Balancing energy and thermal stability: a review of advanced heat-resistant energetic materials
Boqian
Yang
ab,
Honglei
Xia
*ab,
Mingjing
Tang
ac,
Xiujuan
Qi
*ac,
Siwei
Song
ab,
Yi
Wang
 ab and
Qinghua
Zhang
ab and
Qinghua
Zhang
 *ab
*ab
aNational Key Laboratory of Solid Propulsion, Northwestern Polytechnical University, Xi’an, Shanxi 710065, China. E-mail: xia_honglei@nwpu.edu.cn; qinghuazhang@nwpu.edu.cn
bSchool of Astronautics, Northwestern Polytechnical University, Xi’an, Shanxi 710065, China
cSchool of Chemstry and Chemical Engineering, Northwestern Polytechnical University, Xi’an, Shanxi 710065, China
First published on 11th November 2025
Abstract
Energetic materials (EMs) operating in extreme environments, including those encountered in aerospace applications, deep-well mineral extraction, and advanced hypersonic systems, face significant challenges that drive substantial demand for the development of heat-resistant energetic materials (HREMs). However, the inherent trade-off between high thermal stability and high energy density in energetic materials remains a critical bottleneck hindering advancement in this field. Therefore, this review comprehensively summarizes the recent progress in the molecular design, synthesis, and performance of HREMs with thermal decomposition temperatures exceeding 250 °C and detonation velocities exceeding 8500 m s−1. By systematically classifying HREMs into single-ring, fused-ring, bridged, and bridged-fused-ring compounds, this review highlights the key structure–property relationships that determine their thermal stability and detonation performance. Finally, design principles for high-energy HREMs and an outlook on future research directions and challenges are proposed, aiming to contribute to the innovation and development of next-generation HREMs.
1. Introduction
Energetic materials are a class of substances containing explosive groups, or oxidizers and combustibles, which are capable of independent chemical reactions to release energy. Their performances directly determine the destructive power of weapon systems, the operational capability of aerospace devices, and the efficiency of oil and gas extraction.1–4Thermal stability is a critical parameter to evaluate the stability of energetic materials (EMs) under high-temperature conditions. Heat-resistant energetic materials (HREMs) are typically defined as explosives with a thermal decomposition degree exceeding 250 °C and those exhibiting decomposition temperatures above 300 °C are classified as ultra-heat-resistant classification.5–7 In recent years, the rapid advancement of defence technologies, aerospace engineering, and energy exploration—along with the advancement of cutting-edge technologies—has led to increasingly severe operational environments. For instance, hypersonic vehicles operating at Mach 5–20 experience extreme aerodynamic heating; aerospace energetic components and deep-well perforating charges must endure extreme conditions such as high vacuum, thermal cycles, and cosmic radiation.8–10 Therefore, it is critically important for energetic components to maintain reliable performance and structural integrity during prolonged high-temperature exposure, placing unprecedented demands on the thermal stability and energy output of advanced EMs.
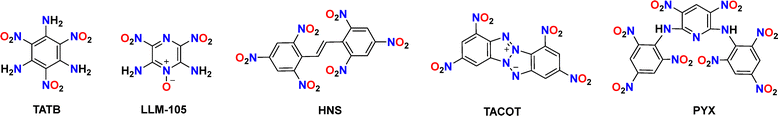
However, the design of HREMs has long faced a core challenge: how to balance the high energy and good thermal stability of explosives? Conventional high-energy compounds—such as hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and hexanitrohexaazaisowurtzitane (CL-20)—have achieved exceptional detonation performance, but they exhibit relatively low thermal stability. In contrast, classical ultra-high HREMs, including 2,2′,4,4′,6,6′-hexanitrostilbene (HNS) and 2,6-bis(picrylamino)-3,5-dinitropyridine (PYX), possess decomposition temperatures exceeding 300 °C, but their energy outputs remain substantially inferior to those of high-energy materials (Fig. 1).11,12 Consequently, overcoming the energy–stability trade-off through rational molecular design has emerged as a pivotal research direction in the field of EMs.
The performance of EMs is closely related to the molecular framework, functional groups and their combination patterns and crystal packing arrangements. To systematically summarize the structure–performance relationships and molecular design strategies of HREMs, we have outlined and analysed the representative HREMs with thermal decomposition temperatures above 250 °C and detonation velocities exceeding 8500 m s−1. Building on this foundation, we refine design principles for high-energy HREMs and offer a perspective on future research directions and challenges, aiming to guide the research and development of next-generation HREMs.
2. Classical HREMs
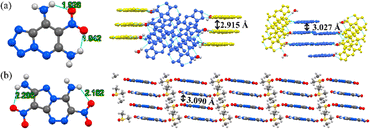
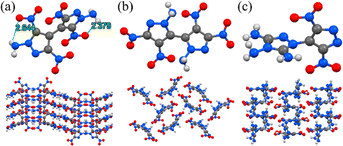
Scheme 1 displays the molecular structures of classical HREMs that have been widely used, including TATB, 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105), HNS, tetranitro-2,3,5,6-dibenzo-1,3a,4,6a-tetraazapentalene (TACOT), and PYX.13 Their detailed physicochemical and energetic performances are summarized in Table 1. The thermal decomposition temperatures of all compounds exceed 300 °C, owing to the introduction of thermally stable molecular skeletons and energetic functional groups. Among them, both TATB and LLM-105 exhibit outstanding detonation performance along with excellent thermal stability (TATB: 8179 m s−1, 360 °C; LLM-105: 8560 m s−1, 342 °C). Structural analysis (Fig. 2) reveals that TATB is completely surrounded by resonance-assisted hydrogen bonds (RAHBs), forming a continuous cyclic hydrogen bond network. RAHBs refer to a type of hydrogen bonds in which both the hydrogen bonding donors and acceptors are connected within a five- or six-membered ring.14 The synergistic effect of adjacent RAHBs results in stronger hydrogen bonding in TATB compared to LLM-105. In contrast, LLM-105 possesses only two nonadjacent RAHBs and two intramolecular hydrogen bonds, which are weaker than those in TATB, as evidenced by their longer hydrogen bond lengths (2.060–2.178 Å) compared to those in TATB (1.692–1.948 Å). Consequently, LLM-105 has a lower thermal decomposition temperature than TATB. LOL-π plots confirm TATB's stronger aromaticity through a more uniform π-electron distribution. In the case of LLM-105, its wave-like stacking forces the adjacent nitro groups into close contact, inducing steric repulsion. This repulsion causes noticeable nitro torsion, thereby severing the electron density isosurface connecting the nitro groups to the framework. Additionally, TATB exhibits graphene-like stacking with an interlayer spacing of 3.155 Å, which is closer than that of LLM-105 (3.227 Å). This reflects stronger π–π interactions which also contributes to its excellent thermal stability. Note that LLM-105 exhibits better detonation performance than TATB due to its higher nitrogen and oxygen content on the nitrogen heterocyclic skeleton and N–O bonds, but its detonation performance is not satisfactory enough. Consequently, developing advanced HREMs with improved energy has attracted significant attention in recent years and continues to be an active area of research.| Comp. | ρ (g cm−3) | D (m s−1) | P (GPa) | OBd (%) | T d (°C) | ISf (J) | FSg (N) |
