Coupling calcination with sulfidation strategy to fabricate a hydrophilic bimetallic transition nanozyme for boosting antibacterial efficiency
Tian-shuo
Wang
a,
Yu-hang
Lin
a,
Lian-xi
Pu
a,
Yin-min
Min
a,
Min
Zhang
 a,
De-ping
Wang
a,
Li-jun
Ding
*b and
Kun
Wang
a,
De-ping
Wang
a,
Li-jun
Ding
*b and
Kun
Wang
 *a
*a
aSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: wangkun@ujs.edu.cn
bSchool of Agricultural Engineering, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: ljding@ujs.edu.cn
First published on 3rd December 2025
Abstract
In this study, we coupled calcination with sulfidation of a Prussian blue analog precursor to construct a hydrophilic CoS2/FeS2@NCF nanozyme. The nanozyme was observed to display exceptionally high peroxidase-like activity, with inhibition rates against E. coli and S. aureus reaching 99.9%. This strategy not only offers a novel approach for synthesizing high-performance nanozymes but also demonstrates their considerable potential in antibacterial applications.
Bacterial infections pose a significant threat to human health.1 Nanozyme-mediated chemodynamic therapy (CDT) has emerged as a promising approach that leverages nanozymes with peroxidase-like (POD-like) activity to catalytically generate reactive oxygen species (ROS) in situ and ultimately induce bacterial death.2 Of the various studied nanozymes, transition-metal sulfide (TMS)-based ones exhibit particularly outstanding POD-like activity,3 and their surface charges can generate electrostatic interactions with bacterial membranes, promoting local ROS production and enhancing sterilization.4 Bimetallic TMS systems (e.g., CoS2, FeS2) further enhance catalytic performance through synergistic effects.5 However, synthesizing bimetallic TMS nanoenzymes in a scalable and controlled manner remains a major challenge.3
Of the diverse strategies developed for synthesizing TMS-based nanozymes, calcination is particularly notable in enabling the conversion of metal–organic frameworks (MOFs) into graphitized nanomaterials, which show significantly enhanced chemical and mechanical stability.6 Complementarily, sulfidation is adept at generating core–shell or surface-modified structures.7 Given their complementary strengths, calcination and sulfidation are frequently integrated to optimize the structural and functional properties of ultimately produced TMS nanozymes.3 In this context, Prussian blue analogues (PBAs) stand out as exceptional sacrificial templates for synthesis of TMSs due to their open frameworks, tunable metal centers, and facile synthesis.8 These findings highlight the rational design of PBA-derived heterostructures and nonmetal-doping strategies as key routes for optimizing nanozyme catalytic performance.
As increasing a substrate's hydrophilicity enhances its solubility, accessibility, and bacterial adhesion, rational design of highly hydrophilic nanozymes is essential to maximize their antibacterial efficacy.9 Building on these foundations, CoFePBA was employed as a precursor to rationally construct heterostructure bimetallic TMS material, specifically CoS2/FeS2@NCF composites, through a calcination–sulfidation strategy (Fig. 1A). Doping a nonmetal heteroatom (e.g., N, C) into the composites facilitated the formation of a conductive network, with this doping not only increasing the density of accessible active sites but also improving overall structural stability. The formation of heterostructure metal sulfides further created highly favorable catalytic centers that have been previously shown to accelerate coupled electron–ion transfer processes.10 As a result, the obtained composites retained the cubic morphology of the parent precursor while simultaneously forming a porous framework and stabilizing the crystalline metal–sulfide phases. In POD-like catalytic assays, CoS2/FeS2@NCF exhibited remarkable POD-like activity, efficiently catalyzing the conversion of H2O2 into highly toxic ˙OH radicals. Owing to its high hydrophilicity, the CoS2/FeS2@NCF material can closely interact with bacterial cell membranes, thereby efficiently generating ˙OH very near the bacteria and inducing oxidative damage. Under the generated sustained ˙OH attack, the bacterial cell membrane was observed to undergo shrinkage and rupture, ultimately leading to leakage of intracellular contents and cell death (Fig. 1B). These radicals enabled the efficient inactivation of both E. coli and S. aureus. Collectively, this study has not only provided a new paradigm for the design of highly active and multifunctional nanozyme platforms but has also broadened their translational potential in antibacterial therapy and other biomedical applications.
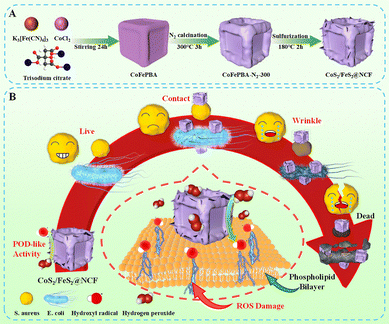 | ||
| Fig. 1 Schematic diagrams of (A) the process used to prepare CoS2/FeS2@NCF and (B) the proposed CoS2/FeS2@NCF antibacterial mechanism. | ||
Thermogravimetric analysis (TGA) under an atmosphere containing both N2 and air was performed to determine the optimal calcination temperature for fabricating the nitrogen-doped carbon bimetallic composite. The analysis results (Fig. S1A) revealed that the mass loss occurred in two main stages: the first stage (below 200 °C) primarily corresponded to the loss of coordinated and crystalline water molecules; the second stage (300–600 °C) was attributed to the progressive decomposition of the organic ligand framework. A significant loss of mass was observed at 300 °C, indicating this temperature to be a critical point for structural decomposition. Therefore, calcination at 300 °C under a N2 atmosphere was selected in this study to form a stable nitrogen-doped carbon framework (NCF) structure,11 thereby enhancing catalytic efficiency. By choosing from different calcination temperatures and atmospheres, PBA can be converted into metal carbides, reducing metal ion leaching to some extent.12 Following calcination, the material was subjected to a sulfidation treatment with Na2S, yielding CoS2/FeS2@NCF. SEM analysis (Fig. S1B–D) showed a nanocube morphology for both the CoFePBA-N2-300 and CoS2/FeS2@NCF composites, with particle dimensions ranging between 150 and 200 nm. The surfaces appeared slightly rough, and the nanozyme framework structure was preserved. To further investigate the bonding nature and interactions within the CoS2/FeS2@NCF heterostructure, FT-IR analysis was performed. The sharp peak at 2140 cm−1 in the precursor data, attributed to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching and characteristic of the precursor cyanide ligands, was not observed for CoS2/FeS2@NCF (Fig. S1E), due to the formation of nitrogen-doped carbon compounds at high temperature. New peaks emerged at 622 cm−1 and 508 cm−1, and were attributed to M–S bonds (M = Co/Fe).13 Subsequently, N2 adsorption–desorption experiments were conducted on the CoS2/FeS2@NCF nanocube and its precursor CoFePBA (Fig. S1F) to investigate changes in pore structure and specific surface area. The CoS2/FeS2@NCF nanocube yielded a type IV adsorption–desorption isotherm, with the average pore size transitioning from a mesoporous value of 37.754 nm for the precursor to a narrower mesoporous value of 12.551 nm for the nanocube. Furthermore, the BET surface area of CoS2/FeS2@NCF (87.68 m2 g−1) was much higher than that of the CoFePBA precursor (11.71 m2 g−1). Pore size distribution analysis revealed that, compared to CoFePBA, the CoS2/FeS2@NCF nanocube derived from thermal decomposition of the precursor crystal framework displayed a relatively porous and loose structure. The abundant pore channels within the particles would increase the contact area between substrates and the nanomaterial,14 while the hollow porous structure would facilitate ion transport, thereby enhancing catalytic performance.15 XRD analysis was performed to identify the crystal structure of the heterostructure metal–sulfide nanocube. After subjecting the precursor to high-temperature carbonization, the collapse of its ligand framework led to the formation of a bimetallic nitrogen-doped carbon compound, with diffraction peaks matching well those of CoFe (PDF#49-1568) (Fig. S1G).16 Subsequent sulfidation yielded the CoS2/FeS2@NCF heterostructure, whose diffraction peaks showed good agreement with the standard patterns of CoS2 (PDF#41-1471) and FeS2 (PDF#06-0710) (Fig. 1H).17 Raman spectroscopy and X-ray photoelectron spectroscopy (XPS) analyses confirmed the successful synthesis of the material (Fig. S2). These characterizations collectively confirmed the successful carbonitriding and sulfidation of the material.
N stretching and characteristic of the precursor cyanide ligands, was not observed for CoS2/FeS2@NCF (Fig. S1E), due to the formation of nitrogen-doped carbon compounds at high temperature. New peaks emerged at 622 cm−1 and 508 cm−1, and were attributed to M–S bonds (M = Co/Fe).13 Subsequently, N2 adsorption–desorption experiments were conducted on the CoS2/FeS2@NCF nanocube and its precursor CoFePBA (Fig. S1F) to investigate changes in pore structure and specific surface area. The CoS2/FeS2@NCF nanocube yielded a type IV adsorption–desorption isotherm, with the average pore size transitioning from a mesoporous value of 37.754 nm for the precursor to a narrower mesoporous value of 12.551 nm for the nanocube. Furthermore, the BET surface area of CoS2/FeS2@NCF (87.68 m2 g−1) was much higher than that of the CoFePBA precursor (11.71 m2 g−1). Pore size distribution analysis revealed that, compared to CoFePBA, the CoS2/FeS2@NCF nanocube derived from thermal decomposition of the precursor crystal framework displayed a relatively porous and loose structure. The abundant pore channels within the particles would increase the contact area between substrates and the nanomaterial,14 while the hollow porous structure would facilitate ion transport, thereby enhancing catalytic performance.15 XRD analysis was performed to identify the crystal structure of the heterostructure metal–sulfide nanocube. After subjecting the precursor to high-temperature carbonization, the collapse of its ligand framework led to the formation of a bimetallic nitrogen-doped carbon compound, with diffraction peaks matching well those of CoFe (PDF#49-1568) (Fig. S1G).16 Subsequent sulfidation yielded the CoS2/FeS2@NCF heterostructure, whose diffraction peaks showed good agreement with the standard patterns of CoS2 (PDF#41-1471) and FeS2 (PDF#06-0710) (Fig. 1H).17 Raman spectroscopy and X-ray photoelectron spectroscopy (XPS) analyses confirmed the successful synthesis of the material (Fig. S2). These characterizations collectively confirmed the successful carbonitriding and sulfidation of the material.
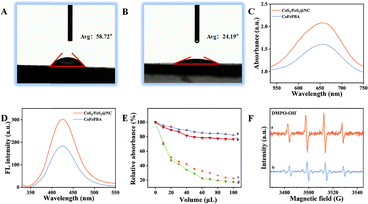
In general, structural modulation of materials significantly influences their hydrophobic/hydrophilic properties and can thereby effectively enhance interactions between substrate molecules and active sites. In the current work, water contact angle measurements revealed values of 58.72° for CoFePBA (Fig. 2A) and 24.19° for CoS2/FeS2@NCF (Fig. 2B), demonstrating the superior hydrophilicity of the sulfide heterostructure. This enhanced wettability originated from increased surface polarity and modified metal electronic states. In general, a strong correlation between catalytic performance and hydrophilicity has been found, with heightened surface affinity facilitating accessibility of substrates to active sites, thereby improving catalytic efficiency.18 Materials with high hydrophilicity have been reported to facilitate more effective contact with Gram-positive bacteria (e.g., S. aureus), thereby enhancing antibacterial activity by strengthening the interfacial affinity between bacterial cells and the material surface.9 Numerous studies have demonstrated intrinsic POD-like activity displayed by metal–sulfide nanoparticles.19,20 In the current work, the POD-like activity of the nanozyme materials was evaluated using the TMB–H2O2 colorimetric reaction system. The nanozymes catalyzed the oxidation of TMB by H2O2, generating blue oxTMB with a characteristic absorption peak at 652 nm in a UV-Vis spectroscopy study. In a set of experiments including adding 100 µL of H2O2 (10 mM), CoS2/FeS2@NCF exhibited significantly stronger absorbance at 652 nm than did the non-calcined/sulfided precursor (Fig. 2C), indicating superior POD-like activity for CoS2/FeS2@NCF. This enhancement originated from the porous nitrogen-doped carbon framework formed during calcination and the stable metal sulfides generated upon sulfidation, both contributing to enhanced substrate adsorption. Furthermore, the coupling of multivalent metal sulfides (Co/Fe) apparently increased the active site density and improved POD-like catalytic activity through synergistic effects. To elucidate the catalytic mechanism of CoS2/FeS2@NCF, its ROS generation capacity was probed using the ˙OH-specific fluorescence method with terephthalic acid (TA) (Fig. 2D). The obtained fluorescence spectra revealed a significantly higher emission intensity at 425 nm (excitation: 315 nm) for the CoS2/FeS2@NCF system than for the CoFePBA precursor. Given the established positive correlation between fluorescence intensity and ˙OH concentration,21 this result directly demonstrated the superior efficiency of the heterostructure in catalytically decomposing H2O2 to generate ˙OH radicals. To identify potential contributions from other ROS species, a systematic analysis was performed using radical scavenger experiments combined with electron spin resonance (ESR) spectroscopy. Ascorbic acid (AA) scavenges both ˙OH and superoxide anion (O2˙−), while thiourea (TH), p-benzoquinone (PBQ), and L-histidine (L-His) selectively quench ˙OH, O2˙−, and singlet oxygen (1O2), respectively. Our results showed a concentration-dependent inhibition: catalytic activity decreased by >80% with increased addition (up to 100 µL) of AA and TH, whereas PBQ and L-His induced negligible effects (Fig. 2E). The similar inhibition profiles of AA and TH indicated ˙OH to be the dominant ROS. ESR spectroscopy results (Fig. 2F) further confirmed this conclusion: using DMPO as a spin trap, the CoS2/FeS2@NC/H2O2 system exhibited the characteristic 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 quartet signal of DMPO-˙OH adducts. The markedly enhanced signal intensity compared to that of the unmodified material provided direct visual evidence for efficient ˙OH generation. To optimize the peroxidase-like activity of CoS2/FeS2@NCF nanocubes, we systematically investigated the effects of key factors such as pH and concentration on the nanocube activity (Fig. S3). Enzymatic kinetic analysis further revealed that, compared to the CoFePBA precursor, the CoS2/FeS2@NCF nanocube material exhibited kinetic parameters indicative of significantly enhanced catalysis of the TMB–H2O2 reaction (Fig. S4).
1 quartet signal of DMPO-˙OH adducts. The markedly enhanced signal intensity compared to that of the unmodified material provided direct visual evidence for efficient ˙OH generation. To optimize the peroxidase-like activity of CoS2/FeS2@NCF nanocubes, we systematically investigated the effects of key factors such as pH and concentration on the nanocube activity (Fig. S3). Enzymatic kinetic analysis further revealed that, compared to the CoFePBA precursor, the CoS2/FeS2@NCF nanocube material exhibited kinetic parameters indicative of significantly enhanced catalysis of the TMB–H2O2 reaction (Fig. S4).
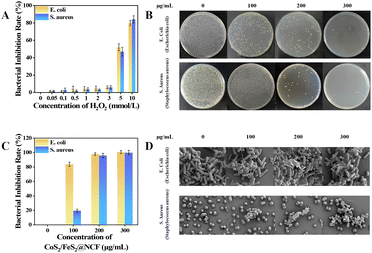
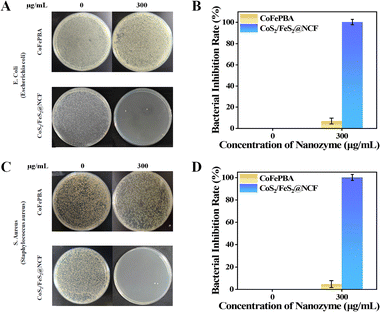
An antibacterial performance evaluation done using the standard plate count method revealed a negligible impact of H2O2 at concentrations below 3 mM on bacterial growth (Fig. 3A). To further assess the disinfection efficacy, gradient concentration reaction systems were established, testing 100 µg mL−1 catalyst + 0.1 mM H2O2, 200 µg mL−1 catalyst + 0.2 mM H2O2, and 300 µg mL−1 catalyst + 0.3 mM H2O2, respectively. As illustrated in Fig. 3B and C, neither the blank control group nor the low-concentration treatment group (100 µg mL−1 catalyst + 0.1 mM H2O2) exhibited significant inhibitory effects against E. coli and S. aureus. Scanning electron microscopy (SEM) observations (Fig. 3D) indicated that the treated bacteria remained structurally intact for these treatment groups: E. coli retained their typical rod-shaped morphology, S. aureus maintained smooth spherical structures, and no obvious damage to the cell membrane was detected, confirming that low-concentration treatment did not induce lethal damage to bacteria.22 In contrast, the system having a higher catalyst concentration, specifically 300 µg mL−1, demonstrated exceptional antibacterial performance, achieving a killing rate exceeding 99.9% against both pathogenic bacteria (Fig. 3B and C). SEM images (Fig. 3D) revealed pronounced cellular morphological aberrations here, including obvious wrinkles on the bacterial surface and rupture of membrane structures, visually demonstrating the lethal damage mediated by ˙OH radicals.9 Experimental data showed that CoS2/FeS2@NCF initiated bacteriostatic effects at 100 µg mL−1, while at 300 µg mL−1, it achieved complete bacterial inactivation through ROS generated via catalysis. As depicted in Fig. 4A and C, comparative analysis on standard agar plates demonstrated that the antibacterial efficacy of the CoS2/FeS2@NCF composite material significantly surpassed that of its precursor, CoFePBA, against E. coli and S. aureus. When the catalyst concentration was increased from 0 to 300 µg mL−1, CoS2/FeS2@NCF exhibited exceptional antibacterial performance (Fig. 4B). In contrast, CoFePBA showed negligible antibacterial effects against the two pathogens (Fig. 4D). In general and notably, many transition-metal–based nanozymes exhibit both peroxidase (POD)-like and catalase (CAT)-like activities with opposing antibacterial effects, but with highly pH-dependent catalytic balance.23 In the current work, under acidic conditions (pH 4.0) favoring Fenton-based POD-like activity while suppressing CAT-like function, UV-Vis spectroscopy, FL spectroscopy, ESR and antibacterial results confirmed that CoS2/FeS2@NCF predominantly followed the POD-like pathway with negligible CAT-like interference. These outcomes underscored that the calcination-sulfurization process (transformation of CoFePBA to CoS2/FeS2@NCF) not only boosted hydrophilicity but also significantly enhanced the bactericidal properties of the material, robustly supporting the conclusion that CoS2/FeS2@NCF nanocubes can elicit remarkable POD-like antibacterial activity through CDT.
In this study, a high-performance bimetallic TMS material, namely CoS2/FeS2@NCF nanocube material, was successfully synthesized using a simple calcination–sulfidation approach, with CoFePBA as the precursor. Structural characterizations confirmed that the nanozyme retained a cubic morphology with a porous architecture, exhibiting a high specific surface area of 87.68 m2 g−1 and abundant redox-active sites, which facilitated efficient substrate adsorption. The reduction in the water contact angle observed upon transforming CoFePBA to CoS2/FeS2@NCF facilitated the interaction between the nanoenzyme and bacteria, significantly enhancing its antibacterial efficacy. The nanozyme displayed superior POD-like activity, as indicated by low Michaelis constants (Km = 0.034 mM for TMB and 0.104 mM for H2O2), signifying strong substrate affinity. Furthermore, at a concentration of 300 µg mL−1, it catalyzed the decomposition of H2O2 to generate hydroxyl radicals (˙OH), which led to membrane disruption, resulting in over 99.9% antibacterial efficiency against both E. coli and S. aureus. The synergistic effect of the two metal sulfides, namely the CoS2 and FeS2, in conjunction with the nitrogen-doped carbon framework, significantly enhanced both catalytic efficiency and stability. This study has highlighted the potential of using PBA-derived TMS nanozymes as multifunctional platforms for antibacterial therapy and offers a novel strategy for designing multifunctional nanozyme materials with tunable catalytic activity and stability.
This work was supported by the National Natural Science Foundation of China (No. 22174055 and 22474051).
Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: additional experimental details, materials, and methods, supplementary figures referenced in the text. See DOI: https://doi.org/10.1039/d5cc05950j.Notes and references
- X. Gao, Y. Yin, H. Wu, Z. Hao, J. Li, S. Wang and Y. Liu, Anal. Chem., 2021, 93, 1569–1577 CrossRef CAS.
- M. Sun, L. Wang, Y. Zhuo, S. Xu, H. Liu, X. Jiang, Z. Lu, X. Wang, Y. Wang, G. Yue, B. Feng, H. Rao and D. Wu, Small, 2024, 20, 2309593 CrossRef CAS.
- Y. Zhang, R. Li, X. Li, P. Zheng, W. Zhu, C. Nie and Q. Pan, ACS Appl. Nano Mater., 2025, 8, 11521–11556 CrossRef CAS.
- H. Han, J. Yang, X. Li, Y. Qi, Z. Yang, Z. Han, Y. Jiang, M. Stenzel, H. Li, Y. Yin, Y. Du, J. Liu and F. Wang, Nano Res., 2021, 14, 2512–2534 CrossRef CAS PubMed.
- S. Liang, T. Chen, Y. Zhao, Y. Ren, M. Li, D. Lu, J. Wang, Y. Dai and Y. Guo, Nanoscale, 2023, 15, 13666–13674 RSC.
- C. Yao, Q. Wang, C. Peng, R. Wang, J. Liu, N. Tsidaeva and W. Wang, Chem. Eng. J., 2024, 479, 147924 CrossRef CAS.
- Y. Gao and L. Zhao, Chem. Eng. J., 2022, 430, 132745 CrossRef CAS.
- J. Je, H. Lim, H. W. Jung and S.-O. Kim, Small, 2022, 18, 2105310 CrossRef CAS.
- X. Yu, K. Huang, Y. Zhang, Y. Jin, Y. Chen, F. Chen and X. Zhang, ACS Appl. Nano Mater., 2024, 7, 3260–3268 CrossRef CAS.
- F. Wang, J. Pan, G. Wu, Z. Hu, A. Fatima and J. Huang, J. Alloys Compd., 2025, 1014, 178762 CrossRef CAS.
- L. Wang, J. Xue, J. Chang, C. Yu, H. Dai, Z. Yao, J. Zhou, G. Sun and W. Huang, J. Mater. Sci., 2021, 56, 13579–13589 CrossRef CAS.
- H. Xie, L. Mao and J. Mao, Chem. Eng. J., 2021, 421, 127826 CrossRef CAS.
- S. Yan, S. Luo, J. Feng, P. Li, R. Guo, Q. Wang, Y. Zhang, Y. Liu and S. Bao, Chem. Eng. J., 2020, 381, 122695 CrossRef CAS.
- G. Xia, C. Wang, P. Jiang, J. Lu, J. Diao and Q. Chen, J. Mater. Chem. A, 2019, 7, 12317–12324 RSC.
- K. Chen, G. Zhang, L. Xiao, P. Li, W. Li, Q. Xu and J. Xu, Small Methods, 2021, 5, 2001056 CrossRef CAS.
- X. Ma, X. Guo, F. Wang, H. Li, Q. Li, M. Liu, X. Chen, J. Yu, Y. Cui, J. Zhang, J. Xu, S. Li and D. Cao, J. Supercond. Nov. Magn., 2022, 35, 345–350 CrossRef CAS.
- Y. Huang, D. Lv, Z. Zhang, Y. Ding, F. Lai, Q. Wu, H. Wang, Q. Li, Y. Cai and Z. Ma, Chem. Eng. J., 2020, 387, 124122 CrossRef CAS.
- T. Yu, J. Wu, Y. Shen, A. Penkova, W. Qi and R. Su, Chem. Eng. J., 2024, 498, 155144 CrossRef CAS.
- B. Luo, J. Cai, Y. Xiong, X. Ding, X. Li, S. Li, C. Xu, A. Yu. Vasil’kov, Y. Bai and X. Wang, Int. J. Biol. Macromol., 2023, 246, 125651 CrossRef CAS.
- H. Cao, Y. Yuan, R. Zhao, W. Shi, J. Jiang, Y. Gao, L. Chen and L. Gao, ACS Appl. Mater. Interfaces, 2024, 16, 30958–30966 CrossRef CAS.
- H. Zhao, K. Li, Y. Zou, Y. Wang, Z. Zhong, Y. Xi and X. Xiao, Talanta, 2024, 273, 125964 CrossRef CAS.
- X. Zhang, G. Sun, S. Jia, H. Xie, Z. Kang, W. Chen, M. Cui, B. Wang, B. Wang, X. Chen and D.-P. Yang, Chem. Eng. J., 2022, 438, 135624 CrossRef CAS.
- S. Zhang, X. J. Gao, Y. Ma, K. Song, M. Ge, S. Ma, L. Zhang, Y. Yuan, W. Jiang, Z. Wu, L. Gao, X. Yan and B. Jiang, Nat. Commun., 2024, 15, 10605 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |