Open Access Article
Open Access ArticleAcid-catalyzed esterification and biotin/fluorescent labeling of glutathione polysulfides based on stability under heating and pH change
Masana
Yazaki
a,
Yaozhuo
Zhang
a,
Lin
Zhou
a,
Ryo
Morise
b,
Takaaki
Akaike
c and
Mieko
Arisawa
 *ac
*ac
aDepartment of Bioscience and Biotechnology, Graduate School of Bioresource and Bioenvironmental Sciences, Kyushu University, Fukuoka, 819-0395, Japan. E-mail: arisawa@agr.kyushu-u.ac.jp
bKumamoto Prefectural Kamoto High School, Kumamoto, 861-0532, Japan
cDepartment of Redox Molecular Medicine, Graduate School of Medicine, Tohoku University, Sendai, 980-8578, Japan
First published on 3rd January 2026
Abstract
Glutathione polysulfides GSSSG and GSSSSG are stable in water up to 80 °C and below pH 9. Their carboxyl groups can be efficiently esterified with TMSCl in alcohols, reacting first at glycine, then glutamic acid. The resulting 3-butyne-1-yl esters allow Cu-catalyzed labeling (e.g., biotin, fluorophore) and can be hydrolyzed by esterases.
The human body contains abundant reduced and oxidized polysulfides derived from cysteine and cystine residues, which have diverse physiological functions.1 For example, polysulfide thiols such as GSSH (G = glutathione) generated from polysulfides are involved in cell signaling;2 polysulfide thiols/polysulfides play critical roles in maintaining myocardial robustness3 and they bind to serum albumin in their oxidized form.4 We previously synthesized GSSSG and related peptide polysulfides by sulfur atom insertion reactions into the disulfide bonds of unprotected peptides using elemental sulfur, and these polysulfides are now employed in biological studies.5 As a result, then, the use of chemically modified derivatives of peptide polysulfides has become a subject of interest, which behave slightly differently from the original GSSSG and can be used to develop useful compounds such as chemical probes and pharmaceuticals. GSSSG containing a highly reactive trisulfide moiety, however, is unstable, readily extruding sulfur atoms, and the control of the chemical reactivity of GSSSG is critical for the derivatization. It is shown here that GSSSG is stable under acidic conditions whereas unstable under basic conditions at pHs higher than 9. GSSSG is thermally stable in water up to 80 °C. GSSSG can be esterified under acid-catalyzed conditions. GSSSG esters can be hydrolyzed through esterase catalysis. The trisulfide group is compatible with Cu-catalyzed cycloaddition reactions. Also described here is the chemical synthesis of biotin- and fluorescent-labeled GSSSG, which can be used to study the behavior of GSSSG and GSSH in biological cells.
The thermal stability of GSSSG 1 was investigated by variable-temperature NMR measurements of an aqueous GSSSG solution at a concentration of 21 mM and 41 mM. The temperature was increased from room temperature to 80 °C in 10 °C increments, and at each temperature, the solution was allowed to stand for 30 min before recording the 1H NMR spectrum. No spectral change was observed in this temperature range, and GSSSG interestingly remained stable at temperatures below 80 °C. Similarly, GSSSSG 2 was also thermally stable at 21 mM, 41 mM, and 83 mM, with no observable changes below 80 °C, demonstrating that its thermal stability is high and independent of concentration.
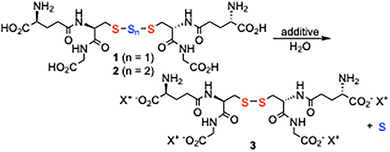
The stability of GSSSG 1/GSSSSG 2 at different pHs was examined in water (Table 1). When GSSSG 1 (20 mg, 0.031 mmol) was dissolved in 0.16 M lithium hydroxide and 1.0 M sodium hydroxide aqueous solutions (pH 14, 0.75 mL) and stirred at room temperature for 10 min, the salts of GSSG 3 with the central sulfur atom extruded were obtained in 78%, 84% yields, respectively. Similarly, 0.16 M sodium hydroxide (pH 13) and 0.16 M sodium carbonate (pH 11) aqueous solutions gave the sodium salt of 3 in 53% and 9% yields, respectively. In 0.16 M ammonia aqueous solution (pH 9), carbonate–bicarbonate buffer (CBB, pH 9), tris–HCl buffer (pH 8), and 1× PBS (pH 7), 1 was quantitatively recovered without sulfur atom extrusion. GSSSSG 2 provided mixtures of salts of 1 and 3 under basic conditions showing a similar tendency with 1, although the extrusion of sulfur atoms of 2 is faster than 1. For example, in an 0.16 M sodium carbonate aqueous solution (0.75 mL, pH 11), 2 provided the sodium salt of 3 with two sulfur atoms extruded in 20% yield and the sodium salt of 1 with one sulfur atom extruded in 70% yield. In contrast, the sulfur extrusion of 1 occurred by 7% under the same conditions. These results indicate that polysulfide stability is affected by pH rather than by the counter cations or anions present. It is shown that GSSSG 1 and GSSSSG 2 are stable under acidic conditions and unstable under basic conditions.6
| Additive | pH | Yield of 3 use of GSSSG 1 | Yield of 3 use of GSSSSG 2 |
|---|---|---|---|
| a n.d. = not detected. b Yield of salt of 1. c CBB = carbonate-bicarbonate buffer. d PBS = phosphate-buffered saline. | |||
| LiOH (0.16 M) | 14 | 78% | 91%, 9%b |
| NaOH (1.0 M) | 14 | 84% | 93%, 7%b |
| NaOH (0.1 M) | 13 | 53% | 81%, 19%b |
| Na2CO3 (0.16 M) | 11 | 9% | 20%, 70%b |
| CBBc (0.1 M) | 9 | n.d.a | n.d.a |
| NH3 aq. (0.16 M) | 9 | n.d.a | n.d.a |
| Tris-HCl buffer (1.0 M) | 8 | n.d.a | n.d.a |
| 1× PBSd | 7 | n.d.a | n.d.a |
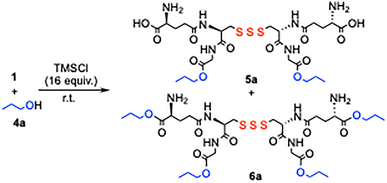
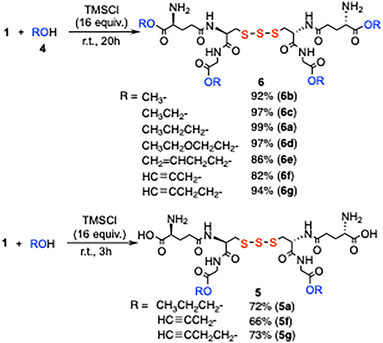
When GSSSG was reacted with 1-propanol (1 mL) and 16 equivalents of TMSCl at room temperature for 20 h, tetraester 6a with all four carboxyl groups 1-propylated was obtained in 99% yield (Table 2, entry 6). HCl was gradually generated from alcohol and TMSCl in the reaction system.7 It was observed that the glycine carboxyl groups were initially esterified over 1 to 3 h, giving diester 5a (entries 2 and 3), and the reaction of glutamic acid carboxyl groups was completed after 20 h (entries 5 and 6). The glycine methylene protons of 5a appeared as two downfield-shifted doublets at δ 4.08 (2H, d, J = 17.7 Hz) and δ 4.02 (2H, d, J = 17.7 Hz), whereas those of GSSSG 1 were observed at δ 3.96 (4H, s), thereby enabling the determination of the 5a structure. The selectivity is advantageous for the precise derivatization of GSSSG 1.
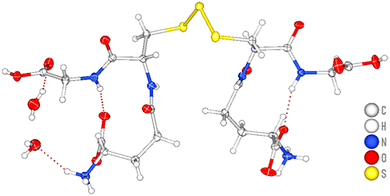
X-ray analysis of a single crystal of GSSSG 1 obtained from water (Fig. 1) revealed a hydrogen bond between the glutamic acid carboxy group and the glycine amino group, indicating that 1 adopts a bicyclic structure through intramolecular hydrogen bonding.8 The structure suggests that the higher reactivity of the glycine carboxy groups in 1 is due to the less steric hindrance on the outer side of the ring structure and/or non-hydrogen bonded nature.
The tetraesterification of GSSSG 1 quantitatively proceeded over 20 h in various alcohols including 2-ethoxyethanol, 3-buten-1-ol, 2-propyn-1-ol, and 3-butyn-1-ol (Scheme 1). When ethanol, 3-butyn-1-ol, and 2-propyn-1-ol were reacted with 1 for 3 h, the diesterified products 5f and 5g were produced in high yields. Note that selective esterification can be carried out efficiently without the extrusion of the sulfur atom.
The esterification of GSSSSG 2 with 4g also effectively proceeded providing tetraester 7g in 94% yield (Scheme 2). N-Acetylcysteine trisulfide NAC2S 8, which was obtained in a large quantity from elemental sulfur and N-acetyl cystine (see SI), was also diesterified with 4g providing diester 9g in 95% yield.
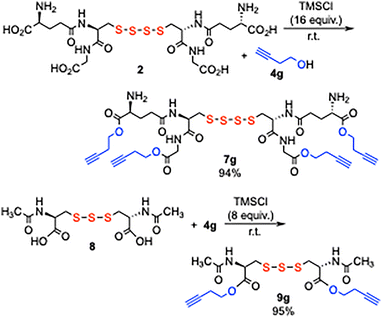 | ||
| Scheme 2 Tetraesterification of tetrasulfide GSSSSG 2 and diesterification of N-acetylcysteine trisulfide 8. | ||
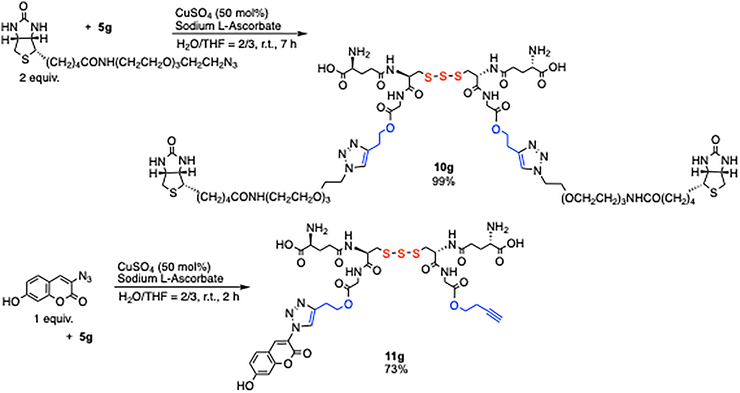
Based on the above results, biotin- and fluorescent-labeled derivatives of GSSSG were synthesized (Scheme 3). The selective acid-catalyzed esterification of the glycine carboxylic acid moiety in GSSSG 1 with 3-butyn-1-ol afforded 5g in 73% yield in 3 h. The subsequent CuSO4-catalyzed cycloaddition of 5g with biotin–PEG3–azide (2 equiv.) quantitatively furnished bis(biotin)-labeled compound 10g. Similarly, the reaction of 5g with 3-azido-7-hydroxycoumarin (1 equiv.) under the same catalytic conditions afforded the mono(coumarin)-labeled compound 11g in 73% yield. Although the reaction was conducted under reducing conditions, no sulfur atom extrusion from the polysulfide 5g was observed under the slightly acidic conditions (pH 5–6).
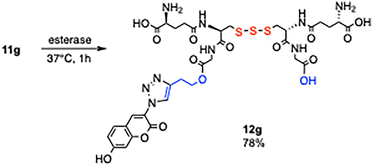
The remaining 3-butyn-1-yl ester in 11g was hydrolyzed using esterase (Scheme 4). A suspension of porcine liver esterase in ammonium sulfate suspension (Sigma-Aldrich, E2884, 300 units) was added to compound 11g (3.0 mg, 3.2 µmol) dissolved in PBS buffer (pH 7, 0.1 mL), and the mixture was incubated at 37 °C for 1 h. The fluorescent-labeled GSSSG derivative 12g was obtained in 78% yield, which exhibited a blue fluorescence emission (Fig. 2. λexmax = 348 nm, λemmax = 429 nm). The results demonstrate that biotin- and fluorescent-labeled derivatives of GSSSG can be prepared with the control of the number and location of the labels.
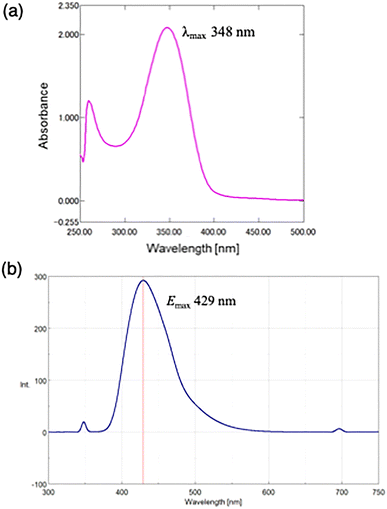 | ||
| Fig. 2 (a) UV-vis and (b) photoluminescence spectra of 12g in DMSO (1.9 µM) at room temperature (excitation at 348 nm). | ||
In summary, GSSSG was shown to be stable below 80 °C and below pH 9 in water, and can be subjected to chemical modifications under these conditions. The site-selective esterification of GSSSG can be conducted through acid catalysis at room temperature without the extrusion of the sulfur atom. From these results, biotin and fluorescent probes can be synthesized by (1) acid-catalyzed selective 3-butyn-1-yl esterification of the glycine carboxylate moiety, (2) Cu-catalyzed azide cycloaddition, and (3) esterase-catalyzed selective hydrolysis of glycine 3-butyn-1-yl ester. The efficient method for the derivatization of unstable GSSSG and related polysulfides has been developed, and the derivatives can be used for biological studies and pharmaceutical applications.
M. A., M. Y. conceived and designed the study. M. Y. led the compound synthesis, data collection, and data analysis, with contributions from Y. Z., L. Z., and R. M., T. A. provided expert advice on the interpretation of the results. M. A. wrote the manuscript with input from all authors. All authors have approved the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.Data availability
Supplementary information (SI): general information, detailed experimental procedures, characterization data for compounds and NMR spectra and X-ray structure. See DOI: https://doi.org/10.1039/d5cc07029e.CCDC 2481054 contains the supplementary crystallographic data for this paper.8
Acknowledgements
The authors thank Dr Taisuke Matsumoto (Evaluation Center of Materials Properties and Function, Institute for Materials Chemistry and Engineering, Kyushu University) for his assistance in X-ray crystallographic analysis. This research was supported by JSPS KAKENHI (Grant Number 21K18947 and 24H01322), JST FOREST Program (Grant Number JPMJFR205X), JST Moonshot R&D (Grant Number JPMJMS2033-13), and Kobayashi foundation. This research was also supported by the grants from JSPS KAKENHI (Grant Numbers JP21H05263, JP23K20040, JP24H00063, and 22K19397), by the Japan Science and Technology Agency (JST) CREST (Grant Number JPMJCR2024), and by the Japan Agency for Medical Research and Development (AMED) Grant Number JP21zf0127001 to TA. Research support for Fellowship for Young Scientists from the JSPS KAKENHI (Grant Number JP25KJ1951) to MY, from the JST SPRING (Grant Number JPMJSP2136) to MY and YS, from the JST FOREST Program (Grant Number JPMJFR205X) to LZ, and the QFC-SP Program to RM is gratefully acknowledged.References
- T. Ida, T. Sawa, H. Ihara, Y. Tsuchiya, Y. Watanabe, Y. Kumagai, M. Suematsu, H. Motohashi, S. Fujii, T. Matsunaga, M. Yamamoto, K. Ono, N. O. Devarie-Baez, M. Xian, J. M. Fukuto and T. Akaike, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 7606 CrossRef CAS PubMed; T. Sawa, H. Motohashi, H. Ihara and T. Akaike, Biomolecules, 2020, 10, 1245 Search PubMed; T. Zhang, H. Tsutsuki, K. Ono, T. Akaike and T. Sawa, J. Clin. Biochem. Nutr., 2021, 68, 5 CrossRef.
- S. Kasamatsu, A. Nishimura, M. Morita, T. Matsunaga, H. A. Hamid and T. Akaike, Molecules, 2016, 21, 1721 CrossRef PubMed; Y. Kimura, S. Koike, N. Shibuya, D. Lefer, Y. Ogasawara and H. Kimura, Sci. Rep., 2017, 7, 10459 CrossRef PubMed.
- A. Nishimura, S. Ogata, X. Tang, K. Hengphasatporn, K. Umezawa, M. Sanbo, M. Hirabayashi, Y. Kato, Y. Ibuki, Y. Kumagai, K. Kobayashi, Y. Kanda, Y. Urano, Y. Shigeta, T. Akaike and M. Nishida, Nat. Commun., 2025, 16, 276 CrossRef CAS PubMed.
- M. Ikeda, Y. Ishima, A. Shibata, V. T. G. Chuang, T. Sawa, H. Ihara, H. Watanabe, M. Xian, Y. Ouchi, T. Shimizu, H. Ando, M. Ukawa, T. Ishida, T. Akaike, M. Otagiri and T. Maruyama, Anal. Chim. Acta, 2017, 969, 18 CrossRef CAS.
- K. Fukumoto, M. Yazaki and M. Arisawa, Org. Lett., 2022, 24, 8176 CrossRef CAS.
- E. M. Brown and N. B. Bowden, ACS Omega, 2022, 7, 11440 CrossRef CAS PubMed.
- Methylation of GSSG: (a) F. S. Palumbo, S. Federico, G. Pitarresi, C. Fiorica and G. Giammona, React. Funct. Polym., 2021, 166, 104986 CrossRef CAS; (b) E. R. Vogel, W. Jackson and D. S. Masterson, Molecules, 2015, 20, 10487 CrossRef CAS PubMed ; Methylation of Cystine: ; (c) J. Li and Y. Sha, Molecules, 2008, 13, 1111 CrossRef CAS PubMed.
- CCDC 2481054: Experimental Crystal Structure Determination, 2026 DOI:10.5517/ccdc.csd.cc2p8r0r.
| This journal is © The Royal Society of Chemistry 2026 |