Metal–organic-framework-derived Fe/Sn bimetallic electrocatalyst for efficient oxygen reduction reaction in rechargeable zinc–air batteries
Benchi
Zhang
a,
Yunjiao
Yang
a,
Guangyang
Lan
a,
Wenchang
Wang
b,
Zhidong
Chen
 *b and
Changhai
Liu
*b and
Changhai
Liu
 *a
*a
aSchool of Materials Science and Engineering, CNPC-CZU Innovation Alliance, Key Laboratory of Materials Surface Science and Technology of Jiangsu Province Higher Education Institutes, Changzhou University, Changzhou, 213164, Jiangsu, China. E-mail: liuch@cczu.edu.cn
bSchool of Petrochemical Engineering, Changzhou University, Changzhou, 213164, Jiangsu, China. E-mail: zdchen@cczu.edu.cn
First published on 21st November 2025
Abstract
Herein, Fe/Sn-NC was synthesized from a derivative of ZIF-8 via thermal treatment, exhibiting optimal ORR performance and highly improved power density and cyclical stability in a zinc–air battery. This work provides new ideas for promoting the progress of clean energy technology.
The oxygen reduction reaction (ORR) is a pivotal process in energy conversion devices such as fuel cells, metal–air batteries (ZABs), as well as in electrolysis of water.1–3 The utilisation of efficient ORR catalysts is imperative for the enhancement of the performance of these technologies. Even though traditional precious metal catalysts, such as platinum, exhibit excellent catalytic performance, their high cost and scarcity limit their widespread application. Consequently, the development of novel, efficient, and economical catalysts has emerged as a pivotal subject in the domain of energy research. In recent years, diatomic catalysts (DACs) have been at the forefront of a new wave of research in the field of electrocatalysis, owing to their distinctive design of diatomic active sites and the augmentation of synergistic effects between metals.4–7 In the field of DACs, two metal atoms are supported on a carrier through the use of ligands, forming a unique catalytic centre with the capacity to provide new reaction mechanisms and active sites. This characteristic enables diatomic catalysts to exhibit superior catalytic performance in oxygen reduction reactions.
In the realm of diatomic catalysts, the Fe-based catalyst has garnered significant attention due to its noteworthy electrocatalytic activity and stability. Iron (Fe), a transition metal, exhibits a complex electronic structure and reasonable valence states.8,9 For instance, Chen et al. reported that Fe–Nb disulfides exhibited optimal oxygen reduction reaction (ORR) activity and outstanding stability, due to the optimised desorption energy of key intermediates (*OH) and suppressed Fe demetallation.10 Furthermore, Zang et al. prepared Fe–Mn bimetallic sites on hollow N-doped carbon nanospheres, which exhibited excellent performance for ORR with a half-wave potential of 0.895 V. The d–d orbital hybridization between Fe–Mn diatomic pairs caused fast electron transfer, optimised their electronic structures, and reduced the energy barriers for *OH desorption.11 As demonstrated in preceding studies, the Sn site functions as an electronic buffer, stabilising the reaction intermediate (e.g., *OOH) and weakening the O–O bond through the Sn–O bond, facilitating its breakage.12–14 The combination of iron (Fe) or tin (Sn) to form a diatomic catalyst has been shown to enhance catalytic activity. This is due to the improved electronic structure of the electrocatalysts for the oxygen reduction reaction (ORR).
In this study, the Fe/Sn-NC catalyst was assembled into ZABs, to explore the performance of the Fe/Sn-NC diatomic catalyst in ORR, and of gaining a deeper understanding of its catalytic mechanism and structure–performance relationship. When ZIF-8 (zeolitic imidazolate framework-8) is utilised as the NC support material, its elevated specific surface area and modifiable pore size not only furnish optimal space and a conducive basic structure for the uniform distribution of Fe ions and Sn atoms but also augment catalytic performance through regulation of the electronic environment. The Fe/Sn-NC DACs demonstrated a high open circuit voltage (1.47 V), high specific capacity (807 mAh g−1), high power density (162 mW cm−2), excellent rate performance, and outstanding cycling stability in ZABs. The present study offers novel insights and methodologies for synthesising highly active sites and elucidating their underlying mechanisms in the ORR process.
The synthesis process of Fe/Sn-NC is depicted in Fig. 1a. The initial step in the process is the stirring of a specific quantity of zinc nitrate and dimethylimidazole at room temperature for a period of 24 h to obtain ZIF-8. Subsequently, Fe3+ and Sn2+ ions are adsorbed onto ZIF-8 to produce the catalyst precursor. Annealing at 900 °C has been demonstrated to be an effective method for removing Zn, concurrently generating numerous pores that contribute to the formation of active sites. The XRD patterns of Fe/Sn-NC and Fe-NC exhibit two peaks at 25.4° and 44.8°, respectively, which can be ascribed to the (002) and (101) crystal faces of graphitic carbon (Fig. 1b). No signals corresponding to Fe or Sn crystals are observed, which indicates that the metal content may be low and the signal peaks are not strong. Fig. 1c and Fig. S1 illustrate the morphology of the as-prepared catalysts. The field emission (FESEM) analysis demonstrates that the subject adopts an indented dodecahedral shape, with a diameter of approximately 500 nm. As demonstrated in Fig. S1a–e, SEM images of each sample reveal that the dimensions remain relatively constant, while the dodecahedron protrudes significantly in vitro. In the absence of Sn, the protrusion diminishes. Conversely, when a small amount of Sn is incorporated, the protrusion begins to invaginate. This phenomenon occurs when the ratio of Fe to Sn is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It has been demonstrated that the degree of invagination increases in proportion to the Sn content. The hypothesis is that increasing the Sn content results in significant deformation of the material or further reaction with Sn, potentially altering the overall surface structure and active site distribution of the catalyst. ZIF-8 provides a substantial supply of nitrogen and carbon sources, offering a greater number of anchoring sites for Fe–N4 moieties. The distinctive pore structure of the material enables the uniform distribution and localised growth of metal ions, preventing the undesirable aggregation of iron atoms. This process leads to the formation of a substantial number of iron active sites within the catalyst, characterised by their high degree of dispersion.
1. It has been demonstrated that the degree of invagination increases in proportion to the Sn content. The hypothesis is that increasing the Sn content results in significant deformation of the material or further reaction with Sn, potentially altering the overall surface structure and active site distribution of the catalyst. ZIF-8 provides a substantial supply of nitrogen and carbon sources, offering a greater number of anchoring sites for Fe–N4 moieties. The distinctive pore structure of the material enables the uniform distribution and localised growth of metal ions, preventing the undesirable aggregation of iron atoms. This process leads to the formation of a substantial number of iron active sites within the catalyst, characterised by their high degree of dispersion.
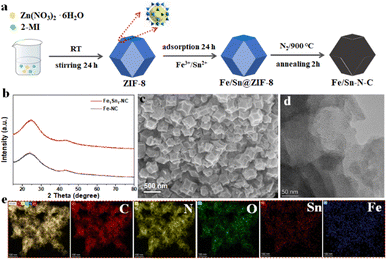 | ||
| Fig. 1 (a) Schematic diagram of the synthesis path for Fe/Sn-NC. (b) XRD patterns of Fe/Sn-NC and Fe-NC samples. (c)–(e) SEM and TEM images and elemental distribution of Fe/Sn-NC. | ||
The high-resolution transmission electron microscopy (HR-TEM) image (Fig. 1c) indicates the absence of metal particles or clusters, suggesting that Fe/Sn–N–C may possess an amorphous structure. The HR-TEM image (Fig. 1d) further corroborates that the carbonized substrate is amorphous carbon, with no crystalline iron or iron compounds detected. This finding serves to substantiate the hypothesis that the iron present within the synthesized Fe/Sn-NC catalyst exists in a monodispersed state. Elemental mapping results indicate that Fe, Sn, C, N, and O atoms are uniformly distributed within the MOF framework, as illustrated in Fig. 1e.
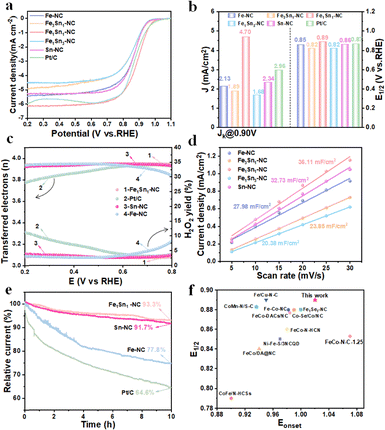
The oxygen reduction activity was evaluated under 0.1 M KOH, and the linear scan curve is shown in Fig. 2a. The half-wave potential of Fe1Sn1 NC is 0.89 V vs. RHE, which is higher than those of Fe-NC (0.85 V), Fe3Sn1-NC (0.82 V), Fe1Sn3-NC (0.82 V), Sn-NC (0.85 V) or Pt/C (0.87 V) catalysts. Furthermore, Fe/Sn-NC demonstrates the highest kinetic current density, confirming that Fe and Sn doping in equal proportions is more favourable for ORR kinetics (Fig. 2b). Concurrently, the initial potential of Fe/Sn-NC is as high as 1.02 V, reflecting the elevated intrinsic activity of the catalyst.
The maximum kinetic energy current density (Jk) of Fe/Sn-NC is calculated according to the K–L equation.21,22 At 0.90 V, the kinetic current density of Fe/Sn-NC is 4.70 mA cm−2, which is higher than that for all other catalysts in this study, indicating that Fe/Sn-NC exhibits a rapid ORR kinetic process (Fig. 2b). It is evident that the calculated results for activity arrangement are largely consistent with the performance test results. The Tafel slope in Fig. S2 for Fe/Sn-NC was found to be 81 mA dec−1, which is lower than that of commercial Pt/C (105 mA dec−1). This further confirms the bimetallic enhanced catalytic performance of Fe/Sn-NC. Furthermore, the rotating ring disk electrode (RRDE) data indicate that the Fe/Sn-NC catalyst demonstrates an electron transfer number of approximately 4 in alkaline media, which is comparable to that of the Pt/C catalyst (Fig. 2c). Within the voltage range of 0.2–0.8 V, the H2O2 yield of Fe1Sn1-NC and Sn-NC remains below 5%, which is a significant improvement on the performance of Pt/C and Fe-NC.23,24 This suggests that O2 is efficiently reduced to H2O through the 4-electron pathway. In order to further investigate the electron transfer mechanism in this process, linear sweep voltammetry (LSV) tests were conducted at different rotational speeds, and the results were used to draw corresponding Koutecký–Levi (K–L) plots (Fig. S3). The results obtained from this study are consistent in their conclusion that Fe/Sn-NC strictly adheres to the 4-electron transfer pathway in the oxygen reduction reaction process, thereby demonstrating its exceptional catalytic efficacy. The electrochemically active surface area (ECSA) is defined as the effective electrochemical surface area of a catalyst. The present study utilised cyclic voltammetry (CV) measurements at varying scan rates to obtain double-layer capacitors (Cdl) of Fe-NC, Fe3/Sn1-NC, Fe/Sn-NC, Fe1/Sn3-NC, and Sn-NC (see Fig. S3). As demonstrated in Fig. 2d, Fe/Sn-NC exhibits the maximum value (36.11 mF cm−2), indicating that Fe/Sn-NC possesses the highest ECSA among all the samples.
In summary, due to the optimal compositional advantage of Fe/Sn-NC, it shows the best ORR activity in alkaline solution, as demonstrated in Fig. S5. The stability of electrocatalysts is pivotal to ensuring efficient and continuous reaction. The present study employed accelerated durability testing (ADT) to evaluate the long-term durability of Fe/Sn-NC in alkaline solution. After 5000 cycles, a mere 10 mV of decay was observed in Fe/Sn-NC, in contrast to the 34 mV decay observed in the reference Pt/C (see Fig. S6 and S7). Moreover, the Fe/Sn-NC catalyst (93.6%) exhibited remarkable stability, as shown in Fig. 2e, during uninterrupted testing for a duration of 10 hours compared with Sn-NC (91.7%), Fe-NC (77.8%) and Pt/C (64.6%). In addition, the morphology of FeSn-NC shows no particle agglomeration during the ADT test (Fig. S8). This outcome lends further credence to the exceptional durability of the catalyst. Furthermore, the performance of this catalyst has been shown to exceed that of the majority of other bimetallic oxygen reduction catalysts (Fig. 2f and Table S1).
The XPS survey spectra confirmed the presence of all the elements (see Fig. S10), and Tables S2, S3 display the concrete content of each element within the resultant catalysts by XPS and ICP-MS analysis. The XPS spectrum of this counterpart (in Fig. S11) shows no peaks for metallic Fe0 or Sn0, indicating that the Fe and Sn elements in the system exist in an ionic state rather than in a metal nanoparticle state. The high-resolution N 1s spectrum of Fe/Sn-NC can be deconvoluted into five peaks, with centres at 403.4, 401.3, 400.4, 398.9 and 398.4 eV. These peaks are consistent with oxidised N, graphitic N, pyrrolic N, M–N and pyridinic N, respectively.15,16 The specific proportions of the different types of nitrogen in each sample are shown in Fig. 3a and Table S4. The content of oxidized N in all samples is comparable in Fig. 3b. It is noteworthy that the content of pyrrolic N increased significantly after the introduction of metal atoms in comparison with the NC sample. This phenomenon can be attributed to the reaction of metal elements with nitrogen atoms when introduced into carbon–nitrogen materials, resulting in the formation of metal–nitrogen (M–N) sites.17,18 This coordination has the potential to modify the electronic structure and chemical environment of N atoms, leading to a substantial augmentation in the content of pyrrolic nitrogen. Pyridinic N has been demonstrated to function as an anchor point for metal atoms. In comparison with Sn-NC and Fe-NC, Fe/Sn-NC is capable of forming a greater number of active sites due to the presence of two metal centres. This results in electron transfer and catalytic reaction capabilities, increasing the probability of reaction and accelerating the reaction rate. In order to demonstrate the effect of the introduction of Sn on oxygen reduction performance, the in situ Raman spectra of Fe/Sn-NC during ORR were measured (Fig. 3c and Fig. S12). The Fe/Sn-NC exhibited an additional peak at ∼1130 cm−1, corresponding to the O–O stretching vibration of superoxol species (O2−) at 0.8 VRHE.19,20 This finding confirms the adsorption of O2− by Fe/Sn sites, which will accelerate the process shown in Fig. 3d: *O + H2O(l) + e− → *OH + OH−. In summary, Raman spectral analysis proved that the diatomic catalyst formed after the introduction of Sn fine-tuned the adsorption free energy of ORR intermediates at the Fe site, enhancing its inherent activity.
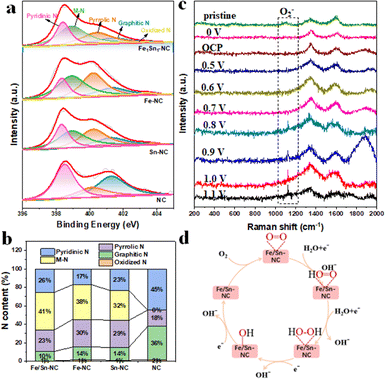 | ||
| Fig. 3 (a) N 1s spectra and (b) ratio of N species for as-prepared catalysts, (c) in situ Raman results of FeSn-NC and (d) possible mechanism. | ||
Catalyst poisoning is a primary factor in the deterioration of catalyst performance. Methanol exhibits high levels of adsorption on the active site surface of the catalyst, which has a significant impact on the electrocatalytic efficiency.25,26 Subsequently, a methanolic resistance test was conducted, and the results demonstrated that, in comparison with 20% Pt/C, the Fe/Sn-NC catalyst exhibited enhanced tolerance to methanol and possesses the potential for application in fuel cells (Fig. S13).
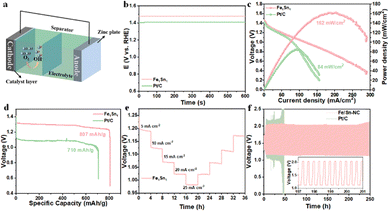
In order to validate the practical application of Fe/Sn-NC electrocatalysts in energy conversion devices, the Fe/Sn-NC catalyst was coated on carbon cloth and assembled into a zinc–air battery, as demonstrated in Fig. 4a. The open circuit voltage of the battery was found to be 1.47 V (Fig. 4b), which is marginally higher than the 1.44 V recorded for the Pt/C catalyst. The findings suggest that the Fe/Sn-NC catalyst is capable of providing high voltage without the need for an external current load, reducing energy loss and enhancing the overall efficiency of the battery. The battery assembled with the Fe/Sn-NC catalyst exhibited remarkable performance, with a power density of 162 mW cm−2 (Fig. 4c), which is nearly double that of the Pt/C catalyst. This phenomenon is ascribed to the elevated specific capacity of the Fe/Sn-NC catalyst, which confers the ability to withstand and facilitate substantial electron transfer during oxidation or reduction processes, ensuring the effective completion of ORR. This efficient energy conversion process is a primary factor contributing to the high specific capacity of ZABs. Specifically, the specific capacity of Fe/Sn-NC in Fig. 4d is 807 mAh g−1, which is significantly higher than that of Pt/C (710 mAh g−1). Furthermore, Fe/Sn-NC demonstrates favourable rate performance (Fig. 4e). In the course of constant current charging and discharging tests conducted at a current of 5 mA cm−2, the Fe/Sn-NC-based battery demonstrated a reduced charging and discharging voltage gap compared to the Pt/C-based battery. Following a 248-hour cycling period at a current density of 5 mA cm−2, the discharge/charge performance of the Fe/Sn-NC-based ZABs exhibited minimal decline, while the performance of the Pt/C-based battery rapidly declined within 50 h (Fig. 4f). This outcome lends further credence to the exceptional stability and efficiency of Fe/Sn-NC in oxygen reduction reactions.
The study revealed that the introduction of Sn can fine-tune the adsorption free energy of ORR intermediates at Fe sites, improving the intrinsic activity of the catalyst. Specifically, the Fe/Sn-NC catalyst demonstrates remarkable efficacy in ORR, exhibiting a half-wave potential of 0.89 V. Furthermore, the catalyst was applied to ZABs, achieving a high power density of 162 mW cm−2. The findings suggest that the Fe/Sn-NC catalyst exhibits not only remarkable catalytic efficacy but also considerable practical application potential.
This work was supported by the National Natural Science Foundation of China (22072093), the Electrolytic Copper Foil Engineering Technology Center of Changzhou University.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc04402b.Notes and references
- S. Li, L. Shi, Y. Guo, J. Wang, D. Liu and S. Zhao, Chem. Sci., 2024, 15, 11188–11228 RSC
.
- T. Vo, J. Gao and Y. Liu, Adv. Funct. Mater., 2024, 34, 2314282 CrossRef CAS
.
- M. Liu, J. Zhang, H. Su, Y. Jiang, W. Zhou, C. Yang, S. Bo, J. Pan and Q. Liu, Nat. Commun., 2024, 15, 1675 CrossRef CAS PubMed
.
- Y. Liu, Y. Yang, X. Lin, Y. Lin, Z. Zhuo, D. Liu, J. Mao and J. Jiang, Nat. Commun., 2025, 16, 5158 CrossRef CAS
.
- N. Xu, Y. Jin, Q. Liu, M. Yu, X. Wang, C. Wang, W. Tu, Z. Zhang, Z. Geng, S. Suenaga, F. Cheng, E. Song, Z. Peng and J. Xu, Angew. Chem., Int. Ed., 2025, 137, e202413749 CrossRef
.
- Z. Huang, M. Li, X. Yang, T. Zhang, X. Wang, W. Song, J. Zhang, H. Wang, Y. Chen, J. Ding and W. Hu, J. Am. Chem. Soc., 2024, 146, 24842–24854 CrossRef CAS
.
- B. Tang, Y. Zhou, Q. Ji, Z. Zhuang, L. Zhang, C. Wang, H. Hu, H. Wang, B. Mei, F. Song, S. Yang, W. Bert, H. Tan, D. Wang and W. Yan, Nat. Synth., 2024, 3, 878–890 CrossRef CAS
.
- W. Zhang, S. Yi, Y. Yu, H. Liu, A. Kucernak, J. Wu and S. Li, J. Mater. Chem. A, 2024, 12, 87–112 RSC
.
- X. Xu, T. Gan and J. Guan, CCS Chem., 2025, 1–18 Search PubMed
.
- R. Sui, B. Liu, C. Chen, X. Tan, C. He, D. Xin, B. Chen, Z. Xu, J. Li, W. Chen, Z. Zhuang, Z. Wang and C. Chen, J. Am. Chem. Soc., 2024, 146, 26442–26453 CrossRef CAS
.
- J. Li, F. Wang, Y. Zhang, R. Wang, S. Zhao and S. Zang, Appl. Catal., B, 2023, 338, 123090 CrossRef CAS
.
- F. Luo, A. Roy, S. Luca, A. David, Z. Andrea, T. Moulay, C. Ismail, M. Tzonka, T. Detre, S. Wagner, J. Wen, F. Dionigi, I. Ulrike, R. Jan, F. Jaouen and S. Peter, Nat. Mater., 2020, 19, 1215–1223 CrossRef CAS
.
- Z. Fan, H. Yang, D. Yang, H. Li, K. Qi, Z. Hua, X. Jia, K. Chen and B. Han, Chem. Commun., 2024, 60, 6202–6205 RSC
.
- A. Rajput, A. Pandey, A. Kundu and B. Chakraborty, Chem. Commun., 2023, 59, 4943–4946 RSC
.
- J. Pels, F. Kapteijn, J. Moulijn, Q. Zhu and K. Thomas, Carbon, 1995, 33, 1641–1653 CrossRef CAS
.
- X. Cui, S. Yang, X. Yan, J. Leng, S. Shuang, P. Ajayan and Z. Zhang, Adv. Funct. Mater., 2016, 26, 5708–5717 CrossRef CAS
.
- B. Sun, S. Zhang, H. Yang, T. Zhang, Q. Dong, W. Zhang, J. Ding, X. Liu, L. Wang, X. Han and W. Hu, Adv. Funct. Mater., 2024, 34, 2315862 CrossRef CAS
.
- C. Yan, L. Lin, G. Wang and X. Bao, Chin. J. Catal., 2019, 40, 23–37 CrossRef CAS
.
- J. Wei, D. Xia, Y. Wei, X. Zhu, J. Li and L. Gan, ACS Catal., 2022, 12, 7811–7820 CrossRef CAS
.
- Y. Li, B. Liu, Y. Chen and Z. Liu, J. Chem. Phys., 2024, 160, 244705 CrossRef CAS PubMed
.
- Q. Wang, H. Guesmi, S. Tingry, D. Cornu, Y. Holade and S. Minteer, ACS Energy Lett., 2022, 7, 952–957 CrossRef CAS
.
- R. Zhou, Y. Zheng, M. Jaroniec and S. Qiao, ACS Catal., 2016, 6, 4720–4728 CrossRef CAS
.
- M. Song, W. Liu, J. Zhang, C. Zhang, X. Huang and D. Wang, Adv. Funct. Mater., 2023, 33, 2212087 CrossRef CAS
.
- C. Zhang, W. Shen, K. Guo, M. Xiong, J. Zhang and X. Lu, J. Am. Chem. Soc., 2023, 145, 11589–11598 CrossRef CAS PubMed
.
- M. Din, M. Idrees, S. Jamil, S. Irfan, G. Nazir, M. Mudassir, M. Saleem, S. Batool, N. Cheng and R. Saidur, J. Energy Chem., 2023, 77, 499–513 CrossRef
.
- M. Ma, Y. Zhang, Y. Ji, Q. Shao, K. Yin, W. Zhu, J. Yang, F. Liao, Z. Fan, Y. Liu, Y. Li, M. Shao and Z. Kang, Appl. Catal., B, 2022, 315, 121549 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2026 |