Synthesis of energetic materials derived from hydroxynitropyrazine
Xiu'e
Jiang
,
Zeyu
Xu
,
Mingren
Fan
,
Ruihui
Wang
,
Yi
Wang
 * and
Qinghua
Zhang
* and
Qinghua
Zhang
 *
*
National Key Laboratory of Solid Propulsion, Northwestern Polytechnical University, Xi'an, Shanxi 710065, China. E-mail: ywang0521@nwpu.edu.cn; qinghuazhang@nwpu.edu.cn
First published on 7th November 2025
Abstract
Energetic materials serve as one of the cornerstone materials for the development of the defense industry, which is why the research and development of novel energetic materials have consistently attracted significant attention. The emergence and advancement of energetic salts have provided breakthrough strategies for the design of a new generation of energetic materials. In this study, hydroxynitropyrazine compounds were selected as energetic frameworks and assembled with organic cations and inorganic metal ions, respectively, leading to the successful synthesis of salts 1, 2 and 3. Their structures were unambiguously confirmed by single-crystal X-ray diffraction analysis. Furthermore, compounds 1–3 all exhibit low sensitivity (IS > 50 J, FS > 360 N), excellent thermal stability (thermal decomposition temperatures ranging from 205 °C to 273 °C), and good detonation performance (detonation velocities reaching 7273–8106 m s−1), making them promising candidate systems for insensitive energetic materials. The successful preparation of these three compounds demonstrates that hydroxynitropyrazine-based energetic frameworks can effectively balance energy and sensitivity.
1. Introduction
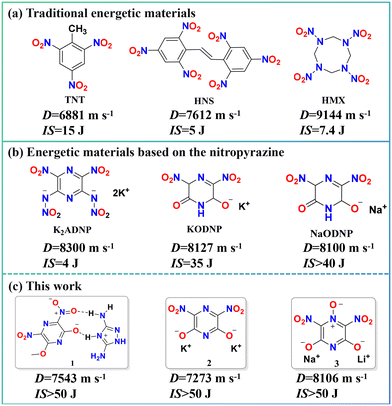
Energetic materials serve as critical functional components in modern weapon systems, determining their overall performance characteristics.1–3 As weapon systems continue to advance toward higher performance standards, the requirements for the comprehensive properties of energetic materials are increasingly demanding. However, energetic compounds are intrinsically metastable substances characterized by an inherent trade-off between energy output and safety.4–6 For instance, conventional 2,4,6-trinitrotoluene (TNT) meets the safety requirements for practical use but exhibits relatively low energy performance, which falls short of the damage power demanded by modern high-technology weaponry. In contrast, high energy-density compounds such as 1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) offer superior detonation performance, yet their high mechanical sensitivity poses considerable safety challenges throughout production, transportation, storage, and usage (Fig. 1a).7–9 Consequently, effectively reconciling the inherent conflict between energy output and safety remains a core scientific challenge in the field of energetic materials.The development of energetic salts has provided efficient pathways to address performance enhancement and the creation of novel energetic compounds.10–12 Energetic salts are energetic compounds composed of energetic cations and energetic anions combined via ionic bonds.13–15 Compared to single-component compounds, energetic salts enable the simultaneous modulation of multiple properties and allow for tailored design and performance optimization by leveraging the extensive library of existing energetic compounds as building blocks.16–18 These materials are primarily constructed by incorporating energetic ions into a unified molecular architecture through intramolecular and intermolecular interactions, including hydrogen bonding and ionic bonding, resulting in energetic assemblies with well-defined structures and customized properties.19–21 Therefore, the rational combination and pairing of energetic cations and anions can effectively balance energy output and safety, leading to energetic materials with comprehensively enhanced performance.
The properties of energetic salts are primarily determined by the selection of energetic frameworks. Therefore, choosing suitable energetic frameworks prior to design and synthesis is crucial for obtaining high-performance energetic materials.22,23 Hydroxynitropyrazine derivatives exhibit outstanding characteristics such as high heat of formation, high density, and high energy, making them a prominent class of energetic frameworks for developing novel energetic salts (Fig. 1b).24–26 Furthermore, the hydroxyl groups on the pyrazine framework can provide multiple ionizable sites, facilitating the formation of diverse assemblies. Simultaneously, the nitro and hydroxyl groups can serve as hydrogen-bond acceptors and donors, respectively, promoting the formation of an extensive hydrogen-bonding network, which effectively enhances the overall performance of the resulting energetic materials. Leveraging these advantages, this study employs hydroxynitropyrazine as an energetic framework (Fig. 1c). On the one hand, novel energetic salts were constructed through hydrogen bond-mediated self-assembly with energetic organic cations; on the other hand, structurally stable energetic metal salts were formed through coordination between the oxygen atoms of hydroxyl groups and inorganic metal ions. This work systematically demonstrates the application potential of the hydroxynitropyrazine framework in self-assembly strategies. The resulting materials were thoroughly characterized in terms of their structure and properties, with a systematic evaluation of their energy and sensitivity characteristics.
2. Results and discussion
2.1. Synthesis
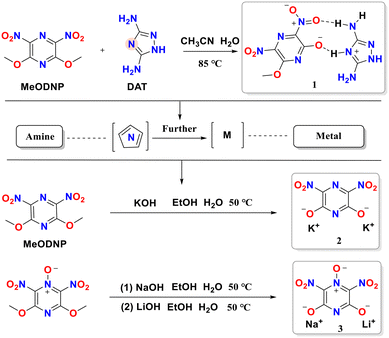
The energetic framework 2,6-dimethoxy-3,5-dinitropyrazine (MeODNP) and its N-oxide (2,6-dimethoxy-3,5-dinitropyrazine-1-oxide) were synthesized according to previously reported methods.27,28 This work first employed the aromatic amine organic base 3,5-diamino-1,2,4-triazole (DAT) to catalyze the hydrolysis of MeODNP. Specifically, both reactants were dissolved in a water–acetonitrile mixed solvent, and the target product was efficiently obtained via a one-pot reaction. This reaction selectively converted the methoxy groups on the pyrazine ring into hydroxyl groups, which act as hydrogen bond donors, while the nitrogen atoms on the DAT ring served as hydrogen bond acceptors, ultimately leading to the successful construction of energetic salt 1 through an extensive hydrogen-bonding network. Furthermore, we treated MeODNP with the more reactive inorganic base KOH for hydrolysis, successfully preparing energetic salt 2. To introduce additional sites and diversify the assembly modes, we further used 2,6-dimethoxy-3,5-dinitropyrazine-1-oxide as the framework and conducted a stepwise reaction by sequentially adding one equivalent of NaOH and LiOH, successfully constructing a heterobimetallic energetic salt 3 (Scheme 1).2.2. Single-crystal structure analysis
The samples of compounds 1–3 suitable for single-crystal diffraction were obtained by direct crystallization from the reaction solution through natural overnight cooling. Detailed crystallographic data for 1·CH3CN·H2O, 2·3H2O and 3·4H2O are provided in the SI.
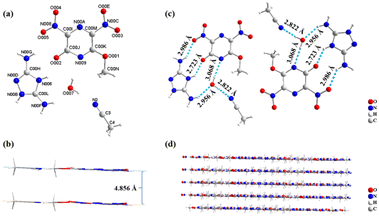
1·CH3CN·H2O (CCDC: 2445286) crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with a crystal density of 1.682 g cm−3 at 150 K with one molecule in each unit cell (Fig. 2a). As shown in Fig. 2b, all non-hydrogen atoms in the structure of 1·CH3CN·H2O (including those from the solvent components) essentially lie in the same plane, with an interplanar distance of 4.856 Å between the two molecular planes. Among them, the non-hydrogen atoms on the pyrazine and triazole rings are also coplanar, respectively, which is proven by the torsion angles C00N–O001–C00K–N009 0.3(3)°, O00E–N00C–C00M–N00A 2.8(3)°, and N00D–N00B–C00L–N00F 179.6(2)° (Table S4). In the planar layered structure, DAT and MeODNP molecules form an extensive hydrogen-bonding network with the solvent molecules (Fig. 2c). This includes the anticipated N006–H006⋯O002 (2.723(2) Å) hydrogen bond between the hydroxyl group of pyrazine and the nitrogen atom on the DAT ring. Additionally, water molecules act as both donors and acceptors to form three hydrogen bonds with acetonitrile, the nitrogen atom on the pyrazine ring, and the amino group of triazole (O007–H00I⋯N2 2.822(6) Å, O007–H00J⋯N009 3.068(3) Å, and N00F–H00F⋯O007 2.956(3) Å). Notably, a strong N00G–H00G⋯O005 2.986(3) Å) hydrogen bond is also observed between the nitro group of pyrazine and the amino group of triazole (Fig. 2c). The molecular planarity of 1·CH3CN·H2O, combined with its hydrogen-bonding networks, extends infinitely, ultimately leading to face-to-face stacking in the crystalline state (Fig. 2d).
, with a crystal density of 1.682 g cm−3 at 150 K with one molecule in each unit cell (Fig. 2a). As shown in Fig. 2b, all non-hydrogen atoms in the structure of 1·CH3CN·H2O (including those from the solvent components) essentially lie in the same plane, with an interplanar distance of 4.856 Å between the two molecular planes. Among them, the non-hydrogen atoms on the pyrazine and triazole rings are also coplanar, respectively, which is proven by the torsion angles C00N–O001–C00K–N009 0.3(3)°, O00E–N00C–C00M–N00A 2.8(3)°, and N00D–N00B–C00L–N00F 179.6(2)° (Table S4). In the planar layered structure, DAT and MeODNP molecules form an extensive hydrogen-bonding network with the solvent molecules (Fig. 2c). This includes the anticipated N006–H006⋯O002 (2.723(2) Å) hydrogen bond between the hydroxyl group of pyrazine and the nitrogen atom on the DAT ring. Additionally, water molecules act as both donors and acceptors to form three hydrogen bonds with acetonitrile, the nitrogen atom on the pyrazine ring, and the amino group of triazole (O007–H00I⋯N2 2.822(6) Å, O007–H00J⋯N009 3.068(3) Å, and N00F–H00F⋯O007 2.956(3) Å). Notably, a strong N00G–H00G⋯O005 2.986(3) Å) hydrogen bond is also observed between the nitro group of pyrazine and the amino group of triazole (Fig. 2c). The molecular planarity of 1·CH3CN·H2O, combined with its hydrogen-bonding networks, extends infinitely, ultimately leading to face-to-face stacking in the crystalline state (Fig. 2d).
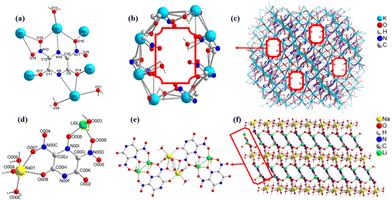
Compound 2·3H2O (CCDC: 2485166) crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with a calculated density of 2.124 g cm−3 at 100 K. The asymmetric unit contains six crystallographically independent K+ ions, a nitropyrazine framework, and three coordinated water molecules. The K+ ions form strong coordination bonds with two nitrogen atoms from the nitropyrazine ligand, with bond lengths of K3–N10 3.032(3) Å and K5–N9 3.289(3) Å. They also coordinate with five oxygen atoms, with bond lengths of K2–O16 2.816(3) Å, K3–O16 2.759(3) Å, K3–O19 2.743(3) Å, K8–O18 3.047(3) Å, K4–O17 2.865(3) Å, K5–O14 2.716(3) Å, and K6–O14 2.785(3) Å, respectively. In addition, coordination occurs with three water molecules, exhibiting bond lengths of K3–O13 2.670(3) Å, K5–O21 2.986(3) Å, and K5–O35 2.845(3) Å (Fig. 3a). As a result, each nitropyrazine ligand acts as a multidentate bridging ligand connecting six K+, extending into a porous three-dimensional network framework (Fig. 3c). The channels within this structure exhibit an irregular octagonal shape (Fig. 3b).
, with a calculated density of 2.124 g cm−3 at 100 K. The asymmetric unit contains six crystallographically independent K+ ions, a nitropyrazine framework, and three coordinated water molecules. The K+ ions form strong coordination bonds with two nitrogen atoms from the nitropyrazine ligand, with bond lengths of K3–N10 3.032(3) Å and K5–N9 3.289(3) Å. They also coordinate with five oxygen atoms, with bond lengths of K2–O16 2.816(3) Å, K3–O16 2.759(3) Å, K3–O19 2.743(3) Å, K8–O18 3.047(3) Å, K4–O17 2.865(3) Å, K5–O14 2.716(3) Å, and K6–O14 2.785(3) Å, respectively. In addition, coordination occurs with three water molecules, exhibiting bond lengths of K3–O13 2.670(3) Å, K5–O21 2.986(3) Å, and K5–O35 2.845(3) Å (Fig. 3a). As a result, each nitropyrazine ligand acts as a multidentate bridging ligand connecting six K+, extending into a porous three-dimensional network framework (Fig. 3c). The channels within this structure exhibit an irregular octagonal shape (Fig. 3b).
Compound 3·4H2O (CCDC: 2485167) crystallizes in the triclinic crystal system with the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Its crystal density, measured at 170 K, is 1.812 g cm−3. The asymmetric unit consists of one energetic framework, one Na+, one Li+, and four water molecules (Fig. 3d). As shown in Fig. 3e, two Na+ act as bridging centers connecting two energetic frameworks, forming a binuclear sodium-bridged motif. Four Li+ are symmetrically distributed on both sides and embedded between two energetic frameworks, thereby extending the molecular framework into a layered structure. Specifically, Na+ coordinates with oxygen atoms provided by both the energetic framework and three water molecules, exhibiting Na–O bond lengths between 2.257(5) and 2.892(5) Å. Meanwhile, Li+ ions coordinate with oxygen atoms from the energetic framework and one water molecule, with Li–O distances in the range of 1.911(11) to 2.024(11) Å. Fig. 3f illustrates a sandwich-like layered packing arrangement of compound 3·4H2O, in which one layer consists of Na+, the middle layer contains energetic frameworks embedded with Li+, and another layer is again composed of Na+.
. Its crystal density, measured at 170 K, is 1.812 g cm−3. The asymmetric unit consists of one energetic framework, one Na+, one Li+, and four water molecules (Fig. 3d). As shown in Fig. 3e, two Na+ act as bridging centers connecting two energetic frameworks, forming a binuclear sodium-bridged motif. Four Li+ are symmetrically distributed on both sides and embedded between two energetic frameworks, thereby extending the molecular framework into a layered structure. Specifically, Na+ coordinates with oxygen atoms provided by both the energetic framework and three water molecules, exhibiting Na–O bond lengths between 2.257(5) and 2.892(5) Å. Meanwhile, Li+ ions coordinate with oxygen atoms from the energetic framework and one water molecule, with Li–O distances in the range of 1.911(11) to 2.024(11) Å. Fig. 3f illustrates a sandwich-like layered packing arrangement of compound 3·4H2O, in which one layer consists of Na+, the middle layer contains energetic frameworks embedded with Li+, and another layer is again composed of Na+.
2.3. Physicochemical and energetic properties
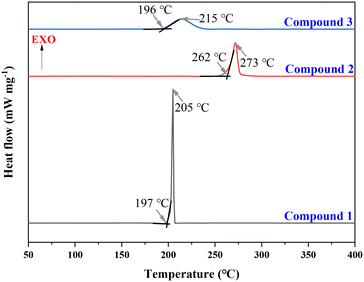
Compounds 1–3 were analyzed by differential scanning calorimetry (DSC) under an argon atmosphere at a heating rate of 10 °C min−1, yielding thermal decomposition temperatures in the range of 205 °C to 273 °C (peak). As shown in Fig. 4, all compounds undergo only a single rapid exothermic decomposition process. Notably, the thermal decomposition temperatures of compounds 1 and 3 are close to that of the classic energetic material 1,3,5-trinitro-1,3,5-triazacyclohexane (RDX) (205 °C). In contrast, compound 2 exhibits the highest thermal stability with a decomposition temperature of 273 °C, significantly higher than that of RDX (205 °C) and approaching that of HMX (279 °C) (Table 1).| Compound | T d °C | ρ g cm−3 | ΔHfc kJ mol−1 | D m s−1 | P/GPae | IS/Jf | FS/Ng |
|---|---|---|---|---|---|---|---|
| a Thermal decomposition temperature (DSC, 10 °C min−1, peak). b Density measured using a gas pycnometer (25 °C). c Heat of formation. d Detonation velocity. e Detonation pressure. f Impact sensitivity. g Friction sensitivity. | |||||||
| 1 | 205 | 1.66 | −71.1 | 7543 | 20.7 | >50 | >360 |
| 2 | 273 | 2.12 | −748.5 | 7273 | 20.9 | >50 | >360 |
| 3 | 215 | 1.89 | −208.7 | 8106 | 27.9 | >50 | >360 |
| TNT 1 | 295 | 1.65 | −67.0 | 6881 | 19.5 | 15 | 353 |
| HMX 8 | 279 | 1.91 | 74.8 | 9144 | 39.2 | 7.4 | 120 |
| RDX 8 | 205 | 1.80 | 70.7 | 8795 | 34.9 | 7.4 | 120 |
Additionally, the heats of formation (ΔHf) of the compounds were calculated using the Gaussian 09 (Revision D.01) software program.29 The detonation performance (detonation velocity and pressure) was further calculated using EXPLO5 software (version 6.04) with the experimental densities and the calculated ΔHf values.30 Compounds 1–3 exhibited detonation velocities in the range of 7273–8106 m s−1 and detonation pressures in the range of 20.7–27.9 GPa, respectively, which are superior to those of the typical energetic material TNT (6881 m s−1). Safety performance was further evaluated using the BAM fall hammer test. The three compounds demonstrated highly insensitive characteristics to impact and friction (IS > 50 J, FS > 360 N), outperforming both TNT (IS = 15 J, FS = 353 N) and RDX (IS = 7.5 J, FS = 120 N).
3. Conclusions
In summary, we selected hydroxynitropyrazines as energetic frameworks and assembled them with organic amines and inorganic metals, respectively, successfully constructing novel energetic salts 1–3. The structures of all three compounds were confirmed by single-crystal X-ray diffraction analysis. Compound 1 exhibits a well-ordered face-to-face stacking mode due to its extensive hydrogen-bonding network; compound 2 exhibits a porous three-dimensional network framework structure; while compound 3 shows a compact sandwich-like layered stacking. Furthermore, the thermal decomposition temperatures of the three compounds range from 205 to 273 °C. Among them, compound 2 possesses the highest decomposition temperature of 273 °C, which is close to that of HMX. Their detonation velocities range from 7273 to 8106 m s−1, significantly higher than that of TNT. Additionally, all three compounds exhibit low mechanical sensitivity (IS > 50 J, FS > 360 N), demonstrating superior safety performance compared to TNT. The successful construction of these three compounds confirms that hydroxynitropyrazine-based frameworks are promising energetic building blocks, providing new insights for the design of novel energetic materials.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ce01009h.CCDC 2445286 (1·CH3CN·H2O), 2485166 (2·3H2O) and 2485167 (3·4H2O) contain the supplementary crystallographic data for this paper.31a–c
Acknowledgements
This work was supported by the China National Science Fund for Distinguished Young Scholars (No. 22325504) and the National Natural Science Foundation of China (No. 22175157) and sponsored by the Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University (No. CX2025002).Notes and references
- H. Gao and J. M. Shreeve, Azole-based energetic salts, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS.
- T. O. Owen and J. Z. Michael, Properties and promise of catenated nitrogen systems as high-energy-density materials, Chem. Rev., 2020, 120, 5682–5744 CrossRef.
- P. Yin, Q. Zhang and J. M. Shreeve, Dancing with energetic nitrogen atoms: versatile N-functionalization strategies for N-heterocyclic frameworks in high energy density materials, Acc. Chem. Res., 2016, 49, 4–16 CrossRef CAS.
- M. Fan, X. Jiang, R. Wang, L. Pan, X. Qi, S. Song, Y. Wang and Q. Zhang, One-step realization of skeleton editing, gem-dinitromethyl functionalization, and zwitterionization in a laser-sensitive 1,3,4-oxadiazole energetic molecule, Org. Lett., 2025, 27(3), 840–845 CrossRef CAS PubMed.
- X. Li, P. Ren, R. Wu, Y. Wang, P. Deng, K. Han, Y. Shang, S. Chen and W. Zhang, An alternative ignition agent concept based on ionic integrated energetic oxidant, Chem. Eng. J., 2025, 510, 161489 CrossRef CAS.
- K. Han, H. Shi, G. He, F. Liao, J. Wang and C. An, Application of pickering emulsion with multilayer dispersion-enhancement synergy of carbon nanotubes in composite energetic materials, Chem. Eng. J., 2025, 505, 159799 CrossRef CAS.
- X. Jiang, D. Yin, S. Song, Y. Wang, M. Fan, R. Wang and Q. Zhang, Achieving ultra-high heat resistance of novel energetic materials through a hydrogen bonding and extended π-conjugation strategy, J. Mater. Chem. A, 2024, 12, 13231–13239 RSC.
- H. Gao, Q. Zhang and J. M. Shreeve, Fused heterocycle-based energetic materials (2012-2019), J. Mater. Chem. A, 2020, 8, 4193–4216 RSC.
- J. Singh, R. J. Staples and J. M. Shreeve, Coordination-driven safer and sustainable energetic materials, J. Mater. Chem. A, 2025, 13, 11475–11485 RSC.
- S. Lal, R. J. Staples and J. M. Shreeve, Energetic performance of trinitromethyl nitrotriazole (TNMNT) and its energetic salts, Chem. Commun., 2023, 59, 11276–11279 RSC.
- M. Gruhne, M. Benz, T. Lorenzen, T. Lenz, T. M. Klapötke and J. Stierstorfer, Hybridization of dinitramide and dicyanamide: evaluation of nitrocyanamide in energetic salts and coordination compounds, Cryst. Growth Des., 2022, 22(1), 200–212 CrossRef CAS.
- M. S. Gruhne, M. Lommel, M. H. H. Wurzenberger, T. M. Klapötke and J. Stierstorfer, Investigation of ethylenedinitramine as a versatile building block in energetic salts, cocrystals, and coordination compounds, Inorg. Chem., 2021, 60(7), 4816–4828 CrossRef CAS.
- Y. Qin, Q. Zeng, J. Zhu, Z. Liu, G. Zhang, X. Duan and B. Wu, Synthesis, thermodynamics and catalytic properties of novel energetic coordination compounds based on nitrotriazolate-formazan ligand, Inorg. Chem. Commun., 2025, 179, 114679 CrossRef CAS.
- S. Li, T. Wang, C. Zhang, Z. Lu, E. Chu, Q. Yu and J. Zhang, Balancing the energy and sensitivity of primary explosives: using isomers to prepare energetic coordination compounds, Inorg. Chem., 2025, 64(4), 2020–2029 CrossRef CAS.
- P. Zhao, Q. Lin, H. Huang, X. Li and J. Yang, Synthesis of energetic salts based on 4-nitro-5-dinitromethyl-1,2,3-1H-triazole, CrystEngComm, 2024, 26, 1730–1737 RSC.
- Ma. Benz, S. M. J. Endraß, T. M. Klapötke, J. Stierstorfer and S. Strey, 1-Amino-5-nitriminotetrazolate as a promising anion in safe yet powerful energetic coordination compounds, Dalton Trans., 2025, 54, 6156–6166 RSC.
- G. Liu, J. Zhao, K. Xue, H. Gao, S. Zhang and C. Zhang, Energetics and ionic-electronic and geometric variabilities of hydroxylammonium-based salts, Cryst. Growth Des., 2023, 23(3), 1821–1831 CrossRef CAS.
- C. Lei, H. Yang and G. Cheng, New pyrazole energetic materials and their energetic salts: combining the dinitromethyl group with nitropyrazole, Dalton Trans., 2020, 49, 1660–1667 RSC.
- T. Wang, Q. Zhang, H. Deng, L. Shang, D. Chen, Y. Li, S. Zhu and H. Li, Evolution of oxidizing inorganic metal salts: ultrafast laser initiation materials based on energetic cationic coordination polymers, ACS Appl. Mater. Interfaces, 2019, 11(44), 41523–41530 CrossRef CAS.
- Q. Lai, Y. Long, P. Yin, J. M. Shreeve and S. Pang, Thinking outside the energetic box: stabilizing and greening high-energy materials with reticular chemistry, Acc. Chem. Res., 2024, 57(19), 2790–2803 CrossRef CAS.
- B. Cheng, F. Yang, J. Zhang, L. Zhang, P. Zhao and Q. Lin, Energetic salts based on a bicyclic nitrogen-rich compound with good molecular stability, Cryst. Growth Des., 2023, 23(3), 1466–1476 CrossRef CAS.
- G. Zhang, C. Xiao, Z. Dong, M. Lv, Y. Liu, X. Hao, Y. Zou, H. Zhang and Z. Ye, Increasing the energy limit of single triazole-tetrazole: HNANTT modified with its energetic coordination polymers, co-crystals, and energetic salts, Cryst. Growth Des., 2023, 23(9), 6756–6764 CrossRef CAS.
- C. Shen, Y. Xu and M. Lu, A series of high-energy coordination polymers with 3,6-bis(4-nitroamino-1,2,5-oxadiazol-3-yl)-1,4,2,5-dioxadiazine, a ligand with multi-coordination sites, high oxygen content and detonation performance: syntheses, structures, and performance, J. Mater. Chem. A, 2017, 5, 18854–18861 RSC.
- J. Singh, A. K. Chinnam, R. J. Staples and J. M. Shreeve, Energetic salts of sensitive N,N'-(3,5-dinitropyrazine-2,6-diyl)dinitramide stabilized through three-dimensional intermolecular interactions, Inorg. Chem., 2022, 61(41), 16493–16500 CrossRef CAS.
- A. K. Yadav, V. D. Ghule and S. Dharavath, Solvent free potassium 3,5-dinitro-6-oxo-1,6-dihydropyrazin-2-olate 3D EMOF as thermally stable energetic material, Chem. – Asian J., 2024, 19, e202400409 CrossRef CAS.
- M. Jujam, A. K. Yadav and S. Dharavath, Crystal engineering of dinitropyrazine-based sodium E-MOFs: toward thermally robust and low-sensitivity energetic materials, Dalton Trans., 2025, 54, 13347–13355 RSC.
- S. Guilloua, G. Jacobb, F. Terriera and R. Goumont, An unexpected synthesis of 7-azidofurazano[3,4-b]tetrazolopyrazine, Tetrahedron, 2009, 65, 8891–8895 CrossRef.
- C. Ma, Z. Liu and Q. Yao, Efficient synthesis of 4-amino-2,6-dichloropyridine and its derivatives, Heterocycl. Commun., 2016, 22, 251–254 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, B. Mennucci VBarone, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Mar-tin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- M. Sućeska, EXPLO5 Program, Brodarski Institut, Zagreb, Croatia, 2013 Search PubMed.
- (a) CCDC 2445286: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2n2j6h; (b) CCDC 2485166: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pf0nt; (c) CCDC 2485167: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pf0pv.
| This journal is © The Royal Society of Chemistry 2026 |