Photo- and thermal catalysis of nitrate to nitrite by manganese ions under light up to 600 nm
Kojiro
Fuku
 *a,
Naohide
Tsuji
b and
Ryosuke
Ueda
b
*a,
Naohide
Tsuji
b and
Ryosuke
Ueda
b
aFaculty of Environmental and Urban Engineering, Kansai University, 3-3-35 Yamate-cho, Suita, Osaka 564-8680, Japan. E-mail: k.fuku@kansai-u.ac.jp
bGraduate School of Science and Engineering, Kansai University, 3-3-35 Yamate-cho, Suita, Osaka 564-8680, Japan
First published on 27th November 2025
Abstract
We report a catalytic system using manganese ion (Mn2+) for the photo- and thermal reduction of nitrate (NO3−) to nitrite ions, in the presence of organic compounds, under ultraviolet and visible light irradiation up to 600 nm. This method enables NO3− reduction utilizing long-wavelength light and a novel Mn2+ catalytic system.
The pollution of rivers, oceans, soil, and groundwater caused by nitrate ions (NO3−) and persistent organic compounds such as aromatic compounds that exist in industrial water, agricultural water and wastewater, has evolved into a serious environmental problem.1 In particular, NO3− (combined with phosphorus-containing compounds) are not only harmful to the human body but also cause eutrophication (overfertilization). The photocatalytic reaction has attracted significant attention because it is an environmentally friendly method for treating NO3−; this type of reaction mainly employs semiconductor photocatalysts.2–4 In the catalysis process, the holes (h+) generated in the photocatalyst by light irradiation oxidize organic compounds or water (H2O), and the simultaneously generated excited electrons (e−) reduce NO3−.2–4 The main objective of the photocatalytic NO3− reduction is the detoxification to nitrogen (N2) (reaction (2)) or conversion to ammonia (NH3) (reaction (3)) in a multi-step, multi-electron process via nitrite ion (NO2−) formation (reaction (1)).
| NO3− + 2H+ + 2e− → NO2− + H2O (+0.84 V vs. RHE) | (1) |
| NO3− + 6H+ + 5e− → 1/2N2 + 3H2O | (2) |
| NO3− + 9H+ + 8e− → NH3 + 3H2O | (3) |
In parallel with NO3− reduction, advances in NOx reduction have been achieved using catalytic approaches.5 Challenges such as selectivity, multi-electron transfer, and by-product suppression are common to both systems. Recent findings in NOx reduction catalysis offer valuable insights for designing efficient NO3− reduction systems.
The introduction of metal co-catalysts in photocatalysts to enable e− trapping and selective e− transfer to achieve multi-electron chemical reduction has been reported in many reaction types (for example, reactions (2) and (3).3 Therefore, improving the efficiency of the two-electron reduction of NO3− to NO2− is highly important to efficient NO3− treatment.
Additionally, research and development have focused on the synthesis and reaction of photocatalysts that respond to visible light, which constitutes a significant portion of sunlight, to harness the inexhaustible sunlight energy. There have been also interesting and promising reports of visible-light-responsive photocatalysts that also achieve conversion of NO3− to N2 or NH3.4 However, there are still few candidate materials for photocatalysts that can utilize visible light in the long-wavelength region, which makes up the majority of sunlight. To achieve efficient conversion of NO3− to N2 or NH3 using solar light energy, the first step is to search for photocatalytic materials that enable efficient reduction of NO3− to NO2− even in visible light in the long-wavelength range.
In this study, we focused on divalent manganese ion (Mn2+), which is transition metal ions that absorb ultraviolet (UV) and visible light (up to ca. 700 nm) as novel photocatalyst to achieve efficient NO3− reduction; Mn2+ exhibits several highly oxidized ionic forms. Many manganese composite oxides and doped materials can be used as photocatalytic materials.6 However, the direct application of Mn2+ to photocatalytic NO3− reduction has not been reported, to the best of our knowledge. In this study, we investigate the potential application of Mn2+ as photo- and thermal catalysts in the reduction of NO3− to NO2− when they are irradiated with UV and visible light (up to 600 nm) while oxidatively degrading phenol, which is a model persistent organic compound. Details of the experimental procedures on the reaction and tracking mechanism are provided in the SI.
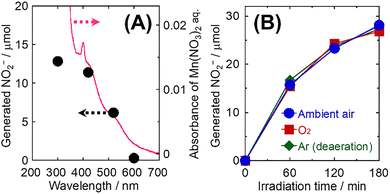
A comparison of the performance in the reduction of NO3− to NO2− at 30 °C in the presence or absence of Mn2+, visible light (λ > 420 nm) irradiation, and phenol is presented in Table 1. An aqueous solution of metal nitrate was used as a source of metal ions and NO3−. In an aqueous solutions of metal nitrates other than manganese nitrate (Mn(NO3)2), no formation of NO2− was observed even under visible light irradiation in the presence of phenol. Regarding Mn2+, the NO2− formation was limited in the absence of visible light irradiation or phenol. The reduction of NO3− to NO2− was significantly achieved in the presence of Mn2+, visible light irradiation, and phenol. To track the specific effects of Mn2+ on the NO3− reduction under visible light irradiation, we investigated the effect of light irradiation wavelength (λ > 300, 420, 520, and 600 nm) on the reduction of NO3− to NO2− in the presence of phenol at 30 °C (Fig. 1(A)). It should be noted that the NO2− formation was achieved even under visible light irradiation in the long-wavelength region (up to 600 nm). In addition, the performance in the reduction of NO3− to NO2− significantly increased as the irradiation wavelength decreased. In this study, the visible light irradiation wavelength was varied by changing the cutoff filters attached to the 300 W Xenon (Xe) illuminator, indicating that the light intensity absorbed by Mn2+ increases as the irradiation wavelength decreases (the UV irradiation was performed without using cutoff filters). The NO2− formation is highly consistent with the absorption property of the Mn(NO3)2 aqueous solution. These wavelength-dependent results indicate that the reduction of NO3− to NO2− is caused by the absorption of light by Mn(NO3)2 in the aqueous solution. Fig. 1(B) shows the variation of the generated NO2− with the irradiation time in the presence of Mn2+, visible light (λ > 420 nm), and phenol at 30 °C under ambient air conditions, oxygen (O2), and oxygen-free argon (Ar). In all reaction conditions, the generated NO2− increased with the irradiation time. Conversely, the variation of the generated NO2− when varying the reaction conditions (i.e., the O2 concentration) was small. Under ambient air conditions, phenol conversion reached ca. 100% within 120 min; additionally, CO2 was generated as a complete degradation product even under mild reaction conditions (Fig. S1). O2 is reduced via photocatalytic two- or four-electron processes to generate hydrogen peroxide (H2O2) or H2O, respectively (reactions (4) and (5)).7 From a thermodynamic perspective of the redox potential, the reduction of NO3− to NO2− (reaction (1)) occurs more readily than that of O2 to H2O2 but less readily than the multi-electron reduction of O2 to H2O.
| O2 + 2H+ + 2e− → H2O2 (+0.68 V vs. RHE) | (4) |
| O2 + 4H+ + 4e− → 2H2O (+1.23 V vs. RHE) | (5) |
| Catalyst and NO3− source | Light irradiation (λ > 420 nm) | Phenol | Generated NO2−/µmol |
|---|---|---|---|
| NaNO3 | ○ | ○ | <0.1 |
| Al(NO3)3 | ○ | ○ | <0.1 |
| Ca(NO3)2 | ○ | ○ | <0.1 |
| Cu(NO3)2 | ○ | ○ | <0.1 |
| Ni(NO3)2 | ○ | ○ | <0.1 |
| Co(NO3)2 | ○ | ○ | 0.4 |
| Mn(NO3)2 | — | ○ | 0.2 |
| ○ | — | 0.7 | |
| ○ | ○ | 28.1 |
However, the results in Fig. 1(B) indicate that the reduction of O2 to H2O2 or H2O using Mn2+ does not occur under the reaction conditions used in this study. Therefore, Mn2+ can be considered a promising visible-light-responsive photocatalyst for the reduction of NO3− to NO2− and the oxidation of persistent organic compounds. These results indicated that Mn2+ exhibits an excited e− level (i.e., potential for the reduction reaction) between +0.68 and +0.84 V vs. RHE and is not subjected to multi-electron reduction reactions of more than four electrons.
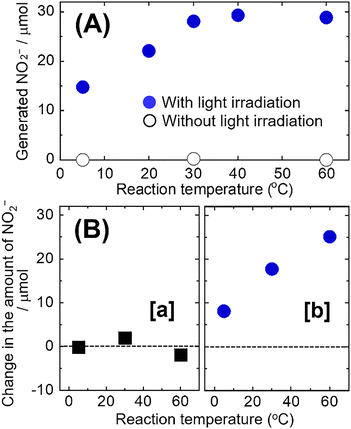
To examine the catalytic properties of Mn2+, in detail, we investigated the effect of the reaction temperature on the reduction of NO3− to NO2− under visible light (λ > 420 nm) irradiation at ambient air conditions (Fig. 2(A) and Fig. S2). The amount of the generated NO2− increased with the reaction time at all reaction temperatures; additionally, it increased linearly as the reaction temperature increased up to 30 °C. Without light irradiation, little formation of NO2− was confirmed regardless of the reaction temperature. These results indicate that Mn2+ acts both as a photo- and thermal catalyst under light irradiation, i.e., it exhibits photo-induced thermal catalytic properties. To investigate these properties, we performed a NO2− degradation (oxidation) test using an ice bath (at ∼5 °C) and an water bath (at 30 and 60 °C) in an aqueous solution containing phenol and NO2− (Fig. 2(B)). Mn(NO3)2 or manganese chloride (MnCl2) was used as an Mn2+ source with or without NO3− under visible light (λ > 420 nm) irradiation at ambient air conditions. In the case of MnCl2 (i.e., in the absence of NO3−), the variation in the amount of the generated NO2− was small at all reaction temperatures, indicating that the photo- and thermal catalytic properties of Mn2+ do not cause NO2− degradation (oxidation) in the presence of phenol. In the case of Mn(NO3)2, the NO2− amount increased significantly (i.e., reduction of NO3− to NO2− was achieved) even in the presence of NO2−. These results indicate that the photo-induced thermal catalysis of Mn2+ facilitates only the reduction of NO3− to NO2− and the oxidation of phenol. Although the precise mechanism remains to be clarified, it is possible that an increase in reaction temperature promotes photoexcitation of Mn2+ species, facilitates charge carrier separation, and enhances interfacial electron transfer kinetics.
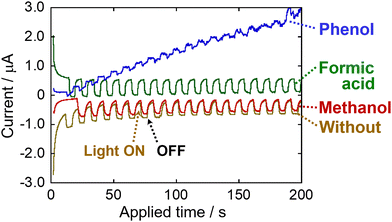
The photocatalysis mechanism of Mn2+ in the reduction of NO3− to NO2− was investigated by examining the effects of the light irradiation wavelength on the photoelectrochemical properties of Mn2+ using a Pt electrode immersed in a 0.1 M Mn(NO3)2 aqueous solution without organic compounds by switching the irradiation light ON and OFF in the range of λ > 300, 420, 520, and 600 nm (Fig. S3(A)). The photoreduction current (i.e., the increase in the cathode current under light irradiation) was observed under all light irradiation conditions. We found that the photoreduction current varies significantly with the irradiation wavelength. As shown in Fig. S3(B), the photoreduction current results are highly consistent with the absorption properties of the Mn(NO3)2 aqueous solution, indicating that this current is generated by the absorption of light by Mn2+. This behavior is similar to that observed in the results showing the effect of the irradiation wavelength on the NO2− generation (Fig. 1(A)). The results in Fig. S3 indicate that irradiating light to the Mn(NO3)2 aqueous solution leads to the reduction of NO3− to NO2− caused by excited e− in Mn2+; additionally, the generated oxidized manganese ion (Mn(2+x)+) species are electrochemically reduced and regenerated to Mn2+ (Fig. S4(A)). In addition, we investigated the effect of adding organic compounds (phenol, methanol, and formic acid) on the photoreduction current by irradiating visible light (λ > 420 nm) to a 0.1 M Mn(NO3)2 aqueous solution (Fig. 3). The photoreduction current varied depending on the type of organic compound; it decreased in the following order: no organic compound ≓ methanol ≓ formic acid ≫ phenol. In particular, the addition of phenol significantly caused decreasing the photoreductive current; this indicates that adding phenol in an Mn(NO3)2 aqueous solution inhibits the electrochemical e− transfer (Fig. S4(A)) to the Mn(2+x)+ species generated by the reduction of NO3− to NO2− under light irradiation, i.e., the Mn(2+x)+ species are preferentially reduced and regenerated by the e− transfer caused by phenol under the electrochemical conditions considered in this study (Fig. S4(B)). In fact, highest reduction performance of NO3− to NO2− was achieved in the presence of phenol among the organic compounds under visible light (λ > 420 nm) irradiation and ambient air conditions at 30 °C (Fig. S5). Furthermore, UV-vis measurements showed negligible absorption overlap between phenol and Mn2+ in the visible region (λ > 420 nm) (Fig. S6), indicating that phenol does not interfere with the photoexcitation process of Mn2+. These results indicate that phenol, which is a model persistent organic (aromatic) compound, acts as an effective reducing agent (e− donor), thereby reducing and regenerating Mn(2+x)+ species in the oxidized state generated by the reduction of NO3− to NO2−.
Based on the above results, the proposed mechanism of the reduction of NO3− to NO2− in an aqueous solution of Mn(NO3)2 and phenol under light irradiation is summarized in Fig. 4. Mn2+ absorbs UV and visible light (up to 600 nm), generating excited e− in Mn2+ that achieve the two-electron reduction reaction from NO3− to NO2−. The Mn(2+x)+ species are reduced and regenerated to Mn2+ by oxidizing the aromatic compound present in the solution. These photocatalytic redox processes are significantly assisted by increasing the reaction temperature.
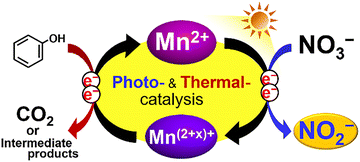 | ||
| Fig. 4 Proposed mechanism for the reduction of NO3− to NO2− in an aqueous solution of Mn(NO3)2 and phenol under light irradiation. | ||
In summary, compared with other metal ions, Mn2+ exhibited specific properties in the reduction of NO3− to NO2− and the oxidative degradation of phenol under visible light irradiation (λ > 420 nm). The wavelength dependence of NO3− reduction under light irradiation was closely linked to the light absorption properties of the Mn(NO3)2 aqueous solution, indicating that Mn2+ can utilize visible light (up to 600 nm). It should be noted that, even though Mn2+ was inactive in the oxidative decomposition of the generated NO2− and the reduction of the coexisting O2, its ability to reduce NO3− to NO2− increased significantly with increasing reaction temperature under light irradiation. The photoelectrochemical properties of the Mn(NO3)2 aqueous solution, which was used as the electrolyte, showed a significant increase in the cathode current under light irradiation. The photoreduction current varied significantly with the light irradiation wavelength, showing good agreement with the aforementioned light absorption properties of the Mn(NO3)2 aqueous solution; additionally, it decreased significantly when adding phenol to the solution. These results suggest Mn2+ could act as a catalyst combining photo- and thermal effects under the conditions used in this study, as it can reduce NO3− to NO2− and oxidatively decompose persistent aromatic organic compounds upon irradiation with UV and visible light (up to 600 nm). This study contributes to the development of promising design guidelines to overcome global environmental problems via the environmentally friendly treatment of NO3− and persistent aromatic compounds contained in wastewater using only inexhaustible visible light energy. The ultimate objective of this study is to detoxify NO3− in wastewater to N2 or convert it into NH3, a valuable chemical product, using the photo- and thermal catalytic properties of Mn2+ as well as its broad light absorption across the UV-visible range. Although the formation of N2 or NH3 was not confirmed under the present conditions, this study focuses on the two-electron reduction of NO3− to NO2−, and we recognize that achieving the multi-electron reduction to N2 or NH3 remains a significant challenge and an essential next step for practical applications. We are currently investigating the integration of Mn2+-based photocatalysis and thermalcatalysis with metal nanoparticle co-catalysts, which are known to facilitate multi-electron transfer reactions through efficient electron trapping and selective transfer. Additionally, we are developing heterogeneous Mn2+ catalysts to enable better control over the catalytic environment, facilitate catalyst separation and recovery after the reaction, and track the valence change of Mn during the reaction. These efforts using the properties of Mn2+ will provide mechanistic insights and design principles for future photocatalytic systems capable of achieving complete NO3− treatment using solar energy.
This work was partially supported by the Environment Research and Technology Development Fund (JPMEERF20233R03) of the Environmental Restoration and Conservation Agency provided by Ministry of the Environment of Japan, and JSPS KAKENHI (Grant Number 22K05293).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this study have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc05059f.Notes and references
- (a) J. Luo, S. Miao, R. Koju, T. P. Joshi, R. Liu, H. Liu and J. Qu, Water Res., 2022, 216, 118292 CrossRef CAS; (b) K. Wick, C. Heumesser and E. Schmid, J. Environ. Manag., 2012, 111, 178–186 CrossRef CAS; (c) M. Munoz, Z. M. de Pedro, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2015, 176–177, 249–265 CrossRef CAS; (d) R. M. Madjar, G. Vasile Scăeţeanu and M. A. Sandu, Water, 2024, 16, 3146 CrossRef CAS.
- (a) W. Hiramatsu, Y. Shiraishi, S. Ichikawa, S. Tanaka, Y. Kawada, C. Hiraiwa and T. Hirai, J. Am. Chem. Soc., 2025, 147, 1968–1979 CrossRef CAS; (b) H. Luo, G. Shu, S. Guo, X. Kuang, C. Zhao, C.-A. Zhou, C. Wang, L. Song, K. Ma and H. Yue, Chem. Commun., 2024, 60, 6627–6630 RSC; (c) J. Li, R. Chen, J. Wang, Y. Zhou, G. Yang and F. Dong, Nat. Commun., 2022, 13, 1098 CrossRef CAS PubMed; (d) J. E. Silveira, A. L. Garcia-Costa, J. Carbajo, A. R. Ribeiro, G. Pliego, W. S. Paz, J. A. Zazo and J. A. Casas, Chem. Eng. J. Adv., 2022, 12, 100387 CrossRef CAS.
- (a) E. Bahadori, A. Tripodi, G. Ramis and I. Rossetti, ChemCatChem, 2019, 11, 4642–4652 CrossRef CAS; (b) H. Gekko, K. Hashimoto and H. Kominami, Phys. Chem. Chem. Phys., 2012, 14, 7965–7970 RSC; (c) M. Oka, Y. Miseki, K. Saito and A. Kudo, Appl. Catal., B, 2015, 179, 407–411 CrossRef CAS.
- (a) Y. Shiraishi, S. Akiyama, W. Hiramatsu, K. Adachi, S. Ichikawa and T. Hirai, JACS Au, 2024, 4, 1863–1874 CrossRef CAS; (b) Y. Shi, Y. Xie, J. Xia, X. Zhang, H. Cheng and J. Chen, Water, 2025, 17, 155 CrossRef CAS; (c) W. Liu, X. Li, X. Chu, S. Zuo, B. Gao, C. Yao, Z. Li and Y. Chen, Chemosphere, 2022, 294, 133763 CrossRef CAS PubMed.
- (a) J. E. Silveira, G. J. Inacio, N. N. Batista, W. P. Morais, M. G. Menezes, J. A. Zazo, J. A. Casas and W. S. Paz, J. Environ. Chem. Eng., 2024, 12, 11998 Search PubMed; (b) J. E. Silveira, A. S. de Souza, F. N. N. Pansini, A. R. Ribeiro, W. L. Scopel, J. A. Zazo, J. A. Casas and W. S. Paz, Sep. Purif. Technol., 2023, 306, 122570 CrossRef CAS; (c) J. E. Silveira, V. G. Garcia, L. T. Pacheco, W. S. Paz, J. A. Casas and J. A. Zazo, Sep. Purif. Technol., 2025, 357, 130217 CrossRef CAS.
- (a) X. Wang, A. Wang and J. Ma, J. Hazard. Mater., 2017, 336, 81–92 CrossRef CAS; (b) R. Yang, Y. Fan, R. Ye, Y. Tang, X. Cao, Z. Yin and Z. Zeng, Adv. Mater., 2021, 33, 2004862 CrossRef CAS; (c) L. A. Chanu, W. J. Singh, K. J. Singh and K. N. Devi, Results Phys., 2019, 12, 1230–1237 CrossRef; (d) K. P. Lucht and J. L. Mendoza-Cortes, J. Phys. Chem. C, 2015, 119, 22838–22846 CrossRef CAS; (e) M. B. Poudel, C. Yu and H. J. Kim, Catalysts, 2020, 10, 546 CrossRef CAS; (f) N. Tamam, M. Aadil, W. Hassan, S. R. Ejaz, Z. M. Najm, I. A. Alsafari, S. Aman, A. V. Trukhanov, M. S. Al-Buriahi and I. Boukhris, Ceram. Int., 2022, 48, 29589–29600 CrossRef CAS.
- (a) Y. Kofuji, Y. Isobe, Y. Shiraishi, H. Sakamoto, S. Tanaka, S. Ichikawa and T. Hirai, J. Am. Chem. Soc., 2016, 138, 10019–10025 CrossRef CAS; (b) K. Fuku, R. Takioka, K. Iwamura, M. Todoroki, K. Sayama and N. Ikenaga, Appl. Catal., B, 2020, 272, 119003 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |