Chiral amplification of prebiotic peptide synthesis induced by chemical–physical interactions on calcite surfaces
Shichao
Yu
a,
Xiangxiao
Zheng
a,
Xiaofan
Guo
b,
Li
Zhang
a,
Yufen
Zhao
 ab and
Jianxi
Ying
ab and
Jianxi
Ying
 *a
*a
aInstitute of Drug Discovery Technology, Qian Xuesen Collaborative Research Center of Astrochemistry and Space Life Sciences, Ningbo University, Ningbo, Zhejiang 315211, China. E-mail: yingjianxi@nbu.edu.cn
bCollege of Chemistry and Chemical Engineering, Xiamen University, No. 422, China
First published on 27th November 2025
Abstract
Understanding how life substances adopted a single configuration under prebiotic conditions is central to exploring the origins of homochirality. This study shows a chiral amplification effect during prebiotic peptide synthesis on calcite surfaces, driven by the synergy between chemical synthesis and physical adsorption. This chemical–physical coupling mechanism offers a unique and promising strategy for promoting the emergence of homochirality.
The origin of homochirality in biomolecules represents a pivotal hallmark of the emergence of life and remains one of the unresolved enigmas in studies of abiogenesis.1 Elucidating the mechanisms underlying peptide chain chirality during precursor synthesis constitutes a central challenge in the study of life's origins.2,3 Chirality is a fundamental phenomenon in nature, arising from the spatial configuration differences among four substituents attached to an asymmetric carbon atom, thereby imparting distinct chiral properties to the molecule.4 The biomolecules that form modern organisms are a typical example of chirality. For instance, proteins are formed from L-amino acids, while nucleic acids are composed of D-configured nucleotides.5,6 Currently, the origin of homochirality in small molecules such as amino acids and nucleotides under prebiotic conditions has received widespread attention.7–9
Before the emergence of life, mineral and other environmental factors have been shown to participate in the assembly of amino acids into peptides and to influence their chirality.10–14 However, compared with studies on the synthesis of chiral building blocks, systematic investigations into how these factors cooperatively promote peptide formation and drive chiral amplification under prebiotic conditions remain limited.15–17 Given the central role of chirality in biological function, elucidating this process is not only crucial for revealing the chemical pathways underlying the origin of life but also for providing key insights into the mechanisms responsible for the emergence of chiral asymmetry in nature. Among these potential environmental factors, mineral surfaces—owing to their unique physicochemical properties—are considered to play an important role in prebiotic chemistry. Previous studies have shown that amino acids of different chirality exhibit distinct adsorption affinities on mineral surfaces, a variation influenced by the crystallographic orientation and local chemical features of the minerals.18,19 Under prebiotic conditions, such selective adsorption could not only enrich specific enantiomers and increase their local concentration, but also orient reactive groups in closer proximity,20 thereby facilitating peptide bond formation and introducing an initial chiral bias that may be amplified in subsequent reactions.21
In this study, we propose a chemical–physical coupling strategy to investigate the potential mechanisms of peptide formation and chiral amplification under prebiotic conditions. Specifically, we constructed an experimental system that simulates the geochemical evolution of the early Earth by reproducing the wet–dry cycles characteristic of ancient hydrothermal fields.22,23 During the drying phase, dehydration-induced concentration and thermal activation at 60 °C promote peptide bond formation between amino acids, whereas in the subsequent rehydration phase at ambient temperature, unreacted substrates redissolve to sustain dynamic cyclic reactions. To further lower the activation barrier under enzyme-free conditions, we introduced trisodium trimetaphosphate (P3m), a compound widely present in volcanic hydrothermal regions, as a natural amino acid activator. Its stability at elevated temperatures makes it highly compatible with prebiotic environments.24,25 In this system, chiral amino acids and the driving force for peptide bond formation are combined with the mirror-symmetric crystal facets of calcite, which provide differentiated adsorption sites for amino acids and peptides.26,27 As a result, the chirality of amino acids and dipeptides ultimately adsorbed on calcite surfaces can serve as important evidence for understanding the origin of homochirality under prebiotic conditions. By integrating chemical and physical processes, this study establishes a plausible pathway for early biomolecule synthesis and the emergence of homochirality.
The experimental results indicate that, through the combined effects of peptide condensation and surface adsorption, the dipeptides ultimately bound to calcite surfaces exhibited significant chiral amplification. As illustrated in Fig. 1, a racemic solution of leucine (an equimolar mixture of L-15N-Leu and D-Leu) can yield two chiral dipeptides, L-15N-Leu2 (3) and D-Leu2 (4). Leveraging the 15N isotope labelling technique, the 1 Da mass-to-charge ratio (m/z) difference between L-15N-amino acids and D-amino acids provided a reliable basis for quantitative analysis. According to quantitative LC-MS results, the enantiomeric excess (ee) value of L-15N-Leu2 (3) eluted from calcite surfaces reached 66.0%. In contrast, the data for amino acids adsorbed on the surface after the reaction showed only a slight increase in ee, from 3.9% in the original solution to 5.8% (Fig. S2–S7).
When the same reactions were performed with alanine, valine, isoleucine, and phenylalanine, a clear chiral amplification was likewise observed. As shown in Table 1, the ee values of the resulting dipeptides were −13.2%, 10.9%, −19.4%, and 5.2%, respectively. Although amino acids also exhibited changes in chirality after the reaction, the differences were minor: the amino acids adsorbed on calcite surfaces showed only slight deviations compared with the original solutions (Fig. S8–S31). Taken together, the five sets of experiments reveal that, while the extent of chiral change differs markedly between adsorbed amino acids and dipeptides, the overall trends remain consistent. This indicates that simple physical adsorption can introduce an initial chiral bias into the environment, whereas the coupling of chemical and physical processes can further amplify this bias.
| Amino acids | The ee value (%) of the origin solution | The ee value (%) of the amino acids in the elute solution | The ee value (%) of the dipeptides in the elute solution |
|---|---|---|---|
| a Represents the ee value (%) of L-enantiomer. b Represents the ee value (%) of D-enantiomer. | |||
| Leu | 3.9a | 5.8a | 66.0a |
| Ala | 1.0b | 1.6b | 13.2b |
| Val | 1.3a | 1.2a | 10.9a |
| Ile | 0.9b | 7.6b | 19.4b |
| Phe | 0.6a | 1.3a | 5.2a |
According to previous studies, calcite exhibits different adsorption coefficients for various amino acids, showing stronger affinity toward acidic amino acids.28,29 However, neutral amino acids are more prevalent and widely distributed in prebiotic environments.30 Or this reason, our investigation focused on neutral amino acids in order to obtain results with broader relevance. As early as 1951, Bernal proposed that biomolecules could undergo polymerization reactions on mineral surfaces.31–33 However, the free enthalpy change of amino acid dimerization is positive, and prolonged polymerization leads to an increase in enthalpy, ultimately inhibiting the formation of peptide chain.32,34,35 Consequently, researchers have long sought strategies to enhance the efficiency of amino acid polymerization and to achieve chiral amplification in peptides. The chemical–physical model proposed in this study addresses both of these critical challenges simultaneously. First, wet–dry cycles provide the driving force for polymerization. During this process, the adsorption properties of calcite surfaces alter the local concentration of amino acids, reducing the distance between the amine and carboxyl groups, thereby enhancing peptide synthesis. From the perspective of chiral amplification, our experimental results demonstrate that calcite surfaces can extend the weak chiral bias observed at the amino acid level into pronounced chiral selection at the dipeptide level. This significant amplification phenomenon offers a plausible pathway for the efficient prebiotic synthesis of peptides and provides important insights into the origin of molecular homochirality.
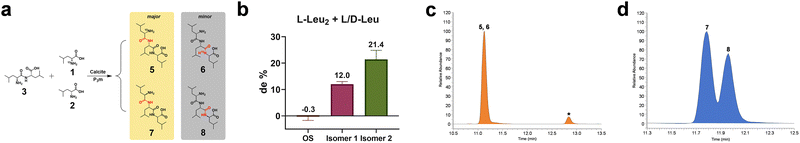
To further explore the universality of the chemical–physical interaction model, we employed L-Leu2 and racemic Leu (an equimolar mixture of L-15N-Leu and D-Leu, with a total concentration equal to that of the dipeptide) as substrates to study the chiral amplification effect of calcite during tripeptide formation under wet–dry cycling. Previous experiments revealed that dipeptides adsorbed on the calcite surface already exhibited pronounced chiral selectivity, leading to the enrichment of one enantiomeric form. When these enriched dipeptides were subsequently used as substrates for tripeptide formation, we found that they preferentially incorporated amino acids of the same chirality. As shown in Fig. 2a, amino acids typically underwent condensation reactions between the amino terminus of a dipeptide and the carboxyl terminus of an amino acid.36,37 Among the four resulting tripeptides, L-15N-Leu-L-Leu2 (5) and D-Leu-L-Leu2 (7) were the most common configurations, whereas L-Leu2-L-15N-Leu (6) and L-Leu2-D-Leu (8) are present in relatively lower amounts. Experimental data showed that the amount of surface-adsorbed L-Leu3 (the sum of 5 and 6) after the reaction was significantly higher than that of the two products incorporating D-Leu, with diastereomeric excess (de) values of 12.0% relative to 7 and 21.4% relative to 8 (Fig. S32–S35).
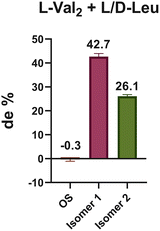
This phenomenon was likewise reproduced in the system of L-Val2 with racemic Leu. As shown in Fig. 3, L-Val2 exhibited a clear chiral preference for L-Leu, leading to pronounced chiral amplification in the surface-bound tripeptides. We found that the de values of L-15N-Leu-L-Val2 was 42.7% relative to L-Val2-D-Leu and 26.1% relative to D-Leu-L-Val2 (Fig. S36–S39). This observation demonstrates a synergistic interplay between surface adsorption and subsequent chain elongation, leading to a stepwise amplification of chiral preference. It further highlights the role of chemical–physical interactions on calcite surfaces in promoting chiral amplification during polypeptide synthesis under prebiotic conditions, thereby revealing the contribution of inorganic environments to the emergence of molecular homochirality.
To further assess the contribution of purely physical or chemical processes, control experiments were performed in the absence of calcite, in which no significant chiral amplification was observed (Fig. S40–S53). In addition, the stability of amino acid adsorption on calcite surfaces was examined. The results showed that calcite exhibited stable adsorption toward five amino acids (Leu, Ala, Val, Ile, and Phe), but no appreciable chiral selectivity was detected in these adsorption processes (Fig. S54–S75).
This study establishes a stepwise model of chiral amplification mediated by calcite surfaces. We show that calcite differentially adsorbs amino acids of distinct configurations, initiating selective enrichment, and that this initial bias is progressively amplified during dipeptide and tripeptide formation through surface chemical–physical interactions. Control experiments confirmed that neither purely physical nor chemical processes alone could account for the observed amplification. Together, these findings highlight the pivotal role of mineral surfaces in promoting chiral amplification under prebiotic conditions and suggest that inorganic environments such as calcite may have contributed significantly to the emergence of molecular homochirality. Extending this interaction model to other prebiotic minerals and biomolecular substrates will provide a broader framework for understanding the chemical origins of life.
S. Y. performed experiments, analyzed the experimental data, and drafted the manuscript. J. Y. designed the experiments and revised the manuscript. J. Y. provided overall supervision of the research. Y. Z. conceived the experiments. X. Z., X. G. and L. Z. carried out the LC-MS experiments.
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LMS25D030002), National Natural Science Foundation of China (No. 42388101, 92256203), Advanced Research Capacity Building Program of Ningbo University (No. GJPY2025040), Technology and Engineering Center for Space Utilization, Chinese Academy of Sciences (No. KJZ-YY-NSM0406), Ningbo Top Talent Project (No. 215-432094250). The Analysis Center of Institute of Drug Discovery Technology is acknowledged for collecting LC-MS data.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc06016h.Notes and references
- D. Kennedy and C. Norman, Science, 2005, 309, 75 CrossRef CAS.
- G. Laurent, D. Lacoste and P. Gaspard, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2012741118 CrossRef CAS.
- S. F. Ozturk and D. D. Sasselov, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2204765119 CrossRef CAS PubMed.
- A. J. Wagner, D. Y. Zubarev, A. Aspuru-Guzik and D. G. Blackmond, ACS Cent. Sci., 2017, 3, 322–328 CrossRef CAS.
- Y. Zheng, K. Mao, S. Chen and H. Zhu, Front. Bioeng. Biotechnol., 2021, 9, 703004 CrossRef.
- H. Elhabashy, F. Merino, V. Alva, O. Kohlbacher and A. N. Lupas, Structure, 2022, 30, 462–475 CrossRef CAS.
- S. F. Ozturk, Z. Liu, J. D. Sutherland and D. D. Sasselov, Sci. Adv., 2023, 9, eadg8274 CrossRef CAS PubMed.
- M. Mauksch, Phys. Chem. Chem. Phys., 2023, 25, 1734–1754 RSC.
- M. Deng, J. Yu and D. G. Blackmond, Nature, 2024, 626, 1019–1024 Search PubMed.
- L. Qiu and R. G. Cooks, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2309360120 CrossRef CAS.
- K. B. Muchowska and J. Moran, Science, 2020, 370, 767–768 CrossRef CAS PubMed.
- C. S. Foden, S. Islam, C. Fernández-García, L. Maugeri, T. D. Sheppard and M. W. Powner, Science, 2020, 370, 865–869 CrossRef CAS.
- M. Keller, E. Blöchl, G. Wächtershäuser and K. O. Stetter, Nature, 1994, 368, 836–838 CrossRef.
- R. M. Hazen and D. S. Sholl, Nat. Mater., 2003, 2, 367–374 CrossRef CAS.
- M. Gleiser and S. I. Walker, Int. J. Astrobiol., 2012, 11, 287–296 CrossRef CAS.
- E. Altamura, A. Comte, A. D’Onofrio, C. Roussillon, D. Fayolle, R. Buchet, F. Mavelli, P. Stano, M. Fiore and P. Strazewski, Symmetry, 2020, 12, 1108 CrossRef CAS.
- C. Chieffo, A. Shvetsova, F. Skorda, A. Lopez and M. Fiore, Astrobiology, 2023, 23, 1368–1382 CrossRef CAS.
- R. M. Hazen and D. A. Sverjensky, Cold Spring Harbor Perspect. Biol., 2010, 2, a002162 Search PubMed.
- R. Li, Q. Deng, L. Han, T. Ouyang, S. Che and Y. Fang, Nat. Commun., 2024, 15, 10130 CrossRef CAS PubMed.
- J. Wu, Z. Zhang, X. Yu, H. Pan, W. Jiang, X. Xu and R. Tang, Chin. Sci. Bull., 2011, 56, 633–639 CrossRef CAS.
- K. P. Bryliakov, Research, 2020, 5689246 CAS.
- T. D. Campbell, R. Febrian, J. T. McCarthy, H. E. Kleinschmidt, J. G. Forsythe and P. J. Bracher, Nat. Commun., 2019, 10, 4508 CrossRef.
- W. Rapin, G. Dromart, B. C. Clark, J. Schieber, E. S. Kite, L. C. Kah, L. M. Thompson, O. Gasnault, J. Lasue, P. Y. Meslin, P. J. Gasda and N. L. Lanza, Nature, 2023, 620, 299–302 CrossRef CAS.
- J. Rabinowitz, J. Flores, R. Kresbach and G. Rogers, Nature, 1969, 224, 795–796 CrossRef CAS.
- Y. Yamagata, H. Watanabe, M. Saitoh and T. Namba, Nature, 1991, 352, 516–519 CrossRef CAS PubMed.
- R. M. Hazen, T. R. Filley and G. A. Goodfriend, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 5487–5490 CrossRef CAS.
- E. D. Dana, Nature, 1922, 110, 210 Search PubMed.
- A. J. Price and E. R. Johnson, Phys. Chem. Chem. Phys., 2020, 22, 16571–16578 RSC.
- J. Gao, H. Li, Z. Sun, J. Song, Y. Liu, C. Jin, Z. Zhang, J. A. Ma and W. Jiang, Langmuir, 2023, 39, 12707–12714 CrossRef CAS PubMed.
- W. Jiang, M. S. Pacella, D. Athanasiadou, V. Nelea, H. Vali, R. M. Hazen, J. J. Gray and M. D. McKee, Nat. Commun., 2017, 8, 15066 CrossRef CAS PubMed.
- V. Erastova, M. T. Degiacomi, D. G. Fraser and H. C. Greenwell, Nat. Commun., 2017, 8, 2033 CrossRef.
- J. F. Lambert, Origins Life Evol. Biospheres, 2008, 38, 211–242 CrossRef CAS.
- A. Rimola, M. Sodupe and P. Ugliengo, J. Am. Chem. Soc., 2007, 129, 8333–8344 CrossRef CAS PubMed.
- E. L. Shock, Geochim. Cosmochim. Acta, 1992, 56, 3481–3491 CrossRef CAS.
- R. B. Martin, Biopolymers, 1998, 45, 351–353 CrossRef CAS.
- J. Ying, P. Chen, Y. Wu, X. Yang, K. Yan, P. Xu and Y. Zhao, Chin. Chem. Lett., 2019, 30, 367–370 CrossRef CAS.
- J. Ying, S. Fu, X. Li, L. Feng, P. Xu, Y. Liu, X. Gao and Y. Zhao, Chem. Commun., 2018, 54, 8598–8601 RSC.
| This journal is © The Royal Society of Chemistry 2026 |