|---|---|---|---|---|---|---|---|
| a Density. b Detonation velocity. c Detonation pressure. d Oxygen balance based on CO for CaHbOcNd: OB (%) = 1600 × (d − a − b/2)/Mw. e Decomposition temperature. f Impact sensitivity evaluated using a standard BAM fall hammer. g Friction sensitivity evaluated using a standard BAM friction tester. h Ref. 12. i Ref. 13. | |||||||
| TATB | 1.93 | 8179 | 30.5 | −18.6 | 350 | 50 | >360 |
| LLM-105 | 1.91 | 8560 | 33.4 | 0 | 342 | 28 | >360 |
| HNS | 1.74 | 7612 | 24.3 | −39.1 | 318 | 5 | 240 |
| TACOT | 1.82 | 7060 | 20.3 | −24.7 | 410 | — | — |
| PYX | 1.76 | 7757 | 25.1 | −24.5 | 360 | 28 | 360 |
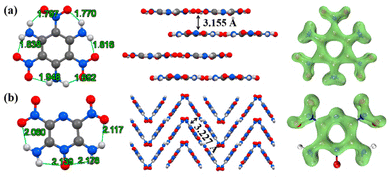 | ||
| Fig. 2 The crystal structure, packing mode and LOL-π plots (isovalue = 0.3) of TATB (a) and LLM-105 (b). | ||
3. Advanced HREMs
3.1. Single-ring HREMs
In 2018, leveraging a materials-genome strategy, our group designed, screened and synthesized a HREM, 2,4,6-triamino-5-nitropyrimidine 1,3-dioxide (ICM-102, 1).15 In order to design high-energy and low-sensitivity EMs, we selected 14 six-membered ring skeletons and listed the different arrangements and combinations of these skeletons with amino, nitro and hydrogen atoms through self-compiled JAVA scripts. Guided by three key “genetic” features (CO2-based oxygen balance value in the range of −80 to −40%, planar aromatic structures with high symmetry and graphite-like crystal packing), identified in reported insensitive highly explosive molecules, we systematically screened for molecules satisfying all these criteria and finally selected ICM-102 as the optimal molecule that meets the conditions. As a result, ICM-102 possesses a high anhydrous density of 1.95 g cm−3, an acceptable thermal decomposition temperature (286 °C), a high detonation velocity (9169 m s−1), and a desired low mechanical sensitivity (IS > 60 J, FS > 360 N). It should be noted that ICM-102 achieves superior detonation performances owing to the selection of the N-oxide pyrimidine skeleton. Similar to TATB and LLM-105, there are also abundant intra- and intermolecular hydrogen bonds in ICM-102, contributing to its good thermal stability. Each ICM-102 molecule engages five adjacent molecules through intermolecular hydrogen bonds, forming a two-dimensional layer structure (as shown in Fig. 3). Each layer interacts with the other layers through π–π interactions with a distance of 3.19 Å to form graphite-like layered crystal packing. This π–π interaction range is smaller than the common range (3.65–4.00 Å), suggesting a stronger π–π interaction, accounting for the extremely low sensitivity of ICM-102.16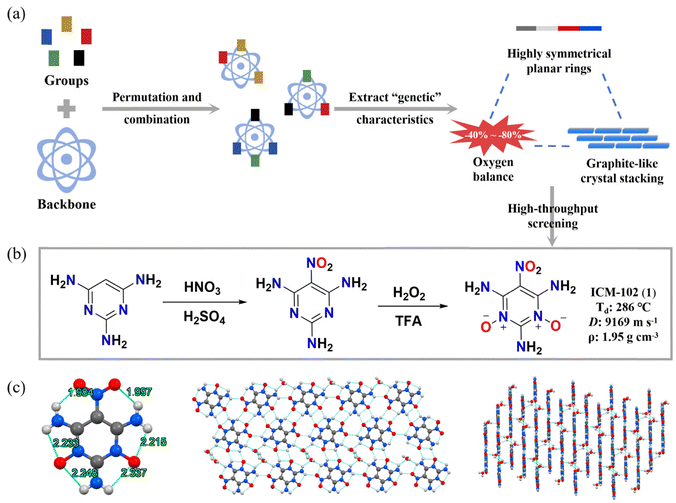 | ||
| Fig. 3 (a) The design strategy of ICM-102; (b) the synthesis method of ICM-102; (b) the crystal structure of ICM-102. | ||
3.2. Fused-ring HREMs
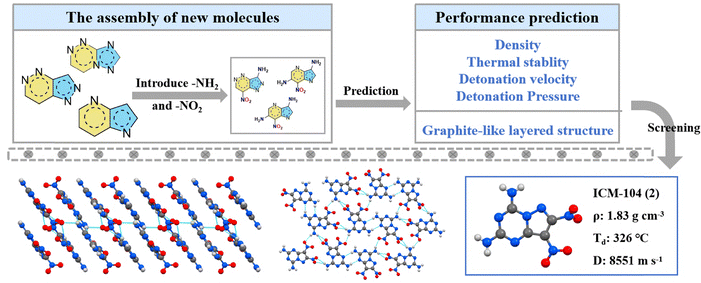
Fused-ring explosives are a class of energetic compounds characterized by molecular skeletons containing two or more rings that share a pair of atoms. Owing to their highly co-planar and conjugated structures, these compounds typically exhibit superior thermal stability and low mechanical sensitivity. Additionally, they benefit from high heats of formation, both of which contribute to improved detonation performance. As a result, fused-ring explosives have attracted extensive research interest in the field of heat-resistant high-energy materials (Scheme 2 and Table 2).17–20In 2022, our group reported a machine learning-assisted high-throughput virtual screening (HTVS) system to guide the discovery of new EMs. Based on this HTVS system, the targeted heat-resistant and insensitive energetic material 7,8-dinitropyrazolo[1,5-a][1,3,5]triazine-2,4-diamine (ICM-104, 2, Td = 326 °C, D = 8551 m s−1) was rapidly screened from 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112 [5,6]biheterocyclic molecular structures (as shown in Fig. 4).21 The system integrates high-throughput molecular generation with machine learning, where the former rapidly produces a vast array of candidate molecules, and the latter consists of a property predictor and a graphite-like structure classifier. Crystallographic analysis confirmed the predicted planar geometry of ICM-104, which exhibits a layered stacking motif. Its excellent thermal stability arises from abundant hydrogen bonds formed by amino and nitro groups, coupled with strong π–π interactions. Furthermore, the HTVS system can be extended to polyheteroaromatic systems, providing new insights into the development of novel energetic materials.22–24
112 [5,6]biheterocyclic molecular structures (as shown in Fig. 4).21 The system integrates high-throughput molecular generation with machine learning, where the former rapidly produces a vast array of candidate molecules, and the latter consists of a property predictor and a graphite-like structure classifier. Crystallographic analysis confirmed the predicted planar geometry of ICM-104, which exhibits a layered stacking motif. Its excellent thermal stability arises from abundant hydrogen bonds formed by amino and nitro groups, coupled with strong π–π interactions. Furthermore, the HTVS system can be extended to polyheteroaromatic systems, providing new insights into the development of novel energetic materials.22–24
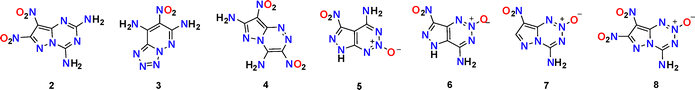
In 2019, Shreeve's group reported 7-nitrotetrazolo[1,5-b]pyridazine-6,8-diamine (3), a remarkable high-energy (D = 8750 m s−1) and thermal stability (Td = 362 °C) EM.25 Shortly, our group reported compound 4 in 2020, which exhibits comparable performance (D = 8727 m s−1, Td = 355 °C).26 The stability of both compounds is supported by their strong hydrogen bonds and π–π interactions. Due to the synergistic effect of the adjacent RAHBs of compound 3, its hydrogen bonds (1.928–1.942 Å) are shorter than those in compound 4 (2.182–2.280 Å), confirming the relative weakness of its hydrogen bond network. Additionally, the larger interlayer spacing in 4 (3.090 Å) versus3 (2.915–3.027 Å) points to diminished π–π interactions. Compound 3 exhibits stronger intra- and intermolecular noncovalent interactions, thus demonstrating a higher thermal decomposition temperature. Furthermore, although compound 4 contains two nitro groups, its nitrogen and oxygen content is only 73.3% and lower than that of compound 3 (73.4%). What's more, the skeleton of compound 3 incorporates an N5 chain, which contributes substantially to its enthalpy of formation. Thus, while their detonation performances are comparable, that of compound 3 is marginally higher (Fig. 5).
