Multifaceted reactions of difluorocarbene with amines: synthesis and mechanistic insights
Jinghan
Bu†
,
Xuanwen
Tao†
,
Mengyi
Huang
,
Zixin
Chen
,
Tao
Zhao
and
Qiang
Yang
 *
*
Key Laboratory of General Chemistry of the National Ethnic Affairs Commission, College of Chemistry and Environment, Southwest Minzu University, Chengdu 610041, China. E-mail: yq09@swun.edu.cn
First published on 5th November 2025
Abstract
Difluorocarbene (:CF2), a highly reactive fluorine-containing intermediate, has emerged as a central research subject in the interdisciplinary domain of modern fluorine chemistry and synthetic methodology, owing to its unique electronic structure and diverse reactivity. The application scope of this intermediate as a versatile “carbon source” is not limited to conventional fluorination reactions but also includes non-traditional transformations. In organic synthesis, difluorocarbene exhibits a remarkable diversity of reactivity with amines. For instance, primary amines undergo nucleophilic attack to form fluoroimine and isocyanide intermediates, which can be further converted into fluorinated heterocyclic compounds, other heterocycles, and nitriles. Secondary amines, in turn, follow nucleophilic substitution pathways to achieve nitrogen-centered difluoromethylation or formylation. Tertiary amines, on the other hand, evolve into ammonium ylides through N-difluoromethylation, subsequently triggering cascade reactions, including cyclization, fluorination, C–N bond cleavage, and coupling processes. Herein, this review systematically summarizes the multifaceted reactivity of difluorocarbene with amines and highlights recent breakthroughs in this rapidly evolving field.
1. Introduction
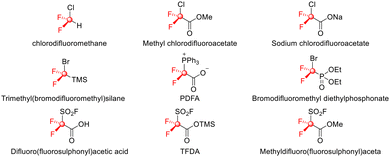
In the contemporary realm of chemical research, fluorine chemistry has emerged as a critical driving force for advancing the development of three major areas: organic synthesis, biomedicine, and functional materials.1–5 This prominence can be attributed to its unique electronic effects imparted by fluorine. In the domain of medicinal chemistry, the precise introduction of fluorine atoms has been shown to significantly optimize drug performance.6–10 Difluorocarbene (:CF2) is a vital fluorinated intermediate whose development has been driven by the increasing demand for fluorinated molecules, and as a result, it has garnered considerable attention from chemists and pharmacologists.Difluorocarbene, a pivotal reactive intermediate in organic synthesis and organofluorine chemistry, was first studied as early as 1947.11 However, it was not until 1960 that the Haszeldine research team first conceptualized difluorocarbene,12 a singlet carbene that features a vacant p orbital. From the perspective of molecular orbital theory, difluorocarbene displays unique dual reactivity. The strong electronegativity of fluorine atoms significantly reduces the electron density at the carbene center through inductive effects, endowing it with characteristic electrophilic reactivity (electrophilicity-dominant mechanism).13–15 Concurrently, the conjugation of lone electron pairs from fluorine atoms with the p orbital of carbene partially alleviates the electron-deficient state at the carbon center, enabling a nucleophilic character under specific conditions (potential nucleophilic pathway).16,17 In recent years, advances in difluorocarbene reagent18–23 research have not only expanded the substrate scope of classic reagents but have also led to the development of novel difluorocarbene-based reagents for difluoromethylation, difluorocyclization, and applications as C1 synthons (Scheme 1).24–34
2. Reactions of difluorocarbene with amines
The pronounced electron-deficient nature of the difluorocarbene center enables it to efficiently trap nucleophiles and form stable chemical bonds through electron transfer or conjugation. Among diverse nucleophilic reagents, amines have emerged as privileged building blocks for functional molecular architectures, owing to their electron-rich nitrogen centers and precisely tunable steric/electronic profiles. In the context of recent advances in green chemistry and precision synthesis, difluorocarbene-mediated amine transformations have achieved significant breakthroughs. Considering the structural diversity of amine compounds and their synergistic interaction modes with difluorocarbene, this review systematically categorizes them into three distinct classes and discusses their respective reaction characteristics and applications.2.1 Functionalization of primary amines mediated by difluorocarbene
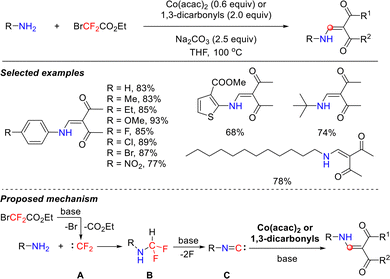
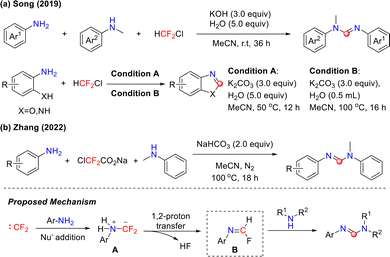
Previous studies on difluorocarbene focused mainly on its combination with nucleophiles, such as organometallic reagents, halide anions, and triphenylphosphine,35 while its interactions with primary amines were rarely reported. In 2018, the Song group pioneered a novel one-pot reaction system in which primary amines reacted with difluorocarbene to generate isocyanide intermediates in situ. These isocyanide intermediates subsequently underwent cross-coupling with 1,3-dicarbonyl compounds, leading to the efficient construction of β-aminoenones (Scheme 2).36 This strategy demonstrated remarkable substrate versatility. For aromatic primary amines, excellent yields were obtained regardless of whether electron-withdrawing or electron-donating groups were present at the para-position of the aryl ring. Furthermore, the reaction scope was successfully extended to various challenging substrates, including heterocyclic primary amines, sterically hindered alkyl primary amines, and long-chain aliphatic primary amines, all achieving favorable reaction outcomes.The reaction mechanism primarily involves the following key steps. First, ethyl bromodifluoroacetate undergoes a concerted debromination–decarboxylation under alkaline conditions, efficiently generating reactive difluorocarbene intermediate A. Subsequently, this difluorocarbene species reacts with primary amines to form metastable difluoromethylamine intermediate B. Owing to its thermodynamic instability under alkaline conditions, intermediate B rapidly undergoes elimination and defluorination, yielding highly reactive isocyanide intermediate C. This intermediate subsequently reacts with either Co(acac)2 or 1,3-dicarbonyl compounds, leading to the efficient construction of β-aminoenones.
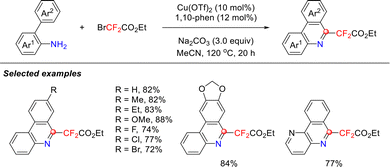
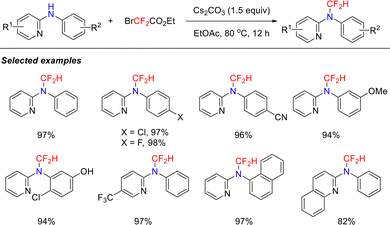
In 2018, building on the successful verification of the isocyanide intermediate generation mechanism, the Song group developed a copper-catalyzed C–H functionalization strategy using BrCF2CO2Et and aromatic primary amines to construct diverse fluorinated heterocyclic compounds (Scheme 3).37 This work represents the first demonstration of BrCF2CO2Et acting as both a C1 synthon and a difluoroalkylation reagent. The reaction demonstrated excellent compatibility with a range of substituents on the Ar2 ring, affording products in 63–88% yields. Notably, the system maintained satisfactory efficiency even when introducing pyridine heterocycles at the Ar1 position. DFT calculations elucidated the reaction mechanism, revealing that alkaline conditions significantly facilitated the formation of the critical isocyanide intermediate.
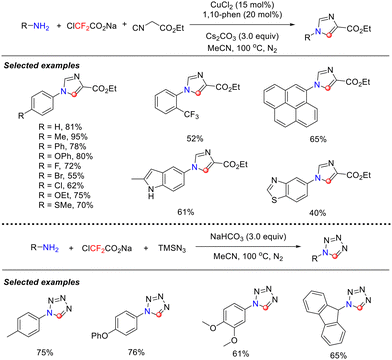
In 2020, building on their previous studies on the in situ generation of isocyanides from primary amines and difluorocarbene, the Song group achieved a significant breakthrough. They successfully developed a novel [3+1+1] cyclization strategy employing ClCF2CO2Na and primary amines (Scheme 4),38 enabling the efficient synthesis of imidazole and tetrazole derivatives. In this work, intermolecular cyclization between in situ-generated isocyanide intermediates and alkyl isocyanides was achieved for the first time through careful optimization of the reaction system. Moreover, structurally diverse tetrazole frameworks were constructed within the same reaction system using trimethylsilyl azide as a nitrogen source. The study demonstrated that this dual-path reaction system exhibited excellent compatibility with various substituted primary amines, showing broad functional group tolerance, encompassing aryl, alkyl, and heterocyclic derivatives.
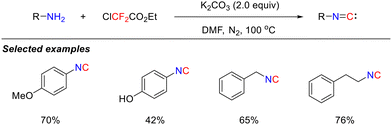
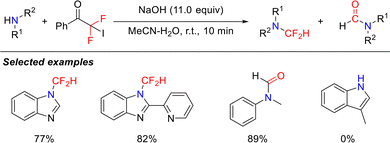
Although isocyanide intermediates participate in the aforementioned reactions, their inherent instability poses significant challenges in isolation. In 2020, the Zhang group addressed this by reporting an efficient and operationally convenient isocyanide synthesis strategy that utilizes primary amines and in situ-generated difluorocarbene, combining high efficiency with system safety (Scheme 5).39 This methodology is compatible with a range of simple primary amines and enables precise late-stage functionalization of the amine residues in complex molecules, such as bioactive compounds, amino acids, and polypeptides. This breakthrough not only significantly broadens the scope of isocyanide chemistry but also pioneers the use of difluorocarbene as a novel C1 synthon in organic synthesis, highlighting its substantial potential.
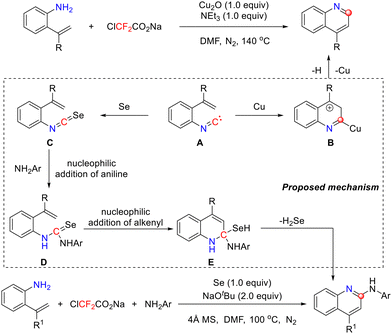
To further explore the application value of isocyanides, in 2022, the same group reported a method that harnesses the condensation of primary amines with difluorocarbene as a source of isocyanides. This approach achieved the modular construction of quinoline and C2-functionalized quinoline derivatives from o-alkenylanilines, demonstrating excellent substrate scope and chemoselectivity (Scheme 6).40 In the Cu2O-mediated system, the isocyanide intermediate undergoes α-regioselective addition with adjacent alkenyl groups, efficiently affording the quinoline framework. In the difluorocarbene precursor/selenium synergistic system, the reaction achieves pathway differentiation through the in situ generation of the selenoisocyanate intermediate. Nucleophilic attack (e.g., by anilines) on this intermediate competitively generates a selenourea species, which subsequently undergoes a cascade of alkenyl nucleophilic addition and β-hydroselenation elimination, ultimately enabling the precise synthesis of 2-aminoquinoline. This multicomponent one-pot synthesis strategy successfully establishes a universal platform for diversifying quinoline compounds at the C2 position using readily available starting materials.
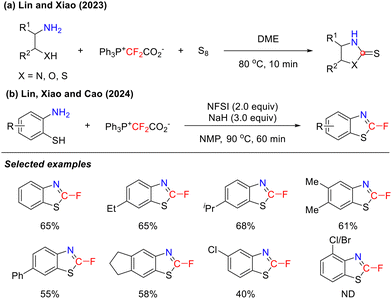
In 2023, the Lin and Xiao group reported that thiocarbonyl fluoride, generated in situ from PPh3+CF2CO2− and sulfur, could serve as a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S source to engage in the cyclization reactions of X–H (X = N, O, or S) substituted aliphatic amines, affording various five-membered heterocycles (Scheme 7(a)).41 However, when arylamines were employed as substrates, the undesirable difluoromethylation of either the N–H bond or the newly formed S–H bond proved difficult to suppress.
S source to engage in the cyclization reactions of X–H (X = N, O, or S) substituted aliphatic amines, affording various five-membered heterocycles (Scheme 7(a)).41 However, when arylamines were employed as substrates, the undesirable difluoromethylation of either the N–H bond or the newly formed S–H bond proved difficult to suppress.
Building on previous studies in heterocycle construction, the research group of Lin, Xiao and Cao successfully developed a novel cyclization strategy using difluorocarbene as the C–F source in the following year. This strategy efficiently afforded 2-fluorobenzothiazole through the cyclization of o-aminothiophenol (Scheme 7(b)).42 This reaction demonstrated excellent functional group compatibility, tolerating various substituents with different electronic properties. Notably, however, when the 3-position of o-aminothiophenol was substituted with Cl or Br, the target product did not form.
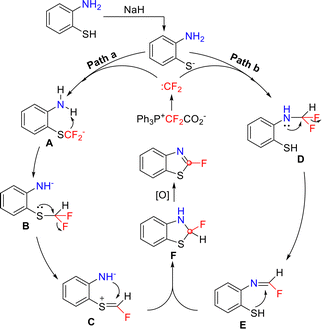
The reaction mechanism for the difluorocarbene-mediated synthesis of fluorinated benzothiazole involves two possible pathways (Scheme 8). Pathway a begins with the deprotonation of the thiol group, generating an ArS− anion. This anion undergoes a nucleophilic attack on the difluorocarbene released from Ph3P+CF2CO2−, affording key intermediate A. Owing to its strong basicity, intermediate A induces deprotonation of the adjacent amino group and is thereby converted into intermediate B. Subsequent electron transfer and concomitant elimination of the fluorine results in intermediate C. In contrast, pathway b begins with difluoromethylation of the amino group, first generating intermediate D. This intermediate undergoes electronic rearrangement and fluorine elimination to form intermediate E, which then undergoes intramolecular nucleophilic cyclization to produce cyclic intermediate F. Finally, oxidation efficiently converts intermediate F into 2-fluorobenzothiazole.
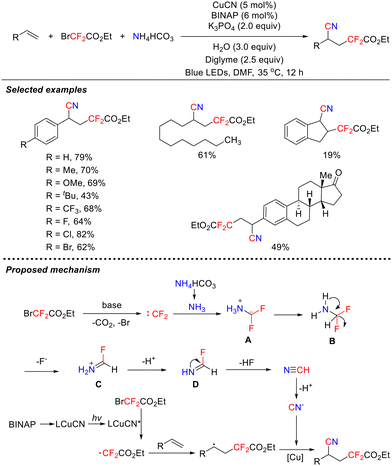
It is noteworthy that in 2021, the research group of Xiao and Jin reported a novel difluorocarbene-based cyanodifluoromethylation of alkenes via a copper catalyst under photocatalytic conditions (Scheme 9).43 This strategy employed BrCF2CO2Et and NH4HCO3 to serve as the carbon and nitrogen sources for the nitrile group, respectively, thereby circumventing the use of toxic nitrile reagents in conventional cyanidation reactions. A key highlight lies in the dual catalytic functions of the copper complex, which serves both as a photosensitizer to drive radical reactions and as a coupling catalyst to facilitate C–CN bond formation. The method demonstrated excellent substrate scope, was compatible with a wide range of structures, including aryl alkenes, alkyl alkenes, and estrone derivatives, and afforded good yields.
In contrast to the isocyanide-dependent pathway for primary amine conversion, this reaction proceeds via a unique difluorocarbene pathway. Mechanistic studies revealed that NH3, released in situ from NH4HCO3, captures electrophilic difluorocarbene to form intermediate A. Intermediate A subsequently undergoes hydrogen transfer, sequential defluorination, and proton dissociation, ultimately generating the critical CN− intermediate. Concurrently, BrCF2CO2Et generates a ˙CF2CO2Et radical under photocatalytic conditions. The radical subsequently couples with CN− mediated by copper catalysis, yielding the desired product.
2.2 Functionalization of secondary amines mediated by difluorocarbene
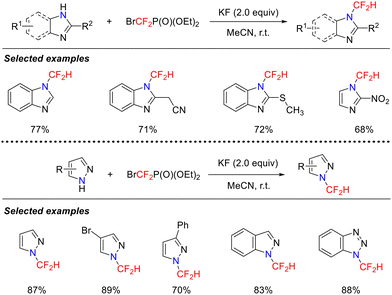
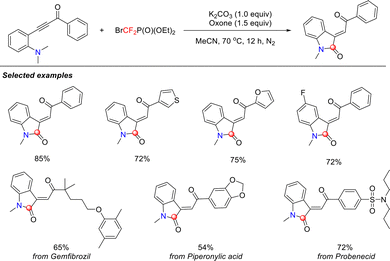
Secondary amines, as highly reactive nitrogen nucleophiles, exhibit unique advantages in the formation of C–N bonds. The amine anion (R2N−), generated by base-promoted deprotonation of the α-hydrogen adjacent to the nitrogen atom, exhibits remarkable nucleophilicity. This property enables it to serve as a key aminating agent in difluorocarbene chemistry for the synthesis of novel fluorine-containing compounds, thereby creating opportunities for the development of fluoro-bioisosteres in medicinal chemistry.In 2018, the He and Li group reported a novel methodology for N-difluoromethylation of imidazole/pyrazole compounds under mild conditions (Scheme 10).44 Using the commercially available and easily operable difluorocarbene precursor BrCF2P(O)(OEt)2, they successfully constructed a series of difluoromethylated heterocycles. This strategy offered a practical, economical, and environmentally friendly approach for the functionalization of pharmaceutically relevant molecules.
In light of the application potential of difluoromethyl frameworks, the Li group successfully developed a base-promoted formylation/N-difluoromethylation method for azaindoles in 2021 (Scheme 11).45 This innovative strategy efficiently constructed the important difluoromethyl 5-formylazaindole scaffold using ethyl difluorobromoacetate as a dual-functional reagent that served as both an aldehyde donor and a difluoromethylating agent. The transformation demonstrated excellent substrate scope and remarkable functional group tolerance. This methodology not only significantly enhanced the synthetic efficiency of IPE10 inhibitors but also pioneered a novel pathway for precision aldehyde functionalization of heterocyclic compounds.
To broaden the substrate scope of difluoromethylation for secondary amines, the Kwong group subsequently developed an efficient difluorocarbene-mediated N-difluoromethylation method for N-aryl-2-aminopyridines (Scheme 12).46 This approach operates under mild conditions without transition-metal catalysts or inert gas protection, thus offering an efficient and environmentally friendly strategy for the late-stage modification of pharmaceutical molecules.
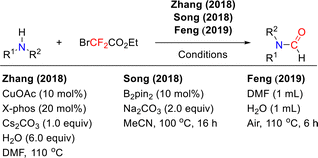
In the reaction system of difluorocarbene with secondary amines, the presence of a proton source typically serves as the key factor for the N-formylation reaction. The crucial role of the proton source in promoting N-formylation has been independently validated by several groups: the Zhang, Song, and Feng groups have each achieved the N-formylation of secondary amines, including both aromatic and aliphatic derivatives, under various reaction systems (Scheme 13).47–49
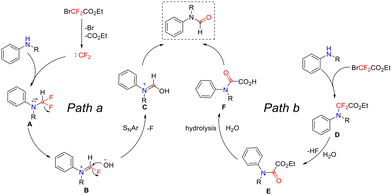
In the reactions discussed above, the Feng group proposed two possible pathways for the difluorocarbene-mediated N-formylation of secondary amines (Scheme 14).49 In path a, ethyl bromodifluoroacetate is first heated in a DMF/H2O mixed solvent, where it undergoes dehalogenation and decarboxylation, releasing a difluorocarbene species. This species then reacts with a secondary amine to form N-difluoromethylated intermediate A. Subsequently, intermediate A eliminates fluorine atoms via a lone-pair-electron-driven rearrangement at the nitrogen atom, yielding reactive intermediate B. This intermediate then undergoes nucleophilic attack by water, forming intermediate C, which finally isomerizes to the target product. Path b begins with the direct amination of ethyl bromodifluoroacetate by secondary amines, generating intermediate D. This intermediate then undergoes defluorination and hydrolysis to form oxoacetate E. Further hydrolysis of E yields oxoacetic acid F, which finally decarboxylates under heating to afford the N-formylation products.
In 2020, the Wang group reported a highly selective transformation of iododifluoroacetophenone with secondary amines under mild conditions (Scheme 15).50 The reaction proceeded within 10 minutes, demonstrating excellent chemoselectivity and substrate compatibility. Experimental results revealed that: (1) benzimidazole-derived secondary amines predominantly yielded N-CF2H derivatives; (2) aromatic secondary amines favored the formation of formamide products; (3) certain indole and alkyl secondary amines did not afford either product. Mechanistic analysis indicated that the thermodynamic stability of the products determined the product distribution. When the target products were insufficiently stable, the initially formed N-difluoromethylated intermediates underwent hydrolysis to N-formamide compounds or further degradation to the starting amines.
The aforementioned reaction system successfully achieved N-difluoromethylation and N-formylation through nucleophilic interactions of secondary amines and water with difluorocarbene. Notably, in the presence of two different amines, difluorocarbene acts as a molecular connector to enable amine tandem reactions, providing a novel strategy for constructing diverse N-containing compounds.
In 2019, the Song group innovatively employed Freon (ClCF2H) as a difluorocarbene precursor and utilized C1 synthons generated via quadruple cleavage to efficiently construct formimidamides and benzo[d]oxazoles, benzo[d]imidazole derivatives (Scheme 16(a)),51 a strategy that demonstrated excellent reaction efficiency and diversity. Subsequently, the Zhang group developed ClCF2CO2Na as an alternative difluorocarbene source and successfully synthesized bis(arylamine)-substituted formamidine compounds (Scheme 16(b)).52 Mechanistic studies revealed that both systems proceed via (E)-N-phenylformamidoyl fluoride intermediate B, deviating from the traditional isocyanide-mediated pathway.
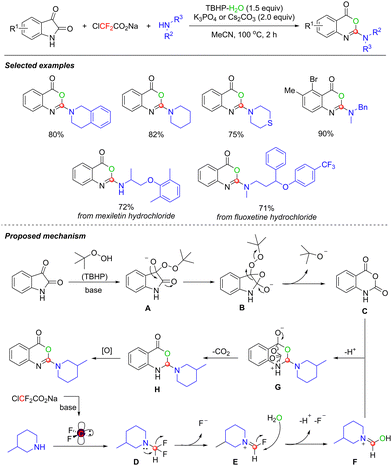
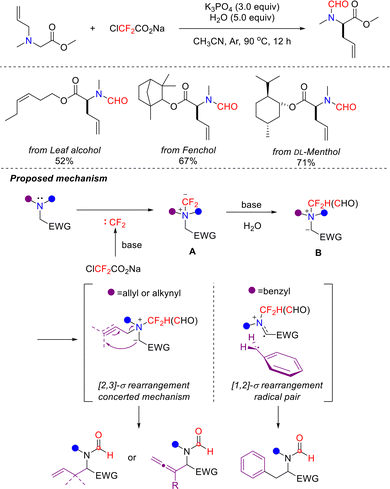
In 2023, building on the use of difluorinated reagents for multiple bond cleavages to release C1 synthons, the groups of Yin and Xu developed a tert-butyl hydroperoxide (TBHP)-promoted method for the efficient synthesis of 2-aminobenzoxazinone derivatives. This method uses ClCF2CO2Na as a difluorocarbene precursor (Scheme 17).53 It demonstrated excellent substrate scope, enabling the efficient transformation of six-membered aliphatic amines and various drug molecules, thereby providing a new pathway for drug modification.
Through a series of mechanistic experiments, the authors proposed a plausible reaction pathway. TBHP first converts isatin into intermediate isatoic anhydride Cvia Baeyer–Villiger oxidation. Simultaneously, difluorocarbene, released from ClCF2CO2Na under alkaline conditions, reacts with piperidine to form intermediate D, which subsequently undergoes defluorination to afford intermediate E. Intermediate E then undergoes nucleophilic addition with H2O to form hydroxyimine intermediate F. Intermediate C then intermolecularly cyclizes with F to generate intermediate G, which transforms into intermediate Hvia decarboxylation. Finally, intermediate H undergoes oxidative dehydrogenation to afford the target product.
Current research on the direct oxidation of difluorocarbene and its conversion into other valuable fluorinated compounds remains insufficient. In 2023, the group of Feng and Wang first used pyridine N-oxide as an oxidizing agent to convert difluorocarbene into difluorophosgene (COF2). The in situ-generated COF2 was then successfully trapped by amines to prepare carbamoyl fluorides (Scheme 18).54 This method offers two major advantages. First, it circumvents the direct handling of toxic gaseous COF2. Second, the mild reaction conditions ensure excellent compatibility with a broad substrate scope. Additionally, the quinoline byproduct generated in this process can be recycled and reused, thereby enhancing the atom economy.
2.3 Functionalization of tertiary amines mediated by difluorocarbene
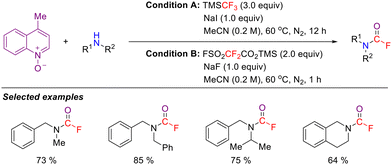
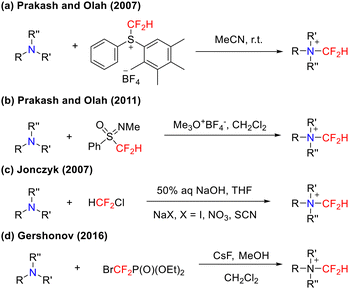
Compared with primary and secondary amines, the nitrogen atom of a tertiary amine possesses enhanced nucleophilicity. Upon reaction with difluorocarbene, tertiary amines rapidly form a difluoromethylammonium ylide intermediate (R3N+–CF2−). This intermediate undergoes polarization-induced C–N bond cleavage through intramolecular charge migration, ultimately enabling the functionalization of the amine compound. This unique reaction pathway offers a novel strategy for the transformation of tertiary amines.As early as 2007, Prakash, Olah, and colleagues first reported the difluoromethylation of tertiary amines using electrophilic S-(difluoromethyl)diaryl sulfonium tetrafluoroborate (Scheme 19(a)).55 Subsequently, the same research group further developed N,N-dimethyl-S-difluoromethyl-S-phenylsulfoximinium tetrafluoroborate as an efficient electrophilic difluoromethylating reagent to synthesize difluoromethyltrialkylammonium salts via an SN2 pathway (Scheme 19(b)).56 In addition, Jonczyk and Gershonov independently reported methods employing HCF2Cl and BrCF2P(O)(OEt)2 as difluoromethylating reagents. Under basic conditions, these reagents generate a difluorocarbene intermediate in situ, which then reacts with tertiary amines to form difluoromethylammonium ylides; subsequent protonation affords the corresponding N-difluoromethylammonium salts in good to excellent yields (Scheme 19(c) and (d)).57,58
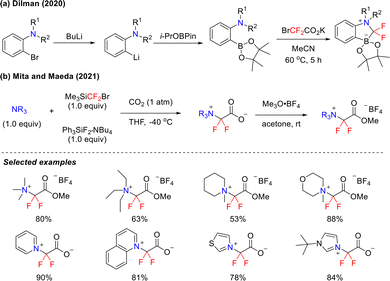
In the nascent stages of research, the capture of difluorocarbene posed significant challenges. In 2020, Dilman and coworkers innovatively developed a bifunctional trapping agent based on ortho-amino aromatic boronate (Scheme 20(a)).59 This reagent efficiently stabilizes difluorocarbene through synergistic interaction between the electrophilic amino group and the nucleophilic boron center. The exceptional stability of the reagent is of particular note, providing a valuable strategy for chemical studies involving difluorocarbene.
As an electrophilic C1 synthon, carbon dioxide (CO2) can efficiently participate in the construction of various compounds. In 2021, Mita and Maeda developed an innovative three-component reaction using tertiary amines, difluorocarbene precursors, and CO2. This strategy successfully afforded a series of difluoroglycine derivatives (Scheme 20(b)).60 The reaction exhibits broad compatibility with various tertiary amines and heterocyclic derivatives, such as pyridine, quinoline, and imidazole, enabling the isolation of high-purity products without the need for column chromatography. Notably, this system not only achieves mild CO2 fixation but also enables the reversible release and capture of difluorocarbene under additive-free conditions at room temperature, offering a novel strategy for green synthesis.
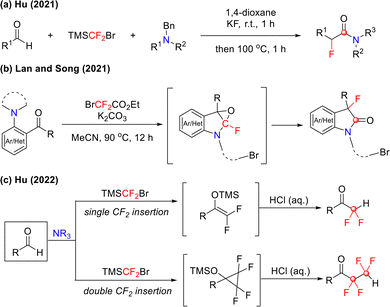
Leveraging the formation of N-difluoromethylammonium salts from tertiary amines and difluorocarbene, Hu and colleagues reported an innovative modular strategy for synthesizing α-fluoroamides in 2021 (Scheme 21(a)).61,62 This protocol used TMSCF2Br as an efficient difluorocarbene precursor, enabling the precise construction of structurally diverse α-fluoroamides via a three-component reaction with aldehydes and tertiary amines. This approach overcame key limitations of traditional routes, such as tedious procedures and low efficiency. Moreover, Lan and Song established a method using ethyl bromodifluoroacetate as the difluorocarbene precursor to couple tertiary amines with ketones, affording 3-fluorinated oxindoles (Scheme 21(b)).63
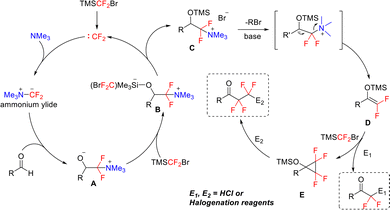
Building upon Hu's previously developed carbene reaction system, in the following year, the team further revealed the unique C–H insertion reactivity of TMSCF2Br reagent, accomplishing precise fluoroalkylation of aldehyde C–H bonds (Scheme 21(c)).64 This transition-metal-free method enables nearly complete selective insertion of mono- and bis-difluoromethylene groups into aldehyde C–H bonds, providing a distinctive approach for simplified access to structurally diverse ketones containing CF2 or CF2CF2 units. Notably, this methodology accomplished the first synthesis of relatively stable 2,2,3,3-tetrafluorocyclopropanol silyl ethers, establishing a new platform for exploring uncharted chemical spaces. The successful insertion of difluoromethylene into aldehyde C–H bonds hinges on the multifaceted role of TMSCF2Br, as illustrated in Scheme 22. The mechanism is initiated with the activation of bromodifluorotrimethylsilane by potassium fluoride, releasing difluorocarbene. This reactive intermediate reacts with tertiary amines to form a difluoromethylammonium ylide (R3N+–CF2−), which subsequently undergoes nucleophilic attack on the aldehyde to yield Intermediate A. Intermediate A then reacts with TMSCF2Br to generate unstable silicate intermediate B. Owing to its inherent instability, intermediate B readily eliminates a bromide ion and difluorocarbene to form intermediate C. Subsequent Hofmann elimination under basic conditions converts intermediate C into key intermediate D, which further reacts with another equivalent of TMSCF2Br to produce intermediate E. Final protonation or halogenation of intermediate D or E affords mono-inserted (CF2H-containing ketones) or bis-inserted (CF2CF2-containing ketones) alkylation products, respectively.
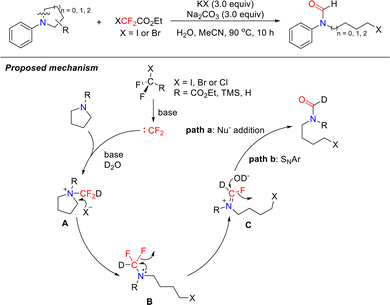
Beyond acyclic systems, tertiary amines are also widely present in heterocyclic scaffolds, such as pyrrolidine, piperidine, piperazine, and morpholine. These nitrogen heterocycles constitute core structural motifs in numerous antibiotics and antitumor agents. To overcome the limitations of traditional cyclic amine functionalization reactions, in 2020, the Song group reported a novel difluorocarbene-mediated cleavage and functionalization of unstrained C(sp3)–N bonds that proceeds without transition metals or oxidants (Scheme 23).65 This transformation enables concurrent carbon and nitrogen difunctionalization via precise C–N bond cleavage and recombination, providing direct access to a diverse array of formamide derivatives, including halogenated, deuterated, and thioether-functionalized products. Notably, this method can be further applied to acyclic systems.
From mechanistic studies, they proposed a plausible reaction pathway. First, the difluoro reagent releases highly reactive difluorocarbene under basic conditions. This carbene rapidly reacts with tertiary amine to form intermediate A. Subsequently, nucleophilic attack by either free halide anions (X−) or exogenous nucleophiles on the α-carbon of intermediate A results in C–N bond cleavage, forming intermediate B. Intermediate B then undergoes electronic rearrangement and defluorination to generate imine intermediate C. Finally, intermediate C undergoes formylation via either nucleophilic addition to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (path a) or nucleophilic aromatic substitution (path b). Key deuterium-labeling experiments confirmed that the hydrogen atom in the formyl group of the final product originates from water present in the system.
N bond (path a) or nucleophilic aromatic substitution (path b). Key deuterium-labeling experiments confirmed that the hydrogen atom in the formyl group of the final product originates from water present in the system.
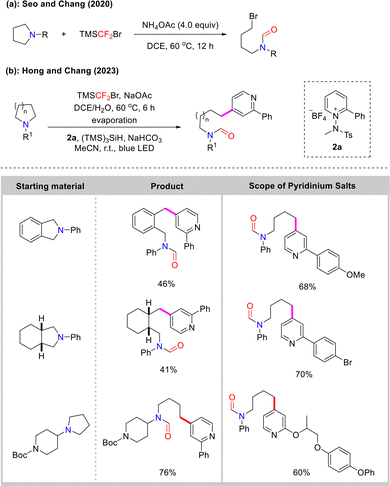
Concurrently, the group of Seo and Chang utilized difluorocarbene, generated from the key reagent TMSCF2Br, to achieve selective C–N bond cleavage in unstrained cyclic tertiary amines, affording bromoformamide products (Scheme 24(a))66 Subsequent functionalization enabled the construction of diverse acyclic frameworks, providing a novel strategy for the structural modification of natural products and pharmaceutical analogs.
In 2023, building on this work, the same group developed a novel one-pot strategy for the ring-opening pyridination of unstrained cyclic amines using visible-light-induced excitation of electron donor–acceptor (EDA) complexes generated from N-amidopyridinium salts. Notably, this method does not require a photocatalyst, enables highly selective C4 functionalization of pyridine, and exhibits broad compatibility with various cyclic amines and pyridine derivatives (Scheme 24(b)).67 Consequently, this method provides a modular and efficient route to C4-functionalized pyridines.
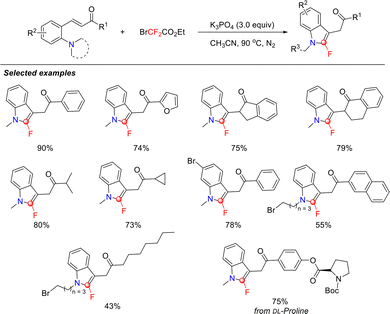
Previous work demonstrated that difluorocarbene can mediate C–N bond cleavage in tertiary amines. Inspired by this, researchers envisioned that integrating both a tertiary amine and a nucleophilic group into a single molecular scaffold would enable intramolecular C–N bond cleavage and coupling cyclization. Translating this concept into practice, in 2021, the Song group developed a metal-free cyclization of ortho-amino styrenes using halogenated difluoroalkylating reagents as difluorocarbene precursors, efficiently affording 2-fluoroindoles (Scheme 25).68,69 The mechanism involves dual-site capture of difluorocarbene, which concurrently constructs one C–N and one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in a single step while introducing a fluorine atom at the indole C2 position. This method displays high efficiency and excellent functional group tolerance, providing a novel strategy for the synthesis of fluorinated indoles.
C bond in a single step while introducing a fluorine atom at the indole C2 position. This method displays high efficiency and excellent functional group tolerance, providing a novel strategy for the synthesis of fluorinated indoles.
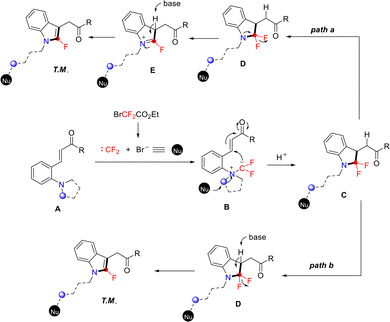
A possible mechanism was proposed, as shown in Scheme 26. Under basic conditions, the halodifluoroalkylating reagent generates a difluorocarbene in situ, which reacts with tertiary amine A to form difluoromethylammonium ylide B. Subsequently, the nucleophile attacks the α-carbon of ylide B, leading to cleavage of the C–N bond. Concurrently, the newly formed difluoromethyl carbanion attacks the intramolecular C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond via Michael addition, affording intermediate C. Intermediate C then converts to the 2-fluoroindole product via two distinct pathways. In path a, base-promoted, aromatization-driven rearrangement of intermediate C furnishes the product. In path b, C undergoes base-induced HF elimination via an E2cb mechanism to afford the final product.
C bond via Michael addition, affording intermediate C. Intermediate C then converts to the 2-fluoroindole product via two distinct pathways. In path a, base-promoted, aromatization-driven rearrangement of intermediate C furnishes the product. In path b, C undergoes base-induced HF elimination via an E2cb mechanism to afford the final product.
Based on their prior expertise in difluoroalkyl reactivity, the Song team subsequently developed a transition-metal-free [4+1] cyclization strategy. This approach employs commercially available and inexpensive difluorocarbene precursor BrCF2P(O)(OEt)2 and ortho-amino aryl alkynones as starting materials to efficiently construct 3-alkenyl-2-oxindole derivatives (Scheme 27).70 The strategy utilizes in situ-generated electrophilic difluorocarbene to mediate C(sp3)–N bond cleavage while simultaneously forging new C–C and C–N bonds. Notably, this methodology requires no transition-metal catalyst, and the difluorocarbene not only mediates C–N bond cleavage but also serves as the carbonyl carbon source.
The strategy of using in situ-generated difluorocarbene as a “scalpel” for the selective cleavage and subsequent functionalization of tertiary amine C–N bonds has witnessed significant progress. However, research aimed at harnessing the unique reactivity of difluorocarbene to induce dissociative rearrangement of quaternary ammonium salts remains limited. A comprehensive investigation into the mechanistic role and reactivity of difluorocarbene in such transformations promises to uncover new avenues for the precise modification of complex amine derivatives and the development of novel rearrangement reactions.
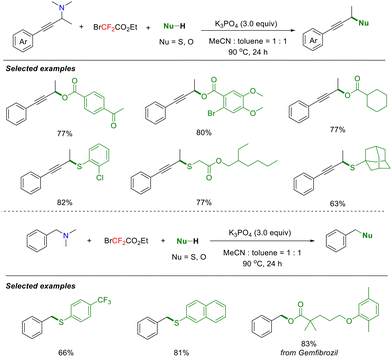
In 2022, the Song group reported a Suzuki–Miyaura-type cross-coupling reaction involving difluorocarbene, offering a new strategy for building C–C bonds (Scheme 28).71 Unlike traditional Suzuki–Miyaura coupling, this transformation requires no metal catalyst and proceeds under mild conditions. This innovative three-component reaction utilizes difluorocarbene to achieve efficient deaminative arylation of tertiary amines with arylboronic acids, thereby overcoming the substrate limitations of traditional coupling and significantly broadening the reaction scope. Based on detailed mechanistic experiments, the authors proposed a plausible mechanism. Initially, the difluoromethylated ammonium salt undergoes protonation or hydrolysis in the presence of H2O to generate intermediate B. Subsequently, intermediate B reacts with an electron-deficient arylboronic acid to form tetracoordinated boron species C, which undergoes 1,2-metallate rearrangement followed by amine dissociation to afford new boronic acid intermediate D. Finally, intermediate D undergoes protodeborylation to afford the final coupling products.
Building on their success with difluorocarbene-mediated Suzuki–Miyaura-type reactions, the Song group extended this strategy by replacing arylboronic acids with carboxylic acids and thiols as nucleophiles. Through mediated selective cleavage of aliphatic tertiary amine C(sp3)–N bonds, difluorocarbene enabled the efficient synthesis of structurally diverse esters and thioethers (Scheme 29).72 This strategy was further applied to the deaminative phosphorylation and sulfonylation of allylamines.73 These diverse transformations center on a key in situ-generated quaternary ammonium ylide intermediate, which undergoes nucleophilic attack to cleave the C(sp3)–N bond, affording differentiated coupling products. Notably, this transition-metal-free process features three key advantages: operational simplicity, broad substrate scope, and excellent functional group tolerance.
Capitalizing on the propensity of in situ-formed difluorocarbene–tertiary amine quaternary ammonium salts to serve as leaving groups, the He group recently developed a concise and efficient biomimetic route to 1,2-cis C-vinyl furanosides (Scheme 30).74 In this method, morpholine serves as a mimic for the lysine residue in C-glycoside synthases, converting unprotected cyclic aldoses into a linear iminium intermediate. This intermediate then undergoes a Petasis reaction with vinylboronic acid into a linear polyhydroxyamine that exhibits high 1,2-anti selectivity. Subsequently, difluorocarbene-induced deaminative cyclization of the tertiary amine efficiently forms the furanoside ring, demonstrating excellent chemoselectivity and stereoselectivity. This strategy exhibits a broad substrate scope, accommodating various allylboronic acids as well as natural mono- and disaccharides, thereby providing a new route for the synthesis of biologically active C-furanoside molecules.
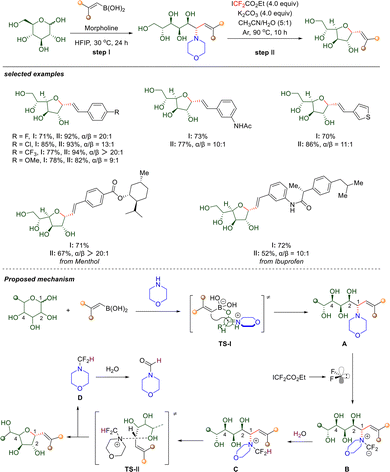 | ||
| Scheme 30 Difluorocarbene-mediated conversion of unprotected aldoses into 1,2-cis C-vinylfuranosides. | ||
Through a series of control experiments, the authors have proposed a plausible reaction mechanism. Initially, morpholine condenses with the carbonyl group of the aldose to form a linear imine intermediate. Concurrently, the C2 hydroxyl group of the aldose coordinates to the boronic acid, generating a borate ester complex. This complex then undergoes intramolecular vinyl migration via transition state TS-I, affording linear polyhydroxyamine intermediate A with high 1,2-anti selectivity. Subsequently, ICF2CO2Et hydrolyzes under basic conditions to release difluorocarbene, which then reacts with the nitrogen atom of the morpholine moiety to form ylide intermediate B. Upon protonation by water, intermediate B is converted into quaternary ammonium species C, thereby activating the C–N bond toward cleavage. In an SN2-type process, the C4 hydroxyl group acts as an intramolecular nucleophile, attacking the electrophilic carbon with inversion of configuration to form the 1,2-cis C-vinyl furanoside. Finally, hydrolysis of the resulting intermediate D releases N-formylmorpholine as a byproduct.
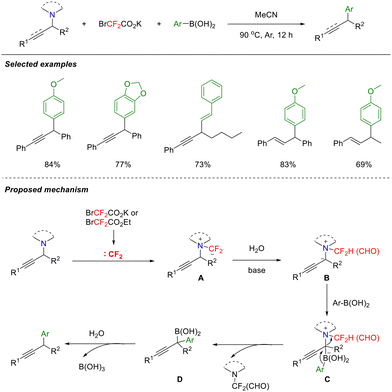
In 2024, to broaden the application of difluorocarbene in organic rearrangements, the Song group developed a novel approach. This method employs difluorocarbene to induce the in situ generation of difluoromethylammonium ylides, thereby overcoming the reliance of traditional Stevens rearrangements on strong bases, high temperatures, or transition-metal catalysts (Scheme 31).75 This strategy offers considerably milder conditions, requiring only a mild base (K3PO4) at 90 °C and maintains excellent compatibility with allyl-, benzyl-, and propargyl-substituted tertiary amines. Notably, the system enables switching between the [1,2]- and [2,3]-rearrangement pathways for benzyl and allyl/propargyl migrations, respectively. Furthermore, applying this method to tertiary amines derived from natural products (e.g., β-citronellol, geranylgeranyl, or menthol) provides direct access to diverse nitrogen-containing compounds, highlighting its utility in medicinal chemistry and natural product synthesis.
The proposed reaction mechanism can be delineated as follows. Under basic conditions, ClCF2CO2Na liberates a highly reactive difluorocarbene, which subsequently reacts with the nitrogen atom of the tertiary amine to form difluoromethyl quaternary ammonium salt A. The quaternary ammonium salt then generates difluoromethylammonium ylide Bvia proton transfer, facilitated by the synergistic action of base and water. This step avoids the high-energy barrier of a direct [1,3]-proton shift. Ammonium ylide B undergoes [1,2]-rearrangement (benzyl migration) to the corresponding product, which may proceed through either a radical pair or a concerted mechanism. The [2,3]-rearrangement, typically involving allyl or propargyl groups, is a concerted σ-migration process that is capable of generating olefin or diene products.
3. Conclusions and outlook
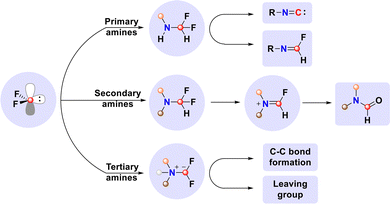
Owing to its unique electronic structure, difluorocarbene exhibits high reactivity toward nucleophiles, among which amines play a central role. Systematic studies reveal that, although primary, secondary, and tertiary amines all undergo N-difluoromethylation with difluorocarbene, their reaction pathways diverge significantly, based on structural features (Scheme 32). Reactions with primary amines afford diverse products, including fluoroimines and nitriles, as well as reactive isocyanide intermediates that enable further functionalization. For secondary amines, the initial N-difluoromethylated adduct undergoes defluorination to a fluoroiminium cation, which hydrolyzes to the formylated product. Notably, a bimolecular pathway involving cooperative capture of difluorocarbene by primary and secondary amines enables efficient construction of amidine frameworks. With tertiary amines, the reaction generates difluoromethylammonium ylide or cation intermediates that exhibit dual reactivity: they can participate in C–C bond formation or serve as leaving groups to trigger cascade processes, including C–N bond cleavage, fluorination, cross-coupling, and rearrangement. This diversity in transformation modes establishes difluorocarbene as a strategic carbon synthon and versatile platform in organic synthesis, opening up new avenues for constructing complex molecular architectures.Despite significant advances in difluorocarbene transformations with common amines, the field still faces three major challenges: (1) utilizing difluorocarbene and amines to construct fluorinated quaternary stereocenters or axially chiral molecules. (2) Exploring novel reactions of difluorocarbene with other nitrogen nucleophiles, such as amides, sulfonamides, and phosphinamides. (3) Developing 18F isotope labeling techniques for bioactive molecules based on reactions between difluorocarbene and amines. Addressing these challenges will expand the applications of the difluorocarbene platform, reveal new reaction types, and provide more innovative and practical synthetic frameworks.
Author contributions
J. Bu and X. Tao designed the review and drafted the manuscript; M. Huang, Z. Chen, and T. Zhao conducted the literature review and critical analysis; Q. Yang supervised the project and critically revised the manuscript. All authors discussed the intellectual content and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software, or code have been included, and no new data were generated or analysed as part of this review.Acknowledgements
We are grateful to the National Natural Science Foundation of China (no. 22001218), the Natural Science Foundation of Sichuan Province (2022NSFSC1259), and the Fundamental Research Funds for the Central Universities (Southwest Minzu University) (ZYN2025267) for their financial support.Notes and references
- R. Britton, V. Gouverneur, J.-H. Lin, M. Meanwell, C. Ni, G. Pupo, J.-C. Xiao and J. Hu, Nat. Rev. Methods Primers, 2021, 1, 47 CrossRef CAS
.
- Y. Ogawa, E. Tokunaga, O. Kobayashi, K. Hirai and N. Shibata, iScience, 2020, 23, 101467 CrossRef CAS PubMed
.
- J. M. Dyke, J. W. Emsley, V. K. Greenacre, W. Levason, F. M. Monzittu, G. Reid and G. De Luca, Inorg. Chem., 2020, 59, 4517–4526 CrossRef CAS
.
- A. Harsanyi and G. Sandford, Green Chem., 2015, 17, 2081–2086 RSC
.
- F. Babudri, G. M. Farinola, F. Naso and R. Ragni, Chem. Commun., 2007, 1003–1022 RSC
.
- C. M. Marshall, J. G. Federice, C. N. Bell, P. B. Cox and J. T. Njardarson, J. Med. Chem., 2024, 67, 11622–11655 CrossRef CAS
.
- E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed
.
- W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS
.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC
.
- K. Müller, C. Faeh and F. O. Diederich, Science, 2007, 317, 1881–1886 CrossRef
.
- J. D. Park, A. F. Benning, F. B. Downing, J. F. Laucius and R. C. McHarness, Ind. Eng. Chem., 1947, 39, 354–358 CrossRef CAS
.
- J. M. Birchall, G. W. Cross and R. N. Haszeldine, Proc. Chem. Soc. London, 1960, 81 CAS
.
- A. D. Dilman and V. V. Levin, Acc. Chem. Res., 2018, 51, 1272–1280 CrossRef CAS PubMed
.
- X. Ma, J. Su and Q. Song, Acc. Chem. Res., 2023, 56, 592–607 CrossRef CAS PubMed
.
- Q. Xie and J. Hu, Acc. Chem. Res., 2024, 57, 693–713 CrossRef CAS
.
- W. Zhou, W.-J. Pan, J. Chen, M. Zhang, J.-H. Lin, W. Cao and J.-C. Xiao, Chem. Commun., 2021, 57, 9316–9329 RSC
.
- Z. Feng, Y.-L. Xiao and X. Zhang, Acc. Chem. Res., 2018, 51, 2264–2278 CrossRef CAS
.
- G. A. Wheaton and D. J. Burton, J. Fluorine Chem., 1976, 8, 97–100 CrossRef CAS
.
- Q.-Y. Chen and S.-W. Wu, J. Org. Chem., 1989, 54, 3023–3027 CrossRef CAS
.
- F. Tian, V. Kruger, O. Bautista, J.-X. Duan, A.-R. Li, W. R. Dolbier Jr. and Q.-Y. Chen, Org. Lett., 2000, 2, 563–564 CrossRef CAS PubMed
.
- F. Wang, W. Zhang, J. Zhu, H. Li, K.-W. Huang and J.-B. Hu, Chem. Commun., 2011, 47, 2411–2413 RSC
.
- Y. Zafrani, G. Sod-Moriah and Y. Segall, Tetrahedron, 2009, 65, 5278–5283 CrossRef CAS
.
- J. Zheng, J.-H. Lin, J. Cai and J.-C. Xiao, Chem. – Eur. J., 2013, 19, 15261–15266 CrossRef CAS PubMed
.
- W. R. Dolbier Jr. and M. A. Battiste, Chem. Rev., 2003, 103, 1071–1098 CrossRef PubMed
.
- M. Fedorynski, Chem. Rev., 2003, 103, 1099–1132 CrossRef CAS PubMed
.
- J. B. I. Sap, C. F. Meyer, N. J. W. Straathof, N. Iwumene, C. W. am Ende, A. A. Trabanco and V. Gouverneur, Chem. Soc. Rev., 2021, 50, 8214–8247 RSC
.
- X. Ma and Q. Song, Chem. Soc. Rev., 2020, 49, 9197–9219 RSC
.
- D. Ge and X.-Q. Chu, Org. Chem. Front., 2022, 9, 2013–2055 RSC
.
- K. Fuchibe and J. Ichikawa, Chem. Commun., 2023, 59, 2532–2540 RSC
.
- H. Sheng, Z. Chen and Q. Song, J. Am. Chem. Soc., 2024, 146, 1722–1731 CrossRef CAS PubMed
.
- H. Sheng, Z. Chen, X. Li, J. Su and Q. Song, Org. Chem. Front., 2022, 9, 3000–3005 RSC
.
- C. Yu, F. Ke, J. Su, X. Ma, X. Li and Q. Song, Org. Lett., 2022, 24, 7861–7865 CrossRef CAS PubMed
.
- S. Wang, X. Li, S. Jin, K. Liu, C. Dong, J. Su and Q. Song, Org. Chem. Front., 2022, 9, 1282–1287 RSC
.
- Y. Wang, S. Mu, X. Li and Q. Song, Chin. Chem. Lett., 2022, 33, 1511–1514 CrossRef CAS
.
- M. J. Tozer and T. F. Herpin, Tetrahedron, 1996, 52, 8619–8683 CrossRef CAS
.
- X. Ma, Y. Zhou and Q. Song, Org. Lett., 2018, 20, 4777–4781 CrossRef CAS PubMed
.
- X. Ma, S. Mai, Y. Zhou, G.-J. Cheng and Q. Song, Chem. Commun., 2018, 54, 8960–8963 RSC
.
- Y. Wang, Y. Zhou and Q. Song, Chem. Commun., 2020, 56, 6106–6109 RSC
.
- Y.-X. Si, P.-F. Zhu and S.-L. Zhang, Org. Lett., 2020, 22, 9086–9090 CrossRef CAS
.
- B.-J. Jiang and S.-L. Zhang, Adv. Synth. Catal., 2022, 364, 2157–2162 CrossRef CAS
.
- J.-L. Hu, M.-X. Fu, J.-C. Xiao and J.-H. Lin, J. Fluorine Chem., 2023, 272, 110212 CrossRef CAS
.
- W.-J. Pan, J. Yu, W.-G. Cao, J.-C. Xiao and J.-H. Lin, Org. Chem. Front., 2024, 11, 120–126 RSC
.
- M. Zhang, J.-H. Lin, C.-M. Jin and J.-C. Xiao, Chem. Commun., 2021, 57, 2649–2652 RSC
.
- T. Mao, L. Zhao, Y. Huang, Y.-G. Lou, Q. Yao, X.-F. Li and C.-Y. He, Tetrahedron Lett., 2018, 59, 2752–2754 CrossRef CAS
.
- Y. Li, N. Sun, C.-L. Zhang and M. Hao, Chin. J. Chem., 2021, 39, 1477–1482 CrossRef CAS
.
- J. Duan, P. Y. Choy, K. B. Ganc and F. Y. Kwong, Org. Biomol. Chem., 2022, 20, 1883–1887 RSC
.
- X.-F. Li, X.-G. Zhang, F. Chen and X.-H. Zhang, J. Org. Chem., 2018, 83, 12815–12821 CrossRef CAS PubMed
.
- X. Ma, S. Deng and Q. Song, Org. Chem. Front., 2018, 5, 3505–3509 RSC
.
- Z.-W. Xu, W.-Y. Xu, X.-J. Pei, F. Tang and Y.-S. Feng, Tetrahedron Lett., 2019, 60, 1254–1258 CrossRef CAS
.
- X.-P. Chen, J. Han, Y.-J. Hu, Y.-F. Li, X.-C. Wang, J.-X. Ran, Z.-H. Wang and F.-H. Wu, Tetrahedron, 2021, 78, 131762 CrossRef CAS
.
- X. Ma, J. Su, X. Zhang and Q. Song, iScience, 2019, 19, 1–13 CrossRef PubMed
.
- Y.-X. Si, C.-C. Feng and S.-L. Zhang, J. Org. Chem., 2022, 87, 13564–13572 CrossRef CAS PubMed
.
- H. Li, Y. Wang, C. Xu, J. Zou, Y. Wu and G. Yin, Org. Chem. Front., 2023, 10, 1988–1993 RSC
.
- H.-J. Tang, X.-M. Shi, X.-Y. Zhu, C.-Q. Wang and C. Feng, Chin. J. Chem., 2023, 41, 2981–2987 CrossRef CAS
.
- G. K. S. Prakash, C. Weber, S. Chacko and G. A. Olah, Org. Lett., 2007, 9, 1863–1866 CrossRef CAS PubMed
.
- G. K. S. Prakash, Z. Zhang, F. Wang, C. Ni and G. A. Olah, J. Fluorine Chem., 2011, 132, 792–798 CrossRef CAS
.
- E. Nawrot and A. Jonczyk, J. Org. Chem., 2007, 72, 10258–10260 CrossRef CAS PubMed
.
- Y. Zafrani, D. Amir, L. Yehezkel, M. Madmon, S. Saphier, N. Karton-Lifshin and E. Gershonov, J. Org. Chem., 2016, 81, 9180–9187 CrossRef CAS
.
- E. A. Ilin, V. O. Smirnov, A. D. Volodin, A. A. Korlyukov and A. D. Dilman, Chem. Commun., 2020, 56, 7140–7142 RSC
.
- H. Hayashi, H. Takano, H. Katsuyama, Y. Harabuchi, S. Maeda and T. Mita, Chem. – Eur. J., 2021, 27, 10040–10047 CrossRef CAS PubMed
.
- A. Liu, C. Ni, Q. Xie and J. Hu, Angew. Chem., Int. Ed., 2022, 61, e202115467 CrossRef CAS PubMed
.
- A. Liu, S. Sun, Q. Xie, R. Huang, T. Kong, C. Ni and J. Hu, Org. Chem. Front., 2023, 10, 5343–5351 RSC
.
- G. Zhang, Q. Shi, M. Hou, K. Yang, S. Wang, S. Wang, W. Li, C. Li, J. Qiu, H. Xu, L. Zhou, C. Wang, S.-J. Li, Y. Lan and Q. Song, CCS Chem., 2022, 4, 1671–1679 CrossRef CAS
.
- A. Liu, C. Ni, Q. Xie and J. Hu, Angew. Chem., Int. Ed., 2023, 62, e202217088 CrossRef CAS
.
- J. Su, X. Ma, Z. Ou and Q. Song, ACS Cent. Sci., 2020, 6, 1819–1826 CrossRef CAS
.
- Y. Kim, J. Heo, D. Kim, S. Chang and S. Seo, Nat. Commun., 2020, 11, 4761 CrossRef CAS PubMed
.
- S. Hong, B. Kweon, W. Lee, S. Chang and S. Hong, Org. Lett., 2023, 25, 2722–2727 CrossRef CAS PubMed
.
- J. Su, X. Hu, H. Huang, Y. Guo and Q. Song, Nat. Commun., 2021, 12, 4986 CrossRef CAS PubMed
.
- H. Sheng, J. Su, X. Li, X. Li and Q. Song, Org. Lett., 2021, 23, 7781–7786 CrossRef CAS PubMed
.
- S. Chen, H. Huang, X. Li, X. Ma, J. Su and Q. Song, Org. Lett., 2023, 25, 1178–1182 CrossRef CAS PubMed
.
- J. Su, C. Li, X. Hu, Y. Guo and Q. Song, Angew. Chem., Int. Ed., 2022, 61, e202212740 CrossRef CAS PubMed
.
- C. Yu, F. Ke, X. Li, X. Li and Q. Song, Green Synth. Catal., 2024 DOI:10.1016/j.gresc.2024.04.009
.
- C. Li, Y. Guo, J. Su, X. Hu, Q. Xuan and Q. Song, Org. Chem. Front., 2024, 11, 3179–3185 RSC
.
- D. Sun, X. Dou, F. Wang, J. Wu, W. Wang and G. He, J. Am. Chem. Soc., 2025, 147, 38879–38888 CrossRef CAS
.
- J. Su, Y. Guo, C. Li and Q. Song, Nat. Commun., 2024, 15, 4794 CrossRef CAS PubMed
.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |