A dual-mode viscosity-activatable probe for the immediate evaluation of photodynamic/photothermal therapy efficacy
Ao-Xiang
Fu†
a,
Li
Li†
a,
Qin-Ting
Liao
a,
Chu-Yu-Hui
Peng
c,
Ning
Yang
a,
Guo-Jiang
Mao
 b,
Juan
Ouyang
b,
Juan
Ouyang
 a,
Liufang
Hu
a,
Fen
Xu
*a and
Chun-Yan
Li
a,
Liufang
Hu
a,
Fen
Xu
*a and
Chun-Yan
Li
 *a
*a
aKey Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Hunan Provincial University Key Laboratory for Environmental and Ecological Health, College of Chemistry, Xiangtan University, Xiangtan, 411105, P. R. China. E-mail: xufen@xtu.edu.cn; lichunyan@xtu.edu.cn
bHenan Key Laboratory of Organic Functional Molecule and Drug Innovation, Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453007, P. R. China
cCollege of Chemical Engineering, Xiangtan University, Xiangtan 411105, P. R. China
First published on 14th November 2025
Abstract
A dual-mode probe, named BC-V, is constructed for viscosity imaging. In high-viscosity solution, the fluorescence and photoacoustic signals are triggered. Under laser irradiation, the photodynamic and photothermal properties are activated. Furthermore, during phototherapy in tumor-bearing mice, therapy efficacy can be immediately evaluated using dual-mode imaging.
Cancer, involving malignant cells, is caused by malignant proliferation of cells and is one of the leading causes of death worldwide.1 Timely and effective treatment is expected to reduce cancer mortality rates, thereby alleviating patients’ suffering. Phototherapy, consisting of photodynamic therapy (PDT) and photothermal therapy (PTT), is a promising therapy method with obvious advantages such as high tumor suppression and non-invasiveness.2 Under laser irradiation, phototherapy agents produce reactive oxygen species for PDT3 or heat for PTT,4 which kill cancer cells and regress tumors. However, excessive irradiation or phototherapy agents can cause side effects, further affecting therapeutic efficacy. Timely monitoring of the treatment process and early evaluation of treatment efficacy are necessary to avoid excessive treatment and achieve precise treatment for cancer patients.5 Although biopsy-based biomarker detection is a classic method for evaluating therapeutic efficacy, it is a highly invasive approach.6 In addition, the current standard for evaluating the efficacy of tumor treatment is to measure the size of tumors through medical imaging techniques, mainly including magnetic resonance imaging (MRI), positron emission tomography (PET), and computed tomography (CT). However, these methods require several weeks or months after treatment to determine significant changes in the tumor.7 Therefore, it is crucial to evaluate the efficacy of tumor treatment early and even immediately through non-invasive methods for the precise management of cancer patients.
Near-infrared fluorescence (FL) imaging is a diagnostic technique with significant advantages, including non-invasiveness, high sensitivity, and low cost.8 The long emission wavelength can avoid background fluorescence interference, but its application in vivo is limited due to its low penetration depth.9 In contrast, photoacoustic imaging (PA), as another diagnostic imaging approach, can provide very high imaging depth.10 Based on this situation, combining FL and PA imaging modes can significantly improve sensitivity and penetration depth, thereby enabling the acquisition of richer, more accurate tumor information.11 Viscosity plays a significant role in physiological and pathological conditions by influencing metabolic processes within the cellular microenvironment.12 Previous studies have shown that cell death is often accompanied by an increase in intracellular viscosity levels due to cytoplasmic condensation and cell contraction.13 Moreover, viscosity is considered a universal biomarker for cell death, independent of the pattern of cancer cell death, further making it highly suitable for early or immediate evaluation of treatment efficacy.14 There are fluorescent probes developed for monitoring viscosity,15 but a dual-mode probe for evaluating the efficacy of tumor treatment immediately through the changes in viscosity is still lacking.
Based on the above considerations, this paper designs a smart probe, BC-V, with dual-mode (fluorescence and photoacoustic) capabilities for immediate monitoring of phototherapy efficacy. The synthetic steps and structural characterization are provided in the (Fig. S1–S5). The absorption spectra of BC-V under different viscosity conditions were measured. As depicted in Fig. 1(A), free BC-V exhibits a primary absorption peak at 675 nm. As the solution viscosity increases, the absorption peak shifts to 690 nm and shows a notable increase. Subsequently, the fluorescence response of BC-V in solutions with various viscosities was investigated (Fig. 1(B)). When BC-V is dissolved in a low-viscosity solution, such as water, it fails to exhibit a pronounced emission. However, in a high-viscosity environment, specifically a 95% glycerol solution, its fluorescence reaches a peak at 790 nm, demonstrating a substantial increase, and the Stokes shift amounts to 100 nm. Upon reaching a solution viscosity of 310.3 cP (95% glycerol), the probe's fluorescence intensity surges 145-fold. Based on the viscosity titration studies, a linear correlation exists between log(F790) and log(viscosity) within the 0.82–310.3 cP range (Fig. S6). The above results show that BC-V can be activated efficiently by viscosity, releasing an intense near-infrared fluorescence signal. In addition, the specificity, effect of pH and the photostability of BC-V were all studied (Fig. S7–S10). Thus, the desirable attributes of high selectivity and photostability render BC-V favorable for tracking viscosity variation in complex biological systems.
It is found that strong NIR absorption appears and increases after probe BC-V reacts with a high-viscosity solution, indicating that probe BC-V will exhibit a favorable PA signal. The PA images of BC-V with varying viscosities were studied, and the results indicate that the PA intensity increases gradually with increasing viscosity. As illustrated in Fig. 1(C), the PA intensity of the solution gradually increases as viscosity rises. Within the 6.47–310.3 cP range, a strong positive linear correlation exists between log(PA intensity) and log(viscosity), which indicates that probe BC-V has an outstanding response to viscosity by PA imaging.
Inspired by the excellent spectral performance above, the photodynamic and photothermal properties of probe BC-V were studied. Dichlorodihydrofluorescein (DCFH) was chosen to detect ROS and assess the ROS-generating ability of BC-V. As shown in Fig. 1(D), the fluorescence intensity of DCFH greatly enhances at 7 min in the presence of BC-V under laser irradiation. As shown in Fig. 1(E), BC-V can be gradually heated from 21.0 °C to 53.8 °C under irradiation with a 660 nm laser within 7 min. The above results indicate that probe BC-V has excellent photodynamic and photothermal properties under laser irradiation (Fig. S11–S16).
Based on the spectral findings, a potential mechanism of BC-V in relation to viscosity was proposed (Fig. 1(F)). Under low-viscosity conditions, free rotation leads to the formation of a twisting intramolecular charge transfer (TICT) state, which, in turn, results in very weak fluorescence. Conversely, in a high-viscosity environment, the formation of the TICT state is impeded. As a result, the rotation is restricted, and strong fluorescence is emitted. The theoretical validation (Fig. S17 and S18) illustrates the formation of the TICT state and the potential of BC-V to act as a photosensitizer.
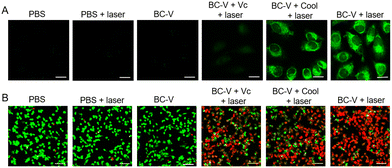
The imaging capabilities of BC-V in detecting viscosity changes within cells were explored (Fig. S19–S21). The results indicate that BC-V can detect increases or decreases in intracellular viscosity. The ability of BC-V to produce reactive oxygen species (ROS) in cells was further confirmed using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). As presented in Fig. 2(A), a minimal fluorescence signal was detected in both groups treated with PBS, regardless of laser irradiation. Moreover, in the group of cells that are treated with BC-V but not exposed to laser irradiation, no fluorescence is detected. Conversely, the cells incubated with BC-V exhibit prominent green fluorescence following laser exposure. Since ascorbic acid (Vc) can scavenge ROS to inhibit the PDT effect, a notable decrease in green fluorescence is detected following the treatment of cells with Vc.
Furthermore, when the cells were irradiated with a laser at 4 °C, eliminating the photothermal therapy (PTT) effect, a distinct green fluorescence remained visible. This demonstrates that the photodynamic therapy (PDT) effect of BC-V remains unaffected by low temperature. These findings suggest that upon laser irradiation, BC-V can generate substantial ROS in cells.
To visualize the phototherapeutic efficacy of BC-V, a double-staining approach with fluorescein calcein-AM/propidium iodide (PI) was used. By differentiating live (green) from dead (red) HeLa cells, the killing effect of the cancer cells was visually assessed. As shown in Fig. 2(B), distinct green fluorescence is observed in both the PBS group and the PBS + laser group, indicating that the laser alone does not exert a killing effect on cancer cells. Simultaneously, the cells treated with BC-V but not irradiated with a laser exhibit significant green fluorescence, further suggesting that the cytotoxic effect of BC-V on cancer cells without laser activation is also insignificant. After the cells were treated with Vc or exposed to low temperature during laser irradiation, a decline in green fluorescence and an uptick in red fluorescence were observed. Conversely, when cells are treated solely with BC-V during laser irradiation, a substantial amount of red fluorescence is detected, while green fluorescence is nearly absent. This suggests that the cells are nearly entirely killed. These findings further imply that the combined effects of photodynamic therapy (PDT) and photothermal therapy (PTT) exhibit greater phototoxicity toward cancer cells than either therapy alone. The phototoxicity of BC-V on HeLa cells was further evaluated by using the CCK-8 method (Fig. S22).
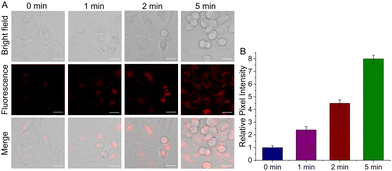
Motivated by BC-V's sensitivity to high viscosity in living cells, the potential of viscosity as a therapeutic effect indicator was assessed in cancer cells. HeLa cells were cultured in the presence of BC-V for 30 min. Subsequently, the cell death processes induced by photodynamic therapy (PDT) and photothermal therapy (PTT) were tracked in real time using fluorescence imaging at various time points during laser irradiation. As depicted in Fig. 3, at 0 min, a faint fluorescence signal is detected in the cells. As the irradiation time progresses, the fluorescence signal steadily intensifies, reaching a peak value at 5 min. Simultaneously, the cell morphology in the bright-field view shows complete shrinkage, indicating cell death. The findings further validate that the cell-death processes triggered by photodynamic therapy (PDT) and photothermal therapy (PTT) are concurrent with an increase in viscosity, endowing BC-V with the potential for the immediate assessment of its therapeutic efficacy.
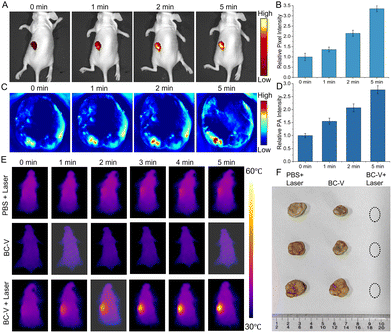
Inspired by the satisfactory therapeutic effects of PTT and PDT in cancer cells, the potential of BC-V for real-time in vivo evaluation of therapeutic effects was explored. After intratumoral injection of BC-V for 2 h, the tumor lesion was irradiated with a 660 nm laser and imaged by fluorescence at multiple time points. The fluorescence signal gradually increases with laser time (Fig. 4(A)). Compared with that at 0 min, the fluorescence at 5 min enhances about 3.4 times, which is attributed to the fact that the ROS and heat generated by PTT and PDT can induce the death of cancer cells and increase viscosity levels (Fig. 4(B)). Also, the tumor lesion was irradiated with 660 nm laser and imaged by PA at different times. The PA signal increases over time and reaches 2.8 times (Fig. 4(C) and (D)). These indicate that viscosity-based fluorescence and photoacoustic intensities are positively correlated with time, enabling BC-V to immediately evaluate PTT and PTT efficacy via dual-mode imaging.
To investigate the synergistic therapeutic efficacy (PDT and PTT) in vivo, the tumor-bearing mice were utilized and randomly divided into 3 groups with different treatments. Fig. 4(E) and Fig. S23 present the photothermal images of the mice at various time points. In contrast to the control groups (PBS + Laser group, BC-V group), the tumor temperature in the BC-V + Laser group increases steadily under continuous laser irradiation, reaching 56.4 °C, which is sufficient for tumor ablation. This proves that the probe BC-V has a highly efficient photothermal effect. Next, tumor volumes in the three groups were measured every 2 days over the 14-day treatment period. Fig. 4(F) and Fig. S24, S25 demonstrate photographs of tumors and tumor-bearing mice during 14 days of PDT and PTT treatment. The tumor volume of the BC-V + Laser group reduces, supporting the effectiveness of phototherapy. The negligible body weight changes in the three mouse groups demonstrate no toxicity and good biocompatibility of BC-V (Fig. S26). In addition, histological analysis of the tumor and main organs was performed using hematoxylin and eosin (H&E) staining (Fig. S27). All these results prove that BC-V can efficiently and safely suppress tumor growth through synergistic phototherapy.
In summary, we designed and synthesized a dual-mode (FI and PA) imaging probe, BC-V, for detecting viscosity levels. BC-V not only exhibits extended near-infrared emission (790 nm) but also shows high selectivity and stability. It is worth noting that BC-V can generate reactive oxygen species and heat under laser irradiation and exhibits a strong synergistic effect with PDT/PTT in cells. During PDT/PTT-induced cell death, cell viscosity increases, suggesting the potential of BC-V for the immediate evaluation of PDT/PTT efficacy. More importantly, efficient PDT/PTT synergistic therapy for tumors in mice has been achieved through BC-V, making it a promising smart probe for cancer treatment and the immediate evaluation of therapeutic efficacy. In a word, this work provides an effective strategy for constructing a viscosity-sensitive dual-mode probe that can be used for PDT/PTT cancer therapy and for the immediate evaluation of therapeutic efficacy.
This work was supported by the National Natural Science Foundation of China (22574141, 22174122, 22404140, 82404835), the Hunan Provincial Natural Science Foundation (2024JJ6420, 2024JJ6444), the Guangdong Provincial Natural Science Foundation (2024A1515011582, 2022A1515110682), the China Postdoctoral Science Foundation Funded Project (2024M760869), the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University (2020YB01), Natural Science Foundation of Henan (No. 252300421451) and Administration of Traditional Chinese Medicine of Hunan Province (B2024119).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental section, synthesis of probes, additional spectral data, response mechanism and supplemental biological assays. See DOI: https://doi.org/10.1039/d5cc06006k.Notes and references
- (a) F. M. White, R. A. Gatenby and C. Fischbach, Cancer Res., 2019, 79, 2107–2110 CrossRef CAS PubMed; (b) H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne and I. Soerjomataram, Ca-Cancer J. Clin., 2021, 71, 209–249 CrossRef.
- (a) S. Z. Pei, H. Y. Li, L. F. Chen, G. Nie, H. L. Wang, C. R. Liu and C. H. Zhang, Anal. Chem., 2024, 96, 5615–5624 CrossRef CAS; (b) X. Zhen, J. J. Zhang, J. G. Huang, C. Xie, Q. Q. Miao and K. Y. Pu, Angew. Chem., Int. Ed., 2018, 57, 7804–7808 CrossRef CAS; (c) H. Q. Huang, Q. L, J. H. Zhu, Y. K. Tong, D. P. Huang, D. H. Zhou, S. R. Long, L. Wang, M. L. Li, X. Q. Chen and X. J. Peng, Aggregate, 2025, 6, e706 CrossRef CAS; (d) H. y Chen, S. j Yan, L. Zhang, B. Zhao, C. Q. Zhu, G. W. Deng and J. Liu, Sens. Actuators, B, 2024, 405, 135346 CrossRef CAS; (e) L. Li, J. g Li, X. Y. Huang, F. Yang, J. X. Wang, L. J. Zhao, J. Liu and G. W. Deng, Sens. Actuators, B, 2025, 443, 138295 CrossRef CAS.
- (a) M. Garcia-Diaz, Y. Y. Huang and M. R. Hamblin, Methods, 2016, 109, 158–166 CrossRef CAS; (b) C. Yang, S. Tian, W. Y. Qiu, L. T. Mo and W. Y. Lin, Anal. Chem., 2023, 95, 16279–16288 Search PubMed; (c) Y. Chen, S. Zhang, T. J. Cheng, W. Lin, L. L. Mao, Z. H. Chen, Y. Yang, H. Q. Huang, J. Q. Li, Z. Y. Ke and Z. K. Cui, J. Mater. Sci. Technol., 2023, 141, 135–148 CrossRef CAS.
- (a) H. T. Sun, Q. Zhang, J. C. Li, S. J. Peng, X. L. Wang and R. Cai, Nano Today, 2021, 37, 101073 CrossRef CAS; (b) Z. R. Jiang, C. L. Zhang, X. Q. Wang, M. Yan, Z. X. Ling, Y. C. Chen and Z. P. Liu, Angew. Chem., Int. Ed., 2021, 60, 22376–22384 CrossRef CAS; (c) Y. Y. Gao, Y. J. Luo and K. Wei, Anal. Chem., 2024, 96, 13557–13565 CrossRef CAS PubMed.
- Q. Zan, L. Fan, W. J. Lu, Y. W. Zhang, Y. N. Huang, X. Yu, Y. H. Han, R. P. Zhang, C. Dong and S. M. Shuang, Adv. Healthcare Mater., 2025, 14, 2402614 CrossRef CAS PubMed.
- F. Bensch, S.-P. Williams, C. Mancao, A. H. Brouwers, B. M. Fine and E. G. E. de Vries, Nat. Med., 2018, 24, 1852–1858 CrossRef CAS.
- (a) L. Mignion, P. Dutta, G. V. Martinez, P. Foroutan, R. J. Gillies and B. F. Jordan, Cancer Res., 2014, 74, 686–694 CrossRef CAS; (b) K. E. Lindsay, S. M. Bhosle, C. Zurla, J. Beyersdorf, K. A. Rogers, D. Vanover, P. Xiao, M. Araínga, L. M. Shirreff, B. Pitard, P. Baumhof, F. Villinger and P. J. Santangelo, Nat. Biomed. Eng., 2019, 3, 371–380 CrossRef CAS PubMed.
- (a) B. Li, W. K. Peng, Z. H. Jin, S. S. Zhang, L. Yang, M. M. Yu and Z. X. Li, Anal. Chem., 2025, 97, 5274–5282 CrossRef CAS PubMed; (b) M. Deng, P. P. Wang, Z. B. Zhai, Y. Liu, D. Cheng, L. W. He and S. J. Li, Anal. Chem., 2025, 97, 2998–3008 CrossRef CAS; (c) D. M. Chen, R. N. Li, Q. Shao, Z. H. Wu, J. H. Cui, Q. Q. Meng and S. S. Li, J. Med. Chem., 2023, 66, 16032–16050 CrossRef CAS.
- (a) F. Li, P. Z. Dong, S. K. Sun, S. M. Zhai, B. X. Zhao and Z. M. Lin, Spectrochim. Acta, Part A, 2024, 318, 124486 CrossRef CAS; (b) H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS; (c) Y. Yue, J. Ai, W. Chi, X. Zhao, F. Huo and C. Yin, Adv. Mater., 2024, 36, 2408450 CrossRef CAS PubMed.
- (a) J. Weber, P. C. Beard and S. E. Bohndiek, Nat. Methods, 2016, 13, 639–650 CrossRef CAS; (b) Z. S. Wu, Y. T. Tang, L. L. Chen, L. T. Liu, H. Q. Huo, J. M. Ye, X. G. Ge, L. C. Su, Z. X. Chen and J. B. Song, Anal. Chem., 2022, 94, 10540–10548 CrossRef CAS PubMed.
- (a) Z. Y. Chen, Z. J. Guo, Y. Bai and W. J. He, J. Am. Chem. Soc., 2019, 141, 17973–17977 CrossRef CAS PubMed; (b) X.-P. Fan, J. Huang, T.-B. Ren, L. Yuan and X.-B. Zhang, Anal. Chem., 2023, 95, 1566–1573 CAS; (c) C. B. Zhang, Z. D. Qiu, L. L. Zhang, S. L. Wang, S. L. Zhao, Q. F. Pang and H. Liang, Anal. Chem., 2022, 94, 6251–6260 CrossRef CAS.
- (a) M. T. Sun, S. H. Wang and D. J. Huang, Talanta, 2021, 225, 121996 CrossRef CAS; (b) C. C. Bian, S. Zhang, J. Y. Fan, S. Chen, M. M. Yu and Z. X. Li, Dyes Pigm., 2023, 216, 111308 CrossRef CAS; (c) J. L. Hao, S. T. Shi, Y. L. Zhang, X. Li, X. W. Ren, J. Gao, S. J. Liu, H. J. Zhang, J. Wu and B. X. Zhang, Sens. Actuators, B, 2025, 433, 137581 CrossRef CAS.
- (a) W. X. Wang, J. J. Chao, Z. Q. Wang, T. Liu, G. J. Mao, B. Yang and C. Y. Li, Adv. Healthcare Mater., 2023, 12, 2301230 CrossRef CAS; (b) B. H. Li, H. K. Liu, Y. L. He, M. Y. Zhao, C. Ge, M. R. Younis, P. Huang, X. Y. Chen and J. Lin, Angew. Chem., Int. Ed., 2022, 61, e202200025 CrossRef CAS PubMed.
- L. Fan, Q. Zan, X. D. Wang, X. Yu, S. H. Wang, Y. W. Zhang, Q. Q. Yang, W. J. Lu, S. M. Shuang and C. Dong, Chem. Eng. J., 2022, 449, 137762 CrossRef CAS.
- (a) J. J. Chao, H. Zhang, Z. Q. Wang, Q. R. Liu, G. J. Mao, D. H. Chen and C. Y. Li, Anal. Chim. Acta, 2023, 1242, 340813 CrossRef CAS; (b) S. J. Li, Y. F. Li, H. W. Liu, D. Y. Zhou, W. L. Jiang, J. O. Yang and C. Y. Li, Anal. Chem., 2018, 90, 9418–9425 CrossRef CAS.
Footnote |
| † Ao-Xiang Fu and Li Li contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |