Recent advances in transition-metal-free deconstructive functionalization of saturated N-, O-, P-, and S-heterocycles
Pengcheng
Li
*a,
Jia-Lin
Tu
 b and
Binbin
Huang
b and
Binbin
Huang
 *c
*c
aSchool of Chemical Engineering, Shandong Institute of Petroleum and Chemical Technology, Shandong Key Laboratory of Green Electricity & Hydrogen Science and Technology, Dongying 257061, China. E-mail: Lipengcheng@sdipct.edu.cn
bCollege of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430072, China
cFaculty of Arts and Sciences, Beijing Normal University, Zhuhai 519085, China. E-mail: binbinhuang@bnu.edu.cn
First published on 14th November 2025
Abstract
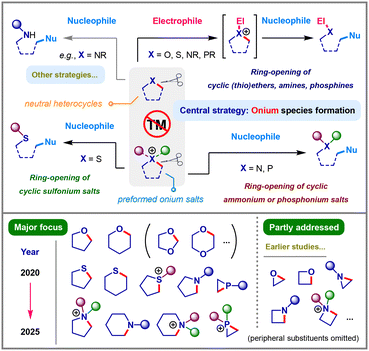
Saturated heterocycles containing oxygen, nitrogen, sulfur, and phosphorus are prevalent structural motifs in natural products, pharmaceuticals, and functional materials. Although significant progress has been made in the direct functionalization of their peripheral C–H bonds, the deconstructive modification of the core heterocyclic skeletons through carbon–heteroatom bond cleavage remains a substantial synthetic challenge, especially for the less strained five- and six-membered ring frameworks. A key strategy to overcome the inherent stability of carbon–heteroatom linkage involves activation of the heteroatom, forming reactive onium species to increase ring electrophilicity, thereby facilitating the following nucleophilic ring-opening. In alignment with the principles of sustainable chemistry, there has been a notable shift from expensive and toxic transition-metal (TM) dependent traditional methodologies toward TM-free approaches for these transformations in recent years. This feature article reviews the major advances in TM-free ring-opening reactions of saturated N-, O-, P-, and S-containing heterocycles over the past five years (2020–2025), while also acknowledging notable earlier studies that have shaped the field.
1. Introduction
Saturated heterocycles containing oxygen, nitrogen, sulfur, and phosphorus are prevalent structural motifs, forming the core scaffolds of countless natural products, pharmaceuticals, and functional materials.1 Their inherent three-dimensional structures and unique physicochemical properties render them versatile building blocks in organic synthesis and medicinal chemistry.2 While significant progress has been achieved in the peripheral functionalization of these saturated rings, primarily through C(sp3)–H bond functionalization,3 the direct modification of their core skeletal framework remains a formidable challenge.Recently, the emerging skeletal editing through transformations such as ring-expansion, ring-contraction, and atom replacement has offered a powerful platform to generate molecular architectures with tailored functions and variable properties.4 In addition to reactions that deliver new heterocyclic structures, deconstructive functionalization, which transforms a simple, readily available heterocyclic precursor into an acyclic skeleton with enhanced structural complexity and additional functional groups, also represents an attractive synthetic strategy.5 However, unlike three- and four-membered heterocycles (e.g., epoxides, aziridines, oxetanes, and azetidines) whose high ring-strain can facilitate facile carbon–heteroatom bond cleavage,6 the less-strained five- and six-membered counterparts lack this intrinsic driving force and possess significantly higher bond dissociation energies,7 rendering them generally inert, both kinetically and thermodynamically, to conventional ring-opening conditions.
To overcome this inherent stability, a central strategy has emerged that focuses on activating the heteroatom within the ring. By treating the neutral heterocycle with a suitable electrophile, a positively charged onium intermediate (such as oxonium, sulfonium, ammonium, or phosphonium) is formed.8 This quaternization dramatically alters the electronic properties of the ring, transforming the otherwise inert C–X bond into a labile linkage by rendering the α-carbons highly electrophilic and susceptible to nucleophilic attack.9 Leveraging this approach, the deconstructive functionalization of cyclic ethers, thioethers, amines, and phosphines can be efficiently achieved either through in situ formation of the corresponding onium intermediates or via preformed onium salts, followed by nucleophilic ring-opening.
While transition-metal (TM) catalysis has played a significant role in enabling such transformations,10,11 a recent paradigm shift toward TM-free approaches has gained considerable momentum. Guided by the principles of sustainable synthetic chemistry, TM-free methods offer distinct advantages, including lower costs, reduced environmental impact, and the avoidance of toxic heavy metal residues in final products.12,13 In the past few years, there has been an explosion of innovative TM-free strategies, particularly those involving Lewis/Brønsted acid activation and in situ generation of reactive species such as carbenes14 and arynes15 under thermal, photochemical, electrochemical, and mechanochemical conditions, facilitating a diverse range of deconstructive functionalization reactions of heterocyclic frameworks.
Within this context, this feature article aims to provide a systematic overview of the recent advances in TM-free deconstructive functionalization of saturated O-, S-, N-, and P- heterocycles via C-heteroatom bond cleavage, with particular emphasis on the onium-based ring-opening of five- and six-membered low-strain rings reported within the past five years (Fig. 1). By categorizing these recent developments according to the specific heteroatom type, we discuss the mechanistic insights and representative substrate scope for each protocol, aiming to highlight both the current trends and existing challenges in this rapidly evolving field, thus inspiring further innovation in the deconstructive diversification of complex molecules.
 | ||
| Fig. 1 Transition-metal-free deconstructive functionalization of various saturated heterocycles: an overview. | ||
2. Transition-metal-free deconstructive functionalization of cyclic (thio)ethers
The deconstructive functionalization of cyclic (thio)ethers has been achieved through various TM-free heteroatom activation strategies, particularly multicomponent transformations for efficient assembly of different molecular frameworks.16 Indeed, both classes of heterocycles share common in situ activation pathways, such as the formation of onium ylides with carbenes,14 leading to further nucleophilic ring-opening. However, the distinct electronic properties and reactivity of oxygen versus sulfur have also spurred the development of specialized activation modes particularly suited to one class of heterocycle. A prominent example is the use of electrophilic arynes,15 a strategy that has proven effective for the ring-opening of cyclic thioethers. Given the existence of both shared mechanisms and these highly tailored strategies, a separate discussion for each class of heterocycle will allow for a clearer and more systematic overview.2.1. Deconstructive functionalization of cyclic ethers
A central challenge in the ring-opening of cyclic ethers, such as tetrahydrofuran (THF), tetrahydropyran (THP), and 1,4-dioxane, is the inert nature of the C–O bond, which necessitates a preliminary activation step. In TM-free systems, this is typically achieved by converting the ether oxygen into an oxonium intermediate. This section provides a systematic overview of recent advancements in the transformations under both photochemical and non-photochemical conditions.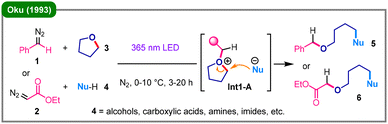 | ||
| Scheme 1 Photoinduced three-component ring-opening of tetrahydrofuran with carbenes and nucleophiles. | ||
Development of efficient methods for the precise incorporation of fluorine atoms into organic molecules has long been a significant focus in organic chemistry, as fluorinated compounds often exhibit desirable properties such as enhanced metabolic stability and solubility.19 For the ring-opening of cyclic ethers with simultaneous fluorine incorporation, previous works by Szabó's group have demonstrated the efficacy of Rh-catalysis, using acceptor-only diazo compounds via rhodium carbenoid intermediates; however, these systems were not compatible with donor–acceptor diazo substrates, which is possibly due to the insufficient reactivity of donor–acceptor carbenoids.20 In 2020, the Koenigs group reported a metal-free three-component fluoro-amino etherification under mild visible-light (470 nm) irradiation (Scheme 2).21 In contrast to conventional visible-light-driven processes that typically require either TM-based or organic photosensitizers,17 this approach utilizes direct photoexcitation of the donor–acceptor diazo substrates (7), thereby enabling the photocatalyst-free denitrogenative carbene transfer processes. The reaction between diazo alkane 7 and N-fluorobenzenesulfonimide (NFSI, 8) within a cyclic ether solvent (9) smoothly delivers product 10 featuring a fluorine atom, a ring-opened ether chain, and a bis(phenylsulfonyl)amino group. Supported by density functional theory (DFT) calculations, the proposed mechanism initiates with the photochemical formation of carbene Int2-A, which then interacts with the oxygen atom of 9 to form a key oxonium ylide intermediate (Int2-B). This ylide is subsequently trapped by the electrophilic fluorine of 8 in the rate-determining step, followed by a rapid intermolecular ring-opening by the resulting sulfonimide anion (Int2-D) to furnish the final product 10. While this method smoothly accommodates six- and seven-membered oxygen heterocycles including 1,4-dioxane, THP, and oxepane, it is not compatible with smaller rings such as THF and oxetane.
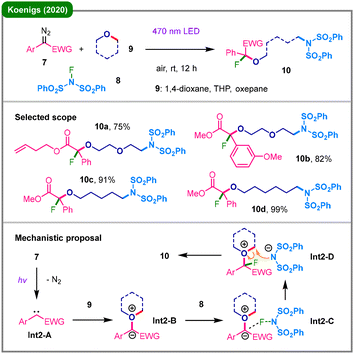 | ||
| Scheme 2 Visible-light-induced three-component fluoro-amino etherification via electrophilic fluorination of an oxonium ylide and nucleophilic ring-opening. | ||
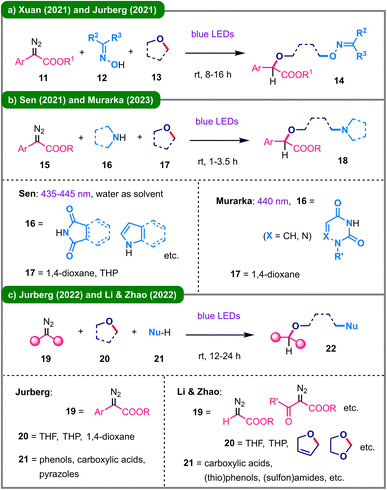
In the subsequent years, this versatile visible-light-driven strategy involving carbene-derived oxonium ylides has been extended to a broader scope of multicomponent reactions, enabling the ring-opening of saturated oxygen-containing heterocycles with the incorporation of diverse functional groups. Given the synthetic utility of oxime derivatives in organic chemistry, in 2021, the Xuan group22 and the Jurberg group23 independently disclosed two additive-free protocols for the deconstructive functionalization of cyclic ethers (13) using aryldiazo esters (11) and oximes (12) under blue light irradiation, leading to the formation of a wide array of oxime ethers (14) (Scheme 3a). This strategy was further applied to achieve N-alkylation of various N-heterocycles (16) (Scheme 3b). Also in 2021, Sen and coworkers developed a three-component reaction in aqueous medium, combining N-heterocycles such as phthalimides and indoles with aryldiazo esters and cyclic ethers including 1,4-dioxane and THP.24 In 2023, Murarka's group demonstrated a similar three-component reaction, effectively functionalizing (aza)uracil derivatives with aryldiazo esters and solvent 1,4-dioxane.25 Subsequently, other protic nucleophiles, such as carboxylic acids and phenols, were successfully incorporated into analogous reaction systems (Scheme 3c). In 2022, Jurberg and colleagues developed a blue light-promoted reaction between aryldiazo esters and different nucleophiles, including carboxylic acids, phenols and pyrazoles, in the presence of cyclic ether solvents.26 Later that year, the research group led by Li and Zhao accomplished a similar transformation, employing acceptor-only diazoacetates in conjunction with diverse N-, O-, and S-nucleophiles along with various cyclic ethers.27
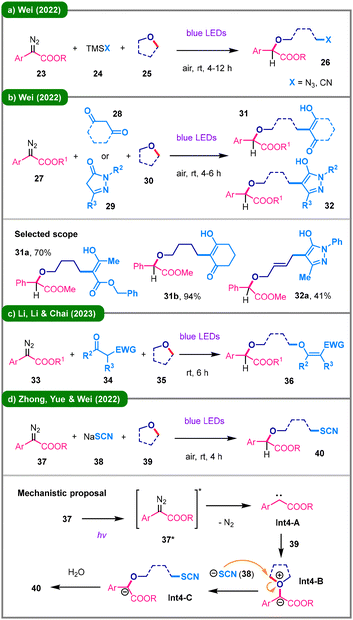
In 2022, Wei's group sequentially reported two three-component reactions employing aryldiazo esters (23 and 27) and cyclic ethers (25 and 30) to generate key oxonium ylide intermediates in situ under visible-light irradiation (Schemes 4a and b). These intermediates are subsequently trapped by N- or C-based nucleophiles, including TMSN3 and TMSCN (24),28 as well as 1,3-dicarbonyl compounds (28) and pyrazolones (29).29 In the following year, Li and colleagues also applied carbonyl compounds (34, mainly 1,3-dicarbonyl ones) as the reaction partners under similar conditions (Scheme 4c).30 In this work, these compounds served as O-nucleophiles, rather than C-centered ones, to access enol ethers (36) via ring-opening of cyclic ether solvents (33) with diazo compounds (35).
Thiocyanates are recognized as versatile intermediates in organic synthesis, serving as precursors to various sulfur-containing derivatives.31 In 2022, Zhong, Yue, Wei, and their coworkers developed a three-component reaction involving α-diazoesters (37), sodium thiocyanate (38), and cyclic ethers (39), affording valuable aliphatic thiocyanates (40) under blue LED irradiation (Scheme 4d).32 The proposed mechanism begins with the photoexcitation of 37 to its excited state (37*), followed by the formation of a carbene intermediate (Int4-A). This carbene activates the oxygen atom of 39, leading to the generation of an oxonium ylide (Int4-B), which undergoes nucleophilic attack by the thiocyanate anion and subsequent protonation to yield the target product 40.
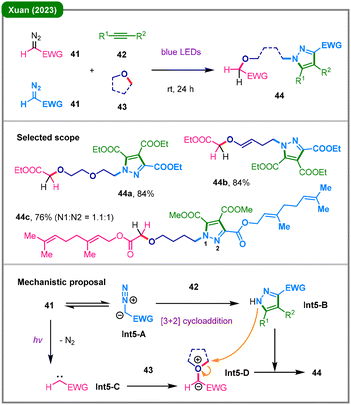
Construction of nitrogen-containing heterocycles via mild photochemical conditions has garnered sustained attention from the synthetic community.33 In a conceptually novel work, the Xuan group reported a visible-light-induced multicomponent transformation in 2023, harnessing the dual reactivity of diazoalkanes in a one-pot cascade (Scheme 5).34 This strategy leverages both the 1,3-dipolar nature and the carbene-precursor reactivity of single acceptor-only diazoalkanes (41), reacting respectively with alkyne (42) and a cyclic ether (43), efficiently constructing complex N-alkylated pyrazoles (44) in a single reaction step. The proposed mechanism involves two convergent pathways: (1) a thermal [3+2] cycloaddition between the diazoalkane (viaInt5-A as a 1,3-dipole) and alkyne 42 to in situ generate a nucleophilic pyrazole intermediate (Int5-B); (2) the photochemical activation of diazoalkane 41 to form a free carbene (Int5-C), which is subsequently trapped by the cyclic ether solvent (43) to form oxonium ylide Int5-D. In the final step, pyrazole Int5-B (exhibiting both N1 and N2 reactivity) acts as a nucleophile to intercept the ylide, triggering the ring-opening to access the final product 44.
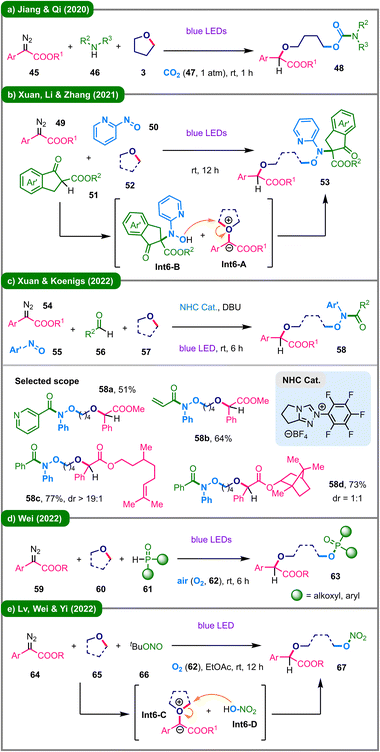
In addition to three-component reactions, several highly desirable four-component transformations involving in situ generation of nucleophiles have also been reported, enabling the rapid construction of molecular complexity. In 2020, the research group led by Jiang and Qi reported that irradiation of a mixture of an aryldiazo ester (45) and a primary or secondary aliphatic amine (46) in THF (3) under a carbon dioxide (47) atmosphere affords an organic carbamate (48) (Scheme 6a).35 The following year, Xuan and coworkers demonstrated a visible-light-promoted synthesis of trisubstituted hydroxylamines (53) from four independent components (Scheme 6b).36 Mechanistically, the reaction proceeds through a convergent pathway: under irradiation, an oxonium ylide (Int6-A) is formed from aryldiazo ester 49 and cyclic ether 52, which is subsequently trapped by a hydroxylamine nucleophile (Int6-B) generated in situ from 2-nitrosopyridine (50) and β-keto ester 51, ultimately furnishing the desired product (53). In 2022, Xuan, Koenigs, and their coworkers disclosed an innovative ring-opening strategy that integrates photoinduced carbene transfer with N-heterocyclic carbene (NHC) organocatalysis (Scheme 6c).37 In this transformation, a hydroxamic acid, generated in situ via non-photochemical NHC-catalyzed condensation of aldehyde 56 and nitrosoarene 55, acts as a nucleophile to intercept the photogenerated oxonium ylide, leading to the formation of ring-opened hydroxamic acid ester 58. The synthetic utility of this method is demonstrated by the successful derivatization of various natural products and pharmaceutical agents.
Oxygenation reactions utilizing ambient air or molecular oxygen as the oxygen source represent sustainable approaches for the synthesis of oxygen-containing compounds.38 In 2022, Wei and colleagues reported a visible-light-driven ring-opening functionalization strategy for constructing organophosphorus compounds via an aerobic four-component reaction involving α-diazoesters (59), cyclic ethers (60), and P(O)H compounds (61) (Scheme 6d).39 A broad range of phosphonates and phosphinates (63) were obtained in moderate to good yields, with incorporation of an oxygen atom derived from atmospheric oxygen (O2, 62). More recently, Lv, Wei, Yi, and coworkers further demonstrated a ring-opening nitrooxylation protocol involving the reaction of an aryldiazo ester (64) with cyclic ether (65) and t-BuONO (TBN, 66) under an oxygen atmosphere (Scheme 6e).40 The proposed mechanism involves the simultaneous formation of an oxonium ylide intermediate (Int6-C) from the diazo compound and cyclic ether, which is then intercepted by the nitrooxylating agent (Int6-D) generated in situ from TBN and O2, resulting in the formation of the nitrate ester product (67).
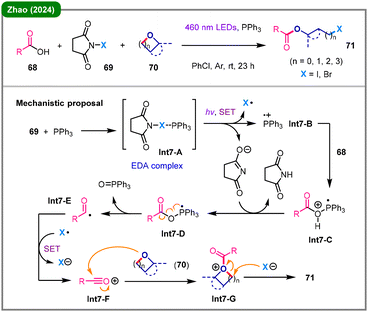
Apart from the above-discussed carbene-based activation modes, TM-free photochemical deconstruction has also been achieved through a radical-cation cascade initiated by formation of a photoactive electron donor–acceptor (EDA) complex.41 In 2024, Zhao and colleagues reported a visible-light-induced ring-opening of cyclic ethers (70) with carboxylic acids (68) to furnish valuable ω-haloalkyl carboxylates (71), employing N-halosuccinimides (NXS, X = I or Br, 69) as the halogen sources (Scheme 7).42 Mechanistically, an EDA complex (Int-7A) is formed through halogen-bonding between 69 and triphenylphosphine, which upon photoexcitation undergoes a single-electron transfer (SET) to generate a key triphenylphosphine radical cation (Int-7B). This radical cation then engages in a cascade with carboxylic acid 68via intermediates Int-7C, Int-7D and Int-7E to form a highly reactive acylium ion (Int-7F). This in situ-generated acylating agent subsequently activates the cyclic ether to form an oxonium intermediate (Int-7G), which is finally intercepted by the halide anion to afford the final product (71).
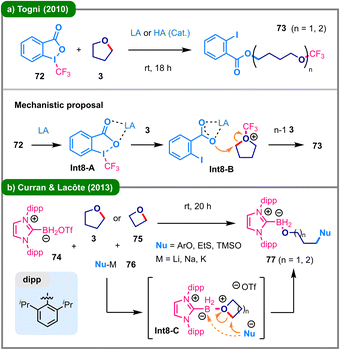 | ||
| Scheme 8 Lewis or Brønsted acid-mediated ring-opening functionalization of cyclic ethers. (a) Ring-opening with Togni's reagent and (b) ring-opening with an NHC-stabilized boryl triflate. | ||
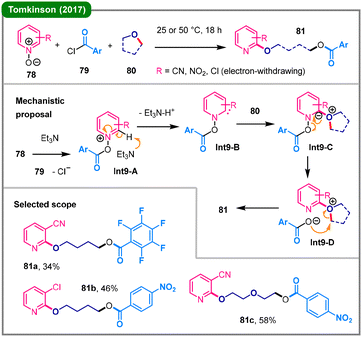
In 2017, Tomkinson and colleagues developed a three-component reaction for the regioselective functionalization of pyridines (Scheme 9).46 Under the standard conditions, various C2-alkoxylated pyridine derivatives (81) were obtained from electron-deficient pyridine N-oxides (78), aroyl chlorides (79), and cyclic ethers (80) in moderate yields. The proposed mechanism commences with the O-acylation of N-oxide 78 with 79 to form the activated intermediate Int9-A, which subsequently undergoes a crucial base-mediated deprotonation at the C2 position, generating a key pyridine-derived carbene intermediate (Int9-B). This carbene is subsequently trapped by cyclic ether 80 to yield a zwitterionic species (Int9-C), which then undergoes a sequence involving the elimination and re-addition of the carboxylate anion (Int9-D), triggering the ring-opening to furnish the final product 81. The presence of an electron-withdrawing group on the pyridine ring of 78 is essential for facilitating the initial deprotonation to form the key carbene intermediate (Int9-B), thereby controlling the unique C2 regioselectivity.
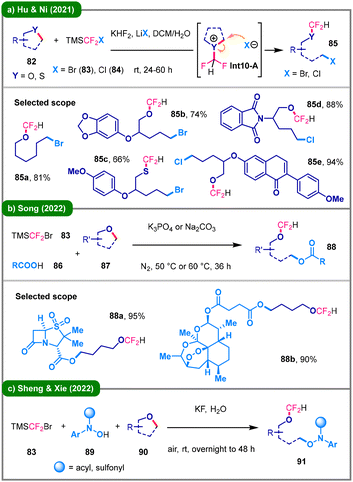
Difluorocarbene,47 a unique reactive intermediate, can be generated under thermal conditions from precursors such as TMSCF2X (X = Cl, Br) to activate cyclic (thio)ethers. The pioneering use of difluorocarbene in this context dates back to 1995, when Uneyama and colleagues reported the deconstructive O-difluoro(phenylseleno)methylation of cyclic (thio)ethers via difluoromethylene oxonium ylide intermediates.48 In 2021, the research group led by Hu and Ni developed a practical strategy for difluorocarbene-induced ring-opening halogenation of (thio)ethers (82), employed as limiting reagents (Scheme 10a).49 This approach utilizes TMSCF2Br (83) or TMSCF2Cl (84) as a dual-function reagent, serving both as a precursor to difluorocarbene and as a source of halide ions. Later in 2022, Song and colleagues described a three-component deconstructive difunctionalization of cyclic ethers (87) using TMSCF2Br (83) and carboxylic acids (86) (Scheme 10b).50 This transformation proceeds efficiently under mild conditions, affording valuable difluoromethyl ether derivatives (88) containing an ester linkage, with an exceptional substrate scope, including late-stage modifications of complex bioactive molecules. Later in the same year, Sheng, Xie, and colleagues reported a difluorocarbene-enabled ring-opening of cyclic ethers (90), employing N-arylhydroxylamines (89) as the O-nucleophiles (Scheme 10c).51
In comparison to their five- and six-membered analogues, strained epoxides and oxetanes exhibit greater susceptibility to ring-opening reactions through activation modes such as TM-free Lewis acid catalysis and borinic acid catalysis.52 A recent study on polymer synthesis by Isono, Satoh, and colleagues in 2022 demonstrated an alkali metal carboxylate-catalyzed ring-opening polymerization of various epoxides and episulfides, enabling access to functional polyethers and polythioethers.53 In 2025, the López group reported a BF3·OEt2-catalyzed nucleophilic ring-opening reaction of various epoxides (93) or oxetane 94 with azulene derivatives (92) under ambient conditions (Scheme 11).54 This intermolecular Friedel–Crafts alkylation proceeds with high regioselectivity, with nucleophilic attack preferentially occurring at the more substituted carbon of the applied epoxide or oxetane, yielding β- or γ-substituted azulenyl alcohols (95 and 96), respectively.
2.2. Deconstructive functionalization of cyclic thioethers
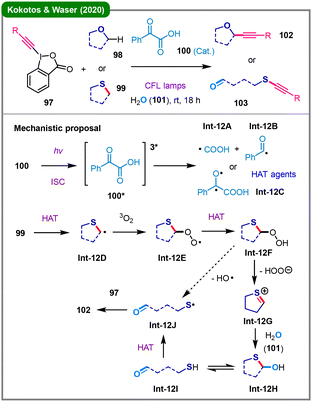
Selective cleavage of five-membered thioethers can be achieved via photosensitized oxidation with singlet oxygen, a process first demonstrated by Ando and colleagues in 1985.55 More recently, leveraging ethynylbenziodoxolones (EBX) reagents, the team led by Kokotos and Waser reported in 2020 a visible-light-mediated alkynylation of saturated O- and S-heterocycles (Scheme 12).56 After screening a broad range of photocatalysts, including Ru-/Ir-based and various aromatic ketone-based ones, the authors found that phenylglyoxylic acid (100) serves as the optimal photoinitiator. This method exhibits significant substrate-dependent reactivity: with cyclic ethers (98), C–H alkynylation occurs to afford products 102; in contrast, when cyclic thioethers (99) are used, deconstructive ring-opening takes place, leading to thioalkynyl aldehydes (103). Mechanistic investigations suggest that photoexcited phenylglyoxylic acid (100*) either undergoes fragmentation to generate active radicals (Int-12A and Int-12B) or produces Int-12C, which initiates a hydrogen atom transfer (HAT) process, forming an α-carbon radical (Int-12D) from 99. A subsequent pathway involves triplet oxygen incorporation and hydrogen atom abstraction, thereby forming intermediate Int-12F. This intermediate undergoes elimination and hydration to form hemithioacetal Int-12H, which then experiences ring-opening to give Int-12I. Finally, alkynylation by EBX reagent 97 delivers the ring-opened product 103.The utilization of in situ generated arynes,15 usually from o-silylaryl triflates (Kobayashi aryne precursors) in the presence a fluoride salt, represents a tailored strategy for the ring-opening of cyclic thioethers through sulfur atom activation. In 2018, Tan, Xu, and coworkers reported the use of o-silylaryl triflates (104) as aryne precursors to achieve deconstructive functionalization of cyclic thioethers (105) in THF at 0 °C (Scheme 13a).57 The combination of KF/18-crown-6 was found to be the optimal choice for the generation of aryne (Int13-A) from 104, while nBu4NF (TBAF) failed to enable such a process. The in situ generated sulfonium ylide, formed from Int13-A and thioether 105, acts as a strong base to deprotonate various protic C-, O-, S-, and N-centered nucleophiles (106), thereby initiating a nucleophilic ring-opening process to afford functionalized thioether products (107). Shortly thereafter, the same group extended this methodology to ring-opening fluorination by employing 2,3-dimethylindole as a proton shuttle mediator.58 In the same year, He's group described a three-component reaction of o-silylaryl triflate arynes (108), cyclic thioethers (109), and aprotic nucleophiles such as KF (110) and TMSNu (111), in the presence of water, efficiently yielding ring-opened products 112 and 113 (Scheme 13b).59 The proposed mechanism involves initial addition of the thioether to the aryne, forming a zwitterionic intermediate (Int13-C), which undergoes intramolecular proton transfer to generate a sulfonium ylide (Int13-D). This ylide is subsequently protonated by water to yield a sulfonium cation (Int13-E), which is then attacked by the nucleophile (110 or 111), resulting in ring-opening and formation of the final product (112 or 113).
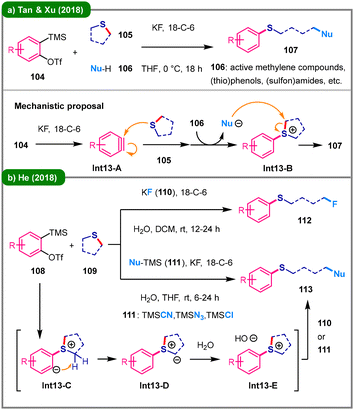 | ||
| Scheme 13 Aryne-mediated deconstructive functionalization of cyclic thioethers. (a) The work of Tan and Xu; (b) He's work. | ||
Boronic acids have emerged as one of the most versatile reagent classes in modern organic synthesis, exhibiting diverse reactivity through C–B bond cleavage.60 In 2023, Tan, Lan, and colleagues reported an innovative approach that utilizes boronic acid (116) as a “hydroxyl synthon” via B–O bond cleavage (Scheme 14).61 In this three-component transformation, an in situ generated aryne (Int14-A), derived from precursor 114, first reacts with cyclic thioether 115 to form a zwitterionic intermediate (Int14-B). Mechanistic studies, including DFT calculations, indicate that this zwitterionic intermediate does not follow traditional pathways to undergo intramolecular proton transfer. Instead, it is directly attacked by the boronate complex via transition state Int14-C, a process that concurrently facilitates B–O and C–S bond cleavage, generating an alkoxide intermediate (Int14-D) and a leaving fluoroboron byproduct. This alkoxide (Int14-D) is protonated during workup to furnish the final product (117).
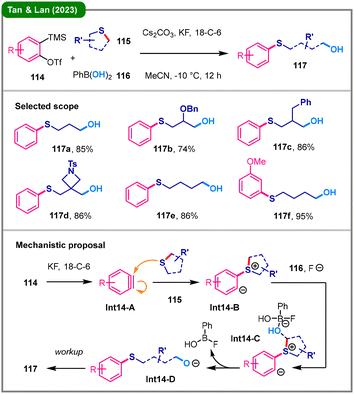 | ||
| Scheme 14 Aryne-induced three-component ring-opening reaction using phenyl boronic acid as a hydroxyl source. | ||
3. Transition-metal-free deconstructive functionalization of cyclic sulfonium salts
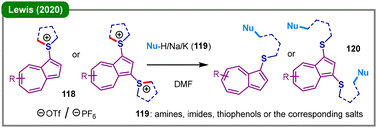
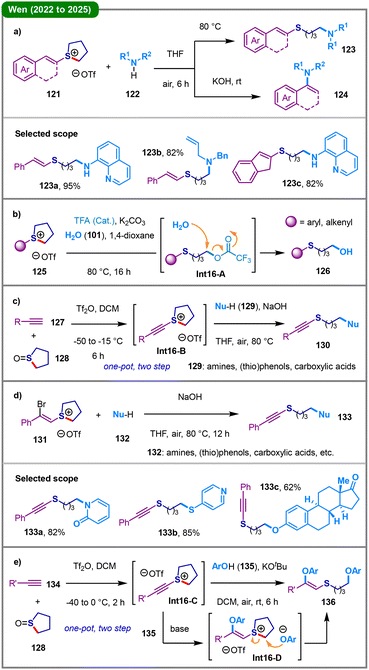
Sulfonium salts are versatile synthetic intermediates in organic synthesis.62 The deconstructive functionalization of intrinsically electrophilic cyclic sulfonium salts has garnered considerable research attention in the past few years, which is typically accomplished through direct nucleophilic ring-opening pathways, delivering linear thioethers containing an aliphatic chain tethered to a nucleophile. In 2020, Lewis and coworkers reported that nucleophilic attack by N- or S-centered nucleophiles (119) at the α-carbon positions of cycloalkyl azulenylsulfonium salts (118) affords the corresponding ring-opened azulenylsulfide products (120) (Scheme 15).63 The precursor compounds (118) can be efficiently synthesized through either single or dual interrupted Pummerer reactions of azulenes with sulfoxides or sulfides at the highly nucleophilic 1- and 3-positions.From 2022 to 2025, Wen's group sequentially established a series of ring-opening protocols of cyclic sulfonium salts (Scheme 16).64–68 In 2022, they reported a condition-controlled, divergent synthesis of two distinct classes of nitrogen-containing compounds from vinyl sulfonium salts (121) and a broad range of N-nucleophiles (122) (Scheme 16a).64 Under thermal conditions (80 °C) in the absence of a base, the reaction proceeds via a nucleophilic ring-opening of the tetrahydrothiophene moiety, involving a C(sp3)–S bond cleavage to afford linear allylic amines (123); when the reaction is conducted at room temperature in the presence of KOH, the pathway switches to a formal C(sp2)–S bond cleavage, leading exclusively to N-vinylazoles (124). Using water (101) as the hydroxyl source, a broad range of aryl and alkenyl sulfonium salts (125) were successfully transformed into the corresponding primary alcohol derivatives (126), in the presence of potassium carbonate and a catalytic amount of trifluoroacetic acid (TFA) at 80 °C (Scheme 16b).65 The plausible mechanism begins with the initial nucleophilic ring-opening of sulfonium salt 125 by the trifluoroacetate anion to form a key ester intermediate (Int16-A), which subsequently undergoes base-mediated hydrolysis to yield alcohol 126. Shortly thereafter, they further developed a base-promoted, selective synthesis of alkynyl sulfides (130) through the nucleophilic ring-opening of alkynyl sulfonium salts (Int18-B), which are typically generated in situ in a one-pot, two-step fashion from terminal alkynes 127 and cyclic sulfoxide 128 (Scheme 16c).66 Almost simultaneously, the same group also demonstrated the synthesis of alkynyl sulfides (133) from α-bromostyrene sulfonium salts (131) via base-mediated elimination of HBr (Scheme 16d).67 More recently, the team led by Wen and Lai reported an overall regio- and Z-stereoselective phenoxythiolation of alkynes (134) with phenols (135) and cyclic sulfoxide (128), allowing the facile synthesis of sulfur-containing aryl alkenylethers (136) under mild one-pot reaction conditions (Scheme 16e).68 Mechanistically, this reaction proceeds through the in situ formation of an alkynyl sulfonium salt (Int16-C), followed by KOtBu-promoted hydrophenoxylation and nucleophilic ring-opening with the phenol partner (Int16-D), ultimately leading to Z-stereoselective alkene product 136.
The preparation of alkyl azides, which are versatile synthetic intermediates for accessing various nitrogen-containing scaffolds, has garnered significant research attention.69 In 2022, Yu, Zhou, and coworkers reported a TM-free protocol for olefinic C–H azidoalkylthiolation through a novel interrupted Pummerer/nucleophilic azidoalkylation cascade (Scheme 17a).70 This strategy exploits vinylsulfonium salts, including ones derived from ketene dithioacetals (137) and styrenes (138), to undergo ring-opening reactions with sodium azide (139) in a mixed solvent of ethanol and water, furnishing a wide range of azidoalkylthiolated alkenes (140 and 141). Mechanistic investigations excluded a radical pathway, suggesting a direct nucleophilic substitution mechanism, where the azide anion attacks the carbon adjacent to the sulfonium sulfur, enabling cleavage of the C(sp3)–S bond. Given the paramount importance of C(sp3)–P bond construction in organic synthesis, catalysis, and materials science,71 in 2024, the same group demonstrated a formal olefinic C–P cross-coupling between alkenyl sulfonium salts and secondary phosphine oxides and H-phosphinates, wherein a couple of ring-opening examples were also included.72 More recently, they continued to develop a cross-coupling reaction between cyclic sulfonium-tethered [2.2]paracyclophane tetrahydro-1H-thiophen-1-ium trifluoromethanesulfonates (142) and secondary phosphine oxides or phosphonates (143) in the presence of base KOtBu, affording a series of thioalkylphosphinylated [2.2]-paracyclophane derivatives (144) through selective C(sp3)–S bond cleavage and C(sp3)–P bond formation (Scheme 17b).73 The practicability of this method was demonstrated by scale-up synthesis and further diversification of the resulting products to access potential P,S-ligands for Pd-catalyzed cross-coupling reactions.
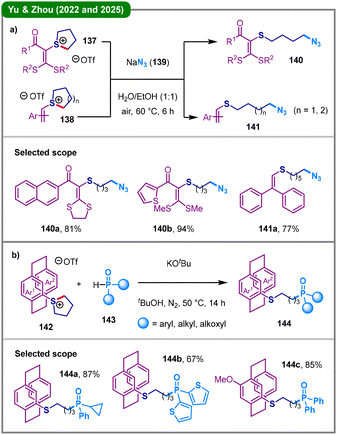 | ||
| Scheme 17 TM-free C–S bond cleavage of cyclic sulfonium salts for C(sp3)–N and C(sp3)–P bond formation. (a) Ring-opening azidation; (b) ring-opening C–P bond formation. | ||
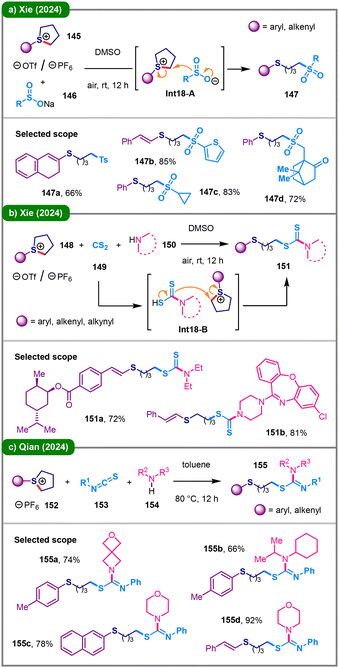
In 2024, Xie and colleagues reported a straightforward method for the ring-opening sulfonylation of cyclic sulfonium salts (145) with sodium sulfinates (146) under ambient conditions (Scheme 18a).74 The nucleophilic sulfinate anion attacks the α-carbon of sulfonium salt 145 with its sulfur center (Int18-A), initiating C(sp3)–S bond cleavage to deliver ring-opened product 147. Shortly thereafter, the same group disclosed an additive-free, three-component reaction approach for synthesizing S-alkyl dithiocarbamates (151) from readily accessible cyclic sulfonium salts (148), CS2 (149) and amines (150) (Scheme 18b).75 The reaction demonstrated a broad substrate scope under ambient conditions, including late-stage functionalization examples, highlighting its potential utility in pharmaceutical chemistry. Also in 2024, Qian and coworkers developed a three-component method for the synthesis of S-alkyl isothioureas (155) under base-free conditions (Scheme 18c).76 This protocol involves the ring-opening of cyclic sulfonium salts (152), including aryl- or alkenyl-substituted ones, upon reaction with various isothiocyanates (153) and amines (154) in toluene at an elevated temperature (80 °C).
The direct incorporation of sulfur or selenium atoms into organic frameworks using inorganic elemental sulfur or selenium has emerged as a highly favorable synthetic approach.77 In 2024, Xie, Peng, and their colleagues reported two protocols for the three-component synthesis of phosphorothioate, selenophosphate, and phosphorodithioate derivatives (159 and 162) from cyclic sulfonium salts (158) under mild, TM-free conditions (Scheme 19a).78 The first protocol involves the reaction of sulfonium salts 156, H-phosphonates (157), and elemental sulfur or selenium (158), affording the corresponding phosphorothioate or selenophosphate products (159). The second approach combines sulfonium salts 156 with phosphorus decasulfide (P4S10, 160) and alcohols (161), enabling the efficient construction of S-alkyl phosphorodithioates 162. Mechanistically, these transformations proceed via nucleophilic ring-opening of the sulfonium salt by key nucleophilic intermediates Int-19B or Int-19D, generated through deprotonation of Int-19A or Int-19C, respectively, which triggers C–S bond cleavage to yield the final products (159 or 162). Later that year, the research group led by Xiong and Zhu developed a three-component reaction of P–H compounds (164) with elemental sulfur/selenium (158) and cyclic vinylsulfonium salts (163), employing NaHCO3 as a base (Scheme 19b).79 This transformation demonstrated remarkable versatility in substrate scope, particularly with respect to organophosphorus compounds containing P–H bonds (including phosphonates and phosphine oxides) and vinylsulfonium salts.
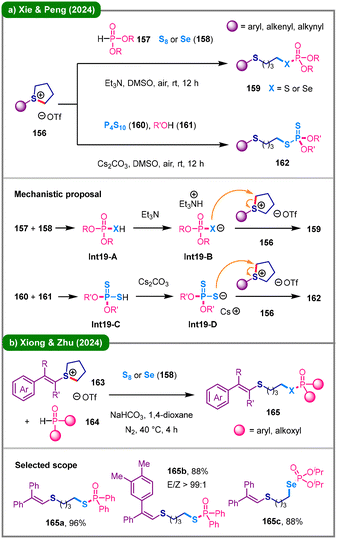 | ||
| Scheme 19 Three-component synthesis of thiophosphorus and selenophosphorus compounds via ring-opening of sulfonium salts. (a) The work of Xie and Peng; (b) the work of Xiong and Zhu. | ||
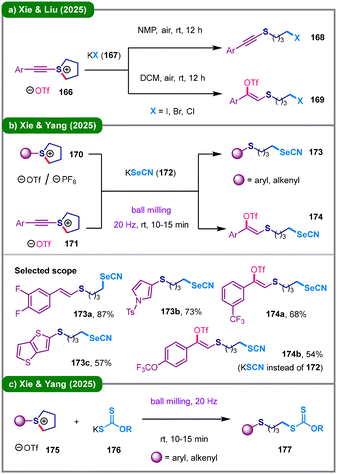
In 2025, Xie and colleagues sequentially disclosed three convenient methods for the deconstructive functionalization of sulfonium salts (Scheme 20).80–82 Xie, Liu, and coworkers introduced a KX (167, X = I, Br, Cl) promoted halogenative ring-opening reaction of alkynyl sulfonium salts (166) (Scheme 20a).80 This protocol enables the switchable synthesis of various halogenated (168) and halosulfonated (169) ring-opening products by simply applying NMP or DCM as the solvent, respectively.
Mechanochemistry has recently emerged as an innovative and sustainable alternative to traditional solvent-based organic synthesis.83 By means of this strategy, Xie, Yang, and their coworkers developed a sustainable strategy for the ring-opening selenocyanation of cyclic sulfonium salts (170 and 171) with potassium selenocyanate (172) under solvent-free conditions (Scheme 20b).81 Under ball milling conditions at 20 Hz, functionalized selenocyanates were efficiently produced via either direct nucleophilic ring-opening of the cyclic sulfonium moieties by the selenocyanate anion (173), or a sequence of intramolecular electrophilic addition of the triflate counterion to the alkyne followed by interception by −SeCN (or −SCN) to yield the final Z-selective vinyl triflate product 174. Short thereafter, the same group further presented a ball-milling-promoted ring-opening xanthylation reaction of sulfonium salts (175) using potassium ethylxanthate (176) as the nucleophile (Scheme 20c).82 This protocol allows for the efficient synthesis of diverse sulfur-containing alkyl xanthates (177) in the absence of organic solvents, demonstrating good functional group compatibility.
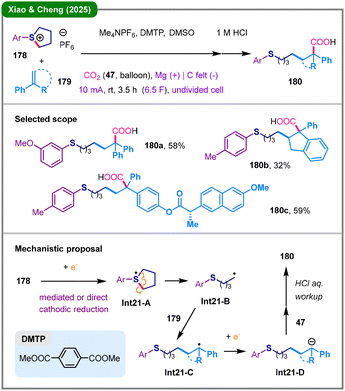
In contrast to conventional synthetic methods which often depend on hazardous chemical oxidants or reductants, organic electrosynthesis utilizes electrons as traceless redox agents to facilitate transformations, offering a more sustainable and environmentally friendly alternative.84 Very recently, the research group led by Xiao and Cheng reported electro-reductive 1,2-thiocarboxylation of aromatic alkenes (179) with cyclic sulfonium salts (178) and carbon dioxide (47), enabling the synthesis of diverse thioether acids (180) in the presence of dimethyl terephthalate (DMTP) as a mediator (Scheme 21).85 Rather than proceeding via a common nucleophilic ring-opening pathway, this transformation initiates with the electro-reductive generation of a sulfur-centered radical (Int21-A) from sulfonium salt 178. This species undergoes radical-mediated ring-opening to form a carbon-centered radical (Int21-B), which subsequently adds across alkene 179, generating benzylic radical Int21-C. This adduct is further reduced and trapped by CO2 (47), ultimately yielding thioether acid 180 upon acidic workup.
4. Transition-metal-free deconstructive functionalization of cyclic amines
The deconstructive functionalization of cyclic amines has been accomplished through a variety of strategies, such as nucleophilic ring-opening of in situ or pre-formed quaternary ammonium species and oxidative or reductive C–N bond cleavage, among others. Two concise reviews published by Ota and Yamaguchi earlier this year summarized recent advances in the deconstructive transformation of cyclic amines, encompassing both TM-catalyzed and TM-free approaches.86 In this section, a broader range of ring-opening strategies for saturated N-heterocycles have been discussed, with a specific focus on sustainable, TM-free methodologies. Among the saturated N-heterocycles of varying ring sizes, three-membered aziridines and four-membered azetidines are recognized as highly versatile synthetic building blocks due to their inherent ring strain and pronounced reactivity in diverse ring-opening functionalization reactions.6 This high reactivity has facilitated the development of numerous TM-free protocols in recent years.87,884.1. Deconstructive functionalization via formation of quaternary ammonium salts
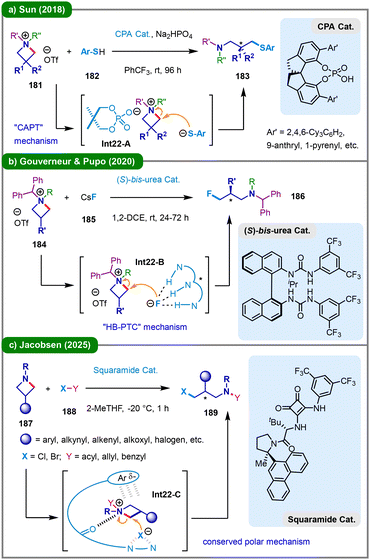
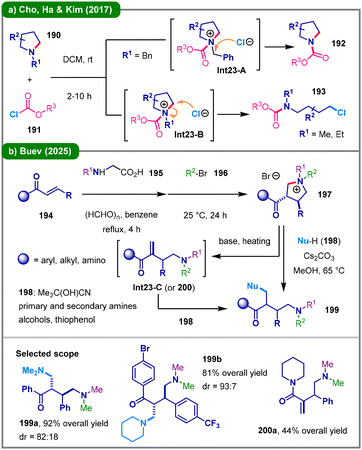
The transition-metal-free enantioselective ring-opening functionalization of azetidinium salts represents a significant advancement in asymmetric synthesis.89 In 2015, the research group led by Sun and Lin achieved the first catalytic enantioselective intermolecular desymmetrization of neutral azetidines through acid-mediated activation at elevated temperature (80 °C).90 This work established a foundational approach for subsequent developments in the field. In 2018, Sun and colleagues expanded this strategy by reporting an enantioselective desymmetrization of prochiral azetidinium salts (181) using various thiol nucleophiles (182), catalyzed by a chiral phosphoric acid (CPA) in the presence of Na2HPO4 at room temperature (Scheme 22a).91 The reaction proceeds via a chiral anion phase-transfer (CAPT) mechanism, wherein the initially insoluble azetidinium salt undergoes ion exchange with the CPA catalyst to form a soluble chiral ion pair in nonpolar solvents. Stereocontrol is achieved through a transition state in which the phosphate anion simultaneously coordinates the ammonium cation and activates the thiol nucleophile via hydrogen bonding, directing nucleophilic attack from the less hindered face opposite to the bulky substituent of the catalyst (Int22-A). In 2020, Gouverneur, Pupo, and their team developed a complementary hydrogen-bonding phase-transfer catalysis (HB-PTC) system employing CsF (185) for the asymmetric nucleophilic ring-opening fluorination of azetidinium salts (184) (Scheme 22b).92 Notably, the neutral N-alkyl-bis(urea) catalyst exhibited superior fluoride-binding affinity compared to azetidinium ions, enabling efficient and highly enantioselective ring opening to afford γ-fluoroamines (186) viaInt22-B. More recently, Tayama's group further advanced the methodology by achieving nucleophilic ring-opening of optically active and diastereomerically pure N-(1-arylethyl)azetidine-2-carboxylic acid-derived tetraalkylammonium salts with fluoride, thereby expanding substrate scope and stereochemical precision.93 In 2025, the Jacobsen group reported a highly enantioselective ring-opening of 3-substituted azetidines (187) with alkyl and acyl halides (188), facilitated by a chiral squaramide-based hydrogen-bond donor catalyst (Scheme 22c).94 This transformation demonstrates broad substrate compatibility, which can be attributed to the catalyst's ability to recognize conserved electrostatic features in the dipolar, enantioselectivity-determining transition state (Int22-C) within the SN2-type ring-opening mechanism.For the five-membered pyrrolidine ring which lacks significant ring strain, cleavage of the C–N bond presents a considerable challenge. In 2017, Cho, Ha, Kim, and colleagues reported that N-alkylpyrrolidines (190) undergo competitive reaction pathways with chloroformates (191), affording either N-dealkylated pyrrolidines (192) or ring-opened 4-chlorobutyl carbamates (193) under ambient conditions (Scheme 23a).95 Notably, pyrrolidines bearing methyl or ethyl substituents on the nitrogen atom predominantly yield ring-opening products 193 in high yields, whereas those with benzyl substituents favor dealkylation (192). In 2025, Buev and co-workers reported a three-step strategy for the α,β-difunctionalization of enones (194) via Hofmann elimination of pyrrolidinium salts (Scheme 23b).96 The process begins with a [3+2] cycloaddition between a non-stabilized azomethine ylide, which is generated in situ from α-amino acid 195, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of 194, followed by alkylation with alkyl halide 196, leading to the formation of a quaternary ammonium salt of 3-acylpyrrolidine (197). Upon heating in a polar solvent such as methanol in the presence of base Cs2CO3, this intermediate reacts with various nucleophiles (198). Specifically, the quaternary ammonium salt first undergoes Hofmann elimination to produce a reactive 2-methylene-4-aminobutan-1-one intermediate (Int23-C), which may also be isolated as product 200. This intermediate acts as a Michael acceptor and is subsequently trapped by the nucleophile, delivering the difunctionalized linear amine product (199) in high yield and with excellent diastereoselectivity.
C double bond of 194, followed by alkylation with alkyl halide 196, leading to the formation of a quaternary ammonium salt of 3-acylpyrrolidine (197). Upon heating in a polar solvent such as methanol in the presence of base Cs2CO3, this intermediate reacts with various nucleophiles (198). Specifically, the quaternary ammonium salt first undergoes Hofmann elimination to produce a reactive 2-methylene-4-aminobutan-1-one intermediate (Int23-C), which may also be isolated as product 200. This intermediate acts as a Michael acceptor and is subsequently trapped by the nucleophile, delivering the difunctionalized linear amine product (199) in high yield and with excellent diastereoselectivity.
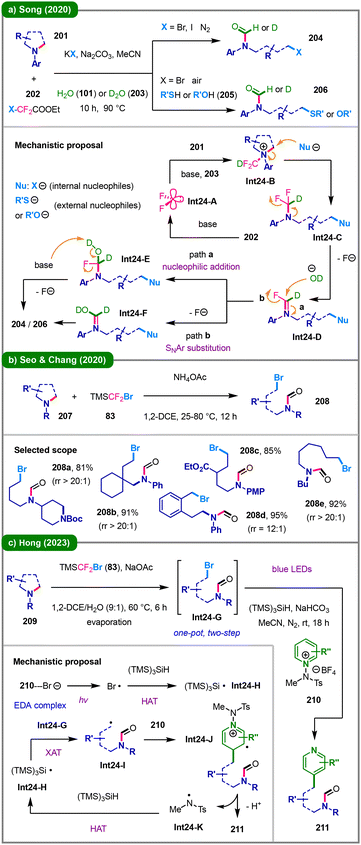
The difluorocarbene strategy has also proven effective in the activation of cyclic amines, generating ammonium intermediates to trigger nucleophilic ring-opening.47 In 2020, the Song group reported a difluorocarbene-induced C–N bond cleavage of various cyclic tertiary amines (201), utilizing halodifluoroacetates (202) as the carbene precursors (Scheme 24a).97 A wide range of functionalized N-formyl products including halides (204) and (thio)ethers (206) were successfully synthesized using H2O/D2O (101/203) as both the H/D and O sources. Mechanistically, an in situ generated difluorocarbene (Int24-A) from 202 is captured by amine 201 and D2O (203) to form a key difluoromethylammonium ylide intermediate (Int24-B). Subsequent nucleophilic attack at the N-α-carbon of Int24-B yields ring-opened intermediate Int24-C, which further undergoes fluoride elimination to afford Int24-D. Hydrolysis of Int24-Dvia either an addition–elimination pathway (path a) or SNAr-type defluorination (path b) delivers the final deuterated N-formyl product (204 or 206). Almost concurrently, Seo, Chang and coworkers also disclosed their difluorocarbene-mediated ring-opening functionalization strategy (Scheme 24b).98 In their work, saturated azacycles, including five- to eight-membered rings (207), react with TMSCF2Br (83) and NH4OAc to afford a range of acyclic N-formyl bromoalkyl amines (208), exhibiting high regioselectivity ratios (rr) when multiple reactive sites are present.
In 2023, Hong's group introduced an innovative one-pot, two-step method for the deconstructive pyridylation of unstrained saturated cyclic amines (Scheme 24c).99 This approach involves C–N bond cleavage via the difluorocarbene strategy to generate intermediates Int24-G, followed by visible-light-induced reactions with N-amidopyridinium salts 210 and (TMS)3SiH, affording various C4-pyridylated acyclic amines (211). The proposed mechanism initiates with the formation of an EDA complex between a bromide anion and the pyridinium salt (210). Light-induced SET within this complex generates a bromine radical, which abstracts a hydrogen atom from (TMS)3SiH, producing a Si-centered radical (Int24-H). This radical participates in a halogen atom transfer (XAT)100 with Int24-G, generating an alkyl radical (Int24-I) to react with amidopyridinium salt 210. The sulfonamidyl radical (Int24-K) formed concomitantly with product 211 acts as a competent HAT agent, capable of regenerating the silyl radical (Int24-H) through reaction with (TMS)3SiH, thereby sustaining the radical chain cycle.
Among various diaryliodonium salts, the ortho-functionalized derivatives constitute a significant subclass, distinguished by their unique structural features and reactivity profiles.101 In 2023, the Olofsson group reported a diarylation strategy coupled with skeletal diversification of unstrained cyclic amines through the development of a novel class of amino-substituted diaryliodonium salts (214) (Scheme 25).102 Upon thermal activation, this salt undergoes intramolecular aryl migration to form a key cyclic diarylammonium intermediate (Int25-A), which can be intercepted by a broad range of external nucleophiles (215) to yield ring-opened diarylamine products (216). Two complementary methodologies were established to achieve this transformation: Method A involves direct nucleophilic ring-opening of the triflate salt using strong nucleophiles such as amines. For less reactive nucleophiles, Method B employs a tosylate salt, in which the tosylate counterion first mediates ring-opening to generate a stable alkyl tosylate intermediate (217), followed by nucleophilic substitution via an SN2 mechanism with the nucleophile. This protocol offers a highly atom-economical route to structurally complex diarylamines from readily accessible cyclic amine precursors, while retaining a synthetically versatile iodine functionality for further derivatization.
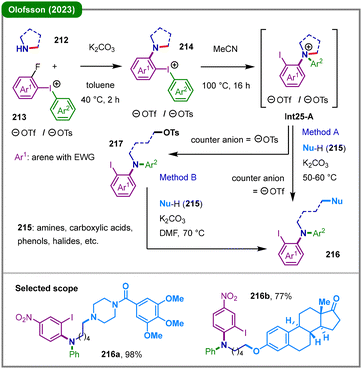 | ||
| Scheme 25 Sequential intramolecular arylation and nucleophilic ring-opening of cyclic amines for diarylamine synthesis. | ||
4.2. Deconstructive functionalization via other strategies
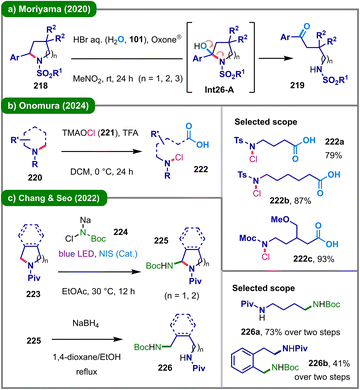
In addition to the formation of quaternary ammonium species, alternative strategies such as oxidative or reductive C–N bond cleavage and Lewis acid-based hydride shuttle catalysis have also been successfully employed to facilitate deconstructive functionalization of cyclic amines. These transformations can be driven by thermal, photochemical, or electrochemical means.The use of strong chemical oxidants or reductants under thermal conditions has proven effective for promoting C–N bond cleavage in saturated cyclic amines. For instance, employing tert-butyl nitrite as both a radical initiator and nitrogen source, the groups of Jia,103 and He and Fan104 independently demonstrated the activation and functionalization of inert C–N bonds in saturated cyclic amines, enabling oxidative ring-opening for the synthesis of structurally diverse molecules. In 2020, the Moriyama group implemented an Oxone®/HBr system to achieve oxidative C–N bond cleavage of substituted cyclic sulfonamides (218), affording N-sulfonyl-protected acyclic amino ketones (219) in high yields (Scheme 26a).105 Oxidation of bromide by Oxone® generates a bromine radical, which acts as an HAT agent to abstract the benzylic N-α-hydrogen from 218.106 Subsequent oxidation and water nucleophilic attack lead to the formation of the key α-hydroxy sulfonamide intermediate (Int26-A) for ring-opening. More recently, the Onomura group utilized tetramethylammonium hypochlorite (TMAOCl, 221) to accomplish oxidative C–N bond cleavage of cyclic amines (220) in the presence of TFA, producing a wide range of N-chloro-ω-amino acids (222) (Scheme 26b).107 In contrast, the group led by Chang and Seo developed a reductive ring-opening protocol for α-aminated cyclic amines (225) generated via photoinduced direct C(sp3)–H amination (Scheme 26c).108 This process initiates with an N-iodosuccinimide (NIS)-mediated, photoinduced α-C–H amination of cyclic amine 223 using N-chloro-N-sodio-carbamate (224). The resulting α-amino intermediate (225) undergoes reductive ring-opening with sodium borohydride to deliver the acyclic diamine product (226).
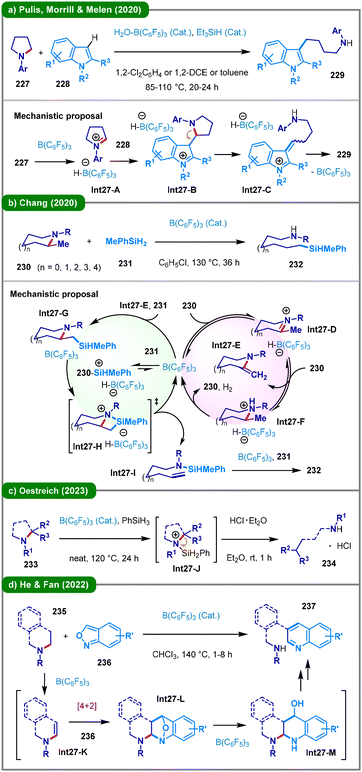
In recent years, hydride shuttle catalysis has emerged as a promising synthetic platform.109 The strategic use of a strong yet sterically hindered borane Lewis acid catalyst, B(C6F5)3,110 prevents coordination with the Lewis basic nitrogen center of cyclic amines, enabling reversible hydride abstraction from the N-α-carbon. In 2020, Pulis, Morrill, Melen, and their colleagues employed B(C6F5)3 to mediate the heterolytic cleavage of α-amino C–H bonds in amines, thereby achieving a C3 alkylation-ring-opening cascade between indoles (228) and cyclic N-aryl pyrrolidines (227) (Scheme 27a).111 According to the proposed mechanism, B(C6F5)3 initiates the reaction by abstracting a hydride from 227, forming a key iminium–borohydride ion pair intermediate (Int27-A). This electrophilic iminium ion is subsequently attacked by the nucleophilic indole (228), resulting in C–C bond formation (Int27-B). The adduct then undergoes an elimination process to generate an α,β-unsaturated iminium ion (Int27-C), which is reduced by the borohydride counterion to yield the final product (229) and regenerate the B(C6F5)3 catalyst, thus completing the catalytic cycle. Shortly after this disclosure, the Chang group introduced a B(C6F5)3-catalyzed cine-silylative ring-opening of α-methyl substituted azacycles (Scheme 27b).112 In this transformation, various α-methyl cyclic amines (230) react with hydrosilanes (231) in the presence of B(C6F5)3 to afford α,ω-aminosilanes (232). The mechanism involves borane-catalyzed dehydrogenation of the amine substrate (230), proceeding via an iminium-borohydride intermediate (Int27-D) to form an exo-enamine (Int27-E). Hydrosilylation of this enamine yields a β-silylazacycle (Int27-G), followed by C–N bond cleavage through a bicyclic silylammonium intermediate (Int27-H). A cis-β-amino elimination then generates a terminally unsaturated aminosilane (Int27-I), which undergoes final hydrosilylation to deliver the product (232). Subsequently, in 2023, the Oestreich group developed a more general method for the B(C6F5)3-catalyzed reductive ring-opening of unstrained cyclic amines (233) (Scheme 27c).113 This approach does not require an α-methyl substituent and enables direct reduction of secondary or tertiary cyclic amines using hydrosilanes, affording acyclic amine hydrochlorides (234) upon acidic workup. Furthermore, He, Fan, and coworkers disclosed a B(C6F5)3-catalyzed α,β-difunctionalization and C–N bond cleavage cascade involving saturated cyclic amines (235) and benzo[c]isoxazoles (236) (Scheme 27d).114 Mechanistically, the transformation proceeds through a sequence of hydrogen-borrowing (Int27-K), [4 + 2] cycloaddition (Int27-L), and subsequent C–N bond cleavage, ultimately yielding quinoline derivatives functionalized with aliphatic secondary amines (237).
Visible-light organophotoredox catalysis115 has been increasingly employed to enable such TM-free transformations. Earlier studies conducted by Wang and Tang in 2018116 and Fu, Du, and Huo in 2020117 leveraged organophotoredox oxidation as a powerful strategy for the deconstructive functionalization of saturated cyclic amines. In 2023, Sarpong, Musaev, Baik, and colleagues reported a bio-inspired photochemical approach for the deconstructive cleavage of C–N bonds in saturated cyclic amines (238), yielding acyclic amino aldehydes (239) (Scheme 28a).118 In this transformation, riboflavin tetraacetate (RFTA) serves as an organophotocatalyst capable of acting as an HAT agent upon photoexcitation, in conjunction with potassium persulfate as a terminal oxidant. Extensive mechanistic studies revealed a photon-controlled “H-atom-then-electron-transfer” pathway, diverging from a more conventional initial single-electron transfer hypothesis. Notably, analogous ring-opening reactions can also be achieved under thermal conditions via copper catalysis or silver-mediated processes employing persulfate, a versatile oxidant that may generate potent HAT species following homolytic cleavage of the peroxy bond.119 In the same year, the group of Yu and Ye developed a pioneering photoinduced reductive carboxylation of various cyclic amines (240) with carbon dioxide (47), enabling the synthesis of diverse α-amino acids (241) (Scheme 28b).120 The reaction proceeds through a consecutive photoinduced electron transfer (ConPET)121 mechanism: upon irradiation, the photocatalyst (PC) is excited and reduced by the sacrificial reductant DIPEA to form a radical anion (PC˙−), which absorbs a second photon to generate a highly reducing excited-state species (*PC˙−). This species transfers an electron to the substrate (240), forming a radical anion intermediate (Int28-A), which undergoes ring-opening to afford a carbon-centered radical (Int28-B). A subsequent photocatalytic cycle reduces this radical to a carbanion (Int28-C), which then reacts with CO2, yielding the final amino acid product (241) after acidic workup. Shortly after this disclosure, Yu, Gui, and coworkers also reported a visible-light-induced Barbier-type reaction of aziridines and azetidines with nonactivated aldehydes via a similar catalytic strategy.122
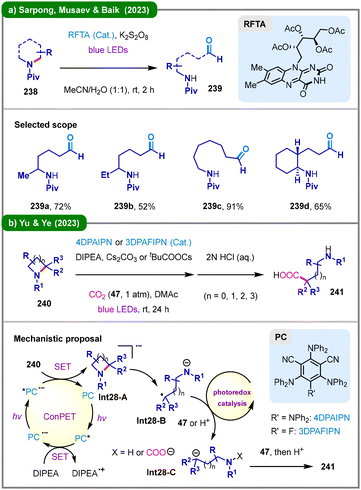 | ||
| Scheme 28 Organophotocatalytic deconstructive C–N bond cleavage of cyclic amines. (a) Oxidative ring-opening using persulfate as the oxidant; (b) reductive ring-opening with CO2 incorporation. | ||
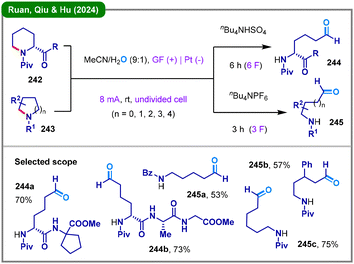
Organic electrosynthesis84 has provided sustainable approaches for the selective ring-opening of cyclic amines. In 2022, Frankowski and colleagues reported an electrochemical transformation of N-acyl pyrrolidines with isocyanides, enabling selective access to either cyclic β-amidoamine products via direct functionalization or linear hydroxybisamides through a ring-opening pathway, depending on the electronic and structural characteristics of the N-acyl activating group.123 In 2024, the research team led by Ruan, Qiu, and Hu developed a versatile electrochemical deconstructive C–N bond cleavage strategy for N-acyl cyclic amines (242 and 243), affording diverse linear amino aldehydes (244 and 245), particularly those tethered to unnatural peptides (Scheme 29).124 This electrochemical system employs a graphite felt (GF) anode and a platinum cathode, using a mixture of acetonitrile and water as the solvent under mild conditions. Notably, the methodology demonstrates significant potential for late-stage functionalization of complex molecules, as evidenced by its successful application to peptide substrates bearing multiple chiral centers and sensitive functional moieties. Mechanistic investigations combining experimental data and DFT calculations reveal that the nature of the N-acyl substituent plays a decisive role in modulating reaction activity and selectivity.
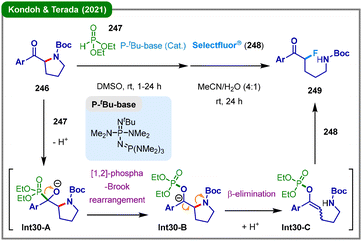
The generation of α-oxygenated carbanions through the [1,2]-phospha-Brook rearrangement125 has been employed to induce C–N cleavage of cyclic amines. In 2021, Kondoh, Terada, and their colleagues reported a formal fluorinative ring-opening reaction enabled by the [1,2]-phospha-Brook rearrangement under Brønsted base catalysis (Scheme 30).126 In this protocol, a mixture of acylpyrrolidine (246) and dialkyl phosphite (247) is treated with a catalytic amount of Brønsted base (P-tBu-base), leading to the formation of alkoxide intermediate Int30-A. This species subsequently undergoes a [1,2]-phospha-Brook rearrangement to generate carbon anion Int30-B, which then participates in β-elimination to yield the ring-opened intermediate Int30-C, concomitantly regenerating the Brønsted base catalyst or its corresponding anion. In the subsequent step, Int31-C is converted into the target α-functionalized ketone 249via reaction with the electrophilic fluorinating agent Selectfluor® (248). Furthermore, the obtained α-fluorinated ketones containing distal amino functionalities can be exploited in the synthesis of piperidine derivatives through reductive intramolecular amination.
5. Transition-metal-free deconstructive functionalization of cyclic phosphonium salts
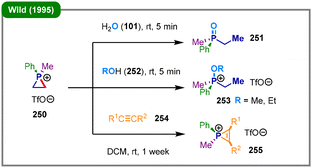
Compared to other saturated heterocycles containing nitrogen, oxygen, and sulfur atoms, phosphine-containing heterocycles have been far less extensively investigated. The deconstructive functionalization of P-heterocycles has primarily been demonstrated in three-membered phosphiranes, which are the phosphorus analogues of epoxides and aziridines. Likewise, phosphiranes possess considerable ring strain, which makes them valuable precursors for synthetic transformations.127 A key approach to unlocking their reactivity involves quaternization of the phosphorus atom to generate highly reactive phosphiranium salts.128 However, this activation strategy presents a fundamental challenge regarding regioselectivity: the resulting phosphiranium cation features two distinct electrophilic centers susceptible to nucleophilic attack—the phosphorus atom and the adjacent ring carbon atoms. Owing to the inherent phosphophilicity of many nucleophiles, particularly those based on oxygen, nucleophilic attack at the phosphorus center is often favored, leading to P–Nu bond formation rather than deconstructive functionalization via C–P bond cleavage.129The field of metal-free phosphiranium chemistry was pioneered by Wild and coworkers in a landmark 1995 study, which reported the first synthesis, structural characterization, and reactivity of a stable phosphiranium salt, 1-methyl-1-phenylphosphiranium triflate (250) (Scheme 31).130 The authors demonstrated that this strained, electrophilic heterocycle readily undergoes ring-opening upon treatment with nucleophiles such as water (101) and alcohols (252). Specifically, reaction with water yielded ethylmethylphenylphosphine oxide (251), whereas exposure to methanol or ethanol produced the corresponding alkoxyphosphonium salts (253). Furthermore, the authors highlighted the synthetic utility of 250 as a phosphenium ion equivalent, showing that it reacts with various internal alkynes (254) to afford substituted phosphirenium salts (255) via formal transfer of the [Me(Ph)P]+ moiety. The significance of this work lies not only in the isolation and characterization of the phosphiranium salt, but also in establishing its fundamental reactivity patterns.
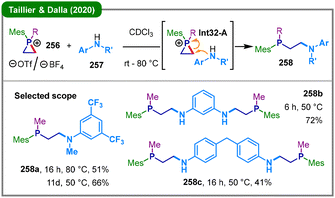
Building upon the above seminal work, where phosphiranium salts undergo ring-opening exclusively through nucleophilic attack at the phosphorus center, Taillier, Dalla, and coworkers reported in 2020 a highly selective method for the challenging carbon-centered ring-opening of these heterocycles (Scheme 32).131 Their approach involves the reaction of quaternary phosphiranium salts (256) bearing a 2,4,6-trimethylphenyl group (Mes), with various aniline nucleophiles (257), directly yielding valuable β-anilino phosphines (258). The transformation enables access to structurally complex products, including mono-adducts and bis-phosphine ligands, in synthetically useful yields. Mechanistically, the key to achieving complete regioselectivity for carbon attack lies in the synergistic effect of sterically demanding substituents on the phosphorus atom and the use of aniline nucleophiles, which exhibit a finely balanced combination of nucleophilicity, pKa, and molecular size. This strategy effectively shields the phosphorus center, thereby directing nucleophilic attack on a ring carbon atom via a proposed SN2-type mechanism through Int32-A. By “taming” the reactivity of phosphiranium salts, this work established a synthetically viable intermolecular C-centered ring-opening and provided a straightforward, reliable route to phosphinoethylamine scaffolds.
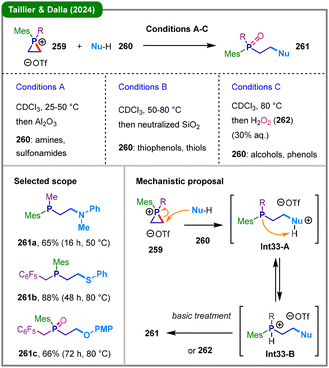
While the previous methodology successfully tamed the reactivity of phosphiranium salts, its applicability was limited to more reactive aniline-based nucleophiles and it failed to achieve productive transformations with weaker nucleophiles. In 2024, the same research group reported a significant advancement by developing a new class of highly electrophilic phosphiranium salts (EPrS, 259) through the incorporation of electron-withdrawing fluorinated benzyl quaternizing groups (Scheme 33).132 The enhancement in electrophilicity enabled the C-centered ring-opening reaction with a broader range of weak protic nucleophiles (260), thereby extending the substrate scope for the first time to include thiols, alcohols, and phenols. The method affords direct access to diverse β-functionalized phosphines or their corresponding oxides (261). Mechanistically, the reaction is proposed to proceed via initial nucleophilic attack (260) at a ring carbon of the phosphiranium salt (259), forming intermediate Int33-A. This species subsequently undergoes equilibration to yield a protiophosphonium intermediate (Int33-B), which, upon basic workup or oxidation by H2O2 (262), delivers the desired product (261).
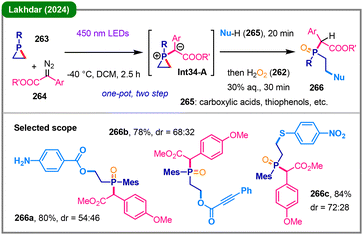
Also in 2024, Lakhdar and colleagues introduced a novel photochemical approach for the C-selective ring-opening of phosphiranes (263) using nucleophiles (265) such as carboxylic acids and thiophenols (Scheme 34).133 This one-pot, two-step protocol involves a blue light-mediated reaction of a diazo compound (264), generating a reactive carbene to interact with phosphirane 263, forming a key phosphiranium ylide intermediate (Int34-A). Subsequent addition of a nucleophile (265), followed by oxidation with H2O2 (262), leads to the formation of ring-opened β-functionalized phosphine oxide products (266). Extensive mechanistic investigations, including DFT calculations and isolation of intermediates, support a stepwise mechanism essential for C-selectivity. The initially formed ylide (Int34-A) acts as a base, deprotonating the nucleophile to generate a phosphiranium cation and the corresponding anionic nucleophile. This activated anion then selectively attacks a carbon atom within the phosphirane ring, avoiding the kinetically favored but thermodynamically disfavored pathway involving nucleophilic attack at the phosphorus center.
6. Summary and outlook
Over the past five years, the transition-metal-free deconstructive functionalization of saturated N-, O-, P-, and S-heterocycles has emerged as a rapidly growing field, providing a powerful and sustainable toolkit for efficient synthetic methodologies.Across the four major classes of heterocycles, distinct ring-opening strategies have been established. The deconstruction of cyclic (thio)ethers has primarily advanced through carbene chemistry, enabling a wide range of multicomponent transformations. Cyclic thioethers have also been effectively activated via aryne-based approaches. The nucleophilic C–S bond cleavage of cyclic sulfonium salts has matured significantly, demonstrating tunable reactivity under simple conditions and benefiting from recent mechanochemical and electrochemical innovations. Ring-opening of cyclic amines has been successfully addressed through multiple strategies, including quaternary ammonium salt formation, oxidative/reductive C–N bond cleavage, and borane-mediated hydride shuttle catalysis. In contrast, phosphirane chemistry remains comparatively underdeveloped, although recent advances in C-selective ring-opening reactions have shown considerable promise.
Despite these significant developments, several critical challenges persist. The future research is expected to focus on the following key areas: (1) stereochemical control: although elegant methods have been developed for smaller rings such as epoxides, aziridines and azetidines, achieving high enantioselectivity in the ring-opening of unstrained five- and six-membered heterocycles remains a substantial challenge, necessitating the development of robust organocatalytic systems. (2) Compatibility with complex molecules: while current protocols generally perform well with simple substrates, they often exhibit limited efficacy with densely functionalized, drug-like structures. Enhancing late-stage functionalization capabilities will be essential for practical applications in pharmaceutical synthesis. (3) Mechanistic understandings: deeper experimental investigations combining kinetic analysis and spectroscopic techniques are anticipated to elucidate the divergent reactivity patterns. (4) Exploration of emerging methodologies: compared to photochemical methods, electrochemical and mechanochemical approaches remain largely underexplored in these transformations. In addition, integration with flow chemistry and machine learning could further accelerate reaction discovery and optimization.
In conclusion, with ongoing advancements in sustainable synthetic chemistry, transition-metal-free deconstructive functionalization of saturated heterocycles is increasingly positioned to become an essential synthetic strategy. It is anticipated that continued efforts from the synthetic community will focus on these transformations to address the current challenges.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
The financial support from the Shandong Institute of Petroleum and Chemical Technology and Beijing Normal University is gratefully acknowledged.Notes and references
- (a) M. D. Delost, D. T. Smith, B. J. Anderson and J. T. Njardarson, J. Med. Chem., 2018, 61, 10996–11020 CrossRef CAS PubMed; (b) S. Pathania, R. K. Narang and R. K. Rawal, Eur. J. Med. Chem., 2019, 180, 486–508 CrossRef CAS; (c) C. M. Marshall, J. G. Federice, C. N. Bell, P. B. Cox and J. T. Njardarson, J. Med. Chem., 2024, 67, 11622–11655 CrossRef CAS PubMed.
- (a) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed; (b) T. J. Ritchie and S. J. F. Macdonald, Drug Discovery Today, 2009, 14, 1011–1020 CrossRef CAS.
- (a) Y. He, Z. Zheng, J. Yang, X. Zhang and X. Fan, Org. Chem. Front., 2021, 8, 4582–4606 RSC; (b) L. Tang, Q. Hu, K. Yang, M. Elsaid, C. Liu and H. Ge, Green Synth. Catal., 2022, 3, 203–211 Search PubMed; (c) J. Zhang and M. Rueping, Nat. Catal., 2024, 7, 963–976 CrossRef CAS; (d) J.-L. Tu and B. Huang, Chem. Commun., 2024, 60, 11450–11465 RSC; (e) Y. Ping, S. Xu and W. Kong, Acc. Chem. Res., 2025, 58, 2477–2495 CrossRef CAS.
- (a) J. Jurczyk, J. Woo, S. F. Kim, B. D. Dherange, R. Sarpong and M. D. Levin, Nat. Synth., 2022, 1, 352–364 CrossRef CAS PubMed; (b) B. W. Joynson and L. T. Ball, Helv. Chim. Acta, 2023, 106, e202200182 CrossRef CAS; (c) R. Sharma, M. Arisawa, S. Takizawa and M. S. H. Salem, Org. Chem. Front., 2025, 12, 1633–1670 RSC; (d) S. F. Kim, C. Amber, G. L. Bartholomew and R. Sarpong, Acc. Chem. Res., 2025, 58, 1786–1800 CrossRef CAS.
- (a) S. P. Morcillo, Angew. Chem., Int. Ed., 2019, 58, 14044–14054 CrossRef CAS; (b) M. Komatsuda and J. Yamaguchi, Chem. Rec., 2023, 23, e202200281 CrossRef CAS; (c) H. A. Maashi, A. H. Husayni, J. Harnedy and L. C. Morrill, Chem. Commun., 2024, 60, 11190–11201 RSC.
- (a) R. D. Bach, J. Am. Chem. Soc., 2004, 126, 4444–4452 CrossRef CAS PubMed; (b) S. H. Krake and S. C. Bergmeier, Tetrahedron, 2010, 66, 7337–7360 CrossRef CAS; (c) B. Alcaide, P. Almendros and C. Aragoncillo, Curr. Opin. Drug. Discovery Devel., 2010, 13, 685–697 CAS; (d) P.-h Chen, B. A. Billett, T. Tsukamoto and G. Dong, ACS Catal., 2017, 7, 1340–1360 CrossRef CAS PubMed; (e) J. M. Lopchuk, K. Fjelbye, Y. Kawamata, L. R. Malins, C.-M. Pan, R. Gianatassio, J. Wang, L. Prieto, J. Bradow, T. A. Brandt, M. R. Collins, J. Elleraas, J. Ewanicki, W. Farrell, O. O. Fadeyi, G. M. Gallego, J. J. Mousseau, R. Oliver, N. W. Sach, J. K. Smith, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, J. Am. Chem. Soc., 2017, 139, 3209–3226 CrossRef CAS PubMed; (f) A. Espinosa Ferao and A. R. Planells, Inorg. Chem., 2020, 59, 11503–11513 CrossRef PubMed; (g) J. Xuan, X.-K. He and W.-J. Xiao, Chem. Soc. Rev., 2020, 49, 2546–2556 RSC; (h) H. Mughal and M. Szostak, Org. Biomol. Chem., 2021, 19, 3274–3286 RSC.
- (a) E. Juaristi, Acc. Chem. Res., 1989, 22, 357–364 CrossRef CAS; (b) E. L. Eliel, Acc. Chem. Res., 1970, 3, 1–8 CrossRef CAS.
- (a) M. H. Holthausen, T. Mahdi, C. Schlepphorst, L. J. Hounjet, J. J. Weigand and D. W. Stephan, Chem. Commun., 2014, 50, 10038–10040 RSC; (b) S. Kaneko, Y. Kumatabara, S. Shimizu, K. Maruoka and S. Shirakawa, Chem. Commun., 2017, 53, 119–122 RSC.
- (a) D. Kaiser, I. Klose, R. Oost, J. Neuhaus and N. Maulide, Chem. Rev., 2019, 119, 8701–8780 CrossRef CAS PubMed; (b) H. J. Dequina and J. M. Schomaker, Trends Chem., 2020, 2, 874–887 CrossRef CAS.
- (a) K. Ouyang, W. Hao, W.-X. Zhang and Z. Xi, Chem. Rev., 2015, 115, 12045–12090 CrossRef CAS PubMed; (b) A. V. Smolobochkin, A. S. Gazizov, A. R. Burilov, M. A. Pudovik and O. G. Sinyashin, Russ. Chem. Rev., 2019, 88, 1104–1127 CrossRef CAS; (c) J. Lou, Q. Wang, P. Wu, H. Wang, Y.-G. Zhou and Z. Yu, Chem. Soc. Rev., 2020, 49, 4307–4359 RSC; (d) J.-L. Tu and B. Huang, Adv. Synth. Catal., 2024, 366, 4618–4633 CrossRef CAS; (e) Z. Jin, T. Yu, H. Wang, P. Jia and Y. Huang, Org. Chem. Front., 2024, 11, 6573–6588 RSC.
- Selected recent reports: (a) G. Wang, D. Han, Y. Li, Y. Nie, S. Muthusamy, Z. Luo and B. Liu, Org. Lett., 2023, 25, 3522–3526 CrossRef CAS PubMed; (b) X. Liu, X. Li, L. Wang, Y. Shi, J. Lv and D. Yang, Org. Chem. Front., 2024, 11, 2195–2200 RSC; (c) J. Ma, X. Li, Y. Chen, Y. Shi, X. Song, J. Lv and D. Yang, Chin. J. Chem., 2024, 42, 1637–1643 CrossRef CAS; (d) K. Aida, M. Hirao, T. Saitoh, T. Yamamoto, Y. Einaga, E. Ota and J. Yamaguchi, J. Am. Chem. Soc., 2024, 146, 30698–30707 CrossRef CAS; (e) Q. Zeng, Y. Gong, X. He, X. Zhang and Z. Lian, Org. Chem. Front., 2024, 11, 6340–6346 RSC; (f) X. Fang, X. Hu, Q.-X. Li, S.-F. Ni and Z. Ruan, Angew. Chem., Int. Ed., 2025, 64, e202418277 CrossRef CAS PubMed; (g) C. Wu, S. Wang, D. Sun, J. Chen, W. Ji, Y. Wang, W. Nam and B. Wang, J. Am. Chem. Soc., 2025, 147, 11432–11445 CrossRef CAS; (h) N. Jiang, H. Chen, Y. Liu and L.-J. Zhong, Chem. Commun., 2025, 61, 11971–11974 RSC; (i) J. Liu, X. Li, G. Chen and H. Luo, Adv. Synth. Catal., 2025, 367, e202500002 CrossRef CAS; (j) L.-J. Zhong, X.-Q. Liu, P.-F. Huang, K.-W. Tang, Q. Zhou and Y. Liu, J. Org. Chem., 2025, 90, 15880–15895 CrossRef CAS.
- (a) J. Alemán and S. Cabrera, Chem. Soc. Rev., 2013, 42, 774–793 RSC; (b) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307–9387 CrossRef CAS PubMed; (c) J. D. Lasso, D. J. Castillo-Pazos and C.-J. Li, Chem. Soc. Rev., 2021, 50, 10955–10982 RSC; (d) T. Dohi, E. E. Elboray, K. Kikushima, K. Morimoto and Y. Kita, Chem. Rev., 2025, 125, 3440–3550 CrossRef CAS PubMed.
- Selected recent reports: (a) K. Aghaie, K. Amiri, M. Rezaei-Gohar, F. Rominger, D. Dar’in, A. Sapegin and S. Balalaie, Chem. Commun., 2024, 60, 2661–2664 RSC; (b) J. Wang, G. Gao, J. Cheng, J. Li, X. Chen, X. Chen, D. Zhang, H. Li, X. Cai and B. Huang, Green Chem., 2024, 26, 5167–5172 RSC; (c) Z. Wang, L. Gan, Z. Song, Y. Liu and J.-P. Wan, Chin. J. Chem., 2024, 42, 3041–3046 CrossRef CAS; (d) N. Miyamoto, K. Kikushima, H. Sasa, T. Katagiri, N. Takenaga, Y. Kita and T. Dohi, Chem. Commun., 2025, 61, 1882–1885 RSC; (e) J. Peng, Q. Chen, X. Wu, R. Zhan, Y. Chen, M. Ji, C. Lu, J. Wang, F. Jiang and B. Huang, Chem. Commun., 2025, 61, 12357–12360 RSC; (f) J. Wang, G. Wei, J. Luo, J. Cheng, D. Zhang, X. Chen, F. Tan, T. Yang, H. Li and B. Huang, Org. Lett., 2025, 27, 10465–10470 CrossRef CAS PubMed; (g) S. Cao, H. Guo, Z. Zhong, D. Chen, W. Song, Y. Liu and J.-P. Wan, Sci. Adv., 2025, 11, eaea4120 CrossRef CAS.
- (a) D. Zhu, L. Chen, H. Fan, Q. Yao and S. Zhu, Chem. Soc. Rev., 2020, 49, 908–950 RSC; (b) Z. Yang, M. L. Stivanin, I. D. Jurberg and R. M. Koenigs, Chem. Soc. Rev., 2020, 49, 6833–6847 RSC; (c) Z. Zhang and V. Gevorgyan, Chem. Rev., 2024, 124, 7214–7261 CrossRef CAS; (d) J. Xiao, D. Jiang, X. Wu, J. Li, K. Liu, B. Huang and W. Wang, Org. Biomol. Chem., 2025, 23, 2024–2040 RSC; (e) F. Zhao, H. Ding, T. Sun, C. Shen, Z.-L. Wang, W. Wei and D. Yi, Chin. Chem. Lett., 2025 DOI:10.1016/j.cclet.2025.111834.
- (a) J. Shi, L. Li and Y. Li, Chem. Rev., 2021, 121, 3892–4044 CrossRef CAS; (b) J. Tan, X. Feng, R. Fan, Z. Zhuang and Y. Guo, Synlett, 2023, 2379–2387 CrossRef CAS; (c) N. Kim, M. Choi, S.-E. Suh and D. M. Chenoweth, Chem. Rev., 2024, 124, 11435–11522 CrossRef CAS PubMed.
- (a) S. Brauch, S. S. van Berkel and B. Westermann, Chem. Soc. Rev., 2013, 42, 4948–4962 RSC; (b) G. A. Coppola, S. Pillitteri, E. V. Van der Eycken, S.-L. You and U. K. Sharma, Chem. Soc. Rev., 2022, 51, 2313–2382 RSC.
- (a) N. Hoffmann, Chem. Rev., 2008, 108, 1052–1103 CrossRef CAS PubMed; (b) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (c) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (d) L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., Int. Ed., 2018, 57, 10034–10072 CrossRef CAS PubMed; (e) L. Buglioni, F. Raymenants, A. Slattery, S. D. A. Zondag and T. Noël, Chem. Rev., 2022, 122, 2752–2906 CrossRef CAS PubMed; (f) J.-L. Tu and B. Huang, Org. Biomol. Chem., 2024, 22, 6650–6664 RSC; (g) J.-L. Tu, Y. Zhu, P. Li and B. Huang, Org. Chem. Front., 2024, 11, 5278–5305 RSC.
- A. Oku, K. Kimura and S. Ohwaki, Acta Chem. Scand., 1993, 47, 391–397 CrossRef CAS.
- (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (b) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214–8264 CrossRef CAS PubMed; (c) J. Wang, H. Gao, C. Shi, G. Chen, X. Tan, X. Chen, L. Xu, X. Cai, B. Huang and H. Li, Chem. Commun., 2021, 57, 12203–12217 RSC.
- (a) W. Yuan and K. J. Szabó, ACS Catal., 2016, 6, 6687–6691 CrossRef CAS; (b) M. Lübcke, W. Yuan and K. J. Szabó, Org. Lett., 2017, 19, 4548–4551 CrossRef.
- F. He, C. Pei and R. M. Koenigs, Chem. Commun., 2020, 56, 599–602 RSC.
- Q. Li, B.-G. Cai, L. Li and J. Xuan, Org. Lett., 2021, 23, 6951–6955 CrossRef CAS PubMed.
- M. L. Stivanin, M. Duarte, L. P. M. O. Leão, F. A. Saito and I. D. Jurberg, J. Org. Chem., 2021, 86, 17528–17532 CrossRef CAS PubMed.
- D. Maiti, R. Das and S. Sen, Green Chem., 2021, 23, 8533–8544 RSC.
- S. K. Hotalal and S. Murarka, Chem. – Asian J., 2024, 19, e202301027 CrossRef PubMed.
- M. L. Stivanin, R. D. C. Gallo, J. P. M. Spadeto, R. A. Cormanich and I. D. Jurberg, Org. Chem. Front., 2022, 9, 1321–1326 RSC.
- K. Zhu, X. Zhou, Y. Ren, L. Dong, G. Zhao, J. Zhao and P. Li, Chem. Commun., 2023, 59, 631–634 RSC.
- Z. Wang, J. Hao, Y. Lv, C. Qu, H. Yue and W. Wei, Asian J. Org. Chem., 2022, 11, e202200484 CrossRef CAS.
- H. Ding, Z. Wang, C. Qu, Y. Lv, X. Zhao and W. Wei, Org. Chem. Front., 2022, 9, 5530–5535 RSC.
- K. Zhu, W.-J. Yu, X. Zhou, C. Xu, G. Zhao, Y. Chai, S.-J. Li, Y. Xu and P. Li, Chem. Commun., 2023, 59, 12605–12608 RSC.
- (a) T. Castanheiro, J. Suffert, M. Donnard and M. Gulea, Chem. Soc. Rev., 2016, 45, 494–505 RSC; (b) H. Chen, X. Shi, X. Liu and L. Zhao, Org. Biomol. Chem., 2022, 20, 6508–6527 RSC.
- Z. Wang, N. Meng, Y. Lv, W. Wei, H. Yue and G. Zhong, Chin. Chem. Lett., 2023, 34, 107599 CrossRef CAS.
- (a) Z.-Y. Xie and J. Xuan, Chem. Commun., 2024, 60, 2125–2136 RSC; (b) B. Huang, Green Chem., 2024, 26, 11773–11796 RSC; (c) B. Huang, Tetrahedron Chem, 2024, 12, 100116 CrossRef CAS; (d) Y. Chen and B. Huang, Org. Chem. Front., 2025, 12, 6614–6630 RSC.
- B.-G. Cai, G.-Y. Xu and J. Xuan, Chin. Chem. Lett., 2023, 34, 108335 CrossRef CAS.
- R. Cheng, C. Qi, L. Wang, W. Xiong, H. Liu and H. Jiang, Green Chem., 2020, 22, 4890–4895 RSC.
- B.-G. Cai, Q. Li, Q. Zhang, L. Li and J. Xuan, Org. Chem. Front., 2021, 8, 5982–5987 RSC.
- B.-G. Cai, Q. Li, C. Empel, L. Li, R. M. Koenigs and J. Xuan, ACS Catal., 2022, 12, 11129–11136 CrossRef CAS.
- C. Tang, X. Qiu, Z. Cheng and N. Jiao, Chem. Soc. Rev., 2021, 50, 8067–8101 RSC.
- C. Qu, J. Hao, H. Ding, Y. lv, X.-E. Zhao, X. Zhao and W. Wei, J. Org. Chem., 2022, 87, 12921–12931 CrossRef CAS PubMed.
- J. Huang, L. Song, H. Yue, Y. Lv, J. Hao, W. Wei and D. Yi, Chem. Commun., 2024, 60, 9801–9804 RSC.
- (a) G. E. M. Crisenza, D. Mazzarella and P. Melchiorre, J. Am. Chem. Soc., 2020, 142, 5461–5476 CrossRef CAS PubMed; (b) T. Tasnim, M. J. Ayodele and S. P. Pitre, J. Org. Chem., 2022, 87, 10555–10563 CrossRef CAS.
- D. K. Jha, S. Acharya, N. Sakkani, S. Chapa, A. Guerra and J. C.-G. Zhao, Org. Lett., 2024, 26, 172–177 CrossRef CAS.
- Selected recent reports: (a) J. Zhang, C. Song, L. Sheng, P. Liu and P. Sun, J. Org. Chem., 2019, 84, 2191–2199 CrossRef CAS PubMed; (b) Q. Huang, K. Dong, W. Bai, D. Yi, J.-X. Ji and W. Wei, Org. Lett., 2019, 21, 3332–3336 CrossRef CAS PubMed; (c) G. Lian, J. Li, P. Liu and P. Sun, J. Org. Chem., 2019, 84, 16346–16354 CrossRef CAS PubMed; (d) B. Liu, J. Li, Y. Hu, Q. Chen, Y. Liu, S. Ji, K. Maruoka, Y. Huo and H.-L. Zhang, J. Org. Chem., 2022, 87, 11281–11291 CrossRef CAS PubMed; (e) C. Wang, Y. Liu and J.-P. Wan, Org. Lett., 2025, 27, 3983–3987 CrossRef CAS PubMed.
- S. Fantasia, J. M. Welch and A. Togni, J. Org. Chem., 2010, 75, 1779–1782 CrossRef CAS PubMed.
- A. Solovyev, E. Lacôte and D. P. Curran, Dalton Trans., 2013, 42, 695–700 RSC.
- D. H. Jones, S. T. Kay, J. A. McLellan, A. R. Kennedy and N. C. O. Tomkinson, Org. Lett., 2017, 19, 3512–3515 CrossRef CAS PubMed.
- (a) X. Ma, J. Su and Q. Song, Acc. Chem. Res., 2023, 56, 592–607 CrossRef CAS PubMed; (b) Q. Xie and J. Hu, Acc. Chem. Res., 2024, 57, 693–713 CrossRef CAS PubMed; (c) J. Krishnan, K. Sahib, K. G. Nair, A. T. Jouhar and R. R. Paul, Chem. Rec., 2025, 25, e202400243 CrossRef CAS PubMed.
- K. Uneyama, K. Maeda, Y. Tokunaga and N. Itano, J. Org. Chem., 1995, 60, 370–375 CrossRef CAS.
- R. Zhang, Q. Li, Q. Xie, C. Ni and J. Hu, Chem. – Eur. J., 2021, 27, 17773–17779 CrossRef CAS PubMed.
- H. Sheng, J. Su, X. Li and Q. Song, CCS Chem., 2022, 4, 3820–3831 CrossRef CAS.
- X. Zhu, J. Wei, C. Hu, Q. Xiao, L. Cai, H. Wang, Y. Xie and R. Sheng, Eur. J. Org. Chem., 2022, e202200629 CrossRef CAS.
- Selected recent reports: (a) H. Yao, J. Liu and C. Wang, Org. Biomol. Chem., 2019, 17, 1901–1905 RSC; (b) G. Wang and M. S. Taylor, Adv. Synth. Catal., 2020, 362, 398–403 CrossRef CAS; (c) D. A. Strassfeld, Z. K. Wickens, E. Picazo and E. N. Jacobsen, J. Am. Chem. Soc., 2020, 142, 9175–9180 CrossRef CAS PubMed; (d) S. P. Desai and M. S. Taylor, Org. Lett., 2021, 23, 7049–7054 CrossRef CAS PubMed; (e) S. Zhang, M. Vayer, F. Noël, V. D. Vuković, A. Golushko, N. Rezajooei, C. N. Rowley, D. Lebœuf and J. Moran, Chem, 2021, 7, 3425–3441 CrossRef CAS; (f) D. A. Strassfeld, R. F. Algera, Z. K. Wickens and E. N. Jacobsen, J. Am. Chem. Soc., 2021, 143, 9585–9594 CrossRef CAS PubMed; (g) T.-Y. Gao, X.-Y. Mo, S.-R. Zhang, Y.-X. Jiang, S.-P. Luo, J.-H. Ye and D.-G. Yu, Chin. Chem. Lett., 2024, 35, 109364 CrossRef CAS; (h) A. G. Savchenko, M. O. Zubkov, V. V. Levin and A. D. Dilman, Org. Lett., 2025, 27, 10831–10835 CrossRef CAS PubMed.
- T. Gao, X. Xia, K. Tajima, T. Yamamoto, T. Isono and T. Satoh, Macromolecules, 2022, 55, 9373–9383 CrossRef CAS.
- G. Brotons, P. García-Martínez, O. Bernardo and L. A. López, Org. Lett., 2025, 27, 9412–9416 CrossRef CAS PubMed.
- T. Takata, K. Ishibashi and W. Ando, Tetrahedron Lett., 1985, 26, 4609–4612 CrossRef CAS.
- E. Voutyritsa, M. Garreau, M. G. Kokotou, I. Triandafillidi, J. Waser and C. G. Kokotos, Chem. – Eur. J., 2020, 26, 14453–14460 CrossRef CAS PubMed.
- T. Zheng, J. Tan, R. Fan, S. Su, B. Liu, C. Tan and K. Xu, Chem. Commun., 2018, 54, 1303–1306 RSC.
- R. Fan, B. Liu, T. Zheng, C. Tan, T. Zeng, K. Xu, S. Su and J. Tan, Chem. Commun., 2018, 54, 7081–7084 RSC.
- H. Jian, Q. Wang, W.-H. Wang, Z.-J. Li, C.-Z. Gu, B. Dai and L. He, Tetrahedron, 2018, 74, 2876–2883 CrossRef CAS.
- R. J. Grams, W. L. Santos, I. R. Scorei, A. Abad-García, C. A. Rosenblum, A. Bita, H. Cerecetto, C. Viñas and M. A. Soriano-Ursúa, Chem. Rev., 2024, 124, 2441–2511 CrossRef CAS PubMed.
- R. Fan, S. Liu, Q. Yan, Y. Wei, J. Wang, Y. Lan and J. Tan, Chem. Sci., 2023, 14, 4278–4287 RSC.
- (a) L. H. S. Smith, S. C. Coote, H. F. Sneddon and D. J. Procter, Angew. Chem., Int. Ed., 2010, 49, 5832–5844 CrossRef CAS PubMed; (b) S. I. Kozhushkov and M. Alcarazo, Eur. J. Inorg. Chem., 2020, 2486–2500 CrossRef CAS PubMed; (c) A. Y. B. Ángel, P. R. O. Campos and E. E. Alberto, Org. Biomol. Chem., 2023, 21, 223–236 RSC; (d) S. Timmann, Z. Feng and M. Alcarazo, Chem. – Eur. J., 2024, 30, e202402768 CrossRef CAS PubMed.
- C. M. López-Alled, F. J. O. Martin, K.-Y. Chen, G. Kociok-Köhn, T. D. James, J. Wenk and S. E. Lewis, Tetrahedron, 2020, 76, 131700 CrossRef.
- L.-H. Zou, B. Liu, C. Wang, Z. Shao, J. Zhou, A. Shao and J. Wen, Org. Chem. Front., 2022, 9, 3231–3236 RSC.
- Z. Wang, Z. Shao, C. Wang and J. Wen, J. Org. Chem., 2024, 89, 3084–3091 CrossRef CAS PubMed.
- Z. Wang, M. Su, H. Xu, J. Zhou and J. Wen, Adv. Synth. Catal., 2024, 366, 2264–2269 CrossRef CAS.
- J. Zhou, Z. Wang, H. Xu, M. Su and J. Wen, Org. Biomol. Chem., 2024, 22, 2953–2957 RSC.
- M. Su, H. Xu, Z. Lai and J. Wen, Org. Chem. Front., 2025, 12, 6088–6093 RSC.
- (a) M. Goswami and B. de Bruin, Eur. J. Org. Chem., 2017, 1152–1176 CrossRef CAS PubMed; (b) A. A. Antonov and K. P. Bryliakov, Tetrahedron Chem, 2024, 12, 100114 CrossRef CAS.
- Y. He, Z. Huang, J. Ma, J. Lin, Y.-G. Zhou and Z. Yu, Adv. Synth. Catal., 2022, 364, 3023–3034 CrossRef CAS.
- J.-L. Tu, Z. Shen and B. Huang, Tetrahedron, 2024, 168, 134352 CrossRef CAS.
- J. Ma, J. Lin, M. Li, L. Wang, K. Wu, Y.-G. Zhou and Z. Yu, Adv. Synth. Catal., 2024, 366, 3664–3669 CrossRef CAS.
- M. Li, J. Ma, L. Wang, K. Wu, Y.-G. Zhou and Z. Yu, J. Org. Chem., 2025, 90, 9593–9607 CrossRef CAS PubMed.
- L.-H. Yang, X.-S. Liu, C. Liu, S.-Y. Wang and L.-Y. Xie, J. Org. Chem., 2024, 89, 12668–12680 CrossRef CAS PubMed.
- L. Yang, J. Shu, Y. Liu, Y.-X. Jin, S.-S. Chen, W. Huang, X.-Q. Xu and L.-Y. Xie, J. Org. Chem., 2024, 89, 15248–15263 CrossRef CAS PubMed.
- C.-W. Qian, W. Xiang and M.-Q. Gu, Chem. – Eur. J., 2025, 31, e202403990 CrossRef CAS PubMed.
- (a) T. B. Nguyen, Adv. Synth. Catal., 2017, 359, 1066–1130 CrossRef CAS; (b) Y.-T. Ma, M.-C. Liu, Y.-B. Zhou and H.-Y. Wu, Adv. Synth. Catal., 2021, 363, 5386–5406 CrossRef CAS.
- L.-H. Yang, L. Chen, B. Li, Y.-X. Jin, Y. Liu, S. Peng and L.-Y. Xie, Org. Chem. Front., 2024, 11, 5731–5740 RSC.
- B. Xiong, M. Li, R. Cao, S. Yue, W. Xu, Y. Liu, L. Zhu and K.-W. Tang, J. Org. Chem., 2025, 90, 275–291 CrossRef CAS PubMed.
- L. Yang, Q.-Z. Yang, J. Shu, Y. Liu, Y.-X. Jin, Q. Liu and L.-Y. Xie, J. Org. Chem., 2025, 90, 8189–8201 CrossRef CAS PubMed.
- S. Peng, L. Tang, N.-D. Meng, H.-Y. Peng, W.-S. Yao, S.-S. Tang, L.-H. Yang and L.-Y. Xie, Tetrahedron, 2025, 185, 134816 CrossRef CAS.
- J. Shu, W. Huang, S.-S. Chen, L.-H. Yang and L.-Y. Xie, Org. Biomol. Chem., 2025, 23, 8392–8396 RSC.
- (a) N. Fantozzi, J.-N. Volle, A. Porcheddu, D. Virieux, F. García and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680–6714 RSC; (b) J. F. Reynes, F. Leon and F. García, ACS Org. Inorg. Au, 2024, 4, 432–470 CrossRef CAS PubMed; (c) P. Pattanayak, S. Saha, T. Chatterjee and B. C. Ranu, Chem. Commun., 2025, 61, 247–265 RSC.
- (a) C. Zhu, N. W. J. Ang, T. H. Meyer, Y. Qiu and L. Ackermann, ACS Cent. Sci., 2021, 7, 415–431 CrossRef CAS PubMed; (b) B. Huang, Z. Sun and G. Sun, eScience, 2022, 2, 243–277 CrossRef CAS; (c) J. Rein, S. B. Zacate, K. Mao and S. Lin, Chem. Soc. Rev., 2023, 52, 8106–8125 RSC; (d) N. Li, R. Sitdikov, A. P. Kale, J. Steverlynck, B. Li and M. Rueping, Beilstein J. Org. Chem., 2024, 20, 2500–2566 CrossRef CAS PubMed; (e) Z. Shen, J.-L. Tu and B. Huang, Org. Chem. Front., 2024, 11, 4024–4040 RSC; (f) Y. Lin, J. Yu and K.-Y. Ye, Org. Chem. Front., 2024, 11, 7243–7248 RSC.
- Y.-F. Huang, L.-J. Wang, M.-J. Luo, C. Cheng and Q. Xiao, Org. Chem. Front., 2025 10.1039/d5qo01313e.
- (a) K. Aida, E. Ota and J. Yamaguchi, Synthesis, 2025, 2278–2288 CAS; (b) E. Ota and J. Yamaguchi, Eur. J. Org. Chem., 2025, e202401322 CrossRef CAS.
- Selected recent reports: (a) P. García-Martínez, O. Bernardo, J. Borge, J. González and L. A. López, Adv. Synth. Catal., 2024, 366, 1007–1012 CrossRef; (b) B. Singh, S. Kashyap, S. Singh, S. Gupta and M. K. Ghorai, J. Org. Chem., 2024, 89, 2247–2263 CrossRef CAS PubMed; (c) B. Zheng and M. S. Taylor, J. Org. Chem., 2025, 90, 6278–6293 CrossRef CAS PubMed; (d) W. Li, X. Yang, M. Miao, F. Sun, H. Yuan, X.-B. Lan, J.-Q. Yu, J. Zhang and Z. An, Org. Chem. Front., 2025, 12, 1266–1273 RSC; (e) E. F. Hicks, L. M. F. Ruiz, B. E. Gaskins, M. K. Takase and B. M. Stoltz, J. Org. Chem., 2025, 90, 10756–10770 CrossRef CAS PubMed; (f) S. Saha, D. Ghosh, D. K. Dhaked and M. M. Guru, Adv. Synth. Catal., 2025, 367, e202500331 CrossRef CAS.
- Selected recent reports: (a) T. N. Nguyen and J. A. May, Org. Lett., 2018, 20, 3618–3621 CrossRef CAS PubMed; (b) L. Zhu, J. Xiong, J. An, N. Chen, J. Xue and X. Jiang, Org. Biomol. Chem., 2019, 17, 3797–3804 RSC; (c) H. Li, Z. Lai, A. Adijiang, H. Zhao and J. An, Molecules, 2019, 24, 459 CrossRef; (d) E. Tayama and K. Kawaib, RSC Adv., 2021, 11, 39607–39618 RSC; (e) E. Tayama, Y. Kurosaki, M. Iida, T. Aida and N. Nakanome, Tetrahedron, 2023, 142, 133528 CrossRef CAS; (f) Z. Li, Y. Wang, D. Liu, L. Ning, M. Pu, L. Lin and X. Feng, Org. Lett., 2023, 25, 7612–7616 CrossRef CAS; (g) H. Maag, D. J. Lemcke and J. M. Wahl, Beilstein J. Org. Chem., 2024, 20, 1671–1676 CrossRef CAS PubMed.
- G. Masson, D. G. Pardo and J. Cossy, Chirality, 2021, 33, 5–21 CrossRef CAS PubMed.
- Z. Wang, F. K. Sheong, H. H. Y. Sung, I. D. Williams, Z. Lin and J. Sun, J. Am. Chem. Soc., 2015, 137, 5895–5898 CrossRef CAS.
- D. Qian, M. Chen, A. C. Bissember and J. Sun, Angew. Chem., Int. Ed., 2018, 57, 3763–3766 CrossRef CAS.
- G. Roagna, D. M. H. Ascough, F. Ibba, A. C. Vicini, A. Fontana, K. E. Christensen, A. Peschiulli, D. Oehlrich, A. Misale, A. A. Trabanco, R. S. Paton, G. Pupo and V. Gouverneur, J. Am. Chem. Soc., 2020, 142, 14045–14051 CrossRef CAS.
- E. Tayama, R. Tsutsumi and D. Uraguchi, Tetrahedron, 2024, 167, 134274 CrossRef CAS.
- J. J. Gair, M. Isomura, C. C. Wagen, D. A. Strassfeld and E. N. Jacobsen, J. Am. Chem. Soc., 2025, 147, 6378–6383 CrossRef CAS PubMed.
- C. Yu, M. A. Shoaib, N. Iqbal, J. S. Kim, H.-J. Ha and E. J. Cho, J. Org. Chem., 2017, 82, 6615–6620 CrossRef CAS PubMed.
- E. M. Buev, V. S. Moshkin and V. Y. Sosnovskikh, Org. Chem. Front., 2025, 12, 1927–1935 RSC.
- J. Su, X. Ma, Z. Ou and Q. Song, ACS Cent. Sci., 2020, 6, 1819–1826 CrossRef CAS PubMed.
- Y. Kim, J. Heo, D. Kim, S. Chang and S. Seo, Nat. Commun., 2020, 11, 4761 CrossRef CAS PubMed.
- S. Hong, B. Kweon, W. Lee, S. Chang and S. Hong, Org. Lett., 2023, 25, 2722–2727 CrossRef CAS.
- F. Juliá, T. Constantin and D. Leonori, Chem. Rev., 2022, 122, 2292–2352 CrossRef PubMed.
- L. Chu, L. Wang and J. Han, Adv. Synth. Catal., 2025 DOI:10.1002/adsc.70135.
- E. Linde and B. Olofsson, Angew. Chem., Int. Ed., 2023, 62, e202310921 CrossRef CAS PubMed.
- (a) K. He, P. Li, S. Zhang, Q. Chen, H. Ji, Y. Yuan and X. Jia, Chem. Commun., 2018, 54, 13232–13235 RSC; (b) Z. Sun, H. Ji, Y. Shao, K. He, Y. Zhang, Y. Yuan and X. Jia, J. Org. Chem., 2019, 84, 12292–12300 CrossRef CAS PubMed.
- (a) Y. He, Z. Zheng, Y. Liu, J. Qiao, X. Zhang and X. Fan, Org. Lett., 2019, 21, 1676–1680 CrossRef CAS; (b) Y. He, Z. Zheng, Y. Liu, J. Qiao, X. Zhang and X. Fan, Chem. Commun., 2019, 55, 12372–12375 RSC.
- Y. Nishiguchi, A. Tomizuka and K. Moriyama, Adv. Synth. Catal., 2020, 362, 5518–5523 CrossRef CAS.
- B. Saxena, R. I. Patela and A. Sharma, RSC Sustainability, 2024, 2, 2169–2189 RSC.
- Y. Kaieda, K. Yamamoto, H. Toguchi, N. Hanazawa, M. Kuriyama and O. Onomura, Synthesis, 2024, 1576–1584 CAS.
- W. Lee, D. Kim, S. Seo and S. Chang, Angew. Chem., Int. Ed., 2022, 61, e202202971 CrossRef CAS PubMed.
- (a) J. P. Gillions, S. A. Elsherbeni, L. Winfrey, L. Yun, R. L. Melen, L. C. Morrill and A. P. Pulis, Synlett, 2023, 2117–2128 CAS; (b) I. Saridakis, I. Klose, B. T. Jones and N. Maulide, JACS Au, 2024, 4, 3358–3369 CrossRef CAS PubMed.
- (a) M. Oestreich, J. Hermeke and J. Mohr, Chem. Soc. Rev., 2015, 44, 2202–2220 RSC; (b) J. L. Carden, A. Dasgupta and R. L. Melen, Chem. Soc. Rev., 2020, 49, 1706–1725 RSC; (c) S. Basak, L. Winfrey, B. A. Kustiana, R. L. Melen, L. C. Morrill and A. P. Pulis, Chem. Soc. Rev., 2021, 50, 3720–3737 RSC.
- S. Basak, A. Alvarez-Montoya, L. Winfrey, R. L. Melen, L. C. Morrill and A. P. Pulis, ACS Catal., 2020, 10, 4835–4840 CrossRef CAS.
- J. Zhang and S. Chang, J. Am. Chem. Soc., 2020, 142, 12585–12590 CrossRef CAS.
- Y. Peng and M. Oestreich, Chem. – Eur. J., 2023, 29, e202203721 CrossRef CAS.
- Y. He, Q. Liu, Z. Du, Y. Xu, L. Cao, X. Zhang and X. Fan, J. Org. Chem., 2022, 87, 14840–14845 CrossRef CAS.
- (a) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed; (b) T. Bortolato, S. Cuadros, G. Simionato and L. Dell’Amico, Chem. Commun., 2022, 58, 1263–1283 RSC.
- H. Wang, Y. Man, K. Wang, X. Wan, L. Tong, N. Li and B. Tang, Chem. Commun., 2018, 54, 10989–10992 RSC.
- Y. Fu, C.-Z. Shi, Q.-S. Xu, Z. Du and C. Huo, Green Chem., 2020, 22, 2264–2269 RSC.
- D. M. Soro, J. B. Roque, J. W. Rackl, B. Park, S. Payer, Y. Shi, J. C. Ruble, A. L. Kaledin, M.-H. Baik, D. G. Musaev and R. Sarpong, J. Am. Chem. Soc., 2023, 145, 11245–11257 CrossRef CAS PubMed.
- J.-L. Tu and B. Huang, RSC Sustainability, 2024, 2, 3222–3234 RSC.
- L. Chen, Q. Qu, C.-K. Ran, W. Wang, W. Zhang, Y. He, L.-L. Liao, J.-H. Ye and D.-G. Yu, Angew. Chem., Int. Ed., 2023, 62, e202217918 CrossRef CAS.
- F. Glaser, C. Kerzig and O. S. Wenger, Angew. Chem., Int. Ed., 2020, 59, 10266–10284 CrossRef CAS.
- Q. Qu, L. Chen, Y. Deng, Y.-Y. Gui and D.-G. Yu, Synlett, 2023, 1385–1390 CrossRef CAS.
- F. Wang and K. J. Frankowski, J. Org. Chem., 2022, 87, 1173–1193 CrossRef CAS PubMed.
- X. Fang, Y. Zeng, Y. Huang, Z. Zhu, S. Lin, W. Xu, C. Zheng, X. Hu, Y. Qiu and Z. Ruan, Nat. Commun., 2024, 15, 5181 CrossRef CAS.
- A. Kondoh and M. Terada, Asian J. Org. Chem., 2023, 12, e202300003 CrossRef CAS.
- A. Kondoh, R. Ojima and M. Terada, Org. Lett., 2021, 23, 7894–7899 CrossRef CAS PubMed.
- F. Mathey, Chem. Rev., 1990, 90, 997–1025 CrossRef CAS.
- J. Gasnot, C. Botella, S. Comesse, S. Lakhdar, C. Alayrac, A.-C. Gaumont, V. Dalla and C. Taillier, Synlett, 2020, 883–888 CAS.
- R. M. Tipker, J. A. Muldoon, J. Jo, C. S. Connors, B. R. Varga, R. P. Hughes and D. S. Glueck, ACS Omega, 2023, 8, 12565–12572 CrossRef CAS PubMed.
- D. C. R. Hockless, M. A. McDonald, M. Pabel and S. B. Wild, J. Chem. Soc., Chem. Commun., 1995, 1995, 257–258 RSC.
- J. Gasnot, C. Botella, S. Comesse, S. Lakhdar, C. Alayrac, A.-C. Gaumont, V. Dalla and C. Taillier, Angew. Chem., Int. Ed., 2020, 59, 11769–11773 CrossRef CAS PubMed.
- M. Ahmad, M.-J. Tranchant, S. Comesse, N. Saffon-Merceron, J. Pilmé, S. Lakhdar, I. Chataigner, V. Dalla and C. Taillier, Nat. Commun., 2024, 15, 8554 CrossRef CAS.
- A. Ghosh, T. H. Van Nguyen, C. Bellanger, S. Chelli, M. Ahmad, N. Saffon-Merceron, C. Taillier, V. Dalla, R. J. Mayer, I. M. Dixon and S. Lakhdar, Angew. Chem., Int. Ed., 2025, 64, e202414172 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |