Open Access Article
Open Access ArticleHydrogen bonding-enabled gold catalysis: ligand effects in gold-catalyzed cycloisomerization of 1,6-enynes in hexafluoroisopropanol (HFIP)
Yuan
Zhao†
 ,
Xinyuan
Ma†
,
Junying
Wang
,
Vladislav A.
Voloshkin
and
Steven P.
Nolan
,
Xinyuan
Ma†
,
Junying
Wang
,
Vladislav A.
Voloshkin
and
Steven P.
Nolan
 *
*
Department of Chemistry, Center for Sustainable Chemistry, Ghent University, Krijgslaan 289, S3, 9000 Ghent, Belgium. E-mail: steven.nolan@ugent.be
First published on 5th January 2026
Abstract
Hydrogen bonding has recently emerged as a powerful yet underexplored tool to enable gold(I) catalysis. Here, we demonstrate that HFIP can directly activate [Au(L)Cl] complexes through hydrogen bonding, in the cycloisomerization of 1,6-enynes under mild conditions without the need for external activators. The influence of ancillary ligands on the catalytic efficiency was systematically examined, revealing that ligand–HFIP interactions play a key role in the activation process. This study provides new insight into hydrogen-bond-assisted gold catalysis and offers a simple strategy for activator-free transformations.
Over the past two decades, gold catalysis has undergone remarkable development and is now regarded as one of most powerful and versatile tools in organic synthesis.1–3 Among gold-based catalysts, gold(I) chloride complexes [Au(L)Cl] are particularly attractive due to their stability and commercial availability. However, because of the inherent inertness of the Au–Cl bond, activation is typically required to initiate catalysis. Silver salts bearing weakly coordinating anions have long served as standard activators for gold(I) chloride complexes. However, their hygroscopic nature, light sensitivity, and the so-called “silver effect” in catalysis often present significant drawbacks and limitations.4–7
Several strategies have been developed to overcome these specific issues in gold catalysis.8 Including the use of alternative alkali metal borates9 and copper salts,10 Brønsted acid activation of basic gold precursors11 and the self-activation of gold(I) chloride complexes bearing specially designed ancillary ligands,8 as well as the use of halogen-bonding donors to activate the Au–Cl bond and generate catalytically active species.12,13 Despite their effectiveness, these strategies share a common limitation: the need for external reagents to promote the Au–Cl bond activation.
Hydrogen bonding can exert a profound influence on gold catalysis.14 In this regard, hexafluoroisopropanol (HFIP) is emerging as a particularly promising medium. Its unique combination of properties—including strong hydrogen bond-donating ability, low nucleophilicity, efficient cation stabilization, enhanced acidity, and conveniently low boiling point (59 °C)—makes it a broadly applicable and easily recyclable solvent for catalytic applications.15–17 For its unique properties, the use of HFIP has exponentially increased in the past decade and has become a solvent of choice in some areas, such as in C–H functionalization chemistry.15 This evolving area encouraged us to explore its use further in gold(I) catalysis. Our group has recently shown that HFIP can promote two distinct cyclization reactions, namely the N-propargyl benzamide cyclization and the alkynoic acid cyclization, without requiring external activators.18,19 In these systems, HFIP plays a dual role by dynamically activating the Au–Cl bond through hydrogen bonding, thereby initiating the catalytic cycle and acting as solvent.
Building on these findings and driven by our ongoing interest in the potential of HFIP in gold catalysis, we sought to explore its broader applicability and performance across a range of gold-catalyzed transformations. The cycloisomerization of 1,6-enynes is a well-established reaction widely employed to evaluate the activity of gold catalysts and the efficiency of activators in homogeneous gold catalysis.20–22 In addition to catalyst performance, this transformation also presents a challenge in terms of controlling product distribution, as it often involves competing rearrangement pathways.23,24 In recent years, various improvements in catalyst design and activator use have been made to the cycloisomerization of 1,6-enynes.12,25–28 However, despite extensive efforts, silver salts remain the most effective activators for this reaction, with no suitable alternatives having been identified to date.
In this context, we report our study on the cycloisomerization of 1,6-enynes in the presence of HFIP, with a focus on the role of ligands in modulating reactivity and selectivity. The representative [Au(NHC)Cl] and [Au(PR3)Cl] complexes employed in this study are illustrated in Scheme 1. Our aim was to explore commonly used ligands in gold catalysis,29 in the hope of enhancing selectivity and provide an alternative to silver-based activation in the cycloisomerization of 1,6-enynes.
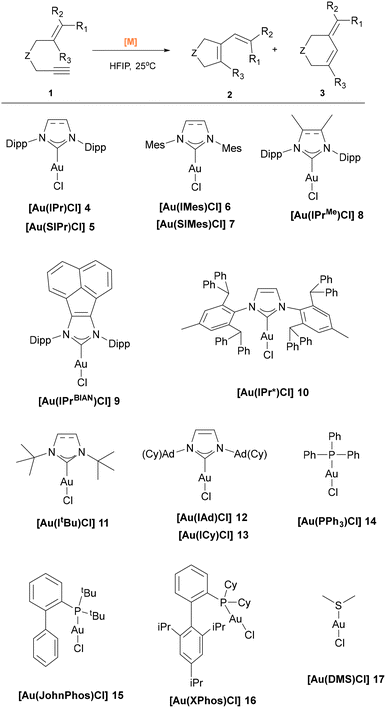 | ||
| Scheme 1 Model reaction and ligand based-catalyst library. Ph = phenyl, Dipp = (2,3-diisopropyl) phenyl, Ad = 1-adamantyl, Cy = cyclohexyl, tBu = tert-butyl. | ||
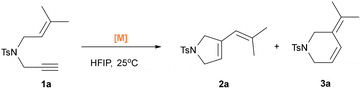
We began our catalytic investigations with the benchmark cycloisomerization of N-Tosyl-1,6-enyne 1a. To our delight, the reaction proceeded smoothly when complex 4 was used, reaching full conversion within 4 hours and affording five-membered lactone 2a and six-membered product 3a with 14.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 selectivity in favor of 2a (Table 1, entry 1). A focused screening of [Au(L)Cl] complexes was then conducted (Table 1). Entries 2–6 show that increasing the steric bulk on the NHC ligand generally leads to diminished reactivity and selectivity. However, complex 10 exhibited good performance, providing high conversion but only moderate selectivity (entry 7). Bulky ligands such as ItBu and IAd resulted in only moderate conversion and poor selectivity (1
1 selectivity in favor of 2a (Table 1, entry 1). A focused screening of [Au(L)Cl] complexes was then conducted (Table 1). Entries 2–6 show that increasing the steric bulk on the NHC ligand generally leads to diminished reactivity and selectivity. However, complex 10 exhibited good performance, providing high conversion but only moderate selectivity (entry 7). Bulky ligands such as ItBu and IAd resulted in only moderate conversion and poor selectivity (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, entries 8 and 9). In contrast, the catalyst bearing the small ICy ligand, complex 13, gave only 39% conversion of 1a (entry 10). Overall, the IPr ligand remains the most effective and reliable among the NHC series.30 Notably, phosphine-based catalysts exhibited reversed selectivity. For example, 14 favored the formation of 3a over 2a in a 1
1 ratio, entries 8 and 9). In contrast, the catalyst bearing the small ICy ligand, complex 13, gave only 39% conversion of 1a (entry 10). Overall, the IPr ligand remains the most effective and reliable among the NHC series.30 Notably, phosphine-based catalysts exhibited reversed selectivity. For example, 14 favored the formation of 3a over 2a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 ratio. Other common phosphine ligands, including 15 and 16, displayed slightly reduced activity and selectivity (entries 12 and 13). Interestingly, silver and copper congener complexes of 4 were catalytically inactive under the same conditions (entries 14 and 15), as was 17 (entry 16). Several Brønsted-basic gold complexes, such as [Au(IPr)(Cbz)] (Cbz = carbazolyl), [Au(IPr)(OH)], and [Au(IPr)(OAc)], gave moderate conversions (31–56%, entries 17–19).
15 ratio. Other common phosphine ligands, including 15 and 16, displayed slightly reduced activity and selectivity (entries 12 and 13). Interestingly, silver and copper congener complexes of 4 were catalytically inactive under the same conditions (entries 14 and 15), as was 17 (entry 16). Several Brønsted-basic gold complexes, such as [Au(IPr)(Cbz)] (Cbz = carbazolyl), [Au(IPr)(OH)], and [Au(IPr)(OAc)], gave moderate conversions (31–56%, entries 17–19).
| Entry | [M] | Combined yield (%) | Selectivity ratio (2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a) 3a) |
|---|---|---|---|
a Unless otherwise noted, the reaction was conducted with 1a (0.2 mmol) and [M] (0.002 mmol, 1 mol%) in HFIP (0.2 mL) in 4 h. 25 °C; the yield and selectivity determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
b DCM instead of HFIP.
c Mixture of DCM and HFIP (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of HFIP.
d Trifluoroethanol (TFE) instead of HFIP.
e 0.5 mmol of phenol instead of HFIP. 1) instead of HFIP.
d Trifluoroethanol (TFE) instead of HFIP.
e 0.5 mmol of phenol instead of HFIP.
|
|||
| 1 | [Au(IPr)Cl] 4 | 99 | 14.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | [Au(SIPr)Cl] 5 | 80 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | [Au(IMes)Cl] 6 | 82 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | [Au(SIMes)Cl] 7 | 89 | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | [Au(IPrMe)Cl] 8 | 70 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | [Au(IPrBIAN)Cl] 9 | 71 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | [Au(IPr*)Cl] 10 | 99 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | [Au(ItBu)Cl] 11 | 72 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | [Au(IAd)Cl] 12 | 76 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
| 10 | [Au(ICy)Cl] 13 | 39 | — |
| 11 | [Au(PPh3)Cl] 14 | 99 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 12 | [Au(JohnPhos)Cl] 15 | 99 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 13 | [Au(XPhos)Cl] 16 | 85 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 14 | [Au(DMS)Cl] 17 | 13 | — |
| 15 | [Ag(IPr)Cl] | 0 | — |
| 16 | [Cu(IPr)Cl] | 0 | — |
| 17 | [Au(IPr)(Cbz)] | 31 | — |
| 18 | [Au(IPr)(OH)] | 56 | — |
| 19 | [Au(IPr)(OAc)] | 47 | — |
| 20 | [Au(IPr)(OC(CF3)2)] | 50 | — |
| 21 | [Au(IPr)(NTf2)] | 39 | — |
| 22 | [Au(IPr)(OTf)] | 65 | — |
| 23b | 4 | 0 | — |
| 24c | 4 | 0 | — |
| 25d | 4 | 20 | — |
| 26e | 4 | 0 | — |
Comparing the catalytic activity of [Au(IPr)(OC(CF3)2)] to that of 4 and to those of the already activated complexes [Au(IPr)(NTf2)] and [Au(IPr)(OTf)], which bear weakly coordinating anions, provides information relating to counterion effects in HFIP assisted systems (entries 20–22). Complex 4 showed no catalytic activity either in neat DCM or in a DCM/HFIP (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture (entries 23 and 24). Finally, trifluoroethanol (TFE), which is a widely used fluorinated alcohol solvent in H-bonding-assisted gold catalysis, was also tested and led to only a 20% yield of the product (entry 25). Replacing HFIP with phenol as an activator also failed to activate the reaction (entry 26). Compared with previously reported systems,22,32 using precatalyst 14 in the HFIP-activated protocol enables cyclization with an efficiency nearly equivalent to that of silver salt-activated reactions for producing the six-membered ring 3a, although requiring slightly longer reaction times. Notably, using simple precatalyst 4 alone enables highly selective formation of the five-membered ring 2a, a result rarely achieved in studies involving silver additives. This highlights the unique effectiveness of HFIP as a hydrogen-bonding solvent in promoting gold-catalyzed cycloisomerization under mild and practical conditions.
1) mixture (entries 23 and 24). Finally, trifluoroethanol (TFE), which is a widely used fluorinated alcohol solvent in H-bonding-assisted gold catalysis, was also tested and led to only a 20% yield of the product (entry 25). Replacing HFIP with phenol as an activator also failed to activate the reaction (entry 26). Compared with previously reported systems,22,32 using precatalyst 14 in the HFIP-activated protocol enables cyclization with an efficiency nearly equivalent to that of silver salt-activated reactions for producing the six-membered ring 3a, although requiring slightly longer reaction times. Notably, using simple precatalyst 4 alone enables highly selective formation of the five-membered ring 2a, a result rarely achieved in studies involving silver additives. This highlights the unique effectiveness of HFIP as a hydrogen-bonding solvent in promoting gold-catalyzed cycloisomerization under mild and practical conditions.
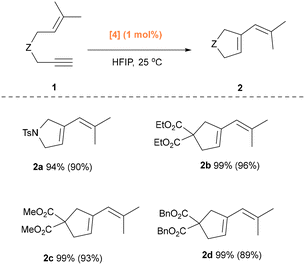
Having performed a thorough screening of ancillary ligands in the HFIP-assisted, gold-catalyzed cycloisomerization reaction of N-Tosyl-1,6-enyne 1a. Next, the selectivity towards the formation of the 5-membered cycle seen with substrate 1a under [Au(IPr)Cl] was also observed with propargylmalonates 1b–1d (Scheme 2). Due to the Thorpe–Ingold effect,31 the reaction times were correspondingly shortened. The reactions of 1,6-enynes 1b and 1c proceeded smoothly to afford the corresponding vinyl cyclopentene derivatives 2b and 2c in quantitative yields within 1 hour. Substrate 1d required 1.5 hours to reach full conversion. It is worth noting that when a phosphine-based catalyst is used in this case, no six-membered ring product was observed. When 1,6-enyne bearing a phenyl substituent on the alkyne was used as the substrate (See SI), no conversion was observed under the standard conditions. This observation is consistent with previous results,19 showcasing that the presence of steric hindrance near the alkyne bond prevents effective binding to a bulky gold catalyst.
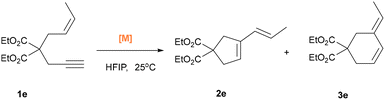
We next examined the reactivity of variously substituted 1,6-enynes under the Au/HFIP catalytic system (Tables 2 and 3). 1e, bearing a methyl group, proceeded smoothly to afford the corresponding vinyl cyclopentene 2e in 1 hour, regardless of whether 4 or 14 were used as catalyst.
| Entry | Substrate | [Au] | Combined yield (%) | Selectivity ratio (2e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3e) 3e) |
|---|---|---|---|---|
| a Unless otherwise noted, the reaction was conducted with 1e (0.2 mmol) and [M] (0.002 mmol, 1 mol%) in HFIP (0.2 mL). 25 °C; the conversion and selectivity determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. Isolated yields are given in parentheses; reaction time: 1 h. | ||||
| 1 | 1e | 4 | 99 (95) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 2 | 14 | 99 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
|
| Entry | Substrate | [Au] | Combined yield (%) | Selectivity ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
|---|---|---|---|---|
| a Unless otherwise noted, the reaction was conducted with 1 (0.2 mmol) and [M] (0.002 mmol, 1 mol%) in HFIP (0.2 mL). 25 °C, 2.5 h. Conversion and selectivity determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. Isolated yields are given in parentheses. | ||||
| 1 | 1f | 4 | 85 (71%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
| 2 | 15 | 46 | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
| 3 | 14 | 54 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3 3.3 |
|
| 4 | 1g | 4 | 28 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 15 | 80 (77%) | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
| 6 | 14 | 71 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
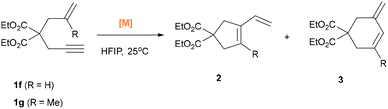
Using the 1,6-enyne 1f without any substituted group, gave a mixture of five-membered lactone 2f and six-membered product 3f, with a selectivity of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 when complex 4 was used. Changing the catalyst to complexes 14 or 15 did not improve the yield or selectivity. Substrate 1g afforded a mixture of 2g and 3g in 80% yield with excellent selectivity (28
1.7 when complex 4 was used. Changing the catalyst to complexes 14 or 15 did not improve the yield or selectivity. Substrate 1g afforded a mixture of 2g and 3g in 80% yield with excellent selectivity (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) when complex 15 was used as the catalyst, whereas only 28% yield and 13
1) when complex 15 was used as the catalyst, whereas only 28% yield and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 selectivity was observed when 4 was employed. However, when a 1,7-enyne was subjected to the reaction conditions, only trace amounts of product were observed, indicating that the longer tether likely weakens the cooperative interaction between the reactive sites and thus severely reduces the reactivity (see SI).
1 selectivity was observed when 4 was employed. However, when a 1,7-enyne was subjected to the reaction conditions, only trace amounts of product were observed, indicating that the longer tether likely weakens the cooperative interaction between the reactive sites and thus severely reduces the reactivity (see SI).
In conclusion, we have developed a Au/HFIP catalytic system that enables the activation of [Au(L)Cl] complexes for the cycloisomerization of 1,6-enynes under external activator-free conditions, providing a silver-free and efficient synthetic strategy. Moreover, in this protocol the simple precatalysts [Au(IPr)Cl] (4), [Au(PPh3)Cl] (14) and [Au(JohnPhos)Cl] (15) exhibit excellent catalytic performance and afford outstanding yields and selectivity. Ongoing studies in our group aim to further explore the broader potential of HFIP in related catalytic transformations.
All authors contributed to the writing and revision of the manuscript. The final version of the manuscript has been approved by all authors.
Conflicts of interest
There are no conflicts to declare.Data availability
The data underlying this study are available in the published article and its supplementary information (SI). Supplementary information: experimental details and NMR spectra for all compounds. See DOI: https://doi.org/10.1039/d5cc07022h.Acknowledgements
We gratefully acknowledge support from the Special Research Fund (BOF) of Ghent University (project grants to SPN) and the Research Foundation – Flanders (FWO) (G0A6823N). YZ, XM and JW thank the China Scholarship Council (CSC) (project No. 202206870016, 201908530217 and 202306870024) for PhD fellowships. Mr Riku Saito is thanked for kindly supplying a sample of [Au(JohnPhos)Cl].References
- R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028–9072 CrossRef CAS.
- A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180–3211 Search PubMed.
- A. S. K. Hashmi, Chem. Rev., 2021, 121, 8309–8310 Search PubMed.
- Z. Lu, J. Han, G. B. Hammond and B. Xu, Org. Lett., 2015, 17, 4534–4537 CrossRef CAS PubMed.
- D. Wang, R. Cai, S. Sharma, J. Jirak, S. K. Thummanapelli, N. G. Akhmedov, H. Zhang, X. Liu, J. L. Petersen and X. Shi, J. Am. Chem. Soc., 2012, 134, 9012–9019 CrossRef CAS PubMed.
- D. Weber and M. R. Gagné, Org. Lett., 2009, 11, 4962–4965 CrossRef CAS PubMed.
- A. Zhdanko and M. E. Maier, ACS Catal., 2015, 5, 5994–6004 Search PubMed.
- A. Franchino, M. Montesinos-Magraner and A. M. Echavarren, Bull. Chem. Soc. Jpn., 2021, 94, 1099–1117 CrossRef CAS.
- M. Wegener, F. Huber, C. Bolli, C. Jenne and S. F. Kirsch, Chem. – Eur. J., 2015, 21, 1328–1336 CrossRef CAS PubMed.
- A. Guérinot, W. Fang, M. Sircoglou, C. Bour, S. Bezzenine-Lafollée and V. Gandon, Angew. Chem., Int. Ed., 2013, 52, 5848–5852 Search PubMed.
- P. Nun, S. Dupuy, S. Gaillard, A. Poater, L. Cavallo and S. P. Nolan, Catal. Sci. Technol., 2011, 1, 58–61 RSC.
- J. Wolf, F. Huber, N. Erochok, F. Heinen, V. Guérin, C. Y. Legault, S. F. Kirsch and S. M. Huber, Angew. Chem., Int. Ed., 2020, 59, 16496–16500 Search PubMed.
- H. F. Jónsson, D. Sethio, J. Wolf, S. M. Huber, A. Fiksdahl and M. Erdelyi, ACS Catal., 2022, 12, 7210–7220 Search PubMed.
- R. Gauthier, N. V. Tzouras, Z. Zhang, S. Bédard, M. Saab, L. Falivene, K. Van Hecke, L. Cavallo, S. P. Nolan and J.-F. Paquin, Chem. – Eur. J., 2022, 28, e202103886 Search PubMed.
- H. F. Motiwala, A. M. Armaly, J. G. Cacioppo, T. C. Coombs, K. R. K. Koehn, V. M. I. Norwood and J. Aubé, Chem. Rev., 2022, 122, 12544–12747 Search PubMed.
- F. Caporaletti, L. Gunkel, M. Á. Fernández-Ibáñez, J. Hunger and S. Woutersen, Angew. Chem., Int. Ed., 2024, 63, e202416091 Search PubMed.
- M. Piejko, J. Moran and D. Lebœuf, ACS Org. Inorg. Au, 2024, 4, 287–300 CrossRef CAS PubMed.
- N. V. Tzouras, L. P. Zorba, E. Kaplanai, N. Tsoureas, D. J. Nelson, S. P. Nolan and G. C. Vougioukalakis, ACS Catal., 2023, 13, 8845–8860 Search PubMed.
- N. V. Tzouras, A. Gobbo, N. B. Pozsoni, S. G. Chalkidis, S. Bhandary, K. V. Hecke, G. C. Vougioukalakis and S. P. Nolan, Chem. Commun., 2022, 58, 8516–8519 RSC.
- A. Munawar, L. T. Maltz, W.-C. Liu and F. P. Gabbaï, Organometallics, 2023, 42, 2742–2746 Search PubMed.
- S. G. Mahamulkar, I. Císařová and U. Jahn, Adv. Synth. Catal., 2018, 360, 4215–4224 CrossRef CAS.
- A. Cervantes-Reyes, F. Rominger, M. Rudolph and A. S. K. Hashmi, Adv. Synth. Catal., 2020, 362, 2523–2533 CrossRef CAS.
- C. Nieto-Oberhuber, M. P. Muñoz, S. López, E. Jiménez-Núñez, C. Nevado, E. Herrero-Gómez, M. Raducan and A. M. Echavarren, Chem. – Eur. J., 2006, 12, 1677–1693 CrossRef CAS PubMed.
- E. García-Padilla, F. Maseras and A. M. Echavarren, ACS Org. Inorg. Au, 2023, 3, 312–320 CrossRef PubMed.
- S. Ito, M. Nanko and K. Mikami, ChemCatChem, 2014, 6, 2292–2297 Search PubMed.
- M. Freytag, S. Ito and M. Yoshifuji, Chem. – Asian J., 2006, 1, 693–700 CrossRef CAS PubMed.
- S. Bastin, C. Barthes, N. Lugan, G. Lavigne and V. César, Eur. J. Inorg. Chem., 2015, 2216–2221 CrossRef CAS.
- K. Muratov and F. Gagosz, Angew. Chem., Int. Ed., 2022, 61, e202203452 CrossRef CAS PubMed.
- A. Collado, D. J. Nelson and S. P. Nolan, Chem. Rev., 2021, 121, 8559–8612 CrossRef CAS PubMed.
- V. A. Voloshkin, L. P. Zorba and S. P. Nolan, Chem. Sci., 2025, 16, 2062–2082 RSC.
- For the Thorpe-Ingold effect, see: R. M. Beesley, C. K. Ingold and J. F. Thorpe, J. Chem. Soc. Trans., 1915, 107, 1080–1106 RSC.
- C. Nieto-Oberhuber, M. P. Munoz, S. Lopez, E. Jimenez-Nunez, C. Nevado, E. Herrero-Gomez, M. Raducan and A. M. Echavarren, Chem. – Eur. J., 2006, 12, 1677–1693 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |