A Mo-guided self-reconstructed highly active NiOOH catalyst for a durable alkaline oxygen evolution reaction
Huihui
Zhi
,
Zhibei
Liao
,
Hao
Jiang
,
Yuan
He
,
Zhanwei
Chen
,
Shaowei
Yang
* and
Hepeng
Zhang
 *
*
Xi’an Key Laboratory of Functional Organic Porous Materials, School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi’an 710129, PR China. E-mail: zhanghepeng@nwpu.edu.cn
First published on 25th November 2025
Abstract
Alkaline water electrolysis (AWE) is vital for green hydrogen production but suffers from sluggish oxygen evolution reaction (OER) kinetics and poor electrode stability. Here, a facile and feasible Mo-guided self-reconstruction strategy was developed to construct an outstanding pure nickel-based OER electrocatalyst (γ-NiOOHMo-SR/NF). Benefiting from the in situ generated abundant high-valent Ni sites and interfacial hydrogen-bonding network, γ-NiOOHMo-SR/NF exhibited excellent OER activity and impressive long-term stability over 1100 h at 1.0 A cm−2, even exceeding 100 h at 3.0 A cm−2, outperforming most reported non-precious metal catalysts. In situ spectroscopic studies confirmed that the interfacial hydrogen-bonding network promoted OH− transport and suppressed local acidification, thereby endowing γ-NiOOHMo-SR/NF with superior OER performance. The present work is instructive for designing highly efficient Ni-based OER electrocatalysts.
The intermittent and unstable nature of renewable energy requires effective strategies to make full use of surplus clean electricity. Water electrolysis offers a practical solution by converting renewable electricity into green hydrogen, addressing both the challenges of electricity grid integration and sustainable hydrogen production.1 Alkaline water electrolysis (AWE) has already reached early industrial application, but it still suffers from high energy consumption due to the slow kinetics of the anode oxygen evolution reaction (OER).2 In addition, the non-precious metal electrodes face serious challenges in maintaining long-term stability under harsh conditions, especially at high current densities.3 Therefore, developing low-cost OER catalysts with low overpotential (η) and high durability is crucial for advancing AWE technology.
Nickel foam (NF) is the most commonly used substrate for OER catalysts due to its low cost and decent resistance to alkaline corrosion. Several strategies have been explored to improve the OER performance of NF, including element doping,4 electronic regulation,5 and phase transformation.6 The main idea behind these strategies is to trigger electrochemical reconstruction of the pre-catalyst during operation, forming highly active NiOOH phases that are recognized as the true active sites for water oxidation under alkaline conditions.7 However, existing methods for the preparation of Ni-based pre-catalysts often involve complex processes, such as high temperature/pressure treatments,8 calcination,9 and electroplating,10 which limit large-scale production. Moreover, poor adhesion between the in situ formed catalyst layer and the NF substrate can lead to material peeling and undermine stability.11
To address the above challenges, herein, we prepared a molybdate-modified Ni pre-catalyst (NiMoO4/NF) via a simple one-step method by immersing clean NF in a 50 °C (NH4)2MoO4 solution for 6 h. During cyclic voltammetry (CV) activation, the pre-catalyst undergoes a Mo-guided in situ self-reconstruction into γ-NiOOH (denoted as γ-NiOOHMo-SR). The transformation generated a hierarchically porous structure with abundant high-valent Ni active sites, which also promoted the formation of an interfacial hydrogen-bonding network. These two features enhanced both the OER catalytic activity and durability of γ-NiOOHMo-SR. As a result, the reconstructed electrode achieves a lower η, far surpassing that of bare NF, and exhibits outstanding long-term stability, maintaining performance for over 1100 h at 1.0 A cm−2 or 100 h at 3.0 A cm−2. These results highlight γ-NiOOHMo-SR/NF as a highly promising anode material for industrial AWE.
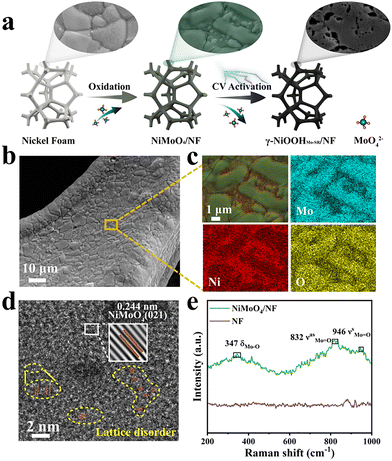
γ-NiOOHMo-SR/NF was rationally synthesized via a Mo-guided self-reconstruction strategy (Fig. 1a). This approach leverages the transient participation of Mo species to guide surface transformation, resulting in a porous structure rich in high-valence Ni active sites that enhance OER kinetics. Moreover, this scalable and energy-efficient synthesis avoids the use of precious metals and maintains a low production cost of 74.6 US$ per m2 (Table S1), making it suitable for industrial application.12
The scanning electron microscopy (SEM) observations (Fig. 1b and Fig. S1) reveal the formation of amorphous coatings preferentially covering the Ni foam surface, while energy dispersive spectroscopy (EDS) mapping (Fig. 1c) manifests the specific distribution of Mo, Ni and O. The high resolution transmission electron microscopy (HRTEM) analysis (Fig. 1d) shows lattice fringes with a spacing of 0.244 nm corresponding to the (021) plane of NiMoO4.6 Moreover, the pre-catalyst surface contained abundant disordered lattice fringes. The active sites derived from the reconstruction of the disordered lattices were confirmed to be more catalytically active.13,14 The Raman spectra (Fig. 1e) display characteristic bands at 347, 832, and 946 cm−1 associated with Mo–O bending and Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, consistent with NiMoO4 species.15 The absence of diffraction peaks in the X-ray diffraction (XRD) pattern (Fig. S2) further supports the amorphous nature of the material. X-ray photoelectron spectroscopy (XPS) analysis of NiMoO4/NF (Fig. S3) confirms the chemical states of Ni and Mo species. The Ni 2p spectrum exhibits two characteristic peaks at 855.8 and 873.5 eV, corresponding to Ni2+ 2p3/2 and Ni2+ 2p1/2, respectively, along with their satellite peaks at 861.5 and 879.4 eV.10 Meanwhile, the Mo 3d spectrum shows two distinct peaks at 232.0 and 235.1 eV, indicating that Mo exists exclusively in the +6 oxidation state (Mo6+) on the catalyst surface.16 Collectively, these characterization studies confirm the successful formation of an amorphous NiMoO4/NF pre-catalyst that serves as a structural template for subsequent self-reconstruction.
O stretching vibrations, consistent with NiMoO4 species.15 The absence of diffraction peaks in the X-ray diffraction (XRD) pattern (Fig. S2) further supports the amorphous nature of the material. X-ray photoelectron spectroscopy (XPS) analysis of NiMoO4/NF (Fig. S3) confirms the chemical states of Ni and Mo species. The Ni 2p spectrum exhibits two characteristic peaks at 855.8 and 873.5 eV, corresponding to Ni2+ 2p3/2 and Ni2+ 2p1/2, respectively, along with their satellite peaks at 861.5 and 879.4 eV.10 Meanwhile, the Mo 3d spectrum shows two distinct peaks at 232.0 and 235.1 eV, indicating that Mo exists exclusively in the +6 oxidation state (Mo6+) on the catalyst surface.16 Collectively, these characterization studies confirm the successful formation of an amorphous NiMoO4/NF pre-catalyst that serves as a structural template for subsequent self-reconstruction.
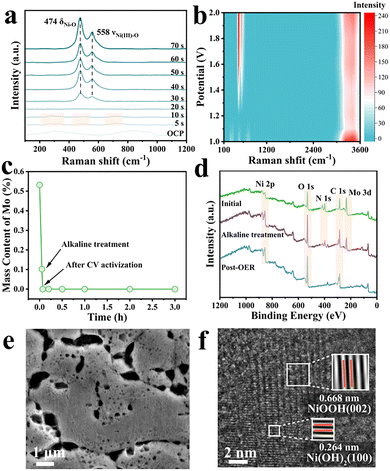
The structural evolution of NiMoO4/NF during activation was investigated using in situ Raman spectroscopy, inductively coupled plasma atomic emission spectrometry (ICP-AES) and XPS. Time-resolved in situ Raman spectroscopy (Fig. 2a) provides direct insight into the dynamic reconstruction process. Within 20 s at 1.4 V vs. RHE (RHE: reversible hydrogen electrode), molybdate-related vibrations rapidly diminish, leaving transient Mo–O–Mo modes (265, 476, and 706 cm−1) that vanish as MoO42− is completely leached.16 Simultaneously, two new peaks at 474 and 558 cm−1 emerge, assigned to Ni–O bending and Ni(III)–O stretching vibrations characteristic of γ-NiOOH.15 The progressive growth and stabilization of these peaks indicate the steady formation of active Ni oxyhydroxide phases during oxygen evolution. The amorphous configuration of NiMoO4 enables its rapid reorganization to γ-NiOOH. Conversely, the in situ Raman spectra of NF showed no discernible signals attributable to γ-NiOOH (Fig. S4). The potential-resolved spectra (Fig. 2b) further show that γ-NiOOH signatures appear only above 1.35 V vs. RHE and intensify with increasing applied potential. The simultaneous enhancement of broad H2O stretching bands (3000–3800 cm−1) reflects the strengthened interaction between interfacial water and active sites, highlighting the dynamic coupling between surface reconstruction and catalytic oxygen evolution.17
ICP-AES measurements (Fig. 2c) further reveal that approximately 80% of Mo species leach into the electrolyte, accompanied by a visible color change from dark green to gray (Fig. S5). After CV activation, Mo was completely removed, and the surface turned black (Fig. S6), indicating a complete phase transformation. Additionally, ICP-AES analysis determines that the catalyst is Fe-free and exclusively Ni-based (Table S2). Consistent with these, the XPS spectra (Fig. 2d and Fig. S7, S8) show the disappearance of Mo signals and the emergence of new Ni3+ peaks at 857.5 and 875.3 eV, aligning with the Ni2+ → Ni3+ oxidation observed in the CV profiles (Fig. S9).10 Both of them match well with the observations of in situ Raman spectroscopy. These results collectively confirm that NiMoO4/NF undergoes self-reconstruction through a Mo-leaching-induced transformation.
Interestingly, the extent of structural reconstruction was found to depend strongly on the initial MoO42− concentration. The SEM image (Fig. 2e) and pore size distribution analysis (Fig. S10) show that an optimal Mo concentration (2.5 g L−1) produces a hierarchically porous structure while maintaining the overall integrity of the catalyst. In contrast, an insufficient Mo content (0.625 g L−1) produces limited porosity (Fig. S11 and S12), and an excessive Mo loading (3.75 g L−1) causes surface collapse (Fig. S13 and S14). For comparison, pure Ni foam shows a negligible morphological change after activation (Fig. S15). Consistent with morphological trends, electrochemical OER measurements (Fig. S16) demonstrate that γ-NiOOHMo-SR/NF-2.5 exhibits the lowest overpotential, the smallest Tafel slope, and the largest double-layer capacitance (Cdl), revealing a strong structure–activity correlation. HRTEM images (Fig. 2f) further identify lattice spacings of 0.668 and 0.264 nm, corresponding to the (002) plane of γ-NiOOH and the (100) plane of Ni(OH)2, respectively, confirming the coexistence of multiple active oxyhydroxide species on the reconstructed surface. Reduction and protonation of γ-NiOOH lead to the formation of the Ni(OH)2 compound.18
Overall, through controlled Mo leaching, the NiMoO4 pre-catalyst undergoes a Mo-guided transformation into porous γ-NiOOHMo-SR, generating abundant active sites and optimized electronic configurations. The Mo-guided self-reconstruction strategy provides a scalable and low-cost route for fabricating efficient OER catalysts under mild conditions.
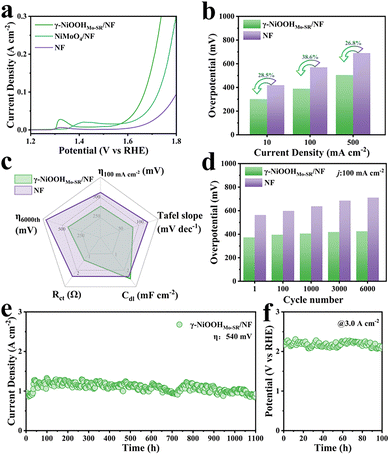
The OER performance of the as-prepared electrocatalysts was evaluated using a standard three-electrode system in an oxygen-saturated 1 M KOH solution. As shown in Fig. 3a, γ-NiOOHMo-SR/NF achieves 10 mA cm−2 at an η of only 302 mV, significantly outperforming bare Ni foam (419 mV). Fig. 3b further quantifies this improvement, revealing reductions in overpotential of 38.6% at 0.1 A cm−2 and 26.8% at 0.5 A cm−2 relative to NF. A comprehensive comparison of OER metrics is presented in Fig. 3c and Fig. S17, where γ-NiOOHMo-SR/NF exhibits a smaller Tafel slope (84.3 mV dec−1), indicating accelerated reaction kinetics, a lower charge transfer resistance (Rct = 1.4 Ω), reflecting faster electron transfer, and a larger double-layer capacitance (Cdl = 2.52 mF cm−2), corresponding to a higher density of active sites. Furthermore, γ-NiOOHMo-SR/NF exhibits a higher ECSA-normalized current density at the same overpotential, confirming its superior intrinsic activity (Fig. S18). As shown in Fig. S19, the turnover frequency (TOF) of γ-NiOOHMo-SR/NF reaches 0.315 s−1 at an η of 500 mV, obviously higher than that of NF (0.027 s−1). The faradaic efficiency is close to 100% at 1.0 A cm−2 (Fig. S20).
The stability of a catalyst is crucial for practical applications. Durability under dynamic operating conditions was first evaluated using CV cycling (Fig. 3d). After 6000 cycles, the η of γ-NiOOHMo-SR/NF increases by only 13.8%, whereas NF degrades by over 26.0%, demonstrating superior operational robustness. Long-term electrolysis tests further confirm this stability: after 1100 h at 1.77 V vs. RHE or 100 h at 3.0 A cm−2, the overpotential of γ-NiOOHMo-SR/NF shows negligible variation (Fig. 3e and f), outperforming most of the reported transition metal-based catalysts (Table S3). The SEM images after 1100 h (Fig. S21) reveal that the catalyst architecture remains largely intact, and the Raman spectra (Fig. S22) confirm the retention of characteristic γ-NiOOH signatures, demonstrating the structural stability of the self-reconstructed catalyst.
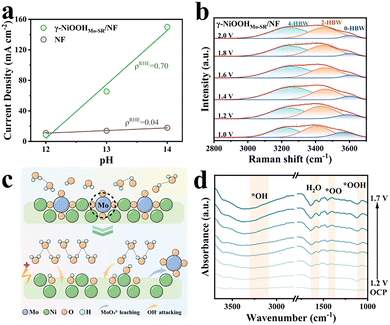
It has been widely accepted that γ-NiOOH is an excellent active catalyst for the OER.15 To gain further insight into the outstanding OER performance of γ-NiOOHMo-SR/NF, the pH dependence of OER activity was evaluated by analyzing the current density at η = 450 mV (ρRHE, ρRHE = ∂![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(j)/∂pH).12 As shown in Fig. 4a and Fig. S23, γ-NiOOHMo-SR/NF exhibits a pronounced increase in activity with increasing pH, whereas NF shows only a slight dependence, indicating that sufficient OH− supply is essential to ensure the electrocatalytic activity for γ-NiOOHMo-SR/NF due to its abundant active sites.8 To explore the surface OH− replenishment of γ-NiOOHMo-SR/NF during the OER process, the water stretching region (3000–3800 cm−1) in the in situ Raman spectra was examined (Fig. 4b). Deconvolution of the broad peak into three Gaussian components identifies tetrahedrally coordinated hydrogen-bonded water (4-HBW, ∼3200 cm−1), doubly coordinated hydrogen-bonded water (2-HBW, ∼3400 cm−1), and free water (0-HBW, ∼3600 cm−1).19 Previous studies have shown that strongly hydrogen-bonded 4-HBW within the interfacial network can enhance mass transport and reduce the energy barrier for proton-coupled electron transfer.1,17 Quantitative analysis reveals that the 4-HBW fraction increases from 40.3% to 53.0% as the potential increases. Therefore, the thus-formed hydrogen-bonding network provides an efficient route for OH− transport, which suppresses local acidification while enhancing the activity and long-term stability. Fig. 4c schematically illustrates the reconstruction process from NiMoO4/NF to γ-NiOOHMo-SR/NF.
log(j)/∂pH).12 As shown in Fig. 4a and Fig. S23, γ-NiOOHMo-SR/NF exhibits a pronounced increase in activity with increasing pH, whereas NF shows only a slight dependence, indicating that sufficient OH− supply is essential to ensure the electrocatalytic activity for γ-NiOOHMo-SR/NF due to its abundant active sites.8 To explore the surface OH− replenishment of γ-NiOOHMo-SR/NF during the OER process, the water stretching region (3000–3800 cm−1) in the in situ Raman spectra was examined (Fig. 4b). Deconvolution of the broad peak into three Gaussian components identifies tetrahedrally coordinated hydrogen-bonded water (4-HBW, ∼3200 cm−1), doubly coordinated hydrogen-bonded water (2-HBW, ∼3400 cm−1), and free water (0-HBW, ∼3600 cm−1).19 Previous studies have shown that strongly hydrogen-bonded 4-HBW within the interfacial network can enhance mass transport and reduce the energy barrier for proton-coupled electron transfer.1,17 Quantitative analysis reveals that the 4-HBW fraction increases from 40.3% to 53.0% as the potential increases. Therefore, the thus-formed hydrogen-bonding network provides an efficient route for OH− transport, which suppresses local acidification while enhancing the activity and long-term stability. Fig. 4c schematically illustrates the reconstruction process from NiMoO4/NF to γ-NiOOHMo-SR/NF.
In situ attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy further tracked the formation of reaction intermediates during the OER (Fig. 4d). The intensity of the *OH signal around 3300 cm−1 increases steadily with potential, indicating efficient penetration of OH− into the surface of γ-NiOOHMo-SR/NF. This observation corroborates the suppression of local acidification and supports the role of the hydrogen-bonding network in facilitating OH− transport. Additional signals at 1400 and 1100 cm−1 correspond to *OO and *OOH species,12 respectively, confirming that the OER proceeds via the adsorbate evolution mechanism (AEM) pathway.9
In summary, we developed a Mo-guided self-reconstruction strategy to construct high-performance pure nickel-based OER catalysts. The in situ self-reconstruction of γ-NiOOHMo-SR/NF during electrochemical activation endowed it with a porous structure with abundant high-valent Ni, which not only promoted numerous OER active sites, but also facilitated the formation of an interfacial hydrogen-bonding network. The network guaranteed efficient OH− transport and suppressed localized acidification, thereby enhancing both the OER catalytic activity and durability. The combination of Mo-guided reconstruction and hydrogen-bond network engineering provides an efficient and scalable strategy for designing durable and highly active OER catalysts, offering promising solutions for industrial AWE and renewable energy conversion.
This work was financially supported by the Innovation Capability Support Program of Shaanxi-Science and Technology Innovation Team Project (No. 2025RS-CXTD-024), the Fundamental Research Funds for the Central Universities (No. G2025KY05240), and the Fundamental Research Foundation of SHCCIG New Materials Technology Research Institute Co., Ltd (No. D5204230171).
Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data are available within the article and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc06040k.References
- R. Fan, S. Lu, F. Wang, Y. Zhang, M. Hojamberdiev, Y. Chai, B. Dong and B. Zhang, Nat. Commun., 2025, 16, 3407 CrossRef CAS.
- Z.-P. Wu, S. Zuo, R. Wu, C. Chen, X. Zhao, Y. Ren, X. T. Xiao, J. Zhang, J. Ruiz-Martínez, Z.-P. Liu, H. Zhang and Y. Han, J. Am. Chem. Soc., 2025, 147, 36763–36773 CrossRef CAS PubMed.
- N. Wang, S. Song, W. Wu, Z. Deng and C. Tang, Adv. Energy Mater., 2024, 14, 2303451 CrossRef CAS.
- L. Wu, M. Ning, X. Xing, Y. Wang, F. Zhang, G. Gao, S. Song, D. Wang, C. Yuan, L. Yu, J. Bao, S. Chen and Z. Ren, Adv. Mater., 2023, 35, 2306097 CrossRef CAS.
- Z. Pei, H. Zhang, Z.-P. Wu, X. F. Lu, D. Luan and X. W. Lou, Sci. Adv., 2023, 9, eadh1320 CrossRef CAS.
- J. Sun, G. Ren, S. Qin, Z. Zhao, Z. Li, Z. Zhang, C. Li and X. Meng, Nano Energy, 2024, 121, 109246 CrossRef CAS.
- D. Y. Chung, P. P. Lopes, P. Farinazzo Bergamo Dias Martins, H. He, T. Kawaguchi, P. Zapol, H. You, D. Tripkovic, D. Strmcnik, Y. Zhu, S. Seifert, S. Lee, V. R. Stamenkovic and N. M. Markovic, Nat. Energy, 2020, 5, 222–230 CrossRef.
- L. Zhou, Y. Shao, F. Yin, J. Li, F. Kang and R. Lv, Nat. Commun., 2023, 14, 7644 CrossRef CAS PubMed.
- Y. Che, J. Shang, Y. Zhang, S. Bo, J. Zhang, X. Qin, X. Liu, H. Sun, W. Zhou, Y. Jiang, X. Chen, S. He, D. Ma, F. Pan and Q. Liu, Adv. Funct. Mater., 2025, 35, 2507544 CrossRef CAS.
- H. Hao, J. Wang, Z. Wang, S. Shen, L. Xu, Z. Lv and B. Wei, Appl. Catal., B, 2025, 363, 124814 CrossRef CAS.
- X. Cui, T. Tang, F. Zhang, L. Sun and B. Zhang, Appl. Catal., B, 2025, 366, 125024 CrossRef CAS.
- J. Sun, S. Zhou, Z. Zhao, S. Qin, X. Meng, C.-H. Tung and L.-Z. Wu, Energy Environ. Sci., 2025, 18, 1952–1962 RSC.
- Z.-P. Wu, H. Zhang, S. Zuo, Y. Wang, S. L. Zhang, J. Zhang, S.-Q. Zang and X. W. Lou, Adv. Mater., 2021, 33, 2103004 CrossRef CAS PubMed.
- Z. Li, J. Yang, R. Gao, S.-M. Xu, X. Kong, X. Hua, P. Zhao, H. Hao, D. O’Hare and Y. Zhao, J. Phys. Chem. Lett., 2024, 15, 2006–2014 CrossRef CAS PubMed.
- R. N. Dürr, P. Maltoni, H. Tian, B. Jousselme, L. Hammarström and T. Edvinsson, ACS Nano, 2021, 15, 13504–13515 CrossRef PubMed.
- W. Du, Y. Shi, W. Zhou, Y. Yu and B. Zhang, Angew. Chem., Int. Ed., 2021, 60, 7051–7055 CrossRef CAS PubMed.
- M. Li, C. Liu, Z. Teng, R. Zhang, X. Liu, J. Zhu, X. Liu, Z. Wu, Y. Chai, J. Chi and L. Wang, Adv. Funct. Mater., 2025, e14517 CrossRef.
- F. Dionigi and P. Strasser, Adv. Energy Mater., 2016, 6, 1600621 CrossRef.
- Q. Wen, J. Duan, W. Wang, D. Huang, Y. Liu, Y. Shi, J. Fang, A. Nie, H. Li and T. Zhai, Angew. Chem., Int. Ed., 2022, 61, e202206077 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |