Open Access Article
Open Access ArticleInterphasic separation of cis/trans perylene-based nanographene isomers driven by selective encapsulation in a supramolecular cage
Carles
Fuertes-Espinosa
*,
Clara
Sabrià
,
Judith
Sala
,
Lorena
Capdevila
,
Ferran
Feixas
 and
Xavi
Ribas
and
Xavi
Ribas
 *
*
Institut de Química Computacional i Catàlisi (IQCC) and Departament de Química, Universitat de Girona, Girona, 17003, Catalonia, Spain. E-mail: xavi.ribas@udg.edu
First published on 5th January 2026
Abstract
The present work describes a supramolecular strategy for the molecular recognition and selective separation of extended PAHs and NGs from isomeric mixtures. This approach consists of a biphasic protocol featuring the selective shuttling of a targeted NG isomer from the organic to the aqueous phase, driven by host–guest interactions with a tetragonal prismatic metal–organic cage (MOC) present in the aqueous layer, while the cage remains permanently confined to the aqueous phase, operating as a static supramolecular receptor throughout the separation process.
Polycyclic aromatic hydrocarbons (PAHs) and nanographenes (NGs) have attracted significant attention in materials science and supramolecular chemistry owing to their distinctive self-assembly1–3 and optoelectronic properties.4,5 Their extended π-conjugated frameworks render them promising candidates for advanced functional materials.6 However, their selective manipulation and separation remain challenging due to strong aggregation, limited solubility, and the structural similarity of related species.7,8 In particular, the isolation of isomerically pure NGs is challenging, as conventional chromatographic methods are typically solvent-intensive, operationally demanding, and often fail to deliver isomerically pure NG fractions.9
In parallel, metal–organic cages (MOCs) have emerged as highly versatile supramolecular platforms, assembled from metal ions and organic ligands, featuring well-defined and tunable cavities.10–13 Their capacity to trap guest molecules within their confined spaces has been exploited in a broad range of applications, including molecular recognition and purification,14,15 catalysis,16 sensing,17 stabilization of reactive intermediates,18 and selective molecular transport.19 The nature of MOC cavities makes them particularly attractive for the recognition of hydrophobic π-conjugated molecules in their confined environments, such as PAHs and fullerenes.20 However, the confinement of NGs within artificial receptors remains challenging.21 Supramolecular strategies that exploit host–guest chemistry for molecular separation are gaining increasing interest.22,23 Recent work by Nitschke and co-workers has shown that phase-transfer of MOCs and their cargoes can be harnessed for practical separation of PAHs.19,24,25 This approach requires repeated in situ anion-exchange steps during the separation cycle, which can lead to the accumulation of salt residues and a progressive decrease in phase-transfer efficiency.25
Our previously reported tetragonal prismatic 4·(BArF)8 cage (Fig. 1a) exhibits strong affinity toward large π-conjugated guests, including fullerenes,26 endohedral metallofullerenes (EMFs),27 and fullertubes.28 The extended planar π-systems of the porphyrin residues in 4·(BArF)8 and PAHs, suggest the potential for favourable π–π stacking interactions. Hence, we hypothesized that 4·(BArF)8 would also serve as a suitable host for extended PAHs and NGs.
Herein, we present a supramolecular strategy for the molecular recognition of extended PAHs and the purification of a perylene-based NGs from a cis–trans isomeric mixture. The method exploits selective phase-transfer driven by host–guest interactions, enabling the targeted isomer to shuttle between immiscible solvents. Specifically, a biphasic system was employed in which a mixture of NG isomers was dissolved in the organic phase, while the anionic cage 4·(SO4)4 remained in the aqueous phase. This static-host biphasic protocol constitutes an operationally simplified alternative to phase-transfer strategies based on anion-driven cage migration, as it eliminates repeated anion-metathesis events during the separation cycle and enables selective isomer separation through a single, operationally simple biphasic extraction.
G-1 and G-2 fluoranthene derivatives, and G-3, a cis-trans mixture of perylene-based NG, were synthesized and characterized following protocols previously reported by our group.29 The selected nanographenes serve as representative scaffolds of structurally sophisticated and elaborated NGs, allowing the evaluation of the 4·(BArF)8 capacity to selectively separate cis/trans isomeric mixtures.
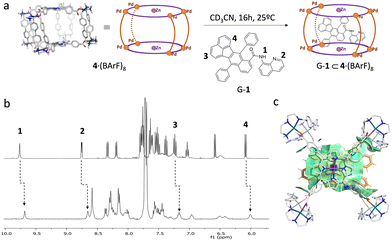
To study the molecular recognition of G-1 within the tetragonal prismatic cage 4·(BArF)8, a stoichiometric amount of G-1 (Fig. S1–S3) stock solution was added into a solution of the host in CD3CN (Fig. 1a).
The host–guest complex G-1⊂4·(BArF)8 was characterized by 1H NMR (Fig. 1b and Fig. S4), high-resolution mass spectrometry (HRMS) (Fig. S5) and NOESY NMR (Fig. S6). In the 1H NMR spectrum, the signals of G-1 underwent upfield shifts attributed to the inclusion-induced shielding effect (Fig. 1b and Fig. S4), consistent with guest encapsulation within the cavity of 4·(BArF)8 in a fast-exchange regime at the NMR timescale. Binding was further evidenced by broadening of the G-1 proton signals (Fig. 1b and Fig. S4 and S7). Concomitant shifts and broadening of the signals of the protons of the metalloporphyrin residues of the cage, together with the NOESY correlations observed, indicated participation of these π-conjugated panels in the guest binding (Fig. S4, S6, S7 and S8). 1H DOSY NMR spectrum showed that host–guest signals present similar diffusion coefficients to free 4·(BArF)8, suggesting the formation of an inclusion complex (Fig. S10). Furthermore, as the G-1⊂4·(BArF)8 adduct equilibrated in fast exchange on the NMR timescale, 1H DOSY NMR analysis of the encapsulated G-1 signals afforded a diffusion coefficient larger than the free G-1, providing further evidence of the formation of a host–guest adduct (Fig. S11). 1H NMR titration experiments provide an association constant Ka = 4.03 (±0.1) × 104 M−1 for the formation of G-1⊂4·(BArF)8 (Fig. S11). Mole-ratio analysis (Fig. S12) supported the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex and ESI-HRMS (Fig. S5) corroborated the stoichiometry, revealing distinct [G-1⊂4]n+ peaks.
1 host–guest complex and ESI-HRMS (Fig. S5) corroborated the stoichiometry, revealing distinct [G-1⊂4]n+ peaks.
The molecular recognition of G-2 (Fig. S20a), featuring an ethylene bridge on the fluoranthene unit (Fig. S13–S15), was investigated using an analogous protocol to that employed for G-1. An equimolar mixture of 4·(BArF)8 and G-2 results in the corresponding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex, as confirmed by mole-ratio analysis (Fig. S16) and ESI-HRMS (Fig. S17). The 1H NMR signals of the porphyrin protons in 4·(BArF)8 broadened and shifted upfield, consistent with altered cage symmetry upon encapsulation of G-2 (Fig. S19 and S20b). Simultaneously, the guest signals exhibited upfield shifts characteristic of an inclusion complex, indicating fast-exchange binding on the NMR timescale (Fig. S20). Notably, the differences in NMR response between G-2 and G-1 were attributed to changes in cage symmetry rather than binding stoichiometry. NOESY correlations indicated participation of the π-conjugated panels of G-2 and the porphyrin residues of the cage in the binding event (Fig. S18). 1H NMR titration experiments revealed an association constant Ka = 5.41 (± 0.1) × 104 M−1 (Fig. S21), slightly higher than to that observed for G-1. Moreover, the diffusion experiments resulted in a diffusion coefficient for the confined G-2 larger than for free G-2 guest, providing further evidence on the formation of G-2⊂4·(BArF)8 host–guest complex (Fig. S22). To get further insight into the non-covalent interactions directing the molecular recognition of G-1 and G-2, molecular dynamics (MD) simulations for G-1⊂4·Cl8 and G-2⊂4·Cl8 host–guest adducts were carried out (Fig. 1c and Fig. S23 and S24). In both cases, a persistent interaction between the oxygen of the carbonyl group of the guests and the Zn atom on the porphyrin of the cage was identified (Fig. S23). The noncovalent interaction (NCI) volumes were also calculated for both systems,30 observing a slightly larger value for G-2 (971.3 ± 78.4 Å3) than for G-1 (901.0 ± 79.1 Å3) (Fig. 1c and Fig. S24). This indicates higher stabilization of the host–guest adduct, which can be correlated with higher affinity of the host towards G-2. The enhanced binding of G-2 compared to G-1 could be tentatively assigned to additional aliphatic CH2–π interactions between the ethylene bridge of G-2 and the aromatic panels of the host cavity (Fig. S25), which could act cooperatively with the dominant π–π interactions.
1 host–guest complex, as confirmed by mole-ratio analysis (Fig. S16) and ESI-HRMS (Fig. S17). The 1H NMR signals of the porphyrin protons in 4·(BArF)8 broadened and shifted upfield, consistent with altered cage symmetry upon encapsulation of G-2 (Fig. S19 and S20b). Simultaneously, the guest signals exhibited upfield shifts characteristic of an inclusion complex, indicating fast-exchange binding on the NMR timescale (Fig. S20). Notably, the differences in NMR response between G-2 and G-1 were attributed to changes in cage symmetry rather than binding stoichiometry. NOESY correlations indicated participation of the π-conjugated panels of G-2 and the porphyrin residues of the cage in the binding event (Fig. S18). 1H NMR titration experiments revealed an association constant Ka = 5.41 (± 0.1) × 104 M−1 (Fig. S21), slightly higher than to that observed for G-1. Moreover, the diffusion experiments resulted in a diffusion coefficient for the confined G-2 larger than for free G-2 guest, providing further evidence on the formation of G-2⊂4·(BArF)8 host–guest complex (Fig. S22). To get further insight into the non-covalent interactions directing the molecular recognition of G-1 and G-2, molecular dynamics (MD) simulations for G-1⊂4·Cl8 and G-2⊂4·Cl8 host–guest adducts were carried out (Fig. 1c and Fig. S23 and S24). In both cases, a persistent interaction between the oxygen of the carbonyl group of the guests and the Zn atom on the porphyrin of the cage was identified (Fig. S23). The noncovalent interaction (NCI) volumes were also calculated for both systems,30 observing a slightly larger value for G-2 (971.3 ± 78.4 Å3) than for G-1 (901.0 ± 79.1 Å3) (Fig. 1c and Fig. S24). This indicates higher stabilization of the host–guest adduct, which can be correlated with higher affinity of the host towards G-2. The enhanced binding of G-2 compared to G-1 could be tentatively assigned to additional aliphatic CH2–π interactions between the ethylene bridge of G-2 and the aromatic panels of the host cavity (Fig. S25), which could act cooperatively with the dominant π–π interactions.
Given the effective encapsulation of G-1 and G-2, we envisioned that 4·(BArF)8 could host extended perylene-based NGs guests, such as cis/trans-G-3 isomeric mixture (Fig. 2a and Fig. S26 and S27). Upon adding an equimolar amount of cis/trans-G-3 into a CD3CN solution of 4·(BArF)8, the chemical shifts of the signals of the host and guest's protons showed marked changes, with some of the peaks undergoing severe broadening (Fig. S28). These observations in the 1H NMR spectrum indicated the formation of a host–guest complex. However, the broadening of the peaks of the cis/trans-G-3 isomeric mixture in the 1H NMR (Fig. S29) precluded its identification when confined (attempts to assign the confined guest signals based on variable temperature 1H NMR, 2D 1H–1H COSY, and NOESY were unsuccessful). Encapsulation/dissociation of the cis/trans-G-3⊂4·(BArF)8 occurs under fast exchange at the NMR time scale, given that signals of the host (in the host–guest adduct) are shifted and broadened; however, a new set of signals corresponding to the encapsulated guest was not observed (Fig. S28 and S29). Stoichiometric analysis (Fig. S30b) indicated the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex. To further confirm the confinement of the NGs mixture, the formation of the cis/trans-G-3⊂4·(BArF)8 complex was studied by ESI-HRMS (Fig. S30a), revealing distinct [cis/trans-G-3⊂4]n+ peaks with a 1
1 inclusion complex. To further confirm the confinement of the NGs mixture, the formation of the cis/trans-G-3⊂4·(BArF)8 complex was studied by ESI-HRMS (Fig. S30a), revealing distinct [cis/trans-G-3⊂4]n+ peaks with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio. To quantify the binding of 4·(BArF)8 towards cis/trans-G-3 mixture, an inverse titration was carried out; the signals attributed to the guest were monitored by 1H NMR upon the sequential addition of a stock solution of the host (Fig. S31). The titration resulted in an association constant Ka = 1.32 (± 0.1) × 105 M−1.
1 binding ratio. To quantify the binding of 4·(BArF)8 towards cis/trans-G-3 mixture, an inverse titration was carried out; the signals attributed to the guest were monitored by 1H NMR upon the sequential addition of a stock solution of the host (Fig. S31). The titration resulted in an association constant Ka = 1.32 (± 0.1) × 105 M−1.
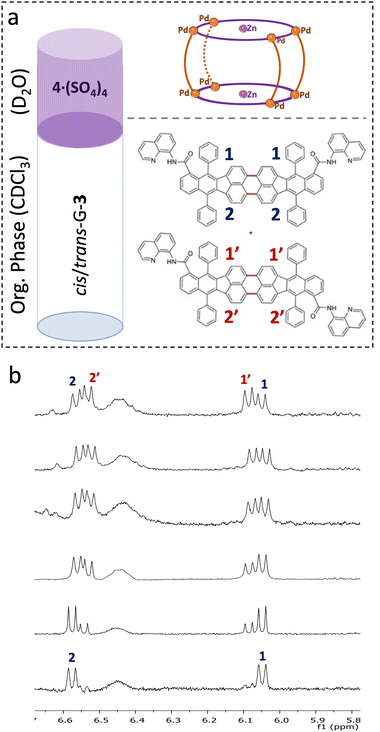
Since conventional chromatographic and crystallization methods lacked sufficient selectivity to separate the cis-G-3 and trans-G-3 isomers, and both cis and trans isomers of G-3 showed host–guest interactions with 4·(BArF)8 in homogeneous solutions (Fig. S31.a), we designed a biphasic protocol to attempt their separation. Many organic guests are soluble in a wide range of water-immiscible organic solvents, making the water/organic system the most versatile setup. Water-soluble cage 4·(SO4)4 was synthesized following previously reported protocols,31 utilizing as a precursor 4·(BArF)8. We evaluated the selectivity of 4·(SO4)4 towards the cis- and trans-G-3 isomers using a biphasic system composed of D2O, containing 4·(SO4)4 (one equivalent per equivalent of trans-G-3), and CDCl3 as the organic layer containing a mixture of cis/trans-G-3 isomers (Fig. 2a). Previous studies suggested that the host–guest properties of 4·(BArF)8 are not strongly affected by the counteranion present in the cage structure.26,31 The organic phase was monitored by 1H NMR over 75 minutes. The spectra revealed a pronounced change in the isomeric ratio—from an initial 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (cis
60 (cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans) composition to 95
trans) composition to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 after 75 minutes—indicating preferential transfer of the trans-G-3 isomer (Fig. 2b) into the aqueous phase. 1H NMR quantification (using mesitylene as an internal standard) of the trans-G-3, indicated that ∼90% of this isomer migrated from the CDCl3 phase into the D2O phase (Fig. 2b). Upon acid treatment of the aqueous phase, the cage was disassembled and the trans-G-3 released (after acid-mediated disassembly, the cage can be reassembled and recovered by basification (∼80%)).32 The 1H NMR spectrum of the released guest revealed >90% of trans-G-3 (Fig. S32), showing only trace amounts of cis-G-3. These results indicate higher affinity of 4·(SO4)4 towards trans-G-3, enabling its selective shuttling into the aqueous phase through host–guest encapsulation within the interphase of the biphasic system. Notably, this purification process does not require cage transfer reactions nor external manipulation, offering a more practical and potentially scalable alternative to conventional phase-transfer purification methods. Conventional chromatographic methods fail to isolate pure trans-G-3 isomer, due to their nearly identical π-surfaces and polarity. This highlights the operational advantage of the biphasic extraction.
5 after 75 minutes—indicating preferential transfer of the trans-G-3 isomer (Fig. 2b) into the aqueous phase. 1H NMR quantification (using mesitylene as an internal standard) of the trans-G-3, indicated that ∼90% of this isomer migrated from the CDCl3 phase into the D2O phase (Fig. 2b). Upon acid treatment of the aqueous phase, the cage was disassembled and the trans-G-3 released (after acid-mediated disassembly, the cage can be reassembled and recovered by basification (∼80%)).32 The 1H NMR spectrum of the released guest revealed >90% of trans-G-3 (Fig. S32), showing only trace amounts of cis-G-3. These results indicate higher affinity of 4·(SO4)4 towards trans-G-3, enabling its selective shuttling into the aqueous phase through host–guest encapsulation within the interphase of the biphasic system. Notably, this purification process does not require cage transfer reactions nor external manipulation, offering a more practical and potentially scalable alternative to conventional phase-transfer purification methods. Conventional chromatographic methods fail to isolate pure trans-G-3 isomer, due to their nearly identical π-surfaces and polarity. This highlights the operational advantage of the biphasic extraction.
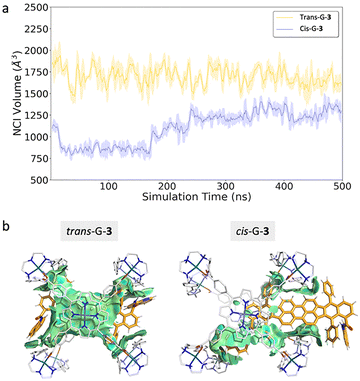
The two isomers (cis- and trans-) of G-3 and their corresponding host–guest complexes were studied by MD simulations (Fig. 3). In the MD simulations corresponding to trans-G-3 ⊂ 4·Cl8, the guest is completely confined within the cage cavity with extensive interactions of the perylene unit with the Zn–porphyrin of the cage (Fig. 3b and Fig. S33). In contrast, for the cis-G-3 ⊂ 4·Cl8, the guest is not completely confined within the cage along all the MD simulation, being sterically excluded from the centre of the cavity, and it is found interacting on the periphery of one of the four cage windows (Fig. 3b and Fig. S33). This suggests that having the 8-aminoquinoline substituents on the same side (as in the cis isomer) makes the NG unable to fit inside the cavity of the host due to steric clashes with the macrocycles of the host. Contrariwise, having the 8-aminoquinoline substituents on different sides (as in the trans isomer) the NG can fit perfectly inside the confined cavity of the cage. The NCI volumes indicated pronounced interactions for the trans isomer, due to the perylene unit interacting with the Zn–porphyrin (1700.8 ± 141.4 Å3 for the trans-G-3), while much weaker interactions for the cis-isomer (971.3 ± 78.4 Å3 for the cis-G-3) (Fig. 3b and Fig. S33), in agreement with the experimental results. Of note, the neat cis/trans-G-3 separation is only achieved upon biphasic phase-to-phase-transfer, whereas no separation is observed in a single-phase encapsulation.
1H NMR titration tests with three commercial PAHs (coronene, pyrene, perylene) show measurable weak binding only for perylene (Fig. S34). Extended perylene derivatives (such as cis/trans-G-3) show better matching with the cage cavity size, exhibiting stronger binding towards 4·(BArF)8. These observations emphasize the importance of the size complementarity between the host and guest for the efficient recognition of NGs.
In summary, we have developed a supramolecular strategy for the selective recognition and phase-transfer purification of extended perylene-based NGs using a tetragonal prismatic MOC. The water-soluble anionic cage 4·(SO4)4 enables the selective shuttling of the trans-G-3 isomer from the organic to the aqueous phase through host–guest interactions operating at the liquid–liquid interface, without requiring cage migration, anion metathesis, or external manipulation. This process achieves efficient isomeric discrimination—transferring over 90% of the trans isomer into the aqueous phase—while avoiding the generation of salt by-products associated with conventional phase-transfer protocols. NMR, HRMS, and MD simulations studies reveal that the trans-G-3 isomer fits fully within the cage cavity through non-covalent interactions with the Zn–porphyrin residues, whereas the cis isomer is sterically excluded. By circumventing repeated cage migration steps and enabling autonomous, selective phase-transfer of the targeted guest, this strategy represents a significant step toward practical and scalable supramolecular separation processes, bridging the gap between fundamental host–guest chemistry and industrial molecular purification.
This work was supported by MCIN Spain (PID2022-136970NB-I00 and TED2021-130573B-I00 to X.R., RYC2020-029552-I, and PID2022-141676NB-I00 to F. F.) and Generalitat de Catalunya (2021SGR00475, 2021SGR00487). C. F. and J. S. thank GenCat for a BdP and FI grant, respectively. C. S. thanks UdG for a PhD grant. X. R. is also grateful for an ICREA-Academia award.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of supplementary information (SI). Supplementary information includes synthesis protocols, spectroscopic characterization, and MD studies. See DOI: https://doi.org/10.1039/d5cc06794d.References
- J. Wu, W. Pisula and K. Müllen, Chem. Rev., 2007, 107, 718–747 Search PubMed.
- A. Narita, X.-Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616–6643 RSC.
- I. Matsumoto, R. Sekiya and T. Haino, Angew. Chem., Int. Ed., 2021, 60, 12706–12711 CrossRef PubMed.
- Y. Gu, Z. Qiu and K. Müllen, J. Am. Chem. Soc., 2022, 144, 11499–11524 Search PubMed.
- Z. Qiu, C.-W. Ju, L. Frédéric, Y. Hu, D. Schollmeyer, G. Pieters, K. Müllen and A. Narita, J. Am. Chem. Soc., 2021, 143, 4661–4667 Search PubMed.
- M. Buendía, J. M. Fernández-García, J. Perles, S. Filippone and N. Martín, Nat. Synth., 2024, 3, 545–553 CrossRef.
- D. Reger, P. Haines, F. W. Heinemann, D. M. Guldi and N. Jux, Angew. Chem., Int. Ed., 2018, 57, 5938–5942 CrossRef PubMed.
- X.-H. Ma, X. Gao, J.-Y. Chen, M. Cao, Q. Dai, Z.-K. Jia, Y.-B. Zhou, X.-J. Zhao, C. Chu, G. Liu and Y.-Z. Tan, J. Am. Chem. Soc., 2024, 146, 2411–2418 CrossRef PubMed.
- V. Kumar, J. L. Páez, S. Míguez-Lago, J. M. Cuerva, C. M. Cruz and A. G. Campaña, Chem. Soc. Rev., 2025, 54, 4922–4947 Search PubMed.
- C. J. T. Cox, J. Hale, P. Molinska and J. E. M. Lewis, Chem. Soc. Rev., 2024, 53, 10380–10408 RSC.
- T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Nat. Mater., 2009, 8, 973–978 CrossRef PubMed.
- M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef PubMed.
- R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 Search PubMed.
- D. Zhang, T. K. Ronson, Y.-Q. Zou and J. R. Nitschke, Nat. Rev. Chem., 2021, 5, 168–182 CrossRef PubMed.
- L.-J. Chen, H.-B. Yang and M. Shionoya, Chem. Soc. Rev., 2017, 46, 2555–2576 RSC.
- M. Morimoto, S. M. Bierschenk, K. T. Xia, R. G. Bergman, K. N. Raymond and F. D. Toste, Nat. Catal., 2020, 3, 969–984 CrossRef.
- Y. Zhou, H. Li, T. Zhu, T. Gao and P. Yan, J. Am. Chem. Soc., 2019, 141, 19634–19643 Search PubMed.
- A. Galan and P. Ballester, Chem. Soc. Rev., 2016, 45, 1720–1737 RSC.
- D. Zhang, T. K. Ronson, R. Lavendomme and J. R. Nitschke, J. Am. Chem. Soc., 2019, 141, 18949–18953 CrossRef CAS PubMed.
- F. J. Rizzuto, L. K. S. von Krbek and J. R. Nitschke, Nat. Rev. Chem., 2019, 3, 204–222 CrossRef.
- H. Wu, Y. Wang, B. Song, H.-J. Wang, J. Zhou, Y. Sun, L. O. Jones, W. Liu, L. Zhang, X. Zhang, K. Cai, X.-Y. Chen, C. L. Stern, J. Wei, O. K. Farha, J. M. Anna, G. C. Schatz, Y. Liu and J. Fraser Stoddart, Nat. Commun., 2021, 12, 5191 CrossRef CAS PubMed.
- S. La Cognata and V. Amendola, Chem. Commun., 2023, 59, 13668–13678 Search PubMed.
- Y.-L. Lu, Y.-P. Wang, K. Wu, M. Pan and C.-Y. Su, Acc. Chem. Res., 2024, 57, 3277–3291 Search PubMed.
- A. B. Grommet and J. R. Nitschke, J. Am. Chem. Soc., 2017, 139, 2176–2179 Search PubMed.
- A. B. Grommet, J. B. Hoffman, E. G. Percástegui, J. Mosquera, D. J. Howe, J. L. Bolliger and J. R. Nitschke, J. Am. Chem. Soc., 2018, 140, 14770–14776 Search PubMed.
- C. García-Simón, M. Garcia-Borràs, L. Gómez, T. Parella, S. Osuna, J. Juanhuix, I. Imaz, D. Maspoch, M. Costas and X. Ribas, Nat. Commun., 2014, 5, 5557 CrossRef PubMed.
- C. Fuertes-Espinosa, A. Gómez-Torres, R. Morales-Martínez, A. Rodríguez-Fortea, C. García-Simón, F. Gándara, I. Imaz, J. Juanhuix, D. Maspoch, J. M. Poblet, L. Echegoyen and X. Ribas, Angew. Chem., Int. Ed., 2018, 57, 11294–11299 CrossRef CAS.
- V. Iannace, C. Sabrià, R. D. Schmitt, F. Feixas, S. Stevenson and X. Ribas, J. Am. Chem. Soc., 2025, 147, 36079–36084 CrossRef CAS PubMed.
- L. Capdevila, J. Sala, C. Berga, A. de Aquino, T. Parella, L. Blancafort, L. Rodríguez, S. Simon and X. Ribas, ChemistryEurope, 2025, 3, e202500102 CrossRef CAS.
- R. A. Boto, F. Peccati, R. Laplaza, C. Quan, A. Carbone, J.-P. Piquemal, Y. Maday and J. Contreras-García, J. Chem. Theo. Comput., 2020, 16, 4150–4158 CrossRef CAS PubMed.
- C. Fuertes-Espinosa, C. García-Simón, M. Pujals, M. Garcia-Borràs, L. Gómez, T. Parella, J. Juanhuix, I. Imaz, D. Maspoch, M. Costas and X. Ribas, Chem, 2020, 6, 169–186 CAS.
- C. Colomban, C. Fuertes-Espinosa, S. Goeb, M. Sallé, M. Costas, L. Blancafort and X. Ribas, Chem. – Eur. J., 2018, 24, 4371–4381 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |