Okawa

Department of Materials Science, Chiba University, 1-33 Yayoicho, Inageku, Chiba 263-8522, Japan. E-mail: shiba@faculty.chiba-u.jp
First published on 18th November 2025
Abstract
Monodisperse particles of zinc gallium oxide have been synthesized via coprecipitation under hydrothermal conditions in the presence of trisodium citrate, which acts as a ligand for the reactant metal ions to establish controlled homogeneous nucleation at an elevated temperature. This procedure enables the particle size to be tuned up to about 200 nm by changing the citrate concentration. Also, the spinel crystal structure can be maintained when the compositional ratio of Ga to Zn in the particle, fGa/Zn, varies, at least in the range of fGa/Zn = 1.3–2.7, depending on the citrate concentration and/or Ga3+/Zn2+ ratio in the reacting solution. The absorption edge of the diffuse reflectance spectra suggests that the present particles possess a band gap of 4.7–4.9 eV, which is almost independent of the synthesis conditions.
Introduction
Particulate materials are one of the important categories of functional materials. Each particle will act as an independent unit of the functionality, and its properties often depend on its size due to its large specific area. Thus, monodisperse particles, which have a very narrow distribution of size, may be one of the ideal forms ensuring the functionality of particulate materials.1 The typical strategy for the synthesis of monodisperse particles is to make the growth history of all the particles the same by separating the short nucleation period from the following growth period, which is known as the LaMer mechanism.2 However, implementing this concept depends on the chemical reactions involved in the synthesis of the target substances. Hence, studies on designing and optimizing the reaction procedure are still essential to obtain monodisperse particles of various substances.Zinc gallium oxide (ZGO; zinc gallate), the target substance in this study, is a gallium compound that has a normal spinel structure, where Zn2+ and Ga3+ ions are located at the tetrahedral and octahedral positions, respectively (space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m).3,4 Although its stoichiometric formula is ZnGa2O4, the atomic ratio of Ga to Zn in ZGO, fGa/Zn, can be varied, while keeping its spinel structure.5
m).3,4 Although its stoichiometric formula is ZnGa2O4, the atomic ratio of Ga to Zn in ZGO, fGa/Zn, can be varied, while keeping its spinel structure.5
ZGO is a wide-bandgap semiconducting material (Eg ∼ 5 eV)6 applied in electronic devices.7–10 ZGO is also expected to be a light-emitting material, typically by doping activating ions such as Mn2+ ions11–13 or lanthanide ions.14,15 Also, Cr3+ and Gd3+ ions are especially interesting due to their persistent luminescence property, which is useful for in vivo imaging.4,16–21 In addition, two or three types of ions are sometimes co-doped to enhance the functionality of the emitted light.22–27 Another application of ZGO is as a photocatalyst, which has been widely studied for splitting water,28,29 reducing CO2,30–32 degrading organic compounds,33–37etc.
There are various reports on the synthesis of ZGO, including physical and chemical methods. The former includes RF sputtering,10 chemical vapor deposition,6,9,38 and pulse laser deposition.13 These procedures enable the production of a thin film structure on a substrate under dry conditions suitable for the fabrication of electronic devices.
In the case of the latter, one of the typical procedures is the solid-phase reaction, in which ZnO and Ga2O3 powders are mixed and heated at ca. 1000 °C to form the ZGO phase.4,7,12,16,39 In this method, ZnGa2O4 may be formed by mixing the raw materials in a stoichiometric ratio. However, sufficient mixing, grinding, and pressing, in addition to high temperature, are essential for the reaction to progress efficiently at an adequate reaction rate.
The calcination of various precursors is also often employed to obtain ZGO solids at lower temperatures than the solid-phase reaction. Gel-like hydroxides formed by adding NH3 to the metal ion solution are typical of the precursor.23,33 Layered double hydroxides (LDH)40 and polyethylene oxide xerogels32 are other types of precursors applied. Duan et al.41 employed citric acid to form precursor gels in the aqueous phase. Li et al.36 utilized metal–acetylacetonate complexes in a homogeneous ethanol solution.
Liquid-phase processes in hydrothermal synthesis are applicable to obtain particulate forms of ZGO based on the following reaction:
| Zn2+ + 2Ga3+ + 8OH− → ZnGa2O4 + 4H2O | (1) |
Given that a series of different-sized monodisperse particles enables us to evaluate their size-dependent properties, including size independence, it is desirable to establish synthesis procedures that generate monodisperse particles with systematically controlled sizes of the target materials. The control of the metal ion composition is also essential if the target multi-metal oxide allows non-stoichiometric compositions. In this sense, the contribution of citrate ions to the formation of ZGO particles is a worthwhile area of study. However, the precise effect of citrate ions has not been demonstrated in a hydrothermal system for the synthesis of monodisperse ZGO particles, despite the effect of the dopant Co2+ ions as a size controller.19 Moreover, the influence of citrate ions on the composition of ZGO has not been evaluated, despite their ability to form complexes with Zn2+ and Ga3+ ions.
Thus, in this study, we propose a procedure for the synthesis of monodisperse spherical ZGO particles using trisodium citrate under hydrothermal conditions, allowing the systematic variation of their particle size and Ga/Zn ratio. The role of citrate ions in the formation process is discussed. Also, the band-gap energy and the fluorescence spectra of the present particles are evaluated.
Experimental
Materials
The chemicals used are Zn(NO3)2·6H2O (99.9%), Ga(NO3)3·nH2O (99.9%), trisodium citrate dihydrate (GR), and NaOH (1 mol L−1, for volumetric titration), which were purchased from FUJIFILM Wako Pure Chemicals, Japan, and used without further purification.Hydrothermal synthesis of ZGO particles
The procedure for the synthesis of the ZGO particles in the present study was typically as follows. Distilled water (8.5 mL), 0.1 mol L−1 Zn(NO3)2 aqueous solution (2.5 mL), and 0.1 mol L−1 Ga(NO3)3 aqueous solution (5 mL) were mixed in a glass vessel and placed in a water bath at 40 °C. Then, under magnetic stirring, 0.25 mol L−1 trisodium citrate (Na3-Cit) aqueous solution (4 mL) and 0.42 mol L−1 NaOH aqueous solution (5 mL) were introduced into it in that order. After being kept at 40 °C for 60 min with stirring, the mixed solution was transferred to a Teflon-lined autoclave and placed in an air oven at 150 °C for 24 h. The formed precipitate was separated from the aqueous phase by centrifuging at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. After removing the supernatant solution, the precipitate was redispersed in distilled water and centrifuged; this process was repeated once more. Then, the precipitate was freeze-dried.
000 rpm for 15 min. After removing the supernatant solution, the precipitate was redispersed in distilled water and centrifuged; this process was repeated once more. Then, the precipitate was freeze-dried.
The reactant concentrations after mixing correspond to CZn = 10 mmol L−1, CGa = 20 mmol L−1, Ccit = 40 mmol L−1, and CNaOH = 84 mmol L−1 for Zn(NO3)2, Ga(NO3)3, Na3-Cit, and NaOH, respectively, under the typical conditions. In the case of varying the CGa/CZn ratio, the total concentration of the metal ions was kept constant as CZn + CGa = 30 mmol L−1; CNaOH = 1.05 × (2CZn + 3CGa) as equivalent to 5% excess to the stoichiometric formation of Zn(OH)2 and Ga(OH)3. Also, the total volume was set to 25 mL in all conditions by changing the volume of distilled water when Ccit and/or CNaOH were varied.
Characterization
The size and shape of the particles were evaluated via TEM (Hitachi H-7650, operated at 100 kV) and FE-SEM (JEOL JSM-6700F, operated at 15 kV; the specimens were coated with Pt). Their crystal structure was identified by powder XRD analysis using a Bruker D8 Advance (Cu Kα, λ = 1.5418 Å).Atomic absorption spectrometry (AAS) was employed to estimate the elemental composition of the particles by determining the Zn2+ and Ga3+ ions remaining in the supernatant solution after the hydrothermal reaction. The supernatant solution (4 mL) was diluted to 20 mL, which also contained HNO3 at 0.3 mol L−1, to apply AAS measurement using an air/acetylene flame with a Varian SpectrAA55 at absorption lines of λ = 213.9 nm and 294.4 nm for Zn and Ga, respectively. TEM-EDX measurement was also applied to the ZGO particles to ensure the AAS determination of their composition.
To estimate the band-gap energy, Eg, a Shimadzu UV-3100PC spectrophotometer with an integral sphere was used to obtain the diffuse reflectance spectra of the ZGO particles dispersed in BaSO4 as the matrix at 1 wt%. Photoluminescent spectra were measured using a JASCO FP-8300 fluorescence spectrometer.
Results and discussion
Formation of monodisperse ZGO particles with Na3-Cit
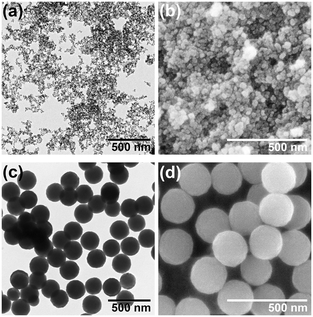
Fig. 1 shows the TEM and FE-SEM images of the ZGO particles hydrothermally synthesized at CGa/CZn = 2 (150 °C, 24 h) in the absence and presence of Na3-Cit. Under the former condition (i.e., Ccit = 0 mmol L−1), the precipitate is in the form of nanoparticles having mean diameter (D) ± one standard deviation (σ) = 9.5 nm ± 2.1 nm (Fig. 1a and b). Conversely, under the latter condition (Ccit = 40 mmol L−1), monodisperse spherical particles with D ± σ = 209 nm ± 20 nm are obtained (Fig. 1c and d; the coefficient of variation, COV, is 9.6%), indicating the effectiveness of Na3-Cit in increasing the size of the ZGO particles. In addition, the relative size distribution is narrower in the presence of Na3-Cit, improving the monodispersity.Fig. 2 indicates the XRD patterns of the ZGO particles shown in Fig. 1. The peaks are located at the same diffraction angles in both patterns and match with that of ZnGa2O4 with a spinel structure (ICDD PDF 01-071-0843). Meanwhile, the peak widths are different, reflecting their particle sizes, implying that the crystallite size increases with the growth of the particles in the presence of Na3-Cit. In fact, the continuous lattice pattern in the HR-TEM image in Fig. S1 in the SI suggests the single crystalline structure of the ZGO particles. According to the diffraction angle, 2θ = 35.56°, of the strongest peak for the 311 reflection in Fig. 2b, the lattice parameter for the spinel cubic cell is estimated as a = 8.37 Å, which is in good agreement with the literature (a = 8.33 Å).3
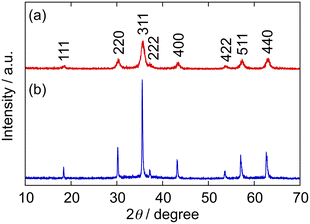 | ||
| Fig. 2 XRD patterns of the precipitates shown in Fig. 1 (CGa/CZn = 2) obtained at Ccit = (a) 0 and (b) 40 mmol L−1 (Cu Kα radiation, λ = 1.5418 Å). The peaks are consistent with those for ZnGa2O4 (ICDD PDF 01-071-0843). | ||
The presence of Na3-Cit affects the composition and the yield of the particles. The AAS measurement indicated that virtually all the Zn2+ ions were converted to the solid phase from the aqueous solution, irrespective of the Ccit conditions. Conversely, 5.1% and 22.4% of unreacted Ga3+ ions were detected in the supernatant solution for Ccit = 0 and 40 mmol L−1 after 24 h reaction at 150 °C, giving non-stoichiometric compositions of the ZGO particles as ZnGa1.90O3.85 and ZnGa1.55O3.33, respectively, due to the reaction condition of CGa/CZn = 2 for these particles. The Ga/Zn atomic ratios in the particles, fGa/Zn, were also confirmed by TEM-EDX analysis; the estimated compositions by TEM-EDX were in good agreement with that obtained from AAS (Fig. S2 in SI). Thus, the yield, y, was calculated to be y = 96.7% and 85.0% for Ccit = 0 and 40 mmol L−1, respectively, by defining y as follows:
| y = (xZnCZn + xGaCGa)/(CZn + CGa) | (2) |
Na3-Cit also affects the behavior of the reacting solution before hydrothermal treatment is applied. In the absence of Na3-Cit, white precipitates were immediately formed upon introducing the NaOH solution into the solution of metal ions. According to the AAS measurement, it was estimated that at this stage, 99.9% of Zn2+ ions and 66.6% of Ga3+ ions coprecipitated from the solution phase containing them at CZn = 10 mmol L−1 and CGa = 20 mmol L−1, respectively. Although the XRD pattern (Fig. S3 in SI) suggests that the white precipitate contains the Zn/Ga-LDH phase,40,47 an amorphous hydroxide phase might also exist, given that the Ga3+ content, fGa/Zn = 1.33, seems to be much higher compared to that expected from general LDHs (typically fM3+/M2+ = 0.2 − 0.5).48,49
On the contrary, for Ccit = 40 mmol L−1, the mixed solution remained transparent even after the addition of NaOH due to the formation of complex species that lower the free Zn2+ ion concentration, preventing coprecipitation before the temperature was elevated to hydrothermal conditions. Although both Zn2+ and Ga3+ ions are amphoteric, the solubilities of their hydroxides are quite different around the reaction pH range (pH 9.0 ± 0.5; including the conditions below). The solubilities of Zn(OH)2 and Ga(OH)3 can be estimated to be 1.5 × 10−6 mol L−1 and 4.0 × 10−2 mol L−1, respectively, at pH 9.0 from the equilibrium constants at room temperature (Fig. S4 in SI).50 Therefore, the central role of Na3-Cit is as a complexing agent for Zn2+ ions51 to achieve controlled precipitation from the homogeneous solution phase at the hydrothermal temperature.
Reflecting the role of Na3-Cit, the addition order of the reactant solutions was critical, where even in the presence of Na3-Cit, tiny ZGO particles, similar to that in Fig. 1a, were formed if the NaOH solution was added prior to the Na3-Cit solution. In this case, the white precipitate remained even after the introduction of Na3-Cit; Na3-Cit did not dissolve the white precipitate effectively once it had formed. This result supports the importance of homogeneous nucleation at the hydrothermal temperature for monodisperse ZGO particles.
Here, it should be noted that no precipitate was formed by hydrothermal treatment in the absence of Zn2+ ions, owing to the low free Ga3+ ion concentration due to the formation of a complex with citrate ions,52 in addition to [Ga(OH)4]−.50 Thus, the present system may be classified as a coprecipitation process, triggered by the release of Zn2+ ions.
Effect of Na3-Cit concentration
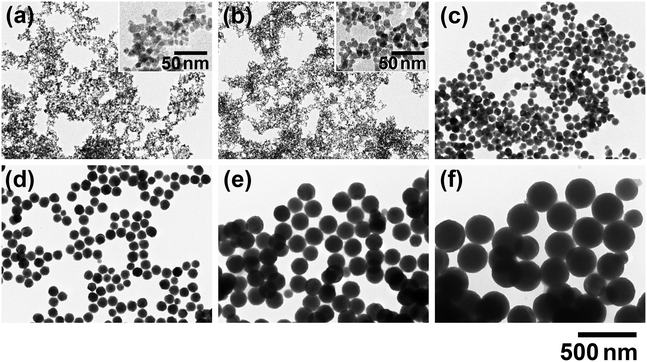
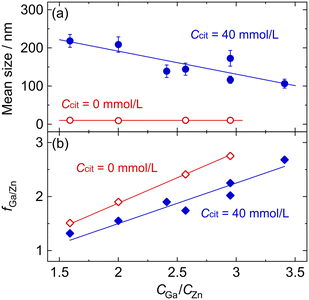
To evaluate the effect of Na3-Cit more precisely, ZGO particles were synthesized under various Ccit conditions in the same manner at 150 °C for 24 h, where CGa/CZn = 2.57 to increase the fGa/Zn ratio to around the stoichiometric value. The TEM images of the ZGO particles are indicated in Fig. 3; the histogram of the particle size for each Ccit condition is shown in Fig. S5 in the SI; the values of D, σ, and COV are listed in Table S1 in SI. Also, Fig. 4 shows the dependence of D and fGa/Zn on Ccit. In the region at Ccit ≥ 25 mmol L−1, the size of the ZGO particles increased up to D ± σ = 240 nm ± 23 nm at Ccit = 60 mmol L−1, depending on Ccit. The particle shape seems spherical under each Ccit condition, although the particles tend to coagulate when Ccit exceeds 60 mmol L−1.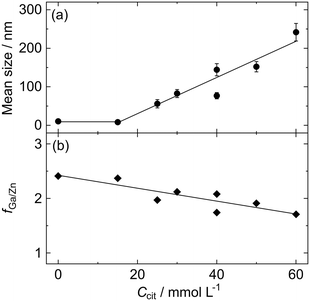 | ||
| Fig. 4 Effect of the Na3-Cit concentration (Ccit) on (a) the mean size and (b) atomic Ga/Zn ratio (fGa/Zn) of the ZGO particles synthesized under the conditions at CGa/CZn = 2.57 and 150 °C for 24 h. | ||
On the contrary, there seems to be no contribution from Na3-Cit to the particle size at Ccit = 15 mmol L−1; the particles (Fig. 3b) are almost the same as that at Ccit = 0 mmol L−1 (Fig. 3a). This should result in the formation of white precipitate upon the addition of NaOH due to the insufficient complex formation with Zn2+. In contrast, at Ccit ≥ 25 mmol L−1, the reacting solutions maintained their transparent state until being transferred to the autoclave, indicating that it is important to maintain the homogenous solution state until elevating the temperature to ensure the effectiveness of Na3-Cit for the controlled synthesis of ZGO particles. However, reflecting the complex formation property, the fGa/Zn value decreased from 2.41 at Ccit = 0 mmol L−1 to 1.71 at Ccit = 60 mmol L−1 (Fig. 4b).
The size of monodisperse particles reflects the number of particles formed from the point of view of the mass-balance relationship. This means that a larger particle size corresponds to a smaller number of spontaneous nuclei. According to the spontaneous nucleation model for monodisperse particles, the number of formed particles, n, is expected to be proportional to the supply rate of the reactant species, Qo, and inversely proportional to the volume increase rate, ![[small upsilon, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e158.gif) , during the nucleation stage as follows:
, during the nucleation stage as follows:
n = QoVm/![[small upsilon, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e158.gif) | (3) |
![[small upsilon, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e158.gif) . In the present case, the former may be more critical. That is, a higher Ccit would make the complexes more stable, inhibiting the release of the Zn2+ and Ga3+ ions. On the contrary, it seems that there is no reason for the increased intrinsic growth rate of ZGO particles in the presence of citrate ions; instead, citrate ions could reduce the growth rate if they adsorb onto the particle surface.
. In the present case, the former may be more critical. That is, a higher Ccit would make the complexes more stable, inhibiting the release of the Zn2+ and Ga3+ ions. On the contrary, it seems that there is no reason for the increased intrinsic growth rate of ZGO particles in the presence of citrate ions; instead, citrate ions could reduce the growth rate if they adsorb onto the particle surface.
Precisely, this equation requires a consistent yield among the conditions for a quantitative discussion, and the present results do not meet this requirement; y = 95.5% at Ccit = 0 mmol L−1, whereas y = 75.9% at Ccit = 60 mmol L−1, reflecting a decrease in fGa/Zn with an increase in Ccit, despite the almost 100% conversion for Zn2+. However, the size control mechanism cannot be explained in terms of the yield difference, given that larger ZGO particles are formed under lower yield conditions, as indicated in Fig. 4. Thus, the discussion on eqn (3) has a certain validity at least qualitatively.
Effect of Ga/Zn ratio in the reacting solution
To evaluate the effect of the Ga/Zn ratio in the reacting solution, ZGO particles were synthesized under various CGa/CZn conditions (CGa/CZn = 1.59–3.41, CZn + CGa = 30 mmol L−1) at Ccit = 0 mmol L−1 and 40 mmol L−1. The spinel ZGO phase precipitated under all the tested conditions.Fig. 5 indicates the particle size, D, and the Ga/Zn ratio in the ZGO particles, fGa/Zn, as a function of CGa/CZn. In the absence of Na3-Cit, the CGa/CZn ratio does not contribute to altering D (= ca. 10 nm) by reflecting the formation of a white precipitate before the hydrothermal treatment, similar to the above-mentioned cases. Alternatively, in the presence of Na3-Cit, an increase in CGa/CZn (i.e., higher CGa and lower CZn) results in a smaller D (i.e., larger n). This result supports that the free Zn2+ ion concentration is the determining parameter for D, according to the comparison of the tendency in Fig. 3 and 4a, in which a smaller D tends to decrease under lower Ccit conditions, which gives a relatively higher concentration of free Zn2+ and Ga3+ ions.
On the contrary, fGa/Zn proportionally depends on CGa/CZn under both Ccit conditions with different slopes, as shown in Fig. 5b. The slopes of 0.94 and 0.75 for Ccit = 0 mmol L−1 and 40 mmol L−1 imply about 6% and 25% of Ga3+ ions remain in the supernatant phase, irrespective of CGa/CZn, under the respective Ccit conditions after the reaction, respectively. As discussed above, this conversion inefficiency of Ga3+ would be due to the larger stability constants of its complexes.
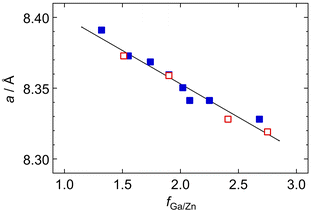
Although the spinel structure was maintained within the CGa/CZn range (Fig. S6 in SI), the lattice parameter for its cubic cell, a, slightly differs among the particles depending on the fGa/Zn value. As shown in Fig. 6, the value of a, estimated from the diffraction angle for the 311 reflection, linearly decreases with fGa/Zn but is independent of the Na3-Cit condition. This result seems to be reasonable given that the ionic radius of Ga3+ is smaller than that of Zn2+, i.e., 0.61 Å and 0.74 Å for 4-coordinate Ga3+ and Zn2+ ions; 0.76 Å and 0.88 Å for 6-coordinate Ga3+ and Zn2+ ions, respectively.54
Band gap estimation from the diffuse reflectance spectra
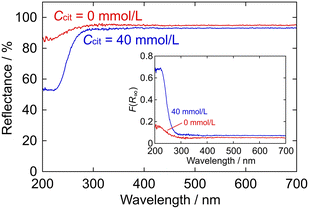
Fig. 7 shows the diffuse reflectance spectra of the ZGO particles (D = 9.5 nm and fGa/Zn = 1.90 for Ccit = 0 mmol L−1; D = 138 nm and fGa/Zn = 1.90 for 40 mmol L−1). The Kubelka–Munk function, F(R∞), converted as follows:| F(R∞) = K/S = (1 − R∞)2/2R∞ | (4) |
To estimate the band gap energy, Eg, [F(R∞)hν]2 is plotted as a function of hν in Fig. 8 based on the Tauc relationship for the direct allowed transition, as follows:
| (αhν)2 = A(hν − Eg), | (5) |
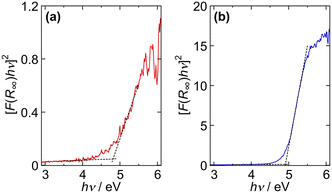 | ||
| Fig. 8 Tauc plots for the ZGO particles using the F(R∞) values in the inset of Fig. 7. The ZGO particles were prepared at (a) Ccit = 0 mmol L−1 (fGa/Zn = 1.90 and D = 9.5 nm) and (b) Ccit = 40 mmol L−1 (fGa/Zn = 1.90 and D = 138 nm). | ||
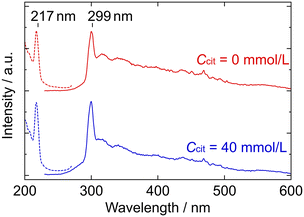
Fig. 9 shows the fluorescence spectra of the ZGO particles, where the wavelength range for the excitation spectra corresponds to the photon energy Ep ≥ 4.6 eV. The excitation and emission peaks are located at 217 nm and 299 nm, respectively; the peak wavelengths are independent of the Ccit conditions, whereas the peak intensity is somewhat higher in the presence of Na3-Cit, which is probably due to the size difference. Bluish-white light fluorescence was observed from each ZGO powder, reflecting the continuous emission spectrum in the visible region.
Among the present ZGO particles, there seems to be almost no difference in their optical properties, except for their diffuse reflectance. Their similar Eg values and FL spectra imply a consistent band structure, at least in the present particle size range (D = 10–200 nm).
Conclusions
The present study has successfully established a procedure for the synthesis of monodisperse zinc gallium oxide (ZGO) nanoparticles, enabling systematic control over their size and composition via the hydrothermal process, utilizing trisodium citrate as a complexing agent. The size of the ZGO particles is tuneable up to about 200 nm based on the citrate concentration, Ccit. The compositional ratio of Ga to Zn in the particles, fGa/Zn, depends on the Ga/Zn ratio in the reacting solution, CGa/CZn, and the Ccit level. At least in the range of fGa/Zn = 1.3–2.7, all the prepared ZGO particles possess a spinel structure; however, their lattice parameter slightly decreased at a higher fGa/Zn. The absorption edges in the diffuse reflectance spectra indicate a band gap energy of Eg = 4.7–4.9 eV, which is almost independent of particle size and composition. The photoluminescence properties are also similar among the particle sizes, where their excitation and emission maxima are located at 217 nm and 299 nm, respectively. Due to the wide-range emission spectrum of the ZGO particles, they exhibit blueish-white light emission under a sufficiently short wavelength of UV light.Author contributions
F. S. designed the study and prepared the manuscript. F. S. and N. K. synthesized and characterized the particles. All the authors discussed the results.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI).Supplementary information: HR-TEM image, TEM-EDX spectra, XRD pattern of the white precipitate, solubility estimations for the metal hydroxides, histograms of size for the ZGO particles, XRD peak shift by the ZGO composition, and Tauc's plots for the ZGO particles of different composition are available. See DOI: https://doi.org/10.1039/d5ce00952a.
Acknowledgements
The XRD patterns were obtained at the Center for Analytical Instrumentation, Chiba University.Notes and references
- T. Sugimoto, Monodisperse Particles, Elsevier, Amsterdam, 2nd edn, 2019 Search PubMed.
- V. K. LaMer and R. H. Dinegare, J. Am. Chem. Soc., 1950, 72, 4847 CrossRef CAS.
- J. Hornstra and E. Keulen, Philips Res. Rep., 1972, 27, 76 CAS.
- M. Allix, S. Chenu, E. Veŕon, T. Poumeyrol, E. A. Kouadri-Boudjelthia and F. Fayon, Chem. Mater., 2013, 25, 1600 CrossRef CAS.
- M. Hirano, M. Imai and M. Inagaki, J. Am. Ceram. Soc., 2000, 83, 977 CrossRef CAS.
- E. Chikoidze, C. Sartel, I. Madaci, H. Mohamed, C. Vilar, B. Ballesteros, F. Belarre, E. del Corro, P. Vales-Castro, G. Sauthier, L. Li, M. Jennings, V. Sallet, Y. Dumont and A. Peŕez-Tomaś, Cryst. Growth Des., 2020, 20, 2535 CrossRef CAS.
- J. Xue, S. Wu and J. Li, J. Am. Ceram. Soc., 2013, 96, 2481 CrossRef CAS.
- Y. Teng, L. X. Song, W. Liu, Z. Y. Xu, Q. S. Wang and M. M. Ruan, J. Mater. Chem. C, 2016, 4, 3113 RSC.
- P.-H. Huang, Y.-C. Shen, C.-Y. Tung, C.-Y. Huang, C. S. Tan and R.-H. Horng, ACS Appl. Electron. Mater., 2020, 2, 3515 CrossRef CAS.
- A. K. Singh, C.-C. Yen, S.-M. Huang, C.-Y. Huang, H.-Y. Chou and D.-S. Wuu, ACS Appl. Electron. Mater., 2024, 6, 1356 CrossRef CAS.
- H. S. Roh, Y. C. Kang, S. B. Park and H. D. Park, Jpn. J. Appl. Phys., 2002, 41, 4559 CrossRef CAS.
- J. S. Kim, S. G. Lee, H. L. Park, J. Y. Park and S. D. Han, Mater. Lett., 2004, 58, 1354 CrossRef CAS.
- T. Dazai, S. Yasui, T. Taniyama and M. Itoh, Inorg. Chem., 2020, 59, 8744 CrossRef CAS.
- T. A. Safeera and E. I. Anila, Scr. Mater., 2018, 143, 94 CrossRef CAS.
- N. D. Hebbar, K. S. Choudhari, N. Pathak, S. A. Shivashankar and S. D. Kulkarni, New J. Chem., 2022, 46, 7032 RSC.
- A. Bessière, S. K. Sharma, N. Basavaraju, K. R. Priolkar, L. Binet, B. Viana, A. J. J. Bos, T. Maldiney, C. Richard, D. Scherman and D. Gourier, Chem. Mater., 2014, 26, 1365 CrossRef.
- T. Luan, J. Liu, X. Yuan and J.-G. Li, Nanoscale Res. Lett., 2017, 12, 219 CrossRef.
- W. Fan, N. Lu, C. Xu, Y. Liu, J. Lin, S. Wang, Z. Shen, Z. Yang, J. Qu, T. Wang, S. Chen, P. Huang and X. Chen, ACS Nano, 2017, 11, 5864 CrossRef CAS PubMed.
- E. Arroyo, B. Medrán, V. Castaing, G. Lozano, M. Ocaña and A. I. Becerro, J. Mater. Chem. C, 2021, 9, 4474 RSC.
- X. Wei, S. V. Kershaw, X. Huang, M. Jiao, C. C. Beh, C. Liu, M. Sarmadi, A. L. Rogach and L. Jing, J. Phys. Chem. Lett., 2021, 12, 7067 CrossRef CAS.
- B. B. Srivastava, S. K. Gupta and Y. Mao, CrystEngComm, 2020, 22, 2491 RSC.
- T. Maldiney, B.-T. Doan, D. Alloyeau, M. Bessodes, D. Scherman and C. Richard, Adv. Funct. Mater., 2015, 25, 331 CrossRef CAS.
- D. P. Dutta, R. Ghildiyal and A. K. Tyagi, J. Phys. Chem. C, 2009, 113, 16954 CrossRef CAS.
- A. Tuerdi and A. Abdukayum, RSC Adv., 2019, 9, 17653 RSC.
- B. B. Srivastava, S. K. Gupta and Y. Mao, J. Mater. Chem. C, 2020, 8, 6370 RSC.
- Y. Liu, T. Zheng, X. Zheng and C. Chen, Sci. Rep., 2023, 13, 14430 CrossRef CAS.
- C. Fang, S. Wang, S. Wei, Q. Xu, Z. Lyu, S. Shen, T. Tan and H. You, Dalton Trans., 2024, 53, 6377 RSC.
- N. Kumagai, L. Ni and H. Irie, Chem. Commun., 2011, 47, 1884 RSC.
- T. Zheng, Y. Xia, X. Jiao, T. Wang and D. Chen, Nanoscale, 2017, 9, 3206 RSC.
- Q. Liu, D. Wu, Y. Zhou, H. Su, R. Wang, C. Zhang, S. Yan, M. Xiao and Z. Zou, ACS Appl. Mater. Interfaces, 2014, 6, 2356 CrossRef CAS.
- Y. Zhang, P. Li, L.-Q. Tang, Y.-Q. Li, Y. Zhou, J.-M. Liu and Z.-G. Zo, Dalton Trans., 2017, 46, 10564 RSC.
- M. Takemoto, Y. Tokudome, K. Teramura, T. Tanaka, A. Nakahira and M. Takahashi, RSC Adv., 2020, 10, 8066 RSC.
- X. Chen, H. Xue, Z. Li, L. Wu, X. Wang and X. Fu, J. Phys. Chem. C, 2008, 112, 20393 CrossRef CAS.
- M. Sun, D. Li, W. Zhang, Z. Chen, H. Huang, W. Li, Y. He and X. Fu, J. Solid State Chem., 2012, 190, 135 CrossRef CAS.
- L. Liu, J. Huang, L. Cao, J. Wu, J. Fei, H. Ouyang, F. Ma and C. Zhou, Mater. Lett., 2013, 95, 160 CrossRef CAS.
- X. Li, X. Zhang, X. Zheng, Y. Shao, M. He, P. Wang, X. Fu and D. Li, J. Mater. Chem. A, 2014, 2, 15796 RSC.
- J. Li, W. Lu, H. Wu, L. Jin, B. Hu, L. Li and Z. Wang, Mater. Sci. Semicond. Process., 2016, 56, 251 CrossRef.
- S. Y. Bae, H. W. Seo, C. W. Na and J. Park, Chem. Commun., 2004, 1834 RSC.
- A. R. Phani, S. Santucci, S. Di Nardo, L. Lozzi, M. Passacantando and P. Picozzi, J. Mater. Sci., 1998, 33, 3969 CrossRef CAS.
- L. Zou, X. Xiang, M. Wei, F. Li and G. Evans, Inorg. Chem., 2008, 47, 1361 CrossRef CAS PubMed.
- X. L. Duan, D. R. Yuan, L. H. Wang, F. P. Yu, X. F. Cheng, Z. Q. Liu and S. S. Yan, J. Cryst. Growth, 2006, 296, 234 CrossRef CAS.
- M. Hirano, J. Mater. Chem., 2000, 10, 469 RSC.
- M. Hirano and N. Sakaida, J. Am. Ceram. Soc., 2002, 85, 1145 CrossRef CAS.
- F. Conrad, Y. Zhou, M. Yulikov, K. Hametner, S. Weyeneth, G. Jeschke, D. Günther, J.-D. Grunwaldt and G. R. Patzke, Eur. J. Inorg. Chem., 2010, 2036 CrossRef CAS.
- M. Hirano and N. Sakaida, J. Ceram. Soc. Jpn., 2003, 111, 0176 CrossRef CAS.
- W. Yang, J. Li, X. Zhang, C. Zhang, X. Jiang and B. Liu, Inorg. Chem., 2019, 58, 549 CrossRef CAS.
- K. Fuda, N. Kudo, S. Kawai and T. Matsunaga, Chem. Lett., 1993, 777 CrossRef CAS.
- R. Von Allmann, Chimia, 1970, 24, 99 CrossRef.
- Y. Zhang, H. Xu and S. Lu, RSC Adv., 2021, 11, 24254 RSC.
- Y. F. Orlov and E. I. Belkina, Russ. J. Inorg. Chem., 2011, 56, 975 CrossRef CAS.
- N. C. Li, A. Lindenbaum and J. M. White, J. Inorg. Nucl. Chem., 1959, 12, 122 CrossRef CAS.
- W. R. Harris and A. E. Martell, Inorg. Chem., 1976, 15, 713 CrossRef CAS.
- T. Sugimoto, F. Shiba, T. Sekiguchi and H. Itoh, Colloids Surf., A, 2000, 164, 183 CrossRef CAS.
- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, Wiley, New York, 6th edn, 1999, p. 1303 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |