Guest water-induced reversible regulation of proton conduction in a two-dimensional nickel(II) coordination polymer
Ao-Na
Sun
,
Fu-Wan
Dong
,
Yi-Chen
,
Si-Chen
Zhang
,
Rui-Han
Liu
,
Junlun
Zhu
 and
Dong
Shao
and
Dong
Shao
 *
*
Hubei Key Laboratory of Processing and Application of Catalytic Materials, Hubei Provincial Engineering Research Center of High Purity Raw Material Processing Technology of Electronic Materials, College of Chemistry and Chemical Engineering, Huanggang Normal University, Huanggang 438000, China. E-mail: shaodong@nju.edu.cn
First published on 19th November 2025
Abstract
Dynamic molecular crystals with switchable proton conduction are highly attractive for understanding and developing high-performance solid-state proton conductors (SSPCs). Herein, a reversible structural switching was achieved between {[Ni2(btca)(tmdp)2(H2O)6]·6H2O}n (Ni·6H2O; H4btca = 1,2,4,5-benzenetetracarboxylic acid; and tmdp = 4,4′-trimethylenedipyridine) and {[Ni2(btca)(tmdp)2(H2O)6]·2H2O}n (Ni·2H2O) via single-crystal-to-single-crystal (SCSC) transformation during partial dehydration and rehydration processes. AC impedance spectroscopy confirmed that the proton conductivity, which is highly dependent on temperature and humidity, is reversibly modulated by partial dehydration/rehydration cycles, switching the material between a superprotonic state (Ni·6H2O, >50 °C, and 95% RH) and a non-superprotonic state (Ni·2H2O, <10−4 S cm−1, and low RH). The tuning of the 1D hydrogen-bonded water chains is responsible for the reversible electrical switching. This work highlights that the guest species can serve as a switch to regulate proton conduction in coordination polymers and suggests an effective strategy for the construction of dynamic SSPCs via a mixed bipyridyl–tetracarboxylate strategy.
Introduction
Proton-conducting materials have garnered significant research interest due to their critical applications in fuel cells, batteries, sensors, and hydrogen separation and purification technologies.1 Over the past two decades, diverse material systems—including ceramic oxides, inorganic solid acids, and organic polymers—have been extensively developed for this purpose.2–5 More recently, metal–organic frameworks (MOFs) have emerged as a promising class of proton-conducting materials owing to their structural tunability and well-defined crystalline architectures, which enable rational design at the molecular level.6–12 Numerous coordination polymers exhibiting high proton conductivity have been realized by incorporating acidic groups (e.g., –COOH, –SO3H) and proton carriers (e.g., H2O, NH3) into their porous frameworks.2 A fundamental understanding of proton conduction mechanisms requires elucidating the correlation between the reorganization of hydrogen bond networks and dynamic proton transport behavior. Consequently, the development of dynamic proton-conducting materials has become a valuable strategy for probing these processes; however, synthesizing such systems remains a considerable challenge.13Dynamic molecular crystals capable of single-crystal-to-single-crystal (SCSC) transformations are of great interest, which offer a unique platform for in situ investigation of proton transport pathways—including their formation, rupture, and reorganization—at the atomic level.14–17 To achieve such dynamic switching, guest solvent molecules serve as readily available and mild stimuli that can diffuse into crystal cavities, inducing molecular conformational changes and modulating packing arrangements. These processes can promote the orderly reorganization of the framework, facilitating SCSC transformations. Among various solvents, water is particularly important due to its abundance and its pivotal role in synthesizing and modulating complex materials.15,18,19 Moreover, water directly influences hydrogen bonding networks, which are critical for proton conduction. Nevertheless, reports correlating water-induced SCSC transformations with proton conductivity remain scarce. Indeed, studies exploring proton conduction mechanisms during SCSC transformations remain highly limited.
Recent studies in our lab are focused on designing and constructing proton conducting molecular materials,20–23 especially exploring dynamic molecular crystals with switchable magnetic properties and proton conduction via a reversible SCSC process.20 For example, we recently reported a dynamic Mn(II) hydrogen-bonded organic framework single crystal that exhibited a dehydration–rehydration induced on–off switching of both magnetic interactions and proton conduction. In our previously reported system, the guest water molecules can be fully removed by heating to high temperatures and rehydration in the air atmosphere. However, dynamic reversible systems involving partial water loss and water absorption have not yet been reported. Following our research focus, herein, we present a dynamic NiII two-dimensional (2D) coordination polymer, in which proton conduction can be reversibly switched through a reversible partial dehydration/rehydration process. Single crystal diffraction analyses indicate a structural switching between {[Ni2(btca)(tmdp)2(H2O)6]·6H2O}n (Ni·6H2O) and {[Ni2(btca)(tmdp)2(H2O)6]·2H2O}n (Ni·2H2O; H4btca = 1,2,4,5-benzenetetracarboxylic acid; and tmdp = 4,4′-trimethylenedipyridine, Fig. 1) that occurred via SCSC. Magnetic studies indicate that the static magnetic properties did not exhibit obvious changes and neither phase showed slow magnetic relaxation. AC impedance spectroscopy revealed that the temperature and humidity-dependent proton conduction behavior was modified by partial dehydration/rehydration. Ni·6H2O acted as a proton conductor above 50 °C under 95% RH, while Ni·2H2O was a non-superprotonic (<10−4) type under low RH conditions. In situ structural studies revealed that the water-driven modification of the 1D hydrogen-bonded water chains is responsible for the observed switchable proton conduction behavior.
Results and discussion
Structure description
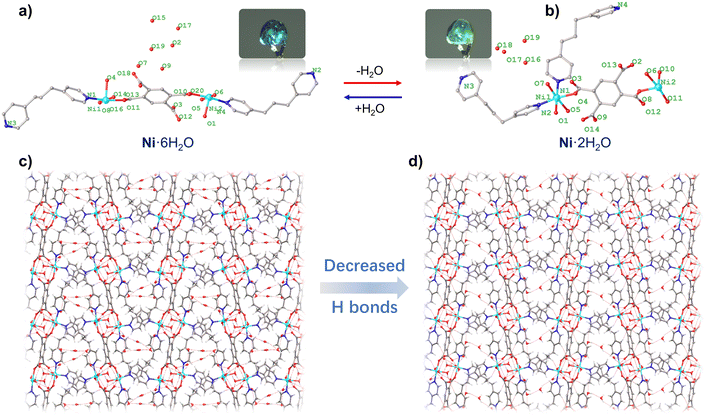
Single-crystal X-ray diffraction (SC-XRD) analyses were conducted for Ni·6H2O and the in situ generated single crystal of Ni·2H2O. Ni·6H2O crystallized in the triclinic P21/c space group while Ni·2H2O crystallized in the triclinic Cc space group (Table 1). In the asymmetric unit of Ni·6H2O (Fig. 2a), two Ni2+ ions were bridged by one fully deprotonated btca4− ligand. The Ni2+ ion was coordinated by a tmdp ligand and three coordinated water molecules. Additionally, there were six lattice water molecules. Structurally, the central (btca4−) component was bonded to two Ni(H2O)3(tmdp) species in an inverse position, and there were six free water molecules in the lattice. By symmetric operation, the Ni2+ ions were located in a six-coordinated N2O4 coordination geometry constructed by two N atoms from two crystallographically different tmdp ligands, one O atom from btca4−, and three O atoms from three coordinated water molecules. Heating to 360 K, the asymmetric unit exhibits a notable decrease of lattice water molecules (Fig. 2b). Notably, the occupancy of four water molecules is 1 (O16), 0.5 (O18), 0.5 (O19), and 0.125 (O17). Thus, two water molecules were crystallized in the lattice. Selected bond lengths and angles for Ni·6H2O and Ni·2H2O are provided in Tables S1–S4. The Ni–N and Ni–O bond lengths are in the range of 2.036–2.123 Å with an average Ni–N/O distance of 2.084, suggesting a high spin state of the Ni2+ ion in Ni·6H2O and Ni·2H2O.24 The continuous shape measure (CShM) analysis of the compounds revealed small deviation values of 0.290, 0349, 0.316, and 0.308 of Ni2+ ions from the ideal octahedral geometry (CShM = 0, Table S5). It can be observed from these results that the partial water loss process did not lead to a significant change in the coordination environment of nickel ions. The striking structural characteristic is the presence of intramolecular hydrogen bonding interactions between the coordination water and the uncoordinated O from the btca4− ligand (Fig. S1 and S2) and the intermolecular hydrogen bonding interactions between the lattice water and the uncoordinated carboxylic acid oxygen (Fig. S1 and S2). Interestingly, the lattice water molecules are connected to each other through multiple and short O–H⋯O hydrogen bonds, leading to the formation of ring-shaped hydrogen-bonded water clusters. Upon the release of guest water, the circled hydrogen-bonded water clusters disappeared (Fig. S2). Nevertheless, the lattice water molecules still form a continuous hydrogen-bonded network structure. The packed structures of Ni·6H2O and Ni·2H2O indicate the formation of 2D layer structures for both compounds with modification of the 3D hydrogen-bonded networks (Fig. 2c and d).| Parameter | Ni·6H2O | Ni·2H2O | Ni·6H2O_re |
|---|---|---|---|
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|. b wR2 = {∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]}1/2 | |||
| Formula | C36H53.5N4Ni2O19.75 | C36H46.25N4Ni2O16.12 | C36H53.5N4Ni2O19.75 |
| Formula weight [g mol−1] | 975.74 | 910.44 | 975.74 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | Cc | P21/c |
| Temperature [K] | 170 | 360 | 170 |
| a [Å] | 9.841(9) | 9.9528(19) | 9.870(11) |
| b [Å] | 19.441(15) | 19.424(4) | 19.429(12) |
| c [Å] | 23.234(17) | 23.595(4) | 23.223(13) |
| α [°] | 90 | 90 | 90 |
| β [°] | 98.01(3) | 99.347(6) | 98.06(3) |
| γ [°] | 90 | 90 | 90 |
| V [Å3] | 4402(6) | 4500.9(15) | 4410(6) |
| Z | 4 | 4 | 4 |
| ρ calcd [g cm−3] | 1.479 | 1.344 | 1.470 |
| μ(Mo-Kα) [mm−1] | 0.937 | 0.905 | 0.935 |
| F(000) | 2046.0 | 1901.0 | 2046.0 |
| R int | 0.0708 | 0.0813 | 0.1512 |
| R 1 /wR2b (I > 2σ(I)) | 0.0359/0.0818 | 0.0700/0.1573 | 0.0457/0.1118 |
| R 1/wR2 (all data) | 0.0467/0.0873 | 0.0776/0.1618 | 0.07003/0.1275 |
| GOF on F2 | 1.032 | 0.0813 | 1.034 |
| Max/min [e Å−3] | 0.41/−0.36 | 0.75/−0.91 | 0.67/−0.94 |
The Ni2+⋯Ni2+ separation linked by btca4− in Ni·6H2O and Ni·2H2O are 11.228 and 11.217 Å, with a relatively long intermolecular distance beyond 7.9 Å. These distances prevent the effective magnetic coupling in Ni·6H2O and Ni·2H2O.25 The topology of the frameworks was also analyzed by ToposPro (Fig. 3).26 The results indicate that both compounds exhibit simple hcb topology with a Schläfli symbol of 63. Compared with the classic regular hcb topology, however, this topology exhibits severe distortion, and such distortion is relatively rare.27 This distortion is presumably attributed to the flexible tmdp ligand.
The reversibility of the SCSC transformation was further investigated. A single crystal of the rehydrated phase, denoted as Ni·6H2O_re, was obtained by exposing a crystal of 1 to ambient atmosphere for 24 hours (Fig. S4). The color of the compound's single crystal changes from pale blue to green, accompanied by the loss of partial lattice water; after absorbing water from the air, the color returns to pale blue, and this transformation can be identified with the naked eye. Single-crystal X-ray diffraction analysis confirmed that the crystal structure of Ni·6H2O_re is consistent with that of the original Ni·6H2O phase (Table 1, Fig. S5). This result unambiguously demonstrates the reversible switchability between the Ni·6H2O and Ni·2H2O states.
Purity and thermal stability analysis
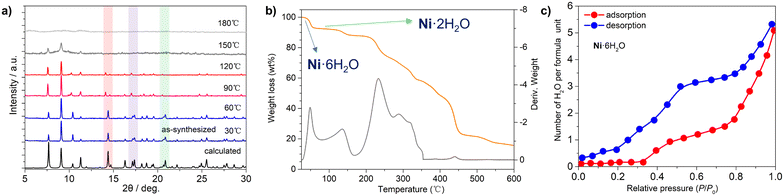
The purity of the synthesized bulk sample of Ni·6H2O for physical measurements was confirmed by powder X-ray diffraction (PXRD) patterns (Fig. S5 and S6). Variable temperature PXRD was also performed to track the phase transformation. As shown in Fig. 4a, we can clearly observe that the positions of the diffraction peaks at 14.4°, 17.1°, and 21.2° undergo significant changes as the temperature rises from 60 °C to 90 °C, confirming the occurrence of a structural phase transition. In the temperature range of 90–120 °C, the partially dehydrated phase maintains stability, with no obvious changes or shifts in the diffraction peak positions. When the temperature is increased to 150 °C, most of the diffraction peaks disappear, which proves that the material has undergone a further structural change. The diffraction results at 180 °C indicate that the material has become amorphous. Thermal gravimetric analysis (TGA) indicated that the water molecules within Ni·6H2O can be removed by heating the sample above 60 °C (Fig. 4b), with the partially dehydrated Ni·2H2O remaining stable up to 130 °C. The weight loss of 7.2% is consistent with the calculated value based on SC-XRD data. As the temperature increases further, the material loses more water (3.2%) and can remain stable up to 220 °C. Both these results support the dehydration-induced structural change.To evaluate the water adsorption behavior of the materials, water adsorption–desorption isotherms of the two compounds at 25 °C over a range of RH values were measured. As shown in Fig. 4c, the adsorption behavior of Ni·6H2O exhibits almost no water adsorption under relatively low humidity conditions; as the RH increases to 40%, the amount of water adsorbed per formula unit increases gradually; under relatively high humidity conditions, the water adsorption amount rises rapidly to 4.5 water molecules per formula unit (corresponding to an adsorption capacity of 52 cm3 g−1). During desorption, the water content of Ni·6H2O decreases rapidly to approximately 3.2 water molecules per formula unit at 55% RH, and eventually, all adsorbed water is completely desorbed under vacuum conditions. In contrast, the water adsorption process of Ni·2H2O proceeds gradually, reaching 8.1 water molecules per formula unit at 100% RH (corresponding to an adsorption capacity of 81 cm3 g−1, Fig. S7). During desorption, Ni·2H2O releases nearly all physisorbed water molecules while retaining its original lattice water content. The final hydration level maintained is comparable to that of Ni·6H2O. These results indicate that the framework material has excellent adaptability to gaseous water molecules.
Magnetic properties
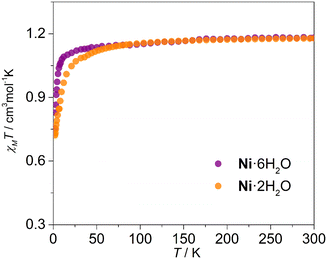
Variable-temperature magnetic susceptibility measurements were first performed on Ni·6H2O under an applied dc field of 1 kOe (Fig. 5). The complex Ni·2H2O was subsequently generated in situ by maintaining the sample at 360 K for 1 hour within the SQUID magnetometer, after which magnetic characterization was repeated. At room temperature, the χMT values for Ni·6H2O and Ni·2H2O are 1.18 and 1.16 cm3 mol−1 K, respectively, notably higher than the spin-only value of 1.0 cm3 mol−1 K expected for an S = 1 system with g = 2.0. This deviation indicates a significant orbital contribution to the magnetic moment and confirms the high-spin state of Ni2+ ions in both compounds.24,25 The χMT values remain nearly constant at 1.05 cm3 mol−1 K from 300 K down to 25 K for Ni·6H2O and to 50 K for Ni·2H2O, below which they gradually decrease to 0.8 and 0.6 cm3 mol−1 K at 2 K, respectively. This divergence in low-temperature magnetic behavior is likely due to differences in weak intermolecular magnetic interactions within their distinct supramolecular architectures. Furthermore, alternating-current (ac) magnetic susceptibility measurements were conducted under both zero and 1 kOe dc fields to investigate potential slow magnetic relaxation in Ni·6H2O and Ni·2H2O. However, no temperature-dependent out-of-phase (χ″) signal was observed for either compound (Fig. S7). The absence of slow relaxation is consistent with the typical paramagnetic behavior observed in octahedral Ni(II) complexes.28Proton conduction
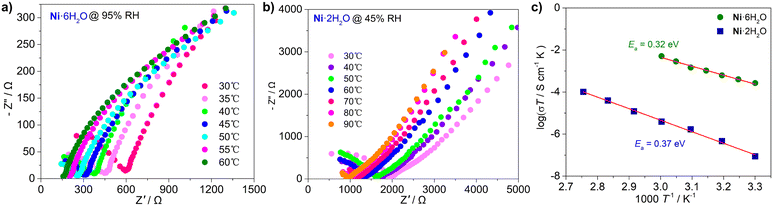
Experimental results confirm complementary roles for water in proton conduction: coordinated water molecules form the structural basis of conduction pathways through hydrogen-bonded networks, while guest water molecules are essential for activating and sustaining high conductivity by dynamically completing and connecting these networks.29–31 The abundance of coordinated and lattice water molecules, coupled with notable hydrogen bond interactions, motivated us to investigate the proton conduction properties of Ni·6H2O and Ni·2H2O. The proton conductivity of Ni·6H2O and Ni·2H2O samples was studied using electrochemical impedance spectroscopy (EIS) at various humidities and temperatures. Under anhydrous conditions at different temperatures, neither Ni·6H2O nor Ni·2H2O exhibited proton conductive properties. Considering the thermal stability of the materials, the test temperature ranges for Ni·6H2O and Ni·2H2O were controlled at 30–60 °C and 30–90 °C, respectively. At 50 °C across different RH ranges, both Ni·6H2O and Ni·2H2O showed obvious proton conductive behavior (Fig. S10 and S11, Tables S8 and S9). Under low-temperature and low-humidity conditions, the proton conductivity of Ni·2H2O was much lower than that of Ni·6H2O. For example, under low humidity (40% RH), the proton conductivities of Ni·6H2O and Ni·2H2O were 2.7 × 10−5 S cm−1 and 4.7 × 10−7 S cm−1, respectively. With further increases in humidity, the proton conductivities of the two compounds rose rapidly: at 95% RH, the proton conductivities of Ni·6H2O and Ni·2H2O reached similar values of 4.7 × 10−3 S cm−1 and 4.1 × 10−3 S cm−1, respectively. Notably, the proton conductivity of Ni·6H2O increased rapidly after 75% relative RH, which is highly consistent with the result that its water adsorption capacity increased sharply at this humidity. This indicates that adsorbed water molecules play a key role in proton transfer. Ni·2H2O also exhibited proton conductive properties similar to those of Ni·6H2O under high humidity, suggesting that Ni·2H2O is converted into Ni·6H2O via water adsorption under high humidity. Therefore, the effective proton conductivity of Ni·2H2O should lie within the low-humidity range. This phenomenon clearly indicates that under conditions of relatively high temperature, high humidity, and electrification, Ni·2H2O forms Ni·6H2O through water adsorption.To investigate the effect of temperature on proton conductivity, we conducted experiments under fixed humidity conditions (95% RH, corresponding to Ni·6H2O and 45% RH, corresponding to Ni·2H2O), with temperature adjustment ranges of 30–60 °C and 30–90 °C, respectively. As shown in Fig. 6a and b, the proton conductivities of both compounds increase with rising temperature (Tables S10 and S11). This phenomenon can be attributed to an increase in the internal energy of the system caused by temperature elevation, which accelerates the migration of proton carriers and facilitates proton transfer along pathways with lower energy barriers.29,30 Although the proton conductivities of Ni·6H2O and Ni·2H2O both show an upward trend with temperature under 95% and 45% RH, respectively, the differences in their conductivity increments are significant. The conductivity of Ni·6H2O increases from 1.1 × 10−5 S cm−1 at 30 °C to 4.6 × 10−4 S cm−1 at 60 °C, with an increment of one order of magnitude. In contrast, the conductivity of Ni·2H2O is 7.1 × 10−7 S cm−1 at 30 °C and increases to 1.2 × 10−6 S cm−1 at 90 °C, showing a relatively small increment. This is because under the low humidity condition of 45% RH, there are not enough water molecules to enter the material. For Ni·6H2O and Ni·2H2O, after the impedance tests, PXRD analysis showed no significant changes in the overall pattern except for slight shifts in some diffraction peaks and the appearance of characteristic peaks in the high-angle region (Fig. S12 and S13), demonstrating the stability and environmental tolerance of the material. To evaluate the long-term durability of the proton conductivity of Ni·6H2O, we measured its impedance at 10 hour intervals. The results indicate that the proton conductivity of Ni·6H2O can be maintained over 3 days without any significant attenuation (Fig. S14).
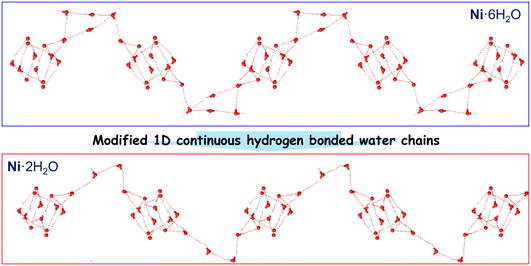
To further quantify the thermal activation behavior and reveal the proton conduction mechanism, we analyzed the impedance data based on the Arrhenius equation (Fig. 6c). The calculated activation energies (Ea) of Ni·6H2O and Ni·2H2O under different relative humidities are 0.32 eV and 0.37 eV, respectively, both lower than 0.40 eV. This indicates that the proton conduction process conforms to the Grotthuss mechanism. Notably, the activation energy of Ni·6H2O is significantly lower than that of Ni·2H2O, reflecting a remarkable difference in their proton conduction capabilities. This difference mainly stems from differences in the hydrogen bond networks within their crystal structures. As shown in Fig. 7, Ni·6H2O forms 1D hydrogen-bonded chains composed of lattice water clusters, coordinated water, and uncoordinated carboxylate oxygen atoms, which are conducive to the “hop” conduction of protons. In the structure of Ni·2H2O formed after the SCSC transformation, however, the number of water molecules decreases, the hydrogen-bond interactions weaken, and the number of vacancies available for proton hopping and proton donors is significantly reduced. This increases the difficulty of proton migration, leading to a higher activation energy and a relatively larger energy barrier.
Conclusions
In summary, we reported the synthesis, crystal structures, magnetic, and electrical properties of a dynamic 2D NiII coordination polymer constructed from mixed bipyridyl–tetracarboxylate ligands. In situ single-crystal diffraction analyses revealed reversible structural switching between the hydrated and its partially dehydrated phases in an SCSC transformation. The structural changes induce a notable switching of temperature- and humidity-dependent proton conduction behavior. Structural studies elucidated that the guest water-driven modification of 1D continuous hydrogen-bonded water chains accounted for the observed switchable proton conduction behavior. However, the magnetic properties of the two phases did not exhibit significant changes. The foregoing results not only provide a rare dynamic proton-conducting material but also highlight the rational design of dynamic solid-state proton conductors through a mixed bipyridyl–tetracarboxylate strategy.Experimental
Materials and synthesis
All reagents were commercially available and used without further purification unless specified otherwise. 1,2,4,5-Benzenetetracarboxylic acid and 4,4′-trimethylenedipyridine were purchased from TCI chemicals (purity: >97.0%, GC). Ni(NO3)2·6H2O was purchased from Alfa Aesar (purity: ≥99.9%), and the solvents used in the synthesis were purchased from Guangzhou Reagent Factory (analytical grade).Conflicts of interest
There are no conflicts to declare.Data availability
Crystallographic data for Ni·6H2O (2491444), Ni·2H2O (2491445), and Ni·6H2O_re (2491446) have been deposited at the CCDC.Supplementary information: experimental details, CShM calculated results, calculated possible hydrogen bonds, PXRD patterns, TGA analysis, additional alternating current impedance data, additional magnetic data, and additional structural parameters for Ni·6H2O and Ni·2H2O. See DOI: https://doi.org/10.1039/d5ce00938c.
CCDC Ni·6H2O (2491444), Ni·2H2O (2491445) and Ni·6H2O_re (2491446) contain the supplementary crystallographic data for this paper.31a–c
Acknowledgements
We acknowledge the Chutian Scholars Program of Hubei Province, Huanggang Normal University (2042021033 and 202210204) and the Hubei Province Natural Science Foundation (2023AFB010 and 2025AFD359).Notes and references
- (a) M. O'Shaughnessy, J. Lim, J. Glover, A. R. Neale, G. M. Day, L. J. Hardwick and A. I. Cooper, J. Am. Chem. Soc., 2025, 147, 15429–15434 CrossRef; (b) M. O'Shaughnessy, J. Glover, R. Hafizi, M. Barhi, R. Clowes, S. Y. Chong, S. P. Argent, G. M. Day and A. I. Cooper, Nature, 2024, 630, 102–108 CrossRef.
- (a) D. W. Lim and H. Kitagawa, Chem. Soc. Rev., 2021, 50, 6349–6368 RSC; (b) C. Wang, Z. Yang, W. Wu, C. Chen, Y. Li, R. Zhang, W. Li, K. Dai and J. Wang, Chem. Mater., 2024, 36, 8255–8263 CrossRef CAS; (c) S. C. Pal and M. C. Das, Adv. Funct. Mater., 2021, 31, 2101584 CrossRef CAS.
- (a) J. Yang, H. Xu, J. Li, K. Gong, F. Yue, X. Han, K. Wu, P. Shao, Q. Fu, Y. Zhu, W. Xu, X. Huang, J. Xie, F. Wang, W. Yang, T. Zhang, Z. Xu, X. Feng and B. Wang, Science, 2024, 385, 1115–1120 CrossRef CAS PubMed; (b) L. Zong, S. Tao, X. Xu, Y. Feng, F. Wen and N. Huang, J. Mater. Chem. A, 2025, 13, 19355–19361 RSC.
- (a) M. O'Shaughnessy, J. Lim, J. Glover, A. R. Neale, G. M. Day, L. J. Hardwick and A. I. Cooper, J. Am. Chem. Soc., 2025, 147, 15429–15434 CrossRef PubMed; (b) C. Liu, L. H. Cao, Z. Y. Zhou, X. Y. Chen and W. Zhang, ACS Appl. Mater. Interfaces, 2025, 17, 40613–40622 CrossRef CAS.
- (a) L. Xu, M. Dang, F. Yang, F. Lang, B. Li, L. Liang, J. Pang and X. H. Bu, Inorg. Chem., 2024, 63, 17747–17754 CrossRef CAS; (b) H. Zhai, J. Chen, C. Meng, X. Yang, Z. Zhou, L. Ai, J. Li, T. S. Miller, M. Perez-Page and S. M. Holmes, Sci. Adv., 2025, 11, eadw5747 CrossRef CAS PubMed; (c) J. Zhu, D. Shao, W. Wen, Z. Tian, X. Zhang and S. Wang, Coord. Chem. Rev., 2024, 518, 216095 CrossRef CAS.
- (a) Y. Zhang, X. Shang, Z. Qi, P. Zhang, C. Zhang and Q. Wang, Dalton Trans., 2025, 54, 11126–11140 RSC; (b) S. L. Yang, G. Li, M. Y. Guo, W. S. Liu, R. Bu and E. Q. Gao, J. Am. Chem. Soc., 2021, 143, 8838–8848 CrossRef CAS; (c) H. K. Lee, Y. Oruganti, J. Lee, S. Han, J. Kim, D. Moon, M. Kim, D.-W. Lim and H. R. Moon, J. Mater. Chem. A, 2024, 12, 795–801 RSC; (d) Y. Oruganti, D. Kim and D.-W. Lim, Cryst. Growth Des., 2024, 24, 1612–1618 CrossRef CAS; (e) B. G. Lee, D. Kim, J. Y. Bae, J. W. Jeong and D.-W. Lim, CrystEngComm, 2025, 27, 5952–5958 RSC.
- (a) Y. Yoshida, T. Yamada, Y. Jing, T. Toyao, K. Shimizu and M. Sadakiyo, J. Am. Chem. Soc., 2022, 144, 8669–8675 CrossRef CAS PubMed; (b) L. Cai, J. Yang, Y. Lai, Y. Liang, R. Zhang, C. Gu, S. Kitagawa and P. Yin, Angew. Chem., Int. Ed., 2023, 62, e202211741 CrossRef CAS.
- (a) C. X. Yu, H. Wu, Z. Shao, M. J. Gao, X. Q. Sun and L. L. Liu, Inorg. Chem., 2025, 64, 3908–3916 CrossRef CAS PubMed; (b) C. Yang, Y. Liu, J. Li, S. Zhuang, F. Wang, Z. Lin, Y. Zhao and W. Huang, Inorg. Chem., 2025, 64, 5271–5283 CrossRef CAS.
- (a) Q. Wu, Q. Li, W. Zou, Z. Zhang, Y. Zhou and Q. Zhao, Chem. Commun., 2025, 61, 1842–1845 RSC; (b) P. M. Unnikrishnan, G. Premanand and S. K. Das, Inorg. Chem., 2025, 64, 3506–3517 CrossRef CAS.
- (a) R. L. Liu, H. M. Ren, S. Zhao, D. Lin, K. Cheng, G. Li and D. Y. Wang, Inorg. Chem., 2025, 64, 1183–1192 CrossRef CAS; (b) R. Liu, X. Li, L. Chen, S. Song, X. Han, H. Zhu, X. Kong, H. Zhou, X. Li, S. Wang, Y. Li, M. Dou, D. Zhong and H. Hao, Inorg. Chem., 2025, 64, 11807–11819 CrossRef CAS.
- (a) P. Li, J. Dong, H. Zhang, J. Li, J. Li, J. Liu, F. Meng and X. Zou, Cryst. Growth Des., 2025, 25, 2155–2162 CrossRef CAS; (b) D. Li, S. Qie, Z. Mu, Y. Yang, W. Hu, R. Xu and M. Hu, Inorg. Chem., 2025, 64, 5672–5681 CrossRef CAS.
- (a) Y. P. Qu, Q. Zou, S. S. Bao and L. M. Zheng, Chin. Chem. Lett., 2024, 35, 108320 CrossRef CAS; (b) J. Chen, B. An, Y. Chen, X. Han, Q. Mei, M. He, Y. Cheng, I. J. Vitorica-Yrezabal, L. S. Natrajan, D. Lee, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, J. Am. Chem. Soc., 2023, 145, 19225–19231 CrossRef CAS.
- F. Xiang, S. Chen, Z. Yuan, L. Li, Z. Fan, Z. Yao, C. Liu, S. Xiang and Z. Zhang, JACS Au, 2022, 2, 1043–1053 CrossRef CAS.
- (a) E. Coronado and G. M. Espallargas, Chem. Soc. Rev., 2013, 42, 1525–1539 RSC; (b) O. Sato, Nat. Chem., 2016, 8, 644–656 CrossRef CAS PubMed.
- (a) D. Shao, L. Shi, L. Yin, B. L. Wang, Z. X. Wang, Y. Q. Zhang and X. Y. Wang, Chem. Sci., 2018, 9, 7986–7991 RSC; (b) D. Shao, L. Shi, F. X. Shen, X. Q. Wei, O. Sato and X. Y. Wang, Inorg. Chem., 2019, 58, 11589–11598 CrossRef CAS PubMed; (c) X. H. Zhao, D. Shao, J. T. Chen, D. X. Gan, J. Yang and Y. Z. Zhang, Sci. China: Chem., 2022, 65, 532–538 CrossRef CAS.
- (a) M. Sadakiyo, T. Yamada, K. Honda, H. Matsui and H. Kitagawa, J. Am. Chem. Soc., 2014, 136, 7701–7707 CrossRef CAS; (b) K. Otsubo, S. Nagayama, S. Kawaguchi, K. Sugimoto and H. Kitagawa, JACS Au, 2022, 2, 109–115 CrossRef CAS; (c) X. Y. Chen, L. H. Cao, Y. Yang, X. T. Bai, F. Zhao, X. J. Cao, M. F. Huang, Y. D. Gao and D. Yang, Chem. – Eur. J., 2023, 29, e202300028 CrossRef CAS.
- (a) Q. Yang, Y. Wang, Y. Shang, J. Du, J. Yin, D. Liu, Z. Kang, R. Wang, D. Sun and J. Jiang, Cryst. Growth Des., 2020, 20, 3456–3465 CrossRef CAS; (b) Y. Wang, M. Zhang, Q. Yang, J. Yin, D. Liu, Y. Shang, Z. Kang, R. Wang, D. Sun and J. Jiang, Chem. Commun., 2020, 56, 15529–15532 RSC; (c) S. Nakatsuka, Y. Watanabe, Y. Kamakura, S. Horike, D. Tanaka and T. Hatakeyama, Angew. Chem., 2020, 59, 1435–1439 CrossRef CAS; (d) A. B. Kanj, A. Chandresh, A. Gerwien, S. Grosjean, S. Bräse, Y. Wang, H. Dube and L. Heinke, Chem. Sci., 2020, 11, 1404–1410 RSC.
- (a) K. Kaushik, S. Mehta, M. Das, S. Ghosh, S. Kamilya and A. Mondal, Chem. Commun., 2023, 59, 13107–13124 RSC; (b) Z. S. Yao, Z. Tang and J. Tao, Chem. Commun., 2020, 56, 2071–2074 RSC; (c) J. J. Zakrzewski, M. Liberka, J. Wang, S. Chorazy and S. Ohkoshi, Chem. Rev., 2024, 124, 5930–6050 CrossRef CAS PubMed.
- (a) Y. Wang, P. Fu, H. Takatsu, C. Tassel, N. Hayashi, J. Cao, T. Bataille, H. J. Koo, Z. Ouyang, M. H. Whangbo, H. Kageyama and H. Lu, J. Am. Chem. Soc., 2024, 146, 8320–8326 CrossRef CAS; (b) Z. K. Liu, K. Sun, J. P. Xue, Z. S. Yao and J. Tao, Dalton Trans., 2024, 53, 7522–7526 RSC.
- (a) D. Shao, W. J. Tang, Z. Ruan, X. Yang, L. Shi, X. Q. Wei, Z. Tian, K. Kumari and S. K. Singh, Inorg. Chem. Front., 2022, 9, 6147–6157 RSC; (b) F. W. Dong, Y. Chen, Q. J. Cai, L. Y. Ma, J. Yang, L. Shi, J. Zhu and D. Shao, Cryst. Growth Des., 2025, 25, 3464–3470 CrossRef CAS; (c) Y. Chen, F. W. Dong, T. Xu, L. Y. Ma, Q. J. Cai, Z. Tian, J. Yang, L. Shi and D. Shao, Cryst. Growth Des., 2025, 25, 7752–7760 CrossRef CAS.
- (a) D. Shao, Y. Zhou, X. Yang, J. Yue, S. Ming, X. Q. Wei and Z. Tian, Dalton Trans., 2022, 51, 18514–18519 RSC; (b) Y. Zhou, H. Xiang, J. Y. Zhu, L. Shi, W. J. You, X. Q. Wei, Z. Tian and D. Shao, Polyhedron, 2022, 228, 116181 CrossRef CAS; (c) Y. L. Zhang, L. Shi, G. Liu, J. Yang and D. Shao, J. Mol. Struct., 2023, 1292, 136131 CrossRef CAS.
- (a) Y. Zhou, S. Moorthy, X. Q. Wei, S. K. Singh, Z. Tian and D. Shao, Dalton Trans., 2023, 52, 909–918 RSC; (b) X. M. Bu, S. Y. Yang, Q. Zhang, L. Z. Han, E. S. Xiang, X. C. Huang, L. Shi and D. Shao, J. Mol. Struct., 2023, 1286, 135544 CrossRef CAS; (c) D. Shao, L. Shi, G. Liu, J. Yue, S. Ming, X. Yang, J. Zhu and Z. Ruan, Cryst. Growth Des., 2023, 23, 5035–5042 CrossRef CAS; (d) D. Q. Wu, Z. Fan, Q. Zhang, L. Y. Yi, Q. Gu, J. Dong, L. Huang, J. Yang, D. Shao and B. Zhai, J. Mol. Struct., 2024, 1306, 137874 CrossRef CAS.
- (a) X. Q. Wei, T. Li, Y. B. Li, J. Yang, L. Shan, J. Zhu, Y. Wan and D. Shao, J. Mol. Struct., 2024, 1308, 138064 CrossRef CAS; (b) J. Dong, L. Huang, L. Shi, J. Yang, Y. Wan and D. Shao, Inorg. Chem., 2024, 63, 17478–17487 CrossRef CAS PubMed; (c) S. Y. Yang, Q. Zhang, Y. L. Zhang, T. S. Tan, J. Zhu, X. Yang, L. Shi, J. Yang and D. Shao, CrystEngComm, 2024, 26, 4181–4189 RSC.
- (a) D. Shao, L. Shi, G. Liu, J. Yue, S. Ming, X. Yang, J. Zhu and Z. Ruan, Cryst. Growth Des., 2023, 23, 5035–5042 CrossRef CAS; (b) Y. L. Zhang, L. Shi, G. Liu, J. Yang and D. Shao, J. Mol. Struct., 2023, 1292, 136131 CrossRef CAS; (c) Y. Zhou, H. Xiang, J. Y. Zhu, L. Shi, W. J. You, X. Q. Wei, Z. Tian and D. Shao, Polyhedron, 2022, 228, 116181 CrossRef CAS.
- (a) Y. Zhou, Y. L. Zhang, Q. Zhang, S. Y. Yang, X. Q. Wei, Z. Tian and D. Shao, Polyhedron, 2022, 225, 116078 CrossRef CAS; (b) X. Q. Wei, Y. Wan, W. J. Tang, J. Dong, L. Huang, J. Yang, X. C. Huang and D. Shao, J. Mol. Struct., 2024, 1305, 137823 CrossRef CAS; (c) X. Q. Wei, T. Li, Y. B. Li, J. Yang, L. Shan, J. Zhu, Y. Wan and D. Shao, J. Mol. Struct., 2024, 1308, 138064 CrossRef CAS; (d) S. T. Wu, Z. Ruan, Z. Tian, L. Shi, J. Yang and D. Shao, CrystEngComm, 2024, 26, 5850–5858 RSC.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576–3586 CrossRef CAS.
- (a) L. Shi, K. O. Kirlikovali, Z. Chen and O. K. Farha, Chem, 2024, 10, 484–503 CrossRef CAS; (b) L. Shi, Z. Xiong, H. Wang, H. Cao and Z. Chen, Chem, 2024, 10, 2464–2472 CrossRef CAS; (c) L. T. Glasby, J. L. Cordiner, J. C. Cole and P. Z. Moghadam, Chem. Mater., 2024, 36, 9013–9030 CrossRef CAS.
- I. Bhowmick, A. J. Roehl, J. R. Neilson, A. K. Rappé and M. P. Shores, Chem. Sci., 2018, 9, 6564–6571 RSC.
- (a) R. Sahoo, S. C. Pal and M. C. Das, ACS Energy Lett., 2022, 7, 4490–4500 CrossRef CAS; (b) S. M. Elahi, S. Chand, W. H. Deng, A. Pal and M. C. Das, Angew. Chem., Int. Ed., 2018, 57, 6662–6666 CrossRef CAS PubMed; (c) S. Chand, S. C. Pal, D. W. Lim, K. Otsubo, A. Pal, H. Kitagawa and M. C. Das, ACS Mater. Lett., 2020, 2, 1343–1350 CrossRef CAS.
- (a) S. C. Pal, D. Mukherjee, Y. Oruganti, B. G. Lee, D. W. Lim, B. Pramanik, A. K. Manna and M. C. Das, J. Am. Chem. Soc., 2024, 146, 14546–14557 CrossRef CAS; (b) R. Sahoo, S. Luo, N. K. Pendyala, S. Chand, Z. H. Fu and M. C. Das, Mater. Chem. Front., 2023, 7, 3373–3381 RSC.
- (a) CCDC 2491444: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmk52; (b) CCDC 2491445: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmk63; (c) CCDC 2491446: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmk74.
| This journal is © The Royal Society of Chemistry 2026 |