| Comp. | ρ (g cm−3) | D (m s−1) | P (GPa) | OBd (%) | T d (°C) | ISf (J) | FSg (N) |
|---|---|---|---|---|---|---|---|
| a Density. b Detonation velocity. c Detonation pressure. d Oxygen balance based on CO for CaHbOcNd: OB (%) = 1600 × (c − a − b/2)/Mw. e Decomposition temperature. f Impact sensitivity evaluated using a standard BAM fall hammer. g Friction sensitivity evaluated using a standard BAM friction tester. h Measured using a gas pycnometer at ambient temperature. i Crystal density at 298 K. j Evaluated using EXPLO5 version 6.02. k Evaluated using EXPLO5 version 6.01. l Onset temperature at a heating rate of 5 °C min−1. m Onset temperature at a heating rate of 10 °C min−1. n Peak temperature at a heating rate of 5 °C min−1. o Peak temperature at a heating rate of 10 °C min−1. | |||||||
| ICM-104 (2) | 1.83 | 8551j | 29.8j | −20.0 | 326 | 35 | >360 |
| 3 | 1.82h | 8750k | 31.5k | −32.6 | 362l | >40 | >360 |
| 4 | 1.90h | 8727k | 32.6k | −20.0 | 355n | >60 | >360 |
| PTO (5) | 1.90h | 8528k | 33.9k | −20.3 | 365m | 356 | 360 |
| 6 | 1.84h | 8731j | 31.2j | −20.3 | 291l | >20 | 300 |
| 7 | 1.87h | 8935j | 34.5j | −20.3 | 302l | >20 | >360 |
| BITE-101(8) | 1.96i | 9317k | 39.3k | 0 | 295 | 18 | 128 |
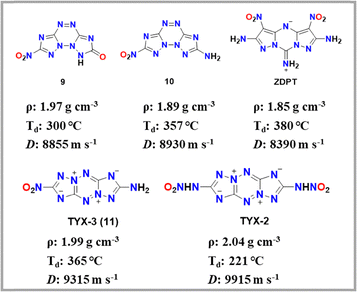
| TYX-3(9) | 1.99h | 9315 | 36.6 | −21.6 | 300o | >40 | >360 |
| 10 | 1.97h | 8855 | 38.7 | −10.8 | 300o | >45 | >600 |
| 11 | 1.89h | 8930 | 38.1 | −21.6 | 357o | >45 | >600 |
In 2022, our group reported 4-amino-5-nitro-7H-pyrazolo[3,4-d][1,2,3]triazine 2-oxide (PTO, 5), which was derived from the classic green primary explosive ICM-103 by replacing its azido group with an amino group (Fig. 6).27,28 This modification significantly increases the thermal decomposition temperature by 205 °C (ICM-103 = 160 °C, PTO = 365 °C), which highlights the destabilizing nature of the azido group in EMs. Although PTO exhibits a reduced enthalpy of formation compared to ICM-103, it retains a considerable detonation velocity due to its well-designed molecular skeleton (D = 8528 m s−1). Subsequently, we reported 4-amino-7-nitro-5H-pyrazolo[4,3-d][1,2,3]triazine 2-oxide (6), an isomer of PTO, exhibiting a detonation velocity of 8731 m s−1 and a thermal decomposition temperature of 355 °C. In terms of structure, owing to the distinct spatial arrangement of nitro and amino groups, PTO exhibits additional intramolecular hydrogen bonds compared to compound 6, indicating stronger intramolecular forces in crystals. In LOL-π plots, the π-electron cloud of PTO is more uniform and abundant compared to that of compound 6, also further demonstrating that PTO exhibits stronger intermolecular weak interactions, thereby enhancing the thermal stability of PTO. The synthesis of compound 6 requires the use of sodium azide, which poses certain hazards, and involves a multi-step synthesis with low efficiency. In contrast, the optimized synthetic route for PTO involves a one-step nitration of the existing 4-aminopyrazolo[3,4-d]-pyrimidine, providing a yield of 47%.29 The high energy, high thermal stability and low sensitivity, combined with an efficient synthesis method, make PTO a very promising high-energy HREM.
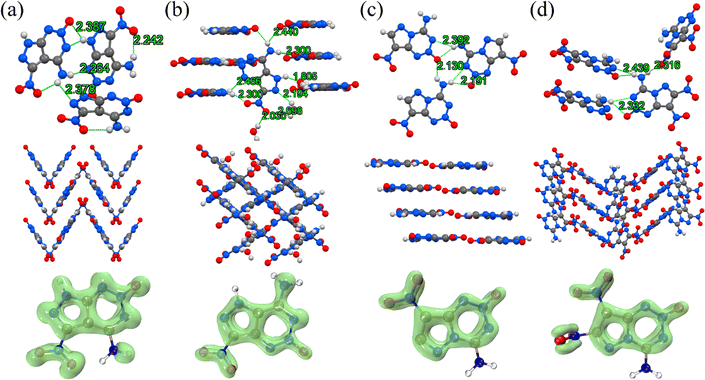 | ||
| Fig. 6 The crystal structure, packing mode and LOL-π plots (isovalue = 0.3) of (a) PTO (5), (b) 6, (c) 7 and (d) BITE-101 (8). | ||
Yang's group developed a heat-resistant explosive, 4-amino-7-nitropyrazolo-[5,1-d][1,2,3,5]-tetrazine 2-oxide (7), which features a pyrazolo-[5,1-d][1,2,3,5]tetrazine skeleton, rather than the pyrazolo[3,4-d][1,2,3]triazine scaffold found in PTO and compound 6, which constitutes the key structural difference.30 It possesses both good thermal stability (302 °C) and high detonation velocity (8935 m s−1). Subsequently, 4-amino-7,8-dinitropyrazolo-[5,1-d][1,2,3,5]-tetrazine 2-oxide (BITE-101, 8) was reported by Pang's group, which has one more nitro group than compound 7, with a thermal stability of 295 °C and a detonation velocity of 9317 m s−1 (Fig. 6).31 By upgrading from a pyrazolo-oxidized triazine to a pyrazolo-oxidized tetrazine skeleton, the enthalpy of formation increases, thereby resulting in compound 7 exhibiting higher energy content than both PTO and compound 6. The introduction of this additional nitro group reduces the planarity of BITE-101 compared to compound 7, resulting in a small torsional angle between the two nitro groups, but brings a higher detonation performance. Four molecules of BITE-101 with different orientations combine to form a wavy-like stacking, and the interlayer spacing between adjacent parallel molecules is 3.165 Å, which is longer than that of compound 7, resulting in reduced π–π interactions. Additionally, the hydrogen bond lengths in BITE-101 are longer than those in compound 7, indicating weaker hydrogen bond interactions. But this difference is relatively small, as evidenced by the LOL-π plot, which indicates that the aromaticity of the two compounds is quite similar. The limited conjugation and π–π interactions in the non-planar BITE-101, along with its weaker hydrogen bonding, result in a thermal stability that is close to but still inferior to that of compound 7. The scale-up preparation of BITE-101 was also investigated by Pang's group, focusing on the optimization of the coupling process, oxidation reaction, and cyclization conditions during synthesis.32 Nevertheless, the overall yield reached only 17.1% at maximum. Although BITE-101 demonstrates relatively good overall properties, its synthetic route remains complex and inefficient, requiring further optimization.
The comparison of compounds 2 to 8 demonstrates the inherent advantage of fused-ring skeletons in balancing energy content and stability. N-oxidized fused-ring compounds exhibit significantly enhanced detonation performance with acceptable thermal stability. Importantly, the pyrazolo-[5,1-d][1,2,3,5]tetrazine 2-oxide skeleton simultaneously delivers higher detonation performance and thermal decomposition temperature compared to the pyrazolo[4,3-d][1,2,3]triazine 2-oxide skeleton, making it a superior structural choice for developing HREMs.
In 2022, Dorofeeva's group reported two compounds based on a tetrazino-bistriazole framework: 7-nitrobis([1,2,4]triazolo)[1,5-b:5′,1′-f][1,2,4,5]tetrazin-2(1H)-one (10) and 7-nitrobis([1,2,4]triazolo)[1,5-b:5′,1′-f][1,2,4,5]tetrazin-2-amine (TYX-3, 11).33 The extensive conjugated system contributes to their high thermal decomposition temperatures, especially when the amino group in TYX-3 leads to a more pronounced improvement in thermal stability. (10: 300 °C; TYX-3: 357 °C). Furthermore, the high nitrogen content and favorable enthalpy of formation endow compounds 10 and TYX-3 with high detonation velocities (10: 8855 m s−1; TYX-3: 8930 m s−1). Unfortunately, single crystals of all compounds could not be obtained due to their extremely poor solubility. Our group also reported a zwitterionic ternary fused-ring heat-resistant explosive, ZDPT, in 2024.36 It exhibits a thermal decomposition temperature of 380 °C and low mechanical sensitivity, demonstrating the promising potential of ternary fused zwitterionic architecture for heat-resistant EMs. However, its detonation velocity (8390 m s−1) is relatively low and did not meet our screening criteria (Fig. 7).
Recently, Liu's group reported 2-amino-7-nitrobis([1,2,4]triazolo)[1,5-b:1′,5′-e][1,2,4,5]tetrazine-5,10-diium-3,8-diide (TYX-3, 9).34 Replacing all functional groups in TYX-3 with nitroamino groups yields TYX-2.35 Despite their similar structure, TYX-3 exhibits a thermal decomposition temperature 132 °C higher than that of TYX-2 (TYX-3: 353 °C; TYX-2: 221 °C), while both compounds maintain favorable detonation velocities (TYX-3: 9315 m s−1; TYX-2: 9915 m s−1). After replacing the nitroamino groups with amino groups, the weakest bond energy of TYX-3 has increased, and additional intermolecular hydrogen bonding interactions are introduced. As a result, the thermal stability of TYX-3 is significantly improved, and the detonation performance remains at an excellent level.
From compounds 9 to 11, compounds based on the tricyclic fused-ring skeleton possess planarity, conjugation, and the ability to resolve the trade-off between energy and thermal stability. Zwitterionic tricyclic fused-ring compounds further combine enhanced detonation performance with high thermal stability. However, caution should be exercised to avoid introducing carbonyl and nitroamino groups, as their presence may compromise thermal stability.
3.3. Bridged HREMs
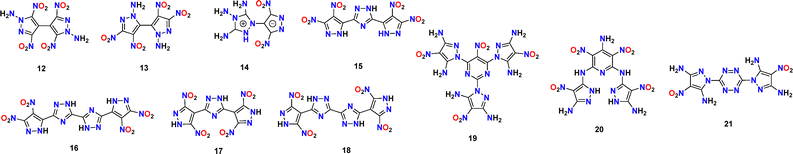
Compared with single-ring explosives, bridged explosives possess more modifiable sites, which provides more possibilities for the design of heat-resistant explosives. The following introduces several high-performance bridged heat-resistant explosives (Scheme 3 and Table 3).37–39In 2023, Pang's group reported two isomers, 1,1-diamino-3,3′,5,5′-tetranitro-4,4′-bipyrazole (12) and 2,2′-diamino-4,4′,5,5′-tetranitro-3,3′-bipyrazole (13), based on a bipyrazole skeleton (Fig. 8).40 While both compounds are high-energy heat-resistant explosives, their isomeric relationship stems solely from the distinct positioning of the nitro groups: a para-arrangement in compound 12versus an ortho arrangement in compound 13. The combination of four nitro groups and N–NH2 groups in both compounds 12 and 13 contributes to their high enthalpy of formation (12: 477 kJ mol−1; 13: 457 kJ mol−1), which results in superior detonation performance (12: 8951 m s−1; 13: 8504 m s−1). Due to compound 12 having the para orientation of the nitro groups in the pyrazole ring, the steric hindrance has reduced. Additionally, compound 12 forms RAHBs with the adjacent amino group, enhancing its thermal stability compared to 13 (12: 305 °C; 13: 252 °C). In contrast, nitro groups at the ortho positions on the pyrazole ring tend to decrease thermal stability.
| Comp. | ρ (g cm−3) | D (m s−1) | P (GPa) | OBd (%) | T d (°C) | ISf (J) | FSg (N) |
|---|---|---|---|---|---|---|---|
| a Density. b Detonation velocity. c Detonation pressure. d Oxygen balance based on CO for CaHbOcNd: OB (%) = 1600 × (c − a − b/2)/Mw. e Decomposition temperature. f Impact sensitivity evaluated using a standard BAM fall hammer. g Friction sensitivity evaluated using a standard BAM friction tester. h Measured using a gas pycnometer at ambient temperature. i Evaluated using EXPLO5 version 6.01. j Onset temperature at a heating rate of 5 °C min−1. k Onset temperature at a heating rate of 10 °C min−1. l Peak temperature at a heating rate of 5 °C min−1. | |||||||
| 12 | 1.88h | 8951i | 35.9i | 0 | 305k | 40 | 360 |
| 13 | 1.76h | 8504i | 31.0i | 0 | 252j | 30 | 360 |
| 14 | 1.82h | 8750i | 31.5i | −40.5 | 362j | >40 | >360 |
| 15 | 1.88h | 8847i | 33.7i | −6.3 | 254j | 30 | >360 |
| 16 | 1.88h | 8729i | 32.8i | −14.3 | 276j | 35 | >360 |
| 17 | 1.86h | 8813i | 33.1i | −6.3 | 300j | 22 | 300 |
| 18 | 1.88h | 8705 | 33.3 | −14.3 | 372l | 26 | >360 |
| 19 | 1.85h | 8637i | 31.1i | −32.1 | 312k | 15 | 288 |
| 20 | 1.90h | 8889i | 33.9i | −27.5 | 365k | 356 | 360 |
| 21 | 1.85h | 8769 | 29.5 | −35.1 | 370l | 40 | >360 |
In 2020, Zhou's group synthesized high-energy, heat-resistant 3,5-dinitro-4-(3,4,5-triamino-4H-1,2,4-triazol-1-ium-1-yl)-4H-pyrazol-4-ide (14), with an exceptional thermal decomposition temperature (Td = 362 °C) and a high detonation velocity (D = 8750 m s−1).41 Similar to compound 12, compound 14 also has a para-nitro-substituted pyrazole ring, which contributes to balancing energy and stability. However, its different structure derives from a triazole ring functionalized with three amino groups. While the triazole ring elevates energy density relative to pyrazole, it compromises stability; this instability is counterbalanced by the stabilizing, albeit energy-attenuating, nature of the multiple amino groups, creating a balanceable effect. Compound 14 exhibits a tighter packing mode, facilitated by an 80.5° torsion between the heterocyclic rings, which further augments the network of intermolecular weak interactions and thermal stability.
In 2020, Cheng's group designed several isomers by using nitrogen heterocyclic molecules as energetic “bridges” by referring to the structure of heat-resistant explosive TKX-55. Among them, 3,5-bis(3,4-dinitro-1H-pyrazol-5-yl)-1H-1,2,4-triazole (15), 5,5′-bis(3,4-dinitro-1H-pyrazol-5-yl)-2H,2′H-3,3′-bi(1,2,4-triazole) (16), 3,5-bis(3,5-dinitro-1H-pyrazol-4-yl)-1H-1,2,4-triazole (17) and 5,5′-bis(3,5-dinitro-1H-pyrazol-4-yl)-1H,1′H-3,3′-bi(1,2,4-triazole) (18) show good performance.42 Their thermal decomposition temperatures are 254, 276, 300 and 372 °C, respectively, and their detonation velocities are 8847, 8729, 8813 and 8705 m s−1, respectively. As shown in Fig. 9, the EMs based on the three-membered ring exhibits inferior thermal stability compared to the EMs based on the four-membered ring. This indicates that enlarging the ring quantity aids in balancing energy and stability. Furthermore, analogous to compounds 12 and 13, a trend is observed among compounds 15–18, where para-nitro substitution on the skeleton confers greater stability than ortho-substitution. Moreover, they indicate that the hydrogen bond force is not an essential element of HREMs. This provides valuable guidance for designing a future combination of energetic groups and molecular backbones.
Cheng's group developed two new HREMs: 1,1′,1″-(5-nitropyrimidine-2,4,6-triyl)tris(4-nitro-1H-pyrazole-3,5-diamine) (19) and N2-(5-amino-4-nitro-1H-pyrazol-3-yl)-N6-(3-amino-4-nitro-1H-pyrazol-5-yl)-3,5-dinitropyridine-2,4,6-triamine (20).43 The thermal decomposition temperatures of compounds 19 and 20 are 312 and 365 °C, respectively, and the detonation velocities are 8637 and 8889 m s−1, respectively. Besides, our group reported two novel compounds with ultra-high thermal stability, viz. 3,6-bis(3,5-diamino-4-nitropyrazol-1-yl)-1,2,4,5-triazine (NPX-01, 21) and 2,4,6-tri(3,5-diamino-4-nitropyrazol-1-yl)-1,3,5-triazine (NPX-02), both of which were synthesized via a simple and efficient one-step reaction (yields: 86–91%) (Fig. 10).44
 | ||
| Fig. 10 The design strategies (a), crystal structures and LOL-π plots (isovalue = 0.5) of compounds (b) 19, (c) 20 and (d) 21. | ||
Notably, NPX-01 possesses a high density (1.85 g cm−3) and detonation performance (D = 8769 m s−1). A comparison of their crystal structures reveals that both compounds feature abundant hydrogen bonds, including RAHBs. What's more, the presence of the –NH– group in compound 20 enables a more continuous intramolecular hydrogen-bonding network. This is corroborated by the LOL-π plot, which shows a more contiguous electron density distribution. Consequently, this continuous network elevates the thermal decomposition temperature. NPX-01 adopts a planar and conjugated structure. It shares a pyrazole ring capable of forming RAHBs with compounds 19 and 20, highlighting its advantageous role in the design of bridged HREMs.
3.4. Bridged fused-ring HREMs
Bridged fused-ring explosives are derived from fused-ring explosives by incorporating various bridging bonds or groups. This hybrid architecture combines the advantageous features of both structural motifs: the extended molecular conjugation of fused-ring systems, which contribute to the high thermal stability and heat of formation, and the capability of incorporating more energetic groups to enhance detonation performance (Scheme 4 and Table 4).| Comp. | ρ (g cm−3) | D (m s−1) | P (GPa) | OBd (%) | T d (°C) | ISf (J) | FSg (N) |
|---|---|---|---|---|---|---|---|
| a Density. b Detonation velocity. c Detonation pressure. d Oxygen balance based on CO for CaHbOcNd: OB (%) = 1600 × (c − a − b/2)/Mw. e Decomposition temperature. f Impact sensitivity evaluated using a standard BAM fall hammer. g Friction sensitivity evaluated using a standard BAM friction tester. h Measured using a gas pycnometer at ambient temperature. i Evaluated using EXPLO5 version 6.02. j Evaluated using EXPLO5 version 6.01. k Onset temperature at a heating rate of 5 °C min−1. l Onset temperature at a heating rate of 10 °C min−1. | |||||||
| 22 | 1.84h | 8884i | 31.4i | −34.5 | 278l | 20 | 360 |
| 23 | 2.00h | 8721i | 30.9i | −14.4 | 281l | 15 | 192 |
| 24 | 1.87h | 8657 | 27.8 | −39.5 | 335l | >60 | >360 |
| 25 | 1.88h | 8755 | 31.7 | −12.9 | 273l | 60 | >360 |
| 26 | 1.85h | 8572j | 31.4j | −14.3 | 315k | >60 | >360 |
| 27 | 1.87h | 9064i | 35.2i | −28.7 | 292k | 16 | >360 |
In 2018, our group reported the first heat-resistant explosives based on bridged fused rings. By introducing two tetrazole rings into the tetrazole-linked 4,8-dihydrodifurazano[3,4-b,e]pyrazine (DFP) fused ring, 4,8-di(1H-tetrazol-5-yl)-difurazano[3,4-b,e]pyrazine (22) and its salts were obtained.45 Single crystals of 22·2DMF suggest that the tetrazole rings form a 10.83° angle with the planar DFP backbone. The DFP skeleton of compound 22 inherently possesses a high enthalpy of formation, and the tetrazolyl group further augments this property. Their synergistic interaction enhances the detonation velocity of 22 to 8884 m s−1. While DFP itself is planar and conjugated, the incorporation of the tetrazolyl group introduces a 10.83° dihedral angle between the tetrazole and the parent ring. This molecular conformation promotes a layered stacking mode and, together with the intermolecular hydrogen bonds, ensures the compound's high stability (Td = 278 °C).
Based on a similar strategy, our group selected a thermally stable fused-ring backbone and synthesized 3,6-dinitro-1,4-di(1H-tetrazol-5-yl)-pyrazolo[4,3-c]pyrazole (DNTPP, 23) by incorporating tetrazole rings.46 As expected, DNTPP exhibited a thermal decomposition temperature of 281 °C and a detonation velocity of 8721 m s−1. Its nitro group is nearly coplanar with the fused ring system, while the tetrazole rings are tilted at a 50.2° dihedral angle relative to it. Crystallographic analysis further reveals that DNTPP forms only intermolecular hydrogen bonds, which link adjacent molecules into a complex stacking architecture. As shown in Fig. 11, there exists a π–π interaction between every parallel molecule. The lower density and heat of formation of DNTPP compared to compound 22 consequently account for its reduced detonation performance.
Subsequently, Cheng et al. developed two high-energy heat-resistant explosives, (8-nitro-3-(1H-tetrazol-5-yl)pyrazolo[5,1-c][1,2,4]triazin-7-amine (24) and 4-amino-7,8-dinitro-3-(1H-tetrazol-5-yl)pyrazolo[5,1-c][1,2,4]triazine-2-oxide (25), by incorporating tetrazole on the fused-ring skeleton, which exhibit thermal decomposition temperatures of 335 and 273 °C and detonation velocities of 8657 and 8755 m s−1, respectively (Fig. 12).47 The parent ring of compound 24 is inherently planar and conjugated, and features a RAHB of 2.40 Å, which enhances its thermal stability. The introduction of a tetrazolyl group further improves its detonation performance. Thus, compound 24 achieves a balance between energy and stability through the strategic combination of its molecular skeleton and functional groups. In contrast, compound 25 is derived from the further oxidation of 24. Its pyrazolo-fused oxidized triazine core inherently possesses the potential to balance energy and stability. Although it lacks specific stabilizing groups, it still meets the criteria for heat-resistant EMs. Moreover, equipped with a tetrazolyl group and two nitro groups, compound 25 exhibits breakthrough detonation performance.
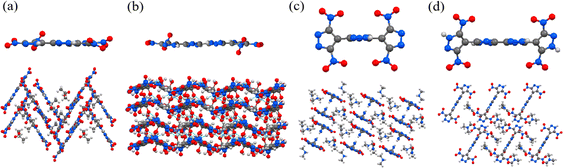
Shreeve's group prepared 6,6′-(hydrazine-1,2-diyl)bis(7-nitrotetrazolo[1,5-b]pyridazin-8-amine) (26) through an efficient reaction of diazonium salts with sodium nitroacetonitrile.48 Due to its excellent thermal stability (Td = 315 °C) and detonation performance (D = 8572 m s−1), compound 26 has the potential to be used as a high-energy heat-resistant explosive. Compound 26 possesses a skeleton composed of two C–C linked pyrazolo-triazine rings, which is planar and conjugated. The adjacent amino and nitro groups in this framework form RAHBs, laying a solid foundation for its high thermal stability. In addition, there is an angle of 59.32° between the two C–C bonded fused rings, which makes compound 26 exhibit a complex stacking pattern. However, there are parallel fused rings in multiple directions, and there are π–π interactions between them. Unfortunately, the synthetic route of compound 26 is nine steps long, the ring-closure reaction conditions are complex, and self-made nitroacetonitrile is required, which severely limits its application (Fig. 13).
 | ||
| Fig. 13 The crystal structures and packing modes of compounds 26 (a), 27 (d) and compounds similar to 27 (b and c). | ||
Inspired by the above work, our group developed a series of heat-resistant explosives by bridging the fused-ring explosives with different catenated nitrogen chains. Among them, 6,6′-(hydrazine-1,2-diyl)bis(7-nitrotetrazolo[1,5-b]pyridazin-8-amine) (27) exhibits the best comprehensive performance (Fig. 13).49 Compound 27 can be obtained in just two steps with a yield of 44%. The introduction of hydrazo bridge enhances the molecular planarity of 27 through the formation of four intramolecular RAHBs, leading to a layered crystal structure with an interlayer spacing of 3.153 Å. These strong and abundant weak interactions contribute to good stability and dense crystal packing. As a result, compound 27 possesses higher decomposition temperature (Td = 292 °C) and detonation performance (D = 9064 m s−1) compared with the other similar compounds.
4. Design strategies for high-energy HREMs
The above discussions reveal that the thermal stability and detonation performance of HREMs are primarily determined by their crystal and molecular structures. Accordingly, the design principles of high-energy HREMs can be summarized as follows (Fig. 14).At the molecular level, the selection of skeletons and energetic functional groups is crucial. Skeletons should be constructed using thermally stable heterocycles—such as pyrazole, triazole, tetrazole, pyrazine, triazine, and tetrazine—interconnected via various bonding strategies (fused, bridged, or fused and bridged). In particular, skeletons such as pyrazolo-oxidized triazine, pyrazolo-oxidized tetrazine, and zwitterionic tricyclic fused-ring systems hold significant potential for the synthesis of HREMs. Simultaneously, the introduced energetic groups should also demonstrate high thermal stability, including amino, C-nitro, N–O, and tetrazole, while the unstable azide, nitroform, and ammonium nitrate groups should be avoided. Furthermore, skeletons and energetic groups with high N/O content are highly desirable, as they exhibit high density and heat of formation, thereby enhancing the detonation performance.
From the aspect of crystal structure, attention should be paid to the compatibility between skeletons and energetic groups. By introducing hydrogen bonding donors and acceptors at appropriate positions within the skeletons, abundant intra/intermolecular hydrogen bonding networks can be constructed within the crystals. Compared to conventional hydrogen bonds, RAHBs formed by amino/imino and nitro groups are preferred due to their stronger bond strength. Moreover, adjacent hydrogen bonding donors and acceptors facilitate the formation of planar molecular structures with extended π-conjugation, leading to regular layered structures through strong π–π interactions. Thus, HREMs composed of highly matched skeletons and energetic groups generally possess good molecular planarity, strong and weak interactions and close crystal packing, all of which contribute to the synergistic enhancement of both thermal stability and detonation performance.
Based on the above strategies, after the core frameworks and functional groups of HREMs were selected, the HTVS system can be employed to screen for molecules with superior performance. The key lies in establishing reliable machine learning models based on well-defined screening criteria, enabling the HTVS system to accurately identify and predict the properties of the designed molecules. Guided by the design strategy of HREMs, using the HTVS system can achieve twice the result with half the effort.
5. Conclusion and outlook
The development of high-energy HREMs represents a pivotal and challenging frontier in materials science and energetic chemistry. This review has reviewed significant progress in advanced HREMs that simultaneously achieve high thermal stability (Td ≥ 250 °C) and good detonation permeance (D ≥ 8500 m s−1). Key strategies—such as incorporating stable and poly-nitrogen heterocyclic skeletons, optimizing hydrogen-bond networks, enhancing molecular planarity and conjugation—have proven effective in balancing thermal stability and detonation performance.Although HREMs have attracted significant research interest in recent years, they constitute less than 30% of all reported single-compound explosives, with merely approximately 11.1% exhibiting a thermal decomposition temperature exceeding 300 °C. In practical applications, factors such as detonation performance, acidic hydrogen content, and synthetic routes must also be considered. Up to now, only four HREMs have been reported that simultaneously satisfy the following criteria: the absence of acidic hydrogen in their molecular structures, detonation velocity exceeding 8800 m s−1, and thermal decomposition temperature above 300 °C (see Fig. 15), but most of these compounds suffer from lengthy synthetic routes, complex reaction conditions, and low efficiency. Consequently, the development of high-energy HREMs remains a highly challenging and essential research direction.
Future research on HREMs should focus on overcoming current technical barriers through three key dimensions: exploration of novel molecular architectures, development of innovative design strategies, and advancement of efficient synthesis methods. First, structural derivatization or de novo scaffold design can be built upon successful paradigms of existing HREMs. Notable examples include tetrazine-based zwitterionic tricyclic fused-ring compounds and pyrazolo-oxidized tetrazine skeletons, which demonstrate exceptional capability of balancing energy content and thermal stability, thereby providing structural blueprints for novel materials design. Second, it is also essential to develop innovative and efficient design strategies for HREMs. For example, the innovative integration of functional groups with robust molecular skeletons, harnessing the synergy of hydrogen bonding and π–π interactions, provides a new pathway to concurrently improve energy performance and thermal stability. Finally, for reported HREMs possessing high energy and excellent thermal stability, there is an urgent need to optimize synthetic routes and processing conditions. Such efforts are crucial to overcoming limitations in yield, cost, and scalable production, thereby facilitating their transition toward practical applications. In addition, advanced computational tools, including machine learning and high-throughput screening, will also increasingly facilitate the rational design of HREMs with tailored performance. Challenges remain in establishing more accurate predictive models for the thermal and detonation behaviours of EMs. It is hoped that this review will offer a comprehensive foundation for understanding HREMs and accelerate the further innovation and advancement in this field.
Author contributions
Boqian Yang: writing – original draft, formal analysis. Honglei Xia: writing – review & editing. Mingjing Tang and Xiujuan Qi: writing – review & validation. Siwei Song and Yi Wang: investigation. Qinghua Zhang: supervision, resources.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was supported by the China National Science Fund for Distinguished Young Scholars (No. 22325504), the National Natural Science Foundation of China (No. 22475174 and 22205221), and the Fundamental Research Funds for the Central Universities (25SH02010013 and G2025KY05025).Notes and references
- Y. Xu, Q. Wang, C. Shen, Q. Lin, P. Wang and M. Lu, Nature, 2017, 549, 78–81 CrossRef CAS PubMed.
- J. Ma, A. Chinnam, G. Cheng, H. Yang, J. Zhang and J. Shreeve, Angew. Chem., Int. Ed., 2021, 60, 5497–5504 CrossRef CAS PubMed.
- W. Huang, Y. Tang, G. Imler, D. Parrish and J. Shreeve, J. Am. Chem. Soc., 2020, 142, 3652–3657 CrossRef CAS.
- C. Li, T. Zhu, C. Lei, G. Cheng, C. Xiao and H. Yang, J. Mater. Chem. A, 2023, 11, 12043–12051 RSC.
- C. Li, T. Zhu, J. Tang, C. Lei, G. Yu, Y. Yang, H. Yang, C. Xiao and G. Cheng, Mater. Horiz., 2024, 11, 5701–5708 RSC.
- S. Wang, S. Li, J. Wang, Z. Wang, H. Yin, Q. Ma and F. Chen, J. Mater. Chem. A, 2025, 13, 8337–8342 RSC.
- C. Lei, H. Yang, Q. Zhang and G. Cheng, ACS Appl. Mater. Interfaces, 2022, 14, 39091–39097 CrossRef CAS PubMed.
- L. Habert, M. Daniel, P. Palmasa and E. Pasquinet, Mater. Chem. A, 2023, 11, 13564–13569 RSC.
- T. Zhu, C. Lei, C. Li, H. Yang, C. Xiao and G. Cheng, J. Mater. Chem. A, 2024, 12, 4678–4683 RSC.
- Y. Dong, M. Li, G. Cheng, W. Huang, Y. Liu, C. Xiao and Y. Tang, J. Mater. Chem. A, 2023, 11, 25992–25999 RSC.
- Y. Wang, X. Song, D. Song, L. Liang, C. An and J. Wang, J. Hazard. Mater., 2016, 312, 73–83 CrossRef CAS PubMed.
- Q. Tariq, M. Tariq, S. Manzoor, M. Xu, Q. Yu and J. Zhang, Chem. Eng. J., 2025, 505, 159133 CrossRef CAS.
- U. Nair, G. Gore, R. Sivabalan, S. Pawar, S. Asthana and S. Venugopalan, J. Hazard. Mater., 2007, 143, 500–505 CrossRef CAS PubMed.
- Y. Cao, T. Jiang, S. Chen, H. Xia, Y. Liu and W. Zhang, J. Mater. Chem. A, 2025, 13, 9127–9134 RSC.
- Y. Wang, Y. Liu, S. Song, Z. Yang, X. Qi, K. Wang, Y. Liu, Q. Zhang and Y. Tian, Nat. Commun., 2018, 9, 2444 CrossRef PubMed.
- C. Lei, G. Cheng, Z. Yi, Q. Zhang and H. Yang, Chem. Eng. J., 2021, 416, 129190 CrossRef CAS.
- J. Zhang, P. Yin, L. Mitchell, D. Parrishc and J. Shreeve, J. Mater. Chem. A, 2016, 4, 7430–7436 RSC.
- X. Yu, J. Tang, C. Lei, H. Yang and G. Cheng, Cryst. Growth Des., 2024, 24, 455–460 CrossRef CAS.
- Y. Liu, X. Zhang, J. Li, A. Kou, H. Ding, S. Pang and C. He, Org. Lett., 2024, 26, 5488–5492 CrossRef CAS PubMed.
- S. Feng, P. Yin, C. He, S. Pang and J. Shreeve, J. Mater. Chem. A, 2021, 9, 12291–12298 RSC.
- S. Song, Y. Wang, F. Chen, M. Yan and Q. Zhang, Engineering, 2022, 10, 99–109 CrossRef CAS.
- R. Wang, Y. Li, L. Pan, M. Fan, Y. Wang, S. Song and Q. Zhang, ACS Appl. Energy Mater., 2025, 8, 6756–6767 CrossRef CAS.
- S. Song, F. Chen, Y. Wang, K. Wang, M. Yan and Q. Zhang, J. Mater. Chem. A, 2021, 9, 21723–21731 RSC.
- S. Song, Y. Wang, X. Tian, W. He, F. Chen, J. Wu and Q. Zhang, J. Phys. Chem. A, 2023, 127, 4328–4337 CrossRef CAS PubMed.
- Y. Tang, C. He, G. Imler, D. Parrish and J. Shreeve, ACS Appl. Energy Mater., 2019, 2, 2263–2267 CrossRef CAS.
- S. Chen, Y. Liu, Y. Feng, X. Yang and Q. Zhang, Chem. Commun., 2020, 56, 1493 RSC.
- M. Deng, Y. Feng, W. Zhang, X. Qi and Q. Zhang, Nat Commun., 2019, 10, 1339 CrossRef PubMed.
- M. Deng, F. Chen, S. Song, S. Huang, Y. Wang and Q. Zhang, Chem. Eng. J., 2022, 429, 132172 CrossRef CAS.
- R. Zhang, Y. Xu, S. Jiang, F. Yang and M. Lu, Org. Lett., 2024, 26, 10119–10123 CrossRef CAS PubMed.
- C. Lei, G. Cheng, Z. Yi, Q. Zhang and H. Yang, Chem. Eng. J., 2021, 416, 129190 CrossRef CAS.
- J. Li, Y. Liu, W. Ma, T. Fei, C. He and S. Pang, Nat Commun, 2022, 13, 5697 CrossRef CAS PubMed.
- J. Li, C. He and S. Pang, Sci. Sin. Tech., 2023, 53, 1567–1573 CrossRef.
- G. Rudakov, V. Sinditskii, I. Andreeva, A. Botnikova, P. Veselkina, S. Kostanyan, N. Yudin, V. Serushkin, G. Cherkaev and O. Dorofeeva, Chem. Eng. J., 2022, 450, 138073 CrossRef CAS.
- B. Tan, X. Yang, J. Dou, J. Su, J. Zhang, S. Song, C. Tang, M. Xu, S. Zeng, W. Li, J. Luan, G. Zhang, Q. Zhang, X. Lu, B. Wang and N. Liu, Def. Technol., 2025, 52, 220–229 CrossRef.
- B. Tan, J. Su, J. Zhang, C. Tang, J. Dou, X. Yang, M. Xu, S. Zeng, W. Li, J. Luan, G. Zhang, S. Song, Q. Zhang, X. Lu, B. Wang and N. Liu, J. Mater. Chem. A, 2025, 13, 25103–25109 RSC.
- R. Lv, L. Jiang, J. Wang, S. Huang, S. Song, L. Wei, Q. Zhang and K. Wang, J. Mater. Chem. A, 2024, 12, 10050 RSC.
- Y. Tang, Z. Yin, A. Chinnam, R. Staples and J. Shreeve, Inorg. Chem., 2020, 59, 17766–17774 CrossRef CAS PubMed.
- X. Jiang, D. Yin, M. Fan, Y. Wang, R. Wang, S. Song and Q. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 49434–49441 CrossRef CAS PubMed.
- J. Ma, A. Chinnam, G. Cheng, H. Yang, J. Zhang and J. Shreeve, Angew. Chem., Int. Ed., 2021, 1, 5497–5504 CrossRef PubMed.
- J. Meng, T. Fei, J. Cai, Q. Lai, J. Zhang, S. Pang and C. He, Mater. Chem. Front., 2023, 7, 5891 RSC.
- C. Li, C. Deng, B. Zhao, M. Wang, M. Zhang and Z. Zhou, Propellants Explos. Pyrotech., 2020, 45, 531–535 CrossRef CAS.
- T. Yan, H. Yang, C. Yang, Z. Yi, S. Zhu and G. Cheng, J. Mater. Chem. A, 2020, 8, 23857 RSC.
- C. Li, T. Zhu, C. Wang, L. Chen, C. Lei, J. Tang, H. Yang, C. Xiao and G. Cheng, J. Mater. Chem. A, 2024, 12, 24188 RSC.
- X. Jiang, D. Yin, S. Song, Y. Wang, M. Fan, R. Wang and Q. Zhang, J. Mater. Chem. A, 2024, 12, 13231 RSC.
- W. Li, Y. Wang, X. Qi, S. Song, K. Wang, Y. Jin, T. Liu and Q. Zhang, Chin. J. Energ. Mater., 2018, 26, 901–909 CAS.
- H. Xia, W. Zhang, Y. Jin, S. Song, K. Wang and Q. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 45914–45921 CrossRef CAS PubMed.
- Z. Cheng, Z. Zhang, Q. Ma, L. Yang, H. Yang, G. Cheng, G. Fan and W. Yang, Chem. Eng. J., 2022, 436, 131990 CrossRef CAS.
- Y. Tang, C. He, G. Imler, D. Parrishb and J. Shreeve, Chem. Commun., 2018, 54, 10566–10569 RSC.
- S. Chen, L. Li, S. Song and Q. Zhang, Cryst. Growth Des., 2023, 23, 4970–4978 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |